Attached files
| file | filename |
|---|---|
| 8-K - 8-K - XENOPORT INC | d729271d8k.htm |
 ©
Copyright 2014 XenoPort, Inc. All rights reserved.
NASDAQ:XNPT
Exhibit 99.1
The Right Strategy and Board to Build
Stockholder Value |
 These slides and the accompanying oral presentation by XenoPort,
Inc.
contain forward-looking statements that involve risks and uncertainties,
including statements relating to the commercial opportunity and value
proposition for HORZIANT; potential future
sales and commercialization
activity for HORIZANT; the XP23829 clinical development program,
including the initiation or conduct of planned or potential future clinical
trials and regulatory submissions and the timing thereof; expected patent
coverage; the anticipated sufficiency of XenoPort's cash reserves to fund
its operations through 2015; and the therapeutic and commercial potential
of XenoPort’s product candidates, including XP23829. XenoPort can give
no assurance with respect to these statements, and we assume no
obligation to update them.
For detailed information about the risks and
uncertainties that could cause actual results to differ materially from those
implied by, or anticipated in, these forward-looking statements, please
refer to the Risk Factors section of our Quarterly Report on Form 10-Q
for the quarter ended March 31, 2014 and filed with the SEC.
2
XenoPort Investor Presentation
May 2014
Safe Harbor Language
Safe Harbor Language |
 XenoPort, Inc., its directors and certain of its executive officers may be deemed
to be participants
in
the
solicitation
of
proxies
from
stockholders
in
connection
with
XenoPort’s
2014
Annual Meeting of Stockholders. XenoPort has filed with the SEC and provided to its
stockholders
a
definitive
proxy
statement
and
a
WHITE
proxy
card
in
connection
with
such
solicitation. XENOPORT STOCKHOLDERS ARE STRONGLY ENCOURAGED TO READ THE
PROXY STATEMENT (INCLUDING ANY AMENDMENTS AND SUPPLEMENTS) AND THE
ACCOMPANYING
WHITE
PROXY
CARD,
AND
ANY
OTHER
RELEVANT
DOCUMENTS WHEN
THEY BECOME AVAILABLE, BECAUSE THEY CONTAIN IMPORTANT INFORMATION.
Information regarding the names of XenoPort’s directors and executive officers
and their respective interests in XenoPort by security holdings or otherwise
is set forth in XenoPort’s definitive proxy statement for the 2014
Annual Meeting of Stockholders, filed with the SEC on April 22, 2014,
including Appendix B thereto. The definitive proxy statement (and amendments
or supplements thereto) and the accompanying WHITE
proxy
card,
and
any
other
relevant
documents
and
other
material
filed
by
XenoPort
with
the
SEC,
are
or
will
be
available
for
no
charge
at
the
SEC’s
website
at
www.sec.gov
and
at
XenoPort’s investor relations website at
http://investor.xenoport.-com/index.cfm. Copies may also be obtained
free of charge by contacting XenoPort Investor Relations by mail at 3410 Central
Expressway, Santa Clara, California 95051 or by telephone at (408)
616-7200. Additional Information
Additional Information
3
XenoPort Investor Presentation
May 2014 |
 Clinton’s Agenda
Clinton’s Agenda
Clinton expressed disagreement with XenoPort Board’s
capital allocation decisions
Proposed in October 2013 and continues to recommend that
XenoPort stop the commercialization of HORIZANT
Insists that XenoPort focus all of its resources on XP23829, a
compound that has yet to enter clinical studies in patients
Insists that Board replace CEO
4
XenoPort Investor Presentation
May 2014 |
 Agenda
Agenda
XenoPort overview
Creating value in HORIZANT
®
XP23829 development execution
Monetizing/creating opportunity for other assets
XenoPort’s Board recommendations
5
XenoPort Investor Presentation
May 2014 |
 XenoPort Overview |
 Background on XenoPort
Background on XenoPort
Founded in 1999; IPO in 2005
92 full-time employees at December 31, 2013
Developed innovative biology/chemistry platform to improve drug efficacy,
tolerability, compliance Discovered and developed 4 patented mid/late stage
or marketed compounds XenoPort Investor Presentation
May 2014
7 |
 $122.5 million of cash, cash
equivalents and short-term
investments at 3/31/14
Operations expected to be funded
through 2015
Additional $25 million in non-dilutive
cash expected in 2014 associated
with licensing agreement
announced 5/15/14*
No debt
All financial data as of March 31, 2014.
8
*Subject to antitrust clearance of transaction
XenoPort Investor Presentation
May 2014
Financials
Financials |
 9
Ronald W. Barrett, Ph.D.
Chief Executive Officer
Founder, former Chief Scientific Officer.
More than 25 years of executive management and R&D
experience in the pharmaceutical industry, Glaxo, Affymax, Abbott
Richard Kim, M.D.
Senior VP, Chief
Medical Officer
More than a decade of
experience in both clinical
development and medical
affairs, including head of
the MS therapeutic area at
Elan and global medical
director, medical affairs at
Biogen Idec
Greg Bates, D.V.M.
Senior VP, Regulatory
Affairs and Quality
25 years regulatory affairs
experience, Pharmacyclics,
Otsuka, Genentech, Syntex.
Vincent Angotti
Executive VP and
Chief Operating
Officer
Over 20 years sales,
marketing, operations
experience. Relaunched
numerous drugs as
executive at Reliant Pharma.
Operations roles at Novartis.
William Harris
Chief Financial Officer
Over 25 years finance experience. CFO at
Coulter (acquired by Corixa Corp). Senior
level finance positions at Gilead. MBA,
Santa Clara University.
Dave Savello, Ph.D.
Senior VP of
Development
Operations
Over 30 years broad pharma
industry experience.
Executive roles in R&D,
operations, regulatory at
Cardinal Health, Guilford,
Glaxo, Boehringer Ingelheim
and 3M Company.
Gianna M. Bosko
Senior VP, Chief Legal Officer
and Secretary
~20 years industry experience. Previously
at Cooley LLP, general corporate and
securities law. J.D., University of Chicago
Law School.
XenoPort Investor Presentation
May 2014
Experienced Management Team
Experienced Management Team |
 10
9 highly-qualified and proven leaders
8 independent directors
3 new directors have joined the Board over the past 5 years
Average tenure of 8.7 years versus average of ~10.1 years
for the S&P 500, ~10.8 years for the S&P 1500, ~10.7 years
for the S&P MidCap index and ~11.5 years for the S&P
SmallCap index¹
22 Board meetings held in 2013 as part of efforts to oversee
XenoPort’s strategy, to monitor progress, to consider strategic
options and to build stockholder value
1
Source:
ISS,
“Director
Tenure
and
Corporate
Governance
Features,”
By
Rob
Yates,
March
25,
2014.
XenoPort Investor Presentation
May 2014
XenoPort’s Current Board Members are
XenoPort’s Current Board Members are
Independent, Qualified and Active
Independent, Qualified and Active |
 John Freund, M.D., Lead Independent Director
Managing Director: Skyline Ventures
Partner: Chancellor Capital (INVESCO). Morgan Stanley Venture Partners
Co-founder: Intuitive Surgical
Former Executive: Acuson
Jeryl Hilleman,
Audit Committee Chair
Ernest Mario, Ph.D.*
Dennis Fenton, Ph.D*.
Catherine Friedman
Paul Berns
Compensation Committee Chair
Ronald Barrett, Ph.D.
Wendell Wierenga, Ph.D.
William Rieflin*
Currently CEO at XenoPort
Former R&D Executive: Glaxo, Affymax
Currently CEO at Anacor
Former CEO: Allos, Bone Care
Former Commercial Executive: Abbott, BASF, BMS
Former Amgen EVP worldwide operations,
manufacturing, process development and quality.
Former Managing Director and Head of West Coast
Healthcare Practice for Morgan Stanley
Currently CFO at Ocera.
Former CFO: Amyris, Symx, Geron, Cytel
Former CEO: Capnia, Reliant, Intrabiotics, ALZA,
Glaxo
Currently CEO at NGM Biopharma
Former President/COO: XenoPort;Tularik
Former CEO: Syrrx
Former Senior R&D Executive at Santarus,
Ambit, Neurocrine, Park Davis, UpJohn
11
XenoPort Investor Presentation
May 2014
*Directors added in past 5 years
Board with Proven Records, Relevant
Board with Proven Records, Relevant
Experience and Broad Skill Sets
Experience and Broad Skill Sets |
 12
XenoPort Phase 3 Trials
4 trials
3 positive
1 negative
•
HORIZANT approved on
the basis of successful
Phase 3 program
XenoPort Phase 2 Trials
11 trials
7 positive
4 negative
•
XP21279 study had trend
to positive outcome that
served as basis for FDA
discussion resulting in
agreement to move to
Phase 3
but…
Only 13% of small molecule drug candidates entering
clinical development are approved.
*
and…
*
Dimasi et al., Clinical Pharmacology & Therapeutics 87, 272-277 (March
2010) XenoPort Investor Presentation
May 2014
XenoPort Success Rate in Mid/Late Stage
XenoPort Success Rate in Mid/Late Stage
Trials Exceeds Industry Norms
Trials Exceeds Industry Norms |
 In
response to HORIZANT Complete Response Letter •
Moved rapidly to restructure around securing approval of HORIZANT and
advancing its clinical-stage assets
•
Conserved resources
•
Reduced headcount by approximately 50%
•
Eliminated research function
•
CEO took voluntary pay cut
•
Spearheaded successful effort to get FDA to approve HORIZANT
In response to AP MS spasticity Phase 3 trial results
•
Moved rapidly to shut-down ongoing AP program expenses and re-focused
around two key assets: XP23829 and HORIZANT
•
Put XP21279 and AP on back burner as XP23829 believed to have higher
value-creation potential
•
Successfully out-licensed AP May 15, 2014*
Management and Board Have Made Tough
Management and Board Have Made Tough
Decisions in Response to Setbacks
Decisions in Response to Setbacks
13
*Subject to antitrust clearance of transaction
XenoPort Investor Presentation
May 2014 |
 Pay
for Performance Philosophy: CEO Compensation Pay for Performance Philosophy: CEO
Compensation Aligned with Performance and Peers
Aligned with Performance and Peers
•
Compensation composed of salary, cash bonus and long-term incentives
•
Pay-for-performance philosophy and practice
Note:
Realizable
pay
for
each
year
is
calculated
as
base
salary
and
bonus
/
NEIP
as
disclosed
in
the
Summary
Compensation
Table,
plus
the
intrinsic
value
of
equity
awards
granted
during
the
year,
based
on
FYE
stock
price;
If
payout
is
not
disclosed,
performance
awards
are
assumed
at
target;
Three
year
average
is
calculated
as
average
cash
compensation plus average intrinsic equity value, based on 2013 FYE stock
price. •
Average realizable compensation is <50% that of peers
•
Realizable compensation has increased and decreased
appropriately with stock price performance
•
Equity awards generally weighted towards stock options --
only deliver value if the stock price increases
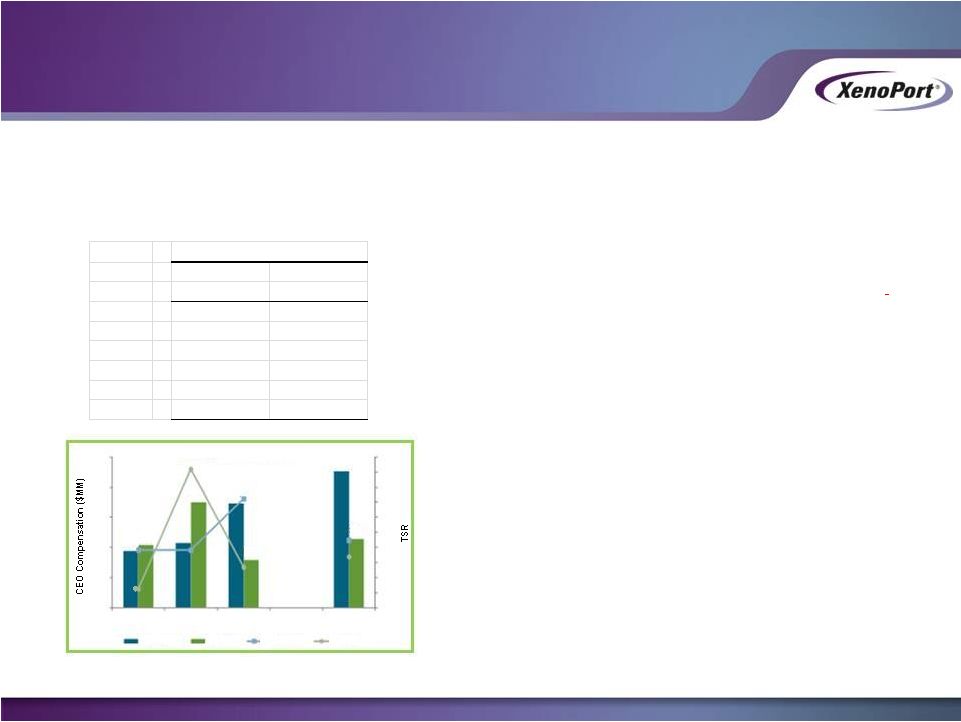
Corp Bonus
Base Salary
Plan Cash
2008
500,000
$
Paid in RSU's
2009
500,000
$
300,000
$
2010
465,937
$
No Bonus
2011
458,522
$
330,136
$
2012
469,985
$
444,136
$
2013
500,000
$
No Bonus
Cash Compensation
XenoPort Investor Presentation
May 2014
14
$0.9
$1.1
$2.3
$1.0
$1.8
$0.8
$1.1
-80%
-60%
-40%
-20%
0%
20%
40%
60%
80%
100%
120%
$0.0
$0.5
$1.0
$1.5
$2.0
$2.5
2011
2012
2013
Average at FYE
2013
Realizable Pay and Performance
Peer Median
XenoPort
Peer Median
XenoPort
$1.7
•
2008:
•
2010:
•
2011:
•
2013:
•
2014:
Dr. Barrett requested and the Board agreed to pay bonus in stock to conserve cash Dr. Barrett requested and the
Board agreed to reduce salary Minimum Corporate Goal score was not met and no bonus paid 280G gross-up benefit
eliminated Minimum
Corporate Goal score was not met and no bonus paid
Dr. Barrett requested and the Board agreed to no increase in salary 50% of long-term incentive
grant performance-based
Stock ownership guidelines implemented for executives and Board * Minimum
Corporate Goal performance required for any bonus payment *
Performance-based equity
grants
in
2010
and
2014 |
 15
Advance development of XP23829 as potential treatment for
psoriasis and/or relapsing forms of multiple sclerosis
Build significant value for HORIZANT
•
Provide funding source to capture the most value for
XP23829
•
Potential revenue through out-licensing deal or through
achieving profitability of commercial effort
•
Hedge risk inherent in XP23829 development
Monetize other assets and create “additional shots on goal”
through partnering
We believe it would be imprudent to focus ONLY on XP23829,
despite its potential. XP23829 is just entering Phase 2 studies
and the
biotechnology business has inherent risk.
XenoPort Investor Presentation
May 2014
Strategy to Build Stockholder Value
Strategy to Build Stockholder Value |
 Existing Stockholders Participated in
Existing Stockholders Participated in
January 2014 Financing
January 2014 Financing
XenoPort regularly meets with stockholders and potential new
investors
XenoPort Board members met with majority of top 15
stockholders in January 2014 prior to financing to get feedback
on Clinton’s demands and XenoPort’s strategy
Majority of largest stockholders participated in subsequent
financing
16
XenoPort Investor Presentation
May 2014 |
 Industry Analysts are Supportive of the
Industry Analysts are Supportive of the
Actions XenoPort is Taking on HORIZANT
Actions XenoPort is Taking on HORIZANT
17
Permission to use quotes was neither sought nor obtained.
XenoPort Investor Presentation
May 2014
“We continue to see HORIZANT ($2.7 million in revenues in Q4 2013) as
contributing modestly to XNPT valuation. However, if XNPT continues to execute
well, we believe HORIZANT could become a profitable business unit and a
modest
contributor
of
cash.”
–
Ladenberg,
2/27/14
“Management has developed a new marketing strategy that shows initial signs
of bearing
fruit.
The
company
has
deployed
40
field
representatives
into
40
territories, and the team has already begun to outperform the marketing effort put
forth
by
GSK.”
–
Jefferies,
4/21/2014
“Horizant
sales
were
$3M
for
the
quarter
with
prescription
trends
continuing
to provide
some
reflection
of
XNPT’s
successful
focused
re-launch,
and
…
its
trajectory
should
enable
the
drug
to
be
cash
flow
positive.”
–
Wells
Fargo,
5/09/14,
XNPT
“HORIZANT appears to be generating positive momentum in markets where
XNPT
is
focused…
We
believe
HORIZANT
revenues
will
continue
to
grow...”
–
Leerink, 5/11/14
Permission to use quotes was neither sought nor obtained.
Industry analysts recognize the value potential of HORIZANT
|
 Creating Value in
HORIZANT
®
(gabapentin enacarbil)
Extended-Release Tablets |
 19
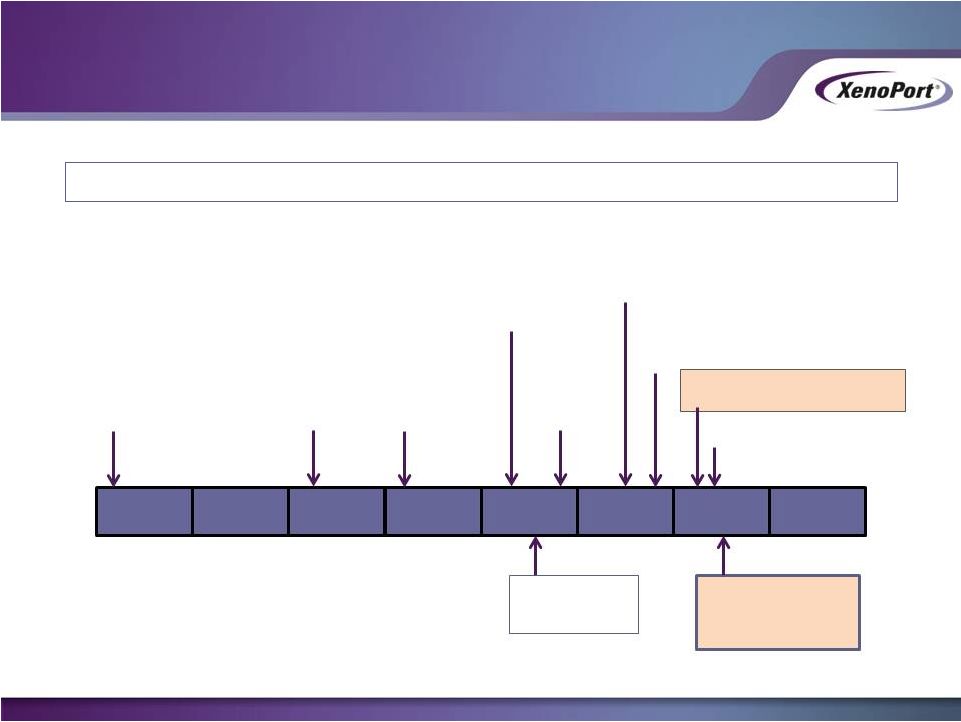
HORIZANT –
Discovered and Developed by XenoPort for RLS prior to 2007
Former Partner
Launches
HORIZANT
Former Partner Experiences
Stockout
XenoPort
Initiates
HORIZANT
Commercialization
2010
2007
2008
2009
2011
2012
2013
2014
XenoPort and Former
Partner
Enter Into Licensing
Agreement
Receive
CRL
From FDA
FDA Approves
HORIZANT for
Moderate-to-Severe
Primary RLS in
Adults
XenoPort
Initiates
Dispute
Process
FDA Approves
HORIZANT for
Management of PHN
In Adults
XenoPort Reacquires
HORIZANT Rights
HORIZANT Agreement
Terminated
Please review the full prescribing and safety information for HORIZANT. The most
common adverse reactions of HORIZANT in RLS patients: somnolence/sedation and
dizziness, and in PHN patients: somnolence, dizziness and headache.
RLS
NDA
Accepted
XenoPort Investor Presentation
May 2014
We Re-Launched HORIZANT
We Re-Launched HORIZANT
Less Than a Year Ago
Less Than a Year Ago |
 Over 5 million adults suffer from
moderate-to-severe primary
restless legs syndrome (RLS)
>6 million annual prescription
Widespread use of dopamine
agonists
Growing awareness of issues
related to dopamine agonist use in
treatment of RLS
Sources: RLS Prevelance-NINDs, NIH, Sleep Medicine, Volume 14, No. 7 ,
2013, Mayo
Clinic
Proceedings,
Volume
88,
No.
9,
2013,
Sleep,
Vol.
35,
No.
8,
2012
20
XenoPort Investor Presentation
May 2014
Moderate-to-Severe Primary RLS
Moderate-to-Severe Primary RLS
Market Opportunity in U.S.
Market Opportunity in U.S. |
 Only non-dopamine agonist approved for treatment of
moderate-to-severe primary RLS in adults
Proven effective in relieving RLS symptoms
HORIZANT is “not interchangeable with other gabapentin
products”
(FDA Label)
Convenient once-a-day dosing
No titration to approved dose
Shows no evidence of augmentation, rebound or impulse
control disorders
Please review the full prescribing and safety information for HORIZANT. The most
common adverse reactions of HORIZANT in RLS patients: somnolence/sedation and
dizziness, and in PHN patients: somnolence, dizziness and headache.
21
XenoPort Investor Presentation
May 2014
HORIZANT Is Differentiated for RLS
HORIZANT Is Differentiated for RLS |
 International
RLS
Study
Group
Task
Force
recommends
that
ligands should be considered for first-line treatment for patients with
RLS
Willis Ekbom Disease Foundation Medical Advisory Board revised
consensus
statement
on
the
management
of
RLS
recommending
ligands should be considered for initial treatment for patients with RLS
American Academy of Sleep Medicine identified gabapentin enacarbil
as the only
Ligand with high level of evidence of efficacy for
patients with RLS
RLS Treatment Guidelines Revised Since
RLS Treatment Guidelines Revised Since
HORIZANT Approval
HORIZANT Approval
22
HORIZANT is the only product in the
ligand class
approved for treatment of RLS.
Please review the full prescribing and safety information for HORIZANT. The most
common adverse reactions of HORIZANT in RLS patients: somnolence/sedation and
dizziness, and in PHN patients: somnolence, dizziness and headache.
XenoPort Investor Presentation
May 2014 |
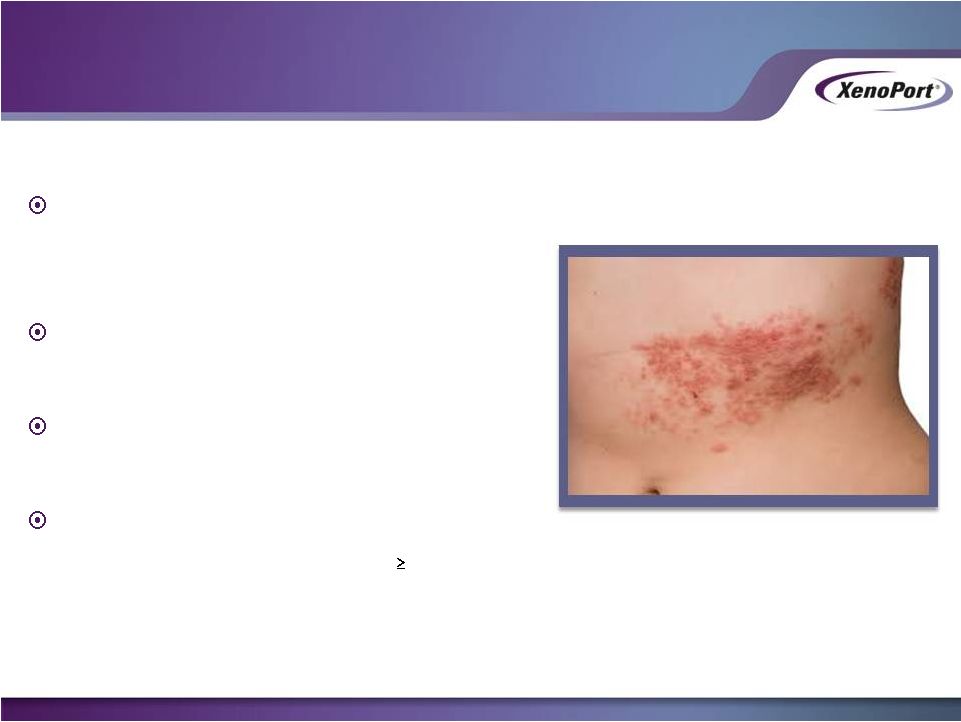 Results from damage that occurs to
the peripheral nerve fibers during a
shingles outbreak
Pain associated with PHN can be very
intense
About 200,000 patients suffer from
PHN in the U.S.
Clear unmet medical need
•
Only ~30% of patients receive
50%
reduction in PHN pain with gabapentin, the
most widely used agent to treat PHN
Sources: Decision Resources, Inc. 2010, Neurontin Product Label
23
XenoPort Investor Presentation
May 2014
Postherpetic Neuralgia (PHN)
Postherpetic Neuralgia (PHN) |
 Simple dosing
•
Three days at 600 mg once a day
•
4
th
day
at
approved
600
mg
twice
daily
Effective at one week
Pharmacokinetic differentiation
•
High bioavailability (75%)
•
Sustained 24-hour gabapentin blood levels (Peak/Trough = 1.5)
•
“Not
interchangeable
with
other
gabapentin
products”
(FDA
label)
Pivotal trial showed 42% of PHN patients experienced
50% pain
intensity score from baseline
24-hour pain reduction
Approved
for
PHN
by
FDA
but
never
launched
by
former
partner
Please review the full prescribing and safety information for HORIZANT. The most
common adverse reactions of HORIZANT in RLS patients: somnolence/sedation and
dizziness, and in PHN patients: somnolence, dizziness and headache. Patients with
renal insufficiency require a modified dose."
24
XenoPort Investor Presentation
May 2014
HORIZANT is Differentiated for PHN
HORIZANT is Differentiated for PHN |
 25
XenoPort Investor Presentation
May 2014
HORIZANT: What Went Wrong with
HORIZANT: What Went Wrong with
Former Partner?
Former Partner?
Six months after former partner’s launch, market research of targeted
customers showed top three reasons for not prescribing HORIZANT:
•
“Wasn’t aware of HORIZANT”
•
“Did not know enough about HORIZANT to decide”
•
“Did not know HORIZANT was approved”
•
Led to former partner’s $3B settlement with U.S. Government
•
Corporate Integrity Agreement
•
New selling model for former partner (no individual incentives for sales)
implemented on the day of HORIZANT launch
Government investigation of former partner’s past marketing practices
•
Partner exited pain development which was a major part of HORIZANT life cycle
planning
Change in former partner’s development strategy
Change
in
former
partner’s
CEO
–
HORIZANT
champions
departed |
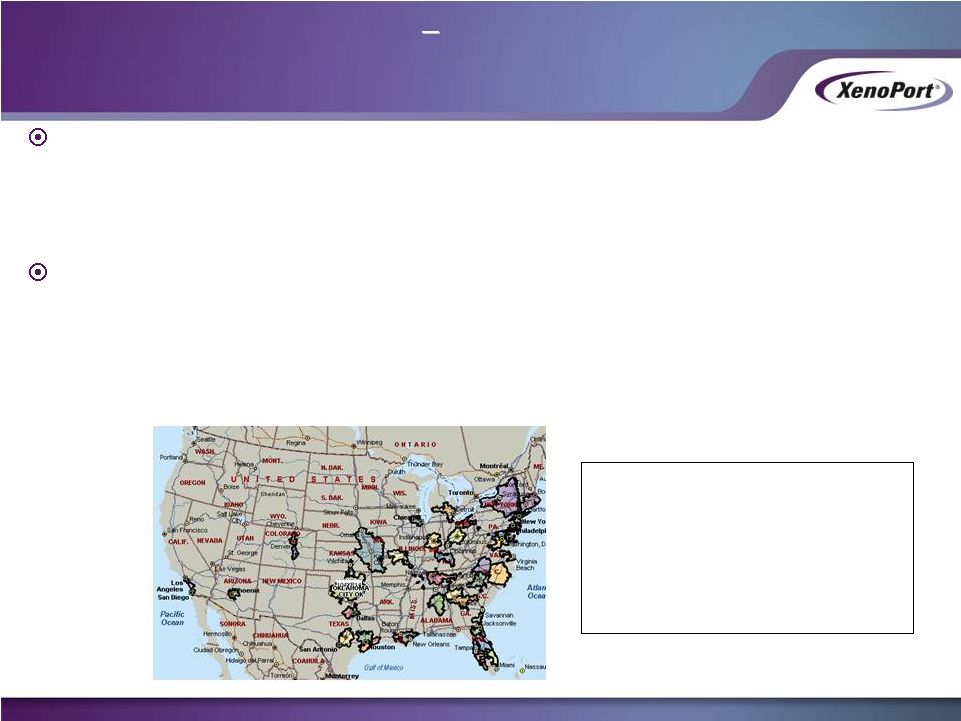 Strategy
•
Measure responsiveness quickly and efficiently
•
Build value in HORIZANT to provide strategic optionality (monetize or grow
business) •
Closely monitor results to make sure continued investment is warranted
Tactics
•
Leverage $40 million and 50 metric tons of active ingredient acquired as part of
settlement
•
Implement state-of-the-art promotional tools
•
Personal promotion and marketing efforts focused in 40 territories
26
•
XenoPort sales
specialists call on ~10%
of the potential market
•
~40 sales reps vs.
former partner’s ~300
XenoPort Investor Presentation
May 2014
XenoPort’s Initial Strategy
XenoPort’s Initial Strategy
Demonstrate that
Demonstrate that
HORIZANT is Promotionally Responsive
HORIZANT is Promotionally Responsive |
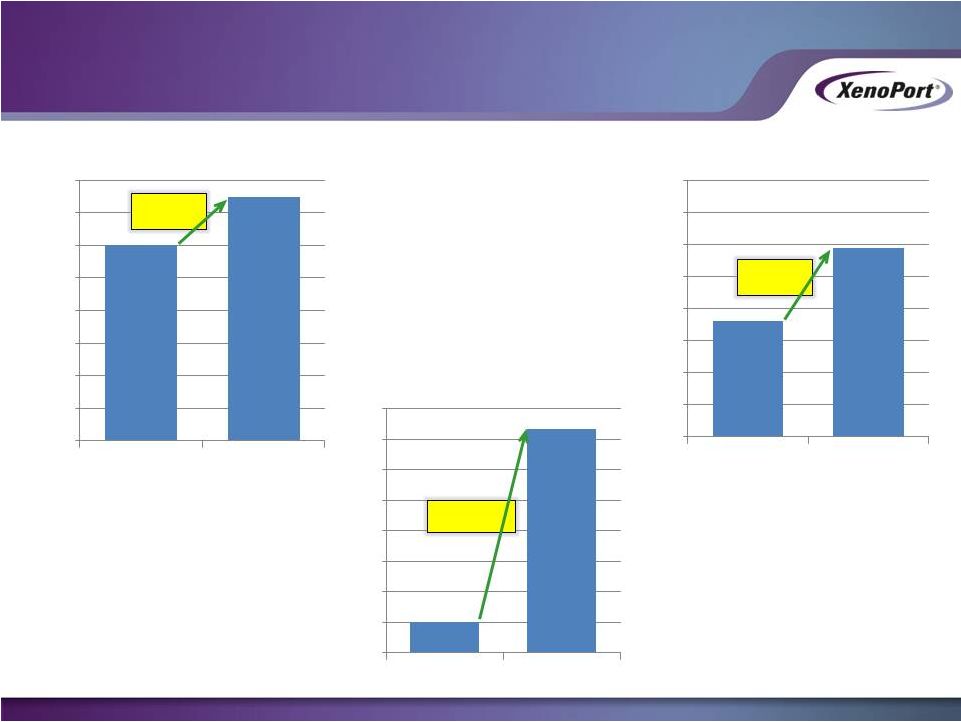 Solid
HORIZANT Prescription Performance Solid HORIZANT Prescription Performance
in 1Q 2014
in 1Q 2014
27
National Prescribed Tablets
XP Territories Prescribed Tablets
Prescribed Tablets per Rep
XenoPort Investor Presentation
May 2014
601,201
746,729
-
100,000
200,000
300,000
400,000
500,000
600,000
700,000
800,000
GSK 1Q13
XP 1Q14
Former
partner
300
sales
reps
XNPT
40
sales
reps
+24%
359,685
588,434
-
100,000
200,000
300,000
400,000
500,000
600,000
700,000
800,000
GSK 1Q13
XP 1Q14
2,024
14,710
-
2,000
4,000
6,000
8,000
10,000
12,000
14,000
16,000
GSK 1Q13
XP 1Q14
+630%
+64% |
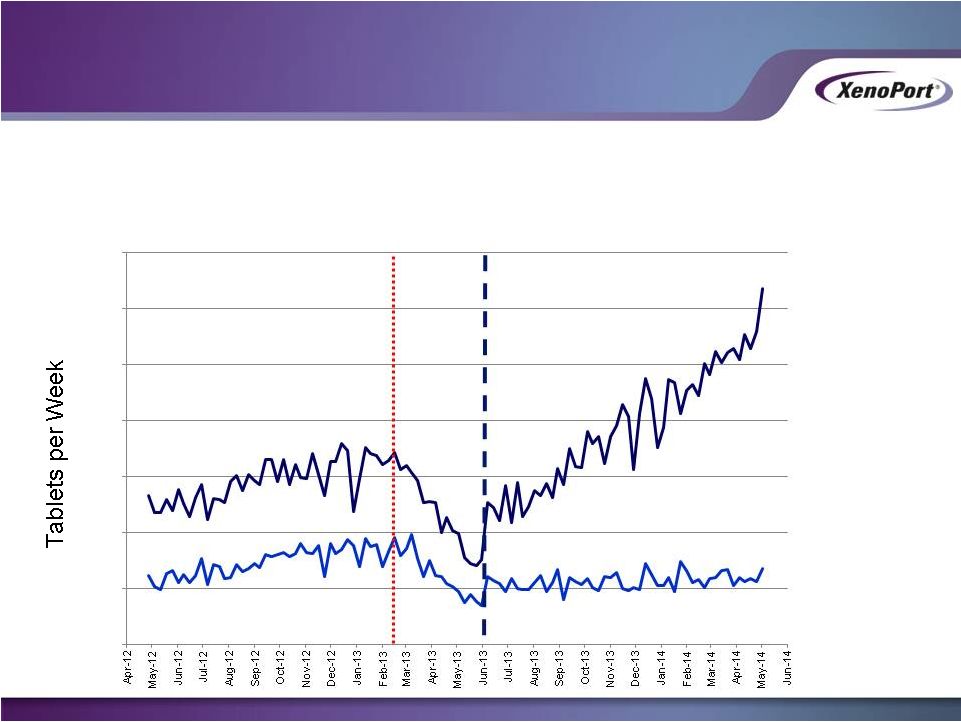 HORIZANT Prescription Growth Coming
HORIZANT Prescription Growth Coming
from XenoPort Promotion
from XenoPort Promotion
28
XenoPort
Promoted
Territories
Non-Promoted
Territories
Previous
Partner
Stockout
XenoPort
Commercialization
Begins
Weekly HORIZANT Prescribed Tablets
XenoPort Investor Presentation
May 2014
63,599
13,506
0
10,000
20,000
30,000
40,000
50,000
60,000
70,000 |
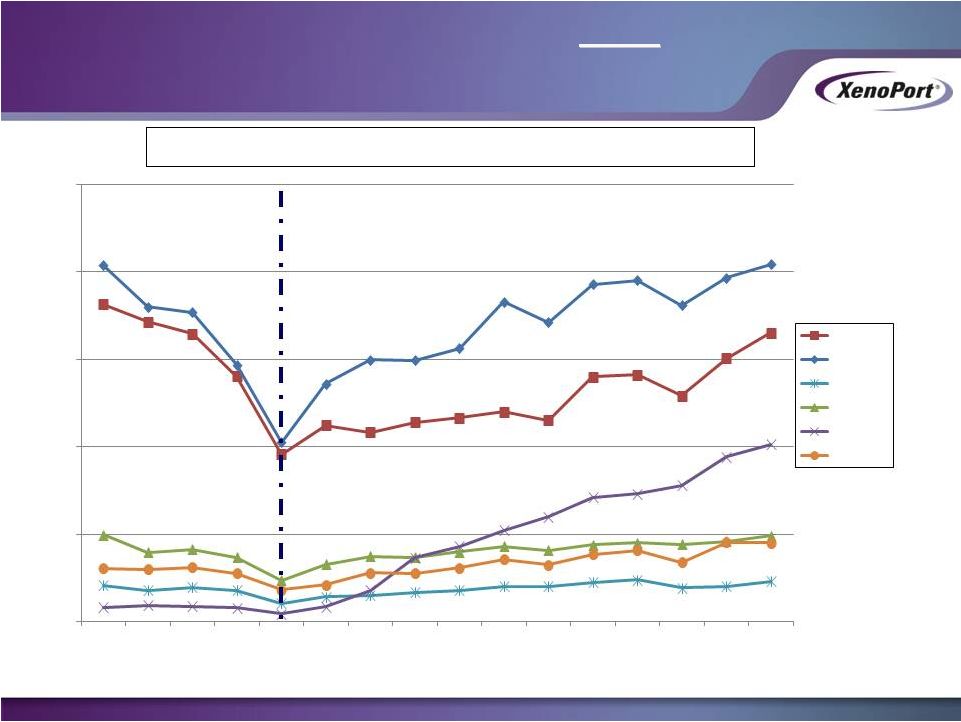 29
Former Partner Never Launched for PHN Indication
Please review the full prescribing and safety information for HORIZANT. The most
common adverse reactions of HORIZANT in RLS patients: somnolence/sedation and
dizziness, and in PHN patients: somnolence, dizziness and headache. Patients
with renal insufficiency require a modified dose." XenoPort Investor
Presentation May 2014
0
500
1000
1500
2000
2500
Jan-13
Feb-13
Mar-13
Apr-13
May-13
Jun-13
Jul-13
Aug-13
Sep-13
Oct-13
Nov-13
Dec-13
Jan-14
Feb-14
Mar-14
Apr-14
Monthly HORIZANT TRx by Specialty
PCP
Neuro
Psych
PUD/Sleep
Pain/Anes
Other
XenoPort
Begins
Promotion
Physicians Now Receiving RLS AND
Physicians Now Receiving RLS AND
PHN
PHN
Promotional Messages
Promotional Messages |
 30
4-Week
Rolling
Average
-
HORIZANT
Prescribed
Tablets
May 31, 2013 to May 9, 2014
XenoPort Investor Presentation
May 2014
69,645 tablets at current WAC price = $489,125 / week
Achieved with 40 sales reps promoting to physicians that
represent ~10% of the potential market
69,645
-
20,000
40,000
60,000
80,000
HORIZANT Gross Demand Sales at ~$25 Million
HORIZANT Gross Demand Sales at ~$25 Million
Annualized Run Rate and Ramping
Annualized Run Rate and Ramping |
 31
Our focused effort has demonstrated promising promotional
responsiveness
HORIZANT is now appropriately priced to be competitive with branded
peers
•
Prescriptions remain robust after price increases
•
This
value
has
accrued
to
XenoPort
stockholders
because
we
still
own
the
product
HORIZANT has patent protection through 2026
HORIZANT current annualized run rate is ~$25 million and ramping
HORIZANT’s poor performance by former partner is explainable
•
We believe XenoPort is building significant value in an asset whose value
was not apparent to the external world when re-acquired.
•
We believe it would be imprudent to discontinue HORIZANT when value
creation curve remains steep.
XenoPort Investor Presentation
May 2014
HORIZANT Summary
HORIZANT Summary |
 XP23829 Development Execution |
 •
Approved in 1990s and widely used for the treatment of psoriasis
in Germany
•
Approved in March 2013 in the United States and February 2013 in
EU for the
treatment of relapsing forms of MS
•
Q1 2014 TECFIDERA revenues were $506 million ($460 million in U.S.; $46
million in sales outside the U.S.)
33
XenoPort Investor Presentation
May 2014
TECFIDERA (dimethylfumarate)
FUMADERM (mixture of dimethylfumarate and monoethyl
fumarate salts)
XP23829 has novel chemical structure that produces the same
active metabolite as TECFIDERA (dimethylfumarate)
Background:
Background:
Fumaric Acid Ester Products
Fumaric Acid Ester Products |
 Lower incidence/less severe GI side effects and flushing
•
Improved compliance; fewer treatment failures
Onset and/or magnitude of efficacy
•
Earlier onset of immunomodulation
Reduced dosing frequency
•
Once a day rather than twice (TECFIDERA) or three times (FUMADERM)
daily
34
XenoPort Investor Presentation
May 2014
Potential Advantages and Areas of
Potential Advantages and Areas of
Differentiation
Differentiation |
 Completed numerous preclinical studies
Most studies included DMF for comparison
Animals studies demonstrated less gastric irritation
Phase 1 data has demonstrated favorable pharmacokinetic and
pharmacodynamic effects
Included direct comparison with TECFIDERA
Demonstrated appropriate MMF exposure
Demonstrated immune biomarker effects with once-a-day dosing
Preparing to initiate a Phase 2 clinical trial in patients with
moderate-to- severe plaque psoriasis by mid-2014
35
•
Early-stage development proceeds in a step-wise fashion and is
less costly than later-stage development.
•
XenoPort has aggressively invested in XP23829 by including DMF
and TECFIDERA in studies to assess potential for differentiation.
XenoPort Investor Presentation
May 2014
Ramping Investment in XP23829 Appropriate for
Ramping Investment in XP23829 Appropriate for
its Stage of Development
its Stage of Development |
 Lymphocyte reductions require further exploration
•
Psoriasis Phase 2 study is designed to establish whether XP23829
differs from
FUMADERM/TECFIDERA and to assess the potential benefits and risks if
different TECFIDERA’s patents likely to be challenged
•
Generic DMF could enter the market and “raise the bar”
for a second generation product
MS and psoriasis markets will be crowded by earliest potential XP23829
launch date
•
Nine approved drugs in U.S. for relapsing forms of MS
•
Generic MS treatments may reach market as early as this year and
change pricing dynamics
•
Seven
approved drugs in U.S. for moderate-to-severe plaque psoriasis
•
Five compounds in Phase 3 development
•
Two oral drugs (apremilast and tofacitinib) expected to enter market in next
year 36
We believe it would be imprudent to focus ONLY on this mid-stage asset,
despite its potential.
XenoPort Investor Presentation
May 2014
XP23829 has Inherent Risk,
XP23829 has Inherent Risk,
Like All Drug Candidates
Like All Drug Candidates |
 Monetizing/Creating Opportunity for Other Assets |
 Exclusive world-wide rights granted to Reckitt Benckiser
Pharmaceuticals
announced
on
May
15,
2014
1
Initial development focus: Alcohol Use Disorder
$20 million up-front plus $5 million on technology transfer
completion
Up to $70 million in development and regulatory milestones
Up to $50 million in commercial milestones
Tiered double-digit royalty payments up to mid-teens on a
percentage basis on potential future net sales in the U.S.
High single-digit royalty payments on potential future sales
outside the U.S.
38
1
Subject to antitrust clearance of transaction
Significant value for an asset that Wall Street had recently valued at $0.
XenoPort Investor Presentation
May 2014
AP Agreement Creates Value for XenoPort
AP Agreement Creates Value for XenoPort
Stockholders
Stockholders |
 XenoPort’s Board Recommendations |
 June 2013: Clinton began buying and selling XenoPort shares.
October
2013:
Clinton
issues
public
letter
attacking
performance
and
leadership, and demanding replacement of XenoPort’s CEO.
Fall –
Winter 2013: Members of XenoPort’s Board and management
have conference calls, in-person meetings and written exchanges with
Clinton to understand and share views. Clinton fails to meet XenoPort’s
request for the financial analysis offered in Clinton’s October
letter. January 2014: Clinton does not respond to management’s offer to
review HORIZANT progress and public guidance on potential time to
reach profitability.
February 2014: Clinton provides notice of intent to nominate competing
slate of director candidates and present other proposals at Annual
Meeting.
40
XenoPort Investor Presentation
May 2014
XenoPort Board Engagement with Clinton
XenoPort Board Engagement with Clinton |
 41
1) Clinton Definitive Proxy, Filed 4/25/14
•
XenoPort’s Board attempted to constructively engage with
Clinton to no avail.
•
The Board is open to stockholder feedback and is committed
to doing what is in the best interest of all stockholders.
XenoPort Investor Presentation
May 2014
February 2014: Members of XenoPort’s Board engage in multiple
discussions with Clinton in an effort to reach a resolution and avoid a
costly and disruptive proxy battle.
March 2014: Clinton does not respond to XenoPort’s modified
settlement proposal.
April 2014: Clinton reduced his position and reportedly now holds
1.6% of XenoPort stock.
1
XenoPort Board Engagement with Clinton
XenoPort Board Engagement with Clinton
(con’t)
(con’t) |
 Clinton’s Agenda for Capital Allocation
Clinton’s Agenda for Capital Allocation
Could Destroy Value
Could Destroy Value
Now is the worst time to abandon HORIZANT commercialization
•
Investment in commercial operations is complete
•
Investments in marketing initiatives and programs are largely committed
•
Positive trajectory of sales is increasing the value of a partnership or
divestiture of HORIZANT
HORIZANT could generate meaningful non-dilutive revenue to fund
further advances of XP23829 development
Diverting funding from HORIZANT to XP23829 will not accelerate its
development
Putting all eggs in one basket is a risky strategy in an inherently risky
industry
42
XenoPort Investor Presentation
May 2014 |
 Clinton’s Agenda to Replace XenoPort CEO and Two
Clinton’s Agenda to Replace XenoPort CEO and Two
Highly-Qualified Directors Could Destroy Value
Highly-Qualified Directors Could Destroy Value
Clinton Agenda: replace the CEO
43
XenoPort Investor Presentation
May 2014
Dr. Barrett has played, and will continue to play, a pivotal role in executing
XenoPort’s strategy
Clinton has not identified or proposed an alternative CEO. Dismissing
current CEO would create a void in the leadership on critical initiatives:
Clinton Agenda: replace three Board members: Dr. Barrett, Ms. Hilleman and Dr.
Wierenga
Initiating by mid-year and conducting a Phase 2 trial for XP23829
Driving education and appropriate use of HORIZANT
Engaging in discussions with potential strategic partners
Replacing these three XenoPort directors removes experience and
expertise that are essential to XenoPort’s future success
|
 Ronald W. Barrett, Ph.D.
Chief Executive Officer of XenoPort since 2001
44
XenoPort Investor Presentation
May 2014
20+ years of executive management experience in the pharmaceutical
industry
Chief Scientific Officer of XenoPort from 1999 to 2001
Various
positions
at
Affymax
Research
Institute,
the
most
recent
of
which was Senior Vice President of Research
Dr. Barrett’s leadership and strategic direction have led to the
discovery and development of our novel product candidates
(including XP23829) and have enabled XenoPort to evolve from a
research-based company to an integrated company with
development and commercialization capabilities.
Director Nominees Provide Expertise
Director Nominees Provide Expertise
Critical to XenoPort’s Success
Critical to XenoPort’s Success |
 Director Nominees Provide Expertise
Director Nominees Provide Expertise
Critical to XenoPort’s Success
Critical to XenoPort’s Success
Jeryl L. Hilleman
Chair of XenoPort Audit Committee
30+ years of finance, marketing and investment experience and an
impressive history of executive leadership
20+ years as CFO of both private and public companies
Ms. Hilleman’s considerable expertise in finance and accounting
allows her to provide important guidance and direction to
XenoPort in relation to its finance, compliance and auditing needs,
as demonstrated by her work in helping to successfully navigate
the accounting complexities and SEC interactions associated with
the re-acquisition of HORIZANT. Her knowledge of XenoPort’s
financials would be difficult to replace.
45
XenoPort Investor Presentation
May 2014 |
 Director Nominees Provide Expertise
Director Nominees Provide Expertise
Critical to XenoPort’s Success
Critical to XenoPort’s Success
Wendell Wierenga, Ph.D.
Executive leadership of pharmaceutical research, clinical development and
regulatory functions for a number of national and multi-national
corporations, including Santarus (acquired by Salix Pharmaceuticals for $2.6
B) and Syrrx (acquired by Takeda for $270M) Director of multiple
publicly-traded biopharmaceutical companies, including Onyx
Pharmaceuticals (acquired by Amgen for $10 B)
Dr. Wierenga’s substantial R&D experience has contributed to the
successful development of our pipeline and the approval of HORIZANT.
His Board experience, including membership of compensation
committees and involvement with M&A processes, provides unique
value to the Board.
46
XenoPort Investor Presentation
May 2014
40 years of leadership of pharmaceutical and biopharmaceutical
companies, including
Participated in the submission of 70+ Investigational New Drug applications
and the filing of 16 New Drug Applications/Biologics License Applications,
and 16 FDA-approved drug products |
 The
Right Strategy and Board to Execute The Right Strategy and Board to Execute
and Deliver Long-Term Value
and Deliver Long-Term Value
XenoPort Is Committed to Realizing the Full Potential of its Assets and Acting
in the Best Interests of XenoPort Stockholders.
We Ask That You Vote FOR ALL
the Board’s Director Nominees
47
XenoPort Investor Presentation
May 2014
Our
Board
is
active
and
engaged,
and
has
the
depth
and
diversity
of
skills
and
expertise needed to oversee the execution of XenoPort’s strategy
We are making meaningful progress commercializing HORIZANT
We are committed to optimizing the value of XP23829
Our Board has concluded that Clinton’s nominees add no relevant skills,
experience or expertise that is not already well represented on the Board
Our Board believes that Clinton’s nominees will push an uninformed
agenda that could undermine the Board’s goal of maximally enhancing
value of XenoPort through
current
investment
in
BOTH
HORIZANT
and
XP23829 |
 48
XenoPort Investor Presentation
May 2014
HORIZANT
®
(gabapentin
enacarbil)
Extended-Release
Tablets
INDICATIONS
HORIZANT
®
(gabapentin
enacarbil)
is
a
prescription
medicine
used
to:
treat
adults
with
moderate-to-severe
primary
Restless
Legs
Syndrome
(RLS).
HORIZANT
is
not
for
people
who
need
to
sleep
during
the
daytime and stay awake at night.
manage pain from damaged nerves (postherpetic neuralgia) that follows healing of
shingles (a painful rash that comes after a herpes zoster infection) in
adults. IMPORTANT SAFETY INFORMATION
Do
not
drive
after
taking
your
dose
of
HORIZANT
until
you
know
how
it
affects
you,
including the
morning after
you
take
it.
Do
not
operate heavy machinery or do other dangerous activities until you know how
HORIZANT affects you. HORIZANT can cause sleepiness, dizziness, slow
thinking, and can affect your coordination. Ask your healthcare provider when it is okay to do these activities.
Do not take other medicines that make you sleepy or dizzy while taking HORIZANT
without talking to your healthcare provider. Taking HORIZANT with these
other medicines may make your sleepiness or dizziness worse. HORIZANT may
cause suicidal thoughts or actions in a very small number of people (about 1 in 500). Pay attention to any changes,
especially sudden changes, in mood, behaviors, thoughts, or feelings. Call your
healthcare provider right away if you have any of these symptoms, especially
if they are new, worse, or worry you: thoughts or actions about suicide,
self-harm, or dying; attempt to commit suicide new or worsening
depression or anxiety; or feeling agitated new or worse restlessness or panic
attacks new or worse trouble sleeping (insomnia); or irritability
acting aggressive, being angry, or violent; acting on dangerous impulses
an extreme increase in activity or talking (mania); other unusual changes in mood
or behavior Do not
stop taking HORIZANT without first talking to your healthcare provider.
Suicidal thoughts or actions can be caused by things other than medicines. If you
have these thoughts or actions, your healthcare provider may check for
other causes. HORIZANT
may
cause
a
serious
or
life-threatening
allergic
reaction
that
may
affect
your
skin
or
other
parts
of
your
body
such
as
your
liver
or blood cells. You may or may not have a rash with these types of reactions. Call
a healthcare provider right away if you have any of the following
symptoms:
skin
rash,
hives,
fever,
swollen
glands
that
do
not
go
away,
swelling
of
your
lips
or
tongue,
yellowing
of
your
skin
or
eyes,
unusual bruising or bleeding, severe fatigue or weakness, unexpected severe muscle
pain, or frequent infections. These symptoms may be the first
signs of a serious reaction. A healthcare provider should examine you to decide if you should continue taking HORIZANT.
HORIZANT:
HORIZANT:
Important Safety Information
Important Safety Information |
 49
XenoPort Investor Presentation
May 2014
HORIZANT
is
not
the
same
medicine
as
gabapentin
(for
example,
Neurontin
®
and
Gralise
®
).
HORIZANT
should
not
be
used
in
their
place.
Do not take these or other gabapentin products while taking HORIZANT.
Before taking HORIZANT, tell your healthcare provider if you:
have or have had kidney problems or are on hemodialysis
have
or
have
had
depression,
mood
problems,
or
suicidal
thoughts
or
behavior
have or have had seizures
have a history of drug abuse
have any other medical conditions
are pregnant or plan to become pregnant. It is not known if HORIZANT will harm your
unborn baby. Talk to your healthcare provider if you are pregnant or plan to
become pregnant while taking HORIZANT. You and your healthcare provider will decide if you should take
HORIZANT while you are pregnant
are breastfeeding or plan to breastfeed. Your body turns HORIZANT into another drug
(gabapentin) that passes into your milk. It is not known if this can harm
your baby. You and your healthcare provider should decide if you will take HORIZANT or breastfeed
drink alcohol
Do not drink alcohol while taking HORIZANT because it may increase the risk of side
effects. Tell
your
healthcare
provider
about
all
the
medicines
you
take,
including
prescription
and
non-prescription
medicines,
vitamins,
and
herbal
supplements. Taking HORIZANT with certain other medicines can cause side effects or
affect how well they work. Do not start or stop other medicines without
talking to your healthcare provider. Do
not
stop
taking
HORIZANT
without
talking
to
your
healthcare
provider
first.
If
you
stop
taking
HORIZANT
suddenly,
you
may
develop
side effects.
The most common side effects of HORIZANT include dizziness, sleepiness, and
headache. Tell your healthcare provider about any side effect that bothers
you or does not go away. These are not all the possible side effects of HORIZANT. For more information, ask your healthcare
provider or pharmacist.
You
are
encouraged
to
report
negative
side
effects
of
prescription
drugs
to
the
FDA.
Visit
www.fda.gov/medwatch,
or
call
1-800-FDA-1088.
See Medication Guide.
HORIZANT:
HORIZANT:
Important Safety Information
Important Safety Information |
