Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Anacor Pharmaceuticals, Inc. | a13-24238_18k.htm |
| EX-99.1 - EX-99.1 - Anacor Pharmaceuticals, Inc. | a13-24238_1ex99d1.htm |
Exhibit 99.2
|
|
Preliminary Results from AN2728 Maximal Use Systemic Exposure (MUSE) Study in Pediatric and Adolescent Patients with Mild-to-Moderate Atopic Dermatitis November 12, 2013 |
|
|
Safe Harbor Statement Certain items in this presentation and other matters discussed today or answers that may be given to questions asked could constitute forward-looking statements, including statements regarding the progress and timing of clinical trials, the safety and efficacy of our product candidates, and estimates of the potential markets for our product candidates. Additional risks and uncertainties are described more fully in Anacor’s 10-K for the year ended December 31, 2012 and subsequent Quarterly Reports on Form 10-Q filed with the Securities and Exchange Commission, including the risk factors set forth in those filings. These statements are subject to risks and uncertainties relating to Anacor’s future financial or business performance. Anacor’s actual results or achievements could differ materially from those anticipated in these forward-looking statements. Please note that Anacor is under no obligation to update any of the forward-looking statements discussed today, except as required by law. |
|
|
Atopic Dermatitis Overview |
|
|
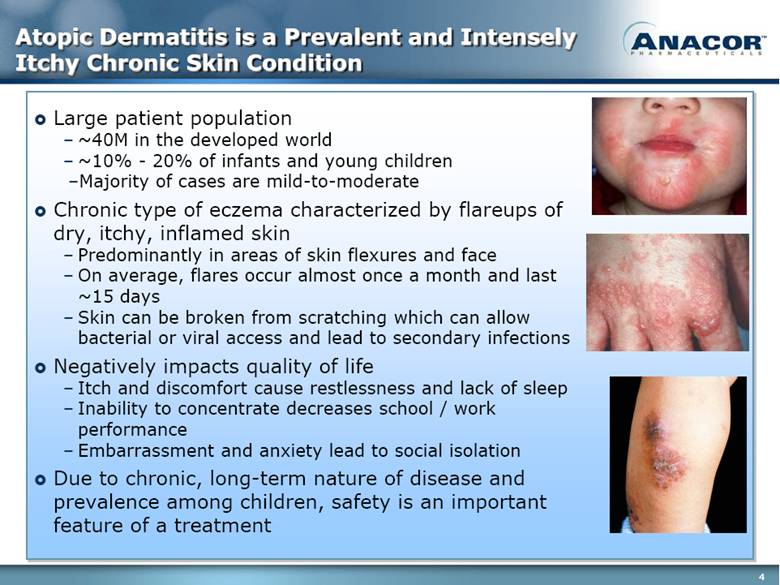
Atopic Dermatitis is a Prevalent and Intensely Itchy Chronic Skin Condition Large patient population ~40M in the developed world ~10% - 20% of infants and young children Majority of cases are mild-to-moderate Chronic type of eczema characterized by flareups of dry, itchy, inflamed skin Predominantly in areas of skin flexures and face On average, flares occur almost once a month and last ~15 days Skin can be broken from scratching which can allow bacterial or viral access and lead to secondary infections Negatively impacts quality of life Itch and discomfort cause restlessness and lack of sleep Inability to concentrate decreases school / work performance Embarrassment and anxiety lead to social isolation Due to chronic, long-term nature of disease and prevalence among children, safety is an important feature of a treatment |
|
|
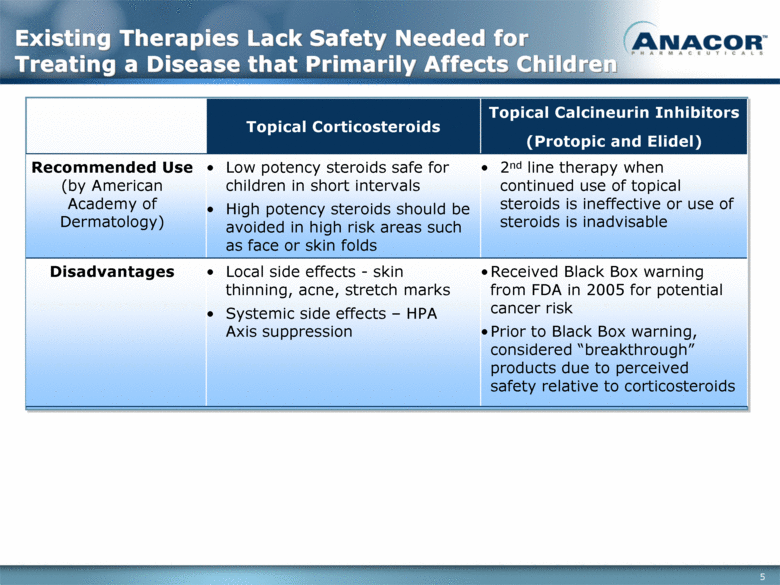
Existing Therapies Lack Safety Needed for Treating a Disease that Primarily Affects Children Topical Corticosteroids Topical Calcineurin Inhibitors (Protopic and Elidel) Recommended Use (by American Academy of Dermatology) Low potency steroids safe for children in short intervals High potency steroids should be avoided in high risk areas such as face or skin folds 2nd line therapy when continued use of topical steroids is ineffective or use of steroids is inadvisable Disadvantages Local side effects - skin thinning, acne, stretch marks Systemic side effects – HPA Axis suppression Received Black Box warning from FDA in 2005 for potential cancer risk Prior to Black Box warning, considered “breakthrough” products due to perceived safety relative to corticosteroids |
|
|
Why Steroid Alternatives are Needed to Treat Atopic Dermatitis Zuberbier et al, Journal of Allergy and Clinical Immunology, July 2006 Risk of side effects increases with: Long duration of use High frequency of applications High potency When applied to intertriginous areas or the face Response to topical steroids may diminish over time Implies need for a change in topical regimen Rotational therapy is often used with intermittent application of steroids or TCIs Parents are often reluctant to use steroids on children In a study of 2,002 subjects (>13 years) and caregivers (of patients 2-13 years), the majority said they would prefer to apply a nonsteroid treatment as early as possible to either prevent a flare from occurring or from getting worse(1) Fear of side effects leads to delayed treatment and poor compliance |
|
|
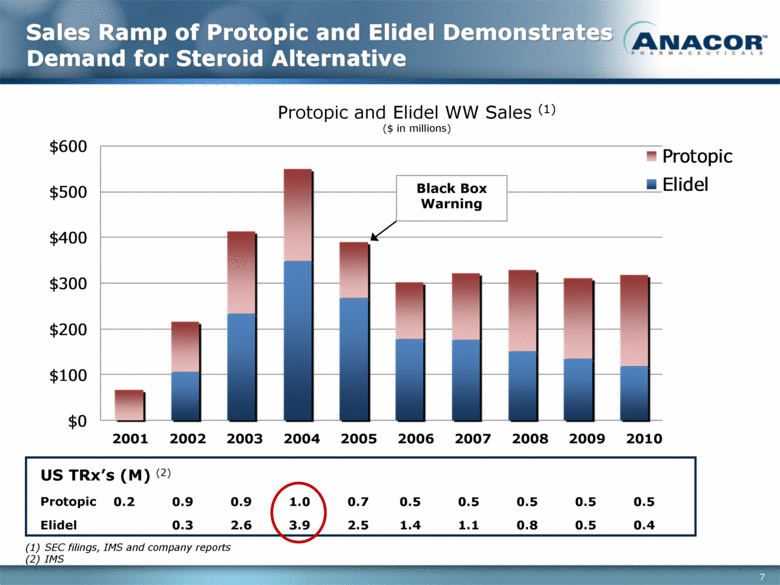
Sales Ramp of Protopic and Elidel Demonstrates Demand for Steroid Alternative Protopic and Elidel WW Sales (1) ($ in millions) Black Box Warning SEC filings, IMS and company reports IMS 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 0.2 0.9 0.9 1.0 0.7 0.5 0.5 0.5 0.5 0.5 0.3 2.6 3.9 2.5 1.4 1.1 0.8 0.5 0.4 Protopic Elidel US TRx’s (M) (2) $0 $100 $200 $300 $400 $500 $600 Protopic Elidel |
|
|
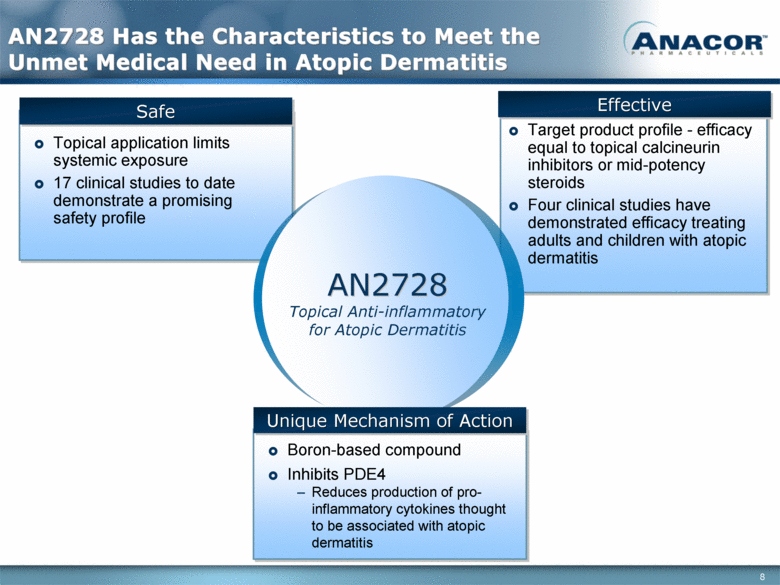
Topical application limits systemic exposure 17 clinical studies to date demonstrate a promising safety profile Safe Effective AN2728 Has the Characteristics to Meet the Unmet Medical Need in Atopic Dermatitis Boron-based compound Inhibits PDE4 Reduces production of pro-inflammatory cytokines thought to be associated with atopic dermatitis Target product profile - efficacy equal to topical calcineurin inhibitors or mid-potency steroids Four clinical studies have demonstrated efficacy treating adults and children with atopic dermatitis AN2728 Topical Anti-inflammatory for Atopic Dermatitis Unique Mechanism of Action |
|
|
AN2728 MUSE Study Summary of Preliminary Data |
|
|
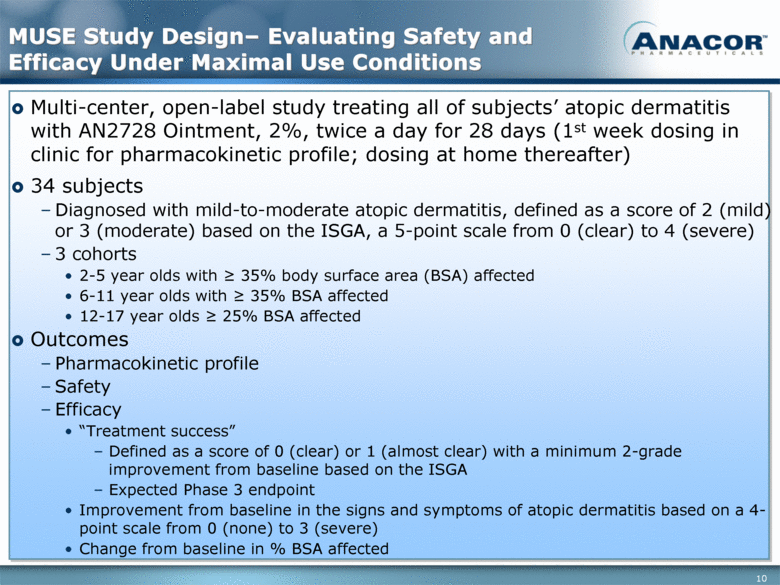
MUSE Study Design– Evaluating Safety and Efficacy Under Maximal Use Conditions Multi-center, open-label study treating all of subjects’ atopic dermatitis with AN2728 Ointment, 2%, twice a day for 28 days (1st week dosing in clinic for pharmacokinetic profile; dosing at home thereafter) 34 subjects Diagnosed with mild-to-moderate atopic dermatitis, defined as a score of 2 (mild) or 3 (moderate) based on the ISGA, a 5-point scale from 0 (clear) to 4 (severe) 3 cohorts 2-5 year olds with > 35% body surface area (BSA) affected 6-11 year olds with > 35% BSA affected 12-17 year olds > 25% BSA affected Outcomes Pharmacokinetic profile Safety Efficacy “Treatment success” Defined as a score of 0 (clear) or 1 (almost clear) with a minimum 2-grade improvement from baseline based on the ISGA Expected Phase 3 endpoint Improvement from baseline in the signs and symptoms of atopic dermatitis based on a 4-point scale from 0 (none) to 3 (severe) Change from baseline in % BSA affected |
|
|
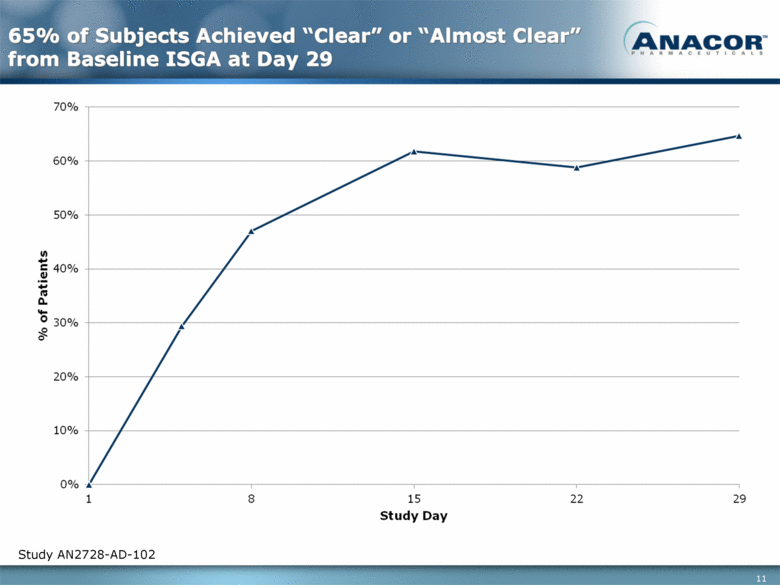
65% of Subjects Achieved “Clear” or “Almost Clear” from Baseline ISGA at Day 29 Study AN2728-AD-102 2.7 0.6 0.6 0.4 0.25 0.75 |
|
|
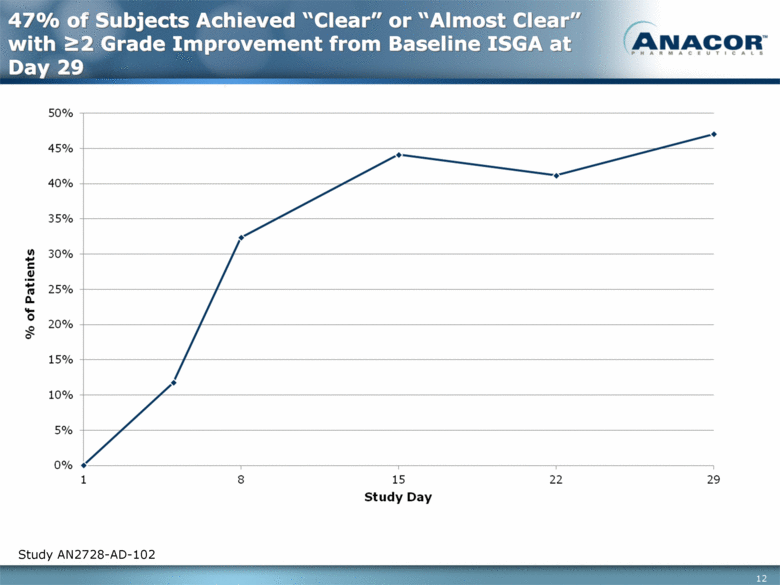
47% of Subjects Achieved “Clear” or “Almost Clear” with >2 Grade Improvement from Baseline ISGA at Day 29 Study AN2728-AD-102 |
|
|
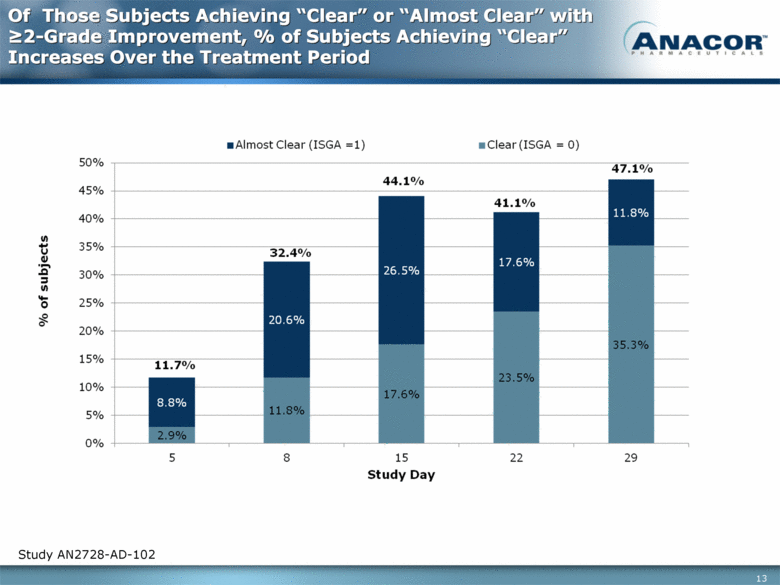
Of Those Subjects Achieving “Clear” or “Almost Clear” with >2-Grade Improvement, % of Subjects Achieving “Clear” Increases Over the Treatment Period Study AN2728-AD-102 |
|
|
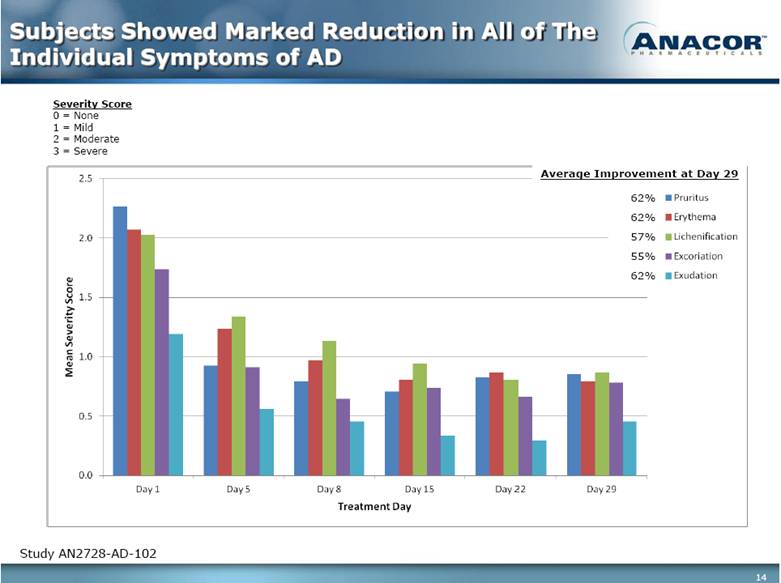
Subjects Showed Marked Reduction in All of The Individual Symptoms of AD Study AN2728-AD-102 Severity Score 0 = None 1 = Mild 2 = Moderate 3 = Severe 62% 62% 57% 55% 62% Average Improvement at Day 29 |
|
|
Reduction in the Individual Symptoms of AD for Patients Judged “Clear” or “Almost Clear” at Day 29 Study AN2728-AD-102 Severity Score 0 = None 1 = Mild 2 = Moderate 3 = Severe Average Improvement at Day 29 86% 85% 74% 91% 100% |
|
|
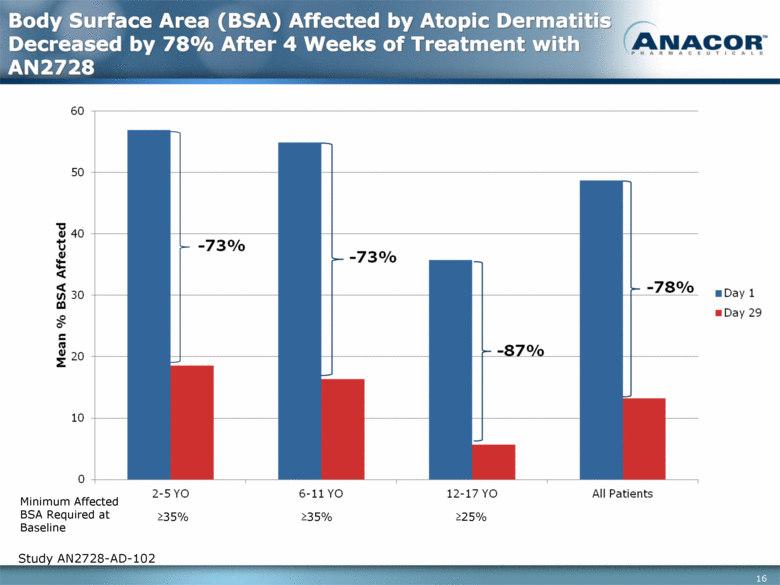
Body Surface Area (BSA) Affected by Atopic Dermatitis Decreased by 78% After 4 Weeks of Treatment with AN2728 Study AN2728-AD-102 Minimum Affected BSA Required at Baseline >35% >35% >25% |
|
|
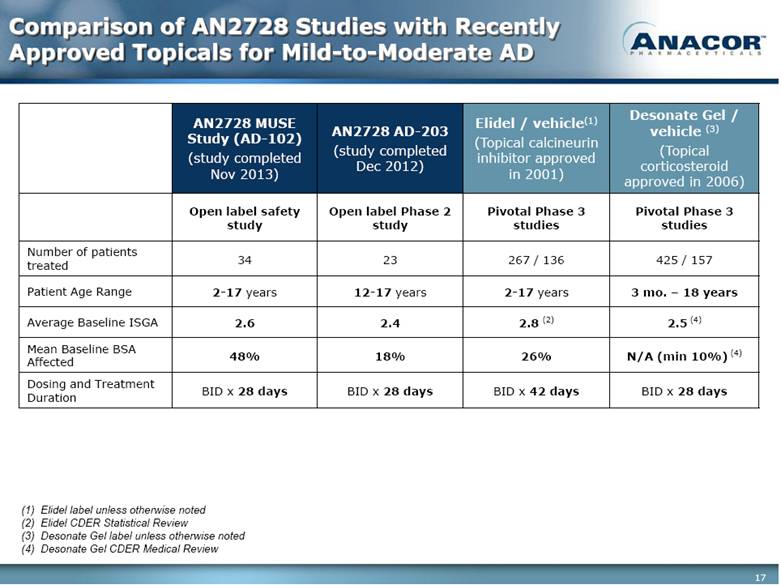
Comparison of AN2728 Studies with Recently Approved Topicals for Mild-to-Moderate AD AN2728 MUSE Study (AD-102) (study completed Nov 2013) AN2728 AD-203 (study completed Dec 2012) Elidel / vehicle(1) (Topical calcineurin inhibitor approved in 2001) Desonate Gel / vehicle (3) (Topical corticosteroid approved in 2006) Open label safety study Open label Phase 2 study Pivotal Phase 3 studies Pivotal Phase 3 studies Number of patients treated 34 23 267 / 136 425 / 157 Patient Age Range 2-17 years 12-17 years 2-17 years 3 mo. – 18 years Average Baseline ISGA 2.6 2.4 2.8 (2) 2.5 (4) Mean Baseline BSA Affected 48% 18% 26% N/A (min 10%) (4) Dosing and Treatment Duration BID x 28 days BID x 28 days BID x 42 days BID x 28 days Elidel label unless otherwise noted Elidel CDER Statistical Review Desonate Gel label unless otherwise noted Desonate Gel CDER Medical Review |
|
|
Based on Comparisons Across Studies AN2728 Compares Favorably to Elidel and Desonate Note: Caution is needed when comparing AN2728’s open-label data with randomized, controlled Phase 3 data for Elidel and Desonate. Results at End of Treatment Period AN2728 MUSE Study (AD-102) AN2728 AD-203 Elidel / vehicle(1) Desonate Gel / vehicle (2) % of Pts Judged “Clear” or “Almost Clear” (ISGA 0 or 1) 65% 74% 35% / 18% 60% / 33% (study 1) (3) 54% / 14% (study 2) (3) % of Pts Judged “Clear” or “Almost Clear” with min 2-grade improvement (Expected Phase 3 endpoint) 47% 35% N/A 44% / 14% (study 1) 28% / 6% (study 2) % of Pts Judged “Clear” 35% 13% 10% / 4% N/A % of Pts with Mild or No Pruritus 76% 87% 57% N/A Mean % Reduction in BSA Affected 78% 55% ~40%-45% N/A Safety Precautions Black Box warning - rare cases of malignancies have been reported Continuous long-term use should be avoided Not to be used on children under the age of 2 Safety not established beyond 4 weeks of use HPA axis suppression, with greater risk in pediatric patients Elidel label unless otherwise noted Desonate Gel label unless otherwise noted Desonate Gel CDER Medical Review |
|
|
Safety, Tolerability and Pharmacokinetics No treatment-emergent serious adverse events occurred One subject discontinued from the study due to an adverse event (application site pain) The most common treatment-related AEs were application site reactions occurring in 12 (35%) subjects Of these 12 subjects the vast majority had a maximum severity of mild or moderate These reactions were generally transient and resolved spontaneously without treatment Pharmacokinetic profile Overall blood levels in pediatrics and adolescents were low and were similar to those previously observed in adults after adjusting for percent BSA treated Cmax-based safety margin is 23-fold to 200-fold AUC-based safety margin is 12-fold to 23-fold |
|
|
Preliminary Phase 3 Trial Design Subject to input from the FDA, Phase 3 study will be initiated in 1H14 with results expected in 1H15 Design Two multi-center, double-blind, placebo-controlled trials ~750 subjects per trial randomized 2:1 (active:vehicle) Twice daily dosing for 28 days Subjects ages 2-17 years with mild-to-moderate atopic dermatitis Trial size primarily determined by FDA requirement for size of safety database With this size, each trial is powered 94% to achieve a P value < 0.05 assuming 20% efficacy for active and 10% for vehicle We believe these efficacy assumptions are conservative based on our study results to date Outcomes Efficacy The primary efficacy endpoint is success in ISGA at Day 29, defined as an ISGA of “Clear” or “Almost Clear” with at least a 2-grade improvement from Baseline Several secondary endpoints planned Safety and local tolerability Reported AEs, tolerability scores, safety laboratory tests, vital signs |
|
|
Preliminary Long-Term Safety Trial Design Long-term safety trial (~500 subjects) to evaluate safety of intermittent use of AN2728 for up to 12 months FDA requires that at least 100 subjects complete 12 months of long-term safety and at least 300 subjects complete 6 months of long-term safety Subjects who complete Phase 3 trial have option to roll into long-term safety trial until ~500 subjects are enrolled Subjects will undergo courses of treatment as needed under the direction of the investigator over the 6-12 month treatment period |
|
|
Next Steps in Development Completion of ongoing TQT study expected by year end 2013 End of Phase 2 meeting with the FDA in 1Q14 Initiate Phase 3 program of AN2728 in 1H14 with results expected in 1H15 Subject to Phase 3 results and timing, file NDA in 2H15 Remaining development costs (including clinical, pre-clinical and manufacturing) through filing of NDA currently estimated to be <$40M |
|
|
Q&A |