Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REGENERON PHARMACEUTICALS, INC. | d659873d8k.htm |
 J.P. Morgan
Healthcare Conference January 2014
Leonard S. Schleifer, M.D., Ph.D.
Chief Executive Officer
Exhibit 99.1 |
 Page 2
| Copyright Regeneron 2014 Safe Harbor Statement
This presentation includes forward-looking statements that involve risks and
uncertainties relating to future events and the future performance of Regeneron
Pharmaceuticals, Inc. (“Regeneron” or the “Company”), and actual events
or results may differ materially from these forward-looking statements. Words such as
“anticipate,” “expect,” “intend,” “plan,”
“believe,” “seek,” “estimate,” variations of such words and similar expressions are intended to identify such forward-looking
statements, although not all forward-looking statements contain these identifying
words. These statements concern, and these risks and uncertainties include,
among others, the nature, timing, and possible success and therapeutic applications of
Regeneron's products, product candidates, and research and clinical programs now
underway or planned; unforeseen safety issues resulting from the administration of products and product candidates in patients, including serious
complications or side effects in connection with the use of Regeneron’s product
candidates in clinical trials; the likelihood and timing of possible regulatory
approval
and
commercial
launch
of
Regeneron's
late-stage
product
candidates
and
new
indications
for
marketed
products,
including
without
limitation
EYLEA
®
,
Alirocumab, Sarilumab, and Dupilumab; ongoing regulatory obligations and oversight and
determinations by regulatory and administrative governmental authorities which may
delay or restrict Regeneron's ability to continue to develop or commercialize Regeneron's products and product candidates; competing
drugs and product candidates that may be superior to Regeneron's products and product
candidates; uncertainty of market acceptance and commercial success of Regeneron's
products and product candidates; the ability of Regeneron to manufacture and manage supply chains for multiple products and product
candidates; coverage and reimbursement determinations by third-party payers, including
Medicare and Medicaid; unanticipated expenses; the costs of developing, producing,
and selling products; the ability of Regeneron to meet any of its sales or other financial projections or guidance and changes to the
assumptions underlying those projections or guidance, including without limitation those
relating to non-GAAP unreimbursed R&D, non-GAAP SG&A and capital
expenditures, the potential for any license or collaboration agreement, including
Regeneron's agreements with Sanofi and Bayer HealthCare, to be cancelled or
terminated without any further product success; and risks associated with third party
intellectual property and pending or future litigation relating thereto. A more
complete description of these and other material risks can be found in Regeneron's filings
with the U.S. Securities and Exchange Commission, including its Form 10-K for the
fiscal year ended December 31, 2012 and its Form 10-Q for the quarterly period ended September 30, 2013, in each case including in the sections
thereof captioned “Item 1A. Risk Factors.” Any forward-looking
statements are made based on management's current beliefs and judgment, and the reader is
cautioned not to rely on any forward-looking statements made by Regeneron.
Regeneron does not undertake any obligation to update publicly any forward-
looking statement, including without limitation any financial projection or guidance,
whether as a result of new information, future events, or otherwise. This
presentation uses non-GAAP net income, non-GAAP unreimbursed R&D, and non-GAAP SG&A, which are financial measures that are not calculated in
accordance with the U.S. Generally Accepted Accounting Principles (“GAAP”).
Regeneron believes that the presentation of these non-GAAP measures is useful to
investors because they exclude, as applicable, (i) non-cash share-based compensation expense which fluctuates from period to period based on factors that
are not within the Company's control, such as the Company's stock price on the dates
share-based grants are issued, (ii) non-cash interest expense related to
the Company's convertible senior notes since this is not deemed useful in evaluating the
Company's operating performance, (iii) non-cash income tax expense, since the
Company does not currently pay, or expect to pay in the near future, significant cash income taxes due primarily to the utilization of net operating loss
and tax credit carry-forwards; therefore, non-cash income tax expense is not deemed
useful in evaluating the Company's operating performance, and (iv) a non- cash tax
benefit as a result of releasing substantially all of the valuation allowance associated with the Company’s deferred tax assets. Non-GAAP unreimbursed
R&D represents non-GAAP R&D expenses reduced by R&D expense reimbursements
from the Company's collaboration partners. Management uses these non- GAAP
measures for planning, budgeting, forecasting, assessing historical performance, and making financial and operational decisions, and also provides
forecasts to investors on this basis. However, there are limitations in the use of
these and other non-GAAP financial measures as they exclude certain expenses that
are recurring in nature. Furthermore, the Company's non-GAAP financial measures may not be comparable with non-GAAP information provided by other
companies. Any non-GAAP financial measure presented by Regeneron should be
considered supplemental to, and not a substitute for, measures of financial
performance prepared in accordance with GAAP. A reconciliation of the Company's GAAP
to non-GAAP results is included at the end of this presentation.
|
 Page 3
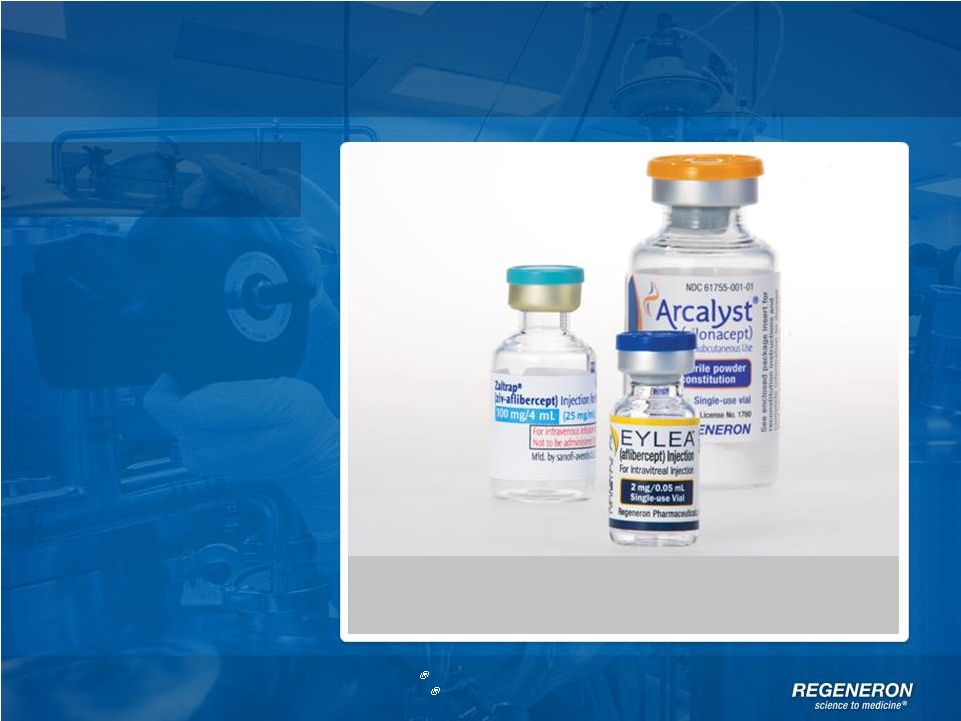
| Copyright Regeneron 2014 Regeneron Today
Products
*EYLEA
ex-U.S is partnered with Bayer HealthCare.
ZALTRAP
is partnered with Sanofi
Three approved products with sales in 50+ countries around
the world* |
 Products
Pipeline
Regeneron Today
Page 4 | Copyright Regeneron 2014
Phase 1
Phase 2
Phase 3
Alirocumab
(REGN727)
PSCK9 Antibody for LDL cholesterol reduction
Sarilumab
(REGN88)
IL-6R Antibody for Rheumatoid arthritis
Dupilumab
(REGN668)
IL-4R Antibody for asthma, atopic dermatitis, nasal polyposis
Sarilumab
(REGN88)
IL-6R Antibody for Non-infectious Uveitis
REGN1033
(GDF8)
Antibody
for
Metabolic
disorders
Fasinumab
(NGF antibody)
on clinical hold
Enoticumab
(REGN421)
DII4 Antibody for Advanced malignancies
Nesvacumab
(REGN910)
Ang2 Antibody for Advanced malignancies
REGN1400
(ErbB3)
Antibody
for
Advanced
malignancies
REGN1154
(undisclosed
target)
REGN1500
(undisclosed
target)
REGN1193
(undisclosed
target)
REGN1908-1909
(undisclosed
target)
REGN2009
(undisclosed
target) |
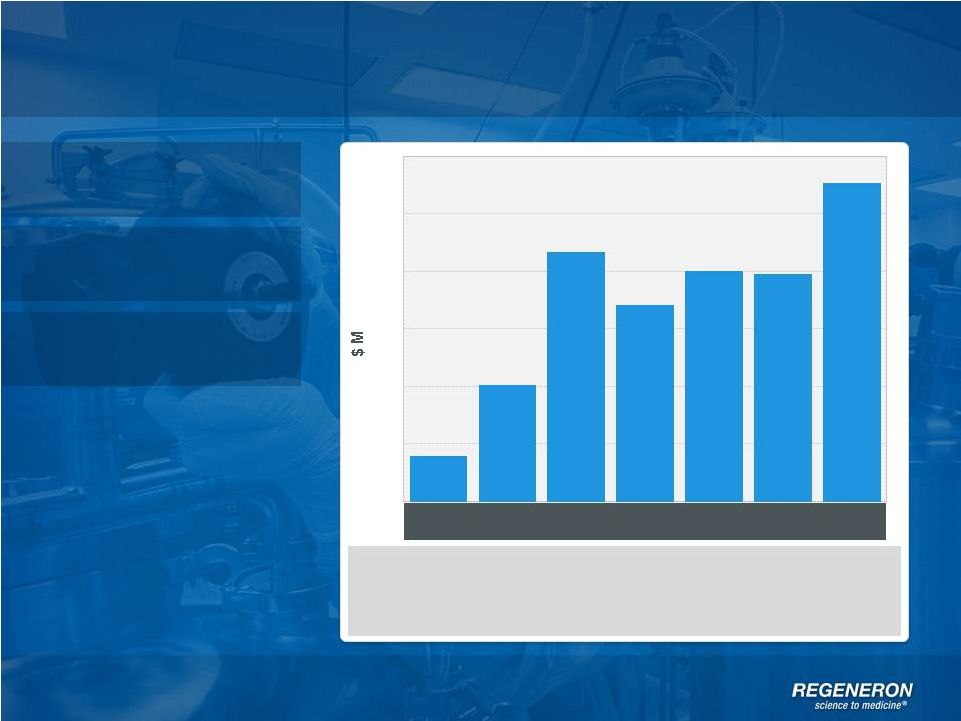 Regeneron
Today Page 5 | Copyright Regeneron 2014
Products
$65M in approval milestones received in 3Q12
$20M in upfront payments to Sanofi for rights to PDGF and ANG2
antibodies in 2Q13
$45M
in
milestone
payments
from
Bayer
for
ex-U.S.
EYLEA
®
in
3Q13
Profits
Pipeline
Non-GAAP Net Income*
Non-GAAP
net
income
excludes
non-cash
share-based
compensation
expense,
non-cash
interest
and
non-cash
income
tax
expense
See page 38 for GAAP to non-GAAP reconciliation
0
50
100
150
200
250
300
1Q12
2Q12
3Q12
4Q12
1Q13
2Q13
3Q13 |
 Awards
Products
Regeneron named top employer in global biopharmaceutical industry by Science
Magazine
for
second
year
in
a
row
Named the best place to work by The Scientist in 2013
CEO and CSO named "Management Team of the Year" by Scrip Intelligence
Dupilumab
named
“Clinical
Advance
of
the
Year”
by
Scrip
Intelligence
Regeneron Today
Page 6 | Copyright Regeneron 2014
Profits
Pipeline |
 Page 7
| Copyright Regeneron 2014 Products
Pipeline
Profits
Awards
People
Regeneron named top employer in global biopharmaceutical
industry by
Science
Magazine
for
second
year
in
a
row
Number of employees grew by 20 percent in 2013 to 2,340
In
four
locations:
Tarrytown,
NY;
Basking
Ridge,
NJ;
Rensselaer,
NY; and Limerick, Ireland
Regeneron Today |
 Regeneron
Building
on
Success
Page 8 | Copyright Regeneron 2014
Organic Growth
Continuing
Top Line Growth
Investment in
R&D and
Technology |
 Regeneron
Building
on
Success
Page 9 | Copyright Regeneron 2014
Significant News January 2014
What’s New for Regeneron
EYLEA
®
& Ophthalmology
Franchise
4Q13 and full year 2013 U.S.
net sales
PDGFR-
clinical and business update
Alirocumab
Sarilumab
Dupilumab
Update on data and filing timelines
Sanofi Collaboration
Amended
investor agreement
Early R&D
Regeneron Genetics Center
Immuno-Oncology
2014 Financial
Guidance
Non-GAAP SG&A,
unreimbursed R&D, and
capital expenditures |
 Regeneron
Building
on
Success
Page 10 | Copyright Regeneron 2014
Continuing
Top Line Growth |
 Regeneron
Building
on
Success
EYLEA
Franchise
Page 11 | Copyright Regeneron 2014
Potential for five major regulatory
submissions or approvals
in next five years |
 Page 12
| Copyright Regeneron 2014 EYLEA
Franchise |
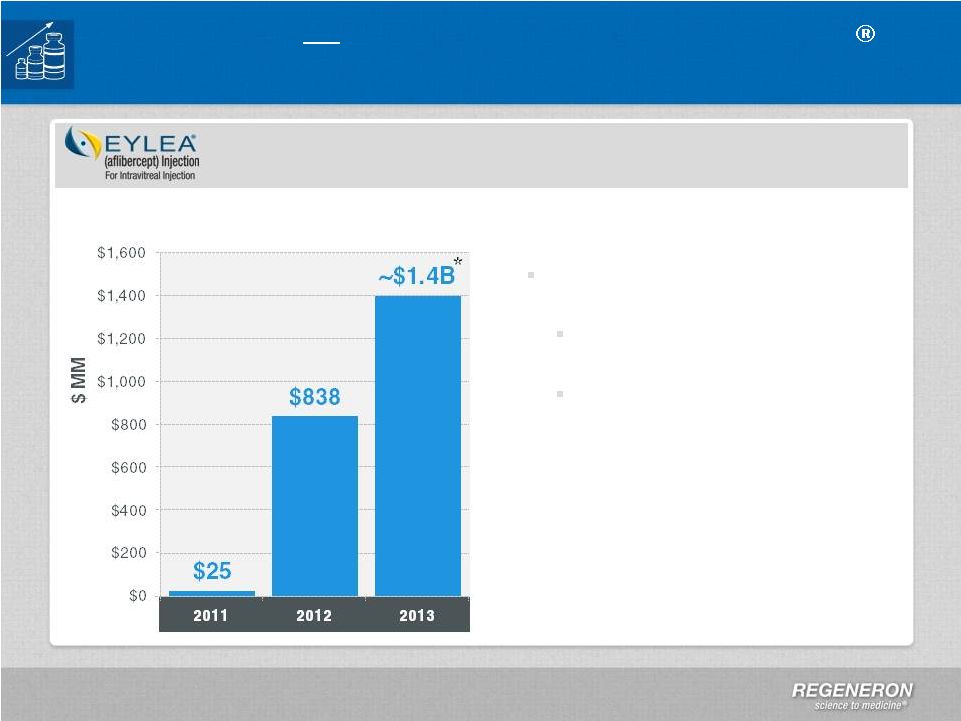 Page
13 | Copyright Regeneron 2014 2013 U.S. Net Sales:
~$1.4 Billion* 4Q13 U.S. Net Sales: ~$400 Million* Distributor inventory increased to
slightly more than two weeks
Inventory has historically been
1-2 weeks
Commercial terms tightened in
January 2014
Full year guidance during 4Q13 call
DME:
PDUFA date of August 18, 2014
BRVO regulatory filing expected in
1Q14
Regeneron
Optimize and Extend EYLEA
U.S.
EYLEA
:
Demographics,
Market
Share,
New
Indications
to
Drive
Growth
DME : Diabetic Macular Edema
BRVO : Branch Retinal vein Occlusion
*
*Preliminary unaudited numbers
Impact of compounding legislation
uncertain
U.S. Net Sales |
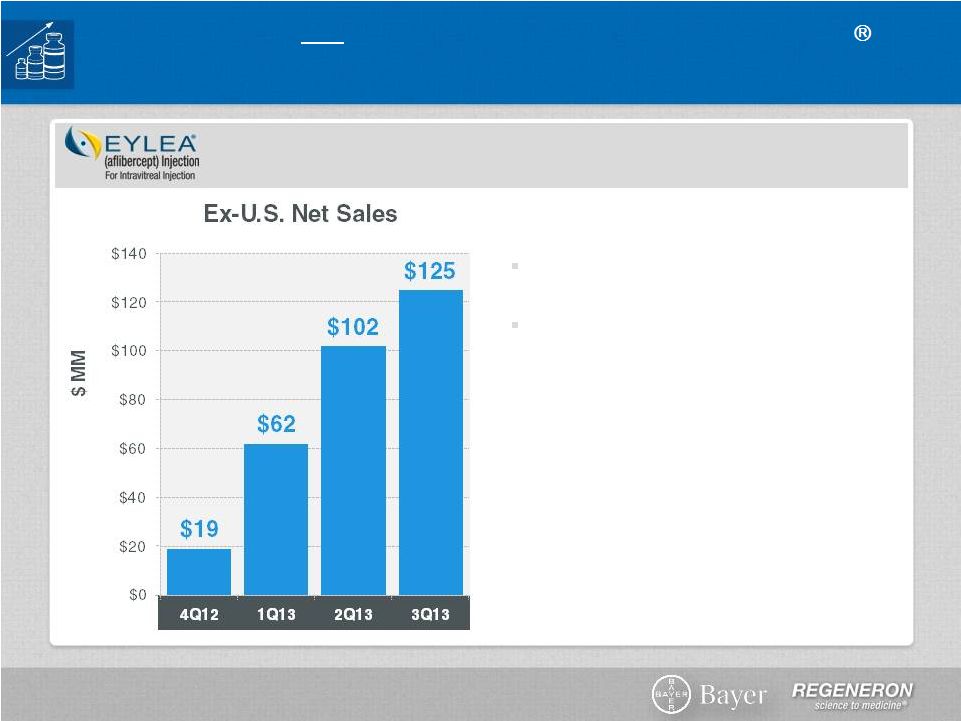 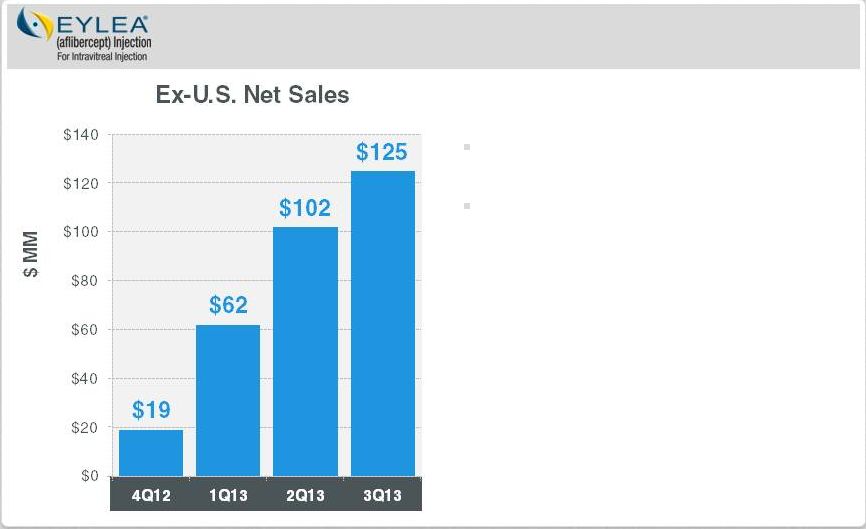 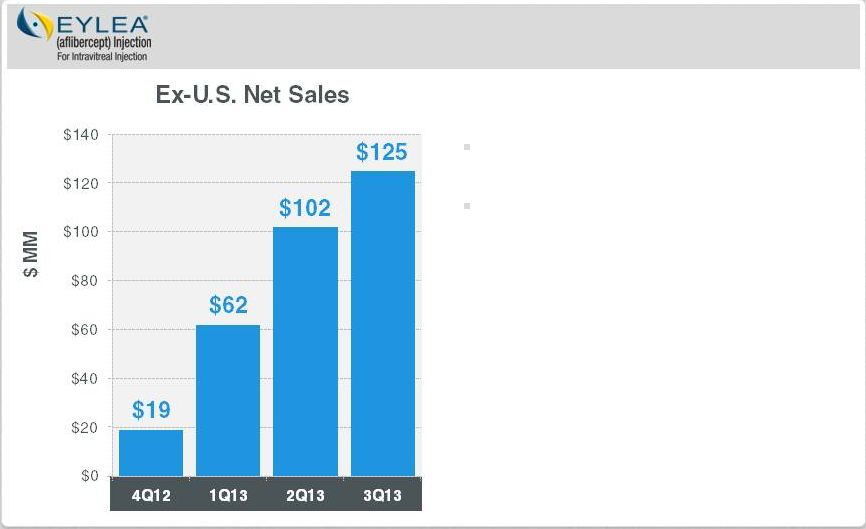 Regeneron
Optimize and Extend EYLEA
Ex-U.S. EYLEA: Launch is Still in Early Innings
Ex-U.S. sales contributing to bottom line
DME submitted in EU
Myopic CNV filed in Japan
Global macular edema following BRVO
submission expected in 2014
DME : Diabetic Macular Edema
AMD: Age-related macular degeneration
CRVO : Central Retinal Vein Occlusion
BRVO : Branch Retinal vein Occlusion
CNV : Choroidal Neovascularization
Ex-U.S. launch by partner, Bayer
HealthCare, continues to do well
In wet-AMD, 40%-50% market share in
Australia, Japan and Germany
Approved for macular edema following
CRVO in EU and Japan
Page 14 | Copyright Regeneron 2014 |
 PDGFR-
/EYLEA
®
co-formulation IND
submitted in December 2013
First patient enrolled in Phase 1
expected in 1Q14
Regeneron owns 100% commercial
rights in U.S.
Bayer collaborating outside the U.S.
Page 15 | Copyright Regeneron 2014
Regeneron
Optimize and Extend EYLEA
Protecting the Long Term Value of The Retina Franchise
PDGFR-
Ophthalmology Research
ANG2
Commitment to remain a leader in
retinal diseases
Investment in internal research and
external collaborations
Intravitreal co-formulation with
EYLEA
®
: IND expected to be
submitted in 2014
$25.5M upfront
$40M in option and milestone
payments
Bayer/REGN share global
development expenses
Bayer responsible for certain third
party royalties and share of
milestones
Companies share profits equally |
 Page 16
| Copyright Regeneron 2014 Late Stage Pipeline |
 Three Late
Stage Programs Regeneron
Advance Late Stage Pipeline
Page 17 | Copyright Regeneron 2014
Late Stage Pipeline Expected to Drive Continued Top-Line Growth
PCSK9 antibody for elevated
cholesterol
IL6R antibody for rheumatoid
arthritis
IL4R antibody for asthma, atopic
dermatitis and nasal polyposis
All three programs—alirocumab, sarilumab and
dupilumab—are part of Sanofi collaboration
Two programs with positive Phase 3 data in
2013: alirocumab & sarilumab
Potential for four major regulatory submissions
or approvals in next five years*:
* Including EYLEA
®
for DME, five major
regulatory submissions or approvals in next five
years
Dupilumab
Sarilumab
Alirocumab
PHASE 3
PHASE 3
PHASE 2
Alirocumab for LDL lowering
Sarilumab for rheumatoid arthritis
Dupilumab for atopic dermatitis
Dupilumab for asthma |
 Page 18
| Copyright Regeneron 2014 Advancing Late Stage Opportunities
Regeneron
Alirocumab
Positive data from the alirocumab clinical trials have been
published in peer-reviewed journals such as the New England
Journal of Medicine
and The Lancet |
 Regeneron
Sarilumab
Page 19 | Copyright Regeneron 2014
Advancing Late Stage Opportunities
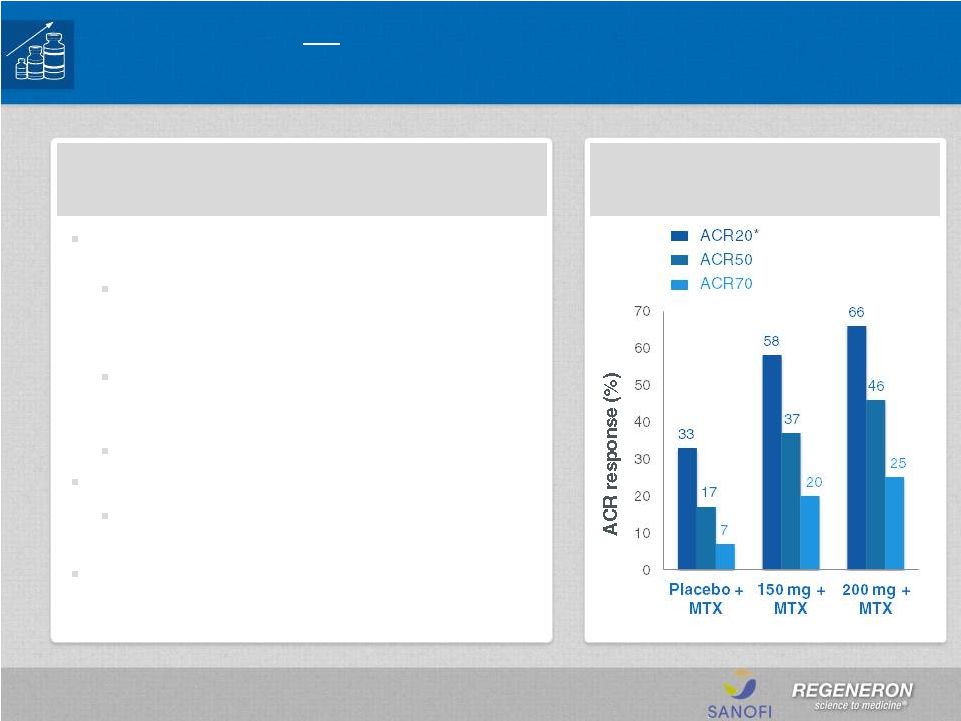
Positive Phase 3 data from MOBILITY (N=1,200)
reported 4Q13
Both sarilumab dose groups—150 mg and 200
mg—given subcutaneously, every other week, in
combination with methotrexate (MTX) achieved
all three co-primary endpoints
Patients receiving 200 mg + MTX showed a 90%
reduction in radiographic progression (mTSS) at
week 52
Data to be presented at a medical conference
Additional Phase 3 data expected in 2015
Ongoing Phase 3 studies are: COMPARE,
TARGET, ASCERTAIN, EXTEND
Regulatory submission expected in 2015
MOBILITY: ACR responses
at 24 weeks
Sarilumab for Rheumatoid Arthritis
Infections were the most frequently reported adverse events and were reported with a higher
incidence in the sarilumab groups vs. placebo, all in combination with MTX (39.6% for
200 mg, 40.1% for the 150 mg group and 31.1% for pbo).
The incidence of serious infections was 4.0% in the 200 mg
+ MTX group, 2.6% in the 150 mg + MTX group, and 2.3% in the placebo + MTX group.
.
*primary endpoint, p<0.0001 vs. pbo |
 Page 20
| Copyright Regeneron 2014 Regeneron
Dupilumab
Advancing Late Stage Opportunities
Positive data from the dupilumab asthma clinical trial have
been published in the New England Journal of Medicine |
 Regeneron
Building on Success
Page 21 | Copyright Regeneron 2014
Investment in
R&D and
Technology |
 Regeneron
Building on Success
Page 22 | Copyright Regeneron 2014
Wholly-Owned
Pipeline of Products
Sanofi Collaboration
Fasinumab*
Phase 2
REGN1400
Phase 1
REGN1154
Phase 1
REGN1500
Phase 1
REGN1193
Phase 1
REGN1908-1909
Phase 1
Innovative Research
& Technologies
Investment in
R&D and
Technology |
 Page 23
| Copyright Regeneron 2014 Sanofi Collaboration |
 Sanofi
Collaboration Regeneron
Sanofi Collaboration
Discovery
$160 million of annual funding
through 2017 (plus possible
tail period through 2020)
Sanofi Antibody Collaboration Continues to Provide R&D Leverage
Page 24 | Copyright Regeneron 2014
*Regeneron
repays
Sanofi
for
50%
of
development
costs
out
of
profits.
Repayment
capped
in
any
year
at
10%
of
Regeneron
share
of
total
antibody
profits
Development
REGN funds 20% of Phase 3
costs for an antibody following
first positive Phase 3 data for
that antibody
Commercialization
Regeneron retains 35% to 45%
45% of profits ex-US*
Co-promotion rights
in US and other major market
countries
Ownership / Investor Agreement
•
Sanofi ownership is currently 15.8M shares or ~16%
•
Sanofi
has
obtained
right
to
nominate
an
independent
director
to
Regeneron
BOD
when they reach 20% ownership
•
Voting rights, lock-up, and standstill limit to 30% ownership
Ownership / Investor Agreement
•
Sanofi ownership is currently 15.8M shares or ~16%
•
Sanofi
has
obtained
right
to
nominate
an
independent
director
to
Regeneron
BOD
when they reach 20% ownership
•
Voting rights, lock-up, and standstill limit to 30% ownership
Sanofi funds approximately 100%
of clinical development cost
Regeneron retains
50% of profits in US* |
 Phase
1 Phase 2
Phase 3
Alirocumab
(REGN727)
PSCK9 Antibody for LDL cholesterol
reduction
Sarilumab
(REGN88)
IL-6R
Antibody for Rheumatoid arthritis
Dupilumab
(REGN668)
IL-4R
Antibody for Asthma, Atopic dermatitis,
nasal polyposis
Sarilumab
(REGN88)
IL-6R
Antibody for Non-infectious Uveitis
REGN1033
GDF8
Antibody
for
Metabolic
disorders
Enoticumab
(REGN421)
DII4
Antibody for Advanced malignancies
Nesvacumab
(REGN910)
Ang2
Antibody for Advanced malignancies
REGN2009
(undisclosed
target)
Page 25 | Copyright Regeneron 2014
Sanofi collaboration a major
contributor to Regeneron R&D
Sanofi is estimated to spend
more than $1 Billion on
collaboration programs in 2014*
Regeneron contribution to
collaboration R&D funding
increasing in 2014
20% funding of alirocumab and
sarilumab Phase 3 trials
Estimated to be ~$115 MM in
2014
Sanofi Antibody Collaboration Continues to Provide R&D Leverage
Regeneron
Sanofi Collaboration
*Regeneron to repay 50% of collaboration clinical development
spending out of antibody profits
Sanofi Collaboration |
 Page 26
| Copyright Regeneron 2014 Pipeline of Wholly-Owned
Antibodies |
 Wholly-Owned Clinical Stage Antibodies
Advancing early stage pipeline of Regeneron-owned antibodies
Fasinumab* (Phase 2, NGF antibody)
REGN1400 (Phase 1, ErbB3, advanced malignancies)
REGN1154 (Phase 1, undisclosed target)
REGN1500 (Phase 1, undisclosed target)
REGN1193 (Phase 1, undisclosed target)
REGN1908-1909 (Phase 1, undisclosed target)
As pipeline advances, R&D expense will increase
Pipeline of Wholly-Owned Antibodies is Growing
Regeneron
Wholly-Owned Antibody Pipeline
*Currently on clinical hold by the FDA
Pipeline compounds address six distinct therapeutic areas
Committed to advance through POC to maximize value |
 Page 28
| Copyright Regeneron 2014 Innovative Research and Novel
Technologies |
 Immuno-Oncology
CD20-CD3 bispecific antibody
expected to enter clinic in
2014
Page 29 | Copyright Regeneron 2014
Regeneron
Innovating
for
the
Future
Investment in Research & Development and Technology
Regeneron Genetics Center
Major new investment in human
genetics research
Collaboration with Geisinger Health
System is cornerstone of population
based approach
Additional collaborations expected
Other Pipeline
Technologies
Long-acting antibodies
Antibody Drug Conjugates
Next Generation
VelocImmune
®
Mice |
 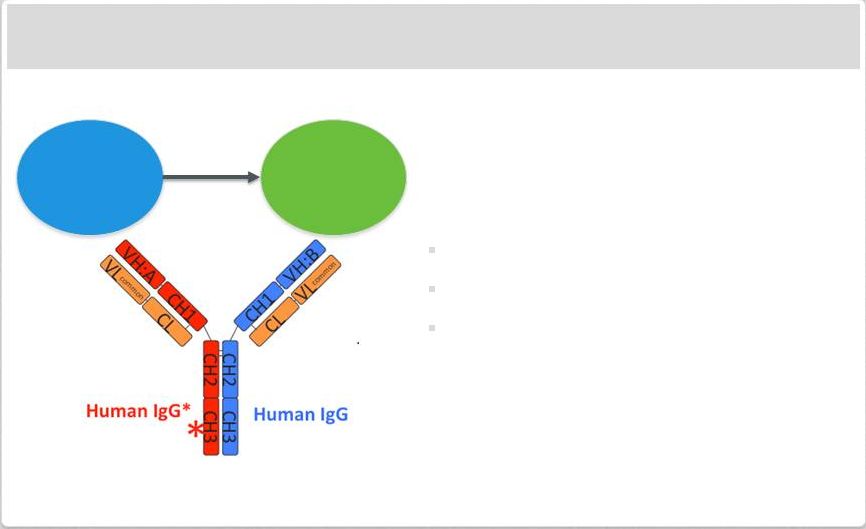 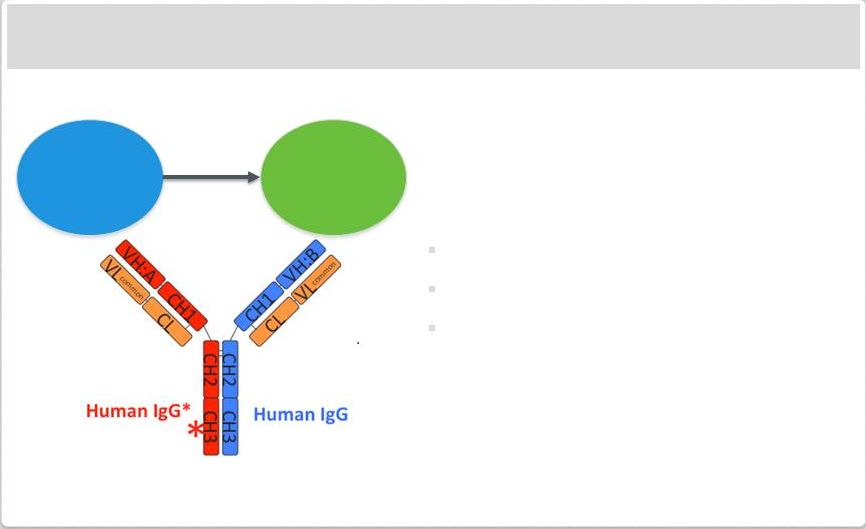 Immuno-Oncology Approach: CD20-CD3 Bispecific Antibody
Bispecific antibody bind T Cells (via CD3)
and tumor (via specific surface marker)
Use of modified VelocImmune
®
mice to
generate fully human bispecific
antibodies provides benefits
High affinity to target
Ease of manufacturing
Typical antibody pharmacodynamics
Initial CD20-CD3 antibody expected to
enter the clinic in 2014
Additional immuno-oncology approaches
in preclinical development
Page 30 | Copyright Regeneron 2014
Investment in Research & Development and Technology
Regeneron
Innovating
for
the
Future
T-cell
Tumor
Cell
Killing |
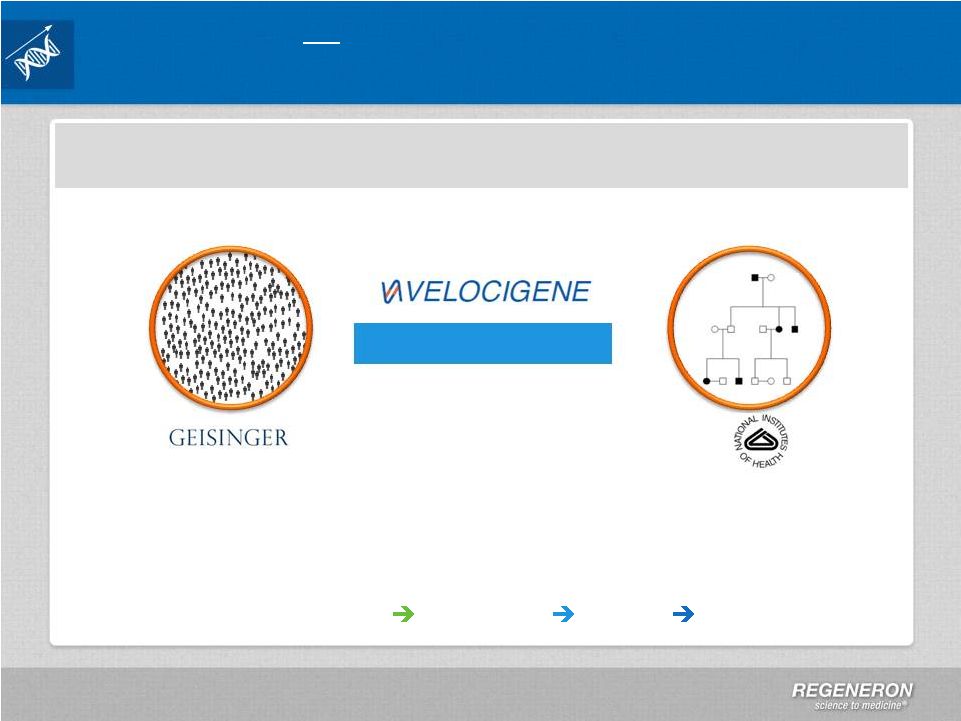 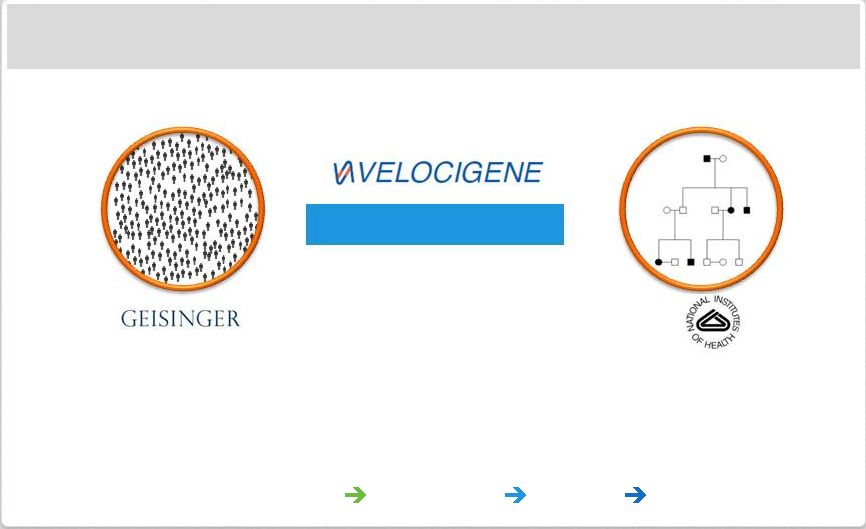 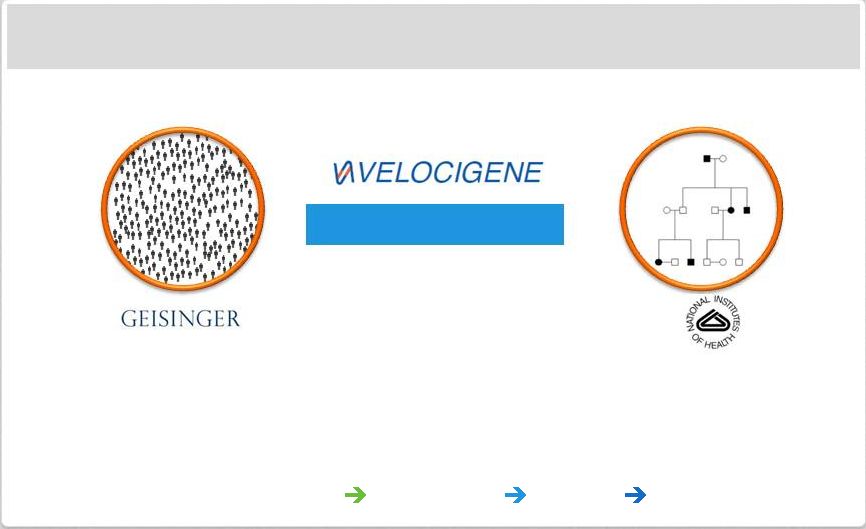 Page 31
| Copyright Regeneron 2014 Regeneron Genetics Center a Major Initiative in Human
Genetics Regeneron
Innovating
for
the
Future
Regeneron Genetics Center
Population-Based
Family-Based
Collaborations:
Geisinger,
NIH,
and
pursuing
more
Approaches:
population
and
family
based,
consortia,
and
functional
studies
Technology:
large
scale
sequencing,
automation,
and
cloud
informatics
Fully Integrated: Sequencing
Informatics
Biology
Drug Development
Targets & Indications |
 Organic
Growth Regeneron
Building
on
Success
Page 32 | Copyright Regeneron 2014 |
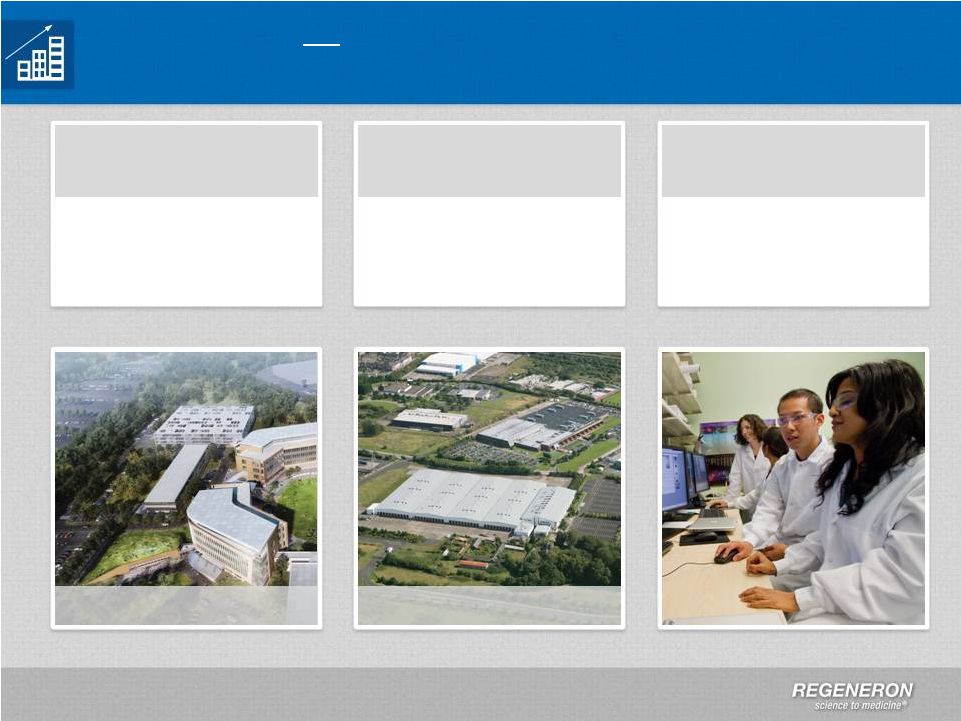 Page
33 | Copyright Regeneron 2014 Regeneron
Organic
Growth
Growth in Manufacturing and at Corporate Headquarters
Two new buildings to
support additional
research and
development
Expansion in
Tarrytown, NY
Expect to increase
headcount to >4,000 by
2018
People
Expansion in Rensselaer,
NY and new facility in
Limerick, Ireland
New Manufacturing
Facilities
Tarrytown, NY
Limerick, Ireland |
 Regeneron
Financial
Guidance
Page 34 | Copyright Regeneron 2014
Financial Guidance
Non-GAAP
SG&A:
$330MM
-
$380MM
Increase in prelaunch commercial expenses, contribution to patient assistance
programs, pharma fee and headcount
Non-GAAP
Unreimbursed
R&D:
$425MM
-
$475MM
~$115 M in expenses related to alirocumab and sarilumab Phase
3 trials
Investment
in
PDGFR
and
Ang2
programs
Growing wholly-owned antibody pipeline
Early technologies, such as Regeneron Genetics Center
Capital
Expenditures:
$350MM
-
$425MM
Manufacturing expansions in Rensselaer and Ireland
R&D and corporate headquarters expansion in Tarrytown
2014 Financial Guidance |
 Continuing
Top Line
Growth
Organic Growth
Regeneron
Building on Success
Page 35 | Copyright Regeneron 2014
Investment in
R&D and
Technology |
 Regeneron
2014 Milestones
Page 36 | Copyright Regeneron 2014
Upcoming Milestones
Clinical
Phase 2 data for dupilumab
in atopic dermatitis in 1H14
Phase 3 trial to start with
dupilumab in AD in 2014
Phase 3 data from
alirocumab ODYSSEY
program in mid-2014
through 3Q14
PDGFR
/EYLEA
coformulation to enter
clinic in 1Q14
CD20-CD3 bispecific
antibody to enter clinic
Commercial
EYLEA U.S. net sales
guidance to be provided on
4Q13 conference call
Regulatory
EYLEA for DME PDUFA
date of August 18, 2014
Filing of EYLEA for BRVO
indication expected in
1Q14
EYLEA for DME regulatory
applications submitted
outside the U.S. |
   Regeneron
News
Page 37 | Copyright Regeneron 2014
Significant News Flow at J.P. Morgan
What’s News for Regeneron at J.P. Morgan 2014
EYLEA
Full year 2013 U.S. Net Sales: ~$1.4
#
Billion
4Q13 U.S. Net Sales: ~$400 Million*
#
Clinical trial to start in 1Q14
Signed a collaboration with Bayer HealthCare for commercial rights outside the U.S.
Alirocumab
Phase 3 data from ODYSSEY program expected mid-year**
Initial regulatory submission ex-US in early 2015, U.S. in 2015
Dupilumab
Phase 2a atopic dermatitis data to be presented at AAAAI in March
Top-line Phase 2b atopic dermatitis data expected in 2Q14
Plan to initiate Phase 3 trial in atopic dermatitis in 2014
Sanofi
Amended
investor
agreement
to
allow
for
Sanofi
to
nominate
a
single
director
to
Regeneron BOD; amended voting rights, lock-up, and stand still agreement
Human Genetics
Announced major effort in human genetics: The Regeneron Genetics Center
Entered into first significant genetics collaboration with Geisinger Health System
Immuno-Oncology
CD20-CD3 bispecific antibody to enter clinic in 2014
2014 Financial
Guidance
Non-GAAP SG&A: $330MM -
$380MM
Non-GAAP unreimbursed R&D: $425MM -
$475MM
Capital expenditures: $350MM -
$425MM
#
Preliminary unaudited numbers
*Includes increase in distributor inventory
**From
all
Phase
3
studies
except
OUTCOMES,
CHOICE
1,
and
CHOICE
2
PDGFR- |
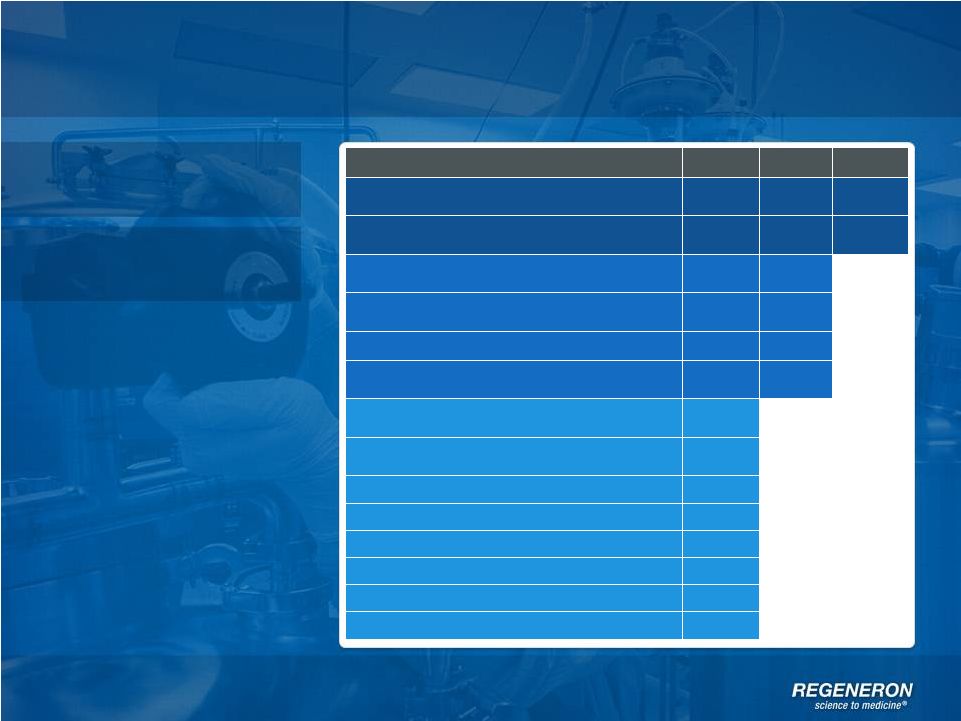
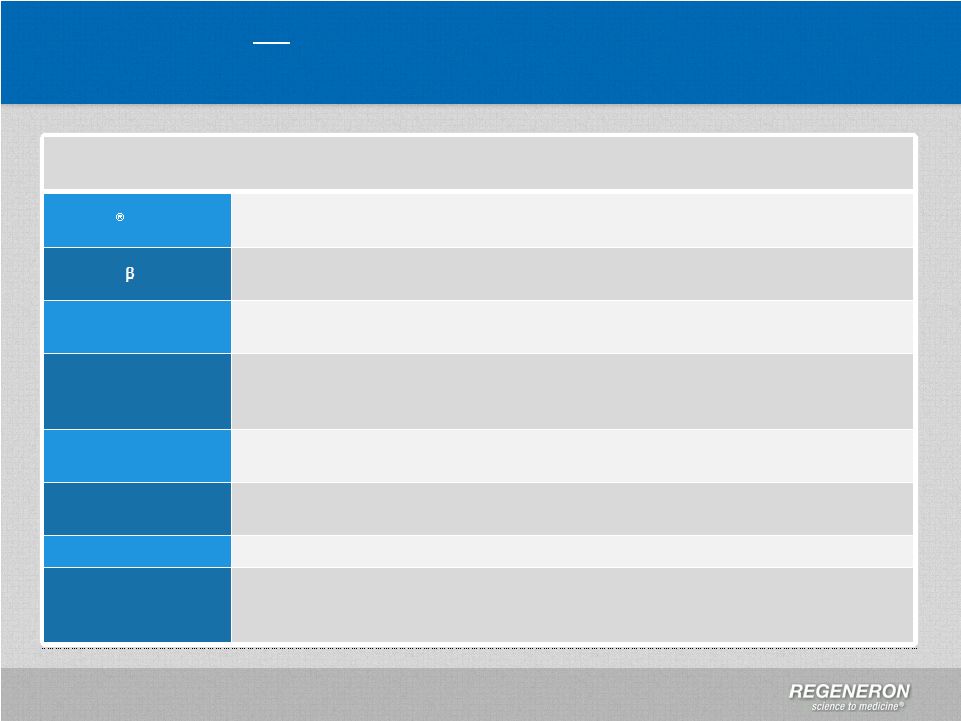
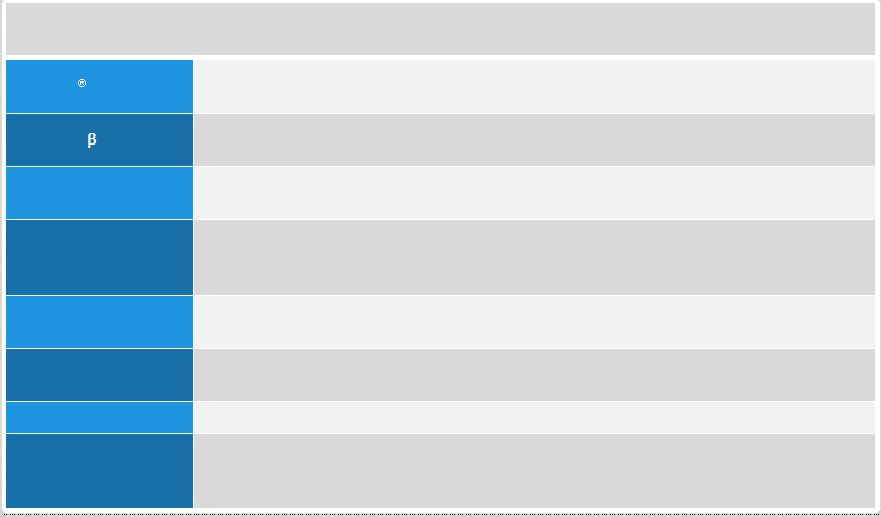
 GAAP to
Non-GAAP Reconciliation 1Q’12
2Q’12
3Q’12
4Q’12
1Q’13
2Q’13
3Q’13
GAAP net income
11,651
76,743
191,468
470,407
98,874
87,376
141,306
Adjustments
R&D: Non-cash share-based compensation expense
10,556
11,442
13,337
18,498
26,761
27,722
28,258
SG&A: Non-cash share-based compensation expense
12,578
7,790
7,030
11,851
25,787
16,344
17,114
COGS: Non-cash share-based compensation expense
111
391
150
422
483
376
373
Interest expense: Non-cash interest related to
convertible senior notes
5,218
5,316
5,499
5,591
5,781
5,535
5,823
Income taxes: Non-cash income tax expense
4,308
(
42,957
60,316
84,378
Income taxes: Release of valuation allowance
(340,156)
(8)
Non-GAAP net income
40,114
101,682
217,484
170,921
200,643
197,669
277,252
Non-GAAP net income per share –
basic
0.43
1.07
2.29
1.79
2.07
2.02
2.82
Non-GAAP net income per share –
diluted
0.37
(3)
0.90
(5)
1.89
(6)
1.47
(9)
1.78
(10)
1.73
(11)
2.40
(12)
Shares used in calculating Non-GAAP net income per
share -
basic
93,446
94,589
95,012
95,691
96,878
97,700
98,226
Shares used in calculating Non-GAAP net income per
share –
diluted
112,495
114,928
115,830
117,237
113,730
115,261
116,068
1)
To exclude non-cash compensation expense related to employee stock option and
restricted stock award 2)
To exclude non-cash interest expense related to the amortization of the debt discount
and debt issuance costs on the Company's 1.875% convertible senior notes
3)
For diluted non-GAAP per share calculations, excludes $1.9 million of interest
expense related to the contractual coupon interest rate on the Company's 1.875%
convertible senior notes, since these securities were dilutive
4)
Weighted average shares outstanding includes the dilutive effect, if any, of
employee stock options, restricted stock awards, convertible senior notes,
and warrants 5)
For diluted non-GAAP per share calculations, excludes $1.9 million of interest
expense for the three months ended June 30, 2012 related to the contractual
coupon interest rate on the Company's 1.875% convertible senior notes, since these
securities were dilutive 6)
For diluted non-GAAP per share calculations, excludes $1.9 million of interest
expense for the three months ended September 30, 2012 related to the contractual
coupon interest rate on the Company's 1.875% convertible senior notes, since these
securities were dilutive 7)
To
exclude
GAAP
income
tax
expense
as
this
amount
is
not
payable
in
cash
8)
To exclude non-cash tax benefit related to releasing substantially all of the
valuation allowance associated with the Company's deferred tax assets
9)
For diluted non-GAAP per share calculations, excludes $1.9 million of interest
expense for the three months ended
December
31,
2012
related to the contractual coupon interest rate on the Company's
1.875% convertible
senior notes, since these securities were dilutive
10)
For diluted non-GAAP per share calculations, excludes $1.9 million of interest
expense for the three month ended March 31, 2013 related to the contractual
coupon interest rate on the Company's 1.875% convertible
senior notes, since these securities were dilutive
11)
For diluted non-GAAP per share calculations, excludes $1.8 million of interest
expense for the three month periods ended June 30, 2013 related to the
contractual coupon interest rate on the Company's 1.875% convertible senior
notes, since these securities were dilutive 12)
For diluted non-GAAP per share calculations, excludes $1.8 million of interest
expense for the three months ended September 30, 2013, related to the
contractual coupon interest rate on the Company's 1.875% convertible senior
notes, since these securities were dilutive (1)
(1)
(1)
(2)
(4)
(7) |
 |
