Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - PALISADE BIO, INC. | v388868_8k.htm |
Exhibit 99.01

Neural Stem Cell Technology Platform Cell Therapy and Pharmaceuticals September 2014 NYSE MKT: CUR

Safe Harbor statements under the Private Securities Litigation Reform Act of 1995 : This presentation contains forward - looking statements as defined in Section 27 A of the Securities Act of 1933 as amended, and section 21 E of the Securities Exchange Act of 1934 , as amended . Such forward - looking statements are based upon Neuralstem , Inc . ’s management’s current expectations, estimates, beliefs, assumptions, and projections about Neuralstem’s business and industry . Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “may,” “will,” “should,” “would,” “potential,” “continue,” and variations of these words (or negatives of these words) or similar expressions, are intended to identify forward - looking statements . In addition, any statements that refer to expectations, projections, or other characterizations of future events or circumstances, including any underlying assumptions, are forward - looking statements . These forward - looking statements are not guarantees of future performance and are subject to certain risks, uncertainties, and assumptions that are difficult to predict . Therefore, our actual results could differ materially and adversely from those expressed in any forward - looking statements as a result of various risk factors . These risks and uncertainties include the risks associated with the effect of changing economic conditions, trends in the products markets, variations in Neuralstem’s cash flow, market acceptance risks, technical development risks and other risk factors detailed in Neuralstem’s Securities and Exchange Commission filings . For links to SEC documents please visit the company’s Web site : neuralstem . com . 2 Neuralstem , Inc. Safe Harbor Statement
Neuralstem Technology – Cells Nurturing, Supporting and Rescuing Remaining Neurons • Regionally specific CNS neural stem cells: brain and spinal cord • P atents worldwide, • cGMP manufacturing • Numerous cell therapy stem cell products • Fully characterized – Expanded under defined conditions: no animal - derived reagents, serum or feeder cells – Reproducible differentiation: constitutive behavior of cells – Physiologically relevant neurons: 50% 5 Midbrain Dopaminergic Spinal Cord Cholingergic Hippocampus GABAergic

Single Platform Technology, Two Programs, Multiple Indications Ischemic Spastic Paraplegia Amyotrophic Lateral Sclerosis Optic Neuritis Lysosomal Diseases Ischemic Stroke Alzheimer’s disease Parkinson’s disease Spinal Cord Injury Traumatic Brain Injury Huntington’s disease Glioblastoma (Brain Cancer) Peripheral Nerve Injury Cerebral Palsy Multiple Sclerosis Diabetic Neuropathy Ischemic Spastic Paraplegia Major Depressive Disorder Traumatic Brain Injury Alzheimer’s disease and Pre - Alzheimer’s Dementia Post - Traumatic Stress Disorder Cognitive complication due to Diabetes Neurodegeneration Stroke Anti - Aging ( Nootropic ) 6 Current Human Clinical Trials Pre - clinical Applications

Neuralstem Patents □ U. S. Pat. No. 8,236,299 (August 2012) □ Transplantation of human n eural c ells for treatment of neurodegenerative conditions □ U.S . Pat. No. 8,058,434 (November 2011) □ Compositions to effect neuronal growth □ U.S . Pat. No. 8,030,492 (October 2011 ) □ Compositions to effect neuronal growth □ U.S. Pat. No. 7,691,629 (April 2010) □ Transplantation of human neural cells for treatment of neurodegenerative conditions □ U.S. Pat. Nos. 7,560,553 and 7,858,628 (July 2009, December 2010) □ Use of fused nicotinamides to promote neurogenesis (div) □ U.S. Pat. No. 7,544,511 (June 2009) □ Stable neural stem cell line methods □ U.S. Pat. No. 6,284,539 (September 2001) □ Method for generating dopaminergic cells derived from neural precursors □ U.S. Pat. No. 6,040,180 (March 2000) □ In vitro generation of differentiated neurons from cultures of mammalian multipotential CNS stem cells □ U.S. Pat. No. 5,753,506 (May 1998) □ Isolation propagation and directed differentiation of stem cells from embryonic and adult central nervous system of mammals ( div ) 5 Neuralstem.com Live Map Includes Worldwide I.P.

Neuralstem Small Molecule Drug Program First - in - class Neurogenic Small Molecule Drugs □ Initially funded by U.S. DOD and NIH grants □ Patented neurogenic small molecule compounds: the ability to recruit endogenous neural stem cells to generate more new neurons in the adult brain □ Lead Drug in Clinical Development: NSI - 189 □ First novel neurogenic compound □ Route of Administration: Oral □ Key biological activity: □ Enhanced neurogenesis □ Increased hippocampal volume □ Neuralstem owns 100% of the commercialization rights for the compounds □ Psychiatric/Small Molecule PI: Maurizio Fava , M.D., Slater Family Professor of Psychiatry at Harvard Medical School and Executive Vice Chair of the Department of Psychiatry at Massachusetts General Hospital 6

MDD 1b trial • ASCP annual meeting: June 17 th • A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - dose Escalation Study Evaluating the Effects of NSI - 189 Phosphate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) • Marlene Freeman, Massachusetts General Hospital • B iomarker data from trial , including Q EEG presented at the International College of Neuropsychopharmacology World Congress in Vancouver on June 24 th 7

Neurogenic NSI - 189/MDD – Phase Ib : All Depression Measures Show Clinically Meaningful Improvement For Duration of Trial • Data Shows First - time Depression Field Findings: large and significant effect size of antidepressant activity, which continued eight weeks after treatment stopped • Large effect was seen in all four commonly used scales employed in the study: SDQ, MADRS, CGI - I and CPFQ • Effect size is a statistical term measuring the overall effect of the treatment; a value above .80 is considered to be a large effect • SDQ data: Combined treatment group showed statistically significant improvement (p=0.02) after 28 days of NSI - 189 treatment compared to randomized, double - blind, placebo control group. Large effect size of 0.90 All: A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound , in Patients with Major Depressive Disorder (MDD ) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D. 20 p=0.02 d=0.90 Study Day -20 0 20 40 60 80 100 Symptoms of Depression Questionnaire 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.03 d=1.10 Day 84 Day 28 Symptoms of Depression Questionnaire (SDQ) p=0.09 d=0.95 Study Day 0 20 40 60 80 100 Montgomery and Asberg Depression Rating Scale 5 10 15 20 25 30 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.19 d=0.84 Montgomery - Asberg Depression Rating Scale (MADRS) Day 28 Day 84

Neurogenic NSI - 189/MDD – Phase Ib : All Cognitive Measures Show Clinically Meaningful Improvement for Duration of Trial • In addition to its antidepressive effects, NSI - 189 showed a significant effect size in cognitive function improvement; cognitive improvement also continued for eight weeks after the last dose • As measured by CPFQ, the assessment scale of cognitive and functioning deficits specifically designed for depressed patients, the NSI - 189 treatment group was significantly better than the placebo group (p=0.01) at Day 28. Large effect size of 0.94 • Effect size is a statistical term measuring the overall effect of the treatment; a value above .80 is considered to be a large effect All : A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phosp hate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D. 21 Day 84 Day 28 Clinical Global Impression Improvement (CGI - I) Cognitive and Physical Functioning Questionnaire (CPFQ) Day 28 Day 84 p=0.22 d=0.57 Study Day 0 20 40 60 80 100 Clinical Global Impression Scale Improvements 1 2 3 4 5 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p=0.09 d=1.13 p=0.01 d=0.94 Study Day -20 0 20 40 60 80 100 Cognitive and Physical Functioning Questionnaire 2.0 2.5 3.0 3.5 4.0 4.5 5.0 Placebo NS-189 NS-189 1x per day NS-189 2x per day NS-189 3x per day p<0.01 d=1.20

Neurogenic NSI - 189/MDD – Phase Ib : All Depressive and Cognitive Measures Show Clinically Meaningful Improvement for Duration of Trial • Quantitative EEG ( qEEG ) measurements, an electrophysical biomarker of depression, showed: – Significantly increased brain wave patterns in the hippocampal region of the brain – Increased electrical coherence in the prefrontal cortical region, which is a pro - cognitive signal • Researchers concluded that these electrophysiological changes are consistent with the neurogenic hypothesis of the drug mechanism, which involves long - term structural changes in the hippocampus • EEG Analysis: High - frequency Alpha at Day 28 – qEEG measurements at Day 28 showed statistical significance between the treatment and the placebo group in the electrical wave patterns emanating from specific areas of the brain, namely the left posterior temporal lobe and parietal region (p<0.02) A Phase 1B, Randomized, Double - Blind, Placebo - Controlled, Multiple - Dose Escalation Study Evaluating the Effects of NSI - 189 Phospha te, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) , presented June 2014, by Maurizio Fava, M.D., Karl Johe, Ph.D., Lev G. Gertsik , MD, Larry Ereshefsky , PharmD , Bettina Hoeppner , Ph.D., Martina Flynn, David Mischoulon , M.D., Ph.D., Gustavo Kinrys , M.D., and Marlene Freeman, M.D. 22 High - frequency alpha at Day 28: 10 - 12 Hz

Neuralstem Cell Therapy Surgical Devices Exclusive Worldwide Licenses □ Spinal Platform and Floating Cannula □ Proprietary breakthrough medical devices □ Expected to be the standard in industry and research community for intraspinal procedures □ Neuralstem holds exclusive worldwide licenses □ Designed by ALS trial neurosurgeon, Nicholas M. Boulis , MD, specifically for the world's first intraspinal delivery of neural stem cells □ To be utilized to deliver Neuralstem cells in the spinal cord safely and effectively for myriad diseases and injuries □ Patent - protected □ U.S. Patent No. 8,092,495 (January 2012) □ Spinal Platform and Method for Delivering a Therapeutic Agent to a Spinal Cord Target □ Issued & Pending Patents: U.S. Patent No. 7,833,217 (November 2010); U.S. Application No. 12/913,527 □ Floating Spinal Cannula and Method of Use 11

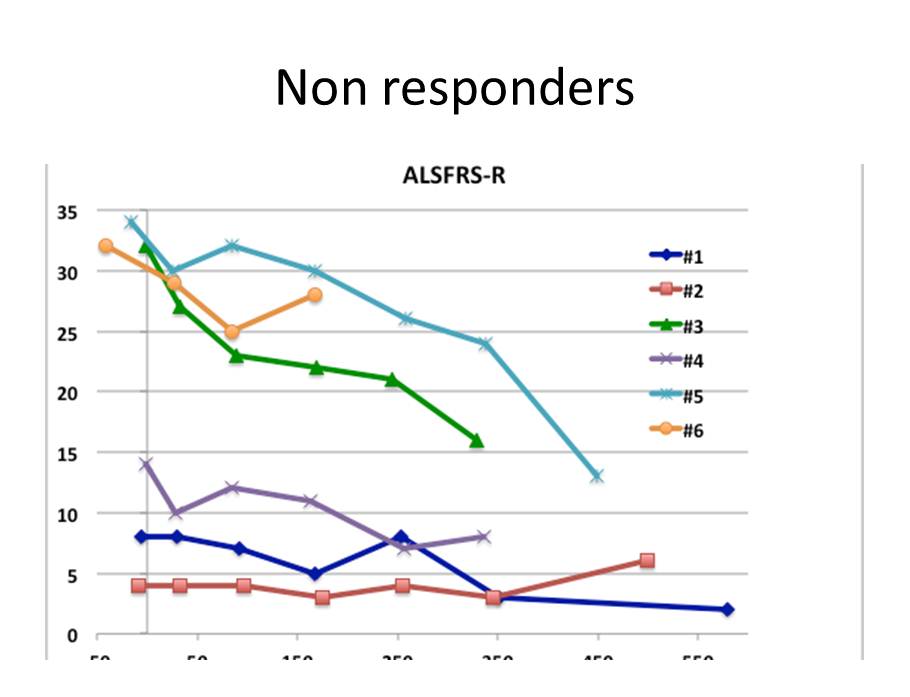
NSI - 566/ALS Phase I Success: Proven Safe, Signs of Efficacy Five of six ambulatory patients showed improvement or very slow progression of the disease post surgery ALS Functional Rating Scale – Revised ( ALSFRS - R) monitors changes in a patient over time with ten measures including speech , swallowing , walking and breathing, total score of 48. 0 baseline: Treatment 9 ALSFRS - R 0 5 10 15 20 25 30 35 40 45 50 -300 -200 -100 0 100 200 300 400 500 600 700 800 ALSFRS - R Score Days After Stem Cell Transplantation #210 #211 #212
Non responders

Neuralstem, Inc. ‘Near Virtual” model Core management functions in house □ Multiple overlap, multiple positions □ Outsource everything that makes sense □ Reduced overhead and fiscal flexibility, higher quality/less control over the calendar Financing □ Strong balance sheet □ SOX compliant □ Money goes to programs not infrastructure Operations □ Maryland – Pennsylvania – California □ China – Korea – Mexico – ( UK – Netherlands) □ Core business only – no bolt on programs – no bolt on businesses 14

Neuralstem , Inc. Collaborators World - Class Clinical P artners □ ALS Trial Clinical Investigators : □ Principal Investigator: Eva L. Feldman, M.D., Ph.D. , Professor of Neurology & Director A. Alfred Taubman Medical Research Inst. of the University of Michigan Medical School; President of American Neurological Assn. □ Site Principal Investigator: Jonathan D. Glass, M.D. , Professor of Neurology & Director Emory ALS Center Emory University □ Co - Investigator & Neurosurgeon: Nicholas M. Boulis M.D., Assist. Professor Neurosurgery Emory University □ ALS Trial Advisory Board: □ Zachary Simmons M.D., Chairman, SMB , Professor of Neurology Penn State University Hershey Medical Center and Director Neuromuscular Program and ALS Center □ Mark Bromberg M.D., Ph.D. Professor of Neurology & Director of the Motor Neuron Disease/ALS Clinic University of Utah School of Medicine □ Lucie Bruijn , Ph.D., Chief Scientist & Sr. VP of Research and Development ALS Association □ Thomas Freeman M.D., Professor, Dept of Neurosurgery & Medical Director, Center for Aging and Brain Repair University of South Florida College of Medicine □ Clifton L. Gooch, M.D., Professor & Chairman Department of Neurology & Director Division of Neuromuscular Disease University of South Florida College of Medicine □ Hiroshi Mitsumoto M.D., D.Sc., Professor of Neurology Columbia University & Director of the Eleanor and Lou Gehrig MDA/ALS Research Center and Neuromuscular Division at the Neurological Institute of New York □ Paul Park M.D., Assistant Professor & Neurosurgeon University of Michigan School of Medicine □ Mike Vogelbaum M.D., Ph.D. , Associate Director, Brain Tumor and Neuro - Oncology Center Cleveland Clinic □ Glioblastoma (Brain Cancer) Principal Investigator John Zhang, M.D., Ph.D., Professor of Neurosurgery, Loma Linda University, CA □ Spinal Cord Injury Lead Collaborator Martin Marsala , M.D., Ph.D., University of California San Diego □ Psychiatric/Small Molecule PI Maurizio Fava, M.D., Slater Family Professor of Psychiatry at Harvard Medical School and Executive Vice Chair of the Department of Psychiatry at Massachusetts General Hospital 15

Neuralstem – Strengths and Future Value Drivers 23 Scientific Strength, Out In Front • Leader: neural stem cell science • Repeated firsts in both platforms, including FDA approval: ◦ ALS & SCI cell therapy trials ◦ MDD lead novel neurogenic compound • Technology platform R&D developed at NIH • Broad, sustainable, worldwide IP protection NSI - 566: Clinical Trial Advancements • FDA - approved ALS Phase II, enrollment completed in August, trial completed in jan. 2015 • FDA - approved cSCI Phase I First patient transplant Sept/Oct 2014 • Stroke Phase I/II treatments initiated in China • Acute SCI IND expected 2014, for Phase I trial in S. Korea • Standing up ALS pilot trials in Mexico, UK , Netherlands NSI - 189: Breakthrough Year, Novel Neurogenics • Phase II Application • Phase Ib MDD trial completed 4Q13 • Data results , reported June 2014, show clinically meaningful improvement across all depressive and cognitive measures for duration of trial • Multiple Phase II trial - ready hippocampus - atrophy applications
