Attached files
| file | filename |
|---|---|
| 8-K - FORM 8K - ORAGENICS INC | d659026d8k.htm |
 Investor Presentation
January 2014
Engineering New
Antibiotics and
Probiotics Through
Synthetic Biology
Exhibit 99.1 |
 1
Certain statements made in this presentation include forward-looking actions that
Oragenics,
Inc.
(“Oragenics,”
or
the
“Company”)
anticipates
based
on
certain
assumptions. These statements are indicated by words such as “expect,”
“anticipate,”
“should”
and similar words indicating uncertainty in facts, figures and
outcomes. Such statements are made pursuant to the Safe Harbor Provisions of the
Private Securities Litigation Reform Act of 1995. While Oragenics believes that
the expectations reflected in such forward-looking statements are
reasonable, it can give no assurance that such statements will prove to be
correct. The risks associated with the Company are detailed in the
Company’s various reports filed by the Company with the Securities and
Exchange Commission. Safe Harbor Statement
1 |
 2
At A Glance -
Oragenics
•
Expertise in bacteria –
especially related to the oral cavity
•
Lantibiotics
–
Capable of killing bacteria, including
antibiotic-resistant strains
•
Next Generation Probiotics –
Designed to be
therapeutically useful
•
ProBiora3
®
-
OTC probiotic used to improve overall oral
health |
 3
At A Glance -
Oragenics
NYSE MKT: OGEN (market cap ~$83M)
Founded in 1999
Based in Tampa, FL
Experienced management team |
 4
Overview
Leverage expertise in lantibiotics and probiotics with
Intrexon’s leading synthetic biology platform via two
exclusive channel collaborations (ECCs) to develop novel
biotherapeutics:
1.
Lantibiotics:
1.
Next Generation Probiotics:
bacteria-based biotherapeutics for
oral cavity, throat, sinus, and esophagus diseases
Market proprietary OTC oral care probiotics (ProBiora3
®
)
novel class of peptide antibacterial compounds
produced by specific strains of bacteria |
 Investor
Presentation January 2014
Engineering New
Antibiotics and
Probiotics Through
Synthetic Biology
Lantibiotics and the Need for New
Antibiotics |
 6
Serious Need for New Antibiotics
Sept. 2013 CDC report highlighted
serious threats of antibiotic-resistant
infections and need for new
antibiotics:
“Antibiotic
Resistance
Threats
in
the
United
States,
2013”
U.S.
Department
of
Health
and
Human
Services
Centers
for
Disease Control and Prevention
>2 million infections and ~23,000 deaths
each year in U.S. caused by resistant
microbes
>$20 billion in direct healthcare spending
from resistance infections
>$35 billion in additional costs due to lost
productivity |
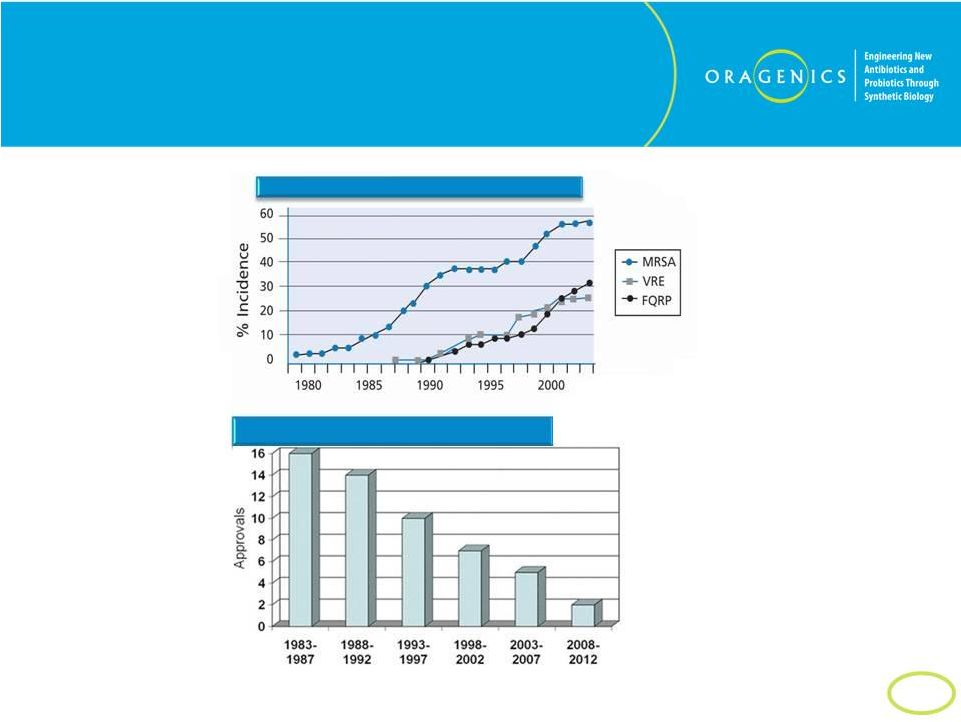 7
Trends Support CDC Concerns
Resistance Increases While Approvals Decrease
Total
Approved
Bacterials
-
US
Resistant Bacterial Strains Spread Rapidly |
 8
Lantibiotics
A Promising Solution to the Growing Health
Crisis of Antibacterial Resistance
Novel class of peptide antibacterial compounds:
•
Naturally-produced by variety of bacterial strains to attack
competing gram-positive bacterial strains
•
>50 known lantibiotics and a very large number of potential analogs
•
Pipeline of new compounds to target resistant infections
•
Development as commercially-viable products previously limited by
technological hurdles |
 9
Lantibiotics
A Promising Solution to the Growing Health
Crisis of Antibacterial Resistance
•
•
Achieved significant increase in production titer yield
Developing robust purification methods compared to
traditional approaches
Completed POC that genetically modified bacteria can produce
MU1140 and analogs
Well-positioned to bring lantibiotics to market:
Intrexon collaboration expected to enable production of
lantibiotics at commercial scale
Progress to date through Intrexon/Oragenics ECC |
 10
Lead Lantibiotic Compound MU1140
Initial focus on MU1140, a lantibiotic shown to be effective against:
•
Excellent therapeutic index
•
Minimal cytotoxicity in vitro using mouse and human cell lines; minimal
immunogenicity
•
Maximum tolerated dose in mice and rats >50 mg/Kg
•
In
vivo
efficacy
observed
in
a
pilot
rat
peritonitis
model
using
S.
aureus
(60xLD50)
•
Apparent synergy with aminoglycosides
Preliminary preclinical data suggests:
•
Methicillin-resistant Staphylococcus aureus (MRSA)
•
Vancomycin-resistant Enterococcus (VRE)
•
Clostridium difficile (C. diff)
•
Resistant Tuberculosis and others |
 11
ECC with Intrexon
Platform for Engineering Bacterial Systems
Through Synthetic Biology
Inventoried DNA Modules
Genetically Modified
Bacterial Cell |
 12
ECC with Intrexon
Development of lantibiotics for treatment of
infectious diseases in humans and animals
Application of engineering principles to the design of
living organisms and their constituent parts (DNA,
proteins, and cells)
Intrexon’s toolset of modular molecular and cellular
systems expected to enable the engineering of specific
functionality into cells to allow industrial scale production
of lantibiotics |
 13
ECC with Intrexon
Lantibiotics –
For Resistant Bacterial Infections
Bacteria
Cell
Extract genes
responsible for
lantibiotic
production
from native
bacteria…
…insert
genes into
modified
bacteria to
maximize
lantibiotic
production…
…and produce MU1140
and pipeline lantibiotics
via fermentation at
commercial scale.
Lantibiotics
(50+ known) |
 14
Matrix of early experiments initiated to screen MU1140 (and several
analogs/homologs) for determining primary efficacy (e.g. MICs, MTD,
primary efficacy in animals, protein binding, etc.)
Fermentation optimization/scale-up purification in progress
IND filing expected by 2H 2015*
2Q13
3Q13
4Q13
1Q14
2Q14
3Q14
4Q14
Animal studies
Animal studies
Pre-IND FDA
meeting
IND*
Plan for MU1140
First of 50+ Known Lantibiotics and Analogs
* Current estimate may change depending on FDA pre-IND meeting
results |
 15
Rising Market Demand for Novel
Antibiotic Platforms
$
Market cap:
approx. $83M
Deal size:
approx. $775M total
approx. $10M upfront
Cubist
Market cap:
approx. $4.2B
Validated Technology
Leads to Deals
Commercial
Oragenics
Cubist
Preclinical
Rib-X and Sanofi
Cubist/Trius/Optimer
Acquisitions
Deal size:
approx. $818M Trius
approx. $808M Optimer
Cubist Acquisitions
(7/31/13)
Approved/Late Stage
Significant valuations for “Single Antibiotic Product” companies –
Oragenics has potential to generate a pipeline of lantibiotics |
  Investor
Presentation January 2014
Engineering New
Antibiotics and
Probiotics Through
Synthetic Biology
Next Generation Probiotics For
Therapeutic Use |
 17
Next Generation Probiotics
Potential to Revolutionize Probiotics for Oral
Diseases
Developing novel probiotics focusing on treatment of patients with
oral cavity, throat, sinus, and esophageal diseases through ECC with
Intrexon
Expect to genetically manipulate bacteria similar to lantibiotics
program
•
Beneficial bacteria naturally part of the human oral cavity microbiome
Genetically-engineered bacterial strains designed to deliver and
release therapeutics locally at disease site to target pain
management, reduce inflammation, and improve patient outcomes
Initial indications:
•
Behçet’s disease
•
Recurrent aphthous stomatitis (aka canker sores) |
 18
Behçet’s disease
Chronic relapsing multi-systemic inflammatory disorder
Characterized by four major symptoms (oral aphthous ulcers,
genital ulcers, skin lesions, and ocular lesions)
Patients often in constant pain and have difficulty eating
~20,000 patients in the U.S.; similar for Europe |
 19
Recurrent Apthous Stomatitis
(aka canker sores)
Painful ulcers; most common oral mucosal disease known
No effective treatments
~200,000 patients in the U.S.
Characterized by multiple, recurrent, small, round, or ovoid ulcers
Usually presents first in childhood or adolescence |
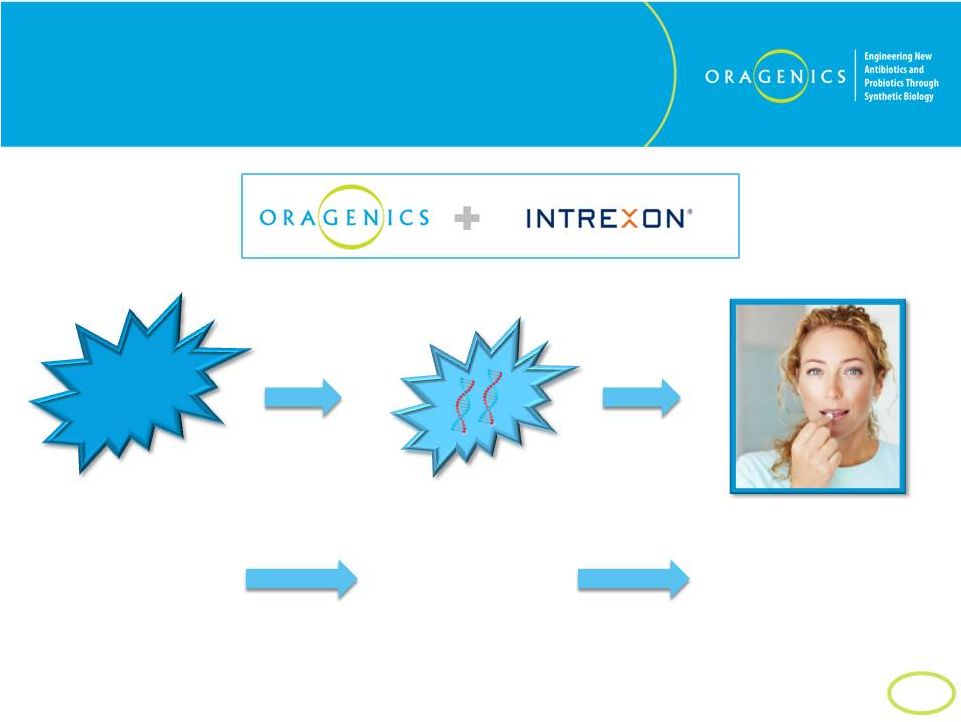 20
Oragenics/Intrexon Solution
GM Probiotics For Oral Health
…insert genes
responsible for
production of
therapeutics
agent(s)…
…deliver new
therapeutic bacteria
into oral cavity to
produce therapeutic
benefits and supplement
natural oral microbiome.
Choose the
appropriate
bacterial strain
to engineer…
Bacteria
Cell |
 Investor
Presentation January 2014
Engineering New
Antibiotics and
Probiotics Through
Synthetic Biology
ProBiora3
®
-
Only OTC Probiotic
Product Specifically Designed for
Oral Health |
 22
Blend
of
3
naturally
occurring,
non-pathogenic
bacteria
–
S.oralis,
S.uberis,
and
S.rattus
–
available
without
prescription
and
taken
daily
“Good”
probiotic bacteria compete for binding sites and nutrients with
“harmful”
bacteria
Release hydrogen peroxide to reduce population of pathogens in mouth
Poor oral health linked to serious health problems, including heart
disease and diabetes
Total consumer oral care market expected to reach $10.9 billion by
2014
1
Also marketed for companion animals
ProBiora3
®
-
OTC Product
First and Only Patented Probiotic Technology for
Oral Care
1
‘The US Market for Oral Care Products (2009)’, published by
Packaged Facts |
 23
Experienced Management Team
John N. Bonfiglio, Ph.D., President, CEO & Director
Mike Sullivan, CFO
Dr. Martin Handfield, VP of Research & Development
Former Tenured Associate Professor, College of Dentistry at The University of
Florida Over 14 years experience with Oragenics
Expert in biomarkers and research surrounding antimicrobials
Over 40 publications and 6 patents
Prior Senior-level financial positions for both publicly and privately held
businesses Significant experience in product licensing and IP issues
Strong background in both domestic and international retail operations
Florida Certified Public Accountant, ex Big 4. MBA from The Florida State
University 30 years of management and pharmaceutical experience
Prior CEO Argos Therapeutics, CEO Immune Response, CEO Peregrine
Pharmaceuticals, COO Cypress Bio
Senior Management positions at Allergan Pharmaceuticals and Baxter Healthcare
MS and PhD in Chemistry, University of California, MBA Pepperdine University
|
 24
Significant Accomplishments in
2013
Novel Antibiotics (Lantibiotics)
Significantly increased yield of MU1140
Identified two new potentially viable purification methods for
commercially producing MU1140
Obtained proof of concept for genetically modified bacteria capable of
higher yields of MU1140 and potentially analogs
Next Generation Probiotics
Established ECC with Intrexon –
announced October 1, 2013
Completed initial research program; commenced activity in 2014
Finance
Raised $3.9M in PIPE with Intrexon to support new ECC
Raised $11M in shelf-takedown
Relisted on NYSE:MKT |
 25
Upcoming Potential Milestones
Novel Antibiotics (Lantibiotics)
Production of new lantibiotic analogs using genetically modified
bacteria –
1H 2014
Animal study results for MU1140/analog –
1H 2014
Pre-IND meeting with FDA -
2H 2014
Next Generation Probiotics (Preliminary Timelines)
Generation of next bacterial prototypes –
2H 2014
GM probiotics producing cytokine therapeutics –
Mid 2015
Proof-of-concept of GM probiotic for therapeutic purposes in animals
– 2H
2015 |
