Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Midatech Pharma US Inc. | dara_8k.htm |
| EX-99.1 - PRESS RELEASE - Midatech Pharma US Inc. | dara_ex991.htm |
EXHIBIT 99.2

NASDAQ: DARA
www.darabio.com
Q3 2013 Financial Results and Corporate Business
Update Conference Call and Webcast
Update Conference Call and Webcast
November 14, 2013

Page *
Forward Looking Statements
All statements in this presentation that are not historical are forward-looking statements within the meaning of the
Securities Exchange Act of 1934, as amended, and are subject to risks and uncertainties. These statements are based on
the current expectations, estimates, forecasts and projections regarding management’s beliefs and assumptions. In some
cases, you can identify forward looking statements by terminology such as “may,” “will,” “should,” “hope,” “expects,”
“intends,” “plans,” “anticipates,” “contemplates,” “believes,” “estimates,” “predicts,” “projects,” “potential,” “continue,”
and other similar terminology or the negatives of those terms. Such forward-looking statements are subject to factors that
could cause actual results to differ materially for DARA from those projected. Important factors that could cause actual
results to differ materially from the expectations described in these forward-looking statements are set forth under the
caption “Risk Factors” in DARA’s most recent Annual Report on Form 10-K, filed with the SEC on March 28, 2013, DARA’s
most recent Quarterly Report on Form 10-Q, filed with the SEC on November 13, 2013, and DARA’s other filings with the SEC
from time to time. Those factors include risks and uncertainties relating to DARA's ability to timely commercialize and
generate revenues or profits from Soltamox®, Gelclair®, Bionect® or other products given that DARA only recently hired its
initial sales force and DARA's lack of history as a revenue-generating company, DARA’s ability to achieve the desired results
from the agreements with Mission and Alamo, FDA and other regulatory risks relating to DARA's ability to market
Soltamox®, Gelclair®, Bionect® or other products in the United States or elsewhere, DARA’s ability to in-license and/or
partner products, DARA's current cash position and its need to raise additional capital in order to be able to continue to
fund its operations, the current regulatory environment in which DARA sells its products, the market acceptance of those
products, dependence on partners, successful performance under collaborative and other commercial agreements,
competition, the strength of DARA's intellectual property and the intellectual property of others, the potential delisting of
DARA's common stock from the NASDAQ Capital Market, risks and uncertainties relating to DARA's ability to successfully
integrate Oncogenerix and other risk factors identified in the documents DARA has filed, or will file, with the Securities and
Exchange Commission ("SEC"). Copies of DARA's filings with the SEC may be obtained from the SEC Internet site at
http://www.sec.gov. DARA expressly disclaims any obligation or undertaking to release publicly any updates or revisions to
any forward-looking statements contained herein to reflect any change in DARA's expectations with regard thereto or any
change in events, conditions, or circumstances on which any such statements are based. DARA BioSciences and the DARA
logo are trademarks of DARA BioSciences, Inc.
Securities Exchange Act of 1934, as amended, and are subject to risks and uncertainties. These statements are based on
the current expectations, estimates, forecasts and projections regarding management’s beliefs and assumptions. In some
cases, you can identify forward looking statements by terminology such as “may,” “will,” “should,” “hope,” “expects,”
“intends,” “plans,” “anticipates,” “contemplates,” “believes,” “estimates,” “predicts,” “projects,” “potential,” “continue,”
and other similar terminology or the negatives of those terms. Such forward-looking statements are subject to factors that
could cause actual results to differ materially for DARA from those projected. Important factors that could cause actual
results to differ materially from the expectations described in these forward-looking statements are set forth under the
caption “Risk Factors” in DARA’s most recent Annual Report on Form 10-K, filed with the SEC on March 28, 2013, DARA’s
most recent Quarterly Report on Form 10-Q, filed with the SEC on November 13, 2013, and DARA’s other filings with the SEC
from time to time. Those factors include risks and uncertainties relating to DARA's ability to timely commercialize and
generate revenues or profits from Soltamox®, Gelclair®, Bionect® or other products given that DARA only recently hired its
initial sales force and DARA's lack of history as a revenue-generating company, DARA’s ability to achieve the desired results
from the agreements with Mission and Alamo, FDA and other regulatory risks relating to DARA's ability to market
Soltamox®, Gelclair®, Bionect® or other products in the United States or elsewhere, DARA’s ability to in-license and/or
partner products, DARA's current cash position and its need to raise additional capital in order to be able to continue to
fund its operations, the current regulatory environment in which DARA sells its products, the market acceptance of those
products, dependence on partners, successful performance under collaborative and other commercial agreements,
competition, the strength of DARA's intellectual property and the intellectual property of others, the potential delisting of
DARA's common stock from the NASDAQ Capital Market, risks and uncertainties relating to DARA's ability to successfully
integrate Oncogenerix and other risk factors identified in the documents DARA has filed, or will file, with the Securities and
Exchange Commission ("SEC"). Copies of DARA's filings with the SEC may be obtained from the SEC Internet site at
http://www.sec.gov. DARA expressly disclaims any obligation or undertaking to release publicly any updates or revisions to
any forward-looking statements contained herein to reflect any change in DARA's expectations with regard thereto or any
change in events, conditions, or circumstances on which any such statements are based. DARA BioSciences and the DARA
logo are trademarks of DARA BioSciences, Inc.
Page *
Page 2

Page 3
Opening Comments
David Drutz, MD, CEO & CMO

Page 4
q Oncology supportive care is an attractive area of focus and
uniquely positions DARA
uniquely positions DARA
q Refined commercial focus has yielded encouraging sales
q Sales force expansion and new product opportunities setting
the stage for growth
the stage for growth
q Continued dialog with FDA orphan division for KRN5500
q DARA is poised for growth
Q3 2013 Overview
DARA’s goal is to establish a leadership position in oncology
supportive care
supportive care

Page 5
Q3 Oncology Supportive Care
Product Portfolio Review and
Recent Events
Product Portfolio Review and
Recent Events
Chris Clement, President & COO

Page 6
qSignificant opportunity / unmet medical need
qCompetitive products not optimal
qLimited existing therapeutic options
qPortfolio approach
qSynergy creates opportunity
qPotential for increased access to caregivers
qFacilitates “branding” the Company
Unique Positioning
Oncology supportive care focus is a differentiating
factor for DARA in the market

Page 7
q Encouraging sales results
• Strong increase in Rx growth for Soltamox®(1) and Gelclair®(2)
q Positive performance indicators for continued growth
• Increased formulary placements
• New specialty pharmacy partners
• Heightened exposure and awareness
q Build upon and drive performance
• Continued execution of plan
• Field force expansion
Q3 Results
Q3 commercial/sales efforts significantly increased focus
on high prescribing oncologists/radiologists (reach/frequency)
(1) Full prescribing information and complete black box warning are available at www.soltamox.com
(2) Full prescribing information is available at www.gelclair.com

Page 8
Sales Force
January 1, 2014 we will have full national coverage with
20 sales representatives

Page 9
Mission is a privately-held pharmaceutical company (750 employees)
manufacturing and selling branded and consumer products WW
manufacturing and selling branded and consumer products WW
Strategic Partnership: Mission Pharmacal/Alamo
qAlamo is a wholly-owned subsidiary
• Experienced CSO (Contract Sales Organization)
• Full service provider
o SFA/Infrastructure
o Time/cost efficient
• Alamo has prior success building similar sales organizations
• History of Impressive sales performance
qGranted marketing rights to three (3) Mission supportive care
products
products
• Ferralet® 90*
• Binosto®*
• Aquoral®*
*Products of Mission Pharmacal, to be sold through the Alamo sales force into the oncology market.
Full prescribing information is available at www.missionpharmacal.com.

Page *
Page 10
Osteoporosis
Anemia
Oral mucositis
Dry mouth
CCIPN*
* Phase 2b - ready drug (KRN5500). Chronic chemotherapy induced peripheral neuropathy (CCIPN)
Partnership with ALAMO/Mission facilitates creation of a
comprehensive oncology supportive care portfolio
comprehensive oncology supportive care portfolio
Comprehensive Portfolio
Dermatitis
Page *
Page 11
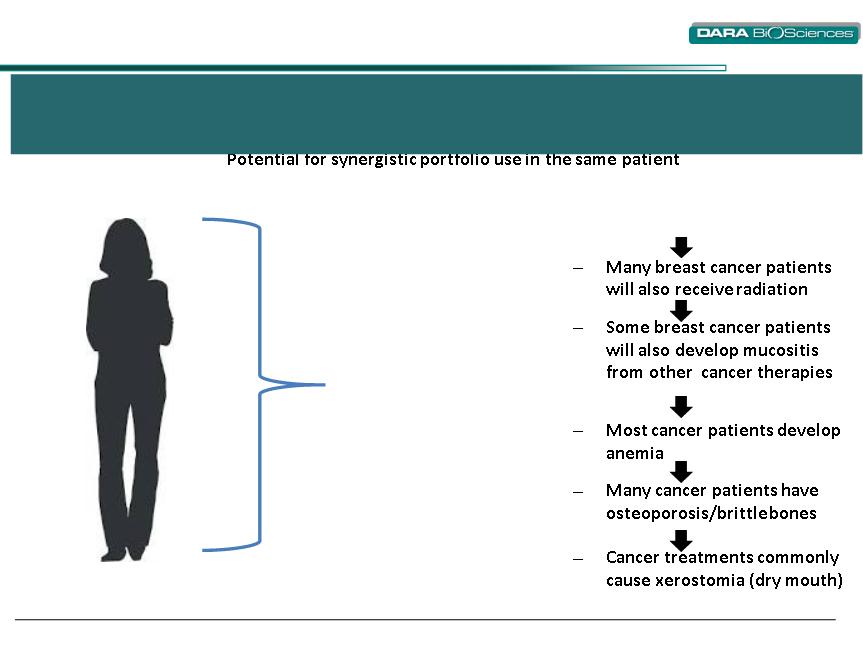
DARA can leverage its newly established portfolio of
oncology supportive care products*
Synergy Creation
Breast cancer patient (illustrative)
• Soltamox®
• Bionect®(1)
• Gelclair®
• Ferralet® 90*
• Binosto®*
• Aquoral®*
─ Breast cancer
(1) Full prescribing information is available at www.bionect.com
* Products of Mission Pharmacal to be sold through the Alamo sales force into the oncology market

Page 12
Oncology Supportive Care
qProviding products meeting market need
qCapitalize on product synergy/portfolio selling
qOpportunity to expand product offerings
qKRN-5500 addressing an area of significant unmet need
qEstablishing a leadership position
Oncology Supportive Care provides DARA a unique
opportunity to create near-term and sustained shareholder value

Page 13
Q3 Financial Summary
Dave Tousley, CFO

Page 14
Closing Comments
David Drutz, CEO & CMO

Page 15
Poised for an exciting 2014
q Unique strategic positioning with a portfolio of synergistic
oncology supportive care products
oncology supportive care products
q Expanded sales force with an experienced/successful CSO
• Revenue growth
q Novel phase 2b-ready drug
• Potential for orphan designation
• Significant unmet medical need
q Catalysts in place to create near and long term value
DARA has significant potential for near and
long term value creation and revenue growth

NASDAQ: DARA
www.darabio.com
Q3 2013 Financial Results and Corporate Business Update
Conference Call and Webcast
Conference Call and Webcast
Rev. 1113
