Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Eloxx Pharmaceuticals, Inc. | v382658_8k.htm |
| EX-10.1 - EX-10.1 - Eloxx Pharmaceuticals, Inc. | v382658_ex10-1.htm |

Senesco Technologies, Inc. Corporate Overview OTC QB: SNTI BIO San Diego June, 2014 1

Certain statements included in this press release are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Actual results could differ materially from such statements expressed or implied herein as a result of a variety of factors, including, but not limited to: the Company’s ability to integrate the Fabrus science and operations; the Company’s ability to continue as a going concern; the Company’s ability to recruit patients for its clinical trial; the ability of the Company to consummate additional financings; the development of the Company’s gene and antibody technology; the approval of the Company’s patent applications; the current uncertainty in the patent landscape surrounding small inhibitory RNA and the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the success of the Company's license agreements; the acceptance by the market of the Company’s products; the timing and success of the Company’s preliminary studies, preclinical research and clinical trials; competition and the timing of projects and trends in future operating performance; and the quotation of the Company’s common stock on an over - the - counter securities market, as well as other factors expressed from time to time in the Company’s periodic filings with the Securities and Exchange Commission (the "SEC"). As a result, this press release should be read in conjunction with the Company’s periodic filings with the SEC. The forward - looking statements contained herein are made only as of the date of this press release, and the Company undertakes no obligation to publicly update such forward - looking statements to reflect subsequent events or circumstances. 2 Safe Harbor Statement

Senesco - Fabrus Update • Fabrus acquisition closed May 16, 2014 • Administrative and financial integration largely complete • Synergies: – targeted delivery of nanoparticles – Advanced biologics drug candidates and platforms • Detailed strategy for combined company to be announced early calendar Q3 3

Senesco is… • A clinical stage biologic therapeutics company with next generation antibody and gene regulation product candidates and platforms • Backed by world renowned investors and advisors – Pfizer – Opko Health – Phillip Frost – Chairman of TEVA, CEO of OPKO – Harlan Waksal – Founder of Imclone – Richard Lerner – Past president Scripps – Christopher Forbes – VP Forbes Media • Using underlying proprietary enabling technologies to discover new product candidates for an internal pipeline or for out - licensing 4

Harlan Waksal , M.D . (chair) Founder, Imclone Systems Phillip Frost, M.D. Chairman TEVA, CEO OPKO Christopher Forbes Vice President, Forbes Media, LLC Vaughn Smider , M.D., Ph.D. Acting CEO, Senesco John Braca Former partner SRone ( Glaxo Smith - Kline) David Rector Principal David Stephen Group Steven Rubin COO, OPKO Board of Directors 5

The Senesco Opportunity • Monoclonal antibodies - >$50B annual sales with 30+ FDA approved molecules in multiple therapeutic areas. – A proven drug class with a straightforward development path. – Senesco has a proprietary technology enabling discovery of first and best in class antibodies against certain large target classes • Nanoparticle gene regulation – Gene and siRNA therapies are an emerging paradigm in disease treatment – Senesco has a proprietary position in eIF5a regulatory pathways, as well as unique nanocage delivery systems 6

Pipeline • SNS01 - T - Nanoparticle for B - cell cancers – siRNA /Gene therapy targeting eIF5a – Induces apoptosis – Phase Ib / IIa trial expected to finish 2H 2014 – Multiple Myeloma, Diffuse Large Cell B - cell Lymphoma, Mantle Cell Lymphoma • FB001 - The world’s first therapeutic antibody against an ion channel – Based on novel cow antibody scaffold – Targets Kv1.3 on T - cells – Autoimmune/Inflammation indications – Unique mechanism of action - completely shuts off key inflammatory cytokines ( TNF a , IL - 17, etc.) without broad immunosuppression . – Phase I expected 2015/16 • FB002 – Anti - Renal Cell Carcinoma – Unique antibody targeting angiogenesis pathways important in anti - VEGF resistance – Potential applications in other cancers – Phase I expected 2016 7

Core Technologies • Monoclonal Antibody Discovery – Fully human antibodies (FB002) – Humanized cow antibody scaffold (FB001) – Addressed libraries for discovery against multipass membrane targets on cells • Broad technology platform targeting eIF5a – Critical cell survival switch (SNS01 - T) • Chimerasome nanocage delivery system 8

These two proteins act as a biological switch to promote cell death or survival CANCER Lysine F5A protein Cell Death amino acid Hypusine eIF5A Survival amino acid eIF5A ▪ Lysine and hypusine modifications control cell death and growth 9 SNS01 - T modulates eIF5A, Which Regulates Cell Growth and Death SNS01 - T

SNS01 - T Study Status • Cohorts 1 - 3 completed • The most frequent AEs are infusion reactions and thrombocytopenia • 2/4 patients in cohort 3 have stable disease after 6 weeks of therapy • Cohort 4 - Dose escalation to 0.375 mg/kg (6 patients). • 3 evaluable patients have completed cohort 4. • Results of cohort 4 expected 2H 2014 10
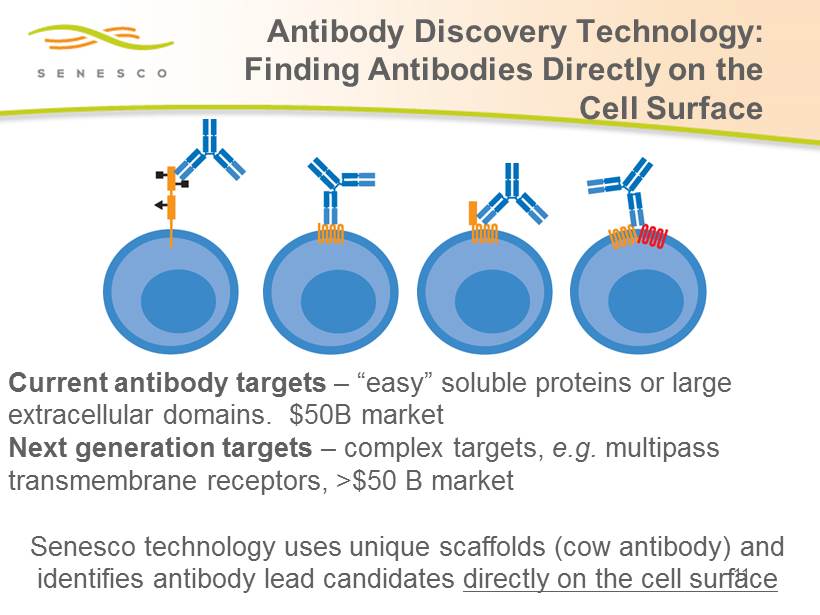
Antibody Discovery Technology: Finding Antibodies Directly on the Cell Surface Current antibody targets – “easy” soluble proteins or large extracellular domains. $50B market Next generation targets – complex targets, e.g. multipass transmembrane receptors, >$50 B market Senesco technology uses unique scaffolds (cow antibody) and identifies antibody lead candidates directly on the cell surface 11

Cow Antibodies: a Novel Scaffold for Targeting Membrane Proteins Wang et.al. (2013) Cell 153 : 1379 - 1393 (featured on the cover) Human Cow Ultralong binding region 12

Engineered Cow Antibodies Targeting Ion Channels Kv1.3 Ion channel IL - 17 (A450, sem ) Inhibition of human effector - memory T - cells Including IL - 17, TNF, and others at pM IC 50 s Autoimmune/Inflammation 13

Future Plans • Advance two antibodies to the clinic in 18 - 24 months • Establish robust pipeline of antibody and nanoparticle biologics • Further develop SNS01 - T • Drug development partnerships • In - license new product candidates and synergistic technologies • Goal: become the preeminent advanced biologics company with best and first in class product candidates and technologies 14

• World - class science, management team, and board • Dr. Harlan Waksal , Founder of ImClone • Dr. Phillip Frost, Chairman of Teva • Near - term clinical trial results from Phase 1b/2a study • Combination with Fabrus provides “product engine” in next generation antibodies • cow antibody coupled with discovery technology enables antibodies against difficult transmembrane targets • Multi - billion opportunity in several therapeutic areas 15 Summary

Corporate Information Senesco Technologies, Inc. 721 Route 202/206, Suite 130 Bridgewater, NJ 08807 Phone: 908 - 864 - 4444 www.senesco.com OTCQB: SNTI 16
