Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ORAMED PHARMACEUTICALS INC. | zk1414375.htm |
| EX-99 - EXHIBIT 99.2 - ORAMED PHARMACEUTICALS INC. | exhibit_99-2.htm |
Exhibit 99.1

Breakthrough
Technology
for a
for a
Brighter Future
1
February 2014

2
Safe Harbor
Certain statements contained in this material are forward-looking statements. These forward-looking statements
are based on the current expectations of the management of Oramed only, and are subject to a number of
factors and uncertainties that could cause actual results to differ materially from those described in the forward-
looking statements, including the risks and uncertainties related to the progress, timing, cost, and results of
clinical trials and product development programs; difficulties or delays in obtaining regulatory approval or patent
protection for our product candidates; competition from other pharmaceutical or biotechnology companies; and
our ability to obtain additional funding required to conduct our research, development and commercialization
activities, and others, all of which could cause the actual results or performance of Oramed to differ materially
from those contemplated in such forward-looking statements. Except as otherwise required by law, Oramed
undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or
circumstances after the date hereof or to reflect the occurrence of unanticipated events. For a more detailed
description of the risks and uncertainties affecting Oramed, reference is made to Oramed's reports filed from time
to time with the Securities and Exchange Commission. which involve known and unknown risks, uncertainties
and other factors which may cause the actual results, performance or achievements of the company, or industry
results, to be materially different from any future results, performance or achievements expressed or implied by
such forward-looking statements. Please refer to the company's filings with the Securities and Exchange
Commission for a comprehensive list of risk factors that could cause actual results, performance or achievements
of the company to differ materially from those expressed or implied in such forward-looking statements. Oramed
undertakes no obligation to update or revise any forward-looking statements.
are based on the current expectations of the management of Oramed only, and are subject to a number of
factors and uncertainties that could cause actual results to differ materially from those described in the forward-
looking statements, including the risks and uncertainties related to the progress, timing, cost, and results of
clinical trials and product development programs; difficulties or delays in obtaining regulatory approval or patent
protection for our product candidates; competition from other pharmaceutical or biotechnology companies; and
our ability to obtain additional funding required to conduct our research, development and commercialization
activities, and others, all of which could cause the actual results or performance of Oramed to differ materially
from those contemplated in such forward-looking statements. Except as otherwise required by law, Oramed
undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or
circumstances after the date hereof or to reflect the occurrence of unanticipated events. For a more detailed
description of the risks and uncertainties affecting Oramed, reference is made to Oramed's reports filed from time
to time with the Securities and Exchange Commission. which involve known and unknown risks, uncertainties
and other factors which may cause the actual results, performance or achievements of the company, or industry
results, to be materially different from any future results, performance or achievements expressed or implied by
such forward-looking statements. Please refer to the company's filings with the Securities and Exchange
Commission for a comprehensive list of risk factors that could cause actual results, performance or achievements
of the company to differ materially from those expressed or implied in such forward-looking statements. Oramed
undertakes no obligation to update or revise any forward-looking statements.

3
Oramed Overview
Proprietary Protein Oral Delivery (POD™) platform technology
For the oral delivery of drugs that are currently only available via injection
Product
Pipeline
Proof of Concept established in preclinical and clinical trials
Publicly traded - NASDAQCM:ORMP
Founded in 2006 by its scientific inventors after more than two
decades of research
§ Oral Insulin (ORMD-0801)
o Type 2 diabetes
o Type 1 diabetes
§ Oral GLP-1 Analog (ORMD-0901)
§ Combination Therapy (ORMD 0801 + 0901)

4
Agenda Overview
Oral Administration
Diabetes
Oramed Pipeline
Corporate Overview
The Challenge
The Oramed Solution
Statistics and Market
Oral Insulin
Oral GLP-1 Analog
Management Team
Scientific Advisory Board
Intellectual Property
Financials

5
Oramed
An Oral Solution
An Oral Solution

Harsh pH
Protease
threat
Mechanical
challenges
Absorption
barrier
6
Fate of proteins/peptides in GIT
Leads to protein breakdown and lack of absorption

Oramed POD™ Technology:
The Solution
7
Oramed’s delivery platform protects proteins and enhances their
absorption, allowing them to reach the bloodstream via the portal
vein, thereby establishing a more physiologic protein gradient
when compared to other delivery systems.
absorption, allowing them to reach the bloodstream via the portal
vein, thereby establishing a more physiologic protein gradient
when compared to other delivery systems.
Protease Inhibitors
Enteric Coating

Versatile
Supports a wide
range of protein
sizes and doses
range of protein
sizes and doses
Simple
Simple blend of
ingredients
ingredients
Versatile
Simple
Competent
Regulatory competence
No NCEs;
widely applied pharmacopoeia
8
Oramed POD™ Technology

Insulin
GLP-1
Analog
Analog
Other
9
Potential Oramed Technology Applications:
Opportunities & Market
Opportunities & Market
$15+ billion 2012 global insulin market
$32 billion projected market for 2018
$2+ billion 2012 global GLP-1 market
Many patients stop treatment as a result of
injection-related side effects
injection-related side effects
Vaccines: $24 billion in 2013 - grew from $5
billion in 2000
billion in 2000
Flu vaccine estimated at $2.9 billion in
2011 to $3.8 billion in 2018
2011 to $3.8 billion in 2018
Interferon: $6.3 billion, 2011 global market

Diabetes:
A Global Epidemic
A Global Epidemic

POPULATION
• 371 million: Number of diabetics worldwide
• 25.8 million in the US - projected to 44.1 million by 2034
• Type 2 diabetes accounts for about 90% of diabetes cases
COST
• $471 billion: estimated annual global economic burden - includes
direct medical costs, disability, reduced productivity
direct medical costs, disability, reduced productivity
• America: approx. $176 billion in direct medical costs and $69 billion in
reduced productivity
reduced productivity
• Projected American economic burden for direct medical costs alone by
2034 - $336 billion (based on current obesity levels, Diabetes Care, 2009).
2034 - $336 billion (based on current obesity levels, Diabetes Care, 2009).
11

Oramed Pipeline

ORMD-0801
Oral Insulin
Oral Insulin

50
37
As of Nov 12, 2013
Total number of
study subjects:
153
Total number of
human doses:
human doses:
1632

15
Portal insulin delivery is physiologic.
Systemic insulin delivery is not.
Systemic insulin delivery is not.
l Blood glucose - insulin secretion
system forms a 'closed-loop'
system forms a 'closed-loop'
l Peripheral insulin promotes glucose
uptake in fat and muscle
uptake in fat and muscle
l First-pass hepatic metabolism
extracts 80% of secreted insulin
extracts 80% of secreted insulin
l Systemic exposure is minimized
portal vein
liver
small intestine
stomach
To systemic
circulation

ORMD-0801
Type 2 Diabetes
(T2DM)
Type 2 Diabetes
(T2DM)

17
Initial Treatment:
• Lifestyle Modification
• Diet & Exercise
Single & Combination Oral
Therapies:
Therapies:
• Reduce insulin resistance
• Stimulate insulin secretion
Final Treatment:
• Insulin Replacement
(injections)
ORMD-0801 is not a substitute for
insulin injections, but rather a
new earlier treatment option
insulin injections, but rather a
new earlier treatment option
Criteria for advancing to next stage:
AIC not at target < 7.0%
Type 2 Diabetes:
Stages & Treatment Options
0
25
50
75
100
IGT
Post-
prandial
hyper-
glycemia
T2DM
phase I
T2DM
phase II
T2DM

18
Fasting Blood Glucose (FBG):
• Measurement of blood glucose levels after a fast (e.g. first thing in the morning)
• Effected by liver regulation of glucose and insulin levels in the body during a fast
Elevated FBG
• Elevated FBG levels are a major issue in T2DM
• Main cause: excessive nocturnal glucose production from liver
• Current treatments for correction of elevated FBG are suboptimal
FBG: Stats
• Approximately 70% of individuals with impaired FBG develop T2DM
• An estimated > 80% of T2DM patients exhibit abnormal FBG and fail to achieve glycemic
control with Metformin or thiazolidinediones (TZDs) preparations
control with Metformin or thiazolidinediones (TZDs) preparations
ORMD-0801: Unique Indication
• Nighttime dose
T2DM

0
20
40
60
80
0
60
120
180
Time (min)
n=4
8 mg
insulin
ORMD-0801: Preclinical - Dogs
• Healthy, non-diabetic, cannulated beagle dogs showed a 60-75% drop in blood
glucose levels within 30-100 minutes of treatment
glucose levels within 30-100 minutes of treatment
• No hypoglycemia or adverse events were observed over the three years of
testing
testing
T2DM
ORMD-0801 (C)
ORMD-0801 (A)
1.5 U NovoRapid
8 mg insulin, no additives

20
40
60
80
-
0
30
60
90
120
NC
0
100
-
10
150
Time (min)
NC; 4 independent test sessions
ORMD-0801; 10 independent sessions
Fasting
n=2
Pre-prandial
0
20
40
60
80
100
120
140
0
50
100
150
Time (min)
-20
n=3
NC; 6 independent test sessions
ORMD-0801; 5 independent sessions
8 mg
insulin
ORMD-0801: Preclinical - Pigs
No hypoglycemia or adverse events were observed
T2DM

ORMD-0801 Trial Results:
A Summary
A Summary
21
• Healthy, non-diabetic, cannulated beagle dogs
showed a 60-75% drop in blood glucose levels
within 30-100 minutes of treatment
• No hypoglycemia or adverse events were
observed over the three years of testing (in dogs)
• Randomized, double-blind, multi-center study
on 29 patients - 21 dosed, 8 placebo,
6 weeks of monitoring
• Showed relevant clinical impact
• Good safety profile
• Safe and well tolerated by all patients
• No SAEs
T2DM Patients
Pre-clinical
T2DM
ORA-D-004
Insulin
CRP
ORMD-0801
placebo
-4
-2
0
2
4
6
8

ORMD-0801
Phase 2a Results

ORMD-0801: Phase 2a FDA Study
Overview:
•30 T2DM patients
•US site
•In-patient setting
•Double blind
•Randomized
•1 week of treatment
23
T2DM
End Points:
•Primary end point:
• Safety and tolerability
•Secondary end points:
• Pharmacodynamic effects on mean night time
glucose
glucose
• Pharmacokinetics on AUC, Cmax, Tmax, T½
• Changes from baseline in FBG, morning fasting
insulin, C-peptide
insulin, C-peptide
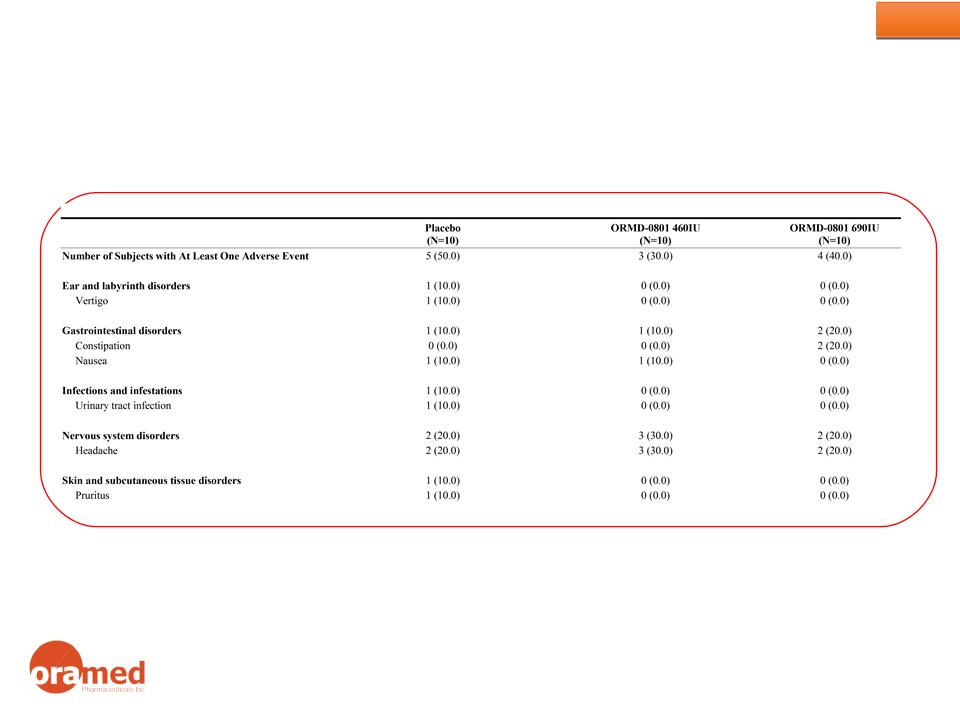
Phase 2a Results: Safety
24
T2DM
No Serious Adverse Events
The study clearly shows that ORMD-0801 is safe and well tolerated

ORMD-0801
Type 1 Diabetes
(T1DM)
Type 1 Diabetes
(T1DM)
25

26
T1DM

50.75
38
49.7
DAY
NIGHT
pretreatment
treatment
Frequency glucose >200mg/dL
20
30
40
50
60
06:00
-
08:59
09:00
-
11:59
12:00
-
13:59
14:00
-
18:59
19:00
-
20:59
21:00
-
23:59
00:00
-
05:59
Time
Design: 8 T1DM, monitor glycemic stability of orally administered ORMD-0801 (1
capsule (8 mg insulin) before meals, three times daily). Glucose monitored with
continuous, blinded glucose monitor
capsule (8 mg insulin) before meals, three times daily). Glucose monitored with
continuous, blinded glucose monitor
27
ORMD-0801: T1DM
DAY
NIGHT
180
200
220
240
260
280
300
pretreatment
treatment
Mean glucose n=8
ê 11.5%
Results: Safe, well tolerated,
reduced glycemia.
reduced glycemia.
T1DM

ORMD-0901
Oral GLP-1
Analog (T2DM)
Oral GLP-1
Analog (T2DM)

Oral GLP-1 Analog (Exenatide)
GLP-1: Hormone Facts
• Secreted by the intestine
• Has effect on the satiety center in the brain
• Has effect on pancreatic β-cells
GLP-1 Analog: Drug Facts
• Good safety profile
• Mimics the natural hormone in the body
• Decreases blood glucose levels - aids in
blood sugar balance
blood sugar balance
• Does not cause hypoglycemia
• Effectively reduces HbA1c
• Preserves beta cell function
• Promotes weight loss
• Current therapy is via injection only
29
• Pre-IND package submitted to
the US FDA Q3 2013
the US FDA Q3 2013
• IND enabling tox studies Q2,
2014
2014
• P1b ex-US study Q2, 2014
ORMD-0901
Oral GLP-1

Oral GLP-1 - ORMD-0901
Blunting of glucose excursions in dogs
Blunting of glucose excursions in dogs
0
20
40
60
80
100
120
S.C.
AG
4
AG
3
-
+
+
+
+
Exenatide
*
*
*
Glucose
Results: Subcutaneous exenatide delivery amounted to a 51% reduction in mean glucose
AUC0-150, while formulations AG4 and AG3 prompted 43% and 29% reductions, respectively
(* p = 0.068, demonstrating a treatment-related trend for the sample size).
ORMD-0901 formulations preserved the biological activity of orally
delivered exenatide. ORMD-0901 successfully curbed blood sugar
excursions following glucose challenge.
delivered exenatide. ORMD-0901 successfully curbed blood sugar
excursions following glucose challenge.
Methods:
Ø Healthy, fasting, cannulated
dogs
Ø Single dose ORMD-0901
formulation
Ø Administered 30 minutes
pre-glucose challenge
Ø Blood samples collected every
15 minutes
30

Mean AUC
Placebo:
148.5±30.5
No Nausea
Insulin:
180.3±106.3
21%
150 mg
exenatide
0
40
60
80
100
120
140
Time (min)
-50
0
100
150
n=4
ORMD-0901
placebo
31
ORMD-0901 - T2DM
Study
•First in Human
•4 healthy volunteers
•Placebo controlled
•Pre-prandial

Pipeline Overview
32
|
Therapy
|
Indication
|
Phase I
|
Phase II
|
Phase III/
Market |
Timeline
|
|
ORMD -
0801 Oral Insulin
|
T2DM
|
|
|
Q4, ‘13: Phase 2a completed
Q2/3, ’14: Phase 2b multi-center study
projected initiation |
|
|
T1DM
|
|
|
|
Q1, ’14: Phase 2a projected initiation
Q1, ’15: Phase 2b multi-center study projected
initiation |
|
|
ORMD-0901
Oral GLP-1
|
T2DM
|
|
|
|
Q2, ’14: Preclinical/IND studies projected
initiation Q2, ’14: Phase 1b ex-US study projected
initiation Q2, ’15: Phase 2 multi-center study projected
initiation |

Corporate
Overview
Overview

Management
34
Nadav Kidron, Esq, MBA
CEO & Director
Experience in various industries, including corporate law and
technology
technology
Miriam Kidron, PhD - CSO & Director
Senior Researcher at the Diabetes Unit of Hadassah Medical
Center for more than 25 years
Center for more than 25 years
Josh Hexter - COO, VP Bus. Dev.
More than 15 years of prominent leadership roles in
biotech and pharma
Yifat Zommer, CPA, MBA - CFO
Extensive experience in corporate financial management
Michael Berelowitz, MD
• Chairman of Oramed SAB
• SVP Clinical Development &
Medical Affairs, Pfizer (former)
Medical Affairs, Pfizer (former)
Harold Jacob, MD
• Chief Medical Officer, Given
Imaging (former)
Imaging (former)
Gerald Ostrov
• CEO, Bausch&Lomb (former)
• Senior level Executive J&J (former)
Leonard Sank
• Entrepreneur and businessman
Board of Directors


36
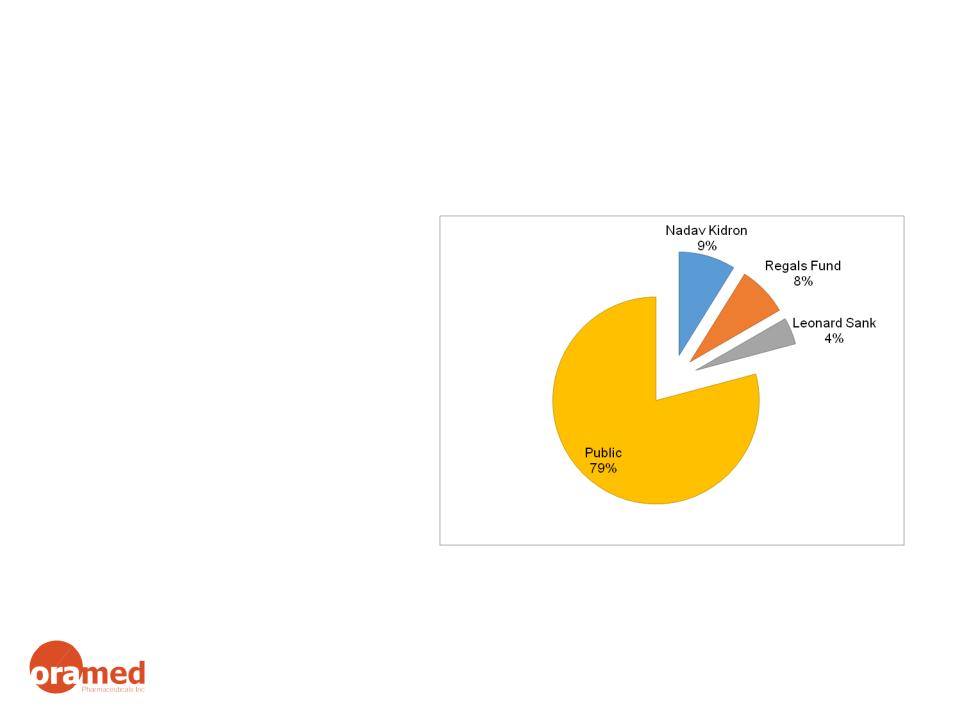
37
Financial Overview*
Ticker: NASDAQ: ORMP
• $43M raised to date **
• No Debt
• Cash and investments: $23.8M
• Shares Issued: 9.7M
• Fully diluted: 11.9M ***
37
* As of January 14, 2014
** Including the shares of D.N.A Biomedical Solutions Ltd.
*** Including outstanding 0.9M options and 1.5M warrants
ORMD-0801
Oral Insulin
ORMD-0901
Oral GLP-1Analog
Anticipated Milestones 2014-2015
•Initiation & Completion of IND-enabling studies
•Initiation & Completion of Phase 1b ex-US study
•Initiation of Phase 2 multi-site study under US IND
T2DM
• Completion of Phase 2a FDA study
• Initiation & Completion of Phase 2b multi-site study
under US IND
under US IND
T1DM
• Initiation & Completion of Phase 2a FDA study
• Initiation & Completion of Phase 2b multi-site study
under US IND
under US IND
Analyst Coverage
39
Oramed is followed by the analysts listed below:
Please note that any opinions, estimates or forecasts regarding Oramed's performance made by
these analysts are theirs alone and do not represent opinions, forecasts or predictions of Oramed
or its management. Oramed does not by its reference above or distribution imply its
endorsement of or concurrence with such information, conclusions or recommendations.
these analysts are theirs alone and do not represent opinions, forecasts or predictions of Oramed
or its management. Oramed does not by its reference above or distribution imply its
endorsement of or concurrence with such information, conclusions or recommendations.
|
Analyst
|
Firm
|
|
Raghuram Selvaraju
|
Aegis Capital Corp.
|
|
Graig Suvannavejh
|
MLV & Co.
|

In Summary
•Product pipeline with the potential to expand to other
indications
•Proprietary technology platform (POD™) for oral
delivery of peptides
•Clear proof of concept
•Strong IP
•Orally ingestible insulin capsule in Phase 2
clinical development under the US FDA
•Significant market opportunity
•World-leading scientific team
•Experienced management team

Breakthrough Technology
for a Brighter Future
Contact :
Nadav Kidron
CEO
nadav@oramed.com
Josh Hexter
COO
josh@oramed.com
41
