Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Fibrocell Science, Inc. | a14-21370_18k.htm |
| EX-99.2 - EX-99.2 - Fibrocell Science, Inc. | a14-21370_1ex99d2.htm |
Exhibit 99.1
|
|
Welcome to Fibrocell Science R&D Day September 24, 2014 |
|
|
Forward-Looking Statements This presentation contains, and our officers and representatives may from time to time make, statements that are “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Examples of forward-looking statements include, among others, statements we make regarding (i) our ability to develop breakthrough therapies for the treatment of skin and connective tissues diseases and (ii) our ability to successfully leverage our relationship with UCLA to expand our proprietary Personalized Biologics platform. These forward-looking statements rely on a number of assumptions concerning future events and are subject to a number of risks, uncertainties, and other factors, many of which are outside of Fibrocell Science’s control. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: (i) uncertainties relating to the initiation and completion of clinical trials; (ii) whether clinical trial results will validate and support the safety and efficacy of azficel-T; and (iii) our ability to establish additional strategic partnerships, as well as those set forth under the caption “Item 1A. Risk Factors” in Fibrocell Science’s most recent Form 10-K filing, as amended, as updated in “Item 1A. Risk Factors” in Fibrocell Science’s most recent Form 10-Q filing. Any forward-looking statement made by us in this presentation is based only on information currently available to us and speaks only as of the date on which it is made. In addition, Fibrocell Science operates in a highly competitive and rapidly changing environment, and new risks may arise. Accordingly, you should not place any reliance on forward-looking statements as a prediction of actual results. Fibrocell Science disclaims any intention to, and undertakes no obligation to, update or revise any forward-looking statement. You are also urged to carefully review and consider the various disclosures in Fibrocell Science’s most recent annual report on Form 10-K, as amended, our most recent Form 10-Q as well as other public filings with the SEC since the filing of Fibrocell Science’s most recent annual report. |
|
|
David Pernock Chairman and CEO Fibrocell Science |
|
|
Agenda 8:00 am Welcome & Introductions—David Pernock, Chairman & CEO, Fibrocell Science 8:10 am The Power of Fibroblasts—John Maslowski, Vice President, Scientific Affairs, Fibrocell Science 8:25 am Intrexon and Fibrocell Science Collaboration—Working Together to Treat RDEB—Suma Krishnan, Senior Vice President, Product Development, Intrexon Corporation 8:40 am Recessive Dystrophic Epidermolysis Bullosa—Alfred T. Lane, MD, MA, Stanford University School of Medicine 9:10 am Vocal Fold Scarring—Edward J. Damrose, MD, FACS, Stanford University School of Medicine 9:25 am Restrictive Burn Scarring—CDR (USN Ret) Nathan S. Uebelhoer, DO, FAAD, Naval Medical Center San Diego, The Aroostook Medical Center 9:40 am Genomic Stability—James Byrne, PhD, Assistant Professor, UCLA 9:55 am Q&A Session 10:25 am Closing |
|
|
The Power of Fibroblasts John Maslowski Vice President, Scientific Affairs Fibrocell Science |
|
|
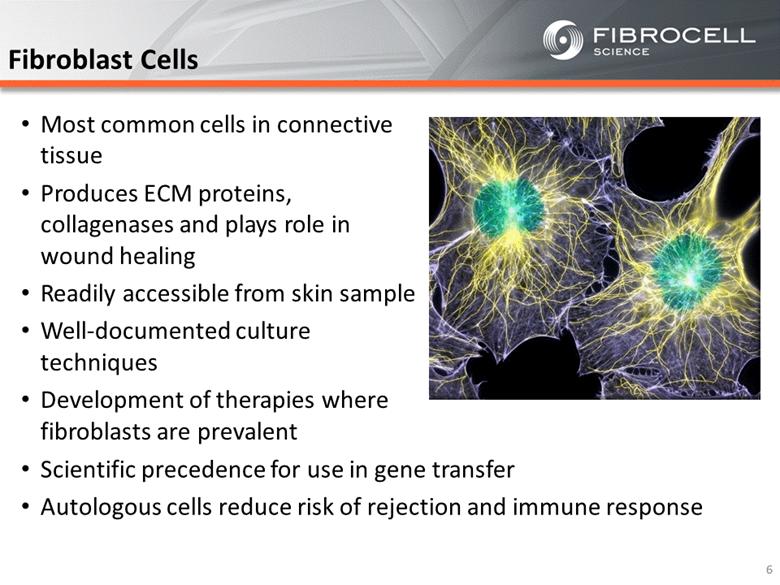
Most common cells in connective tissue Produces ECM proteins, collagenases and plays role in wound healing Readily accessible from skin sample Well-documented culture techniques Development of therapies where fibroblasts are prevalent Fibroblast Cells 6 Scientific precedence for use in gene transfer Autologous cells reduce risk of rejection and immune response |
|
|
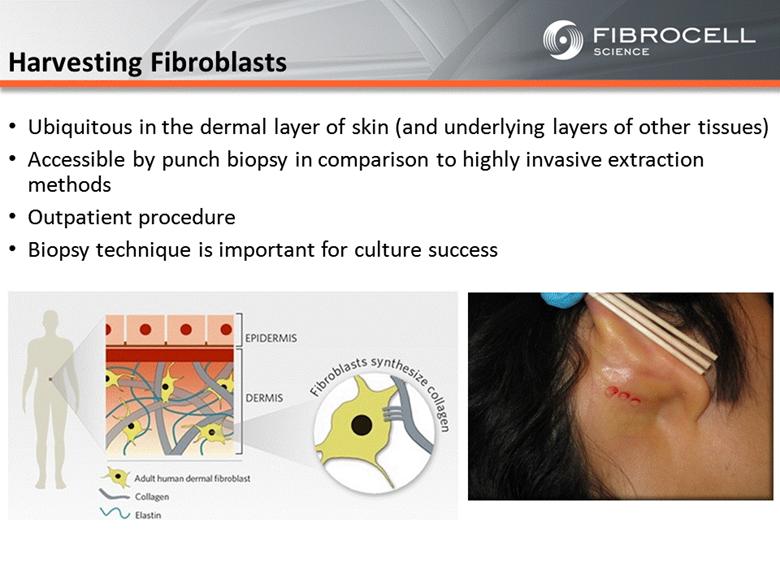
Harvesting Fibroblasts Ubiquitous in the dermal layer of skin (and underlying layers of other tissues) Accessible by punch biopsy in comparison to highly invasive extraction methods Outpatient procedure Biopsy technique is important for culture success |
|
|
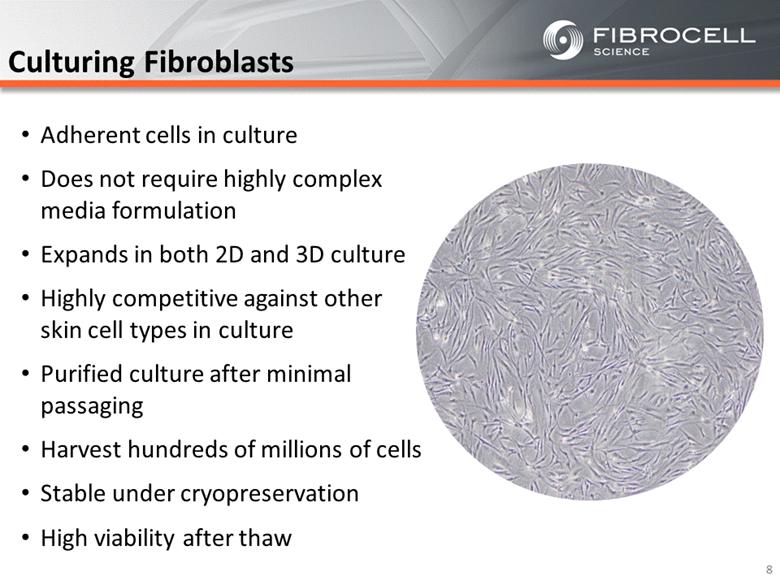
Culturing Fibroblasts Adherent cells in culture Does not require highly complex media formulation Expands in both 2D and 3D culture Highly competitive against other skin cell types in culture Purified culture after minimal passaging Harvest hundreds of millions of cells Stable under cryopreservation High viability after thaw 8 |
|
|
Meeting Regulatory Requirements Regulated as a biologic High bar for quality and control Strict tracking controls Extensive testing requirements for safety, viability, identity and potency Achieved BLA approval in June 2011 |
|
|
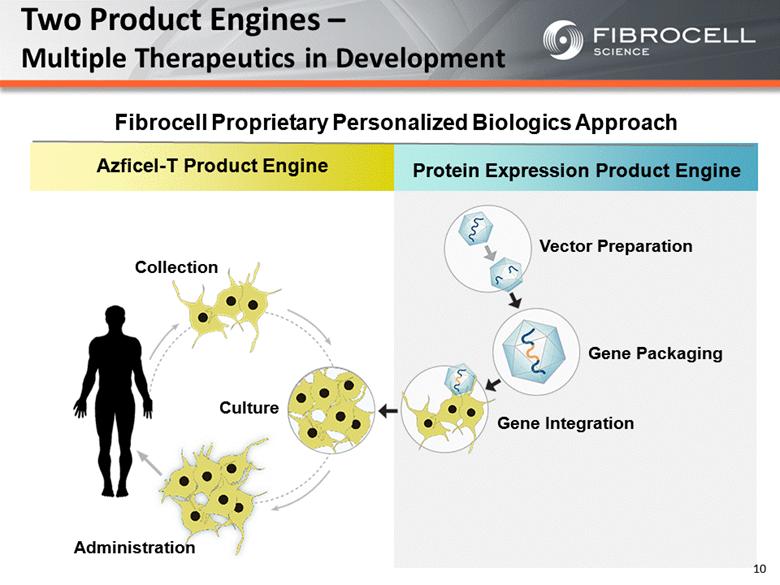
Protein Expression Product Engine Azficel-T Product Engine Two Product Engines – Multiple Therapeutics in Development Collection Culture Administration Vector Preparation Gene Packaging Gene Integration Fibrocell Proprietary Personalized Biologics Approach 10 |
|
|
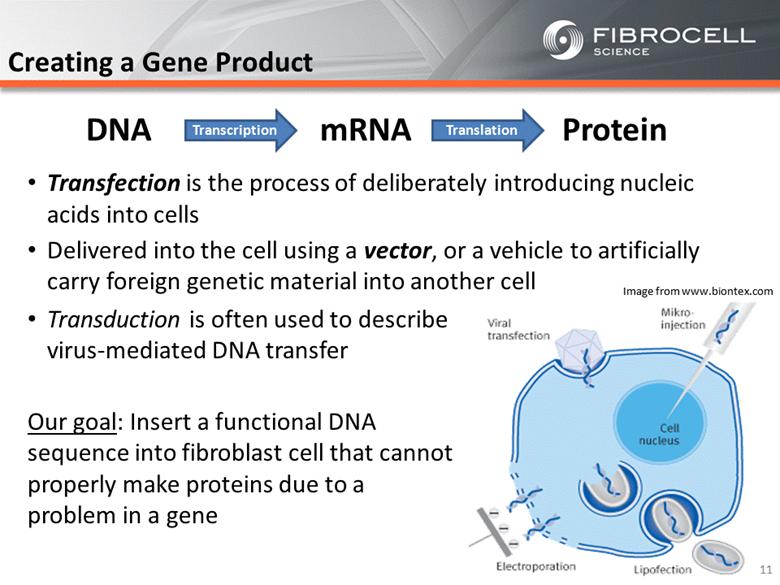
Creating a Gene Product Transfection is the process of deliberately introducing nucleic acids into cells Delivered into the cell using a vector, or a vehicle to artificially carry foreign genetic material into another cell Transduction is often used to describe virus-mediated DNA transfer Our goal: Insert a functional DNA sequence into fibroblast cell that cannot properly make proteins due to a problem in a gene Image from www.biontex.com 11 DNA mRNA Protein Transcription Translation |
|
|
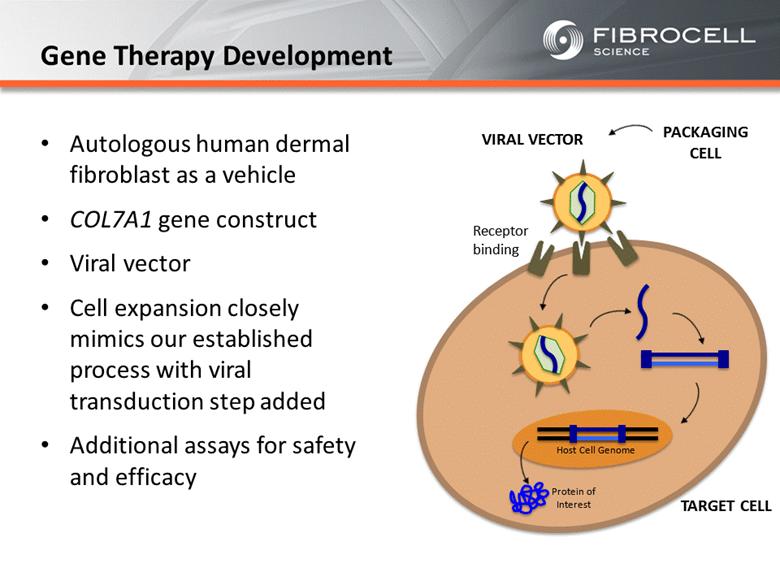
Receptor binding TARGET CELL VIRAL VECTOR Host Cell Genome Protein of Interest PACKAGING CELL Autologous human dermal fibroblast as a vehicle COL7A1 gene construct Viral vector Cell expansion closely mimics our established process with viral transduction step added Additional assays for safety and efficacy Gene Therapy Development |
|
|
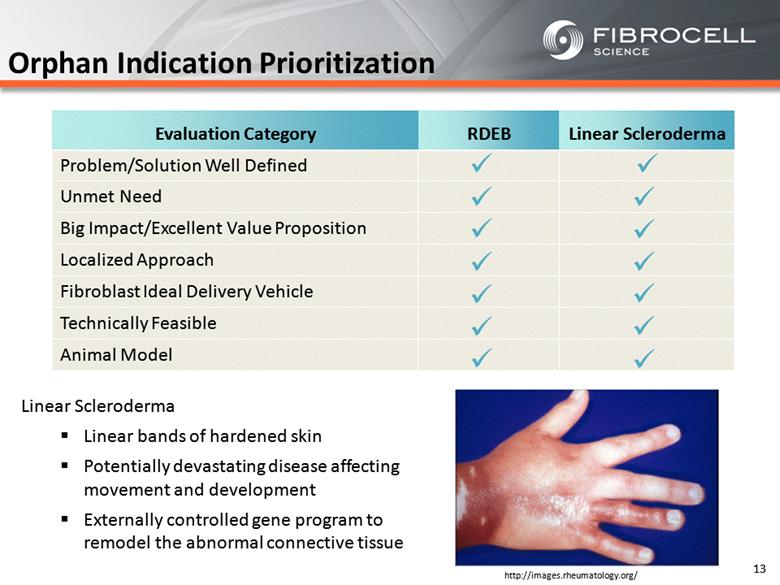
Orphan Indication Prioritization RDEB Linear Scleroderma Problem/Solution Well Defined Unmet Need Big Impact/Excellent Value Proposition Localized Approach Fibroblast Ideal Delivery Vehicle Technically Feasible Animal Model ü ü ü ü ü ü ü ü ü ü ü ü ü ü Evaluation Category RDEB Linear Scleroderma 13 http://images.rheumatology.org/ Linear Scleroderma Linear bands of hardened skin Potentially devastating disease affecting movement and development Externally controlled gene program to remodel the abnormal connective tissue |
|
|
Benefits of the Fibroblast Cell Platform Plays a significant role in the basic biology of skin and other connective tissues Allows for the targeting of biological solutions for conditions of the skin and other connective tissues Autologous nature reduces rejection and immunogenicity risks Local delivery minimizes systemic risks to surrounding organs Entry into orphan indications 14 |
|
|
15 Suma Krishnan Senior Vice President, Product Development Intrexon Corporation |
|
|
Intrexon and Fibrocell Collaboration: Working Together to Treat RDEB Suma Krishnan Sr. Vice President, Product Development Fibrocell Science R&D Day Yale Club, New York September 24, 2014 |
|
|
Forward-Looking Statements Special Note Regarding Forward-Looking Statements Some of the matters discussed in this presentation may constitute forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements relate to expectations, beliefs, projections, future plans and strategies, anticipated events or trends and similar expressions concerning matters that are not historical facts. The forward-looking statements are based on Intrexon’s beliefs, assumptions and expectations of its future performance, taking into account all information currently available to it. These beliefs, assumptions and expectations can change as a result of many possible events or factors, not all of which are known to Intrexon or are within its control. The following factors, among others, could cause actual results to vary from the forward-looking statements: These risks and uncertainties include, but are not limited to, (i) our ability to enter into new exclusive channel collaborations (ECCs) and joint ventures; (ii) developments concerning our existing ECCs; (iii) competition from existing technologies and products or new technologies and products that may emerge; (iv) our ability, and the ability of our collaborators, to protect our intellectual property and other proprietary rights and technologies; (v) the ability of our collaborators to secure any necessary regulatory approvals to commercialize any products developed under the ECCs; (vi) the rate and degree of market acceptance of any products developed by a collaborator under an ECC; and (vii) our expectations relating to AquaBounty and Trans Ova Genetics. Intrexon does not undertake any obligation to update any forward-looking statements to reflect circumstances or events that occur after the date on which such statements were made except as required by law. Additional information about factors affecting Intrexon is available in Intrexon’s Annual Report on Form 10-K for the year ended December 31, 2013, and Quarterly Report on Form 10-Q for the period ended June 30, 2014, and other filings with the SEC, which are available at www.sec.gov. © 2014 Intrexon Corp. All rights reserved. Intrexon Corporation is sharing the following materials for informational purposes only. Such materials do not constitute an offer to sell or the solicitation of an offer to buy any securities of Intrexon. Any offer and sale of Intrexon’s securities will be made, if at all, only upon the registration and qualification of such securities under all applicable federal and state securities laws or pursuant to an exemption from such requirements. The attached information has been prepared in good faith by Intrexon. However, Intrexon makes no representations or warranties as to the completeness or accuracy of any such information. Any representations or warranties as to Intrexon shall be limited exclusively to any agreements that may be entered into by Intrexon and to such representations and warranties as may arise under law upon distribution of any prospectus or similar offering document by Intrexon. |
|
|
Intrexon Technology Overview |
|
|
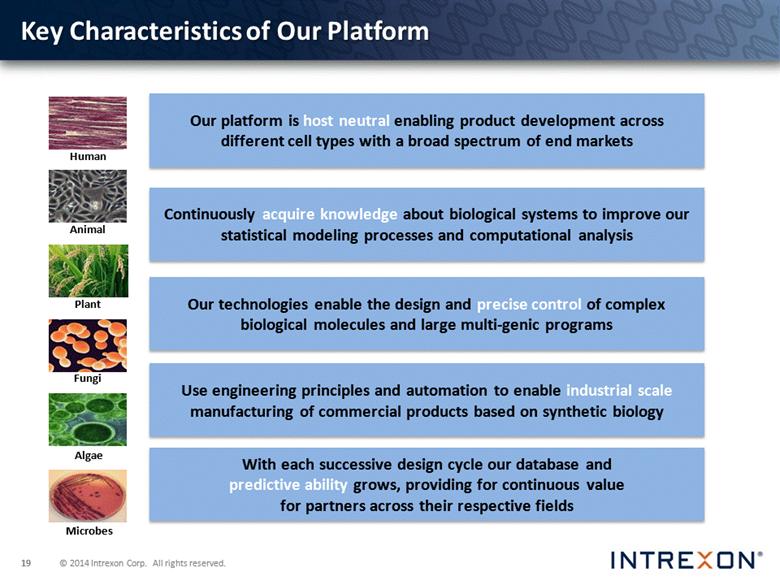
Human Animal Plant Our platform is host neutral enabling product development across different cell types with a broad spectrum of end markets Continuously acquire knowledge about biological systems to improve our statistical modeling processes and computational analysis Our technologies enable the design and precise control of complex biological molecules and large multi-genic programs Use engineering principles and automation to enable industrial scale manufacturing of commercial products based on synthetic biology With each successive design cycle our database and predictive ability grows, providing for continuous value for partners across their respective fields Human Animal Plant Fungi Algae Microbes Key Characteristics of Our Platform |
|
|
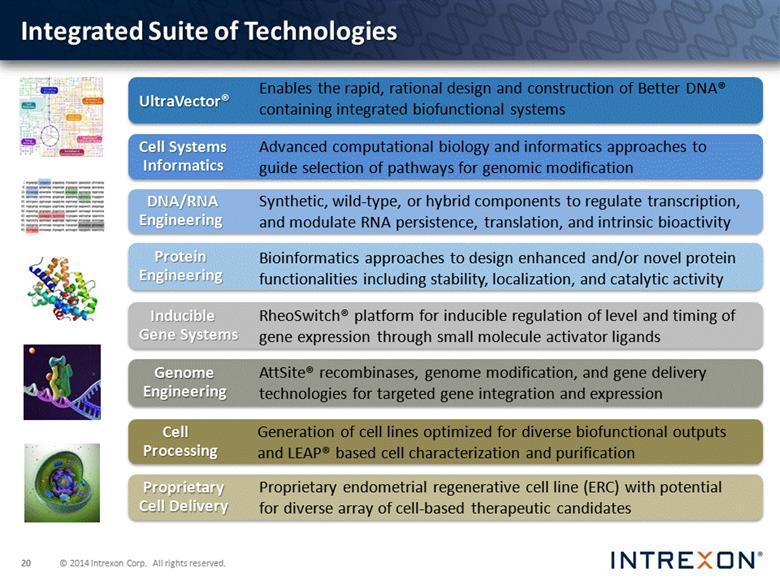
UltraVector® Integrated Suite of Technologies Cell Systems Informatics Enables the rapid, rational design and construction of Better DNA® containing integrated biofunctional systems Inducible Gene Systems Genome Engineering Cell Processing Proprietary Cell Delivery DNA/RNA Engineering Protein Engineering Advanced computational biology and informatics approaches to guide selection of pathways for genomic modification Synthetic, wild-type, or hybrid components to regulate transcription, and modulate RNA persistence, translation, and intrinsic bioactivity Bioinformatics approaches to design enhanced and/or novel protein functionalities including stability, localization, and catalytic activity RheoSwitch® platform for inducible regulation of level and timing of gene expression through small molecule activator ligands AttSite® recombinases, genome modification, and gene delivery technologies for targeted gene integration and expression Generation of cell lines optimized for diverse biofunctional outputs and LEAP® based cell characterization and purification Proprietary endometrial regenerative cell line (ERC) with potential for diverse array of cell-based therapeutic candidates |
|
|
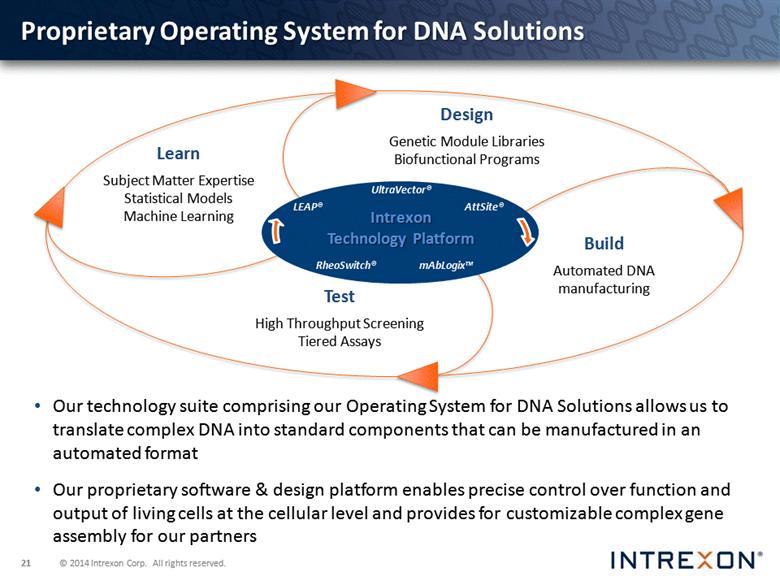
Proprietary Operating System for DNA Solutions Our technology suite comprising our Operating System for DNA Solutions allows us to translate complex DNA into standard components that can be manufactured in an automated format Our proprietary software & design platform enables precise control over function and output of living cells at the cellular level and provides for customizable complex gene assembly for our partners UltraVector® RheoSwitch® LEAP® AttSite® mAbLogix™ Intrexon Technology Platform |
|
|
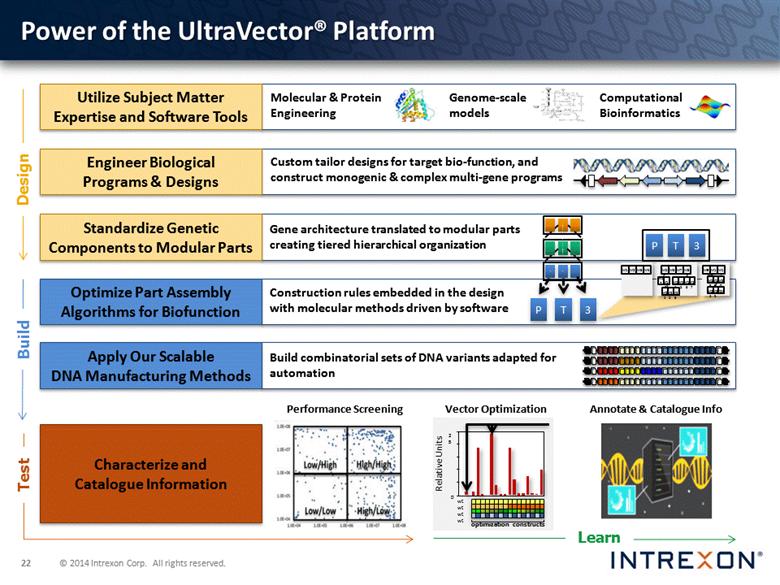
Standardize Genetic Components to Modular Parts Power of the UltraVector® Platform Utilize Subject Matter Expertise and Software Tools Engineer Biological Programs & Designs Optimize Part Assembly Algorithms for Biofunction Apply Our Scalable DNA Manufacturing Methods Characterize and Catalogue Information Molecular & Protein Engineering Computational Bioinformatics Genome-scale models Annotate & Catalogue Info Performance Screening Custom tailor designs for target bio-function, and construct monogenic & complex multi-gene programs Construction rules embedded in the design with molecular methods driven by software Build combinatorial sets of DNA variants adapted for automation Design Build Test Learn Gene architecture translated to modular parts creating tiered hierarchical organization P T M9 M10 M11 M5 M6 M7 M8 M1 M2 M3 M4 3 M12 M13 M21 M22 M23 M14 M15 M16 M17 M18 M19 M20 M24 M25 M26 P T 3 0 25 optimization constructs Vector Optimization Relative Units wt wt wt wt |
|
|
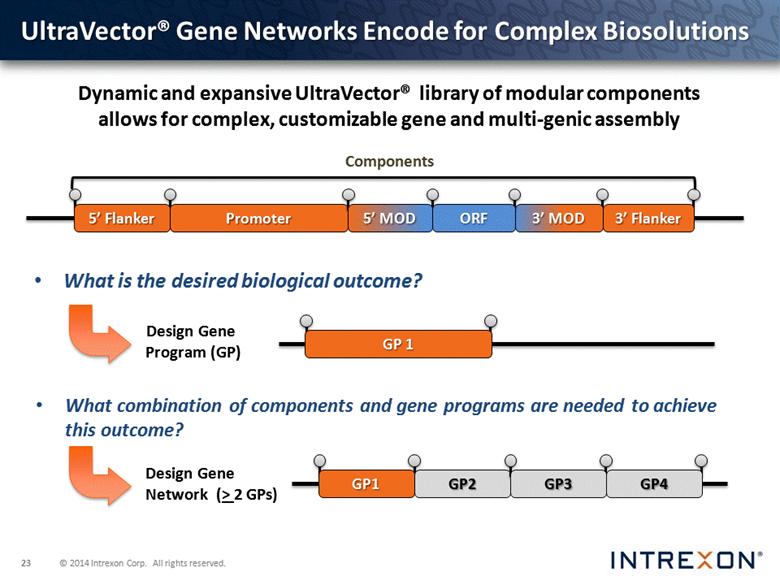
UltraVector® Gene Networks Encode for Complex Biosolutions Design Gene Network (> 2 GPs) ORF 5’ MOD What combination of components and gene programs are needed to achieve this outcome? What is the desired biological outcome? Components Dynamic and expansive UltraVector® library of modular components allows for complex, customizable gene and multi-genic assembly GP 1 Design Gene Program (GP) |
|
|
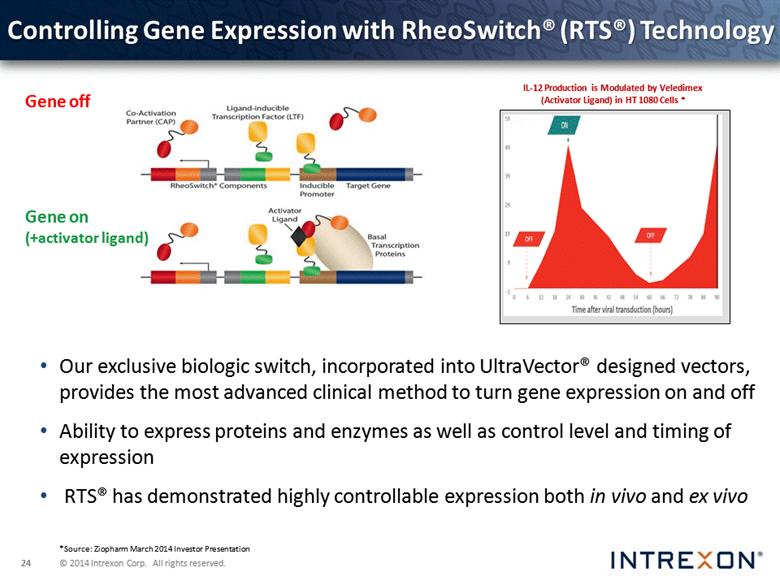
Controlling Gene Expression with RheoSwitch® (RTS®) Technology Our exclusive biologic switch, incorporated into UltraVector® designed vectors, provides the most advanced clinical method to turn gene expression on and off Ability to express proteins and enzymes as well as control level and timing of expression RTS® has demonstrated highly controllable expression both in vivo and ex vivo Gene off Gene on (+activator ligand) IL-12 Production is Modulated by Veledimex (Activator Ligand) in HT 1080 Cells * *Source: Ziopharm March 2014 Investor Presentation |
|
|
Overview of Recessive Dystrophic Epidermolysis Bullosa (RDEB) and Genetically Modified Human Dermal Fibroblasts (GM-HDF) |
|
|
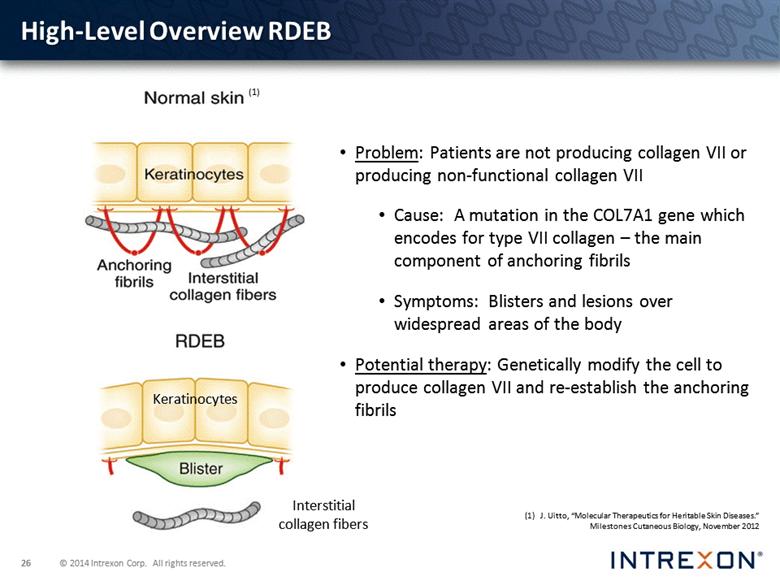
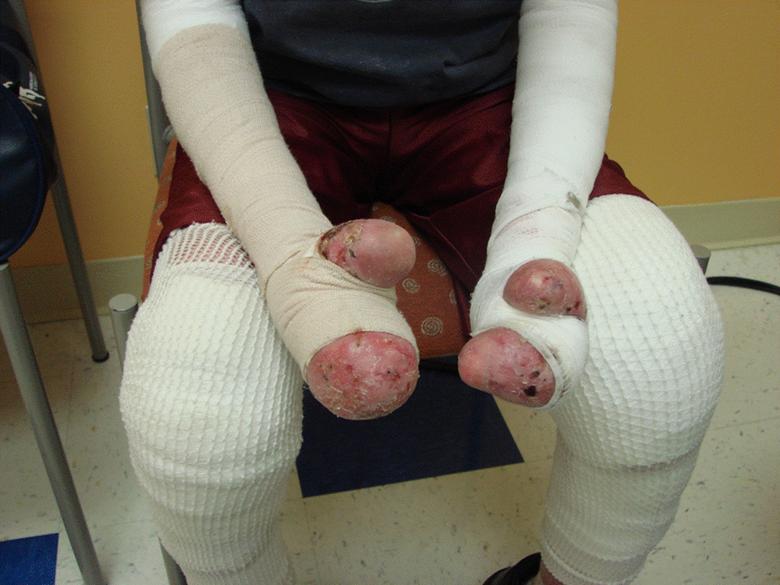
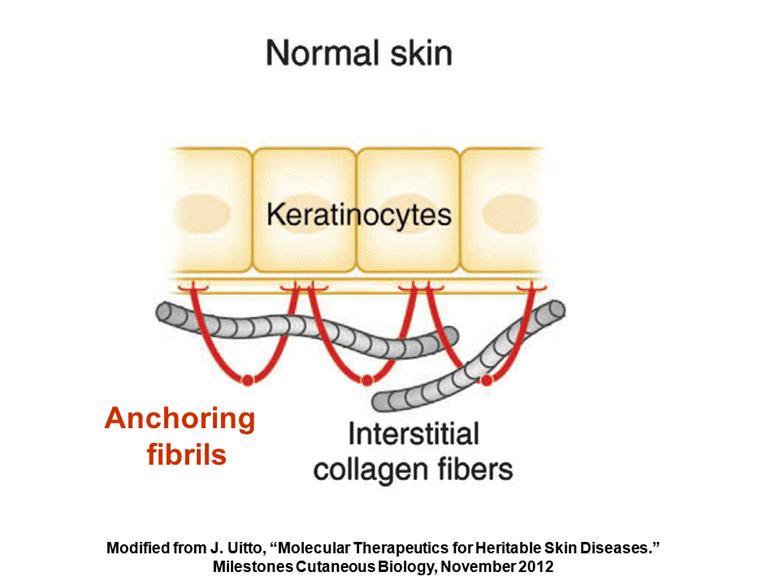
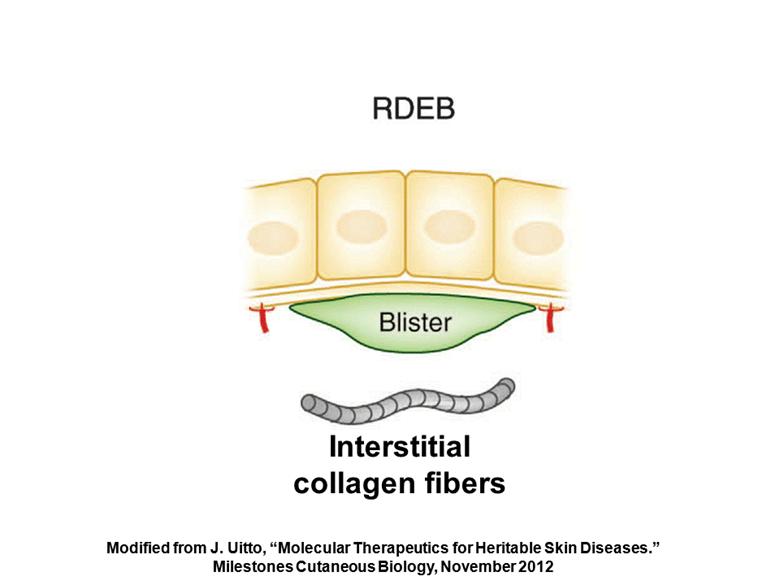
Problem: Patients are not producing collagen VII or producing non-functional collagen VII Cause: A mutation in the COL7A1 gene which encodes for type VII collagen – the main component of anchoring fibrils Symptoms: Blisters and lesions over widespread areas of the body Potential therapy: Genetically modify the cell to produce collagen VII and re-establish the anchoring fibrils Keratinocytes Interstitial collagen fibers (1) J. Uitto, “Molecular Therapeutics for Heritable Skin Diseases.” Milestones Cutaneous Biology, November 2012 (1) High-Level Overview RDEB |
|
|
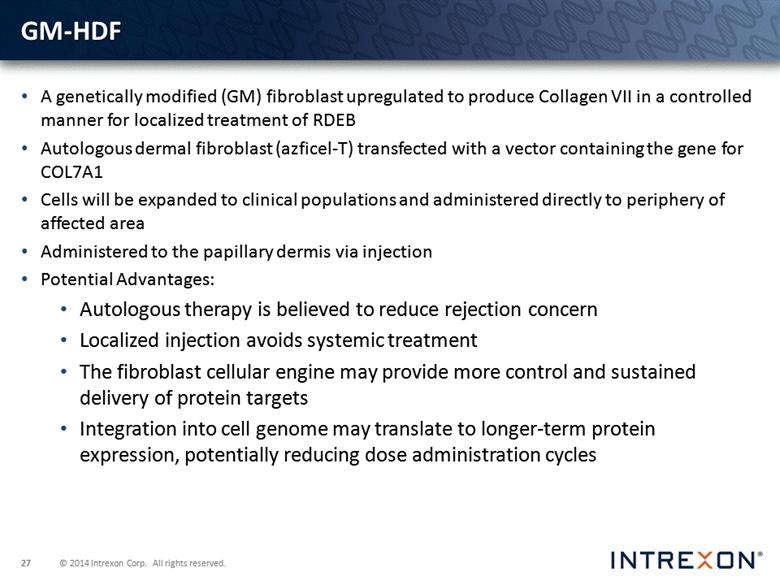
GM-HDF A genetically modified (GM) fibroblast upregulated to produce Collagen VII in a controlled manner for localized treatment of RDEB Autologous dermal fibroblast (azficel-T) transfected with a vector containing the gene for COL7A1 Cells will be expanded to clinical populations and administered directly to periphery of affected area Administered to the papillary dermis via injection Potential Advantages: Autologous therapy is believed to reduce rejection concern Localized injection avoids systemic treatment The fibroblast cellular engine may provide more control and sustained delivery of protein targets Integration into cell genome may translate to longer-term protein expression, potentially reducing dose administration cycles |
|
|
No clinical trials have been or are currently being conducted with genetically modified human dermal fibroblasts. 10 June 2014: Fibrocell received orphan drug designation from FDA. 4 August 2014: Fibrocell met with FDA for a Pre-IND meeting to discuss the development path for GM-HDF. Regulatory |
|
|
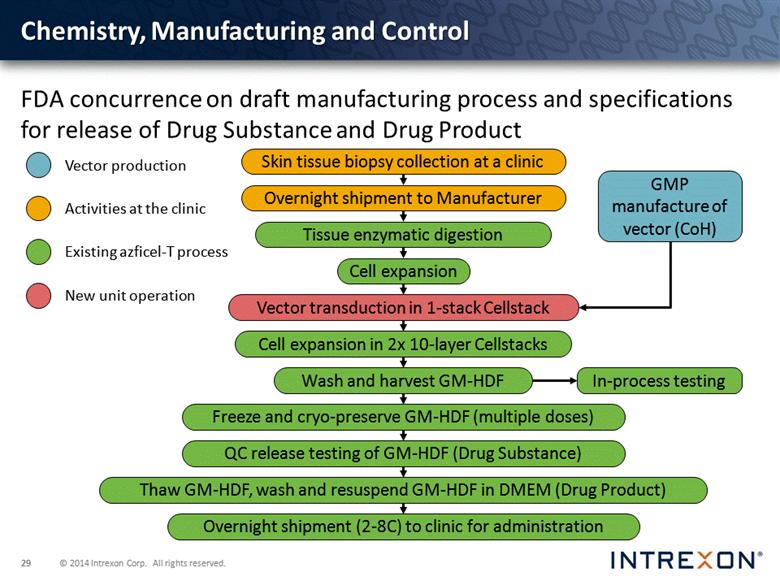
Chemistry, Manufacturing and Control FDA concurrence on draft manufacturing process and specifications for release of Drug Substance and Drug Product GMP manufacture of vector (CoH) In-process testing Skin tissue biopsy collection at a clinic Overnight shipment to Manufacturer Tissue enzymatic digestion Cell expansion Cell expansion in 2x 10-layer Cellstacks Wash and harvest GM-HDF Freeze and cryo-preserve GM-HDF (multiple doses) QC release testing of GM-HDF (Drug Substance) Overnight shipment (2-8C) to clinic for administration Thaw GM-HDF, wash and resuspend GM-HDF in DMEM (Drug Product) Vector transduction in 1-stack Cellstack |
|
|
Preclinical and Clinical FDA concurrence on preclinical development plan obtained during Pre-IND meeting Materials and cells are being prepared for the conduct of the studies at Stanford University Three evaluations to be conducted in two studies: Multiple doses of GM-HDF in an in vitro organ culture study. Multiple doses of GM-HDF in vivo on RDEB human skin, which would be grown in vitro prior to grafting. GM-HDF administered to normal human skin on the xenograft SCID mouse model Initial clinical protocol will be a small phase 1 (~5 subjects) in adults. Feedback received from FDA is being incorporated. |
|
|
Alfred T. Lane MD, MA Professor of Dermatology and Pediatrics, Emeritus (Active) Stanford University School of Medicine |
|
|
Genetically Corrected Fibroblast Therapy for Dystrophic Epidermolysis Bullosa Alfred T. Lane MD, MA Professor of Dermatology and Pediatrics, Emeritus (Active) Stanford University School of Medicine |
|
|
DEB Dystrophic scarring Epidermolysis breaking apart of skin Bullosa means blister |
|
|
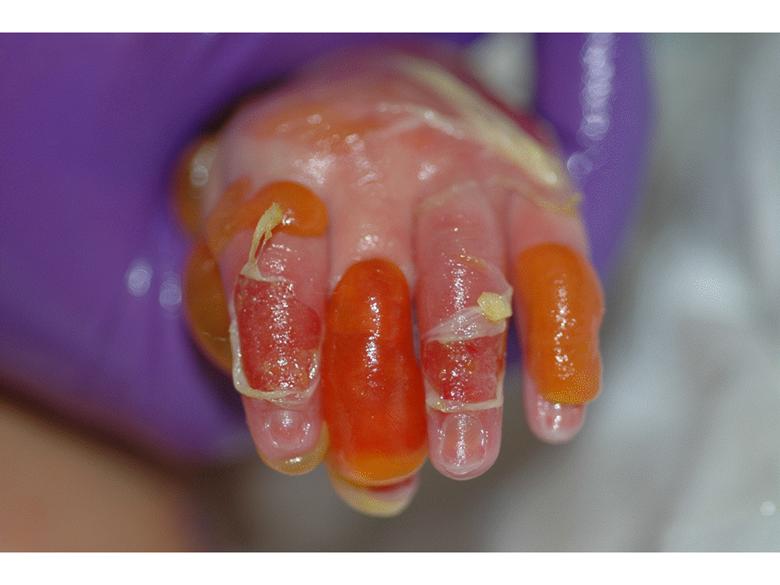
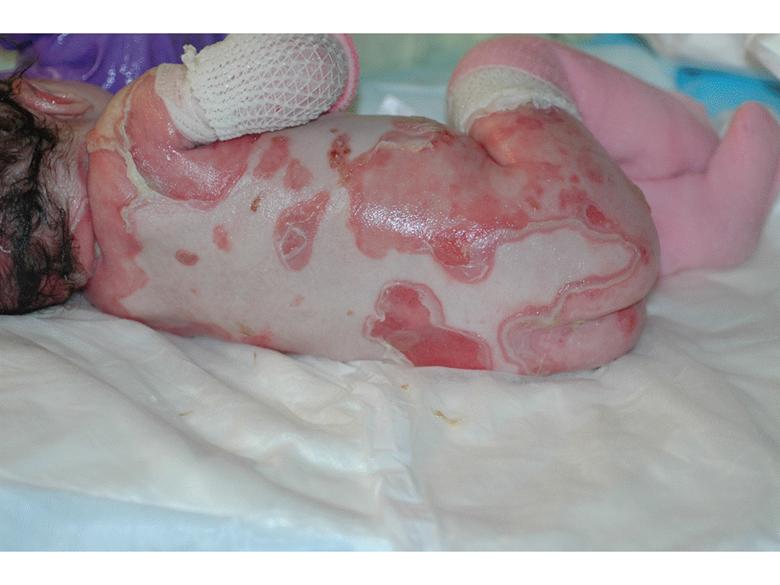
RDEB Blisters present soon after birth Diagnosis is made by absence of type VII collagen in the upper most dermis at junction of epidermis Many years of painful, expanding wounds that never heal Death from malnutrition, infection or squamous cell carcinoma (SCC) |
|
|
Estimated incidence of RDEB is 2-4 infants per million births USA: 4 million annual births leads to 8-16 RDEB infants a year Worldwide: 75 million annual births leads to 150-300 RDEB infants a year |
|
|
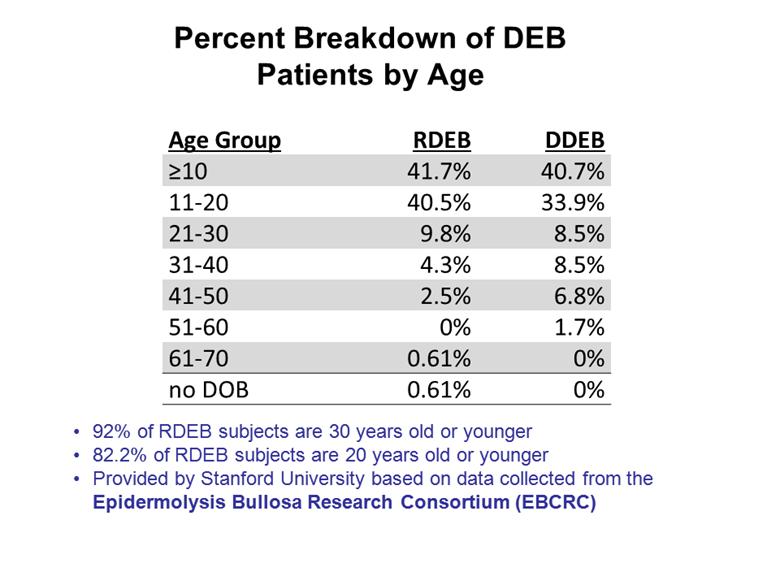
Percent Breakdown of DEB Patients by Age 2 Age Group RDEB DDEB >10 41.7% 40.7% 11-20 40.5% 33.9% 21-30 9.8% 8.5% 31-40 4.3% 8.5% 41-50 2.5% 6.8% 51-60 0% 1.7% 61-70 0.61% 0% no DOB 0.61% 0% 92% of RDEB subjects are 30 years old or younger 82.2% of RDEB subjects are 20 years old or younger Provided by Stanford University based on data collected from the Epidermolysis Bullosa Research Consortium (EBCRC) |
|
|
[LOGO] |
|
|
[LOGO] |
|
|
[LOGO] |
|
|
[LOGO] |
|
|
[LOGO] |
|
|
[LOGO] |
|
|
[LOGO] |
|
|
[LOGO] |
|
|
Major Improvements in EB Care |
|
|
[LOGO] |
|
|
[LOGO] |
|
|
Current EB Care |
|
|
[LOGO] |
|
|
Current EB Care Wound dressings Estimated cost of bandages is $1,000 up to $20,000 a month |
|
|
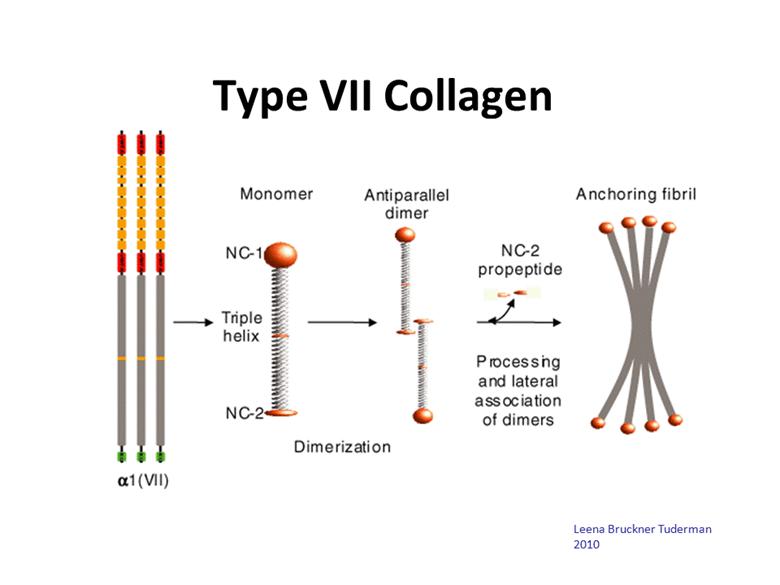
Type VII Collagen Leena Bruckner Tuderman 2010 |
|
|
Modified from J. Uitto, “Molecular Therapeutics for Heritable Skin Diseases.” Milestones Cutaneous Biology, November 2012 Anchoring fibrils |
|
|
Interstitial collagen fibers Modified from J. Uitto, “Molecular Therapeutics for Heritable Skin Diseases.” Milestones Cutaneous Biology, November 2012 |
|
|
Advantages of Cell Based Therapies for EB Skin is an easy target Autologous cells have lower risks of rejection Potential lifelong therapy |
|
|
Stanford Phase 1 Trial 5 adult subjects Expression of type VII collagen at 12 weeks and 6 months Restoration of anchoring fibrils by Electron Microscopy |
|
|
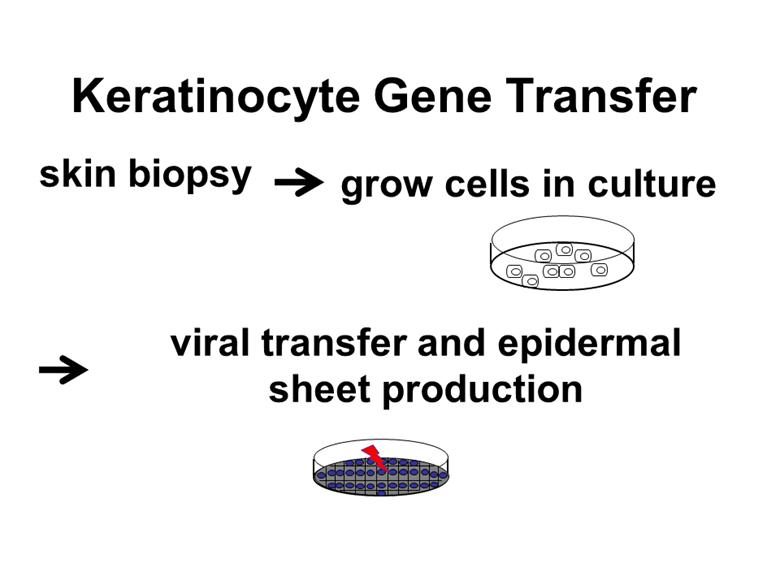
skin biopsy grow cells in culture viral transfer and epidermal sheet production Keratinocyte Gene Transfer |
|
|
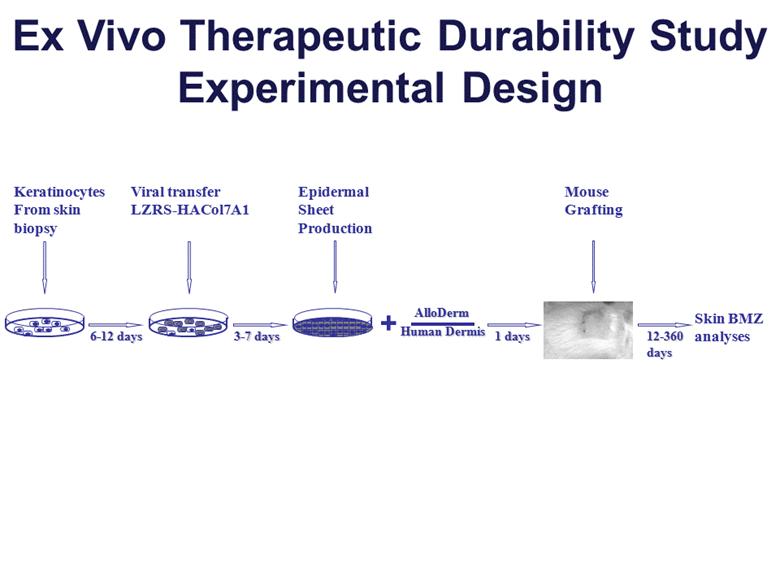
Ex Vivo Therapeutic Durability Study Experimental Design + Skin BMZ analyses Epidermal Sheet Production Keratinocytes From skin biopsy Viral transfer LZRS-HACol7A1 Mouse Grafting 6-12 days 3-7 days 1 days 12-360 days Human Dermis AlloDerm |
|
|
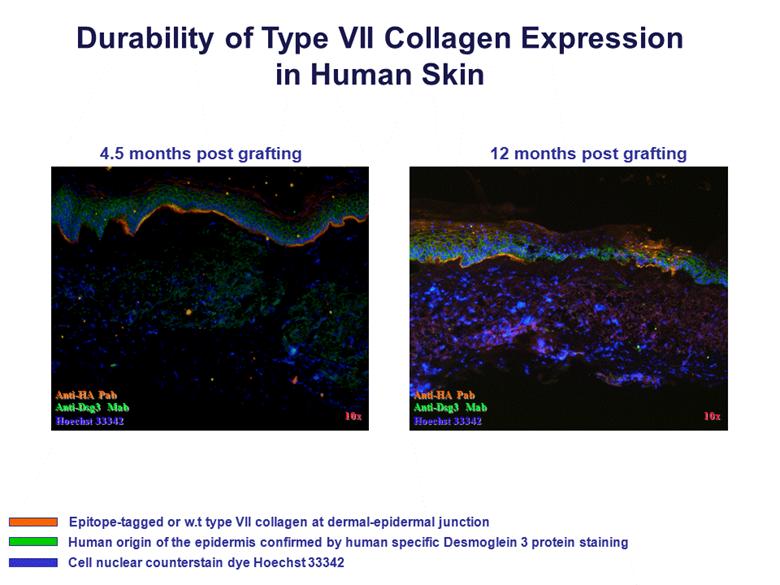
Durability of Type VII Collagen Expression in Human Skin Anti-HA Pab Anti-Dsg3 Mab Hoechst 33342 10x 10x Anti-HA Pab Anti-Dsg3 Mab Hoechst 33342 4.5 months post grafting 12 months post grafting Epitope-tagged or w.t type VII collagen at dermal-epidermal junction Human origin of the epidermis confirmed by human specific Desmoglein 3 protein staining Cell nuclear counterstain dye Hoechst 33342 |
|
|
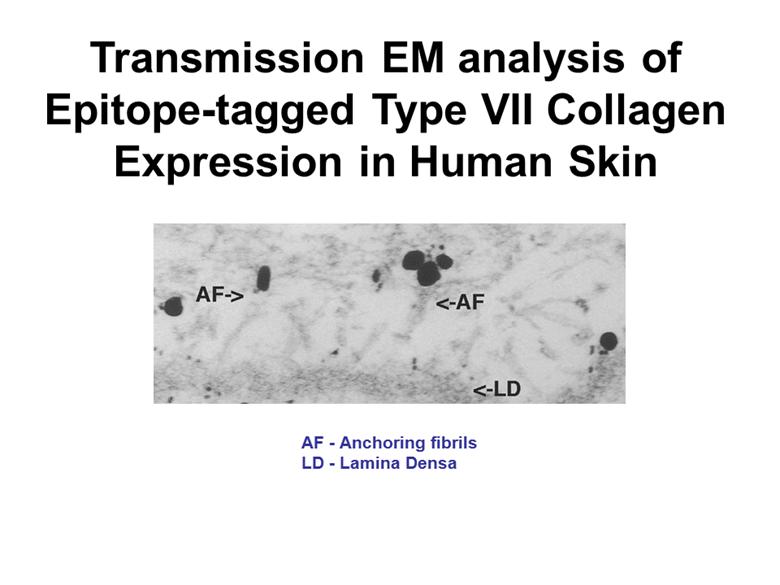
Transmission EM analysis of Epitope-tagged Type VII Collagen Expression in Human Skin AF - Anchoring fibrils LD - Lamina Densa |
|
|
LZRSE-Col7A1 Engineered Autologous Epidermal Sheet (LEAES) |
|
|
Long Term Safety Issues to be Monitored Skin Cancer Auto-immunity Toxicity |
|
|
Fibrocell is the scientific leader in autologous fibroblasts for treating skin and connective tissue diseases |
|
|
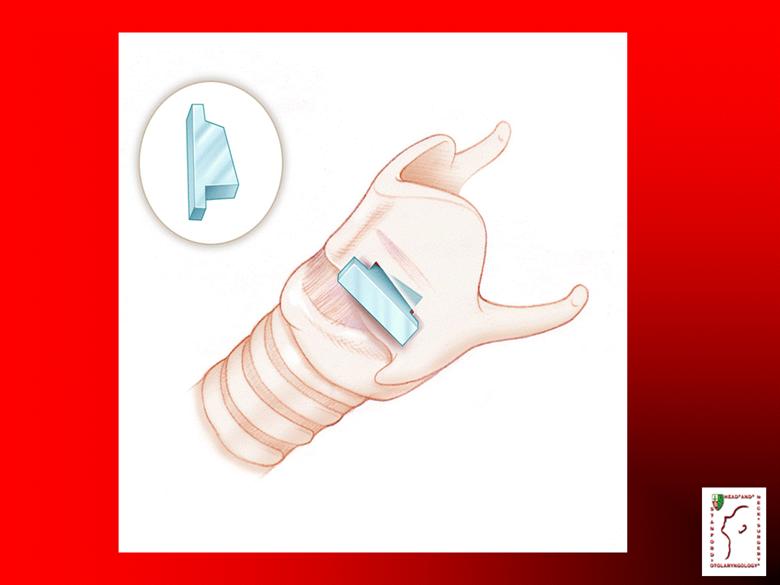
Fibroblasts or Keratinocytes? Fibroblasts can be injected into fingers, hands, mouth, and esophagus Early treatment can avoid scar formation Fibroblast injections are easier than keratinocyte grafting |
|
|
Fibrocell Science Inc. Viral vector inserted into fibroblasts Autologous cells reduce risk of patient rejection Fibroblasts are easily injected into non-wounded or wounded skin |
|
|
Pre-Clinical Design Skin generated with RDEB keratinocytes grown on genetically corrected RDEB fibroblasts Corrected and generated skin grown on an immune suppressed mouse Document presence of type VII collagen at the basement membrane zone and anchoring fibrils |
|
|
Successful molecular therapy for EB may lead to development of therapies for other skin diseases as well as diseases affecting other organs |
|
|
Edward J. Damrose, MD, FACS Associate Professor of Otolaryngology at Stanford University School of Medicine Chief of Laryngology |
|
|
A Phase II, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of Azficel-T for the Treatment of Vocal Fold Scarring and Age-Related Dysphonia |
|
|
Background Associate Professor of Otolaryngology at Stanford University School of Medicine Chief of Laryngology Specialization in voice disorders 2750 patient visits per year 300 surgical procedures 900 office-based laryngeal procedures |
|
|
Incidence & Epidemiology True incidence unknown but grossly underestimated Lifetime risk of a voice disorder is 30% 28 million people with daily voice problem $2.5 billion annual costs for teachers |
|
|
[LOGO] |
|
|
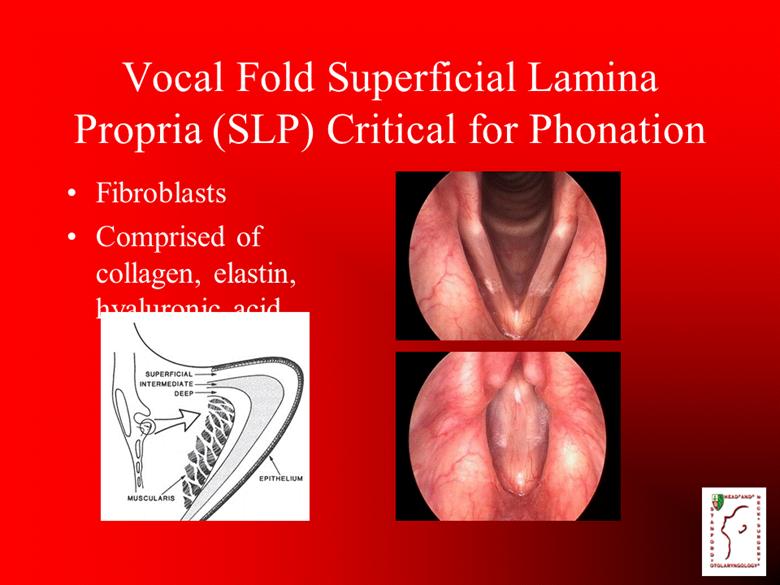
Vocal Fold Superficial Lamina Propria (SLP) Critical for Phonation Fibroblasts Comprised of collagen, elastin, hyaluronic acid |
|
|
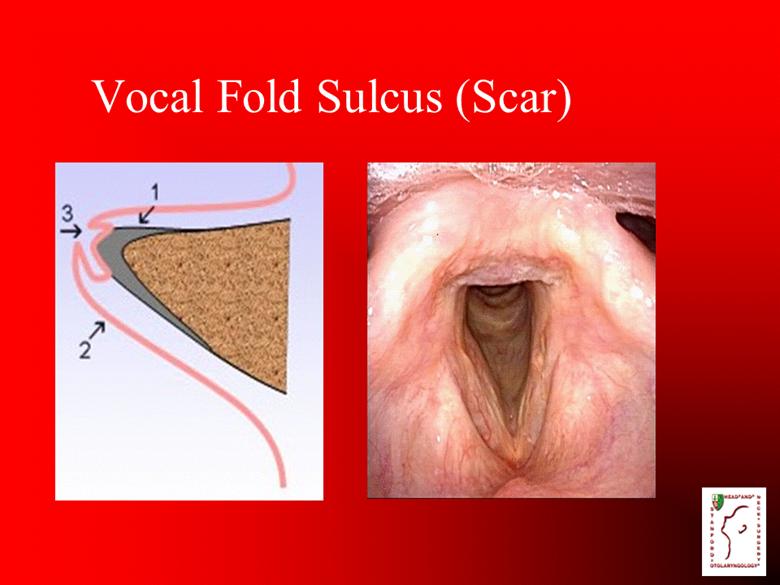
Vocal Fold Sulcus (Scar) |
|
|
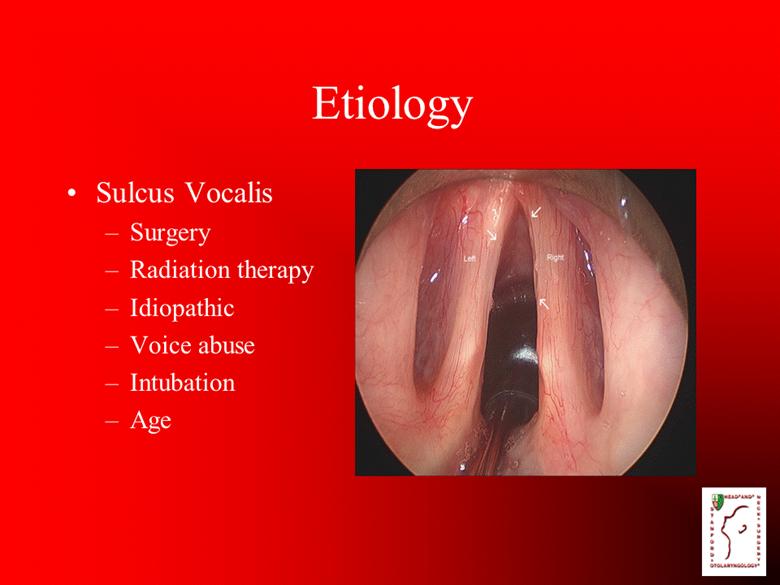
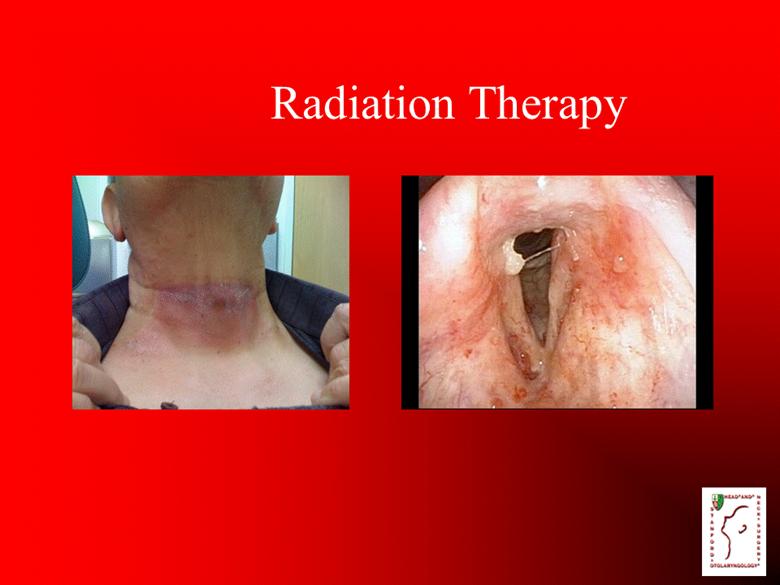
Etiology Sulcus Vocalis Surgery Radiation therapy Idiopathic Voice abuse Intubation Age |
|
|
Radiation Therapy |
|
|
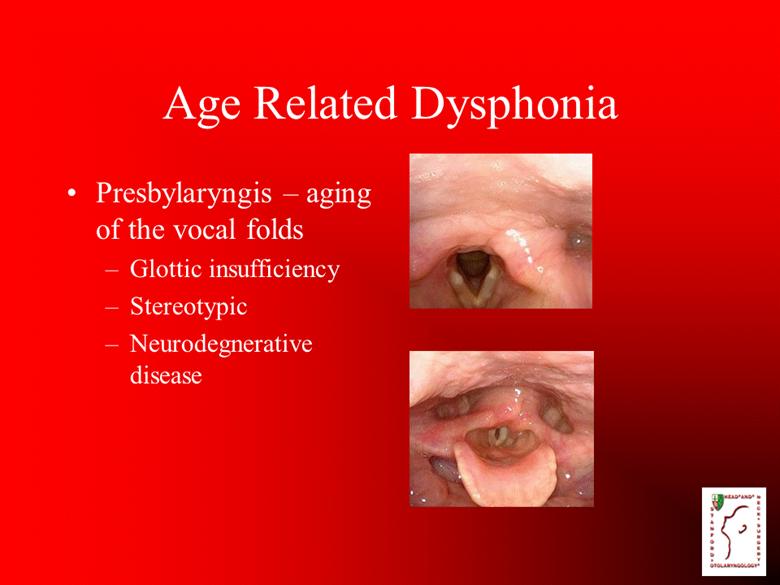
Age Related Dysphonia Presbylaryngis – aging of the vocal folds Glottic insufficiency Stereotypic Neurodegnerative disease |
|
|
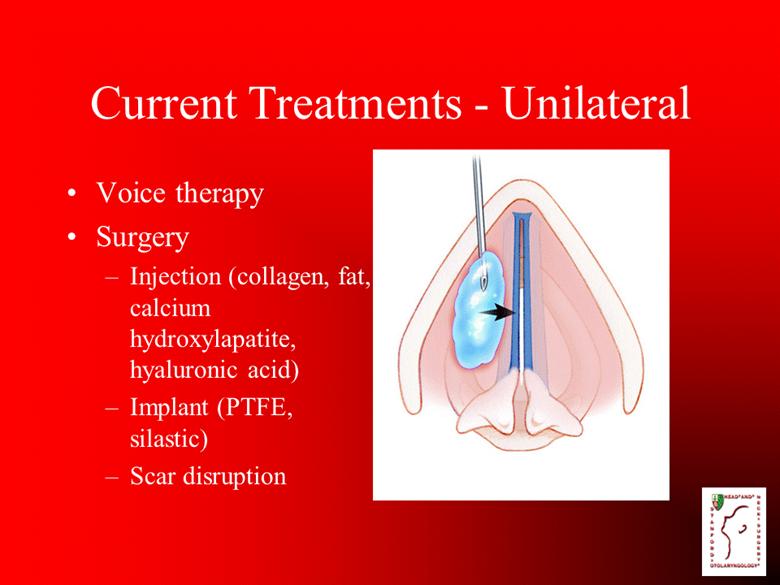
Current Treatments - Unilateral Voice therapy Surgery Injection (collagen, fat, calcium hydroxylapatite, hyaluronic acid) Implant (PTFE, silastic) Scar disruption |
|
|
[LOGO] |
|
|
[LOGO] |
|
|
[LOGO] |
|
|
Current Therapy Inadequate Unable to replace SLP Improve vocal fold closure but not vibration Transient Results unpredictable Highly specialized training |
|
|
Three sites Idiopathic sulcus vocalis or age-related dysphonia Failure of alternative therapies Unilateral or bilateral involvement with poor mucosal vibration 20 patients aggregate |
|
|
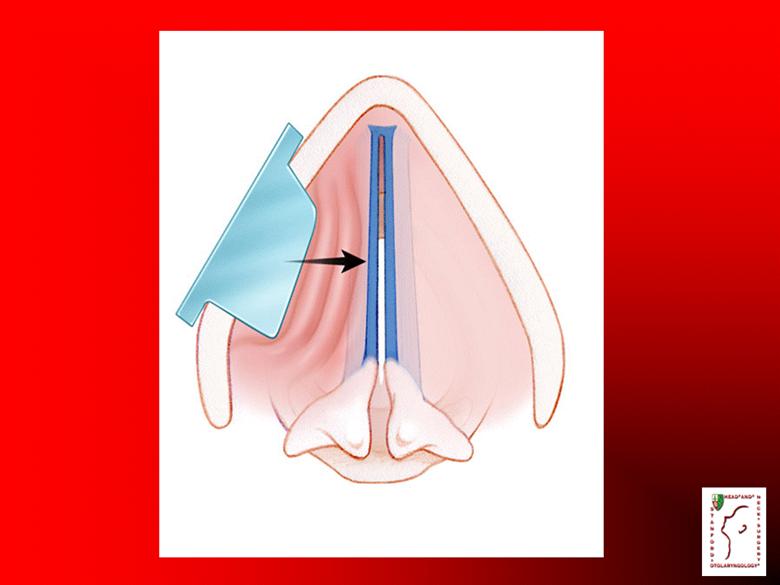
Protocol Post auricular skin biopsy Fibroblasts harvested and prepared Double-blind, placebo controlled Three injections to each affected vocal fold, two weeks apart |
|
|
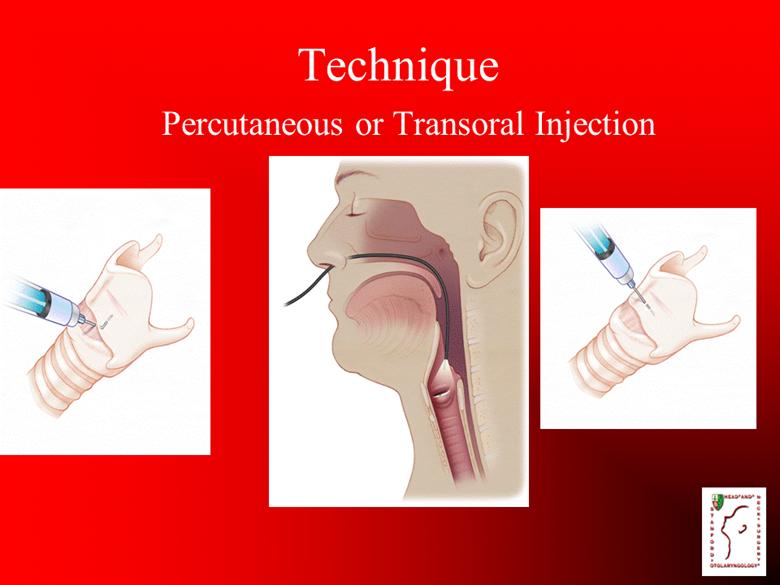
Technique Percutaneous or Transoral Injection |
|
|
Vocal Fold Injection Office-based procedure Topical and/or local anesthesia Injection into affected side Minimal bleeding Well-tolerated |
|
|
Efficacy Assessments Independent evaluator (speech language pathologist) Primary Mucosal wave grade (videostroboscopy) Voice Handicap Index GRBAS Secondary Visual Analogue Scale Impression of voice quality questionnaire Voice recording for acoustic and perceptual analysis |
|
|
Key Points Azficel-T could offer a potential cure for scarred, degenerated vocal folds Potential advance in laryngeal surgery with the potential to help millions of people Study design is robust and outcome measurements are thorough and well-conceived May be the most well-designed assessment of a vocal fold injectable ever described in the otolaryngologic literature |
|
|
Update: Restrictive Burn Scarring Program |
|
|
Update: Restrictive Burn Scarring Program Enrollment challenges stem from the length of a placebo-controlled study when other non-therapeutic options exist, such as surgery and physical therapy 7 of 21 patients have been enrolled Fibrocell took steps to address the issue: Expanded the number of sites Lessened enrollment criteria Alternative strategies to derive value more quickly are being evaluated |
|
|
CDR (USN Ret) Nathan S. Uebelhoer, DO, FAAD Dermatologic and Laser Surgeon Naval Medical Center San Diego The Aroostook Medical Center |
|
|
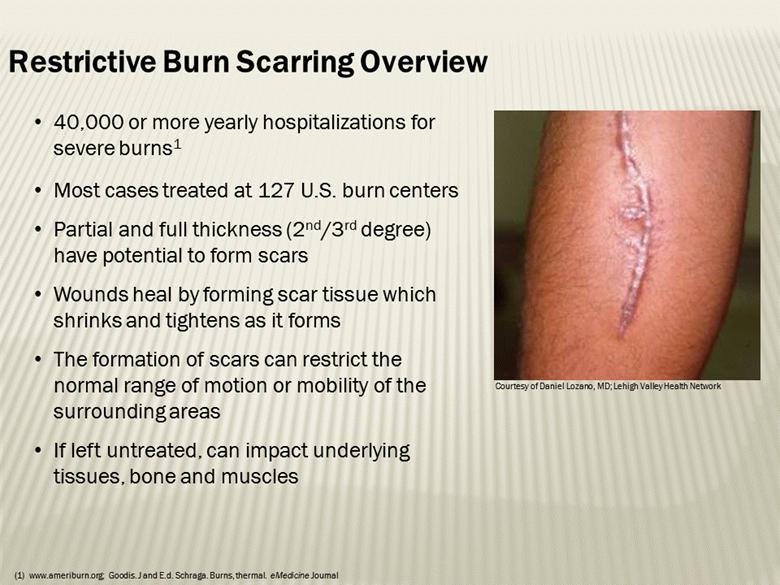
40,000 or more yearly hospitalizations for severe burns1 Most cases treated at 127 U.S. burn centers Partial and full thickness (2nd/3rd degree) have potential to form scars Wounds heal by forming scar tissue which shrinks and tightens as it forms The formation of scars can restrict the normal range of motion or mobility of the surrounding areas If left untreated, can impact underlying tissues, bone and muscles Restrictive Burn Scarring Overview Courtesy of Daniel Lozano, MD; Lehigh Valley Health Network www.ameriburn.org; Goodis. J and E.d. Schraga. Burns, thermal. eMedicine Journal |
|
|
Iwuagwu, FC, Wilson D, and Bailie F. The use of skin grafts in postburn contracture release: A 10-year review. Plast Reconstr Surg. 1999; 103:1198-1204. Schneider JC, Holavanahali R, Helm P, Goldstein R, Kowalske K. Contractures in burn injury: defining the problem. J Burn Care Res. 2006; 27:508-514. Patient Impact 3 “Burn scarring and contracture affecting function remain the most frustrating late complications of burn injury”1 Restrictive burn scars can have significant consequences to quality of life, including pain and reduced functionality In a prospective survey of burn survivors at one burn center, more than one-third of patients had developed at least one restrictive scar affecting a major joint at the time of hospital discharge2 Most frequently restricted large joint was the shoulder, followed by the elbow and the knee Restrictive burn scars may result in reduced mobility, limited reach, difficulties with grasping and manipulating objects, or impaired mobility of the neck and face Contractures of the upper extremities may impact activities of daily living, such as grooming, dressing, eating, and bathing and fine motor tasks2 |
|
|
Current Methods of Treatment No FDA approved drug or biologic therapies available Physical and occupational therapy Training for activities of daily living (ADL) Splinting Massage Pressure garments Surgery to release the contracture Flap procedures (Z-plasty) Skin grafting (full or partial thickness) Tissue expansion Although patient status limits how early such operations can be conducted, surgery on scars matured over 2 years may prolong recovery unnecessarily (Sheridan, 2004) A lack of healthy, unburned skin “can be a major issue during burn reconstruction” (Chester, 2004) 4 |
|
|
Role of Fibroblasts 5 Human dermal fibroblasts play an essential role in wound healing, migrating to the site of injury Injection into mature burn scars may stimulate a wound healing response Potentially breaks down existing, disorganized extracellular matrix as cells are stimulated to migrate into the injection site Potentially regenerates new, organized matrix within the wound Fibrocell has previous experience with injection of autologous fibroblasts into acute wounds and scars in case studies |
|
|
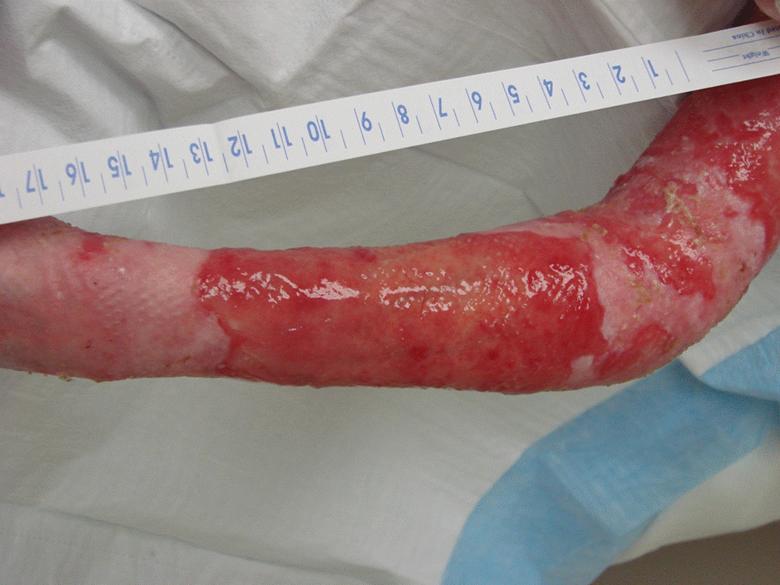
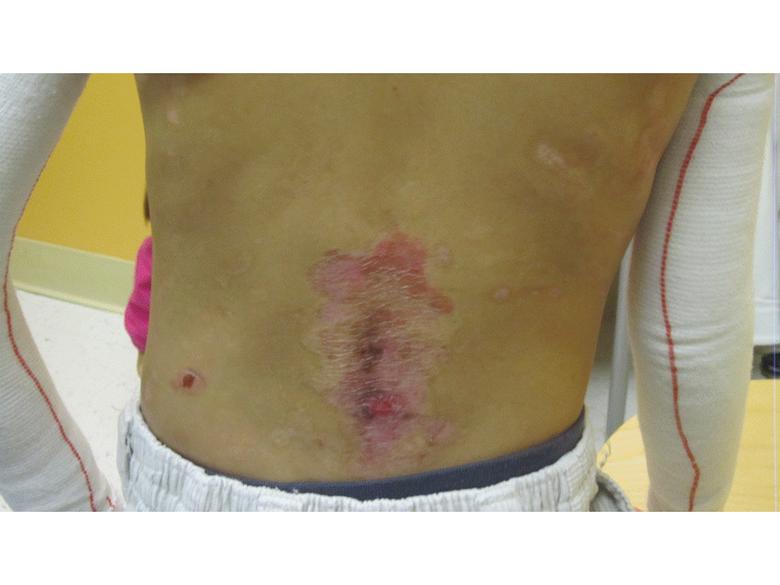
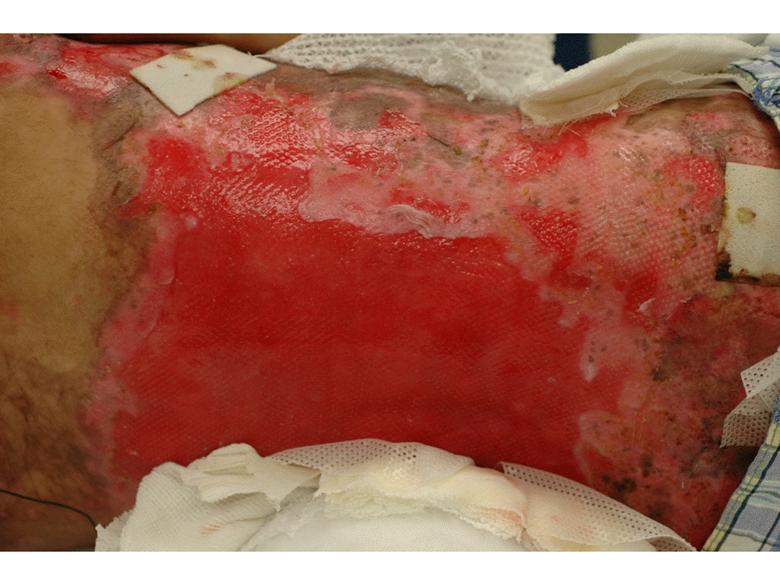
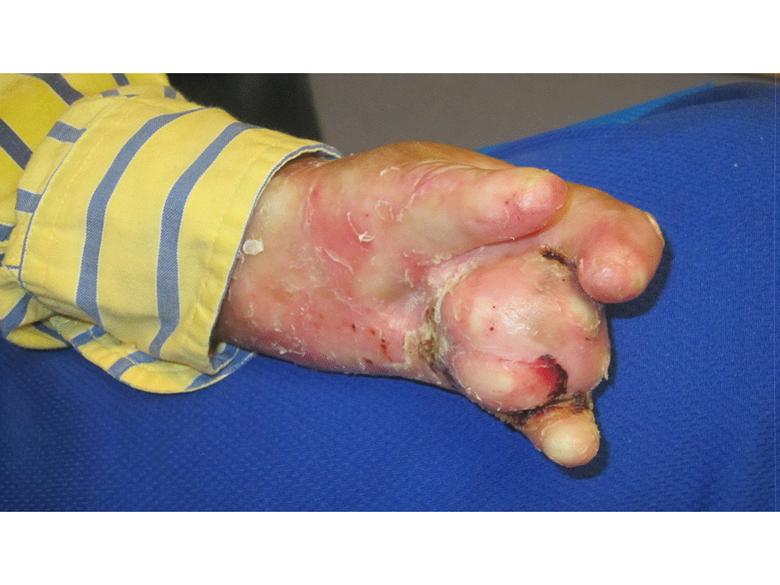
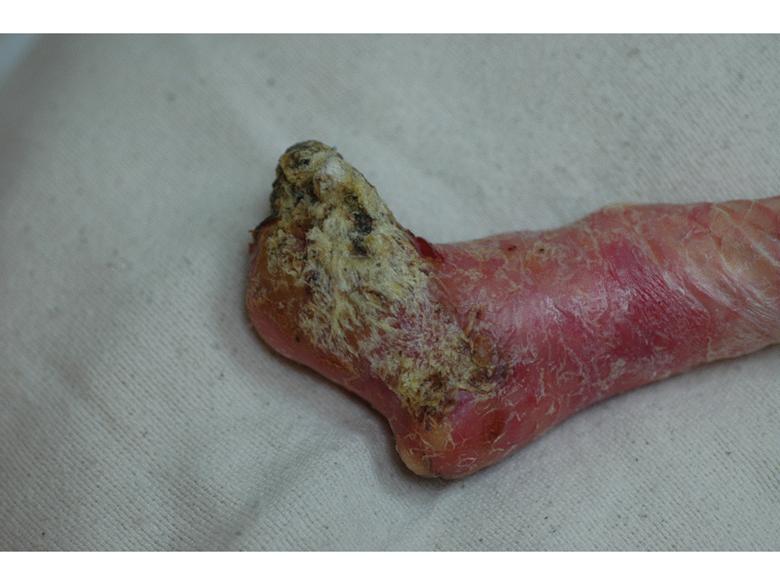
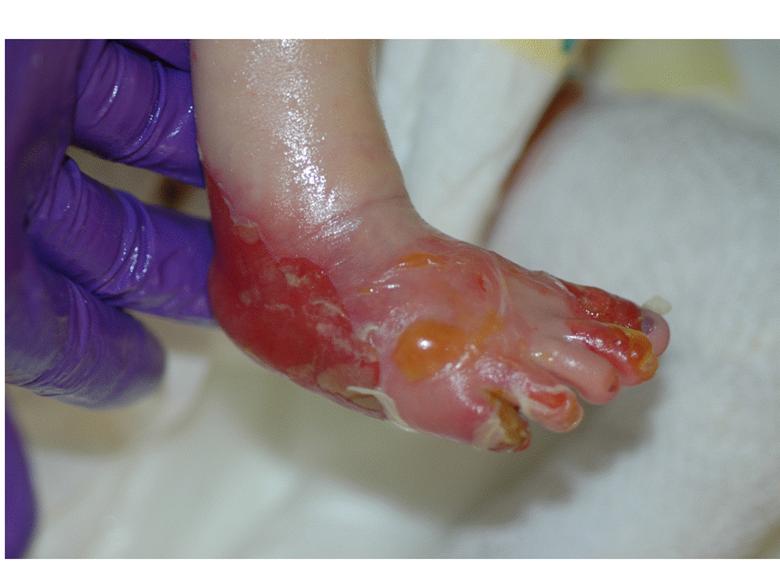
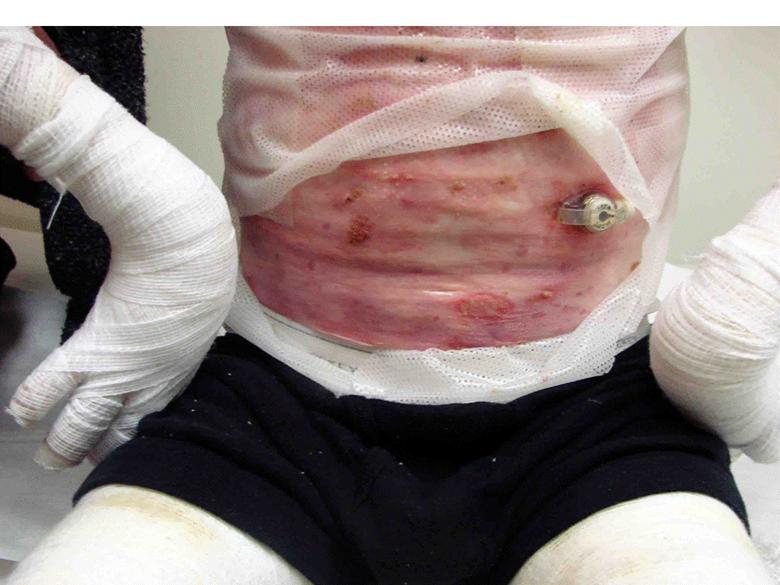
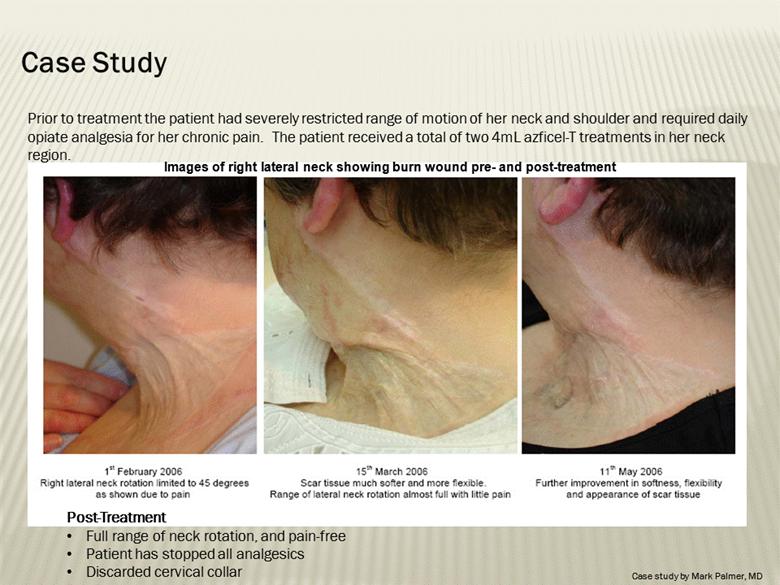
Images of right lateral neck showing burn wound pre- and post-treatment Case Study Prior to treatment the patient had severely restricted range of motion of her neck and shoulder and required daily opiate analgesia for her chronic pain. The patient received a total of two 4mL azficel-T treatments in her neck region. Post-Treatment Full range of neck rotation, and pain-free Patient has stopped all analgesics Discarded cervical collar Case study by Mark Palmer, MD |
|
|
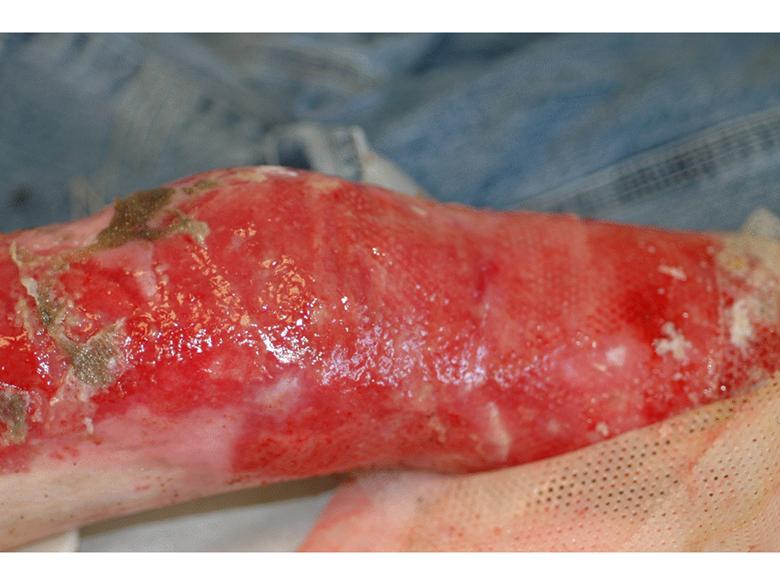
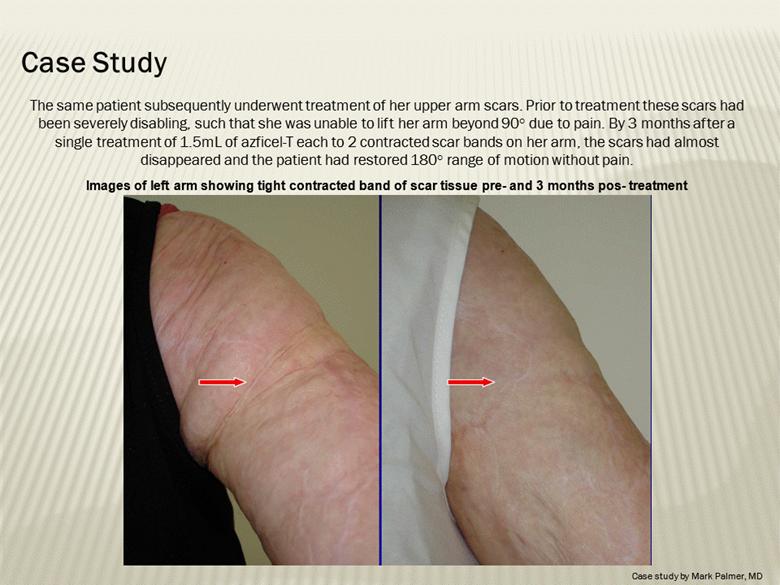
The same patient subsequently underwent treatment of her upper arm scars. Prior to treatment these scars had been severely disabling, such that she was unable to lift her arm beyond 90° due to pain. By 3 months after a single treatment of 1.5mL of azficel-T each to 2 contracted scar bands on her arm, the scars had almost disappeared and the patient had restored 180° range of motion without pain. Images of left arm showing tight contracted band of scar tissue pre- and 3 months pos- treatment Case Study Case study by Mark Palmer, MD |
|
|
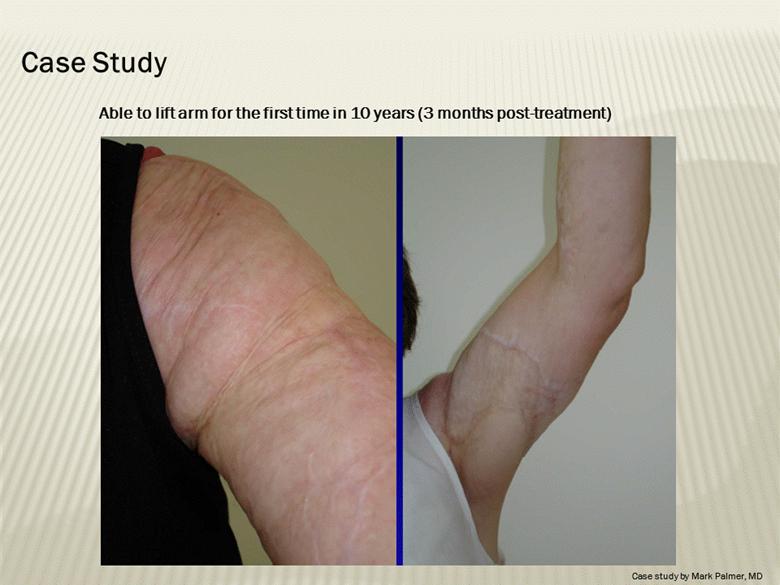
Case Study Able to lift arm for the first time in 10 years (3 months post-treatment) Case study by Mark Palmer, MD |
|
|
Double-blind, randomized, placebo-controlled, 21 subjects Trial Initiated; 7 subjects enrolled Multi-center trial: 8 centers 6 month efficacy endpoint Subjects inclusion criteria: Unilateral restrictive burn scars of a jointed area 20-60% restriction in normal range of motion Validated Measurement Scales: Range of Motion Measurement(1) Brief Pain Index (BPI)(2) Scar Appearance – Vancouver Scar Scale(3) Restrictive Burn Scarring Phase II 9 Standard ROM measuring techniques (Parry, et al 2010) Brief Pain Inventory (BPI) numerical scale (0 to 10) (Cleeland and Ryan 1994; Tan, et al 2004) Vancouver Scar Scale (Baryza and Baryza 1995; Nedelec, et al 2000) |
|
|
Conclusion Restrictive burn scars have a major impact on the quality of life for patients Current therapies are limited to physical manipulation, pressure garments and surgery Autologous fibroblasts may provide a non-surgical approach for improvement of range of motion for joints affected by restrictive burn scars 10 |
|
|
James Byrne, PhD Assistant Professor, UCLA Consultant, Fibrocell Science, Inc. Founder, Clinical Investigations for Dermal Mesenchymally-Obtained Derivatives (CIDMOD) Initiative |
|
|
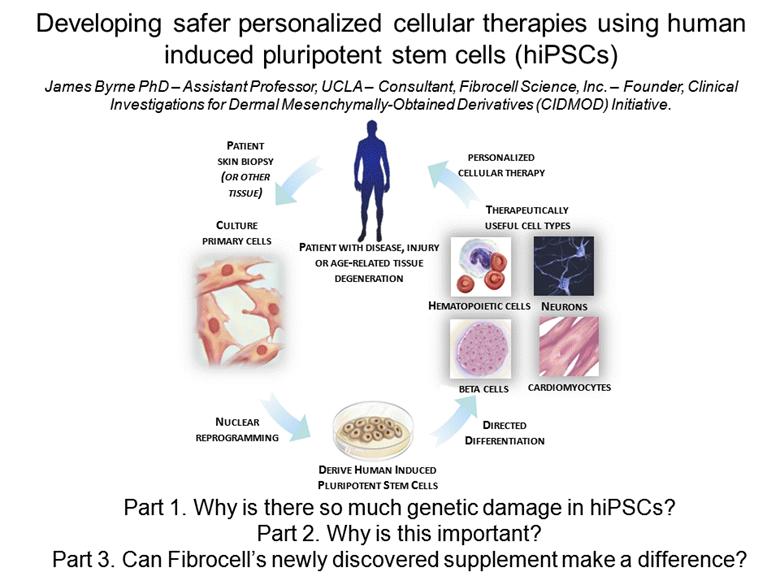
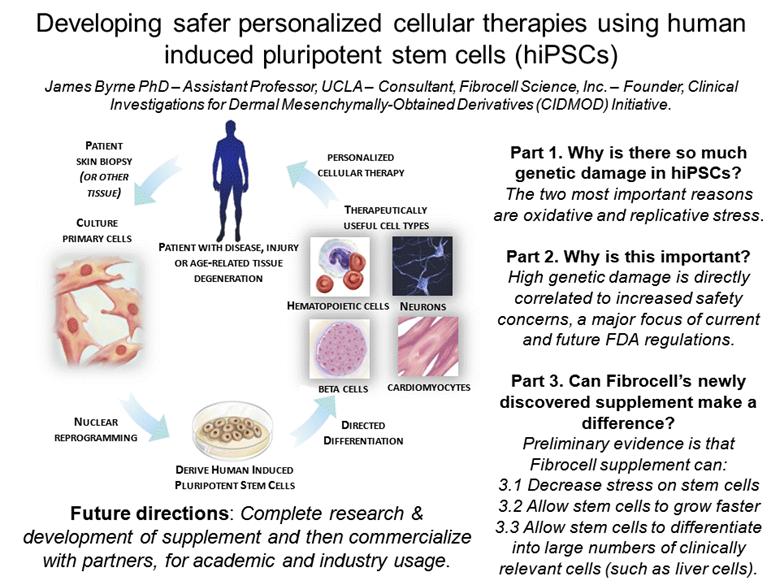
PATIENT SKIN BIOPSY (OR OTHER TISSUE) PERSONALIZED CELLULAR THERAPY CULTURE PRIMARY CELLS PATIENT WITH DISEASE, INJURY OR AGE-RELATED TISSUE DEGENERATION NUCLEAR REPROGRAMMING BETA CELLS NEURONS CARDIOMYOCYTES HEMATOPOIETIC CELLS DERIVE HUMAN INDUCED PLURIPOTENT STEM CELLS DIRECTED DIFFERENTIATION THERAPEUTICALLY USEFUL CELL TYPES Developing safer personalized cellular therapies using human induced pluripotent stem cells (hiPSCs) James Byrne PhD – Assistant Professor, UCLA – Consultant, Fibrocell Science, Inc. – Founder, Clinical Investigations for Dermal Mesenchymally-Obtained Derivatives (CIDMOD) Initiative. Part 1. Why is there so much genetic damage in hiPSCs? Part 2. Why is this important? Part 3. Can Fibrocell’s newly discovered supplement make a difference? |
|
|
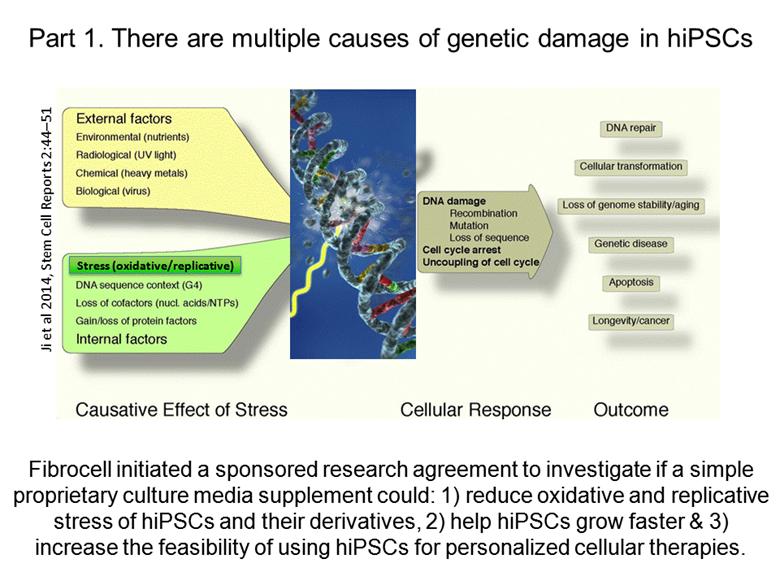
Part 1. There are multiple causes of genetic damage in hiPSCs Fibrocell initiated a sponsored research agreement to investigate if a simple proprietary culture media supplement could: 1) reduce oxidative and replicative stress of hiPSCs and their derivatives, 2) help hiPSCs grow faster & 3) increase the feasibility of using hiPSCs for personalized cellular therapies. Stress (oxidative/replicative) Ji et al 2014, Stem Cell Reports 2:44–51 |
|
|
Part 2. Why is this important? Safety is the number 1 concern of the FDA with regards to personalized hiPSC-based therapeutics. Any technology that significantly increases the safety of personalized hiPSC-based therapeutics would likely become broadly adopted. Current Market for hiPSC-based regenerative market: Extremely large any degenerative disease, disorder or injury. Current spending on stem cell media/supplements is (after salary costs) the LARGEST proportion of budget for any stem cell lab. Future Market for our supplement: depend on scientific, political and regulatory developments - CIDMOD Initiative - Over $500,000,000 left to spend on stem cell research in CA from CIRM alone! |
|
|
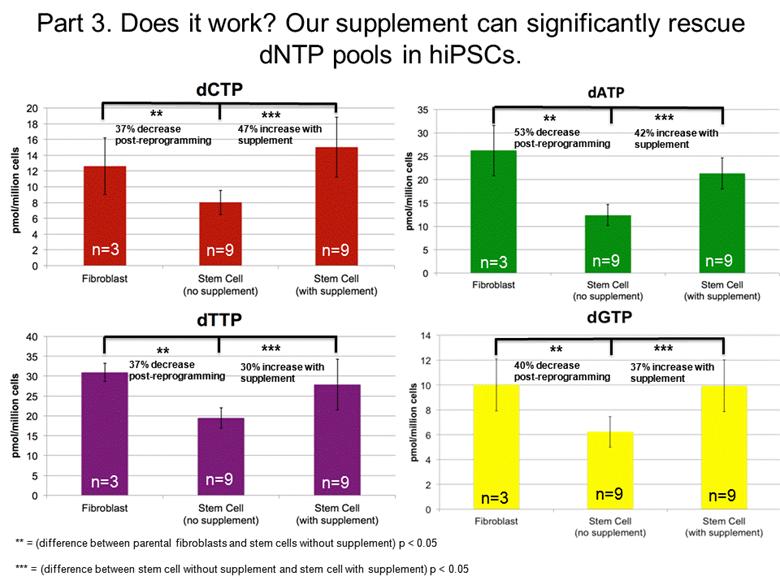
** ** ** ** *** *** *** *** n=3 n=3 n=3 n=3 n=9 n=9 n=9 n=9 n=9 n=9 n=9 n=9 ** = (difference between parental fibroblasts and stem cells without supplement) p < 0.05 *** = (difference between stem cell without supplement and stem cell with supplement) p < 0.05 37% decrease post-reprogramming 47% increase with supplement 53% decrease post-reprogramming 37% decrease post-reprogramming 40% decrease post-reprogramming 30% increase with supplement 42% increase with supplement 37% increase with supplement Part 3. Does it work? Our supplement can significantly rescue dNTP pools in hiPSCs. |
|
|
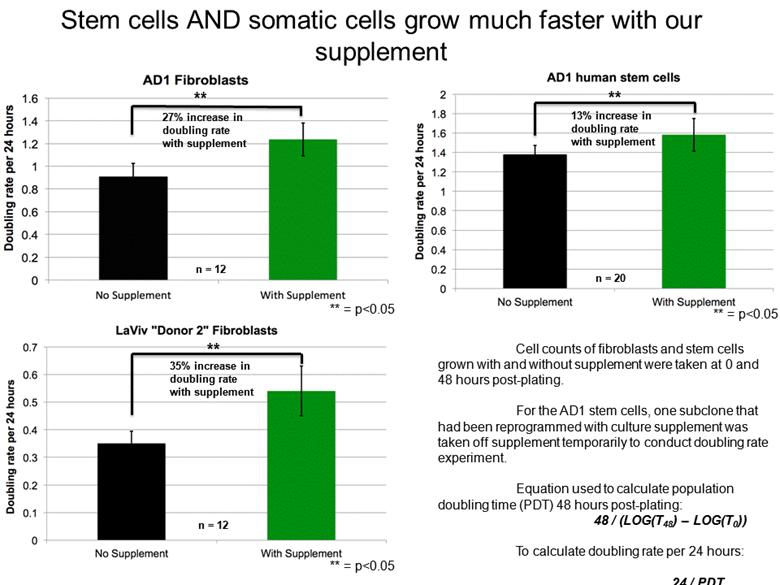
n = 12 27% increase in doubling rate with supplement ** ** = p<0.05 ** 35% increase in doubling rate with supplement ** = p<0.05 n = 12 ** 13% increase in doubling rate with supplement n = 20 Cell counts of fibroblasts and stem cells grown with and without supplement were taken at 0 and 48 hours post-plating. For the AD1 stem cells, one subclone that had been reprogrammed with culture supplement was taken off supplement temporarily to conduct doubling rate experiment. Equation used to calculate population doubling time (PDT) 48 hours post-plating: 48 / (LOG(T48) – LOG(T0)) To calculate doubling rate per 24 hours: 24 / PDT ** = p<0.05 Stem cells AND somatic cells grow much faster with our supplement |
|
|
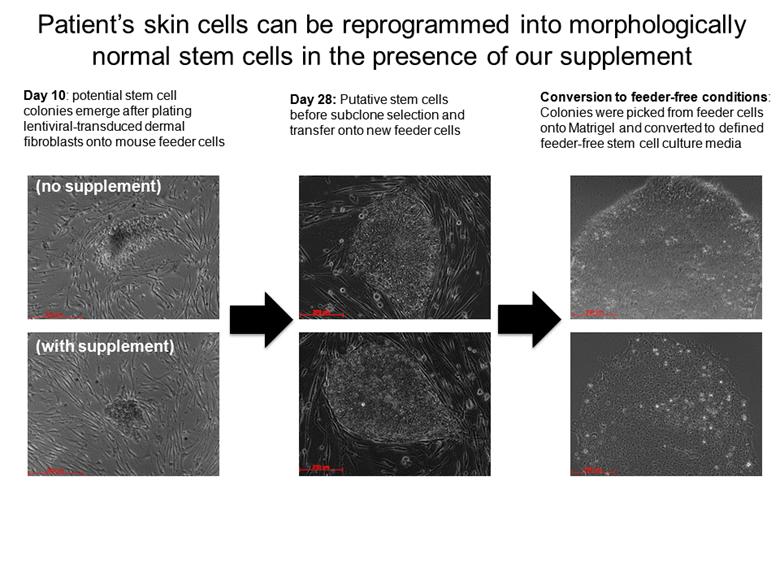
Patient’s skin cells can be reprogrammed into morphologically normal stem cells in the presence of our supplement Day 10: potential stem cell colonies emerge after plating lentiviral-transduced dermal fibroblasts onto mouse feeder cells (no supplement) (with supplement) Day 28: Putative stem cells before subclone selection and transfer onto new feeder cells Conversion to feeder-free conditions: Colonies were picked from feeder cells onto Matrigel and converted to defined feeder-free stem cell culture media |
|
|
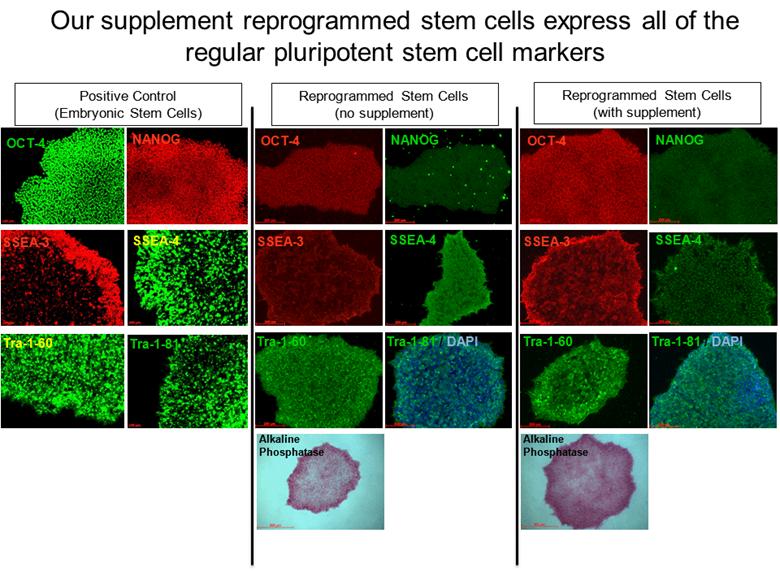
Our supplement reprogrammed stem cells express all of the regular pluripotent stem cell markers OCT-4 SSEA-4 SSEA-3 NANOG Alkaline Phosphatase Tra-1-81 / DAPI Tra-1-60 OCT-4 SSEA-4 SSEA-3 NANOG Alkaline Phosphatase Tra-1-81 / DAPI Tra-1-60 OCT-4 SSEA-4 SSEA-3 NANOG Tra-1-81 / Tra-1-60 Positive Control (Embryonic Stem Cells) Reprogrammed Stem Cells (no supplement) Reprogrammed Stem Cells (with supplement) |
|
|
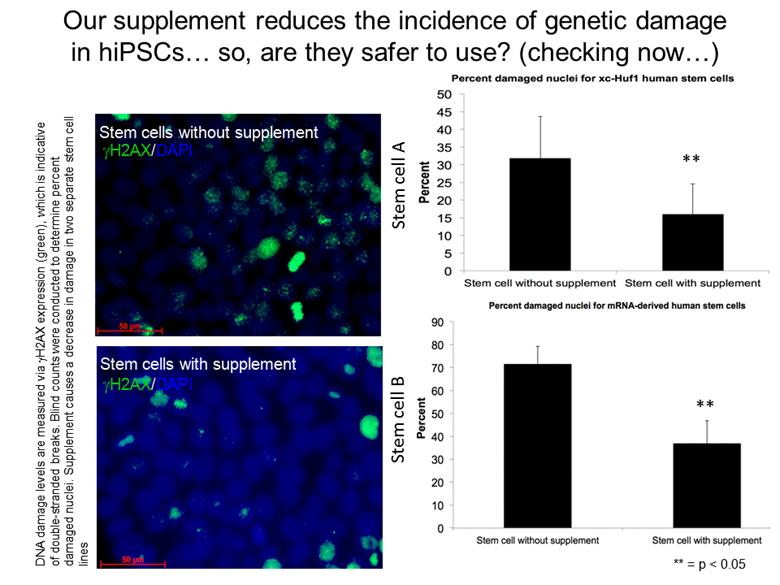
Our supplement reduces the incidence of genetic damage in hiPSCs so, are they safer to use? (checking now) Stem cells without supplement gH2AX/DAPI gH2AX/DAPI Stem cells with supplement ** ** ** = p < 0.05 Stem cell A Stem cell B DNA damage levels are measured via gH2AX expression (green), which is indicative of double-stranded breaks. Blind counts were conducted to determine percent damaged nuclei. Supplement causes a decrease in damage in two separate stem cell lines |
|
|
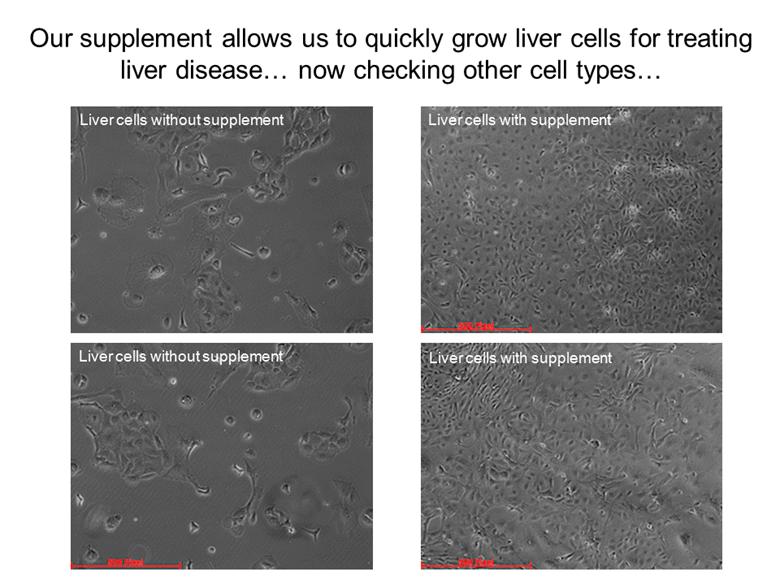
Liver cells with supplement Liver cells with supplement Liver cells without supplement Liver cells without supplement Our supplement allows us to quickly grow liver cells for treating liver disease now checking other cell types |
|
|
CULTURE PRIMARY CELLS PATIENT SKIN BIOPSY (OR OTHER TISSUE) PERSONALIZED CELLULAR THERAPY PATIENT WITH DISEASE, INJURY OR AGE-RELATED TISSUE DEGENERATION NUCLEAR REPROGRAMMING BETA CELLS NEURONS CARDIOMYOCYTES HEMATOPOIETIC CELLS DERIVE HUMAN INDUCED PLURIPOTENT STEM CELLS DIRECTED DIFFERENTIATION THERAPEUTICALLY USEFUL CELL TYPES Developing safer personalized cellular therapies using human induced pluripotent stem cells (hiPSCs) James Byrne PhD – Assistant Professor, UCLA – Consultant, Fibrocell Science, Inc. – Founder, Clinical Investigations for Dermal Mesenchymally-Obtained Derivatives (CIDMOD) Initiative. Part 1. Why is there so much genetic damage in hiPSCs? The two most important reasons are oxidative and replicative stress. Part 2. Why is this important? High genetic damage is directly correlated to increased safety concerns, a major focus of current and future FDA regulations. Part 3. Can Fibrocell’s newly discovered supplement make a difference? Preliminary evidence is that Fibrocell supplement can: 3.1 Decrease stress on stem cells 3.2 Allow stem cells to grow faster 3.3 Allow stem cells to differentiate into large numbers of clinically relevant cells (such as liver cells). Future directions: Complete research & development of supplement and then commercialize with partners, for academic and industry usage. |
|
|
Question & Answer Session |
|
|
Thank You for Joining Us Today |