Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ASENSUS SURGICAL, INC. | d627741d8k.htm |
| EX-99.2 - EX-99.2 - ASENSUS SURGICAL, INC. | d627741dex992.htm |
| EX-99.1 - EX-99.1 - ASENSUS SURGICAL, INC. | d627741dex991.htm |
 Advancing Surgery
Through Innovation
Exhibit 99.3 |
 Any
statements contained in this presentation that do not describe historical facts, including
statements about the beliefs and expectations of SafeStitch Medical, Inc.
(“SafeStitch”) and TransEnterix, Inc. (“TransEnterix”), may
constitute forward-looking statements as that term is defined by the United
States Private Securities Litigation Reform Act of 1995. These forward-looking
statements can be identified by terminology such as "will," "expect,"
"anticipate," "future," "intend," "plan,"
"believe," "estimate," "confident" and similar statements. Any forward-looking statements
contained herein are based on current expectations, but are subject to a number of risks and
uncertainties that may cause actual results to differ materially
from expectations.
Potential risks and uncertainties include the risks outlined in this presentation and
SafeStitch’s filings with the U.S. Securities and Exchange Commission, including
the benefits and opportunities of surgical robotics, whether the merger between
SafeStitch and TransEnterix will be successful, whether the combined company will be
successful in 2014 and beyond, the pace of adoption of our
product technology by surgeons, the outcome of coverage and reimbursement decisions by the
government and third party payors, the success and market opportunity of our
continuing and new product development efforts, including the SurgiBot system, the
effect on our business of existing and new regulatory requirements, and other
economic and competitive factors. SafeStitch and TransEnterix caution readers
not to place undue reliance upon any forward-looking statements, which speak only
as of the date made. We do not undertake or accept any obligation or undertaking to
release publicly any updates or revisions to any forward-looking statement to reflect any change
in our expectations or any change in events, conditions or circumstances on which any such
statement is based.
Cautionary Statement
2 |
 Financial Overview
Joe Slattery
Executive Vice President & CFO
3 |
 Advancing
Surgery Through Innovation |
 Investment
Highlights Focus
Technology
Solution
Market
Team
•
Surgical robotics
•
Developing patient-side robotic platform: SurgiBot
•
Addresses unmet needs in today’s robotic offerings
•
Large
addressable
market
–
~2M
US
procedures
•
Strong management team
•
Cost effective
•
Broad procedure applicability |
 Company
History 6
•
TransEnterix
founded
•
FDA 510(K) clearance for
Spider Surgical System
•
CE Mark clearance and release
of Spider Surgical System
•
>3,000 SPIDER cumulative
procedures performed to date
•
Began SurgiBot development
•
SurgiBot preclinical labs
•
Merger with SafeStitch
Medical
2006
2009
2010
2012
2013 |
 •
Transaction closed on September 4, 2013
•
About SafeStitch
–
Publicly-traded, development stage medical device company (OTCBB: SFES)
–
Obesity and GERD-focused, flexible endoluminal instrumentation
•
Board and investors with demonstrated success in leading high-growth
healthcare companies
•
$30M in financing raised from existing TransEnterix and SafeStitch
shareholders
•
Combined entity will be called TransEnterix, Inc.
Merger with SafeStitch Medical
7 |
 Surgical
Progress = Less Invasive 8
Open
Surgery
Rigid
Laparoscopy
(1989)
Flexible
Laparoscopy
(2010) |
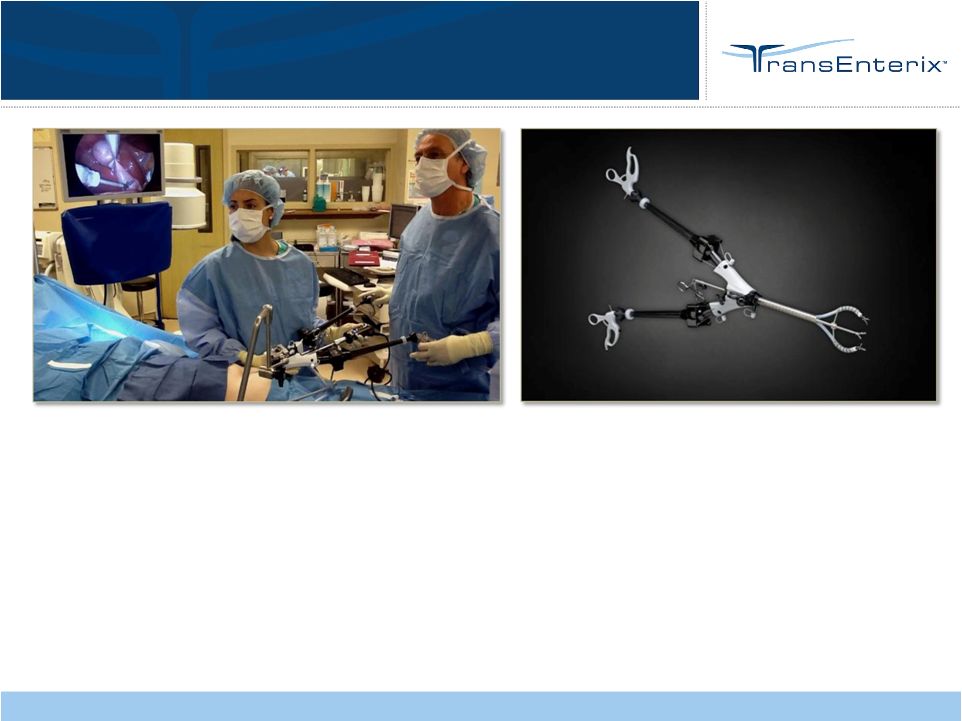 •
•
•
•
•
•
Spider Surgical System
Enabling Flexible Laparoscopy
9
Internal (intra-abdominal)
triangulation
Flexible, articulating instruments
for dissection and retraction
True left/right instrumentation
Lower profile port of access
Over 3,000 procedures
performed
Broad procedural mix |
 Innovative,
Minimally Invasive Robotic Platform
SurgiBot
TM |
 Benefits of Surgical Robotics
11
Benefits of
Surgical
Robotics
Improved
dissection and
retraction strength
Enhanced precision
and control
Improved surgeon
ergonomics
Advanced
visualization |
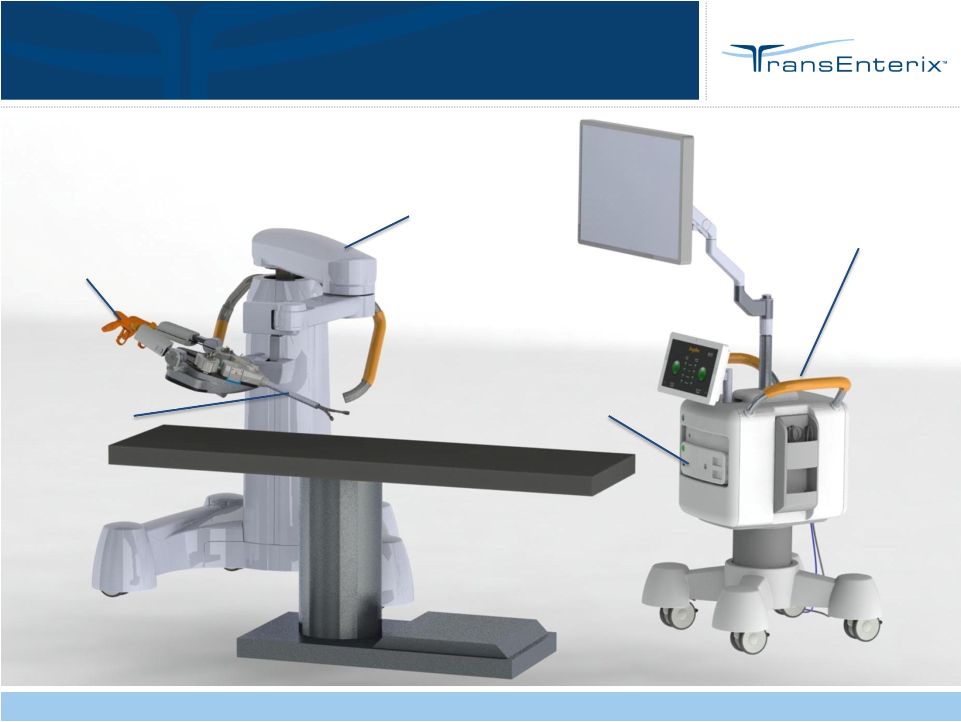 Confidential
12
Positioning Arm
Cart
Endodrive
Flex
Instruments
3DHD Optics
SurgiBot
First Patient-side Robotic Platform |
 SurgiBot
First Patient-side Robotic Platform
13
Surgeon scrubbed-in
Flexible, articulating channels &
instruments
Flexible & steerable 3DHD
Small, mobile platform
Easily repositioned for multi-
quadrant surgery
Cost effective platform |
 14
Benefits to Key Stakeholders
Surgeons
•
Precision of robotic surgery
•
Advanced 3D visualization
•
Scrubbed-in patient-side
•
Ergonomics/reduced fatigue
Hospitals and Surgical Centers
•
Attractive ROI for many health care facilities,
relative to competing robotic platforms
•
Strategic asset for surgeon and patient
recruitment
Patients
•
Advances minimally
invasive surgery |
 Large
Addressable Market 15
Source: CDC
Over 5,000 Hospitals in the United States
# of Beds
1,566
2,054
930
458
Under 50
50-200
200-400
Over 400
Additionally over
surgery centers in US
5,000 |
 Hospital
Market Opportunity Confidential
16
Hospitals
without
Robotic Capability
•
Current robotic offerings
are not cost effective
•
Price
•
Procedure volume
•
Surgery centers –
untapped market for
robotics
•
Large underpenetrated
OUS opportunity
Hospitals
with
Robotic Capability
•
Invested in the strategic
and competitive value of
robotic surgery
•
ROI expectations
changing/under pressure
•
Potential for
diversification of robotic
solutions
•
Procedures
•
Price
•
Facilities |
 17
Bariatric Surgery
Cholecystectomy
Colorectal
Gynecology/Urology
Targeted US
Procedures
160K
1,140K
363K
230K
Source: Millennium Research Group US Markets Laparoscopic Devices 2014
Note: Company Estimate of Outside US addressable market = US opportunity
~2M US
Laparoscopic
Procedures
Procedure Market Opportunity |
 Recurring
Revenue Model 18
Capital Equipment
Service
Instruments and
Accessories |
 Commercial Pathway
19
•
Design &
Pre-clinical Testing
•
Pre-submission
FDA Meeting
•
First Human
Procedures
•
Regulatory
Submissions
•
Regulatory
Clearances
•
Global Launch
2013
2014
2015 |
 Prior Experience
Todd M. Pope
President and Chief Executive Officer
Joe Slattery
EVP and Chief Financial Officer
Richard Mueller
Chief Operating Officer
Mohan Nathan
Vice President of Global Marketing
Tammy Carrea
Vice President, Quality and Regulatory Affairs
Management Team
20 |
 •
Phillip Frost, M.D.
–
CEO and Chairman of OPKO Health
–
Chairman of Teva Pharmaceuticals
•
Jane Hsiao, Ph.D., MBA
–
Former Chairman of SafeStitch
–
Vice Chairman and CTO of OPKO Health
•
Aftab Kherani, M.D.
–
Principal of Aisling Capital
•
Richard Pfenniger, Jr.
–
Former Chairman/CEO of Continucare
–
Former CEO of Whitman Education Group
•
Paul LaViolette
–
Chairman of TransEnterix
–
Partner, SV Life Sciences
•
Todd Pope
–
President and Chief Executive Officer, TransEnterix
•
Dennis Dougherty
–
Founder, Intersouth Partners
•
David Milne
–
Managing Partner, SV Life Sciences
•
William Starling
–
Managing Director, Synergy Life Science Partners
21
Board of Directors
Healthcare Investors
Board of Directors and Healthcare
Investors |
 Investment
Highlights 22
Focus
Technology
Solution
Market
Team
•
Surgical robotics
•
Developing patient-side robotic platform: SurgiBot
•
Addresses unmet needs in today’s robotic offerings
•
Large
addressable
market
–
~2M
US
procedures
•
Strong management team
•
Cost effective
•
Broad procedure applicability |
 Advancing
Surgery Through Innovation |
