Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GALECTIN THERAPEUTICS INC | d674384d8k.htm |
 Corporate Presentation
February 10, 2014
NASDAQ: GALT
www.galectintherapeutics.com
©
2014 Galectin
Therapeutics
NASDAQ:GALT
EXHIBIT 99.1 |
 Forward Looking Statements
This presentation contains, in addition to historical information, statements that
look forward in time or that express management’s beliefs, expectations
or hopes. Such statements are forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. These statements relate to future
events or future financial performance, and use words such as "may,"
"estimate," "could," "expect" and others. They are based on our current expectations and
are subject to risks and uncertainties that could cause actual results to differ
materially from those described in the statements.
These
statements
include
our
plans,
expectations
and
goals
regarding
drugs
in
development,
clinical
trials
and
regulatory approval for any of our drugs or treatments, the anticipated timeline
for clinical trials and results, related market opportunities for our drugs,
potential benefits of our drugs, efforts related to partnering opportunities with other companies,
estimates regarding cash and spending, liquidity and funding requirements for
clinical trials, and estimates regarding those impacted by NASH, liver
fibrosis and cirrhosis. The risks and uncertainties impacting these statements include that our
plans, expectations and goals regarding drugs in development, clinical trials and
regulatory approval are subject to factors beyond
our
control.
Our
clinical
trials
may
not
begin
or
produce
positive
results
in
a
timely
fashion,
if
at
all,
and
any
necessary changes during the course of such trials could prove time consuming and
costly. We may have difficulty in enrolling candidates for testing and we
may not be able to achieve the desired results. Upon receipt of regulatory approval
for any drug or treatment, we may face competition with other drugs and treatments
that are currently approved or those that are currently in development,
which could have an adverse impact on our ability to achieve revenues from the
approved indication. Plans regarding development, approval and marketing of any of
our drugs are subject to change at any time based on the changing needs of
our company as determined by management and regulatory agencies. Estimates
regarding the potential benefits of our drugs and the potential market for any of
our drugs may be inaccurate and, to the extent the estimates are correct, we
may not be successful in achieving revenues from any such drugs, as the successful
marketing of any approved drugs will be subject to strong competition within the
health care industry and patient and physician acceptance of our drugs as
safe, affordable and effective. Our ongoing discussions with other companies may
not lead to partnering opportunities, and if we are unable to partner with other
companies and/or raise additional capital, we will likely be unable to
complete future stages of clinical trials and ultimately produce revenue from our drugs in
development. Funding from potential sources of capital, including the
potential exercise of warrants, may not materialize. To date, we have
incurred operating losses since our inception, and our ability to successfully develop and market drugs
may be impacted by our ability to manage costs and finance our continuing
operations. For a discussion of additional factors impacting our business,
see our most recent Annual Report on Form 10-K and our subsequent filings with the SEC.
You should not place undue reliance on forward-looking statements. Although
subsequent events may cause our views to change, we disclaim any obligation
to update forward-looking statements. 2
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Agenda
•
The Company and Key Team Members
•
Galectin Inhibitors
•
Fibrosis Program –
our primary focus
•
Cancer Immunotherapy
•
Summary
©
2014 Galectin Therapeutics
NASDAQ:GALT
3 |
 What
We Do •
Clinical stage biopharmaceutical company targeting
fibrotic diseases and cancer with novel compounds that
inhibit galectin proteins (galectin-3)
•
Galectin proteins are important in the development and promotion
of many inflammatory, fibrotic and neoplastic diseases
•
Currently in clinical trials in liver fibrosis and cancer
•
Liver fibrosis indication: NASH (Fatty Liver Disease) with
advanced liver fibrosis
•
Cancer immunotherapy indication: Metastatic melanoma
4
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Key
Facts – As of February 7, 2014
5
Trading Symbol
Nasdaq: GALT
Corporate Headquarters
Norcross, GA (suburb of Atlanta)
Stock Price; 52 Week Range
$13.91 $2.61 -
$17.88
Shares Outstanding
21.5 million
Daily Volume (50 day average)
555,231 shares
Market Capitalization
$299 million
Debt
$0
Cash & Equivalents
$35 million
Estimated Spending in 2014
$14.5 million
Fiscal Year Ends
December 31
Accounting Firm
McGladrey LLP
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Experienced Leadership Team
6
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Agenda
•
The Company and Key Team Members
•
Galectin Inhibitors
•
Fibrosis Program –
our key focus
•
Cancer Immunotherapy
•
Summary
7
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Galectin Proteins: Bind galactose
residues on glycoproteins
8
•
Galectin-3 is most important in pathological situations, is widely expressed, but
highest in immune cells (macrophages)
•
Under normal physiological situations, galectin-3 is expressed at low
levels •
In areas of acute or chronic inflammation and fibrogenesis, the gal-3 expression
is markedly increased. The majority of cancers express high levels of
galectin-3 ©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Our
drugs are natural complex carbohydrates that bind to galectin-3 and
block interactions with natural ligands
©
2014 Galectin Therapeutics
NASDAQ:GALT
9
Galacturonic Acid
Rhamnose
Galactose
Mannose
GR-MD-02
(simplified schematic)
•
Produced from
apple pectin
•
Method of use
patents in liver
fibrosis, fatty liver
disease, and
diabetic kidney
disease (others
pending)
•
Composition of
matter pending
GM-CT-01
(simplified schematic)
•
Produced from guar
gum
•
Method of use
patents in liver
fibrosis and cancer
(others pending)
•
Composition of
matter patent |
 Agenda
•
The Company and Key Team Members
•
Galectins and Disease
•
Fibrosis Program –
our key focus
•
Cancer Immunotherapy
•
Summary
10
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Fundamental Science on Target is Strong:
Galectin-3 is critically important in the
development of organ fibrosis
•
Galectin-3 null mice (no galectin-3) are resistant to fibrosis
due to toxin-induced liver toxicity
•
Galectin-3 null mice are also resistant to fibrosis in:
•
Fatty liver disease
•
Lung fibrotic disease
•
Kidney fibrotic disease
11
Normal mouse
No gal-3 mouse
Red stain is collagen,
the principal
component of fibrotic
tissue
Henderson, et al 2006
Mice treated with liver toxin to induce fibrosis
Normal mice develop
fibrosis whereas
those without gal-3
do not
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Company’s Galectin Inhibitors Reverse
Cirrhosis in Rat Model
12
GR-MD-02
Vehicle
GM-CT-01
•
Animal model presented a very
high
hurdle
for
drug
treatment:
Cirrhosis induced with high dose
toxin and continued throughout drug
treatment
•
Treatment with four weekly doses
Broad bands of
collagen with nodule
formation (N)
indicates advanced
fibrosis and cirrhosis
Reduction in
collagen with thin
and broken bands
(arrow) indicates
resolving fibrosis
and cirrhosis
©
2014 Galectin Therapeutics
NASDAQ:GALT |
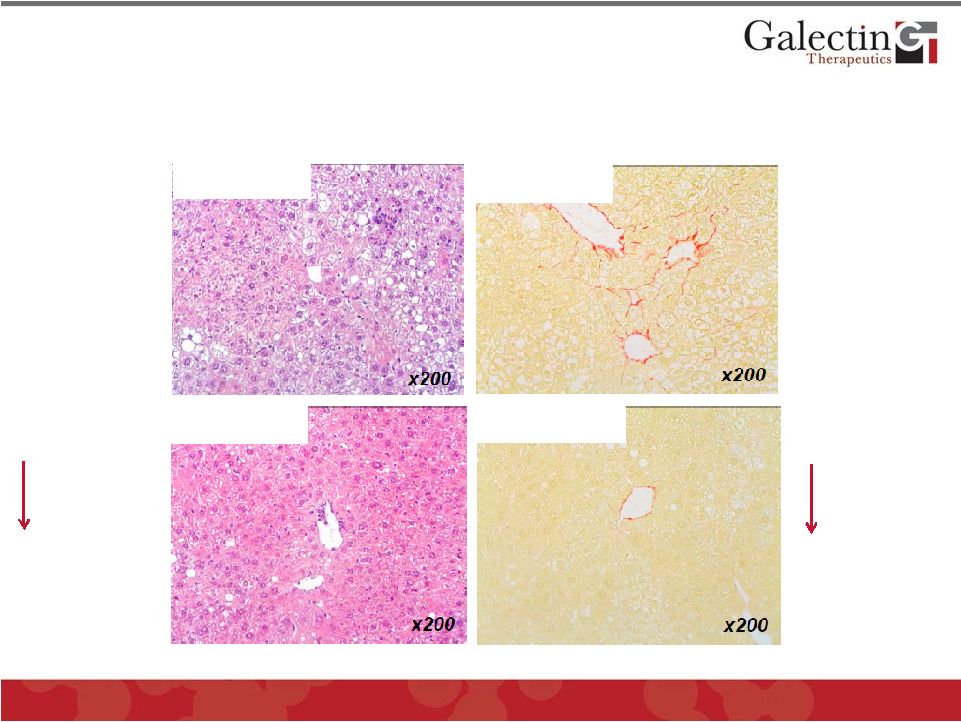 Galectin Inhibitor GR-MD-02 Improved Fat, Liver Cell
Death, Inflammation, and Fibrosis in Mouse Model of
Fatty Liver Disease (NASH) with Fibrosis
13
Fat
Cell death
Inflammation
Fat
Cell death
Inflammation
Red =
Collagen
Red =
Collagen
©
2014 Galectin Therapeutics
NASDAQ:GALT
Control
GR-MD-02
GR-MD-02
Control |
 Potential Use in Lung Fibrosis: GR-MD-02
Reduces Fibrosis in Mouse Model
14
Vehicle
Normal
GR-MD-02
Marked reduction in
area and severity of
fibrosis without
aggregation into larger
formations
Large areas of
confluent fibrosis.
Lung fibrosis induced by tracheal
instillation of bleomycin followed by
four infusions of either vehicle or
GR-MD-02
©
2014 Galectin Therapeutics
NASDAQ:GALT |
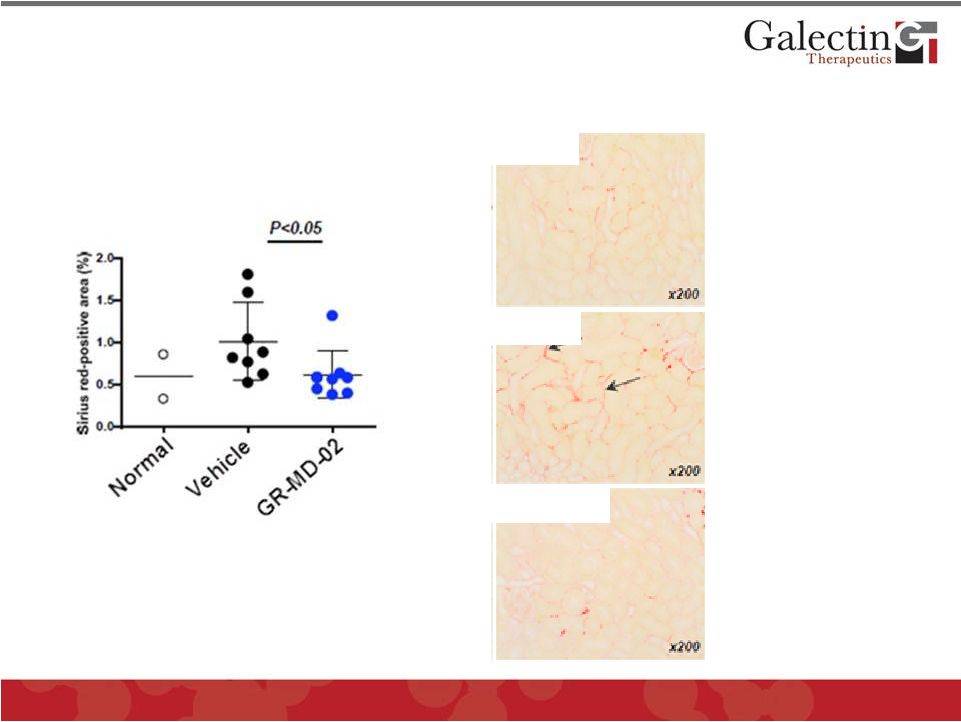 Potential Use in Kidney Fibrosis:
GR-MD-02 Reduces Fibrosis in Diabetic Mouse
15
Vehicle
Normal
GR-MD-02
Reduction in interstitial
fibrosis
Arrows show areas
of interstitial fibrosis
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Liver
Fibrosis Development Program NASH (Non-Alcoholic SteatoHepatitis): Very
Large Unmet Medical Need
•
Multiple liver diseases lead to fibrosis which leads to liver failure,
medical complications, and death
•
There is no approved medical therapy for liver fibrosis
•
Only current therapy is liver transplantation
•
First indication is fatty liver disease with fibrosis (non-alcoholic
steatohepatitis, or NASH).
•
Prevalence of NASH in U.S. is between 9-15 million people
•
Approximately 30% will develop cirrhosis; estimated prevalence of
patients with advanced fibrosis is approximately 6 million.
•
NASH cirrhosis projected to be primary reason for liver transplant
•
IND for GR-MD-02 with advanced fibrosis: Jan. 30, 2013
•
Fast Tract Designation received in August 2013
•
Phase 1 trial: First cohort enrollment completed. Data to be
reported around end of first quarter 2014.
16
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Development Program: Targeting
Therapy In The Progression of NASH
17
Obesity/Insulin Resistance/Diabetes
Steatosis (fatty liver)
NASH (inflammation, cell death)
Stage 1
2 3 Fibrosis
Stage 4
Cirrhosis
Decades
•
No certainty of progression from early to late disease in an individual
•
Late disease much closer to clinical outcomes
•
Because of effect on inflammation in NASH and ability to reduce existing fibrosis,
our clinical program targets NASH patients with late disease
Early Disease
Late Disease
Clinical Outcomes
Complications
Transplant
Death
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Phase
1 Clinical Trial of GR-MD-02 in NASH (Fatty Liver Disease) with
Advanced Fibrosis 18
0
28
35
42
56
70
Day
Infusion
-1
Biomarkers
Biomarkers
Patient inclusion: Biopsy proven NASH with advanced fibrosis (stage 3)
Design: Each cohort has 8 patients (6 active, 2 placebo, blinded)
Dose: Starting dose of 2 mg/kg which is within the presumptive therapeutic range;
next two cohort doses 4 mg/kg and 8 mg/kg.
Primary
endpoints:
Safety;
Pharmacokinetics
Secondary
endpoints:
Serum
biomarkers
to
assess
for
potential
treatment
Timing
of
expected
data
from
each
cohort
Cohort 1: Mar-Apr 2014
Cohort 2: Jul-Aug 2014
Cohort 3: Oct–Nov 2014
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Key
Biomarkers for Assessing Potential Efficacy in Phase 1 Clinical Trial
•
Most important biomarkers are clinically validated composite
scores that have been shown to correlate with fibrosis
•
ELF
(Enhanced
Liver
Fibrosis)
Score:
Includes
measurement
of
hyaluronic acid, tissue inhibitor of metalloproteinase-1, and PIIINP
(amino terminal propeptide of type III pro-collagen)
•
Fibrotest:
age
and
gender,
alpha-2-macroglobulin,
haptoglobin,
apolipoprotein A1, GGTP, total bilirubin
•
Other fibrosis markers: TGF-ß, lumican, Matrix metalloproteinase-1,
-3, and -9
•
Biomarkers associated with NASH—ballooning degeneration of
hepatocytes
•
Cytokeratin-18 (M30 and M65 antibody tests)
•
A variety of inflammatory biomarkers are also being explored
19
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 20
Phase 2 Clinical Trial Plans
•
Patient
inclusion:
Biopsy
proven
NASH
with
advanced
fibrosis
(stage 3 and stage 4 with no complications of cirrhosis)
•
Design:
Randomized,
placebo
controlled,
and
double
blind.
•
Dose:
Likely
two
dosage
groups
•
Treatment
Duration:
TBD
•
Primary
endpoint:
Liver
biopsy:
Collagen
proportional
area
•
Galectin human NASH biopsy study done to guide design
•
Timeline:
Start
around
end
of
2014;
Top
line
data
dependent
on
trial
design: expectation is 1H 2016.
•
Secondary
endpoints:
•
Liver Biopsy: NASH Activity Score and Fibrosis Stage
•
Liver function testing: HepQuant (bile acid clearance test)
•
Imaging methods—Fibroscan and/or MR-elastography
•
Serum biomarkers based on analysis of Phase 1 data: Fibrotest
and ELF Score key biomarkers
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Comparison of Galectin-3 Inhibitors
21
Galectin Thera.
La Jolla Pharm.
Galecto Biotech
Drug
GR-MD-02
GCS-100
TD 139
Drug
Characteristics
Mod. apple pectin:
MW: 20-70K;
aqueous solution*
Mod. citrus pectin;
MWt >100K
colloidal solution**
Modified lactose, a
disaccharide
Cell culture effects
No cytotoxicity or
apoptosis; inhibits
cytokine
production *
Kills cells through
apoptosis**; no
effect on cytokines*
Shown to reduce
TGF-
signaling
Human side effects
TBD; in phase 1
DLT was Grade 3
maculopapular
rash (vasculitis).
Animal studies.***
Unknown, pre-
clinical
Clinical
Development
NASH with
advanced fibrosis
Kidney
insufficiency
Pre-clinical; Lung
fibrosis; inhalation
Dosing in clinical
program
Phase 1 starting
dose= 2 mg/kg
Highest dose= 0.75
mg/kg
#
TBD
*GALT patents
**LJPC patents
***GCS-100 published abstract
#
LJPC press release
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Competition in NASH
22
NASH (inflammation, cell death)
Stage 1
2 3 Fibrosis
Stage 4
Cirrhosis
Early Disease
Late Disease
Clinical Outcomes
Complications
Transplant
Death
©
2014 Galectin Therapeutics
NASDAQ:GALT
•
Focus is on improving
NAFLD Activity Score (8
points total):
•
Fat (3 pts.),
Ballooning (2 pts.),
Inflammation (3 pts.)
•
PIVENS (pioglitazone and
vitamin E
•
FLINT (NIDDK and
Intercept)
•
Genfit trial
•
Focus is on stopping
progression or reversing
fibrosis
•
Galectin Therapeutics
Trials
•
Gilead Trials
•
LOL-2 (Lysyl oxidase-like-2)
mAb (GS-6624): Monoclonal
antibody that blocks the
enzyme which cross links
collagen fibers |
 Fibrosis Strategy Summary
•
NASH with Advanced Fibrosis: Evidence of efficacy of
GR-MD-02 from well controlled phase 2 clinical trial
•
Other Organ Fibrosis: Potential for partnering
opportunities
•
Lung
fibrosis
–
pre-clinical
results
suggest
possible
use
in
Idiopathic Pulmonary Fibrosis
•
Kidney fibrosis
•
Ongoing discussions with large pharmaceutical
companies
•
Discussions
will
provide
foundation
for
partnering
opportunities
at
the most opportune time
23
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Agenda
•
The Company and Key Team Members
•
Galectin Inhibitors
•
Fibrosis Program –
our key focus
•
Cancer Immunotherapy
•
Summary
24
©
2014 Galectin Therapeutics
NASDAQ:GALT |
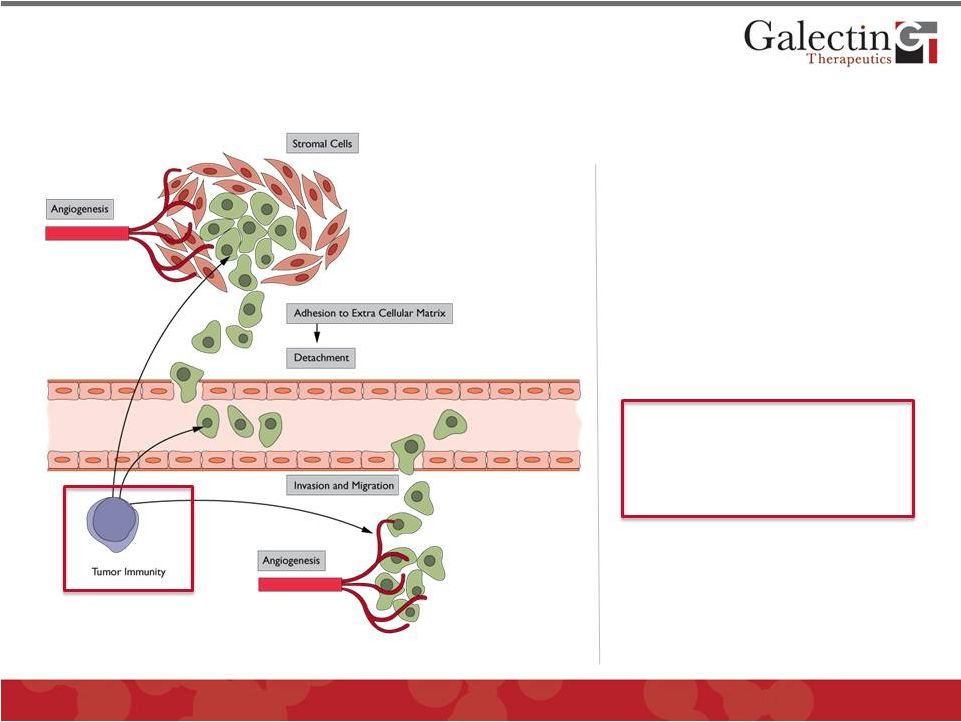 The
Vast Majority of Cancers Secrete Large Amounts of Galectins Which Have Multiple
Roles in Tumor Pathogenesis
•
Tumor cell invasion:
extracellular matrix
adhesion & detachment
•
Metastasis:
cell invasion and migration
•
Angiogenesis
•
Tumor immunity
has
recently been shown to be
critically affected by
galectins
25
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 26
Checkpoint Inhibitor Blockade
•
CTLA4 receptor mAb: Yervoy®
(Ipilimumab, BMS)
•
Anti-PD-1 (nivolumab BMS; lambrolizumab Merck)
•
Anti PD-L1 (MPDL3280A , Roche)
May 22, 2013
©
2014 Galectin Therapeutics
NASDAQ:GALT
•
In Development:
•
Marketed:
Cancer Immunotherapy Named Top Scientific Breakthrough
of 2013 by Science Magazine
|
 Cancer Therapy Strategy
•
Focus on cancer immunotherapy based on the hypothesis that
galectin inhibitors will enhance efficacy of immunotherapies
•
Metastatic melanoma is initial cancer indication
•
In US 76,000 new diagnoses and 9,100 deaths annually
•
5% five year survival for metastatic disease
•
Even with newly approved drugs, still a substantial unmet medical need
•
We have sought collaborations with institutions that have:
•
Demonstrated clinical trial expertise in melanoma
•
Tumor immunology basic science research
•
Ability to conduct clinical trials and assist in funding
•
Two collaborations have been established
•
Ludwig Cancer Institute, Brussels Belgium
•
Robert W. Franz Cancer Research Center, Earle A. Chiles Research
Institute (EACRI) Providence-Portland Medical Center, Portland Oregon
27
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 CD8+
T-Cells
Cytokines (kill tumor cells)
T-Cells
28
Tumor
Cells
Potential sites of action for galectin
inhibition in tumor immunology
Galectin-3
CD8+
T-Cells
Clonal
Expansion
Immunotherapies
Checkpoint Inhibitor
Blockage:
anti-CTLA4
anti-PD1
Tumor Vaccines
Potential for galectin
inhibitors to enhance
anti-tumor immune
response
Potential for galectin inhibitors
to enhance anti-tumor activity
of T-cells by blocking
“Galectin Effect”
Galectin-3
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Checkpoint inhibitors plus GR-MD-02 boosts anti-
tumor immunity, reduce tumor size and increase
survival in mouse model of prostate cancer (similar
results in breast cancer, melanoma and sarcoma)
29
*p<0.05
aCTLA-4 = anti-CTLA-4 mAb [ipilimumab in humans (Yervoy, BMS)]
aPD-1 = anti-PD-1 mAb [positive results in clinical trials, BMS,
Merck] Unpublished data 2013: Stefanie N. Linch, Melissa J. Kasiewicz, Peter G.
Traber, and William L. Redmond, Galectin Therapeutics and Earle A. Chiles
Research Institute (EACRI), Portland Oregon ©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Phase
1B Clinical Trial in patients with advanced melanoma using GR-MD-02 in
combination with Yervoy®
(ipilimumab): March 2014 Start
30
1
23
64
85
Day
Infusion: GR-MD-02 followed by Yervoy at standard doses
Patient inclusion: Advanced melanoma with indication for Yervoy Rx
Design: 3+3 dose escalation; 10 patients treated with MTD
Dose: Starting dose of 1 mg/kg
Endpoints:
Followed every 12 weeks for survival
Bx
Bx
©
2014 Galectin Therapeutics
NASDAQ:GALT
43
•
Safety; Pharmacokinetics
•
Tumor response: immune response RECIST criteria
•
Biological responses including memory CD4+ T-cells, memory CD8+ T-cells,
melanoma specific T-cells, and composition of tumor immune infiltrate
from tumor biopsies when available. |
 Cancer Therapy Strategy: Summary
•
Two immunotherapy agents have been approved for use to
date, with many more vaccines and activators in development
•
Our strategy is to leverage world class expertise in basic tumor
immunology and in the conduct of melanoma clinical trials.
•
Providence Portland Medical Center and Earle A. Chiles
accepted
for
phase
1B
clinical
trial
in
patients
with
advanced
melanoma treated with a combination of Yervoy and GR-MD-02
•
Initial funding of clinical trial by PPMC/EACRI. Galectin is providing
GR-MD-02 study drug, reference to its IND, and PK analysis
•
Ongoing discussions with large pharmaceutical companies in
the immunotherapy space to seek a partnering opportunity to
take beyond proof of concept from initial clinical trials
31
©
2014 Galectin Therapeutics
NASDAQ:GALT
: Ongoing pre-clinical studies; IND
Research Institute (EACRI) |
 Agenda
•
The Company and Key Team Members
•
Galectins and Disease
•
Fibrosis Program –
our key focus
•
Cancer Immunotherapy
•
Summary
32
©
2014 Galectin Therapeutics
NASDAQ:GALT |
 Summary of Development Program
•
Liver Fibrosis
•
First indication: GR-MD-02 in NASH with advanced fibrosis
•
Phase 1 clinical trial underway; interim data expected March-April
2014
•
Other Organ Fibrosis: Studies to demonstrate broad
application of drugs in organ fibrosis; seek partner
•
Cancer Therapy: Combination immunotherapy to enhance
the ability of the immune system to recognize and kill tumor
cells in metastatic melanoma
•
Leverage world class expertise in basic tumor immunology and in
the conduct of melanoma clinical trials.
•
Ongoing discussions with large pharmaceutical companies
to provide foundation for partnering opportunities at the
most opportune time
33
©
2014 Galectin Therapeutics
NASDAQ:GALT |
