Attached files
| file | filename |
|---|---|
| EX-5.1 - EX-5.1 - Sintx Technologies, Inc. | d753469dex51.htm |
| EX-23.1 - EX-23.1 - Sintx Technologies, Inc. | d753469dex231.htm |
Table of Contents
As filed with the Securities and Exchange Commission on July 16, 2014
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Amedica Corporation
(Exact name of registrant as specified in its charter)
| Delaware | 3841 | 84-1375299 | ||
| (State or other jurisdiction of incorporation or organization) |
Primary Standard Industrial Classification Code Number) |
(IRS Employer Identification No.) |
1885 West 2100 South
Salt Lake City, UT 84119
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Eric K. Olson
President and Chief Executive Officer
Amedica Corporation
1885 West 2100 South
Salt Lake City, UT, 84119
(801) 839-3500
(Name, address, including zip code, and telephone number, including area code, of agent for service)
with copies to:
Anthony E. Hubbard, Esq.
Mintz, Levin, Cohn, Ferris,
Glovsky and Popeo, P.C.
One Financial Center
Boston, MA 02111
(617) 542-6000
Approximate date of commencement of proposed sale to the public: From time to time following the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act:
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x | |||
CALCULATION OF REGISTRATION FEE
|
| ||||||||
| Title of securities to be registered | Amount to Be Registered (1) |
Proposed maximum offering price per share (2) |
Proposed maximum aggregate offering price (2) |
Amount of Registration Fee | ||||
| Common Stock, par value $0.01 per share |
2,326,409 (3) | $4.32 | $10,050,087 | $1,294 | ||||
|
| ||||||||
|
| ||||||||
| (1) | In the event of a stock split, stock dividend or other similar transaction involving the registrant’s common stock, in order to prevent dilution, the number of shares of common stock registered hereby shall be automatically increased to cover the additional common shares in accordance with Rule 416(a) under the Securities Act of 1933. |
| (2) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act of 1933. Based on the average of the high and low sales prices of the registrant’s common stock ($4.32 per share) on the NASDAQ marketplace on July 15, 2014. |
| (3) | Consists of (a) 773,333 shares of common stock issuable to the selling stockholder upon conversion of the principal amount of, and accrued or accruable interest on, the registrant’s privately placed outstanding senior convertible note; (b) 933,334 shares of common stock issuable upon conversion of the principal amount of, and accruable interest on, an additional, fixed-price senior convertible note to be issued in a private placement to the selling stockholder within ten days of the effectiveness of this resale registration statement, subject only to conditions outside of the selling stockholder’s control or that the selling stockholder cannot cause not to be satisfied, none of which are related to the market price of the common stock; (c) 50,853 shares of common stock issued to the selling stockholder in connection with the registrant entering into a securities purchase agreement dated June 30, 2014; and (d) 568,889 shares of common stock issued or issuable upon exercise of certain warrants dated June 30, 2014, issued to the selling stockholder in connection with the securities purchase agreement. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. The selling stockholders may not sell or offer these securities until the registration statement filed with the Securities and Exchange Commission is declared effective. This prospectus is not an offer to sell these securities and neither Amedica Corporation nor the selling stockholder is soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to completion, dated July 16, 2014
PROSPECTUS
2,326,409 Shares of Common Stock
of
Amedica Corporation
This prospectus relates to the sale of up to 2,326,409 shares of our common stock by MG Partners II Ltd., or the Selling Stockholder, consisting of:
| • | 1,706,667 shares issued or issuable upon conversion of an aggregate principal amount of $6.4 million of our senior convertible notes, including accrued interest, subject to adjustment; |
| • | 50,853 shares issued to the Selling Stockholder in connection with a securities purchase agreement dated June 30, 2014; and |
| • | 568,889 shares issued or issuable to the Selling Stockholder upon exercise of warrants at an exercise price of $4.65 per share, subject to adjustment pursuant to the terms of the warrant. |
The shares offered by this prospectus may be sold from time to time by the selling stockholder at prevailing market prices or prices negotiated at the time of sale. See “Plan of Distribution” and “Selling Stockholder.” The shares offered by this prospectus were issued or are issuable upon conversion or exercise of securities issued to the selling stockholder in transactions exempt from registration under the Securities Act of 1933, or the Securities Act.
We will not receive any cash proceeds from the sale of shares by the selling stockholder, but to the extent that the warrants are exercised in whole or in part for cash, we will receive payment for the exercise price. We will pay the expenses of registering the shares.
Our common stock is listed on the NASDAQ Capital Market under the symbol “AMDA.” The last reported sale price of our common stock on July 15, 2014 was $4.25 per share. The Selling Stockholder will sell at prevailing market prices per share (as quoted on the NASDAQ), at the time of sale, at fixed prices, at varying prices determined at the time of sale, or at negotiated prices.
Investing in our common stock involves a high degree of risk. These risks are described under the caption “Risk Factors” that begins on page 8 of this prospectus.
Neither the United States Securities and Exchange Commission, or the SEC, nor any state securities commission has approved or disapproved of the common stock that may be offered under this prospectus, nor have any of these regulatory authorities determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2014.
Table of Contents
| Page | ||||
| 1 | ||||
| 8 | ||||
| 28 | ||||
| 29 | ||||
| 29 | ||||
| 30 | ||||
| 30 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
31 | |||
| 48 | ||||
| 70 | ||||
| 74 | ||||
| 80 | ||||
| 83 | ||||
| 85 | ||||
| 89 | ||||
| 90 | ||||
| 91 | ||||
| 91 | ||||
| 91 | ||||
| 91 | ||||
You should rely only on the information contained in this prospectus or contained in any prospectus supplement or free writing prospectus filed with the SEC. Neither we nor the Selling Stockholder has authorized anyone to provide you with additional information different from that contained in this prospectus filed with the SEC. The Selling Stockholder is not making an offer to sell, or soliciting offers to buy, shares of our common stock in any jurisdiction where the offer is not permitted. The information contained in this prospectus is accurate only as of date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock.
This prospectus includes statistical and other industry and market data that we obtained from industry publications and research surveys and studies conducted by third parties. Industry publications and third party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe these industry publications and third party research, surveys and studies are reliable, we have not independently verified such data.
For investors outside the United States: neither we nor the Selling Stockholder has done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside the United States.
Table of Contents
This summary highlights information contained elsewhere in this prospectus and other information incorporated by reference herein. Because it is only a summary, it does not contain all of the information that you should consider before investing in shares of our common stock and it is qualified in its entirety by, and should be read in conjunction with the more detailed information appearing elsewhere in this prospectus. You should read the entire prospectus carefully, especially “Risk Factors” and other information incorporated by reference herein. Unless the context requires otherwise, references to “Amedica,” “we,” “our” and “us” in this prospectus refer to Amedica Corporation and its subsidiary.
Amedica Corporation
Our Company
We are a commercial biomaterial company focused on using our silicon nitride technology platform to develop, manufacture and sell a broad range of medical devices. We currently market spinal fusion products and are developing products for use in total hip and knee joint replacements. We believe our silicon nitride technology platform enables us to offer new and transformative products in the orthopedic and other medical device markets. We believe we are the first and only company to use silicon nitride in medical applications and over 14,000 of our silicon nitride spine products have been implanted in patients.
Biomaterials are synthetic or natural materials available in a variety of forms that are used in virtually every medical specialty. We believe our silicon nitride biomaterial has superior characteristics compared to commonly used biomaterials in the markets we are targeting, including polyetheretherketone, or PEEK, which is the most common biomaterial used for interbody spinal fusion products. Specifically, we believe our silicon nitride has the following key attributes: promotion of bone growth; hardness, strength and resistance to fracture; resistance to wear; non-corrosive; anti-infective properties; and superior diagnostic imaging compatibility.
We currently market our Valeo family of silicon nitride interbody spinal fusion devices in the United States and Europe for use in the cervical and thoracolumbar areas of the spine. We believe our Valeo devices have a number of advantages over existing products due to silicon nitride’s key characteristics, resulting in faster and more effective fusion and reduced risk of infection. As of December 31, 2013, the rate of adverse events reported to the U.S. Food and Drug Administration, or FDA, for our implanted Valeo interbody spinal fusion devices is 0.1%.
In addition to our silicon nitride-based spinal fusion products, we market a complementary line of non-silicon nitride spinal fusion products which allows us to provide surgeons and hospitals with a broader range of products. These products include three lines of spinal fusion devices and three types of orthobiologics, which are used by surgeons to help promote bone growth and fusion in spinal fusion procedures. Although our non-silicon nitride products have accounted for approximately 57% of our product revenues for the three months ended March 31, 2014 and 66% and 74% or more of our product revenues for the years ended December 31, 2013 and 2012, we believe the continued promotion and potential for adoption of our silicon nitride products and product candidates, if approved, provides us the greatest opportunity to grow our business in new and existing markets and achieve our goal to become a leading biomaterial company.
We are also incorporating our silicon nitride technology into components for use in total hip and knee replacement product candidates that we are, or plan on, developing in collaboration with a strategic partner. We believe that our silicon nitride total hip and knee product candidates will provide competitive advantages over current products made with traditional biomaterials. We believe our silicon nitride technology platform can be used for developing products in other markets and have developed prototypes for use in the dental, sports medicine and trauma markets. As a result of some of the key characteristics of our silicon nitride, we also believe our coating technology may be used to enhance our metal products as well as commercially available metal spinal fusion, joint replacement and other medical products.
We operate a 30,000 square foot manufacturing facility located at our corporate headquarters in Salt Lake City, Utah, and we are the only vertically integrated silicon nitride orthopedic medical device manufacturer in the world. We market and sell our products to surgeons and hospitals in the United States and select markets in Europe and South America through our established network of more than 50 independent sales distributors who are managed by our experienced in-house sales and marketing management team.
Market Opportunity
Our products and product candidates target the interbody spinal fusion and total hip and knee joint replacement markets. According to iData Research, Inc., in 2012, the markets for spinal implants in the United States and in combined major European markets were $5.3 billion and $1.0 billion, respectively. Interbody spinal fusions accounted for over $1.2 billion and $172.2 million of these markets, respectively. Additionally, Orthopedic Network News reported that the U.S. markets for the components of total hip and knee replacement product candidates that we are initially developing were $455.0 million and $1.5 billion, respectively.
1
Table of Contents
Our Silicon Nitride Technology Platform
We believe our silicon nitride, an advanced ceramic, is ideally suited for use in many medical applications and has the following characteristics that make it superior to other biomaterials, which do not possess all of these characteristics:
| • | Promotes Bone Growth. The biomaterials used in interbody spinal fusion devices should promote bone growth in and around the device to further support fusion and stability. Our silicon nitride has an inherent surface chemistry and topography which creates an ideal environment for the promotion of new bone growth. |
| • | Hard, Strong and Resistant to Fracture. The biomaterials used in interbody spinal fusion devices and joint replacement implants should be strong and resistant to fracture during implantation of the device and withstand the static and dynamic forces exerted on the spine or to adequately bear the significant loads placed on joints during daily activities. Biomaterials used in joint replacements should also be resistant to deformation, which is referred to as hardness. We believe our silicon nitride is hard, strong and resistant to fracture. |
| • | Anti-Infective. Infection is a serious problem in orthopedic surgery and treating device-related infection generally requires extensive repeat surgery, including replacement, or revision, surgery, which extends patient suffering and increases costs. We have demonstrated in in vitro and in vivo studies that our silicon nitride has inherent anti-infective properties, which reduce the risk of infection in and around a silicon nitride device. We demonstrated that live bacteria counts were between 8 to 30 times lower on silicon nitride than PEEK and up to 8 times lower on silicon nitride than titanium, another commonly used biomaterial. |
| • | Imaging Compatible. The biomaterials used in interbody spinal fusion devices should be visible through, and not inhibit the effective use of, common surgical and diagnostic imaging techniques, such as x-ray, CT and MRI. Our silicon nitride interbody spinal fusion devices are semi-radiolucent and clearly visible in x-rays, and produce no distortion under MRI and no scattering under CT. These characteristics enable an exact view of the device for precise intra-operative placement and post-operative bone fusion assessment in spinal fusion procedures. We believe these qualities provide surgeons with greater certainty of outcomes with our silicon nitride devices than with other biomaterials, such as PEEK and metals. |
| • | Resistant to Wear. The biomaterials used in joint replacement procedures should have sufficient hardness and toughness, as well as extremely smooth surfaces, to effectively resist wear. Because the articulating implants move against each other, they are subject to friction and cyclic loading, which frequently lead to abrasive wear and fatigue failure. We believe joint implants incorporating our silicon nitride components will have comparable or higher resistance to wear than the two most commonly used combinations of biomaterials in total hip replacement implants. |
| • | Non-Corrosive. Biomaterials should be non-corrosive and should not cause adverse patient reactions. Metal placed in the human body corrodes over time and also results in the release of metal ions that can cause serious adverse reactions and conditions. Our silicon nitride does not have the drawbacks associated with the corrosive nature of metal within the body nor does it result in the release of metal ions into the body. |
We produce silicon nitride in four forms: (1) a fully dense, load-bearing solid, referred to as MC2; (2) a porous bone-like cancellous structured form, referred to as CSC; (3) a composite incorporating both our solid MC2 material and our porous CSC material intended to promote an ideal environment for bone growth; and (4) a coating for application onto other biomaterials. This capability provides us with the ability to utilize our silicon nitride in distinct ways depending on its intended application, which, together with our silicon nitride’s key characteristics, distinguishes us from manufacturers of other biomaterials and our products from products using other biomaterials.
Our Competitive Strengths
We believe we can use our silicon nitride technology platform to become a leading biomaterial company and have the following principal strengths:
| • | Sole Provider of Silicon Nitride Medical Devices. We believe we are the only company that designs, develops, manufactures and sells medical grade silicon nitride-based products. |
| • | In-House Manufacturing Capabilities. We operate a 30,000 square foot manufacturing facility located at our corporate headquarters in Salt Lake City, Utah. This state-of-the-art facility allows us to rapidly design and produce silicon nitride products and control the entire manufacturing process from raw material to finished goods. We have also entered to a manufacturing, development and supply agreement with Kyocera Industrial Ceramics Corporation, or Kyocera, under which Kyocera will become a second qualified manufacturer of our silicon nitride-based spinal fusion products and product candidates. |
2
Table of Contents
| • | Established Commercial Infrastructure. We market and sell our products to surgeons and hospitals in the United States and select markets in Europe and South America through our established network of more than 50 independent sales distributors who are managed by our experienced in-house sales and marketing management team. |
| • | Portfolio of Non-Silicon Nitride Products. We offer a full suite of spinal fusion products, which increases our access to surgeons and hospitals and allows us to more effectively market our silicon nitride spinal fusion products to our customers. |
| • | Highly Experienced Management and Surgeon Advisory Team. We have recently assembled a senior management team with over 150 years of collective experience in the healthcare industry. Members of our management team have experience in product development, launching of new products into the orthopedics market and selling to hospitals through direct sales organizations, distributors, manufacturers and other orthopedic companies. We also collaborate with a network of leading surgeon advisors in the design, development and use of our products and product candidates. |
Our Strategy
Our goal is to become a leading biomaterial company focused on using our silicon nitride technology platform to develop, manufacture and commercialize a broad range of medical devices. Key elements of our strategy to achieve this goal are the following:
| • | Drive Further Adoption of our Silicon Nitride Interbody Spinal Fusion Devices. We believe that increasing the awareness of our silicon nitride technology by educating surgeons about its key benefits, and the design improvements to our silicon nitride products and related instruments, will accelerate the adoption of our products and ultimately help improve patient outcomes. To drive further awareness of our products and the associated benefits offered by our silicon nitride technology, we will continue to educate surgeons through multiple channels, including industry conferences and meetings, media outlets and through our sales and marketing efforts. |
| • | Continue to Implement our Design and Build Program. In 2013, we initiated a commercialization strategy, referred to as our Design and Build Program, in which we collaborate with influential surgeons to develop customized silicon nitride spinal fusion products and instruments. We first sell these products to the designing surgeons and a team of evaluating surgeons. After evaluation and acceptance by these surgeons, we plan to introduce these products more broadly into the market. The first products designed under this program were sold for initial evaluation in the third quarter of 2013. |
| • | Enhance our Commercial Infrastructure. We expect to increase the productivity of our sales and marketing team by continuing to engage experienced independent sales distributors with strong orthopedic surgeon relationships. For example, in October 2013, we entered into a new European sales agent agreement with K2M, Inc., one of the largest privately held spinal device companies in the world. We may also establish distribution collaborations in the United States and abroad when access to large or well-established sales and marketing organizations may help us gain access to new markets, increase sales in our existing markets or accelerate market penetration for selected products. |
| • | Develop Silicon Nitride for Total Joint Components. We are incorporating our silicon nitride technology into silicon nitride-coated metal components for use in total hip and knee replacement product candidates that we plan on developing in collaboration with a strategic partner. We also have designs for solid silicon nitride components and we will make a decision in the future about whether to pursue the development of these components. In December 2013, we participated in a pre-submission meeting with the FDA to finalize the regulatory requirements for a 510(k) clearance of our silicon nitride-coated total joint components in the United States. The FDA reviewers confirmed that the regulatory pathway would be a standard 510(k) clearance with supporting biomechanical testing. In response, we intend to develop silicon nitride-coated metal joint replacement components and then, together with a strategic partner, initiate biomechanical testing with our silicon nitride-coated metal components for use in total hip and knee replacement procedures to support a 510(k) submission to the FDA. We intend to pursue clearance of a total hip replacement product first and, if clearance is obtained, we intend to commercially launch silicon nitride-coated metal products for use in total hip replacement by the second half of 2015. |
| • | Apply our Silicon Nitride Technology Platform to Other Opportunities. Our silicon nitride technology platform is adaptable and we believe it may be used to develop products to address other significant opportunities, such as in the dental, sports medicine and trauma markets. We have manufactured prototypes of dental implants, sports medicine and trauma products, and we have developed a process to coat metals with our silicon nitride to enhance current medical devices and instruments. We plan to collaborate with other companies to develop and commercialize any future products in those areas or we may develop any one of them by ourselves should sufficient resources become available. |
3
Table of Contents
Risks Associated with Our Business
Our business is subject to a number of risks that you should be aware of before making an investment decision. These risks are discussed more fully in the section of this prospectus entitled “Risk Factors” immediately following this prospectus summary. You should read these risks before you invest in our common stock. We may be unable, for many reasons, including those that are beyond our control, to implement our business strategy. In particular, risks associated with our business include:
| • | our accumulated deficit was $144.6 million as of March 31, 2014, and we expect we will continue to incur additional, and possibly increasing, losses, which, among other things, raises doubts about our ability to continue as a going concern; |
| • | our success depends on our ability to successfully commercialize silicon nitride-based medical devices, which to date have experienced only limited market acceptance and may not be widely accepted by hospitals and surgeons in the future; |
| • | we may not be able to increase the productivity of our sales and marketing infrastructure to successfully penetrate the spinal fusion market; |
| • | our long-term success depends substantially on our ability to obtain regulatory clearance or approval of our product candidates and then successfully commercializing these product candidates; |
| • | the orthopedic market is highly competitive and we may not be able to compete effectively against the larger, well-established companies that dominate this market or emerging and small innovative companies; and |
| • | we and our former independent registered public accounting firm have identified material weaknesses and a significant deficiency in our internal control over financial reporting, which increases the risk of material misstatements in our future financial statements. |
Corporate Information
We were incorporated in Delaware in 1996 under the name Amedica Corp. and have since changed our name to Amedica Corporation. Effective September 20, 2010, we acquired all of the outstanding shares of US Spine, Inc. which then became our wholly-owned subsidiary, which is our only subsidiary. Our principal executive offices are located at 1885 West 2100 South, Salt Lake City, Utah 84119, and our telephone number is (801) 839-3500. Our web site address is www.amedicacorp.com. The information on, or that may be accessed through, our web site is not incorporated by reference into this prospectus and should not be considered a part of this prospectus.
Certain monetary amounts, percentages and other figures included in this prospectus have been subject to rounding adjustments. Accordingly, figures shown as totals in certain tables may not be the arithmetic aggregation of the figures that precede them, and figures expressed as percentages in the text may not total 100% or, as applicable, when aggregated may not be the arithmetic aggregation of the percentages that precede them.
“Amedica,” “CSC,” “MC2,” “Valeo” and “rethink what’s possible” are registered U.S. trademarks of Amedica Corporation. “US Spine” is a registered U.S. trademark of our subsidiary, US Spine, Inc. All other trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners. Trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or TM symbols for convenience. Such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Recent Developments
Hercules Loan and Security Agreement
On June 30, 2014, we entered into a Loan and Security Agreement with Hercules Technology Growth Capital, Inc., or Hercules Technology, as administrative and collateral agent for the lenders thereunder and as lender, and Hercules Technology III, LP, as lender. The Loan and Security Agreement provides us with a $20 million term loan that matures on January 1, 2018, and which is secured by substantially all of our assets. Proceeds of the loan were used to repay in full and terminate our prior senior secured credit facility with General Electric Capital Corporation and the remainder of these loan proceeds will be used for general corporate purposes.
4
Table of Contents
This term loan bears interest at the rate of the greater of either (i) the prime rate plus 7.7%, and (ii) 10.95%. Interest accrues from the date of the Loan and Security Agreement with interest payments due monthly commencing on July 1, 2014. Principal payments are required commencing August 1, 2015 and are to be made in thirty equal installments, with the remainder due at maturity; provided, however, in the event that we meet certain conditions set forth in the Loan and Security Agreement, the interest only period may be extended through February 1, 2016, reducing the number of required principal payments to twenty-four. Additionally, under certain circumstances, we may, or Hercules Technology may require that we repay a portion of the principal in the form of shares of our common stock. The conversion price to be used for the calculation of the amount of shares to be delivered in such instance is $5.72 per share. The Loan and Security Agreement contains representations and warranties, affirmative, negative and financial covenants, and events of default customary for financings of this type, including, among other things, limitations on certain other indebtedness, loans and investments, liens, mergers, asset sales and transactions with affiliates, including a minimum liquidity covenant that requires us maintain cash and cash equivalents of not less than $6.0 million through August 15, 2014, and not less than $9.0 million thereafter. We will need to obtain additional funding during the fourth quarter of 2014 to maintain compliance with the financial and liquidity covenants under the Loan and Security Agreement through the next twelve months. Furthermore, if we are unable to access additional funds prior to becoming non-compliant with the financial or liquidity covenants, the entire remaining balance of the Loan and Security Agreement could become immediately due and payable at the option of Hercules Technology.
In addition, we issued a warrant to the Hercules Technology, or the Hercules Warrant, to purchase 516,129 shares of our common stock, subject to adjustment as set forth in the Hercules Warrant; provided, further, that the number of shares of our common stock for which the Hercules Warrant may be exercisable may be increased to 623,655 in the event that we do not meet certain conditions set forth in the Hercules Warrant.
Under the Loan and Security Agreement, we will incur a $500,000 penalty in the event that the registration statement related to this prospectus is not declared effective by the SEC on or before August 15, 2014.
Private Placement of Senior Convertible Notes and Warrant to Magna
On June 30, 2014, we entered into a Securities Purchase Agreement with MG Partners II Ltd., an affiliate of Magna, or the Selling Stockholder. Pursuant to the terms of the Securities Purchase Agreement, we sold to the Selling Stockholder an initial unsecured senior convertible note with an original principal amount of $2.9 million, or the Initial Convertible Note, for a purchase price of $2.5 million. Additionally, the Selling Stockholder is irrevocably bound to purchase, an additional unsecured senior convertible note with an original principal amount of $3.5 million, or the Additional Convertible Note, for a fixed purchase price of $3.5 million, subject only to conditions outside of the Selling Stockholder’s control or that the Selling Stockholder cannot cause not to be satisfied, none of which are related to the market price of shares of our common stock, no later than ten calendar days after the effective date of a registration statement registering the shares issued in connection with the Securities Purchase Agreement. The Initial Convertible Note and Additional Convertible Note are collectively referred to as the Convertible Notes.
With respect to the Initial Convertible Note, $150,000 of the outstanding principal amount (together with any accrued and unpaid interest with respect to such portion of the principal amount) will be automatically extinguished if (1) we have properly filed a registration statement registering the shares issued in connection with the Securities Purchase Agreement with the SEC on or prior to August 14, 2014 and (2) no event of default, or an event that with the passage of time or giving of notice would constitute an event of default, has occurred on or prior to such date. In addition, $250,000 of the outstanding principal amount of the Initial Convertible Note (together with any accrued and unpaid interest with respect to such portion of the principal amount) will be automatically extinguished if (1) the registration statement is declared effective by the SEC on or prior to the earlier of (i) October 13, 2014 and (ii) the fifth trading day after the SEC notifies us that the registration statement is not subject to further review, and this prospectus is available for use by the Selling Stockholder and (2) no event of default, or an event that with the passage of time or giving of notice would constitute an event of default, has occurred on or prior to such date. The Securities Purchase Agreement and the Convertible Notes also contain representations and warranties, covenants and events of default customary for financings of this type, including, among other things, limitations on certain other indebtedness, dividends and distributions, sales and transfers of assets and transactions with affiliates.
The Convertible Notes mature on June 30, 2016 (subject to extension as provided in the Convertible Notes) and accrue interest at an annual rate of 6.0%. The Convertible Notes are convertible at any time after issuance, in whole or in part, at the Selling Stockholder’s option, into shares of common stock at an initial fixed conversion price equal to $3.75 per share, or the Initial Fixed Price. If, however, the closing sale price of our common stock is below 110% of the Initial Fixed Price, or $4.13 per share, for two consecutive trading days, either:
| (1) | we may redeem the Convertible Notes in full at a 127.5% premium of the entire principal and interest remaining on the Convertible Notes within sixty (60) days of such occurrence; or |
| (2) | beginning on the 61st day following such occurrence, the Selling Stockholder will be able to convert the Convertible Notes, in whole or in part, at the lessor of: (i) the Initial Fixed Price or (ii) a price equal to 80% of the lowest daily volume weighted average price, or VWAP, of our common stock during the five trading days prior to conversion. |
5
Table of Contents
In addition, we issued to the Selling Stockholder a warrant, or the Magna Warrant, to purchase up to 568,889 shares of our common stock at an exercise price of $4.65 per share. The Magna Warrant expires on June 30, 2016 and is exercisable as to 257,778 shares immediately and will become exercisable as of the date of issuance of the Additional Convertible Note with respect to the remaining 311,111 shares.
At no time will the Selling Stockholder be entitled to convert any portion of the Convertible Notes or exercise any portion of the Magna Warrant to the extent that after such conversion or exercise, the Selling Stockholder (together with its affiliates) would beneficially own more than 4.99% of the outstanding shares of our common stock as of such date, or the Maximum Percentage. The Maximum Percentage may be raised to any other percentage not in excess of 9.99% at the option of the Selling Stockholder upon at least 61 days’ prior notice to us, or lowered to any other percentage, at the option of the Selling Stockholder, at any time.
We also issued 50,853 shares of our common stock to the Selling Stockholder which represents 4% of the total purchase price for the Convertible Notes under the Securities Purchase Agreement calculated using a per share price of $4.7195, the VWAP of the Common Stock per share on June 27, 2014, in connection with our execution of the Securities Purchase Agreement.
See “Description of Securities—Senior Convertible Notes”. The resale by Selling Stockholder of the shares issued in connection with, or issuable upon conversion of, the Convertible Notes and exercise of the Magna Warrant are covered by this prospectus.
6
Table of Contents
THE OFFERING
| Common stock that may be offered by the Selling Stockholder | 2.326,409 shares of our common stock. See “Selling Stockholder” on page 89. | |
| Common stock outstanding before this offering | 12,411,207 Shares as of July 16, 2014 | |
| Common stock outstanding after this offering (assuming full conversion of the Convertible Notes and full exercise of the Magna Warrant) | 14,686,763 shares | |
| Use of proceeds | We will not receive any of the proceeds from the sale or other disposition of the shares of our common stock covered by this prospectus by the Selling Stockholders. However, we may receive proceeds upon the cash exercise of the Magna Warrant, the underlying shares of which are offered by this prospectus. | |
| Risk factors | See “Risk Factors” beginning on page 8 and other information included in this prospectus for a discussion of the risks of investing in our common stock. | |
| NASDAQ Capital Market symbol | AMDA | |
7
Table of Contents
An investment in shares of our common stock involves a high degree of risk. You should carefully read and consider the risks described below, as well as the other information in this prospectus and other information incorporated by reference herein, before deciding to invest in our common stock. The occurrence of any of the following risks could have a material adverse effect on our business, financial condition, results of operations or cash flows. In that case, the trading price of our common stock could decline, and you could lose all or part of your investment.
Risks Related to Our Business and Strategy
We have incurred net losses since our inception and anticipate that we will continue to incur substantial net losses for the foreseeable future. We may never achieve or sustain profitability.
We have incurred substantial net losses since our inception. For the years ended December 31, 2013 and 2012 we incurred a net loss of $8.2 million and $35.0 million, respectively, and used cash in operations of $9.2 million and $9.7 million, respectively. We have an accumulated deficit of $144.6 million at March 31, 2014. Our losses have resulted principally from costs incurred in connection with our sales and marketing activities, research and development activities, manufacturing activities, general and administrative expenses associated with our operations, impairments on intangible assets and interest expense. Even if we are successful in launching additional products into the market, we expect to continue to incur substantial losses for the foreseeable future as we continue to sell and market our current products and research and develop, and seek regulatory approvals for, our product candidates.
If sales revenue from any of our current products or product candidates that receive marketing clearance from the FDA or other regulatory body is insufficient, if we are unable to develop and commercialize any of our product candidates, or if our product development is delayed, we may never become profitable. Even if we do become profitable, we may be unable to sustain or increase our profitability on a quarterly or annual basis.
Our success depends on our ability to successfully commercialize silicon nitride-based medical devices, which to date have experienced only limited market acceptance.
We believe we are the first and only company to use silicon nitride in medical applications. To date, however, we have had limited acceptance of our silicon nitride-based products and our product revenue has been derived substantially from our non-silicon nitride products. In order to succeed in our goal of becoming a leading biomaterial technology company utilizing silicon nitride, we must increase market awareness of our silicon nitride interbody spinal fusion products, continue to implement our sales and marketing strategy, enhance our commercial infrastructure and commercialize our silicon nitride joint replacement components and other products. If we fail in any of these endeavors or experience delays in pursuing them, we will not generate revenues as planned and will need to curtail operations or seek additional financing earlier than otherwise anticipated.
Our current products and our future products may not be accepted by hospitals and surgeons and may not become commercially successful.
Although we received 510(k) regulatory clearance from the FDA for our first silicon nitride spinal fusion products in 2008, we have not been able to obtain significant market share of the interbody spinal fusion market to date, and may not obtain such market share in the future. Even if we receive regulatory clearances or approvals for our product candidates in development, these product candidates may not gain market acceptance among orthopedic surgeons and the medical community. Orthopedic surgeons may elect not to use our products for a variety of reasons, including:
| • | lack or perceived lack of evidence supporting the beneficial characteristics of our silicon nitride technology; |
| • | limited long-term data on the use of silicon nitride in medical devices; |
| • | lower than expected clinical benefits in comparison with other products; |
| • | the perception by surgeons that there are insufficient advantages of our products relative to currently available products; |
| • | hospitals may choose not to purchase our products; |
| • | group purchasing organizations may choose not to contract for our products, thus limiting availability of our products to hospital purchasers; |
| • | the price of our products, which may be higher than products made of the other commonly used biomaterials in the interbody spinal fusion market and total joint market; |
8
Table of Contents
| • | lack of coverage or adequate payment from managed care plans and other third-party payors for the procedures that use our products; |
| • | Medicare, Medicaid or other third-party payors may limit or not permit reimbursement for procedures using our products; |
| • | ineffective marketing and distribution support; |
| • | the time and resources that may be required for training, or the inadequate training, of surgeons in the proper use of our products; |
| • | the development of alternative biomaterials and products that render our products less competitive or obsolete; and |
| • | the development of or improvement of competitive products. |
If surgeons do not perceive our silicon nitride products and product candidates as superior alternatives to competing products, we will not be able to generate significant revenues, if any.
Even if surgeons are convinced of the superior characteristics of our silicon nitride products and our product candidates that we successfully introduce compared to the limitations of the current commonly used biomaterials, surgeons may find other methods or turn to other biomaterials besides silicon nitride to overcome such limitations. For instance, with respect to interbody spinal fusion products, surgeons or device manufacturers may use more effective markers for enhancing the imaging compatibility of PEEK devices, more effective antibiotics to prevent or treat implant-related infections, and more effective osteoconductive and osteoinductive materials when implanting an interbody spinal fusion device. Device manufacturers may also coat metal with existing traditional ceramics to reduce the risk of metal wear particles and corrosion in total joint replacement implants. Additionally, surgeons may increase their use of metal interbody spinal fusion devices if there is an increasing perception that PEEK devices are limited by their strength and resistance to fracture.
If we are unable to increase the productivity of our sales and marketing infrastructure we will not be able to penetrate the spinal fusion market.
We market and sell our products to surgeons and hospitals in the United States and select markets in Europe and South America using a network of independent third-party distributors who have existing surgeon relationships. We manage this distribution network through our in-house sales and marketing management team. We may also establish distribution collaborations in the United States and abroad in instances where access to a large or well-established sales and marketing organization may help to expand the market or accelerate penetration for selected products.
We cannot assure you that we will succeed in entering into and maintaining productive arrangements with an adequate number of distributors that are sufficiently committed to selling our products. The establishment of a distribution network is expensive and time consuming. As we launch new products and increase our marketing effort with respect to existing products, we will need to continue to hire, train, retain and motivate skilled independent distributors with significant technical knowledge in various areas, such as spinal fusion and total hip and knee joint replacement. In addition, the commissions we pay our distributors have increased over time, which has resulted in higher sales and marketing expenses, and those commissions and expenses may increase in the future. Furthermore, current and potential distributors may market and sell the products of our competitors. Even if the distributors market and sell our products, our competitors may be able, by offering higher commission payments or other incentives, to persuade these distributors to reduce or terminate their sales and marketing efforts related to our products. The distributors may also help competitors solicit business from our existing customers. Some of our independent distributors account for a significant portion of our sales volume, and, if we were to lose them, our sales could be adversely affected.
Even if we engage and maintain suitable relationships with an adequate number of distributors, they may not generate revenue as quickly as we expect them to, commit the necessary resources to effectively market and sell our products, or ultimately succeed in selling our products. We have been unable to obtain meaningful market share in the interbody spinal fusion device market with our current silicon nitride products to date and we may not be successful in increasing the productivity of our sales and marketing team and distribution network to gain meaningful market share for our silicon nitride products, which could adversely affect our business and financial condition.
The orthopedic market is highly competitive and we may not be able to compete effectively against the larger, well-established companies that dominate this market or emerging and small innovative companies that may seek to obtain or increase their share of the market.
The markets for spinal fusions and total hip and knee implant products are intensely competitive, and many of our competitors are much larger and have substantially more financial and human resources than we do. Many have long histories and strong reputations within the industry, and a relatively small number of companies dominate these markets. In 2012, Medtronic, Inc.; DePuy Synthes Companies, a group of Johnson & Johnson companies; Stryker Corporation; Biomet, Inc.; Zimmer Holdings, Inc.; and Smith & Nephew plc, accounted for more than 65% of orthopedic sales worldwide.
9
Table of Contents
These companies enjoy significant competitive advantages over us, including:
| • | broad product offerings, which address the needs of orthopedic surgeons and hospitals in a wide range of procedures; |
| • | products that are supported by long-term clinical data; |
| • | greater experience in, and resources for, launching, marketing, distributing and selling products, including strong sales forces and established distribution networks; |
| • | existing relationships with spine and joint reconstruction surgeons; |
| • | extensive intellectual property portfolios and greater resources for patent protection; |
| • | greater financial and other resources for product research and development; |
| • | greater experience in obtaining and maintaining FDA and other regulatory clearances and approvals for products and product enhancements; |
| • | established manufacturing operations and contract manufacturing relationships; |
| • | significantly greater name recognition and widely recognized trademarks; and |
| • | established relationships with healthcare providers and payors. |
Our products and any product candidates that we may introduce into the market may not enable us to overcome the competitive advantages of these large and dominant orthopedic companies. In addition, even if we successfully introduce additional product candidates incorporating our silicon nitride biomaterial into the market, emerging and small innovative companies may seek to increase their market share and they may eventually possess competitive advantages, which could adversely impact our business. Our competitors may also employ pricing strategies that could adversely affect the pricing of our products and pricing in the spinal fusion and total joint replacement market generally.
Moreover, many other companies are seeking to develop new biomaterials and products which may compete effectively against our products in terms of performance and price. For example, Smith & Nephew has developed a ceramic-coated metal, known as Oxinium, which may overcome certain of the limitations of metal joint replacement products and could directly compete with our silicon nitride and silicon nitride-coated product candidates.
We have significant customer concentration, so that economic difficulties or changes in the purchasing policies or patterns of our key customers could have a significant impact on our business and operating results.
A small number of customers account for a substantial portion of our product revenues. Our customers are primarily hospitals and surgical centers. At December 31, 2013, our largest customer, Bon Secours St. Mary’s Hospital, or St. Mary’s, had a receivable balance of approximately 15% of our total trade accounts receivable. In addition, St. Mary’s accounted for 14% of our product revenues for each of the years ended December 31, 2013 and 2012. Sales of our products to our customers, including St. Mary’s, are not based on long-term, committed-volume purchase contracts, and we may not continue to receive significant revenues from St. Mary’s or any customer. Because of our significant customer concentration, our revenue could fluctuate significantly due to changes in economic conditions, the use of competitive products, or the loss of, reduction of business with, or less favorable terms with St. Mary’s or any of our other significant customers. A significant portion of St. Mary’s’ purchases have been of our non-silicon nitride products, so it may be able to purchase competitive similar products from others. A reduction or delay in orders from St. Mary’s or any of our other significant customers, or a delay or default in payment by any significant customer, could materially harm our business and results of operations.
The manufacturing process for our silicon nitride products is complex and requires sophisticated state-of-the-art equipment, experienced manufacturing personnel and highly specialized knowledge. If we are unable to manufacture our silicon nitride products on a timely basis consistent with our quality standards, our results of operation will be adversely impacted.
In order to control the quality, cost and availability of our silicon nitride products, we developed our own manufacturing capabilities. We operate a 30,000 square foot manufacturing facility which is certified under the ISO 13485 medical device manufacturing standard for medical devices and operates under the FDA’s quality systems regulations, or QSRs. All operations with the exceptions of raw material production, cleaning, packaging and sterilization are performed at this facility.
10
Table of Contents
In order to mitigate the risk associated with us being the sole manufacturer of our silicon nitride medical device products, in June 2014, we entered into a manufacturing development and supply agreement with Kyocera Industrial Ceramics Corporation, or Kyocera. We have initiated the FDA required actions and processes in order to qualify Kyocera as a second source supplier of our silicon nitride products. We expect to update our material master file and submit a special 510(k) with the FDA in July 2014 to qualify Kyocera as a second source supplier of our silicon nitride products. We expect to begin receiving production parts from Kyocera in July 2014 for distribution in the fourth quarter of this year assuming that the FDA accepts and approves our special 510(k) submission. Although we expect this arrangement with Kyocera will lead to Kyocera becoming a secondary qualified manufacturer, if Kyocera fails to become a qualified manufacturer of these products and product candidates, we will continue to be the sole manufacturer of these products and will need to seek other potential secondary manufacturers. Our reliance solely on our internal resources to manufacture our silicon nitride products entails risks to which we would not be subject if we had secondary suppliers for their manufacture, including:
| • | the inability to meet our product specifications and quality requirements consistently; |
| • | a delay or inability to procure or expand sufficient manufacturing capacity to meet additional demand for our products; |
| • | manufacturing and product quality issues related to the scale-up of manufacturing; |
| • | the inability to produce a sufficient supply of our products to meet product demands; |
| • | the disruption of our manufacturing facility due to equipment failure, natural disaster or failure to retain key personnel; and |
| • | our inability to ensure our compliance with regulations and standards of the FDA including QSRs and corresponding state and international regulatory authorities. |
Any of these events could lead to a reduction in our product sales, product launch delays, failure to obtain regulatory clearance or approval or impact our ability to successfully sell our products and commercialize our products candidates.
We depend on a limited number of third-party suppliers for key raw materials used in the manufacturing of our silicon nitride products, and the loss of these third-party suppliers or their inability to supply us with adequate raw materials could harm our business.
We rely on a limited number of third-party suppliers for the raw materials required for the production of our silicon nitride products and product candidates. Our dependence on a limited number of third-party suppliers involves several risks, including limited control over pricing, availability, quality, and delivery schedules for raw materials. We have no supply agreements in place with any of our suppliers and cannot be certain that our current suppliers will continue to provide us with the quantities of raw materials that we require or that satisfy our anticipated specifications and quality requirements. Any supply interruption in limited or single sourced raw materials could materially harm our ability to manufacture our products until a new source of supply, if any, could be identified and qualified. We may be unable to find a sufficient alternative supply channel within a reasonable time or on commercially reasonable terms. Any performance failure on the part of our suppliers could delay the production of our silicon nitride products and product candidates and delay the development and commercialization of our product candidates, including limiting supplies necessary for commercial sale, clinical trials and regulatory approvals, which could have a material adverse effect on our business.
Use of third-party manufacturers increases the risk that we will not have adequate supplies of our non-silicon nitride products or instrumentation sets.
The majority of our product revenue is currently generated by sales of non-silicon nitride products. Our reliance on a limited number of third-party manufacturers to supply us with our non-silicon nitride products and instruments exposes us to risks that could delay our sales, or result in higher costs or lost product revenues. In particular, our manufacturers could:
| • | encounter difficulties in achieving volume production, quality control and quality assurance or suffer shortages of qualified personnel, which could result in their inability to manufacture sufficient quantities of our commercially available non-silicon nitride products to meet market demand for those products, or they could experience similar problems that result in the manufacture of insufficient quantities of our non-silicon nitride product candidates; and |
| • | fail to follow and remain in compliance with the FDA-mandated QSRs, compliance which is required for all medical devices, or fail to document their compliance to QSRs, either of which could lead to significant delays in the availability of materials for our non-silicon nitride products or instrumentation sets. |
If we are unable to obtain adequate supplies of our non-silicon nitride products and related instrumentation sets that meet our specifications and quality standards, it will be difficult for us to compete effectively. We have no supply agreements in place with our manufacturers and they may change the terms of our future orders or choose not to supply us with products or instrumentation sets in
11
Table of Contents
the future. Furthermore, if a third-party manufacturer from whom we purchase fails to perform its obligations, we may be forced to purchase products or related instrumentation from other third-party manufacturers, which we may not be able to do on reasonable terms, if at all. In addition, if we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines. The delays associated with the verification of a new manufacturer or the re-verification of an existing manufacturer could negatively affect our ability to produce and distribute our non-silicon nitride products or instruments in a timely manner.
In order to be successful, we must expand our available product lines of silicon nitride-based medical devices by commercializing new product candidates, but we may not be able to do so in a timely fashion and at expected costs, or at all.
Although we are currently marketing our silicon nitride interbody spinal fusion implants, in order to be successful, we will need to expand our product lines to include other silicon nitride devices. Therefore, we are developing silicon nitride product candidates for total hip and knee replacement procedures and are exploring the application of our silicon nitride technology for other potential applications. However, we have yet to commercialize any silicon nitride products beyond our spinal fusion products. To succeed in our commercialization efforts, we must effectively continue product development and testing, obtain regulatory clearances and approvals, and enhance our sales and marketing capabilities. We may also have to write down significant inventory if existing products are replaced by new products. Because of these uncertainties, there is no assurance that we will succeed in bringing any of our current or future product candidates to market. If we fail in bringing our product candidates to market, or experience delays in doing so, we will not generate revenues as planned and will need to curtail operations or seek additional financing earlier than otherwise anticipated.
We will depend on one or more strategic partners to develop and commercialize our total joint replacement product candidates, and if our strategic partners are unable to execute effectively on our agreements with them, we may never become profitable.
Pursuant to a joint development and license agreement with Orthopaedic Synergy, Inc., or OSI, we are dependent on OSI’s ability to execute product development plans, obtain regulatory approvals, and sell, distribute and market our jointly developed product candidate for total hip and total knee joint replacement implants that use our MC2 silicon nitride technology. We would similarly be reliant on other strategic partners to develop and commercialize a total hip or knee joint replacement product candidate that utilizes silicon nitride-coated components, although we have not yet entered into an agreement with any strategic partner to develop products with these silicon nitride-coated components and may be unable to do so on agreeable terms. In order to succeed in our joint commercialization efforts, we and OSI, and any future partners must execute effectively on all elements of a combined business plan, including continuing to establish sales and marketing capabilities, manage certified, validated and effective commercial-scale manufacturing operations, conduct product development and testing, and obtain regulatory clearances and approvals for our product candidate. If we or any of our strategic partners fail in any of these endeavors, or experience delays in pursuing them, we will not generate revenues as planned and will need to curtail operations or seek additional financing earlier than otherwise anticipated.
The use of physician-owned distributorships could result in increased pricing pressure on our products or harm our ability to sell our products to physicians who own or are affiliated with those distributorships and the sale of our products through such distributorships may expose us to regulatory enforcement risk.
Physician-owned distributorships, or PODs, are medical device distributors that are owned, directly or indirectly, by physicians. These physicians derive a proportion of their revenue from selling or arranging for the sale of medical devices for use in procedures they perform on their own patients at hospitals that agree to purchase from or through the POD, or that otherwise furnish ordering physicians with income that is based directly or indirectly on those orders of medical devices.
We may sell and distribute our products through a limited number of PODs. The number of PODs in the orthopedic industry may continue to grow as physicians search for ways to increase their incomes. These companies and the physicians who own, or partially own, them have significant market knowledge and access to the surgeons and hospitals that may potentially purchase our products and the physicians who own these PODs will have financial incentives to purchase from these distributorships. As a result, growth in this area may reduce our ability to compete effectively for business.
On March 26, 2013, the Department of Health and Human Services Office of Inspector General issued a Special Fraud Alert on Physician-Owned Entities and identified PODs as “inherently suspect” under the federal Anti-Kickback Statute. While the PODs themselves may be the target of any government enforcement efforts in this area, it is possible that regulatory scrutiny may extend to other entities that have relationships with PODs, including us. We are not aware that we are currently subject to any such scrutiny. However, the cost of defending such enforcement actions, if brought (even without merit), as well as any sanctions, if imposed, could have a material adverse effect on our business.
12
Table of Contents
If hospitals and other healthcare providers are unable to obtain coverage or adequate reimbursement for procedures performed with our products, it is unlikely our products will be widely used.
In the United States, the commercial success of our existing products and any future products will depend, in part, on the extent to which governmental payors at the federal and state levels, including Medicare and Medicaid, private health insurers and other third-party payors provide coverage for and establish adequate reimbursement levels for procedures utilizing our products. Because we typically receive payment directly from hospitals and surgical centers, we do not anticipate relying directly on payment from third-party payors for our products. However, hospitals and other healthcare providers that purchase our orthopedic products for treatment of their patients generally rely on third-party payors to pay for all or part of the costs and fees associated with our products as part of a “bundled” rate for the associated procedures. The existence of coverage and adequate reimbursement for our products and the procedures performed with them by government and private payors is critical to market acceptance of our existing and future products. Neither hospitals nor surgeons are likely to use our products if they do not receive adequate reimbursement for the procedures utilizing our products.
Many private payors currently base their reimbursement policies on the coverage decisions and payment amounts determined by the Centers for Medicare and Medicaid Services, or CMS, which administers the Medicare program. Others may adopt different coverage or reimbursement policies for procedures performed with our products, while some governmental programs, such as Medicaid, have reimbursement policies that vary from state to state, some of which may not pay for the procedures performed with our products in an adequate amount, if at all. A Medicare national or local coverage decision denying coverage for one or more of our products could result in private and other third-party payors also denying coverage for our products. Third-party payors also may deny reimbursement for our products if they determine that a product used in a procedure was not medically necessary, was not used in accordance with cost-effective treatment methods, as determined by the third-party payor, or was used for an unapproved use. Unfavorable coverage or reimbursement decisions by government programs or private payors underscore the uncertainty that our products face in the market and could have a material adverse effect on our business.
Many hospitals and clinics in the United States belong to group purchasing organizations, which typically incentivize their hospital members to make a relatively large proportion of purchases from a limited number of vendors of similar products that have contracted to offer discounted prices. Such contracts often include exceptions for purchasing certain innovative new technologies, however. Accordingly, the commercial success of our products may also depend to some extent on our ability to either negotiate favorable purchase contracts with key group purchasing organizations and/or persuade hospitals and clinics to purchase our product “off contract.”
The healthcare industry in the United States has experienced a trend toward cost containment as government and private payors seek to control healthcare costs by paying service providers lower rates. While it is expected that hospitals will be able to obtain coverage for procedures using our products, the level of payment available to them for such procedures may change over time. State and federal healthcare programs, such as Medicare and Medicaid, closely regulate provider payment levels and have sought to contain, and sometimes reduce, payment levels. Private payors frequently follow government payment policies and are likewise interested in controlling increases in the cost of medical care. In addition, some payors are adopting pay-for-performance programs that differentiate payments to healthcare providers based on the achievement of documented quality-of-care metrics, cost efficiencies, or patient outcomes. These programs are intended to provide incentives to providers to deliver the same or better results while consuming fewer resources. As a result of these programs, and related payor efforts to reduce payment levels, hospitals and other providers are seeking ways to reduce their costs, including the amounts they pay to medical device manufacturers. We may not be able to sell our implants profitably if third-party payors deny or discontinue coverage or reduce their levels of payment below that which we project, or if our production costs increase at a greater rate than payment levels. Adverse changes in payment rates by payors to hospitals could adversely impact our ability to market and sell our products and negatively affect our financial performance.
In international markets, medical device regulatory requirements and healthcare payment systems vary significantly from country to country, and many countries have instituted price ceilings on specific product lines. We cannot assure you that our products will be considered cost-effective by international third-party payors, that reimbursement will be available or, if available, that the third-party payors’ reimbursement policies will not adversely affect our ability to sell our products profitably. Any failure to receive regulatory or reimbursement approvals would negatively impact market acceptance of our products in any international markets in which those approvals are sought.
Prolonged negative economic conditions in domestic and international markets may adversely affect us, our suppliers, partners and consumers, and the global orthopedic market which could harm our financial position.
Global credit and financial markets have been experiencing extreme disruptions over the past several years, including severely diminished liquidity and availability of credit, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. Credit and financial markets and confidence in economic conditions
13
Table of Contents
might deteriorate further. Our business may be adversely affected by the recent economic downturn and volatile business environment and continued unpredictable and unstable market conditions. In addition, there is a risk that one or more of our current suppliers may not continue to operate. Any lender that is obligated to provide funding to us under any future credit agreement with us may not be able to provide funding in a timely manner, or at all, when we require it. The cost of, or lack of, available credit or equity financing could impact our ability to develop sufficient liquidity to maintain or grow our company. These negative changes in domestic and international economic conditions or additional disruptions of either or both of the financial and credit markets may also affect third-party payors and may have a material adverse effect on our business, results of operations, financial condition and liquidity.
In addition, we believe that various demographics and industry-specific trends will help drive growth in the orthopedics markets, but these demographics and trends are uncertain. Actual demand for orthopedic products generally, and our products in particular, could be significantly less than expected if our assumptions regarding these factors prove to be incorrect or do not materialize, or if alternative treatments gain widespread acceptance.
We have a new senior management team and are dependent on our senior management team, engineering team, sales and marketing team and surgeon advisors, and the loss of any of them could harm our business.
We have recently assembled a new senior management team. They have worked together in their new positions with us for a limited time and may not be able to successfully implement our strategy. In addition, we have not entered into employment agreements, other than severance agreements, with any of the members of our senior management team. There are no assurances that the services of any of these individuals will be available to us for any specified period of time. The successful integration of our new senior management team, the loss of members of our senior management team, sales and marketing team, engineering team and key surgeon advisors, or our inability to attract or retain other qualified personnel or advisors could have a material adverse effect on our business, financial condition and results of operations.
If we experience significant disruptions in our information technology systems, our business, results of operations and financial condition could be adversely affected.
The efficient operation of our business depends on our information technology systems. We rely on our information technology systems to effectively manage our sales and marketing, accounting and financial functions; manufacturing processes; inventory; engineering and product development functions; and our research and development functions. As such, our information technology systems are vulnerable to damage or interruption including from earthquakes, fires, floods and other natural disasters; terrorist attacks and attacks by computer viruses or hackers; power losses; and computer systems, or Internet, telecommunications or data network failures. The failure of our information technology systems to perform as we anticipate or our failure to effectively implement new systems could disrupt our entire operation and could result in decreased sales, increased overhead costs, excess inventory and product shortages, all of which could have a material adverse effect on our reputation, business, results of operations and financial condition.
Risks Related to Our Capital Resources and Impairments
We will require additional financing and our failure to obtain additional funding could force us to delay, reduce or eliminate our product development programs or commercialization efforts.
We currently have limited committed sources of capital and we have limited liquidity. Our cash and cash equivalents as of June 30, 2014, December 31, 2013 and December 31, 2012, were $XX million, $2.3 million and $2.7 million, respectively. We require substantial future capital in order to continue to conduct the research and development and regulatory clearance and approval activities necessary to bring our products to market, to establish effective marketing and sales capabilities. Our existing capital resources, including the net proceeds from our initial public offering of shares of our common stock and financing through Hercules Technology and the Selling Stockholder are not sufficient to enable us to fund the completion of the development and commercialization of all of our product candidates. We cannot determine with certainty the duration and completion costs of the current or future development and commercialization of our product candidates for spinal fusion procedures, joint replacement and coated metals or if, when, or to what extent we will generate revenues from the commercialization and sale of any of these product candidates for which we obtain regulatory approval. We may never succeed in achieving regulatory approval for certain or all of these product candidates. The duration, costs and timing of clinical trials and development of our spinal fusion, joint replacement and coated metal product candidates will depend on a variety of factors, including:
| • | the scope, rate of progress, and expense of our ongoing, as well as any additional, clinical trials and other research and development activities; |
| • | future clinical trial results we may must or choose to conduct; |
| • | potential changes in government regulation; and |
| • | the timing and receipt of any regulatory approvals. |
14
Table of Contents
A change in the outcome of any of these variables with respect to the development of spinal fusion, joint replacement or coated metal product candidates could mean a significant change in the costs and timing associated with the development of these product candidates.
In addition, the repayment of the Loan and Security Agreement and the liquidity covenant limit our ability to use our cash and cash equivalents to fund our operations and may restrict our ability to continue development of our product candidates. Additionally, the Loan and Security Agreement restricts our ability to incur additional pari passu indebtedness, which may reduce our ability to seek additional financing. The Convertible Notes issued to the Selling Stockholder also contain other covenants and events of default customary for financings of that type, including, among other things, limitations on certain other indebtedness, dividends and distributions, sales and transfers of assets and transactions with affiliates. If adequate funds are not available on a timely basis, we may terminate or delay the development of one or more of our product candidates, or delay activities necessary to commercialize our product candidates. Additional funding may not be available to us on acceptable terms, or at all. Any additional equity financing, if available, may not be available on favorable terms and will most likely be dilutive to our current stockholders, and debt financing, if available, may involve more restrictive covenants. Our ability to access capital when needed is not assured and, if not achieved on a timely basis, will materially harm our business, financial condition and results of operations.
As a result of our debt obligations, we will need additional funds to meet our operational needs and capital requirements for product development, clinical trials and commercialization. The timing and amount of our future capital requirements will depend on many factors, including:
| • | our ability to satisfy our obligation to pay principal and interest on the Loan and Security Agreement; |
| • | our ability to comply with the minimum liquidity covenant related to the Loan and Security Agreement; |
| • | the level of sales of our current products and the cost of revenue and sales and marketing; |
| • | the extent of any clinical trials that we will be required to conduct in support of the regulatory clearance of our total hip and knee replacement product candidates; |
| • | the scope, progress, results and cost of our product development efforts; |
| • | the costs, timing and outcomes of regulatory reviews of our product candidates; |
| • | the number and types of products we develop and commercialize; |
| • | the costs of preparing, filing and prosecuting patent applications and maintaining, enforcing and defending intellectual property-related claims; and |
| • | the extent and scope of our general and administrative expenses. |
If we do not adhere to the financial covenants set forth in the Loan and Security Agreement with Hercules Technology, we will be in default of the Loan and Security Agreement.
In June 2014 we entered into a Loan and Security Agreement with Hercules Technology Growth Capital, Inc., or Hercules Technology, as administrative and collateral agent for the lenders thereunder and as lender, and Hercules Technology III, LP, as lender. The Loan and Security Agreement provides us with a $20 million term loan with a maturity date of January 1, 2018 and is secured by substantially all of our assets and is described in more detail in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this prospectus. Proceeds of the loan were used to repay in full and terminate our prior credit facility with General Electric Capital Corporation, or GE Capital, and the remainder of the loan proceeds will be used for general corporate purposes.
The Loan and Security Agreement contains a minimum liquidity covenant that requires us to maintain cash and cash equivalents and availability under the Loan and Security Agreement of not less than $6.0 million through August 15, 2014 and not less than $9.0 million thereafter. We will need to obtain additional funding during the fourth quarter of 2014 to maintain compliance with this minimum liquidity covenant under the Loan and Security Agreement through the next twelve months. Furthermore, if we are unable to access additional funds prior to becoming non-compliant with the liquidity covenant, the entire remaining balance of the Loan and Security Agreement could become immediately due and payable at the option of Hercules Technology.
Hercules Technology could declare a default under the Loan and Security Agreement upon the occurrence of a material adverse effect, as defined under the credit facility, thereby requiring us to either repay the outstanding indebtedness immediately or attempt to reverse the declaration of default through negotiation or litigation. Any declaration of an event of default could significantly harm our business and prospectus and could cause the price of our common stock to decline.
15
Table of Contents
Raising additional capital by issuing securities or through debt financings or licensing arrangements may cause dilution to existing stockholders, restrict our operations or require us to relinquish proprietary rights.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or products or grant licenses on terms that are not favorable to us. Any of these events could adversely affect our ability to achieve our product development and commercialization goals and have a material adverse effect on our business, financial condition and results of operations.
Our former independent registered public accounting firm has included an explanatory paragraph relating to our ability to continue as a going concern in its report on our audited financial statements. We may be unable to continue to operate without the threat of liquidation for the foreseeable future.
Our report from our former independent registered public accounting firm for the year ended December 31, 2013 includes an explanatory paragraph stating that our recurring losses from operations and our need to obtain additional financing in order to satisfy our debt obligations and to be compliant with covenants under our debt obligations through 2014 raise substantial doubt about our ability to continue as a going concern. If we are unable to obtain sufficient additional funding, our business, prospects, financial condition and results of operations will be materially and adversely affected and we may be unable to continue as a going concern. If we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our consolidated financial statements, and it is likely that investors will lose all or a part of their investment. Future reports from our independent registered public accounting firm may also contain statements expressing doubt about our ability to continue as a going concern. If we seek additional financing to fund our business activities in the future and there remains doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding on commercially reasonable terms or at all.
An impairment charge could have a material adverse effect on our financial condition and results of operations.
We are required to test acquired goodwill for impairment on an annual basis. Goodwill represents the excess of the amount paid over the fair value of the net assets at the date of the acquisition. We have chosen to complete our annual impairment reviews of goodwill at the end of each calendar year. We also are required to test goodwill for impairment between annual tests if events occur or circumstances change that would more likely than not reduce our enterprise fair value below its book value. In addition, we are required to test our finite-lived intangible assets for impairment if events occur or circumstances change that would indicate the remaining net book value of the finite-lived intangible assets might not be recoverable. These events or circumstances could include a significant change in the business climate, including a significant sustained decline in our market value, legal factors, operating performance indicators, competition, sale or disposition of a significant portion of our business and other factors.
If the fair market value of our reporting unit is less than its book value, we could be required to record an impairment charge. The valuation of a reporting unit requires judgment in estimating future cash flows, discount rates and other factors. In making these judgments, we evaluate the financial health of our business, including such factors as industry performance, changes in technology and operating cash flows. Changes in our forecasts or decreases in the value of our common stock could cause book values of our reporting unit to exceed its fair value, which may result in goodwill impairment charges. The amount of any impairment could be significant and could have a material adverse effect on our reported financial results for the period in which the charge is taken.
Risks Related to Regulatory Approval of Our Products and Other Government Regulations
Our long-term success depends substantially on our ability to obtain regulatory clearance or approval and thereafter commercialize our product candidates; we cannot be certain that we will be able to do so in a timely manner or at all.
The process of obtaining regulatory clearances or approvals to market a medical device from the FDA or similar regulatory authorities outside of the United States can be costly and time consuming, and there can be no assurance that such clearances or approvals will be granted on a timely basis, or at all. The FDA’s 510(k) clearance process generally takes one to six months from the date of submission, depending on whether a special or traditional 510(k) premarket notification has been submitted, but can take significantly longer. An application for premarket approval, or PMA, must be submitted to the FDA if the device cannot be cleared through the 510(k) clearance process or is not exempt from premarket review by the FDA. The PMA process almost always requires one or more clinical trials and can take two to three years from the date of filing, or even longer. In some cases, including in the case of our interbody spinal fusion devices which incorporate our CSC technology and our MC2 silicon nitride femoral head component, the FDA requires clinical data as part of the 510(k) clearance process.
16
Table of Contents
It is possible that the FDA could raise questions about our spinal fusion products, our spinal fusion product candidates and our total hip and knee joint replacement product candidates and could require us to perform additional studies on our products and product candidates. Even if the FDA permits us to use the 510(k) clearance process, we cannot assure you that the FDA will not require either supporting data from laboratory tests or studies that we have not conducted, or substantial supporting clinical data. If we are unable to use the 510(k) clearance process for any of our product candidates, are required to provide clinical data or laboratory data that we do not possess to support our 510(k) premarket notifications for any of these product candidates, or otherwise experience delays in obtaining or fail to obtain regulatory clearances, the commercialization of our product candidates in the United States will be delayed or prevented, which will adversely affect our ability to generate additional revenues. It also may result in the loss of potential competitive advantages that we might otherwise attain by bringing our products to market earlier than our competitors. Additionally, although the FDA allows modifications to be made to devices that have received 510(k) clearance with supporting documentation, the FDA may disagree with our decision to modify our cleared devices without submission of a new 510(k) premarket notification, subjecting us to potential product recall, field alerts and corrective actions. Any of these contingencies could adversely affect our business.
Similar to our compliance with U.S. regulatory requirements, we must obtain and comply with international requirements in order to market and sell our products outside of the United States and we may only promote and market our products, if approved, as permitted by applicable regulatory authorities.
The safety of our products is not yet supported by long-term clinical data, and they may prove to be less safe and effective than our laboratory data indicate.
We obtained FDA clearance for each of our products that we currently market, and we have sought and intend to seek FDA clearance or approval through the FDA’s 510(k) or PMA process and, where applicable, CE marking for our product candidates. The 510(k) clearance process is based on the FDA’s agreement that a new product candidate is substantially equivalent to an already marketed product for which a PMA was not required. While most 510(k) premarket notifications do not require clinical data for clearance, the FDA may request that such data be provided. Long-term clinical data or marketing experience obtained after clearance may indicate that our products cause unexpected complications or other unforeseen negative effects. If this happens, we could be subject to the withdrawal of our marketing clearance and other enforcement sanctions by the FDA or other regulatory authority, product recalls, significant legal liability, significant negative publicity, damage to our reputation and a dramatic reduction in our ability to sell our products, any one of which would have a material adverse effect on our business, financial condition and results of operations.
We expect to be required to conduct clinical trials to support regulatory approval of some of our product candidates. We have no experience conducting clinical trials, they may proceed more slowly than anticipated, and we cannot be certain that our product candidates will be shown to be safe and effective for human use.
In order to commercialize our product candidates in the United States, we must submit a PMA for some of these product candidates, which will require us to conduct clinical trials. We also plan to provide the FDA with clinical trial data to support some of our 510(k) premarket notifications. We will receive approval or clearance from the FDA to commercialize products requiring a clinical trial only if we can demonstrate to the satisfaction of the FDA, through well-designed and properly conducted clinical trials, that our product candidates are safe and effective and otherwise meet the appropriate standards required for approval or clearance for specified indications. Clinical trials are complex, expensive, time consuming, uncertain and subject to substantial and unanticipated delays. Before we may begin clinical trials, we must submit and obtain approval for an investigational device exemption, or IDE, that describes, among other things, the manufacture of, and controls for, the device and a complete investigational plan. Clinical trials generally involve a substantial number of patients in a multi-year study. Because we do not have the experience or the infrastructure necessary to conduct clinical trials, we will have to hire one or more contract research organizations, or CROs, to conduct trials on our behalf. CRO contract negotiations may be costly and time consuming and we will rely heavily on the CRO to ensure that our trials are conducted in accordance with regulatory and industry standards. We may encounter problems with our clinical trials and any of those problems could cause us or the FDA to suspend those trials, or delay the analysis of the data derived from them.
A number of events or factors, including any of the following, could delay the completion of our clinical trials in the future and negatively impact our ability to obtain FDA approval for, and to introduce our product candidates:
| • | failure to obtain financing necessary to bear the cost of designing and conducting clinical trials; |
| • | failure to obtain approval from the FDA or foreign regulatory authorities to commence investigational studies; |
| • | conditions imposed on us by the FDA or foreign regulatory authorities regarding the scope or design of our clinical trials; |
| • | failure to find a qualified CRO to conduct our clinical trials or to negotiate a CRO services agreement on favorable terms; |
17
Table of Contents
| • | delays in obtaining or in our maintaining required approvals from institutional review boards or other reviewing entities at clinical sites selected for participation in our clinical trials; |
| • | insufficient supply of our product candidates or other materials necessary to conduct our clinical trials; |
| • | difficulties in enrolling patients in our clinical trials; |
| • | negative or inconclusive results from clinical trials, or results that are inconsistent with earlier results, that necessitate additional clinical studies; |
| • | failure on the part of the CRO to conduct the clinical trial in accordance with regulatory requirements; |
| • | our failure to maintain a successful relationship with the CRO or termination of our contractual relationship with the CRO before completion of the clinical trials; |
| • | serious or unexpected side effects experienced by patients in whom our product candidates are implanted; or |
| • | failure by any of our third-party contractors or investigators to comply with regulatory requirements or meet other contractual obligations in a timely manner. |
Our clinical trials may need to be redesigned or may not be completed on schedule, if at all. Delays in our clinical trials may result in increased development costs for our product candidates, which could cause our stock price to decline and limit our ability to obtain additional financing. In addition, if one or more of our clinical trials are delayed, competitors may be able to bring products to market before we do, and the commercial viability of our product candidates could be significantly reduced.
Our current and future relationships with third-party payors and current and potential customers in the United States and elsewhere may be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm administrative burdens and diminished profits and future earnings.
Our current and future arrangements with third-party payors and current and potential customers, including providers and physicians, as well as PODs, as discussed above, may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act, which may constrain the business or financial arrangements and relationships through which we sell, market and distribute our products. In addition, we may be subject to transparency laws and patient privacy regulations by U.S. federal and state governments and by governments in foreign jurisdictions in which we conduct our business. The applicable federal, state and foreign healthcare laws and regulations that may affect our ability to operate include:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal healthcare programs, such as Medicare and Medicaid; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, including the federal False Claims Act, which impose criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose obligations on covered healthcare providers, health plans, and healthcare clearinghouses, as well as their business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | the Physician Payments Sunshine Act, which requires (i) manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to CMS information related to certain “payments or other transfers of value” made to physicians, which is defined to include doctors, dentists, optometrists, podiatrists and chiropractors, and teaching hospitals, with data collection beginning on August 1, 2013, (ii) applicable manufacturers and applicable group purchasing organizations to report annually to CMS ownership and investment interests held in such entities by physicians and their immediate family members, with data collection beginning on August 1, 2013, (iii) manufacturers to submit reports to CMS by March 31, 2014 and the 90th day of each subsequent calendar year, and (iv) disclosure of such information by CMS on a publicly available website beginning in September 2014; and |
18
Table of Contents
| • | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require medical device companies to comply with the medical device industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require medical device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, fines, imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, which could have a material adverse effect on our business. If any of the physicians or other healthcare providers or entities with whom we expect to do business, including our collaborators, are found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also materially affect our business.
In July 2012, we received a subpoena from the Department of Justice seeking the production of documents, including documents related to our relationship with a particular customer and various entities, including a company distributor, and individuals associated with that distributor. In April 2013, we received a second subpoena requesting similar records. We cooperated with the Department of Justice’s requests and provided the records requested by the two subpoenas. We have had no further communications with the Department of Justice since responding to its second request in June 2013. While we do not believe that we are the target of the government’s investigation, if we are found to have violated one or more applicable laws, we could be subject to the risks and consequences discussed above. In addition, responding to any additional requests or actions of the Department of Justice in connection with this investigation may be expensive and time-consuming.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain regulatory approval or clearance of our product candidates and affect the prices we may obtain for our products.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay clearance and/or approval of our product candidates, restrict or regulate post-clearance and post-approval activities and affect our ability to profitably sell our products and any product candidates for which we obtain marketing approval or clearance.
In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of our products. Delays in receipt of or failure to receive regulatory clearances or approvals for our new products would have a material adverse effect on our business, results of operations and financial condition. In addition, the FDA is currently evaluating the 510(k) process and may make substantial changes to industry requirements, including which devices are eligible for 510(k) clearance, the ability to rescind previously granted 510(k) clearances and additional requirements that may significantly impact the process.
Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and expanding access. In the United States, the medical device industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively the ACA, a sweeping law intended, among other things, to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms.
19
Table of Contents
Among the provisions of the ACA of importance to our products and product candidates are:
| • | a 2.3% medical device excise tax on the U.S. sales of most medical devices, which currently includes and we expect will continue to include U.S. sales of our current products and products candidates that receive clearance or approval; |
| • | expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, and new government investigative powers and enhanced penalties for non-compliance; |
| • | new requirements under the federal Open Payments program and its implementing regulations; |
| • | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and |
| • | creation of an independent payment advisory board that will submit recommendations to reduce Medicare spending if projected Medicare spending exceeds a specified growth rate. |
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. For example, on August 2, 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee on Deficit Reduction did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, or ATRA, which, among other things, reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. On March 1, 2013, the President signed an executive order implementing the Budget Control Act’s 2% Medicare payment reductions, and on April 1, 2013, these reductions went into effect. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our financial operations.
We expect that the ACA, as well as other healthcare reform measures that have been and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for our products. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may affect our ability to generate revenue and profits or commercialize our product candidates.
In the European Union and some other international markets, the government provides health care at a low cost to consumers and regulates prices of healthcare products, patient eligibility or reimbursement levels to control costs for the government-sponsored health care system. Many countries are reducing their public expenditures and we expect to see strong efforts to reduce healthcare costs in international markets, including patient access restrictions, suspensions on price increases, prospective and possibly retroactive price reductions and other recoupments and increased mandatory discounts or rebates and recoveries of past price increases. These cost control measures could reduce our revenues. In addition, certain countries set prices by reference to the prices in other countries where our products are marketed. Thus, our inability to secure adequate prices in a particular country may not only limit the marketing of our products within that country, but may also adversely affect our ability to obtain acceptable prices in other markets. This may create the opportunity for third-party cross border trade or influence our decision to sell or not to sell a product, thus adversely affecting our geographic expansion plans and revenues.
The U.S. federal medical device excise tax may materially adversely affect our business and results of operations, and we may be subject to increased taxes in other jurisdictions.
The ACA imposed a 2.3% federal medical device excise tax on the sales in the United States of most medical devices. Most if not all of our products will be subject to this tax. This excise tax became effective in 2013 and has forced, and will continue to force us to identify ways to reduce spending in other areas to offset the expected earnings impact due to the tax. We do not expect to be able to pass along the cost of this tax to hospitals, which continue to face cuts to their Medicare reimbursement due to the ACA and the recently enacted ATRA. Nor do we expect to be able to offset the cost of the tax through higher sales volumes resulting from the expansion of health insurance coverage because of the demographics of the current uninsured population in the United States. While it is still too early to fully understand and predict the ultimate impact of the medical device tax on our business, ongoing implementation of this legislation and any similar taxes imposed in other jurisdictions could have a material adverse effect on our results of operations and cash flows.
20
Table of Contents
Risks Related to Our Intellectual Property and Litigation
If the combination of patents, trade secrets and contractual provisions that we rely on to protect our intellectual property is inadequate, our ability to commercialize our orthopedic products successfully will be harmed, and we may not be able to operate our business profitably.
Our success depends significantly on our ability to protect our proprietary rights to the technologies incorporated in our products. We currently have 34 issued U.S. patents, 38 pending U.S. patent applications, 11 granted foreign patents and 18 pending foreign patent applications. Our issued patents begin to expire in 2014, with the last of these patents expiring in 2031. We rely on a combination of patent protection, trade secret laws and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology. However, these may not adequately protect our rights or permit us to gain or keep any competitive advantage.
The issuance of a patent is not conclusive as to its scope, validity or enforceability. The scope, validity or enforceability of our issued patents can be challenged in litigation or proceedings before the U.S. Patent and Trademark Office, or the USPTO, or foreign patent offices. In addition, our pending patent applications include claims to numerous important aspects of our products under development that are not currently protected by any of our issued patents. We cannot assure you that any of our pending patent applications will result in the issuance of patents to us. The USPTO or foreign patent offices may deny or require significant narrowing of claims in our pending patent applications. Patents issued as a result of the pending patent applications, if any, may not provide us with significant commercial protection or be issued in a form that is advantageous to us. Proceedings before the USPTO or foreign patent offices could result in adverse decisions as to the priority of our inventions and the narrowing or invalidation of claims in issued patents. The laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States, if at all.
Our competitors may successfully challenge and invalidate or render unenforceable our issued patents, including any patents that may issue in the future, which could prevent or limit our ability to market our products and could limit our ability to stop competitors from marketing products that are substantially equivalent to ours. In addition, competitors may be able to design around our patents or develop products that provide outcomes that are comparable to our products but that are not covered by our patents.
We have also entered into confidentiality and assignment of intellectual property agreements with all of our employees, consultants and advisors as one of the ways we seek to protect our intellectual property and other proprietary technology. However, these agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements.
In the event a competitor infringes upon any of our patents or other intellectual property rights, enforcing our rights may be difficult, time consuming and expensive, and would divert management’s attention from managing our business. There can be no assurance that we will be successful on the merits in any enforcement effort. In addition, we may not have sufficient resources to litigate, enforce or defend our intellectual property rights.
We have no patent protection covering the composition of matter for our solid MC2 silicon nitride or the process we use for manufacturing our MC2 silicon nitride, and competitors may create silicon nitride formulations substantially similar to ours.
Although we have a number of U.S. and foreign patents and pending applications relating to our MC2 silicon nitride products or product candidates, we have no patent protection either for the composition of matter for our silicon nitride or for the processes of manufacturing MC2 silicon nitride. As a result, competitors may create silicon nitride formulations substantially similar to ours, and use their formulations in products that may compete with our silicon nitride products, provided they do not violate our issued product patents. Although we have, and will continue to develop, significant know-how related to these processes, there can be no assurance that we will be able to maintain this know-how as trade secrets, and competitors may develop or acquire equally valuable or more valuable know-how related to the manufacture of silicon nitride.
We could become subject to intellectual property litigation that could be costly, result in the diversion of management’s time and efforts, require us to pay damages, prevent us from marketing our commercially available products or product candidates and/or reduce the margins we may realize from our products that we may commercialize.
The medical devices industry is characterized by extensive litigation and administrative proceedings over patent and other intellectual property rights. Whether a product infringes a patent involves complex legal and factual issues, and the determination is often uncertain. There may be existing patents of which we are unaware that our products under development may inadvertently infringe. The likelihood that patent infringement claims may be brought against us increases as the number of participants in the orthopedic market increases and as we achieve more visibility in the market place and introduce products to market.
21
Table of Contents
Any infringement claim against us, even if without merit, may cause us to incur substantial costs, and would place a significant strain on our financial resources, divert the attention of management from our core business, and harm our reputation. In some cases, litigation may be threatened or brought by a patent holding company or other adverse patent owner who has no relevant product revenues and against whom our patents may provide little or no deterrence. If we were found to infringe any patents, we could be required to pay substantial damages, including triple damages if an infringement is found to be willful, and royalties and could be prevented from selling our products unless we obtain a license or are able to redesign our products to avoid infringement. We may not be able to obtain a license enabling us to sell our products on reasonable terms, or at all, and there can be no assurance that we would be able to redesign our products in a way that would not infringe those patents. If we fail to obtain any required licenses or make any necessary changes to our technologies or the products that incorporate them, we may be unable to commercialize one or more of our products or may have to withdraw products from the market, all of which would have a material adverse effect on our business, financial condition and results of operations.
In addition, in order to further our product development efforts, we have entered into agreements with orthopedic surgeons to help us design and develop new products, and we expect to enter into similar agreements in the future. In certain instances, we have agreed to pay such surgeons royalties on sales of products which incorporate their product development contributions. There can be no assurance that surgeons with whom we have entered into such arrangements will not claim to be entitled to a royalty even if we do not believe that such products were developed by cooperative involvement between us and such surgeons. In addition, some of our surgeon advisors are employed by academic or medical institutions or have agreements with other orthopedic companies pursuant to which they have agreed to assign or are under an obligation to assign to those other companies or institutions their rights in inventions which they conceive or develop, or help conceive or develop.
There can be no assurance that one or more of these orthopedic companies or institutions will not claim ownership rights to an invention we develop in collaboration with our surgeon advisors or consultants on the basis that an agreement with such orthopedic company or institution gives it ownership rights in the invention or that our surgeon advisors on consultants otherwise have an obligation to assign such inventions to such company or institution. Any such claim against us, even without merit, may cause us to incur substantial costs, and would place a significant strain on our financial resources, divert the attention of management from our core business and harm our reputation.
We may be subject to damages resulting from claims that we, our employees, or our independent sales agencies have wrongfully used or disclosed alleged trade secrets of our competitors or are in breach of non-competition agreements with our competitors or non-solicitation agreements.
Many of our employees were previously employed at other orthopedic companies, including our competitors and potential competitors. Many of our distributors and potential distributors sell, or in the past have sold, products of our competitors. We may be subject to claims that either we, or these employees or distributors, have inadvertently or otherwise used or disclosed the trade secrets or other proprietary information of our competitors. In addition, we have been and may in the future be subject to claims that we caused an employee or sales agent to break the terms of his or her non-competition agreement or non-solicitation agreement. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail in defending such claims, in addition to paying money damages, we may lose valuable intellectual property rights or personnel. A loss of key personnel or their work product could hamper or prevent our ability to commercialize products, which could have an adverse effect on our business, financial condition and results of operations.
If our silicon nitride products or our product candidates conflict with the rights of others, we may not be able to manufacture or market our products or product candidates, which could have a material and adverse effect on us.
Our commercial success will depend in part on not infringing the patents or violating the other proprietary rights of third parties. Issued patents held by others may limit our ability to develop commercial products. All issued patents are entitled to a presumption of validity under the laws of the United States. If we need suitable licenses to such patents to permit us to develop or market our product candidates, we may be required to pay significant fees or royalties and we cannot be certain that we would even be able to obtain such licenses. Competitors or third parties may obtain patents that may cover subject matter we use in developing the technology required to bring our products to market, that we use in producing our products, or that we use in treating patients with our products. We know that others have filed patent applications in various jurisdictions that relate to several areas in which we are developing products. Some of these patent applications have already resulted in patents and some are still pending. If we were found to infringe any of these issued patents or any of the pending patent applications, when and if issued, we may be required to alter our processes or product candidates, pay licensing fees or cease activities. If use of technology incorporated into or used to produce our product candidates is challenged, or if our processes or product candidates conflict with patent rights of others, third parties could bring legal actions against us, in Europe, the United States and elsewhere, claiming damages and seeking to enjoin manufacturing and marketing of the affected products. Additionally, it is not possible to predict with certainty what patent claims may issue from pending applications. In the United States, for example, patent prosecution can proceed in secret prior to issuance of a patent, provided such application is not filed in foreign jurisdiction. For U.S. patent applications that are also filed in foreign jurisdictions, such patent applications will not publish until 18 months from the filing date of the application. As a result, third parties may be able to obtain patents with claims relating to our product candidates which they could attempt to assert against us. Further, as we develop our products, third parties may assert that we infringe the patents currently held or licensed by them, and we cannot predict the outcome of any such action.
22
Table of Contents
There has been extensive litigation in the medical devices industry over patents and other proprietary rights. If we become involved in any litigation, it could consume a substantial portion of our resources, regardless of the outcome of the litigation. If these legal actions are successful, in addition to any potential liability for damages, we could be required to obtain a license, grant cross-licenses and pay substantial royalties in order to continue to manufacture or market the affected products.
We cannot assure you that we would prevail in any legal action or that any license required under a third party patent would be made available on acceptable terms, or at all. Ultimately, we could be prevented from commercializing a product, or forced to cease some aspect of our business operations, as a result of claims of patent infringement or violation of other intellectual property rights, which could have a material and adverse effect on our business, financial condition and results of operations.
Risks Related to Potential Litigation from Operating Our Business
We may become subject to potential product liability claims, and we may be required to pay damages that exceed our insurance coverage.
Our business exposes us to potential product liability claims that are inherent in the design, testing, manufacture, sale and distribution of our currently marketed products and each of our product candidates that we are seeking to introduce to the market. The use of orthopedic medical devices can involve significant risks of serious complications, including bleeding, nerve injury, paralysis, infection, and even death. Any product liability claim brought against us, with or without merit, could result in the increase of our product liability insurance rates or in our inability to secure coverage in the future on commercially reasonable terms, if at all. In addition, if our product liability insurance proves to be inadequate to pay a damage award, we may have to pay the excess of this award out of our cash reserves, which could significantly harm our financial condition. If longer-term patient results and experience indicate that our products or any component of a product causes tissue damage, motor impairment or other adverse effects, we could be subject to significant liability. A product liability claim, even one without merit, could harm our reputation in the industry, lead to significant legal fees, and result in the diversion of management’s attention from managing our business.
Any claims relating to our improper handling, storage or disposal of biological or hazardous materials could be time consuming and costly.
Although we do not believe that the manufacture of our silicon nitride or non-silicon nitride products will involve the use of hazardous materials, it is possible that regulatory authorities may disagree or that changes to our manufacturing processes may result in such use. Our business and facilities and those of our suppliers and future suppliers may therefore be subject to foreign, federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous materials and waste products. We may incur significant expenses in the future relating to any failure to comply with environmental laws. Any such future expenses or liability could have a significant negative impact on our business, financial condition and results of operations.
Risks Related to Our Common Stock
The price of our common stock is volatile and is likely to will continue to fluctuate due to reasons beyond our control.
The volatility of orthopedic company stocks, including shares of our common stock, often do not correlate to the operating performance of the companies represented by such stocks or our operating performance. Some of the factors that may cause the market price of our common stock to fluctuate include:
| • | our ability to sell our current products and the cost of revenue; |
| • | our ability to develop, obtain regulatory clearances or approvals for, and market new and enhanced product candidates on a timely basis; |
| • | changes in governmental regulations or in the status of our regulatory approvals, clearances or future applications; |
| • | our announcements or our competitors’ announcements regarding new products, product enhancements, significant contracts, number and productivity of distributors, number of hospitals and surgeons using products, acquisitions or strategic investments; |
| • | announcements of technological or medical innovations for the treatment of orthopedic pathology; |
| • | delays or other problems with the manufacturing of our products, product candidates and related instrumentation; |
| • | volume and timing of orders for our products and our product candidates, if and when commercialized; |
23
Table of Contents
| • | changes in the availability of third-party reimbursement in the United States and other countries; |
| • | quarterly variations in our or our competitors’ results of operations; |
| • | changes in earnings estimates or recommendations by securities analysts, if any, who cover our common stock; |
| • | failure to meet estimates or recommendations by securities analysts, if any, who cover our stock; |
| • | changes in the fair value of our common stock warrant liability resulting from changes in the market price of our common stock, which may result in significant fluctuations in our quarterly and annual operating results; |
| • | changes in healthcare policy in the United States and internationally; |
| • | product liability claims or other litigation involving us; |
| • | sales of an substantial aggregate number of shares of our common stock, including potential sales by longstanding holders of our securities, commencing on August 12, 2014, following the expiration of the lock-up period related to our initial public offering; |
| • | sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders; |
| • | disputes or other developments with respect to intellectual property rights; |
| • | changes in accounting principles; |
| • | changes to tax policy; and |
| • | general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors. |
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent our stockholders from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the company that issued the stock. If our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit regardless of the merits of the case or the eventual outcome. Such a lawsuit also would divert the time and attention of our management from running our company.
Securities analysts may not continue to provide coverage of our common stock or may issue negative reports, which may have a negative impact on the market price of our common stock.
Since completing our initial public offering of shares of our common stock in February 2014, a limited number of securities analysts have begun providing research coverage of our common stock. If securities analysts do not continue to cover our common stock, the lack of research coverage may cause the market price of our common stock to decline. The trading market for our common stock may be affected in part by the research and reports that industry or financial analysts publish about our business. If one or more of the analysts who elect to cover us downgrade our stock, our stock price would likely decline rapidly. If one or more of these analysts cease coverage of us, we could lose visibility in the market, which in turn could cause our stock price to decline. In addition, under the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, and a global settlement among the Securities and Exchange Commission, or the SEC, other regulatory agencies and a number of investment banks, which was reached in 2003, many investment banking firms are required to contract with independent financial analysts for their stock research. It may be difficult for a company such as ours, with a smaller market capitalization, to attract independent financial analysts that will cover our common stock. This could have a negative effect on the market price of our stock.
The low trading volume of our common stock may adversely affect the price of our shares.
Although our common stock is listed on the NASDAQ Capital Market, our common stock has experienced low trading volume. The average daily trading volume of our common stock from April 1, 2014 to June 30, 2014, as reported by NASDAQ, was approximately 23,000 shares. Limited trading volume may subject our common stock to greater price volatility and may make it difficult for investors to sell shares of our common stock at a price that is attractive to them.
24
Table of Contents
If our executive officers, directors and principal stockholders choose to act together, they will be able to exert significant influence over us and our significant corporate decisions and may act in a manner that advances their best interests and not necessarily those of other stockholders.
Our executive officers, directors, and beneficial owners of 5% or more of our outstanding common stock and their affiliates beneficially own approximately 40% of our outstanding common stock as of July 8, 2014. As a result, these persons, acting together, will have the ability to significantly influence the outcome of all matters requiring stockholder approval, including the election and removal of directors and any merger, consolidation, or sale of all or substantially all of our assets, and they may act in a manner that advances their best interests and not necessarily those of other stockholders, by among other things:
| • | delaying, deferring or preventing a change in control of our company; |
| • | entrenching our management and/or our board of directors; |
| • | impeding a merger, consolidation, takeover or other business combination involving our company; |
| • | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our company; or |
| • | causing us to enter into transactions or agreements that are not in the best interests of all stockholders. |
Future sales of our common stock in the public market may cause our stock price to decline and impair our ability to raise future capital through the sale of our equity securities.
There are a substantial number of shares of our common stock held by stockholders who owned shares of our capital stock prior to our initial public offering that will be able to be sold in the public market in the near future. Sales by such stockholders of a substantial number of shares could significantly reduce the market price of our common stock. Moreover, 2,581,941 outstanding shares of common stock, which were converted from convertible preferred stock to common stock upon the completion of our initial public offering, or IPO, and outstanding warrants to purchase 72,939 shares of common stock, which were converted from preferred stock warrants into common stock warrants upon the completion of our IPO, and 12,363 outstanding shares of common stock, assuming the exercise of common stock warrants, are deemed to be “restricted securities” pursuant to Rule 144 under the Securities Act of 1933, as amended, or the Securities Act, and will be eligible for sale in reliance on Rule 144, subject to the lock-up agreements covering substantially all of these securities which will expire on August 12, 2014. These shares of common stock, totaling 2,667,243 shares, assuming the exercise of the common stock warrants, represent approximately 21% of the total number of shares of our common stock outstanding. In addition, warrants to acquire approximately 633,669 shares of our common stock and 8,627,454 shares of our outstanding common stock which are deemed to be “restricted securities” pursuant to Rule 144 under the Securities Act, also will be eligible for sale in reliance on Rule 144, subject to the lock-up agreements covering substantially all of these securities which will expire on August 12, 2014. A holder of warrants to acquire shares of our common stock will be able to net exercise such warrants by surrendering a portion of that holder’s warrants as payment of the exercise price rather than paying the exercise price in cash. As of December 31, 2013, holders of warrants to acquire approximately 633,669 shares of our common stock would be eligible to rely on Rule 144 for the resale of such shares if the warrants are net exercised, subject to the lock-up agreements. Additionally, all of our outstanding RSUs at July 8, 2014 will vest on August 12, 2014, resulting in approximately an additional 1,827,516 shares eligible to be sold in the public market.
In addition, once the registration statement to which this prospectus relates is declared effective by the SEC, the Selling Stockholder will be eligible to sell up to 2,326,409 shares of our common stock following conversion of the Convertible Notes and exercise of the Magna Warrant.
We have also registered all shares of our common stock that we may issue pursuant to our 2003 Stock Option Plan and our Amended and Restated 2012 Equity Incentive Plan, or the 2012 Plan. Shares issued by us upon exercise of options granted under these equity plans are eligible for sale in the public market, subject to the lock-up agreements entered into in connection with the IPO. If any of these holders cause a large number of securities to be sold in the public market, the sales could reduce the trading price of our common stock. These sales also could impede our ability to raise future capital.
Anti-takeover provisions in our organizational documents and Delaware law may discourage or prevent a change in control, even if an acquisition would be beneficial to our stockholders, which could affect our stock price adversely and prevent attempts by our stockholders to replace or remove our current management.
Our restated certificate of incorporation and restated bylaws contain provisions that could discourage, delay or prevent a merger, acquisition or other change in control of our company or changes in our board of directors that our stockholders might consider favorable, including transactions in which you might receive a premium for your shares. These provisions also could limit the price
25
Table of Contents
that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. Stockholders who wish to participate in these transactions may not have the opportunity to do so. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove management. These provisions:
| • | allow the authorized number of directors to be changed only by resolution of our board of directors; |
| • | provide for a classified board of directors, such that not all members of our board will be elected at one time; |
| • | prohibit our stockholders from filling board vacancies, limit who may call stockholder meetings, and prohibit the taking of stockholder action by written consent; |
| • | prohibit our stockholders from making certain changes to our restated certificate of incorporation or restated bylaws except with the approval of holders of 75% of the outstanding shares of our capital stock entitled to vote; |
| • | require advance written notice of stockholder proposals that can be acted upon at stockholders meetings and of director nominations to our board of directors; and |
| • | authorize our board of directors to create and issue, without prior stockholder approval, preferred stock that may have rights senior to those of our common stock and that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our board of directors. |
In addition, we are subject to the provisions of Section 203 of the Delaware General Corporation Law, which may prohibit certain business combinations with stockholders owning 15% or more of our outstanding voting stock. Any delay or prevention of a change in control transaction or changes in our board of directors could cause the market price of our common stock to decline.
We do not intend to pay cash dividends.
We have never declared or paid cash dividends on our capital stock and we do not anticipate paying any cash dividends in the foreseeable future. We currently intend to retain all available funds and any future earnings for debt service and use in the operation and expansion of our business. Each of the Hercules Secured Credit Facility and the Convertible Notes issued to, and which may be issued to, the Selling Stockholder contain a negative covenant which prohibits us from paying dividends to our stockholders without the prior written consent of Hercules Technology and the Selling Stockholder, respectively. In addition, the terms of any future debt or credit facility may preclude us from paying any dividends.
Risks Related to our Senior Convertible Notes
Adjustments to the conversion price for our Convertible Notes issued to the Selling Stockholder will dilute the ownership interests of our existing stockholders.
The Convertible Notes are convertible at any time after issuance, in whole or in part, at the holder’s option, into shares of common stock at an initial fixed conversion price equal to $3.75 per share, or the Initial Fixed Price. If, however, the closing sale price of our common stock is below 110% of the Initial Fixed Price, or $4.13 per share, for two consecutive trading days, either: (1) we can redeem the Convertible Notes in full at a 127.5% premium of the entire principal and interest remaining on the Convertible Notes within sixty (60) days or such occurrence; or (2) on the 61st day following such occurrence, the Selling Stockholder will be able to convert the Convertible Notes, in whole or in part, at the lessor of: (i) the Initial Fixed Price or (ii) a price equal to 80% of the lowest daily volume weighted average price per share during the five trading days prior to conversion. Accordingly, if the closing market price of our common stock decreases to less than $4.13 per share for two consecutive trading days, the number of shares issuable upon conversion of the Convertible Notes will increase, and may result in the issuance of a significant number of additional shares of our common stock upon conversion.
Risks Related to Public Companies
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 and a “smaller reporting company” and the reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (1) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, (2) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and (3) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
26
Table of Contents
Additionally, under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We are electing to delay such adoption of new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. As a result of this election, our financial statements may not be comparable to the financial statements of other public companies.
We may take advantage of these exemptions until we are no longer an emerging growth company. Under the JOBS Act, we may be able to maintain emerging growth company status for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700 million as of any June 30 before the end of such five-year period or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31. Additionally, if we issue more than $1.0 billion in non-convertible debt during any three-year period before that time, we would cease to be an emerging growth company immediately.
We are also currently a “smaller reporting company” as defined in the Securities Exchange Act of 1934, and in the event that we are still considered a smaller reporting company at such time as we cease being an emerging growth company, we will be required to provide additional disclosure in our SEC filings. However, similar to emerging growth companies, smaller reporting companies are able to provide simplified executive compensation disclosures in their filings, are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting, and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. We cannot predict whether investors will find our common stock less attractive because of our reliance on any of these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
We incur substantial costs as a result of being a public company and our management expects to devote substantial time to public company compliance programs.
As a public company, we incur significant legal, insurance, accounting and other expenses, including costs associated with public company reporting. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment will result in increased general and administrative expenses and may divert management’s time and attention from product development and commercialization activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us, and our business may be harmed. These laws and regulations could make it more difficult and costly for us to obtain director and officer liability insurance for our directors and officers, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified executive officers and qualified members of our board of directors, particularly to serve on our audit and compensation committees. In addition, if we are unable to continue to meet the legal, regulatory and other requirements related to being a public company, we may not be able to maintain the listing of our common stock on The NASDAQ Capital Market, which would likely have a material adverse effect on the trading price of our common stock.
Our internal control over financial reporting does not currently meet the standards required by Section 404 of the Sarbanes-Oxley Act, and failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could result in material misstatements of our annual or interim financial statements and have a material adverse effect on our business and share price.
As a new public company, we are not currently required to make a formal assessment of the effectiveness of our internal control over financial reporting for purposes of compliance with the SEC’s rules that implement Section 404 of the Sarbanes Oxley Act. We are, however, required to comply with certain of these rules, which will require management to certify financial and other information in our quarterly and annual reports and provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our second annual report. This assessment will need to include the disclosure of any material weaknesses or significant deficiencies in our internal control over financial reporting identified by our management or our former independent registered public accounting firm. A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. A “significant deficiency” is a deficiency, or a combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness, yet important enough to merit attention by those responsible for oversight of our financial reporting, including the audit committee of the board of directors.
Our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal control over financial reporting until the later of our second annual report or the first annual report required to be filed with the SEC following the date we are no longer an “emerging growth company” as defined in the JOBS Act. However, in connection with our audits for the years ended December 31, 2013 and 2012, and their review of our interim financial statements, our former independent registered public accounting firm noted four material weaknesses and one significant deficiency in our internal control over financial reporting.
27
Table of Contents
One material weakness related to our improper recording and disclosure of non-routine transactions due to deficiencies in the design and operation of our controls to account for non-routine transactions as part of the financial close process. We plan to remedy this by increasing the size and expertise of our internal accounting team.
Another material weakness was identified related to the deficiency in the design and operation of our controls to account for inventory. In addition to increasing the size and expertise of our accounting team, we plan to address this deficiency by physically counting inventory held by certain of our distributors on a more frequent basis and monitoring more closely the movement of inventory between locations.
The third material weakness related to deficiencies in our income tax accounting. We intend to implement a formal process for accounting for income taxes, including evaluating the tax treatment of certain transactions on permanent and temporary book/tax differences, and the effect on the income tax provision and related deferred tax accounting balances.
The fourth material weakness relates to deficiencies in the design and operation of our controls to appropriately identify and evaluate transactions for appropriate cut-off at the end of the financial reporting period and the level of precision and timeliness of our financial close process. In addition to increasing the size and expertise of our accounting team, we plan to remedy this by implementing a formal financial close process related to financial reporting.
Additionally, our former independent registered public accounting firm identified a significant deficiency related to the design and operation of our controls to manage the safeguarding of assets, particularly our instruments that we provide to surgeons and hospitals on consignment. We plan to implement a formal process for tracking and monitoring fixed assets as they are deployed for use at various locations.
We cannot assure you that our plans will sufficiently address the identified deficiencies, nor can we assure you that there will not be material weaknesses or significant deficiencies in our internal controls in the future. Additionally, in the event that our internal control over financial reporting is perceived as inadequate, or that we are unable to produce timely or accurate financial statements, investors may lose confidence in our operating results and the trading price of our common stock could decline.
Finally, as a private company, we were not previously required to prepare quarterly financial statements, nor were we required to generate financial statements in the time periods mandated for public companies by the SEC’s reporting requirements. We believe that we will need to expand our accounting resources, including the size and expertise of our internal accounting team, to effectively execute a quarterly close process and on an appropriate time frame for a public company. If we are unsuccessful or unable to sufficiently expand these resources, we may not be able to produce U.S. GAAP-compliant financial statements on a time frame required to comply with our reporting requirements under the Exchange Act, and the financial statements we produce may contain material misstatements, either of which could cause investors to lose confidence in our financial reports and our financial reporting generally, which could lead to a material decline in the trading price of our common stock.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that are based on our management’s beliefs and assumptions and on information currently available to us. The forward-looking statements are contained principally in, but not limited to, the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| • | our ability to achieve sufficient market acceptance of any of our products or product candidates; |
| • | our perception of the growth in the size of the potential market for our products and product candidates; |
| • | our estimate of the advantages of our silicon nitride technology platform; |
| • | our ability to become a profitable biomaterial technology company; |
| • | our ability to comply with, or receive waivers from compliance with the covenants, made in the Hercules Secured Credit Facility and the Convertible Notes; |
| • | our estimates regarding our needs for additional financing and our ability to obtain such additional financing on suitable terms; |
| • | our ability to succeed in obtaining FDA clearance or approvals for our product candidates; |
| • | our ability to receive CE Marks for our product candidates; |
28
Table of Contents
| • | the timing, costs and other limitations involved in obtaining regulatory clearance or approval for any of our product candidates and product candidates and, thereafter, continued compliance with governmental regulation of our existing products and activities; |
| • | our ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others; |
| • | our ability to obtain sufficient quantities and satisfactory quality of raw materials to meet our manufacturing needs; |
| • | the availability of adequate coverage reimbursement from third-party payors in the United States; |
| • | our estimates regarding anticipated operating losses, future product revenue, expenses, capital requirements and liquidity; |
| • | our ability to maintain and continue to develop our sales and marketing infrastructure; |
| • | our ability to enter into and maintain suitable arrangements with an adequate number of distributors; |
| • | our manufacturing capacity to meet future demand; |
| • | our ability to establish Kyocera as a secondary manufacturing source for our silicon nitride products; |
| • | our ability to develop effective and cost efficient manufacturing processes for our products; |
| • | our reliance on third parties to supply us with raw materials and our non-silicon nitride products and instruments; |
| • | the safety and efficacy of products and product candidates; |
| • | the timing of and our ability to conduct clinical trials; |
| • | potential changes to the healthcare delivery systems and payment methods in the United States or internationally; |
| • | any potential requirement by regulatory agencies that we restructure our relationships with referring surgeons; |
| • | our ability to develop and maintain relationships with surgeons, hospitals and marketers of our products; and |
| • | our ability to attract and retain a qualified management team, engineering team, sales and marketing team, distribution team, design surgeons, surgeon advisors and other qualified personnel and advisors. |
In some cases, you can identify forward-looking statements by terms such as “may,” “could,” “will,” “should,” “would,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “project” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the heading “Risk Factors” and elsewhere in this prospectus. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward-looking statements.
Any forward-looking statement in this prospectus reflects our current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our operations, results of operations, industry and future growth. Except as required by law, we assume no obligation to publicly update or revise any forward-looking statements contained in this prospectus, whether as a result of new information, future events or otherwise. The Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act of 1933, as amended, or the Securities Act, do not protect any forward-looking statements that we make in connection with this offering.
We will not receive any proceeds from the resale of the shares of common stock offered by the Selling Stockholder. In addition, to the extent the Magna Warrant exercised in whole or in part for cash, we will receive payment for the exercise price. If all 568,889 shares of common stock underlying the Magna Warrant included in this prospectus were exercised for cash on July 8, 2014, we would have received approximately $2.6 million.
We intend to apply any proceeds received in connection with the exercise of the Magna Warrant (i) to fund research and development and commercialization activities of our product candidates, including the funding of clinical trials we plan to conduct for our product candidates, and (ii) to continue to build sales, marketing and distribution capabilities for our silicon nitride technology platform, including the costs of inventory and instruments.
MARKET PRICE OF AND DIVIDEND POLICY
Market Information
Our shares of common stock are currently quoted on the NASDAQ Capital Market under the symbol “AMDA”.
29
Table of Contents
The following table sets forth the high and low closing bid prices of our common stock, as reported by NASDAQ Capital Markets, for the periods indicated:
| 2014 | ||||||||
| High | Low | |||||||
| First Quarter ( February 13 to March 31) |
$ | 9.37 | $ | 5.30 | ||||
| Second Quarter |
$ | 6.25 | $ | 4.40 | ||||
Dividend Policy
We have never paid or declared any cash dividends on our common stock, and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development and expansion of our business.
The shares of our common stock to be sold by the Selling Stockholder pursuant to this prospectus are shares of common stock that will be issued to our stockholders upon exercise of the Convertible Notes and the Magna Warrant. Accordingly, there will be no dilution to our existing stockholders from these sales, other than from the exercise of the Convertible Notes and the Magna Warrant.
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes incorporated by reference herein. The selected consolidated statement of comprehensive loss data for the years ended December 31, 2013 and 2012 and selected consolidated balance sheet data as of December 31, 2013 and 2012 were derived from our audited consolidated financial statements that are incorporated by reference herein. The selected consolidated statement of comprehensive loss data for the three months ended March 31, 2014 and 2013 and selected consolidated balance sheet data as of March 31, 2014 were derived from our unaudited consolidated financial statements that are incorporated by reference herein. In the opinion of management, the unaudited consolidated financial statements were prepared on a basis consistent with our audited consolidated financial statements incorporated by reference herein and include all adjustments necessary for the fair presentation of the financial information contained in those statements. The historical results presented below are not necessarily indicative of financial results to be achieved in future periods, and the results for the three months ended March 31, 2014 are not necessarily indicative of results to be expected for the full year.
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| (unaudited) | (unaudited) | (audited) | (audited) | |||||||||||||
| (in thousands except share and per share amounts) | ||||||||||||||||
| Consolidated Statement of Comprehensive Loss Data: |
||||||||||||||||
| Product revenue |
$ | 5,780 | $ | 5,253 | $ | 22,314 | $ | 23,065 | ||||||||
| Total cost of revenue |
1,649 | 1,946 | 7,045 | 6,466 | ||||||||||||
| Total operating expenses |
8,187 | 6,080 | 25,604 | 45,701 | ||||||||||||
| Net loss from operations |
(4,056 | ) | (2,773 | ) | (10,335 | ) | (29,102 | ) | ||||||||
| Net loss |
(4,713 | ) | (3,534 | ) | (8,286 | ) | (35,035 | ) | ||||||||
| Net loss per share: |
||||||||||||||||
| Basic and diluted |
$ | (0.79 | ) | $ | (8.45 | ) | (15.52 | ) | (100.52 | ) | ||||||
| Weighted average common shares outstanding : |
||||||||||||||||
| Basic and diluted |
5,967,400 | 418,172 | 534,073 | 348,550 | ||||||||||||
| As of March 31, | As of December 31, | |||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (unaudited) | (audited) | (audited) | ||||||||||
| (in thousands) | ||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||
| Cash, restricted cash and cash equivalents |
$ | 13,691 | $ | 2,671 | $ | 3,001 | ||||||
| Inventories, net |
11,628 | 10,084 | 8,826 | |||||||||
| Total assets |
43,848 | 34,327 | 33,455 | |||||||||
| Current debt |
16,132 | 17,925 | 20,466 | |||||||||
| Total liabilities |
23,386 | 25,932 | 28,264 | |||||||||
| Convertible preferred stock |
— | 161,456 | 153,474 | |||||||||
| Accumulated deficit |
(144,636 | ) | (139,923 | ) | (131,637 | ) | ||||||
| Total stockholders’ equity (deficit) |
20,462 | (153,061 | ) | (148,283 | ) | |||||||
30
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with “Selected Consolidated Financial Data,” our consolidated financial statements and related notes incorporated by reference herein. This discussion and analysis contains forward-looking statements based upon current beliefs, plans, expectations, intentions and projections that involve risks, uncertainties and assumptions, such as statements regarding our plans, objectives, expectations, intentions and projections. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We are a commercial biomaterial company focused on using our silicon nitride technology platform to develop, manufacture and sell a broad range of medical devices. We currently market spinal fusion products and are developing products for use in total hip and knee joint replacements. We believe our silicon nitride technology platform enables us to offer new and transformative products in the orthopedic and other medical device markets. We believe we are the first and only company to use silicon nitride in medical applications and over 14,000 of our intervertebral fusion devices have been implanted in patients.
We currently market our Valeo MC2 silicon nitride interbody spinal fusion devices in the United States and Europe for use in the cervical and thoracolumbar areas of the spine. We believe our Valeo devices have a number of advantages over existing products due to silicon nitride’s key characteristics, resulting in faster and more effective fusion and reduced risk of infection. Our first generation Valeo silicon nitride device received 510(k) regulatory clearance and a CE Mark in 2008. Based on surgeon feedback, we developed a second generation of Valeo AL, PL and OL products with design enhancements that improve surgeon control during implantation and stability post procedure. In 2013, we initiated a targeted launch of our second generation Valeo AL, PL and OL interbody fusion devices and expect to complete the full launch in the third quarter of 2014. We are also completing the development of our second generation cervical and TL Valeo interbody spinal fusion devices and expect these to be launched in the fourth quarter of 2014. We also market our Valeo composite interbody spinal fusion device made from both our solid MC2 and porous CSC silicon nitride in the Netherlands, Spain and Germany. We are currently conducting a prospective clinical trial in Europe, named CASCADE, comparing our Valeo composite silicon nitride interbody devices to PEEK interbody devices to obtain additional data to support 510(k) clearance in the United States. The trial is 100% enrolled. We expect results to be available in the second half of 2014. If this trial is successful, we plan to file a 510(k) submission with the FDA by mid-2015. In addition, in the first half of 2013, we initiated a Design and Build Program focused on collaborating with influential surgeons to develop customized silicon nitride spinal fusion products and instruments. The first products designed under this program were sold in the third quarter of 2013.
In addition to our silicon nitride-based spinal fusion products, we market a complementary line of non-silicon nitride spinal fusion products which allows us to provide surgeons and hospitals with a broader range of products. These products include three lines of spinal fusion devices and five types of orthobiologics, which are used by surgeons to help promote bone growth and fusion in spinal fusion procedures. Although our non-silicon nitride products have accounted for approximately 57% of our product revenues for the three months ended March 31, 2014 and 66% and 74% or more of our product revenues for the years ended December 31, 2013 and 2012, respectively, we believe the continued promotion and potential for adoption of our silicon nitride products and product candidates, if approved, provides us the greatest opportunity to grow our business in new and existing markets and achieve our goal of becoming a leading commercial biomaterial company.
We market and sell our products to surgeons and hospitals in the United States and select markets in Europe and South America through our established network of more than 50 independent sales distributors. A substantial portion of our product revenue has historically been derived from sales in the United States.
We plan to use our silicon nitride technology platform to expand our product offerings. We are incorporating our silicon nitride technology into components for use in total hip and knee replacement product candidates that we are, or plan on, developing in collaboration with a strategic partner. In addition, we believe our silicon nitride technology platform can be used for developing products in other markets and have developed prototypes for use in the dental, sports medicine and trauma markets. We believe our coating technology may be used to enhance our metal products as well as commercially-available metals, such as those used in spinal fusion, joint replacement and other medical products.
31
Table of Contents
Recent Developments
Hercules Loan and Security Agreement
In June 2014 we entered into a Loan and Security Agreement with Hercules Technology Growth Capital, Inc., or. Hercules Technology, as administrative and collateral agent for the lenders thereunder and as lender, and Hercules Technology III, LP, as lender. The Loan and Security Agreement provides us with a $20 million term loan that matures on January 1, 2018, and which is secured by substantially all of our assets. Proceeds of this loan were used to repay in full and terminate our prior credit facility with General Electric Capital Corporation, or GE Capital, and the remainder of these loan proceeds will be used for general corporate purposes.
The Loan and Security Agreement includes a minimum liquidity covenant that requires us to maintain cash and cash equivalents under the Loan and Security Agreement of not less than $6.0 million through August 15, 2014, and not less than $9.0 million thereafter. We will need to obtain additional funding during the fourth quarter of 2014 to maintain compliance with the financial and liquidity covenants under the Loan and Security Agreement through the next twelve months. Furthermore, if we are unable to access additional funds prior to becoming non-compliant with the liquidity covenant, the entire remaining balance of the Loan and Security Agreement could become immediately due and payable at the option of Hercules Technology.
In addition, we issued a warrant to Hercules Technology, or the Hercules Warrant, to purchase 516,129 shares of our common stock, subject to adjustment as set forth in the Hercules Warrant; provided, further, that the number of shares of our common stock for which the Hercules Warrant may be exercisable may be increased to 623,655 in the event that we do not meet certain conditions set forth in the Hercules Warrant.
Under the Loan and Security Agreement, we will incur a $500,000 penalty in the event that the registration statement related to this prospectus is not declared effective by the SEC on or before August 15, 2014.
Private Placement of Senior Convertible Notes and Warrant to Magna
On June 30, 2014, we entered into a Securities Purchase Agreement with the Selling Stockholder pursuant to which, we sold to the Selling Stockholder an initial unsecured senior convertible note with an original principal amount of $2.9 million, or the Initial Convertible Note, for a purchase price of $2.5 million. Additionally, the Selling Stockholder is irrevocably bound to purchase, an additional unsecured senior convertible note with an original principal amount of $3.5 million, or the Additional Convertible Note, for a fixed purchase price of $3.5 million, subject only to conditions outside of the Selling Stockholder’s control or that the Selling Stockholder cannot cause not to be satisfied, none of which are related to the market price of shares of our common stock, no later than ten calendar days after the effective date of the registration statement registering the shares issued in connection with the Securities Purchase Agreement. The Initial Convertible Note and the Additional Convertible Note are collectively referred to as the Convertible Notes.
With respect to the Initial Convertible Note, $150,000 of the outstanding principal amount (together with any accrued and unpaid interest with respect to such portion of the principal amount) will be automatically extinguished if (1) we have properly filed a registration statement registering the shares covered by this prospectus with the SEC on or prior to August 14, 2014 and (2) no event of default, or an event that with the passage of time or giving of notice would constitute an event of default, has occurred on or prior to such date. In addition, $250,000 of the outstanding principal amount of the Initial Convertible Note (together with any accrued and unpaid interest with respect to such portion of the principal amount) will be automatically extinguished if (1) the registration statement is declared effective by the SEC on or prior to the earlier of (i) October 13, 2014 and (ii) the fifth trading day after the SEC notifies us that the registration statement is not subject to further review, and this prospectus is available for use by the Selling Stockholder and (2) no event of default, or an event that with the passage of time or giving of notice would constitute an event of default, has occurred on or prior to such date.
The Convertible Notes mature on June 30, 2016 (subject to extension as provided in the Initial Convertible Note) and accrue interest at an annual rate of 6.0%. The Convertible Notes are convertible at any time after issuance, in whole or in part, at the Selling Stockholder’s option, into shares of common stock at an initial fixed conversion price equal to $3.75 per share, or the Initial Fixed Price. If, however, the closing sale price of our common stock is below 110% of the Initial Fixed Price, or $4.13 per share, for two consecutive trading days, either:
| (1) | we may to redeem the Convertible Notes in full at a 127.5% premium of the entire principal and interest remaining on the Convertible Notes within sixty (60) days of such occurrence; or |
| (2) | the Selling Stockholder will be able to convert the Convertible Notes, in whole or in part, at the lessor of: (i) the Initial Fixed Price or (ii) a price equal to 80% of the lowest daily volume weighted average price, or VWAP, of our common stock during the five trading days prior to conversion beginning on the 61st day following such occurrence,. |
32
Table of Contents
In addition, we issued a warrant to the Selling Stockholder, or the Magna Warrant, to purchase up to 568,889 shares of our common stock at an exercise price of $4.65 per share. The Magna Warrant expires on June 30, 2016 and is exercisable as to 257,778 shares immediately and will become exercisable as of the date of issuance of the Additional Convertible Note with respect to the remaining 311,111 shares.
We also issued 50,853 shares of our common stock to the Selling Stockholder which represents 4% of the total purchase price for the Convertible Notes under the Securities Purchase Agreement calculated using a per share price of $4.7195, representing the VWAP of the Common Stock per share on June 27, 2014 in connection with our execution of the Securities Purchase Agreement.
Components of our Results of Operations
We manage our business within one reportable segment, which is consistent with how our management reviews our business, makes investment and resource allocation decisions and assesses operating performance.
Product Revenue
We derive our product revenue primarily from the sale of spinal fusion devices and related products used in the treatment of spine disorders. Our product revenue is generated from sales to two types of customers: (1) surgeons and hospitals; and (2) stocking distributors. Most of our products are sold on a consignment basis through a network of independent sales distributors; however, we also sell our products to independent stocking distributors. Product revenue is recognized when all four of the following criteria are met: (1) persuasive evidence that an arrangement exists; (2) delivery of the products has occurred; (3) the selling price of the product is fixed or determinable; and (4) collectability is reasonably assured. We generate the majority of our revenue from the sale of inventory that is consigned to independent sales distributors that sell our products to surgeons and hospitals. For these products, we recognize revenue at the time we are notified the product has been used or implanted and all other revenue recognition criteria have been met. For all other transactions, we recognize revenue when title and risk of loss transfer to the stocking distributor, and all other revenue recognition criteria have been met. We generally recognize revenue from sales to stocking distributors at the time the product is shipped to the distributor. Stocking distributors, who sell the products to their customers, take title to the products and assume all risks of ownership at time of shipment. Our stocking distributors are obligated to pay within specified terms regardless of when, if ever, they sell the products. Our policy is to classify shipping and handling costs billed to customers as an offset to total shipping expense in the statement of operations, primarily within sales and marketing. In general, our customers do not have any rights of return or exchange.
We believe our product revenue from the sale of our silicon nitride based products and our non-silicon nitride products will increase due to our sales and marketing efforts and as we introduce new silicon nitride based products into the market, such as our second generation Valeo interbody spinal fusion products in the United States. We expect that our product revenue will continue to be primarily attributable to sales of our products in the United States, though, as we expand our sales and marketing efforts and market additional products abroad, such as our spinal fusion device incorporating our CSC, we expect international sales will increase.
Cost of Revenue
The expenses that are included in cost of revenue include all direct product costs if we obtained the product from third-party manufacturers and our in-house manufacturing costs for the products we manufacture. We obtain our non-silicon nitride products, including our metal and orthobiologic products, from third-party manufacturers, while we currently manufacture our silicon-nitride products in-house.
Specific provisions for excess or obsolete inventory and, beginning in 2013, the 2.3% excise tax on the sale of medical devices in the United States, are also included in cost of revenue. In addition, we pay royalties attributable to the sale of specific products to some of our surgeon advisors that assisted us in the design, regulatory clearance or commercialization of a particular product, and these payments are recorded as cost of revenue.
Gross Profit
Our gross profit measures our product revenue relative to our cost of revenue. While we expect our cost of revenue to increase in absolute terms as our sales volume increases, we believe our gross profit will be higher as we realize manufacturing efficiencies associated with our silicon nitride-based products.
33
Table of Contents
Research and Development Expenses
Our net research and development costs are expensed as incurred. Research and development costs consist of engineering, product development, clinical trials, test-part manufacturing, testing, developing and validating the manufacturing process, manufacturing, facility and regulatory-related costs. Research and development expenses also include employee compensation, employee and non-employee stock-based compensation, supplies and materials, consultant services, and travel and facilities expenses related to research activities. To the extent that certain research and development expenses are directly related to our manufactured products, such expenses and related overhead costs are allocated to inventory.
We expect to incur additional research and development costs as we continue to develop new spinal fusion products such as our second generation Valeo products, our product candidates for total joint replacements, such as our total hip replacement product candidate, and our silicon nitride-coated metals which may increase our research and development expenses.
Sales and Marketing Expenses
Sales and marketing expenses consist of salaries, benefits and other related costs, including stock-based compensation, for personnel employed in sales, marketing, medical education and training. In addition, our sales and marketing expenses include commissions and bonuses, generally based on a percentage of sales, to our sales managers and independent sales distributors. We provide our products in kits or banks that consist of a range of device sizes and separate instruments necessary to complete the surgical procedure. We generally consign our instruments to our distributors or our hospital customers that purchase the device used in spinal fusion surgery. Our sales and marketing expenses include depreciation of the surgical instruments.
We expect our sales and marketing expenses to continue to increase, including instrument set depreciation, as we introduce new products, such as our second generation Valeo spinal fusion products into the United States, and seek to enhance our commercial infrastructure, including increasing our marketing efforts and further educating our distributors. Additionally, we expect our commissions to continue to increase in absolute terms over time but remain approximately the same or decrease as a percentage of product revenue.
General and Administrative Expenses
General and administrative expenses primarily consist of salaries, benefits and other related costs, including stock-based compensation, for certain members of our executive team and other personnel employed in finance, legal, compliance, administrative, information technology, customer service, executive and human resource departments. General and administrative expenses include allocated facility expenses, related travel expenses and professional fees for accounting and legal services.
We expect our general and administrative expenses will increase due to costs associated with transitioning from a private to a public company and as we continue to grow our business.
RESULTS OF OPERATIONS
Three Months Ended March 31, 2014 and 2013
The following is a tabular presentation of our condensed consolidated operating results for the three months ended March 31, 2014 compared to our condensed consolidated operating results for the three months ended March 31, 2013 (in thousands):
| Three Months Ended March 31, | ||||||||||||||||
| 2014 | 2013 | $ Change | % Change | |||||||||||||
| Product revenue |
$ | 5,780 | $ | 5,253 | $ | 527 | 10 | % | ||||||||
| Total cost of revenue |
1,649 | 1,946 | (297 | ) | (15 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
4,131 | 3,307 | 824 | 25 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
591 | 1,056 | (465 | ) | (44 | %) | ||||||||||
| General and administrative |
3,075 | 1,382 | 1,693 | 123 | % | |||||||||||
| Sales and marketing |
4,521 | 3,642 | 879 | 24 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
8,187 | 6,080 | 2,107 | 35 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(4,056 | ) | (2,773 | ) | (1,283 | ) | 46 | % | ||||||||
| Other income (expense), net |
(657 | ) | (761 | ) | 104 | 14 | % | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss before income taxes |
(4,713 | ) | (3,534 | ) | (1,179 | ) | (33 | %) | ||||||||
| Provision for income taxes |
— | — | — | (N/A | ) | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (4,713 | ) | $ | (3,534 | ) | $ | (1,179 | ) | (33 | %) | |||||
|
|
|
|
|
|
|
|||||||||||
34
Table of Contents
Product Revenue
The following table sets forth our product revenue from sales of the indicated product category for the three months ended March 31, 2014 and 2013 (in thousands):
| Three Months Ended March 31, | ||||||||||||||||
| 2014 | 2013 | $ Change | % Change | |||||||||||||
| Silicon Nitride |
$ | 2,487 | $ | 1,768 | $ | 719 | 41 | % | ||||||||
| Non-Silicon Nitride |
3,293 | 3,485 | (192 | ) | (6 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total Product Revenue |
$ | 5,780 | $ | 5,253 | $ | 527 | 10 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
Total product revenue during the three months ended March 31, 2014 increased $0.5 million or 10% as compared to the same period in 2013. This increase was primarily driven by increased sales of our silicon nitride products, which increased by $0.7 million or 41%, for the three months ended March 31, 2014 as compared to the same period in 2013 primarily due to our continued focus and investment in sales and marketing efforts of our silicon nitride products. The increase in sales of silicon nitride products was partially offset by a decrease in non-silicon nitride sales of $0.2 million or 6% during the three months ended March 31, 2014 as compared to the same period in 2013, as sales and marketing was primarily focused on silicon nitride product sales as opposed to non-silicon nitride product sales.
The following table sets forth, for the periods indicated, our product revenue by geographic area (in thousands):
| Three Months Ended March 31, | ||||||||||||||||
| 2014 | 2013 | $ Change | % Change | |||||||||||||
| Domestic |
$ | 5,775 | $ | 5,196 | $ | 579 | 11 | % | ||||||||
| International |
5 | 57 | (52 | ) | (91 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total Product Revenue |
$ | 5,780 | $ | 5,253 | $ | 527 | 10 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
International revenue decreased 91% as we are primarily focused on increasing sales domestically and we expect international sales to remain nominal for the foreseeable future.
Cost of Revenue and Gross Profit
Cost of revenue was $1.6 million during the three months ended March 31, 2014 as compared to $1.9 million for the same period in 2013, a decrease of $0.3 million, or 15%. This decrease was primarily related to a decrease in excess and obsolete inventory costs of $0.1 million related to our first generation Valeo products, which were reserved in 2013, and a decrease in product costs of $0.2 million as we continue to gain production efficiencies. As a result, gross profit as a percentage of product revenue increased by 8% to 71% for three months ended March 31, 2014 from 63% for the same period in 2013.
Research and Development Expenses
Research and development expenses were $0.6 million in the three months ended March 31, 2014 as compared to $1.1 million for the same period in 2013, a decrease of $0.5 million, or 44%. This decrease was primarily due to our allocation, in 2014, of an additional $1.6 million of overhead costs to inventory as a result of the ramp-up phase for our second generation Valeo products, which overhead costs had previously been allocated to research and development expenses during the three months ended March 31, 2013. The increase in additional overhead allocated to inventory was partially offset by our continued investment in research and development.
General and Administrative Expenses
General and administrative expenses were $3.1 million during the three months ended March 31, 2014 as compared to $1.4 million for the same period in 2013, an increase of $1.7 million, or 123%. This increase was primarily due to an increase in stock-based compensation of $0.8 million, a $0.4 million increase in accounting and consulting expenses, a $0.3 million increase in legal and patent expense and a $0.1 million increase in employee compensation.
Sales and Marketing Expenses
Sales and marketing expenses were $4.5 million during the three months ended March 31, 2014 as compared to $3.6 million for the same period in 2013, an increase of $0.9 million, or 24%. This increase was primarily due to an increase in commission expenses of $0.4 million as our sales increased and overall sales commission rate increased in the three months ended March 31, 2014 as compared to 2013 and an increase in stock compensation of $0.4 million. Marketing expenses also increased by $0.2 million as we continue to focus on marketing our silicon nitride products.
35
Table of Contents
Other Income (Expense), Net
Other expense during the three months ended March 31, 2014 decreased $0.1 million or 14% as compared to the same period in 2013. This decrease in other expense was primarily due to a lower net increase in fair value of our stock warrant liabilities as a result of a portion of the warrants being repriced during the three months ended March 31, 2013. This decrease was partially offset by an increase in interest expense of $0.1 million as a result of increased non-cash interest expense from amortization of debt modification fees.
Outstanding common stock warrants that we issued in connection with certain of our private offerings prior to our IPO include price protection provisions, and the exercise price of such warrants was reduced to $3.75 per share as a result of our issuance of the Initial Convertible Note to the Selling Stockholder on June 30, 2014. We anticipate recording a non-cash expense for the second quarter of 2014 of approximately $0.5 million because of the reduction of the exercise price of these warrants. The exercise price of outstanding warrants that include price protection provisions may be further adjusted if the conversion price of the Convertible Notes issued to the Selling Stockholder is reduced in the event the closing sale price of our common stock is below $4.13 per share for two consecutive trading days.
Year Ended December 31, 2013 Compared to the Year Ended December 31, 2012
The following table sets forth, for the periods indicated, our results of operations for the years ended December 31, 2013 and 2012 (in thousands):
| Year Ended December 31, | ||||||||||||||||
| 2013 | 2012 | $ Change | % Change | |||||||||||||
| Product revenue |
$ | 22,314 | $ | 23,065 | $ | (751 | ) | (3 | %) | |||||||
| Total cost of revenue |
7,045 | 6,466 | 579 | 9 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
15,269 | 16,599 | (1,330 | ) | (8 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
3,461 | 6,013 | (2,552 | ) | (42 | %) | ||||||||||
| General and administrative |
5,759 | 7,313 | (1,554 | ) | (21 | %) | ||||||||||
| Sales and marketing |
16,384 | 17,094 | (710 | ) | (4 | %) | ||||||||||
| Impairment loss on intangible assets |
— | 15,281 | (15,281 | ) | (N/A | ) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
25,604 | 45,701 | (20,097 | ) | (44 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(10,335 | ) | (29,102 | ) | 18,767 | (64 | %) | |||||||||
| Other income (expense), net |
2,049 | (6,659 | ) | 8,708 | 131 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss before income taxes |
(8,286 | ) | (35,761 | ) | 27,475 | 77 | % | |||||||||
| Income tax benefit |
— | 726 | 726 | (N/A | ) | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (8,286 | ) | $ | (35,035 | ) | $ | 26,749 | 76 | % | ||||||
|
|
|
|
|
|
|
|||||||||||
Product Revenue
The following table sets forth our product revenue from sales of the indicated product category for the years ended December 31, 2103 and 2012 (in thousands):
| Year Ended December 31, | ||||||||||||||||
| 2013 | 2012 | $ Change | % Change | |||||||||||||
| Silicon Nitride |
$ | 7,667 | $ | 6,578 | $ | 1,089 | 17 | % | ||||||||
| Non-Silicon Nitride |
14,647 | 16,487 | (1,840 | ) | (11 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total Product Revenue |
$ | 22,314 | $ | 23,065 | $ | (751 | ) | (3 | %) | |||||||
|
|
|
|
|
|
|
|||||||||||
Total product revenue was $22.3 million in 2013 as compared to $23.1 million in 2012, a decrease of $0.8 million or 3%. This decrease in total product revenue was primarily attributable to our restructuring of our sales and marketing teams during the first quarter of 2013, resulting from changes in our distribution network, the timing of the launch of our second generation Valeo products and a one-time sale of non-silicon nitride products to an international customer in the 2012 period with no corresponding sale in 2013. Sales of our silicon nitride products increased by $1.1 million, or 17%, in 2013 as compared to 2012. Non-silicon nitride sales decreased $1.8 million, or 11%, in 2013 compared to 2012, as the new sales team was primarily focused on silicon nitride product sales as opposed to non-silicon nitride product sales.
36
Table of Contents
The following table sets forth, for the periods indicated, our product revenue by geographic area (in thousands):
| Year Ended December 31, | ||||||||||||||||
| 2013 | 2012 | $ Change | % Change | |||||||||||||
| Domestic |
$ | 22,203 | $ | 21,847 | $ | 356 | 2 | % | ||||||||
| International |
111 | 1,218 | (1,107 | ) | (91 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total Product Revenue |
$ | 22,314 | $ | 23,065 | $ | (751 | ) | (3 | %) | |||||||
|
|
|
|
|
|
|
|||||||||||
Product revenue attributable to sales in the United States was $22.2 million in 2013, an increase of $0.4 million, or 2%, as compared to 2012. Product revenue attributable to international sales was $0.1 million in 2013, a decrease of $1.1 million, or 91%, as compared to 2012. The decrease was primarily attributable to a one-time sale of non-silicon nitride products to an international customer in 2012.
Cost of Revenue
Cost of revenue was $7.0 million in 2013 as compared to $6.5 million in 2012, an increase of $0.5 million, or 8%. This increase was primarily related to an increase in excess and obsolete inventory costs of $0.3 million related to our first generation Valeo products and the new 2.3% medical device excise tax in the United States, which totaled $0.4 million in 2013. The increases were partially offset by lower costs of our orthobiologic products in 2013 as compared to 2012.
Gross Profit
Gross profit as a percentage of product revenue decreased by 4% to 68% for 2013 from 72% for the same period in 2012, primarily as a result of the U.S. medical device excise tax of 2.3% on product revenue which became effective in January 2013 and an increase in excess and obsolete inventory costs of $0.3 million related to our first generation Valeo products.
Research and Development Expenses
Research and development expenses were $3.5 million in 2013 as compared to $6.0 million in 2012, a decrease of $2.5 million, or 42%. This decrease was primarily due to our allocation, in 2013, of an additional $2.9 million of overhead costs to inventory as a result of the ramp-up phase for our second generation Valeo products, which overhead costs had been allocated to research and development expenses in 2012.
General and Administrative Expenses
General and administrative expenses were $5.8 million in 2013 as compared to $7.3 million in 2012, a decrease of $1.5 million, or 21%. This decrease was primarily due to decreases of $1.4 million in amortization expense and $1.1 million in legal and patent expense, partially offset by a $0.8 million increase in employee compensation and $0.2 million increase in accounting and consulting expenses.
Sales and Marketing Expenses
Sales and marketing expenses were $16.4 million in 2013 as compared to $17.1 million in 2012, a decrease of $0.7 million, or 4%. This decrease was primarily due to a decrease of $0.8 million in marketing expenses and $0.5 million decrease in depreciation and maintenance expense associated with instrumentations. These decreases were partially offset by an increase of $0.6 million in commission expenses, primarily to our sales distributors, to support increased silicon nitride sales volume.
37
Table of Contents
Impairment Loss on Intangible Assets
Impairment loss on intangible assets was $15.3 million in 2012 relating to assets we obtained in our acquisition of US Spine, Inc. (“US Spine”). The amount of the impairment loss was determined during management’s annual impairment review and resulted from lower sales of certain products and lower sales to customers acquired in the US Spine transaction than originally expected. There was not an impairment loss on intangible assets during 2013.
Other Income (Expense), Net
Other income was $2.0 million in 2013 as compared to other expenses of $6.7 million in 2012, a decrease of $8.7 million, or 131%. This decrease in other expense was primarily due to a net decrease in fair value of $4.7 million of our common and preferred stock warrant liabilities as a result of our lower stock price at December 31, 2013 as compared to December 31, 2012. Furthermore, there was a decrease of $3.8 million in interest expense due to our senior secured notes being converted into shares of Series F convertible preferred stock in December 2012.
Liquidity and Capital Resources
For the three months ended March 31, 2014 and 2013, we incurred a net loss of $4.7 million and $3.5 million, respectively, and used cash in operations of $2.0 million and $1.1 million, respectively. For the years ended December 31, 2013 and 2012, we incurred a net loss of $8.3 million and $35.0 million, respectively, and used cash in operations of $9.9 million and $9.7 million, respectively. We have an accumulated deficit of $144.6 million as of March 31, 2014. To date, our operations have been principally financed from proceeds from the issuance of convertible preferred stock and common stock, convertible debt and bank debt and, to a lesser extent, cash generated from product sales.
In order to finance the continued growth in product sales, to invest in further product development and to otherwise satisfy obligations as they mature, we completed an initial public offering of our common stock (“IPO”), in which we sold and issued 3,682,900 shares, including 182,900 shares sold pursuant to the exercise by the underwriters of their over-allotment option, in February 2014, at an issuance price of $5.75 per share, less underwriting discounts and commissions. As a result of the IPO, we received proceeds of approximately $15.4 million, net of approximately $5.8 million in IPO related costs. As of March 31, 2014, we had approximately $13.7 million in cash and cash equivalents and restricted cash.
In June 2014 we entered into a Loan and Security Agreement with Hercules Technology as administrative and collateral agent for the lenders thereunder and as lender, and Hercules Technology III, LP, as lender. The Loan and Security Agreement provides for a $20 million term loan that matures on January 1, 2018 and is secured by substantially all of our assets. Proceeds of the loan were used to repay in full and terminate our prior credit facility with GE Capital and the remainder of the proceeds of this loan will be used for general corporate purposes.
This term loan bears interest at the rate of the greater of either (i) the prime rate plus 7.7%, and (ii) 10.95%. Interest accrues from the date of the Loan and Security Agreement with interest payments due monthly commencing on July 1, 2014. Principal payments are required commencing August 1, 2015 and are to be made in thirty equal installments, with the remainder due at maturity; provided, however, in the event that we meet certain conditions set forth in the Loan and Security Agreement the interest only period may be extended through February 1, 2016, reducing the number of required principal payments to twenty-four. Additionally, under certain circumstances we may, or Hercules Technology may require that we, repay a portion of the principal in the form of shares of our common stock. The conversion price used for the calculation of the amount of shares to be delivered in such instance is $5.72 per share.
Under the Loan and Security Agreement, we will incur a $500,000 penalty in the event that the registration statement related to this prospectus is not declared effective by the SEC on or before August 15, 2014.
The Loan and Security Agreement contains certain covenants related to restrictions on payments to certain company affiliates, financial reporting requirements and a minimum liquidity covenant that requires us to maintain cash and cash equivalents of no less than $6.0 million through August 15, 2014 and not less than $9.0 million thereafter.
We will need to obtain additional funding during the fourth quarter of 2014 to maintain compliance with the financial and liquidity covenants related to the Loan and Security Agreement through the next twelve months. Furthermore, if we are unable to access additional funds prior to becoming non-compliant with the financial or liquidity covenants, the entire remaining balance of the Loan and Security Agreement could become immediately due and payable at the option of Hercules Technology.
The repayment of the Loan and Security Agreement and the liquidity covenant limit our ability to use our cash and cash equivalents to fund our operations and may restrict our ability to continue development of our product candidates. Additionally, the Loan and Security Agreement restricts our ability to incur additional pari passu indebtedness, which may reduce our ability to seek additional financing. If adequate funds are not available on a timely basis, we may terminate or delay the development of one or more of our
38
Table of Contents
product candidates, or delay activities necessary to commercialize our product candidates. Additional funding may not be available to us on acceptable terms, or at all. Any additional equity financing, if available, may not be available on favorable terms and will most likely be dilutive to our current stockholders, and debt financing, if available, may involve more restrictive covenants. Our ability to access capital when needed is not assured and, if not achieved on a timely basis, will materially harm our business, financial condition and results of operations. As a result of our debt obligations, we will need additional funds to meet our operational needs and capital requirements for product development, clinical trials and commercialization.
On June 30, 2014, we entered into a Securities Purchase Agreement with MG Partners II Ltd., an affiliate of Magna, or the Selling Stockholder. Pursuant to the terms of the Securities Purchase Agreement, we sold the Selling Stockholder an initial unsecured senior convertible note with an original principal amount of $2.9 million, or the Initial Convertible Note, for a purchase price of $2.5 million. Additionally, the Selling Stockholder is irrevocably bound to purchase, an additional unsecured senior convertible note with an original principal amount of $3.5 million, or the Additional Convertible Note, for a fixed purchase price of $3.5 million, subject only to conditions outside of the Selling Stockholder’s control or that the Selling Stockholder cannot cause not to be satisfied, none of which are related to the market price of our common stock, no later than ten calendar days after the effective date of the registration statement registering the shares issued in connection with the Securities Purchase Agreement. The Initial Convertible Note and Additional Convertible Note are collectively referred to as the Convertible Notes.
With respect to the Initial Convertible Note, $150,000 of the outstanding principal amount (together with any accrued and unpaid interest with respect to such portion of the principal amount) will be automatically extinguished if (1) we have properly filed a registration statement registering the shares to which this prospectus relates with the SEC on or prior to August 14, 2014 and (2) no event of default, or an event that with the passage of time or giving of notice would constitute an event of default, has occurred on or prior to such date. In addition, $250,000 of the outstanding principal amount of the Initial Convertible Note (together with any accrued and unpaid interest with respect to such portion of the principal amount) will be automatically extinguished if (1) the registration statement is declared effective by the SEC on or prior to the earlier of (i) October 13, 2014 and (ii) the fifth trading day after we receive notification from the SEC that the registration statement is not subject to further review, and this prospectus is available for use by the Selling Stockholder and (2) no event of default, or an event that with the passage of time or giving of notice would constitute an event of default, has occurred on or prior to such date.
The Convertible Notes mature on June 30, 2016 (subject to extension as provided in the Initial Convertible Note) and accrue interest at an annual rate of 6.0%. The Convertible Notes are convertible at any time after issuance, in whole or in part, at the Selling Stockholder’s option, into shares of common stock at an initial fixed conversion price equal to $3.75 per share, or the Initial Fixed Price. If, however, the closing sale price of our common stock is below 110% of the Initial Fixed Price , or $4.13 per share, for two consecutive trading days, either:
| (1) | we may redeem the Convertible Notes in full at a 127.5% premium of the entire principal and interest remaining on the Convertible Notes within sixty (60) days of such occurrence; or |
| (2) | beginning on the 61st day following such occurrence, the Selling Stockholder will be able to convert the Convertible Notes, in whole or in part, at the lessor of: (i) the Initial Fixed Price or (ii) a price equal to 80% of the lowest daily volume weighted average price, or VWAP, of our common stock during the five trading days prior to conversion. |
In addition, we issued to the Selling Stockholder a warrant, or the Magna Warrant, to purchase up to 568,889 shares of our common stock at an exercise price of $4.65 per share. The Magna Warrant expires on June 30, 2016 and is exercisable as to 257,778 shares immediately and will become exercisable as of the date of issuance of the Additional Convertible Note with respect to the remaining 311,111 shares.
At no time will the Selling Stockholder be entitled to convert any portion of the Convertible Notes or exercise any portion of the Magna Warrant to the extent that after such conversion or exercise, the Selling Stockholder (together with its affiliates) would beneficially own more than 4.99% of the outstanding shares of our common stock as of such date, or the Maximum Percentage. The Maximum Percentage may be raised to any other percentage not in excess of 9.99% at the option of the Selling Stockholder upon at least 61 days’ prior notice to us, or lowered to any other percentage, at the option of the Selling Stockholder, at any time.
We also issued 50,853 shares of our common stock to the Selling Stockholder which represents 4% of the total purchase price for the Convertible Notes under the Securities Purchase Agreement calculated using a per share price of $4.7195, the VWAP of the Common Stock per share on June 27, 2014 in connection with our execution of the Securities Purchase Agreement. We are seeking additional financing through the issuance of common stock and/or debt, to satisfy our debt obligations, meet our working capital requirements, make continued investment in research and development and make capital expenditures needed for us to maintain and expand our business. We may not be able to obtain additional financing on terms favorable to us, if at all. It is also possible that we may allocate significant amounts of capital toward solutions or technologies for which market demand is lower than anticipated and, as a result, abandon such efforts. If we are unable to obtain adequate financing or financing on terms satisfactory to us when we require it, or if we expend capital on projects that are not successful, our ability to continue to support our business growth and to respond to business challenges could be significantly limited, or we may even have to scale back our operations. If we raise additional funds through further issuances of equity or convertible debt securities, our existing stockholders could suffer significant dilution, and any new equity securities we issue could have rights, preferences and privileges superior to those of holders of our common stock.
39
Table of Contents
Going Concern
Our ability to access capital when needed is not assured and, if not achieved on a timely basis, will materially harm our business, financial condition and results of operations. These uncertainties create substantial doubt about our ability to continue as a going concern. Our former independent registered public accounting firm included an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern in their report on our annual financial statements for the fiscal year ended December 31, 2013. The financial information throughout this prospectus have been prepared on a basis which assumes that we will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The financial information and statements presented in this prospectus or incorporated by reference herein do not include any adjustments that may result from the outcome of this uncertainty.
Cash Flows
Three Months Ended March 31, 2014 and 2013
The following table summarizes, for the periods indicated, cash flows from operating, investing and financing activities (in thousands):
| Three Months Ended March 31, | ||||||||
| 2014 | 2013 | |||||||
| Net cash used in operating activities |
$ | (2,001 | ) | $ | (1,115 | ) | ||
| Net cash (used in) provided by investing activities |
(590 | ) | 216 | |||||
| Net cash provided by financing activities |
13,569 | 307 | ||||||
|
|
|
|
|
|||||
| Net cash provided (used) |
$ | 10,978 | $ | (592 | ) | |||
|
|
|
|
|
|||||
Net Cash Used in Operating Activities
Net cash used in operating activities was $2.0 million during the three months ended March 31, 2014, compared to $1.1 million for the same period in 2013, a decrease of $0.8 million, or 79%. The increase in cash used in operating activities during 2014 was primarily attributable to a $2.0 million increase in the change in inventory as we built up our inventory, $0.9 million reduction in accounts payable and accrued liabilities and an overall increase in operational expenditures for sales and marketing and general and administrative activities as we continue to focus our efforts on growth. These amounts were partially offset by a $2.6 million decrease in other current assets primarily due to a reduction of deferred offering costs.
Net Cash Provided by Investing Activities
Net cash used in investing activities was $0.6 million during the three months ended March 31, 2014, compared to $0.2 million provided by investing activities during the same period in 2013, a decrease of $0.8 million. The decrease in net cash from investing activities during 2014 was primarily attributable to purchases of property and equipment of $0.5 million and an increase in restricted cash of $0.1 million. No proceeds were received from maturities of marketable securities during 2014 as compared to the same period in 2013 when we received proceeds of $0.5 million.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $13.6 million during the three months ended March 31, 2014, compared to $0.3 million provided during the same period in 2013, an increase of $13.3 million. This increase in net cash provided by financing activities in 2014 was primarily attributable to receiving $15.4 million in net proceeds from the issuance of common stock in our IPO offset by long-term debt principal payments of $1.8 million. In 2013, we received $2.9 million in proceeds from the issuance of common stock in connection with the exercise of common warrants and options, which was offset by net payments of $2.6 million on our revolving credit facility.
40
Table of Contents
Year Ended December 31, 2013 Compared to the Year Ended December 31, 2012
The following table summarizes, for the periods indicated, cash flows from operating, investing and financing activities (in thousands):
| Year Ended December 31, | ||||||||
| 2013 | 2012 | |||||||
| Net cash used in operating activities |
$ | (9,949 | ) | $ | (9,730 | ) | ||
| Net cash provided by investing activities |
253 | 4,275 | ||||||
| Net cash provided by financing activities |
9,234 | 4,865 | ||||||
|
|
|
|
|
|||||
| Net cash used |
$ | (462 | ) | $ | (590 | ) | ||
|
|
|
|
|
|||||
Net Cash Used in Operating Activities
Net cash used in operating activities was $9.9 million in 2013, compared to $9.7 million used in 2012, an increase of $0.2 million, or 2%. The cash used in operating activities for 2013 was primarily attributable to a $4.2 million increase in the change in prepaid expenses and other current assets primarily due to the deferred offering costs and a $2.6 million increase in the change in inventory as we built up our inventory. These amounts were partially offset by a $1.2 million decrease in trade accounts receivable mostly due to improved collection and cash management efforts and a $3.3 million increase in accounts payable and accrued liabilities.
Net Cash Provided by Investing Activities
Net cash provided by investing activities was $0.3 million in 2013, compared to $4.3 million provided in 2012, a decrease of $4.0 million, or 93%. The decrease in net cash provided by investing activities was primarily attributable to a $7.5 million decrease in the proceeds from maturities of marketable securities and a $1.7 million increase in the purchase of property and equipment, which were partially offset by a $5.1 million decrease in purchases of marketable securities.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $9.2 million in 2013, compared to $4.9 million provided in 2012, an increase of $4.3 million, or 90%. This increase in net cash provided by financing activities was primarily attributable to an $8.9 million increase in net proceeds from the issuance of convertible preferred stock and a $2.9 million increase in proceeds from the issuance of common stock in connection with the exercise of common stock warrants and options, partially offset by a $4.8 million decrease in net proceeds from the issuance of convertible debt and a $3.1 million increase in net payments on our GE Secured Lending Facility.
Indebtedness
In December 2012, we entered into a senior secured credit facility with GE Capital, which consisted of an $18.0 million term loan and up to $3.5 million revolving credit facility. We pledged all of our assets as collateral for the loans. The revolving line of credit was secured by our accounts receivable, based on certain defined criteria. This credit facility provided by GE Capital was paid in full on June 30, 2014 and there is no longer any outstanding obligation on this indebtedness.
On June 30, 2014 we entered into a Loan and Security Agreement with Hercules Technology as administrative and collateral agent for the lenders thereunder and as lender, and Hercules Technology III, LP, as lender. The Loan and Security Agreement provides for a $20 million term loan with a maturity date of January 1, 2018 and is secured by substantially all of our assets. The proceeds of the loan were used to repay in full and terminate our prior credit facility with GE Capital and the remainder of the proceeds will be used for general corporate purposes.
Also, on June 30, 2014, we entered into a Securities Purchase Agreement with the Selling Stockholder, pursuant to which we sold an initial unsecured senior convertible note with an original principal amount of $2.9 million, or the Initial Convertible Note, for a purchase price of $2.5 million. Additionally, the Selling Stockholder is irrevocably bound to purchase, an additional unsecured senior convertible note with an original principal amount of $3.5 million, or the Additional Convertible Note, for a fixed purchase price of $3.5 million, subject only to conditions outside of the Selling Stockholder’s control or that the Selling Stockholder cannot cause not to be satisfied, none of which are related to the market price of shares of our common stock, no later than ten calendar days after the effective date of a registration statement registering the shares issued in connection with the Securities Purchase Agreement. The Initial Convertible Note and the Additional Convertible Note are collectively referred to as the Convertible Notes.
The Convertible Notes mature on June 30, 2016 (subject to extension as provided in the Convertible Notes) and accrue interest at an annual rate of 6.0%. The Convertible Notes are convertible at any time after issuance, in whole or in part, at the Selling Stockholder’s option, into shares of common stock at an initial fixed conversion price equal to $3.75 per share, or the Initial Fixed Price. If, however, the closing sale price of our common stock is below 110% of the Initial Fixed Price, or $4.13 per share, for two consecutive trading days, either:
| (1) | we may redeem the Convertible Notes in full at a 127.5% premium of the entire principal and interest remaining on the Convertible Notes within sixty (60) days of such occurrence; or |
41
Table of Contents
| (2) | beginning on the 61st day following such occurrence, the Selling Stockholder will be able to convert the Convertible Notes, in whole or in part, at the lessor of: (i) the Initial Fixed Price or (ii) a price equal to 80% of the lowest daily volume weighted average price, or VWAP, of our common stock during the five trading days prior to conversion. |
See above in this “Management’s Discussion and Analysis of our Financial Condition and Results of Operation – Recent Developments” for additional information about the Loan and Security Agreement and the Convertible Notes.
Contractual Obligations and Commitments
The following table summarizes our outstanding contractual obligations as of March 31, 2014 (in thousands):
| Total | Less Than 1 Year |
1-3 Years | 4-5 Years | After 5 Years |
||||||||||||||||
| Long-term debt (1) |
$ | 16,200 | $ | 5,400 | $ | 10,800 | $ | — | $ | — | ||||||||||
| Operating leases |
5,324 | 651 | 1,793 | 1,900 | 980 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual obligations |
$ | 21,524 | $ | 6,051 | $ | 12,593 | $ | 1,900 | $ | 980 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Does not include the $720,000 final payment fee we must pay upon prepayment in full or scheduled maturity of the term loan, the $15,000 per year annual management fee, the amendment fees of up to $1.1 million, which were paid in June 2014, or monthly interest payments. |
The information above reflects only payment obligations that are fixed and determinable. Our commitments for long-term debt relate to our former term loan and revolving credit facility with GE Capital and our commitment to our operating lease for our corporate headquarters and manufacturing facility in Salt Lake City, Utah. The above table does not include any of the contractual obligations with respect to royalties payable upon sales of certain of our products as none of our arrangements contain minimum royalty payments. We also do not have contractually minimum purchase commitments for the supply of any of our raw materials, products or instruments.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, as defined in Item 303(a)(4) of Regulation S-K.
Seasonality and Backlog
Our business is generally not seasonal in nature. However, our sales may be influenced by summer vacation and winter holiday periods during which we believe fewer spinal fusion surgeries are conducted. Our sales generally consist of products that are in stock with us or maintained at hospitals or with our sales distributors. Accordingly, we do not have a backlog of sales orders.
Critical Accounting Policies and Estimates
The preparation of the consolidated financial statements requires us to make assumptions, estimates and judgments that affect the reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities as of the date of the consolidated financial statements, and the reported amounts of product revenues and expenses during the reporting periods. Certain of our more critical accounting policies require the application of significant judgment by management in selecting the appropriate assumptions for calculating financial estimates. By their nature, these judgments are subject to an inherent degree of uncertainty. On an ongoing basis, we evaluate our judgments, including those related to inventories, recoverability of long-lived assets and the fair value of our common stock. We use historical experience and other assumptions as the basis for our judgments and making these estimates. Because future events and their effects cannot be determined with precision, actual results could differ significantly from these estimates. Any changes in those estimates will be reflected in our consolidated financial statements as they occur. As an “emerging growth company,” we have elected to delay the adoption of new or revised accounting standards until those standards would otherwise apply to private companies. As a result, our financial statements may not be comparable to those of other public companies. While our significant accounting policies are more fully described in the footnotes to our consolidated financial statements incorporated by reference herein, we believe that the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results. The critical accounting policies addressed below reflect our most significant judgments and estimates used in the preparation of our consolidated financial statements.
42
Table of Contents
Revenue Recognition
We derive our product revenue primarily from the sale of spinal fusion devices and related products used in the treatment of spine disorders. Our product revenue is generated from sales to two types of customers: (1) surgeons and hospitals; and (2) stocking distributors. Most of our products are sold on a consignment basis through a network of independent sales distributors; however, we also sell our products to independent stocking distributors. Product revenue is recognized when all four of the following criteria are met: (1) persuasive evidence that an arrangement exists; (2) delivery of the products has occurred; (3) the selling price of the product is fixed or determinable; and (4) collectability is reasonably assured. We generate the majority of our revenue from the sale of inventory that is consigned to independent sales distributors that sell our products to surgeons and hospitals. For these products, we recognize revenue at the time we are notified the product has been used or implanted and all other revenue recognition criteria have been met. For all other transactions, we recognize revenue when title and risk of loss transfer to the stocking distributor, and all other revenue recognition criteria have been met. We generally recognize revenue from sales to stocking distributors at the time the product is shipped to the distributor. Stocking distributors, who sell the products to their customers, take title to the products and assume all risks of ownership at time of shipment. Our stocking distributors are obligated to pay within specified terms regardless of when, if ever, they sell the products. Our policy is to classify shipping and handling costs billed to customers as an offset to total shipping expense in the statement of operations, primarily within sales and marketing. In general, our customers do not have any rights of return or exchange.
Accounts Receivable and Allowance for Doubtful Accounts
The majority of our accounts receivable is composed of amounts due from hospitals or surgical centers. Accounts receivable are carried at cost less an allowance for doubtful accounts. On a regular basis, we evaluate accounts receivable and estimate an allowance for doubtful accounts, as needed, based on various factors such as customers’ current credit conditions, length of time past due, and the general economy as a whole. Receivables are written off against the allowance when they are deemed uncollectible.
Inventories
Inventories are stated at the lower of cost or market, with cost for manufactured inventory determined under the standard cost method which approximates the first-in first-out method. Manufactured inventory consists of raw material, direct labor and manufacturing overhead cost components. Inventories purchased from third-party manufacturers are stated at the lower of cost or market using the first-in, first out method. We review the carrying value of inventory on a periodic basis for excess or obsolete items and record an expense for the identified items as necessary. We have made adjustments to, and it is reasonably possible that we may be required to make further adjustments to, the carrying value of inventory in future periods. We hold some consigned inventory at distributors and other customer locations where revenue recognition criteria have not yet been met.
Long-Lived Assets, Indefinite-Lived Intangibles and Goodwill
Periodically we assess potential impairment of our long-lived assets, which include property, equipment, and acquired intangible assets. We perform an impairment review whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors we consider important which could trigger an impairment review include, but are not limited to, significant under-performance relative to historical or projected future operating results, significant changes in the manner of use of the acquired assets or our overall business strategy, and significant industry or economic trends. When we determine that the carrying value of a long-lived asset may not be recoverable based upon the existence of one or more of the above indicators, we determine the recoverability by comparing the carrying amount of the asset to net future undiscounted cash flows that the asset is expected to generate and recognize an impairment charge equal to the amount by which the carrying amount exceeds the fair market value of the asset. We amortize intangible assets on a straight-line basis over their estimated useful lives.
For indefinite lived intangible assets that are not subject to amortization, the impairment test consists of a comparison of the fair value of an intangible asset with its carrying amount. If the carrying amount of an intangible asset exceeds its fair value, an impairment loss is recognized in an amount equal to that excess.
Our management noted that certain US Spine product sales and sales to certain acquired US Spine customers during the one-year period ended December 31, 2012 had been less than expected relative to the forecasted revenues at the time of our acquisition of US Spine. This indicator prompted us to question whether the carrying value of our long-lived and indefinite lived intangible assets would be recoverable. We compared the carrying amount of the assets to net future undiscounted cash flows that the intangible assets are expected to generate, and concluded that an impairment existed. We estimated the fair values of the intangible assets and recognized an impairment loss of approximately $15.3 million in the year ended December 31, 2012. No impairment loss was recognized in the year ended December 31, 2013. Should conditions change such that our estimates of associated undiscounted cash flows would not support the unamortized carrying value of specific assets, we could have further impairments.
The income approach used in our 2012 impairment analysis considered management’s business plans and projections as the basis for expected cash flows for the next ten years and a 5% residual growth rate thereafter. We also used a weighted average discount rate of 17%, a weighted average revenue growth rate ranging from (58)% to 10% and an EBITDA margin ranging from approximately 9% to 12.4% for the analysis.
43
Table of Contents
Our long-lived assets include surgical instruments used by spine surgeons during surgical procedures to facilitate the implantation of our products. There are no contractual terms with respect to the usage of our instruments by our customers. Surgeons are under no contractual commitment to use our instruments. We maintain ownership of these instruments and, when requested, we allow the surgeons to use the instruments to facilitate implantation of our related products. We do not currently charge for the use of our instruments and there are no minimum purchase commitments of our products. As our surgical instrumentation is used numerous times over several years, often by many different customers, instruments are capitalized as property and equipment once they have been placed in service. Once placed in service, instruments are carried at cost, less accumulated depreciation. Depreciation is computed using the straight-line method based on average estimated useful lives. Estimated useful lives of surgical instruments are three years and are determined based on a variety of factors including reference to associated product life cycles. As instruments are used as tools to assist surgeons, depreciation of instruments is recognized as a sales and marketing expense. Instrument depreciation expense was $1.0 million and $1.2 million for the years ended December 31, 2013 and 2012, respectively.
We review our long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. An impairment loss would be recognized when estimated future undiscounted cash flows relating to the assets are less than the assets’ carrying amount. An impairment loss is measured as the amount by which the carrying amount of an asset exceeds its fair value.
We test goodwill for impairment annually at the end of each fiscal year, or whenever events or changes in circumstances indicate that goodwill may be impaired. We initially assess qualitative factors to determine whether the existence of events or circumstances leads to a determination that it is more-likely-than-not that the fair value of a reporting unit is less than its carrying amount. For goodwill impairment testing purposes, we consider the value of our equity, including the value of our convertible preferred stock, in the total carrying value of our single reporting unit. If, after assessing the totality of events or circumstances, we determine it is more-likely-than-not that the fair value of our reporting unit is less than its carrying amount, then we perform a first step analysis by comparing the carrying amount of net assets to the fair value of our single reporting unit. If the fair value is determined to be less than the carrying amount, a second step analysis is performed to compute the amount of impairment as the difference between the implied estimated fair value of goodwill and the carrying amount.
At December 31, 2013, the balance of goodwill resulting from the US Spine acquisition was $6.2 million. We measure the fair value of our reporting unit for purposes of our impairment test utilizing the income approach. The income approach is calculated based on management’s best estimates of future cash flows which depend primarily upon revenue growth, discount rate, terminal value and long-term growth rate and total operating expenses. There is a certain degree of uncertainty associated with these key assumptions and there are potential events and circumstances that could reasonably be expected to affect these key assumptions, such as (i) significant decline in product revenue or failure to increase revenue in future years, (ii) failure of the new Design and Build Program to increase revenue as expected, (iii) significant increases in the manufacturing costs or acquisition costs of our inventory and (iv) lack of clearance or approval from the FDA for any of our future product candidates.
Income Taxes
We recognize deferred tax assets and liabilities for the future tax consequences attributable to the differences between the financial statement carrying value of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates in effect for the fiscal year in which those temporary differences are expected to be recovered or settled. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
We operate in various tax jurisdictions and are subject to audit by various tax authorities. We provide for tax contingencies whenever it is deemed probable that a tax asset has been impaired or a tax liability has been incurred for events such as tax claims or changes in tax laws. Tax contingencies are based upon their technical merits relative tax law and the specific facts and circumstances as of each reporting period. Changes in facts and circumstances could result in material changes to the amounts recorded for such tax contingencies.
We recognize uncertain income tax positions taken on income tax returns at the largest amount that is more-likely than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained.
Our policy for recording interest and penalties associated with uncertain tax positions is to record such items as a component of our income tax provision. For the years ended December 31, 2013 and 2012, we did not record any material interest income, interest expense or penalties related to uncertain tax positions or the settlement of audits for prior periods.
44
Table of Contents
Stock-Based Compensation Expense
Common Stock Valuation
Historically, our board of directors has determined the fair value of the common stock with assistance from management and based upon information available at the time of grant. The valuation of our common stock requires us to make complex and subjective judgments. We considered a combination of valuation methodologies, including income, market and transaction approaches. The most significant factors considered by our board of directors when determining the fair value of our common stock were as follows:
| • | external market and economic conditions affecting the medical device industry; |
| • | prices at which we sold shares of our convertible preferred stock to third-party investors; |
| • | the superior rights and preferences of securities senior to our common stock, such as our preferred stock, at the time of each grant; |
| • | our need for future financing to fund commercial operations; |
| • | the lack of marketability of our common stock; |
| • | third-party valuations of our common stock; |
| • | our historical operating and financial performance; |
| • | the status of our research and development efforts; |
| • | the status of our new product releases to the spine market; |
| • | the likelihood of achieving a liquidity event, such as an initial public offering or sale of our company; and |
| • | estimates and analysis provided by management. |
We have regularly obtained third-party valuations to assist our board of directors in determining the fair value of our common stock for each stock option grant and other stock-based awards, on an annual basis since 2007.
Significant Factors and Assumptions Used in Determining Fair Value of Common Stock
The $5.75 per share issuance price of the common stock sold in our IPO in February 2014 was used as the estimated fair value of the shares of our common stock as of and for the quarter ended December 31, 2013 due to the close proximity to the valuation dates and there were no significant changes that would indicate a decrease or increase in the stock price. The price of $17.53 per share was used as the estimated fair value of our common shares for estimated fair value calculations done as of and prior to September 30, 2013. The price of $17.53 per share was based on a third party valuation performed in September 2012. The valuation technique used in the third party valuation was a hybrid of the discounted cash flow method and the guideline public company method. The significant assumptions used in determining the $17.53 per share estimated fair value of our common shares using a hybrid of the discounted cash flow method and the guideline public company methodology were as follows:
| Weighted-average cost of capital (WACC) |
17 | % | ||
| Revenue growth rate (range) |
32.5% to 5 | % | ||
| Compounded average revenue growth rate |
17.7 | % | ||
| EBITDA margin (range) |
(23.8)% to 32.7 | % |
Stock-Based Compensation
We apply the fair value recognition provisions of Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, Topic 718, Compensation-Stock Compensation, or ASC 718. Determining the amount of stock-based compensation to be recorded requires us to develop estimates of the fair value of stock options and other equity awards as of their grant date. Stock-based compensation expense is recognized ratably over the requisite service period, which in most cases is the vesting period of the award. Calculating the fair value of stock-based awards requires that we make highly subjective assumptions. Use of this valuation methodology requires that we make assumptions as to the volatility of our common stock, the expected term of our stock options, the risk free rate of return for a period that approximates the expected term of our stock options and our expected dividend yield. Because we are a privately-held company with no trading history, we utilize the historical stock price volatility from a representative group of public companies to estimate expected stock price volatility. We selected companies from the medical device industry, specifically those who are focused on the design, development and commercialization of products for the treatment of spine disorders, and who have similar characteristics to us, such as stage of life cycle and size. We intend to continue to utilize the historical volatility of the same or similar public companies to estimate expected volatility until a sufficient amount of historical information regarding the price of our publically traded stock becomes available. We use the simplified method as prescribed by the Securities and Exchange Commission Staff Accounting Bulletin No. 107, Share-based Payment, to calculate the expected term of stock option grants to employees as we do not have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected
45
Table of Contents
term of stock options granted to employees. We utilize a dividend yield of zero because we have never paid cash dividends and have no current intention to pay cash dividends. The risk-free rate of return used for each grant is based on the U.S. Treasury yield curve in effect at the time of grant for instruments with a similar expected life. No options were granted to employees during the year ended December 31, 2013. The following assumptions were used in the calculation to estimate the fair value of options granted to employees using the Black-Scholes-Merton valuation model during the year ended December 31, 2012:
| Weighted-average risk-free interest rate |
1.14 | % | ||
| Weighted-average expected life (in years) |
5.34 | |||
| Expected dividend yield |
0 | % | ||
| Weighted-average expected volatility |
72 | % |
No options were granted to non-employees during the year ended December 31, 2012. The following assumptions were used in the Black-Scholes-Merton valuation model related to non-employee stock options granted during the year ended December 31, 2013:
| Weighted-average risk-free interest rate |
2.74 | % | ||
| Weighted-average expected life (in years) |
10.0 | |||
| Expected dividend yield |
0 | % | ||
| Weighted-average expected volatility |
52 | % |
The estimated fair value of stock-based awards for employee and non-employee director services are expensed over the requisite service period. Option awards issued to non-employees, excluding non-employee directors, are recorded at their fair value as determined in accordance with authoritative guidance, are periodically revalued as the options vest and are recognized as expense over the related service period. As a result, the charge to operations for non-employee awards with vesting conditions is affected each reporting period by changes in the fair value of our common stock.
We are required to estimate the level of forfeitures expected to occur and record stock-based compensation expense only for those awards that we ultimately expect will vest. We estimate our forfeiture rate based on the type of award, employee class and historical experience. Through December 31, 2013, actual forfeitures have not been material.
In February 2013, our employees elected to exchange 93,968 options to purchase our common stock for restricted stock units, or RSUs, pursuant to a one-time tender offer authorized by our board of directors. The RSUs were issued under the 2012 Plan and have three-year terms and vest upon the earlier of a change in control or upon the expiration of the lock-up period for our IPO on August 12, 2014. The incremental fair value on the date of the exchange between the original stock options and the RSUs issued of approximately $0.8 million will be recognized over a period of six months upon completion of the IPO.
At December 31, 2013, we had 168,832 unvested RSUs, the majority of which will vest upon the earlier of a change in control or on August 12, 2014, the expiration of the lock-up period for our IPO. Subsequent to December 31, 2013, our board approved grants on January 27, 2014, totaling 1,692,980 RSUs issued on April 1, 2014 upon effectiveness of the filing of a registration statement on Form S-8 and which will vest upon the expiration of the lock-up period for our IPO. We will take compensation charges upon vesting of RSUs based upon their grant date fair value. The estimated aggregate value to be recognized as compensation expense in 2014 for the RSUs granted on January 27, 2014 and outstanding RSUs at December 31, 2013 is expected to be approximately $11.4 million, including the $0.8 million expense as a result of the exchange described above.
Common Stock Warrant Liability and Preferred Stock Warrant Liability
As of December 31, 2013, we had warrants outstanding to purchase shares of our Series C, Series D, Series E and Series F convertible preferred stock and common stock. Freestanding warrants that are related to the purchase of redeemable preferred stock are classified as liabilities and recorded at fair value regardless of the timing of the redemption feature or the redemption price or the likelihood of redemption. The warrants are subject to re-measurement at each balance sheet date and any change in fair value is recognized as a component of other income (expense), net in our statement of comprehensive loss. We measure the fair value of our warrants to purchase our convertible preferred stock using a Black-Scholes-Merton option pricing model. The warrants to purchase shares of our common stock contain a provision requiring a reduction to the exercise price in the event we issue common stock, or securities convertible into or exercisable for common stock, at a price per share lower than the warrant exercise price. The anti-dilution feature requires the warrants to be classified as liabilities and re-measured at fair value at each balance sheet date. The fair value of the warrants to purchase common stock on the date of issuance and on each re-measurement date is classified as a liability and is estimated using the Black-Scholes-Merton valuation model. Any modifications to the warrant liabilities are recorded in earnings during the period of the modification. The significant assumptions used in estimating the fair value of our warrant liabilities include the exercise price, volatility of the stock underlying the warrant, risk-free interest rate, estimated fair value of the stock underlying the warrant and the estimated life of the warrant.
46
Table of Contents
Upon consummation of our IPO in February 2014, all classes of our convertible preferred stock were converted into common stock. Upon conversion of the underlying classes of convertible preferred stock, pursuant to the terms of the preferred stock warrants, the remaining warrants to purchase our Series C, Series D, Series E and Series F convertible preferred stock were classified as a component of equity and no longer be subject to re-measurement. However, the common stock warrant liability will continue to be required to be re-measured at each balance sheet date, until such time that the common stock warrants are exercised or expire.
Jumpstart Our Business Startups Act of 2012
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012, or JOBS Act, was enacted. Section 107 of the JOBS Act, provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are electing to delay such adoption of new or revised accounting standards, and as a result, we may not comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. As a result of this election, our financial statements may not be comparable to the financial statements of other public companies. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we intend to rely on certain of these exemptions, including without limitation, (1) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (2) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the consolidated financial statements, known as the auditor discussion and analysis. We may be able to remain an “emerging growth company” until the earliest of (a) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more, (b) the last day of our fiscal year following the fifth anniversary of the date of our IPO, (c) the date on which we have issued more than $1 billion in non-convertible debt during the previous three years or (d) the date on which we are deemed to be a large accelerated filer under the rules of the SEC. Additionally, we are also currently a “smaller reporting company” as defined in the Securities Exchange Act of 1934, and in the event that we are still considered a smaller reporting company at such time as we cease being an emerging growth company, we will be exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting.
Quantitative and Qualitative Disclosures About Market Risk
We are exposed to market risk in the ordinary course of our business. We do not hold or issue financial instruments for trading purposes. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market exposure is primarily a result of fluctuations in interest rates, however, we do not believe there is material exposure to interest rate risk. We also do not believe we are exposed to material risk resulting from fluctuations in foreign currency exchange rates due to the level of our international sales.
Controls and Procedures
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. GAAP. We are currently in the process of reviewing, documenting and testing our internal control over financial reporting. We have not performed an evaluation of our internal control over financial reporting, such as required by Section 404 of the Sarbanes-Oxley Act, nor have we engaged an independent registered public accounting firm to perform an audit of our internal control over financial reporting as of any balance sheet date or for any period reported in our financial statements. Presently, we are not an accelerated filer, as such term is defined by Rule 12b-2 of the Securities Exchange Act of 1934, as amended, and therefore, our management is not presently required to perform an annual assessment of the effectiveness of our internal control over financial reporting. This requirement will first apply to our Annual Report on Form 10-K for the year ending December 31, 2014. Our independent public registered accounting firm will first be required to attest to the effectiveness of our internal control over financial reporting for our Annual Report on Form 10-K for the first year we are no longer an “emerging growth company” under the JOBS Act. However, in connection with our audits for the years ended December 31, 2013 and 2012, and the review of our interim financial statements, our former independent registered public accounting firm noted four material weaknesses and one significant deficiency in our internal control over financial reporting. See “Risk Factors—Our internal control over financial reporting does not currently meet the standards required by Section 404 in the Sarbanes-Oxley Act, and failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could result in material misstatements of our annual or interim financial statements and have a material adverse effect on our business and share price,” for a discussion of these matters.
47
Table of Contents
Overview
We are a commercial biomaterial company focused on using our silicon nitride technology platform to develop, manufacture and sell a broad range of medical devices. We currently market spinal fusion products and are developing products for use in total hip and knee joint replacements. We believe our silicon nitride, an advanced ceramic, technology platform enables us to offer new and transformative products in the orthopedic and other medical device markets. We believe we are the first and only company to use silicon nitride in medical applications and over 14,000 of our silicon nitride spine products have been implanted in patients.
Biomaterials are synthetic or natural materials available in a variety of forms that are used in virtually every medical specialty. We believe our silicon nitride biomaterial has superior characteristics compared to commonly used biomaterials in the markets we are targeting, including polyetheretherketone, or PEEK, which is the most common biomaterial used for interbody spinal fusion products. Specifically, we believe our silicon nitride has the following key attributes: promotion of bone growth; hardness, strength and resistance to fracture; resistance to wear; non-corrosive properties; anti-infective properties; and superior diagnostic imaging compatibility.
We produce our silicon nitride advanced ceramic in four forms: (1) a fully dense, load-bearing solid, referred to as MC2; (2) a porous bone-like cancellous structured form, referred to as CSC; (3) a composite incorporating both our solid MC2 material and our porous CSC material intended to promote an ideal environment for bone growth; and (4) a coating for application onto other biomaterials. This capability provides us with the ability to utilize our silicon nitride in distinct ways depending on its intended application, which, together with our silicon nitride’s key characteristics, distinguishes us from manufacturers of other biomaterials and our products from products using other biomaterials.
According to iData Research, Inc., or iData, in 2012, the markets for spinal implants in the United States and in combined major European markets were $5.2 billion and $1.0 billion, respectively. Interbody spinal fusions accounted for over $1.2 billion and $172.2 million of these markets, respectively. Additionally, Orthopedic Network News reported that the U.S. markets for the components of total hip and knee replacement product candidates that we are initially developing were $455.0 million and $1.5 billion, respectively.
We currently market our Valeo MC2 silicon nitride interbody spinal fusion devices in the United States and Europe for use in the cervical and thoracolumbar areas of the spine. We believe our Valeo devices have a number of advantages over existing products due to silicon nitride’s key characteristics, resulting in faster and more effective fusion and reduced risk of infection. Our first generation Valeo silicon nitride device received 510(k) regulatory clearance and a CE Mark in 2008. Based on surgeon feedback, we developed a second generation of Valeo AL, PL and TL products with design enhancements that improve surgeon control during implantation and stability post procedure. In the first half of 2013, we initiated a targeted launch of our second generation AL, PL and TL Valeo interbody fusion devices and expect to complete the full launch in the second quarter of 2014. We are also completing the development of our second generation cervical Valeo Interbody fusion device and expect this to be launched as a beta in the second half of 2014. We also market our Valeo composite interbody spinal fusion device made from both our solid MC2 and porous CSC silicon nitride in the Netherlands, Spain and Germany. This device may reduce or eliminate the need for allograft bone, which is taken from human cadavers, and other biomaterials to act as a scaffold to support bone growth as part of the surgical procedure. We are currently conducting a prospective clinical trial in Europe, named CASCADE, comparing our Valeo composite silicon nitride interbody devices to PEEK interbody devices to obtain additional data to support 510(k) clearance of this product in the United States. The trial is 100% enrolled. We expect results to be available in the second half of 2014. If this trial is successful, we plan to file a 510(k) submission with the U.S. Food and Drug Administration, or FDA, by mid-2015. In addition, in the first half of 2013, we initiated a Design and Build Program focused on collaborating with influential surgeons to develop customized silicon nitride spinal fusion products and instruments and the first products designed under this program were sold in the third quarter of 2013. As of December 31, 2013, the rate of adverse events reported to the FDA for our implanted Valeo interbody spinal fusion devices is 0.1%.
In addition to our silicon nitride-based spinal fusion products, we market a complementary line of non-silicon nitride spinal fusion products which allows us to provide surgeons and hospitals with a broader range of products. These products include three lines of spinal fusion devices and five types of orthobiologics, which are used by surgeons to help promote bone growth and fusion in spinal fusion procedures. Although our non-silicon nitride products have accounted for approximately 57% of our product revenues for the three months ended March 31, 2014 and 66% and 74%or more of our product revenues for the years ended December 31, 2013 and 2012, respectively, we believe the continued promotion and potential for adoption of our silicon nitride products and product candidates, if approved, provides us the greatest opportunity to grow our business in new and existing markets and achieve our goal to become a leading biomaterial company.
We are also incorporating our silicon nitride technology into components for use in total hip and knee replacement product candidates that we are, or plan on, developing in collaboration with a strategic partner. If approved by the FDA, we believe that our silicon nitride total hip and knee product candidates will provide competitive advantages over current products made with traditional biomaterials.
48
Table of Contents
We also believe our silicon nitride technology platform can be used for developing products in other markets and have developed prototypes for use in the dental, sports medicine and trauma markets. In addition, as a result of some of the key characteristics of our silicon nitride, including the promotion of bone growth, resistance to wear, non-corrosiveness and anti-infective properties, we believe our silicon nitride coating may be used to enhance our metal products as well as commercially available metal spinal fusion, joint replacement and other medical products.
We have recently put in place a senior management team with over 150 years of collective experience in the healthcare industry. Members of our management team have experience in product development, launching new products into the orthopedics market and selling to hospitals through direct sales organizations, distributors, manufacturers and other companies in the orthopedic space. We operate a 30,000 square foot manufacturing facility located at our corporate headquarters in Salt Lake City, Utah, and we are the only vertically integrated silicon nitride orthopedic medical device manufacturer in the world. We market and sell our products to surgeons and hospitals in the United States and select markets in Europe and South America through our established network of more than 50 independent sales distributors who are managed by our in-house sales and marketing management team.
Biomaterials
Biomaterials are synthetic or natural biocompatible materials that are used in virtually every medical specialty to improve or preserve body functionality. Various types of biomaterials are used as essential components in medical devices, drug delivery systems, replacement and tissue repair technologies, prostheses and diagnostic technologies.
There are four general categories of biomaterials:
| • | Metals. Metals commonly used as biomaterials include titanium, stainless steel, cobalt, chrome, gold, silver and platinum, and alloys of these metals. Examples of medical uses of metals include the repair or stabilization of fractured bones, stents, surgical instruments, bone and joint replacements, spinal fusion devices, dental implants and restorations and heart valves. According to MarketsandMarkets, a global market research firm, metals represented approximately 31% of the worldwide sales of all biomaterials in 2012. |
| • | Polymers. Polymers are synthetic compounds consisting of similar molecules linked together that can be created to have specific properties. Polymers commonly used as biomaterials include nylon, silicon rubber, polyester, polyethylene, cross-linked polyethylene (a stronger version), polymethylmethacrylate, polyvinyl chloride and polyetheretherketone, which is commonly referred to as PEEK. Examples of medical uses of polymers include soft-tissue replacement, sutures, drug delivery systems, joint replacements, spinal fusion devices and dental restorations. Polymers represented approximately 29% of the worldwide sales of all biomaterials in 2012. |
| • | Ceramics. Ceramics are hard, non-metallic, non-corrosive, heat-resistant materials made by shaping and then applying high temperatures. Traditional ceramics commonly used as biomaterials include carbon, oxides of aluminum, zirconium and titanium, calcium phosphate and zirconia-toughened alumina. Examples of medical uses of ceramics include repair, augmentation or stabilization of fractured bones, bone and joint replacements, spinal fusion devices, dental implants and restorations, heart valves and surgical instruments. Ceramics represented approximately 26% of the worldwide sales of all biomaterials in 2012. |
| • | Natural biomaterials. Natural biomaterials are derived from human donors, animal or plant sources and include human bone, collagen, gelatin, cellulose, chitin, alginate and hyaluronic acid. Examples of medical uses of natural biomaterials include the addition or substitution of hard and soft tissue, cornea protectors, vascular grafts, repair and replacement of tendons and ligaments, bone and joint replacements, spinal fusion devices, dental restorations and heart valves. Natural biomaterials represented approximately 14% of the worldwide sales of all biomaterials in 2012. |
According to MarketsandMarkets, orthopedics accounted for approximately $15.0 billion, or 34%, of the $44.0 billion total biomaterials market in 2012. Within orthopedics, biomaterials are extensively used in spinal fusion procedures, hip and knee replacements and the repair or stabilization of fractured bones.
Market Opportunity
Overview
We believe our silicon nitride technology platform provides us with numerous competitive advantages in the orthopedic biomaterials market. We market interbody spinal fusion devices and related products and are developing products for use as components in total hip and knee joint replacements. We believe we can also utilize our silicon nitride technology platform to develop future products in additional markets, such as the dental, sports medicine and trauma markets.
49
Table of Contents
According to iData, in 2012, the markets for spinal implants in the United States and in combined major European markets were $5.3 billion and $1.0 billion, respectively. Interbody spinal fusion products accounted for over $1.2 billion and $172.2 million of these markets, respectively. In 2012, there were approximately 300,000 interbody spinal fusion procedures conducted in the United States, of which the significant majority utilized interbody devices comprised of PEEK and bone, with occasional use of metals and other materials including ceramics. The market for interbody spinal fusion devices has shifted over time as new biomaterials with superior characteristics have been incorporated into these devices and have launched into the market. For example, in the 1990s, metals quickly penetrated the interbody spinal fusion market because of the limitations of devices available at that time made from allograft bone and, more recently, products made of PEEK rapidly penetrated the market because of the limitations of devices available at that time made from metal or allograft bone. Similarly, we believe our silicon nitride interbody spinal fusion products address the key limitations of other biomaterials currently used in interbody spinal fusion devices and demonstrate superior characteristics needed to improve clinical outcomes.
Additionally, Orthopedic Network News reported that the U.S. markets for total hip and knee replacements in 2012 were $2.7 billion and $4.0 billion, respectively. According to Orthopedic Network News, in 2012, there were more than 470,000 total hip replacement procedures and 734,000 total knee replacement procedures conducted in the United States. Orthopedic Network News also reported that in 2012, the U.S. markets for the components of total hip and knee replacement product candidates that we are initially developing were $455.0 million and $1.5 billion, respectively. The combinations of biomaterials most commonly used in joint replacement implants are metal-on-cross-linked polyethylene and traditional ceramic-on-cross-linked polyethylene.
We believe that the main drivers for the growth of the orthopedic biomaterials market, and, in particular, the spinal fusion and joint replacement markets, are the following:
| • | Favorable and Changing Demographics. With the growing number of elderly people, age-related ailments are expected to rise sharply, which we believe will increase the demand and need for biomaterials and devices with improved performance capabilities. Also, middle-aged and older patients increasingly expect to enjoy active lifestyles, and consequently demand effective treatments for painful spine and joint conditions, including better performing and longer-lasting interbody spinal fusion devices and joint replacements. |
| • | Introduction of New Technologies. Better performing and longer-lasting biomaterials, improved diagnostics, and advances in surgical procedures allow for surgical intervention earlier in the continuum of care and better outcomes for patients. We believe surgical options using better performing and longer-lasting biomaterials will gain acceptance among surgeons and younger patients and drive accelerated growth and increase the size of the spinal fusion and joint replacement markets. |
| • | Market Expansion into New Geographic Areas. MarketsandMarkets anticipates that demand for biomaterials and the associated medical devices will increase as the applications in which biomaterials are used are introduced to and become more widely accepted in underserved countries, such as China. |
The Interbody Spinal Fusion Market
The human spinal canal is made up of 33 interlocking bones, referred to as vertebrae, separated by 23 intervertebral discs comprised of a hard outer ring made of collagen with a soft inner core, that act as shock absorbers between vertebrae. Disorders of the spine can result from degenerative conditions, deformities and trauma or tumor-related damage. Spinal fusion is the standard of care used to treat most spinal disorders and typically involves the placement of an interbody device between vertebrae to reestablish spacing between vertebrae and alignment of the spine. Generally, the interbody device is stabilized by screws and, in some procedures, plates or rods. To enhance bone attachment, surgeons often pack the interbody device with a biomaterial that induces bone growth. Following successful treatment, new bone tissue grows in and around the interbody device over time, which helps fuse the vertebrae and create long-term stability of the interbody device, leading to the alleviation of pain and increase in mobility.
We selected this market as the first application for our silicon nitride technology because of its size, the limitations of currently available products and the key characteristics silicon nitride possesses which are critical for a superior interbody spinal fusion device.
| • | Promotion of Bone Growth. The biomaterial should be both osteoconductive and create an osteoinductive environment to promote bone growth in and around the interbody device to further support fusion and stability. Osteoconduction occurs when material serves as a scaffold to support the growth of new bone in and around the material. Osteoinduction involves the stimulation of osteoprogenitor cells to develop, or differentiate, into osteoblasts, which are cells that are needed for bone growth. |
| • | Strength and Resistance to Fracture. The biomaterial should be strong and resistant to fracture during implantation of the device and to successfully restore intervertebral disc space and spinal alignment during the fusion process. The biomaterial should have high flexural strength, which is the ability to resist breakage during bending, and high compressive strength, which is the ability to resist compression under pressure, to withstand the static and dynamic forces exerted on the spine during daily activities over the long term. |
50
Table of Contents
| • | Anti-Infective. Spinal fusion devices can become colonized with bacteria, which may limit fusion to adjacent vertebrae or cause serious infection. Treating device-related infection is costly and generally requires repeat surgery, including surgery to replace the device, referred to as revision surgery, which may extend hospital stays, suffering and disability for patients. A biomaterial that has anti-infective properties can reduce the incidence of bacteria colonization in and around the interbody device that can lead to infection, revision surgery and associated increased costs. Publicly available articles report infection rates following implantation of traditional spinal fusion devices ranging from 3% to 18%. |
| • | Imaging Compatibility. The biomaterial should be visible through, and not inhibit the effective use of, common surgical and diagnostic imaging techniques, such as x-ray, CT and MRI. These imaging techniques are used by surgeons during and after spinal fusion procedures to assist in the proper placement of interbody devices and to assess the quality of post-operative bone fusion. |
Limitations of Biomaterials used in Interbody Spinal Fusion Devices
The three biomaterials most commonly used in interbody spinal fusion devices are PEEK, human cadaver bone, also referred to as allograft bone, and metals. We believe these materials do not possess the key characteristics required to form the optimal interbody spinal fusion device and are susceptible to potential fracture, implant-related infection, pain, limited fusion and instability, which have resulted in revision surgeries.
PEEK (polyetheretherketone)
PEEK is the most frequently used biomaterial for interbody spinal fusion devices and accounted for almost half of the devices implanted in the United States in 2012. We believe that the rate of revision surgery for PEEK interbody spinal fusion devices is approximately 6%. We believe this is caused by the following limitations of PEEK:
| • | Restricts Bone Growth. Due to PEEK’s hydrophobic nature, the human body may recognize PEEK as a foreign substance and, therefore, may encapsulate the device with fibrous tissue. Although it is still possible for bone to grow through the device, bone may not adhere to the surface of the device if this tissue develops. |
| • | Lacks Strength and Resistance to Fracture. PEEK lacks sufficient flexural strength, compressive strength and resistance to fracture necessary to reduce the risk of deformity or fracture during the fusion process. In addition, PEEK devices may fracture during implantation in certain interbody spinal fusion procedures. For example, in December 2012, Zimmer Spine recalled its PEEK Ardis® Interbody System Inserter, a surgical instrument used to implant a PEEK interbody spinal fusion device, because it resulted in the PEEK implants being susceptible to breakage when too much lateral force was applied to the inserter during implantation. |
| • | Lacks Anti-Infective Properties. PEEK does not have any inherent anti-infective properties. In fact, a biofilm may form around a PEEK device that allows the colonization of bacteria, which can lead to infection. |
| • | Lacks Imaging Compatibility. PEEK is invisible on x-rays. As a result, manufacturers of PEEK devices add metal markers to their devices so surgeons can see the location of the devices by x-ray. These markers, however, do not show the full outline of the device, which makes it difficult to assess the accuracy of the placement of the device. In addition, the metal markers cause artifacts on CT and MRI that can compromise the quality of the image. |
Allograft Bone
Allograft bone is the second most frequently used biomaterial in interbody spinal fusion devices and accounted for over 40% of the devices implanted in the United States in 2012. Allograft bone has the following limitations:
| • | Limited Promotion of Bone Growth. Allograft bone has limited osteoinductive characteristics and therefore may not effectively promote bone growth in and around the interbody device. |
| • | Lacks Strength and Resistance to Fracture. Generally, allograft bone is not as strong as live bone within the body or other materials used in interbody devices. In addition, techniques used to sterilize allograft bone, like gamma irradiation, can cause the allograft to become brittle and more likely to fracture. |
| • | Lacks Anti-Infective Properties and Risk of Disease Transmission. In addition to not having inherent anti-infective properties, allograft bone exposes patients to a greater risk of disease transmission and auto-immune response. |
51
Table of Contents
In addition, allograft bone is subject to inconsistent quality and size, which may require surgeons to make compromises on the fit of the device during surgery.
Metals
We believe metal interbody devices accounted for less than 10% of the devices implanted in the United States in 2012. Metals have the following limitations:
| • | Limited Promotion of Bone Growth. Metals have limited osteoinductive characteristics and therefore do not effectively promote bone growth in and around the interbody device. |
| • | Lack Anti-Infective Properties. Metals do not have inherent anti-infective properties and do not suppress the colonization of bacteria in and around the device which can lead to infection. |
| • | Lack Imaging Compatibility. Metals are opaque in x-rays and can cause significant imaging artifacts in CTs and MRIs. This can make it difficult for surgeons to detect the extent and quality of bone growth in and around the device in post-operative diagnostic imaging procedures. |
The Hip and Knee Joint Replacement Market
Total joint replacement involves removing the diseased or damaged joint and replacing it with an artificial implant consisting of components made from several different types of biomaterials. The key components of a total hip implant include an artificial femoral head, consisting of a ball mounted on an artificial stem attached to the femur, and a liner, which is placed inside a cup affixed into the pelvic bone. The femoral head and liner move against each other to replicate natural motion in what is known as an articulating implant. Total knee replacement implants also use articulating components and are comprised of the following four main components: a femoral condyle, which is a specially shaped bearing that is affixed to the lower end of the femur; a tibial tray that is affixed to the upper end of the tibia; a tibial insert that is rigidly fixed to the tibial tray and serves as the surface against which the femoral condyle articulates; and a patella, or knee cap, which also articulates against the femoral condyle.
Implants for total hip and knee replacements are primarily differentiated by the biomaterials used in the components that articulate against one another. The combinations of biomaterials most commonly used in hip and knee replacement implants in the United States are metal-on-cross-linked polyethylene and traditional ceramic-on-cross-linked polyethylene. The use of hip replacement implants incorporating metal-on-metal and traditional ceramic-on-traditional ceramic biomaterials experienced a steep decline in the United States over the last several years due to their significant limitations. We believe that the most common currently used biomaterials in joint replacement implants also have limitations, and do not possess all of the following key characteristics required for optimal total joint replacement implants:
| • | Resistance to Wear. The biomaterials should have sufficient hardness and toughness, as well as extremely smooth surfaces, to effectively resist wear. Because the articulating implants move against each other, they are subject to friction, which frequently lead to abrasive wear and the release of small wear particles. This may cause an inflammatory response which results in osteolysis, or bone loss. Surgeons have identified osteolysis as a leading cause of joint implant failure, resulting in the need for revision surgery to replace the failed implant. One of the most commonly used combinations of biomaterials, metal-on-cross-linked polyethylene, as well as metal-on-metal implants tend to generate a large number of metal wear particles, which can cause osteolysis and a moderate to severe allergic reaction to the metal, referred to as metal sensitivity. While less common, metal implants may also cause a serious condition called metallosis. Both metal sensitivity and metallosis can result in revision surgery. |
| • | Non-Corrosive. The biomaterials should be non-corrosive and should not cause adverse patient reactions. Metal placed in the human body corrodes over time and also results in the formation of metal ions, which leads to metal sensitivity in approximately 10% to 15% of the population and, less commonly, metallosis. As a result, there are significant increased risks from using metal-on-cross-linked polyethylene and metal-on-metal implants. |
| • | Hardness, Strength and Resistance to Fracture. The biomaterials should be hard, strong and resistant to fracture to adequately bear the significant loads placed on joints like the hip and knee during daily activities. We believe there are strength limitations associated with traditional ceramic-on-cross-linked polyethylene and traditional ceramic-on-traditional ceramic implants. |
| • | Anti-Infective. The biomaterials should have anti-infective properties to reduce the risk of bacteria colonization in and around the components that can lead to infection, revision surgeries and associated increased costs. Anti-infective properties reduce the risk of bacteria colonization in and around the components and reduce the likelihood of infection, revision surgeries and associated increased costs. None of the most commonly used biomaterials in joint replacement implants have anti-infective properties. |
52
Table of Contents
Our Silicon Nitride Technology Platform
We believe we are the first and only company to use silicon nitride in medical applications. Silicon nitride is a chemical compound comprised of the elements silicon and nitrogen, with the chemical formula Si3N4. Silicon nitride, an advanced ceramic, is lightweight, resistant to fracture and strong, and is used in many demanding mechanical, thermal and wear applications, such as in space shuttle bearings, jet engine components and body armor.
We believe our silicon nitride is ideally suited for use in many medical applications and has the following characteristics that make it superior to other biomaterials, including PEEK, bone, metal and traditional ceramics, which do not possess all of these characteristics:
| • | Promotes Bone Growth. Our silicon nitride is osteoconductive through its inherent surface topography that provides support for new bone growth. We also believe our silicon nitride promotes an ideal environment for osteoinduction. As a hydrophilic material, silicon nitride attracts protein cells and nutrients that stimulate osteoprogenitor cells to differentiate into osteoblasts, which are needed for bone growth. Our silicon nitride also has an inherent surface chemistry that is more similar to bone than PEEK and metals are. As a result, we believe our silicon nitride has superior osteoconductive and osteoinductive properties when compared to other biomaterials, including those commonly used in interbody spinal fusion devices, such as PEEK, allograft bone and metal. These properties are highlighted in an in vivo study, where we measured the force required to separate devices from the spine after being implanted for three months, which indicates the level of osteointegration. In the absence of bacteria, the force required to separate our silicon nitride from its surrounding bone was approximately three times that of PEEK, and nearly two times that of titanium. In the presence of bacteria, the force required to separate our silicon nitride from its surrounding bone was over five times that of titanium, while there was effectively no separation force required for PEEK, indicating essentially no osteointegration. |
| • | Hard, Strong and Resistant to Fracture. Our silicon nitride is hard, strong and possesses superior resistance to fracture over traditional ceramics and greater strength than polymers currently on the market. For example, our silicon nitride’s flexural strength is more than five times that of PEEK and our silicon nitride’s compressive strength is over twenty times that of PEEK. Unlike PEEK interbody spinal fusion devices, we believe our silicon nitride interbody spinal fusion devices can withstand the forces exerted during implantation and daily activities over the long term. |
| • | Anti-Infective. We have demonstrated in in vitro and in vivo studies that silicon nitride has inherent anti-infective properties, which reduce the risk of infection in and around a silicon nitride device. PEEK, traditional ceramics, metals and bone do not have inherent anti-infective characteristics. These properties were highlighted in an in vitro study, where live bacteria counts were between 8 and 30 times lower on our silicon nitride than PEEK and up to 8 times lower on our silicon nitride than titanium. In addition to improving patient outcomes, we believe the anti-infective properties of our silicon nitride should make it an attractive biomaterial to hospitals and surgeons who are not reimbursed by third-party payors for the treatment of hospital-acquired infections. Additionally, silicon nitride is synthetic and, therefore, there is a lower risk of disease transmission through cross-contamination or of an adverse auto-immune response, sometimes associated with the use of allograft bone. |
| • | Imaging Compatible. Our silicon nitride interbody spinal fusion devices are semi-radiolucent and clearly visible in x-rays, and produce no distortion under MRI and no scattering under CT. These characteristics enable an exact view of the device for precise intra-operative placement and post-operative bone fusion assessment in spinal fusion procedures. We believe these qualities provide surgeons with greater certainty of outcomes with our silicon nitride devices than with other biomaterials, such as PEEK and metals. |
| • | Resistant to Wear. We believe our silicon nitride joint implant product candidates will have comparable or higher resistance to wear than metal-on-cross-linked polyethylene and traditional ceramic-on-cross-linked polyethylene joint implants, the two most commonly used total hip replacement implants. Also, debris associated with metal implants increases the risk of metal sensitivity and metallosis. Wear debris is a primary reason for early failures of metal and polymer articulating joint components. |
| • | Non-Corrosive. Our silicon nitride does not have the drawbacks associated with the corrosive nature of metal within the body, including metal sensitivity and metallosis, nor does it result in the release of metal ions into the body. As a result, we believe our silicon nitride products will have lower revision rates and fewer complications than comparable metal products. |
Our Forms of Silicon Nitride
The chemical composition of our in-house formulation of silicon nitride, processing and manufacturing experience allow us to produce silicon nitride in four distinct forms. This capability provides us with the ability to utilize our silicon nitride in a variety ways depending on its intended application, which, together with our silicon nitride’s key characteristics, distinguishes us from manufacturers of products using other biomaterials.
53
Table of Contents
We currently produce silicon nitride for use in our commercial products and product candidates in the following forms:
| • | Solid Silicon Nitride, or MC2. This form of silicon nitride is a fully dense, load-bearing solid, and is used for devices that require high strength, toughness, fracture resistance and low wear, including for interbody spinal fusion devices, hip and knee replacement implants and dental implants. |

| • | Porous Silicon Nitride, or CSC. While this form of silicon nitride has a chemical composition that is identical to that of MC2, the CSC form of silicon nitride has a porous structure, which is engineered to mimic cancellous bone, the spongy like bone tissue that typically makes up the interior of human bones. Our porous silicon nitride has interconnected pores ranging in size between about 90 and 600 microns, which is similar to that of cancellous bone. This form of silicon nitride can be used for the promotion of bone in-growth and attachment. Our porous silicon nitride is used as a substitute for the orthobiologics currently used to fill interbody devices in an effort to stimulate fusion and as a bone void filler, and as a porous scaffold for medical devices. |

| • | Composite Silicon Nitride. This form of silicon nitride is a combination, or composite, of MC2 and CS C forms of silicon nitride. This composite may be used to manufacture devices and implants that mimic the structure of natural bone by incorporating both a fully dense, load-bearing solid MC2 component on the outside and a porous CSC component intended to promote bone in-growth on the inside. This composite form of silicon nitride is used in interbody spinal fusion devices and can be used in components for total hip and knee replacement implants. |
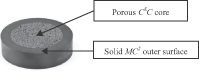
| • | Silicon Nitride Coating. With a similar chemical composition as our other forms of silicon nitride, this form of silicon nitride can be applied as an adherent coating to metallic substrates, including cobalt-chromium, titanium and steel alloys. We believe applying silicon nitride as a coating may provide a highly wear-resistant articulation surface, such as on femoral heads, which may reduce problems associated with metal or polymer wear debris. We also believe that the silicon nitride coating can be applied to devices that require firm fixation and functional connections between the device or implant and the surrounding tissue, such as hip stems and screws. The use of silicon nitride coating may also create an anti-infective barrier between the device and the adjacent bone or tissue. |

Our Competitive Strengths
We believe we can use our silicon nitride technology platform to become a leading biomaterial company and have the following principal strengths:
| • | Sole Provider of Silicon Nitride Medical Devices. We believe we are the only company that designs, develops, manufactures and sells medical grade silicon nitride-based products. Due to its key characteristics, we believe our silicon nitride enables us to offer new and transformative products across multiple medical specialties. In addition, with the FDA clearance of our silicon nitride Valeo products, we are one of only three companies that have developed and manufacture a ceramic for use in FDA cleared orthopedic medical devices in the United States. |
| • | In-House Manufacturing Capabilities. We operate a 30,000 square foot manufacturing facility located at our corporate headquarters in Salt Lake City, Utah. This operation complies with the FDA’s quality system regulation, or QSR, and is certified under the International Organization for Standardization’s, or ISO, standard 13485 for medical devices. This state-of- |
54
Table of Contents
| the-art facility allows us to rapidly design and produce silicon nitride products and control the entire manufacturing process from raw material to finished goods. We have also entered into a cooperative research and development agreement with Kyocera Industrial Ceramics Corporation, or Kyocera, under which we will work with Kyocera to determine its ability to become a second qualified manufacturer of our silicon nitride-based spinal fusion products and product candidates. |
| • | Established Commercial Infrastructure. We market and sell our products to surgeons and hospitals in the United States, and select markets in Europe and South America through our established network of more than 50 independent sales distributors who are managed by our experienced in-house sales and marketing management team. As a result, our product revenue is driven by end-user prices, unlike other biomaterial companies that sell their products at lower prices to OEMs who then sell their products to the end user. Our control over the sales and marketing processes also allows us greater flexibility to selectively collaborate with distributors when we believe their experience or geographic reach can be beneficial to us. |
| • | Portfolio of Non-Silicon Nitride Products. In addition to designing, developing, manufacturing and commercializing silicon nitride interbody spinal fusion devices, we sell a complementary line of non-silicon nitride spinal fusion products. We offer a full suite of spinal fusion products, which increases our access to surgeons and hospitals, and allows us to more effectively market our silicon nitride spinal fusion products to our customers. Product revenue from the sale of these non-silicon nitride products also supports further development of our silicon nitride products and product candidates. |
| • | Highly Experienced Management and Surgeon Advisory Team. We have recently assembled a senior management team with over 150 years of collective experience in the healthcare industry. Members of our management team have experience in product development, launching new products into the orthopedics market and selling to hospitals through direct sales organizations, distributors, manufacturers and other orthopedic companies. We also collaborate with a network of leading surgeon advisors in the design and use of our products and product candidates. |
Our Strategy
Our goal is to become a leading biomaterial company focused on using our silicon nitride technology platform to develop, manufacture and commercialize a broad range of medical devices. Key elements of our strategy to achieve this goal are the following:
| • | Drive Further Adoption of our Silicon Nitride Interbody Spinal Fusion Devices. We believe that increasing the awareness of our silicon nitride technology by educating surgeons about its key benefits, and design improvements to our silicon nitride products and related instruments, will accelerate the adoption of our products and ultimately help improve patient outcomes. We continue to innovate with further design enhancements in the introduction of our second-generation interbody spinal fusion devices. We are currently selling this new line of AL, PL and OL Valeo to select surgeons and expect to complete the full launch of the line in the United States in the third quarter of 2014. We are also completing the development of our second generation cervical and TL Valeo interbody spinal fusion devices and expect to launch these in the fourth quarter of 2014. To drive further awareness of our products and the associated benefits offered by our silicon nitride technologies, we will continue to educate surgeons through multiple channels including industry conferences and meetings, media outlets and through our sales and marketing efforts. We also plan to facilitate the publication of data from bench testing and clinical outcome case studies. |
| • | Continue to Implement our Design and Build Program. In the first half of 2013, we initiated a commercialization strategy, referred to as our Design and Build Program, in which we collaborate with influential surgeons to develop customized silicon nitride spinal fusion products and instruments. We first sell these products for use by the designing surgeons and a team of evaluating surgeons for their review based on their individual preferences focused on ease of use of the product and instrumentation and patient outcomes as compared to the previous products and instruments used by the surgeon. After the enhanced products are sold and evaluated and, if accepted by these surgeons, we plan to introduce these products more broadly into the market. The first products designed under this program were sold for initial evaluation in 2013. |
| • | Enhance our Commercial Infrastructure. We expect to increase the productivity of our sales and marketing infrastructure to help us further penetrate the interbody spinal fusion market by continuing to engage experienced independent sales distributors with strong orthopedic surgeon relationships. For example, in October 2013, we entered into a new European sales agent agreement with K2M, Inc., one of the largest privately held spinal device companies in the world. We also periodically conduct programs to ensure that our distributors are knowledgeable about how the characteristics of our silicon nitride devices meet the demands of a range of spinal fusion procedures, and we regularly update our distributors about studies, test results, reviews and other developments that demonstrate the competitive advantages of our silicon nitride devices. We may also establish distribution collaborations in the United States and abroad when access to large or well-established sales and marketing organizations may help us gain access to new markets, increase sales in our existing markets, or accelerate market penetration for selected products. |
| • | Develop Silicon Nitride for Total Joint Components. We are incorporating our silicon nitride technology into silicon nitride-coated components for use in total hip and knee replacement product candidates that we plan on developing in collaboration with a strategic partner. We also have designs for solid silicon nitride components and we will make a decision in the future |
55
Table of Contents
| about whether to pursue the development of these components. In December 2013, we participated in a pre-submission meeting with the FDA to finalize the regulatory strategy for a 510(k) clearance of our silicon nitride-coated total joint components in the United States. The FDA reviewers confirmed that the regulatory pathway would be a standard 510(k) clearance with supporting biomechanical testing. In response, we intend to develop silicon nitride-coated metal joint replacement components and then, together with a strategic partner, initiate biomechanical testing with our silicon nitride-coated metal components for use in total hip and knee replacement procedures to support a 510(k) submission to the FDA. We intend to pursue clearance of a total hip replacement product first, and if clearance is obtained, we intend to commercially launch products for use in total hip replacement by the second half of 2015. |
| • | Apply our Silicon Nitride Technology Platform to Other Opportunities. Our silicon nitride technology platform is adaptable and we believe it may be used to develop products to address other significant opportunities, such as in the dental, sports medicine and trauma markets. We have manufactured prototypes of dental implants, sports medicine and trauma products, and we have developed a process to coat metals with our silicon nitride to enhance current medical devices and instruments. We plan to collaborate with other companies to develop and commercialize any future products in those areas or we may develop any one of them by ourselves if sufficient resources should become available. |
Our Products and Product Candidates
We currently market a family of silicon nitride interbody spinal fusion devices and other non-silicon nitride spinal fusion products for use in cervical and lumbar spinal fusion surgical procedures to treat patients who suffer from degenerative, diseased and traumatic spine conditions. We are also developing multiple silicon nitride components for use in our total hip and knee replacement product candidates.
Spinal Fusion Products and Product Candidates
Our Valeo Silicon Nitride Products and Product Candidates
Our first generation Valeo silicon nitride spinal fusion device received 510(k) regulatory clearance and a CE mark in 2008. Based on surgeon feedback, we developed a second generation of Valeo products. In 2012, we received 510(k) clearance to market this second generation family of Valeo interbody spinal fusion devices, and we launched them with a select number of surgeons in 2013. Our second generation Valeo interbody spinal fusion devices offer distinct improvements over the first generation. The instrumentation of the second generation devices allow for better control of the device during implantation. The device allows for improved stability and potentially improved fusion after implantation and is offered in a broader selection of sizes. We expect to complete the full launch of our second generation AL, PL and OL Valeo interbody spinal fusion devices in the United States in the third quarter of 2014. We are also completing the development of our second generation cervical and TL Valeo interbody spinal fusion devices and expect to launch these in the fourth quarter of 2014.
Our current products are:
| Valeo Interbody Fusion Devices |
Generation | |
| AL: Anterior Lumbar |
1st and 2nd | |
| PL: Posterior Lumbar |
1st and 2nd | |
| OL: Oblique Lumbar |
1st and 2nd | |
| C: Cervical |
1st | |
| TL: Transforaminal Lumbar |
1st and 2nd | |
| CORP: Corpectomy |
1st |
We are also in the process of finishing the development of a Valeo stand-alone anterior lumbar intervertebral fusion device made from our MC2 silicon nitride. The Valeo stand-alone product candidate, which incorporates fixation screws, will allow surgeons to perform less invasive procedures. We believe this may result in better patient outcomes compared to other spinal fusion procedures. We anticipate seeking 510(k) clearance for this product candidate in the second quarter of 2014, and, if cleared by the FDA, we anticipate launching our Valeo stand-alone product candidate in the United States in the second half of 2014.
56
Table of Contents
In 2009, we received a CE Mark to commercialize our Valeo interbody spinal fusion devices made from our composite silicon nitride. The porous CSC center structure of these devices is designed to facilitate bone growth into the device, which we believe will allow surgeons to reduce or eliminate the use of allograft bone and other osteoconductive biomaterials. We are currently marketing these devices in the Netherlands, Spain and Germany. Additionally, we are conducting a prospective clinical trial in Europe, named CASCADE, comparing our Valeo composite silicon nitride interbody devices to PEEK interbody devices to obtain additional safety and efficacy data to support the 510(k) clearance in the United States. The trial is 100% enrolled. We expect results to be available in the second half of 2014. If this trial is successful, we plan to file a 510(k) submission with the FDA by mid-2015.

Valeo Composite (MC2 + C2C)
Our Non-Silicon Nitride Spinal Fusion Products
We sell a line of complementary non-silicon nitride spinal fusion products to provide surgeons and hospitals with a broader range of products. Product revenue from the sale of our non-silicon nitride spinal fusion products further supports development of our silicon nitride products and product candidates. We plan to enhance our metal spinal fusion products with a silicon nitride coating. The following table lists our marketed non-silicon nitride spinal products.
| CATEGORY |
PRODUCT NAME |
BIOMATERIAL | ||
| Facet Fixation System |
Facet Gun Max/Facet Bolt | Metal | ||
| Javelin: MIS Locking Facet System | ||||
| Lumbar Spine Fixation |
Preference Classic Spine System | Metal | ||
| Preference 2 Spine System | ||||
| Preference 2 Complex Spine System | ||||
| Orthobiologics |
Preference Element Bone Graft Substitute | Allograft | ||
| Valeo BP: Synthetic Bone Putty | ||||
| PROCET: Facet Fusion Allograft Implant | ||||
| Interbody Spinal Fusion Device |
Phantom Plus PLIF/TLIF IBFD | PEEK | ||
| Phantom Plus Cervical Spacer | ||||
57
Table of Contents
Our Total Hip and Knee Joint Replacement Product Candidates
Our Total Hip Implant Product Candidates
We have developed two designs of femoral heads for use in our total hip replacement product candidates. Our first design is a silicon nitride-coated metal femoral head, for total joint replacement, which we plan to develop with a medical device partner. The second design is a femoral head that is made from our solid MC2 silicon nitride and we are collaborating with Orthopaedic Synergy, Inc. to develop a total hip replacement product candidate using this design. These femoral heads are expected to articulate against a cross-linked polyethylene liner, fixed into a metal acetabular cup. We intend to initially advance our process to develop silicon nitride-coated femoral heads and then, together with a strategic partner, initiate biomechanical testing with our silicon nitride-coated femoral head for use in total hip replacement procedures to support a 510(k) submission to the FDA. If clearance is obtained, we intend to commercially launch products for use in total hip replacement by the second half of 2015. Although we have designs for solid silicon nitride components, we have not yet determined if we will pursue the development of these components.
Our Total Knee Implant Product Candidates
We have developed two designs of femoral condyle components for use in our total knee replacement product candidates. The first design utilizes our silicon nitride coating and we plan to partner with a medical device company to incorporate this design into a total knee replacement product candidate. The second design is made from our solid MC2 silicon nitride and we are collaborating with Orthopaedic Synergy Inc. to develop a total knee joint replacement for this design. The femoral condyle component will attach to the lower end of the femur. The femoral condyle is expected to articulate against a cross-linked polyethylene tibial insert that will attach to the tibial tray at the upper end of the tibia, which we expect will be made from metal. We have successfully made prototypes of both designs. We intend to develop silicon nitride-coated femoral condyle components and then, together with a strategic partner, initiate biomechanical testing with our components for use in knee replacement procedures to support a 510(k) submission to the FDA. If clearance is obtained we intend to commercialize our products for use in total knee replacement surgeries post-FDA clearance. Although we have designs for solid silicon nitride components, we have not yet determined if we will pursue the development of these components.
Other Product Opportunities
Our silicon nitride technology platform is adaptable and we believe it may be used to develop products to address other significant opportunities, such as in the dental, sports medicine and trauma markets.
We also believe our coating technology may be used to enhance our marketed metal products as well as other commercially available metal spinal fusion and joint replacement products. We have produced feasibility prototypes of dental implants, other components for use in total hip implants in addition to our total hip and knee implant product candidates discussed above, a suture anchor for sports medicine and prototypes of silicon nitride-coated plates for potential trauma applications. We have also developed a process to apply our silicon nitride as a coating on other biomaterials.
The FDA has not evaluated any of these potential products and we are not currently advancing the development of any of these product candidates. We plan to collaborate with medical device companies to complete the development of and commercialize any product candidates we advance in these areas or develop any one of them ourself if sufficient resources should become available.
Supporting Data
We and a number of independent third parties have conducted extensive biocompatibility, biomechanical, in vivo and in vitro testing on our silicon nitride to establish its safety and efficacy in support of regulatory clearance of our biomaterial, products and product candidates. We have also completed additional testing of our silicon nitride products and product candidates. The results of this testing have been published in peer review publications. Additionally, we have initiated prospective randomized clinical trials in humans in vivo and in vitro to support and expand our understanding of our silicon nitride’s performance relative to other biomaterials and medical devices. We believe our product development strategy is consistent with the manner in which other biomaterials have been successfully introduced into the market and adopted as the standard of care. Listed below is an overview of some of the key testing completed on our silicon nitride biomaterial, products and product candidates to date, as well as other information about our silicon nitride and other biomaterials.
58
Table of Contents
Biocompatibility
Before our silicon nitride was first used in commercial products in 2008, we conducted a series of biocompatibility tests following the guidelines of the FDA and ISO and submitted the results to the FDA as part of the regulatory clearance process. These tests confirmed that our silicon nitride products meet required biocompatibility standards for human use.
Promotion of Bone Growth
In 2012, we conducted two separate studies at Brown University, the results of which suggest that the chemistry and inherent surface topography of our solid MC2 silicon nitride provides an optimal environment for bone growth onto and around the device.
The first study was a series of in vitro analyses of protein adsorption, or presence on the surface of the biomaterial, onto silicon nitride, PEEK and titanium. The results of this study indicated that adsorption of two key proteins necessary for bone growth (fibronectin and vitronectin) were up to eight times greater on our silicon nitride than on PEEK, and up to four times greater than on titanium. A third important protein (laminin) had up to two times greater adsorption on our silicon nitride than on PEEK, and up to two-and-one-half times greater adsorption than on titanium.
The second study was an in vivo investigation of the osteointegration characteristics of these same three biomaterials after they had been surgically implanted into the skulls of laboratory rats. This study included an examination of the effect of Staphylococcus epidermidis bacteria on osteointegration. At time intervals of up to three months after implantation of the biomaterial, the amount of new bone growth within the surgical site and in direct contact with the implanted biomaterial was evaluated. In the absence of bacteria, new bone formation within the surgical site surrounding our silicon nitride was approximately 69%, compared with 36% and 24% for titanium and PEEK, respectively. Similarly, bone in direct contact, or apposition, with our silicon nitride, titanium and PEEK was 59%, 19% and 8%, respectively. As is common, in the presence of bacteria, new bone formation within the surgical site was suppressed, but still significantly greater for our silicon nitride than for the other two biomaterials. Observed new bone growth within the surgical site surrounding our silicon nitride was 41%, compared with 26% and 21% for titanium and PEEK, respectively. At the implant interface, the bone apposition for our silicon nitride, titanium and PEEK was 23%, 9% and 5%, respectively. To further characterize the extent of osteointegration, the force needed to separate each implant from its surrounding bone was measured. A larger force needed to separate the implant is an indication of improved osteointegration. At three months after implantation, in the absence of bacteria, the force required to separate our silicon nitride from its surrounding bone was approximately three times that of PEEK, and nearly two times that of titanium. In the presence of bacteria, there was effectively no separation force required for PEEK, indicating essentially no osteointegration. Our silicon nitride required over five times the force to separate it from its surrounding bone in the presence of bacteria in comparison to titanium.
In 2008, we conducted an animal study in which we evaluated the level of osteointegration of our porous CSC silicon nitride with a knee-defect model in adult sheep. At three months after implantation, three out of five of the silicon nitride implants had extensive new bone formation at and into the implant surface, showing that the bone had grown into our CS C silicon nitride to a depth of 3 millimeters, or mm. This animal study demonstrated the rapid osteointegration potential of our CSC silicon nitride.
Hardness, Strength and Resistance to Fracture
Comparative Information
As shown in the table of comparative information publicly available about various biomaterials below:
| • | the hardness, or a material’s resistance to deformity, of silicon nitride is comparable to traditional ceramics, but is substantially higher than either polymers or metals; |
| • | the strength of silicon nitride is comparable or higher than metals and traditional ceramics, and is about sixteen to fifty-five times stronger than highly-cross-linked polyethylene, and four to eight times stronger than PEEK; and |
| • | silicon nitride has the highest fracture resistance of any medical ceramic material and is three to eleven times more resistant to fracture than PEEK or highly-cross-linked polyethylene. This is due to the interwoven microstructure of silicon nitride. Metals have the highest fracture resistance. |
59
Table of Contents
Comparison of Mechanical Properties Among Orthopedic Biomaterials
| Material |
Hardness (GPa)(1) |
Strength (MPa)(1) |
Fracture Resistance (MPa.m1/2)(1) | |||
| Silicon Nitride |
13 - 16 | 800 - 1200 | 8 - 11 | |||
| Aluminum Oxide Ceramic |
14 - 19 | 300 - 500 | 3 - 5 | |||
| Zirconia-Toughened Alumina Ceramic |
12 - 19 | 700 - 1150 | 5 - 10 | |||
| PEEK |
0.09 - 0.28 | 160 - 180 | 2 - 3 | |||
| Highly-Cross-Linked Polyethylene Polymer |
0.03 -0.07 | 22 - 48 | 1 - 2 | |||
| Cobalt-Chromium Metal |
3 - 4 | 700 - 1000 | 50 - 100 | |||
| Titanium Alloy Metal |
3 - 4 | 920 - 980 | 75 |
| (1) | GPa is a giga-pascal. MPa is a mega-pascal. Pascals are a measure of pressure. MPa.m1/2 is mega-pascal times a square root meter and is a measure related to the energy required to initiate fracture of a material. |
We believe that the combination of high hardness, strength and fracture resistance positions our silicon nitride as an ideal biomaterial for many medical applications.
Burst Strength
In 2006, we conducted in-house comparative “burst strength” tests on femoral heads made from our silicon nitride produced by a contract manufacturer to our specifications and femoral heads made from one of the strongest commercially available ceramics, BIOLOX® delta (zirconia-toughened alumina). These tests were performed on three designs of 28 mm femoral heads using accepted testing protocols. The tests involved applying a load to each femoral head while mounted on a cobalt-chromium simulated hip implant stem, until the head burst. This enabled us to directly compare the strength of the femoral heads made of the two biomaterials. The results also provided an indication of each biomaterial’s resistance to fracture. The results of these tests are shown in the chart below.
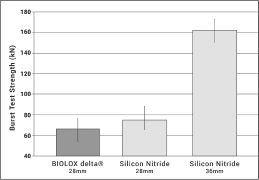
The average burst test strength for the silicon nitride femoral heads in these tests was 75 kilonewtons, or kNs, compared with 65 kN for BIOLOX® delta, or about a 15% improvement. The burst strengths observed in our tests for BIOLOX® delta femoral heads are comparable to those observed by an independent party testing the same design BIOLOX® delta femoral heads as we did. We also conducted burst strength tests of 36 mm femoral heads made from our silicon nitride which showed those femoral heads had burst strengths that averaged 164 kN.
Resistance to Wear
In 2011, we commissioned an independent laboratory to conduct a wear study using our silicon nitride femoral heads. We tested our 28 mm silicon nitride femoral heads articulated against cross-linked polyethylene acetabular liners and our 40 mm silicon nitride femoral heads articulated against cross-linked polyethylene acetabular liners using well-established protocols in a hip simulator for their wear performance over 5 million cycles. We then compared the results for our silicon nitride product candidates to the results for the cobalt chrome femoral head and publicly available data from other commonly paired products. The results and comparison showed that:
| • | our silicon nitride-on-cross-linked polyethylene had approximately half the wear rate of that publicly reported for cobalt chrome-on-cross-linked polyethylene articulating hip components; and |
| • | our silicon nitride-on-cross-linked polyethylene had comparable wear to that publicly reported for traditional ceramic-on-cross-linked polyethylene articulating hip components. |
60
Table of Contents
Anti-Infective Properties
The results of the two studies at Brown University in 2012, demonstrate that our solid MC2 silicon nitride has anti-infective properties. The objective of the in vitro study was to determine how our silicon nitride, PEEK and titanium interact with bacteria, protein and bone cells without the use of antibiotics and compared the growth of five different types of bacteria on silicon nitride, PEEK and titanium over time. Live bacteria counts were between 8 to 30 times lower on silicon nitride than PEEK and up to 8 times lower on silicon nitride than titanium.
In the in vivo study, bacteria were applied to the biomaterials before implantation. Three months after implantation, no infection was observed with silicon nitride, whereas both PEEK and titanium showed infection. The data demonstrate that our silicon nitride inhibits biofilm formation and bacterial colonization around the biomaterial.
Imaging Compatibility
In 2007, we conducted a study to compare the imaging characteristics of test blanks made of PEEK, the metals titanium and tantalum, and silicon nitride using a cadaver human vertebral body. Images of the vertebral body and the blanks were obtained using x-ray, CT and MRI under identical conditions. We assessed the radiolucent characteristics of the blanks in x-ray images quantitatively, assessed the presence of scatter in CT qualitatively and assessed distortion in MRI quantitatively. In x-ray, the metal blanks did not permit visualization of the underlying bone of the vertebral body, while PEEK was transparent, rendering its location difficult to determine. The silicon nitride blank had an intermediate radiolucency that rendered it visible and allowed a visual assessment of the underlying bone of the vertebral body. CT and MRI of the metal blanks indicated the presence of distortion while silicon nitride and PEEK exhibited no scattering.
Sales and Marketing
We market and sell our products to surgeons and hospitals through our established network of more than 50 independent sales distributors who are managed by our experienced 14 person in-house sales and marketing management team. Our sales efforts to date have been in the United States and selected markets in Europe and South America. To supplement our independent sales distributors, in select international markets, such as Europe, Japan, Australia and Canada, we may also seek to establish collaborations with leading orthopedic companies where we believe that a large, well-established partner may provide better access to those markets. For example, in October 2013, we entered into a European sales agent agreement with K2M, Inc., one of the largest privately held spinal device companies in the world. In addition, we may establish collaborations in the United States under circumstances where access to a larger sales and marketing organization may help to expand the market or accelerate penetration for selected products.
In the first quarter of 2013, we restructured the leadership of our sales and marketing team and hired a Senior Vice President of Global Sales, a Vice President of Marketing and a Senior Vice President, Strategic Marketing. This new leadership team has reviewed our entire sales and marketing practices and have implemented steps to improve the performance of these departments.
In addition to leveraging the strong existing surgeon relationships of our distribution network, we market our products through a combination of initiatives that are designed to establish and increase awareness of our silicon nitride products and their benefits over alternative products. We attend and make presentations at major industry events, including society meetings sponsored by the North American Spine Society, the America Academy of Orthopaedic Surgeons and the Congress of Neurological Surgeons, to educate surgeons and distributors about our products and product candidates. We advertise in trade journals and publications, and offer unique pricing strategies, including product bundling and incentivizing our distribution network to create and maintain long-term relationships with surgeons and hospitals. We also use surgeon advisors to assist in product development and to help implement awareness campaigns aimed at educating surgeons about our products. As part of these campaigns, we provide educational materials for hospitals and surgeons. We also conduct regional training seminars where our product managers, trainers, engineers, sales and marketing staff members work together with our surgeon advisors to educate surgeons and our distribution network in the use of our products.
Manufacturing
Silicon Nitride Manufacturing
To control the quality, cost and availability of our silicon nitride products and product candidates, we operate our own manufacturing facility. Our 54,000 square foot corporate building includes a 30,000 square foot ISO 13485 certified medical device manufacturing space. It is equipped with state-of-the-art, powder processing, spray drying, pressing and computerized machining equipment, sintering furnaces, and other testing equipment that enables us to control the entire manufacturing process for our silicon nitride products and product candidates. To our knowledge, we are the only vertically integrated silicon nitride orthopedic medical device
61
Table of Contents
manufacturer in the world. All operations with the exceptions of raw material production, cleaning, packaging and sterilization are performed in-house. We purchase raw materials, consisting of silicon nitride ceramic powder and dopant chemical compounds, from several vendors which are ISO registered and approved by us. These raw materials are characterized and tested in our facility in accordance with our specifications and then blended to formulate our silicon nitride. We believe that there are multiple vendors that can supply us these raw materials and we continually monitor the quality and pricing offered by our vendors to ensure high quality and cost-effective supply of these materials. A flowchart of the silicon nitride manufacturing process is shown below.
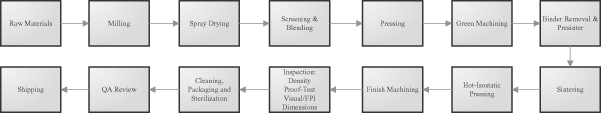
In order to mitigate the risk associated with us being the sole manufacturer of our silicon nitride medical device products, in June 2014, we entered into a manufacturing development and supply agreement with Kyocera Industrial Ceramics Corporation, or Kyocera. We have initiated the FDA required actions and processes in order to qualify Kyocera as a second source supplier of our silicon nitride products. We expect to update our material master file and submit a special 510(k) with the FDA in July 2014 to qualify Kyocera as a second source supplier of our silicon nitride products. We expect to begin receiving production parts from Kyocera in July 2014 for distribution in the fourth quarter of this year assuming that the FDA accepts and approves our special 510(k) submission. Although we expect this arrangement with Kyocera will lead to Kyocera becoming a secondary qualified manufacturer, if Kyocera fails to become a qualified manufacturer of our silicon nitride-based spinal fusion products and product candidates, we will continue to be the sole manufacturer of our silicon nitride products and will need to seek other potential secondary manufacturers.
Non-Silicon Nitride and Instruments Manufacturing
We obtain our non-silicon nitride spinal fusion products and instruments from third-party manufacturers. We also plan to rely on third-party manufacturers for the supply of the metal components of our silicon nitride hip and knee joint replacement product candidates. We only use manufacturers that operate under QSR and are ISO 13485 certified. Our in-house quality control group examines subcontracted components to ensure that they meet our required specifications. We believe that the use of third-party sources for non-silicon nitride spinal fusion products and instruments will reduce our capital investment requirements and allow us to strategically focus our resources on the manufacture of our silicon nitride products and product candidates.
Intellectual Property
We rely on a combination of patents, trademarks, trade secrets and other forms of intellectual property, nondisclosure agreements, proprietary information ownership agreements and other measures to protect our intellectual property rights. We believe that in order to have a competitive advantage, we must continue to develop and maintain the proprietary aspects of our technologies.
As of June 30, 2014, we had 34 issued U.S. patents, 38 pending U.S. patent applications, 11 granted foreign patents and 18 pending foreign patent applications. Our issued patents begin to expire in 2014, with the last of these patents expiring in 2031. The first core patents do not expire until 2022; these include US 6,881,229 and US 6,790,233.
We have seven U.S patents, one European patent, and related pending applications, directed to articulating implants using our high-strength, high toughness doped silicon nitride MC2 ceramic. The issued patents, which include US 6,881,229; US 7,666,229; US 8,123,812; US 7,780,738; US 7,695,521; US 7,776,085; US 8,133,284; and EP 1408874, begin to expire in 2022. We also have two U.S. patents, two European patents, and related pending applications, related to our CSC technology that are directed to implants that have both a dense load-bearing, or cortical, component and a porous, or cancellous, component, together with a surface coating. The issued patents, which include US 6,790,233; US 6,846,327; EP 1389978; and EP 2055267, begin to expire in 2022.
We also have three U.S. patents that we acquired in July 2012 from Dytech Corporation Ltd., or Dytech, directed to manufacturing processes for the production of porous ceramics for use in our orthopedic implants. These patents, which include US 5,563,106; US 5,705,448; and US 6,617,270, expire between 2014 and 2019. Under our acquisition agreement with Dytech, Dytech granted to us a perpetual, irrevocable and exclusive license, including the right to grant sublicenses, to certain improvements and know-how related to the acquired patents. In return, we are required to pay Dytech a low single-digit royalty on net sales of products sold by us, our affiliates, or our licensees that are covered by one or more valid claims of these patents, and a percentage of any non-royalty licensing income we may receive in the event we grant a license to others.
62
Table of Contents
Our remaining issued patents and pending applications are directed to additional aspects of our products and technologies including, among other things:
| • | designs for cervical plates; |
| • | designs for pedicle screws; |
| • | designs for cervical disc implants; |
| • | designs for intervertebral fusion devices; |
| • | designs for facet fixation devices; |
| • | designs for hip implants; and |
| • | designs for knee implants. |
We also expect to rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our intellectual property position. However, trade secrets are difficult to protect. We seek to protect the trade secrets in our proprietary technology and processes, in part, by entering into confidentiality agreements with commercial partners, collaborators, employees, consultants, scientific advisors and other contractors and into invention assignment agreements with our employees and some of our commercial partners and consultants. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of the technologies that are developed.
Competition
The main alternatives to our silicon nitride biomaterial include: PEEK, which is predominantly manufactured by Invibio, BIOLOX® delta, which is a traditional ceramic manufactured by CeramTec, allograft bone, metals and coated metals.
We believe our main competitors in the orthopedic implant market, which utilize a variety of competitive biomaterials, include: Medtronic, Inc.; DePuy Synthes Companies, a group of Johnson & Johnson companies; Stryker Corporation; Biomet, Inc.; Zimmer Holdings, Inc.; Smith & Nephew plc; and Aesculap Inc. Presently, these companies buy ceramic components on an OEM basis from manufacturers such as CeramTec, Kyocera and CoorTek, Inc., among others. We anticipate that these and other orthopedic companies and OEMs will seek to introduce new biomaterials and products that compete with ours.
Competition within the industry is primarily based on technology, innovation, product quality, and product awareness and acceptance by surgeons. Our principal competitors have substantially greater financial, technical and marketing resources, as well as significantly greater manufacturing capabilities than we do, and they may succeed in developing products that render our implants and product candidates non-competitive. Our ability to compete successfully will depend upon our ability to develop innovative products with advanced performance features based on our silicon nitride technologies.
Government Regulation of Medical Devices
Governmental authorities in the United States, at the federal, state and local levels, and other countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, promotion, advertising, distribution, marketing and export and import of products such as those we are commercializing and developing. Failure to obtain approval or clearance to market our products and products under development and to meet the ongoing requirements of these regulatory authorities could prevent us from continuing to market or develop our products and product candidates.
United States
Pre-Marketing Regulation
In the United States, medical devices are regulated by the FDA. Unless an exemption applies, a new medical device will require either prior 510(k) clearance or approval of a premarket approval application, or PMA, before it can be marketed in the United States. The information that must be submitted to the FDA in order to obtain clearance or approval to market a new medical device varies depending on how the medical device is classified by the FDA. Medical devices are classified into one of three classes on the basis of the controls deemed by the FDA to be necessary to reasonably ensure their safety and effectiveness. Class I devices, which are those that have the lowest level or risk associated with them, are subject to general controls, including labeling, premarket notification and adherence to the QSR. Class II devices are subject to general controls and special controls, including performance standards. Class III devices, which have the highest level of risk associated with them, are subject to most of the previously identified requirements as well as to premarket approval. Most Class I devices and some Class II devices are exempt from the 510(k) requirement, although manufacturers of these devices are still subject to registration, listing, labeling and QSR requirements.
63
Table of Contents
A 510(k) premarket notification must demonstrate that the device in question is substantially equivalent to another legally marketed device, or predicate device, that did not require premarket approval. In evaluating the 510(k), the FDA will determine whether the device has the same intended use as the predicate device, and (a) has the same technological characteristics as the predicate device, or (b) has different technological characteristics, and (i) the data supporting the substantial equivalence contains information, including appropriate clinical or scientific data, if deemed necessary by the FDA, that demonstrates that the device is as safe and as effective as a legally marketed device, and (ii) does not raise different questions of safety and effectiveness than the predicate device. Most 510(k)s do not require clinical data for clearance, but the FDA may request such data. The FDA’s goal is to review and act on each 510(k) within 90 days of submission, but it may take longer based on requests for additional information. In addition, requests for additional data, including clinical data, will increase the time necessary to review the notice. If the FDA does not agree that the new device is substantially equivalent to the predicate device, the new device will be classified in Class III, and the manufacturer must submit a PMA. Since July 2012, however, with the enactment of the Food and Drug Administration Safety and Innovation Act, or FDASIA, a de novo pathway is directly available for certain low to moderate risk devices that do not qualify for the 510(k) pathway due to lack of a predicate device. Modifications to a 510(k)-cleared medical device may require the submission of another 510(k) or a PMA if the changes could significantly affect the safety or effectiveness or constitute a major change in the intended use of the device.
Modifications to a 510(k)-cleared device frequently require the submission of a traditional 510(k), but modifications meeting certain conditions may be candidates for FDA review under a Special 510(k). If a device modification requires the submission of a 510(k), but the modification does not affect the intended use of the device or alter the fundamental scientific technology of the device, then summary information that results from the design control process associated with the cleared device can serve as the basis for clearing the application. A Special 510(k) allows a manufacturer to declare conformance to design controls without providing new data. When the modification involves a change in material, the nature of the “new” material will determine whether a traditional or Special 510(k) is necessary. For example, in its Device Advice on How to Prepare a Special 510(k), the FDA uses the example of a change in a material in a finger joint prosthesis from a known metal alloy to a ceramic that has not been used in a legally marketed predicate device as a type of change that should not be submitted as a Special 510(k). However, if the “new” material is a type that has been used in other legally marketed devices within the same classification for the same intended use, a Special 510(k) is appropriate. The FDA gives as an example a manufacturer of a hip implant who changes from one alloy to another that has been used in another legally marketed predicate. Special 510(k)s are typically processed within 30 days of receipt.
The PMA process is more complex, costly and time consuming than the 510(k) clearance procedure. A PMA must be supported by extensive data including, but not limited to, technical, preclinical, clinical, manufacturing, control and labeling information to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. After a PMA is submitted, the FDA has 45 days to determine whether it is sufficiently complete to permit a substantive review. If the PMA is complete, the FDA will file the PMA. The FDA is subject to performance goal review times for PMAs and may issue a decision letter as a first action on a PMA within 180 days of filing, but if it has questions, it will likely issue a first major deficiency letter within 150 days of filing. It may also refer the PMA to an FDA advisory panel for additional review, and will conduct a preapproval inspection of the manufacturing facility to ensure compliance with the QSR, either of which could extend the 180-day response target. While the FDA’s ability to meet its performance goals has generally improved during the past few years, it may not meet these goals in the future. A PMA can take several years to complete and there is no assurance that any submitted PMA will ever be approved. Even when approved, the FDA may limit the indication for which the medical device may be marketed or to whom it may be sold. In addition, the FDA may request additional information or request the performance of additional clinical trials before it will reconsider the approval of the PMA or as a condition of approval, in which case the trials must be completed after the PMA is approved. Changes to the device, including changes to its manufacturing process, may require the approval of a supplemental PMA.
If a medical device is determined to present a “significant risk,” the manufacturer may not begin a clinical trial until it submits an investigational device exemption, or IDE, to the FDA and obtains approval of the IDE from the FDA. The IDE must be supported by appropriate data, such as animal and laboratory testing results and include a proposed clinical protocol. These clinical trials are also subject to the review, approval and oversight of an institutional review board, or IRB, which is an independent and multi-disciplinary committee of volunteers who review and approve research proposals, and the reporting of adverse events and experiences, at each institution at which the clinical trial will be performed. The clinical trials must be conducted in accordance with applicable regulations, including but not limited to the FDA’s IDE regulations and current good clinical practices. A clinical trial may be suspended by the FDA, the IRB or the sponsor at any time for various reasons, including a belief that the risks to the study participants outweigh the benefits of participation in the trial. Even if a clinical trial is completed, the results may not demonstrate the safety and efficacy of a device, or may be equivocal or otherwise not be sufficient to obtain approval.
64
Table of Contents
Post-Marketing Regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
| • | compliance with the QSR, which require manufacturers to follow stringent design, testing, control, documentation, record maintenance, including maintenance of complaint and related investigation files, and other quality assurance controls during the manufacturing process; |
| • | labeling regulations, which prohibit the promotion of products for uncleared or unapproved or “off-label” uses and impose other restrictions on labeling; and |
| • | medical device reporting obligations, which require that manufacturers investigate and report to the FDA adverse events, including deaths, or serious injuries that may have been or were caused by a medical device and malfunctions in the device that would likely cause or contribute to a death or serious injury if it were to recur. |
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
| • | warning letters; |
| • | fines, injunctions, and civil penalties; |
| • | recall or seizure of our products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | refusal to grant 510(k) clearance or PMA approvals of new products; |
| • | withdrawal of 510(k) clearance or PMA approvals; and |
| • | criminal prosecution. |
To ensure compliance with regulatory requirements, medical device manufacturers are subject to market surveillance and periodic, pre-scheduled and unannounced inspections by the FDA, and these inspections may include the manufacturing facilities of our subcontractors.
International Regulation
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ. For example, the primary regulatory authority with respect to medical devices in Europe is that of the European Union. The European Union consists of 28 countries and has a total population of over 500 million people. The unification of these countries into a common market has resulted in the unification of laws, standards and procedures across these countries, which may expedite the introduction of medical devices like those we are offering and developing. Norway, Iceland, Lichtenstein and Switzerland are not members of the European Union, but have transposed applicable European medical device laws into their national legislation. Thus, a device that is marketed in the European Union may also be recognized and accepted in those four non-member European countries as well.
The European Union has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices. Devices that comply with the requirements of relevant directives will be entitled to bear CE Conformity Marking, indicating that the device conforms to the essential requirements of the applicable directives and, accordingly, can be commercially distributed throughout the European Union. Actual implementation of these directives, however, may vary on a country-by-country basis. The CE Mark is a mandatory conformity mark on medical devices distributed and sold in the European Union and certifies that a medical device has met applicable requirements.
The method of assessing conformity varies, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a “Notified Body.” Notified Bodies are independent testing houses, laboratories, or product certifiers authorized by the European Union member states to perform the required conformity assessment tasks, such as quality system audits and device compliance testing. An assessment by a Notified Body based within the European Union is required in order for a manufacturer to distribute the product commercially throughout the European Union. Medium and higher risk devices require the intervention of a Notified Body which will be responsible for auditing the manufacturer’s quality system. The Notified Body will also determine whether or not the product conforms to the requirements of the applicable directives. Devices that meet the applicable requirements of E.U. law and have undergone the appropriate conformity assessment routes will be granted CE “certification.”
65
Table of Contents
The CE Mark is mandatory for medical devices sold not only within the countries of the European Union but more generally within most of Europe. As many of the European standards are converging with international standards, the CE Mark is often used on medical devices manufactured and sold outside of Europe (notably in Asia that exports many manufactured products to Europe). CE Marking gives companies easier access into not only the European market but also to Asian and Latin American markets, most of whom recognize the CE Mark on medical device as a mark of quality and adhering to international standards of consumer safety, health or environmental requirements.
In September 2012, the European Commission adopted a proposal for a regulation which, if adopted, will change the way that most medical devices are regulated in the European Union, and may subject our products to additional requirements.
Compliance with Healthcare Laws
We must comply with various U.S. federal and state laws, rules and regulations pertaining to healthcare fraud and abuse, including anti-kickback and false claims laws, rules, and regulations, as well as other healthcare laws in connection with the commercialization of our products. Fraud and abuse laws are interpreted broadly and enforced aggressively by various state and federal agencies, including the U.S. Department of Justice, the U.S. Office of Inspector General for the Department of Health and Human Services and various state agencies.
We have entered into agreements with certain surgeons for assistance with the design of our products, some of whom we anticipate may make referrals to us or order our products. A majority of these agreements contain provisions for the payments of royalties and/or stock options. In addition, some surgeons currently own shares of our stock. We have structured these transactions with the intention of complying with all applicable laws, including fraud and abuse, data privacy and security, and transparency laws. Despite this intention, there can be no assurance that a particular government agency or court would determine our practices to be in full compliance with such laws. We could be materially impacted if regulatory or enforcement agencies or courts interpret our financial arrangements with surgeons to be in violation of healthcare laws, including, without limitation, fraud and abuse, data privacy and security, or transparency laws.
The U.S. federal Anti-Kickback Statute prohibits persons, including a medical device manufacturer (or a party acting on its behalf), from knowingly or willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for a service or product or the purchasing, ordering, arranging for, or recommending the ordering of, any service or product for which payment may be made by Medicare, Medicaid or any other federal healthcare program. This statute has been interpreted to apply to arrangements between medical device manufacturers on one hand and healthcare providers on the other. The term “remuneration” is not defined in the federal Anti-Kickback Statute and has been broadly interpreted to include anything of value, such as cash payments, gifts or gift certificates, discounts, waiver of payments, credit arrangements, ownership interests, the furnishing of services, supplies or equipment, and the provision of anything at less than its fair market value. Courts have broadly interpreted the scope of the law, holding that it may be violated if merely “one purpose” of an arrangement is to induce referrals, irrespective of the existence of other legitimate purposes. The Anti-Kickback Statute prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain business arrangements from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from federal Anti-Kickback Statute liability. The reach of the Anti-Kickback Statute was broadened by the recently enacted Patient Protection and Affordable Care Act of 2010 and the Health Care and Education Affordability Reconciliation Act of 2010, collectively, the Affordable Care Act or ACA, which, among other things, amends the intent requirement of the federal Anti-Kickback Statute such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the ACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act (discussed below) or the civil monetary penalties statute, which imposes fines against any person who is determined to have presented or caused to be presented claims to a federal healthcare program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. In addition to the federal Anti-Kickback Statute, many states have their own anti-kickback laws. Often, these laws closely follow the language of the federal law, although they do not always have the same scope, exceptions, safe harbors or sanctions. In some states, these anti-kickback laws apply not only to payments made by government healthcare programs but also to payments made by other third-party payors, including commercial insurance companies.
66
Table of Contents
Sales, marketing, consulting, and advisory arrangements between medical device manufacturers and sales agents and physicians are subject to the Anti-Kickback Statute and other fraud and abuse laws. Government officials have focused recent enforcement efforts on, among other things, the sales and marketing activities of healthcare companies, including medical device manufacturers, and have brought cases against individuals or entities whose personnel allegedly offered unlawful inducements to potential or existing customers in an attempt to procure their business. We expect these activities to continue to be a focus of government enforcement efforts. Settlements of these cases by healthcare companies have involved significant fines and penalties and in some instances criminal plea agreements. We are also aware of governmental investigations of some of the largest orthopedic device companies reportedly focusing on consulting and service agreements between these companies and orthopedic surgeons. These developments are ongoing and we cannot predict the effects they will have on our business.
The federal False Claims Act imposes liability on any person that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the False Claims Act allow a private individual to bring civil actions on behalf of the federal government alleging that the defendant has submitted a false claim, or has caused such a claim to be submitted, to the federal government, and to share in any monetary recovery. There are many potential bases for liability under the False Claims Act. Liability arises, primarily, when a person knowingly submits, or causes another to submit, a false claim for reimbursement to the federal government. The False Claims Act has been used to assert liability on the basis of inadequate care, kickbacks, and other improper referrals, and allegations as to misrepresentations with respect to the services rendered. Qui tam actions have increased significantly in recent years, causing greater numbers of healthcare companies, including medical device manufacturers, to defend false claim actions, pay damages and penalties, or be excluded from participation in Medicare, Medicaid or other federal or state healthcare programs as a result of investigations arising out of such actions. In addition, various states have enacted similar laws analogous to the False Claims Act. Many of these state laws apply where a claim is submitted to any third-party payor and not merely a federal healthcare program. We are unable to predict whether we would be subject to actions under the False Claims Act or a similar state law, or the impact of such actions. However, the cost of defending such claims, as well as any sanctions imposed, could adversely affect our financial performance. The Health Insurance Portability and Accountability Act of 1996, or HIPAA, also created several new federal crimes, including healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third party payors. The false statements statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services.
In addition, we may be subject to, or our marketing or research activities may be limited by, data privacy and security regulation by both the federal government and the states in which we conduct our business. For example, HIPAA and its implementing regulations established uniform federal standards for certain “covered entities” (healthcare providers, health plans and healthcare clearinghouses) governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of protected health information. The American Recovery and Reinvestment Act of 2009, commonly referred to as the economic stimulus package, included expansion of HIPAA’s privacy and security standards called the Health Information Technology for Economic and Clinical Health Act, or HITECH, which became effective on February 17, 2010. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to “business associates”—independent contractors or agents of covered entities that create, receive, maintain, or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. These laws also require the reporting of breaches of protected health information to affected individuals, regulators and in some cases, local or national media. HIPAA and HITECH impose strict limits on our physician collaborators’ ability to use and disclose patient information on our behalf.
There are also an increasing number of state “sunshine” laws that require manufacturers to provide reports to state governments on pricing and marketing information. Several states have enacted legislation requiring medical device companies to, among other things, establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales and marketing activities, and to prohibit or limit certain other sales and marketing practices. In addition, a federal law known as the Physician Payments Sunshine Act, now requires medical device manufacturers to track and report to the federal government certain payments and other transfers of value made to physicians and teaching hospitals and ownership or investment interests held by physicians and their immediate family members. The first reporting period will cover only payments or transfers of value made and ownership or investment interests held by physicians and their immediate family members from August 1, 2013 to December 31, 2013. The federal government will disclose the reported information on a publicly available website beginning in September 2014. For calendar year 2014, the Physician Payments Sunshine Act will require medical device manufacturers to report payments and transfers of values made and ownership or investment interests held by physicians and their immediate family members for the full calendar year. These laws may adversely affect our sales, marketing, and other activities by imposing administrative and compliance burdens on us. If we fail to track and report as required by these laws or to otherwise comply with these laws, we could be subject to the penalty provisions of the pertinent state and federal authorities.
67
Table of Contents
Clinical research is heavily regulated by FDA regulations for the protection of human subjects (21 C.F.R. 50 and 56) and also the regulations of the U.S Department of Health and Human Services, or the Common Rule (45 C.F.R 46). Both FDA human subject regulations and the Common Rule impose restrictions on the involvement of human subjects in clinical research and require, among other things, the balancing of the risks and benefits of research, the documented informed consent of research participants, initial and ongoing review of research by an IRB. Similar regulations govern research conducted in foreign countries. Compliance with human subject protection regulations is costly and time consuming. Failure to comply could substantially and adversely impact our research program and the development of our products.
Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the federal and state laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including criminal and significant civil monetary penalties, damages, fines, imprisonment, exclusion from participation in government healthcare programs, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre-marketing product clearances and approvals, private “qui tam” actions brought by individual whistleblowers in the name of the government or refusal to allow us to enter into supply contracts, including government contracts, and the curtailment or restructuring of our operations. Public disclosure of privacy and data security violations could cause significant reputational harm. Any of these events could adversely affect our ability to operate our business and our results of operations. To the extent that any of our products are sold in a foreign country, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws, implementation of corporate compliance programs, as well as laws and regulations requiring transparency of pricing and marketing information and governing the privacy and security of health information, such as the E.U.’s Directive 95/46 on the Protection of Individuals with regard to the Processing of Personal Data, or the Data Directive, and the wide variety of national laws implementing the Data Directive.
Healthcare Reform
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs.
In March 2010, President Obama signed into law the ACA, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers and impose additional health policy reforms. Among other things, the ACA imposes a 2.3% medical device excise tax on sales of many medical devices in the United States which became effective on January 1, 2013. Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with healthcare practitioners and a significant number of provisions are not yet, or have only recently become, effective. Although it is too early to determine the full effect of the ACA, the new law appears likely to place downward pressure on pricing of medical devices, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. For example, on August 2, 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee on Deficit Reduction did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, or ATRA, which, among other things, reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. On March 1, 2013, the President signed an executive order implementing the Budget Control Act’s 2% Medicare payment reductions, and on April 1, 2013, these reductions went into effect. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our financial operations.
We expect that the ACA, as well as other healthcare reform measures that have been and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for our products. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may affect our ability to generate revenue and profits or commercialize our product candidates.
68
Table of Contents
Third-Party Reimbursement
Because we typically receive payment directly from hospitals and surgical centers, we do not anticipate relying directly on payment for any of our products from third-party payors, such as Medicare, Medicaid, private insurers, and managed care companies. However, our business will be affected by policies administered by federal and state healthcare programs, such as Medicare and Medicaid, as well as private third-party payors, which often follow the policies of the state and federal healthcare programs. For example, our business will be indirectly impacted by the ability of a hospital or medical facility to obtain coverage and third-party reimbursement for procedures performed using our products. Many hospitals and clinics in the United States belong to group purchasing organizations (that typically incentivize their hospital members to make a relatively large proportion of purchases from a limited number of vendors of similar products that have contracted to offer discounted prices). Such contracts often include exceptions for purchasing certain innovative new technologies, however. Accordingly, the commercial success of our products may also depend to some extent on our ability to either negotiate favorable purchase contracts with key group purchasing organizations or persuade hospitals and clinics to purchase our product “off contract.” These third-party payors may deny reimbursement if they determine that a device used in a procedure was not medically necessary; was not used in accordance with cost-effective treatment methods, as determined by the third-party payor; or was used for an unapproved use. A national or local coverage decision denying Medicare coverage for one or more of our products could result in private insurers and other third party payors also denying coverage. Even if favorable coverage and reimbursement status is attained for our products, less favorable coverage policies and reimbursement rates may be implemented in the future. The cost containment measures that third-party payors and providers are instituting, both within the United States and abroad, could significantly reduce our potential revenues from the sale of our products and any product candidates. We cannot provide any assurances that we will be able to obtain and maintain third party coverage or adequate reimbursement for our products and product candidates in whole or in part.
For inpatient and outpatient procedures, including those that will involve use of our products, Medicare and many other third-party payors in the United States reimburse hospitals at a prospectively determined amount. This amount is generally based on one or more diagnosis related groups, or DRGs, associated with the patient’s condition for inpatient treatment and generally based on ambulatory payment classifications, or APCs, associated with the procedures performed as an outpatient at an ambulation surgicenter. Each DRG or APC is associated with a level of payment and may be adjusted from time to time, usually annually. Prospective payments are intended to cover most of the non-physician hospital costs incurred in connection with the applicable diagnosis and related procedures. Implant products, such as those we plan to sell, represent part of the total procedure costs while labor, hospital room and board, and other supplies and services represent the balance of those costs. However, the prospective payment amounts are typically set independently of a particular hospital’s actual costs associated with treating a particular patient and implanting a device. Therefore, the payment that a hospital would receive for a particular hospital visit would not typically take into account the cost of our products.
Medicare has established a number of DRGs for inpatient procedures that involve the use of products similar to ours. Although Medicare has authority to create special DRGs for hospital services that more properly reflect the actual costs of expensive or new-technology devices implanted as part of a procedure, it has declined to do so in the past, and we do not expect that it will do so with respect to our current products and product candidates. Medicare’s DRG and APC classifications may have implications outside of Medicare, as many other U.S. third-party payors often use Medicare DRGs and APCs for purposes of determining reimbursement.
We believe that orthopedic implants generally have been well received by third-party payors because of the ability of these implants to greatly reduce long-term healthcare costs for patients with degenerative joint disease. However, coverage and reimbursement policies vary from payor to payor and are subject to change. As discussed above, hospitals that purchase medical devices for treatment of their patients generally rely on third-party payors to reimburse all or part of the costs and fees associated with the procedures performed with these devices. Both government and private third-party coverage and reimbursement levels are critical to new product acceptance. Neither hospitals nor surgeons are likely to use our products if they do not receive reimbursement for the procedures adequate to cover the cost of our products.
While it is expected that hospitals will be able to obtain coverage for procedures using our products, the level of payment available to them for such procedures may change over time. State and federal healthcare programs, such as Medicare and Medicaid, closely regulate provider payment levels and have sought to contain, and sometimes reduce, payment levels. Commercial insurers and managed care plans frequently follow government payment policies, and are likewise interested in controlling increases in the cost of medical care. These third-party payors may deny payment if they determine that a procedure was not medically necessary, a device used in a procedure was not used in accordance with cost-effective treatment methods, as determined by the third-party payor, or was used for an unapproved use.
In addition, some payors are adopting pay-for-performance programs that differentiate payments to healthcare providers based on the achievement of documented quality-of-care metrics, cost efficiencies, or patient outcomes. These programs are intended to provide incentives to providers to find ways to deliver the same or better results while consuming fewer resources. As a result of these programs, and related payor efforts to reduce payment levels, hospitals and other providers are seeking ways to reduce their costs, including the amounts they pay to medical device suppliers. Adverse changes in payment rates by payors to hospitals could adversely impact our ability to market and sell our products and negatively affect our financial performance.
69
Table of Contents
In international markets, healthcare payment systems vary significantly by country and many countries have instituted price ceilings on specific product lines. There can be no assurance that our products will be considered cost-effective by third-party payors, that reimbursement will be available or, if available, that the third-party payors’ reimbursement policies will not adversely affect our ability to sell our products profitably.
Member countries of the European Union offer various combinations of centrally financed healthcare systems and private health insurance systems. The relative importance of government and private systems varies from country to country. Governments may influence the price of medical devices through their pricing and reimbursement rules and control of national healthcare systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may be marketed only once a reimbursement price has been agreed upon. Some of these countries may require, as condition of obtaining reimbursement or pricing approval, the completion of clinical trials that compare the cost-effectiveness of a particular product candidate to currently available therapies. Some E.U. member states allow companies to fix their own prices for devices, but monitor and control company profits. The choice of devices is subject to constraints imposed by the availability of funds within the purchasing institution. Medical devices are most commonly sold to hospitals or healthcare facilities at a price set by negotiation between the buyer and the seller. A contract to purchase products may result from an individual initiative or as a result of a competitive bidding process. In either case, the purchaser pays the supplier, and payment terms vary widely throughout the European Union. Failure to obtain favorable negotiated prices with hospitals or healthcare facilities could adversely affect sales of our products.
Employees
As of July 8, 2014, we had 95 employees, including ten part-time temporary employees, of which 19 are employed in administration, 18 in operations, 41 in manufacturing and research and development, and 17 in sales and marketing. We believe that our success will depend, in part, on our ability to attract and retain qualified personnel. We have never experienced a work stoppage due to labor difficulties and believe that our relations with our employees are good. None of our employees are represented by labor unions.
Facilities
Our 54,000 square foot corporate office and manufacturing facilities are located in Salt Lake City, Utah. We occupy these facilities pursuant to a lease that expires in January 2020. We may extend the lease for two additional periods of five years each. We believe that our existing facilities are adequate for our current and projected needs for the foreseeable future.
Legal Matters
We are currently not a party to any material legal proceedings. However, our industry is characterized by frequent claims and litigation, including claims regarding intellectual property and product liability. As a result, we may be subject to various legal proceedings in the future.
Directors and Executive Officers
Our current directors and executive officers and their respective ages and positions are as follows:
| Name |
Age | Position | ||||
| Max E. Link, Ph.D. |
73 | Chairman of the Board of Directors | ||||
| Eric K. Olson |
51 | Director, President and Chief Executive Officer | ||||
| Jay M. Moyes |
60 | Director and Chief Financial Officer | ||||
| B. Sonny Bal, M.D. |
51 | Director | ||||
| David W. Truetzel |
57 | Director | ||||
| Jeffrey S. White |
60 | Director | ||||
| James P. Abraham |
55 | Senior Vice President, Global Sales | ||||
| Kevin Davis |
48 | Chief Operating Officer | ||||
| Bryan J. McEntire |
62 | Chief Technology Officer | ||||
| Kevin Ontiveros |
53 | Chief Legal Officer, Chief Compliance Officer and Corporate Secretary | ||||
| Vytas Rupinskas |
62 | Vice President, Marketing | ||||
| Christopher R. Whitfield |
47 | Chief Commercial Officer | ||||
70
Table of Contents
The following is a brief summary of the background of each of our current directors and executive officers.
Max E. Link, Ph.D. has served as the chairman of our board of directors since October 2003. Dr. Link was chairman of the board of directors and Chief Executive Officer of Centerpulse AG, a medical implant company from March 2002 to October 2003. Prior to joining Centerpulse, Dr. Link was Chief Executive Officer of Corange (Bermuda), the parent company of Boehringer Mannheim Corporation and chairman of the board of directors and chief executive officer of Sandoz Pharma, Ltd., now part of Novartis Corporation, a manufacturer of pharmaceutical products. Dr. Link is chairman of the boards of directors of three publicly listed biopharmaceutical companies, Alexion Pharmaceuticals, Inc., Celsion Corporation and CytRx Corporation. Dr. Link holds a Ph.D. in Economics from University of St. Gallen (Switzerland).
We believe that Dr. Link is qualified to serve as a member of our board of directors because of his significant experience leading companies in our industry as well as the depth of his institutional knowledge of our company.
Eric K. Olson has served as our Chief Executive Officer and President and as a director since February 2012. Prior to serving us in this capacity, Mr. Olson served as our Senior Vice President of Global Marketing from June 2011 through February 2012. From December 2007 to June 2011, Mr. Olson was the Executive Vice President of Sales & Marketing for Axial Biotech, Inc., a molecular diagnostics company. Mr. Olson has also held senior sales and marketing positions with Medtronic, Inc. and Smith & Nephew. Mr. Olson holds a B.S. in Behavioral Science and Health Administration from the University of Utah, and has also completed a master’s-level internship program at the same institution.
We believe that Mr. Olson’s position as the Chief Executive Officer and President of our Company uniquely qualifies him to serve on our board of directors due to his intimate knowledge of our day-to-day operations. Additionally, Mr. Olson possesses a wealth of industry experience related to our business.
Jay M. Moyes has served on our board of directors since November 2012 and as our Chief Financial Officer since October 2013. Since November 2007 Mr. Moyes has been the managing member of Drayton Investments, LLC, a partnership focused on investing in private healthcare related companies and real estate financing. In April 2012, he joined the board of directors of Puma Biotechnology Inc., a biopharmaceutical company. Since May 2006, he has been a member of the board of directors and chairman of the audit committee of Osiris Therapeutics, Inc., a publicly held stem cell therapeutics company. Mr. Moyes is also a director of BioCardia, Inc., a medical device company, and Integrated Diagnostics Inc., a molecular diagnostics company. From May 2008 through July 2009, Mr. Moyes served as the Chief Financial Officer of XDx, Inc., a privately held molecular diagnostics company. Prior to that, he served as the Chief Financial Officer of Myriad Genetics, Inc., a publicly held healthcare diagnostics company, from June 1996 until his retirement in November 2007, and as its Vice President of Finance from July 1993 until July 2005. From 1991 to 1993, Mr. Moyes served as Vice President of Finance and Chief Financial Officer of Genmark, Inc., a privately held genetics company. He held various positions with the accounting firm of KPMG LLP from 1979 through 1991, most recently as a Senior Manager. He also served as a member of the Board of Trustees of the Utah Life Science Association from 1999 through 2006. He holds an M.B.A from the University of Utah and received his B.A. in Economics from Weber State University.
In addition to serving as our Chief Financial Officer, we believe that Mr. Moyes’ experience working with biotechnology companies through their transformation from emerging growth to established, publicly-traded companies qualify him to serve on our board of directors.
B. Sonny Bal, M.D. has served on our board of directors since February 2012. Dr. Bal is Professor & Chief of Adult Reconstruction at the University of Missouri, Columbia, specializing in hip and knee replacement surgery. He also is an Adjunct Professor of Material Sciences at the University of Missouri at Rolla. Dr. Bal is a member of the American Academy of Orthopaedic Surgeons and the American Association of Hip and Knee Surgeons. Dr. Bal received his M.D. degree from Cornell University and an M.B.A. from Northwestern University, and a J.D. from the University of Missouri. Dr. Bal is a licensed attorney and co-founder of the Bal Brenner law firm in North Carolina.
We believe that Dr. Bal’s expertise in orthopedic surgery and his specialty in hip and knee replacement surgery qualifies him to serve on our board of directors.
David W. Truetzel has served on our board of directors since our acquisition of US Spine, Inc. in September 2010. Mr. Truetzel has been the general partner of Augury Capital Partners a private equity fund that invests in life sciences and information technology companies, which he co-founded in 2006. Mr. Truetzel is a director of Enterprise Bank, Inc., Verifi, Inc., a provider of electronic payment solutions, Clearent, LLC, a credit card processing provider, and Paranet, LLC, an IT services provider. Mr. Truetzel holds a B.S. in Business Administration from Saint Louis University, an M.B.A. from The Wharton School and is a licensed C.P.A.
71
Table of Contents
We believe that Mr. Truetzel’s financial and managerial expertise qualify him to serve on our board of directors.
Jeffrey S. White has served on our board of directors since January 2014. Since January 2013, Mr. White has served as Principal at Medtech Advisory Group LLC, a firm he founded that advises early and mid-stage medical technology firms. Mr. White is currently a director of Residency Select LLC, a company which offers psychometric assessment, training and compliance products to medical and surgical residency programs. From May 2006 to December 2012 he served as Global Director of Business Development for Synthes Inc., a global orthopedic firm that was acquired by Johnson and Johnson in 2012. Mr. White has served as Chief Executive Officer and co-founder of several start-up surgical device firms and has previously held executive level positions at Richard-Allan Medical Industries Inc., a medical device manufacturer, which was acquired by Urohealth Systems Inc. and United States Surgical Corporation, unit of Covidien plc. Mr. White holds a B.S. in Biology from Union College in Schenectady NY.
We believe that Mr. White’s experience as an executive and founder of medical device companies qualifies him to serve on our board of directors.
James P. Abraham joined us as Senior Vice President, Global Sales, in January 2013. From January 2007 to December 2013 Mr. Abraham worked in various capacities at Stryker Corporation, a medical equipment company including as Senior Director of Sales. He also previously served as Senior Vice President of Sales and Marketing for IsoTis Orthobiologics, Inc., a company which specializes in human tissue and synthetic grafting and injectable bone growth stimulation. Mr. Abraham holds a B.S. in Business Administration from Creighton University.
Kevin Davis has served as our Chief Operating Officer since June 2012. From December 2011 to June 2012, he served as our President of Manufacturing. From March 2011 to December 2011, he served as our Vice President of Strategy and Business Development. From March 2009 to March 2011, he served as our Cost Accountant, Financial Systems. Mr. Davis was the Chief Financial Officer, from April 2007 to March 2009, of Nevada Chemicals, Inc., a sodium cyanide chemical company and served as one of its directors. Mr. Davis graduated from the University of Utah with a B.S. in Accounting.
Bryan J. McEntire has served as our Chief Technology Officer since May 2012. From June 2004 to May 2012 he served as our Vice President of Manufacturing and as our Vice President of Research from December 2006 to May 2012. Mr. McEntire has worked in various advanced ceramic product development, quality engineering and manufacturing roles at Applied Materials, Inc., Norton Advanced Ceramics, a division of Saint-Gobain Industrial Ceramics Corporation, Norton/TRW Ceramics and Ceramatec, Inc., a small producer of ionic-conducting and structural ceramic components located in Salt Lake City, Utah. Mr. McEntire holds a B.S. degree in Materials Science and Engineering and an M.B.A. from the University of Utah.
Kevin Ontiveros has served as our Chief Legal Officer and Chief Compliance Officer since December 2012. Mr. Ontiveros was previously a practicing attorney at Life Science Law PC from February 2011 to December 2012 and Stoel Rives LLP from January 2009 to January 2011, where he provided legal and business counsel on a wide range of matters, including technology licensing transactions, corporate financing opportunities (including public and private equity and debt offerings), public company SEC reporting compliance, and clinical trial, manufacturing, distribution, and research and development agreements. Mr. Ontiveros served as the Vice President-Legal Affairs, General Counsel and Corporate Secretary for ImaRx Therapeutics, Inc. from March 2007 to December 2008 and as the Vice President-Corporate Law and Assistant Corporate Secretary for NPS Pharmaceuticals, Inc. from April 1996 to March 2007. Mr. Ontiveros earned his B.A. from the University of Arizona, his J.D. from the University of Utah School of Law and his L.L.M. in Taxation from the University of Florida.
Vytas Rupinskas is our Vice President of Marketing, a position he has held since December 2012. From September 2005 to March 2012, Mr. Rupinskas served as the Director of Product Management for Leads & Accessories for the Neuromodulation Division of St. Jude Medical, Inc., and Marketing Manager for St. Jude Medical, a provider of implantable medical devices. Prior to his tenure at St. Jude Medical, Mr. Rupinskas served as Senior Product Manager at Exactech, Inc., a provider of implant devices and surgical instruments and held various senior global marketing and international sales management positions at DePuy Orthopaedics, Inc., a medical device company, and its affiliates DePuy International Ltd. and DePuy Spine, Inc. Mr. Rupinskas is a graduate of the University of Illinois with a B.S. degree in Liberal Arts and Sciences and an M.S. in Mechanical Engineering.
Christopher R. Whitfield has served as our Chief Commercial Officer since November 2013. From March 2012 to September 2012, Mr. Whitfield served as the Executive Vice President, Sales and Marketing of Pioneer Surgical Technologies and from October 2009 to March 2012 as its Vice President, Marketing. From October 2008 to September 2009, he served as the West Area Vice President, Sales for Zimmer Spine, a division of Zimmer, Inc. From September 2007 to October 2008, he served as the Senior Director, Marketing of Abbot Spine, Inc., from January 2007 to September 2007 as its Director, Product Management and from June 2005 to January 2007 he served as its Group Manager, Product Marketing. Mr. Whitfield received a B.S. degree in Business Administration, Marketing and Management from the University of Texas at Austin.
72
Table of Contents
Board Composition
Our restated certificate of incorporation and restated bylaws provide that the authorized number of directors may be changed only by resolution of the board of directors. Seven directors are currently authorized. In accordance with our restated certificate of incorporation, our board of directors is divided into three classes with staggered three-year terms. At each annual meeting of stockholders, the successors to the directors whose terms then expire will be elected to serve until the third annual meeting following such election. Our directors are divided among the three classes as follows:
| • | The Class I directors are Max E. Link, Ph.D. and Jay M. Moyes and their terms will expire in 2015 our annual meeting of stockholders; |
| • | The Class II directors are Eric K. Olson and David W. Truetzel, and their terms will expire in 2016 at our annual meeting of stockholders; and |
| • | The Class III directors are B. Sonny Bal, M.D. and Jeffrey S. White, and their terms will expire in 2017 at our annual meeting of stockholders. |
Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the total number of directors.
Director Independence
Our board of directors has reviewed the materiality of any relationship between us and each of our directors, either directly or indirectly. Based on this review, the board of directors has determined that Max E. Link, Ph.D., David W. Truetzel, Jeffrey S. White and B. Sonny Bal, M.D. are “independent directors” as defined by the SEC and NASDAQ. The rules of The NASDAQ Capital Market require that a majority of our board of directors be independent directors, as defined by the rules of The NASDAQ Capital Market. We currently have a board of directors consisting of a majority of independent directors.
Committees of the Board of Directors
Our board of directors has an audit committee, a compensation committee and a nominating and corporate governance committee, each of which has the composition and responsibilities described below. The rules of The NASDAQ Capital Market require that the audit committee consist of at least three members of our board of directors, each of whom must be independent, as established under the rules of The NASDAQ Capital Market and the SEC.
Audit Committee
Our audit committee is composed of David W. Truetzel (Chairman), B. Sonny Bal, M.D. and Jeffrey White, each of whom is independent within the meaning of the rules of the SEC and the listing standards of The NASDAQ Capital Market. Our board of directors has determined Mr. Truetzel qualifies as a financial expert as defined in SEC rules. Our independent auditors and management periodically meet privately with our audit committee. Our audit committee is authorized to:
| • | approve and retain the independent auditors to conduct the annual audit of our books and records; |
| • | review the proposed scope and results of the audit; |
| • | review and pre-approve the independent auditor’s audit and non-audit services rendered; |
| • | approve the audit fees to be paid; |
| • | review accounting and financial controls with the independent auditors and our financial and accounting staff; |
| • | review and approve transactions between us and our directors, officers and affiliates; |
| • | recognize and prevent prohibited non-audit services; |
| • | establish procedures for complaints received by us regarding accounting matters; |
| • | oversee internal audit functions; and |
| • | prepare the report of the audit committee that SEC rules require to be included in our annual meeting proxy statement. |
Compensation Committee
The rules of The NASDAQ Capital Market require that the compensation committee consist of at least two members of our board of directors, each of whom must be independent, as established under the rules of The NASDAQ Capital Market and the SEC. Our compensation committee is composed of Jeffrey White (Chairman), David W. Truetzel and B. Sonny Bal, M.D., each of whom is independent within the meaning of the rules of the SEC and The NASDAQ Capital Market.
73
Table of Contents
Our compensation committee is authorized to:
| • | annually evaluate the performance of and review and recommend to our board of directors the compensation arrangements for management, including the compensation for our president and chief executive officer; |
| • | establish and review general compensation policies with the objective to attract and retain superior talent, to reward individual performance and to achieve our financial goals; |
| • | determine or review and make recommendations to our board of directors with respect to director compensation; |
| • | evaluate and assess potential compensation advisors and retain and approve their compensation; and |
| • | administer our stock incentive plans. |
Nominating and Governance Committee
Our nominating and governance committee is composed of Max E. Link, Ph.D. (Chairman) and B. Sonny Bal, M.D., each of whom is independent within the meaning of the rules of the SEC and The NASDAQ Capital Market. Our nominating and governance committee is authorized to:
| • | develop and recommend to the board of directors criteria for board and committee membership; |
| • | establish procedures for identifying and evaluating board of director candidates, including nominees recommended by stockholders; |
| • | identify individuals qualified to become members of the board of directors and recommend such persons to the board of directors to be nominated for election as directors and/or to each of the board of directors’ committees; |
| • | develop and recommend to the board of directors a set of corporate governance principles applicable to our company; and |
| • | oversee the evaluation of the board of directors and management. |
Compensation Committee Interlocks and Insider Participation
No member of our compensation committee has at any time been an employee of ours. None of our executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
Code of Business Conduct and Ethics
We have adopted a code of business ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Our code of business conduct and ethics is available on our website at www.amedica.com. We intend to disclose any amendments to the code, or any waivers of its requirements on our website.
EXECUTIVE AND DIRECTOR COMPENSATION
The following discussion relates to the compensation of our “named executive officers,” including our Chief Executive Officer and President, Eric K. Olson, and our two most highly compensated executive officers (other than our chief executive officer), Jay M. Moyes, our Chief Financial Officer, and Bryan J. McEntire, our Chief Technology Officer.
Summary Compensation Table
The following table sets forth information about certain compensation awarded or paid to our named executive officers for the 2012 and 2013 fiscal years.
| Name and Principal Position |
Year | Salary ($) |
Bonus ($) |
Stock Awards ($)(1) |
Option Awards ($)(2) |
All Other Compensation ($)(3) |
Total ($) |
|||||||||||||||||||||
| Eric K. Olson |
|
2013 2012 |
|
|
315,192 240,423 |
|
|
— 20,000 |
|
|
442,000 — |
(4)
|
|
— 325,000 |
|
|
34,075 — |
(5) (6) |
|
791,267 585,423 |
| |||||||
| Jay M. Moyes |
|
2013 2012 |
|
|
50,000 — |
(7)
|
|
100,000 — |
(8)
|
|
340,202 — |
(9)
|
|
— — |
|
|
30,288 8,043 |
(10) (11) |
|
520,490 8,043 |
| |||||||
| Bryan J. McEntire |
|
2013 2012 |
|
|
228,543 217,995 |
|
|
— 5,000 |
|
|
299,200 — |
(12)
|
|
— 94,550 |
|
|
33,246 8,680 |
|
|
560,989 326,225 |
| |||||||
| (1) | Amounts shown reflect the aggregate grant date fair value of restricted stock units, or RSUs, granted to the named executive officer computed in accordance with the Financial Accounting Standards Board, Accounting Standards Codification Topic 718, Compensation — Stock Compensation, or FASB ASC Topic 718. These amounts may not correspond to the actual value that will be recognized by the named executive officers. The grant date fair value of performance-based RSUs is determined based on the probable outcome of such performance conditions as of the grant date. The grant date fair value of the performance-based RSUs assuming the maximum potential value that can be achieved is $442,000 for Mr. Olson, $340,202 for Mr. Moyes and $299,200 for Mr. McEntire. Assumptions used in the calculations of these amounts are included in Note 8 to our financial statements incorporated by reference herein. |
74
Table of Contents
| (2) | Amount shown for Mr. Olson reflects the grant date fair value of options awarded in 2012 determined in accordance with FASB ASC Topic 718. The amount shown for Mr. McEntire reflects the incremental fair value of stock options issued in exchange for outstanding stock options with exercise prices over $25.77 per share in March 2012. These amounts exclude the value of estimated forfeitures. Assumptions used in the calculations of these amounts are included in Note 8 to our financial statements incorporated by reference herein. |
| (3) | Amount reflects the aggregation of any matching of 401(k) contributions, group term life premiums paid by us and accrued vacation in instances where each such amount is less than $10,000, unless otherwise noted. |
| (4) | Amount includes the grant date fair value of (i) 23,279 RSUs issued to Mr. Olson in exchange for the cancellation of options to purchase 23,279 shares of our common stock held by Mr. Olson and (ii) 1,940 RSUs that were issued to Mr. Olson in June 2013. |
| (5) | Includes $26,923 of accrued vacation. |
| (6) | Mr. Olson did not contribute money to our 401(k) plan in 2012. Therefore we paid no matching 401(k) amounts nor did we provide him with any other additional compensation in 2012. |
| (7) | Amount reflects the pro-rated amount of Mr. Moyes’s annual salary of $325,000 that was paid to Mr. Moyes since the beginning of his employment with us through the end of the 2013 fiscal year. |
| (8) | Amount reflects the signing bonus that was paid to Mr. Moyes upon the commencement of his employment. |
| (9) | Amount reflects the grant date fair value of (i) 58,197 RSUs that were issued to Mr. Moyes upon the commencement of his employment and (ii) 582 RSUs that were issued to Mr. Moyes as a non-employee director prior to the commencement of his employment. |
| (10) | Amount includes the $27,185 of board member attendance fees paid to Mr. Moyes for his service as a member of our board of directors prior to the commencement of his employment with us. |
| (11) | Amount reflects fees paid to Mr. Moyes for service on our board of directors in the 2012 fiscal year. |
| (12) | Amount reflects the grant date fair value of (i) 16,101 RSUs that were issued to Mr. McEntire in February 2013 in exchange for the cancellation of options to purchase 16,101 shares of our common stock held by him and (ii) 970 RSUs that were issued to Mr. McEntire in June 2013. |
Narrative Disclosure to Summary Compensation Table
Base Salaries. The base salaries for our named executive officers were determined by our compensation committee after reviewing a number of factors, including:
| • | the responsibilities associated with the position held by each of our executive officers and where that position fits within our overall corporate structure; |
| • | the seniority of the individual executive’s position; |
| • | the base salary level of each executive officer in prior years; |
| • | our overall financial position; and |
| • | for executive officers other than our Chief Executive Officer, recommendations made by our Chief Executive Officer. |
2012 Compensation
Our board of directors approved a salary of $250,000 for Mr. Olson and $228,545 for Mr. McEntire, effective as of January 1, 2012. Mr. Moyes was not employed by us at this time and as such received no compensation from us in 2012, other than fees for serving as a non-employee director. Our board of directors reduced the salaries of our named executive officers by ten percent effective as of May 31, 2012 in order to conserve cash. On December 12, 2012, Mr. McEntire’s salary was restored to its original 2012 rate and Mr. Olson’s salary was increased to $300,000. Our board, on the recommendation of our compensation committee decided to give this base salary increase to Mr. Olson in recognition of his significant contributions to our company, including helping to increase our revenue above $23.0 million, aligning our sales and marketing team’s salaries with our revenues and our receipt of 510(k) clearance for our second generation Valeo products while under his leadership. As a result of these salary changes in 2012, Messrs. Olson and McEntire received the following salary amounts:
| Name |
Initial 2012 Base Salary (1)($) |
10% Reduced 2012 Base Salary (2) |
Restored/New 2012 Base Salary (3) |
|||||||||
| Eric K. Olson |
250,000 | 225,000 | 300,000 | |||||||||
| Bryan J. McEntire |
228,545 | 205,691 | 228,545 | |||||||||
| (1) | Annual base salary rate from January 1, 2012 to May 31, 2012 |
| (2) | Annual base salary rate from June 1, 2012 to December 12, 2012 |
| (3) | Base salary as of December 12, 2012. |
75
Table of Contents
Annual Cash Bonuses. We have historically awarded discretionary cash bonuses to our executive officers. These bonuses are intended to reward our executive officers for the achievement of key strategic and business outcomes. Accordingly, each of Messrs. Olson and McEntire was awarded a cash bonus for 2012 equal to $20,000 and $5,000, respectively. Mr. Moyes was not employed with us in 2012 and as such did not receive a cash bonus for 2012. Our compensation committee has established a set of corporate objectives pursuant to which they may award our executive officers cash bonuses for their performance during 2013. These cash bonuses are discretionary and no cash bonuses were awarded for 2013 performance. Our compensation committee has not yet determined corporate objectives for 2014 to which they may award our executive officers cash bonuses for their performance during 2014.
Long-Term Incentives. All options granted to our executive officers have been granted under the 2003 Stock Option Plan, or the 2003 Plan. These options vest over a period of time, generally four years. Upon termination of employment for any reason other than cause, our vested stock options granted to our named executive officers do not terminate and instead remain outstanding for their full-term of ten years. In the future, our compensation committee, with the approval of our board and stockholders, may grant to our named executive officers under the Amended and Restated 2012 Equity Incentive Plan, or the 2012 Plan, incentive stock options, non-qualified stock options, restricted and unrestricted stock awards, or stock-based awards, including RSUs and other stock based awards. See “—Equity Incentive Plans—2012 Plan” below for additional details about the 2012 Plan.
In March 2012, our board approved the cancellation of stock options held by current employees and members of our board with exercise prices above $25.77 per share and replaced such options with new options for an equivalent number of shares with exercise prices of $25.77 per share and ten-year terms to expiration, which were fully vested as of the date of grant. Mr. McEntire exchanged 8,147 options for an equal number of options at this time. None of Mr. Olson’s options were cancelled because he held no options that had exercise prices over $25.77. Mr. Moyes was not a member of our board of directors or our employee at that time.
2013 Compensation
In January 2013, we offered to each employee and director that held options to acquire shares of our common stock awarded under the 2003 Plan the opportunity to exchange such options for RSUs to be issued under the 2012 Plan on a one-for-one basis. As a result of the exchange offer, 93,968 RSUs were issued under the 2012 Plan in February 2013. Messrs. Olson and McEntire exchanged stock options and received 23,279 and 16,101 RSUs, respectively. Mr. Moyes did not have options eligible for conversion. The RSUs expire three years from the date of grant and will vest upon the earlier of (i) August 12, 2014 or (ii) the date of closing of a change in control provided, in each case, that the individual is providing services to us on such date.
In June 2013, in lieu of granting stock options, our board approved a grant of RSUs under the 2012 Plan to our named executive officers. Messrs. Olson and McEntire received 1,940 and 970 RSUs, respectively. The RSUs, which were awarded based on our 2012 performance, will vest upon the earlier of (i) August 12, 2014 or (ii) the date of a closing of a change in control, in each case, provided that the executive officer is providing services to us on such date and such event occurs within three years from the grant date.
On October 27, 2013, our board approved the hiring of Mr. Moyes as our Chief Financial Officer at an annual base salary of $325,000 and provided Mr. Moyes with a signing bonus of $100,000 which was paid on his first day of employment. In addition, if Mr. Moyes’s employment is terminated by us for any reason other than cause, he shall be entitled to receive a pro-rated portion of his annual bonus for the year in which the termination occurs. In connection with his hiring, our board approved a grant of 58,197 RSUs to Mr. Moyes under the 2012 Plan, of which (i) 19,399 RSUs vested on his first day of employment, (ii) 19,399 RSUs vested on January 27, 2014 and (iii) 19,399 RSUs vested on February 12, 2014. In addition, the release date of the shares for any vested RSUs will be postponed until the first to occur of (i) a change in control or (ii) six months after a separation of Mr. Moyes’s service with us for any reason in compliance with Section 409A of the Internal Revenue Code.
Our board authorized salary increases for each of our named executive officers effective as of October 30, 2013. Accordingly, Mr. Olson’s salary was increased from $300,000 to $350,000, and Mr. McEntire’s salary was increased from $228,545 to $235,000. Mr. Moyes did not receive a salary increase as he had just begun his employment with us at the time of the salary increases.
2014 Compensation
On January 27, 2014, our board approved grants of RSUs to our named executive officers in the aggregate amount of 807,965 shares, as detailed below, subject to stockholder approval of an amendment to the 2012 Plan to increase the number of shares authorized for issuance under the 2012 Plan, which approval was obtained in February 2014 and which were RSUs were issued the named executive officers on April 1, 2014, upon the effectiveness of a registration statement on Form S-8 which registered the shares underlying the RSUs:
| Name and Title |
Grant Amount | |||
| Eric K. Olson |
417,079 | |||
| Jay M. Moyes |
299,713 | |||
| Bryan J. McEntire |
91,175 | |||
76
Table of Contents
Outstanding Equity Awards at Fiscal Year-End
The following table shows information regarding equity awards held by our named executive officers as of December 31, 2013:
| Option Awards | Stock Awards | |||||||||||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
Equity Incentive Plan Awards: Number of Unearned Shares, Units, or Other Rights That Have Not Vested (#)(1) |
Equity Incentive Value of Unearned Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(2) |
||||||||||||||||||
| Eric K. Olson |
— | — | — | — | 25,219 | 145,007 | ||||||||||||||||||
| Jay M. Moyes |
— | — | — | — | 39,380 | 226,434 | ||||||||||||||||||
| Bryan J. McEntire |
7,760 | — | 6.44 | 6/8/2014 | 17,071 | 98,159 | ||||||||||||||||||
| (1) | Mr. Olson and M. McEntire’s RSUs vest upon the earlier of (i) August 12, 2014 or (ii) the date of a closing of a change in control provided, in each case, that the individual providing services to us on such date, and expire three years after the date of grant. 19,399 of Mr. Moyes RSUs vested on January 27, 2014 and 19,399 vested on February 12, 2014. The delivery of the vested RSUs will be postponed until the first to occur of (i) a change in control or (ii) six months after a separation of Mr. Moyes service with us for any reason in compliance with Section 409A of the Internal Revenue Code. |
| (2) | Reflects the value calculated by multiplying the number of unvested RSUs by the value of our common stock on December 31, 2013, which we estimate was approximately $5.75. |
Equity Compensation Plan Information
The following table summarizes our equity compensation plans as of December 31, 2013:
| Plan category |
(a) Number of securities to be issued upon exercise of outstanding options, warrants, and rights |
(b) Weighted- average exercise price of outstanding options, warrants and rights |
(c) Number of securities remaining available for future issuances under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
| Equity compensation plans approved by security holders |
294,775 | $ | 28.90 | 91,659 | ||||||||
| Equity compensation plans not approved by security holders |
— | $ | 0.00 | N/A | ||||||||
|
|
|
|
|
|||||||||
| Total |
294,775 | $ | 28.90 | 91,659 | ||||||||
|
|
|
|
|
|||||||||
Retirement Benefits
401(k) Plan
We offer our executive officers, including our named executive officers, retirement benefits, including participation in our tax-qualified profit sharing plan that includes a “cash-or-deferred” or 401(k) feature in the same manner as other employees. The plan is intended to satisfy the requirements of Section 401 of the Internal Revenue Code. Our employees may elect to reduce their current
77
Table of Contents
compensation by up to the statutorily prescribed annual limit and have a like amount contributed to the plan. In addition, we may make discretionary and/or matching contributions to the plan in amounts determined annually by our board. We currently elect to match the contributions of our employees who participate in our 401(k) plan as follows: a match of 100% on the first 3% of compensation contributed by a plan participant and a match of 50% on amounts above 3%, up to 5%, of compensation contributed by a plan participant.
Potential Payments Upon Termination or Change in Control
We have entered into certain agreements and maintain certain plans that may require us to make certain payments and/or provide certain benefits to the executive officers named in the Summary Compensation Table in the event of a termination of employment or change in control.
Pursuant to severance agreements that we have entered into with each of our named executive officers (other than Mr. Moyes, who would receive severance payments pursuant to the terms of his employment agreement as more fully described below), upon the consummation of a change in control, all outstanding options, restricted stock and other such rights held by the executives will fully vest. Additionally, if a change in control occurs and at any time during the one-year period following the change in control (i) we or our successor terminate the executive’s employment other than for cause (but not including termination due to the executive’s death or disability) or (ii) the executive terminates his employment for good reason, then such executive has the right to receive payment consisting of a lump sum payment equal to two times his highest annual salary with us during the preceding three-year period, including the year of such termination and including bonus payments (measured on a fiscal year basis), but not including any reimbursements and amounts attributable to stock options and other non-cash compensation. “Change in control” is defined in the severance agreements as occurring upon: (i) any “person” (as such term is used in Sections 13(d) and 14(d) of the Exchange Act) becoming the “beneficial owner” (as defined in Rule 13d-3 under the Exchange Act), directly or indirectly, of securities representing 50% or more of the total voting power represented by our then outstanding voting securities (excluding securities held by us or our affiliates or any of our employee benefit plans) pursuant to a transaction or a series of related transactions which our board did not approve; (ii) a merger or consolidation of our company, other than a merger or consolidation which would result in our voting securities outstanding immediately prior thereto continuing to represent at least 50% of the total voting securities or such surviving entity or parent of such corporation outstanding immediately after such merger or consolidation; or (iii) the approval by our stockholders of an agreement for the sale or disposition of all or substantially all of our assets. As defined in the severance agreements, “cause” means: (i) the executive’s commission of a felony (other than through vicarious liability or through a motor vehicle offense); (ii) the executive’s material disloyalty or dishonesty to us; (iii) the commission by the executive of an act of fraud, embezzlement or misappropriation of funds; (iv) a material breach by the executive of any material provision of any agreement to which the executive and we are party, which breach is not cured within 30 days after our delivery to the executive of written notice of such breach; or (v) the executive’s refusal to carry out a lawful written directive from our board. “Good reason” as defined in the severance agreements means, without the executive’s consent: (i) a change in the principal location at which the executive performs his duties to a new work location that is at least 50 miles from the prior location; or (ii) a material change in the executive’s compensation, authority, functions, duties or responsibilities, which would cause his position with us to become of less responsibility, importance or scope than his prior position, provided, however, that such material change is not in connection with the termination of the executive’s employment with us for any reason.
In the event that an officer entitled to receive or receives payment or benefit under the severance agreements described above, or under any other plan, agreement or arrangement with us, or any person whose action results in a change in control or any other person affiliated with us and it is determined that the total amount of payments will be subject to excise tax under Section 4999 of the Internal Revenue Code, or any similar successor provisions, we will be obligated to pay such officer a “gross up” payment to cover all taxes, including any excise tax and any interest or penalties imposed with respect to such taxes due to such payment.
Pursuant to the terms of Mr. Moyes’s employment arrangement, if: (i) Mr. Moyes is terminated by us without cause, (ii) he terminates his employment for good reason, or (iii) in the event a change in control occurs and Mr. Moyes is not offered continuing employment by the acquiring company or if such continuing employment is terminated without cause or if he terminates such continuing employment with good reason at any time during the twelve months following the change in control, we will be required to pay Mr. Moyes a lump sum equal to the sum of: (i) two times his annual salary in effect on the date of termination, (ii) any unpaid bonus through the end of his employment for the prior year that had been earned by Mr. Moyes but not paid plus a pro rata portion of his performance bonus and (iii) two times his sign on bonus. In addition, provided Mr. Moyes properly elects for continuation coverage, we will pay health insurance premiums for Mr. Moyes, his spouse and any covered dependents for a period of 24 months following the termination of his employment. Pursuant to the terms of the employment arrangement, “good reason” means, without Mr. Moyes’s consent, the occurrence of any one or more of the following events: (i) a material diminution of Mr. Moyes’s authority, functions, duties or responsibilities; (ii) a relocation of Mr. Moyes’s principal workplace to a new location more than 50 miles from the prior location; (iii) the material diminution of Mr. Moyes’s annual base salary, other than in the event of a reduction in compensation of all of our executive officers, generally, so long as the reduction to Mr. Moyes’s base salary is no more than the average reduction; or (iv) a material breach by us of the terms of the employment agreement. “Change in control” has the same meaning as previously described under the severance agreements for the other executive officers.
78
Table of Contents
In connection with a change in control, if Mr. Moyes will be required to pay any excise tax pursuant to Section 4999 of the Internal Revenue Code or any similar successor provisions then we will make an additional “gross up” payment to Mr. Moyes in an amount to cover such excise tax and the taxes associated with each payment.
Director Compensation
The following table shows the total compensation paid or accrued during the fiscal year ended December 31, 2013 to each of our non-employee directors except Mr. Jeffrey S. White who was not a member of our board of directors in 2013. Fees paid to Mr. Moyes for his service as a director prior to his employment with us are included in the Summary Compensation Table.
| Name |
Fees Earned or Paid in Cash($) |
Stock Awards ($)(1)(2) |
Option Grants ($)(3) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||
| Max E. Link, Ph.D. |
38,500 | 10,200 | — | — | 48,700 | |||||||||||||||
| B. Sonny Bal, M.D. |
26,000 | 10,200 | — | — | 36,200 | |||||||||||||||
| Gregg R. Honigblum(4) |
24,151 | 10,200 | — | — | 34,351 | |||||||||||||||
| Rohit Patel(5) |
30,909 | 10,200 | — | 12,250 | (6) | 53,359 | ||||||||||||||
| George Singer(7) |
17,522 | 10,200 | — | — | 27,722 | |||||||||||||||
| David Truetzel |
43,751 | 10,200 | — | — | 53,951 | |||||||||||||||
| (1) | As part of the annual grant to directors, each director received 582 RSUs. These RSUs expire three years from the date of grant and will only vest upon the earlier of (a) August 12, 2014, the date of expiration of the lock-up period imposed in connection with the closing of the initial public offering of shares of our common stock or (b) the date of a closing of a change of control. |
| (2) | Amount shown reflects the grant date fair value of the RSUs awarded in 2013 determined in accordance with the Financial Accounting Standards Board, Accounting Standards Codification Topic 718, Compensation-Stock Compensation. Assumptions used in the calculation of these amounts are included in Note 8 to our financial statements incorporated by reference herein. |
| (3) | No stock options were granted to directors during 2013. However, as of December 31, 2013, our directors held the following aggregate number of stock options: Dr. Link, 3,783; Dr. Bal, 2,813; and Mr. Honigblum, 4,171. Mr. Singer, did not hold any stock options as of December 31, 2013. All stock options are fully vested. |
| (4) | Mr. Honigblum resigned from our board of directors in September 2013. |
| (5) | Mr. Patel resigned from our board of directors in September 2013. |
| (6) | We paid Mr. Patel $12,250 in consulting fees related to a litigation matter against our former Chief Executive Officer, Ben Shappley, in 2013. |
| (7) | Mr. Singer resigned from our board of directors in September 2013. |
We compensate each of the non-employee members of our Board in accordance with the following annual retainer and meeting fees (paid on a quarterly basis):
| • Board member Annual Retainer |
$ | 20,000 | ||
| • Board Chair Annual Additional Retainer |
$ | 10,000 | ||
| • Committee Chair Annual Retainer |
$ | 7,500 | ||
| • Committee member Annual Retainer |
$ | 3,750 | ||
| • Board meeting-in person attendance |
$ | 1,500 | ||
| • Board meeting-telephonic attendance |
$ | 1,000 | ||
| • Committee meeting attendance |
$ | 1,500 | ||
| • Committee meeting-telephonic attendance |
$ | 1,000 |
In addition to cash compensation, the non-employee members of our Board have historically been awarded an annual stock option grant in the amount of 582 shares of our common stock. In 2013, in lieu of stock options, non-employee members of the board were each granted 582 RSUs. In January 2013, we offered to each director that held options to acquire shares awarded under the 2003 Plan the opportunity to exchange such options for an equal number of RSUs. Messrs. Patel and Truetzel each exchanged stock options and received 9,021 RSUs and 2,716 RSUs, respectively, under the 2012 Plan. These RSUs expire three years from the date of grant and will only vest upon continued service with us and if either of the following events occurs prior to the expiration date: (i) August 12, 2014, the date of the expiration of the lock-up period imposed on the directors after the completion of the closing of the initial public offering of the shares of our commons stock or (ii) upon a “change in control”. “Change of Control” is defined in the restricted stock unit agreements entered into in connection with the awarding of the RSUs, as occurring upon: (i) any “person” (as such term is used in Sections 13(d) and 14(d) of the Exchange Act) becoming the “beneficial owner” (as defined in Rule 13d-3 under the Exchange Act), directly or indirectly, of securities representing 50% or more of the total voting power represented by our then outstanding voting securities (excluding for this purpose any such voting securities held by the us our affiliates or by any of our employee benefit plans) pursuant to a transaction or a series of related transactions which the Board of Directors does not approve; or (ii) a merger or consolidation of our company, whether or not approved by the Board of Directors, other than a merger or consolidation which would result in our voting securities outstanding immediately prior thereto continuing to represent (either by remaining outstanding or by
79
Table of Contents
being converted into voting securities of the surviving entity or the parent of such corporation) more than 50% of the total voting power represented by our voting securities of the surviving entity or parent of such corporation, as the case may be, outstanding immediately after such merger or consolidation; or (iii) the sale or disposition by us of all or substantially all of our assets in a transaction requiring stockholder approval.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following includes a summary of transactions since January 1, 2011 to which we have been a party, in which the amount involved in the transaction exceeded $120,000, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our common stock, on an as converted basis, or any affiliates of the foregoing persons or entities, in each case, evaluated at the time of the transaction, had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under “Executive and Director Compensation.” Our board of directors approved our participation in each transaction described below.
Transactions with MSK Investments, LLC and its Affiliates following our Acquisition of US Spine, Inc.
Settlement Agreement with MSK Investments, LLC
In September 2010, we acquired US Spine, Inc., or US Spine. In this transaction, US Spine became our wholly owned subsidiary as we acquired all of the outstanding capital stock of US Spine. In May 2012, we entered into a settlement agreement with James G. Koman and with MSK Investments, LLC, or MSK, a company controlled by Mr. Koman, on its own behalf and acting in its capacity as stockholders’ representative for the former stockholders of US Spine, to resolve certain disputes. Pursuant to the settlement agreement, in lieu of the issuance of up to 6,250,000 shares of our Series E convertible preferred stock upon the achievement of certain earnout milestones, which we refer to as the US Spine Earnout, we issued (a) 842,443 shares of our Series E convertible preferred stock to the former stockholders of US Spine, of which 39,249 shares were issued to MSK and its affiliates, and (b) 2,557,562 shares of our Series C convertible preferred stock to MSK. We also agreed to release the 1,806,250 shares of our Series E convertible preferred stock from an escrow arrangement established at the time of our acquisition of US Spine, of which 1,380,654 were received by MSK and its affiliates. MSK and Mr. Koman also agreed to certain standstill covenants in our favor that expire on May 10, 2015. Spinal Management LLC, of which David Truetzel is a 50% co-owner, also received a commission that was paid in 42,122 and 127,878 of the shares of our Series E convertible preferred stock and Series C convertible preferred stock, respectively, issued under the settlement agreement.
Restructuring and Payment of the US Spine Note
In October 2012, we restructured the terms of a promissory note issued in favor of MSK at the time of our acquisition of US Spine, or the US Spine Note, to extend the maturity date of the second $3.0 million installment from September 2012 to December 2012. We made payments to MSK of $500,000 on October 31, 2012 and $2,500,000 on December 17, 2012 in connection with this restructuring.
Restructuring and Conversion of Senior Secured Subordinated 6%/8% Convertible Promissory Notes
Between March 2011 and May 2011, we issued an aggregate principal amount of $24.8 million of Senior Secured Subordinated 6%/8% Convertible Promissory Notes, or the Senior Secured Notes, and warrants to purchase 288,685 shares of our common stock at an exercise price of $51.55 per share to 85 accredited investors. In connection with the initial closing of this offering, we received a commitment from Hampshire Med Tech Partners, LP to purchase an additional $5.0 million Senior Secured Note by no later than the first anniversary of the initial closing of that offering. Pursuant to this commitment, we issued an additional $5.0 million Senior Secured Note to Hampshire Med Tech Partners, LP in February 2012.
In December 2012, we amended the terms of the Senior Secured Notes and the holders thereof converted all of their Senior Secured Notes into an aggregate of 14,887,500 shares of our Series F convertible preferred stock. We also amended the terms of the warrants to purchase shares of our common stock issued in connection with the issuance of the Senior Secured Notes to lower the exercise prices thereof from $51.55 per share to $25.77 per share. As a result, we issued an aggregate of 6,131,250 shares of our Series F convertible preferred stock to the following directors, officers and beneficial owners of more than 5% of our common stock, on an as converted basis, and their affiliates:
| Name |
Number of Shares of Series F Convertible Preferred Stock |
|||
| Max E. Link, Ph.D. |
25,000 | |||
| David Truetzel(1) |
12,500 | |||
| Allan R. Lyons(2) |
475,000 | |||
| Gregg R. Honigblum(3) |
6,250 | |||
| Karl Kipke(4) |
5,000,000 | |||
| B. Sonny Bal, M.D.(5) |
12,500 | |||
| Kevin Murphy |
600,000 | |||
| (1) | Includes 12,500 shares that were issued to Truetzel Revocable Trust, of which Mr. Truetzel and his spouse are the sole beneficiaries. |
80
Table of Contents
| (2) | Shares were issued to Vestal. Mr. Lyons is the managing member and sole owner of 21st Century Strategic Investment Planning, LLC, the general partner of Vestal. |
| (3) | Represents 50% of the 12,500 shares that were issued to Creation Capital. Mr. Honigblum is a 50% owner and a managing member of Creation Capital. Mr. Honigblum resigned from our board of directors in September 2013. |
| (4) | Shares were issued to Hampshire Med Tech Partners, LP. Mr. Kipke is the managing member of Hampshire Med Tech, its general partner. |
| (5) | Shares were issued to Dr. Bal’s father. |
Warrant Restructuring and Private Placement of Common Stock
In March 2013, we amended the terms of certain of the common stock warrants issued in connection with the issuance of the Senior Secured Notes to further lower the exercise prices thereof from $25.77 per share to $17.53 per share. We then issued an aggregate of 178,516 shares of our common stock to 33 accredited investors upon exercise of the amended common stock warrants and the sale of additional shares of our common stock to other investors in the offering at $17.53 per share. We also issued to investors who exercised their common stock warrants new warrants to purchase an aggregate of 76,455 shares of our common stock at an exercise price of $17.53 per share. We issued an aggregate of 53,347 shares of our common stock and new warrants to purchase up to 17,773 shares of our common stock at an exercise price of $17.53 per share to the following directors, officers and beneficial owners of more than 5% of our common stock, on an as converted basis, and their affiliates:
| Name |
Common Stock upon Exercise of Warrants |
New Common Stock | New Common Stock Warrants |
|||||||||
| Allan R. Lyons(1) |
9,214 | — | 9,214 | |||||||||
| Kevin Murphy |
8,558 | — | 8,558 | |||||||||
| Karl Kipke(2) |
— | 53,347 | — | |||||||||
| (1) | Represents the exercise of common stock warrants by, and issuance of common stock warrants to, Vestal. Mr. Lyons is the managing member and sole owner of 21st Century Strategic Investment Planning, LLC, the general partner of Vestal. |
| (2) | Represents 53,347 shares of common stock purchased by Hampshire Med Tech Partners, LP. Mr. Kipke is the managing member of Hampshire Med Tech, its general partner. |
Private Placement of Series F Convertible Preferred Stock
In August 2013 and September 2013, we issued an aggregate of 94.8 units, each unit consisting of 50,000 shares of our Series F convertible preferred stock and a warrant to acquire 970 shares of our common stock at an exercise price of $25.77 per share, to 45 accredited investors at $100,000 per unit. This resulted in our issuance of an aggregate of 4,740,000 shares of our Series F convertible preferred stock and warrants to purchase an aggregate of 91,951 shares of our common stock, including an aggregate of 1,125,000 shares of our Series F convertible preferred stock and warrants to purchase an aggregate of 21,824 shares of our common stock to the following directors, officers and beneficial owners of more than 5% of our common stock, on an as converted basis, and their affiliates:
| Name |
Number of Units | Purchase Price | Number of Shares of Series F Convertible Preferred Stock |
Common Stock Warrants |
||||||||||||
| Max E. Link, Ph.D. |
2.0 | $ | 200,000 | 100,000 | 1,940 | |||||||||||
| B. Sonny Bal, M.D. |
1.5 | $ | 150,000 | 75,000 | 1,455 | |||||||||||
| David W. Truetzel(1) |
1.0 | $ | 100,000 | 50,000 | 970 | |||||||||||
| Jay M. Moyes(2) |
0.5 | $ | 50,000 | 25,000 | 485 | |||||||||||
| George Singer(3) |
1.0 | $ | 100,000 | 50,000 | 970 | |||||||||||
| Allan R. Lyons(4) |
3.5 | $ | 350,000 | 175,000 | 3,395 | |||||||||||
| James G. Koman(5) |
1.0 | $ | 100,000 | 50,000 | 970 | |||||||||||
| Kevin Murphy(6) |
12.0 | $ | 1,200,000 | 600,000 | 11,639 | |||||||||||
| (1) | Investment made by Truetzel Revocable Trust, of which Mr. Truetzel and his spouse are the sole beneficiaries. |
81
Table of Contents
| (2) | Investment made by Drayton Investments, LLC, of which Mr. Moyes is a managing member. |
| (3) | Consists of 50% of the investment made by Singer Bros. LLC. Mr. Singer is a 50% owner and a managing member of Singer Bros. LLC. Mr. Singer resigned from our board of directors in September 2013. |
| (4) | Investment made by Vestal. Mr. Lyons is the managing member and sole owner of 21st Century Strategic Investment Planning, LLC, the general partner Vestal. |
| (5) | Investment made by MSK, of which Mr. Koman is the managing member. |
| (6) | In connection with the sale and issuance of certain of the units in this financing, we also issued to TGP Securities, Inc., an entity controlled by Mr. Murphy, warrants to purchase 9,311 shares of our common stock at an exercise price of $56.70 per share and paid a cash commission of $480,000 to TGP Securities, Inc., neither of which are reflected in the table. |
Transactions with Creation Capital, LLC and Creation Capital Advisors, LLC
Mr. Gregg R. Honigblum, the Chief Executive Officer and a 50% co-owner of each of Creation Capital LLC, or Creation Capital, and Creation Capital Advisors, LLC, or Creation Advisors, served on our board of directors from 2006 until September 2013. In connection with the private placement of our Senior Secured Notes Creation Capital served as our placement agent In February 2012, when we issued an additional $5.0 million Senior Secured Note to Hampshire Med Tech Partners, LP, we paid Creation Capital an $212,500 as commissions.
In June 2012, we entered into a financial advisor consulting agreement with Creation Advisors, pursuant to which we agreed to extend the termination date of certain Series C convertible preferred stock warrants previously issued to Creation Capital from February 2013 to February 2018.
In connection with the conversion of our Senior Secured Notes in December 2012, we agreed to pay Creation Advisors a strategic financial advisory fee in the amount of approximately $447,000. We agreed to pay half of the advisory fee, approximately $223,000 in December 2012 and the remaining half within 24 months, which we paid in September 2013. Karl Kipke, who beneficially owns more than 5% of our common stock, received $60,000 from Creation Advisors in 2012, as a consultant for Creation Advisors, for advising us at this time on our financing options.
In connection with the warrant restructuring and private placement of common stock in March 2013, we paid Creation Advisors a strategic financial advisory fee of approximately $250,000. In October 2013, we entered into a one-year consulting agreement for financial advisory services with Creation Advisors in which Creation Advisors will receive compensation of up to $180,000 in cash (payable $15,000 per month). We paid $45,000, under this agreement, during the year ended December 31, 2013 and $45,000 during the three months ended March 31, 2014. This agreement was terminated in March 2014 and as consideration for termination of the agreement, we paid $60,000 and issued 50,000 restricted shares of our common stock, then valued at $372,000, to Creation Advisers.
Equity Grants
We have granted options to purchase shares of our common stock and RSUs to our executive officers and directors. See “Executive and Director Compensation.”
Change in Control Agreements
We have entered into severance agreements with our executive officers as described in the section of this prospectus entitled “Executive and Director Compensation—Potential Payments Upon Termination or Change in Control.”
Indemnification Arrangements
Our restated certificate of incorporation and restated bylaws provide that we will indemnify our directors and officers to the fullest extent permitted by Delaware law. In addition, we have entered into indemnification agreements with each of our directors and executive officers. A stockholder’s investment in our common stock may decline in value to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to any indemnification provisions.
Policy for Approval of Related Person Transactions
We believe that all the transactions described above were made on terms no less favorable to us than those that could have been obtained from unaffiliated third parties. With the exception of transactions in which related parties participated on the same terms as those of other participants who were not related parties, our board of directors reviewed and approved the transactions with each
82
Table of Contents
related party, namely our directors, executive officers and beneficial owners of more than 5% of our common stock, on an as converted basis, and affiliates of our directors, executive officers and 5% stockholders, and reviewed the material facts as to a related party’s relationship or interest in a transaction that were disclosed to our board of directors prior to our board of directors’ consideration of a transaction with a related party. The transactions involving related parties were approved by our board of directors, including all of our directors who were not interested in these transactions.
All related party transactions must be approved by our audit committee. Pursuant to the written charter of our audit committee, the audit committee is responsible for reviewing and approving, prior to our entry into any transaction involving related parties, all transactions in which we are a participant and in which any parties related to us has or will have a direct or indirect material interest.
In reviewing and approving these transactions, the audit committee shall obtain, or shall direct our management to obtain on its behalf, all information that the committee believes to be relevant and important to a review of the transaction prior to its approval. Following receipt of the necessary information, a discussion shall be held of the relevant factors, if deemed to be necessary by the committee, prior to approval. If a discussion is not deemed to be necessary, approval may be given by written consent of the committee. No related party transaction shall be entered into prior to the completion of these procedures.
The audit committee or its chairman, as the case may be, shall approve only those related party transactions that are determined to be in, or not inconsistent with, the best interests of us and our stockholders, taking into account all available facts and circumstances as the committee or the chairman determines in good faith to be necessary. No member of the audit committee shall participate in any review, consideration or approval of any related party transaction with respect to which the member or any of his or her immediate family members is the related party.
The following table sets forth certain information regarding the beneficial ownership of our common stock as of July 8, 2014 by:
| • | each of our current directors; |
| • | the executive officers named in the summary compensation table; |
| • | all of our directors and executive officers as a group; and |
| • | each stockholder known by us to own beneficially more than 5% of our common stock. |
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Shares of common stock that may be acquired by an individual or group within 60 days of July 8, 2014, pursuant to the exercise or vesting of options or warrants or conversion of convertible promissory notes, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Percentage of shares beneficially owned is based on 12,411,207 shares issued and outstanding on July 8, 2014.
83
Table of Contents
Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them, based on information provided to us by such stockholders. The address for each director and executive officer listed is: c/o Amedica Corporation, 1885 West 2100 South, Salt Lake City, Utah 84119.
| Shares Beneficially Owned | ||||||||
| Name and Address of Beneficial Owner |
Number | Percentage | ||||||
| Five Percent Stockholders: |
||||||||
| Karl Kipke (1) Hampshire Group LLC 500 Plaza on the Lake, Suite #103 Austin, TX 78746 |
1,459,349 | 11.7 | % | |||||
| MG Partners II Ltd. (2) 5 Hanover Square New York, NY 10004 |
2,326,409 | 15.8 | % | |||||
| Directors and Named Executive Officers: |
||||||||
| Max E Link, PhD (3) |
83,276 | * | ||||||
| B. Sonny Bal, M.D. (4) |
63,894 | * | ||||||
| David W. Truetzel (5) |
56,517 | * | ||||||
| Jeffrey S.White |
— | * | ||||||
| Eric K. Olson (6) |
442,298 | 3.4 | % | |||||
| Jay M. Moyes (7) |
366,766 | 2.9 | % | |||||
| Bryan J. McEntire (8) |
108,246 | * | ||||||
| All executive officers and directors as a group (12 persons) (9) |
1,614,509 | 12.8 | % | |||||
| * | Represents beneficial ownership of less than 1% of the shares of our common stock. |
| (1) | Consists of: (i) 16,179 warrants that are currently exercisable held by Mr. Kipke; (ii) 1,303,347 shares and 96,994 common stock warrants held by Hampshire Med Tech Partners, LP; (iii) 25,915 shares held by Hampshire Healthcare Partners, LP; and (iv) 16,914 shares held by Hampshire Asset Management, LLC. Hampshire Med Tech is the general partner of Hampshire Med Tech Partners, LP and Special Opportunities is the general partner of Hampshire Healthcare Partners, LP. Mr. Kipke is the managing member of each of Hampshire Med Tech and Special Opportunities and the president of Hampshire Asset Management, LLC. |
| (2) | Consists of 50,853 shares of our common stock, 568,889 common stock warrants and 1,706,667 shares issuable upon conversion of convertible notes. Magna Gibraltar Investments LLC, a Delaware limited liability company, or Magna Gibraltar, owns 50% of the outstanding shares of the Selling Stockholder and, through representation on the board of directors of the Selling Stockholder, controls the Selling Stockholder. Pursuant to a shareholders agreement relating to the ownership of the Selling Stockholder, the board of directors of the Selling Stockholder, acting by majority vote, has sole power to vote or to direct the vote and sole power to dispose or to direct the disposition of all securities owned directly by the Selling Stockholder, including, without limitation, the common stock issuable upon conversion of the Convertible Notes and the exercise of the Magna Warrant. The board of directors of the Selling Stockholder consists of three individuals, two of which are appointed by Magna Gibraltar. The two directors initially appointed by Magna Gibraltar are Joshua Sason and Michael Abitebol. |
Hanover Holdings I, LLC, a New York limited liability company, or Hanover, owns all of the membership interests in Magna Gibraltar. Magna Group Capital Management, LLC, a Delaware limited liability company (“Magna Group”), is a manager of Magna Gibraltar. Mr. Sason is the Chief Executive Officer and managing member of each of Hanover and Magna Group and owns all of the membership interests in each of Hanover and Magna Group. Mr. Abitebol is the Chief Operating Officer and a manager of Magna Gibraltar. None of Magna Gibraltar, Hanover, Magna Group, Mr. Sason or Mr. Abitebol directly owns any shares of Common Stock. None of the Selling Stockholder, Magna Gibraltar, Hanover or Magna Group is a registered broker-dealer, and none of the Selling Stockholder, Magna Gibraltar, Hanover or Magna Group nor any of their respective affiliates is an affiliate or an associated person of a registered broker-dealer.
The address of the principal business office of each of Magna Gibraltar, Hanover, Magna Group, Mr. Sason and Mr. Abitebol is: 5 Hanover Square, New York, New York 10004. The address of the principal business office of the Selling Stockholder is: 57/63 Line Wall Road, Gibraltar.
| (3) | Consists of 70,085 shares of our common stock, 3,783 common stock options, 6,984 RSUs and 2,424 common stock warrants. |
| (4) | Consists of 18,750 shares of our common stock held by Dr. Bal, 33,893 shares of our common stock held by Dr. Bal and his spouse, 2,813 common stock options, 6,984 RSUs and 1,454 common stock warrants. |
| (5) | Consists of 5,337 shares of our common stock held by Mr. Truetzel, 26,689 shares of our common stock held by Truetzel Revocable Trust of which Mr. Truetzel and his spouse are the sole beneficiaries, 23,279 RSUs and 1,212 common stock warrants. |
| (6) | Consists of 442,298 RSUs. |
| (7) | Consists 1,534 shares of our common stock, 6,250 shares held by Drayton Investments, LLC, 358,498 RSUs and 484 common stock warrants held by Drayton Investments, LLC. Mr. Moyes is a managing member of Drayton Investments, LLC. |
| (8) | Consists of 108,246 RSUs. |
| (9) | Consists of 162,538 shares of our common stock, 1,439,801 RSUs, 6,596 common stock options and 5,574 common stock warrants. |
84
Table of Contents
We are authorized to issue 250,000,000 shares of common stock, $0.01 par value per share, and 130,000,000 shares of preferred stock, $0.01 par value per share. As of July 8, 2014, there were 12,411,207 shares of common stock outstanding, which were held of record by 598 stockholders, no shares of preferred stock outstanding, 1,827,516 RSUs outstanding, 301,489 common stock options outstanding and 1,705,707 common stock warrants outstanding. The following description summarizes the most important terms of our capital stock. Because it is only a summary, it does not contain all the information that may be important to you. For a complete description you should refer to our restated certificate of incorporation and restated bylaws, copies of which have been incorporated by reference herein, and to the applicable provisions of the Delaware General Corporation Law.
Common Stock
Holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, and do not have cumulative voting rights. Accordingly, the holders of a majority of the shares of our common stock entitled to vote can elect all of the directors standing for election. Subject to preferences that may be applicable to any outstanding shares of preferred stock, holders of our common stock are entitled to receive ratably such dividends, if any, as may be declared from time to time by our board of directors out of funds legally available for dividend payments. All outstanding shares of our common stock are fully paid and nonassessable, and the shares of our common stock to be sold pursuant to this prospectus will be fully paid and nonassessable. The holders of common stock have no preferences or rights of conversion, exchange, pre-emption or other subscription rights. There are no redemption or sinking fund provisions applicable to our common stock. In the event of any liquidation, dissolution or winding-up of our affairs, holders of our common stock will be entitled to share ratably in our assets that are remaining after payment or provision for payment of all of our debts and obligations and after liquidation payments to holders of outstanding shares of preferred stock, if any.
Preferred Stock
The preferred stock, if issued, would have priority over our common stock with respect to dividends and other distributions, including the distribution of assets upon liquidation. Our board of directors has the authority, without further stockholder authorization, to issue from time to time shares of preferred stock in one or more series and to fix the terms, limitations, relative rights and preferences and variations of each series. Although we have no present plans to issue any shares of preferred stock, the issuance of shares of preferred stock, or the issuance of rights to purchase such shares, could decrease the amount of earnings and assets available for distribution to the holders of common stock, could adversely affect the rights and powers, including voting rights, of the common stock, and could have the effect of delaying, deterring or preventing a change in control of us or an unsolicited acquisition proposal.
Warrants
As of July 8, 2014 we had the following warrants outstanding to purchase a total of 1,705,707 shares of our common stock:
| • | warrants to purchase in the aggregate 52,325 shares of our common stock at an exercise price of $56.70 per share, terminating in February 2018; |
| • | warrants to purchase 25,020 shares of our common stock at an exercise price of $56.70 per share, terminating in September 2015; |
| • | a warrant to purchase 2,204 shares of our common stock at an exercise price of $56.70 per share, terminating in April 2015; |
| • | warrants to purchase in the aggregate 67,499 shares of common stock at an exercise price of $51.55 per share, terminating in December 2022; |
| • | warrants to purchase in the aggregate 22,064 shares of common stock at an exercise price of $85.06 per share, issued in June and August 2008, and terminating seven years from the date of issuance; |
| • | a warrant to purchase 2,910 shares of common stock at an exercise price of $45.11 per share, issued in February 2010 and terminating on February 17, 2017; |
| • | warrants to purchase in the aggregate 288,685 shares of common stock at an exercise price of $3.75 per share, originally issued between March and May 2011, and terminating seven years from the date of issuance, which have a price protection provision for any securities issued by us at a price below $3.75 per share (excluding shares granted to our employees or consultants); |
| • | warrants to purchase in the aggregate 193 shares of common stock at an exercise price of $51.55 per share, issued on November 15, 2011, and terminating three years from date of issuance; |
85
Table of Contents
| • | warrants to purchase in the aggregate 57,557 shares of common stock at an exercise price of $3.75 per share, issued on May 9, 2011, and terminating five years from the date of issuance, which have a price protection provision for any securities issued by us at a price below $3.75 per share (excluding shares granted to our employees or consultants); |
| • | a warrant to purchase 970 shares of common stock at an exercise price of $51.55 issued on March 17, 2011; |
| • | warrants to purchase in the aggregate 91,951 shares of common stock at an exercise price of $3.75 per share, issued in August and September 2013, and terminating five years from the date of issuance, which have a price protection provision for any securities issued by us at a price below $3.75 per share (excluding shares granted to our employees or consultants); |
| • | warrants to purchase 9,311 shares of common stock at an exercise price of $3.75 per share, issued in August and September 2013, and terminating five years from the date of issuance, which have a price protection provision for any securities issued by us at a price below $3.75 per share (excluding shares granted to our employees or consultants); |
| • | warrants to purchase in the aggregate 568,889 shares of common stock at an exercise price of $4.65 per share, issued in June 2014 and terminating two years from the date of issuance; and |
| • | warrants to purchase in the aggregate 516,129 shares of common stock at an exercise price of $4.65 per share, issued in June 2014 and terminating five years from the date of issuance, which have a price protection provision for any securities issued by us at a price below $4.65 per share (excluding shares granted to our employees or consultants or convertible notes granted to Magna). |
These warrants provide for adjustments of the exercise price and the number of shares underlying the warrants upon the occurrence of certain events, including stock dividends, stock splits, reclassifications or other changes in our corporate structure. The holders of certain of these warrants have registration rights that are outlined below under the heading “—Registration Rights.”
Senior Convertible Notes
On June 30, 2014, we entered into a Securities Purchase Agreement with the Selling Stockholder, pursuant to which we sold to the Selling Stockholder an initial unsecured senior convertible note with an original principal amount of $2.9 million, or the Initial Convertible Note, for a purchase price of $2.5 million. Additionally, the Selling Stockholder is irrevocably bound to purchase, an additional unsecured senior convertible note with an original principal amount of $3.5 million, or the Additional Convertible Note, for a fixed purchase price of $3.5 million, subject only to conditions outside of the Selling Stockholder’s control or that the Selling Stockholder cannot cause not to be satisfied, none of which are related to the market price of shares of our common stock, no later than ten calendar days after the effective date of a registration statement registering the shares issued in connection with the Securities Purchase Agreement. The Initial Convertible Note and Additional Convertible Note are collectively referred to as the Convertible Notes.
With respect to the Initial Convertible Note, $150,000 of the outstanding principal amount (together with any accrued and unpaid interest with respect to such portion of the principal amount) will be automatically extinguished if (1) we have properly filed a registration statement registering the shares to which this prospectus relates with the SEC on or prior to August 14, 2014 and (2) no event of default, or an event that with the passage of time or giving of notice would constitute an event of default, has occurred on or prior to such date. In addition, $250,000 of the outstanding principal amount of the Initial Convertible Note (together with any accrued and unpaid interest with respect to such portion of the principal amount) will be automatically extinguished if (1) the registration statement is declared effective by the SEC on or prior to the earlier of (i) October 13, 2014 and (ii) the fifth trading day after the SEC notifies us that the registration statement is not subject to further review, and this prospectus is available for use by the Selling Stockholder and (2) no event of default, or an event that with the passage of time or giving of notice would constitute an event of default, has occurred on or prior to such date. The Securities Purchase Agreement and the Convertible Notes also contain representations and warranties, covenants and events of default customary for financings of this type, including, among other things, limitations on certain other indebtedness, dividends and distributions, sales and transfers of assets and transactions with affiliates.
The Convertible Notes mature on June 30, 2016 (subject to extension as provided in the Convertible Notes) and accrue interest at an annual rate of 6.0%. The Convertible Notes are convertible at any time after issuance, in whole or in part, at the Selling Stockholder’s option, into shares of common stock at an initial fixed conversion price equal to $3.75 per share, or the Initial Fixed Price. If, however, the closing sale price of our common stock is below 110% of the Initial Fixed Price, or $4.13 per share, for two consecutive trading days, either:
| (1) | we may redeem the Convertible Notes in full at a 127.5% premium of the entire principal and interest remaining on the Convertible Notes within 60 days of such occurrence; or |
| (2) | beginning on the 61st day following such occurrence, the Selling Stockholder will be able to convert the Convertible Notes, in whole or in part, at the lessor of: (i) the Initial Fixed Price or (ii) a price equal to 80% of the lowest daily volume weighted average price, or VWAP, of our common stock during the five trading days prior to conversion. |
86
Table of Contents
As long as the Convertible Notes are outstanding and for so long as the Selling Stockholder or its affiliates beneficially own any of the shares of Common Stock issuable upon conversion of the Convertible Notes or exercise of the Warrant, they may not engage in any “short sale” transactions in the Common Stock. In addition, the Selling Stockholder has agreed that unless otherwise mutually agreed upon, the Selling Stockholder, upon conversion of the Convertible Notes, shall not sell more than the greater of: (i) $125,000 of Common Stock, in any five consecutive trading day period, or (ii) 15% of the daily trading volume of the Common Stock on any given trading day. If, however, on any given trading day more than $250,000 of the Common Stock is traded, the Selling Stockholder may trade up to 33% of the daily trading volume on that day. The foregoing restrictions are referred to as the Investor Restrictions. The Investor Restrictions will be removed on any day the price of the stock trades below $2.50.
At no time will the Selling Stockholder be entitled to convert any portion of the Convertible Notes or exercise any portion of the Magna Warrant to the extent that after such conversion or exercise, the Selling Stockholder (together with its affiliates) would beneficially own more than 4.99% of the outstanding shares of our common stock as of such date, or the Maximum Percentage. The Maximum Percentage may be raised to any other percentage not in excess of 9.99% at the option of the Selling Stockholder upon at least 61 days’ prior notice to us, or lowered to any other percentage, at the option of the Selling Stockholder, at any time.
The Initial Convertible Note includes and, if issued, the Additional Convertible Note will include, customary event of default provisions. The Initial Convertible Note provides and, if issued, the Additional Convertible Note will provide for a default interest rate of 18%. Upon the occurrence of an event of default, Investor may require the Company to pay in cash the “Event of Default Redemption Price” which is defined in the Convertible Notes to mean the greater of (i) the product of (A) the amount to be redeemed multiplied by (B) 135% (or 100% if an insolvency related event of default) and (ii) the product of (X) the conversion price in effect at that time multiplied by (Y) the product of (1) 127.5% (or 100% if an insolvency related event of default) multiplied by (2) the greatest closing sale price of the common stock on any trading day during the period commencing on the date immediately preceding such event of default and ending on the date we make the entire payment required to be made under the default provision.
We have the right to prepay the Convertible Notes, in whole or in part, for an amount equal to 127.5% of the outstanding principal remaining.
Under the Securities Purchase Agreement, if at any time the aggregate number of shares issued or issuable in connection with the Securities Purchase Agreement is 15% or more of the total shares of the Common Stock outstanding on the date of the Securities Purchase Agreement, then at the next special or annual meeting of stockholders of the Company, the Company is required to take all action necessary to obtain the approval of its stockholders of the issuance of all such shares in accordance with the rules of the NASDAQ Stock Market. If, despite the Company’s commercially reasonable efforts such stockholder approval is not obtained, the Company is required to continue to seek stockholder approval at each special or annual meeting of stockholders of the Company convened such meeting until such stockholder approval is obtained. In addition, until stockholder approval is obtained, the Company may not issue any shares in connection with the Securities Purchase Agreement in an aggregate amount that exceeds 19.99% of the issued and outstanding Common Stock on June 30, 2014.
Registration Rights
On June 30, 2014, we entered into a registration rights agreement with the Selling Stockholder that provides it with certain registration rights subject to the rules and regulations of the SEC. These registration rights are subject to certain conditions and limitations, including our right to limit the number of shares included in any such registration under certain circumstances. We are generally required to pay all expenses incurred in connection with registrations effected in connection with the registration rights below, excluding selling expenses such as broker commissions and underwriting discounts. The registration rights may be transferred to any transferee or assign of the Selling Stockholder who agrees to be bound by the terms of the registration rights agreement.
Resale Registration Rights. We are required to file a registration statement on Form S-1, on or before August 14, 2014, covering the resale of all or a portion of the shares of our common stock issuable to the Selling Stockholder upon conversion of the Conversion Notes, the Magna Warrant and the 50,853 shares issued to the Selling Stockholder in connection with the execution of the Securities Purchase Agreement. We are further required to use commercially reasonable efforts to have the registration statement declared and thereafter remain effective no later than the earlier of: (i) October 13, 2014 and (ii) the fifth trading day after the SEC has notified us that the registration statement is not subject to further review, and this prospectus is available for use by the Selling Stockholder. In the event that all registrable securities are not covered by the registration statement, we may be required to file additional registration statements covering the remainder of the registrable securities, not in excess of one registration statement in any rolling six month period.
Piggyback Rights. If, at any time prior to the six month anniversary of the date of the Securities Purchase Agreement there is not an effective registration statement covering the registrable securities, we propose to register shares of our common stock under the Securities Act in connection with a public offering of common stock, we will, prior to such filing, give written notice to the Selling Stockholder of our intention to do so. Upon the timely written request of the Selling Stockholder after receipt of such notice, we shall cause all securities which we have been requested by the Selling Stockholder to register to be registered under the Securities Act to the extent not already registered pursuant to an effective registration statement. These piggyback registration rights do not apply to registrations of our securities that we initiate that are (i) issuable in connection with our acquisition of another entity or business or (ii) incidental to any of our equity compensation, employee stock purchase or other employee benefit plans or any sales agent/distributor equity incentive program that we may implement.
87
Table of Contents
Effects of Anti-Takeover Provisions of Our Restated Certificate of Incorporation, Our Restated Bylaws and Delaware Law
The provisions of (1) Delaware law, (2) our restated certificate of incorporation and (3) our restated bylaws discussed below could discourage or make it more difficult to prevail in a proxy contest or effect other change in our management or the acquisition of control by a holder of a substantial amount of our voting stock. It is possible that these provisions could make it more difficult to accomplish, or could deter, transactions that stockholders may otherwise consider to be in their best interests or our best interests. These provisions are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and in the policies formulated by the board of directors and to discourage certain types of transactions that may involve an actual or threatened change in control of our company. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. These provisions also are intended to discourage certain tactics that may be used in proxy fights. These provisions also may have the effect of preventing changes in our management.
Delaware Statutory Business Combinations Provision. We are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. For purposes of Section 203, a “business combination” is defined broadly to include a merger, asset sale or other transaction resulting in a financial benefit to the interested stockholder, and, subject to certain exceptions, an “interested stockholder” is a person who, together with his or her affiliates and associates, owns (or within three years prior, did own) 15% or more of the corporation’s voting stock.
Classified Board of Directors; Appointment of Directors to Fill Vacancies; Removal of Directors for Cause. Our restated certificate of incorporation provides that our board of directors will be divided into three classes as nearly equal in number as possible. Each year the stockholders will elect the members of one of the three classes to a three-year term of office. All directors elected to our classified board of directors will serve until the election and qualification of their respective successors or their earlier resignation or removal. The board of directors is authorized to create new directorships and to fill any positions so created and is permitted to specify the class to which any new position is assigned. The person filling any of these positions would serve for the term applicable to that class. The board of directors (or its remaining members, even if less than a quorum) is also empowered to fill vacancies on the board of directors occurring for any reason for the remainder of the term of the class of directors in which the vacancy occurred. Members of the board of directors may only be removed for cause and only by the affirmative vote of holders of at least 75% of our outstanding voting stock. These provisions are likely to increase the time required for stockholders to change the composition of the board of directors. For example, in general, at least two annual meetings will be necessary for stockholders to effect a change in a majority of the members of the board of directors.
Authorization of Blank Check Preferred Stock. Our restated certificate of incorporation provides that our board of directors is authorized to issue, without stockholder approval, blank check preferred stock. Blank check preferred stock can operate as a defensive measure known as a “poison pill” by diluting the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our board of directors.
Advance Notice Provisions for Stockholder Proposals and Stockholder Nominations of Directors. Our restated bylaws provide that, for nominations to the board of directors or for other business to be properly brought by a stockholder before a meeting of stockholders, the stockholder must first have given timely notice of the proposal in writing to our Secretary. For an annual meeting, a stockholder’s notice generally must be delivered not less than 45 days nor more than 75 days prior to the anniversary of the mailing date of the proxy statement for the previous year’s annual meeting. For a special meeting, the notice must generally be delivered no less than 60 days nor more than 90 days prior to the special meeting or ten days following the day on which public announcement of the meeting is first made. Detailed requirements as to the form of the notice and information required in the notice are specified in our restated bylaws. If it is determined that business was not properly brought before a meeting in accordance with our bylaw provisions, this business will not be conducted at the meeting.
Special Meetings of Stockholders. Special meetings of the stockholders may be called only by our board of directors pursuant to a resolution adopted by a majority of the total number of directors.
No Stockholder Action by Written Consent. Our restated certificate of incorporation does not permit our stockholders to act by written consent. As a result, any action to be effected by our stockholders must be effected at a duly called annual or special meeting of the stockholders.
Super-Majority Stockholder Vote required for Certain Actions. The Delaware General Corporation Law provides generally that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s certificate of incorporation or bylaws, unless the corporation’s certificate of incorporation or bylaws, as the case may be, requires a greater percentage. Our restated certificate of incorporation requires the affirmative vote of the holders of at least 75% of our outstanding
88
Table of Contents
voting stock to amend or repeal any of the provisions discussed in this section of this prospectus entitled “Effect of Anti-Takeover Provisions of Our Restated Certificate of Incorporation, Our Restated Bylaws and Delaware Law” or to reduce the number of authorized shares of common stock or preferred stock. This 75% stockholder vote would be in addition to any separate class vote that might in the future be required pursuant to the terms of any preferred stock that might then be outstanding. A 75% vote is also required for any amendment to, or repeal of, our restated bylaws by the stockholders. Our restated bylaws may be amended or repealed by a simple majority vote of the board of directors.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer and Trust Company. The transfer agent and the registrar’s address is 59 Maiden Lane, New York, New York 10038.
Listing
Our common stock trades on The NASDAQ Capital Market under the symbol “AMDA.”
The shares of our common stock to which this prospectus relates consist of 1,706,667 shares of our common stock issued or issuable to the Selling Stockholder, upon conversion of an aggregate principal amount of $6.4 million of our senior convertible notes, including accrued interest, subject to adjustment; 50,853 shares of our common stock issued in connection with the issuance of the senior convertible notes; and 568,889 shares of our common stock issuable upon the exercise of the Magna Warrant at an exercise price of $4.65 per share, subject to adjustment as provided pursuant to the terms of the warrant. We issued the Convertible Notes, shares of our common stock and the Magna Warrant to the Selling Stockholder in an offer exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act.
We may require the Selling Stockholder to suspend the sales of the common stock covered by this prospectus if we determine in good faith that the disclosure of any material event that has occurred and is continuing would be materially detrimental to us or our business.
The table below sets forth:
| • | the name of the Selling Stockholder; |
| • | the number of shares of common stock, and the percentages of outstanding common stock, beneficially owned by the Selling Stockholder as of July 8, 2014 (except as otherwise indicated), prior to the Selling Stockholder’s offering of the shares of common stock for resale pursuant to this prospectus; |
| • | the maximum number of shares of common stock that may be offered for resale by the Selling Stockholder pursuant to this prospectus; and |
| • | the number of shares of common stock, and the percentages of outstanding common stock, to be beneficially owned by the Selling Stockholder after the sale of the shares of common stock offered pursuant to this prospectus, assuming all such offered shares are sold by the Selling Stockholder and that the Selling Stockholder does not acquire any additional shares of common stock. |
The number of shares disclosed in the table below as ‘‘beneficially owned’’ are those beneficially owned as determined under the rules of the SEC. Such information is not necessarily indicative of ownership for any other purpose.
We obtained the information in the table below from the Selling Stockholder (other than the information regarding the percentages of outstanding common stock beneficially owned by the Selling Stockholder). Except as may be noted below, the Selling Stockholder does not, or within the past three years has not had, any material relationship with us or any of our affiliates.
We cannot advise you as to whether the Selling Stockholder will in fact sell any or all of such shares of common stock. In addition, the Selling Stockholder may have sold, transferred or otherwise disposed of, or may sell, transfer or otherwise dispose of, at any time and from time to time, the shares in transactions exempt from the registration requirements of the Securities Act after the date on which the Selling Stockholder provided the information set forth in the table below. Only the Selling Stockholder referenced in the table below may sell the securities offered hereby, except as described under “Plan of Distribution” and otherwise permitted by law. Changed information regarding the Selling Stockholder will be presented in a prospectus supplement or post-effective amendment to the registration statement of which this prospectus forms a part if and when required. Except as may be indicated below, the Selling Stockholder is not a registered broker-dealer or an affiliate of a broker-dealer.
89
Table of Contents
The number of shares of common stock underlying the convertible notes and warrants assumes no adjustment in the number of shares issuable upon conversion or exercise thereof as a result of stock splits and stock dividends, and conversion price or exercise price adjustments pursuant to the terms of the agreements.
| Beneficial Ownership of Common Stock Prior to Offering |
Common Stock Being Offered Pursuant to this Prospectus |
Beneficial Ownership of Common Stock After Offering |
||||||||||||||||||
| Name of Selling Stockholder |
Shares | Percentage | Shares | Percentage | ||||||||||||||||
| MG Partners II Ltd. |
2,326,409 | 15.8 | % | 2,326,409 | — | — | ||||||||||||||
We are registering the shares covered by this prospectus on behalf of the Selling Stockholder. All costs, expenses and fees connected with the registration of these shares will be borne by us. Any brokerage commissions and similar expenses connected with selling the shares will be borne by the Selling Stockholder. The Selling Stockholder may offer and sell the shares covered by this prospectus from time to time in one or more transactions. The term “Selling Stockholder” includes pledgees, donees, transferees and other successors-in-interest who may acquire shares through a pledge, gift, partnership distribution or other non-sale related transfer from the Selling Stockholder. The Selling Stockholder will act independently of the Company in making decisions with respect to the timing, manner and size of each sale and they may sell the shares on one or more exchanges, in the over-the-counter market or in privately negotiated transactions at prevailing market prices at the time of sale, at fixed prices, at varying prices determined at the time of the sale or at negotiated prices. These transactions include:
| • | ordinary brokerage transactions and transactions in which the broker solicits purchasers; |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its own account pursuant to this prospectus; |
| • | exchange or over-the-counter distributions in accordance with the rules of the exchange or other market; |
| • | block trades in which the broker-dealer attempts to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | a combination of any such method of sale; and |
| • | any other method permitted pursuant to applicable law. |
In connection with distributions of the shares or otherwise, the Selling Stockholder may:
| • | enter into option or other transactions with broker-dealers or other financial institutions which require the delivery to them of the shares covered by this prospectus, which they may in turn resell; and |
| • | pledge the shares to broker-dealers or other financial institutions, which, upon a default, they may in turn resell. |
The Selling Stockholder may also sell any of the shares under Rule 144 rather than with this prospectus if the sale meets the requirements of that rule.
In effecting sales, the Selling Stockholder may engage broker-dealers or agents, who may in turn arrange for other broker-dealers to participate. Broker-dealers or agents may receive commissions, discounts or concessions from the Selling Stockholder and/or from the purchasers of the shares for whom the broker-dealers may act as agents or to whom they sell as principal, or both. The compensation to a particular broker-dealer may be in excess of customary commissions. To our knowledge, there is currently no plan, arrangement or understanding between the Selling Stockholder and any broker-dealer or agent regarding the sale of any of the shares by the Selling Stockholder.
The Selling Stockholder, any broker-dealers or agents and any participating broker-dealers that act in connection with the sale of the shares covered by this prospectus may be “underwriters” under the Securities Act with respect to those shares and will be subject to the prospectus delivery requirements of the Securities Act. Any profit that the Selling Stockholder realizes, and any compensation that any broker-dealer or agent may receive in connection with any sale, including any profit realized on resale of the shares acquired as principal, may constitute underwriting discounts and commissions. If the Selling Stockholder is deemed to be an underwriter, the Selling Stockholder may be subject to certain liabilities under statutes including, but not limited to, Section 11, 12 and 17 of the Securities Act and Section 10(b) and Rule 10b-5 under the Exchange Act.
90
Table of Contents
The securities laws of some states may require the Selling Stockholder to sell the shares in those states only through registered or licensed brokers or dealers. These laws may also require that we register or qualify the shares for sale in those states unless an exemption from registration and qualification is available and the Selling Stockholder and we comply with that exemption. In addition, the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of the shares in the market and to the activities of the Selling Stockholder and its affiliates. Regulation M may restrict the ability of any person engaged in the distribution of the shares to engage in market-making activities with respect to the shares. All of the foregoing may affect the marketability of the shares and the ability of any person to engage in market-making activities with respect to the shares.
We have agreed to indemnify the Selling Stockholder and certain of its affiliates against liabilities, including liabilities under the Securities Act and state securities laws, relating to the registration of the shares offered by this prospectus.
The validity of the issuance of the common stock offered by us in this offering will be passed upon for us by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., Boston, Massachusetts.
The consolidated financial statements of Amedica Corporation at December 31, 2013 and 2012, and for each of the two years in the period ended December 31, 2013, incorporated by reference herein have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon (which contains an explanatory paragraph describing conditions that raise substantial doubt about our ability to continue as a going concern as described in Note 1 to the consolidated financial statements) incorporated by reference herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the common stock offered by this prospectus. This prospectus, which is part of the registration statement, omits certain information, exhibits, schedules and undertakings set forth in the registration statement. For further information pertaining to us and our common stock, reference is made to the registration statement and the exhibits and schedules to the registration statement. Statements contained in this prospectus as to the contents or provisions of any documents referred to in this prospectus are not necessarily complete, and in each instance where a copy of the document has been filed as an exhibit to the registration statement, reference is made to the exhibit for a more complete description of the matters involved.
You may read and copy all or any portion of the registration statement without charge at the public reference room of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Copies of the registration statement may be obtained from the SEC at prescribed rates from the public reference room of the SEC at such address. You may obtain information regarding the operation of the public reference room by calling 1-800-SEC-0330. In addition, registration statements and certain other filings made with the SEC electronically are publicly available through the SEC’s web site at http://www.sec.gov. The registration statement, including all exhibits and amendments to the registration statement, has been filed electronically with the SEC.
We file periodic reports and other information with the SEC. Such periodic reports and other information are available for inspection and copying at the public reference room and website of the SEC referred to above. We maintain a website at http://www.Amedica.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information and other content contained on our website are not part of the prospectus.
INCORPORATION OF DOCUMENTS BY REFERENCE
We have elected to incorporate by reference certain information in this prospectus pursuant to General Instruction VII of Form S-1 in accordance with the Securities Exchange Act of 1934. We have previously filed the following documents with the SEC and are incorporating them by reference into this prospectus, except for information furnished under Item 2.02 or Item 7.01 of Form 8-K, and any exhibits relating to such information, which is neither deemed filed nor incorporated by reference herein:
| • | our Annual Report on Form 10-K for the fiscal year ended December 31, 2013 filed on March 31, 2014; |
| • | our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 filed on May 15, 2014; |
91
Table of Contents
| • | our Current Reports on Form 8-K filed on Form 8-K filed on February 20, 2014, April 18, 2014, June 5, 2014 and July 1, 2014; and |
| • | the description of our common stock contained in our Registration Statement on Form 8-A filed on February 7, 2014, including any amendment or report filed for the purpose of updating such description. |
A statement contained in a document incorporated by reference into this prospectus shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus, any prospectus supplement or in any other subsequently filed document which is also incorporated in this prospectus modifies or replaces such statement. Any statements so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You may request a copy of any or all of the documents incorporated herein by reference. These documents will be provided to you at no cost, by contacting: Investor Relations, Amedica Corporation, 1885 West 2100 South, Salt Lake City, UT 84119. You may also read and copy our annual, quarterly and current reports, and other SEC filings at our website, http://www.amedica.com, and at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling the SEC at (800) SEC-0330. Our SEC filings are also available to the public on the SEC’s website at www.sec.gov.
92
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution. |
The following table sets forth an itemization of the various costs and expenses, all of which we will pay, in connection with the sale of the securities being registered. All of the amounts shown are estimated except the SEC Registration Fee.
| SEC Registration Fee |
$ | * | ||
| Legal Fees and Expenses |
* | |||
| Accounting Fees and Expenses |
* | |||
| Blue Sky Fees and Expenses |
* | |||
| Miscellaneous |
* | |||
|
|
|
|||
| Total |
$ | * | ||
|
|
|
| * | To be provided by amendment. |
| Item 14. | Indemnification of Directors and Officers. |
Our amended and restated certificate of incorporation and amended and restated bylaws provide that each person who was or is made a party or is threatened to be made a party to or is otherwise involved (including, without limitation, as a witness) in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he or she is or was one of our directors or officers or is or was serving at our request as a director, officer, employee or agent of another corporation, or of a partnership, joint venture, trust or other enterprise, including service with respect to an employee benefit plan, whether the basis of such proceeding is alleged action in an official capacity as a director, officer or trustee or in any other capacity while serving as a director, officer or trustee, shall be indemnified and held harmless by us to the fullest extent authorized by the Delaware General Corporation Law against all expense, liability and loss (including attorneys’ fees, judgments, fines, Employee Retirement Insurance Security Act excise taxes or penalties and amounts paid in settlement) reasonably incurred or suffered in connection with legal proceedings. These provisions limit the liability of our directors and officers to fullest extent permitted under Delaware law. A director or officer will not receive indemnification if he or she is found not to have acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interest.
Section 145 of the Delaware General Corporation Law permits a corporation to indemnify any director or officer of the corporation against expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with any action, suit or proceeding brought by reason of the fact that such person is or was a director or officer of the corporation, if such person acted in good faith and in a manner that he reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, if he or she had no reasonable cause to believe his or her conduct was unlawful. In a derivative action, (i.e., one brought by or on behalf of the corporation), indemnification may be provided only for expenses actually and reasonably incurred by any director or officer in connection with the defense or settlement of such an action or suit if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, except that no indemnification shall be provided if such person shall have been adjudged to be liable to the corporation, unless and only to the extent that the court in which the action or suit was brought shall determine that such person is fairly and reasonably entitled to indemnity for such expenses despite such adjudication of liability.
Pursuant to Section 102(b)(7) of the Delaware General Corporation Law, Article Eighth of our amended and restated certificate of incorporation eliminates the liability of a director to us or our stockholders for monetary damages for such a breach of fiduciary duty as a director, except for liabilities arising:
| • | from any breach of the director’s duty of loyalty to us or our stockholders; |
| • | from acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
| • | under Section 174 of the Delaware General Corporation Law; or |
| • | from any transaction from which the director derived an improper personal benefit. |
We carry insurance policies insuring our directors and officers against certain liabilities that they may incur in their capacity as directors and officers. We have entered into indemnification agreements with certain of our executive offices and directors. These agreements, among other things, indemnify and advance expenses to our directors and officers for certain expenses, including attorney’s fees, judgments, fines and settlement amounts incurred by any such person in any action or proceeding, including any action by us arising out of such person’s services as our director or officer, or any other company or enterprise to which the person provides services at our request. We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and officers. We have entered into agreements to indemnify all of our directors and officers.
II-1
Table of Contents
Additionally, reference is made to the Underwriting Agreement filed as Exhibit 1.1 hereto, which provides for indemnification by the underwriters of Amedica Corporation, our directors and officers who sign the registration statement and persons who control Amedica Corporation, under certain circumstances.
| Item 15. | Recent Sales of Unregistered Securities. |
Since January 1, 2011, we have sold the following securities that were not registered under the Securities Act.
(a) Issuances of Capital Stock and Warrants
The sale and issuance of the securities set forth below were deemed to be exempt from registration under the Securities Act by virtue of Section 4(2) or Rule 506 promulgated under Regulation D promulgated thereunder and Section 3(a)(9). Each of the recipients of securities in these transactions was an accredited investor within the meaning of Rule 501 of Regulation D under the Securities Act and had adequate access, through employment, business or other relationships, to information about us. No underwriters were involved in these transactions.
| • | Between March 4, 2011 and May 9, 2011 and on February 15, 2012, we issued aggregate principal amount of $29.8 million of Senior Secured Subordinated 6%/8% Convertible Promissory Notes, or the Senior Secured Notes, and warrants to purchase an aggregate of 6,250,000 shares of our common stock at an exercise price of $2.00 per share to 85 accredited investors. In connection with this sale, on May 9, 2011, we issued a warrant to purchase 57,577 shares of our common stock at an exercise price of $56.70 per share to a Creation Capital, LLC. |
| • | On May 10, 2012, in connection with entering into a settlement agreement, we issued 800,316 shares of our Series E convertible preferred stock and 2,557,562 shares of our Series C convertible preferred stock to the former stockholders of US Spine and 42,122 and 127,878 shares of our Series E convertible preferred stock to the advisor to US Spine as a further transaction fee payment. |
| • | On December 17, 2012, in connection with entering into a commercial lending transaction, we issued warrants to purchase 270,000 shares of our Series F convertible preferred stock at an exercise price of $2.00 per share to two of our institutional lenders. |
| • | On December 17, 2012, we issued 14,887,500 shares of our Series F convertible preferred stock upon conversion of all of the outstanding Senior Secured Notes. In connection with this conversion, on December 17, 2012, we issued a warrant to purchase 183,290 shares of our Series E convertible preferred stock at an exercise price of $2.20 per share to Creation Capital. |
| • | In March 2013, we issued an aggregate of 178,516 shares of our common stock to 33 accredited investors upon exercise of warrants and the sale of additional shares of our common stock to other investors at $17.53 per share for an aggregate purchase price of $3,128,802. We also issued each investor purchasing shares of our common stock through the exercise of warrants new warrants to purchase an aggregate of 76,455 shares of our common stock at an exercise price of $17.53 per share. |
| • | In 2013, we issued a total of 13,191 shares of our common stock at $17.53 per share to sales agents for equity incentive awards earned in 2011, 2012 and 2013 in connection with our obligations under a settlement agreement dated May 1, 2010. |
| • | On August 30, 2013 and September 20, 2013, we issued and sold a total of 94.8 units, each unit consisting of 50,000 shares of our Series F convertible preferred stock and a warrant to acquire 970 shares of our common stock at an exercise price of $25.77 per share, to 45 accredited investors at $100,000 per unit for an aggregate purchase price of $9,480,000. The purchase of these units resulted in our issuance of 4,740,000 shares of our Series F convertible preferred stock and warrants to purchase 91,951 shares of our common stock. In connection with this offering, we issued warrants to purchase an aggregate of 9,311 shares of our common stock, at an exercise price of $56.70 per share, to TGP Securities, Inc. |
| • | On March 20, 2014, we issued 50,000 shares of our common stock to a service provider for services previously rendered with respect to certain corporate development activities. |
| • | On June 30, 2014, we issued a warrant to purchase 516,129 shares of our common stock to Hercules Technology in connection with a Loan and Security Agreement. |
| • | On June 30, 2014, we issued a warrant to purchase warrants to purchase up to 568,889 shares of our common stock to the Selling Stockholder pursuant to a Securities Purchase Agreement. |
II-2
Table of Contents
(b) Certain Grants and Exercises of Stock Options
| • | In 2011, we granted options to purchase a total of 48,210 shares of our common stock to participants in our 2003 Stock Option Plan, at a weighted-average price of $25.77 per share. In 2011, we issued 667 shares of common stock upon the exercise of options to purchase such shares of common stock at a weighted-average price of $5.41 per share. |
| • | In 2012, we granted options to purchase a total of 90,450 shares of our common stock to participants in the 2003 Plan, at a weighted-average price of $25.77 per share. In 2012, we issued 450 shares of common stock upon the exercise of options to purchase such shares of common stock at a weighted-average price of $11.60 per share. |
| • | From January 1, 2013 through March 31, 2014, we granted a total of 1,928,046 RSUs and 179,699 options at exercise prices ranging from $5.68 to $25.77. During the same period, 60,719 options to purchase shares of common stock were exercised. |
Option grants, RSU grants and the issuances of common stock upon exercise of such options were exempt pursuant to Rule 701 and Section 4(2) of the Securities Act of 1933.
II-3
Table of Contents
| Item 16. | Exhibits and Financial Statement Schedules. |
(a) Exhibits
| Exhibit |
Exhibit Description |
Filed with this |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File/Reg. Number | |||||
| 3.1 | Restated Certificate of Incorporation of the Registrant | Form 8-K (Exhibit 3.1) |
2/20/14 | 001-33624 | ||||||
| 3.2 | Restated Bylaws of the Registrant | Form 8-K (Exhibit 3.1) |
2/20/14 | 001-33624 | ||||||
| 4.1 | Form of Common Stock Certificate of the Registrant | Amendment No. 3 to Form S-1 (Exhibit 4.1) |
1/29/14 | 333-192232 | ||||||
| 4.2 | Warrant Agreement by and between the Registrant and Creation Capital LLC, dated as of February 24, 2006 | Form S-1 (Exhibit 4.5) |
11/8/13 | 333-192232 | ||||||
| 4.3 | Series D Warrant Agreement by and between the Registrant and Creation Capital LLC, dated as of April 27, 2007 | Form S-1 (Exhibit 4.6) |
11/8/13 | 333-192232 | ||||||
| 4.4 | Common Stock Warrant Agreement by and between the Registrant and Creation Capital LLC, dated as of April 30, 2008 | Form S-1 (Exhibit 4.7) |
11/8/13 | 333-192232 | ||||||
| 4.5 | Series E Warrant Agreement by and between the Registrant and Creation Capital LLC, dated as of September 14, 2010 | Form S-1 (Exhibit 4.8) |
11/8/13 | 333-192232 | ||||||
| 4.6 | Form of Warrant to Purchase Shares of Common Stock of the Registrant, issued on May 9, 2011 | Amendment No. 3 to Form S-1 (Exhibit 4.9) |
1/29/14 | 333-192232 | ||||||
| 4.7 | Warrant to Purchase 156,978 Shares of Series F Convertible Preferred Stock by and between the Registrant and GE Capital Equity Investments, Inc., dated as of December 17, 2012 | Form S-1 (Exhibit 4.10) |
11/8/13 | 333-192232 | ||||||
| 4.8 | Warrant to Purchase 113,022 Shares of Series F Convertible Preferred Stock by and between the Registrant and Zions First National Bank, dated as of December 17, 2012 | Form S-1 (Exhibit 4.11) |
11/8/13 | 333-192232 | ||||||
| 4.9 | Form of Warrant to Purchase Shares of Common Stock of the Registrant, issued on March 4, 2011 and May 9, 2011 | Form S-1 (Exhibit 4.12) |
11/8/13 | 333-192232 | ||||||
| 4.10 | Form of Amendment to Warrant to Purchase Shares of Common Stock of the Registrant, dated as of December 18, 2012 | Form S-1 (Exhibit 4.13) |
11/8/13 | 333-192232 | ||||||
| 4.11 | Form of Amendment No. 2 to Warrant to Purchase Shares of Common Stock of the Registrant, dated as of February 1, 2013 | Form S-1 (Exhibit 4.14) |
11/8/13 | 333-192232 | ||||||
| 4.12 | Warrant to Purchase Shares of Common Stock of the Registrant by and between the Registrant and the University of Utah Research Foundation, dated as of February 17, 2010 | Form S-1 (Exhibit 4.15) |
11/8/13 | 333-192232 | ||||||
| 4.13 | Form of Warrant to Purchase Shares of Common Stock of the Registrant, issued on April 18, 2011, November 15, 2011, November 16, 2011, February 22, 2012, February 29, 2012 and March 7, 2012 | Form S-1 (Exhibit 4.16) |
11/8/13 | 333-192232 | ||||||
II-4
Table of Contents
| Exhibit |
Exhibit Description |
Filed with this |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File/Reg. Number | |||||
| 4.14 | Form of Warrant to Purchase Shares of Common Stock of the Registrant, issued on August 30, 2013 and September 20, 2013, as amended | Amendment No. 2 to Form S-1 (Exhibit 4.17) |
12/20/13 | 333-192232 | ||||||
| 4.15 | Form of Amendment to Warrant to Purchase Common Stock of the Registrant, dated as of December 23, 2013 | Amendment No. 3 to Form S-1 (Exhibit 4.17.1) |
1/29/14 | 333-192232 | ||||||
| 4.16 | Warrant to Purchase Shares of Common Stock of the Registrant by and between the Registrant and Zions First National Bank, dated as of March 17, 2011 | Form S-1 (Exhibit 4.18) |
11/8/13 | 333-192232 | ||||||
| 4.17 | Series E Warrant Agreement by and between the Registrant and Zions First National Bank, dated as of April 7, 2010 | Amendment No. 2 to Form S-1 (Exhibit 4.19) |
12/20/13 | 333-192232 | ||||||
| 4.18 | Form of Warrant to Purchase Shares of Common Stock of the Registrant, issued to TGP Securities, Inc. on August 30, 2013 and September 20, 2013, as amended | Amendment No. 2 to Form S-1 (Exhibit 4.20) |
12/20/13 | 333-192232 | ||||||
| 4.19 | Form of Amendment to Warrant to Purchase Shares of Common Stock of the Registrant, issued to TGP Securities, Inc., dated as of December 23, 2013 | Amendment No. 3 to Form S-1 (Exhibit 4.21) |
1/29/14 | 333-192232 | ||||||
| 4.21 | Form of Magna Senior Convertible Note | Form 8-K (Exhibit 4.1) |
7/1/2014 | 001-33624 | ||||||
| 4.22 | Magna Warrant to Purchase Common Stock | Form 8-K (Exhibit 4.2) |
7/1/2014 | 001-33624 | ||||||
| 4.3 | Hercules Warrant to Purchase Common Stock | Form 8-K (Exhibit 4.3) |
7/1/2014 | 001-33624 | ||||||
| 5.1 | Opinion of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., counsel to Amedica Corporation, with respect to the legality of the securities being registered | X | ||||||||
| 10.1 | Securities Purchase Agreement by and between the Registrant and MG Partners II Ltd, dated as of June 30, 2014 | Form 8-K (Exhibit 10.1) |
7/1/2014 | 001-33624 | ||||||
| 10.2 | Registration Rights Agreement by and between the Registrant and MG Partners II Ltd., dated as of June 30, 2014 | Form 8-K (Exhibit 10.2) |
7/1/2014 | 001-33624 | ||||||
| 10.3 | Loan and Security Agreement by and among the Registrant, its subsidiary, Hercules Technology Growth Capital, Inc., and Hercules Technology III, L.P., dated as of June 30, 2014 | Form 8-K (Exhibit 10.3) |
7/1/2014 | 001-33624 | ||||||
| 10.4 | Joint Development and License Agreement by and between the Registrant and Orthopaedic Synergy, Inc., dated as of February 8, 2010** | Amendment No. 3 to Form S-1 (Exhibit 10.7) |
1/29/14 | 333-192232 | ||||||
| 10.5 | Distribution Agreement by and between the Registrant and Orthopaedic Synergy, Inc., dated as of February 22, 2010, and First Amendment and Addendum thereto, dated as of November 1, 2012** | Amendment No. 3 to Form S-1 (Exhibit 10.8) |
1/29/14 | 333-192232 | ||||||
| 10.6 | Centrepointe Business Park Lease Agreement Net by and between the Registrant and Centrepointe Properties, LLC, dated as of April 21, 2009 | Form S-1 (Exhibit 10.10) |
11/8/13 | 333-192232 | ||||||
| 10.7 | First Addendum to Centrepointe Business Park Lease Agreement Net by and between the Registrant and Centrepointe Properties, LLC, dated as of January 31, 2012 | Form S-1 (Exhibit 10.11) |
11/8/13 | 333-192232 | ||||||
II-5
Table of Contents
| Exhibit |
Exhibit Description |
Filed with this |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File/Reg. Number | |||||
| 10.8 | Form of Severance and Change of Control Agreement* | Amendment No. 2 to Form S-1 (Exhibit 10.12) |
12/20/13 | 333-192232 | ||||||
| 10.9 | Employment Term Sheet by and between the Registrant and Jay M. Moyes, dated as of October 29, 2013* | Amendment No. 2 to Form S-1 (Exhibit 10.13) |
12/20/13 | 333-192232 | ||||||
| 10.10 | Restricted Stock Unit Agreement, by and between the Registrant and Jay Moyes, dated as of October 30, 2013* | Amendment No. 3 to Form S-1 (Exhibit 10.13.1) |
1/29/14 | 333-192232 | ||||||
| 10.11 | Form of Indemnification Agreement by and between the Registrant and its officers and directors* | Amendment No. 2 to Form S-1 (Exhibit 10.14) |
12/20/13 | 333-192232 | ||||||
| 10.12 | Amedica Corporation Amended and Restated 2012 Equity Incentive Plan* | Amendment No. 4 to Form S-1 (Exhibit 10.15) |
2/12/14 | 333-192232 | ||||||
| 10.13 | Form of 2012 Stock Option Grant Notice and Stock Option Agreement* | Amendment No. 4 to Form S-1 (Exhibit 10.16) |
2/12/14 | 333-192232 | ||||||
| 10.14 | Form of 2012 Restricted Stock Award and Restricted Stock Unit Agreement* | Amendment No. 4 to Form S-1 (Exhibit 10.17) |
2/12/14 | 333-192232 | ||||||
| 10.15 | Amedica Corporation 2003 Stock Option Plan* | Form S-1 (Exhibit 10.18) |
11/8/13 | 333-192232 | ||||||
| 10.16 | Form of 2003 Non-Qualified Stock Option Agreement and Notice of Exercise of Non-Qualified Stock Option thereunder* | Form S-1 (Exhibit 10.19) |
11/8/13 | 333-192232 | ||||||
| 10.17 | Form of 2003 Incentive Stock Option Agreement and Notice of Exercise of Incentive Stock Option thereunder* | Form S-1 (Exhibit 10.20) |
11/8/13 | 333-192232 | ||||||
| 16.1 | Letter of Ernst & Young, dated April 18, 2014 | Form S-1 (Exhibit 21.1) |
11/8/13 | 333-192232 | ||||||
| 21.1 | List of Subsidiaries of the Registrant | Form S-1 (Exhibit 21.1) |
11/8/13 | 333-192232 | ||||||
| 23.1 | Consent of Independent Registered Public Accounting Firm | X | ||||||||
| 23.2 | Consent of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo P.C. (included as part of Exhibit 5.1) | X | ||||||||
| 24.1 | Power of Attorney (included on the signature page to this registration statement) | X | ||||||||
| * | Management contract or compensatory plan or arrangement. | |||||||||
| ** | Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment and then filed separately with the SEC. | |||||||||
II-6
Table of Contents
| Item 17. | Undertakings |
| (a) | The undersigned registrant hereby undertakes: |
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or Controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
II-7
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Salt Lake City, Utah on July 16, 2014.
| AMEDICA CORPORATION | ||
| By: | /s/ Eric K. Olson | |
| Eric K. Olson Chief Executive Officer and President | ||
POWER OF ATTORNEY
We, the undersigned directors and officers of Amedica Corporation (the “Company”), hereby severally constitute and appoint Eric K. Olson and Kevin Ontiveros, and each of them singly, our true and lawful attorneys, with full power to them, and to each of them singly, to sign for us and in our names in the capacities indicated below, the registration statement on Form S-1 filed herewith, and any and all pre-effective and post-effective amendments to said registration statement, and any registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, in connection with the registration under the Securities Act of 1933, as amended, of equity securities of the Company, and to file or cause to be filed the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as each of us might or could do in person, and hereby ratifying and confirming all that said attorneys, and each of them, or their substitute or substitutes, shall do or cause to be done by virtue of this Power of Attorney.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities held on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Eric K. Olson Eric K. Olson |
Chief Executive Officer, President and Director (principal executive officer) |
July 16, 2014 | ||
| /s/ Jay M. Moyes Jay M. Moyes |
Chief Financial Officer and Director (principal financial and accounting officer) |
July 16, 2014 | ||
| /s/ Max E. Link Max E. Link, Ph.D. |
Chairman of the Board of Directors |
July 16, 2014 | ||
| /s/ B. Sonny Bal B. Sonny Bal, M.D. |
Director |
July 16, 2014 | ||
| /s/ David W. Truetzel David W. Truetzel |
Director |
July 16, 2014 | ||
| /s/ Jeffrey S. White Jeffrey S. White |
Director |
July 16, 2014 | ||
II-8
