Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - GALECTIN THERAPEUTICS INC | d701335d8k.htm |
| EX-99.2 - EX-99.2 - GALECTIN THERAPEUTICS INC | d701335dex992.htm |
 GT-020 Phase 1 Clinical Trial:
Results of First Cohort
Release: March 31, 2014
Webcast: April 1, 2014
NASDAQ: GALT
www.galectintherapeutics.com
©
2014 Galectin Therapeutics inc.
Exhibit 99.1 |
 Forward-Looking Statement
This
presentation
contains,
in
addition
to
historical
information,
forward-looking
statements
within
the
meaning
of
the
Private
Securities
Litigation
Reform
Act
of
1995.
These
statements
relate
to
future
events
or
future
financial
performance,
and
use
words
such
as
“may,”
“estimate,”
“could,”
“expect”
and
others.
They
are
based
on
our
current
expectations
and
are
subject
to
factors
and
uncertainties
which
could
cause
actual
results
to
differ
materially
from
those
described
in
the
statements.
These
statements
include
those
regarding
potential
therapeutic
benefits
of
GR-MD-02
and
expectations
regarding
the
clinical
trial,
including
the
future
enrollment
of
patients
and
the
timing
of
results
from
the
second
cohort.
Factors
that
could
cause
our
actual
performance
to
differ
materially
from
those
discussed
in
the
forward-looking
statements
include,
among
others,
that
results
from
the
first
cohort
of
Phase
1
may
differ
materially
from
future
results,
and
there
is
no
guarantee
that
the
current
clinical
trial
will
lead
to
positive
outcomes
or
that
GR-MD-02
will
ever
be
approved
by
the
FDA.
We
may
experience
delays
in
the
current
trial,
and
we
may
have
difficulty
enrolling
patients
and
processing
the
resulting
data.
Future
phases
or
future
clinical
studies
may
not
begin
or
produce
positive
results
in
a
timely
fashion,
if
at
all,
and
could
prove
time
consuming
and
costly.
Plans
regarding
development,
approval
and
marketing
of
any
of
our
drugs
are
subject
to
change
at
any
time
based
on
the
changing
needs
of
our
company
as
determined
by
management
and
regulatory
agencies.
Regardless
of
the
results
of
current
or
future
studies,
we
may
be
unsuccessful
in
developing
partnerships
with
other
companies
or
obtaining
capital
that
would
allow
us
to
further
develop
and/or
fund
any
studies
or
trials.
To
date,
we
have
incurred
operating
losses
since
our
inception,
and
our
ability
to
successfully
develop
and
market
drugs
may
be
impacted
by
our
ability
to
manage
costs
and
finance
our
continuing
operations.
For
a
discussion
of
additional
factors
impacting
our
business,
see
our
Annual
Report
on
Form
10-K
for
the
year
ended
December
31,
2013,
and
our
subsequent
filings
with
the
SEC.
You
should
not
place
undue
reliance
on
forward-looking
statements.
Although
subsequent
events
may
cause
our
views
to
change,
we
disclaim
any
obligation
to
update
forward-looking
statements.
2
©
2014 Galectin Therapeutics | NASDAQ:GALT |
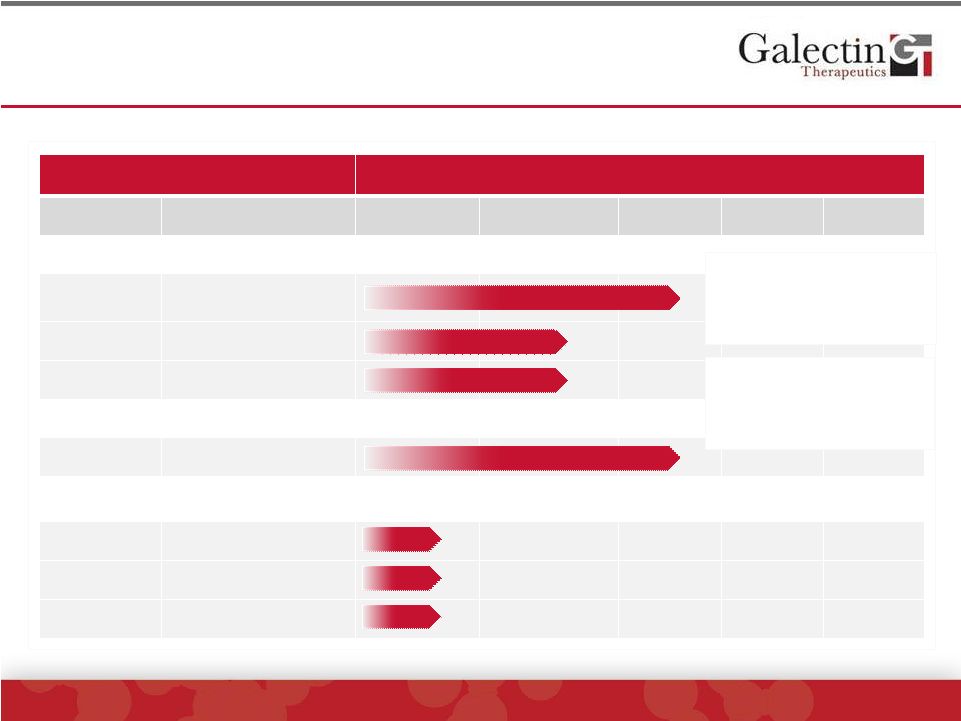 Our
Pipeline Of Galectin-3 Inhibitors ©
2014 Galectin Therapeutics | NASDAQ:GALT
3
Clinical Focus
Stage of Development
Drug
Indication
Discovery
Pre-clinical
Phase 1
Phase 2
Phase 3
Fibrosis
GR-MD-02
Fatty liver disease with
advanced fibrosis
Lung fibrosis
Kidney fibrosis
Cancer Immunotherapy
GR-MD-02
Melanoma
Galectin-3 Inhibitors
GR-MD-03
Subcutaneous
GR-MD-04
Oral
G-XXX*
Oral
Report on first
cohort of Phase 1
Clinical Trial
*Galectin Sciences, LLC
Timely Reporting:
Last bloods: 3-7-14
Last visit: 3-21-14 |
 Summary of Findings
•
GR-MD-02 was safe and well tolerated at 2 mg/kg (80 mg/m
2
) with no drug-
related adverse events
•
Pharmacokinetics was consistent between individuals and after single and
multiple doses; exposure was 40% of lowest dose used in NASH animal
model; this was a therapeutic dose
•
Key composite biomarkers of fibrosis improved after four doses of
GR-MD-02 •
Key inflammatory cytokines were decreased after four doses of
GR-MD-02 •
Patients with greater cellular injury as indicated by elevated ALT levels, had a
marked decrease in CK-18, a cell death biomarker
•
Galectin-3 blood levels do not correlate with disease activity and are not a
biomarker of drug effect in patients with NASH with advanced fibrosis
In addition to being safe and well tolerated, GR-MD-02 improved
biomarkers of fibrosis, inflammation and liver cell injury in patients with
NASH with advanced fibrosis
4
©
2014 Galectin Therapeutics | NASDAQ:GALT |
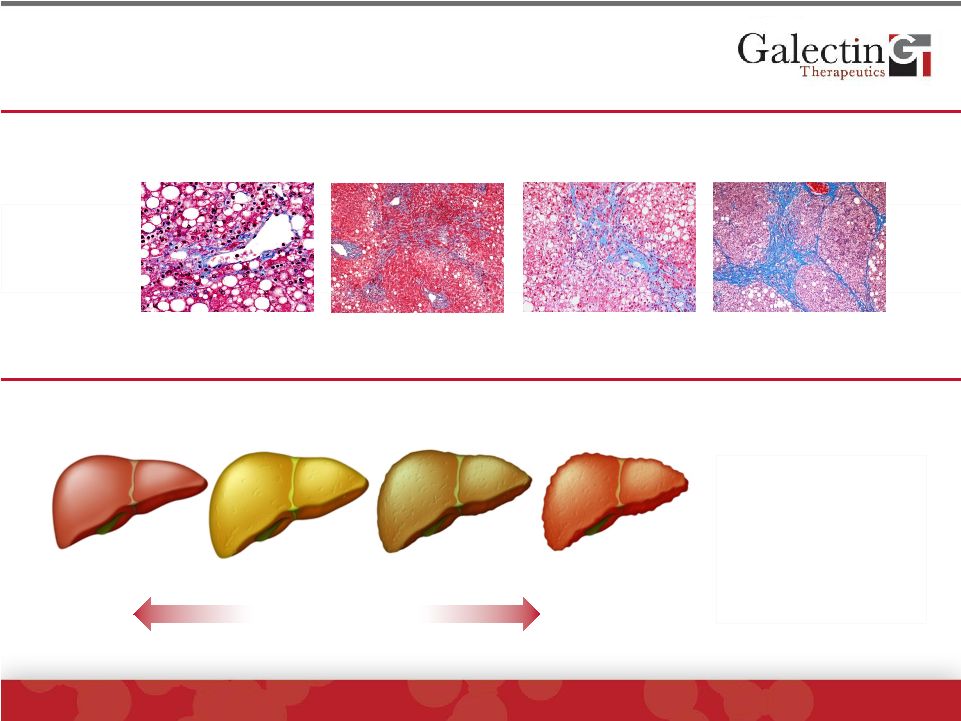 All
Chronic Liver Diseases Lead To Fibrosis Example: Liver Fibrosis In Fatty Liver
Disease (NASH) ©
2014 Galectin Therapeutics | NASDAQ:GALT
5
Stage 1
Stage 2
Stage 3
Stage 4
Patient
Liver
biopsy
Healthy
Fatty
Fibrosis
Cirrhosis
Liver failure
Bleeding
Encephalopathy
Edema
Asymptomatic
Only therapy for
patients with
cirrhosis is liver
transplantation
Bridging Fibrosis
Cirrhosis
Portal/Central
Pericellular/Central
(High Mag)
Blue=fibrosis
Occurs over decades |
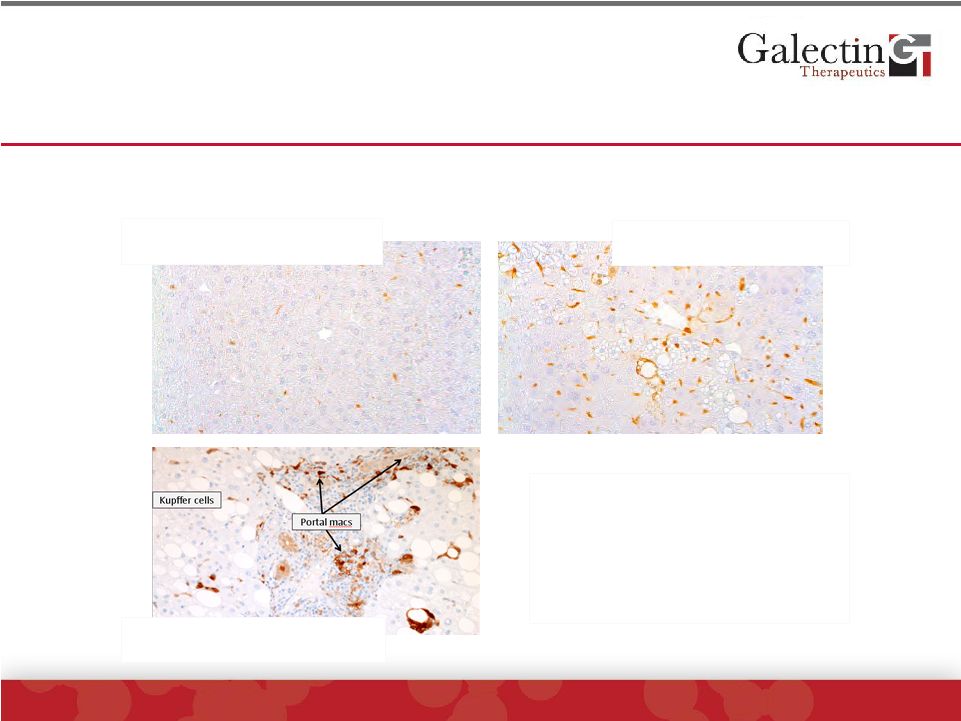 Galectin-3 is Expressed In Liver
Macrophages And Is Markedly Increased In
Human and Mouse NASH
©
2014 Galectin Therapeutics | NASDAQ:GALT
6
Normal Mouse Liver
NASH Mouse Liver
•
Kupffer cells= liver resident
macrophages
•
Portal macs=macrophages
located in portal regions
Immunohistochemistry for Gal-3 (brown pigment indicates gal-3)
NASH Human Liver |
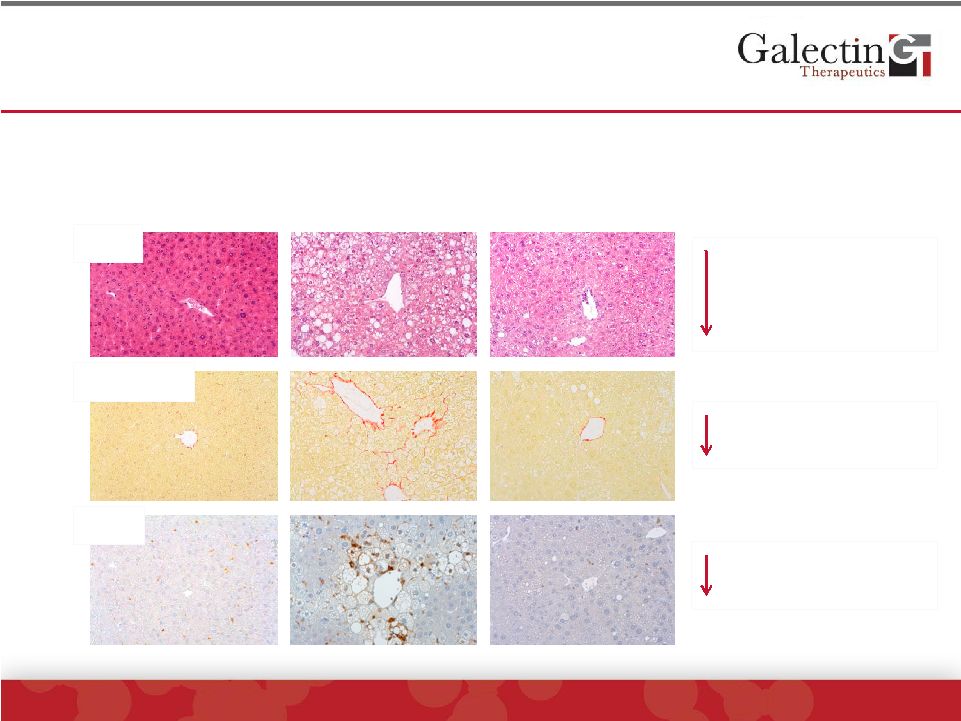 GR-MD-02, A Galectin-3 Inhibitor, Has Therapeutic
Effect On NASH With Fibrosis In Mouse Model
©
2014 Galectin Therapeutics | NASDAQ:GALT
7
Normal
NASH:Control
NASH:GR-MD-02
GR-MD-02 Effects
NAFLD Activity Score
•
Fat
•
Cell death
•
Inflammation
Collagen
(Fibrosis)
Galectin-3
Protein
Improvement is linked to decreased tissue Galectin-3
H&E
Sirius Red
Gal-3 |
 GR-MD-02 Is A Galectin-3 Inhibitor That Reduces
Collagen Synthesis And Increases Collagen
Degradation In Pre-Clinical Models
©
2014 Galectin Therapeutics | NASDAQ:GALT
8
Restoration to Normal
Fibrosis results from increased
collagen and other matrix protein
synthesis with little to no change in
collagen degradation.
Liver Fibrotic Tissue Homeostasis
Normal
In the normal liver, collagen and
matrix protein synthesis matches
degradation to provide appropriate
amount of extracellular matrix.
Collagen Synthesis
Collagen Degradation
=
Collagen Synthesis
Collagen
Degradation
Fibrosis
Collagen
Synthesis
Fibrosis can resolve either by a
reduction in collagen synthesis or an
increase in degradation. The
combination would increase rate of
resolution.
+
Collagen
Degradation
+/- |
 GR-MD-02 Is Being Developed For The Indication Of
NASH With Advanced Fibrosis (Stage 3 and 4)
9
©
2014 Galectin Therapeutics | NASDAQ:GALT
Obesity/Insulin Resistance/Diabetes
Steatosis (fatty liver)
NASH (inflammation, cell death)
Stage 1 2 3
Fibrosis
Stage 4
Cirrhosis
•
No certainty of progression from early to late disease in an individual
•
Late disease much closer to clinical outcomes
•
Surrogates of clinical outcomes are better developed for late disease
•
GR-MD-02 reduces inflammation, ballooning and fat in NASH and reduces
existing fibrosis and reverses cirrhosis in animal models
Early Disease
Late Disease
Clinical Outcomes
Complications
Transplant
Death
Targeting Late Disease |
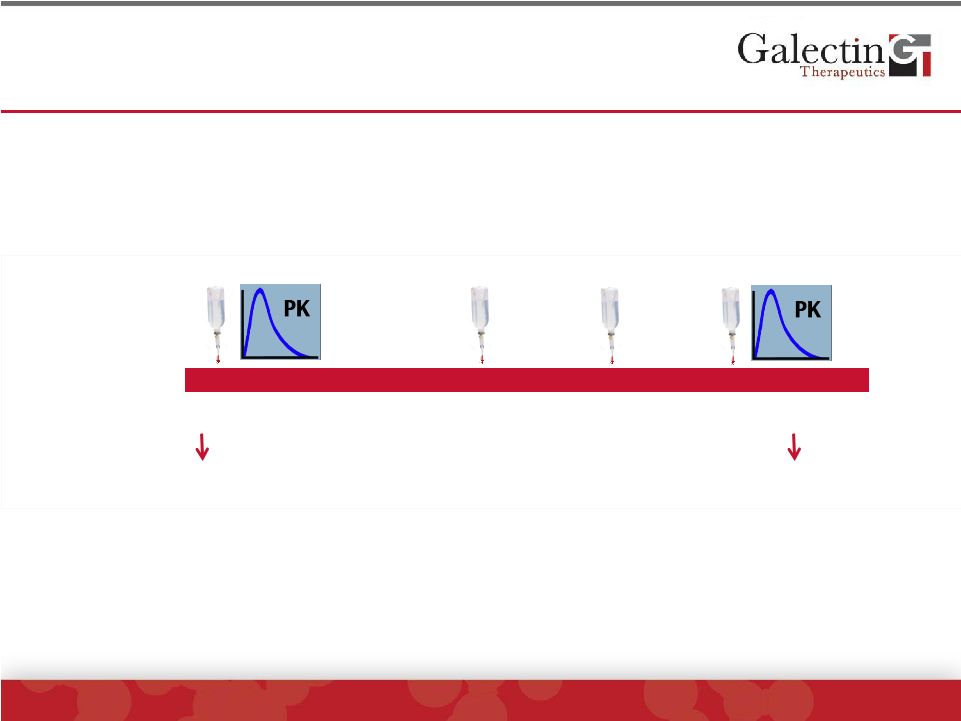 Phase
1 Clinical Trial Of GR-MD-02 In NASH With Advanced Fibrosis: Report On
Cohort 1 ©
2014 Galectin Therapeutics | NASDAQ:GALT
10
0
28
35
42
56
70
Day
Infusion
-1
Biomarkers
Biomarkers
Patient inclusion:
Biopsy proven NASH with advanced fibrosis (stage 3)
Design:
Cohort has 8 patients (6 active, 2 placebo, blinded)
Dose:
Starting dose of 2 mg/kg lean body weight (equivalent to 80 mg/m
2
);
Infusions at days 0, 28, 35 and 42.
Primary endpoints:
Safety
Pharmacokinetics
Secondary endpoints:
Disease-related serum biomarkers to assess for
potential treatment effect |
 Patient Characteristics & Safety
Patient Characteristics
•
6 women and 2 men
•
Ages 40-64 (mean=54)
•
Mean body mass index (BMI)=39
(obese >30)
•
Diabetes Mellitus in 6 patients
•
Patients had a liver biopsy within one year of
enrollment
•
All patients had definitive pathological
diagnosis of NASH
•
7 patients had stage 3 fibrosis
(bridging); 1 patient had stage 4 fibrosis
•
All patients enrolled completed full protocol
through final follow-up visit at day 70.
•
Last subject, last blood draw was 3-7-14;
Last subject, last visit was 3-21-14
11
©
2014 Galectin Therapeutics | NASDAQ:GALT
Patient Safety
•
There were no Serious Adverse Events
•
There were no Treatment Emergent
Adverse Events in patients receiving GR-
MD-02 that were attributed to the drug
•
One patient receiving GR-MD-02 had
several mild AE’s that were judged by
investigator to be unrelated to drug
•
Two patients receiving placebo had mild
AE’s that were judged by investigator as
possibly related
•
There were no treatment emergent
laboratory or ECG findings
GR-MD-02 at a dose of 2 mg/kg
(80 mg/m
2
) was safe and well tolerated |
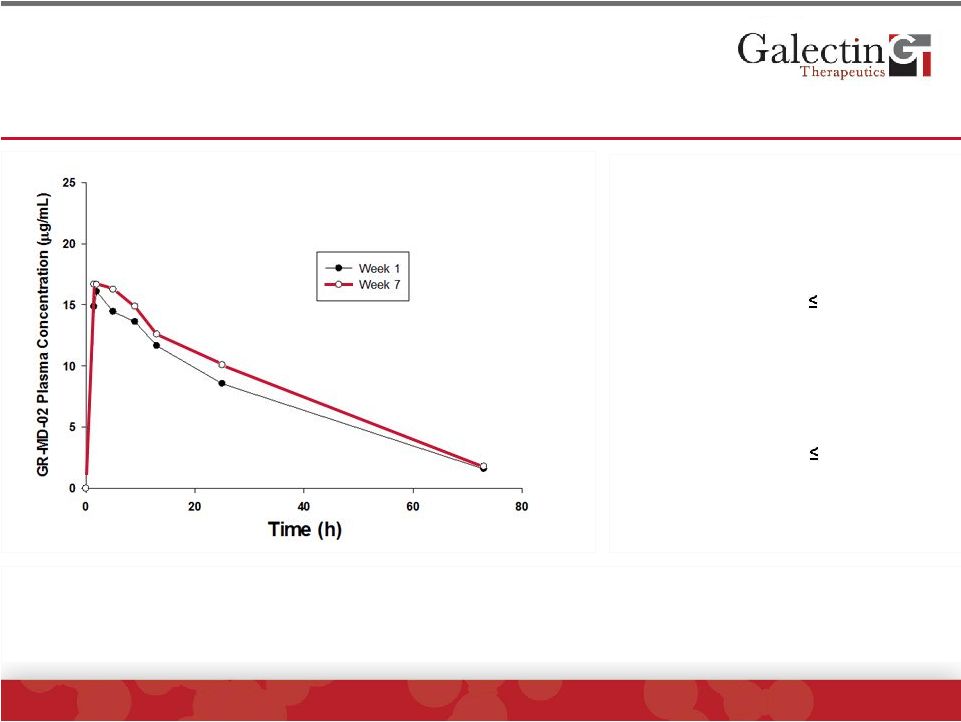 Pharmacokinetics: GR-MD-02 Blood Levels Were
Consistent Between Individuals And Not Significantly
Different After Single Or Multiple Infusions
©
2014 Galectin Therapeutics | NASDAQ:GALT
12
First Infusion (week 1)
Fourth Infusion (week 7)
The AUC in humans given 2 mg/kg was approximately 40% of the AUC
of
the lowest therapeutic dose in the mouse NASH model
C
max
= 16.3 µg/mL
T
1/2
= 19.9 h
AUC = 572.6 h*µg/mL
V
ss
= 5.2 L
Variability
15%
C
max
= 17.7 µg/mL
T
1/2
= 20.5 h
AUC = 645.4 h*µg/mL
V
ss
= 4.7 L
Variability
24%
Mean GR-MD-02 Plasma Concentration-Time
Profiles of 6 Patients on Weeks 1 and 7
Accumulation ratio ~1.16
(95% CI 0.85 to 1.47) |
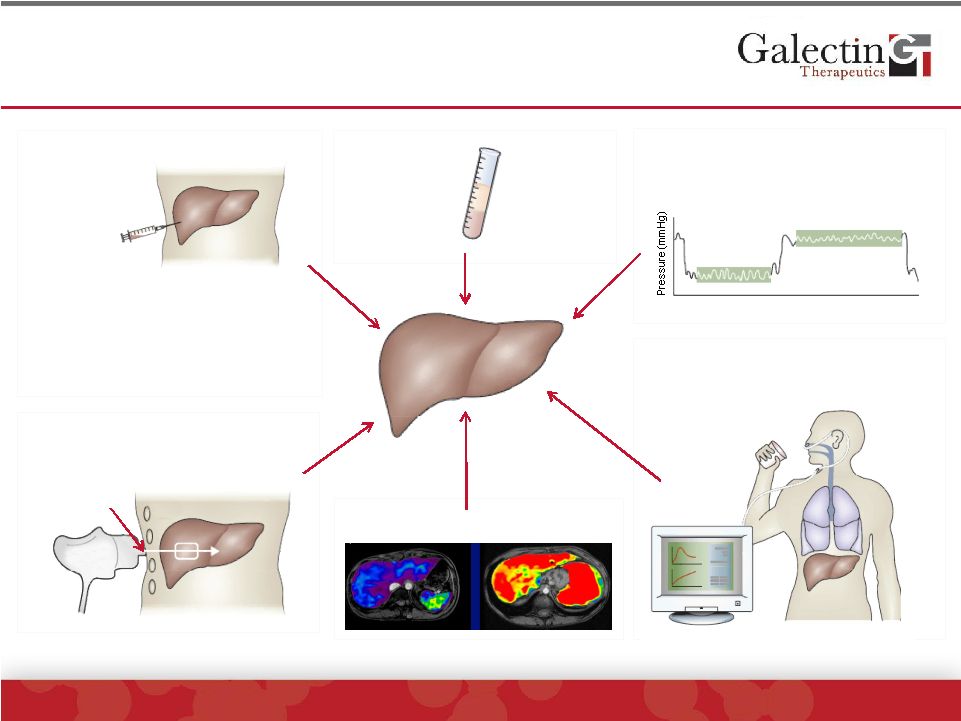 Assessment Methods for Liver Fibrosis
©
2014 Galectin Therapeutics | NASDAQ:GALT
13
Functional metabolic and
shunt tests (eg. HepQuant™)
Labeled
substrate
WHVP
FHVP
Time (s)
Vibrator
Probe
Ribs
Transducter
Serum
markers
Liver
biopsy
Transient Elastography
(FibroScan™)
Hepatic venous pressure
gradient (HVPG)
Source: Nat Rev Gastroenterol Hepatol. 2010 Aug;7(8):425-36.Epub 2010 Jun
29. MR-Elastography
•
Potential serious complications
•
1/50,000
th
of liver
•
High sampling variability
•
41% discordance of 1 fibrosis
stage in NASH*
*Ratziu, et al. Gastro. 2005 |
 Major
Pathological Processes in NASH ©
2014 Galectin Therapeutics | NASDAQ:GALT
14
Steato-Hepatitis (NASH Activity)
•
Ballooning of liver cells (cell
death/apoptosis)
key
hallmark
•
Fat in liver cells (steatosis)
•
Immune cell infiltration (inflammation)
Fibrosis/Cirrhosis
•
Increase in collagen/matrix
•
Disruption of architecture
•
Liver cell nodules
Do Not Always Correlate in Same Patient
•
Can have high NASH activity score with minimal fibrosis
•
Can have advanced fibrosis/cirrhosis with minimal NASH activity
We measured biomarkers of both major pathological processes
|
 Serum
Biomarkers Of Fibrosis In NASH ©
2014 Galectin Therapeutics | NASDAQ:GALT
15
Composite Scores
FibroTest™
(FibroSURE™)
•
Indirect
biomarker
of
fibrosis
•
Age and gender, Alpha-2-
macroglobulin, Haptoglobin,
Apolipoprotein A1, GGTP, Total
bilirubin
Individual Markers
Exploratory*
•
TGF-
•
Lumican
•
Osteopontin
•
Matrix Metalloproteinases
ELF (Enhanced Liver Fibrosis)
Score
•
Direct
biomarker
of
fibrosis
•
Hyaluronic acid
•
TIMP1 (tissue inhibitor of
metalloproteinase-1)
•
P3NP (amino terminal propeptide
of type III pro-collagen)
Hyaluronic Acid
•
Matrix polysaccharide
•
Direct marker
•
Correlates to fibrosis
* Indicates that there is some evidence
that suggests they are increased in
fibrosis, but not confirmed in sufficient
number of patients or studies
For
more
information
and
references
on
biomarkers:
http://bit.ly/1jzFK50 |
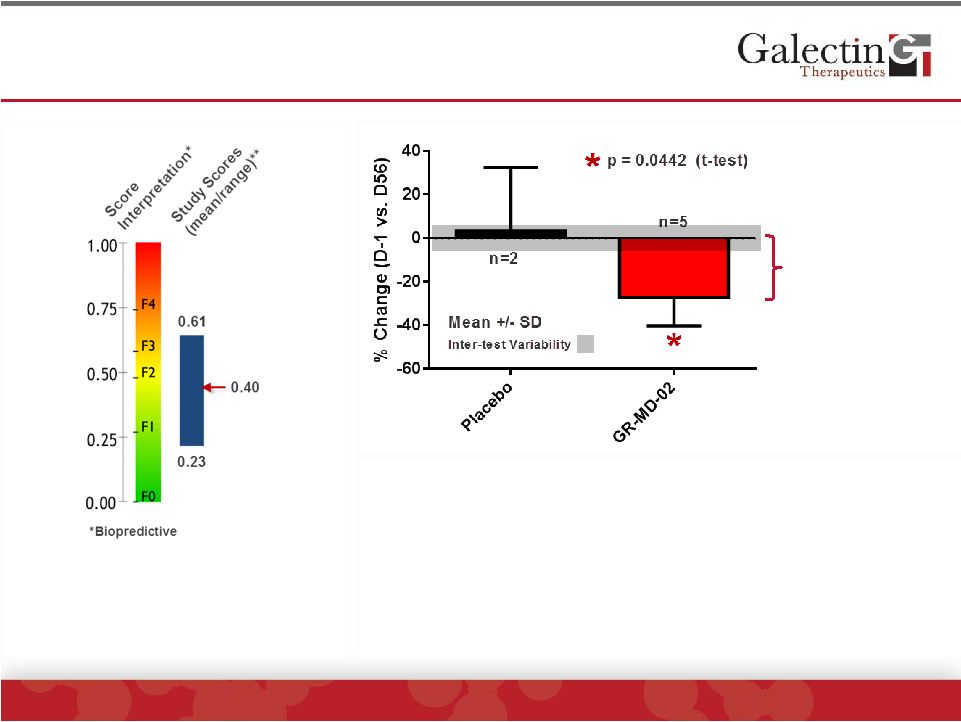 FibroTest
™
(FibroSURE
™
) Scores Significantly
Decreased In GR-MD-02 Treated Patients
©
2014 Galectin Therapeutics | NASDAQ:GALT
16
Equivalent to a 10%
change on scale
**One patient on GR-MD-02 had
scores < 0.08 which was highly
discordant with biopsy (stage 3).
Patient had high haptoglobin which is
known for false negative test.
FibroTest
™
has been shown to:
•
Correlate with stage of fibrosis
•
Assess fibrosis regression
•
Assess fibrosis progression
•
Predict liver-related mortality
Note:
While
the
numbers
are
small,
exploratory
statistics
have
been
performed
to
evaluate
differences
using
a
one-sided
t-test
and
confirmed
using
a
non-parametric
test,
Mann-Whitney |
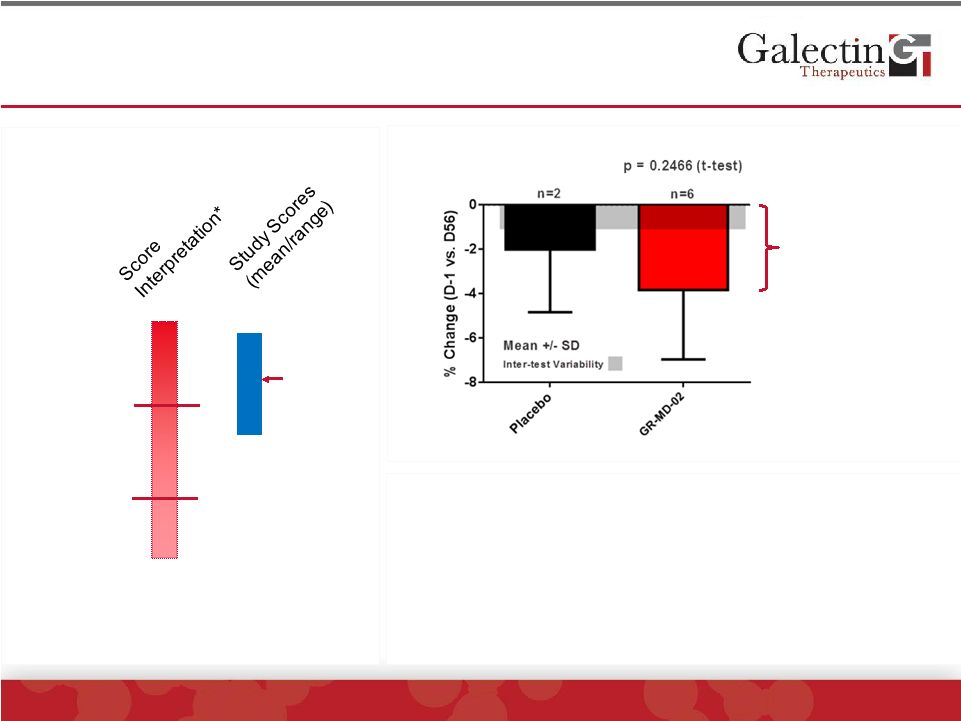 ELF
Score Tended To Decrease In GR-MD-02 Treated Patients
©
2014 Galectin Therapeutics | NASDAQ:GALT
17
7.7
9.8
Severe
Fibrosis
Moderate
Fibrosis
Mild/No
Fibrosis
10.1
8.8
*Siemens Diagnostics
Equivalent to a 0.5
change on scale
ELF score has been shown to:
•
Correlate with stage of fibrosis
•
Assess progression (0.032 increase/yr in PBC)
•
Predict mortality (a one unit change in score
correlates to a 2-fold change in liver related mortality)
11.5 |
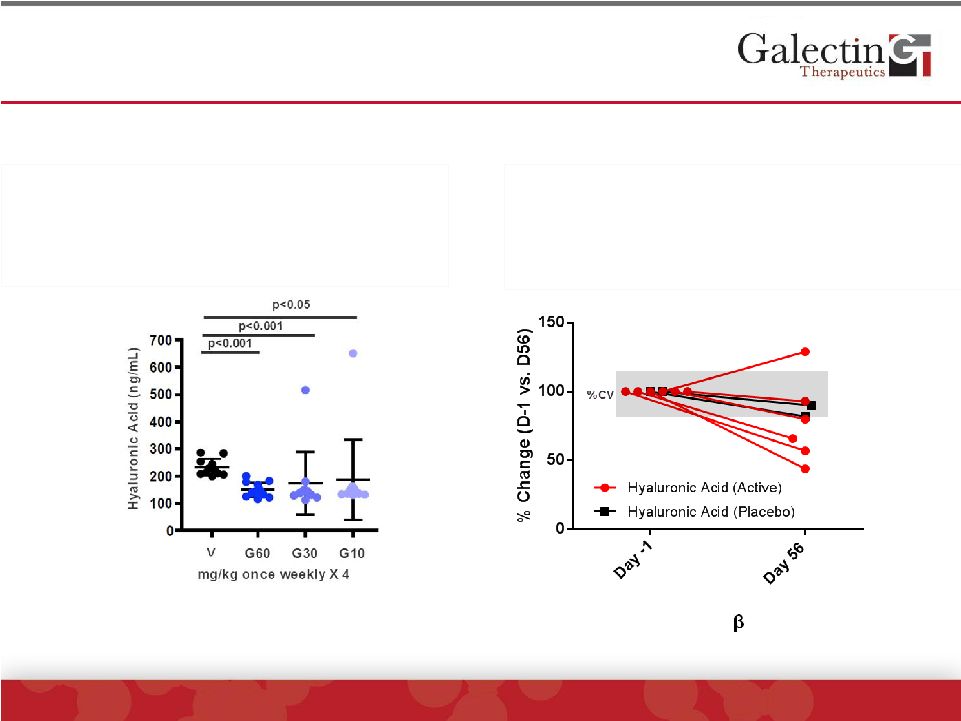 Hyaluronic Acid (HA) Levels Were Decreased In A
Subset Of Patients On GR-MD-02
©
2014 Galectin Therapeutics | NASDAQ:GALT
18
•
3 of 6 patients treated with GR-MD-02 had
significant reductions in HA
•
No change in placebo patients
•
Multiple clinical studies have shown that
HA levels correlate with liver fibrosis
•
HA levels measured in NASH mice
treated with GR-MD-02
•
HA levels decreased at all three doses
compared to vehicle-treated controls
•
Some animals had variable levels
Animal Study
Study Results
No
consistent
elevation
and/or
changes
in
Osteopontin,
TGF-
or
MMPs;
Lumican presented in later slides |
 Serum
Biomarkers of NASH Inflammation and Injury ©
2014 Galectin Therapeutics | NASDAQ:GALT
19
Cellular Injury
Serum Transaminases
•
ALT and AST
•
Enzymes released from liver cells
•
2/3 of NASH patients have normal
levels at any given time
•
Entire spectrum of disease can be
seen with normal levels
Cytokeratin 18
•
A circulating biomarker of
cell death
•
Predictive of NASH
severity
Cell Death (Apoptosis)
Key cytokines*
•
IL-6
•
IL-8
•
TNF-
Exploratory**
•
INF-
•
Endothelin-1
•
IP-10
•
VEGF
•
CD40-ligand
Inflammatory Cytokines
* Evidence of association with human NASH and importance
in pathogenesis, particularly as products of macrophages
** Some evidence of association with human and/or animal
NASH in at least one publication
For
more
information
and
references
on
biomarkers:
http://bit.ly/1jzFK50 |
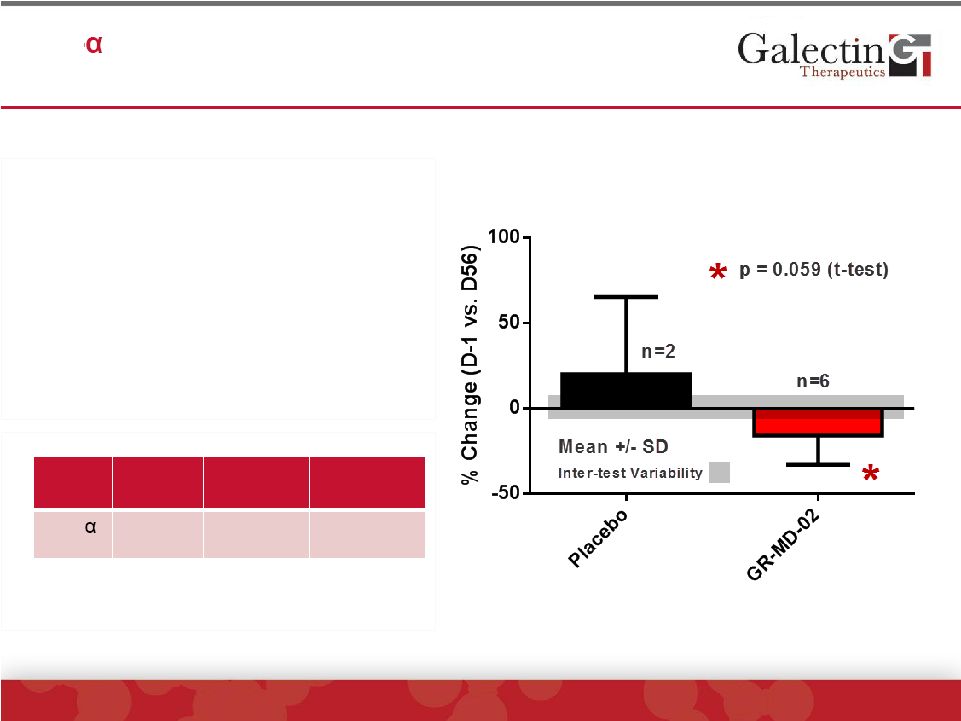
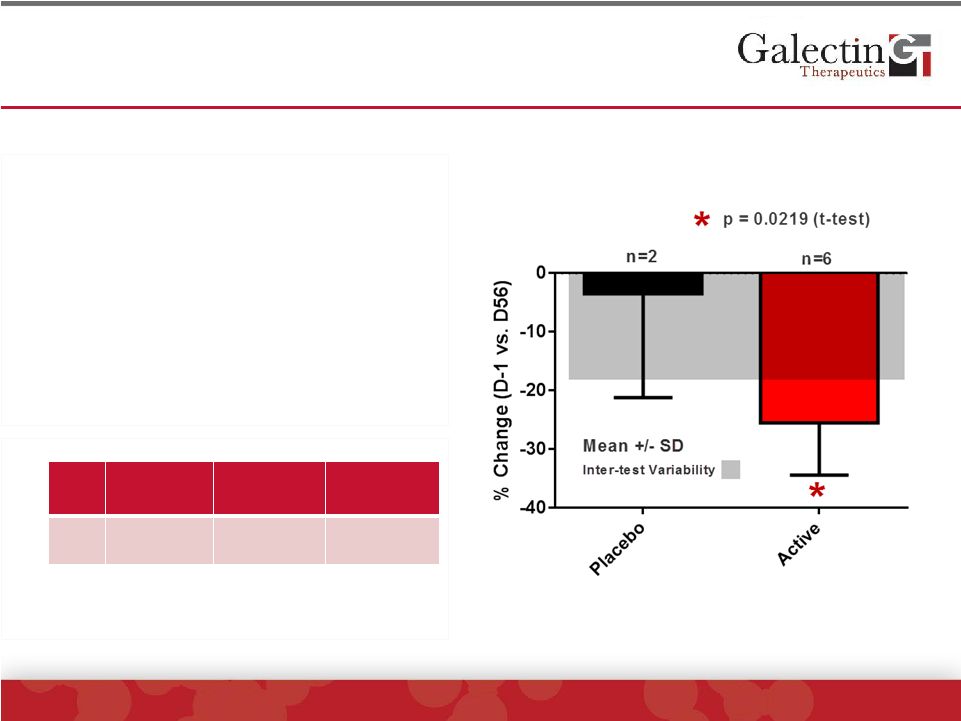 Interleukin-8 Levels Were Significantly Reduced In
GR-MD-02 Treated Patients
©
2014 Galectin Therapeutics | NASDAQ:GALT
20
•
Pro-inflammatory cytokine
expressed in macrophages
•
Elevated serum levels in NASH
•
Study patients had elevated serum
levels
•
GR-MD-02 treated patients had
significant reduction when
compared to placebo
Study
Cohort*
NAFLD**
Obese
Controls**
IL-8
pg/mL
28.0 ±
8.6
24.1 ±
38.5
7.8 ±
3.6
*Baseline levels
**Jarrar, et al. Aliment. Pharmacol. Ther. 2007 |
 ©
2014 Galectin Therapeutics | NASDAQ:GALT
21
TNF-
Levels
Were
Significantly
Reduced
In
GR-MD-02 Treated Patients
•
Pro-Inflammatory cytokine and
promotes lipid accumulation
•
Elevated serum levels in NASH
•
Study patients had elevated serum
levels
•
GR-MD-02 treated patients had
significant reduction when
compared to placebo
Study
Cohort*
NAFLD**
Obese
Controls**
TNF-
pg/mL
23 ±
5.8
6.0 ±
16.6
1.9 ±
0.3
*Baseline levels
**Jarrar, et al. Aliment. Pharmacol. Ther. 2007 |
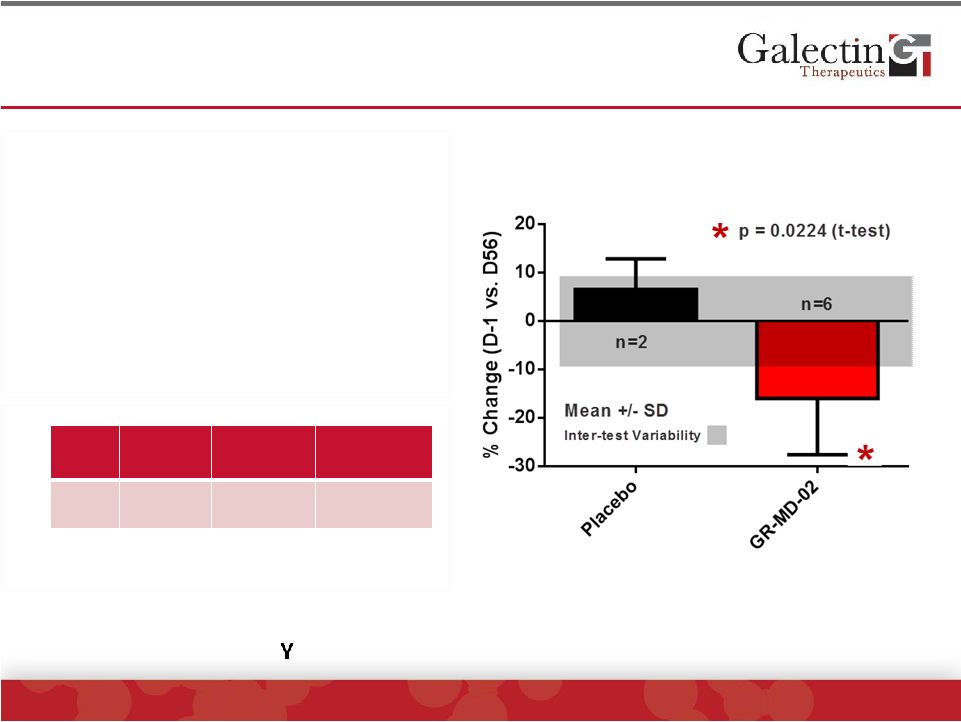 Interleukin-6 Levels Were Significantly Reduced In
GR-MD-02 Treated Patients
©
2014 Galectin Therapeutics | NASDAQ:GALT
22
•
Pro-Inflammatory cytokine
secreted by T cells and
macrophages.
•
Increased serum levels in NASH
•
Levels not increased in patients
•
GR-MD-02 treated patients had
significant reduction when
compared to placebo
Study
Cohort*
NAFLD**
Obese
Controls**
IL-6
pg/mL
6.1 ±
2.5
23.1±72.9
7.6±6.3
*Baseline levels
**Jarrar, et al. Aliment. Pharmacol. Ther, 2007
Exploratory cytokines were not elevated and/or did not change including
INF-
, Endothelin-1, IP-10, VEGF, CD40-ligand |
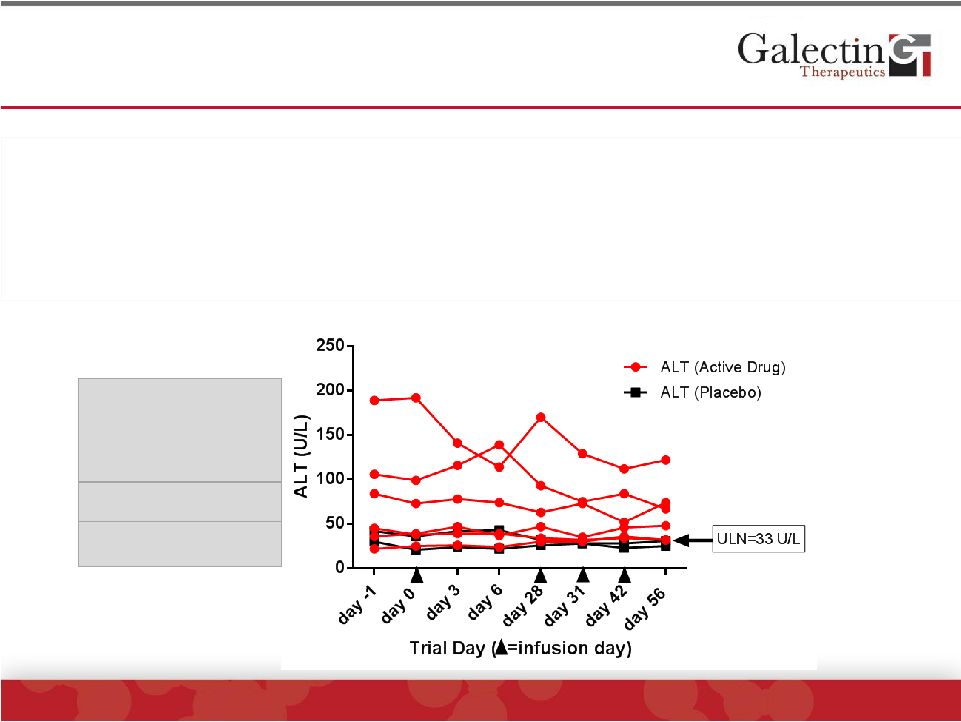 Markedly Elevated Alanine Aminotransferase (ALT)
Levels Decreased With GR-MD-02 Treatment
©
2014 Galectin Therapeutics | NASDAQ:GALT
23
•
Typical for NASH patients, there was a broad range of baseline ALT levels
•
Those with ALT levels below 50 U/L had no change with therapy
•
Two patients with ALT above 100 U/L, both of whom received active drug, had
reductions of 39 U/L and 67 U/L
•
One
patient
with
ALT
between
50
and
100
had
minimal
reduction
of
10
U/L
100-200
(2 patients)
50-100
(1 patient)
0-50
(5 patients) |
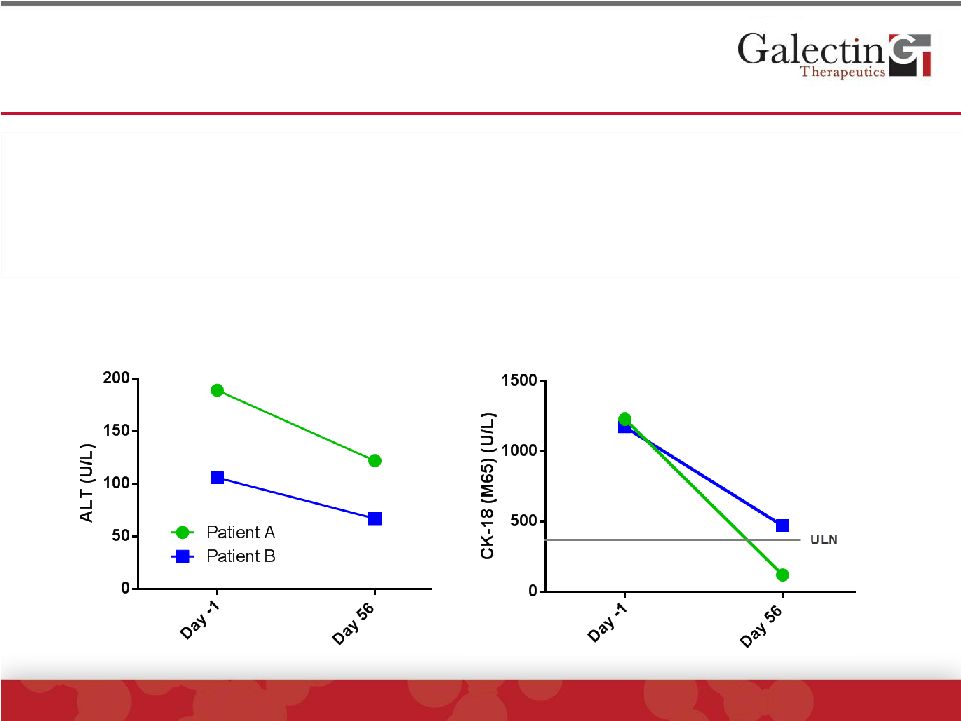 Cell
Death Biomarker CK18 Was Reduced In Two Patients With Highest ALT Levels
©
2014 Galectin Therapeutics | NASDAQ:GALT
24
CK-18 (M65)
ALT
•
CK-18, a biomarker of cell death of hepatocytes, was markedly
reduced in the two patients with ALT greater than 100 U/L
|
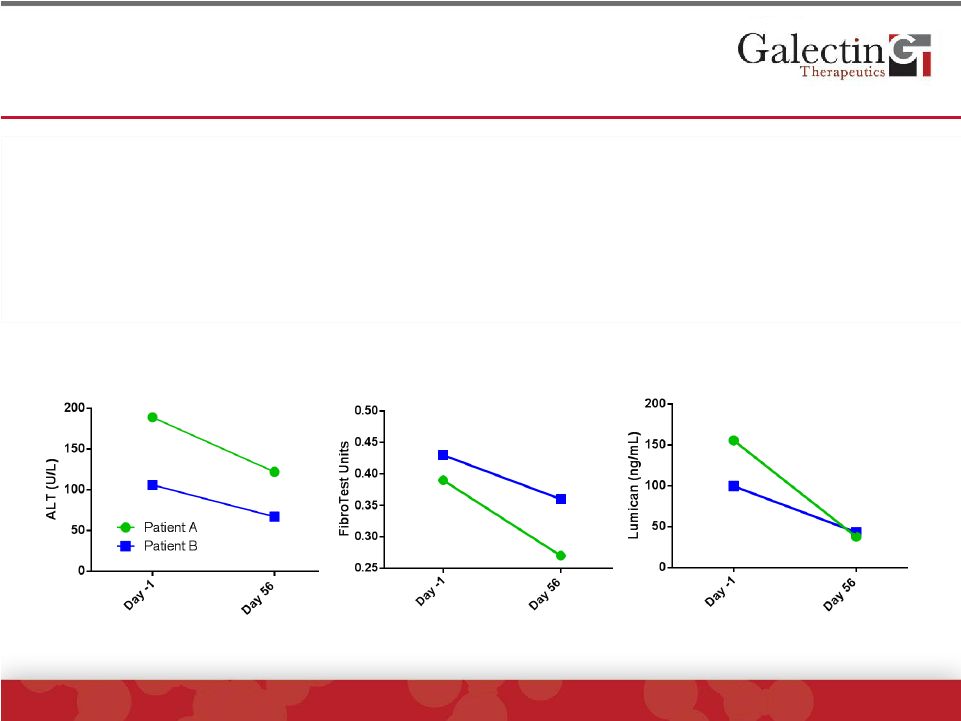 Fibrosis Biomarkers Were Reduced In The Two
Patients Receiving GR-MD-02 With Highest ALT*
©
2014 Galectin Therapeutics | NASDAQ:GALT
25
Lumican
ALT
FibroTest™
•
FibroTest™
scores were markedly decreased in the high ALT
patients after treatment with GR-MD-02
•
Lumican, a matrix protein that is involved in fibrogenesis in the liver,
was elevated in all patients, but was highest and had the greatest
decrease with treatment in the two patients with high ALT levels
*Patient
with
intermediate
ALT
not
included
in
analysis
because
of
false
negative
FibroTest™
score |
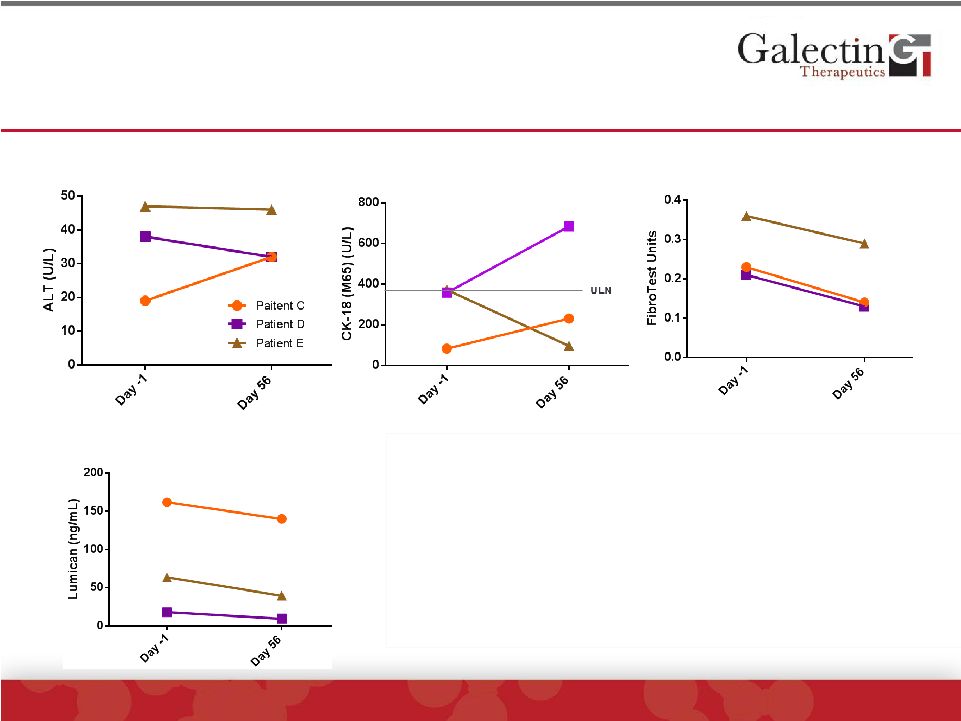 Patients With Low ALT Levels Receiving GR-MD-02
Had Improvement In Fibrosis Markers But Not Cell
Death Markers
©
2014 Galectin Therapeutics | NASDAQ:GALT
26
ALT
FibroTest™
CK-18 (M65)
Lumican
•
The three GR-MD-02 treated patients with
low ALT levels did not have changes in ALT
•
These three patients had lower CK-18 levels
which did not decrease with therapy
•
Fibrosis markers of FibroTest™
and
Lumican did improve with treatment |
 GR-MD-02 Treatment Appears To Improve Both Major
Pathological Processes In NASH
©
2014 Galectin Therapeutics | NASDAQ:GALT
27
•
Improvement in Fibrosis Biomarkers: There was a statistically significant
reduction in Fibrotest™
and trends towards a reduction in ELF score and
hyaluronic acid
•
Improvement in Inflammation Biomarkers: There were statistically significant
reductions in IL-6, IL-8 and TNF-a, all important cytokines in
NASH •
Improvement in Cell Death Biomarkers:
A patient subset with high ALT levels
indicative of more cellular injury had improvement in CK-18
Steato-Hepatitis (NASH Activity)
•
Ballooning of liver cells (cell
death/apoptosis)
key
hallmark
•
Fat in liver cells (steatosis)
•
Immune cell infiltration (inflammation)
Fibrosis/Cirrhosis
•
Increase in collagen/matrix
•
Disruption of architecture
•
Liver cell nodules |
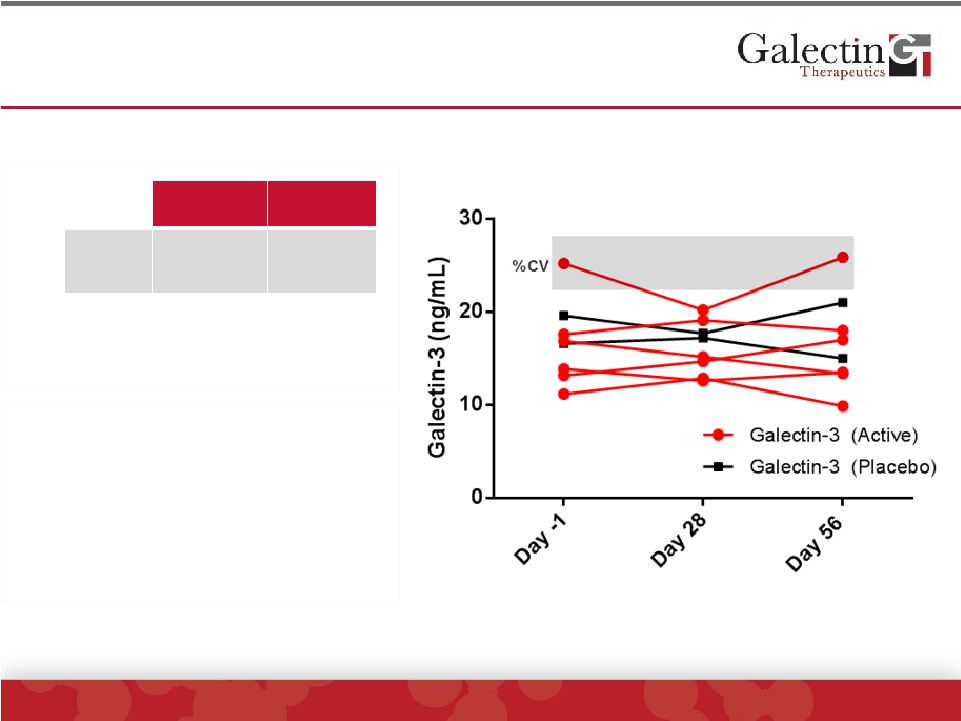 Patients Had A Normal Range Of Blood Galectin-3
Levels At Baseline And No Change With Treatment
©
2014 Galectin Therapeutics | NASDAQ:GALT
28
Timing of gal-3 levels
•
Before 1
st
infusion
•
28 day after 1
st
infusion
•
14 days after 4
th
infusion
Cohort
Range*
Normal
Range**
Gal-3
(ng/mL)
13.2 to 25.2
5.4 to 26.2
*Baseline values
**Range of values in a non-
diseased population published by
manufacturer (BG Medicine) |
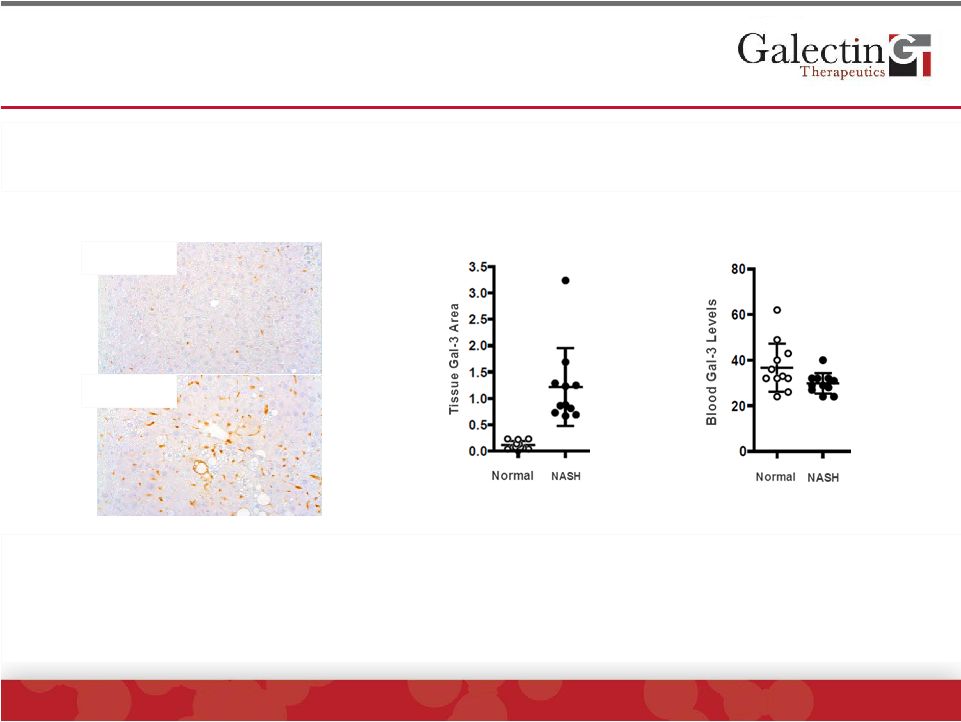 Blood
and Tissue Levels Of Galectin-3 Are Not Correlated In Mouse NASH Model Nor
Human NASH ©
2014 Galectin Therapeutics | NASDAQ:GALT
29
Marked changes in expression of galectin-3 in liver macrophages are not
reflected in changes in blood galectin-3
Liver Gal-3 Staining
Blood Level
Liver Tissue Level
Normal
NASH
•
No
evidence
for
correlation
between
blood
galectin-3
levels
and
disease
activity
or
fibrosis
stage
in
patients
with
NASH
1
•
Blood
galectin-3
levels
in
humans
are
correlated
with
obesity
1,2
and
diabetes
2
1
Yilmax, et al. Clinical Biochemistry 2011
2
Weigert, et al. J Clin Endocrinol Metab, 2010 |
 Summary of Findings
•
GR-MD-02
was
safe
and
well
tolerated
at
2
mg/kg
(80
mg/m
2
)
with
no
drug-
related
adverse
events
•
Pharmacokinetics was consistent between individuals and after single and
multiple doses; exposure was 40% of lowest dose used in NASH animal
model; this was a therapeutic dose
•
Key composite biomarkers of fibrosis improved after four doses of
GR-MD-02 •
Key inflammatory cytokines were decreased after four doses of
GR-MD-02 •
Patients with greater cellular injury as indicated by elevated ALT levels, had a
marked decrease in CK-18, a cell death biomarker
•
Galectin-3 blood levels do not correlate with disease activity and are not a
biomarker of drug effect in patients with NASH with advanced fibrosis
In addition to being safe and well tolerated, GR-MD-02 improved
biomarkers of fibrosis, inflammation and liver cell injury in patients with
NASH with advanced fibrosis
30
©
2014 Galectin Therapeutics | NASDAQ:GALT |
 Next
Steps: Continuation of Phase 1 Trial ©
2014 Galectin Therapeutics | NASDAQ:GALT
31
•
The dose of GR-MD-02 will be increased to 4 mg/kg (160 mg/m
2
) in the
second cohort of 8-10 patients
•
Eight clinical sites in the US are now active to facilitate rapid enrollment of
cohort 2
•
FibroScan™, a FDA-approved ultrasonic measure of liver tissue elasticity,
has been added to the protocol for cohort 2. FibroScan™
will be performed at
baseline and after the four doses in as many patients as possible to gain
experience with this method of fibrosis assessment.
•
Results from Cohort 2 are expected to be reported in July-August 2014 time
frame.
•
Planning for phase 2 clinical trials is ongoing. The results of the first cohort
suggest that 2 mg/kg is a safe, well-tolerated dose that has indication
of anti- fibrotic and anti-inflammatory effect. Therefore, this
defines at least one potential dose level for phase 2 clinical trials.
|
