Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REVA Medical, Inc. | a14-17783_28k.htm |
Exhibit 99.1
|
|
REVA’s Bioresorbable Scaffold Program Update Alexandre Abizaid, MD, PhD, FACC Instituto Dante Pazzanese de Cardiologia Sao Paulo, Brazil |
|
|
REVA’s Technology Transition to the Fantom Scaffold 2011: ReZolve® Slide & Lock Platform Non-deformable design based upon slide & lock technology Currently being evaluated in 112 patients in the RESTORE II Study Preliminary results Acute technical success > 95% Uniform scaffolding with reduction in strut thickness desired to improve deliverability and laminar flow Clinical follow-up ongoing through 9-month primary endpoint 2014: REVA Transition to the Fantom™ Platform Designed to be deliverable, visible, strong, and easy to use |
|
|
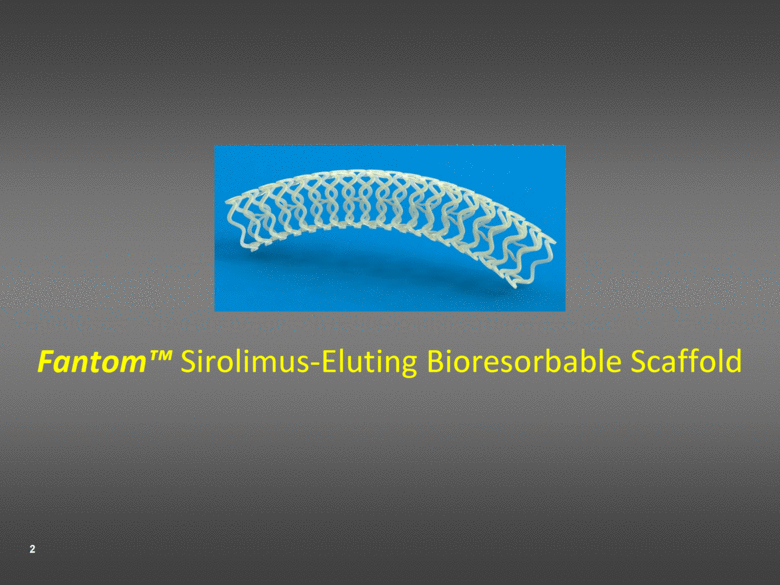
Fantom™ Sirolimus-Eluting Bioresorbable Scaffold |
|
|
REVA Polymer Family High-performance biomaterials Based on phenyl ring structure Competitive Polymers Polylactide-based Based on Alkane structure Polymer Foundation for the Fantom Scaffold REVA Polymer Family vs. Competitive Polymers Phenyl ring structure provides high strength, radiopaque materials without compromising structural properties |
|
|
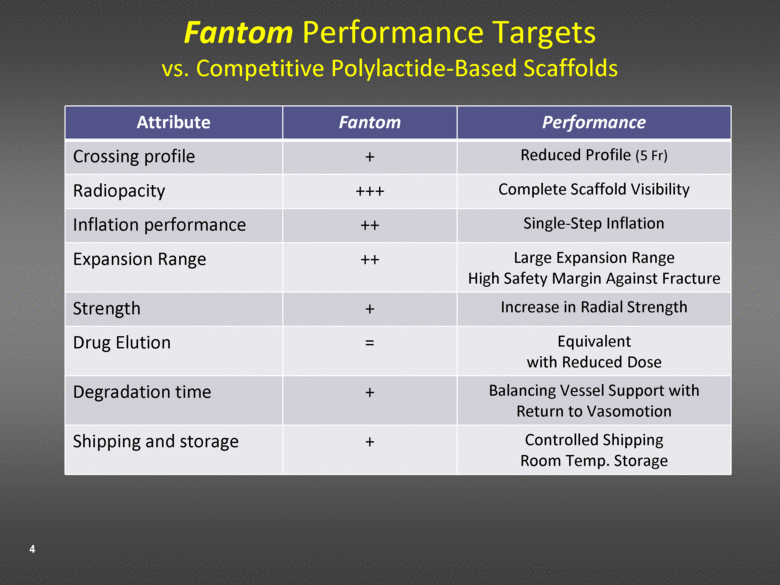
Fantom Performance Targets vs. Competitive Polylactide-Based Scaffolds Attribute Fantom Performance Crossing profile + Reduced Profile (5 Fr) Radiopacity +++ Complete Scaffold Visibility Inflation performance ++ Single-Step Inflation Expansion Range ++ Large Expansion Range High Safety Margin Against Fracture Strength + Increase in Radial Strength Drug Elution = Equivalent with Reduced Dose Degradation time + Balancing Vessel Support with Return to Vasomotion Shipping and storage + Controlled Shipping Room Temp. Storage |
|
|
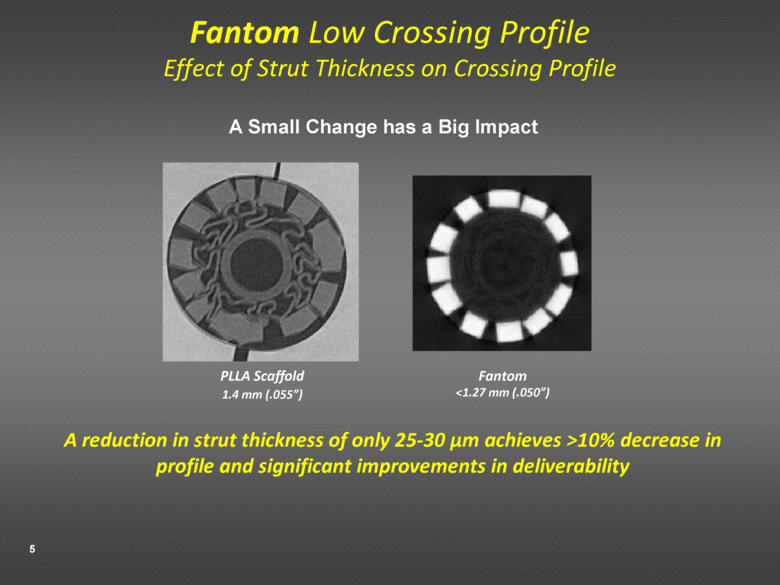
Fantom Low Crossing Profile Effect of Strut Thickness on Crossing Profile A reduction in strut thickness of only 25-30 µm achieves >10% decrease in profile and significant improvements in deliverability PLLA Scaffold 1.4 mm (.055”) Fantom <1.27 mm (.050”) A Small Change has a Big Impact |
|
|
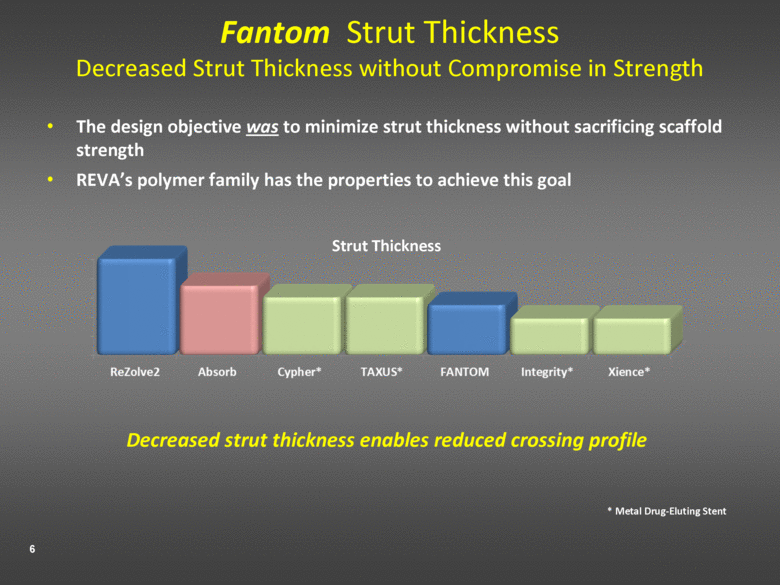
Fantom Strut Thickness Decreased Strut Thickness without Compromise in Strength The design objective was to minimize strut thickness without sacrificing scaffold strength REVA’s polymer family has the properties to achieve this goal Decreased strut thickness enables reduced crossing profile Strut Thickness * Metal Drug-Eluting Stent |
|
|
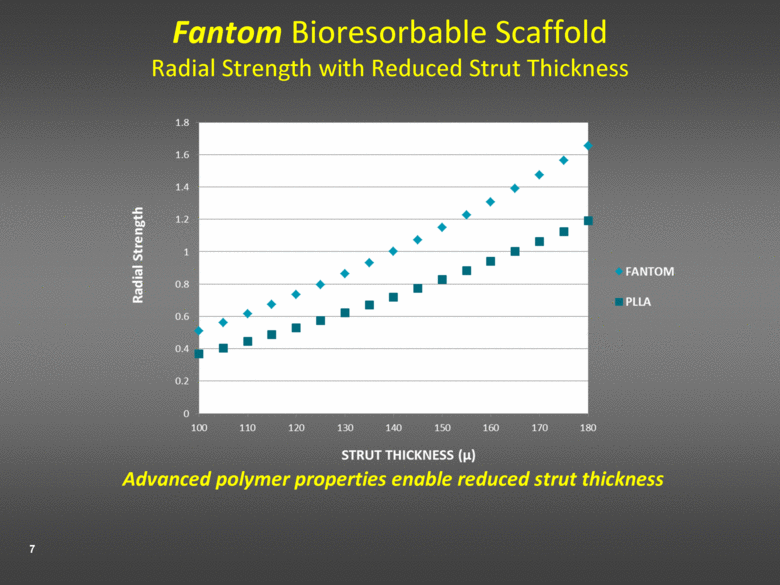
Fantom Bioresorbable Scaffold Radial Strength with Reduced Strut Thickness Advanced polymer properties enable reduced strut thickness |
|
|
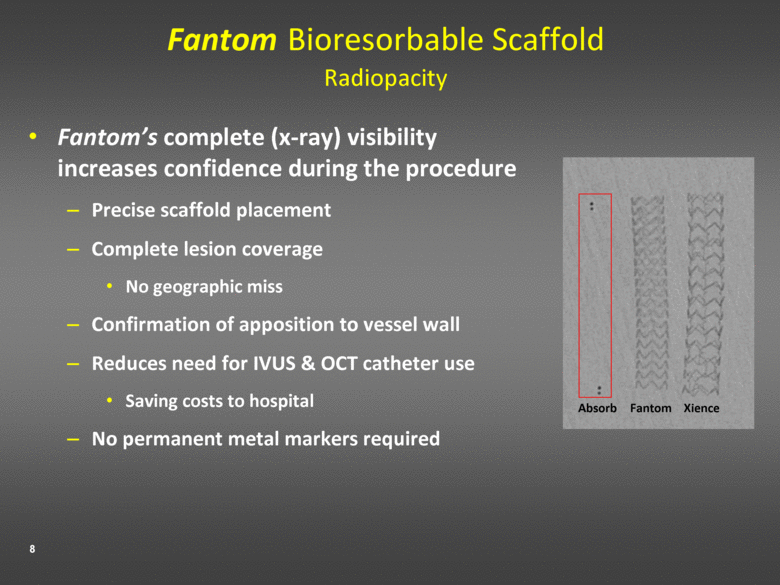
Fantom Bioresorbable Scaffold Radiopacity Fantom’s complete (x-ray) visibility increases confidence during the procedure Precise scaffold placement Complete lesion coverage No geographic miss Confirmation of apposition to vessel wall Reduces need for IVUS & OCT catheter use Saving costs to hospital No permanent metal markers required Absorb Fantom Xience |
|
|
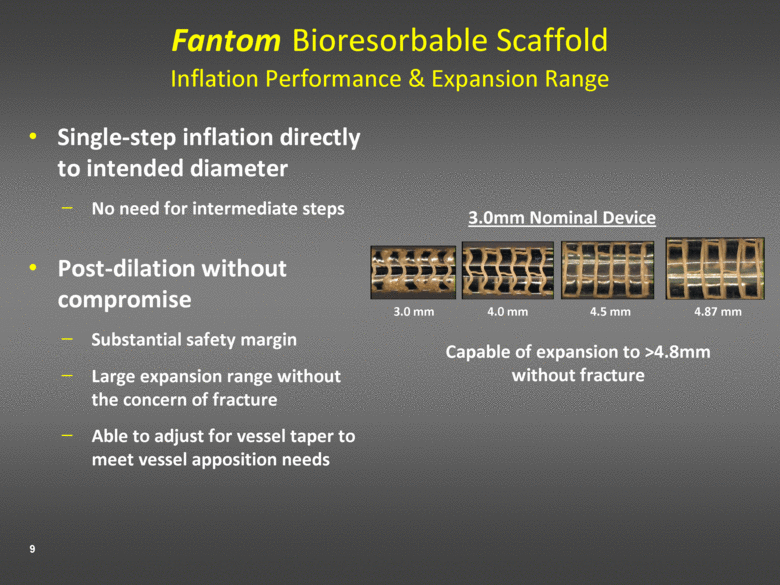
Fantom Bioresorbable Scaffold Inflation Performance & Expansion Range Single-step inflation directly to intended diameter No need for intermediate steps Post-dilation without compromise Substantial safety margin Large expansion range without the concern of fracture Able to adjust for vessel taper to meet vessel apposition needs 3.0mm Nominal Device Capable of expansion to >4.8mm without fracture 4.0 mm 4.5 mm 4.87 mm 3.0 mm |
|
|
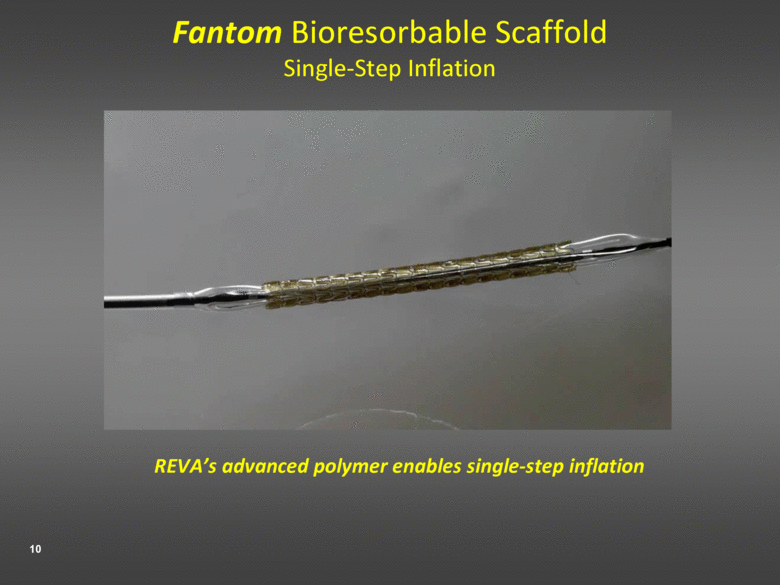
Fantom Bioresorbable Scaffold Single-Step Inflation REVA’s advanced polymer enables single-step inflation |
|
|
Fantom Bioresorbable Scaffold Degradation & Resorption Degradation Greater than 80% degradation within 1 year Full restoration of natural vasomotion Eliminates undesirable shear stress induced by a permanent implant Resorption Complete resorption within ~3 years 0 10 20 30 40 50 60 70 80 90 100 0 2 4 6 8 10 12 Percentage Months Degradation Profile Molecular Weight Loss |
|
|
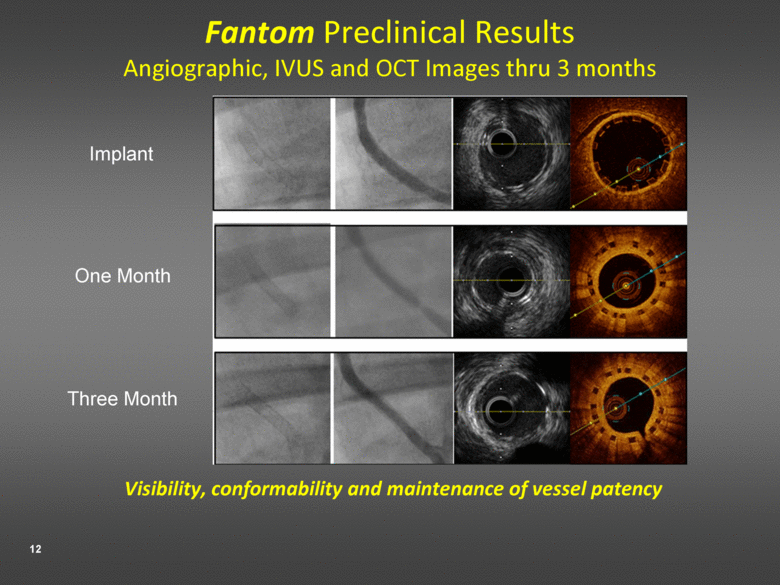
Fantom Preclinical Results Angiographic, IVUS and OCT Images thru 3 months Visibility, conformability and maintenance of vessel patency Implant One Month Three Month |
|
|
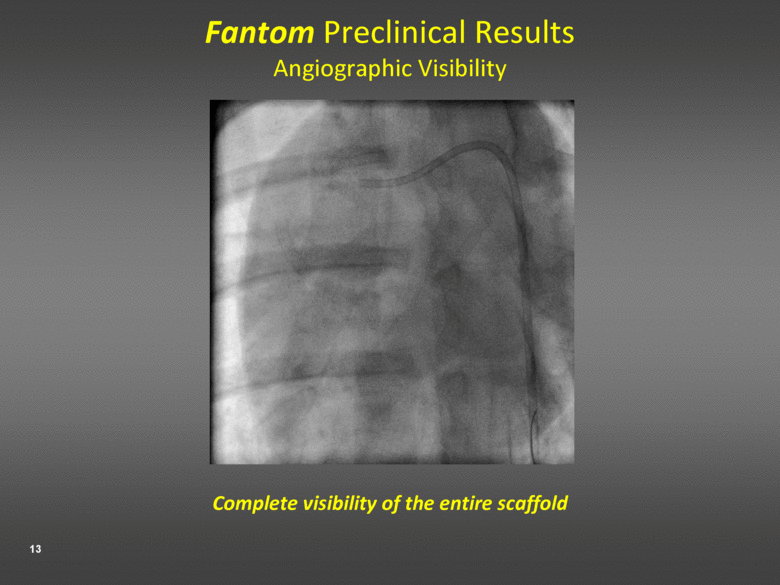
Fantom Preclinical Results Angiographic Visibility Complete visibility of the entire scaffold |
|
|
Fantom Clinical Plan First Patient Implants anticipated Q4’14 All CE sites fully enrolling by early 2015 Up to 125 patients Over 20 clinical investigational centers AUS, BE, BR, DK, FR, DE, NL and PL Primary endpoints MACE and late lumen loss at six months |
|
|
Fantom Bioresorbable Scaffold Overview Visibility Strength Ease-of-Use Improved deliverability with reduced profile Superior scaffold visibility Expansion with one smooth and continuous inflation No requirement for “stepped” inflation Post-dilation without compromise Scaffold strength to treat challenging lesions No procedural time limitations No special storage requirements Human Clinical Evaluation Q4 2014 |