Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ignyta, Inc. | d734860d8k.htm |
| EX-99.1 - EX-99.1 - Ignyta, Inc. | d734860dex991.htm |
 Phase
1 Open Label, Dose Escalation Study of RXDX-101, an Oral Pan-Trk,
ROS1, and ALK Inhibitor, in Patients with Advanced Solid
Tumors with Relevant Molecular Alterations
Filippo G. De Braud
1
, Lorenzo Pilla
1
, Monica Niger
1
, Silvia
Damian
1
, Benedetta Bardazza
1
, Antonia Martinetti
1
, Giuseppe
Pelosi
1
, Giovanna Marrapese
2
, Laura Palmeri
2
, Giulio Cerea
2
,
Emanuele Valtorta
2
, Silvio Veronese
2
, Andrea Sartore-Bianchi
2
,
Elena Ardini
3
, Marcella Martignoni
4
, Arturo Galvani
3
, Paul
Pearson
5
, David Luo
5
, James L. Freddo
5
,
and Salvatore Siena
2
1
Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy;
2
Ospedale Niguarda Ca' Granda, Milan, Italy;
3
Nerviano Medical Sciences, Nerviano, Italy;
4
CLInical Organization for Strategies & Solutions (CLIOSS),
NMS Group, Nerviano, Italy;
5
Ignyta, Inc., San Diego, CA
Exhibit 99.2 |
 Disclosures
Consultant or Advisory Role
Research Funding
Presented by: Filippo De Braud
•
Novartis ; Servier ; Amgen ; Pierre Fabre
•
Bristol-Myers Squibb ; Boehringer Ingelheim;
•
Roche
•
Nerviano Medical Sciences; Novartis ; Servier ; Amgen ;
Pierre Fabre; Bristol-Myers Squibb ; Boehringer
Ingelheim; Roche |
 RXDX-101: Next Generation Kinase Inhibitor
RXDX-101 is a potent, selective, orally available ATP-competitive
inhibitor of 5 target oncogenic drivers
In vitro,
RXDX-101
demonstrates
inhibition
of
targets
as
well
as
downstream
effectors
in the MAPK and PI3K/AKT pathways
-
Active against the most common ALK mutants responsible for crizotinib
resistance In
vivo,
RXDX-101
demonstrates
tumor
eradication
and
tumor
growth
inhibition
in
multiple models
-
Crosses the blood brain barrier, enabling targeting of CNS lesions
3
Target
TrkA
TrkB
TrkC
ROS1
ALK
IC50 (nM)
1.7
0.1
0.1
0.2
1.6
Presented by: Filippo De Braud |
 Trk
Signaling: Activation Drives Cell Growth and Survival
Brodeur GM, et al. Clin Cancer Res 2009
NTRK1
encodes the TrkA receptor tyrosine kinase
Trk family of neurotrophin receptors (TrkA, TrkB, TrkC) play a role in
development of the central and peripheral nervous system
4
Presented by: Filippo De Braud
TrkA activates
PI3K/AKT, PKC and
ERK1/2 pathways,
which promote cell
growth and survival |
 Trk
Rearrangements in Human Malignancy TM
Kinase Domain
NTRK1
MPRIP-NTRK1
CD74-NTRK1
NSCLC
TPM3-NTRK1
TFG-NTRK1
TPR-NTRK1
Papillary Thyroid
Cancer
TPM3-NTRK1
Colorectal Cancer
Presented by: Filippo De Braud
5
NTRK1, NTRK2 or NTRK3 fusions have also been identified
in Glioblastoma, AML and secretory breast cancer |
 RXDX-101: Robust Activity In Vitro and In Vivo
against TrkA Rearranged Colorectal Cancer
6
KM-12 is a human CRC line driven by a constitutively active TrkA fusion
protein RXDX-101 potently inhibits TrkA phosphorylation and downstream
signaling RXDX-101 induces in vivo tumor regression and durable
tumor stabilization Continuous dosing treatments started at
Day 9, bid; 21 treatments completed
Presented by: Filippo De Braud |
 NCI-H2228 (ALK-driven NSCLC) cells were injected intracranially;
Mice were treated orally with RXDX-101 at 120 mg/kg BID for 10 days
RXDX-101 Effective in
NSCLC Brain Metastases Model
7
RXDX-101
120 mg/kg os BID
Vehicle
Presented by: Filippo De Braud
Pre-Treatment
After 10 D of treatment
Median
Survival
Control
33.5 days
RXDX-101
56.5 days
P-value:
<5x10e
-4 |
 ALKA-372-001: Phase I Dose Escalation Study of
RXDX-101 in Adult Patients with Advanced Solid Tumors
•
Patient population: advanced metastatic solid tumors, with
alterations to TrkA, ROS1 or ALK
•
Dosing schedule: oral administration once daily 4 days a
week (4 days on, 3 days off) for 3 consecutive weeks
followed by 1 week of rest, repeated in 28 day cycles
•
Dose cohorts: 100, 200, 400, 800, 1200, 1600 mg/m
2
•
Primary objectives: DLT, MTD, RP2D
•
Secondary objectives: safety, PK, antitumor activity by
RECIST
•
N= 20 enrolled, 19 dosed
Presented by: Filippo De Braud |
 Demographics and Baseline Characteristics
Presented by: Filippo De Braud |
 *
7 had failed prior treatment w/ crizotinib Histology and Molecular
Alteration Presented by: Filippo De Braud |
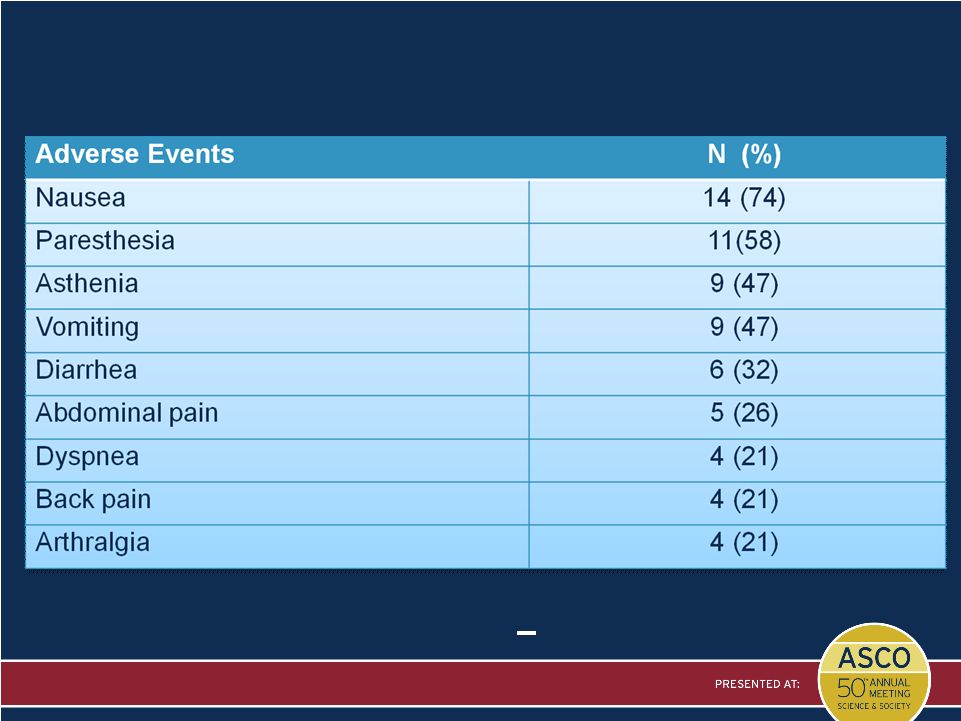 Most
Common Adverse Events* * Frequency > 20%
Presented by: Filippo De Braud |
 Grade 3 / 4 Adverse Events
* Possibly drug related
No DLT observed at any dose level
No drug related SAEs
Presented by: Filippo De Braud |
 RXDX-101 Steady State PK Profile
Suitable for Once Daily Dosing
Exposure to RXDX-101
increased in an
approximate dose
proportional manner up
to doses of 800 mg/m
2
Mean plasma half-life
ranged from 17-
44
hours
Presented by: Filippo De Braud |
 Observed Clinical Responses in Each of TrkA,
ROS1 and ALK Patients
* Confirmed PR
Presented by: Filippo De Braud
13 additional patients had progressive disease |
       Presented by: Filippo De Braud
Partial Response in Patient with
TrkA Rearranged Colorectal Cancer
Patient
screened
and
treated
at
Ospedale
Niguarda
Ca’
Granda,
Milan,
Italy
Cycle 1
April 23, 2014
Pre Treatment
March 20, 2014 |
  Partial Response in Patient with
ALK+ Neuroblastoma
Presented by: Filippo De Braud
Cycle 15
Dose escalated to 1200 mg/m
2
Pre Treatment
February 5, 2013
April 14, 2014 |
 RXDX-101: Novel Multi-Kinase Inhibitor with
Promising Early Clinical Data
•
RXDX-101 is a potent, selective inhibitor of key oncogenic
kinases: Trk family, ROS1 and ALK
•
Observed partial responses in patients with each of TrkA,
ROS1 and ALK alterations
•
Well tolerated at doses up to 1600 mg/m
2
on an
intermittent schedule
•
PK profile suitable for appropriate target coverage with
once daily dosing
•
Currently testing continuous daily dosing
Presented by: Filippo De Braud |
 Acknowledgements
•
Participating patients and their families
•
Fondazione IRCCS Istituto Nazionale dei Tumori,
Milan, Italy
•
Ospedale Niguarda Ca' Granda
(PI: Salvatore Siena, MD), Milan, Italy
•
Nerviano Medical Sciences, Nerviano, Italy
•
Clinical Organization for Strategies & Solutions
•
Ignyta, Inc., San Diego, CA
Presented by: Filippo De Braud |
