Attached files
| file | filename |
|---|---|
| EX-4.7 - EX-4.7 - Anacor Pharmaceuticals, Inc. | a2203077zex-4_7.htm |
| EX-4.8 - EX-4.8 - Anacor Pharmaceuticals, Inc. | a2203077zex-4_8.htm |
| EX-23.1 - EX-23.1 - Anacor Pharmaceuticals, Inc. | a2203077zex-23_1.htm |
| EX-31.1 - EX-31.1 - Anacor Pharmaceuticals, Inc. | a2203077zex-31_1.htm |
| EX-32.1 - EX-32.1 - Anacor Pharmaceuticals, Inc. | a2203077zex-32_1.htm |
| EX-31.2 - EX-31.2 - Anacor Pharmaceuticals, Inc. | a2203077zex-31_2.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
Index to Financial Statements
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| (Mark One) | ||
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the Fiscal Year Ended December 31, 2010 |
||
or |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
Commission File Number: 001-34973 |
||
ANACOR PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
2834 (Primary Standard Industrial Classification Code Number) |
25-1854385 (I.R.S. Employer Identification No.) |
||
1020 East Meadow Circle Palo Alto, CA 94303-4230 (Address of Principal Executive Offices) (Zip Code) |
||||
(650) 543-7500 (Registrant's Telephone Number, Including Area Code) |
||||
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class: | Name of Each Exchange on which Registered | |
|---|---|---|
| Common Stock, par value $0.001 per share | The NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer ý (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No ý
The registrant's common stock was not publicly traded as of the last business day of the registrant's most recently completed second fiscal quarter. The number of outstanding shares of the registrant's common stock on February 28, 2011 was 27,996,928.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates information by reference to the definitive proxy statement for the Company's Annual Meeting of Stockholders to be held on or about May 26, 2011, to be filed within 120 days of the registrant's fiscal year ended December 31, 2010.
Anacor Pharmaceuticals, Inc.
Form 10-K
Index
i
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements, including statements regarding the progress and timing of clinical trials, the safety and efficacy of our product candidates, actions to be taken by our partner, GlaxoSmithKline LLC, or GSK, our ability to enter into and maintain additional collaborations, our ability to scale and support commercial activities, the goals of our research and development activities, estimates of the potential markets for our product candidates, availability of drug product, our expected future revenues, operations and expenditures and projected cash needs. The forward-looking statements are contained principally in the sections entitled "Business," "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations." These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that could cause our actual results, levels of activity, performance or achievement to differ materially from those expressed or implied by these forward-looking statements.
Forward-looking statements include all statements that are not historical facts. In some cases, you can identify forward-looking statements by terms such as "may," "will," "should," "could," "would," "expects," "plans," "anticipates," "believes," "estimates," "projects," "predicts," "potential," or the negative of those terms, and similar expressions and comparable terminology intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. These forward-looking statements represent our estimates and assumptions only as of the date of this Annual Report on Form 10-K and, except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise after the date of this Annual Report on Form 10-K.
Overview
We are a biopharmaceutical company focused on discovering, developing and commercializing novel small-molecule therapeutics derived from our boron chemistry platform. We have demonstrated that our organization, utilizing our boron chemistry platform, is highly productive and efficient at creating novel clinical product candidates. We have discovered, synthesized and developed five molecules that are currently in clinical development, and we believe that our organization and boron chemistry platform have the potential to continue to yield clinical candidates at a similar pace and efficiency in the future. While drug development is often uncertain and occasionally uneven, our current portfolio of product candidates and our ability to efficiently fill our own pipeline provide us with a unique opportunity to create a valuable and sustainable biopharmaceutical company.
We believe that our expertise in boron chemistry enables us to identify compounds that interact with known drug targets in novel ways and also inhibit drug targets that have been historically difficult to inhibit with traditional chemistry. We have applied this expertise across a range of fungal, inflammatory, bacterial and parasitic diseases that represent significant unmet medical needs. We have discovered and advanced into clinical development multiple differentiated product candidates that we believe have significant disease-modifying potential and attractive pharmaceutical properties. We believe that our product candidates may offer significant improvements over the standard of care for diseases that represent large, well-defined commercial opportunities.
The productivity of our internal discovery capability has enabled us to generate a pipeline of both topical and systemic boron-based compounds. We currently have five product candidates in clinical development. Our three lead product candidates include two topically administered dermatologic compounds—AN2690, an antifungal for the treatment of onychomycosis, and AN2728, an anti-inflammatory for the treatment of psoriasis and atopic dermatitis, as well as a systemic antibiotic for the treatment of infections caused by Gram-negative bacteria—GSK2251052, or GSK '052 (formerly referred to as AN3365). In addition, we are developing AN2718 as a topical antifungal product candidate for the treatment of onychomycosis and skin fungal infections and AN2898 as a topical anti-inflammatory product candidate for the treatment of psoriasis and atopic dermatitis.
We have entered into and are seeking partnerships to expand the therapeutic application and commercial value of our boron chemistry platform. In October 2007, we entered into a research and development collaboration, option and license agreement with GlaxoSmithKline LLC, or GSK, for the discovery, development and worldwide commercialization of boron-based systemic anti-infectives. In July 2010, GSK exercised its option to license GSK '052, and we are actively conducting research to discover additional systemic anti-infectives with GSK. In August 2010, we entered into a collaboration with Eli Lilly and Company, or Lilly, under which we are collaborating to discover products for a variety of animal health applications. In February 2011, we entered into a research and development option and license agreement with Medicis Pharmaceutical Corporation, or Medicis, to discover and develop compounds directed against a target for the potential treatment of acne. In addition, we are applying our boron chemistry platform to the development of treatments for various neglected diseases in collaboration with leading not-for-profit organizations, including the Drugs for Neglected Diseases initiative, Medicines for Malaria Ventures, Sandler Center for Basic Research in Parasitic Diseases and the Institute for OneWorld Health.
1
Our Clinical Pipeline
The following table summarizes the current status and the anticipated next steps in the development plans for our clinical-stage product candidates:
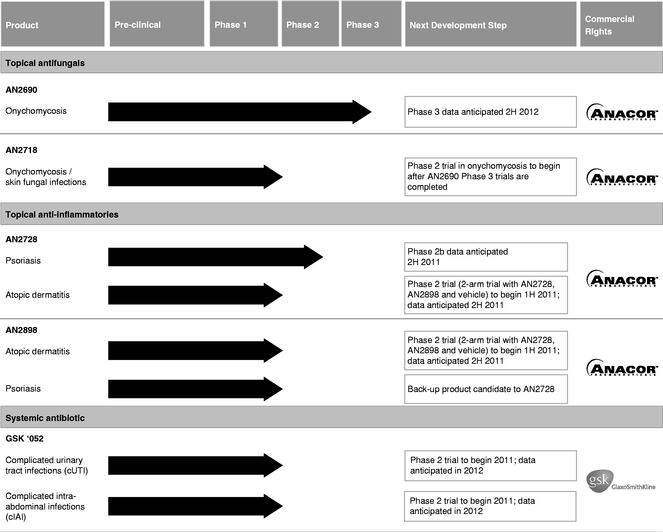
AN2690 is our lead topical antifungal product candidate for the treatment of onychomycosis, a fungal infection of the nail and nail bed. Onychomycosis affects approximately 35 million people in the United States, and new prescriptions to treat this disease continue to grow. Lamisil (terbinafine), a systemic drug approved for onychomycosis, had worldwide peak sales of $1.2 billion in 2004, before generic entry. According to IMS Health, for the 12-month period ending June 30, 2010, 1.4 million new prescriptions were filled in the United States for both branded and generic versions of terbinafine. Despite its high labeled efficacy (38%), we believe the usage of branded and generic terbinafine has been limited due to safety concerns related to liver toxicity. The leading topical drug for onychomycosis, Penlac Nail Lacquer (ciclopirox), had U.S. sales of $125.0 million in 2002, before generic entry. According to IMS Health, for the 12-month period ending June 30, 2010, over 350,000 new prescriptions were filled in the United States for branded or generic ciclopirox. While ciclopirox has been shown to be safe due in part to its topical administration, we believe the usage of branded and generic ciclopirox has been limited due to its low labeled efficacy (5.5%-8.5%).
We believe AN2690 can potentially offer significant improvements over the standards of care for onychomycosis by combining the safety of a topical drug with significant efficacy. Due to its unique boron chemistry, AN2690 has demonstrated enhanced nail penetration properties, a novel mechanism
2
of action with potent antifungal activity and, due in part to its topical administration, no observed systemic side effects in human dosing. AN2690 inhibits an essential fungal enzyme, leucyl-transfer RNA synthetase, or LeuRS, required for protein synthesis. The inhibition of protein synthesis leads to termination of cell growth and cell death, eliminating the fungal infection. We reported positive results from three Phase 2 clinical trials and held an end-of-Phase 2 meeting with the United States Food and Drug Administration, or FDA. In August 2010, we filed a Special Protocol Assessment, or SPA, request with the FDA in order to reach agreement on key endpoint measures and trial design to be used in our first of two identical planned Phase 3 clinical trials of AN2690. We have received the FDA's agreement on what we believe are the major parameters associated with the design and conduct of our first Phase 3 trial for AN2690. We initiated the Phase 3 clinical trial in onychomycosis in the fourth quarter of 2010. We are currently enrolling patients at clinical sites in the United States and also plan to enroll patients at clinical sites in Canada and Mexico in 2011.
AN2728 is our lead topical anti-inflammatory product candidate for the treatment of psoriasis, a chronic inflammatory skin disease that affects approximately 7.5 million people in the United States and over 100 million people worldwide. Approximately 80% of psoriasis patients have mild-to-moderate disease, which is mainly treated with topical corticosteroids and vitamin D analogs. However, topical corticosteroids and vitamin D analogs are limited in their use by patients due to their long-term safety and/or their tolerability profile. In spite of these limitations, according to IMS Health, approximately 3.9 million prescriptions were filled for these topical therapies to treat psoriasis in the United States in 2009.
We believe that AN2728 has the potential to be an effective psoriasis treatment with an attractive safety profile in a topical application, and thus provide an alternative to treatment with topical corticosteroids and vitamin D analogs. Due to its boron-based structure, AN2728 binds with the active site of the enzyme phosphodiesterase-4 (PDE4) in a novel manner, thus inhibiting its activity. This mechanism subsequently reduces the production of TNF-alpha, IL-12, IL-23 and other pro-inflammatory cytokines that are precursors of the inflammation associated with psoriasis. In June 2010, we successfully completed a Phase 2b dose-ranging trial to evaluate the safety and efficacy of AN2728. We have initiated a final Phase 2b trial for AN2728 in psoriasis that matches the anticipated design of our planned Phase 3 trials in which patients will be randomized to receive either AN2728 or vehicle. Following the completion of this Phase 2b trial, we plan to initiate a Phase 3 trial in the second half of 2011. We are also exploring the activity of AN2728 for the treatment of atopic dermatitis, and plan to initiate a Phase 2 trial in this indication in the first half of 2011. This Phase 2 clinical trial will be designed as a two-arm trial evaluating the efficacy and safety of AN2728 and AN2898 compared to vehicle.
GSK '052 is our lead systemic antibiotic for the treatment of infections caused by Gram-negative bacteria. Gram-negative bacterial infections are increasing in prevalence, especially in hospitals, and represent a serious public health challenge due to their growing resistance to currently available drug therapies. According to the New England Journal of Medicine, it is estimated that there were 1.7 million hospital-acquired Gram-negative and Gram-positive infections and approximately 100,000 associated deaths in the United States alone in 2002. The New England Journal of Medicine also indicates that Gram-negative bacteria are responsible for more than 30.0% of hospital-acquired infections and account for approximately 70.0% of hospital-acquired infections in the intensive care unit. IMS Health estimates there were 40 million days of Gram-negative therapy administered in the United States in 2009. Gram-negative bacterial infections are becoming a major global health issue where their growing resistance to currently available drug therapies is rapidly increasing. Furthermore, recently approved Gram-negative antibiotics have been limited to new versions of existing antibiotics, which carry the risk of rapid resistance development from pre-existing mechanisms of resistance. Preclinical studies suggest that GSK '052 could be a novel approach for the treatment of infections caused by a broad range of
3
Gram-negative bacteria, including E. coli, Klebsiella pneumoniae, Citrobacter spp., S. marcescens, P. vulgaris, Providentia spp., Pseudomonas aeruginosa and Enterobacter spp.
Due to its unique boron-based chemical structure, GSK '052 specifically targets the bacterial enzyme leucyl-transfer RNA synthetase, or LeuRS, which is required for protein synthesis. The inhibition of protein synthesis leads to termination of cell growth and cell death, eliminating the bacterial infection. Since GSK '052 is the first antibiotic to target LeuRS, bacteria have not developed resistance to it. In preclinical safety, pharmacology and toxicology studies, GSK '052 showed robust activity against multi-resistant Gram-negative bacteria with no cross resistance to existing classes of antibiotics. In a Phase 1 proof-of-concept trial, GSK '052 demonstrated a promising safety profile and linear dose-proportional pharmacokinetic properties, reaching blood levels that were many times higher than the anticipated efficacious dose. If approved, we believe GSK '052 would be the first new class of antibacterial agents to treat serious hospital-acquired Gram-negative infections in thirty years. In addition, we believe GSK '052 has the potential to be the first antibiotic specifically targeting infections caused by Gram-negative bacteria that can be administered by both IV and oral routes, which would allow patients to continue on the same antibiotic therapy they received in the hospital once they are discharged.
Following the completion of the Phase 1 trial, GSK exercised its option to obtain an exclusive license to develop and commercialize GSK '052 and has assumed responsibility for further development of the product candidate and any resulting commercialization. Following the exercise of the option in July 2010, we received a fee of $15.0 million. We are eligible to receive further development milestones up to $69.0 million, commercial milestones up to $175.0 million and double-digit tiered royalties with the potential to reach the mid-teens on annual net sales. We believe GSK currently plans to develop GSK '052 as a potential treatment for complicated urinary tract infections, or cUTI, complicated intra-abdominal infections, or cIAI, and other Gram-negative bacterial infections, such as hospital-acquired and ventilator-associated pneumonia, or HAP/VAP. We anticipate that GSK will initiate Phase 2 trials of GSK '052 in patients with cUTI and cIAI in 2011.
Our clinical pipeline also includes two additional product candidates that may extend and expand the market opportunity of our dermatology portfolio. AN2718, our second topical antifungal product candidate, has the potential to treat onychomycosis and fungal infections of the skin. We expect to initiate a Phase 2 trial of AN2718 in onychomycosis after we have completed Phase 3 trials with AN2690. AN2898, our second topical anti-inflammatory product candidate, has the potential to treat psoriasis and atopic dermatitis. We expect to initiate a two-arm Phase 2 trial with AN2898 and AN2728 compared to vehicle in atopic dermatitis in the first half of 2011.
Boron Chemistry Platform
All of our current research and development programs, including our five clinical product candidates, are based on compounds that have been internally discovered using our boron chemistry platform. Boron is a naturally occurring element that is ingested frequently through consumption of fruits, vegetables, milk and coffee. Boron has two attributes that we believe confer compounds with drug-like properties. First, boron-based compounds have a unique geometry that allows them to have two distinct shapes, giving boron-based drugs the ability to interact with biological targets in novel ways and to address targets not amenable to intervention by traditional carbon-based compounds. Second, boron's enhanced reactivity as compared to carbon allows us to design molecules that can hit targets that are difficult to inhibit with carbon chemistry.
Despite the ubiquity of boron in the environment, researchers have faced challenges in evaluating boron-based compounds as product candidates due to previously limited understanding of the physical properties necessary to provide boron-based compounds with the chemical and biological attributes required of pharmaceutical therapies as well as difficulty in chemical synthesis.
4
We have developed expertise and an understanding of the interactions of boron-based compounds with key biological targets relevant to treating disease. This know-how is primarily related to methods for modulating boron's reactivity to optimize reactions with the target and minimize unwanted chemical reactivity. Our advances have enabled the efficient optimization of disease-modifying properties for our lead compounds and their rapid progression from the research stage into clinical trials.
Additionally, we have discovered new methods of synthesis of boron compounds, allowing for the creation of new compound families with broad chemical diversity and retention of drug-like properties. These new compound families expand the universe of biological targets that can be addressed by small-molecule, boron-based compounds. We have been in operation since 2002 and began generating clinical candidates in our second year. Since that time, we have discovered and synthesized thousands of boron-containing molecules, and of these, five are currently in clinical development. The rate of discovery of molecules and promotion to clinical development has occurred at a relatively constant rate.
We believe our focus on boron-based chemistry provides us with multiple advantages in the small-molecule drug discovery process. These advantages include:
- •
- Novel access to biological targets. Due to the unique
geometry and reactivity of boron-based molecules, our boron-based compounds are able to modulate existing biological targets and can address targets not amenable to intervention by traditional
carbon-based compounds. This may enable us to treat diseases that have not been effectively addressed by carbon-based compounds, for example, by developing antibiotic or antifungal therapies that kill
pathogens that have become resistant to existing drugs.
- •
- Broad utility across multiple disease areas. Our compounds
have exhibited extensive preclinical activity in multiple disease areas, including fungal, inflammatory and bacterial diseases, which are our core areas of focus, as well as in parasitic, cancer and
ophthalmic indications and for applications in animal health.
- •
- Rapid and efficient synthesis of drug-like
compounds. Our proprietary technological advances in the synthesis of boron-based compounds, coupled with our rational drug design
capabilities, have enabled us to rapidly create large families of boron-based compounds with drug-like properties. We believe that these advances have made manufacturing of boron-based
compounds economical on a commercial scale.
- •
- Unencumbered intellectual property landscape. We believe the intellectual property landscape for boron-based pharmaceutical products is relatively unencumbered compared to that of carbon-based products, providing an attractive opportunity for us to build our intellectual property portfolio.
Our Strategy
Our objective is to discover, develop and commercialize proprietary boron-based drug compounds with superior efficacy, safety and convenience for the treatment of a variety of diseases. The key elements of our strategy to achieve this objective are to:
- •
- Drive rapid, efficient discovery of novel boron-based
compounds. We believe the unique characteristics of boron and the expertise we have developed allow us to design novel product
candidates that target a broad range of diseases and drive a rapid and efficient drug development process. We have discovered and advanced five compounds that are currently in clinical development
during our first nine years of operations and, in addition, have other active research and development programs ongoing.
- •
- Focus development activities in our core therapeutic areas. We intend to focus our development activities in our core therapeutic areas of fungal, inflammatory and bacterial diseases. To fully
5
- •
- Commercialize our products ourselves in specialty markets in the United
States. We intend to build a sales force to focus on domestic specialty markets, such as dermatology. We have entered into and will continue to seek commercialization partners
for products in non-specialty and international markets.
- •
- Leverage partnerships for non-core therapeutic
areas. We believe boron chemistry has utility in a broad range of diseases outside of our core therapeutic areas. To maximize the value
of our boron chemistry platform and to provide non-dilutive capital to support development in our core therapeutic areas, we have entered into and will continue to seek partnerships early
in development for compounds in non-core areas, such as parasitic, cancer and ophthalmic indications and for applications in animal health.
- •
- Expand and protect our intellectual property. We intend to expand and aggressively prosecute our intellectual property in the area of boron chemistry and boron-based compounds. Since a relatively limited amount of research has been done in the area of boron-based drug development, we believe that we can establish a defensible and valuable intellectual property portfolio.
leverage our boron chemistry platform, we have established and will continue to pursue development partnerships in these therapeutic areas.
Our Product Candidates
Our Topical Antifungal Programs
AN2690 for Onychomycosis
Our most advanced product candidate is AN2690, a topical treatment for onychomycosis, which is a fungal infection of the nail and nail bed. We reported positive results from three Phase 2 clinical trials, have completed an end-of-Phase 2 meeting with the FDA, and filed a Special Protocol Assessment request with the FDA in order to reach agreement on key endpoint measures and trial design to be used in our planned Phase 3 clinical trials of AN2690 in onychomycosis. We initiated Phase 3 trials in the fourth quarter of 2010, at clinical sites in the United States and are enrolling at clinical sites in Canada and Mexico in 2011.
Onychomycosis Market
Onychomycosis is primarily caused by dermatophytes, which are fungi that infect the skin, hair or nails. The infection involves the nail plate, the nail bed and, in some cases, the skin surrounding the nail plate. Onychomycosis causes nails to deform, discolor, thicken, become brittle and split and separate from the nail bed. Toenails affected by onychomycosis can become so thick that routine trimming of the nails becomes difficult. The condition can also cause pain when individuals wear shoes, leading to difficulties walking. Onychomycosis can also lead to social embarrassment due to the unsightly appearance of the infected nails and because it may be perceived to be an active infection and contagious.
According to Podiatry Today, 35 to 36 million people in the United States have onychomycosis. Over 95% of onychomycosis infections are infections of the toenail, according to a report in U.S. Dermatology Review. According to the manufacturer of Lamisil, 47% of those affected by onychomycosis are not receiving treatment. For those who do seek treatment, options include debridement, oral or topical drugs or a combination of debridement and drug therapies. Debridement consists of scraping, cutting away or removal of the affected nail. Onychomycosis may persist or worsen if not treated. Onychomycosis often recurs in susceptible individuals because the fungi that cause onychomycosis are present in many common locations such as floors, the soil, socks and shoes. Consequently, the nail can
6
be reinfected, and additional courses of treatment are frequently required even after successful treatment.
Lamisil (terbinafine), a systemic drug approved for onychomycosis, had worldwide peak sales of $1.2 billion in 2004, before generic entry. According to IMS Health, for the 12-month period ending June 30, 2010, 1.4 million new prescriptions were filled in the United States for both branded and generic versions of terbinafine. Despite its high labeled efficacy (38%), we believe the usage of branded and generic terbinafine has been limited due to safety concerns related to liver toxicity. Penlac Nail Lacquer (ciclopirox), the only U.S. approved topical agent for onychomycosis, had U.S. sales of $125.0 million in 2002, before generic entry. According to IMS Health, for the 12-month period ending June 30, 2010, over 350,000 new prescriptions were filled in the United States for branded or generic ciclopirox. While ciclopirox has been shown to be safe due in part to its topical administration, we believe the usage of branded and generic ciclopirox has been limited due to its low labeled efficacy (5.5%-8.5%).
Limitations of Current Onychomycosis Therapies
Current therapies for onychomycosis include debridement and drug therapies. Debridement is time consuming and only marginally effective in eliminating the fungal infection. Drug therapies are available in two types, either oral therapies such as Lamisil, or topical therapies such as Penlac. According to the Lamisil product label, 38% of patients treated in clinical trials with a 12-week course of therapy achieved 100% clear nail and mycological cure. However, Lamisil has been associated with rare cases of liver failure, some leading to death or liver transplant. We believe this risk of liver failure limits acceptance of this therapy by both physicians and patients. Patients are recommended to undergo liver function tests prior to initiating Lamisil treatment and those patients with pre-existing liver disease cannot be treated with it.
Penlac is approved for use in onychomycosis in conjunction with frequent debridement. In the two clinical trials cited on Penlac's product label, even with frequent debridement, only 5.5% and 8.5% of patients treated with Penlac achieved 100% clear nail and mycological cure, respectively. We believe that a significant barrier to effective treatment by current topical therapies is the difficulty of formulating the drug product to penetrate through the nail plate and reach the site of infection.
Our Solution: AN2690
By addressing the limitations of current therapies, we believe AN2690 has a potential safety and efficacy profile that can make it a best-in-class therapy for the treatment of onychomycosis. We have completed three Phase 2 clinical trials in which AN2690 had no observed systemic side effects. We believe that the clinical data that we have generated to date demonstrates the potential advantages of AN2690 relative to Lamisil and Penlac.
We have designed AN2690, our topical antifungal, with three distinguishing characteristics:
- •
- Enhanced nail penetration properties. We utilized our
expertise in medicinal chemistry to design AN2690 with enhanced nail penetration properties, allowing for improved delivery of the compound through the nail plate to the nail bed, the site of
onychomycosis infection. Preclinical studies utilizing human nails indicate that AN2690 is able to penetrate the nail plate 250 times more effectively than Penlac.
- •
- Novel mechanism of action with potent antifungal activity. We have utilized our expertise in boron-based chemistry to design AN2690 with potent antifungal activity. AN2690 inhibits an essential fungal enzyme, leucyl-transfer RNA synthetase, or LeuRS, required for protein synthesis. The inhibition of protein synthesis leads to termination of cell growth and cell death, eliminating the fungal infection. We have shown that this inhibitory activity requires the presence
7
- •
- No detected systemic side effects after topical dosing. We have conducted clinical trials to assess systemic absorption of AN2690. The results of these trials found that topical treatment with AN2690 resulted in low or no detectable levels of drug in the blood or urine. No treatment-related systemic side effects have been observed in any of our clinical trials, and we believe it is unlikely that treatment of onychomycosis with AN2690 will result in significant systemic side effects.
of boron within the compound, as the replacement of the boron atom with a carbon atom in AN2690 inactivated the molecule. The unique boron-based mechanism of action underlying AN2690 was detailed in the June 22, 2007 issue of the journal Science.
We have completed three Phase 2 clinical trials which demonstrated that AN2690 is efficacious as defined by the percentage of patients achieving clear nail growth and negative fungal culture. We believe that these data have demonstrated that AN2690's efficacy should be in a range that is at least twice that of Penlac and may approach that of Lamisil. In addition, in our Phase 1 and Phase 2 clinical trials, we have shown that AN2690 achieves significant nail penetration, results in little or no systemic exposure and is well-tolerated across a range of doses.
AN2690 Phase 3 Development Program
In the fourth quarter of 2010, we initiated Phase 3 clinical trials for AN2690 in onychomycosis in the United States. The AN2690 Phase 3 program consists of two double-blind, vehicle-controlled trials enrolling approximately 600 patients each. Vehicle refers to the formulation without the active ingredient. Two-thirds of the patients are randomized to receive AN2690 at the 5.0% concentration, or dose, compared to one-third who receive vehicle once daily for 48 weeks. The primary efficacy endpoint is a composite endpoint measuring complete cure of the great toenail at week 52, which is consistent with the FDA requirement for Lamisil. Complete cure requires both a mycologic cure and a completely clear nail. Mycologic cure is achieved when the fungus present in the nail plate is killed by treatment. Achieving a clear nail requires a complete elimination of the diseased portion of the nail by replacement with a new healthy growing nail and nail bed. Given the slow rate of nail growth, which is approximately one to two millimeters per month, the industry standard for conducting Phase 3 clinical trials of onychomycosis is to evaluate the nails over a 12-month period, in order to allow sufficient time for patients to grow a new nail. An end-of-Phase 2 meeting with the FDA was completed, and in August 2010 we filed an SPA request with the FDA in order to reach agreement on key endpoint measures and trial design to be used in our planned Phase 3 trials of AN2690 in onychomycosis. We have received the FDA's agreement on what we believe are the major parameters associated with the design and conduct of our Phase 3 trials for AN2690. We initiated Phase 3 trials in the fourth quarter of 2010 at clinical sites in the United States, and we also plan to enroll patients at clinical sites in Canada and Mexico in 2011. Guidance meetings have also been completed with the European Medicines Agency and regulatory authorities in Japan. Based upon these discussions, we anticipate that our Phase 3 development program will help support approval in these regions, although an additional comparator trial will likely be required in these regions.
AN2690 Phase 2 Clinical Development Program
Our Phase 2 clinical trials of AN2690 have enabled us to define multiple well-tolerated, efficacious doses and a dose-response relationship. We have also demonstrated that topical application to the toenails has led to little or no detectable systemic drug exposure in blood or urine. Results from these trials support the selection of the 5.0% dose of AN2690 for the Phase 3 clinical trials and have enabled appropriate statistical calculations for the design of those trials.
8
The following chart summarizes our Phase 2 clinical trials:
Study Number
|
Type | Dosing | Patients | Trial Objectives | Completed | |||||
|---|---|---|---|---|---|---|---|---|---|---|
200/200a |
Double-blind | Vehicle; 2.5%; 5.0%; 7.5% | 187 | Evaluate safety and efficacy compared to vehicle | August 2007 | |||||
201 |
Open-label |
5.0%; 7.5% |
60 |
Evaluate safety and efficacy |
February 2007 |
|||||
201 |
Open-label |
5.0% |
29 |
Evaluate safety and efficacy of longer treatment period |
July 2008 |
|||||
203 |
Open-label |
1.0%; 5.0% |
60 |
Evaluate efficacy of lower doses and less frequent dosing |
August 2007 |
In our Phase 2 clinical trials, we enrolled onychomycosis patients representative of a wide clinical spectrum of the disease. We believe that the results of these trials indicate that AN2690 may effectively treat patients who are representative of the population that would seek treatment for onychomycosis and collectively support Phase 3 clinical trial initiation.
Study 200/200a: Double-Blind—Safety and Efficacy Compared to Vehicle
The primary objectives of Study 200/200a were to demonstrate that the efficacy of AN2690 could be differentiated from vehicle, and to select the appropriate dose of AN2690 for Phase 3 clinical trials. A total of 187 patients were enrolled at multiple sites in Mexico and the United States. Enrolled patients were randomly assigned to be dosed with the vehicle or one of the following concentrations of AN2690 solution: 2.5%, 5.0% or 7.5%. The double-blind nature of the trial ensured that neither the patients nor the investigators were aware of which of the four treatment options was being applied. Patients dosed themselves daily for the first three months and three times per week for the remaining three months of the six-month treatment period. After an additional six months of follow-up, complete and partial responders were identified and post-treatment effects were assessed.
The study's primary endpoint was the number of patients with at least two millimeters of clear nail growth and negative fungal culture at the end of the six-month treatment period. At this time point, 27% of patients receiving the 2.5% dose, 26% of patients receiving the 5.0% dose and 32% of patients receiving the 7.5% dose were observed to achieve the trial's primary endpoint, compared to 14% of patients receiving the vehicle. Overall, significantly more patients treated with AN2690 achieved the primary endpoint, compared with the vehicle (P-value of 0.03). Of 187 enrolled patients, four subjects experienced five episodes of skin irritation, and four of these episodes were observed in patients receiving AN2690 at the highest dose level. Based upon these results, we believe the 5% dose will offer patients the best combination of efficacy and tolerability and therefore, we selected this dose for advancement into our Phase 3 clinical trials.
Study 201: Open-Label (first, second and third cohorts)—Safety and Efficacy
The objective of Study 201 was to determine the safety and efficacy of 5.0% and 7.5% topical solutions of AN2690 in treating onychomycosis. A total of 60 patients were enrolled at multiple sites in Mexico in the first two cohorts. Half received a 5.0% daily dose and the other half received a 7.5% daily dose for a period of six months, with a subset of patients receiving an additional six months of follow-up assessment. The trial was open-label, such that both investigators and the patients knew which dose was being administered. The study's primary endpoint was the number of patients with at least two millimeters of clear nail growth at six months and a negative fungal culture. At the end of the six-month treatment period, 43% of patients receiving the 5.0% dose and 53% of patients receiving the 7.5% dose were observed to have achieved the trial's primary endpoint.
9
The primary objective of the third cohort of Study 201 was to evaluate the safety of a longer treatment period of AN2690 in onychomycosis. In this open-label trial, patients received a 5.0% dose once daily for up to 360 days. A total of 29 patients were enrolled in this cohort, which indicated that 5.0% AN2690 solution applied daily for 360 days was well-tolerated by most subjects. At the end of the 12-month period, tolerability was shown to be similar to what was previously seen with 24 weeks of treatment. In addition, 14% and 24% of the patients treated in the third cohort reached the endpoint of at least two millimeters of clear nail growth and negative fungal culture at six months and twelve months, respectively. Seven percent of the third cohort patients demonstrated a completely clear nail and negative fungal culture after twelve months of dosing. The percentage of patients reaching the primary endpoint at six months in the third cohort was significantly lower than the percentage of patients reaching the primary endpoint in the first and second cohorts.
Study 203: Open-Label—Efficacy of Lower Doses and Less Frequent Dosing
The objective of Study 203 was to determine safety and efficacy of lower dosing and less frequent dosing of AN2690 in treating onychomycosis. Two groups of 30 patients were enrolled at multiple sites in the United States. Half applied a 1.0% daily dose for six months and the other half applied a 5.0% daily dose for the first month, then three times per week for the remaining five months of treatment. A subset of each treatment group was followed for an additional six months after the end of treatment. The study's primary endpoint was the number of patients with at least two millimeters of clear nail growth at six months and a negative fungal culture. At the end of the six-month treatment period, 30% of patients receiving the 1.0% dose and 50% of patients receiving the 5.0% dose achieved the primary endpoint.
AN2690 Preclinical Development Program
We believe we have completed all of the preclinical toxicology studies required for marketing approval, including a chronic (nine-month) dermal minipig study, two-year mouse and rat carcinogenicity studies and definitive characterization of ADME (absorption, distribution, metabolism, and excretion) in animals.
Former Collaboration with Schering
In February 2007, we entered into an exclusive license, development and commercialization agreement with Schering-Plough Corporation, or Schering, for the development and worldwide commercialization of AN2690. Under the agreement, Schering paid us a $40.0 million upfront fee and $9.5 million for our development-related transition activities and assumed sole responsibility for development and commercialization of AN2690. In addition, Schering invested $10.0 million in a preferred stock financing completed in December 2008. In October 2009, the end-of-Phase 2 meeting with the FDA was completed. In November 2009, Schering merged with Merck & Co., Inc., or Merck, and in May 2010, pursuant to a mutual termination and release agreement, we regained the exclusive worldwide rights to AN2690. Merck did not retain any rights to this compound. Since regaining rights to AN2690 in May 2010, Merck has transferred back to us all materials and documents relating to AN2690 and has paid us $5.8 million.
AN2718 for Topical Fungal Infections
AN2718 is our second topical antifungal in clinical development for onychomycosis and fungal infections of the skin and utilizes the same mechanism as AN2690. AN2718 appears to be well-suited to target organisms that cause common skin and topical fungal infections, including Trichophyton and Candida fungi. Based on preclinical studies and in comparison to AN2690, we believe that AN2718 has greater potency against the dermatophytes T. mentagrophytes and T. rubrum and results in similar nail
10
penetration. These studies suggest that, like AN2690, AN2718 also has significantly greater nail penetration than Penlac.
Our Phase 1 data for AN2718 has also indicated that AN2718 has a low skin irritation profile across multiple doses and that it would thus be suitable for the treatment of skin fungal infection. This Phase 1 data, which we announced in March 2009, was from a 21-day skin irritation trial. In the trial, we compared AN2718 gel at 1.5%, 2.5%, 5.0% and 7.5%, and AN2718 cream at 0.3% and 1.0% to their respective vehicles. All doses of AN2718 gel, cream and vehicle were applied to the skin of normal volunteers for 21 days using semi-occlusive, or semi-air and water-tight, adhesive patches. Application sites were then evaluated daily for signs of irritation. The irritation indices for all AN2718 doses were very low and comparable to vehicle. We currently intend to initiate a Phase 2 trial of AN2718 for onychomycosis following the completion of Phase 3 trials of AN2690.
Our Topical Anti-Inflammatory Programs
AN2728 for Psoriasis and Atopic Dermatitis
AN2728 is our lead topical anti-inflammatory product candidate for the treatment of psoriasis and atopic dermatitis, chronic inflammatory diseases that affect millions of people worldwide. We have achieved positive results from three Phase 1b, one Phase 2a, one Phase 2 and one Phase 2b clinical trials. In February 2011, we initiated a final Phase 2b trial to evaluate the safety and efficacy of AN2728 in patients with mild-to-moderate psoriasis. We anticipate that the design of this trial will be similar to that of our planned Phase 3 trials in which patients will be randomized to receive either AN2728 or vehicle. The objective of this Phase 2b trial is to demonstrate efficacy in a patient-to-patient trial design using the anticipated FDA-required endpoints and to inform the design and powering of our planned two Phase 3 trials for AN2728. Following the completion of this Phase 2b trial, we plan to have an end-of-Phase 2 meeting with the FDA and subsequently advance AN2728 into Phase 3 development in the second half of 2011.
In addition, in the first half of 2011, we expect to initiate a Phase 2 trial of AN2898 outside of the United States in patients with atopic dermatitis, which will be designed as a two-arm trial evaluating the efficacy and safety of AN2898 and AN2728 compared to vehicle. Prior to initiation of clinical trials of AN2898 in the United States, we will file an investigational new drug application, or IND, with the FDA for AN2898 for psoriasis and/or atopic dermatitis.
Psoriasis Market
Psoriasis is a chronic inflammatory skin disease that affects approximately 7.5 million people in the U.S and over 100 million people worldwide. Patients can be categorized as mild, moderate or severe, with approximately 80% of patients having mild to moderate forms of the disease. Psoriasis is characterized by thickened patches of inflamed, red skin covered with thick, silvery scales typically found at the elbows, knees, scalp and genital area. The disorder ranges from a single, small, localized lesion in some patients to a severe generalized eruption. Patients with mild-to-moderate psoriasis are typically treated with a combination of topical therapies, while patients with moderate-to-severe psoriasis are typically treated with a combination of topical and systemic therapies. The recent introductions of new systemic biologic therapies have provided new treatment options for patients with moderate to severe disease and have greatly expanded amounts spent on drugs to treat psoriasis. According to LeadDiscovery, sales of psoriasis drugs in the seven major pharmaceutical markets (United States, Japan, France, Germany, Italy, Spain and the United Kingdom) were $2.5 billion in 2008. In 2009, over 4.5 million prescriptions were written for patients with psoriasis in the United States, with approximately 3.9 million of these prescriptions written for topical therapies.
11
Limitations of Current Psoriasis Therapies
Most psoriasis patients use more than one type of treatment at any given time and may rotate treatments over time as their disease severity changes or they develop complications. Although current treatments attempt to decrease the severity of the disease, none of them cures the disease. Currently available treatments can be classified as topical, oral, injectable or phototherapy. According to IMS Health, 84% of all prescriptions for psoriasis within the United States in 2009 were for topical treatments. The most common topical treatments are corticosteroids, vitamin D derivatives, such as Dovonex (calcipotriene), topical retinoids, such as Tazorac (tazarotene), and crude coal tar preparations. Taclonex is also a treatment for psoriasis and is a combination of calcipotriene and the high potency corticosteroid betamethasone dipropionate. The most common oral treatments are the immunosuppressive drug methotrexate and the oral retinoid acetretin. A number of injectable biologic drugs in the market include Amevive, Enbrel, Humira, Remicade and Stelara. The majority of these drugs are monoclonal antibodies, complex protein molecules, some of which act by the inhibition of TNF-alpha. In addition to topicals, orals and injectables, psoriasis is also treated with ultraviolet light exposure. Typically, physicians initiate treatment by prescribing topical therapies to treat mild or moderate forms of psoriasis, followed by light therapy or oral treatments if the patient's disease does not improve. For patients who do not respond to oral treatments or light therapy, or for those who have moderate-to-severe psoriasis, physicians will prescribe injectable biologic treatments.
Current topical therapies have demonstrated varying levels of efficacy. However, their use has been limited due to issues of safety and tolerability. Long-term use of topical corticosteroids is associated with atrophy, or thinning, of the skin and has the potential to suppress the body's ability to make normal amounts of endogenous corticosteroids, which limit the duration of safe treatment with these therapies. Vitamin D derivatives can cause skin irritation, and some patients report burning sensations associated with their use. Topical retinoids can also cause skin irritation and have been shown to cause birth defects. Thus, their use must be avoided during pregnancy. Oral and injectable drugs have greater activity than topical therapies, but also have well-documented and significant systemic side effects, such as liver toxicity, increase in blood fats and suppression of the immune system. In addition, injectable biologic drugs are very expensive, costing tens of thousands of dollars annually. Ultraviolet light treatments can be effective, but require multiple visits to the doctor's office each week and may increase patients' risk of developing skin cancer.
Atopic Dermatitis Market
Atopic dermatitis is a chronic rash characterized by inflammation and itchiness. In 2007, Datamonitor reported that atopic dermatitis affected approximately 40 million people across the seven major pharmaceutical markets. The condition most commonly appears in childhood, with 20% of children in the United States affected, and it can persist into adulthood. Skin that is broken and chafed from itching allows bacterial or viral access, which leads to secondary infections.
Limitations of Current Atopic Dermatitis Therapies
Current atopic dermatitis treatments attempt to reduce inflammation and itchiness to maintain the protective integrity of the skin. Combinations of antibiotics, antihistamines, topical corticosteroids and topical immunomodulators, such as Elidel and Protopic, are the current standard of care. However, these have limited utility because of lack of efficacy and side effects. While not approved by the FDA for treatment of atopic dermatitis, ultraviolet light has been used to treat this disease.
Our Solution: AN2728
We believe that AN2728 will have comparable efficacy to that of topical corticosteroids and vitamin D analogs in treating psoriasis, but with a better safety and tolerability profile thus allowing for
12
longer duration of treatment. AN2728 is a novel boron-containing small molecule that inhibits PDE4 and reduces the production of TNF-alpha, a precursor of the inflammation associated with psoriasis, as well as other cytokines, including IL-12 and IL-23, which are proteins believed to be involved in the inflammation process and immune responses. If approved, AN2728 would be the first topical non-steroidal treatment that inhibits TNF-alpha release. Because AN2728 has a novel mechanism of action, it can potentially be combined with topical corticosteroids and vitamin D analogs for patients with mild-to-moderate psoriasis. In addition, patients with severe psoriasis who combine topical and systemic therapies may use AN2728. We believe the anti-inflammatory characteristics of AN2728 may also prove effective for the treatment of patients with atopic dermatitis.
AN2728 Phase 2b and Phase 3 Development Programs
In February 2011, we initiated a final Phase 2b trial to evaluate the safety and efficacy of AN2728 in patients with mild-to-moderate psoriasis. We anticipate that the design of this trial will be similar to that of our planned Phase 3 trials in which patients will be randomized to receive either AN2728 or vehicle. The objective of this Phase 2b trial is to demonstrate efficacy in a patient-to-patient trial design using the anticipated FDA-required endpoints and to inform the design and powering of our planned two Phase 3 trials for AN2728. Following the completion of this Phase 2b trial, we plan to have an end-of-Phase 2 meeting with the FDA and subsequently advance AN2728 into Phase 3 development with the first of two planned Phase 3 trials anticipated to commence in the second half of 2011. We anticipate that we will have data from at least one of our two Phase 3 trials for AN2728 by the second half of 2012.
AN2728 Phase 1 and Phase 2 Development Program
AN2728 has demonstrated initial tolerability and activity against psoriatic lesions in three Phase 1b, one Phase 2a, one Phase 2 and one Phase 2b clinical trials. For AN2728, all three of our completed Phase 1b microplaque trials showed significant activity over vehicle. Subsequently, our three completed Phase 2 trials confirmed the results of the microplaque studies when applied by the patient.
The following chart summarizes our AN2728 clinical trials to date which compared AN2728 to existing topical treatments for psoriasis or vehicle:
Study Number
|
Type | Dosing | Patients | Trial Duration |
Trial Objectives |
Completed | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Microplaque Phase 1b |
Open-label | 5.0% Betnesol-V, Protopic, Vehicles | 12 | 12 days | Evaluate safety and efficacy compared to Betnesol-V, Protopic and vehicles | March 2007 | ||||||
Microplaque Phase 1b |
Open-label |
0.5%, 2.0%, 5.0%, Betnesol-V, Protopic, Vehicle |
12 |
12 days |
Evaluate safety and efficacy of multiple doses compared to Betnesol-V, Protopic and vehicle |
December 2007 |
||||||
Microplaque Phase 1b |
Open-label |
0.3%, 1.0%, 2.0%, Betnesol-V, Vehicle |
12 |
12 days |
Evaluate safety and efficacy of multiple doses compared to Betnesol-V and vehicle |
March 2008 |
||||||
Phase 2a |
Double-blind |
Vehicle; 5.0% |
35 |
4 weeks |
Evaluate safety and efficacy compared to vehicle |
March 2008 |
||||||
Phase 2 |
Double-blind |
Vehicle; 5.0% |
30 |
12 weeks |
Evaluate optimal duration of therapy |
December 2008 |
||||||
Phase 2b dose-ranging |
Double-blind |
Vehicle; 0.5%, 2.0% once and twice daily |
145 |
12 weeks |
Evaluate optimal dose and duration of therapy |
June 2010 |
13
Phase 1b Clinical Trials
We have completed three Phase 1b clinical trials of AN2728. These trials utilized a microplaque design in which each patient had small areas of a large psoriatic lesion treated with various medications for 12 days. Each of the treatment areas was evaluated at days one, eight and twelve. The primary endpoint for each of these trials was the change in thickness of the psoriatic lesion as measured by sonography, and the secondary endpoint was improvement based on clinical score as evaluated by a physician. No treatment-related adverse events were observed in these trials.
In March 2007, we completed our first Phase 1b microplaque clinical trial of AN2728 in patients with psoriasis. The study enrolled 12 patients for a 12-day treatment and compared AN2728 5.0% ointment, AN2728 5.0% cream, Betnesol-V cream (betamethasone vallerate, a mid potency corticosteroid) and Protopic ointment (tacrolimus, an immunomodulator), vehicle cream and vehicle ointment. This study demonstrated that AN2728 caused a significant reduction in the thickness of psoriatic lesions compared to both vehicles, at a P-value of 0.025. The mean percent reduction in infiltrate thickness for AN2728 was 54%, as compared to 48% for Protopic and 72% for Betnesol-V. The results of the secondary endpoint paralleled the results of the primary endpoint.
In December 2007, we completed our second Phase 1b microplaque clinical trial of AN2728 in patients with psoriasis. This study was a dose-ranging study designed to compare 0.5%, 2.0% and 5.0% AN2728 ointment, Betnesol-V cream, Protopic, and the ointment vehicle. Based on the primary endpoint, which was the change in the thickness of the inflammatory infiltrate, all three concentrations were significantly better than the ointment vehicle (P-values less than 0.003). The mean percent reductions for 5.0%, 2.0% and 0.5% AN2728 ointment were 36%, 35%, and 26%, respectively. The percent reductions for Betnesol-V and Protopic were 59% and 34%, respectively.
In March 2008, we completed our third Phase 1b microplaque trial in patients with psoriasis. This was a study designed to compare 0.3%, 1% and 2% AN2728, Betnesol-V and vehicle. The study enrolled 12 patients for a 12-day treatment. In the study, 1% and 2% AN2728 demonstrated significant improvement over vehicle and 0.3% AN2728 demonstrated a strong trend in superiority over vehicle.
Phase 2 Clinical Trials
Psoriasis is often bilateral and symmetrical, meaning that patients with psoriasis commonly have similar areas of psoriasis on opposing sides of their body. Each of our Phase 2 studies of AN2728 for the treatment of psoriasis were designed as a bilateral study in which patients treat one of their plaques with one treatment and a similar plaque or the other side of the body with a comparison treatment. This allowed patients to serve as their own control.
In March 2008, we completed a Phase 2a bilateral trial of AN2728 to characterize the safety profile and to assess efficacy. In this trial, patients, in a double-blinded fashion, treated one of their areas of psoriasis with AN2728 5.0% ointment and a matching area on the opposite side of the body with the vehicle alone. The trial treated 35 patients with mild-to-moderate psoriasis. The primary endpoint was the proportion of patients in whom the AN2728-treated area improved more than the vehicle-treated area based on the overall target plaque severity score, or OTPSS. The OTPSS is a scoring system used by investigators that characterizes severity of disease, which ranges from zero (no evidence of disease) to eight (very severe). Based on the OTPSS after four weeks, the trial achieved its primary endpoint, with 69% of the AN2728 treated plaques demonstrating a lower score than the vehicle treated plaques as compared to 6% of the vehicle treated plaques (P-value less than 0.001). Significant differences were also noted after two weeks and three weeks and in all secondary endpoints, including scaling, erythema and plaque elevation. In addition to no serious adverse events, there were no treatment-related adverse reactions or application site reactions.
14
In December 2008, we completed a Phase 2 double-blind bilateral trial comparing 5.0% AN2728 ointment and vehicle using a design similar to our Phase 2a study but using a longer treatment duration. In this Phase 2 study, patients with mild-to-moderate psoriasis applied AN2728 and vehicle twice daily for 12 weeks in order to define the optimal duration of therapy. Results showed statistically significant reductions in the OTPSS, as well as in the individual signs of psoriasis, such as erythema, scale and plaque thickness at several time points. Compared to those treated with vehicle, psoriasis plaques treated with AN2728 achieved a lower OTPSS in a significantly greater proportion of patients after as few as two weeks of treatment, with optimal responses seen at six and eight weeks (P-value less than 0.001 and 0.01, respectively). Thirteen percent of the treated plaques cleared completely and 43% of the plaques achieved clear or almost clear with a two-grade improvement from baseline. Treatments were generally well-tolerated with the most common side effect being irritation at the application site. During the course of the trial, one serious adverse event was reported in a patient who developed a rash after receiving an injection of penicillin outside of the trial for a sore throat and needed to be hospitalized for this non-life threatening reaction.
In June 2010, we completed a 145-patient randomized, double-blind, vehicle-controlled, multicenter, Phase 2b bilateral dose-ranging trial to evaluate the safety and efficacy of 0.5% and 2.0% AN2728 ointment, applied either once or twice daily for 12 weeks for the treatment of mild-to-moderate psoriasis. Compared to those treated with vehicle, psoriasis plaques treated with AN2728 achieved greater improvement in the OTPSS in a significantly higher proportion of patients after six weeks in the 2.0% AN2728 twice daily dosing group (P-value less than 0.001), which was the primary endpoint of the trial. A dose response was also observed across the four dosing groups for this outcome. Additionally, of those plaques treated for 12 weeks with 2.0% AN2728 twice daily, 54% achieved complete or near complete clearance with at least a two-grade improvement from their baseline severity score.
Based on these results, we initiated and have completed enrollment in a double-blind, Phase 2b trial for AN2728 in February 2011. The Phase 2b trial is designed to evaluate the safety and efficacy of AN2728 in patients with mild to moderate plaque-type psoriasis and is being conducted under anticipated Phase 3 conditions. This double-blind, randomized, vehicle-controlled trial has enrolled 68 patients with mild to moderate plaque-type psoriasis at ten centers in the United States. Patients have been randomized to 2% AN2728 topical ointment or vehicle in a 2:1 ratio and will apply the ointment twice daily for 12 weeks. Given its size, the trial is not designed to demonstrate statistical differentiation of AN2728 from vehicle, but to inform the size and design of the two planned pivotal Phase 3 trials for AN2728. Following the completion of this Phase 2b trial, we plan to have an end-of-Phase 2 meeting with the FDA and subsequently advance AN2728 into Phase 3 development with the first of two planned Phase 3 trials anticipated to commence in the second half of 2011. We anticipate that we will have data from at least one of our two Phase 3 trials for AN2728 by the second half of 2012.
AN2898 for Psoriasis and Atopic Dermatitis
AN2898 is our second anti-inflammatory product candidate for psoriasis and atopic dermatitis. Like AN2728, AN2898 is a novel boron-containing small molecule that inhibits PDE4 and reduces the production of both TNF-alpha, a precursor of the inflammation associated with psoriasis, and other cytokines, including IL-12 and IL-23. AN2898 has a similar mechanism of action to that of AN2728 and appears to have activity in a larger set of animal models, which may predict greater clinical efficacy than AN2728 in atopic dermatitis.
In February 2009, we initiated a Phase 1b study evaluating AN2898 in 12 patients with plaque-type psoriasis for 12 days. This was a microplaque study conducted in a similar fashion as the AN2728 microplaque trials. Achieving its primary endpoint, this study demonstrated that AN2898 caused a significant reduction in the thickness of psoriatic lesions compared to vehicle, at a P-value less than
15
0.0001. The mean percent reduction in infiltrate thickness on day 12 for AN2898 was 39%, as compared to 60% for Betnesol-V, the positive control. The results relative to the secondary endpoint (clinical response) paralleled those of the primary endpoint.
In a cumulative irritation trial completed in the first quarter of 2009, AN2898 ointment at 5.0% and its vehicle were applied daily to the skin of normal volunteers under occlusive, adhesive patches for four consecutive days. Application sites were evaluated daily for signs of irritation. No irritation potential was seen for 5.0% AN2898 ointment or the vehicle.
In the first half of 2011, we expect to initiate a Phase 2 trial of AN2898 outside of the United States in patients with atopic dermatitis, which will be designed as a two-arm trial evaluating AN2898 and AN2728 compared to vehicle. Prior to initiation of clinical trials of AN2898 in the United States, we will file an IND with the FDA for AN2898 for psoriasis and/or atopic dermatitis. We are also evaluating AN2898 for the treatment of psoriasis. However, we expect AN2728 to remain our lead product candidate in psoriasis since it has reached a more advanced stage of clinical development.
Our Systemic Antibiotic Program
GSK '052 for Gram-Negative Infections
GSK '052 (formerly AN3365) is our lead systemic antibiotic product candidate for the treatment of infections caused by Gram-negative bacteria. In July 2010, GSK exercised its option to obtain an exclusive license to develop and commercialize GSK '052. GSK has assumed responsibility for further development of the product candidate and any resulting commercialization. We anticipate that GSK will initiate Phase 2 trials of GSK '052 in patients with cUTI and cIAI in 2011.
Gram-Negative Infection Market
Gram-negative infections are a type of bacterial infection caused by a broad class of bacteria called Gram-negative bacteria and are most commonly acquired and treated in the hospital setting. Many commonly used antibiotics do not work against Gram-negative bacteria and resistance to existing therapies continues to be a growing problem. According to the New England Journal of Medicine, it is estimated that there were 1.7 million hospital-acquired Gram-negative and Gram-positive infections and approximately 100,000 associated deaths in the United States alone in 2002. The New England Journal of Medicine also indicates that Gram-negative bacteria are responsible for more than 30.0% of hospital-acquired infections and account for approximately 70.0% of hospital-acquired infections in the intensive care unit. IMS Health estimates that there were 40 million days of Gram-negative therapy administered in the United States in 2009. According to the Archives of Internal Medicine, hospital-acquired infections related to sepsis and pneumonia alone caused $8.1 billion in additional hospital costs in 2006.
Limitations of Current Gram-Negative Antibiotics
Traditionally, Gram-negative infections have been treated with antibiotics, particularly beta-lactams, including penicillins, cephalosporins and carbapenems, and quinolones, including flouroquinolones. However, the effectiveness of existing antibiotics has been declining due to increasingly prevalent drug resistance. Bacteria develop resistance to drugs through genetic mutations or by acquiring genes from other bacteria that have become resistant. For example, in a recent survey of resistance rates of Gram-negative bacteria to current therapies in the United States, the resistance of E. coli to fluoroquinolones has been dramatically increasing. For example, resistance of E. coli to ciprofloxacin increased from 4% in 1999 to 30% in 2008 and resistance of E. coli to levofloxacin increased from 10% in 2003 to 30% in 2008. Over the same period, resistance of another Gram-negative bacteria, Klebsiella pneumoniae, to third generation cephalosporins, such as ceftriaxone and ceftazidime, increased from virtually no resistance to 15%. The same survey also showed that by 2008, 17%-19% of Pseudomonas aeruginosa were resistant to fluoroquinolones, 10%-70% were resistant to third generation
16
cephalosporins and 7%-15% were resistant to carbapenems, such as meropenem and imipenem. Therefore, there is an ongoing need for novel antibiotics to combat the widespread proliferation of antibiotic resistance, particularly for Gram-negative bacteria. Additionally, currently marketed products have side effect profiles that can include nausea, diarrhea, vomiting, rash, insomnia, and potential liver toxicity. Also, currently approved antibiotics specifically targeting infections caused by Gram-negative bacteria are only available in either IV or oral formulations, but not both, so patients cannot continue on the same antibiotic therapy they received in the hospital once they are discharged.
Our Solution: GSK '052
GSK '052 is a novel boron-based, small molecule product candidate that targets the bacterial enzyme leucyl tRNA synthetase. The inhibition of protein synthesis leads to termination of cell growth and cell death, eliminating the bacterial infection. Since GSK '052 is the first antibiotic to target LeuRS, bacteria have not developed resistance to it. Preclinical studies suggest that GSK '052 could be a novel approach for the treatment of infections caused by Gram-negative bacteria, including E. coli, Klebsiella pneumoniae, Citrobacter spp., S. marcescens, P. vulgaris, Providentia spp., Pseudomonas aeruginosa and Enterobacter spp. GSK '052 has demonstrated a favorable profile in preclinical safety and toxicology studies. Results from a Phase 1 proof-of-concept trial showed that GSK '052 demonstrated a promising safety profile and linear dose-proportional pharmacokinetic properties, reaching blood levels that were multiple times higher than the anticipated efficacious dose. We believe, if approved, GSK '052 would be the first new class antibacterial to treat serious hospital-acquired Gram-negative infections in thirty years. In addition, we believe GSK '052 has the potential to be the first antibiotic specifically targeting infections caused by Gram-negative bacteria that can be administered by both IV and oral routes, which, for the first time, would allow patients to continue on the same antibiotic therapy they received in the hospital once they are discharged.
GSK '052 Development Program
In November 2009, we initiated a Phase 1 dose-escalating clinical study for GSK '052, to evaluate the safety, tolerability and pharmacokinetics of GSK '052 in healthy volunteers. The randomized, double-blind, placebo-controlled, dose-escalation study enrolled 72 subjects. Participants in this study received GSK '052 in single or multiple doses for treatment durations of up to 14 days and included doses that achieve blood levels that are approximately four times the expected efficacious blood levels based on our preclinical studies. In June 2010, we reported Phase 1 results showing that GSK '052 appeared to be safe and well-tolerated. In July 2010, GSK exercised its option to obtain an exclusive license to develop and commercialize GSK '052. Upon exercise of the option, we received a fee of $15.0 million. We are eligible to receive further development milestones up to $69.0 million, commercial milestones up to $175.0 million and double-digit tiered royalties with the potential to reach the mid-teens on annual net sales. GSK has assumed responsibility for further development of the product candidate and any resulting commercialization. We believe GSK currently plans to develop GSK '052 as a potential treatment for cUTI, cIAI, and other Gram-negative bacterial infections, such as HAP/VAP. We anticipate that GSK will initiate Phase 2 trials of GSK '052 in patients with cUTI and cIAI in 2011.
Research Activities
Other Systemic Anti-Infective Programs in Collaboration with GSK
Under our collaboration with GSK, we are conducting research on additional systemic anti-infectives in three target-based project areas in addition to GSK '052. The collaborative research term of the agreement is six years, subject to a two-year extension if agreed to by both parties.
17
Internal Research Activities
Our internal research activities include follow-on research to our existing compounds as well as investigation of novel activity of our boron chemistry platform in multiple therapeutic applications. Key efforts currently include the further development of topical and systemic PDE4 inhibitors for the treatment of inflammatory diseases, the development of novel anti-infectives against targets not covered in our existing GSK collaboration, and work on novel kinase inhibitors.
Neglected Diseases Initiative
Neglected diseases are defined as diseases that disproportionately affect the world's poorest people, including tuberculosis, or TB, malaria, visceral leishmaniasis, Chagas disease, human African trypanosomiasis, or African sleeping sickness, and filarial worms. Despite the fact that these diseases cause significant morbidity and mortality worldwide, and that the current standards of care are difficult to administer, have significant toxicities and are increasingly becoming less effective due to resistance, there has been little investment in developing new therapies for these diseases due to the absence of a reasonable expectation of a financial return.
Our boron chemistry platform appears to be particularly well suited for the treatment of these types of infectious diseases, and we feel a responsibility to apply our technology to the development of new treatments. Until such time as we are profitable, however, we are committed to doing that research only when we can use grants and other non-dilutive sources of funding in a cash-neutral manner.
In recent years, a number of foundations and governments have created public-private partnerships to address this gap by funding promising technologies that may result in new drugs. In December 2007, we established a partnership with the Drugs for Neglected Diseases initiative, or DNDi, to develop new therapeutics for African sleeping sickness, visceral leishmaniasis and Chagas disease. In May 2009, we established a collaboration with the Global Alliance for TB Drug Development. In April 2010, we entered into a research collaboration with the Medicines for Malaria Venture, or MMV, to identify lead compounds for the treatment of prophylaxis of malaria and in March 2011 we also entered into a development agreement with MMV to develop our compound AN3661 for the treatment of malaria. In December 2010, we entered into a collaboration with the Sandler Center for Drug Discovery at the University of California at San Francisco to discover new drug therapies for the treatment of river blindness, a parasitic disease that is the second leading cause of infectious blindness worldwide. In March 2011, we announced the establishment of our joint research agreement with the Institute for OneWorld Health to discover antibacterial compounds for treating diarrheal diseases.
Our work in this area also allows us the potential benefits of expanding the chemical diversity of our boron compounds, understanding new properties of our boron compounds, receiving future incentives, such as the potential grant of a priority review voucher by the FDA, and, ultimately if a drug is approved, potential revenue in some regions.
Collaboration with GSK
In October 2007, we entered into a research and development collaboration, option and license agreement with GSK for the discovery, development and worldwide commercialization of boron-based systemic anti-infectives. Under the agreement, we are currently working to identify and develop multiple product candidates in three target-based project areas. The collaborative research term of the agreement is six years, subject to an extension of up to two years if agreed to by both parties.
In each project, GSK has the option to obtain an exclusive license to develop, commercialize and market worldwide certain product candidates once they have achieved certain proof-of-concept criteria. We will be primarily responsible for the discovery and development of each product candidate from the
18
research stage until GSK exercises an option for such product candidate, at which point GSK will assume sole responsibility for the further development and commercialization of such product candidate on a worldwide basis, including the responsibility to obtain regulatory approvals on a country-by-country basis. During the research term, we are committed to use diligent efforts to discover and optimize compounds pursuant to agreed research plans and to provide specified resources, including certain numbers of full-time equivalent scientists, on a project-by-project basis. Each party is responsible for its own research and development costs.
Pursuant to the agreement, GSK paid us a $12.0 million non-refundable, non-creditable upfront fee in October 2007. In addition, GSK invested $30.0 million in a preferred stock financing completed in December 2008.
In July 2010, GSK exercised its option to obtain an exclusive license to develop and commercialize GSK '052 (formerly AN3365), our lead systemic antibiotic for the treatment of infections caused by Gram-negative bacteria. We believe GSK currently plans to develop GSK '052 as a potential treatment for cUTI, cIAIs and other Gram-negative bacterial infections, such as HAP/VAP. Upon exercise of the option, we received a licensing fee of $15.0 million. We are eligible to receive further development milestones up to $69.0 million, commercial milestones up to $175.0 million and double-digit tiered royalties with the potential to reach the mid-teens on annual net sales. GSK has assumed responsibility for further development of the product candidate and any resulting commercialization.
In addition to assuming sole responsibility for the costs of further development and commercialization of the compounds for which it exercises an option to license, GSK is obligated to make payments to us if certain development, regulatory and commercial milestones are met on a compound-by-compound basis. In addition, GSK is obligated to make payments to us if certain milestones are met, which range from up to $252.8 million to $330.5 million in the aggregate per product candidate. Milestone payments may be lower for designated programs depending upon: whether GSK makes the selection of the product candidate before or after initiation of Phase I clinical trial dosing (10%-15% reduction if selected before such dosing); if certain target product profile characteristics are not achieved (20%-40% reduction); and whether the product candidate is designated after the initial two product candidate designations in a program (50% reduction). GSK is further obligated to pay us double-digit tiered royalties with the potential to reach the mid-teens on annual net sales of products containing optioned compounds in jurisdictions where there is a valid patent claim covering composition of matter or method of use of the product and lesser royalties for sales in jurisdictions where there is no such valid patent claim. Such royalties shall continue until the later of expiration of such valid patent claims or ten years from the first commercial sale on a product-by-product and country-by-country basis. To date, in addition to the $12.0 million upfront payment and $15.0 million option exercise fee, we have received $10.1 million for achievement of performance milestones, including GSK '052 milestones for lead declaration, candidate selection and first patient dosing in a Phase 1 clinical trial. We have also received milestone payments for lead declarations in two other GSK programs.
The agreement provides for a joint research committee to oversee the research collaboration, and a joint patent subcommittee responsible for coordination of intellectual property developed by the collaboration, including patent application filing. We and GSK have appointed an equal number of members to each such committee and decisions are made on a consensus basis, except that ultimate decision-making authority with respect to the establishment of proof-of-concept criteria, the design of proof-of-concept trials for each research compound and determining whether proof-of-concept criteria have been met, is vested in GSK. Unless earlier terminated, the agreement will continue in effect until expiration of all payment obligations under the agreement. GSK retains the unilateral right to terminate the agreement in its entirety upon six months prior written notice to us and immediately with respect to any project. Either party may also terminate the agreement, on a project-by-project basis or in its entirety, for any uncured material breach of the agreement by the other party. Either party may
19
also terminate the agreement upon specified actions relating to insolvency of the other party. In the event of unilateral termination by GSK, all rights granted by us to GSK with respect to the project to which such termination applies would terminate and we would retain the rights to any compounds relating to such project. In the event of termination by GSK for cause, GSK would have a perpetual exclusive license under our intellectual property to develop and commercialize any project compounds to which such termination applies, subject to GSK's payment to us of specified royalties on sales of such compounds. In the event of termination by us for cause, we would have a perpetual exclusive license under GSK's intellectual property to develop and commercialize any project compound to which such termination applies, subject to payment by us to GSK of specified royalties on sales of such compounds.
For nine years following the effective date of the agreement, subject to certain exceptions, we may not research, optimize, develop or commercialize outside of the collaboration any compounds that we progressed through the project, or any other compounds directed against the same target, unless GSK's option to such compound terminated without being exercised or GSK later terminates its license to such compound after exercising such option. If we choose to develop such compounds after expiration of GSK's option or termination of GSK's license, we may be required under certain circumstances to make certain regulatory milestone and royalty payments to GSK for such compounds.
Upon a change of control of either party, the agreement would remain in effect. Upon a change of control of Anacor, GSK has the right to elect to exercise any remaining options to license and commercialize compounds then under development pursuant to the research collaboration. Depending upon the status of compound development and the project under which the compound is being developed, the exercise by GSK of its license options in connection with a change of control of Anacor could result in lower milestone payments and royalty rates being payable by GSK upon commercialization of such compound.
Collaboration with Eli Lilly and Company
In August 2010, we entered into a research agreement with Eli Lilly and Company, or Lilly, under which we will collaborate to discover products for a variety of animal health applications and Lilly will be responsible for worldwide development and commercialization of compounds advancing from these efforts. The collaboration combines our boron-based technology platform and drug research capabilities with Lilly's expertise in the area of animal health. We received an upfront payment of $3.5 million and we will receive a minimum of $6.0 million in research funding with the potential of up to $12.0 million in research funding, if successful. In addition, we will be eligible to receive payments upon the achievement of development and regulatory milestones, as well as tiered royalties escalating from high single digit to in the tens commercial royalties on sales depending in part upon the mix of products sold. Such royalties continue through the later of expiration of our patent rights or six years from the first commercial sale on a product-by-product and country-by-country basis.
Unless earlier terminated, the agreement continues in effect until the termination of royalty payment obligations. The agreement allows for termination by Lilly upon written notice, with certain additional payments to us and a notice period that has a duration dependent on whether the notice is delivered prior to the first regulatory approval of a product under the agreement or thereafter. In addition, either party may terminate for the other party's uncured material breach of the agreement. In the event of termination by us for material breach by Lilly or termination upon written notice by Lilly, Lilly would assign to us certain trademarks and regulatory materials used in connection with the products under the agreement and grant to us an exclusive license under Lilly's patent rights covering such products, and we would pay to Lilly a reasonable royalty on sales of such products should we desire an exclusive license. In the event of termination for material breach by us, Lilly will be entitled to a return of all research funding payments for expenses we have not incurred or irrevocably committed.
20
Collaboration with Medicis
In February 2011, we entered into a research and development option and license agreement with Medicis to discover and develop boron-based small molecule compounds directed against a target for the potential treatment of acne.
Under the terms of the agreement, we received a $7.0 million upfront payment from Medicis and will be primarily responsible, during a defined research collaboration term, for discovering and conducting early development of product candidates which utilize our proprietary boron chemistry platform. Medicis will have an option to obtain an exclusive license for products covered by the agreement. We will be eligible for future research, development, regulatory and sales milestones of up to $153.0 million, as well as high single-digit to in the tens royalties on sales of products that Medicis licenses pursuant to its option. Following option exercise, Medicis will be responsible for further development and commercialization of the licensed products on a worldwide basis.
If Medicis exercises its option for a product candidate, the agreement will continue in effect until the expiration of royalty payment obligations, which obligations will run through the later of patent or regulatory exclusivity and 7 years from first commercial sale, on a product-by-product basis. Upon the expiration of such payment obligations for a product under the agreement, Medicis will retain an exclusive, fully paid and royalty-free right and license in such product. The agreement allows for at-will termination by Medicis upon written notice, and either party may terminate for the other party's uncured material breach of the agreement or specified activities related to insolvency. In the event of at-will termination by Medicis or termination by us for material breach or insolvency activities by Medicis, all rights granted by us to Medicis would revert to us, and Medicis would be required to grant to us a non-exclusive license under its patent rights covering products under the agreement. If we materially breach the agreement prior to the completion of the research collaboration term and exercise of the option, Medicis would be entitled to either terminate the agreement or continue with the agreement and terminate the research collaboration term, in which case Medicis would have a right to reduce its financial obligations to us or recover its costs to mitigate the damages resulting from such breach.
We have agreed not to research or develop, with respect to the target that is the subject of the agreement, any small molecule products in a specified field for use in humans for a period of 11 years. Medicis has agreed not to research or develop with respect to the target that is the subject of the agreement any boron-containing compound for 9 years from the date of the agreement or, if a certain milestone is not met, for 4 years from the date of the agreement.
Sales and Marketing
Our strategy is to develop a sales force targeting dermatologists and other specialty markets in the United States and to collaborate with other companies for sales into primary care markets. In addition, we plan to enter into agreements with third parties to commercialize our products outside of the United States.
We expect that if AN2690 and AN2718 are approved, primary care physicians will write the majority of prescriptions for these compounds in the United States, and we expect half of overall sales will be within the United States. Accordingly, we plan to enter into a partnership for AN2690 after the completion of Phase 3 clinical trials. We also anticipate that, if AN2690, AN2718, AN2728 and AN2898 are approved, dermatologists will write a significant number of AN2690 and AN2718 prescriptions, as well as the majority of prescriptions for AN2728 and AN2898, which we expect to address with our own sales force in the United States. If AN2728 and AN2898 are approved, we intend to sell these products for psoriasis and atopic dermatitis to dermatologists in the United States, and license commercialization rights to these product candidates to third parties for sales outside of the United States.
21
Intellectual Property
Previous efforts to produce boron-based drugs have been centered largely on boronic acids as serine protease inhibitors, such as the oncology treatment Velcade. Our research concentrates on different biological targets and uses novel boron-based compounds where we believe there is little existing intellectual property held by others. All of our intellectual property related to our product candidates was initially developed by us or our subcontractors. In the course of our development and commercialization collaborations, additional intellectual property relating to our product candidates may be developed by us and our collaborators, such as GSK.
As of February 15, 2011, we were the owner of record of 8 issued U.S. patents (U.S. Pat. No. 7,390,806; U.S. Pat. No. 7,888,356; U.S. Pat. No. 7,465,836; U.S. Pat. No. 7,393,856; U.S. Pat. No. 7,652,000; U.S. Pat. No. 7,582,621; U.S. Pat. No. 7,767,657; and U.S. Pat. No. 7,816,344) and 4 non-U.S. patents (2 South African patents, 1 Australian patent and 1 New Zealand patent). We are actively pursuing, either solely or with a collaborator, 28 U.S. patent applications (10 provisional and 18 non-provisional), 16 international (PCT) patent applications and 103 non-U.S. patent applications in at least 39 jurisdictions, including Australia, Brazil, Canada, China, Europe, Hong Kong, India, Israel, Japan, Mexico, New Zealand, Russia, South Africa, and South Korea. Of these actively pursued applications, 3 U.S. patent applications (2 provisional and 1 non-provisional) and 1 international (PCT) patent application are solely owned by a collaborator as of February 15, 2011.
Our patent filings seek to protect innovations created by us, such as composition of matter (compound or pharmaceutical formulation), method of use, and process of production. As of February 15, 2011, claims have issued in the United States (U.S. Pat. No. 7,582,621 and 7,767,657), Australia, New Zealand, and South Africa which cover, among other embodiments, methods of using AN2690 to treat onychomycosis as well as pharmaceutical formulations containing AN2690. As of February 15, 2011, claims have issued in Australia and South Africa which cover, among other embodiments, methods of using AN2718 to treat onychomycosis as well as pharmaceutical formulations containing AN2718. Due to the existence of an expired U.S. patent relating to a non-pharmaceutical use of the compounds, we are not pursuing a claim solely covering AN2690 or AN2718 as a compound. U.S. Pat. No. 7,816,344 claims GSK '052 as a compound as well as part of a pharmaceutical formulation. U.S. Pat. No. 7,390,806 and U.S. Pat. No. 7,465,836 claim compounds which do not relate to our current product candidates. U.S. Pat. No. 7,465,836 and U.S. Pat. No. 7,888,356 claim pharmaceutical formulations which do not relate to our current product candidates. U.S. Pat. No. 7,465,836, U.S. Pat. No. 7,393,856, U.S. Pat. No. 7,652,000 and U.S. Pat. No. 7,888,356 claim methods of using compounds which do not relate to our current product candidates.
In 2011, we are electing to allow certain non-U.S. patents and non-U.S. patent applications that do not relate to our current product candidates to expire or become abandoned. In 2011, we estimate that we will allow 22 non-U.S. patents and 49 non-U.S. patent applications that do not relate to our clinical product candidates to expire or become abandoned and have not included such patents and patent applications in the totals above.
Our agreement with GSK provides that we will retain all of our right, title and interest in intellectual property for which we possess the right to license or sublicense as of the effective date of the agreement and throughout the term of the agreement. Each party will remain the sole owner of intellectual property developed by its personnel under the research collaboration. Intellectual property that is jointly developed by GSK and us under the collaboration will be jointly owned by GSK and us, subject to exclusive licenses that may be granted to GSK or us pursuant to the terms of the agreement.
Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection of our current and future product candidates and the methods used to manufacture them, as well as successfully defending these patents against third-party challenges. Our ability to stop third parties from making, using, selling, offering to sell or importing our products
22
depends on the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities. There can be no assurance that our pending patent applications will result in issued patents.
Manufacturing and Supply
Our current product candidates are low molecular weight molecules that are synthesized chemically and, therefore, we believe they are easier to manufacture at relatively lower cost than biologic drugs from cell-based sources. All of our current clinical drug product manufacturing activities are in compliance with current Good Manufacturing Practices, or cGMP, and are outsourced to qualified third parties with oversight by our employees. We have limited in-house, non-GMP manufacturing capacity for research activities. We rely on third-party cGMP manufacturers for scale-up of the active ingredient, process development work and to produce sufficient quantities of product candidates for use in clinical trials. We intend to continue to rely on third-party cGMP manufacturers for any future clinical trials and large-scale commercialization of all of our compounds for which we have manufacturing responsibility. We have established multiple sources of supply for the reagents necessary for the manufacture of our compounds. We believe this manufacturing strategy will enable us to direct financial resources to the development and commercialization of products rather than diverting resources to establishing a manufacturing infrastructure.
Prior to entering into the agreement with Schering in 2007, we had successfully outsourced AN2690 cGMP manufacturing to a contract manufacturer at a scale that would have been sufficient to conduct Phase 3 clinical trials. Under our agreement with Schering, the formulation for AN2690 was finalized and manufactured at one of Schering's GMP facilities. In November 2009, Schering merged with Merck and in May 2010, we regained the worldwide rights to AN2690. As part of the transition from Merck to us, we believe that we received sufficient drug product to initiate and sufficient active ingredient to complete our planned Phase 3 clinical trials of AN2690. We have transferred AN2690 manufacturing to a third party that operates in compliance with cGMP regulations and will need to scale up and manufacture additional active ingredient and drug product in order to complete our NDA submission. We outsource global process development work and product manufacturing to third parties for AN2728, AN2718 and AN2898.
Competition
Onychomycosis
If approved for the treatment of onychomycosis, we anticipate AN2690 and AN2718 would compete with other approved onychomycosis therapeutics including:
- •
- Systemic treatments: Lamisil, also known by its generic
name, terbinafine, is marketed by Novartis. Terbinafine is available from several generic manufacturers. Sporanox, also known by its generic name, itraconazole, is marketed by Johnson & Johnson
and is available as a generic as well.
- •
- Topical treatments: Penlac, also known by its generic name, ciclopirox, is marketed by sanofi-aventis. Several versions of ciclopirox are available as generics.
In addition to approved onychomycosis therapeutics, the marketing of several over-the-counter products is directed toward persons suffering from onychomycosis, even though none of these products is FDA-approved for onychomycosis treatment.
There are also several pharmaceutical product candidates under development that could potentially be used to treat onychomycosis and compete with AN2690. Product candidates in late-stage development include a novel topical triazole in Phase 3 development by Dow Pharmaceutical Sciences, or DPS, a wholly-owned subsidiary of Valeant Pharmaceuticals International, an undisclosed topical
23
product candidate in Phase 3 development by Promius Pharma, LLC, a wholly-owned subsidiary of Dr. Reddy's Laboratories, and a topical reformulation of terbinafine in Phase 3 development by Celtic Pharma Management L.P. There are also several companies pursuing various devices for onychomycosis, including laser technology. In 2010, a laser device marketed by PinPointe USA, Inc. was cleared by the FDA for onychomycosis. In addition, there are a number of earlier stage therapeutics and devices in various stages of development for the treatment of onychomycosis.
Psoriasis
If approved for the treatment of psoriasis, we anticipate AN2728 and AN2898 would compete with other marketed psoriasis therapeutics including:
- •
- Prescription topical treatments: Several branded products
exist for the topical treatment of mild-to-moderate psoriasis. Taclonex, a combination of calcipotriene and the high potency corticosteroid betamethasone dipropionate, and
Dovonex, also known by its generic name, calcipotriene, are currently marketed by LEO Pharma. Tazorac, also known by its generic name, tazarotene, is currently marketed by Allergan. Vectical, also
known by its generic name, calcitriol, is currently marketed by Galderma. Generic products for the treatment of mild-to-moderate psoriasis include corticosteroids, such as
triamcinolone and betamethasone, as well as the natural product derivative anthralin.
- •
- Systemic treatments: Among the product prescribed for the
treatment of moderate-to-severe psoriasis are the following: oral products, such as methotrexate, cyclosporine and acitretin; injected biologic products, such as Enbrel,
marketed by Amgen, Remicade, marketed by Johnson & Johnson, Stelara and Simponi, marketed by Centocor Ortho Biotech, Amevive, marketed by Astellas, and Humira, marketed by Abbott.
- •
- Other treatments: Various light-based treatments are also used to treat psoriasis, including the 380 nanometer excimer laser and pulsed dye lasers. In addition, there are several non-prescription or over-the-counter topical treatments utilized to treat psoriasis, including salicylic acid and coal tar, as well as bath solutions and moisturizers.
In addition to the marketed psoriasis therapeutics, there are product candidates in Phase 3 development that could potentially be used to treat psoriasis and compete with AN2728 and AN2898, including an injectable therapy from Abbott, and oral therapies from Celgene and Isotechnika. In addition to these, a number of topical, oral and injectable product candidates are in various stages of development for the treatment of psoriasis.
Atopic Dermatitis
If approved for the treatment of atopic dermatitis, we anticipate AN2728 and AN2898 would compete with other marketed atopic dermatitis therapeutics. Current atopic dermatitis treatments attempt to reduce inflammation and itchiness to maintain the protective integrity of the skin. Combinations of antibiotics, antihistamines, topical corticosteroids and topical immunomodulators, such as Elidel (pimecrolimus) and Protopic (tacrolimus), are the current standard of care. In addition, while the use of ultraviolet light is not approved by the FDA, it has been applied to the treatment of atopic dermatitis.
Gram-Negative Infections
If approved for the treatment of infections caused by Gram-negative bacteria, we anticipate GSK '052 would compete with other marketed broad spectrum and Gram-negative antibiotics.
- •
- Marketed antibiotics: There are a variety of marketed broad spectrum as well as Gram-negative antibiotics targeting Gram-negative infections. Current marketed products for Gram-negative
24
- •
- Antibiotics in development: The need for new antibiotics will continue to grow due to evolving resistance to existing antibiotics. There are several earlier stage compounds in clinical development including Forest and AstraZeneca's ceftazidime/NXL-104, which is in Phase 2 development, and Cubist's CXA-201, which is also in Phase 2 development. We expect that GSK '052 will face competition from new antibiotics entering the market to address this ongoing need.
infections include piperacillin/tazobactam (Pfizer's Zosyn/Tazocin), imipenem/cilastatin (Merck's Primaxin/Tienam), meropenem (Cubist Pharmaceuticals/AstraZeneca's Merrem/Meronem), levofloxacin (Johnson & Johnson's Levaquin, sanofi-aventis's Tavanic and Daiichi Sankyo's Cravit) and ciprofloxacin, or cipro, marketed under multiple brand names. Next-generation cephalosporins, carbapenems, quinolones and beta-lactam/beta-lactamase inhibitors, such as Johnson & Johnson's Doribax and Pfizer's Tygacil, are expected to capture significant market share.
Government Regulation
Federal Food, Drug and Cosmetic Act
Prescription drug products are subject to extensive pre- and post-market regulation by the FDA, including regulations that govern the testing, manufacturing, safety, efficacy, labeling, storage, record keeping, advertising and promotion of such products under the Federal Food, Drug and Cosmetic Act, or FFDCA, and its implementing regulations. The process of obtaining FDA approval and achieving and maintaining compliance with applicable laws and regulations requires the expenditure of substantial time and financial resources. Failure to comply with applicable FDA or other requirements may result in refusal to approve pending applications, a clinical hold, warning letters, civil or criminal penalties, recall or seizure of products, partial or total suspension of production or withdrawal of the product from the market. FDA approval is required before any new drug or dosage form, including a new use of a previously approved drug, can be marketed in the United States. All applications for FDA approval must contain, among other things, information relating to safety and efficacy, pharmaceutical formulation, stability, manufacturing, processing, packaging, labeling and quality control.
New Drug Applications (NDAs)
The FDA's new drug approval process generally involves:
- •
- completion of preclinical laboratory and animal safety testing in compliance with the FDA's Good Laboratory Practice, or
GLP, regulations and the International Conference on Harmonisation, or ICH, guidelines;
- •
- submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may
begin in the United States;
- •
- performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the
proposed drug product for each intended use;
- •
- satisfactory completion of an FDA pre-approval inspection of the facility or facilities at which the product
is manufactured to assess compliance with the FDA's cGMP regulations; and
- •
- submission to and approval by the FDA of an NDA.
The preclinical and clinical testing and approval process requires substantial time, effort and financial resources, and we cannot guarantee that any approvals for our product candidates will be granted on a timely basis, if at all. Preclinical tests include laboratory evaluation of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animals. The results of preclinical tests, together with manufacturing information and analytical data, are submitted as part of an IND
25
application to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions about the submission, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can begin. Our submission of an IND may not result in FDA authorization to commence clinical trials. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development. Further, an independent institutional review board, or IRB, covering each medical center proposing to conduct clinical trials must review and approve the plan for any clinical trial before it commences at that center and it must monitor the study until completed. The FDA, the IRB or the Sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. As a separate amendment to an IND, a sponsor may submit a request for a Special Protocol Assessment, or SPA, from the FDA. Under the SPA procedure, a sponsor may seek the FDA's agreement on the design and size of a clinical trial intended to form the primary basis of an effectiveness and safety claim. If the FDA agrees in writing, the study design may not be changed after the trial begins, except in limited circumstances, such as when a substantial scientific issue essential to determining the safety and effectiveness of a product candidate is identified. If the outcome of the trial is successful, the sponsor will ordinarily be able to rely on it as the primary basis for approval with respect to effectiveness and safety. Clinical testing also must satisfy extensive Good Clinical Practice, or GCP, regulations, which include requirements that all research subjects provide informed consent and that all clinical studies be conducted under the supervision of one or more qualified investigators.
For purposes of an NDA submission and approval, human clinical trials are typically conducted in the following sequential phases, which may overlap:
- •
- Phase 1: Trials are initially conducted in a
limited population to test the product candidate for safety, dose tolerance, absorption, metabolism, distribution and excretion, generally in healthy humans.
- •
- Phase 2: Trials are generally conducted in a
limited patient population to identify possible adverse effects and safety risks, to determine the initial efficacy of the product for specific targeted indications and to determine dose tolerance and
optimal dosage. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more extensive Phase 3 clinical trials. In some
cases, a sponsor may decide to run what is referred to as a "Phase 2b" evaluation, which is a second, confirmatory Phase 2 clinical trial that could, if positive and accepted by the FDA,
serve as a pivotal trial in the approval of a product candidate.
- •
- Phase 3: These are commonly referred to as pivotal
studies. When Phase 2 evaluations demonstrate that a dose range of the product is effective and has an acceptable safety profile, Phase 3 clinical trials are undertaken in large patient
populations to further evaluate dosage, to provide substantial evidence of clinical efficacy and to further test for safety in an expanded and diverse patient population at multiple,
geographically-dispersed clinical trial sites. Generally, replicate evidence of safety and effectiveness needs to be demonstrated in two adequate and well-controlled Phase 3
clinical trials of a product candidate for a specific indication. These studies are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product
labeling.
- •
- Phase 4: In some cases, the FDA may condition approval of an NDA for a product candidate on the sponsor's agreement to conduct additional clinical trials to further assess the drug's safety and/or efficacy after NDA approval. Such post-approval trials are typically referred to as Phase 4 clinical trials.
26
Progress reports detailing the results of the clinical studies must be submitted at least annually to the FDA and safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events. Concurrent with clinical studies, sponsors usually complete additional animal safety studies and must also develop additional information about the chemistry and physical characteristics of the product and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final product. Moreover, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
The results of product development, preclinical studies and clinical trials, along with the aforementioned manufacturing information, are submitted to the FDA as part of an NDA. NDA's must also contain extensive manufacturing information. Under the Prescription Drug User Fee Act, or PDUFA, the FDA agrees to specific goals for NDA review time through a two-tiered classification system, Standard Review and Priority Review. Standard Review is applied to a drug that offers at most, only minor improvement over existing marketed therapies. Standard Review NDAs have a goal of being completed within a ten-month timeframe, although a review can take a significantly longer amount of time. A Priority Review designation is given to drugs that offer major advances in treatment, or provide a treatment where no adequate therapy exists. A Priority Review means that the time it takes the FDA to review an NDA is reduced such that the goal for completing a Priority Review initial review cycle is six months. It is likely that our product candidates will be granted Standard Reviews. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA may refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
The FDA may deny approval of an NDA if the applicable regulatory criteria are not satisfied, or it may require additional clinical data or additional pivotal Phase 3 clinical trials. Even if such data are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data from clinical trials are not always conclusive and the FDA may interpret data differently than we do. Once issued, product approval may be withdrawn by the FDA if ongoing regulatory requirements are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require testing, including Phase 4 clinical trials, Risk Evaluation and Mitigation Strategies (REMS) and surveillance programs to monitor the effect of approved products that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved label. Further, if there are any modifications to the drug, including changes in indications, labeling or manufacturing processes or facilities, approval of a new or supplemental NDA may be required, which may involve conducting additional preclinical studies and clinical trials.
Drug Price Competition and Patent Term Restoration Act of 1984
Under the Drug Price Competition and Patent Term Restoration Act of 1984, known as the Hatch-Waxman Amendments, a portion of a product's patent term that was lost during clinical development and application review by the FDA may be restored. The Hatch-Waxman Amendments also provide for a statutory protection, known as nonpatent market exclusivity, against the FDA's acceptance or approval of certain competitor applications.
Patent term restoration can compensate for time lost during product development and the regulatory review process by returning up to five years of patent life for a patent that covers a new
27
product or its use. This period is generally one-half the time between the effective date of an IND (falling after issuance of the patent) and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Patent term restorations, however, cannot extend the remaining term of a patent beyond a total of 14 years. The application for patent term extension is subject to approval by the United States Patent and Trademark Office in conjunction with the FDA. It takes at least six months to obtain approval of the application for patent term extension. Up to five years of interim one year extensions are available if a product is still undergoing development or FDA review at the time of its expiration.
The Hatch-Waxman Amendments also provide for a period of statutory protection for new drugs that receive NDA approval from the FDA. If a new drug receives NDA approval as a new chemical entity, meaning that the FDA has not previously approved any other new drug containing the same active moiety, then the Hatch-Waxman Amendments prohibit submission of an Abbreviated New Drug Application, or ANDA, or a 505(b)(2) NDA (each as described below) by another company for a generic version of such drug, or modification to the previously approved version, with some exceptions, for a period of five years from the date of approval of the NDA. The statutory protection provided pursuant to the Hatch-Waxman Amendments will not prevent the filing or approval of a full NDA. In order to gain approval of a full NDA, however, a competitor would be required to conduct its own preclinical investigations and clinical trials. If NDA approval is received for a new drug containing an active ingredient that was previously approved by the FDA but the NDA is for a drug that includes an innovation over the previously approved drug and if such NDA approval was dependent upon the submission to the FDA of new clinical investigations, other than bioavailability studies, then the Hatch-Waxman Amendments prohibit the FDA from making effective the approval of an ANDA or a 505(b)(2) NDA for a generic version of such drug or modification of the previously approved version for a period of three years from the date of the NDA approval. This three year exclusivity, however, only covers the innovation associated with the NDA to which it attaches. Thus, the three year exclusivity does not prohibit the FDA, with limited exceptions, from approving ANDAs or 505(b)(2) NDAs for drugs containing the same active ingredient but without the new innovation.
While the Hatch-Waxman Amendments provide certain patent term restoration and exclusivity protections to innovator drug manufacturers, it also permits the FDA to approve ANDAs for generic versions of their drugs. The ANDA process permits competitor companies to obtain marketing approval for a drug with the same active ingredient for the same uses but does not require the conduct and submission of clinical trials demonstrating safety and effectiveness for that product. Instead of safety and effectiveness data, an ANDA applicant needs only to submit data demonstrating that its product is bioequivalent to the innovator product as well as relevant chemistry, manufacturing and product data. The Hatch-Waxman Amendments also instituted a third type of drug application that requires the same information as an NDA including full reports of clinical and preclinical studies except that some of the information from the reports required for marketing approval comes from studies that the applicant does not own or for which the applicant does not have a legal right of reference. This type of application, a "505(b)(2) NDA," permits a manufacturer to obtain marketing approval for a drug without needing to conduct or obtain a right of reference for all of the required studies.
Finally, the Hatch-Waxman Amendments require, in some circumstances, an ANDA or a 505(b)(2) NDA applicant to notify the patent owner and the holder of the approved NDA of the factual and legal basis of the applicant's opinion that the patent listed by the holder of the approved NDA in FDA's Approved Drug Products with Therapeutic Equivalence Evaluations manual is not valid or will not be infringed (the patent certification process). Upon receipt of this notice, the patent owner and the NDA holder have 45 days to bring a patent infringement suit in federal district court and obtain a 30-month stay against the company seeking to reference the NDA. The NDA or patent holder could still file a patent suit after the 45 days, but if they did, they would not have the benefit of a 30-month stay. Alternatively, after this 45-day period, the applicant may file a declaratory judgment action,
28
seeking a determination that the patent is invalid or will not be infringed. The discovery, trial and appeals process in such suits can take several years. If such a suit is timely commenced, the Hatch-Waxman Amendments provide a 30-month stay on the approval of the competitor's ANDA or 505(b)(2) NDA. If the litigation is resolved in favor of the competitor or the challenged patent expires during a 30-month stay period, unless otherwise extended by court order, the stay is lifted and the FDA may approve the application. The patent owner and the NDA holder have the opportunity to trigger only a single 30-month stay per ANDA or 505(b)(2) NDA. Once the ANDA or 505(b)(2) NDA applicant has notified the patent owner and the NDA holder of the infringement, the applicant cannot be subjected to another 30-month stay, even if the applicant becomes aware of additional patents that may be infringed by its product.
International Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our future products. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Under European Union regulatory systems, marketing authorizations may be submitted either under a centralized or a mutual recognition procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The mutual recognition procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may submit an application to the remaining member states. Within 90 days of receiving the applications and assessment report, each member state must decide whether to recognize approval.
Third-Party Payor Coverage and Reimbursement
Although none of our product candidates has been commercialized for any indication, if they are approved for marketing, commercial success of our product candidates will depend, in part, upon the availability of coverage and reimbursement from third-party payors at the federal, state and private levels. Government payor programs, including Medicare and Medicaid, private health care insurance companies and managed-care plans have attempted to control costs by limiting coverage and the amount of reimbursement for particular drug treatments. The U.S. Congress and state legislatures from time to time propose and adopt initiatives aimed at cost-containment. Ongoing federal and state government initiatives directed at lowering the total cost of health care will likely continue to focus on health care reform, the cost of prescription pharmaceuticals and on the reform of the Medicare and Medicaid payment systems. Examples of how limits on drug coverage and reimbursement in the United States may cause reduced payments for drugs in the future include:
- •
- changing Medicare reimbursement methodologies;
- •
- fluctuating decisions on which drugs to include in formularies;
- •
- revising drug rebate calculations under the Medicaid program; and
- •
- reforming drug importation laws.
Indeed, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively, the Healthcare Reform Act, which was recently signed into law in March of 2010, substantially changes the way health care is financed by both
29
governmental and private insurers, and significantly impacts pharmaceutical manufacturers. The Healthcare Reform Act includes, among other things, the following measures:
- •
- Annual, non-deductible fees on any entity that manufactures or imports certain prescription drugs;
- •
- Increases in Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program for both branded and generic
drugs;
- •
- A new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical
effectiveness research;
- •
- New requirements for manufacturers to discount drug prices to eligible patients by 50 percent at the pharmacy level
and for mail order services in order for their outpatient drugs to be covered under Medicare Part D; and
- •
- An increase in the number of entities eligible for discounts under the Public Health Service pharmaceutical pricing program.
Additionally, some third-party payors also require pre-approval of coverage for new or innovative drug therapies before they will reimburse health care providers who use such therapies. While we cannot predict whether any proposed cost-containment measures will be adopted or otherwise implemented in the future, the announcement or adoption of these proposals could have a material adverse effect on our ability to obtain adequate prices for our product candidates and operate profitably.
Manufacturing Requirements
We rely, and expect to continue to rely, on third parties for the production of clinical and eventually, commercial, quantities of our products. We and our third-party manufacturers must comply with applicable FDA regulations relating to FDA's cGMP regulations. The cGMP regulations include requirements relating to organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, records and reports and returned or salvaged products. The manufacturing facilities for our products must meet cGMP requirements to the satisfaction of the FDA pursuant to a pre-approval inspection before we can use them to manufacture our products. We and our third-party manufacturers are also subject to periodic inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including warning letters, the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations and civil and criminal penalties. Adverse experiences with the product must be reported to the FDA and could result in the imposition of market restriction through labeling changes or in product removal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following approval.
Other Regulatory Requirements
With respect to post-market product advertising and promotion, the FDA imposes a number of complex regulations on entities that advertise and promote pharmaceuticals, which include, among others, standards for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. The FDA has very broad enforcement authority under the FFDCA, and failure to abide by these regulations can result in penalties, including the issuance of a warning letter directing entities to correct deviations from FDA
30
standards, a requirement that future advertising and promotional materials be pre-cleared by the FDA and state and federal civil and criminal investigations and prosecutions.
We are also subject to various laws and regulations regarding laboratory practices, the experimental use of animals and the use and disposal of hazardous or potentially hazardous substances in connection with our research. In each of these areas, as above, the FDA has broad regulatory and enforcement powers, including the ability to levy fines and civil penalties, suspend or delay issuance of approvals, seize or recall products and withdraw approvals, any one or more of which could have a material adverse effect on us.
Legal Proceedings
We are not currently a party to any material legal proceedings.
Employees
As of December 31, 2010, we had 65 full-time employees, 29 of whom held Ph.D. or M.D. degrees and 53 of whom were engaged in full-time research and development activities. We plan to continue to expand our product development programs. To support this growth and to support public company requirements, we will need to expand our managerial, development, finance and other functions. None of our employees are represented by a labor union and we consider our employee relations to be good.
Facilities
Our corporate headquarters are located in Palo Alto, California, where we lease a 36,960 square-foot building with laboratory and office space. The lease will terminate in March 2018.
We also lease approximately 15,300 square feet of laboratory and office space in another building in Palo Alto, California under a lease agreement that terminates in December 2011. We may terminate the lease by providing four months' written notice.
Corporate and Available Information
Our principal corporate offices are located at 1020 East Meadow Circle, Palo Alto, California 94303-4230 and our telephone number is (650) 543-7600. We were incorporated in Delaware in December 2000 and began business operations in March 2002. Our internet address is www.anacor.com. We make available on our website, free of charge, our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission, or the SEC. Our SEC reports can be accessed through the Investors section of our internet website. Further, a copy of this Annual Report on Form 10-K is located at the SEC's Public Reference Rooms at 100 F Street, N.E., Washington, D. C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains reports, proxy and information statements and other information regarding our filings at http://www.sec.gov. The information found on our internet website is not incorporated by reference into this Annual Report on Form 10-K or any other report we file with or furnish to the SEC.
31
Investing in our common stock involves a high degree of risk. You should carefully consider the following risk factors, as well as the other information in this Annual Report on Form 10-K, before deciding whether to invest in shares of our common stock. The occurrence of any of the following adverse developments described in the following risk factors could harm our business, financial condition, results of operations or prospects. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Relating to Our Financial Position and Need for Additional Capital
We have never been profitable. Currently, we have no products approved for commercial sale, and to date we have not generated any revenue from product sales. As a result, our ability to curtail our losses and reach profitability is unproven, and we may never achieve or sustain profitability.
We are not profitable and do not expect to be profitable in the foreseeable future. We have incurred net losses in each year since our inception, including net losses of approximately $21.6 million, $24.8 million and $10.1 million for 2008, 2009 and 2010, respectively, and as of December 31, 2010, we had an accumulated deficit of approximately $111.2 million. We have devoted most of our financial resources to research and development, including our preclinical development activities and clinical trials. We have not completed development of any product candidate and we have therefore not generated any revenues from product sales. We expect to incur increased expenses as we continue Phase 3 clinical trials of AN2690, commence Phase 3 clinical trials of AN2728, advance our other product candidates and expand our research and development programs. We also expect an increase in our expenses associated with preparing for commercialization of our product candidates and creating additional infrastructure to support operations as a public company. As a result of the foregoing, we expect to continue to experience net losses and negative cash flows for the foreseeable future. These losses and negative cash flows have had, and will continue to have, an adverse effect on our stockholders' equity and working capital.
Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve or maintain profitability. In addition, our expenses could increase more than currently anticipated if we are required by the United States Food and Drug Administration, or FDA, to perform studies in addition to those that we currently expect. To date, we have financed our operations primarily through the sale of equity securities, debt arrangements, government contracts and grants and the payments under our agreements with GlaxoSmithKline LLC, or GSK, Schering Corporation, or Schering, Eli Lilly and Company, or Lilly, and Medicis Pharmaceutical Corporation, or Medicis. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. Revenues from our collaboration with GSK are uncertain because GSK may not continue to develop GSK2251052, or GSK '052 (formerly known as AN3365) the product candidate recently licensed by GSK, milestones under our agreements with GSK may not be achieved, GSK may not exercise its option to license additional product candidates that may be identified pursuant to our collaboration with them, these product candidates may not receive regulatory approval or, if they are approved, such product candidates may not be accepted in the market. In addition, we may not be able to enter into other collaborations that will generate significant cash. If we are unable to develop and commercialize one or more of our product candidates, or if revenues from any product candidate that receives marketing approval are insufficient, we will not achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability.
32
We have a limited operating history and we expect a number of factors to cause our operating results to fluctuate on a quarterly and annual basis, which may make it difficult to predict our future performance.
Our operations to date have been primarily limited to developing our technology and undertaking preclinical studies and clinical trials of our product candidates. We have not yet obtained regulatory approvals for any of our product candidates. Consequently, any predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history and/or approved products on the market. Our financial condition and operating results have varied significantly in the past and will continue to fluctuate from quarter-to-quarter or year-to-year due to a variety of factors, many of which are beyond our control. Factors relating to our business that may contribute to these fluctuations include the following risk factors, as well as other factors described elsewhere in this Annual Report on Form 10-K:
- •
- our ability to obtain additional funding to develop our product candidates;
- •
- the need to obtain and maintain regulatory approval for AN2690, AN2728, AN2898, GSK '052 or any of our other product
candidates;
- •
- delays in the commencement, enrollment and the timing of clinical testing;
- •
- the success of our clinical trials through all phases of clinical development, including our Phase 3 clinical
trials of AN2690 and AN2728;
- •
- any delays in regulatory review and approval of product candidates in clinical development;
- •
- potential side effects of our product candidates that could delay or prevent commercialization or cause an approved drug
to be taken off the market;
- •
- our ability to develop systemic product candidates;
- •
- market acceptance of our product candidates;
- •
- our ability to establish an effective sales and marketing infrastructure;
- •
- competition from existing products or new products that may emerge;
- •
- the ability of patients or healthcare providers to obtain coverage of or sufficient reimbursement for our products;
- •
- our ability to receive approval and commercialize our product candidates outside of the United States;
- •
- our dependency on third-party manufacturers to supply or manufacture our products;
- •
- our ability to establish or maintain collaborations, licensing or other arrangements;
- •
- our ability and third parties' abilities to protect intellectual property rights;
- •
- costs related to and outcomes of potential intellectual property litigation;
- •
- our ability to adequately support future growth;
- •
- our ability to attract and retain key personnel to manage our business effectively;
- •
- our ability to build our finance infrastructure and improve our accounting systems and controls;
- •
- potential product liability claims;
- •
- potential liabilities associated with hazardous materials; and
- •
- our ability to maintain adequate insurance policies.
33
Due to the various factors mentioned above, and others, the results of any quarterly or annual periods should not be relied upon as indications of future operating performance.
If we are unable to raise capital when needed, we would be forced to delay, reduce or eliminate our product development programs.
Developing pharmaceutical products, including conducting preclinical studies and clinical trials, is expensive. We expect our research and development expenses to increase in connection with our ongoing activities, particularly our Phase 3 clinical trials of AN2690 and AN2728. If the FDA requires that we perform additional studies beyond those that we currently expect, our expenses could increase beyond what we currently anticipate and the timing of any potential product approval may be delayed. Under the GSK agreement, we are required to use diligent efforts to identify and optimize product candidates and to provide certain numbers of personnel and other resources under drug development projects. We currently have no commitments or arrangements for any additional financing to fund our research and development programs other than through our loan facility, research funding under our collaboration with Lilly, reimbursements from our various collaborations with leading not-for-profit organizations related to our neglected diseases initiatives and contingent milestone or royalty payments from GSK, Lilly or Medicis, which we may not receive. We believe our existing cash, cash equivalents and short-term investments and interest thereon will be sufficient to fund our projected operating requirements for at least the next 12 months. However, we may need to raise substantial additional capital in the future to complete the development and commercialization of our product candidates.
Until we can generate a sufficient amount of revenue from our products, if ever, we expect to finance future cash needs through public or private equity offerings, debt financings or corporate collaborations and licensing arrangements. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available, we may be required to delay, reduce the scope of or eliminate one or more of our research or development programs or our commercialization efforts. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience additional dilution, and debt financing, if available, may involve restrictive covenants. To the extent that we raise additional funds through collaborations and licensing arrangements, it may be necessary to relinquish some rights to our technologies or our product candidates or grant licenses on terms that may not be favorable to us. We may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time.
Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed elsewhere in this "Risk Factors" section. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect.
Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to:
- •
- the initiation, progress, timing, costs and results of preclinical studies and clinical trials for our product candidates
and potential product candidates, including initiation and conduct of Phase 3 clinical trials for AN2690 and AN2728;
- •
- the success of our collaborations with GSK, Lilly and Medicis and the attainment of milestones and royalty payments, if
any, under those agreements;
- •
- the number and characteristics of product candidates that we pursue;
- •
- the terms and timing of any future collaboration, licensing or other arrangements that we may establish;
34
- •
- the outcome, timing and cost of regulatory approvals;
- •
- the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
- •
- the effect of competing technological and market developments;
- •
- the cost and timing of completion of commercial-scale outsourced manufacturing activities;
- •
- the cost of establishing sales, marketing and distribution capabilities for any product candidates for which we may
receive regulatory approval; and
- •
- the extent to which we acquire or invest in businesses, products or technologies.
Raising funds through lending arrangements may restrict our operations or produce other adverse results.
Our current loan and security agreement with Oxford Finance Corporation and Horizon Technology Finance Corporation, which we entered into in March 2011, contains a variety of affirmative and negative covenants, including required financial reporting, limitations on certain dispositions of assets, limitations on the incurrence of additional debt and other requirements. To secure our performance of our obligations under this loan and security agreement, we granted a security interest in substantially all of our assets, other than intellectual property assets, to the lenders. Our failure to comply with the covenants in the loan and security agreement, the occurrence of a material impairment in our prospect of repayment or in the perfection or priority of the lender's lien on our assets, as determined by the lenders, or the occurrence of certain other specified events could result in an event of default that, if not cured or waived, could result in the acceleration of all or a substantial portion of our debt, potential foreclosure on our assets, and other adverse results.
Risks Relating to the Development, Regulatory Approval and Commercialization of Our Product Candidates
We cannot be certain that AN2690, AN2728, AN2898, GSK '052 or any of our other product candidates will receive regulatory approval, and without regulatory approval our product candidates will not be able to be marketed.
We have invested a significant portion of our efforts and financial resources in the development of our most advanced product candidates, especially AN2690. Our ability to generate revenue related to product sales, which we do not expect will occur for at least the next several years, if ever, will depend on the successful development and regulatory approval of our product candidates.
In August 2010, we filed a Special Protocol Assessment request with the FDA in order to reach agreement on key endpoint measures and trial design to be used in our first of two identical planned Phase 3 clinical trials of AN2690. We have received the FDA's agreement on what we believe are the major parameters associated with the design and conduct of our first Phase 3 trial for AN2690, and we commenced Phase 3 clinical trials in the fourth quarter of 2010. We may conduct lengthy and expensive Phase 3 clinical trials of AN2690 only to learn that this drug candidate is not a safe or effective treatment, in which case these clinical trials may not lead to regulatory approval for AN2690. Similarly, our clinical development programs for AN2728, AN2898 and our other product candidates and GSK's development programs for GSK '052 may not lead to regulatory approval from the FDA and similar foreign regulatory agencies. This failure to obtain regulatory approvals would prevent our product candidates from being marketed and would have a material and adverse effect on our business.
We currently have no products approved for sale and we cannot guarantee that we will ever have marketable products. The development of a product candidate, including preclinical and clinical testing, manufacturing, quality systems, labeling, approval, record-keeping, selling, promotion, marketing and distribution of products, is subject to extensive regulation by the FDA in the United States and
35
regulatory authorities in other countries, with regulations differing from country to country. We are not permitted to market our product candidates in the United States until we receive approval of a new drug application, or NDA, from the FDA. We have not submitted an NDA for any of our product candidates. Obtaining approval of an NDA is a lengthy, expensive and uncertain process. An NDA must include extensive preclinical and clinical data and supporting information to establish the product candidate's safety and effectiveness for each indication. The approval application must also include significant information regarding the chemistry, manufacturing and controls for the product. The FDA review process typically takes years to complete and approval is never guaranteed. If a product is approved, the FDA may limit the indications for which the product may be used, include extensive warnings on the product labeling or require costly ongoing requirements for post-marketing clinical studies and surveillance or other risk management measures to monitor the safety or efficacy of the product candidate. Markets outside of the United States also have requirements for approval of drug candidates with which we must comply prior to marketing. Obtaining regulatory approval for marketing of a product candidate in one country does not ensure we will be able to obtain regulatory approval in other countries but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in other countries. Also, any regulatory approval of any of our products or product candidates, once obtained, may be withdrawn. If AN2690, AN2728, AN2898, GSK '052 or any of our other product candidates do not receive regulatory approval, we may not be able to generate sufficient revenue to become profitable or to continue our operations.
Delays in the commencement, enrollment and completion of clinical trials could result in increased costs to us and delay or limit our ability to obtain regulatory approval for our product candidates.
Delays in the commencement, enrollment and completion of clinical trials could increase our product development costs or limit the regulatory approval of our product candidates. We do not know whether clinical trials of AN2690, AN2728, AN2898, GSK '052 or other product candidates will begin on time or, if commenced, will be completed on schedule or at all. The commencement, enrollment and completion of clinical trials can be delayed for a variety of reasons, including:
- •
- inability to reach agreements on acceptable terms with prospective clinical research organizations, or CROs, and trial
sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
- •
- regulatory objections to commencing a clinical trial;
- •
- inability to identify and maintain a sufficient number of trial sites, many of which may already be engaged in other
clinical trial programs, including some that may be for the same indication as our product candidates;
- •
- withdrawal of clinical trial sites from our clinical trials as a result of changing standards of care or the ineligibility
of a site to participate in our clinical trials;
- •
- inability to obtain institutional review board, or IRB, approval to conduct a clinical trial at prospective sites;
- •
- difficulty recruiting and enrolling patients to participate in clinical trials for a variety of reasons, including meeting
the enrollment criteria for our study and competition from other clinical trial programs for the same indication as our product candidates; and
- •
- inability to retain patients in clinical trials due to the treatment protocol, personal issues, side effects from the therapy or lack of efficacy, particularly for those patients receiving either a vehicle without the active ingredient or a placebo. For example, our Phase 3 clinical trials of AN2690, commenced in December 2010, have a treatment duration of 48 weeks, and it may be difficult to retain patients for this entire period.
36
In addition, a clinical trial may be suspended or terminated by us, our current or any future partners, the FDA or other regulatory authorities due to a number of factors, including:
- •
- failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols;
- •
- failed inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities;
- •
- unforeseen safety issues or any determination that a clinical trial presents unacceptable health risks; or
- •
- lack of adequate funding to continue the clinical trial due to unforeseen costs resulting from enrollment delays, requirements to conduct additional trials and studies, increased expenses associated with the services of our CROs and other third parties or other reasons.
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those currently contemplated, we may be delayed in obtaining, or may not be able to obtain, marketing approval for these product candidates. In addition, if our current or any future partners assume development of our product candidates, they may suspend or terminate their development and commercialization efforts, including clinical trials for our product candidates, at any time. For example, with the license of GSK '052, GSK now controls the development and commercialization of GSK '052.
Changes in regulatory requirements and guidance may occur and we or any partners may need to amend clinical trial protocols to reflect these changes with appropriate regulatory authorities. Amendments may require us or any partners to resubmit clinical trial protocols to IRBs for re-examination, which may impact the costs, timing or successful completion of a clinical trial. If we or any of our partners experience delays in the completion of, or if we or our partners terminate, clinical trials, the commercial prospects for our product candidates will be harmed, and our ability to generate revenue from sales of our products will be prevented or delayed. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate.
Clinical failure can occur at any stage of clinical development. Because the results of earlier clinical trials are not necessarily predictive of future results, any product candidate we, GSK or our potential future partners advance through clinical trials may not have favorable results in later clinical trials or receive regulatory approval.
Clinical failure can occur at any stage of our clinical development. Clinical trials may produce negative or inconclusive results, and we or our partners may decide, or regulators may require us, to conduct additional clinical or preclinical testing. In addition, data obtained from tests are susceptible to varying interpretations, and regulators may not interpret our data as favorable as we do, which may delay, limit or prevent regulatory approval. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate. Frequently, product candidates that have shown promising results in early clinical trials have subsequently suffered significant setbacks in later clinical trials. In addition, the design of a clinical trial can determine whether its results will support approval of a product and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We have limited experience in designing clinical trials and may be unable to design and execute a clinical trial to support regulatory approval. Further, clinical trials of potential products often reveal that it is not practical or feasible to continue development efforts. If AN2690, AN2728, AN2898, GSK '052 or our other product candidates are found to be unsafe or lack efficacy, we will not be able to obtain regulatory approval for them and our business would be harmed. For example, if the results of our current Phase 3 clinical trials of AN2690 and planned Phase 3 clinical
37
trials of AN2728 do not achieve the primary efficacy endpoints or demonstrate an acceptable safety level, the prospects for approval of AN2690 and AN2728 would be materially and adversely affected. A number of companies in the pharmaceutical industry, including those with greater resources and experience than us, have suffered significant setbacks in Phase 3 clinical trials, even after seeing promising results in earlier clinical trials.
In some instances, there can be significant variability in safety and/or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, adherence to the dosing regimen, particularly for self-administered topical drugs, and other trial protocols and the rate of dropout among clinical trial participants. We do not know whether any Phase 2, Phase 3 or other clinical trials we or any partners may conduct will demonstrate consistent and/or adequate efficacy and safety to obtain regulatory approval to market our product candidates.
We have never conducted a Phase 3 clinical trial or submitted an NDA before, and may be unable to do so for AN2690, AN2728 and other product candidates we are developing.
We are conducting the Phase 3 clinical trials of AN2690 and plan to conduct the Phase 3 clinical trials of AN2728. The conduct of Phase 3 clinical trials and the submission of a successful NDA is a complicated process. We have not conducted a Phase 3 clinical trial before, have limited experience in preparing, submitting and prosecuting regulatory filings, and have not submitted an NDA before. Consequently, we may be unable to successfully and efficiently execute and complete these planned clinical trials in a way that leads to NDA submission and approval of AN2690, AN2728 and other product candidates we are developing. We may require more time and incur greater costs than our competitors and may not succeed in obtaining regulatory approvals of products that we develop. Failure to commence or complete, or delays in, our planned clinical trials, would prevent us from or delay us in commercializing AN2690, AN2728 and other product candidates we are developing.
Our product candidates may have undesirable side effects which may delay or prevent marketing approval, or, if approval is received, require them to be taken off the market or otherwise limit their sales.
Unforeseen side effects from any of our product candidates could arise either during clinical development or, if approved, after the approved product has been marketed. For example, a small number of patients who received AN2690 treatment experienced some skin irritation around their toenails during clinical trials of AN2690 for onychomycosis. In addition, a small number of patients who received AN2728 treatment experienced some skin irritation during clinical trials of AN2728 for psoriasis, and one serious adverse event was reported in a patient who developed a rash after receiving an injection of penicillin outside the trial for a sore throat and needed to be hospitalized for this non-life threatening reaction. GSK '052 is being developed for the systemic treatment of infections caused by Gram-negative bacteria and is still in the early stages of clinical development. The range and potential severity of possible side effects from systemic therapies is greater than for topically administered drugs. The results of future clinical trials may show that our product candidates cause undesirable or unacceptable side effects, which could interrupt, delay or halt clinical trials, resulting in delay of, or failure to obtain, marketing approval from the FDA and other regulatory authorities.
If any of our product candidates receives marketing approval and we or others later identify undesirable or unacceptable side effects caused by such products:
- •
- regulatory authorities may require the addition of labeling statements, specific warnings, a contraindication or field
alerts to physicians and pharmacies;
- •
- we may be required to change the way the product is administered, conduct additional clinical trials or change the labeling of the product;
38
- •
- we may have limitations on how we promote the product;
- •
- sales of the product may decrease significantly;
- •
- regulatory authorities may require us to take our approved product off the market;
- •
- we may be subject to litigation or product liability claims; and
- •
- our reputation may suffer.
Any of these events could prevent us, GSK or our potential future partners from achieving or maintaining market acceptance of the affected product or could substantially increase commercialization costs and expenses, which in turn could delay or prevent us from generating significant revenues from the sale of our products.
All of our product candidates require regulatory review and approval prior to commercialization. Any delay in the regulatory review or approval of any of our product candidates will harm our business.
All of our product candidates require regulatory review and approval prior to commercialization. Any delays in the regulatory review or approval of our product candidates would delay market launch, increase our cash requirements and result in additional operating losses.
The process of obtaining FDA and other required regulatory approvals, including foreign approvals, often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. Furthermore, this approval process is extremely complex, expensive and uncertain. We, GSK or our potential future partners may be unable to submit any NDA in the United States or any marketing approval application or other foreign applications for any of our products. If we or our partners submit any NDA, including any amended NDA or supplemental NDA, to the FDA seeking marketing approval for any of our product candidates, the FDA must decide whether to either accept or reject the submission for filing. We cannot be certain that any of these submissions will be accepted for filing and reviewed by the FDA, or that the marketing approval application submissions to any other regulatory authorities will be accepted for filing and review by those authorities. We cannot be certain that we or our partners will be able to respond to any regulatory requests during the review period in a timely manner without delaying potential regulatory action. We also cannot be certain that any of our product candidates will receive favorable recommendations from any FDA advisory committee or foreign regulatory bodies or be approved for marketing by the FDA or foreign regulatory authorities. In addition, delays in approvals or rejections of marketing applications may be based upon many factors, including regulatory requests for additional analyses, reports, data and studies, regulatory questions regarding data and results, changes in regulatory policy during the period of product development and the emergence of new information regarding our product candidates or other products.
Data obtained from preclinical studies and clinical trials are subject to different interpretations, which could delay, limit or prevent regulatory review or approval of any of our product candidates. In addition, as a routine part of the evaluation of any potential drug, clinical trials are generally conducted to assess the potential for drug-to-drug interactions that could impact potential product safety. To date, we have not been requested to perform drug-to-drug interaction studies on our topical product candidates, but any such request, which would be more typical with a systemic product candidate, may delay any potential product approval and may increase the expenses associated with clinical programs. Furthermore, regulatory attitudes towards the data and results required to demonstrate safety and efficacy can change over time and can be affected by many factors, such as the emergence of new information, including on other products, policy changes and agency funding, staffing and leadership. We do not know whether future changes to the regulatory environment will be favorable or unfavorable to our business prospects.
39
In addition, the environment in which our regulatory submissions may be reviewed changes over time. For example, average review times at the FDA for NDAs have fluctuated over the last ten years, and we cannot predict the review time for any of our submissions with any regulatory authorities. Review times can be affected by a variety of factors, including budget and funding levels and statutory, regulatory and policy changes. Moreover, in light of widely publicized events concerning the safety risk of certain drug products, regulatory authorities, members of Congress, the Government Accounting Office, medical professionals and the general public have raised concerns about potential drug safety issues. These events have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products and establishment of risk evaluation and mitigation strategies, or REMS, that may, for instance, restrict distribution of drug products. The increased attention to drug safety issues may result in a more cautious approach by the FDA to clinical trials. Data from clinical trials may receive greater scrutiny with respect to safety, which may make the FDA or other regulatory authorities more likely to terminate clinical trials before completion, or require longer or additional clinical trials that may result in substantial additional expense and a delay or failure in obtaining approval or may result in approval for a more limited indication than originally sought.
Our use of boron chemistry to develop pharmaceutical product candidates is novel and may not prove successful in producing approved products. Undesirable side effects of any of our product candidates, or of boron-based drugs developed by others, may extend the time period required to obtain regulatory approval or harm market acceptance of our product candidates, if approved.
All of our product development activities are centered around compounds containing boron. The use of boron chemistry to develop new drugs is largely unproven. If boron-based compounds developed by us or others have significant adverse side effects, regulatory authorities could require additional studies of our boron-based compounds, which could delay the timing of and increase the cost for regulatory approvals of our product candidates. Additionally, adverse side effects for other boron-based compounds could affect the willingness of third-party payors and medical providers to provide reimbursement for or use our boron-based drugs and could impact market acceptance of our products.
Additionally, there can be no assurance that boron-based products will be free of significant adverse side effects. During clinical trials, a small number of our patients who received AN2690 experienced some skin irritation around their toenails and a few patients who received AN2728 experienced some skin irritation around their psoriasis plaques. In addition, during the course of a clinical trial of AN2728 for psoriasis, one serious adverse event was reported in a patient who developed a rash after receiving an injection of penicillin outside of the trial for a sore throat and needed to be hospitalized for this non-life-threatening reaction. If boron-based drug treatments result in significant adverse side effects, they may not be useful as therapeutic agents. If we are unable to develop products that are safe and effective using our boron chemistry platform, our business will be materially and adversely affected.
If any of our product candidates for which we receive regulatory approval do not achieve broad market acceptance, the revenues that are generated from their sales will be limited.
The commercial success of AN2690, AN2728, AN2898, GSK '052 or our other product candidates will depend upon the acceptance of these products among physicians, patients and the medical community. The degree of market acceptance of our product candidates will depend on a number of factors, including:
- •
- limitations or warnings contained in a product's FDA-approved labeling;
- •
- changes in the standard of care for the targeted indications for any of our product candidates;
- •
- limitations in the approved indications for our product candidates;
40
- •
- lower demonstrated clinical safety and efficacy compared to other products;
- •
- lack of significant adverse side effects;
- •
- ineffective sales, marketing and distribution support;
- •
- lack of availability of reimbursement from managed care plans and other third-party payors;
- •
- timing of market introduction and perceived effectiveness of competitive products;
- •
- lack of cost-effectiveness;
- •
- availability of alternative therapies at similar or lower cost, including generics and
over-the-counter products;
- •
- adverse publicity about our product candidates or favorable publicity about competitive products;
- •
- convenience and ease of administration of our products; and
- •
- potential product liability claims.
If our product candidates are approved, but do not achieve an adequate level of acceptance by physicians, health care payors and patients, sufficient revenue may not be generated from these products, and we may not become or remain profitable. In addition, efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never be successful.
We have never marketed a drug before, and if we are unable to establish an effective sales force and marketing infrastructure, or enter into acceptable third-party sales and marketing or licensing arrangements, we may not be able to commercialize our product candidates successfully.
We plan to develop a sales and marketing infrastructure to market and sell our products in certain U.S. specialty markets. We currently do not have any sales, distribution and marketing capabilities, the development of which will require substantial resources and will be time consuming. These costs may be incurred in advance of any approval of our product candidates. In addition, we may not be able to hire a sales force in the United States that is sufficient in size or has adequate expertise in the medical markets that we intend to target. If we are unable to establish our sales force and marketing capability, our operating results may be adversely affected. In addition, we plan to enter into sales and marketing or licensing arrangements with third parties for non-specialty markets in the United States and for international sales of any approved products. If we are unable to enter into any such arrangements on acceptable terms, or at all, we may be unable to market and sell our products in these markets.
We expect that our existing and future product candidates will face competition and most of our competitors have significantly greater resources than we do.
The pharmaceutical industry is highly competitive, with a number of established, large pharmaceutical companies, as well as many smaller companies. Most of these companies have significant financial resources, marketing capabilities and experience in obtaining regulatory approvals for product candidates. There are many pharmaceutical companies, biotechnology companies, public and private universities, government agencies and research organizations actively engaged in research and development of products that may target the same markets as our product candidates. We expect any future products we develop to compete on the basis of, among other things, product efficacy, price, lack of significant adverse side effects and convenience and ease of treatment.
Compared to us, many of our potential competitors have substantially greater:
- •
- resources, including capital, personnel and technology;
41
- •
- research and development capability;
- •
- clinical trial expertise;
- •
- regulatory expertise;
- •
- intellectual property portfolios;
- •
- expertise in prosecution of intellectual property rights;
- •
- manufacturing and distribution expertise; and
- •
- sales and marketing expertise.
As a result of these factors, our competitors may obtain regulatory approval of their products more rapidly than we are able to or may obtain patent protection or other intellectual property rights that limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are more effective, more widely used and less costly than ours and may also be more successful than us in manufacturing and marketing their products.
The dermatology market is competitive, which may adversely affect our ability to commercialize our dermatological product candidates.
If AN2690 is approved for the treatment of onychomycosis, we anticipate that it would compete with other marketed nail fungal therapeutics including Lamisil, Sporanox, Penlac and generic versions of those compounds. AN2690 will also compete against over-the-counter products and possibly various devices under development for onychomycosis. If approved for the treatment of psoriasis, AN2728 will compete against a number of approved topical treatments, including Taclonex (a combination of calcipotriene and the high potency corticosteroid betamethasone dipropionate), Dovonex (calcipotriene), Tazorac (tazarotene) and generic versions, where available. AN2728 would also compete against systemic treatments for psoriasis, which include oral products such as methotrexate and cyclosporine and injected biologic products such as Enbrel, Remicade, Stelara, Simponi, Amevive, and Humira. A number of other treatments are used for psoriasis, including light based treatments and non-prescription topical treatments. In atopic dermatitis, our product candidates, if approved, would compete with other marketed atopic dermatitis therapeutics, which typically include combinations of antibiotics, antihistamines, topical corticosteroids and topical immunomodulators, such as Elidel (pimecrolimus) and Protopic (tacrolimus).
Even if a generic product or an over-the-counter product is less effective than our product candidates, a less effective generic or over-the-counter product may be more quickly adopted by health insurers and patients than our competing product candidates based upon cost or convenience. In addition, each of our product candidates may compete against product candidates currently under development by other companies.
Reimbursement decisions by third-party payors may have an adverse effect on pricing and market acceptance. If there is not sufficient reimbursement for our products, it is less likely that our products will be widely used.
Successful commercialization of pharmaceutical products usually depends on the availability of adequate coverage and reimbursement from third-party payors. Patients or healthcare providers who purchase drugs generally rely on third-party payors to reimburse all or part of the costs associated with such products. Adequate coverage and reimbursement from governmental payors, such as Medicare and Medicaid, and commercial payors, such as HMOs and insurance companies, can be central to new product acceptance.
Current treatments for onychomycosis are often not reimbursed by third-party payors. We do not know the extent to which AN2690 will be reimbursed if it is approved. Reimbursement decisions by
42
third-party payors may have an effect on pricing and market acceptance. Our other product candidates, such as AN2728 and GSK '052, will also be subject to uncertain reimbursement decisions by third-party payors. Our products are less likely to be used if they do not receive adequate reimbursement.
The market for our product candidates may depend on access to third-party payors' drug formularies, or lists of medications for which third-party payors provide coverage and reimbursement. Industry competition to be included in such formularies results in downward pricing pressures on pharmaceutical companies. Third-party payors may refuse to include a particular branded drug in their formularies when a competing generic product is available.
All third-party payors, whether governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. In addition, in the United States, no uniform policy of coverage and reimbursement for medicines exists among all these payors. Therefore, coverage of and reimbursement for drugs can differ significantly from payor to payor and can be difficult and costly to obtain.
Healthcare policy changes, including the Healthcare Reform Act, may have a material adverse effect on us.
Healthcare costs have risen significantly over the past decade. On March 23, 2010, the President signed one of the most significant healthcare reform measures in decades. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively, the Healthcare Reform Act, substantially changes the way healthcare is financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. The Healthcare Reform Act contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud and abuse, which will impact existing government healthcare programs and will result in the development of new programs, including Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program. We anticipate that if we obtain approval for our products, some of our revenue may be derived from U.S. government healthcare programs, including Medicare. Furthermore, beginning in 2011, the Healthcare Reform Act imposed a non-deductible excise tax on pharmaceutical manufacturers or importers who sell "branded prescription drugs," which includes innovator drugs and biologics (excluding orphan drugs or generics) to U.S. government programs. We expect that the Healthcare Reform Act and other healthcare reform measures that may be adopted in the future could have a material adverse effect on our industry generally and our ability to successfully commercialize our products or could limit or eliminate our spending on development projects.
In addition to the Healthcare Reform Act, there will continue to be proposals by legislators at both the federal and state levels, regulators and third-party payors to keep these costs down while expanding individual healthcare benefits. Certain of these changes could impose limitations on the prices we will be able to charge for any products that are approved or the amounts of reimbursement available for these products from governmental agencies or third-party payors or may increase the tax requirements for life sciences companies such as ours. While it is too early to predict what effect the recently enacted Healthcare Reform Act or any future legislation or regulation will have on us, such laws could have a material adverse effect on our business, financial position and results of operations.
We expect that a substantial portion of the market for our products will be outside the United States. Our product candidates may never receive approval or be commercialized outside of the United States.
We plan to enter into sales and marketing arrangements with third parties for international sales of any approved products. To market and commercialize any product candidates outside of the United States, we, GSK or any other third parties that are marketing or selling our products must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and
43
additional administrative review periods. The regulatory approval process in other countries may include all of the risks detailed above regarding failure to obtain FDA approval in the United States as well as other risks. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. Failure to obtain regulatory approval in other countries or any delay or setback in obtaining such approval could have the same adverse effects detailed above regarding FDA approval in the United States. As described above, such effects include the risks that our product candidates may not be approved for all indications requested, or at all, which could limit the uses of our product candidates and have an adverse effect on product sales and potential royalties, and that such approval may be subject to limitations on the indicated uses for which the product may be marketed or require costly post-marketing follow-up studies.
Even if our product candidates receive regulatory approval, we may still face future development and regulatory difficulties.
Even if regulatory approval is obtained for any of our product candidates, regulatory authorities may still impose significant restrictions on a product's indicated uses or marketing or impose ongoing requirements for potentially costly post-approval studies. Given the number of high profile adverse safety events with certain drug products, regulatory authorities may require, as a condition of approval, costly risk evaluation and mitigation strategies, which may include safety surveillance, restricted distribution and use, patient education, enhanced labeling, expedited reporting of certain adverse events, pre-approval of promotional materials and restrictions on direct-to-consumer advertising. For example, any labeling approved for any of our product candidates may include a restriction on the term of its use, or it may not include one or more of our intended indications. Furthermore, any new legislation addressing drug safety issues could result in delays or increased costs during the period of product development, clinical trials and regulatory review and approval, as well as increased costs to assure compliance with any new post-approval regulatory requirements. Any of these restrictions or requirements could force us or our partners to conduct costly studies.
Our product candidates will also be subject to ongoing regulatory requirements for the labeling, packaging, storage, advertising, promotion, record-keeping and submission of safety and other post-market information on the drug. In addition, approved products, manufacturers and manufacturers' facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to current Good Manufacturing Practices, or cGMP. As such, we and our contract manufacturers are subject to continual review and periodic inspections to assess compliance with cGMP. Accordingly, we and others with whom we work must continue to expend time, money, and effort in all areas of regulatory compliance, including manufacturing, production, and quality control. We will also be required to report certain adverse reactions and production problems, if any, to the FDA, and to comply with certain requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product's approved label. As such, we may not promote our products for indications or uses for which they do not have approval.
If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or disagrees with the promotion, marketing, or labeling of a product, a regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If our product candidates fail to comply with applicable regulatory requirements, a regulatory agency may:
- •
- issue warning letters;
44
- •
- mandate modifications to promotional materials or require us to provide corrective information to healthcare
practitioners;
- •
- require us or our partners to enter into a consent decree, which can include imposition of various fines, reimbursements
for inspection costs, required due dates for specific actions and penalties for noncompliance;
- •
- impose other civil or criminal penalties;
- •
- suspend regulatory approval;
- •
- suspend any ongoing clinical trials;
- •
- refuse to approve pending applications or supplements to approved applications filed by us, GSK or our potential future
partners;
- •
- impose restrictions on operations, including costly new manufacturing requirements; or
- •
- seize or detain products or require a product recall.
Guidelines and recommendations published by various organizations may affect the use of our products.
Government agencies may issue regulations and guidelines directly applicable to us, GSK or our potential future partners and our product candidates. In addition, professional societies, practice management groups, private health/science foundations, and organizations involved in various diseases from time to time publish guidelines or recommendations to the health care and patient communities. These various sorts of recommendations may relate to such matters as product usage, dosage, route of administration and use of related or competing therapies. Changes to these recommendations or other guidelines advocating alternative therapies could result in decreased use of our products, which may adversely affect our results of operations.
Risks Related to Our Dependence on Third Parties
We are dependent on our existing third party collaborations to fund additional development opportunities and expect to continue to expend resources in our current collaborations with GSK, Lilly and Medicis. These research collaborations may fail to successfully identify product candidates, or our collaboration partners may elect not to license, develop or commercialize any of the resulting compounds, including, in the case of GSK, compounds in development by us with respect to which GSK has option rights as well as a compound (GSK '052) for which GSK has sole development and commercialization rights. In the event our collaborator does not elect to exercise its option or elects not to develop or commercialize our collaboration candidate products, our operating results and financial condition could be materially and adversely affected.
We currently have three ongoing collaboration agreements: our most advanced arrangement, an October 2007 research and development collaboration, option and license agreement with GSK for the discovery, development, manufacture and worldwide commercialization of novel systemic anti-infectives for viral and bacterial diseases utilizing our boron-based chemistry; an August 2010 collaborative research, license and commercialization agreement with Lilly for the discovery, development, and worldwide commercialization of animal health products for specific applications; and a February 2011 research and development option and license agreement with Medicis for the discovery, development, and worldwide commercialization of novel boron-based compounds directed against a specific target for the treatment of acne.
During the research terms of the collaborations, we (and in some cases also our partner) are committed to use our diligent efforts to discover and develop compounds and to provide specified resources, including certain numbers of full-time equivalent scientists, on a project-by-project basis. We are either reimbursed our research costs, or each party is responsible for its own research costs, but in
45
all cases we expect to continue to expend resources on the collaborations. If we fail to successfully identify product candidates or, in some cases, demonstrate proof-of-concept for those product candidates we identify, our operating results and financial condition could be materially and adversely affected. In addition, we may mutually agree with our collaboration partner not to pursue all of the research activities contemplated under the applicable agreement. Our collaboration partners have the option, but are not required, to exclusively license or select for further development certain product candidates under the agreement once the product candidate meets specified criteria, subject to continuing obligations to make milestone payments and royalty payments on commercial sales, if any, of such licensed compounds. Typically, the collaboration partner is obligated to make payments to us upon the achievement of certain initial discovery and developmental milestones, but further, more significant milestone payments are payable only on compounds that the partner chooses to license or develop, such as GSK '052 in the case of GSK. If we devote significant resources to a research project and our collaboration partner elects not to exercise its option with respect to or develop any resulting product candidates, our financial condition could be materially and adversely affected. In certain cases, if our partner does not exercise a given option or terminates development, we may request a license to develop and commercialize products containing the relevant compounds. If we make such request, we will be obligated to pay certain milestones and royalties if we elect to develop and commercialize such products.
If our collaboration partner elects to license or develop a compound, like GSK has with GSK '052, the partner assumes sole responsibility for further development, regulatory approval and commercialization of such compound. For example, under the GSK collaboration, we developed and GSK exercised an exclusive license to GSK '052, which is our lead systemic antibiotic for the treatment of infections caused by Gram-negative bacteria. We are dependent on GSK for the further development and commercialization of this compound. Thus, even with respect to compounds that our partner chooses to develop, the timing of development and future payments to us, including milestone and royalty payments, will depend on the extent to which such licensed compounds advance through development, regulatory approval and commercialization by our partner. Additionally, our partner can choose to terminate the agreement with a specified notice period or its license to any compounds at any time with no further obligation to develop and commercialize such compounds. In such event, we would not be eligible to receive further payments for the affected compounds. We would retain rights to develop and market any such product candidates. However, we would be required to fund further development and commercialization ourselves or with other partners if we continue to pursue these product candidates, and in some cases would owe our previous partner royalties to continue to develop and commercialize any such product candidates.
If our partner does not devote sufficient resources to the research, development and commercialization of compounds identified through our research collaboration, including GSK in the case of GSK '052, or is ineffective in doing so, our operating results could be materially and adversely affected. In particular, if our partner independently develops products that compete with our compounds, it could elect to advance such products and not develop or commercialize our product candidates, even while complying with applicable exclusivity provisions. We cannot assure you that our collaboration partners will fulfill their obligations under the agreements or develop and commercialize compounds identified by the research collaborations, including GSK in the case of GSK '052. If our partners fail to fulfill their obligations under the agreements or terminate the agreements, we would need to obtain the capital necessary to fund the development and commercialization of the returned compounds, enter into alternative arrangements with a third party or halt our development efforts in these areas. We could also become involved in disputes with our partners, which could lead to delays in or termination of the research collaborations or the development and commercialization of identified product candidates and time-consuming and expensive litigation or arbitration. If our partners terminate or breach their agreements with us or otherwise do not advance the compounds identified by
46
our research collaborations, our chances of successfully developing or commercializing such compounds could be materially and adversely affected.
We may not be successful in establishing and maintaining development and commercialization collaborations, which could adversely affect our ability to develop certain of our product candidates and our financial condition and operating results.
Developing pharmaceutical products, conducting clinical trials, obtaining regulatory approval, establishing manufacturing capabilities and marketing approved products is expensive. Consequently, we plan to establish collaborations for development and commercialization of product candidates and research programs. For example, if AN2690, AN2728, AN2898 or any of our other product candidates receives marketing approval, we intend to enter into sales and marketing arrangements with third parties for non-specialty markets in the United States and for international sales, and to develop our own sales force targeting dermatologists and other specialty markets in the United States. If we are unable to enter into any such arrangements on acceptable terms, if at all, we may be unable to market and sell our products in these markets. We expect to face competition in seeking appropriate collaborators. Moreover, collaboration arrangements are complex and time consuming to negotiate, document and implement and they may require substantial resources to maintain. We may not be successful in our efforts to establish and implement collaborations or other alternative arrangements for the development of our product candidates. When we partner with a third party for development and commercialization of a product candidate, we can expect to relinquish some or all of the control over the future success of that product candidate to the third party. Our collaboration partner may not devote sufficient resources to the commercialization of our product candidates or may otherwise fail in their commercialization. The terms of any collaboration or other arrangement that we establish may not be favorable to us. In addition, any collaboration that we enter into may be unsuccessful in the development and commercialization of our product candidates. In some cases, we may be responsible for continuing preclinical and initial clinical development of a partnered product candidate or research program, and the payment we receive from our collaboration partner may be insufficient to cover the cost of this development. If we are unable to reach agreements with suitable collaborators for our product candidates we would face increased costs, we may be forced to limit the number of our product candidates we can commercially develop or the territories in which we commercialize them and we might fail to commercialize products or programs for which a suitable collaborator cannot be found. If we fail to achieve successful collaborations, our operating results and financial condition will be materially and adversely affected.
We depend on third-party contractors for a substantial portion of our operations and may not be able to control their work as effectively as if we performed these functions ourselves.
We outsource substantial portions of our operations to third-party service providers, including chemical synthesis, biological screening and manufacturing. Our agreements with third-party service providers and clinical research organizations are on a study-by-study basis and are typically short-term. In all cases, we may terminate the agreements with notice and are responsible for the supplier's previously incurred costs.
Because we have relied on third parties, our internal capacity to perform these functions is limited. Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. In addition, the use of third-party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated. There is a limited number of third-party service providers that specialize or have the expertise required to achieve our business objectives. Identifying, qualifying and managing performance of third-party service providers can be difficult, time consuming and cause delays in our development programs. We currently have a small number of
47
employees, which limits the internal resources we have available to identify and monitor our third-party providers. To the extent we are unable to identify, retain and successfully manage the performance of third-party service providers in the future, our business may be adversely affected.
We have no experience manufacturing our product candidates on a large clinical or commercial scale and have no manufacturing facility. As a result, we are dependent on numerous third parties for the manufacture of our product candidates and our supply chain, and if we experience problems with any of these suppliers, the manufacturing of our product candidates or products could be delayed.
We do not own or operate facilities for the manufacture of our product candidates. We have a small number of personnel with experience in drug product manufacturing. We currently outsource all manufacturing and packaging of our preclinical and clinical product candidates to third parties and intend to continue to do so. Under our agreement with Schering, the formulation for AN2690 was finalized and manufactured at one of Schering's GMP facilities. In November 2009, Schering merged with Merck, and in May 2010, we regained the worldwide rights to AN2690. As part of the transition from Merck to us, we received sufficient drug product to initiate and sufficient active ingredient to complete our Phase 3 clinical trials of AN2690. We have transferred manufacturing of additional supplies of AN2690 to a third-party manufacturer that operates in compliance with cGMP regulations and will need to scale up and manufacture additional active ingredient and drug product in order to complete our NDA submission. The inability to manufacture sufficient supplies of the drug product could adversely effect clinical trial enrollment or timing or, if AN2690 is approved, product commercialization. We also outsource active ingredient process development work and product manufacturing to third parties for AN2728, AN2718 and AN2898. We do not currently have any agreements with third-party manufacturers for the long-term commercial supply of our product candidates, including AN2690. We may encounter technical difficulties or delays in the transfer of AN2690 manufacturing on a commercial scale to a third-party manufacturer. We may be unable to enter into agreements for commercial supply with third party manufacturers, or may be unable to do so on acceptable terms. We may not be able to establish additional sources of supply for our products. Such suppliers are subject to regulatory requirements, covering manufacturing, testing, quality control and record keeping relating to our product candidates and subject to ongoing inspections by the regulatory agencies. Failure by any of our suppliers to comply with applicable regulations may result in long delays and interruptions to our product candidate supply while we seek to secure another supplier that meets all regulatory requirements.
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured the product candidates ourselves, including:
- •
- the possible breach of the manufacturing agreements by the third parties because of factors beyond our control; and
- •
- the possibility of termination or nonrenewal of the agreements by the third parties because of our breach of the manufacturing agreement or based on their own business priorities.
Any of these factors could cause the delay of clinical trials, regulatory submissions, required approvals or commercialization of our products, cause us to incur higher costs and prevent us from commercializing our product candidates successfully. Furthermore, if any of our product candidates are approved and contract manufacturers fail to deliver the required commercial quantities of finished product on a timely basis and at commercially reasonable prices and we are unable to find one or more replacement manufacturers capable of production at a substantially equivalent cost, in substantially equivalent volumes and quality and on a timely basis, we would likely be unable to meet demand for our products and could lose potential revenue. It may take several years to establish an alternative source of supply for our product candidates and to have any such new source approved by the FDA.
48
If we lose our relationships with contract research organizations, our drug development efforts could be delayed.
We are substantially dependent on third-party vendors and contract research organizations for preclinical studies and clinical trials related to our drug discovery and development efforts. If we lose our relationship with any one or more of these providers, we could experience a significant delay in both identifying another comparable provider and then contracting for its services, which could adversely affect our development efforts. We may be unable to retain an alternative provider on reasonable terms, if at all. Even if we locate an alternative provider, it is likely that this provider will need additional time to respond to our needs and may not provide the same type or level of services as the original provider. In addition, any contract research organization that we retain will be subject to the FDA's regulatory requirements and similar foreign standards and we do not have control over compliance with these regulations by these providers. Consequently, if these practices and standards are not adhered to by these providers, the development and commercialization of our product candidates could be delayed, which could severely harm our business and financial condition.
Risks Relating to Our Intellectual Property
It is difficult and costly to protect our proprietary rights, and we may not be able to ensure their protection.
Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection of our current and future product candidates and the methods used to manufacture them, as well as successfully defending these patents against third-party challenges. Our ability to stop third parties from making, using, selling, offering to sell or importing our products is dependent upon the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities.
The patent positions of pharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in pharmaceutical patents has emerged to date in the United States or in many foreign jurisdictions. Changes in either the patent laws or in interpretations of patent laws in the United States and foreign jurisdictions may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be enforced in the patents that may be issued from the applications we currently or may in the future own or license from third parties. Further, if any patents we obtain or license are deemed invalid and unenforceable, it could impact our ability to commercialize or license our technology.
The degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
- •
- others may be able to make compounds that are similar to our product candidates but that are not covered by the claims of
our patents;
- •
- we might not have been the first to make the inventions covered by our pending patent applications;
- •
- we might not have been the first to file patent applications for these inventions;
- •
- others may independently develop similar or alternative technologies or duplicate any of our technologies;
- •
- any patents that we obtain may not provide us with any competitive advantages;
- •
- we may not develop additional proprietary technologies that are patentable; or
- •
- the patents of others may have an adverse effect on our business.
49
As of February 15, 2011, we are the owner of record of 8 issued U.S. patents and 4 non-U.S. patents with claims to boron-containing compounds and methods of using these compounds in various indications. We are actively pursuing, either solely or with a collaborator, 28 U.S. patent applications (10 provisional and 18 non-provisional), 16 international (PCT) patent applications and 103 non-U.S. patent applications in at least 39 jurisdictions. Of these actively pursued applications, 3 U.S. patent applications (2 provisional and 1 non-provisional) and 1 international (PCT) patent application are solely owned by a collaborator as of February 15, 2011. In 2011, we estimate that we will allow 22 non-U.S. patents and 49 non-U.S. patent applications that do not relate to our clinical product candidates to expire or become abandoned and have not included such patents and patent applications in the totals above.
Due to the patent laws of a country, or the decisions of a patent examiner in a country, or our own filing strategies, we may not obtain patent coverage for all of the product candidates or methods involving these candidates in the parent patent application. We plan to pursue divisional patent applications and/or continuation patent applications in the United States and many other countries to obtain claim coverage for inventions which were disclosed but not claimed in the parent patent application.
We also may rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, outside scientific collaborators and other advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Our patent applications would not prevent others from taking advantage of the chemical properties of boron to discover and develop new therapies, including therapies for the indications we are targeting. If others seek to develop boron-based therapies, their research and development efforts may inhibit our ability to conduct research in certain areas and expand our intellectual property portfolio.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights and we may be unable to enforce or protect our rights to, or use, our technology.
If we choose to go to court to stop another party from using the inventions claimed in any patents we obtain, that individual or company has the right to ask the court to rule that such patents are invalid or should not be enforced against that third party. These lawsuits are expensive and would consume time and resources and divert the attention of managerial and scientific personnel even if we were successful in stopping the infringement of such patents. In addition, there is a risk that the court will decide that such patents are not valid and that we do not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of such patents is upheld, the court will refuse to stop the other party on the ground that such other party's activities do not infringe our rights to such patents. In addition, the U.S. Supreme Court has recently modified some tests used by the U.S. Patent Office in granting patents over the past 20 years, which may decrease the likelihood that we will be able to obtain patents and increase the likelihood of challenge of any patents we obtain or license.
Furthermore, a third party may claim that we or our manufacturing or commercialization partners are using inventions covered by the third party's patent rights and may go to court to stop us from engaging in our normal operations and activities, including making or selling our product candidates. These lawsuits are costly and could affect our results of operations and divert the attention of managerial and scientific personnel. There is a risk that a court would decide that we or our commercialization partners are infringing the third party's patents and would order us or our partners
50
to stop the activities covered by the patents. In that event, we or our commercialization partners may not have a viable way around the patent and may need to halt commercialization of the relevant product with it. In addition, there is a risk that a court will order us or our partners to pay the other party damages for having violated the other party's patents. In the future, we may agree to indemnify our commercial partners against certain intellectual property infringement claims brought by third parties. The pharmaceutical and biotechnology industries have produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our products or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid, and we may not be able to do this. Proving invalidity is difficult. For example, in the United States, proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents.
Because some patent applications in the United States may be maintained in secrecy until the patents are issued, because patent applications in the United States and many foreign jurisdictions are typically not published until eighteen months after filing, and because publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered by our pending applications, or that we were the first to invent the technology. Our competitors may have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent application may have priority over our patent applications, which could further require us to obtain rights to issued patents covering such technologies. If another party has filed a U.S. patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the U.S. Patent and Trademark Office to determine priority of invention in the United States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful if, unbeknownst to us, the other party had independently arrived at the same or similar invention prior to our own invention, resulting in a loss of our U.S. patent position with respect to such inventions.
Patents covering the composition of matter of AN2690 and AN2718 that were owned by others have expired. Our patent applications and patents include or support claims on other aspects of AN2690 or AN2718, such as pharmaceutical formulations containing AN2690 or AN2718, methods of using AN2690 or AN2718 to treat disease and methods of manufacturing AN2690 or AN2718. Without patent protection on the composition of matter of AN2690 or AN2718, our ability to assert our patents to stop others from using or selling AN2690 or AN2718 in a non-pharmaceutically acceptable formulation may be limited.
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
We do not have exclusive rights to intellectual property we developed under U.S. federally funded research grants and contracts in connection with certain of our neglected diseases initiatives, and, in the case of U.S. funded research, we could ultimately lose the rights we do have under certain circumstances.
Some of our intellectual property rights related to compounds that are not in clinical development as of February 15, 2011 were initially developed in the course of research funded by the U.S. government. As a result, the U.S. government may have certain rights to intellectual property embodied in our current or future products pursuant to the Bayh-Dole Act of 1980. Government rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right to require us to grant exclusive licenses to any of these inventions to a third
51
party if they determine that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations. The U.S. government also has the right to take title to these inventions if we fail to disclose the invention to the government and fail to file an application to register the intellectual property within specified time limits. In addition, the U.S. government may acquire title in any country in which a patent application is not filed within specified time limits.
Some of our intellectual property rights related to boron-containing compounds that are not in clinical development as of February 15, 2011 were developed through a collaboration with the Drugs for Neglected Diseases initiative, or DNDi. We accept research funding from DNDi. We have a co-exclusive, royalty-free, sublicensable license with DNDi to make, use, import and manufacture a product for treatment of human African trypanosomiasis, Chagas disease, and cutaneous and visceral leishmaniasis in humans in all countries of the world, specifically excluding Japan, Australia, New Zealand, Russia, China, and all countries of North America and Europe, or DNDi Territory. We also grant to DNDi an exclusive, royalty-free, sublicensable license to distribute, including uses by, or on behalf of, a public sector agency, a product containing molecules synthesized under the research plan for treatment of human African trypanosomiasis, Chagas disease, and cutaneous and visceral leishmaniasis in humans in the DNDi Territory. As a result, we may not be able to realize any revenue in the DNDi Territory for any human therapeutics that we discover for these diseases. As of February 15, 2011, the boron-containing compounds being studied in this collaboration are structurally distinct from our five clinical product candidates. As of February 15, 2011, none of our five clinical product candidates are being considered for use in the DNDi collaboration.
Some of our intellectual property rights related to boron-containing compounds that are not in clinical development as of February 15, 2011 were developed through a collaboration with the Global Alliance for TB Drug Development, or TB Alliance. We accept research funding from the TB Alliance, and we provide the TB Alliance with a non-exclusive, worldwide, royalty-free, sublicensable license to make, have made, use, sell, offer for sale and import for the purpose of treating tuberculosis, boron-containing small molecule compounds that have a high level of effectiveness against the bacteria associated with tuberculosis, but a low level of effectiveness against certain other bacteria which are associated with other human diseases. As a result, we may not be able to realize any revenue from any human therapeutics generated under the agreement for tuberculosis. As of February 15, 2011, the boron-containing compounds under development in this collaboration are structurally distinct from our five clinical product candidates. As of February 15, 2011, none of our five clinical product candidates are being considered for use in the TB Alliance collaboration.
Some of our intellectual property rights related to boron-containing compounds that are not in clinical development as of February 15, 2011 were developed through a collaboration with Medicines for Malaria Venture, or MMV. We accept research funding from MMV, and we provide MMV with a worldwide, royalty-free non-exclusive license (without the right to sublicense, except with prior Anacor written approval) to intellectual property rights arising under the collaboration to develop human therapeutics for the treatment of malaria under the agreement. If the research is successful, we are obligated to negotiate in good faith with MMV a preclinical and clinical development agreement and compound license. As of February 15, 2011, the boron-containing compounds under development in this collaboration are structurally distinct from our five clinical product candidates. As of February 15, 2011, none of our five clinical product candidates are being considered for use in the MMV collaboration.
52
Risks Related to Employee Matters and Managing Growth
We will need to expand our operations and increase the size of our company, and we may experience difficulties in managing growth.
As we increase the number of product development programs we have underway and advance our product candidates through preclinical studies and clinical trials, we will need to increase our product development, scientific and administrative headcount to manage these programs. In addition, to meet our obligations as a public company, we will need to increase our general and administrative headcount. Our management, personnel and systems currently in place may not be adequate to support this future growth. Our need to effectively manage our operations, growth and various projects requires that we:
- •
- successfully attract and recruit new employees with the expertise and experience we will require;
- •
- manage our clinical programs effectively, which we anticipate being conducted at numerous clinical sites;
- •
- develop a marketing and sales infrastructure; and
- •
- continue to improve our operational, financial and management controls, reporting systems and procedures.
If we are unable to successfully manage this growth, our business may be adversely affected.
We may not be able to manage our business effectively if we are unable to attract and retain key personnel.
We may not be able to attract or retain qualified management, finance, scientific and clinical personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses, particularly in Northern California. If we are not able to attract and retain necessary personnel to accomplish our business objectives, we may experience constraints that will significantly impede the achievement of our development objectives, our ability to raise additional capital and our ability to implement our business strategy.
Our industry has experienced a high rate of turnover of management personnel in recent years. We are highly dependent on the development, regulatory, commercialization and business development expertise of our executive officers and key employees. If we lose one or more of our executive officers or key employees, our ability to implement our business strategy successfully could be seriously harmed. We have entered into change of control and severance agreements with each of our officers as part of our retention efforts. Replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain regulatory approval of and commercialize products successfully. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these additional key personnel. Our failure to retain key personnel could materially harm our business.
If we fail to maintain proper and effective internal controls, our ability to produce accurate financial statements on a timely basis could be impaired, which would adversely affect our business and our stock price.
As a new public company, following our November 2010 initial public offering, we operate in an increasingly demanding regulatory environment, which requires us to comply with the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, and the related rules and regulations of the Securities and Exchange Commission, expanded disclosure requirements, accelerated reporting requirements and more complex accounting rules. Company responsibilities required by the Sarbanes-Oxley Act include establishing adequate internal control over financial reporting and disclosure controls and procedures. Effective internal controls are necessary for us to produce reliable financial reports and are important
53
to help prevent financial fraud. The growth of our operations and our initial public offering created a need for additional resources within the accounting and finance functions in order to produce timely financial information and to create the level of segregation of duties customary for a U.S. public company.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Our management does not expect that our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system's objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within our company will have been detected.
We will be required for the first time to comply with Section 404 of the Sarbanes-Oxley Act, or Section 404, in connection with our Annual Report on Form 10-K for the year ending December 31, 2011. We expect to expend significant resources in developing the necessary documentation and testing procedures required by Section 404. We cannot be certain that the actions we will be taking to improve our internal control over financial reporting will be sufficient, or that we will be able to implement our planned processes and procedures in a timely manner. In addition, if we are unable to produce accurate financial statements on a timely basis, investors could lose confidence in the reliability of our financial statements, which could cause the market price of our common stock to decline and make it more difficult for us to finance our operations and growth.
Other Risks Relating to Our Business
We face potential product liability exposure, and if successful claims are brought against us, we may incur substantial liability for a product candidate and may have to limit its commercialization.
The use of our product candidates in clinical trials and the sale of any products for which we may obtain marketing approval expose us to the risk of product liability claims. Product liability claims may be brought against us or our partners by participants enrolled in our clinical trials, patients, health care providers or others using, administering or selling our products. If we cannot successfully defend ourselves against any such claims, we would incur substantial liabilities. Regardless of merit or eventual outcome, product liability claims may result in:
- •
- withdrawal of clinical trial participants;
- •
- termination of clinical trial sites or entire trial programs;
- •
- costs of related litigation;
- •
- substantial monetary awards to patients or other claimants;
- •
- decreased demand for our product candidates and loss of revenues;
- •
- impairment of our business reputation;
- •
- diversion of management and scientific resources from our business operations; and
- •
- the inability to commercialize our product candidates.
We have obtained limited product liability insurance coverage for our clinical trials domestically and in selected foreign countries where we are conducting clinical trials. Our coverage is currently limited to $5.0 million per occurrence and $5.0 million in the aggregate per year. As such, our insurance coverage may not reimburse us or may not be sufficient to reimburse us for any expenses or
54
losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to product liability. We intend to expand our insurance coverage for products to include the sale of commercial products if we obtain marketing approval for our product candidates in development, but we may be unable to obtain commercially reasonable product liability insurance for any products approved for marketing. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
Our operations involve hazardous materials, which could subject us to significant liabilities.
Our research and development processes involve the controlled use of hazardous materials, including chemicals. Our operations produce hazardous waste products. We cannot eliminate the risk of accidental contamination or discharge or injury from these materials. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of these materials. We could be subject to civil damages in the event of exposure of individuals to hazardous materials. In addition, claimants may sue us for injury or contamination that results from our use of these materials and our liability may exceed our total assets. We have general liability insurance of up to $5.0 million per occurrence, with an annual aggregate limit of $6.0 million, which excludes pollution liability. This coverage may not be adequate to cover all claims related to our biological or hazardous materials. Furthermore, if we were to be held liable for a claim involving our biological or hazardous materials, this liability could exceed our insurance coverage, if any, and our other financial resources. Compliance with environmental and other laws and regulations may be expensive and current or future regulations may impair our research, development or production efforts.
Our insurance policies are expensive and protect us only from some business risks, which will leave us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. For example, we do not carry earthquake insurance. In the event of a major earthquake in our region, our business could suffer significant and uninsured damage and loss. Some of the policies we currently maintain include general liability, employment practices liability, property, auto, workers' compensation, products liability and directors' and officers' insurance. We do not know, however, if we will be able to maintain existing insurance with adequate levels of coverage. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our cash position and results of operations.
Risks Relating to Owning Our Common Stock
Our executive officers, directors and principal stockholders have the ability to control all matters submitted to our stockholders for approval.
As of February 15, 2011 our executive officers, directors and stockholders who own more than 5% of our outstanding common stock, together beneficially own shares representing more than 80% of our common stock. As a result, if these stockholders were to choose to act together, they would be able to control all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, will control the election of directors and approval of any merger, consolidation, sale of all or substantially all of our assets or other business combination or reorganization. This concentration of voting power could delay or prevent an acquisition of us on terms that other stockholders may desire. The interests of this group of stockholders may not always coincide with your interests or the interests of other stockholders and they may act in a manner that advances their best interests and not necessarily those of other stockholders,
55
including seeking a premium value for their common stock, and might affect the prevailing market price for our common stock.
We do not anticipate paying cash dividends, and accordingly, stockholders must rely on stock appreciation for any return on their investment.
We do not anticipate paying cash dividends in the future. As a result, only appreciation of the price of our common stock, which may never occur, will provide a return to stockholders. Investors seeking cash dividends should not invest in our common stock. In addition, our ability to pay cash dividends is currently prohibited by the terms of our loan and security agreement with Oxford Finance Corporation and Horizon Technology Finance Corporation.
Our share price may be volatile and could decline significantly.
The market price of shares of our common stock could be subject to wide fluctuations in response to many risk factors listed in this section, and others beyond our control, including:
- •
- results of our clinical trials;
- •
- results of clinical trials of our competitors' products;
- •
- regulatory actions with respect to our products or our competitors' products;
- •
- actual or anticipated fluctuations in our financial condition and operating results;
- •
- actual or anticipated changes in our growth rate relative to our competitors;
- •
- actual or anticipated fluctuations in our competitors' operating results or changes in their growth rate;
- •
- competition from existing products or new products that may emerge;
- •
- announcements by us, our collaborators or our competitors of significant acquisitions, strategic partnerships, joint
ventures, collaborations or capital commitments;
- •
- issuance of new or updated research or reports by securities analysts;
- •
- fluctuations in the valuation of companies perceived by investors to be comparable to us;
- •
- share price and volume fluctuations attributable to inconsistent trading volume levels of our shares;
- •
- additions or departures of key management or scientific personnel;
- •
- disputes or other developments related to proprietary rights, including patents, litigation matters and our ability to
obtain patent protection for our technologies;
- •
- announcement or expectation of additional financing efforts;
- •
- sales of our common stock by us, our insiders or our other stockholders;
- •
- market conditions for biopharmaceutical stocks in general; and
- •
- general economic and market conditions.
Furthermore, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political and market conditions such as recessions, interest rate changes or international currency fluctuations, may
56
negatively impact the market price of shares of our common stock. You may not realize any return on an investment in us and may lose some or all of your investment.
We may be subject to securities litigation, which is expensive and could divert management attention.
Our share price may be volatile, and in the past companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management's attention from other business concerns, which could seriously harm our business.
A significant portion of our total outstanding shares of common stock is restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur in the future. These sales, or the perception in the market that the holders of a large number of shares of common stock intend to sell shares, could reduce the market price of our common stock. Sales of 14,614,227 shares of our common stock are currently restricted under securities laws or as a result of lock-up agreements but will be able to be resold beginning upon expiration of the lock-up agreements, which will occur in May 2011, unless extended under certain circumstances. Moreover, holders of an aggregate of 13,318,572 shares of our common stock have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders.
As a public company we are subject to additional expenses and administrative burden.
As a new public company, we incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, our administrative staff are required to perform additional tasks. For example, we must adopt additional internal controls and disclosure controls and procedures, retain a transfer agent and bear all of the internal and external costs of preparing and distributing periodic public reports in compliance with our obligations under the securities laws.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act and related regulations implemented by the Securities and Exchange Commission and the NASDAQ Global Market, are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. We are currently evaluating and monitoring these rules and proposed changes to rules, and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment will result in increased general and administrative expenses and may divert management's time and attention from product development activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed. In the future, it may be more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
57
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and our bylaws may delay or prevent an acquisition of us. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management team. In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits, with some exceptions, stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us. Finally, our charter documents establish advanced notice requirements for nominations for election to our board of directors and for proposing matters that can be acted upon at stockholder meetings. Although we believe these provisions together provide for an opportunity to receive higher bids by requiring potential acquirers to negotiate with our board of directors, they would apply even if the offer may be considered beneficial by some stockholders.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
We lease a 36,960 square-foot building consisting of office and laboratory space in Palo Alto, California, which serves as our corporate headquarters. The lease term commenced in March 2008 and has scheduled annual rent increases through the lease expiration in March 2018. We also have a lease for a 15,300 square-foot building consisting of office and laboratory space in Palo Alto, California, which we extended through December 2011 and is cancelable with four months' notice. We believe that the facilities that we currently lease are adequate for our needs for the immediate future and that, should it be needed, additional space can be leased to accommodate any future growth.
We are not currently a party to any material litigation or other material legal proceedings.
58
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock began trading on the NASDAQ Global Market under the symbol "ANAC" on November 24, 2010. Prior to this date, there was no public market for our common stock. The following table sets forth the high and low closing prices of our common stock for the period indicated since our initial public offering, or IPO, as reported on the NASDAQ Global Market.
| |
High | Low | |||||
|---|---|---|---|---|---|---|---|
November 24, 2010 to December 31, 2010 |
$ | 5.37 | $ | 4.90 | |||
As of February 28, 2011, there were 27,996,928 shares of our common stock issued and outstanding with approximately 67 stockholders of record. A significantly larger number of stockholders may be "street name" or beneficial holders, whose shares of record are held by banks, brokers and other financial institutions.
59
The following stock performance graph compares our total stock return with the total return for (i) the NASDAQ Composite Index and the (ii) the NASDAQ Biotechnology Index for the period from November 24, 2010 (the date our common stock commenced trading on the NASDAQ Global Market) through December 31, 2010. The figures represented below assume an investment of $100 in our common stock at the closing price of $5.09 on November 24, 2010 and in the NASDAQ Composite Index and the NASDAQ Biotechnology Index on November 24, 2010 and the reinvestment of dividends into shares of common stock. The comparisons in the table are required by the Securities and Exchange Commission, or SEC, and are not intended to forecast or be indicative of possible future performance of our common stock. This graph shall not be deemed "soliciting material" or be deemed "filed" for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any of our filings under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
$100 investment in stock or index
|
Ticker | 11/24/10 | Nov 10 | Dec 10 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Anacor |
ANAC | 100.00 | 98.23 | 105.50 | ||||||||
NASDAQ Composite Index |
IXIC | 100.00 | 98.23 | 104.32 | ||||||||
NASDAQ Biotechnology Index |
NBI | 100.00 | 97.76 | 104.51 | ||||||||
We have never declared or paid any cash dividends. We currently expect to retain earnings for use in the operation and expansion of our business, and therefore do not anticipate paying any cash dividends in the foreseeable future. In addition, our ability to pay cash dividends is currently prohibited by the terms of our loan and security agreement with Oxford Finance Corporation and Horizon Technology Finance Corporation.
60
Under the Registration Statement on Form S-1, as amended (File No. 333-169322) that was declared effective on November 23, 2010, we registered the offering and sale of an aggregate of 13,800,000 shares of our common stock, including 1,800,000 shares pursuant to an over-allotment option granted to the underwriters. 13,382,651 shares of common stock registered under the Registration Statement, which included 1,382,651 shares of our common stock sold pursuant to the exercise an over-allotment option granted to the underwriters, were sold at a price to the public of $5.00 per share. The aggregate offering price for shares sold in the offering was $66,913,255. Citigroup Global Markets Inc. and Deutsche Bank Securities, Inc. acted as joint book running managers for the offering, and Cowen and Company, LLC and Wedbush PacGrow Life Sciences acted as co-managers for the offering. The offering commenced on November 24, 2010 and closed on November 30, 2010. The sale of shares pursuant to the over-allotment occurred on December 28, 2010.
Concurrent with the closing of the IPO on November 30, 2010, we completed a private placement offering to affiliates of an existing investor for 2,000,000 shares of common stock at a price of $5.00 per share (see Recent Sale of Unregistered Securities). As a result of our IPO and concurrent private placement offering, we raised a total of $76.9 million in gross proceeds, and approximately $71.0 million in net proceeds after deducting underwriting discounts of $3.2 million and estimated offering expenses of $2.7 million paid by us. We did not pay, directly or indirectly, any offering expenses to any of our directors or officers or persons owning ten percent or more our securities or to their associates, or to our affiliates, other than payments in the ordinary course of business to officers for salaries and to non-employee directors as compensation for board or board committee service.
There has been no material change in our planned use of proceeds from the IPO from that described in the final prospectus filed with the SEC pursuant to Rule 424(b) on November 24, 2010. We invested the funds received in a variety of capital preservation instruments, including short- and long-term interest-bearing obligations, direct or guaranteed obligations of the U.S. government, certificates of deposit and money market funds.
RECENT SALE OF UNREGISTERED SECURITIES
On November 30, 2010, we sold and issued 2,000,000 shares of common stock to Venrock Healthcare Capital Partners, L.P. and VHCP Co-Investment Holdings, LLC (affiliates of an existing investor), or Venrock, at a price of $5.00 per share in a private placement offering for aggregate gross proceeds of $10.0 million pursuant to a common stock purchase agreement dated as of November 23, 2010. The private placement offering closed concurrently with the closing of our IPO of shares of our common stock. The sale and issuance of shares of common stock in the private placement was made in reliance on Rule 506 promulgated under the Securities Act of 1933, as amended, and was made without general solicitation or advertising. Each purchaser represented that it was an accredited investor with access to information about us sufficient to evaluate the investment and that the common stock being acquired constituted restricted securities. Pursuant to a registration rights agreement, we granted certain registration rights pursuant to which, under certain conditions, we will register shares of common stock held by Venrock affiliates, including the shares of common stock sold in the private placement offering, for resale on a registration statement on Form S-3.
61
ITEM 6. SELECTED FINANCIAL DATA
The following selected data are derived from our financial statements which have been audited by Ernst & Young LLP, independent registered public accounting firm. Ernst & Young LLP's report on the financial statements for the year ended December 31, 2010 appears elsewhere herein. The data in the tables below should be read together with our financial statements and accompanying notes and "Management's Discussion and Analysis of Financial Condition and Results of Operations" appearing elsewhere in this Annual Report on Form 10-K. The selected financial data in this section is not intended to replace our financial statements and the accompanying notes. Our historical results are not necessarily indicative of our future results.
The statements of operations data for 2006 and 2007 and the balance sheet data as of December 31, 2006, 2007 and 2008 were derived from our audited financial statements not included in this report. The statements of operations data for 2008, 2009 and 2010 and the balance sheet data as of December 31, 2009 and 2010 were derived from our audited financial statements appearing elsewhere in this report.
| |
Year Ended December 31, | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | 2009 | 2010 | |||||||||||||
| |
(in thousands, except share and per share data) |
|||||||||||||||||
Statement of Operations Data: |
||||||||||||||||||
Revenues: |
||||||||||||||||||
Contract revenue |
$ | — | $ | 20,738 | $ | 19,776 | $ | 9,793 | $ | 8,448 | ||||||||
Contract revenue-related party |
— | 500 | 5,300 | 8,850 | 17,909 | |||||||||||||
Government contract and grant revenue |
861 | 51 | — | — | 1,467 | |||||||||||||
Total revenues |
861 | 21,289 | 25,076 | 18,643 | 27,824 | |||||||||||||
Operating expenses: |
||||||||||||||||||
Research and development |
16,627 | 24,597 | 36,189 | 34,083 | 29,866 | |||||||||||||
General and administrative |
3,629 | 7,924 | 10,171 | 7,054 | 7,452 | |||||||||||||
Total operating expenses |
20,256 | 32,521 | 46,360 | 41,137 | 37,318 | |||||||||||||
Loss from operations |
(19,395 | ) | (11,232 | ) | (21,284 | ) | (22,494 | ) | (9,494 | ) | ||||||||
Interest income |
311 | 1,469 | 500 | 154 | 25 | |||||||||||||
Interest expense |
(369 | ) | (1,268 | ) | (2,298 | ) | (2,434 | ) | (2,005 | ) | ||||||||
Other income (expense) |
(136 | ) | (580 | ) | 1,413 | (80 | ) | 1,416 | ||||||||||
Loss before income tax benefit |
(19,589 | ) | (11,611 | ) | (21,669 | ) | (24,854 | ) | (10,058 | ) | ||||||||
Income tax benefit |
— | — | 44 | 15 | — | |||||||||||||
Net loss |
$ | (19,589 | ) | $ | (11,611 | ) | $ | (21,625 | ) | $ | (24,839 | ) | $ | (10,058 | ) | |||
Net loss per common share—basic and diluted |
$ | (15.87 | ) | $ | (8.48 | ) | $ | (15.39 | ) | $ | (17.55 | ) | $ | (2.71 | ) | |||
Weighted-average number of common shares used in calculating net loss per common share—basic and diluted(1) |
1,234,538 | 1,368,999 | 1,405,140 | 1,415,083 | 3,705,505 | |||||||||||||
- (1)
- Please see Note 2 to our audited financial statements for an explanation of the method used to calculate basic and diluted net loss per common share attributable to common stockholders and the number of shares used in the computation of the per share amounts.
| |
As of December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | 2009 | 2010 | |||||||||||
| |
(in thousands) |
|||||||||||||||
Balance Sheet Data: |
||||||||||||||||
Cash, cash equivalents and short-term investments |
$ | 5,236 | $ | 32,491 | $ | 47,305 | $ | 14,503 | 78,591 | |||||||
Working capital (deficit) |
2,304 | 4,189 | 29,836 | 3,633 | 66,687 | |||||||||||
Total assets |
6,517 | 37,873 | 54,515 | 17,945 | 83,964 | |||||||||||
Notes payable |
7,863 | 8,262 | 14,289 | 10,724 | 7,741 | |||||||||||
Convertible preferred stock |
37,637 | 37,637 | 87,473 | 87,473 | — | |||||||||||
Accumulated deficit |
(43,047 | ) | (54,658 | ) | (76,283 | ) | (101,122 | ) | (111,180 | ) | ||||||
Total stockholders' equity (deficit) |
(42,530 | ) | (53,211 | ) | (72,911 | ) | (95,284 | ) | 56,393 | |||||||
62
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis together with our financial statements and the notes to those statements included elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth under "Risk Factors" and elsewhere in this Annual Report on Form 10-K, our actual results may differ materially from those anticipated in these forward-looking statements.
Overview
We are a biopharmaceutical company focused on discovering, developing and commercializing novel small-molecule therapeutics derived from our boron chemistry platform. We have discovered, synthesized and developed five molecules that are currently in clinical development.
Our three lead programs are: AN2690, a topical antifungal for the treatment of onychomycosis, a fungal infection of the nail and nail bed; AN2728, a topical anti-inflammatory for the treatment of psoriasis and atopic dermatitis; and GSK '052, a systemic antibiotic for the treatment of infections caused by Gram-negative bacteria. Our most advanced product candidate is AN2690. We initiated our Phase 3 clinical trials for AN2690 in the fourth quarter of 2010. In June 2010, we completed a Phase 2b dose-ranging trial in psoriasis for AN2728, and we initiated a final Phase 2b clinical trial in psoriasis in the first quarter of 2011. In June 2010, we completed a Phase 1 trial of GSK2251052, or GSK '052 (formerly referred to as AN3365), and achieved proof-of-concept as defined under our collaboration agreement with GlaxoSmithKline LLC, or GSK. In July 2010, GSK exercised its option to obtain an exclusive license to develop and commercialize GSK '052 and paid us an option exercise fee of $15.0 million in August 2010. In addition to our three lead programs, we have two other clinical product candidates, AN2718, our second topical antifungal product candidate, and AN2898, our second topical anti-inflammatory product candidate. We also have a pipeline of internally discovered topical and systemic boron-based compounds in development. In August 2010, we entered into a collaboration with Eli Lilly and Company, or Lilly, under which we will collaborate to discover products for a variety of animal health applications and in February 2011, we entered into a research and development collaboration with Medicis Pharmaceutical Corporation, or Medicis, to discover and develop compounds directed against a target for the potential treatment of acne.
In February 2007, we entered into an exclusive license, development and commercialization agreement with Schering Corporation, or Schering, for the development and worldwide commercialization of AN2690. Pursuant to the agreement, Schering paid us a $40.0 million non-refundable, non-creditable upfront fee and assumed sole responsibility for development and commercialization of AN2690. In addition, in accordance with the agreement, Schering invested $10.0 million in a preferred stock financing completed in December 2008. The agreement also obligated Schering to pay all of the remaining costs for development and commercialization of AN2690, including paying us for our development-related activities to transition AN2690 to Schering. In November 2009, Schering merged with Merck & Co., Inc., or Merck, and in May 2010, we entered into a mutual termination and release agreement with Merck. Under this agreement we regained the exclusive worldwide rights to AN2690, Merck paid us $5.8 million and we released each other from any and all claims, liabilities or other types of obligations under the 2007 agreement. Merck did not retain any rights to this compound.
In October 2007, we entered into a research and development collaboration, option and license agreement with GSK for the discovery, development and worldwide commercialization of boron-based systemic anti-infectives. Under the agreement, we are currently working to identify and develop multiple product candidates in three target-based project areas.
63
In each project, GSK has the option to obtain an exclusive license to develop, commercialize and market worldwide a specified number of product candidates once such candidates have achieved certain proof-of-concept criteria. We are primarily responsible for the discovery and development of each product candidate from the research stage until GSK exercises an option for such product candidate, at which point GSK will assume sole responsibility for the further development and commercialization of such product candidate on a worldwide basis. During the research term, we are committed to use reasonable efforts to discover and optimize compounds pursuant to agreed research plans and to provide specified resources, including certain numbers of full-time equivalent scientists, on a project-by-project basis. Each party is responsible for its own research and development costs.
Pursuant to the agreement, GSK paid us a $12.0 million non-refundable, non-creditable upfront fee in October 2007. In addition, GSK is obligated to make payments to us if certain development, regulatory and commercial milestones are met on a compound-by-compound basis, which range from up to $252.8 million to $330.5 million in the aggregate per product candidate, depending on the product profile of the candidate. Milestone payments may be lower for designated programs depending upon: whether GSK makes the selection of the product candidate before or after initiation of Phase I clinical trial dosing (10%-15% reduction if selected before such dosing); if certain target product profile characteristics are not achieved (20%-40% reduction); and whether the product candidate is designated after the initial two product candidate designations in a program (50% reduction). GSK is further obligated to pay us tiered double-digit royalties with the potential to reach the mid-teens on annual net sales of products containing optioned compounds in jurisdictions where there is a valid patent claim covering composition of matter or method of use of the product and lesser royalties for sales in jurisdictions where there is no such valid patent claim. Such royalties shall continue until the later of expiration of such valid patent claims or ten years from the first commercial sale on a product-by-product and country-by-country basis. GSK also invested $30.0 million in a preferred stock financing completed in December 2008, at which time GSK became a related party. Subsequently, in November 2010, GSK invested an additional $5.0 million in our common stock in connection with our initial public offering, or IPO. From execution of the agreement through December 31, 2010, in addition to the $12.0 million upfront payment, we have received $25.1 million for achievement of performance milestones, including milestones related to GSK '052 for lead declaration, candidate selection, first patient dosing in a clinical trial and an option to obtain an exclusive license to develop and commercialize GSK '052. GSK has assumed responsibility for further development of GSK '052 and any resulting commercialization.
In August 2010, we entered into a collaboration agreement with Lilly, under which the companies will collaborate to discover products for a variety of animal health applications. Lilly will be responsible for worldwide development and commercialization of compounds advancing from these efforts. We received a non-refundable, non-creditable upfront payment of $3.5 million in September 2010 and will receive a minimum of $6.0 million in research funding with the potential of up to $12.0 million in research funding, if successful. In addition, we will be eligible to receive payments upon the achievement of specified development and regulatory milestones, as well as tiered royalties escalating from high single digit to in the tens royalties on sales, depending in part upon the mix of products sold.
In February 2011, we entered into a research and development agreement with Medicis to discover and develop boron-based small molecule compounds directed against a target for the potential treatment of acne. Under the terms of the agreement, we received a $7.0 million upfront payment from Medicis in February 2011 and will be primarily responsible for discovering and conducting early development of product candidates that utilize our proprietary boron chemistry platform. Medicis will have an option to obtain an exclusive license for products covered by the agreement. We will be eligible for future research, development, regulatory and sales milestones of up to $153.0 million, as well as high single-digit to in the tens royalties on sales by Medicis. Medicis will be responsible for further development and commercialization of the licensed products on a worldwide basis.
64
We began business operations in March 2002. To date, we have not generated any revenue from product sales and have never been profitable. As of December 31, 2010, we have an accumulated deficit of $111.2 million. We have funded our operations primarily through the sale of equity securities, upfront payments and milestone payments under our agreements with Schering, GSK and Lilly, government contracts and grants and borrowings under debt arrangements. We expect to incur losses in future periods. The size of our future losses will depend, in part, on the rate of growth of our expenses, our ability to enter into additional licensing, research and development agreements and future payments earned under our agreements with GSK, Lilly, Medicis or any such future partners. Our intent is to enter into licensing and development agreements with additional partners to further develop certain of our product candidates and to fund other areas of our research. If the GSK, Lilly and/or Medicis agreements are terminated or we are unable to enter into other collaboration agreements, we may incur additional operating losses and our ability to expand and continue our research and development activities and move our product candidates into later stages of development may be limited. We will need to seek additional capital through collaborations, equity and debt financings to fund our operations.
Financial Operations Overview
Revenues
Our recent revenues are comprised primarily of collaboration agreement-related revenues and government grants, while historically we also had government contract revenues. Collaboration agreement-related revenues have included license fees, development reimbursements and development milestones. In addition, we have received a termination and release payment with respect to our previous agreement with Schering. Government grant revenues have consisted of grant funding received from government entities and government contract revenues have included cost and cost plus fixed fee reimbursements for allowable costs.
We have generated approximately $109.9 million in revenue from inception through December 31, 2010. Through 2004, we had recognized cumulative revenues of $16.1 million through our contract with the U.S. Department of Defense for the development of antibiotics against infective anthrax. From 2005 through 2007, we recognized $1.0 million in revenue from a National Institutes of Health, or NIH, grant for the identification of targets for certain antifungal compounds, which included work on the mechanism of action of AN2690. In March 2007, we received from Schering a $40.0 million upfront fee that was recognized ratably over the estimated period during which we performed development-related activities to transition AN2690 to Schering. The estimated period was based on the research transition plan jointly developed by Schering and us. In addition to the upfront fee, we were paid for our development-related activities, which included certain preclinical and clinical activities. Through December 31, 2010, we recognized revenue of $49.5 million under the Schering agreement, which was comprised of the full recognition of the $40.0 million upfront fee and $9.5 million for our development-related transition activities. In November 2009, Schering merged with Merck and in May 2010, upon termination of the 2007 agreement, we regained the exclusive worldwide rights to AN2690 and received a $5.8 million payment from Merck, which was recognized as revenue during 2010.
In October 2007, we received a $12.0 million upfront fee under our agreement with GSK, which we are recognizing ratably over the six-year research term. Through December 31, 2010, we recognized $32.5 million under this agreement which was comprised of $6.5 million related to the upfront fee, $25.1 million for achievement of performance milestones and $0.9 million in reimbursement for patent costs, procurement of drug material and performance of certain clinical studies for GSK '052. In the future, revenue under our agreement with GSK may include fees for our GSK product candidates achieving development, regulatory and sales milestones and product royalties.
65
In September 2010, we received a non-creditable, non-refundable upfront fee of $3.5 million under our agreement with Lilly, which we are recognizing over the four-year research term on a straight-line basis. Under this agreement, we will receive a minimum of $6.0 million in research funding with the potential of up to $12.0 million in research funding, if successful. Through December 31, 2010, we have recognized $1.4 million under this agreement, which was comprised of $0.3 million related to the upfront fee and $1.1 million in research funding.
From inception through December 31, 2010, we have recognized $2.1 million of revenue for research relating to animal health indications and for research performed under our agreements with not-for-profit organizations for neglected diseases.
In October 2010, we were awarded $1.5 million from the United States Department of the Treasury under the Qualifying Therapeutic Discovery Project Program to support research for six projects with the potential to produce new therapies. The full amount was recognized as government grant revenue in 2010.
Research and Development Expenses
Research and development expenses consist primarily of costs associated with research activities, as well as costs associated with our product development efforts, including preclinical studies and clinical trials. Research and development expenses, including those paid to third parties, are recognized as incurred. Research and development expenses include:
- •
- external research and development expenses incurred pursuant to agreements with third-party manufacturing organizations,
contract research organizations and investigational sites;
- •
- employee and consultant-related expenses, which include salaries, benefits, stock-based compensation and consulting fees;
- •
- third-party supplier expenses; and
- •
- facilities, depreciation and other allocated expenses, which include direct and allocated expenses for rent and maintenance of facilities, amortization or depreciation of leasehold improvements, equipment and laboratory supplies and other expenses.
Our expenses associated with preclinical studies and clinical trials are based upon the terms of the service contracts, the amount of the services provided and the status of the related activities. We expect that research and development expenses will increase significantly in the future as we progress our product candidates through clinical development, conduct our research and development activities under our agreements with GSK, Lilly and Medicis, advance our discovery research projects into the preclinical stage, and continue our early-stage research.
The table below sets forth our research and development expenses for 2008, 2009 and 2010, and for the period from January 1, 2004 through December 31, 2010 for our five clinical-stage product candidates, other work conducted under the GSK agreement, work on neglected diseases and other programs, including programs that are currently inactive. Prior to January 1, 2004, we did not separately track expenses for AN2690 or any other product candidates due to the early stage of their development. A portion of our research and development costs, including indirect costs relating to our
66
product candidates, are not tracked on a program basis and are allocated based on the personnel resources assigned to each program.
| |
Year Ended December 31, |
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Total from January 1, 2004 to December 31, 2010 |
|||||||||||||
| |
2008 | 2009 | 2010 | |||||||||||
| |
(in thousands) |
|||||||||||||
AN2690, including NIH grant expenses |
$ | 2,253 | $ | 363 | $ | 4,824 | $ | 32,173 | ||||||
AN2728 |
4,978 | 3,124 | 6,839 | 20,792 | ||||||||||
AN2718 |
3,167 | 1,112 | 169 | 7,730 | ||||||||||
AN2898 |
2,962 | 1,023 | 670 | 4,859 | ||||||||||
GSK programs: |
||||||||||||||
GSK '052 |
3,389 | 7,803 | 2,156 | 13,348 | ||||||||||
Other GSK programs |
14,804 | 14,515 | 5,237 | 36,723 | ||||||||||
Neglected diseases |
172 | 1,643 | 2,502 | 4,318 | ||||||||||
Other programs |
4,464 | 4,500 | 7,469 | 46,029 | ||||||||||
Total research and development expenses |
$ | 36,189 | $ | 34,083 | $ | 29,866 | $ | 165,972 | ||||||
We expect our research and development expenses to increase in future periods. Our costs associated with AN2690 will increase as we advance our Phase 3 clinical trials. We expect costs associated with AN2728 and AN2898 to increase as we expand the clinical and preclinical activities for these programs. Due to the option exercise by GSK for GSK '052 in July 2010, GSK has assumed all future development costs for this product candidate and therefore our expenses associated with GSK '052 are expected to be minimal in the future. In addition, spending for our early-stage research programs, including our other GSK programs, will be dependent upon our success in developing and advancing new product candidates for these programs. We also expect costs associated with our early stage research activities to increase in future periods primarily as a result of our recent collaborations with Lilly and Medicis and work on neglected diseases.
We cannot determine with certainty the duration and completion costs of the current or future clinical trials of our product candidates or if, when, or to what extent we will generate revenues from the commercialization and sale of any of our product candidates. We or our partners may never succeed in achieving marketing approval for any of our product candidates. The duration, costs and timing of clinical trials and development of our product candidates will depend on a variety of factors, including the uncertainties of future clinical and preclinical studies, uncertainties in clinical trial enrollment rates and significant and changing government regulation and whether our current or future collaborators are committed to and make progress in programs licensed to them. For example, the timing to complete development of GSK '052 will be controlled by GSK because they have exercised their option to license this product candidate. In addition, the probability of success for each product candidate will depend on numerous factors, including competition, manufacturing capability and commercial viability. Consequently, we are unable to provide a meaningful estimate of the period in which material cash inflows will be received. We will determine which programs to pursue and how much to fund each program in response to the scientific and clinical success of each product candidate, as well as an assessment of each product candidate's commercial potential.
Our strategy includes entering into additional collaborations with third parties for the development and commercialization of some of our product candidates. To the extent that third parties have control over preclinical development or clinical trials for some of our product candidates, we will be dependent upon their efforts for the progress of such product candidates. We cannot forecast with any degree of certainty which of our product candidates, if any, will be subject to future collaborations or how such arrangements would affect our development plans or capital requirements.
67
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for our personnel, including stock-based compensation and travel expenses, in executive, finance, business development and other administrative functions. Other general and administrative expenses include facility-related costs not otherwise included in research and development expenses, consulting costs associated with financial services, professional fees for legal services, including patent-related expenses, and auditing and tax services. We expect that general and administrative expenses will increase in the future as we expand our operating activities, hire additional staff and incur additional costs associated with operating as a public company.
Critical Accounting Policies and Significant Judgments and Estimates
Our management's discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenues and expenses during the reporting periods. We base our estimates on historical experience and on various other factors that we believe to be reasonable under the circumstances and review our estimates on an ongoing basis. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 2 of our financial statements included in this Annual Report on Form 10-K, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our financial statements.
Revenue Recognition
Our contract revenues are generated primarily through research and development collaboration agreements, which typically may include non-refundable, non-creditable upfront fees, funding for research and development efforts, payments for achievement of specified development, regulatory and sales milestones, and royalties on product sales of licensed products.
For multiple element arrangements, we evaluate the components of each arrangement as separate elements based on certain criteria. Accordingly, revenues from collaboration agreements are recognized based on the performance requirements of the agreements. We recognize revenue when persuasive evidence of an arrangement exists; transfer of technology has been completed, services are performed or products have been delivered; the fee is fixed and determinable; and collection is reasonably assured.
Upfront payments for licensing our intellectual property generally are not separable from the activity of providing research and development services because the license does not have stand-alone value separate from the research and development services provided. Such upfront payments are recorded as deferred revenue in the balance sheet and are recognized as contract revenue over the contractual or estimated performance period, which is consistent with the term of the research and development obligations contained in the research and development collaboration agreement. We regularly review the basis for our estimates, and we may change the estimates if circumstances change. These changes can significantly increase or decrease the amount of revenue recognized. To date, we have not experienced significant changes in our estimates.
Payments resulting from our research and development efforts under license agreements or government grants are recognized as the activities are performed and are presented on a gross basis. Revenue is recorded gross because we act as a principal, with discretion to choose suppliers, bear credit
68
risk and perform part of the services. The costs associated with these activities are reflected as a component of research and development expense in the statements of operations and approximate revenue recognized.
Substantive, at-risk milestone payments are recognized as revenue when the milestone is achieved and collectibility is assured. When payments are not for substantive and at-risk milestones, revenue is recognized over the estimated remaining term of the service period.
Royalties based on reported sales of licensed products will be recognized based on contract terms when reported sales are reliably measurable and collectability is reasonably assured. To date, we have not earned any royalty revenue from product sales.
Preclinical Study and Clinical Trial Accruals and Deferred Advance Payments
We estimate our preclinical study and clinical trial expenses based on the services performed pursuant to contracts with research institutions and clinical research organizations that conduct these activities on our behalf. In recording service fees, we estimate the time period over which the related services will be performed and compare the level of effort expended through the end of each period to the cumulative expenses recorded and payments made for such services and, as appropriate, accrue additional service fees or defer any non-refundable advance payments until the related services are performed. If the actual timing of the performance of services or the level of effort varies from the estimate, we will adjust our accrual or deferred advance payment accordingly. If we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. To date, we have not experienced significant changes in our estimates of preclinical study and clinical trial accruals.
Stock-Based Compensation
Employee stock-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the employee's requisite service period (generally the vesting period). We use the Black-Scholes option-pricing model as the most appropriate fair-value method for our stock-based awards. For options granted prior to January 1, 2006, we used the graded-vested (multiple option) method for expense attribution and, prior to January 1, 2006, recognized option forfeitures as they occurred. For options granted after January 1, 2006, we use the straight-line (single option) method for expense attribution and estimate forfeitures and recognize expense only for those shares expected to vest.
We account for equity instruments issued to nonemployees based on their fair values on the measurement dates using the Black-Scholes option-pricing model. The fair values of the options granted to nonemployees are remeasured as they vest. As a result, the noncash charge to operations for nonemployee options with vesting is affected each reporting period by changes in the fair value of our common stock.
We recorded noncash stock-based compensation expense for employee and nonemployee stock option grants of $2.0 million, $2.5 million, and $2.8 million during 2008, 2009 and 2010, respectively. Based on stock options outstanding as of December 31, 2010, we had unrecognized stock-based compensation expense for employees, net of estimated forfeitures, of $3.6 million, which will be recognized over a weighted-average period of 1.9 years. We expect to continue to grant stock options in the future, which will increase our stock-based compensation expense in future periods. If any of the assumptions used in the Black-Scholes model change significantly, stock-based compensation expense may differ materially in the future from that recorded in the current period.
As of December 31, 2010, we had outstanding vested options to purchase 1,275,609 shares of our common stock and unvested options to purchase 667,643 shares of our common stock with intrinsic
69
values of $3.0 million and $0.1 million, respectively, based on the differences between the exercise prices of the underlying options and the year-end closing price of our common stock of $5.37 per share.
Fair Values of Preferred Stock Warrants
Prior to the our IPO, outstanding warrants to purchase shares of our convertible preferred stock were freestanding warrants that were exercisable into convertible preferred stock that was subject to redemption and were therefore classified as liabilities in the balance sheet at fair value. The initial liability recorded was adjusted for changes in the fair values of our preferred stock warrants during each reporting period and was recorded as a component of other income (expense) in the statement of operations for that period.
We estimated the fair values of these warrants using the Black-Scholes option-pricing model based on inputs for the estimated fair value of the underlying convertible preferred stock at the valuation measurement date, the remaining contractual terms of the warrant, risk-free interest rates, expected dividend rates and expected volatility of the price of the underlying convertible preferred stock. We estimated the fair value of the convertible preferred stock using valuation analysis methods, inputs and assumptions consistent with those we used for our common stock valuations, giving consideration to the preferences and other terms of the convertible preferred stock. Our estimates were based, in part, on subjective assumptions.
Upon closing of our IPO and the conversion of the underlying preferred stock to common stock, all outstanding warrants to purchase shares of our preferred stock were automatically converted into warrants to purchase shares of our common stock. The aggregate fair value of our warrants upon the closing of the IPO was reclassified from liabilities to additional paid-in capital, a component of stockholders' equity (deficit), and we ceased recording any related periodic fair value adjustments as all such amounts are recorded in additional paid in capital.
Results of Operations
Comparison of Years Ended December 31, 2009 and 2010
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | Increase/ (decrease) |
|||||||
| |
(in thousands) |
|||||||||
Contract revenue |
$ | 9,793 | $ | 8,448 | $ | (1,345 | ) | |||
Contract revenue-related party |
8,850 | 17,909 | 9,059 | |||||||
Government grant revenue |
— | 1,467 | 1,467 | |||||||
Research and development expenses(1) |
34,083 | 29,866 | (4,217 | ) | ||||||
General and development expenses(1) |
7,054 | 7,452 | 398 | |||||||
Interest income |
154 | 25 | (129 | ) | ||||||
Interest expense |
2,434 | 2,005 | (429 | ) | ||||||
Other income (expense) |
(80 | ) | 1,416 | 1,496 | ||||||
- (1)
- Includes the following stock-based compensation expenses:
Research and development expenses |
$ | 1,557 | $ | 1,631 | $ | 74 | |||||
General and administrative expenses |
893 | 1,155 | 262 |
70
Contract revenue. During 2009, we recognized the final $8.7 million of the $40.0 million non-refundable, non-creditable, upfront fee under our license agreement with Schering as we completed all development-related transition activities. We also recognized $0.3 million of revenue from these transition activities performed by us. In addition, we recognized $0.8 million of revenue for research performed under other agreements, including under agreements with not-for-profit organizations for neglected diseases. During 2010, we recognized in full the $5.8 million fee that we received from Merck in connection with the termination of the Schering license agreement. We also recognized $1.1 million for research funding and $0.3 million of the $3.5 million non-refundable, non-creditable upfront fee we received under the Lilly agreement and $1.3 million of revenue for research performed under other agreements, including under agreements with not-for-profit organizations for neglected diseases.
Contract revenue-related party. During each of 2009 and 2010, we recognized $2.0 million of the $12.0 million non-refundable, non-creditable upfront fee received from GSK. We also recognized $6.8 million and $15.0 million in 2009 and 2010, respectively, for development milestones earned under this collaboration. In addition, during 2010, we recognized $0.9 million of revenue for reimbursement of patent and clinical trial costs related to GSK '052.
Government grant revenue. During 2010, we recognized the full $1.5 million of the Qualifying Therapeutic Discovery Project grant funds awarded to us in October 2010 by the United States Department of the Treasury for eligible investments we made in six of our projects during 2009 and 2010.
Research and development expenses. The decrease in research and development expenses for 2010 compared to 2009 was primarily due to decreases of $9.3 million and $5.6 million in our other GSK programs and GSK '052, respectively. In the latter part of 2009, we redirected some of our resources away from our early-stage GSK programs to focus our efforts more on GSK '052. This led to the reduction in spending on the other GSK programs in 2010. In 2009, we were in the midst of multiple development activities for GSK '052 whereas during 2010, our activities were limited to conducting the Phase 1 clinical trial for this compound. Furthermore, GSK became solely responsible for expenditures related to GSK '052 following their licensing of GSK '052 in July 2010. These two factors resulted in an overall reduction in GSK '052 expenses from 2009 to 2010.
Our GSK program spending decreases were partially offset by increases of $4.5 million, $3.7 million and $3.0 million in our AN2690, AN2728 and other research programs, respectively. Due to Merck notifying us in January 2010 that they were terminating our licensing agreement with Schering, we were actively transitioning AN2690 materials and documents from Schering back to us during 2010, as well as preparing to initiate the Phase 3 clinical trials, which commenced in the fourth quarter of 2010. In contrast, during the prior year, we were winding down our AN2690 development work in anticipation of Schering beginning Phase 3 trials later that year. During 2010, we were conducting our first Phase 2b trial for AN2728, completing some preclinical studies and initiating manufacturing activities in anticipation of starting additional preclinical studies and our final Phase 2b clinical trial in psoriasis for AN2728. This caused us to incur higher expenses for AN2728 in 2010 than during 2009 when we were winding down our initial Phase 2b clinical trial and initiating startup activities for our final Phase 2b psoriasis trial for AN2728.
Our efforts on our internal early-stage research projects increased during 2010 as compared to 2009 with increased expenses of $1.7 million for animal health-related research, including under our collaboration with Lilly, and $1.3 million more for other early-stage research projects.
General and administrative expenses. The increase in general and administrative expenses in the 2010 as compared to 2009 was primarily due to higher stock-based compensation expenses, increased fees for audit services and an increase in bonus expenses as we achieved a higher percentage of our
71
corporate goals in 2010 than in 2009. These increases were only partially offset by lower corporate legal expenses.
Interest income. The decrease in interest income was due to the reduction in investment balances and lower yields.
Interest expense. Interest expense decreased in 2010 due to the lower outstanding balance on our debt and the increase in the term over which we are now amortizing the final payment and debt discounts as a result of our January 2010 loan restructuring. This decrease was partially offset by additional interest expenses associated with the January 2010 loan restructuring in the form of accrued interest for the loan modification fee and amortization of the additional debt discount associated with the new warrant issued at that time.
Other income (expense). The increase in other income (expense) in 2010 compared to 2009 reflected the net post-issuance reduction in the fair values of our preferred stock warrants during 2010 in contrast with 2009 when the fair values of these warrants increased.
Comparison of Years Ended December 31, 2008 and 2009
| |
Year Ended December 31, |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Increase/ (decrease) |
|||||||||
| |
2008 | 2009 | ||||||||
| |
(in thousands) |
|||||||||
Contract revenue |
$ | 19,776 | $ | 9,793 | $ | (9,983 | ) | |||
Contract revenue-related party |
5,300 | 8,850 | 3,550 | |||||||
Research and development expenses(1) |
36,189 | 34,083 | (2,106 | ) | ||||||
General and administrative expenses(1) |
10,171 | 7,054 | (3,117 | ) | ||||||
Interest income |
500 | 154 | (346 | ) | ||||||
Interest expense |
2,298 | 2,434 | 136 | |||||||
Other income (expense) |
1,413 | (80 | ) | (1,493 | ) | |||||
- (1)
- Includes the following stock-based compensation expenses:
Research and development expenses |
$ | 1,263 | $ | 1,557 | $ | 294 | |||||
General and administrative expenses |
728 | 893 | 165 |
Contract revenue. During 2008, under our license agreement with Schering, we recognized $17.5 million of the $40.0 million non-refundable, non-creditable, upfront fee received in March 2007 and $2.3 million of revenue from development-related transition activities performed by us. During 2009, we recognized the remaining $8.7 million of the upfront fee and $0.3 million of revenue from transition activities. Transition activities were completed in June 2009 in accordance with our earlier estimates. During 2009, we also recognized $0.8 million of revenue for research performed under our other research agreements, including under agreements with not-for-profit organizations for neglected diseases.
Contract revenue-related party. In each of 2008 and 2009, we recognized $2.0 million of the $12.0 million non-refundable, non-creditable upfront fee received in October 2007 from GSK. We also recognized $3.3 million and $6.8 million in 2008 and 2009, respectively, for development milestones earned under the GSK collaboration.
Research and development expenses. The decrease in research and development expenses for 2009 compared to 2008 was due to decreases of $1.9 million, $1.9 million, $2.l million and $1.9 million in our AN2690, AN2728, AN2718 and AN2898 programs, respectively. In 2009, our transition activities for
72
AN2690 were winding down in preparation for the Phase 3 trials to be initiated by Schering. For our other clinical programs, the decreases were due to our decision to prioritize our 2009 spending on the GSK programs. Partially offsetting these decreases was an increase of $4.1 million in our GSK programs. In 2008, we were in the early research stage for our GSK programs, while in 2009, we advanced the development of these programs, including moving GSK '052 into preclinical and clinical development. In addition, partially offsetting these decreases was a $1.5 million increase in our neglected disease programs in 2009 compared to 2008.
General and administrative expenses. The decrease in general and administrative expenses for 2009 compared to 2008 was primarily due to the $2.4 million expensing of offering costs in 2008 when we withdrew our registration statement for an initial public offering of common stock with the Securities and Exchange Commission. In addition, salaries and benefits expenses declined by $0.6 million, primarily related to the resignations of two of our officers.
Interest income. The decrease in interest income was attributable to lower yields on our investment balances during 2009 as compared to those earned in 2008.
Interest expense. Interest expense increased in 2009 due to a full year's interest expense for the additional $7 million borrowing that occurred in April 2008 offset by lower interest expense on the original $8 million borrowing. Debt discount amortization related to the warrants associated with the debt increased due to the additional shares which became exercisable in January 2009 and the full year's amortization of the discount associated with the shares that became exercisable in May 2008.
Other income (expense). The decrease in other income (expense) reflects the increase in the fair values of the preferred stock warrants during 2009 in contrast with 2008 when other income (expense) reflected a decrease in the fair values of these warrants.
Income Taxes
At December 31, 2010, we had net operating loss carryforwards for federal income tax purposes of $95.5 million and federal research and development tax credit carryforwards of $4.6 million. Our utilization of the net operating loss and tax credit carryforwards may be subject to annual limitations due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. The annual limitations may result in the expiration of net operating losses and credits prior to utilization. We recorded a valuation allowance to offset in full the benefit related to the deferred tax assets because realization of this benefit was uncertain.
Liquidity and Capital Resources
Since our inception, we have financed our operations primarily through our initial public offering in November 2010, the private placement of equity securities, funding from our agreements with Schering, GSK and Lilly, government contract and grant funding and debt arrangements. In 2010, we received $61.0 million in net proceeds from the public sale of our common stock and $10.0 million from a concurrent private placement of our common stock. Through December 31, 2010, we have received aggregate net proceeds of $87.5 million from the issuance of convertible preferred stock which converted into common stock upon our initial public offering. In 2007, we received a $40.0 million non-refundable, non-creditable upfront fee from Schering and a $12.0 million non-refundable, non-creditable upfront fee from GSK. In 2010, we received a $3.5 million non-refundable, non-creditable upfront fee from Lilly and $1.1 million in research funding. In addition, from 2007 through 2010, we received $9.5 million and $26.0 million in reimbursements for development activities and milestone fees from Schering and GSK, respectively. In May 2010, we also received $5.8 million from Merck, in connection with the termination of our 2007 agreement with Schering. Through December 31, 2010, we have received $18.5 million from government contract and grant revenues and
73
borrowed $15.0 million under a loan agreement. We have also earned interest on our cash, cash equivalents and short-term investments.
The following table sets forth the primary sources and uses of cash for each of the periods presented below (in thousands):
| |
Year Ended December 31, |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Net cash used in operating activities |
$ | (39,390 | ) | $ | (27,824 | ) | $ | (3,182 | ) | |
Net cash provided by (used in) investing activities |
21,250 | (6,440 | ) | (51,153 | ) | |||||
Net cash provided by (used in) financing activities |
56,119 | (4,457 | ) | 67,494 | ||||||
Net increase (decrease) in cash and cash equivalents |
$ | 37,979 | $ | (38,721 | ) | $ | 13,159 | |||
The use of cash by operating activities in 2008 and 2009 resulted primarily from our net losses adjusted for non-cash items and changes in operating assets and liabilities, including the amortization of the deferred revenue amounts that arose in 2007 from the $52.0 million upfront license payments received under our collaboration agreements with Schering and GSK. The cash used in operating activities for 2010 resulted primarily from our net loss adjusted for non-cash items and changes in operating assets and liabilities, including the amortization of the deferred revenue that arose in 2007 from the $12.0 million upfront license payment received from GSK and an increase in accrued liabilities due to increased preclinical and clinical activities late in the year and liabilities arising from our initial public offering. The decrease in cash used by our operating activities in 2009 as compared with 2008 resulted primarily from a decline in deferred revenue amortization as we recognized only the remaining six-month portion of the Schering upfront payment in 2009 versus a full year of amortization in the prior year. The winding down of our AN2690 transition development obligations under the Schering agreement and our decision to defer certain development activities for our clinical stage programs to preserve our cash resources also contributed to the 2009 decrease. The decrease in cash used by operating activities during 2010 versus 2009 was primarily a result of the receipt of a $15.0 million milestone payment from GSK, a $5.8 million payment from Merck in connection with their termination of our agreement with Schering and a $3.5 million upfront license fee payment from Lilly in 2010 compared to the receipt of $9.8 million in milestone payments from GSK in 2009. A decrease in our spending on our GSK programs, only partially offset by increases in our development and clinical spending for AN2690 and AN2728, also contributed to the net decrease in cash used by operating activities in 2010.
During 2008, 2009 and 2010, our investing activities provided (used) cash of $21.3 million, $(6.4) million, and $(51.2) million, respectively. The cash provided by investing activities for 2008 was due primarily to the net result of maturities and purchases of short-term investments, which was partially offset by purchases of property and equipment of $2.1 million. The cash used by investing activities for 2009 was due primarily to the net result of purchases, maturities and sales of short-term investments. The cash used by investing activities for 2010 was primarily the net result of purchases and maturities of short-term investments as we invested the net proceeds from our initial public offering and our concurrent private placement.
During 2008, 2009 and 2010, our financing activities provided (used) cash of $56.1 million, $(4.5) million and $67.5 million, respectively. The cash provided by financing activities in 2008 was due primarily to the sale and issuance of 2,774,512 shares of Series E convertible preferred stock for total net proceeds of $46.8 million. Proceeds of $3.0 million from the issuance of convertible promissory notes, which along with the related accrued interest were converted into 177,771 shares of Series E convertible preferred stock, and proceeds of $7.0 million from the issuance of notes payable, net of $0.7 million in principal payments on notes payable, also contributed to the cash provided by financing
74
activities in 2008. Cash used by financing activities in 2009 was primarily a result of principal payments on notes payable. The cash provided by financing activities in 2010 was primarily due to our initial public offering of common stock. The Company sold 13,382,651 shares of common stock, including the over-allotment of 1,382,651 shares granted to the underwriters for net proceeds of $61.0 million. Concurrent with the closing of the offering, 2,000,000 common shares were sold through a private placement with net proceeds of $10.0 million. Partially offsetting these proceeds were principal payments on notes payable of $3.1 million and a loan restructuring fee payment of $0.5 million in 2010.
As of December 31, 2010, we had approximately $78.6 million in cash, cash equivalents and short-term investments. We believe that our existing cash, cash equivalents and short-term investments as of December 31, 2010, along with interest thereon, will be sufficient to meet our anticipated cash requirements for at least the next twelve months. However, our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially.
Our future capital requirements are difficult to forecast and will depend on many factors, including:
- •
- the initiation, progress, timing, costs and results of preclinical studies and clinical trials for our product candidates
and potential product candidates, including initiation and conduct of Phase 3 clinical trials for AN2690 and AN2728;
- •
- the success of our collaborations with GSK, Lilly and Medicis and the attainment of milestones and royalty payments, if
any, under those agreements;
- •
- the number and characteristics of product candidates that we pursue;
- •
- the terms and timing of any future collaboration, licensing or other arrangements that we may establish;
- •
- the outcome, timing and cost of regulatory approvals;
- •
- the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
- •
- the effect of competing technological and market developments;
- •
- the cost and timing of completion of commercial-scale outsourced manufacturing activities;
- •
- the cost of establishing sales, marketing and distribution capabilities for any product candidates for which we may
receive regulatory approval; and
- •
- the extent to which we acquire or invest in businesses, products or technologies.
75
Contractual Obligations
The following table summarizes our contractual obligations at December 31, 2010 and the effect such obligations are expected to have on our liquidity and cash flow in future years.
| |
Payments due by period (in thousands) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Total | Less than 1 year |
1-3 years | 3-5 years | More than 5 years |
|||||||||||
Contractual obligations: |
||||||||||||||||
Operating leases |
$ | 11,708 | $ | 1,815 | $ | 2,955 | $ | 3,150 | $ | 3,788 | ||||||
Notes payable (including interest) due in 2011 as of December 31, 2010 (See Notes Payable below as these amounts were refinanced in 2011.) |
8,430 | 8,430 | — | — | — | |||||||||||
Total contractual obligations |
$ | 20,138 | $ | 10,245 | $ | 2,955 | $ | 3,150 | $ | 3,788 | ||||||
Operating Leases
We lease a 36,960 square-foot building in Palo Alto, California consisting of office and laboratory space which serves as our corporate headquarters. The lease commenced in April 2008 and will terminate in March 2018.
We also have a lease for a 15,300 square-foot building consisting of office and laboratory space in Palo Alto, California, which was extended through December 2011 and is cancelable with four months' notice.
Notes Payable
On March 18, 2011, we entered into a loan and security agreement with new lenders to provide up to $30.0 million, available in three tranches of $10.0 million each (see Note 16 to the financial statements). The first $10.0 million tranche was drawn on March 18, 2011, at which time we repaid $6.6 million of the remaining obligations under our existing loan with Lighthouse Capital Partners V, L.P., or Lighthouse. At December 31, 2010, $6.2 million of the existing note payable balance has been classified as a long-term liability in the balance sheet. Future payments related to the first $10.0 million tranche of the new loan are $0.7 million, $2.9 million, $3.8 million, $3.8 million and $1.8 million in 2011, 2012, 2013, 2014 and 2015, respectively, and are not included as contractual obligations at December 31, 2010 in the table above.
As of December 31, 2010, our loan agreement with Lighthouse, required twelve remaining payments of principal and interest through December 1, 2011 and a $1.5 million final payment also due on December 1, 2011. From January 1, 2011 to March 1, 2011, we made loan payments to Lighthouse totaling $1.7 million prior to paying our remaining obligations to Lighthouse from the proceeds of the first tranche borrowed under our new March 2011 loan and security agreement.
Contracts
We enter into contracts in the normal course of business with clinical research organizations for clinical trials and clinical supply manufacturing and with vendors for preclinical research studies, research supplies and other services and products for operating purposes. These contracts generally provide for termination on notice, and therefore we believe that our noncancelable obligations under these agreements are not material.
76
Off-Balance Sheet Arrangements
Since inception, we have not engaged in the use of off-balance sheet arrangements, such as structured finance, special purpose entities or variable interest entities.
Recent Accounting Pronouncements
In October 2009, the FASB issued an Accounting Standards Update (ASU) No. 2009-13, which requires companies to allocate revenue in multiple element arrangements based on an element's estimated selling price if vendor-specific or other third-party evidence of value is not available. The standard is effective beginning January 1, 2011, although earlier application is permitted. We are currently evaluating both the timing and the impact of the pending adoption of this standard on our financial statements.
In April 2010, the FASB amended ASU 2010-17 which provides guidance on the milestone method of revenue recognition for research or development arrangements. An entity can make an accounting policy election to recognize a payment that is contingent upon the achievement of a substantive milestone in its entirety in the period in which the milestone is achieved. The milestone method is not required and is not the only acceptable method of revenue recognition for milestone payments. The standard is effective on a prospective basis for annual periods, and interim periods within those years, on or after June 15, 2010, although earlier application is permitted. We do not expect the adoption of this standard to have a material impact on our financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The primary objective of our investment activities is to preserve our capital for the purpose of funding operations while at the same time maximizing the income we receive from our investments without significantly increasing risk. To achieve these objectives, our investment policy allows us to maintain a portfolio of cash equivalents and short-term investments in a variety of high credit quality securities, including U.S. government instruments, commercial paper, money market funds and corporate debt securities. Our investment policy prohibits us from holding auction rate securities or derivative financial instruments. To the extent that the investment portfolios of companies whose commercial paper is included in our investment portfolio may be subject to interest rate risks, which could be negatively impacted by reduced liquidity in auction rate securities or derivative financial instruments, we may also be subject to these risks. However, our investments as of December 31, 2010 are comprised of U.S treasury securities, federal agency securities with a minimum rating of AAA or A1, and U.S. government guaranteed corporate bonds with a remaining average maturity of the entire portfolio of one hundred fifty-five days. Due to the short-term nature of our investments, we believe that there is no material exposure to interest rate risk and we are not aware of any material exposure to market risk.
77
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Index to Financial Statements
Contents
78
Report of Independent Registered Public Accounting Firm
The
Board of Directors and Stockholders
Anacor Pharmaceuticals, Inc.
We have audited the accompanying balance sheets of Anacor Pharmaceuticals, Inc. (the Company) as of December 31, 2009 and 2010 and the related statements of operations, convertible preferred stock and stockholders' equity (deficit) and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Anacor Pharmaceuticals, Inc. at December 31, 2009 and 2010, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles.
| /s/ ERNST & YOUNG LLP |
Palo
Alto, California
March 29, 2011
79
Anacor Pharmaceuticals, Inc.
Balance Sheets
(In Thousands, Except Share and Per Share Data)
| |
December 31 | |||||||
|---|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | ||||||
Assets |
||||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 8,584 | $ | 21,743 | ||||
Short-term investments |
5,919 | 56,848 | ||||||
Contract receivable |
73 | 1,066 | ||||||
Contract receivable—related party |
— | 18 | ||||||
Government grant receivable |
— | 108 | ||||||
Prepaid expenses and other current assets |
620 | 1,537 | ||||||
Total current assets |
15,196 | 81,320 | ||||||
Property and equipment, net |
2,462 | 1,844 | ||||||
Restricted investments |
196 | 197 | ||||||
Other assets |
91 | 603 | ||||||
Total assets |
$ | 17,945 | $ | 83,964 | ||||
Liabilities, convertible preferred stock and stockholders' equity (deficit) |
||||||||
Current liabilities: |
||||||||
Accounts payable |
$ | 1,746 | $ | 2,138 | ||||
Accrued liabilities |
3,628 | 6,963 | ||||||
Accrued liabilities—related party |
— | 125 | ||||||
Notes payable |
2,866 | 1,522 | ||||||
Preferred stock warrants liability |
1,273 | — | ||||||
Deferred revenue |
2,050 | 3,885 | ||||||
Total current liabilities |
11,563 | 14,633 | ||||||
Deferred rent |
835 | 902 | ||||||
Notes payable, less current portion |
7,858 | 6,219 | ||||||
Deferred revenue, less current portion |
5,500 | 5,817 | ||||||
Commitments and contingencies |
||||||||
Convertible preferred stock: $0.001 par value; authorized: 55,672,227 shares and no shares at December 31, 2009 and 2010, respectively; issued and outstanding: 10,909,478 shares and no shares at December 31, 2009 and 2010, respectively; aggregate liquidation value of $87,920 and none at December 31, 2009 and 2010, respectively; |
87,473 | — | ||||||
Stockholders' equity (deficit): |
||||||||
Preferred stock, $0.001 par value; authorized: no shares and 10,000,000 shares at December 31, 2009 and 2010, respectively; issued and outstanding: no shares at December 31, 2009 and 2010 |
— | — | ||||||
Common stock, $0.001 par value; authorized: 75,500,000 shares and 100,000,000 shares at December 31, 2009 and 2010; issued and outstanding: 1,443,696 shares and 27,996,878 shares at December 31, 2009 and 2010, respectively. |
1 | 28 | ||||||
Additional paid-in capital |
5,835 | 167,559 | ||||||
Accumulated other comprehensive income (loss) |
2 | (14 | ) | |||||
Accumulated deficit |
(101,122 | ) | (111,180 | ) | ||||
Total stockholders' equity (deficit) |
(95,284 | ) | 56,393 | |||||
Total liabilities, convertible preferred stock and stockholders' equity (deficit) |
$ | 17,945 | $ | 83,964 | ||||
See accompanying notes.
80
Anacor Pharmaceuticals, Inc.
Statements of Operations
(In Thousands, Except Share and Per Share Data)
| |
Year Ended December 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Revenues: |
||||||||||
Contract revenue |
$ | 19,776 | $ | 9,793 | $ | 8,448 | ||||
Contract revenue—related party |
5,300 | 8,850 | 17,909 | |||||||
Government grant revenue |
— | — | 1,467 | |||||||
Total revenues |
25,076 | 18,643 | 27,824 | |||||||
Operating expenses: |
||||||||||
Research and development |
36,189 | 34,083 | 29,866 | |||||||
General and administrative |
10,171 | 7,054 | 7,452 | |||||||
Total operating expenses |
46,360 | 41,137 | 37,318 | |||||||
Loss from operations |
(21,284 | ) | (22,494 | ) | (9,494 | ) | ||||
Interest income |
500 | 154 | 25 | |||||||
Interest expense |
(2,298 | ) | (2,434 | ) | (2,005 | ) | ||||
Other income (expense) |
1,413 | (80 | ) | 1,416 | ||||||
Loss before income tax benefit |
(21,669 | ) | (24,854 | ) | (10,058 | ) | ||||
Income tax benefit |
44 | 15 | — | |||||||
Net loss |
$ | (21,625 | ) | $ | (24,839 | ) | $ | (10,058 | ) | |
Net loss per common share—basic and diluted |
$ | (15.39 | ) | $ | (17.55 | ) | $ | (2.71 | ) | |
Weighted-average number of common shares used in calculating net loss per common share—basic and diluted |
1,405,140 | 1,415,083 | 3,705,505 | |||||||
See accompanying notes.
81
Anacor Pharmaceuticals, Inc.
Statements of Convertible Preferred Stock and Stockholders' Equity (Deficit)
(In Thousands, Except Share Data)
| |
Convertible Preferred Stock |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Preferred Stock | Common Stock | |
Accumulated Other Comprehensive Income |
|
Total Stockholders' Equity (Deficit) |
|||||||||||||||||||||||||||
| |
Additional Paid-In Capital |
Accumulated Deficit |
|||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||
Balance at December 31, 2007 |
7,957,195 | $ | 37,637 | — | $ | — | 1,402,595 | $ | 1 | $ | 1,366 | $ | 80 | $ | (54,658 | ) | $ | (53,211 | ) | ||||||||||||||
Issuance of Series E convertible preferred stock, net of financing costs |
2,774,512 | 46,825 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||
Issuance of Series E convertible preferred stock upon conversion of promissory notes and accrued interest |
177,771 | 3,011 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||
Issuance of common stock upon exercise of stock options |
— | — | — | — | 5,921 | — | 14 | — | — | 14 | |||||||||||||||||||||||
Stock-based compensation on options issued to consultants |
— | — | — | — | — | — | 282 | — | — | 282 | |||||||||||||||||||||||
Employee stock-based compensation expense |
— | — | — | — | — | — | 1,709 | — | — | 1,709 | |||||||||||||||||||||||
Comprehensive loss: |
|||||||||||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (21,625 | ) | (21,625 | ) | |||||||||||||||||||||
Change in unrealized gain on marketable securities |
— | — | — | — | — | — | — | (80 | ) | — | (80 | ) | |||||||||||||||||||||
Comprehensive loss |
(21,705 | ) | |||||||||||||||||||||||||||||||
Balance at December 31, 2008 |
10,909,478 | 87,473 | — | — | 1,408,516 | 1 | 3,371 | — | (76,283 | ) | (72,911 | ) | |||||||||||||||||||||
Issuance of common stock upon exercise of stock options |
— | — | — | — | 35,180 | — | 14 | — | — | 14 | |||||||||||||||||||||||
Stock-based compensation on options issued to consultants |
— | — | — | — | — | — | 193 | — | — | 193 | |||||||||||||||||||||||
Employee stock-based compensation expense |
— | — | — | — | — | — | 2,257 | — | — | 2,257 | |||||||||||||||||||||||
Comprehensive loss: |
|||||||||||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (24,839 | ) | (24,839 | ) | |||||||||||||||||||||
Change in unrealized gain on marketable securities |
— | — | — | — | — | — | — | 2 | — | 2 | |||||||||||||||||||||||
Comprehensive loss |
(24,837 | ) | |||||||||||||||||||||||||||||||
Balance at December 31, 2009 |
10,909,478 | 87,473 | — | — | 1,443,696 | 1 | 5,835 | 2 | (101,122 | ) | (95,284 | ) | |||||||||||||||||||||
Conversion of preferred stock to common stock |
(10,909,478 | ) | (87,473 | ) | — | — | 11,120,725 | 11 | 87,462 | — | — | 87,473 | |||||||||||||||||||||
Issuance of common stock upon initial public offering, net of issuance costs |
— | — | — | — | 12,000,000 | 12 | 54,583 | — | — | 54,595 | |||||||||||||||||||||||
Issuance of common stock in a private placement offering concurrent with the closing of the initial public offering, net of issuance costs |
— | — | — | — | 2,000,000 | 2 | 9,982 | — | — | 9,984 | |||||||||||||||||||||||
Issuance of common stock upon exercise of overallotment by underwriters, net of issuance costs |
— | — | — | — | 1,382,651 | 1 | 6,428 | — | — | 6,429 | |||||||||||||||||||||||
Issuance of common stock upon exercise of stock options |
— | — | — | — | 49,806 | 1 | 55 | — | — | 56 | |||||||||||||||||||||||
Reclassification of preferred stock warrants liability to additional paid-in capital in conjunction with the conversion of the convertible preferred stock to common stock upon initial public offering |
— | — | — | — | — | — | 428 | — | — | 428 | |||||||||||||||||||||||
Stock-based compensation on options issued to consultants |
— | — | — | — | — | — | 496 | — | — | 496 | |||||||||||||||||||||||
Employee stock-based compensation expense |
— | — | — | — | — | — | 2,290 | — | — | 2,290 | |||||||||||||||||||||||
Comprehensive loss: |
|||||||||||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (10,058 | ) | (10,058 | ) | |||||||||||||||||||||
Change in unrealized gain on marketable securities |
— | — | — | — | — | — | — | (16 | ) | — | (16 | ) | |||||||||||||||||||||
Comprehensive loss |
(10,074 | ) | |||||||||||||||||||||||||||||||
Balance at December 31, 2010 |
— | $ | — | — | $ | — | 27,996,878 | $ | 28 | $ | 167,559 | $ | (14 | ) | $ | (111,180 | ) | $ | 56,393 | ||||||||||||||
See accompanying notes.
82
Anacor Pharmaceuticals, Inc.
Statements of Cash Flows
(In Thousands)
| |
Year Ended December 31 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||||
Operating activities |
||||||||||||
Net loss |
$ | (21,625 | ) | $ | (24,839 | ) | $ | (10,058 | ) | |||
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
Depreciation and amortization |
536 | 752 | 725 | |||||||||
Amortization of debt issuance costs |
542 | 771 | 701 | |||||||||
Write-off of deferred initial public offering costs |
2,136 | — | — | |||||||||
Stock-based compensation |
1,991 | 2,450 | 2,786 | |||||||||
Change in fair value of preferred stock warrants liability |
(1,403 | ) | 80 | (1,415 | ) | |||||||
Amortization of premium on short-term investments |
(291 | ) | 322 | 100 | ||||||||
Accrual of final payment on notes payable |
459 | 424 | 480 | |||||||||
Changes in assets and liabilities: |
||||||||||||
Contract receivable |
759 | 194 | (993 | ) | ||||||||
Contract receivable—related party |
(3,000 | ) | 3,000 | (18 | ) | |||||||
Government grant receivable |
— | — | (108 | ) | ||||||||
Prepaid and other current assets |
(126 | ) | (10 | ) | (895 | ) | ||||||
Other assets |
(45 | ) | — | (558 | ) | |||||||
Accounts payable |
(1,793 | ) | (311 | ) | 392 | |||||||
Accrued liabilities |
1,199 | (89 | ) | 3,335 | ||||||||
Accrued liabilities—related party |
— | — | 125 | |||||||||
Deferred revenue |
(19,455 | ) | (10,677 | ) | 2,152 | |||||||
Deferred rent |
726 | 109 | 67 | |||||||||
Net cash used in operating activities |
(39,390 | ) | (27,824 | ) | (3,182 | ) | ||||||
Investing activities |
||||||||||||
Purchases of short-term investments |
(1,474 | ) | (32,039 | ) | (56,936 | ) | ||||||
Maturities of short-term investments |
24,850 | 23,785 | 5,890 | |||||||||
Sales of short-term investments |
— | 2,016 | — | |||||||||
Acquisition of property and equipment |
(2,126 | ) | (202 | ) | (107 | ) | ||||||
Net cash provided by (used in) investing activities |
21,250 | (6,440 | ) | (51,153 | ) | |||||||
Financing activities |
||||||||||||
Proceeds from the issuance of convertible preferred stock |
46,825 | — | — | |||||||||
Proceeds from initial public offering, net of issuance costs of $5,889 |
— | — | 61,024 | |||||||||
Proceeds from the issuance of common stock from private placement concurrent with initial public offering, net of issuance costs of $16 |
— | — | 9,984 | |||||||||
Proceeds from the exercise of stock options by employees and consultants |
14 | 14 | 56 | |||||||||
Proceeds from convertible promissory notes |
3,000 | — | — | |||||||||
Proceeds from notes payable |
7,000 | — | — | |||||||||
Principal payments on notes payable |
(720 | ) | (4,471 | ) | (3,120 | ) | ||||||
Payment of loan restructuring fee |
— | — | (450 | ) | ||||||||
Net cash provided by (used in) financing activities |
56,119 | (4,457 | ) | 67,494 | ||||||||
Net increase (decrease) in cash and cash equivalents |
37,979 | (38,721 | ) | 13,159 | ||||||||
Cash and cash equivalents at beginning of period |
9,326 | 47,305 | 8,584 | |||||||||
Cash and cash equivalents at end of period |
$ | 47,305 | $ | 8,584 | $ | 21,743 | ||||||
Supplemental schedule of noncash financing activities |
||||||||||||
Conversion of promissory notes and accrued interest to convertible preferred stock |
$ | 3,011 | $ | — | $ | — | ||||||
Conversion of preferred stock to common stock and additional paid-in capital |
$ | — | $ | — | $ | 87,473 | ||||||
Reclassification of preferred stock warrants liability to additional paid-in capital |
$ | — | $ | — | $ | 428 | ||||||
Supplemental disclosure of cash flow information |
||||||||||||
Interest paid, including payment of loan restructuring fee |
$ | 1,288 | $ | 1,238 | $ | 1,273 | ||||||
Fair value of warrants to purchase convertible preferred stock issued in connection with notes payable |
$ | 1,216 | $ | 256 | $ | 570 | ||||||
See accompanying notes.
83
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements
1. The Company
Nature of Operation
Anacor Pharmaceuticals, Inc. (the Company) was incorporated in the state of Delaware on December 14, 2000 and began business operations in March 2002. In November 2010, the Company completed an initial public offering (IPO) of its common stock (see Note 11). The Company is a biopharmaceutical company focused on discovering, developing and commercializing novel small-molecule therapeutics derived from its boron chemistry platform. The Company has discovered, synthesized and developed five molecules that are currently in clinical development. Its three lead programs are AN2690, a topical antifungal for the treatment of onychomycosis; AN2728, a topical anti-inflammatory for the treatment of psoriasis and atopic dermatitis; and GSK2251052 (GSK '052, formerly referred to as AN3365), a systemic antibiotic for the treatment of infections caused by Gram-negative bacteria. In addition to its three lead programs, the Company has two other clinical product candidates, AN2718, its second topical antifungal product candidate, and AN2898, its second topical anti-inflammatory product candidate. The Company also has a pipeline of internally discovered topical and systemic boron-based compounds in development.
The Company is also conducting research on additional systemic antibiotics under an October 2007 collaboration agreement with GlaxoSmithKline LLC (GSK). The Company entered into the six-year research and development collaboration, option and license agreement with GSK for the discovery, development and worldwide commercialization of boron-based systemic therapeutics (see Note 8). As a result of the purchase of shares of the Company's Series E convertible preferred stock in December 2008, GSK became a related party (see Notes 8 and 9).
In August 2010, the Company entered into a research agreement with Eli Lilly and Company (Lilly), under which the Company will collaborate to discover products for a variety of animal health applications using its boron-based technology platform and drug research capabilities and Lilly will be responsible for worldwide development and commercialization of compounds advancing from these efforts (see Note 8).
In February 2007, the Company entered into a worldwide license, development and commercialization agreement with Schering Corporation (Schering), a wholly owned subsidiary of Schering-Plough, for the development and commercialization of AN2690 (see Note 8). In November 2009, Schering merged with Merck & Co., Inc. (Merck) and in May 2010, Anacor regained the exclusive worldwide rights to AN2690. Merck did not retain any rights to this compound.
Management believes that the Company's cash, cash equivalents and short-term investments as of December 31, 2010 will be sufficient to fund the Company's operations through December 31, 2011. The Company has incurred cumulative losses of $111.2 million through December 31, 2010 and management expects the Company to incur losses for the next several years. The Company's ultimate success depends on the outcome of its research and development activities. The Company may seek to raise funds through the sale of equity or debt securities or through external borrowings. In addition, the Company may enter into additional collaborative arrangements or strategic partnerships for the development and commercialization of its compounds. If, over the next several years, adequate funds are not available, the Company may be required to delay, reduce the scope of, or eliminate one or more of its development programs.
84
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of the financial statements in accordance with U.S. generally accepted accounting principles requires the Company to make estimates and judgments in certain circumstances that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. In preparing these financial statements, management has made its best estimates and judgments of certain amounts included in the financial statements, giving due consideration to materiality. On an ongoing basis, the Company evaluates its estimates, including those related to revenue recognition, fair values of financial instruments in which we invest, convertible preferred stock and common stock, income taxes, preclinical study and clinical trial accruals and other contingencies. Management bases its estimates on historical experience or on various other assumptions that it believes to be reasonable under the circumstances. Actual results could differ from these estimates.
Cash, Cash Equivalents and Short-Term Investments
The Company considers all highly liquid investments with a maturity of three months or less at the time of purchase to be cash and cash equivalents. Investments with a maturity date of more than three months, but less than twelve months, from the date of purchase are considered short-term investments and are classified as current assets. The Company's short-term investments in marketable securities are classified as available for sale (see Note 3). Securities available for sale are carried at estimated fair value, with unrealized gains and losses reported as part of accumulated other comprehensive income or loss, a separate component of stockholders' equity (deficit). The Company has estimated the fair value amounts by using available market information. The cost of available-for-sale securities sold is based on the specific-identification method.
Fair Value of Financial Instruments
The carrying amounts of certain of the Company's financial instruments, including cash and cash equivalents, short-term investments, restricted investments, contract receivables and accounts payable, approximate their fair value due to their short maturities. Based on the borrowing rates available to the Company for loans with similar terms and average maturities, the carrying value of the Company's long-term notes payable approximate their fair values at December 31, 2009 and 2010.
Fair value is considered to be the price at which an asset could be exchanged or a liability transferred (an exit price) in an orderly transaction between knowledgeable, willing parties in the principal or most advantageous market for the asset or liability. Where available, fair value is based on or derived from observable market prices or other observable inputs. Where observable prices or inputs are not available, valuation models are applied. These valuation techniques involve management estimation and judgment, the degree of which is dependent on the price transparency for the instruments or market and the instruments' complexity.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist of cash, cash equivalents, short-term investments and restricted investments. Substantially all the Company's cash, cash equivalents and restricted investments are held by two financial institutions that management believes are of high credit quality. Such deposits may, at times, exceed federally insured limits. At December 31, 2009 and 2010, 91% and 62%, respectively, of the cash and cash equivalents
85
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
were held in a money market fund invested in U.S. Treasuries, securities guaranteed as to principal and interest by the U.S. government and repurchase agreements in respect of such securities. The Company's short-term investments at December 31, 2009 and 2010 were held in securities guaranteed as to principal and interest by the U.S. government. The Company has not experienced any losses on its deposits of cash, cash equivalents, short-term investments and restricted investments.
Customer Concentration
The Company's revenues consist primarily of contract revenue from collaboration agreements with Schering and GSK. Collaborators have accounted for significant revenues in the past and may not provide contract revenues in the future under existing agreements and/or new collaboration agreements, which may have a material affect on the Company's operating results.
Collaborators whose contract revenue or contract revenue—related party accounted for 10% or more of total revenues were as follows:
| |
Year Ended December 31 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Schering/Merck(1) |
79 | % | 48 | % | 21 | % | ||||
GSK (related party) |
21 | % | 47 | % | 64 | % | ||||
- (1)
- Agreement with Schering was terminated in May 2010 (see Note 8).
Contract Receivables and Related Valuation Account
The Company's contract receivables are primarily composed of amounts due under collaboration agreements and the Company believes that the credit risks associated with these collaborators are not significant. To date, the Company has not written-off any bad debt and, accordingly, does not have an allowance for doubtful accounts at December 31, 2009 and 2010.
The contract receivable at December 31, 2009 includes $0.1 million of reimbursable development costs due from Schering. The contract receivable at December 31, 2010 consists of $0.8 million in research funding due from Lilly (see Note 8) and $0.3 million due related to other contracts. The contract receivable—related party at December 31, 2010 represents reimbursable patent costs of $18,000 due from GSK (see Note 8). No amount was due from GSK at December 31, 2009.
Restricted Investments
Under its facility lease agreements, the Company is required to secure letters of credit. At December 31, 2009 and 2010, the Company had approximately $0.2 million of restricted investments to secure these letters of credit.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization. Depreciation and amortization are calculated using the straight-line method over the estimated useful
86
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
lives of the respective assets, generally three to five years. Leasehold improvements are amortized over the shorter of the remaining lease term or the estimated useful economic lives of the related assets.
Impairment of Long-Lived Assets
Losses from impairment of long-lived assets used in operations are recorded when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets' carrying amounts. The Company regularly evaluates its long-lived assets for indicators of possible impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. An impairment loss would be recognized when estimated undiscounted future cash flows expected to result from the use of an asset and its eventual disposition are less than its carrying amount. Impairment, if any, is assessed using discounted cash flows.
Revenue Recognition
The Company's contract revenues are generated primarily through research and development collaboration agreements, which typically may include non-refundable, non-creditable upfront fees, funding for research and development efforts, payments for achievement of specified development, regulatory and sales milestones and royalties on product sales of licensed products.
For multiple element arrangements, the Company evaluates the components of each arrangement as separate elements based on certain criteria. Accordingly, revenues from collaboration agreements are recognized based on the performance requirements of the agreements. The Company recognizes revenue when persuasive evidence of an arrangement exists; transfer of technology has been completed, services are performed or products have been delivered; the fee is fixed and determinable; and collection is reasonably assured.
Upfront payments for licensing the Company's intellectual property generally are not separable from the activity of providing research and development services because the license does not have stand-alone value separate from the research and development services provided. Such upfront payments are recorded as deferred revenue in the balance sheet and are recognized as contract revenue over the contractual or estimated performance period, which is consistent with the term of the research and development obligations contained in the research and development collaboration agreement.
Payments resulting from the Company's research and development efforts under license agreements or government grants are recognized as the activities are performed and are presented on a gross basis. Revenue is recorded gross because the Company acts as a principal, with discretion to choose suppliers, bears credit risk and performs part of the services. The costs associated with these activities are reflected as a component of research and development expense in the statements of operations and approximate revenue recognized.
Substantive, at-risk milestone payments are recognized as revenue when the milestone is achieved and collectibility is reasonably assured. When payments are not for substantive and at-risk milestones, revenue will be recognized over the estimated remaining term of the service period.
Royalties based on reported sales of licensed products will be recognized based on contract terms when reported sales are reliably measurable and collectibility is reasonably assured. To date, none of
87
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
the Company's products have been approved and therefore the Company has not earned any royalty revenue from product sales.
Research and Development Expenses
All research and development expenses, including those funded by third parties, are expensed as incurred. Research and development expenses include, but are not limited to, salaries, benefits, stock-based compensation, lab supplies, allocated overhead, fees for professional service providers and costs associated with product development efforts, including preclinical studies and clinical trials.
Preclinical Study and Clinical Trial Accruals and Deferred Advance Payments
The Company estimates preclinical study and clinical trial expenses based on the services performed pursuant to contracts with research institutions and clinical research organizations that conduct these activities on its behalf. In recording service fees, the Company estimates the time period over which the related services will be performed and compares the level of effort expended through the end of each period to the cumulative expenses recorded and payments made for such services and, as appropriate, accrues additional service fees or defers any non-refundable advance payments until the related services are performed. If the actual timing of the performance of services or the level of effort varies from the estimate, the Company will adjust its accrual or deferred advance payment accordingly. If the Company later determines that it no longer expects the services associated with a deferred non-refundable advance payment to be rendered, the deferred advance payment will be charged to expense in the period that such determination is made.
Fair Values of Preferred Stock Warrants
Prior to the Company's IPO, outstanding warrants to purchase shares of its Series D and Series E convertible preferred stock were freestanding warrants that were exercisable into convertible preferred stock that was subject to redemption and were therefore classified as liabilities in the balance sheet at fair value. The initial liability recorded was adjusted for changes in the fair values of the Company's preferred stock warrants during each reporting period and was recorded as a component of other income (expense) in the statement of operations for that period.
Upon closing of the Company's IPO and the conversion of the underlying preferred stock to common stock, all outstanding warrants to purchase shares of preferred stock were automatically converted into warrants to purchase shares of the Company's common stock. The aggregate fair value of these warrants upon the closing of the IPO was $0.4 million which was reclassified from liabilities to additional paid-in capital, a component of stockholders' equity (deficit), and the Company ceased recording any related periodic fair value adjustments. The Company estimated the fair values of these warrants using the Black-Scholes option-pricing model, based on the inputs for the estimated fair value of the underlying convertible preferred stock at the valuation measurement date, the remaining contractual term of the warrant, risk-free interest rates, expected dividend rates and expected volatility of the price of the underlying convertible preferred stock. These estimates were based on subjective assumptions.
88
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
Stock-Based Compensation
Employee stock-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the employee's requisite service period (generally the vesting period). The Company uses the Black-Scholes option-pricing model to estimate the fair value of its stock-based awards. For options granted prior to January 1, 2006, the Company used the graded-vested (multiple-option) method for expense attribution and, prior to January 1, 2006, recognized option forfeitures as they occurred. For options granted after January 1, 2006, the Company uses the straight-line (single-option) method for expense attribution, estimates forfeitures and recognizes expense only for those shares expected to vest.
The Company accounts for equity instruments issued to nonemployees based on their fair values on the measurement dates using the Black-Scholes option-pricing model. The fair values of the options granted to nonemployees are remeasured as they vest. As a result, the noncash charge to operations for nonemployee options with vesting is affected each reporting period by changes in the fair value of the Company's common stock.
Income Taxes
The Company utilizes the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax reporting bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Company recorded a full valuation allowance at each balance sheet date presented. Based on the available evidence, the Company believes that it is more likely than not that it will be unable to utilize all of its deferred tax assets in the future. The Company intends to maintain the full valuation allowances until there is sufficient evidence to support the reversal of the valuation allowances.
The Company accounts for uncertain tax positions in the financial statements when it is more likely than not that the position will be sustained upon examination by the tax authorities. Such tax positions must initially and subsequently be measured as the largest amount of tax benefit that has a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority assuming full knowledge of the position and relevant facts. The Company's policy is to recognize any interest and penalties related to income taxes in income tax expense.
Net Loss per Common Share
Basic net loss per common share is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period, without consideration for common stock equivalents. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted-average number of common share equivalents outstanding for the period determined using the treasury-stock method. For purposes of this calculation, potentially dilutive securities consisting of convertible preferred stock, stock options and warrants are considered to be common stock equivalents and were excluded in the calculation of diluted net loss per share because their effect would be antidilutive.
89
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
The following table presents the calculation of basic and diluted net loss per share of common stock (in thousands, except share and per share data):
| |
Year Ended December 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Historical net loss per common share |
||||||||||
Numerator: |
||||||||||
Net loss |
$ | (21,625 | ) | $ | (24,839 | ) | $ | (10,058 | ) | |
Denominator: |
||||||||||
Weighted-average number of common shares used in calculating net loss per common share—basic and diluted |
1,405,140 | 1,415,083 | 3,705,505 | |||||||
Net loss per common share—basic and diluted |
$ | (15.39 | ) | $ | (17.55 | ) | $ | (2.71 | ) | |
Comprehensive Loss
Comprehensive loss and its components are reported in the statements of convertible preferred stock and stockholders' equity (deficit). Comprehensive loss consists of net loss and unrealized gains or losses on marketable securities.
Recent Accounting Pronouncements
In October 2009, the FASB issued an Accounting Standards Update (ASU) No. 2009-13, which requires companies to allocate revenue in multiple element arrangements based on an element's estimated selling price if vendor-specific or other third-party evidence of value is not available. The standard is effective for the Company beginning January 1, 2011, although earlier application is permitted. The Company is currently evaluating both the timing and the impact of the pending adoption of this standard on its financial statements.
In April 2010, the FASB amended ASU 2010-17 which provides guidance on the milestone method of revenue recognition for research or development arrangements. An entity can make an accounting policy election to recognize a payment that is contingent upon the achievement of a substantive milestone in its entirety in the period in which the milestone is achieved. The milestone method is not required and is not the only acceptable method of revenue recognition for milestone payments. The standard is effective on a prospective basis for annual periods, and interim periods within those years, on or after June 15, 2010, although earlier application is permitted. The Company does not expect the adoption of this standard to have a material impact on its financial statements.
90
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
3. Marketable Securities
At December 31, 2009, the amortized cost and fair value of marketable securities, with gross unrealized gains and losses, were as follows (in thousands):
| |
Amortized Cost | Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Money market fund |
$ | 7,844 | $ | — | $ | — | $ | 7,844 | ||||||
Federal agency securities |
5,917 | 2 | — | 5,919 | ||||||||||
Total marketable securities |
$ | 13,761 | $ | 2 | $ | — | $ | 13,763 | ||||||
Reported as: |
||||||||||||||
Cash and cash equivalents |
$ | 7,844 | ||||||||||||
Short-term investments |
5,919 | |||||||||||||
Total marketable securities |
$ | 13,763 | ||||||||||||
At December 31, 2010, the amortized cost and fair value of marketable securities, with gross unrealized gains and losses, were as follows (in thousands):
| |
Amortized Cost | Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Money market fund |
$ | 13,413 | $ | — | $ | — | $ | 13,413 | ||||||
U.S. treasury securities |
14,363 | — | (2 | ) | 14,361 | |||||||||
Federal agency securities |
29,259 | 1 | (11 | ) | 29,249 | |||||||||
U.S. government guaranteed corporate bonds |
21,184 | 1 | (3 | ) | 21,182 | |||||||||
Total marketable securities |
$ | 78,219 | $ | 2 | $ | (16 | ) | $ | 78,205 | |||||
Reported as: |
||||||||||||||
Cash and cash equivalents |
$ | 21,357 | ||||||||||||
Short-term investments |
56,848 | |||||||||||||
Total marketable securities |
$ | 78,205 | ||||||||||||
All marketable securities held at December 31, 2009 and 2010 had original maturities at the date of purchase of less than one year.
The Company recognized gross realized gains of $4,000 in 2009 from the sale of marketable securities. There were no realized gains or losses recognized from the sale of marketable securities in 2008 or 2010.
91
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
4. Balance Sheet Detail
Property and Equipment
Property and equipment consists of the following (in thousands):
| |
December 31 | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | |||||
Laboratory equipment |
$ | 2,910 | $ | 2,944 | |||
Furniture and fixtures |
171 | 171 | |||||
Computer equipment and software |
823 | 886 | |||||
Leasehold improvements |
1,271 | 1,278 | |||||
|
5,175 | 5,279 | |||||
Less: accumulated depreciation and amortization |
(2,713 | ) | (3,435 | ) | |||
Property and equipment, net |
$ | 2,462 | $ | 1,844 | |||
Accrued Liabilities
Accrued liabilities consist of the following (in thousands):
| |
December 31 | ||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | |||||
Accrued compensation |
$ | 1,212 | $ | 1,656 | |||
Accrued preclinical study and clinical trial costs |
1,433 | 3,310 | |||||
Other |
983 | 1,997 | |||||
Accrued liabilities |
$ | 3,628 | $ | 6,963 | |||
Accrued Liabilities—Related Party
The accrued liabilities—related party balance of $0.1 million at December 31, 2010 consists of the unused portion of an advance for GSK '052 clinical trial costs due to GSK. This amount will be repaid in 2011. No amount was due to GSK at December 31, 2009.
5. Fair Value Measurements
In measuring fair value, the Company evaluates valuation techniques such as the market approach, the income approach and the cost approach. A three-level valuation hierarchy that prioritizes the inputs to valuation techniques is used to measure fair value based upon whether such inputs are observable or unobservable.
Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions made by the reporting entity. The three-level hierarchy for the inputs to valuation techniques is briefly summarized as follows:
- •
- Level 1—Observable inputs such as quoted prices (unadjusted) for identical instruments in active markets;
92
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
5. Fair Value Measurements (Continued)
- •
- Level 2—Observable inputs such as quoted prices for similar instruments in active markets, quoted prices for identical or similar
instruments in markets that are not active, or model-derived valuations
whose significant inputs are observable; and
- •
- Level 3—Unobservable inputs that reflect the reporting entity's own assumptions.
The Company's financial assets and financial liabilities subject to fair value measurements on a recurring basis and the levels of the inputs used in such measurements are as follows (in thousands):
| |
Fair Value Measurements on a Recurring Basis at December 31, 2009 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Level 1 | Level 2 | Level 3 | Total | ||||||||||
| |
Quoted Prices in Active Markets for Identical Items |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
Balance at December 31, 2009 |
||||||||||
Financial assets: |
||||||||||||||
Money market fund |
$ | 7,844 | $ | — | $ | — | $ | 7,844 | ||||||
Federal agency securities |
— | 5,919 | — | 5,919 | ||||||||||
Total marketable securities |
$ | 7,844 | $ | 5,919 | $ | — | $ | 13,763 | ||||||
Financial liabilities: |
||||||||||||||
Preferred stock warrants liability |
$ | — | $ | — | $ | 1,273 | $ | 1,273 | ||||||
| |
Fair Value Measurements on a Recurring Basis at December 31, 2010 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Level 1 | Level 2 | Level 3 | Total | ||||||||||
| |
Quoted prices in Active Markets for Identical Items |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
Balance at December 31, 2010 |
||||||||||
Financial assets: |
||||||||||||||
Money market fund |
$ | 13,413 | $ | — | $ | — | $ | 13,413 | ||||||
U.S. treasury securities |
— | 14,361 | — | 14,361 | ||||||||||
Federal agency securities |
— | 29,249 | — | 29,249 | ||||||||||
U.S. government guaranteed corporate bonds |
— | 21,182 | — | 21,182 | ||||||||||
Total marketable securities |
$ | 13,413 | $ | 64,792 | $ | — | $ | 78,205 | ||||||
The fair values of the money market fund were derived from quoted market prices in an active market. Federal agency securities are valued using third-party pricing sources that apply applicable inputs and other relevant data into their models to estimate fair value.
93
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
5. Fair Value Measurements (Continued)
The following table presents changes in liabilities measured at fair value on a recurring basis using Level 3 inputs (in thousands):
| |
Preferred Stock Warrants Liability |
|||
|---|---|---|---|---|
Balance at December 31, 2008 |
$ | 937 | ||
Fair value of January 2009 increase in number of shares available for purchase under warrant issued in connection with 2008 borrowing (see Note 6) |
256 | |||
Increase in fair value included in other expense |
80 | |||
Balance at December 31, 2009 |
1,273 | |||
Fair value of new warrant issued in connection with January 2010 loan modification (see Note 6) |
570 | |||
Decrease in fair value included in other income |
(1,415 | ) | ||
Reclassification of preferred stock warrants liability to additional paid-in capital in conjunction with the conversion of the convertible preferred stock to common stock upon the IPO |
(428 | ) | ||
Balance at December 31, 2010 |
$ | — | ||
The Company's valuation technique to measure the fair value of the preferred stock warrants liability was based on valuations prepared by the Company, with the assistance of independent consultants, using unobservable inputs, including the Company's assumptions about projected revenues, expenses, cash flows, risk-based rates of return on projected cash flows, expected volatility and marketability discounts applicable to the Company's common and convertible preferred stock. The Company remeasured the fair value of the preferred stock warrants liability at each reporting period using current assumptions, with decreases (increases) in fair value recorded as other income (expense). The preferred stock warrants liability was reclassified to additional paid-in capital, a component of stockholders equity (deficit) subsequent to the Company's IPO in November 2010 (see Note 10).
6. Notes Payable
In 2006, the Company entered into a loan agreement for $8.0 million (the Original Loan). The related notes are secured by all assets of the Company except intellectual property. The notes required interest-only payments for the period from initial borrowing through December 2007 at prime plus 2% resulting in interest rates that ranged from 9.50% to 10.25% per annum, followed by 30 monthly payments of principal and interest at an interest rate of 9.25% per annum. A final payment of $0.8 million due one month after the 30 monthly principal and interest payments, or in June 2010, was being accrued over the term of the loan into interest expense. A debt discount, related to the initial fair value of a warrant for shares of Series D convertible preferred stock issued in conjunction with the Original Loan, is being amortized to interest expense over the term of the notes (see Note 10).
In May 2008, the terms of the Original Loan were modified effective April 1, 2008, in conjunction with a new $7.0 million borrowing from the same lender. For the $7.3 million Original Loan balance outstanding at March 31, 2008, the Company, in accordance with the new terms, was required to make
94
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
6. Notes Payable (Continued)
interest-only payments through April 2009 at 9.75% per annum, followed by 24 monthly payments of principal and interest at an interest rate of 9.25% per annum. An amended final payment of $1.1 million comprised of the original final payment of $0.8 million and a new loan amendment fee of $0.3 million, was due one month after the 24 monthly principal and interest payments, or April 2011. The portion of this amended final payment not already accrued at March 31, 2008 is being accrued over the remaining revised term of the loan into interest expense.
For the additional $7.0 million borrowed by the Company in May 2008, the related note required interest-only payments through April 2009 at 12% per annum, followed by 24 monthly payments of principal and interest at an interest rate of 9.25%. A final payment of $0.4 million due at the end of the 36-month term, or April 2011, is being accrued over the term of the loan into interest expense. A debt discount, related to the initial fair value of a warrant for shares of Series D convertible preferred stock issued in conjunction with the additional $7.0 million borrowing in May 2008 and the January 2009 increase in the number of shares available for purchase under this warrant, is being amortized to interest expense over the term of the new note (see Note 10). The note is secured by all assets of the Company except intellectual property.
In January 2010, the Company negotiated changes to the existing loan agreement and obtained an interest-only payment period of 6 months beginning on January 1, 2010. During the interest-only period, principal payments were deferred and interest was paid at a 9.25% annual interest rate. Monthly payments of principal and interest at the same annual interest rate began on July 1, 2010 and run through December 1, 2011. A restructuring fee of $0.5 million was paid on July 1, 2010. On December 1, 2011, a $1.5 million final payment, which includes all previous final payments, is due. Both the $0.5 million restructuring fee and the portion of the $1.5 million final payment not already accrued at December 31, 2009 are being accrued into interest expense over the remaining revised term of the loan. In conjunction with the restructuring, the Company issued the debt-holder a warrant to purchase 40,623 shares of the Company's Series E convertible preferred stock at an exercise price of $16.936 per share. The warrant is exercisable until January 1, 2017.
In connection with the closing of the IPO in November 2010, all three of the preferred stock warrants automatically converted to common stock warrants (see Note 10).
Future payments at December 31, 2010 are as follows (in thousands):
Total minimum payments due in 2011 as of December 31, 2010; |
|||||
refinanced in 2011 (see below) |
$ | 8,430 | |||
Less amount representing interest |
1,741 | ||||
Notes payable, gross |
6,689 | ||||
Unamortized discount on notes payable |
(236 | ) | |||
Accretion of the final payment |
1,288 | ||||
|
7,741 | ||||
Less current portion of notes payable, including unamortized discount |
1,522 | ||||
Notes payable, less current portion |
$ | 6,219 | |||
On March 18, 2011, the Company entered into a loan and security agreement with new lenders to provide up to $30.0 million, available in three tranches of $10.0 million each (see Note 16). The first
95
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
6. Notes Payable (Continued)
$10.0 million tranche was drawn on March 18, 2011, at which time the Company repaid $6.6 million of the remaining obligations under its existing loan. As of December 31, 2010, $6.2 million of the existing note payable balance has been classified, and is reflected in the table above, as a long-term liability in the balance sheet.
The Company recorded interest expense related to all borrowings, including the amortization of the debt discounts, the amortization of direct financing costs related to the Original Loan and the interest expense related to the accretion of the final payments of $2.3 million, $2.4 million and $2.0 million for 2008, 2009 and 2010, respectively. The annual effective interest rate after consideration of the January 2010 restructuring, including the amortization of the debt discounts and accretion of the final payments is 21.42%.
7. Commitments and Contingencies
Operating Leases
The Company leases a 36,960 square-foot building consisting of office and laboratory space in Palo Alto, California, for its corporate headquarters. The lease term commenced in March 2008 and has scheduled annual rent increases through the lease expiration in March 2018. Rent expense is recognized on a straight-line basis over the term of the lease. The Company is also responsible for certain operating expenses. The lease provided a $0.4 million allowance from the landlord for tenant improvements. This amount has been included in deferred rent in the accompanying December 31, 2009 and 2010 balance sheets and is being amortized over the term of the lease, on a straight-line basis, as a reduction to rent expense. Under the lease agreement, the Company provided a security deposit in the amount of $0.1 million in the form of a standing letter of credit.
The Company also continues to lease a 15,300 square-foot building consisting of office and laboratory space in Palo Alto, California. The operating lease, which would have expired in December 2009, was extended for one year to December 31, 2010. In September 2010, the Company exercised its option to extend the lease for another one-year extension to December 31, 2011. The lease is cancelable with four months' notice. The Company currently provides a $0.1 million standing letter of credit as a security deposit under its lease agreement, which it will continue to provide with any lease extensions.
Rent expense under all facility operating leases was $1.5 million, $1.8 million and $1.8 million for 2008, 2009, and 2010, respectively. Deferred rent of $0.8 million and $0.9 million at December 31, 2009 and 2010, respectively, is included in the accompanying balance sheets and represents the difference between recorded rent expense and the cash payments related to the operating lease, plus the unamortized portion of the tenant improvement allowance.
96
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
7. Commitments and Contingencies (Continued)
At December 31, 2010, future minimum cash payments under facility operating leases with initial terms in excess of one year are as follows (in thousands):
2011 |
$ | 1,815 | |||
2012 |
1,454 | ||||
2013 |
1,501 | ||||
2014 |
1,550 | ||||
2015 |
1,600 | ||||
Thereafter |
3,788 | ||||
Total future minimum lease payments |
$ | 11,708 | |||
Guarantees and Indemnifications
The Company, as permitted under Delaware law and in accordance with its bylaws, indemnifies its officers and directors for certain events or occurrences, subject to certain limits, while the officer or director is or was serving at the Company's request in such capacity. The term of the indemnification period is equal to the officer's or director's lifetime.
The maximum amount of potential future indemnification is unlimited; however, the Company currently holds director and officer liability insurance. This insurance limits the Company's exposure and may enable it to recover a portion of any future amounts paid. The Company believes that the fair value of these indemnification obligations is minimal. Accordingly, the Company has not recognized any liabilities relating to these obligations for any period presented.
The Company has certain agreements with contract research organizations with which it does business that contain indemnification provisions pursuant to which the Company typically agrees to indemnify the party against certain types of third-party claims. The Company accrues for known indemnification issues when a loss is probable and can be reasonably estimated. The Company would also accrue for estimated incurred but unidentified indemnification issues based on historical activity. There were no accruals for or expenses related to indemnification issues for any period presented.
8. License, Research, Development and Commercialization Agreements
GSK
In October 2007, the Company entered into a research and development collaboration, option and license agreement with GSK for the discovery, development, and worldwide commercialization of boron-based systemic therapeutics. The collaborative research term of the agreement is six years, subject to an extension of up to two years if agreed to by both parties. Under the agreement, the Company is currently working to identify and develop multiple product candidates in three target-based project areas. In each project, GSK has the option to obtain an exclusive license to develop, commercialize and market worldwide a specified number of product candidates once they have achieved certain proof-of-concept criteria.
Upon exercise of an option, GSK assumes sole responsibility for the further development and commercialization of the applicable product candidate. GSK is obligated to make payments to the Company if certain development, regulatory and commercial milestones are met on a
97
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
8. License, Research, Development and Commercialization Agreements (Continued)
compound-by-compound basis. Additionally, the Company is eligible to receive commercial milestones for each approved drug based on sales of the drug. Milestone payments may be lower for designated programs depending upon: whether GSK makes the selection of the product candidate before or after initiation of Phase I clinical trial dosing (10%-15% reduction if selected before such dosing); if certain target product profile characteristics are not achieved (20%-40% reduction); and whether the product candidate is designated after the initial two product candidate designations in a program (50% reduction). GSK is further obligated to pay the Company royalties on annual net sales of products. Such royalties shall continue until the later of expiration of such valid patent claims or ten years from the first commercial sale on a product-by-product and country-by-country basis. To date, no products have been approved and therefore no royalty fees have been earned under this agreement.
During the research term, the Company is committed to use diligent efforts to discover and optimize compounds pursuant to agreed research plans and to provide specified resources, including certain numbers of full-time-equivalent scientists, on a project-by-project basis. Each party is responsible for its own research and development costs. The Company will be primarily responsible for the discovery and development of each product candidate from the research stage until GSK exercises an option for such product candidate.
Unless earlier terminated, the agreement will continue in effect until expiration of all payment obligations under the agreement. GSK retains the unilateral right to terminate the agreement in its entirety upon six months prior written notice to the Company and immediately with respect to any project. Either party may also terminate the agreement, on a project-by-project basis or in its entirety for any uncured material breach of the agreement by the other party. Either party may also terminate the agreement upon specified actions relating to insolvency of the other party. In the event of unilateral termination by GSK, all rights granted by the Company to GSK with respect to the project to which such termination applies would terminate and the Company would retain the rights to any compounds relating to such project. In the event of termination by GSK for cause, GSK would have a perpetual exclusive license under the Company's intellectual property to develop and commercialize any project compounds to which such termination applies, subject to GSK's payment to the Company of specified royalties on sales of such compounds. In the event of termination by the Company for cause, the Company would have a perpetual exclusive license under GSK's intellectual property to develop and commercialize any project compound to which such termination applies, subject to payment by the Company to GSK of specified royalties on sales of such compounds.
Pursuant to the agreement, GSK paid the Company a $12.0 million non-refundable, non-creditable upfront fee in October 2007, which is being recognized over the six-year research term on a straight-line basis. GSK '052 is the Company's most advanced product candidate under the collaboration. In July 2010, GSK notified the Company that the Company's GSK '052 product candidate had achieved proof-of-concept criteria and that GSK was exercising its option to obtain an exclusive license to develop, commercialize and market worldwide the GSK '052 compound. In connection with the exercise of this option, GSK paid the Company $15.0 million in August 2010 and will assume sole responsibility for the further development and commercialization of GSK '052. In addition, GSK is obligated to make payments to the Company if GSK '052 meets certain development, regulatory and commercial milestones and to pay royalties on any future product sales of GSK '052.
98
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
8. License, Research, Development and Commercialization Agreements (Continued)
The Company also had the right to require GSK to purchase $10.0 million of the Company's capital stock. In December 2008, GSK purchased $30.0 million of the Company's Series E convertible preferred stock and became a related party. Upon closing of the IPO in November 2010, all of the outstanding shares of the Company's convertible preferred stock owned by GSK were automatically converted into 1,771,374 shares of its common stock (see Note 9). In connection with the IPO in November 2010, GSK purchased an additional 1,000,000 shares of common stock of the Company at $5.00 per share.
Revenues recognized under this agreement in 2008, 2009 and 2010 were as follows (in thousands):
| |
Year Ended December 31 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | ||||||||
Contract revenue—related party: |
|||||||||||
Amortization of upfront fee |
$ | 2,000 | $ | 2,000 | $ | 2,000 | |||||
Milestone fees |
3,300 | 6,825 | 15,000 | ||||||||
Reimbursement for patent costs, clinical trial costs and procurement of drug material |
— | 25 | 909 | ||||||||
Total contract revenue—related party |
$ | 5,300 | $ | 8,850 | $ | 17,909 | |||||
Lilly
In August 2010, the Company entered into a research agreement with Lilly under which the companies will collaborate to discover products for a variety of animal health applications. Lilly will be responsible for worldwide development and commercialization of compounds advancing from these efforts. The Company received an upfront payment of $3.5 million in September 2010, which is being recognized over a four-year research term on a straight-line basis, and the Company will receive a minimum of $6.0 million in research funding with the potential of up to $12.0 million in research funding, if successful. In addition, the Company will be eligible to receive payments upon the achievement of specified development and regulatory milestones, as well as tiered royalties escalating from high single digit to in the tens royalties on sales, depending in part upon the mix of products sold. Such royalties continue through the later of expiration of the Company's patent rights or six years from the first commercial sale on a product-by-product and country-by-country basis.
Unless earlier terminated, the agreement continues in effect until the termination of royalty payment obligations. The agreement allows for termination by Lilly upon written notice, with certain additional payments to the Company and a notice period that has a duration dependent on whether the notice is delivered prior to the first regulatory approval of a product under the agreement or thereafter. In addition, either party may terminate for the other party's uncured material breach of the agreement. In the event of termination by the Company for material breach by Lilly or termination upon written notice by Lilly, Lilly would assign to the Company certain trademarks and regulatory materials used in connection with the products under the agreement and grant to the Company an exclusive license under Lilly's patent rights covering such products, and the Company would pay to Lilly a reasonable royalty on sales of such products should the Company desire an exclusive license. In the event of termination for material breach by the Company, Lilly will be entitled to a return of all research funding payments for expenses the Company has not incurred or irrevocably committed.
99
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
8. License, Research, Development and Commercialization Agreements (Continued)
Revenues recognized under this 2010 agreement were as follows (in thousands):
| |
2010 | ||||
|---|---|---|---|---|---|
Contract revenue: |
|||||
Amortization of upfront fee |
$ | 308 | |||
Research funding |
1,057 | ||||
Total contract revenue |
$ | 1,365 | |||
Schering
In February 2007, the Company entered into an exclusive license, development and commercialization agreement with Schering for the development and worldwide commercialization of AN2690. Under the agreement, Schering assumed sole responsibility for development and commercialization of AN2690.
Pursuant to the agreement, in March 2007, Schering paid the Company a $40.0 million non-refundable, non-creditable upfront fee, which was recognized on a straight-line basis over the estimated 27.5 month period ended in June 2009 during which the Company performed development-related activities under its transitional responsibilities (the Transition Period). During the Transition Period, Schering reimbursed the Company for costs incurred by the Company to perform specified development-related activities. No milestones or royalty fees were earned under this agreement.
In November 2009, Schering merged with Merck. In May 2010, the Company entered into a mutual termination and release agreement with Merck pursuant to which the 2007 agreement was terminated. Under the May 2010 agreement, the Company regained the exclusive worldwide rights to AN2690, Merck provided for the transfer to the Company of all material and information related to AN2690, Merck paid $5.8 million to the Company, and the parties released each other from any and all claims, liabilities or other types of obligations relating to the 2007 agreement. Merck did not retain any rights to this compound.
Revenues recognized in 2008, 2009 and 2010 under the agreement with Schering and the related mutual termination and release agreement with Merck were as follows (in thousands):
| |
Year Ended December 31 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | ||||||||
Contract revenue: |
|||||||||||
Amortization of upfront fee |
$ | 17,454 | $ | 8,728 | $ | — | |||||
Payment from Merck in connection with termination and release agreement |
— | — | 5,750 | ||||||||
Reimbursement for development-related activities |
2,257 | 277 | 29 | ||||||||
Total contract revenue |
$ | 19,711 | $ | 9,005 | $ | 5,779 | |||||
100
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
9. Convertible Preferred Stock
The authorized, issued and outstanding shares of convertible preferred stock at December 31, 2009 were as follows (in thousands, except share data):
| |
Shares Authorized |
Shares Issued and Outstanding |
Aggregate Liquidation Preference |
Carrying Amounts (net of Financing Costs) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Series A-1 |
4,228,329 | 845,663 | $ | 3,000 | $ | 2,943 | |||||||
Series A-2 |
1,198,046 | 239,608 | 850 | 850 | |||||||||
Series B |
3,339,341 | 667,866 | 4,000 | 3,972 | |||||||||
Series C |
28,089,885 | 5,617,968 | 25,000 | 24,837 | |||||||||
Series D |
3,716,626 | 586,090 | 5,070 | 5,035 | |||||||||
Series E |
15,100,000 | 2,952,283 | 50,000 | 49,836 | |||||||||
|
55,672,227 | 10,909,478 | $ | 87,920 | $ | 87,473 | |||||||
The Company recorded the convertible preferred stock at fair value on the dates of issuance, net of issuance costs. The Company had classified the convertible preferred stock outside of stockholders' equity (deficit) because the shares contained redemption features that were not solely within its control. Upon closing of the IPO in November 2010, 10,909,478 outstanding shares of convertible preferred stock were automatically converted into 11,120,725 shares of common stock, and the related carrying value of $87.5 million was reclassified to additional paid-in capital. At December 31, 2010, no convertible preferred shares were authorized, issued or outstanding.
10. Preferred Stock Warrants Liability and Common Stock Warrants
In connection with the debt financing entered into in June 2006 (see Note 6), the Company issued a warrant to purchase Series D convertible preferred stock for $8.65 per share (Warrant #1). Under Warrant #1, 30,058 shares were initially available for purchase upon execution of the loan agreement in June 2006. In connection with the July and December 2006 borrowings, the number of shares available for purchase under Warrant #1 was increased by an additional 30,057 shares. The Company assigned an initial fair value of $0.4 million to the warrant, of which $0.2 million related to the June 2006 issuance, which was accounted for as capitalized debt financing cost, and $0.2 million related to the July and December 2006 share increases, which were accounted for as discounts on the related borrowings. The Company is amortizing the capitalized debt financing costs and the debt discounts over the amended term of the note (see Note 6). This warrant expires in 2013.
In connection with the additional $7.0 million borrowing in May 2008, the Company issued a second warrant to purchase up to 97,109 shares of the Series D convertible preferred stock for $8.65 per share (Warrant #2). Upon funding of the additional loan proceeds, there were 64,739 shares available for purchase under Warrant #2, all of which were immediately exercisable. On January 1, 2009, the number of shares available for purchase under Warrant #2 increased by 32,370, and these shares were immediately exercisable. The Company assigned an initial fair value of $1.2 million to Warrant #2 for the 64,739 shares that were available for purchase when the warrant was issued in May 2008 and a fair value of $0.3 million for the January 2009 increase in shares available for purchase under Warrant #2, both of which were accounted for as discounts on the related borrowings. The Company is amortizing the debt discounts over the amended term of the note (see Note 6). Warrant #2 expires in 2015.
101
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
10. Preferred Stock Warrants Liability and Common Stock Warrants (Continued)
In conjunction with the restructuring of the Company's debt in January 2010, the Company issued a warrant to purchase 40,623 shares of the Company's Series E convertible preferred stock for $16.936 per share (Warrant #3). The Company assigned an initial fair value of $0.6 million to Warrant #3, which was accounted for as a discount on the related borrowings. The debt discount is being amortized over the amended term of the note (see Note 6). Warrant #3 expires in 2017.
The initial values of the three warrants were determined using the Black-Scholes model and the following assumptions:
| |
Warrant #1 | Warrant #2 | Warrant #2 | Warrant #3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2008 | 2009 | 2010 | |||||||||
Dividend yield |
0 | % | 0 | % | 0 | % | 0 | % | |||||
Volatility |
73 | % | 74 | % | 75 | % | 70 | % | |||||
Weighted-average expected life (in years) |
6.5 - 7.0 | 7.0 | 6.3 | 7.0 | |||||||||
Risk-free interest rate |
4.7 - 5.1 | % | 3.4 | % | 1.87 | % | 3.4 | % | |||||
During 2008, 2009 and 2010, the Company's interest expense included $0.5 million, $0.8 million and $0.7 million, respectively, for the amortization of the financing costs and debt discounts.
The preferred stock warrants liability was adjusted to its fair value at the end of each reporting period using the Black-Scholes option-pricing model to determine such fair value. During 2008, 2009 and 2010, the Company recorded other expense (income) for the increase (decrease) in the fair value of the warrants of $(1.4) million, $0.1 million and $(1.4) million, respectively. The fair value of the warrants was estimated to be $1.3 million at December 31, 2009. In connection with the closing of the IPO in November 2010, all three of the preferred stock warrants automatically converted into common stock warrants and the preferred stock warrants liability, $0.4 million as of the conversion date, was reclassified to additional paid-in capital. Accordingly, there was no preferred stock warrants liability at December 31, 2010.
At December 31, 2010, the following common stock warrants were issued and outstanding:
Warrant
|
Shares Subject to Warrants |
Exercise Price per Share |
Expiration | |||||
|---|---|---|---|---|---|---|---|---|
Warrant #1 |
60,115 | $ | 8.65 | June 2013 | ||||
Warrant #2 |
97,109 | $ | 8.65 | May 2015 | ||||
Warrant #3 |
40,623 | $ | 16.936 | January 2017 | ||||
Total warrants |
197,847 | |||||||
11. Stockholders' Equity (Deficit)
Initial Public Offering
In November 2010, the Company completed its IPO of common stock pursuant to a registration statement that was declared effective on November 23, 2010. The Company sold 13,382,651 shares of its common stock, which included 1,382,651 shares of the Company's common stock sold pursuant to an over-allotment option granted to the underwriters, at a price to the public of $5.00 per share. As a
102
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
11. Stockholders' Equity (Deficit) (Continued)
result of the IPO, the Company raised a total of $66.9 million in gross proceeds, and approximately $61.0 million in net proceeds after deducting underwriting discounts and commissions of $3.2 million and estimated offering expenses of $2.7 million. Costs directly associated with the Company's IPO were capitalized and recorded as deferred offering costs prior to the closing of the IPO. These costs have been recorded as a reduction of the proceeds received to determine the amount to be recorded in additional paid-in capital. Upon the closing of the IPO, 10,909,478 shares outstanding of the Company's convertible preferred stock automatically converted into 11,120,725 shares of its common stock.
Concurrent with the closing of the IPO, the Company sold 2,000,000 shares of its common stock through a private placement offering at a price of $5.00 per share. Gross proceeds raised in the private placement were $10.0 million with estimated issuance costs of $16,000.
Reverse Stock Split
In November 2010, the Company filed a certificate of amendment of amended and restated certificate of incorporation effecting a 1-for-5 reverse stock split of the Company's issued and outstanding shares of common stock and convertible preferred stock. The par value of the common and convertible preferred stock was not adjusted as a result of the reverse stock split. All issued and outstanding common stock, convertible preferred stock, warrants for common stock, warrants for preferred stock, and per share amounts contained in the Company's financial statements have been retroactively adjusted to reflect this reverse stock split for all periods presented.
Preferred Stock
In November 2010, the Company amended and restated its certificate of incorporation to authorize 10,000,000 shares of preferred stock upon the completion of the IPO of its common stock. At December 31, 2010, there was no preferred stock outstanding.
Common Stock
In November 2010, the Company amended and restated its certificate of incorporation to increase the authorized common stock to 100,000,000 shares upon the completion of the IPO of its common stock.
The following table presents common stock reserved for future issuance for the following equity instruments as of December 31, 2010:
Warrants to purchase common stock |
197,847 | ||||
Options: |
|||||
Outstanding under the 2001 Equity Incentive Plan |
1,943,252 | ||||
Reserved for future grants under the 2010 Equity Incentive Plan |
932,670 | ||||
Reserved for future issuance under the 2010 Employee Stock Purchase Plan |
250,000 | ||||
Total common stock reserved for future issuance |
3,323,769 | ||||
103
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
11. Stockholders' Equity (Deficit) (Continued)
2010 Equity Incentive Plan
In November 2010, the Company's board of directors approved the Company's 2010 Equity Incentive Plan (2010 Plan), which became effective on the date of the IPO of the Company's common stock. A total of 929,832 shares of common stock were initially reserved for future issuance under the 2010 Plan; and shares subject to outstanding stock awards granted under the Company's 2001 Equity Incentive Plan (2001 Plan) that expire or terminate for any reason prior to their exercise or settlement and would otherwise return to the 2001 Plan reserve will be added to the shares reserved under the 2010 Plan. In addition, the number of shares of common stock available for issuance under the 2010 Plan will automatically increase on January 1st of each year for ten years commencing on January 1, 2012 by an amount equal to 4% of the total number of shares outstanding on December 31st of the preceding calendar year, or a lesser number of shares of common stock as determined by the Company's board of directors. The maximum number of all shares that may be issued under the 2010 Plan is 2,875,922 shares. At December 31, 2010, the Company had 932,670 shares of common stock reserved for issuance under the 2010 Plan.
The 2010 Plan provides for the grant of awards to employees, directors and consultants in the form of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance awards and other awards. Incentive stock options may be granted to employees and nonstatutory options may be granted to employees, directors or consultants at exercise prices of no less than 100% of the fair value of the common stock on the grant date as determined by the board of directors. Incentive stock options may only be granted for ten years from the date the 2010 Plan was approved by the board of directors. Options typically vest as determined by the board of directors, at the rate of 25% at the end of the first year with the remaining balance vesting monthly over the next three years, but may be granted with different vesting terms. Options granted under the 2010 Plan expire no later than ten years after the date of grant. The stock issuable under the 2010 Plan may be shares of authorized but unissued or reacquired common stock of the Company, including shares repurchased by the Company on the open market or otherwise.
2010 Employee Stock Purchase Plan
The Company's board of directors adopted the 2010 Employee Stock Purchase Plan (ESPP) in November 2010, and the Company's stockholders approved the ESPP in November 2010. The ESPP became effective upon the IPO of the Company's common stock. A total of 250,000 shares of common stock were initially reserved for future issuance under the ESPP and are pursuant to purchase rights granted to the Company's employees or to employees of any of the Company's designated affiliates. The number of shares of common stock reserved for issuance under the ESPP will automatically increase on January 1st of each year for ten years commencing on January 1, 2012 by the least of (i) 1% of the total number of shares outstanding on December 31st of the preceding calendar year, (ii) 80,000 shares or (iii) the number of shares of common stock as determined by the Company's board of directors. The ESPP permits eligible employees to purchase common stock at a discount by contributing, normally through payroll deductions, up to 10% of their base compensation during defined offering periods. The price at which the stock is purchased is equal to the lower of 85% of the fair market value of the common stock at the first date of an offering period, or 85% of the fair value of the common stock on the date of purchase. No shares were purchased under the ESPP in 2010. At December 31, 2010, all 250,000 shares remain available for future issuance under the ESPP.
104
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
11. Stockholders' Equity (Deficit) (Continued)
2001 Equity Incentive Plan
In connection with the Company's IPO, no further options or stock purchase rights will be granted under the 2001 Plan. Any shares remaining for issuance under the 2001 Plan as of the IPO date became available for issuance under the 2010 Plan. All outstanding stock awards granted under the 2001 Plan will remain subject to the terms of the 2001 Plan; however, shares that expire, terminate or are forfeited prior to exercise or settlement of such shares become available for issuance under the 2010 Plan. At December 31, 2010, there were 1,943,252 shares of common stock reserved for future issuance under the 2001 Plan.
Under the 2001 Plan, the board of directors was authorized to grant options or stock purchase rights to employees, directors and consultants. Options granted were either incentive stock options or nonstatutory stock options. Incentive stock options were granted to employees with exercise prices of no less than the fair value, and nonstatutory options were granted to employees or consultants at exercise prices of no less than 85% of the fair value of the common stock on the grant date as determined by the board of directors. Options vest as determined by the board of directors, generally at the rate of 25% at the end of the first year, with the remaining balance vesting monthly over the next three years. Options granted under the Plan expire no later than ten years after the date of grant. The Plan provides for the new issuance of common stock of the Company upon the exercise of stock options. At December 31, 2009 and 2010, there were no shares subject to repurchase by the Company.
Stock Option Repricing
Effective April 1, 2009, the Company's board of directors approved the reduction of the exercise prices of certain outstanding stock options previously granted to employees and nonemployees of the Company who were still providing services to the Company as of that date. The Company repriced options to purchase 664,371 shares of the Company's common stock that included both vested and unvested stock options granted from June 2007 through August 2008 with original exercise prices ranging from $12.75 to $22.25 per share. The Company's board of directors adjusted all of the original exercise prices for the repriced options to $7.25 per share and determined the fair value of the underlying shares of common stock to be $5.00 per share on the date of the repricing. No other terms of the repriced options were modified and these repriced stock options will continue to vest according to their original vesting schedules and will retain their original expiration dates.
The modification was treated as a one-for-one exchange of the previously issued stock options for new stock options with an exercise price of $7.25. The Company recorded a one-time stock-based compensation charge of $0.2 million for the incremental value of the vested options. In addition, the Company will record additional stock-based compensation charges of $0.5 million for the incremental value of the unvested repriced options, which will be recognized over the remaining vesting period of the new stock options. The 2009 share numbers for options granted and options canceled in the table below include the 664,371 option shares that were exchanged in connection with the April 2009 repricing.
105
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
11. Stockholders' Equity (Deficit) (Continued)
Stock Option Activity
The following table summarizes stock option activity:
| |
Shares Available for Grant |
Outstanding Options Number of Shares |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value (in thousands) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at December 31, 2007 |
234,642 | 1,056,816 | $ | 5.30 | |||||||||||||
Additional shares authorized for grant |
400,000 | — | |||||||||||||||
Options granted |
(494,296 | ) | 494,296 | $ | 21.42 | ||||||||||||
Options exercised |
— | (5,921 | ) | $ | 2.55 | $ | 96 | ||||||||||
Options canceled |
164,075 | (164,075 | ) | $ | 14.10 | ||||||||||||
Balance at December 31, 2008 |
304,421 | 1,381,116 | $ | 10.00 | |||||||||||||
Options granted |
(909,661 | ) | 909,661 | $ | 6.64 | ||||||||||||
Options exercised |
— | (35,180 | ) | $ | 0.40 | $ | 162 | ||||||||||
Options canceled |
672,481 | (672,481 | ) | $ | 19.95 | ||||||||||||
Balance at December 31, 2009 |
67,241 | 1,583,116 | $ | 4.07 | 7.1 | $ | 2,495 | ||||||||||
Additional shares authorized for grant |
1,275,400 | — | |||||||||||||||
Options granted |
(480,761 | ) | 480,761 | $ | 6.59 | ||||||||||||
Options exercised |
— | (49,806 | ) | $ | 1.12 | $ | 203 | ||||||||||
Options canceled |
70,790 | (70,819 | ) | $ | 6.21 | ||||||||||||
Balance at December 31, 2010 |
932,670 | 1,943,252 | $ | 4.69 | 6.7 | $ | 3,111 | ||||||||||
At December 31, 2010: |
|||||||||||||||||
Options vested and expected to vest |
1,908,614 | $ | 4.66 | 6.7 | $ | 3,104 | |||||||||||
Exercisable |
1,275,609 | $ | 3.82 | 5.6 | $ | 3,001 | |||||||||||
The aggregate intrinsic value of options outstanding at December 31, 2010 is calculated as the difference between the exercise price of the underlying options and the Company's common stock closing price for the 1,038,981 shares that had exercise prices that were lower than the closing stock price of $5.37 at December 31, 2010. The weighted-average fair values of options granted to employees in 2008, 2009 and 2010 were $14.22, $1.49 and $4.13 per share, respectively. Total options granted to employees during 2009 were 844,861 shares, including 614,571 shares underlying options that were exchanged in connection with the April 2009 repricing.
106
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
11. Stockholders' Equity (Deficit) (Continued)
Stock options by exercise price at December 31, 2010 are as follows:
| |
Options Outstanding | Options Exercisable | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Exercise Price
|
Number | Weighted- Average Remaining Contractual Term (Years) |
Weighted- Average Exercise Price |
Number | Weighted- Average Exercise Price |
|||||||||||
$0.35 |
27,020 | 1.7 | $ | 0.35 | 27,020 | $ | 0.35 | |||||||||
$0.60 |
587,745 | 4.5 | $ | 0.60 | 587,745 | $ | 0.60 | |||||||||
$1.90 |
4,813 | 5.6 | $ | 1.90 | 4,813 | $ | 1.90 | |||||||||
$5.00 |
419,403 | 8.9 | $ | 5.00 | 121,621 | $ | 5.00 | |||||||||
$7.25 |
606,889 | 6.2 | $ | 7.25 | 470,475 | $ | 7.25 | |||||||||
$7.55 |
297,382 | 9.6 | $ | 7.55 | 63,935 | $ | 7.55 | |||||||||
|
1,943,252 | 6.7 | $ | 4.69 | 1,275,609 | $ | 3.82 | |||||||||
Stock-Based Compensation
The Company estimates the fair value of stock options granted on the date of grant using the Black-Scholes option-pricing model, which requires the use of highly subjective and complex assumptions to determine the fair value of stock-based awards. As a newly public company, sufficient relevant historical data does not exist to support the volatility of the Company's common stock prices or the expected term of its stock options. Therefore, the Company has used the average historical volatilities of a peer group of publicly-traded companies within its industry to determine a reasonable estimate of its expected volatility. In addition, the Company has opted to use the simplified method for estimating the expected term of options granted to employees.
The risk-free interest rate assumptions are based on the yield of U.S. Treasury Constant Maturity rate with durations similar to the expected term of the related awards. The expected dividend yield assumption is based on the Company's historic and expected absence of dividend payouts.
For options granted prior to January 1, 2006, the graded-vested (multiple-option) method continues to be used for expense attribution related to the portion of those options that were unvested as of January 1, 2006. The straight-line (single-option) method is being used for expense attribution of all awards granted on or after January 1, 2006.
The fair values of employee stock options granted under the Plan were estimated at the date of grant using the Black-Scholes model with the following assumptions:
| |
Year Ended December 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Dividend yield |
0 | % | 0 | % | 0 | % | ||||
Volatility |
73 | % | 70 - 76 | % | 71 - 73 | % | ||||
Weighted-average expected life (in years) |
6.1 | 4.9 - 6.1 | 6.1 | |||||||
Risk-free interest rate |
2.8 | % | 1.7 - 2.1 | % | 1.8 - 1.9 | % | ||||
Prior to its IPO, the Company had historically granted stock options at exercise prices not less than the fair market value of its common stock as determined by the board of directors based on input
107
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
11. Stockholders' Equity (Deficit) (Continued)
from management. The determination of estimated fair value of its common stock on the date of grant had been based on a number of objective and subjective factors including: recent sales of convertible preferred stock to investors; comparable rights and preferences of other outstanding equity securities; progress of research and development efforts and milestones attained; results of operations, financial position and levels of debt and available capital resources of the Company; perspective provided by valuation analyses of the Company's stock performed by management; and the likelihood of a liquidity event such as an IPO or the sale of the Company given prevailing market and biotechnology sector conditions.
Employee stock-based compensation expense recognized in 2008, 2009 and 2010 was calculated based on awards ultimately expected to vest and has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Employee stock-based compensation expense was as follows (in thousands):
| |
Year Ended December 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Research and development |
$ | 1,002 | $ | 1,451 | $ | 1,530 | ||||
General and administrative |
707 | 806 | 760 | |||||||
Total |
$ | 1,709 | $ | 2,257 | $ | 2,290 | ||||
At December 31, 2010, the Company had $3.6 million of total unrecognized compensation expense, net of estimated forfeitures, related to outstanding stock options that will be recognized over a weighted-average period of 1.9 years.
Stock Options Granted to Nonemployees
During 2008, 2009 and 2010, the Company granted nonemployees 25,000, 64,800, and 67,500 option shares, respectively, to purchase common stock. Included in the 2009 grants to nonemployees were 49,800 option shares that were exchanged in connection with the April 2009 repricing.
In connection with grants of stock options to nonemployees, the Company recorded stock-based compensation expense as follows (in thousands):
| |
Year Ended December 31 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Research and development |
$ | 261 | $ | 106 | $ | 101 | ||||
General and administrative |
21 | 87 | 395 | |||||||
Total |
$ | 282 | $ | 193 | $ | 496 | ||||
Stock-based compensation expense related to stock options granted to nonemployees is recognized as the stock options vest. The stock-based compensation expense related to nonemployees will fluctuate as the fair value of the Company's common stock fluctuates. The Company believes that the fair values of the stock options are more reliably measurable than the fair values of the services received. The fair value of nonemployee options in 2008, 2009 and 2010 was estimated using the Black-Scholes method
108
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
11. Stockholders' Equity (Deficit) (Continued)
with the following weighted-average assumptions: expected life is equal to the remaining contractual term of the award as of the measurement date; risk-free rate is based on the U.S. Treasury Constant Maturity rate with a term similar to the expected life of the option at the measurement date; expected dividend yield of 0%; and an average volatility ranging from 70% to 80%. The Company has used the average historical volatilities of a peer group of publicly-traded companies within its industry to estimate volatility.
12. 401(k) Plan
The Company sponsors a 401(k) Plan to which eligible employees may elect to contribute, on a pretax basis, subject to certain limitations. The Company does not match any employee contributions.
13. Income Taxes
The income tax benefits for 2008 and 2009 were federal refundable credits of $44,000 and $15,000, respectively. The American Recovery and Reinvestment Act of 2009 allowed corporations without current tax liabilities to obtain refunds for certain research tax credit and alternative minimum tax credit carryforwards by electing to forego the 50% additional first year depreciation for new property acquired after June 30, 2008 and placed in service before January 1, 2010. This Act extended the provisions of the Housing Assistance Tax Act of 2008 (H.R. 3221) which was set to expire for assets placed in service before January 1, 2009. For tax years 2008 and 2009, the Company elected to obtain refunds for a portion of its research and development tax credit carryforwards from taxable years before January 1, 2006.
A reconciliation of the expected income tax benefit computed using the federal statutory income tax rate to the Company's effective income tax rate is as follows:
| |
Year Ended December 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2008 | 2009 | 2010 | |||||||
Income tax computed at federal statutory tax rate |
34.0 | % | 34.0 | % | 34.0 | % | ||||
State taxes, net of federal benefit |
7.8 | % | 7.0 | % | 9.0 | % | ||||
Stock-based compensation |
(1.5 | )% | (1.5 | )% | (4.2 | )% | ||||
Research and development credits |
3.9 | % | 3.4 | % | 6.3 | % | ||||
Change in valuation allowance |
(46.0 | )% | (42.6 | )% | (49.7 | )% | ||||
Government grant credit |
— | % | — | % | 4.5 | % | ||||
Other |
2.0 | % | (0.2 | )% | 0.1 | % | ||||
Total |
0.2 | % | 0.1 | % | 0.0 | % | ||||
109
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
13. Income Taxes (Continued)
Significant components of the Company's deferred tax assets for federal and state income taxes were as follows (in thousands):
| |
December 31 | |||||||
|---|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | ||||||
Deferred tax assets: |
||||||||
Federal and state net operating losses |
$ | 34,026 | $ | 38,046 | ||||
Federal and state research and development credit carryforwards |
4,353 | 5,290 | ||||||
Deferred revenues |
2,988 | 2,191 | ||||||
Stock-based compensation |
1,141 | 1,777 | ||||||
Other |
1,331 | 1,593 | ||||||
Total deferred tax assets |
43,839 | 48,897 | ||||||
Valuation allowance |
(43,839 | ) | (48,897 | ) | ||||
Net deferred tax assets |
$ | — | $ | — | ||||
Due to the Company's lack of earnings history, the deferred assets have been fully offset by a valuation allowance at December 31, 2009 and 2010. The increase in the valuation allowance on the deferred tax assets was $10.0 million, $10.6 million and $5.1 million for 2008, 2009 and 2010, respectively.
At December 31, 2010, the Company had net operating loss carryforwards for federal and state income tax purposes of approximately $95.5 million and $95.6 million, respectively. These federal and state carryforwards will begin to expire in the years 2021 and 2013, respectively. The Company also had federal research and development tax credit carryforwards of approximately $4.6 million, which expire beginning in 2024 if not utilized. In addition, state research and development tax credit carryforwards of approximately $3.0 million will carry over indefinitely.
Internal Revenue Code Section 382 places a limitation (the Section 382 Limitation) on the amount of taxable income, that can be offset by net operating loss carryforwards after a change in control (generally, a greater than 50% change in ownership). Typically, after a control change, a company cannot deduct operating loss carryforwards in excess of the Section 382 Limitation. Due to these changes in ownership provisions, utilization of the net operating loss and tax credit carryforwards may be subject to an annual limitation regarding their utilization against taxable income in future periods.
The Company adopted the provisions of the standard for accounting for uncertainties in income taxes on January 1, 2007. Upon adoption, the Company recognized no material adjustment in the liability for unrecognized tax benefits. At December 31, 2009 and 2010, the Company had approximately $1.3 million and $1.5 million, respectively, of unrecognized tax benefits, none of which would currently affect the Company's effective tax rate if recognized due to the Company's deferred tax assets being fully offset by a valuation allowance. The Company does not believe there will be any material changes in its unrecognized tax positions over the next twelve months.
110
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
13. Income Taxes (Continued)
The activity related to unrecognized tax benefits was as follows (in thousands):
| |
Year Ended December 31 |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
2009 | 2010 | ||||||
Balance at the beginning of the year |
$ | 932 | $ | 1,254 | ||||
Additions based on tax positions related to current year |
345 | 273 | ||||||
Additions for tax positions of prior years |
— | — | ||||||
Reductions for tax positions of prior years |
(23 | ) | (2 | ) | ||||
Settlements |
— | — | ||||||
Balance at the end of the year |
$ | 1,254 | $ | 1,525 | ||||
If applicable, the Company would classify interest and penalties related to uncertain tax positions in income tax expense. Through December 31, 2010, there has been no interest expense or penalties related to unrecognized tax benefits. The tax years 2001 through 2010 remain open to examination by one or more major taxing jurisdictions to which the Company is subject. There are no income tax examinations currently in progress.
14. U.S. Government Grant
In October 2010, the Company was awarded $1.5 million in grant funds by the United States Department of the Treasury under the Qualifying Therapeutic Discovery Project program to support research for six projects with the potential to produce new therapies. The qualifying expenses under this program were incurred by the Company during 2009 and 2010. The Company recognized $1.5 million as government grant revenue for the year ended December 31, 2010. At December 31, 2010, $0.1 million is due to the Company and is included in government grant receivable.
15. Quarterly Financial Data (Unaudited)
The following table summarizes the unaudited quarterly financial data for the last two fiscal years (in thousands, except per share data):
Selected Quarterly Data:
| |
Quarter Ended | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
March 31, 2009 |
June 30, 2009 |
September 30, 2009 |
December 31, 2009 |
|||||||||
Total revenues |
$ | 5,454 | $ | 5,585 | $ | 829 | $ | 6,775 | |||||
Net income (loss) |
$ | (5,935 | ) | $ | (5,496 | ) | $ | (9,484 | ) | $ | (3,924 | ) | |
Basic net income (loss) per share |
$ | (4.21 | ) | $ | (3.90 | ) | $ | (6.72 | ) | $ | (2.74 | ) | |
Diluted net income (loss) per share |
$ | (4.21 | ) | $ | (3.90 | ) | $ | (6.72 | ) | $ | (2.74 | ) | |
111
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
15. Quarterly Financial Data (Unaudited) (Continued)
| |
Quarter Ended | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
March 31, 2010 |
June 30, 2010 |
September 30, 2010 |
December 31, 2010 |
|||||||||
Total revenues |
$ | 710 | $ | 6,674 | $ | 16,897 | $ | 3,543 | |||||
Net income (loss) |
$ | (8,154 | ) | $ | (1,471 | ) | $ | 7,062 | $ | (7,495 | ) | ||
Basic net income (loss) per share |
$ | (5.53 | ) | $ | (0.99 | ) | $ | 4.73 | $ | (0.73 | ) | ||
Diluted net income (loss) per share |
$ | (5.53 | ) | $ | (0.99 | ) | $ | 0.49 | $ | (0.73 | ) | ||
Income (loss) per share amounts for the 2009 and 2010 quarters and full years have been computed separately. Accordingly, quarterly amounts may not add to the annual amounts because of differences in the weighted average shares outstanding during each quarter.
16. Subsequent Events
New Agreements
Medicis
On February 10, 2011, the Company entered into a research and development agreement with Medicis Pharmaceutical Corporation (Medicis) to discover and develop boron-based small molecule compounds directed against a target for the potential treatment of acne.
Under the terms of the agreement, the Company received a $7.0 million upfront payment from Medicis in February 2011 and will be primarily responsible for discovering and conducting early development of product candidates which utilize the Company's proprietary boron chemistry platform. Medicis will have an option to obtain an exclusive license for products covered by the agreement. The Company will be eligible to receive payments upon the achievement of specified development, regulatory and sales milestones, as well as high single-digit to in the tens royalties on sales by Medicis. Medicis will be responsible for further development and commercialization of the licensed products on a worldwide basis.
Medicines for Malaria Venture
On March 17, 2011, the Company entered into a development agreement with Medicines for Malaria Ventures (MMV) to develop Anacor's compound AN3661 for the treatment of malaria. Under the agreement, Anacor and MMV will work together on the development of AN3661 through human proof-of-concept studies. AN3661 was developed as part of a research agreement signed by the two parties in April 2010 to identify new compounds for the treatment of malaria using Anacor's boron chemistry. AN3661 is the first candidate arising out of the research agreement to move into preclinical development. The Company expects to receive upfront funding associated with this preclinical development under this agreement of approximately $1.8 million in the second quarter of 2011. In addition, the Company received approximately $0.6 million in funding in February 2011 under the original research agreement.
Notes Payable
On March 18, 2011, the Company entered into a loan and security agreement with new lenders to provide up to $30.0 million available in three tranches of $10.0 million each. The first $10.0 million
112
Anacor Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
16. Subsequent Events (Continued)
tranche was drawn at the closing of the transaction, at which time the Company repaid $6.6 million of the remaining obligations under its existing loan. The second and third tranches of $10.0 million each are available upon the achievement of either the full enrollment of the Phase 3 trials for AN2690 or the initiation of a Phase 3 trial for AN2728. Upon achievement of either of these development milestones, the second tranche of $10.0 million is available for drawdown through March 31, 2012 and the third tranche of $10.0 million is available for drawdown through September 30, 2012. The interest rate for each tranche will be fixed upon drawdown at a rate equal to the greater of 9.4% or 9.1% plus the 3-month U.S. LIBOR rate. Payments under the loan agreement are interest only until April 30, 2012 followed by equal monthly payments of interest and principal through April 1, 2015. In addition, a final payment equal to 5.5% of the amounts drawn ($550,000 as of the first tranche) will be due on the earlier of April 1, 2015, or termination. The Company paid a financing fee of $0.3 million associated with the new loan. Future payments related to the first $10.0 million tranche of the new loan are $0.7 million, $2.9 million, $3.8 million, $3.8 million and $1.8 million in 2011, 2012, 2013, 2014 and 2015, respectively.
The loan agreement includes standard affirmative and restrictive covenants, but does not include any covenants to attain or maintain certain financial metrics, and also included standard events of default, including payment defaults, breaches of covenants following any applicable cure period, a material impairment in the perfection or priority of the lenders' security interest or in the value of the collateral, and a material impairment of the prospect of repayment of the loans. Upon the occurrence of an event of default and following any applicable cure periods, a default interest rate of an additional 5% may be applied to the outstanding loan balances, and the lenders may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the loan agreement.
The loan is secured by all assets of the Company except intellectual property. Under the loan agreement, the Company also agreed to certain restrictions regarding the pledging or encumbrance of its intellectual property.
In connection with the new loan agreement and the first tranche drawdown of $10.0 million, the Company issued initial warrants to the lenders to purchase 80,527 shares of its common stock (equal to 5.5% of the amount drawn) at an exercise price of $6.83 per share. The warrants are immediately exercisable, may be exercised on a cashless basis and will expire on March 18, 2018. The number of shares subject to the warrants will increase by an amount equal to 5.5% of the amount drawn at each subsequent tranche, divided by the exercise price per share for that tranche (the lower of the 10-day average share price prior to the drawdown or the price per share on the day prior to the drawdown), up to a maximum aggregate exercise price of $1.65 million.
The Company granted certain piggyback registration rights pursuant to which, under certain conditions, the Company will register its shares of common stock issuable upon exercise of the warrants held by the lenders.
113
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As required by SEC Rule 13a-15(b), we carried out an evaluation, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K. Based on the foregoing, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level.
Exemption from Management's Report on Internal Control Over Financial Reporting for the Fiscal Year Ended December 31, 2010
As a newly public company and under the applicable rules of the Securities and Exchange Commission, we are not required to include a report of management's assessment regarding internal control over financial reporting or an attestation report of an Independent Registered Public Accounting Firm in this Annual Report on Form 10-K.
Changes in Internal Control Over Financial Reporting
None.
None.
114
Certain information required by Part III is omitted from this Report on Form 10-K and is incorporated herein by reference to our definitive Proxy Statement for our 2011 Annual Meeting of Stockholders (the "Proxy Statement"), which we intend to file pursuant to Regulation 14A of the Securities Exchange Act of 1934, as amended, within 120 days after December 31, 2010.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item concerning our directors is incorporated by reference to the information set forth in the section titled "Directors and Corporate Governance" in our Proxy Statement. Information required by this item concerning our executive officers is incorporated by reference to the information set forth in the section entitled "Executive Officers of the Company" in our Proxy Statement. Information regarding Section 16 reporting compliance is incorporated by reference to the information set forth in the section entitled "Section 16(a) Beneficial Ownership Reporting Compliance" in our Proxy Statement.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item regarding executive compensation is incorporated by reference to the information set forth in the sections titled "Executive Compensation" in our Proxy Statement.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item regarding security ownership of certain beneficial owners and management is incorporated by reference to the information set forth in the section titled "Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters" and "Equity Compensation Plan Information" in our Proxy Statement.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item regarding certain relationships and related transactions and director independence is incorporated by reference to the information set forth in the sections titled "Certain Relationships and Related Transactions" and "Election of Directors", respectively, in our Proxy Statement.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this item regarding principal accountant fees and services is incorporated by reference to the information set forth in the section titled "Principal Accountant Fees and Services" in our Proxy Statement.
115
Item 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
- (a)
- The
following documents are filed as part of this report:
- (1)
- FINANCIAL STATEMENTS
- (2)
- FINANCIAL STATEMENT SCHEDULES
Financial Statements—See Index to Consolidated Financial Statements at Item 8 of this Annual Report on Form 10-K.
Financial statement schedules have been omitted in this Annual Report on Form 10-K because they are not applicable, not required under the instructions, or the information requested is set forth in the consolidated financial statements or related notes thereto.
- (b)
- Exhibits. The exhibits listed in the accompanying index to exhibits are filed as part of, or incorporated by reference into, this Annual Report on Form 10-K.
116
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Palo Alto, State of California, on the 29th day of March 2011.
| ANACOR PHARMACEUTICALS, INC. | ||||
By: |
/s/ DAVID P. PERRY David P. Perry President and Chief Executive Officer |
|||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints David P. Perry and Geoffrey M. Parker, jointly and severally, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her, and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises hereby ratifying and confirming all that said attorneys-in-fact and agents, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature
|
Title
|
Date
|
|||
|---|---|---|---|---|---|
| /s/ DAVID P. PERRY David P. Perry |
President, Chief Executive Officer and Director (Principal Executive Officer) |
March 29, 2011 | |||
/s/ GEOFFREY M. PARKER Geoffrey M. Parker |
Senior Vice President, Chief Financial Officer (Principal Financial and Accounting Officer) |
March 29, 2011 |
|||
/s/ MARK LESCHLY Mark Leschly |
Chairman of the Board of Directors |
March 29, 2011 |
|||
/s/ ZHI HONG, PH.D. Zhi Hong, Ph.D. |
Director |
March 29, 2011 |
117
Signature
|
Title
|
Date
|
|||
|---|---|---|---|---|---|
| /s/ ANDERS D. HOVE, M.D. Anders D. Hove, M.D. |
Director | March 29, 2011 | |||
/s/ PAUL H. KLINGENSTEIN Paul H. Klingenstein |
Director |
March 29, 2011 |
|||
/s/ RICHARD J. MARKHAM Richard J. Markham |
Director |
March 29, 2011 |
|||
/s/ LUCY SHAPIRO, PH.D. Lucy Shapiro, Ph.D. |
Director |
March 29, 2011 |
118
| Exhibit Number |
Description | |
|---|---|---|
| 3.1(1) | Amended and Restated Certificate of Incorporation of the Registrant. | |
3.2(1) |
Amended and Restated Bylaws of the Registrant. |
|
4.1 |
Reference is made to Exhibits 3.1 through 3.2. |
|
4.2(2) |
Form of the Registrant's Common Stock Certificate. |
|
4.3(2) |
Amended and Restated Investors' Rights Agreement among the Registrant and certain of its security holders, dated as of December 24, 2008, and amendment thereto, dated July 22, 2010. |
|
4.4(2) |
Amended and Restated Preferred Stock Purchase Warrant to purchase shares of Series D convertible preferred stock issued to Lighthouse Capital Partners V, L.P., dated as of May 1, 2008. |
|
4.5(2) |
Preferred Stock Purchase Warrant to purchase shares of Series D convertible preferred stock issued to Lighthouse Capital Partners V, L.P., dated as of May 1, 2008. |
|
4.6(2) |
Preferred Stock Purchase Warrant to purchase shares of Series E convertible preferred stock issued to Lighthouse Capital Partners V, L.P., dated as of January 1, 2010. |
|
4.7 |
Common Stock Purchase Agreement among the Registrant and certain of its security holders, dated as of November 23, 2010. |
|
4.8 |
Registration Rights Agreement among the Registrant and certain of its security holders, dated as of November 23, 2010. |
|
10.1(2)+ |
Form of Amended and Restated Indemnification Agreement between the Registrant and each of its directors and executive officers. |
|
10.2(2)+ |
Anacor Pharmaceuticals, Inc. 2001 Equity Incentive Plan, as amended, and forms of agreement thereunder. |
|
10.3(2)+ |
Anacor Pharmaceuticals, Inc. 2010 Equity Incentive Plan, and forms of agreement thereunder. |
|
10.4(2)+ |
Anacor Pharmaceuticals, Inc. 2010 Employee Stock Purchase Plan. |
|
10.5(2)+ |
Anacor Pharmaceuticals, Inc. Employee Bonus Plan. |
|
10.6(2)+ |
Letter Agreement between the Registrant and Kirk R. Maples, Ph.D., dated as of August 1, 2002. |
|
10.7(2)+ |
Letter Agreement between the Registrant and David P. Perry, dated as of November 21, 2002, and amendment thereto, dated as of August 30, 2005. |
|
10.8(2)+ |
Letter Agreement between the Registrant and Jacob J. Plattner, Ph.D., dated as of October 21, 2003. |
|
10.9(2)+ |
Letter Agreement between the Registrant and Irwin Heyman, Ph.D., dated as of December 16, 2005. |
|
10.10(2)+ |
Letter Agreement between the Registrant and James Marconi, dated as of October 29, 2007. |
|
10.11(2)+ |
Letter Agreement between the Registrant and Lee Zane, dated as of November 30, 2007. |
119
| Exhibit Number |
Description | |
|---|---|---|
| 10.12(2) | Letter Agreement between the Registrant and Stephen J. Benkovic, Ph.D., dated as of May 24, 2009. | |
10.13(2)+ |
Letter Agreement between the Registrant and Geoffrey M. Parker, dated as of September 10, 2010. |
|
10.14(2) |
Consulting Agreement between the Registrant and Stephen J. Benkovic, Ph.D., dated as of May 24, 2007, and amendments thereto, dated effective as of April 1, 2008, January 1, 2009 and January 1, 2010. |
|
10.15(2)+ |
Consulting Agreement between the Registrant and Geoffrey M. Parker, dated as of December 8, 2009 and amendments thereto, dated effective as of April 9, 2010 and July 10, 2010. |
|
10.16(2) |
Advisory Board Agreement between the Registrant and Lucy Shapiro, Ph.D., dated effective as of October 1, 2005, and amendments thereto, dated effective as of October 1, 2007 and January 1, 2010. |
|
10.17(2)+ |
Form of Change of Control and Severance Agreement for Senior Vice Presidents of the Registrant, and amendment thereto. |
|
10.18(2)+ |
Form of Change of Control and Severance Agreement for Vice Presidents of the Registrant, and amendment thereto. |
|
10.19(2) |
Change of Control Agreement between the Registrant and Stephen J. Benkovic, Ph.D., dated as of September 28, 2006. |
|
10.20(2) |
Change of Control Agreement between the Registrant and Lucy Shapiro, Ph.D., dated as of October 25, 2006. |
|
10.21(2)+ |
Change of Control and Severance Agreement between the Registrant and David P. Perry, dated as of August 21, 2007, and amendment thereto, dated as of December 30, 2008. |
|
10.22(2) |
Lease between Balzer Family Investments, L.P. (formerly HDP Associates, LLC) and the Registrant, dated as of October 16, 2002, and amendments thereto, dated as of January 21, 2003, August 1, 2005, September 23, 2008, March 31, 2009 and September 30, 2010. |
|
10.23(2) |
Lease between California Pacific Commercial Corporation and the Registrant, dated as of October 5, 2007. |
|
10.24(2) |
Loan and Security Agreement No. 5251 between Lighthouse Capital Partners V, L.P. and the Registrant, dated as of June 30, 2006, and amendments thereto dated as of February 26, 2008, May 1, 2008 and January 23, 2010. |
|
10.25(2)† |
Research and Development Collaboration, Option and License Agreement between GlaxoSmithKline LLC and the Registrant, dated as of October 5, 2007, and amendments thereto, dated as of December 15, 2008, May 20, 2009 and July 21, 2009. |
|
10.26(2)† |
Collaborative Research, License & Commercialization Agreement between Eli Lilly and Company and Registrant, dated as of August 25, 2010. |
|
23.1 |
Consent of Independent Registered Public Accounting Firm. |
|
24.1 |
Power of Attorney (see signature page). |
|
31.1 |
Certification of Principal Executive Officer required by Rule 13a-14(a) or Rule 15d-14(a). |
|
31.2 |
Certification of Principal Financial Officer required by Rule 13a-14(a) or Rule 15d-14(a). |
120
| Exhibit Number |
Description | |
|---|---|---|
| 32.1 | Certifications required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350). |
- +
- Indicates
management contract or compensatory plan.
- †
- Confidential
treatment has been received with respect to certain portions of this exhibit. Omitted portions have been filed separately with the
Securities and Exchange Commission.
- ††
- Confidential
treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed
separately with the Securities and Exchange Commission.
- (1)
- Filed
as an exhibit to the Registrant's Current Report on Form 8-K filed on December 6, 2010, and incorporated herein by
reference.
- (2)
- Filed as an exhibit to the Registrant's Registration Statement on Form S-1 (File No. 333-169322), effective November 23, 2010, and incorporated herein by reference.
121
