Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Cellectar Biosciences, Inc. | v380716_8k.htm |
| EX-99.2 - EXHIBIT 99.2 - Cellectar Biosciences, Inc. | v380716_ex99-2.htm |
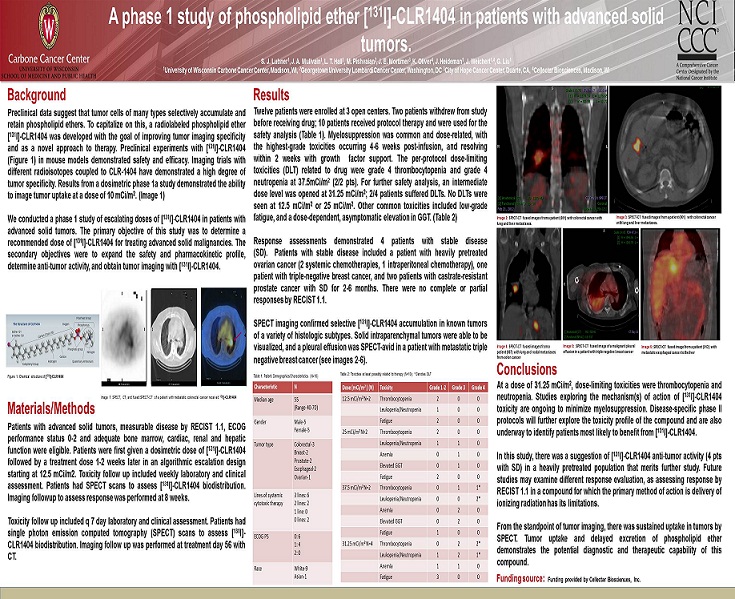
Background Materials/Methods Results Conclusions Funding source: Funding provided by Cellectar Biosciences, Inc. A phase 1 study of phospholipid ether [ 131 I] - CLR1404 in patients with advanced solid tumors. S. J. Lubner 1 , J. A. Mullvain 1 , L. T. Hall 1 , M. Pishvaian 2 , J. E. Mortimer 3 , K. Oliver 4 , J. Heideman 1 , J. Weichert 1,4 , G. Liu 1 1 University of Wisconsin Carbone Cancer Center, Madison, WI, 2 Georgetown University Lombardi Cancer Center, Washington, DC 3 City of Hope Cancer Center, Duarte, CA, 4 Cellectar Biosciences, Madison, WI Preclinical data suggest that tumor cells of many types selectively accumulate and retain phospholipid ethers . To capitalize on this, a radiolabeled phospholipid ether [ 131 I] - CLR 1404 was developed with the goal of improving tumor imaging specificity and as a novel approach to therapy . Preclinical experiments with [ 131 I] - CLR 1404 (Figure 1 ) in mouse models demonstrated safety and efficacy . Imaging trials with different radioisotopes coupled to CLR - 1404 have demonstrated a high degree of tumor specificity . Results from a dosimetric phase 1 a study demonstrated the ability to image tumor uptake at a dose of 10 mCi/m 2 . (Image 1 ) We conducted a phase 1 study of escalating doses of [ 131 I] - CLR 1404 in patients with advanced solid tumors . The primary objective of this study was to determine a recommended dose of [ 131 I] - CLR 1404 for treating advanced solid malignancies . The secondary objectives were to expand the safety and pharmacokinetic profile, determine anti - tumor activity, and obtain tumor imaging with [ 131 I] - CLR 1404 . At a dose of 31 . 25 mCi/m 2 , dose - limiting toxicities were thrombocytopenia and neutropenia . Studies exploring the mechanism(s) of action of [ 131 I] - CLR 1404 toxicity are ongoing to minimize myelosuppression . Disease - specific phase II protocols will further explore the toxicity profile of the compound and are also underway to identify patients most likely to benefit from [ 131 I] - CLR 1404 . In this study, there was a suggestion of [ 131 I] - CLR 1404 anti - tumor activity ( 4 pts with SD) in a heavily pretreated population that merits further study . Future studies may examine different response evaluation, as assessing response by RECIST 1 . 1 in a compound for which the primary method of action is delivery of ionizing radiation has its limitations . From the standpoint of tumor imaging, there was sustained uptake in tumors by SPECT . Tumor uptake and delayed excretion of phospholipid ether demonstrates the potential diagnostic and therapeutic capability of this compound . Twelve patients were enrolled at 3 open centers . Two patients withdrew from study before receiving drug ; 10 patients received protocol therapy and were used for the safety analysis (Table 1 ) . Myelosuppression was common and dose - related, with the highest - grade toxicities occurring 4 - 6 weeks post - infusion, and resolving within 2 weeks with growth factor support . The per - protocol dose - limiting toxicities (DLT) related to drug were grade 4 thrombocytopenia and grade 4 neutropenia at 37 . 5 mCi/m 2 ( 2 / 2 pts) . For further safety analysis, an intermediate dose level was opened at 31 . 25 mCi/m 2 ; 2 / 4 patients suffered DLTs . No DLTs were seen at 12 . 5 mCi/m 2 or 25 mCi/m 2 . Other common toxicities included low - grade fatigue, and a dose - dependent, asymptomatic elevation in GGT . (Table 2 ) Response assessments demonstrated 4 patients with stable disease (SD) . Patients with stable disease included a patient with heavily pretreated ovarian cancer ( 2 systemic chemotherapies, 1 intraperitoneal chemotherapy), one patient with triple - negative breast cancer, and two patients with castrate - resistant prostate cancer with SD for 2 - 6 months . There were no complete or partial responses by RECIST 1 . 1 . SPECT imaging confirmed selective [ 131 I] - CLR 1404 accumulation in known tumors of a variety of histologic subtypes . Solid intraparenchymal tumors were able to be visualized, and a pleural effusion was SPECT - avid in a patient with metastatic triple negative breast cancer (see images 2 - 6 ) . Image 2: SPECT - CT fused images from a patient (001) with colorectal cancer with lung and liver metastases. Patients with advanced solid tumors, measurable disease by RECIST 1 . 1 , ECOG performance status 0 - 2 and adequate bone marrow, cardiac, renal and hepatic function were eligible . Patients were first given a dosimetric dose of [ 131 I] - CLR 1404 followed by a treatment dose 1 - 2 weeks later in an algorithmic escalation design starting at 12 . 5 mCi/m 2 . Toxicity follow up included weekly laboratory and clinical assessment . Patients had SPECT scans to assess [ 131 I] - CLR 1404 biodistribution . Imaging followup to assess response was performed at 8 weeks . Toxicity follow up included q 7 day laboratory and clinical assessment . Patients had single photon emission computed tomography (SPECT) scans to assess [ 131 I] - CLR 1404 biodistribution . Imaging follow up was performed at treatment day 56 with CT . Characteristic N Median age 55 (Range 40 - 70) Gender Male - 5 Female - 5 Tumor type Colorectal - 3 Breast - 2 Prostate - 2 Esophageal - 2 Ovarian - 1 Lines of systemic cytotoxic therapy 3 lines: 6 2 lines: 2 1 line: 0 0 lines: 2 ECOG PS 0: 6 1: 4 2: 0 Race White - 9 Asian - 1 Dose (mCi/m 2 ) (N) Toxicity Grade 1 - 2 Grade 3 Grade 4 12.5 mCi/m 2 N=2 Thrombocytopenia 2 0 0 Leukopenia/Neutropenia 1 0 0 Fatigue 2 0 0 25 mCi/m 2 N=2 Thrombocytopenia 2 0 0 Leukopenia/Neutropenia 1 1 0 Anemia 0 1 0 Elevated GGT 0 1 0 Fatigue 2 0 0 37.5 mCi/m 2 N=2 Thrombocytopenia 0 1 1* Leukopenia/Neutropenia 0 0 2* Anemia 0 2 0 Elevated GGT 0 2 0 Fatigue 1 0 0 31.25 mCi/m 2 N=4 Thrombocytopenia 0 2 2* Leukopenia/Neutropenia 1 2 1* Anemia 1 1 0 Fatigue 3 0 0 Table 1: Patient Demographics/Characteristics (N=10) Table 2: Toxicities at least possibly related to therapy (N=10); * Denotes DLT Image 4: SPECT - CT fused images from a patient (007) with lung and nodal metastases from colon cancer Image 5: SPECT - CT fused image of a malignant pleural effusion in a patient with triple negative breast cancer Image 6: SPECT - CT fused image from a patient (012) with metastatic esophageal cancer to the liver Image 3: SPECT - CT fused images from a patient (001) with colorectal cancer with lung and liver metastases. Figure 1: Chemical structure of [ 131 I] - CLR1404 Image 1: SPECT, CT, and fused SPECT - CT of a patient with metastatic colorectal cancer received 131 I] - CLR1404
