Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Cellectar Biosciences, Inc. | v380716_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Cellectar Biosciences, Inc. | v380716_ex99-1.htm |

A Novel “Diapeutic” Molecular Agent for Combined Oncologic Diagnosis and Therapy in a Broad Spectrum of Human Cancers: Preliminary Clinical Experience with CLR1404 Pickhardt PJ, Lee MH, Longino M, Pinchuk A, Banach M, Grudzinsk J, Titz B, Jaskowiak C, Traynor AM, Kuo JS, Weichert JP, Hall LT From the Departments of Radiology, Medical Physics, Internal Medicine, Neurologiical Surgery, and Carbone Cancer Center, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA; and Cellectar Biosciences, Madison, Wisconsin, USA.

Disclosure of Potential COI The following co - authors either have or recently had a financial relationship with the following commercial organizations: • PJ Pickhardt: Viatronix, Braintree, Mindways , VirtuoCTC , Cellectar • M Longino, A Pinchuk, M Banach , J Grudzinski , B Titz , C Jaskowiak: Cellectar • JP Weichert is Founder & Chief Science Officer of Cellectar Funding for the imaging studies were supported by the NCI (R01 - 158800), UW Institute for Clinical and Translational Research pilot grant (9U54TR000021), and Cellectar Biosciences Presented by: Perry J. Pickhardt, MD

Background Presented by: Perry J. Pickhardt, MD O(CH 2 ) n OPOCH 2 CH 2 N + Me 3 CH 3 (CH 2 ) 16 CO O O - O I X X= 125,124,131 I • CLR1404 – an alkylphosphocholine analog • Capitalizes on over - abundance of phospholipid ethers present in most cancer cells

Background Presented by: Perry J. Pickhardt, MD Tumor targeting Imaging label • Tumor - targeting not affected by iodine label • PET tumor imaging with 124 I - CLR1404 • Molecular radiotherapy with 131 I - CLR1404 • Potential for both imaging diagnosis and therapy = “ diapeutic ” (same agent) • Distinct from a “ theranostic ” approach
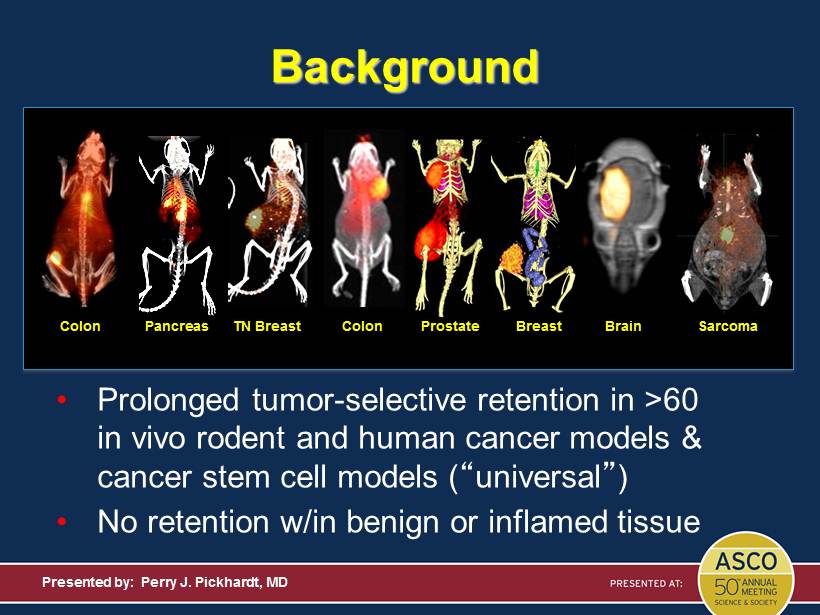
Background Presented by: Perry J. Pickhardt, MD • Prolonged tumor - selective retention in >60 in vivo rodent and human cancer models & cancer stem cell models ( “ universal ” ) • No retention w/in benign or inflamed tissue Colon TN Breast Pancreas Prostate Breast Colon Brain Sarcoma

Background Presented by: Perry J. Pickhardt, MD • Significant tumor growth reduction and survival benefit from a single injection of 131 I - CLR1404 in a wide range of human tumor xenograft models • Weichert JP et al. Sci Trans Med (in press)

Purpose R eport our initial experience with CLR1404 for localization and imaging in a broad spectrum of cancers in early human trials • PET/CT imaging with 124 I - CLR1404 - Oncologic imaging; compare with 18 FDG PET • SPECT/CT imaging with 131 I - CLR1404 - Therapeutic form of this “ diapeutic ” agent Presented by: Perry J. Pickhardt, MD

Methods • IRB - approved prospective imaging protocols • All patients gave signed informed consent • Early phase trials with 124 I - CLR1404 PET and subtherapeutic 131 I - CLR1404 SPECT • Main inclusion criterion: biopsy - proven refractory advanced solid malignancy - Separate trial of primary brain tumors excluded Presented by: Perry J. Pickhardt, MD

Methods • 124 I - CLR1404 PET/CT scans: • 64 - detector - row PET/CT scanner (Discovery VCT, GE Healthcare, Waukesha, WI) • Serial imaging out to 5 - 10 days following the injection of up to 5 mCi of 124 I - CLR1404 • 2D acquisition mode • No correction employed for the 124 I cascade gammas • Low - dose non - contrast MDCT for attenuation correction and lesion localization using 140 kV p and tube current modulation (70 mA average ) Presented by: Perry J. Pickhardt, MD

Methods • 131 I - CLR1404 SPECT/CT scans: • Serial imaging ( Infinia /Hawkeye , GE Healthcare) out 21 days • Phase I dosimetry trial not designed to show therapeutic benefit • Non - contrast low - dose CT was performed using 140 kV p and 2.5 mA Presented by: Perry J. Pickhardt, MD

Methods • Review of imaging studies: • All PET/CT and SPECT/CT studies were reviewed on PACS workstation (McKesson) with fusion software (Mirada XD3) • Correlation with concurrent 18 FDG PET/CT in most cases • Additional relevant cross - sectional imaging studies were also reviewed Presented by: Perry J. Pickhardt, MD

Initial Findings Study Cohort: 22 patients with metastatic cancer • Mean age, 60.4 years; 12M, 10F • Complex prior treatment histories • Tumor types: bronchogenic carcinoma (n=7), colorectal cancer (n=4), prostate cancer (n=3), triple - negative breast cancer (n=2), esophageal cancer (n=2), head & neck squamous cell carcinoma (n=2), pancreatic cancer (n=1), and melanoma (n=1 ) Presented by: Perry J. Pickhardt, MD

Initial Findings 124 I - CLR1404 PET/CT in 14 patients and 131 I - CLR1404 SPECT/CT in 9 patients • Preferential uptake of 124 I - and 131 I - CLR1404 within metastatic foci with all cancer subtypes • Persistent retention within metastatic sites, coupled with progressive washout of background activity, favored delayed imaging (6 - 21 days after single injection). Presented by: Perry J. Pickhardt, MD

Initial Findings 124 I - CLR1404 PET/CT in 14 patients and 131 I - CLR1404 SPECT/CT in 9 patients • CLR1404 uptake was evident in pulmonary, nodal, skeletal, hepatic, CNS, and other sites of active metastatic disease • Potential advantages in oncologic imaging over FDG PET included both fewer false - negatives and fewer post - treatment false - positives Presented by: Perry J. Pickhardt, MD

70M with bronchogenic carcinoma 124 I - CLR1404 PET 18 F - FDG PET Post - Contrast MR 124 I - CLR1404 PET

60F with recurrent malignant melanoma 124 I - CLR1404 PET 18 F - FDG PET Follow - up MR Post - Contrast MR

48M with colorectal carcinoma 131 I - CLR1404 SPECT/CT Post - Contrast CT

57F with colorectal carcinoma 131 I - CLR1404 SPECT Post - Contrast CT 131 I - CLR1404 SPECT/CT Post - Contrast CT
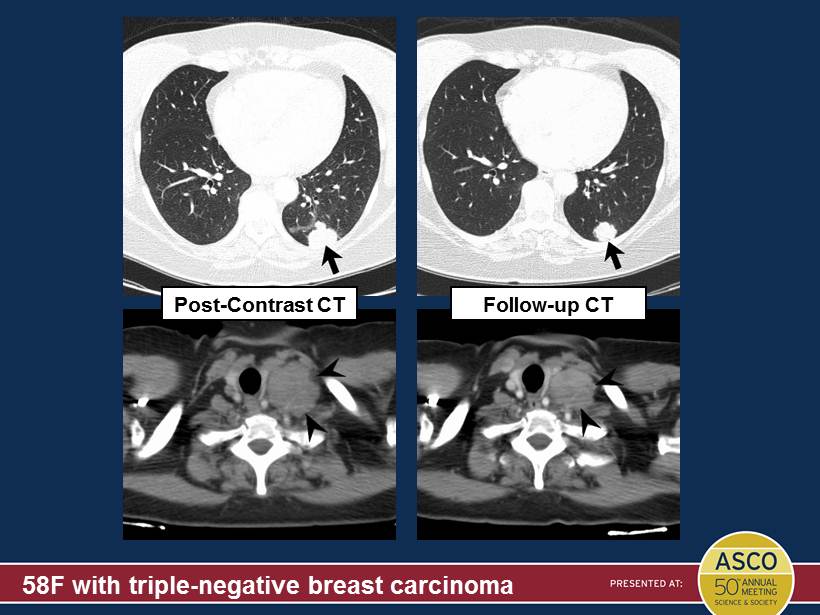
58F with triple - negative breast carcinoma Post - Contrast CT Follow - up CT

65M with bronchogenic carcinoma 124 I - CLR1404 PET/CT 18 F - FDG PET/CT 124 I - CLR1404 PET/CT
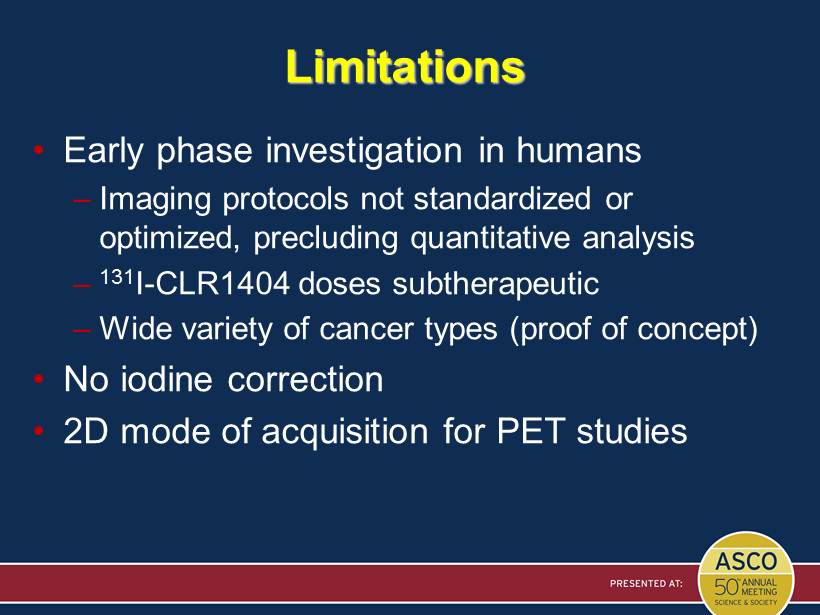
Limitations • Early phase investigation in humans – Imaging protocols not standardized or optimized, precluding quantitative analysis – 131 I - CLR1404 doses subtherapeutic – Wide variety of cancer types (proof of concept) • No iodine correction • 2D mode of acquisition for PET studies

Further Research Opportunities • Selective tumor uptake of CLR1404 with prolonged retention within a broad spectrum of historically difficult - to - treat metastatic cancers – Regardless of the site of metastatic disease • Potential advantages over FDG PET observed: – Detection in cases of FDG false - negatives – Lack of uptake in cases of FDG false - positives – 124 I - CLR1404 may improve accuracy for oncologic PET imaging
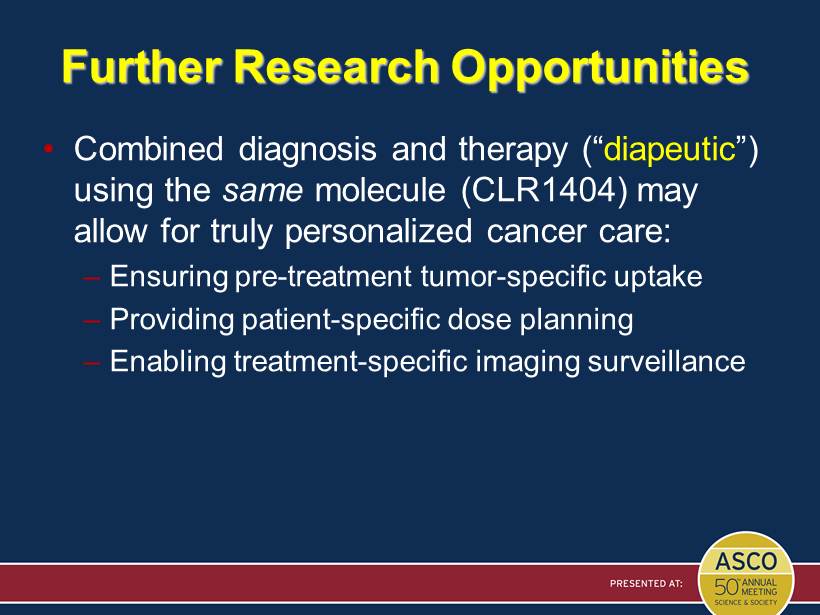
Further Research Opportunities • Combined diagnosis and therapy (“ diapeutic ” ) using the same molecule (CLR1404) may allow for truly personalized cancer care: – Ensuring pre - treatment tumor - specific uptake – Providing patient - specific dose planning – Enabling treatment - specific imaging surveillance

Diapeutic Treatment Paradigm 124 I - CLR404 PET/CT Distribution, Quantification, & Personal Dose Calculation 131 I - CLR1404 Therapy Dose Injection Monitor Response w/ 124 I - CLR404 PET/CT

Thank You
