Attached files
| file | filename |
|---|---|
| 8-K - 8-K - InspireMD, Inc. | v389230_8k.htm |
| EX-99.2 - EXHIBIT 99.2 - InspireMD, Inc. | v389230_ex99-2.htm |

Joachim Schofer (PI) Piotr Musialek (Co - PI) On behalf of the CARENET Investigators Evaluation of PET Mesh Covered Stent in Patients with Carotid Artery Disease T he CARENET - Trial ( CAR otid E mbolic protection using micro NET ) Joachim Schofer, MD,PhD , Hamburg University CardiovascularCenter , Hamburg Germany Piotr Musialek , MD, PhD, Jagiellonian University Medical College at John Paul II Hospital, Krakow, Poland, Ralf Kolvenbach , MD , PhD, Augusta Hospital, Dusseldorf, Germany, Horst Sievert, MD, PhD, Cardiovascular Center Frankfurt, Frankfurt, Germany

Disclosure Statement of Financial Interest • Grant/Research Support • InspireMD Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company

Conventional Carotid Stent Plaque protrusion may lead to early and late distal embolization
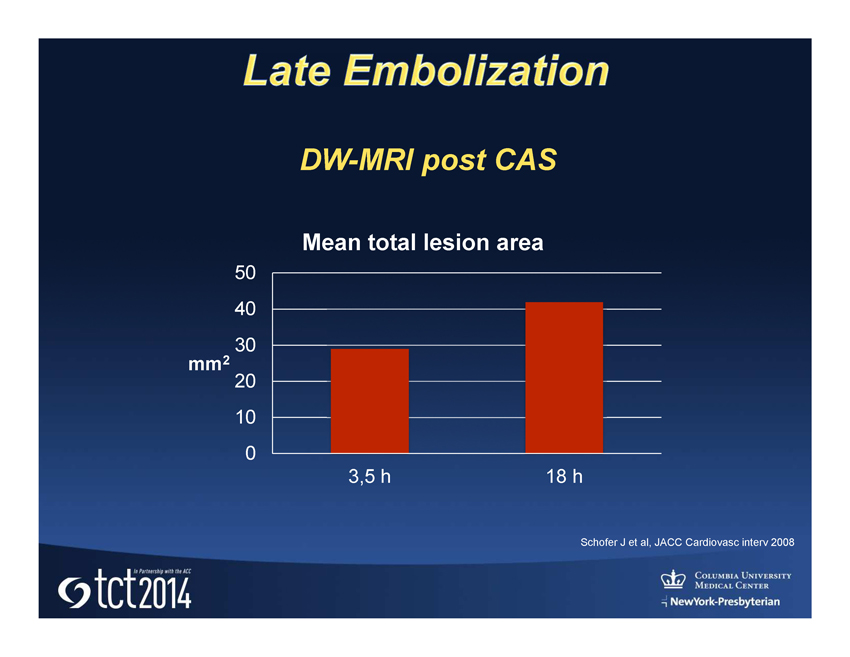
Schofer J et al, JACC Cardiovasc interv 2008 0 10 20 30 40 50 3,5 h 18 h mm 2 Mean total lesion area DW - MRI post CAS

CGuard TM Carotid Embolic Prevention System Specifications Device Features Stent type Nitinol Self - Expanding MicroNet Aperture Size 150 - 180µ Guidewire 0.014” Foreshortening <10% Sizes Diameter( 6mm - 10mm) x Length (20mm – 60mm) Delivery System (OD) 6F (2.1mm) CE marked. Not approved for sale in the US
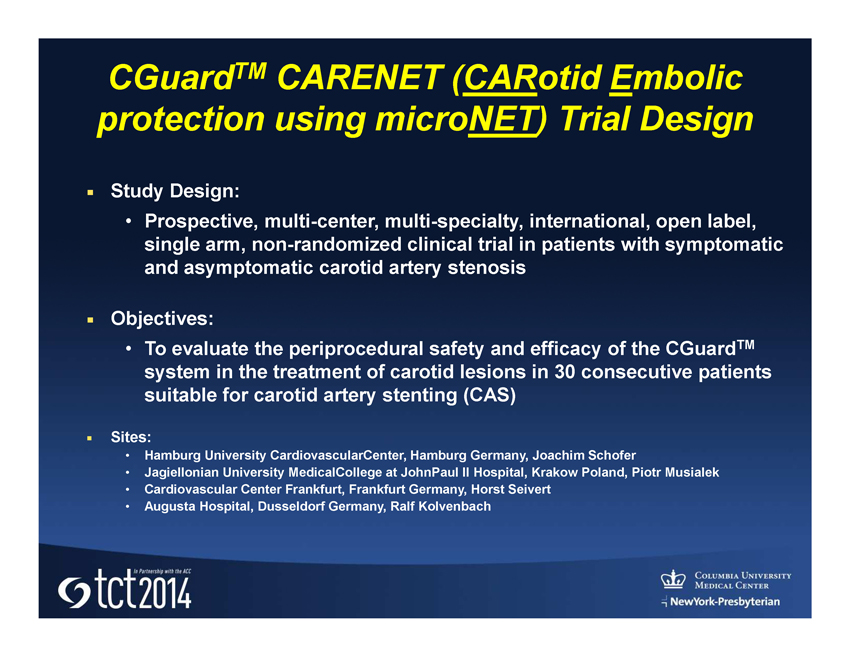
CGuard TM CARENET ( CAR otid E mbolic protection using micro NET ) Trial Design ■; Study Design: • Prospective, multi - center, multi - specialty, international, open label, single arm, non - randomized clinical trial in patients with symptomatic and asymptomatic carotid artery stenosis ■; Objectives: • To evaluate the periprocedural safety and efficacy of the CGuard TM system in the treatment of carotid lesions in 30 consecutive patients suitable for carotid artery stenting (CAS) ■; Sites: • Hamburg University CardiovascularCenter , Hamburg Germany, Joachim Schofer • Jagiellonian University MedicalCollege at JohnPaul II Hospital, Krakow Poland, Piotr Musialek • Cardiovascular Center Frankfurt, Frankfurt Germany, Horst Seivert • Augusta Hospital, Dusseldorf Germany, Ralf Kolvenbach

■; Study Population: • Symptomatic pts (w/ history of a transient ischemic attack, stroke, or amaurosis fugax within the last 6 mos on the ipsilateral side) w/carotid stenosis ≥ 50% • Asymptomatic pts w/ carotid stenosis ≥ 80% both as diagnosed by angiography using NASCET methodology ■; Primary Endpoint : • 30 day MACE (death, stroke, MI) ■; Key secondary Endpoints: • Technical success • Periprocedural complications (including device - related) • Incidence, number and volume of new lesions assessed by DW MRI during pre - procedure, 24 - 48 hours post - procedure, and at 30 days (+/ - 3 days ) • Peak systolic velocity (PSV) and end diastolic velocity (EDV) assessment by ultrasound examination at 30 days, 6 mos , and 1 year CGuard TM CARENET ( CAR otid E mbolic protection using micro NET ) Trial Design

Baseline Characteristics (n=30) Age 71.6 ± 7.6 Male 63.4% Symptomatic 33.3% (10) BMI (kg/m 2 ) 26.4 ± 3.9 Hypertension 83.3% (25) Dyslipidemia 90% (27) Diabetics 23.3% (7) Smoker: Current Former 13.4% (4) 36.6% (11) Prior MI 26.7% (8) Prior TIA 13.3% (4)

Procedure Results Femoral access 100% (30) Target vessel Left ICA Right ICA 33.3% (10) 66.6% (20) Protection used Distal protection Proximal protection 100% 96.6% (29) 3.4% (1) Pre dilatation 70.9% (22) Post dilatation 77.4% (24) Post dilatation Pressure (ATM) 13.6 ± 4.5 Device success 100% (30) Stent deployed 100% (30) Stent diameter (Mean) 8.23mm ± 0.8 Stent length (Mean) 34.8 mm ± 5.0 Second stent used 3.33% (1)

Pre & Post Procedure Carotid Angiogram in Patient with right ICA Stenosis

Clinical Outcomes Post Procedure Discharge 30 days Device success 100% NA NA MACE 0% 0% 0% Death 0% 0% 0% MI 0% 0% 0% Stroke 0% 0% 0%

Angiographic Assessment Baseline Final Lesion location (internal) 100% NA Lesion length (mm) 16.94 ± 4.7 NA RVD (mm) 6.18 5.89 MLD (mm) 1.25 4.82 Diameter stenosis (%) 79.9 % ± 5.0% 16.9% ± 6.5% (in stent) ECA stenosis (%) 18.0% 22.1% TIMI flow in ECA Normal 100.0% 100.0%

CARENET with Distal Protection DW - MRI @ 24 - 48 hrs CARENET CGuard with only Distal EPD (N=26 * ) Incidence of New Lesions 46% Lesions ( per patient) 1.62 ± 2.68 Volume (per patient) 0.061 ± 0.11 cm 3 • *3 pts unable to undergo MRI (1 = pacemaker; 2 = claustrophobia) • 1 pt with proximal protection had 78 new lesions. New ischemic lesions had no clinical or neurological impact, all lesions been resolved at 30 days.

CARENET Comparison DW - MRI @ 24 - 48 hrs CARENET (Filter group) N=26 PROFI 1 (Filter group) N=31 ICSS 2 (Filter group) N=37 Incidencs of New Lesions 48% 87% 73% Avg Lesion Volume 0.06 cm 3 0.59 cm 3 NA 1 JACC, April 2012 2 Lancet, March 2010

Conclusions • CARENET trial demonstrated the safety of the CGuard TM Technology with zero MACCE at 30 days • The procedural success was 100% • Compared to published DW - MRI data of non - mesh covered carotid stents, the incidence of new ischemic lesions was reduced by almost 50% and the average lesion volume per patient 10 times smaller • These initial clinical results suggest that the MicroNet TM covered CGuard TM offers unique clinical benefits for patients undergoing CAS 14

• Back up slides

Histopathology in Pig Carotid Artery 16

Preprocedure MRI 48 - Hr MRI 30 - Day MRI Subjects with Lesions 2 (8.0%) 11 (46.0%) 2 (8.32%) Number of new lesions 12 42 2 Avg. Lesions (per patient) 0.5 + 1.72 1.71 + 4.77 0.08 + 0.28 Total Lesion Volume (cm 3 ) 0.33 + 1.4 0.16 + 0.31 0.13 + 0.54 Ischemic Lesions (Pre, 48hr Post, 30 day Post)

» Co - Cr Bare metal stent wrapped with an expandable MicroNet TM » Single strand Polyethylene Terephthalate (PET) fiber » Attached only to proximal and distal crowns of the stent » Deploys exactly like a typical balloon expandable stent » Thousand’s of MGuard TM coronary embolic protection systems have been implanted for AMI / STEMI indications CE Mark and commercial (coronary indication) throughout world (Not available for sale in US) History - MGuard TM Prime EPS DESIGNED FOR EMBOLIC PROTECTION

» Co - Cr Bare metal stent wrapped with an expandable MicroNet TM » Single strand Polyethylene Terephthalate (PET) fiber » Attached only to proximal and distal crowns of the stent » Deploys exactly like a typical balloon expandable stent » Thousand’s of MGuard TM coronary embolic protection systems have been implanted for AMI / STEMI indications CE Mark and commercial (coronary indication) throughout world (Not available for sale in US) History - MGuard TM Prime EPS DESIGNED FOR EMBOLIC PROTECTION Mesh (pore size 150 - 180 µ) reduces the risk of distal embolization providing protection during and post procedure
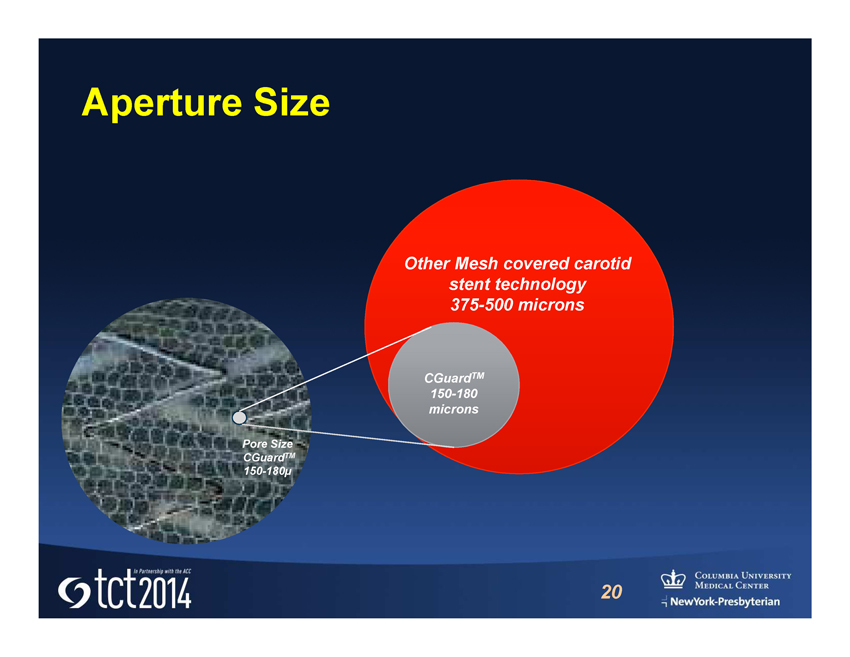
Aperture Size 20 CGuard TM 150 - 180 microns Other Mesh covered carotid stent technology 375 - 500 microns Pore Size CGuard TM 150 - 180µ
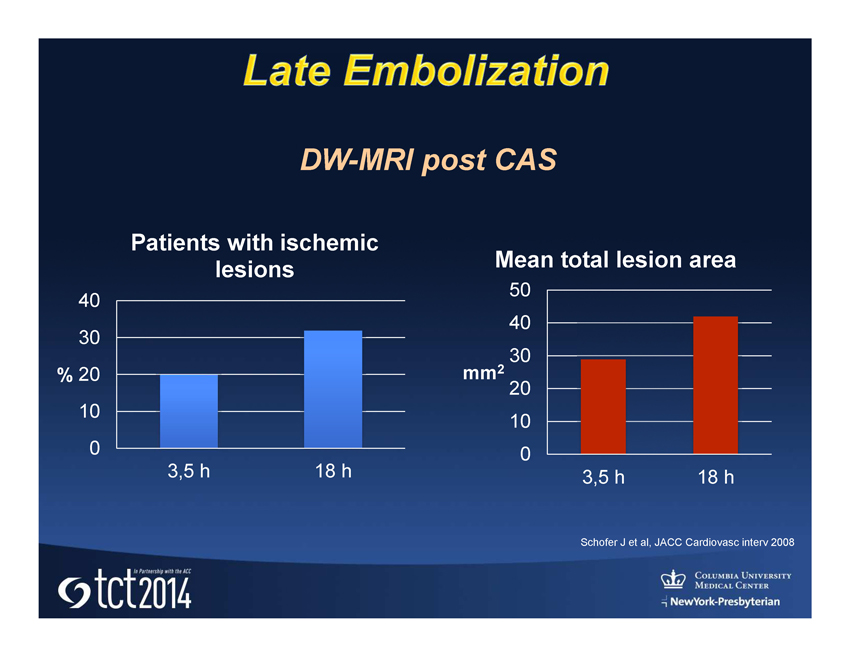
Schofer J et al, JACC Cardiovasc interv 2008 0 10 20 30 40 3,5 h 18 h % Patients with ischemic lesions 0 10 20 30 40 50 3,5 h 18 h mm 2 Mean total lesion area DW - MRI post CAS

CARENET with Distal Protection DW - MRI @ 24 - 48 hrs CARENET CGuard with only Distal EPD (N=26 * ) Incidence of New Lesions 46% Lesions ( per patient) 1.62 ± 2.68 Number of new lesion 42 Volume (per patient) 0.061 ± 0.11 cm 3 • *3 pts unable to undergo MRI (1 = pacemaker; 2 = claustrophobia) • 1 pt with proximal protection had 78 new lesions. New ischemic lesions had no clinical or neurological impact, all lesions been resolved at 30 days.

Conclusions • CARENET trial met primary endpoint of zero MACE (no death, stroke, and MI) at 30 days • The procedural success was 100% • Compared to published historical control groups of non - mesh covered carotid stents, in the CARENET trial the incidence of new ischemic lesions as assessed by DW - MRI was reduced by almost 50% and the average lesion volume per patient was 10 times smaller • These initial clinical results suggest that the MicroNet TM covered CGuard TM offers unique clinical benefits for patients undergoing CAS 23
