Attached files
| file | filename |
|---|---|
| 8-K - 8-K - HERON THERAPEUTICS, INC. /DE/ | d726242d8k.htm |
| EX-99.1 - EX-99.1 - HERON THERAPEUTICS, INC. /DE/ | d726242dex991.htm |
 Company Update
May 2014
Exhibit 99.2 |
 2
Legal Disclaimer
This
presentation
contains
"forward-looking
statements"
as
defined
by
the
Private
Securities
Litigation
Reform
Act
of
1995.
These
forward-looking
statements
involve
risks
and
uncertainties,
including
uncertainties
associated
with
timely
development,
approval,
launch
and
acceptance
of
new
products,
satisfactory
completion
of
clinical
studies,
establishment
of
new
corporate
alliances,
progress
in
research
and
development
programs
and
other
risks
and
uncertainties
identified
in
the
Company's
filings
with
the
Securities
and
Exchange
Commission.
Actual
results
may
differ
materially
from
the
results
expected
in
our
forward
looking
statements.
We
caution
investors
that
forward-looking
statements
reflect
our
analysis
only
on
their
stated
date.
We
do
not
intend
to
update
them
except
as
required
by
law.
2 |
 3
Barry D. Quart, PharmD
Chief Executive Officer
Ardea Biosciences
Agouron Pharmaceuticals
Pfizer
Robert Rosen
President &
Chief Commercial Officer
Bayer Healthcare
Sanofi-Synthèlabo
Imclone
Stephen Davis
Chief Operating Officer
Ardea Biosciences
Neurogen
Mark Gelder, M.D.
Senior Vice President &
Chief Medical Officer
GE Healthcare
Bayer Healthcare
Wyeth
Michael Adam, PhD
Senior Vice President
Regulatory Affairs and Quality
Pfizer
Agouron Pharmaceuticals
Bristol-Myers Squibb
Paul Marshall
Senior Vice President
Technical Operations
Amylin
Amgen
Baxter International
Thomas Ottoboni, PhD
Vice President
Pharmaceutical Development
Talima Therapeutics
Point Biomedical
InSite Vision
Brian Drazba
Vice President, Finance &
Chief Financial Officer
ISTA Pharmaceuticals
Insight Health Corp
Arthur Andersen & Co
Senior Management
3 |
 4
Highlights
•
Lead product candidate, SUSTOL™
(formerly known as APF530), is a long-acting,
injectable product for the prevention of chemotherapy-induced nausea and
vomiting (CINV)
–
Incorporates
widely
used
5-HT3
antagonist
granisetron
(Kytril
®
)
with
a
5-day
delivery
profile
–
Reduces both acute-
and delayed-onset CINV with a single injection
–
Patent coverage into 2024; however, effective exclusivity actually longer due to
polymer •
SUSTOL
shown
to
be
non-inferior
to
market
leader
Aloxi
®
–
1,341-patient, randomized, controlled, Phase 3 study
•
On-going 1000 patient study in patients receiving highly emetogenic
chemotherapy
(HEC) is designed to obtain a “delayed HEC”
indication
–
No 5-HT3 agent has the delayed HEC indication
–
About 500,000 units of Aloxi are used annually in HEC patients
•
SUSTOL targets a large market opportunity, with approximately 7 million doses of
chemotherapy annually in US alone*
•
Plans
to
leverage
our
Biochronomer™
drug
delivery
technology,
development
capacity
and
commercial
expertise
for
other
opportunities:
–
Long-acting anesthetic for post-surgical pain
–
Double and triple-combination for CINV is under evaluation, with potential for
several others *TDR August 2006 internal report |
 SUSTOL CLINICAL SUMMARY |
 6
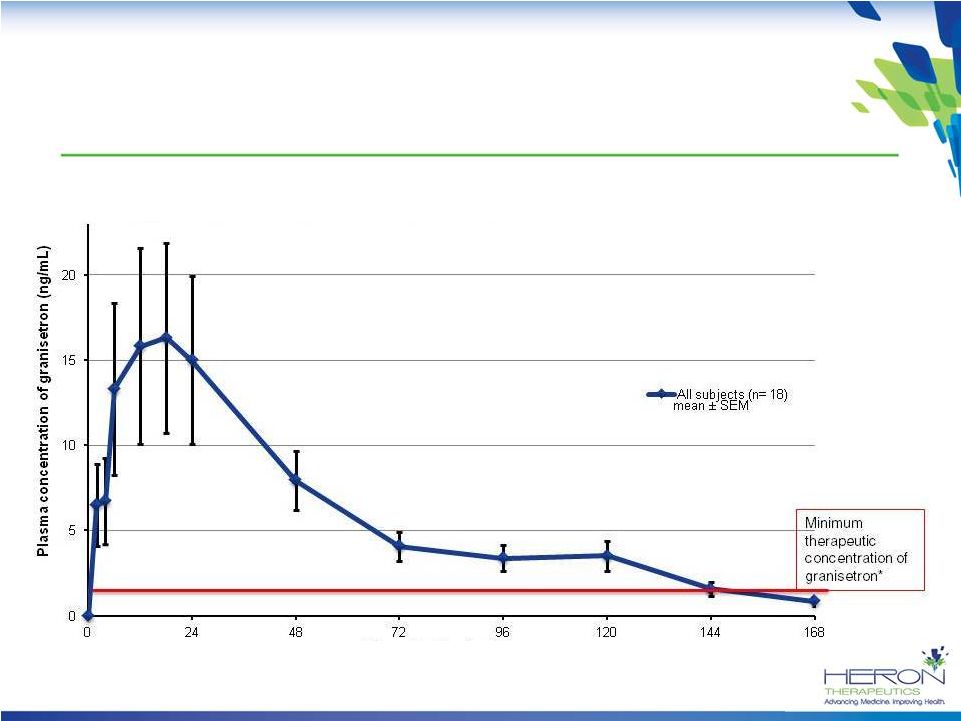
5-Day Profile: APF530 Pharmacokinetics
Granisetron is released rapidly following injection of APF530 and continues to be
released over a 5-day period, providing long-acting coverage for
CINV *Data from patent application 20120258164 for transdermal granisetron
Time after Dosing (h) |
 7
SUSTOL Pivotal Phase 3 Study
Overview
•
Randomized, controlled, multi-center study
•
1,341 patients in primary efficacy population
•
Two doses of APF530 (5 mg and 10 mg granisetron)
compared to the approved dose of Aloxi (results from 10
mg dose group presented)
•
Patients stratified by type of chemotherapy regimen:
moderately emetogenic (MEC) or highly emetogenic (HEC)
•
Primary end point compared complete response between
groups in both the acute (day 1) and delayed (days 2-5)
phase
–
Complete response defined as no emesis and no rescue medications
–
A ±15% margin was used to establish non-inferiority
|
 8
Primary Efficacy Results: Complete
Response
Patients Receiving Moderately
Emetogenic Chemotherapy
Acute
Delayed
Difference in Complete Response
APF530-Aloxi (97.5% CI) |
 9
Primary Efficacy Results: Complete
Response
Patients Receiving Highly
Emetogenic Chemotherapy
Difference in Complete Response
APF530-Aloxi (98.33% CI)
Acute
Delayed |
 10
Safety Summary
•¹ Safety
results with the 5 mg dose of APF530 studied in separate arm of the phase 3 study are not included
•² >90%
of injection site reactions were reported as mild; one patient discontinued due to injection site reaction |
 11
Improves
Difference
Between
SUSTOL
and
Aloxi
in
HEC
Patients
Protocol Specified HEC Population
ASCO 2011 Guideline HEC Population
FDA-Requested
ASCO
2011
Reanalysis
Acute
Delayed
Acute
Delayed |
 12
CR Rates by Treatment
Chemotherapeutic Regimen
APF530 10 mg
Aloxi 0.25 mg
Moderately
Emetogenic
Acute
Cyclophosphamide/Doxorubicin
70.7%
65.7%
All other regimens
84.4%
85.0%
Delayed
Cyclophosphamide/Doxorubicin
47.4%
46.3%
All other regimens
72.9%
70.0%
Highly
Emetogenic
Acute
Cisplatin regimens
81.1%
75.5%
Carboplatin/Paclitaxel
85.4%
89.8%
All other regimens
75.4%
67.6%
Delayed
Cisplatin regimens
66.0%
60.4%
Carboplatin/Paclitaxel
70.8%
71.4%
All other regimens
65.2%
57.4%
Largest
Differences
Between
Arms
is
Seen
With
Most
Difficult
Chemo
Regimens
1
•
1
Data from post-hoc analysis. Not statistically significant.
•
Highlighted HEC regimens were considered HEC in both protocol specified Hesketh
and 2011 ASCO Guidelines |
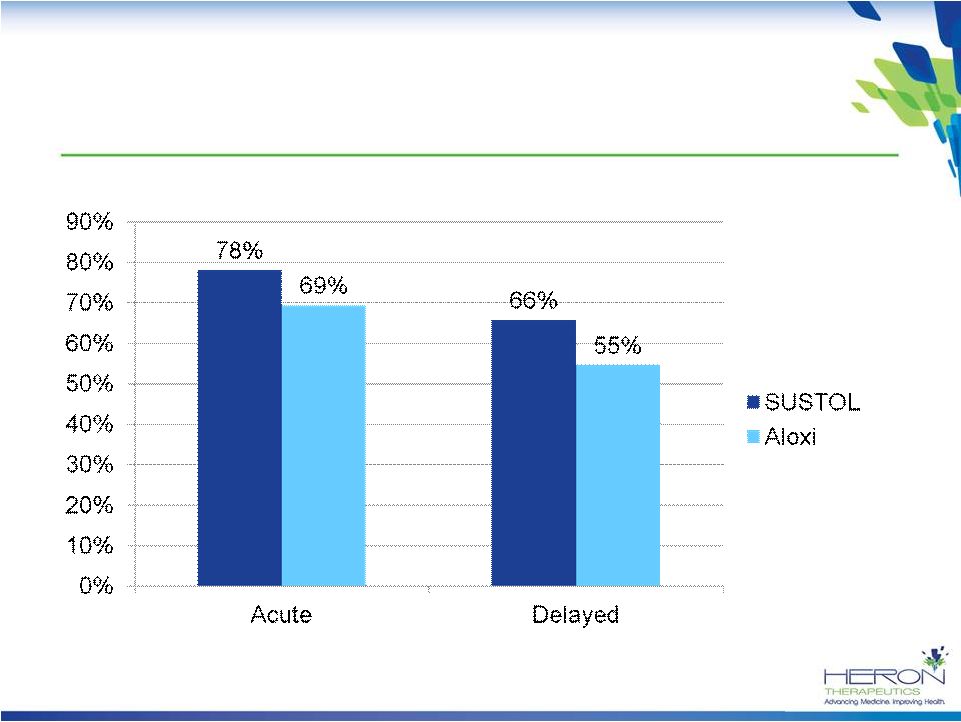 13
Response Rates With Chemotherapy Classified
as HEC by Both Hesketh and 2011 ASCO*
*Cisplatin,
carmustine, dacarbazine, dactinomycin, mechlorethamine, streptozotocin
SUSTOL is 9-11% Better Than Aloxi in the Most Emetogenic Chemotherapy
|
 14
Summary of Clinical Results
•
Biochronomer polymer-based drug delivery technology releases
granisetron over 5 days to prevent CINV
•
Large, randomized, Phase 3 study conducted: SUSTOL showed non-
inferiority to Aloxi
–
For both acute-
and delayed-onset CINV with both moderately and
highly emetogenic chemotherapy
–
Further analysis of the data shows SUSTOL to be 9-11% superior to
Aloxi in the most emetogenic chemotherapy
•
SUSTOL was well-tolerated
–
Incidence of adverse events comparable to Aloxi
–
Injection site reactions where predominately mild
•
Efficacy was maintained with reanalysis using ASCO 2011 guidelines
and through multiple cycles of chemotherapy
•
TQT study showed APF530 has no clinically significant effect on QT;
differentiated from Zofran(ondansetron) and Anzemet(dolasetron)
|
 SUSTOL LIFE-CYCLE MANAGEMENT
PLANS TO OBTAIN POST-APPROVAL
INDICATION FOR “DELAYED HEC” |
 16
On-Going Phase 3 “Delayed”
HEC Study Design
Cycle 1
1000 patients
scheduled to receive
HEC* randomized
1:1
Ondansetron 0.15 mg/kg
IV (up to 16 mg IV) d1 +
Fosaprepitant 150 mg IV d1
+ Placebo SC d1 + DEX
SUSTOL SC d1 +
Fosaprepitant 150 mg IV d1
+ Placebo IV d1 + DEX
1)
All subjects will receive dexamethasone 12 mg IV on day 1 and 8 mg PO on days 2-4
2)
All subjects will be allowed to receive “rescue”
medications as needed at the discretion of their
treating physician
* HEC agents as defined in the 2011 ASCO CINV Guidelines
•
Study design has been accepted by FDA for obtaining expanded
indication
•
Study is powered to show superiority (10% difference) to three drug
“standard of care”
for HEC
•
Study planned to complete late 2014 |
 17
New SUSTOL Study Has a High Likelihood of
Success Based on Previous Results
^^Average
Complete
Response
rate
improvement
when
adding
an
NK-1
RA
to
a
5-HT3
RA
and
Dex
is
~15
-
20%
in
the
delayed
HEC
*Poll-Bigelli;
Cancer,
97:12,
3090,
2003
**Projection
of
what
would
happen
with
a
20%
increased
response
by
addition
of
fosaprepitant
to
Sustol
+
Dex
Projected
Response
with addition
of NK1
^^
Study
powered
to show 10%
difference:
65% vs 75%
APF530 + Dex
+ Fosaprepitant**
APF530+Dex
Ondansetron + Dex
+ Fosaprepitant*
Ondansetron + Dex*
Standard of Care
Phase 3 Study
HEC Study
67%
65%
45%
Study powered for a 10% difference between arms
20% difference is expected with the addition of fosaprepitant,
87%
75% |
 18
SUSTOL Has the Potential to be the Next
Generation 5-HT3 Receptor Antagonist
5-HT3
RAs
1
st
generation
2
nd
generation
3
rd
generation
Products
ondansetron
granisetron
palonosetron
SUSTOL
Duration of
action
Short acting
~ 8 hr half-life
Longer acting
~40 hr half-life
Long acting
PK profile 5-7 days
Indications
Prevention of CINV in
emetogenic chemo including
high-dose cisplatin
MEC –
acute & delayed CINV
HEC –
acute CINV
MEC –
acute & delayed CINV
HEC –
acute & delayed CINV*
*Obtaining delayed HEC will be based on completion of new clinical trial
|
 SUSTOL REGULATORY
STATUS |
 20
SUSTOL NDA Status
•
Submitted NDA in May 2009 under 505(b)(2) filing pathway
•
Received Complete Response Letter in March 2010
•
FDA raised major issues in multiple areas
Resubmitted NDA in September 2012
•
CMC: correction of PAI issues and revision of one in-vitro release
method
•
Requirement for Human Factors Validation Study with commercial
product
•
Re-analysis of the existing Phase 3 study using the ASCO 2011
guidelines for categorization of MEC and HEC
-
Received Complete Response Letter March 2013 raising three main
issues: |
 21
How We Are Addressing the CRL
•
Chemistry, Manufacturing, and Controls
–
Sites with PAI issues have been eliminated from the supply chain, with work
transferred to a well-established site with no PAI issues
•
Transition
is
complete,
with
secondary
benefit
of
improvement
in
the
COGS
–
New in-vitro release method has been developed and validated
–
Multiple validation batches of finished product have now been completed
•
Human Factors Validation Study
–
Will be completed shortly
•
Re-analysis of Phase 3 using new ASCO 2011 Guidelines
–
Re-analysis complete
–
Complete dataset and programs supplied to FDA and found acceptable
•
Re-submission is planned for mid-2014 |
 SUSTOL COMMERCIAL
OPPORTUNITY |
 23
U.S. CINV Market Dynamics
Source: WK 07/2013 |
 24
HEC Regimens Represent a Significant
Market Opportunity for SUSTOL
1
IntrinsiQ data from July 2012 –
June 2013
HEC regimens account for ~20% (500K)
of palonosetron administrations
Of all HEC administrations, ~20% are given
without concomitant IV 5-HT3 –
inconsistent with clinical guidelines |
 POST-OPERATIVE PAIN PROGRAM |
 |
 27
Goals for Pain Program
•
Develop products that provide a clear advantage
compared to available therapies
•
Take advantage of the FDA’s current focus on reducing
the use of opiates
•
Main goals of therapy for our post-operative pain program
–
Significantly reduce:
•
pain intensity for 3-4 days post-operatively
•
opiate use
•
length of hospital stay
•
hospital readmissions due to pain |
 28
Biochronomer Bupivacaine/Meloxicam
Significantly
Superior
to
Exparel
®
at
24-72
Hours
1.
Study #1; All studies used the post-operative pain model in pigs from Castle et
al, 2013 EPJ 2.
Study #2 compared <½
expected human dose of Biochronomer bupivacaine/meloxicam formulation to the
human dose of Exparel (40% smaller incision used with Exparel)
Pig Post-Operative Pain Model
(n=4 pigs, except at 120 hrs for Study #2: preliminary results from 2 animals)
|
 29
A New Formulation of Bupivacaine + Meloxicam
Has Been Added to Our Pain Program
•
While both Biochronomer ropivacaine and bupivacaine formulations
produce significant pain relief for 72-96 hrs, and function better than
Exparel, neither provide complete elimination of pain for the critical first
2-3 days in the pig post-operative pain model
•
In order to achieve near complete control of pain, it is necessary to target
the hyperalgesia caused by
inflammatory during the first several days
•
A combination product using our Biochronomer technology was developed
•
Bupivacaine was selected for the combination product due to improved co-
formulation characteristics
•
Meloxicam was selected as the NSAID due to its high potency, good local
tolerability and minimal effects of platelets
–
Local administration of meloxicam is very good and did not differ from placebo,
even when administered daily for 4 weeks (British Journal of
Rheumatology 1996;35 (suppl. l): 44-50)
–
The very low dose of meloxicam in our formulation is less than half of the
no-effect dose for altering Thomboxane B2 formation or platelet
aggregation (Journal of Clinical Pharmacology,
2002;42:881-886) |
 30
30
Only One Day of Pain Reduction Observed
With 60-hour Continuous Infusion of
Bupivacaine
Post-Herniorrhaphy
1
Mean Worst Pain Scores
Daily Use of Hydrocodone
1. Schurr et al. Surgery 2004;136:761-9
•
Continuous infusion of
bupivacaine failed to show
significant benefits after 24 hr¹
•
Biochronomer formulations of
either bupivacaine or ropivacaine
showed reduced analgesic
effects days 2-4, even though
pharmacokinetics data showed
drug was continually being
released during that period
•
Inflammation is known to reduce
the effectiveness of local
anesthetics
•
Biochronomer co-formulation of
local anesthetic and NSAID
developed to overcome this
limitation of local anesthetics |
 31
Next Steps for Post-Operative Pain
Program
•
Combination formulation has been selected
•
Starting Phase 1 enabling toxicology shortly
•
Initiate Phase 1 with combination product in Fall
•
Assuming positive results from Phase 1, initiate Phase
2 program before the end of this year
•
Continue development of Biochronomer ropivacaine
alone formulation focused on nerve block, where the
inflammatory component of pain is not relevant |
 32
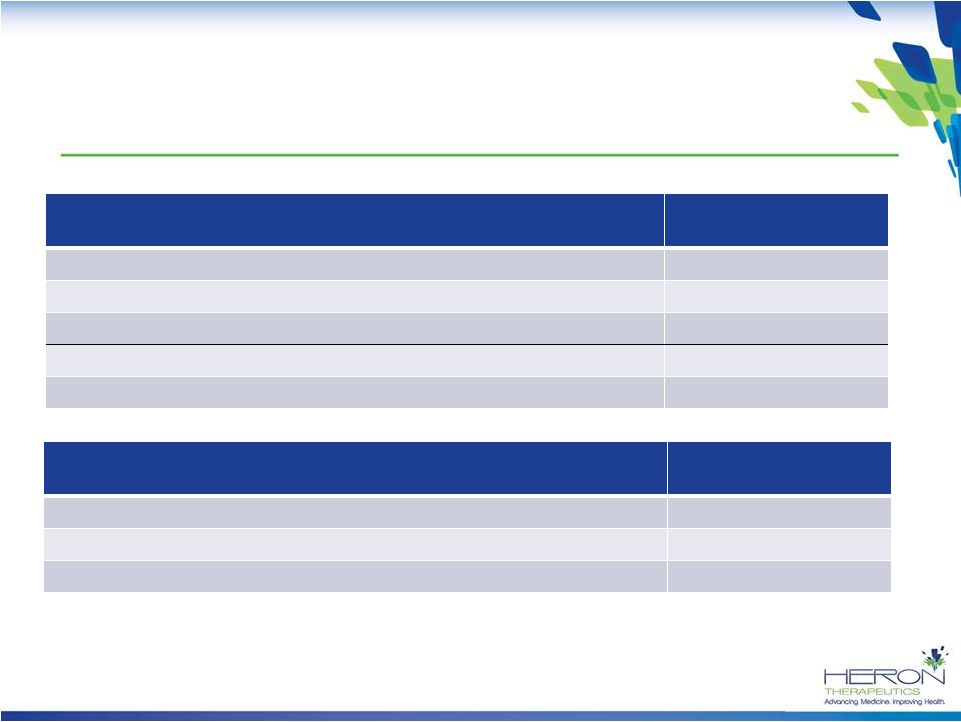
Financial Summary
Summary Statement of Operations
(In thousands, except per share data)
Three Months Ended
March 31, 2014
Revenue
$
– Operating expenses
17,322
Other income (expenses)
(216)
Net loss
$ (17,538)
Net loss per share¹
$ (0.74)
Condensed Balance Sheet Data
(In thousands)
March 31, 2014
Cash and cash equivalents
$ 57,475
Total assets
$ 62,793
Total stockholders’
equity
$ 55,409
1
Based on 23.7 million weighted average common shares outstanding for the period ended
March 31, 2014 (1-for-20 reverse stock split in JAN2014).
|
