Table of Contents
As filed with the Securities and Exchange Commission on November 7, 2013
Registration No. 333-191737
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 4
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
OXFORD IMMUNOTEC GLOBAL PLC
(Exact name of registrant as specified in its charter)
| England and Wales | 2835 | Not applicable | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
94C Innovation Drive,
Milton Park, Abingdon
OX14 4RZ
United Kingdom
Tel: +44 (0)1235 442780
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Peter Wrighton-Smith, Ph.D.
Chief Executive Officer
Oxford Immunotec Global PLC
94C Innovation Drive
Milton Park, Abingdon
OX14 4RZ
United Kingdom
Tel: +44 (0)1235 442780
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Michael D. Beauvais Ropes & Gray LLP Prudential Tower 800 Boylston Street Boston, MA 02199 (617) 951-7000 |
Patricia Randall General Counsel Oxford Immunotec Global PLC 700 Nickerson Road Suite 200 Marlborough, MA 01752 (508) 481-4648 |
Deanna L. Kirkpatrick Davis Polk & Wardwell LLP 450 Lexington Avenue New York, NY 10017 (212) 450-4000 |
Approximate date of commencement of proposed sale to public: As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | x (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of each class of securities to be registered |
Proposed maximum aggregate offering price(1)(2) |
Amount of registration fee(3) | ||
| Ordinary Shares, £0.001 nominal value |
$86,250,000 | $11,109 | ||
|
| ||||
|
| ||||
| (1) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) of the Securities Act of 1933, amended. |
| (2) | Includes shares that may be sold upon exercise of the underwriters’ option to purchase additional shares. See “Underwriting.” |
| (3) | Previously paid. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED NOVEMBER 7, 2013
PRELIMINARY PROSPECTUS
shares

Oxford Immunotec Global PLC
Ordinary Shares
$ per share
This is the initial public offering of our ordinary shares. We are selling ordinary shares. We currently expect the initial public offering price to be between $ and $ per ordinary share.
We have granted the underwriters an option to purchase up to additional ordinary shares to cover overallotments.
We have applied for listing of our ordinary shares on The NASDAQ Global Market under the symbol “OXFD.”
We are an emerging growth company as that term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our ordinary shares involves a high degree of risk. See “Risk factors” beginning on page 13.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed on the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| Per share | Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds to us, before expenses |
$ | $ | ||||||
| (1) | We have agreed to reimburse the underwriters for certain FINRA-related expenses. See “Underwriting.” |
The underwriters expect to deliver the ordinary shares to purchasers on or about , 2013.
| J.P. Morgan | Piper Jaffray | |||||||||
| Cowen and Company |
Baird | |||||||||
, 2013
Table of Contents
| Page | ||||
| 1 | ||||
| 13 | ||||
| 45 | ||||
| 46 | ||||
| 47 | ||||
| 48 | ||||
| 50 | ||||
| 52 | ||||
| Management’s discussion and analysis of financial condition and results of operations |
55 | |||
| 83 | ||||
| 116 | ||||
| 124 | ||||
| 139 | ||||
| 142 | ||||
| 145 | ||||
| 160 | ||||
| 162 | ||||
| 171 | ||||
| 176 | ||||
| 177 | ||||
| 177 | ||||
| 177 | ||||
We and the underwriters have not authorized anyone to provide any information other than that contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We and the underwriters are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus. Our business, financial condition, results of operations and prospects may have changed since that date.
Until , 2013, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
For investors outside of the United States: we have not and the underwriters have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. Persons outside of the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the ordinary shares and the distribution of this prospectus outside of the United States.
i
Table of Contents
This summary highlights information contained in other parts of this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before investing in our ordinary shares and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus. You should read the entire prospectus carefully, especially “Risk factors” and our financial statements and the related notes, before deciding to buy our ordinary shares. Unless the context requires otherwise (i) prior to completion of the Scheme of Arrangement (as defined below), which was effected on October 2, 2013, references in this prospectus to the “Company,” “we,” “us” and “our” refer to Oxford Immunotec Limited and its consolidated subsidiaries and (ii) following completion of the Scheme of Arrangement, references in this prospectus to the “Company,” “we,” “us” and “our” refer to Oxford Immunotec Global PLC and its consolidated subsidiaries, including Oxford Immunotec Limited.
Overview
We are a global, commercial-stage diagnostics company committed to improving patient care by providing advanced, innovative tests in the field of immunology. Our proprietary T-SPOT® technology platform allows us to measure the responses of specific immune cells, known as T cells, to inform the diagnosis, prognosis and monitoring of patients with immunologically controlled diseases. T cells are a central component of the human body’s immune system, and are implicated in the control and progression of many medical conditions, including certain types of infectious diseases, cancers and autoimmune diseases.
The initial product we have developed using our T-SPOT technology platform is our T-SPOT.TB test, which is used to test for latent Tuberculosis (TB) infection, or LTBI. Our T-SPOT.TB test has been approved for sale in over 50 countries, including the United States, where we have received pre-market approval (PMA) from the Food and Drug Administration (FDA), in Europe, where we have obtained a CE mark, as well as Japan and China. Our T-SPOT.TB test has been included in clinical guidelines (that is, guidelines issued by governmental agencies and professional societies covering recommended or suggested uses of available diagnostics) for TB screening in 17 countries, including the United States, several European countries and Japan. In addition, we have established reimbursement for our test in the United States, as well as a Current Procedural Terminology, or CPT, code that is used only for our test. We believe that many payors rely upon CPT codes to determine the amount they pay providers. Outside the United States, we have established reimbursement in several countries where reimbursement applies, including Japan, Switzerland and Germany. Our customers benefit from the existence of reimbursement mechanisms as it provides more certainty of the amount they will be paid for performing our test, as described in the section under the heading “Business—Funding and reimbursement.” We believe the annual global market opportunity for our T-SPOT.TB test is well in excess of $1 billion, assuming we can largely displace the skin test described below in the developed world.
Tuberculosis remains a significant global public health problem. According to the World Health Organization, or the WHO, approximately two billion people globally have LTBI, and on average each carries a 10% lifetime risk of progressing to active TB disease. In 2011, approximately 9 million people contracted active TB disease, of which approximately 1.5 million people died.
A central component of TB control strategies worldwide, particularly in developed markets, is to screen large numbers of people in high-risk groups for LTBI. These screening programs seek to identify infected people so that treatment can be administered to prevent these individuals from
1
Table of Contents
subsequently progressing to active TB disease and infecting others. In total, we estimate that there are 22 million LTBI tests performed each year in the United States in a variety of settings, including hospitals, public health departments, physicians’ offices and clinics. Outside the United States, we estimate the total number of tests to be 28 million each year, for a combined market size of 50 million LTBI tests annually.
The vast majority of these tests are performed using the 100-year-old Tuberculin Skin Test, or the TST. Our T-SPOT.TB test is designed to replace the TST and has several important advantages over the TST including higher sensitivity (a measure of our test’s ability to correctly identify infected subjects) and specificity (a measure of our test’s ability to identify uninfected subjects), simpler test administration, and the ability to reduce costs for healthcare institutions.
Sales of our T-SPOT.TB test are growing rapidly. As of September 30, 2013, we had cumulatively sold over two million T-SPOT.TB tests, with approximately one million tests sold over the 12 months ended September 30, 2013. Over the last three years we have grown our revenue from $4.3 million in 2009 to $20.7 million in 2012, a compound annual growth rate of 69%. We attribute the growing commercial success of our T-SPOT.TB test to the following factors:
| • | Compelling advantages over the TST. Our T-SPOT.TB test enables better TB control due to its clinical, logistical and health-economic advantages. The cost-effectiveness of our T-SPOT.TB test versus the TST has been demonstrated in multiple studies and has been persuasive in the adoption of our test. |
| • | Broad regulatory approval and scientific validation. Our T-SPOT.TB test is approved for sale in over 50 countries, giving us a substantial accessible market. The performance of our T-SPOT.TB test has been validated in over 300 peer-reviewed publications in scientific journals. |
| • | Supportive guidelines. Our T-SPOT.TB test has been recognized in clinical guidelines for TB screening in 17 countries, including the United States, several European countries and Japan. |
| • | Established payment mechanisms. We have established reimbursement in several key countries, including the United States, Japan, Switzerland and Germany. |
| • | Large underpenetrated market. Our T-SPOT.TB test addresses the estimated global market of 50 million tests per annum, which we believe represents a market opportunity for us of well in excess of $1 billion. Our penetration of this market is in its early stages. We estimate that over 90% of testing is still performed with the TST, giving us a significant opportunity for long-term growth through displacement of the TST. |
| • | Flexible business model. We offer our T-SPOT.TB test in two formats to accommodate customer preference and maximize sales. Our in vitro diagnostic kit format (a test performed outside the body), which is available globally, allows customers to perform the test in their own institutions. In our service format, which we offer in the United States and the United Kingdom, we perform our T-SPOT.TB test on samples sent by customers to our laboratory facilities. Our service offering provides us with direct customer contact and, therefore, unique market insights. |
| • | Recurring revenue. Once a customer begins using our T-SPOT.TB test instead of the TST, our experience is that the customer rarely goes back to using the TST. This purchasing pattern |
2
Table of Contents
| allows us to continually leverage our sales force to generate new business, rather than to maintain existing customers. |
We are a global business with 151 employees, including sales and marketing teams, on three continents, and laboratories in the United States and the United Kingdom. We sell to customers in over 40 countries and derived 50% of our revenue from outside the United States for the year ended December 31, 2012. Our current customer base is comprised of over 1,000 active customers, consisting of hospitals, public health departments, commercial testing laboratories, importers and distributors. Our revenue for the year ended December 31, 2011 was $12.6 million, for the year ended December 31, 2012 was $20.7 million, and for the nine months ended September 30, 2013 was $28.6 million. Our net loss for the year ended December 31, 2011 was $13.1 million, for the year ended December 31, 2012 was $14.9 million and for the nine months ended September 30, 2013 was $5.3 million.
Current TB skin test and its limitations
The primary test currently used for TB screening is the 100-year-old TST. The TST is administered by injecting an extract from cultured M. tuberculosis, called Tuberculin or Purified Protein Derivative, known as PPD, into the skin of a subject’s forearm using a needle and syringe. The injection of PPD into the skin of a subject previously infected with TB stimulates the immune response, including T cells, causing the formation of a hard lump at the site of the injection. Because it takes time for this reaction to occur, the subject must return in 48 to 72 hours after the PPD injection to have the result read. The test result is graded by feeling for the boundaries of the swelling, marking these boundaries with a pen and then measuring the diameter with a ruler.
The TST suffers from several limitations, including the following:
| • | Antiquated technique results in substantial test variability. The technique of administering the PPD injection and reading the TST is inherently variable. For example, variation in the size of the swelling due to administration of the injection averages approximately 15%, while variation in test reading among experienced operators is also estimated at approximately 15%. |
| • | Multiple patient visits required. The TST requires that the patient return in 48 to 72 hours after the time of injection. This requirement presents a significant logistical challenge. Additionally, non-return rates can be as high as 30%, resulting in considerable time and money being wasted to persuade the subjects to be rescreened as well as the duplicated materials costs and time associated with retesting. |
| • | False negatives. False-negative results to the TST are common due to a number of factors relating to the quality of the PPD used and the patient receiving the injection. Specifically, the PPD may be improperly stored, improperly diluted or contaminated. In addition, infections (including active TB disease) can suppress the TST response, leading to a false negative. False negatives are also prominent among newborns and elderly subjects. Many other conditions can also cause false-negative TST results, including HIV, certain live-virus vaccinations and common immunosuppressive drugs such as steroids and biologics. |
| • | False positives. False-positive results to the TST are common and are attributed to the presence in PPD of antigens that are shared with other mycobacteria. As a result, the TST can |
3
Table of Contents
| cross-react in those patients who are infected with non-tuberculous mycobacteria as well as those patients who have received the Bacille Calmette-Guerin, or BCG, vaccine for TB, which is the most widely administered vaccine in the world. |
| • | “Boosting” of results. The TST result can also be “boosted,” which occurs when an infected subject’s reaction to an initially false-negative skin test causes increased sensitivity in a subsequent test such that the subject tests positive. The misinterpretation of a boosted reaction as a new infection with M. tuberculosis can result in unnecessary additional testing for the subject, unnecessary treatment and unnecessary testing of other people. |
Our solution
Our T-SPOT.TB test is a highly sensitive and specific, single-cell based method for identifying LTBI. It is a single-tube blood test that directly measures antigen-specific T cells that indicate LTBI. We believe our T-SPOT.TB test has a number of compelling advantages that make it a superior alternative to the 100-year-old TST, including:
| • | In head-to-head studies, our T-SPOT.TB test is frequently found to have higher sensitivity than the TST. In addition, and unlike the TST, our T-SPOT.TB test is not significantly affected by immune-suppression. |
| • | Our T-SPOT.TB test is more specific than the TST, primarily because the antigens in our T-SPOT.TB test do not cross-react in individuals who have had the BCG vaccination or who have been infected with most other non-tuberculous mycobacteria. |
| • | Our T-SPOT.TB test requires a simple blood draw and therefore does not require specifically trained healthcare workers to administer the test. |
| • | There is no requirement for a return visit in 48 to 72 hours to obtain our T-SPOT.TB test result. This makes the testing process more convenient for patients and avoids the costs of readministering the test to those who fail to return to have the TST read. |
| • | Our T-SPOT.TB test does not suffer from the “boosting” phenomenon that can affect the TST because there is no injection of immunogenic substances into the body. Consequently, with our T-SPOT.TB test, screening of new healthcare workers can be condensed to a single visit, rather than the two-step testing that is recommended when using the TST, which entails four visits. |
| • | The combination of our T-SPOT.TB test’s greater accuracy and its logistical benefits means that the adoption of our T-SPOT.TB test can improve patient care while reducing costs for institutions. |
Our strategy
Our near-term objective is to increase adoption of our T-SPOT.TB test for screening and detecting persons with LTBI. Our longer-term objective is to leverage our proprietary T-SPOT technology platform, immunology domain expertise and regulatory experience, to cost-effectively introduce other high-value immunology-based diagnostic tests. To achieve these objectives, our strategy is to:
| • | Accelerate adoption of our T-SPOT.TB test in proven market segments in the United States. |
| • | Expand into other market segments in the United States. |
4
Table of Contents
| • | Expand our sales presence outside the United States. |
| • | Expand our addressable market outside the United States. |
| • | Launch new diagnostic tests. |
Our technology platform
Our proprietary T-SPOT technology platform allows us to efficiently measure marker-specific T cell responses at a single-cell level and thereby inform the diagnosis, prognosis and monitoring of patients with immunologically controlled diseases. By measuring T cells, we can provide additional data to clinicians that are not available through other methods, such as molecular diagnostics. For example, LTBI cannot be diagnosed by a molecular test (that is, a test relying on the identification of genomic material from the TB bacterium).
Our research and development efforts are focused on developing new diagnostic tests that use our quantitative T cell measurement technology. T cells are a central component of the human body’s immune system and are implicated in the control and progression of many medical conditions, including certain types of infectious diseases, cancers and autoimmune diseases. Therefore, we believe that our technology platform has potential to be deployed more broadly for other diseases and conditions. Our initial focus is on assays that would help transplant physicians better manage patients at risk of rejection and infection. Because the antigens in this context are largely known, reducing the lead time required for antigen discovery, we believe that we may be able to develop a test for use in the transplant market more quickly and with less development risk. In addition, because we already have sales penetration in hospitals where such centers are generally located, we believe that we may be able to efficiently build upon our existing sales and marketing infrastructure in order to introduce a test in this market. Given that intensive patient monitoring is required in the first few years post-surgery, we believe that this can be a significant market for our tests. We believe our market opportunity in the transplant segment could be as high as $500 million annually.
Risks associated with our business
An investment in our ordinary shares involves a high degree of risk. Among these important risks are the following:
| • | We have a history of losses and anticipate that we will incur continued losses for at least the next few years. We cannot be certain that we will achieve or sustain profitability. |
| • | We are currently a single-product company that is heavily dependent on the successful further commercialization of our T-SPOT.TB test, and if we encounter delays or difficulties in the commercialization of this product, our business could be harmed. |
| • | The commercial success of our T-SPOT.TB test will depend upon the degree of market acceptance by hospitals and public health departments, as well as physicians and others in the medical community. |
| • | The success of our T-SPOT.TB test depends on the continued demand for diagnostic products for tuberculosis. |
5
Table of Contents
| • | New market opportunities may not develop as quickly as we expect, limiting our ability to market and sell our T-SPOT.TB test successfully. |
| • | Our T-SPOT.TB test competes with other diagnostic testing methods that may be more widely accepted than our test, and may compete with new diagnostic tests that may be developed by others in the future, which could impair our ability to maintain and grow our business and remain competitive. |
| • | If we are unable to maintain and expand our network of direct sales representatives and independent distributors, we may not be able to generate anticipated sales. |
For additional information about the risks we face, please see the section of this prospectus captioned “Risk factors.”
Implications of being an emerging growth company
As a company with less than $1.0 billion in revenue during our most recently completed fiscal year, we qualify as an emerging growth company as defined in Section 2(a) of the Securities Act of 1933, as amended, or the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies that are not emerging growth companies. These provisions include:
| • | only two years of audited financial statements in addition to any required unaudited interim financial statements, with correspondingly reduced disclosure in the “Management’s discussion and analysis of financial condition and results of operations” section of this prospectus; |
| • | reduced disclosure about our executive compensation arrangements; |
| • | no non-binding shareholder advisory votes on executive compensation or golden parachute arrangements; and |
| • | exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would no longer be an emerging growth company if we have more than $1.0 billion in annual revenue as of the end of our fiscal year, we have more than $700.0 million in market value of our shares held by non-affiliates as of the end of our second fiscal quarter or we issue more than $1.0 billion of non-convertible debt over a three-year-period. We may choose to take advantage of some or all of these reduced disclosure obligations.
The JOBS Act permits emerging growth companies to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are choosing to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
6
Table of Contents
Scheme of arrangement
On October 2, 2013, Oxford Immunotec Global PLC completed a scheme of arrangement under the laws of England and Wales, or the Scheme of Arrangement, which was approved by the High Court of Justice in England and Wales, whereby holders of equity interests in Oxford Immunotec Limited, a private limited company incorporated in England and Wales, including holders of ordinary shares, preferred ordinary shares, options and warrants, exchanged their interests in Oxford Immunotec Limited for identical interests in Oxford Immunotec Global PLC, a public limited company incorporated in England and Wales, which then became the parent company of Oxford Immunotec Limited.
Exchange rate information
Throughout this prospectus, we present certain figures that have been converted to U.S. Dollars. Unless otherwise noted, these figures have been converted using the exchange rate as set forth in the H.10 statistical release of the Federal Reserve Board. The rates represent the noon buying rate in New York for cable transfers payable in foreign currencies as of August 30, 2013. No representation is made that the foreign currency amounts referred to in this prospectus could have been or could be converted into U.S. Dollars at any particular rate or at all.
The following table sets forth information concerning exchange rates between the foreign currencies indicated and the U.S. Dollar on August 30, 2013. These rates are provided solely for your convenience and are not necessarily the exchange rates that we will use in the preparation of our periodic reports or any other information to be provided to you.
| Currency: | Noon Buying Rate: | |
| Pound Sterling (U.S.$/£) |
1.5468 | |
| Yen (¥/U.S.$) |
98.2200 | |
| Euro (U.S.$/€) |
1.3196 | |
| Renminbi (RMB/U.S.$) |
6.1193 |
Corporate information
Oxford Immunotec Global PLC was incorporated in England and Wales in 2013. Our principal executive offices are located at 94C Innovation Drive, Milton Park, Abingdon, OX14 4RZ, United Kingdom, and our telephone number is +44 (0) 1235 442 780. Our internet website is www.oxfordimmunotec.com. The information on, or that can be accessed through, our website is not part of this prospectus, and you should not rely on any such information in making the decision whether to purchase our ordinary shares.
We use “T-SPOT®,” “T-Cell Xtend®,” “Oxford Diagnostic Laboratories®,” “ODL®,” the Oxford Immunotec logo, our laboratory logo and other marks as trademarks in the United States and other countries. This prospectus contains references to our trademarks and service marks and to those belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or ™ symbols, but such references are not intended to indicate in any way that we will not assert, to the fullest extent under applicable law, our rights to these trademarks and trade names. We do not intend our use or display of other entities’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other entity.
7
Table of Contents
The offering
| Ordinary shares offered by us |
shares |
| Ordinary shares to be outstanding after this offering |
shares |
| Option to purchase additional shares |
The underwriters have an option for a period of 30 days to purchase up to additional ordinary shares to cover overallotments. |
| Use of proceeds |
We estimate that the net proceeds from this offering will be approximately $ million, or approximately $ million if the underwriters exercise their option to purchase additional shares in full, at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds of this offering: (1) to hire additional sales, marketing and customer service personnel and expand marketing programs both in the United States and outside the United States; (2) to fund research and development programs dedicated to development of new diagnostic tests in the field of immunology; (3) to repay indebtedness outstanding under our senior secured term debt and revolving credit facility; and (4) for working capital and other general corporate purposes. See “Use of proceeds.” |
| Dividend policy |
We have never paid or declared any cash dividends on our ordinary shares, and we do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. See “Dividend policy.” |
| Risk factors |
You should read the “Risk factors” section of this prospectus for a discussion of factors to consider carefully before deciding to invest in our ordinary shares. |
| Proposed NASDAQ Global Market symbol |
“OXFD” |
The number of ordinary shares to be outstanding after this offering is based on ordinary shares outstanding as of , 2013 and excludes the following:
| • | ordinary shares issuable upon exercise of share options outstanding as of , 2013 at a weighted-average exercise price of $ per share; |
| • | ordinary shares issuable upon the exercise of warrants outstanding as of , 2013 at a weighted-average exercise price of $ per share; and |
| • | ordinary shares reserved for future issuance under our equity incentive plans as of , 2013. |
8
Table of Contents
Unless otherwise indicated, this prospectus reflects and assumes the following:
| • | completion of the Scheme of Arrangement; |
| • | a 1-for- reverse share split of our ordinary shares to be effected on , 2013; |
| • | the conversion of all outstanding preferred ordinary shares and A ordinary shares into ordinary shares, which will occur automatically immediately prior to the closing of this offering; |
| • | the adoption of our articles of association, to be effective upon the closing of this offering; and |
| • | no exercise by the underwriters of their option to purchase up to additional ordinary shares in this offering. |
9
Table of Contents
Summary consolidated financial data
The following tables summarize our consolidated financial and other data. The consolidated statement of operations data for the years ended December 31, 2011 and 2012 and the consolidated balance sheet data as of December 31, 2011 and 2012 have been derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statement of operations data for the nine months ended September 30, 2012 and 2013 and the consolidated balance sheet data as of September 30, 2013 have been derived from our unaudited consolidated financial statements appearing elsewhere in this prospectus.
On October 2, 2013, we completed the Scheme of Arrangement. Prior to the Scheme of Arrangement, our business was conducted by Oxford Immunotec Limited and its consolidated subsidiaries. Following the Scheme of Arrangement, our business has been conducted by Oxford Immunotec Global PLC and its consolidated subsidiaries, including Oxford Immunotec Limited.
We have prepared the unaudited consolidated interim financial information presented below on the same basis as our audited consolidated financial statements. The unaudited consolidated financial information includes all adjustments, consisting only of normal recurring adjustments that are necessary for a fair presentation of our financial position and results of operations for these periods. Our historical results are not necessarily indicative of the results that may be expected in the future and our results for any interim period are not necessarily indicative of the results that may be expected for a full fiscal year. You should read the summary of our financial data set forth below together with our financial statements and the related notes to those statements, as well as “Management’s discussion and analysis of financial condition and results of operations” appearing elsewhere in this prospectus. The summary financial data in this section are not intended to replace our financial statements and the accompanying notes.
10
Table of Contents
| Year ended December 31, | Nine months ended September 30, |
|||||||||||||||
| (in thousands, except share and per share data) (unaudited) |
2011 | 2012 | 2012 | 2013 | ||||||||||||
|
|
||||||||||||||||
| Consolidated statement of operations data: |
||||||||||||||||
| Revenue |
$ | 12,641 | $ | 20,685 | $ | 15,406 | $ | 28,559 | ||||||||
| Cost of revenue |
8,417 | 12,424 | 9,123 | 14,165 | ||||||||||||
|
|
|
|||||||||||||||
| Gross profit |
4,224 | 8,261 | 6,283 | 14,394 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
1,780 | 1,947 | 1,232 | 1,583 | ||||||||||||
| Sales and marketing |
10,536 | 11,177 | 7,895 | 9,557 | ||||||||||||
| General and administrative |
5,232 | 8,068 | 5,784 | 8,457 | ||||||||||||
|
|
|
|||||||||||||||
| Total operating costs |
17,548 | 21,192 | 14,911 | 19,597 | ||||||||||||
|
|
|
|||||||||||||||
| Loss from operations |
(13,324 | ) | (12,931 | ) | (8,628 | ) | (5,203 | ) | ||||||||
| Other (expense) income |
101 | (2,103 | ) | (1,944 | ) | (98 | ) | |||||||||
|
|
|
|||||||||||||||
| Loss before provision for income taxes |
(13,223 | ) | (15,034 | ) | (10,572 | ) | (5,301 | ) | ||||||||
| Income tax provision (benefit) |
(119 | ) | (151 | ) | 21 | 35 | ||||||||||
|
|
|
|||||||||||||||
| Net loss |
$ | (13,104 | ) | $ | (14,883 | ) | $ | (10,593 | ) | $ | (5,336 | ) | ||||
|
|
|
|||||||||||||||
| Net loss per share attributable to ordinary shareholders, basic and diluted |
$ | (1.61 | ) | $ | (1.26 | ) | $ | (1.02 | ) | $ | (0.35 | ) | ||||
|
|
|
|||||||||||||||
| Weighted-average shares used to compute net loss attributable to ordinary shareholders, basic and diluted |
8,150,146 | 11,825,803 | 10,338,893 | 15,129,791 | ||||||||||||
|
|
|
|||||||||||||||
| Pro forma (loss) per share, basic and diluted (1) |
||||||||||||||||
|
|
|
|
|
|||||||||||||
| Pro forma weighted-average number of shares, basic and diluted |
||||||||||||||||
|
|
|
|
|
|||||||||||||
| Supplemental financial metric: |
||||||||||||||||
| Adjusted EBITDA(2) |
$ | (12,519 | ) | $ | (12,131 | ) | $ | (8,057 | ) | $ | (4,296 | ) | ||||
|
|
||||||||||||||||
11
Table of Contents
| As of December 31, | As of September 30, 2013 |
|||||||||||||||||
|
|
|
|
|
|||||||||||||||
| 2011 | 2012 | Actual | Pro forma(1) |
|||||||||||||||
|
|
||||||||||||||||||
| Consolidated balance sheet data: |
| |||||||||||||||||
| Cash and cash equivalents |
$ | 2,334 | $ | 12,578 | $ | 13,035 | $ | |||||||||||
| Total assets |
9,639 | 25,483 | 31,538 | |||||||||||||||
| Total liabilities |
4,413 | 8,534 | 17,335 | |||||||||||||||
| Total shareholders’ equity |
$ | 5,226 | $ | 16,949 | $ | 14,203 | $ | |||||||||||
| Shares outstanding: |
||||||||||||||||||
| Preferred ordinary shares |
37,642,730 | 48,955,690 | 55,435,513 | |||||||||||||||
| Ordinary shares |
8,492,175 | 14,442,575 | 15,677,098 | |||||||||||||||
|
|
||||||||||||||||||
| (1) | The pro forma data gives effect to the Scheme of Arrangement, which was completed on October 2, 2013, the 1-for- reverse share split of our ordinary shares to be effected on , 2013, the conversion of all outstanding preferred ordinary shares and A ordinary shares into an aggregate of ordinary shares upon closing of this offering, the repayment of indebtedness outstanding under our senior secured term debt and our issuance and sale of ordinary shares at an assumed initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| (2) | Adjusted EBITDA is a non-GAAP financial measure that we calculate as profit (loss), adjusted for tax benefit and impairment (expense), unrealized exchange fluctuations, interest expense, interest income, depreciation and amortization and share-based compensation. We believe that Adjusted EBITDA provides useful information to investors in understanding and evaluating our operating results in the same manner as our management and Board of Directors. Our presentation of Adjusted EBITDA is not made in accordance with U.S. GAAP, and our computation of Adjusted EBITDA may vary from others in the industry. Our use of Adjusted EBITDA has limitations as an analytical tool, and you should not consider it in isolation or as a substitute for analysis of our results as reported under U.S. GAAP. For example, Adjusted EBITDA does not reflect the impact of earnings or charges resulting from matters that we consider not to be indicative of our ongoing operations. |
The following table presents a reconciliation of net income (loss), the most comparable U.S. GAAP financial measure, to Adjusted EBITDA for each of the periods indicated:
| Year ended December 31, |
Nine months ended September 30, |
|||||||||||||||
| (in thousands) | 2011 | 2012 | 2012 | 2013 | ||||||||||||
|
|
||||||||||||||||
| Reconciliation of (loss) profit to Adjusted EBITDA |
||||||||||||||||
| Net loss |
$ | (13,104 | ) | $ | (14,883 | ) | $ | (10,593 | ) | $ | (5,336 | ) | ||||
|
|
|
|||||||||||||||
| Income tax provision (benefit) |
(119 | ) | (151 | ) | 21 | 35 | ||||||||||
| Interest income |
(1 | ) | (1 | ) | — | — | ||||||||||
| Interest expense |
4 | 1,478 | 1,452 | 256 | ||||||||||||
| Depreciation and amortization |
630 | 801 | 580 | 863 | ||||||||||||
|
|
|
|||||||||||||||
| EBITDA |
(12,590 | ) | (12,756 | ) | (8,540 | ) | (4,182 | ) | ||||||||
|
|
|
|||||||||||||||
| Reconciling items: |
||||||||||||||||
| Share-based compensation expense |
125 | 79 | 59 | 77 | ||||||||||||
| Unrealized exchange gains (losses) |
(54 | ) | 546 | 424 | (191 | ) | ||||||||||
|
|
|
|||||||||||||||
| Adjusted EBITDA |
$ | (12,519 | ) | $ | (12,131 | ) | $ | (8,057 | ) | $ | (4,296 | ) | ||||
|
|
||||||||||||||||
12
Table of Contents
Investing in our ordinary shares involves a high degree of risk. You should carefully consider the risks and uncertainties described below together with all of the other information contained in this prospectus, including our consolidated financial statements and the related notes appearing at the end of this prospectus, before deciding whether to invest in our ordinary shares. If any of the following risks actually occurs, our business, prospects, operating results and financial condition could suffer materially, the trading price of our ordinary shares could decline and you could lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business.
Risks related to our business.
We have a history of losses and anticipate that we will incur continued losses for at least the next few years. We cannot be certain that we will achieve or sustain profitability.
We were founded in 2002 and to date we have engaged primarily in development, clinical testing and marketing of our T-SPOT.TB test. We have never been profitable. For the fiscal years ended December 31, 2011 and 2012, we had net losses of $13.1 million and $14.9 million, respectively, and we had an accumulated deficit at December 31, 2012 of $91.0 million. For the nine months ended September 30, 2012 and 2013, we had net losses of $10.6 million and $5.3 million, respectively, and we had an accumulated deficit at September 30, 2013 of $96.3 million. Substantially all of our operating losses in these periods resulted from costs incurred in connection with sales and marketing of our T-SPOT.TB test, general and administrative costs associated with our operations and our research and development programs. Additionally, as a result of our anticipated future significant expenses relating to expansion of our sales and marketing capabilities, further commercialization of our T-SPOT.TB test, and research and development, we expect to continue to incur significant operating losses for at least the next few years, even though we generate revenue from our T-SPOT.TB test. Because of the numerous risks and uncertainties associated with developing and commercializing diagnostic products, we are unable to predict the magnitude of these future losses. Our historic losses, combined with expected future losses, have had and will continue to have an adverse effect on our cash resources, shareholders’ deficit and working capital. We expect our research and development expenses to be substantial for at least the next few years as we work to develop other product candidates based on our T-SPOT technology.
Our ability to become profitable depends upon our ability to generate revenue. In 2004, we began to generate revenue from the sale and marketing of our T-SPOT.TB test, but we may not be able to generate sufficient revenue to attain profitability. Our ability to generate profits on sales of our T-SPOT.TB test is subject to market acceptance in market segments we currently serve as well as in new market segments and new geographies. In addition, we may be compelled to sell our T-SPOT.TB test at lower prices if, for example, our customers or prospective customers are unwilling to pay for our tests at current pricing levels or as a result of increased competition generally. Any price erosion would impede our ability to generate revenue. If we are unable to generate sufficient revenue, we will not become profitable and may be unable to continue operations without continued funding.
13
Table of Contents
We are currently a single-product company that is heavily dependent on the successful further commercialization of our T-SPOT.TB test and, if we encounter delays or difficulties in the further commercialization of this product, our business could be harmed.
Our success is heavily dependent upon the successful further commercialization of our T-SPOT.TB test. Our business could be materially harmed if we encounter difficulties in the further commercialization of this product, including, among others: failure to achieve sufficient market acceptance by hospitals and public health departments as well as physicians, third-party payors and others in the medical community; the inability to compete with other diagnostic methods, including the TST; the inability to maintain and expand our sales, marketing and distribution networks; the inability to manage anticipated growth; the inability to obtain and/or maintain necessary regulatory approvals; and the inability to effectively protect our intellectual property.
The commercial success of our T-SPOT.TB test will depend upon the degree of market acceptance by hospitals and public health departments, as well as physicians and others in the medical community.
Our T-SPOT.TB test may not gain sufficient market acceptance by hospitals and public health departments. If this product does not achieve an adequate level of acceptance by such customer groups, we may not generate enough revenue to become profitable. The degree of market acceptance of our T-SPOT.TB test will depend on a number of factors, including:
| • | clinical guidelines relative to the screening for, and diagnosis and monitoring of, TB infection; |
| • | the efficacy and potential advantages of our T-SPOT.TB test over alternative tests; |
| • | the willingness of our target customers to accept and adopt our T-SPOT.TB test; |
| • | the ability to offer attractive pricing for our T-SPOT.TB test; |
| • | the strength of marketing and distribution support and the timing of market introduction of competitive products; and |
| • | outcomes from clinical studies and other publicity concerning our T-SPOT.TB test or competing products. |
Our efforts to educate physicians and other members of the medical community on the benefits of our T-SPOT.TB test may require significant resources and may never be successful. Such efforts to educate the marketplace may require more resources than are required by conventional technologies marketed by our competitors. In particular, continuing to gain market acceptance for our T-SPOT.TB test in nascent markets could be challenging. In certain markets, including, for example, Japan and China, our potential for future growth is difficult to forecast. If we were to incorrectly forecast our ability to penetrate these markets, expenditures that we make may not result in the benefits that we expect, which could harm our results of operations. Moreover, in the event that our T-SPOT.TB test is the subject of guidelines, clinical studies or scientific publications that are unhelpful or damaging, or otherwise call into question the benefits of our T-SPOT.TB test, we may have difficulty in convincing prospective customers to adopt our test.
14
Table of Contents
The success of our T-SPOT.TB test depends on the continued demand for diagnostic products for tuberculosis.
Even if we achieve market acceptance, our success will depend on continued demand for diagnostic products for tuberculosis. Tuberculosis screening policies could change such that tests are conducted less frequently or in fewer instances. For example, healthcare institutions facing increased cost control requirements could determine to reduce employee testing. In addition, various institutions or governing bodies may decide that the incidence of TB has dropped sufficiently within their screening population so as to permit reduced testing (e.g., U.S. military guidelines were recently updated such that testing may now be required in fewer instances than under previous guidelines). If there are widespread testing policy changes that substantially reduce testing in the markets we serve, our business could be materially and adversely affected.
New market opportunities may not develop as quickly as we expect, limiting our ability to market and sell our T-SPOT.TB test successfully.
We intend to take steps to increase the presence of our T-SPOT.TB test in new markets both in the United States and outside the United States. We believe these opportunities will take substantial time to develop or mature and we cannot be certain that these market opportunities will develop as we expect. The future growth and success of our T-SPOT.TB test in these markets depends on many factors beyond our control, including recognition and acceptance by the scientific community in that market and the prevalence and costs of competing methods of tuberculosis screening. If the markets for our T-SPOT.TB test do not develop as we expect, our business may be adversely affected.
Our T-SPOT.TB test competes with other diagnostic testing methods that may be more widely accepted than our test, and may compete with new diagnostic tests that may be developed by others in the future, which could impair our ability to maintain and grow our business and remain competitive.
The clinical diagnostics market is highly competitive, and we must be able to compete effectively against existing and future competitors in order to be successful. In selling our T-SPOT.TB test, we compete primarily with existing diagnostic technologies, particularly the TST, which is widely used as a test for diagnosing tuberculosis. In addition, we compete with the QuantiFERON®-TB Gold In-Tube test1, or QFN, which, like our T-SPOT.TB test, employs an interferon-gamma release assay, or IGRA, method for diagnosing tuberculosis. If we are unable to differentiate our diagnostic tests from those of our competitors, our business may be materially and adversely affected. In addition, improvements in these technologies or the development of new technologies for diagnosing tuberculosis and the introduction of products that compete with our T-SPOT.TB test could adversely impact our ability to sell our T-SPOT.TB test or the sales price of the test. This could impact our ability to market our test and/or secure a distribution partner, both of which could have a substantial impact on the value of our T-SPOT.TB test.
We also face competition in the development, manufacture, marketing and commercialization of diagnostic products from a variety of other sources, such as academic institutions, government agencies, research institutions and other life sciences companies. These competitors are working to develop and market other diagnostic tests, systems, products and other methods of detecting, preventing or reducing tuberculosis.
| 1 | QuantiFERON is a registered trademark of Qiagen N.V. |
15
Table of Contents
Among the many experimental diagnostics being developed around the world, there may be diagnostics unknown to us that may compete with our T-SPOT.TB test. Many of our potential competitors have much greater capital resources, manufacturing, research and development resources and production facilities than we do. Competitors with greater resources may be able to offer tests and/or services at prices at which we are unable to compete and more quickly develop improvements than we are. Many of them may also have more experience than we have in preclinical testing and clinical trials of new diagnostic tests.
In our service offering, we also may face competition from commercial laboratories, including large national and regional laboratories, which may be able to offer access to TB testing. These laboratories may have perceived advantages over our solution, including phlebotomy services, established payor relationships and dedicated courier services. For example, as we seek to further penetrate the physicians’ office segment of the U.S. market, we may find that physicians have established relationships with commercial laboratories that offer physicians additional services, such as phlebotomy, and a wider range of available laboratory tests that a physician may choose to order in addition to a TB test. Further, some commercial laboratories may be able to offer their services at lower cost to physicians’ patients due to the reimbursement arrangements these laboratories may have established with third-party payors. These factors may make it difficult for us to convince physicians to use our test and service offering.
The markets for our T-SPOT.TB test are subject to changing technology, new product introductions and product enhancements, and evolving industry standards. The introduction or enhancement of products embodying new technology or the emergence of new industry standards could render existing products obsolete or result in short product life cycles or our inability to sell our T-SPOT.TB test without offering a significant discount.
If we are unable to maintain and expand our network of direct sales representatives and independent distributors, we may not be able to generate anticipated sales.
We have limited experience marketing and selling our T-SPOT.TB test. Our operating results are directly dependent upon the sales and marketing efforts of not only our employees, but also our independent distributors. We expect our direct sales representatives and independent distributors to develop long-lasting relationships with the providers they serve. If our direct sales representatives or independent distributors fail to adequately promote, market and sell our product, our sales could significantly decrease.
We face significant challenges and risks in managing our geographically dispersed sales and distribution network and retaining the individuals who make up that network. If a substantial number of our direct sales representatives were to leave us within a short period of time, or if a substantial number of our independent distributors were to cease to do business with us within a short period of time, our sales could be adversely affected. If any significant independent distributor were to cease to distribute our product, our sales could be adversely affected. In such a situation, we may need to seek alternative independent distributors or increase our reliance on our direct sales representatives, which may not prevent our sales from being adversely affected. If a direct sales representative or independent distributor were to depart and be retained by one of our competitors, we may be unable to prevent them from helping competitors solicit business from our existing customers, which could further adversely affect our sales. Because of the intense competition for their services, we may be unable to recruit additional qualified independent distributors or to hire additional qualified direct sales representatives to work with us. We may also not be able to enter into agreements with them on favorable or commercially
16
Table of Contents
reasonable terms, if at all. Failure to hire or retain qualified direct sales representatives or independent distributors would prevent us from expanding our business and generating sales. See “—Certain of our customers account for a significant portion of our revenue.”
As we launch new products and increase our sales, marketing and distribution efforts with respect to our T-SPOT.TB test, we will need to expand the reach of our sales, marketing and distribution networks. Our future success will depend largely on our ability to continue to hire, train, retain and motivate skilled direct sales representatives and independent distributors with significant technical knowledge in various areas. New hires require training and take time to achieve full productivity. If we fail to train new hires adequately, or if we experience high turnover in our sales force in the future, we cannot be certain that new hires will become as productive as may be necessary to maintain or increase our sales.
If we are unable to expand our sales and marketing capabilities domestically and internationally, we may not be able to effectively commercialize our product, which would adversely affect our business, results of operations and financial condition.
Health insurers and other third-party payors may decide not to cover, or may discontinue reimbursing, our T-SPOT.TB test or any other diagnostic tests we may develop in the future, or may provide inadequate reimbursement, which could jeopardize our ability to expand our business.
Although for many of our current customers, including those in the hospital and public health segments, the cost of screening their employees for tuberculosis is not reimbursable, our business is somewhat impacted, and in the future may be more greatly impacted, by the level of reimbursement from third-party payors. In the United States, the regulatory process allows diagnostic tests to be marketed regardless of any coverage determinations made by payors. For new diagnostic tests, each third-party payor makes its own decision about which tests it will cover, how much it will pay and whether it will continue reimbursing the test. Clinicians may order diagnostic tests that are not reimbursed by third-party payors if the patient is willing to pay for the test without reimbursement, but coverage determinations and reimbursement levels and conditions are important to the commercial success of a diagnostic product.
The Centers for Medicare & Medicaid Services, or CMS, establishes reimbursement payment levels and coverage rules for Medicare. CMS currently covers our T-SPOT.TB test. If CMS were to place significant restrictions on the use of our tests, reduce payment amounts or eliminate coverage altogether, our ability to generate revenue from our diagnostic tests could be limited. For example, payment for diagnostic tests furnished to Medicare beneficiaries is made based on a fee schedule set by CMS. Payments under these fee schedules have decreased in recent years and may decrease further in the future.
In addition, state Medicaid plans and private commercial payors establish rates and coverage rules independently. As a result, the coverage determination process is often a time-consuming and costly process that requires us to provide scientific and clinical support for the use of our tests to each payor separately, with no assurance that coverage or adequate reimbursement will be obtained. Even if one or more third-party payors decides to reimburse for our tests, that payor may reduce utilization or stop or lower payment at any time, which could reduce our revenue. We cannot predict whether or when third-party payors will cover our tests or offer adequate reimbursement to make them commercially attractive. Clinicians may decide not to order our tests if inadequate third-party payments result in additional costs to the patient.
17
Table of Contents
We are also subject to foreign reimbursement schemes in the international markets we serve, including Germany, Switzerland, France and Japan. Decisions by health insurers or other third-party payors in these markets not to cover, or to discontinue reimbursing could materially and adversely affect our business.
If we do not achieve, sustain or successfully manage our anticipated growth, our business and growth prospects may be harmed.
We have experienced significant revenue growth in a relatively short period of time. We may not achieve similar growth rates in future periods. Investors should not rely on our operating results for any prior periods as an indication of our future operating performance. If we are unable to maintain adequate revenue growth, our financial results could suffer and our share price could decline. Furthermore, growth will place significant strains on our management and our internal systems and processes, as well as potentially those of our suppliers.
Further development and commercialization of our T-SPOT.TB test and other diagnostic product candidates will require us to expand our sales, marketing and distribution networks. If we cannot effectively manage our expanding operations and our costs, we may not be able to continue to grow or we may grow at a slower pace and our business could be adversely affected.
We depend upon a limited number of suppliers, and certain components of our product may only be available from a sole source or limited number of suppliers.
Our T-SPOT.TB test is generally assembled by us from supplies we obtain from a limited number of suppliers. Critical components required to assemble our tests may only be available from a sole or limited number of component suppliers. For example, we source key components of our T-SPOT.TB test from EMD Millipore Corporation, Stemcell Technologies Inc., Mabtech AB, MicroCoat Biotechnologie GmbH and Life Technologies Corporation, any of whom would be difficult to replace. Even if the key components that we source are available from other parties, the time and effort involved in obtaining any necessary regulatory approvals for substitutes could impede our ability to replace such components timely or at all. The loss of a sole or key supplier would impair our ability to deliver products to our customers in a timely manner and would adversely affect our sales and operating results and negatively impact our reputation. Our business would also be harmed if any of our suppliers could not meet our quality and performance specifications and quantity and delivery requirements.
Certain of our customers account for a significant portion of our revenue.
We sell our T-SPOT.TB test through a direct sales force in the United States, certain European countries and Japan. In Japan, while we maintain end-user relationships through our direct sales force, we sell through a single importer of record, Riken Genesis Co., Ltd., or Riken. In other parts of the world, we sell through distributors. For example, in China, we sell through a single distributor, Shanghai Fosun Long March Medical Science Co. Ltd., or Fosun. For the nine months ended September 30, 2013, sales to Fosun and through Riken together accounted for 34% of our total revenue, with Fosun accounting for 17%. In the event that either of these customers or any other significant customer substantially reduces its purchases of our products, particularly if this occurs without adequate advance notice to enable us to secure alternate importation or distribution arrangements, our results of operations could be materially and adversely affected.
18
Table of Contents
We or our suppliers may experience development or manufacturing problems or delays that could limit the growth of our revenue or increase our losses.
We may encounter unforeseen situations in the manufacturing and assembly of our T-SPOT.TB test that would result in delays or shortfalls in our production. Our suppliers may also face similar delays or shortfalls. In addition, our or our suppliers’ production processes and assembly methods may have to change to accommodate any significant future expansion of our manufacturing capacity, which may increase our or our suppliers’ manufacturing costs, delay production of our product, reduce our product margin and adversely impact our business. If we are unable to keep up with demand for our product by successfully manufacturing and shipping our product in a timely manner, our revenue could be impaired, market acceptance for our product could be adversely affected and our customers might instead purchase our competitors’ products. In addition, developing manufacturing procedures for new products would require developing specific production processes for those products. Developing such processes could be time consuming, and any unexpected difficulty in doing so can delay the introduction of a product.
We currently perform our tests for our service offering exclusively in one laboratory facility in the United States and one laboratory in the United Kingdom. If these or any future facilities or our equipment were damaged or destroyed, or if we experience a significant disruption in our operations for any reason, our ability to continue to operate our business could be materially harmed.
We currently perform our T-SPOT.TB test for our service offering in the United States exclusively in a single laboratory facility in Memphis, Tennessee, and in the United Kingdom exclusively in a single laboratory facility in Abingdon, England. If these or any future facilities were to be damaged, destroyed or otherwise unable to operate, whether due to fire, floods, hurricanes, storms, tornadoes, other natural disasters, employee malfeasance, terrorist acts, power outages, or otherwise, or if performance of our laboratories is disrupted for any other reason, we may not be able to perform our tests or generate test reports as promptly as our customers expect, or possibly not at all. Building or finding a replacement facility could be difficult, expensive and time consuming and any new laboratory would need to satisfy the various certification, accreditation and licensing requirements to which our current laboratory facilities are subject, including, for example, CLIA requirements in the United States. If we are unable to perform our tests or generate test reports within a timeframe that meets our customers’ expectations, our business, financial results and reputation could be materially harmed.
As of September 30, 2013, we maintain insurance coverage totaling $6.5 million against damage to our property and equipment and an additional $22 million to cover business interruption and research and development restoration expenses, subject to deductibles and other limitations. If we have underestimated our insurance needs with respect to an interruption, or if an interruption is not subject to coverage under our insurance policies, we may not be able to cover our losses. Even if we cover our losses, our business, financial results and reputation could be materially harmed.
Billing complexities associated with obtaining payment or reimbursement for our tests may negatively affect our revenue, cash flow and profitability.
Although third-party payors account for only 4% of our total revenue for the year ended December 31, 2012, we currently rely in part, and may in the future more heavily rely, on obtaining third-party payment or reimbursement for our test. Billing for diagnostic tests is complex. We or our customers receive payment from individual patients and from a variety of
19
Table of Contents
payors, such as commercial insurance carriers, including managed care organizations and governmental programs, primarily Medicare and Medicaid in the United States. Each payor typically has different billing requirements, and the billing requirements of many payors have become increasingly stringent.
Among the factors complicating our billing of third-party payors are:
| • | disputes among payors as to which party is responsible for payment; |
| • | disparity in coverage among various payors; |
| • | disparity in information and billing requirements among payors; and |
| • | incorrect or missing billing information, which is required to be provided by the ordering physician. |
These billing complexities, and the related uncertainty in obtaining payment for our tests, could negatively affect our revenue, cash flow and profitability.
We may require substantial additional resources to fund our operations. We may not be able to obtain additional capital resources on favorable terms and if we cannot find additional capital resources, we may have difficulty operating our business. Raising additional capital may also cause dilution to our existing shareholders.
As of September 30, 2013, we had cash and cash equivalents of $13.0 million and working capital of $16.3 million. We believe that after this offering we will have sufficient resources to fund our projected operations for at least the next few years. However, changing circumstances may cause us to consume capital significantly faster or slower than we currently anticipate. We have based these estimates on assumptions that may prove to be wrong, and we could exhaust our available financial resources sooner than we currently anticipate. In order to fund our strategic plans, we may need to enter into a strategic collaboration or raise additional capital. We may seek to raise additional capital through the issuance of equity or debt securities in the public or private markets, or through a collaborative arrangement or sale of assets. Additional financing opportunities may not be available to us, or if available, may not be on favorable terms. Further, to the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a shareholder.
Our future capital requirements will depend on many factors, including revenue generated from the sale of our T-SPOT.TB test, margins, operating expenses and our ability to control costs associated with our operations, and the costs of filing, prosecuting, maintaining, defending and enforcing any patent claims and other intellectual property rights. The availability of additional capital will also depend on many factors, including the market price of our ordinary shares and the availability and cost of additional equity capital from existing and potential new investors, our ability to retain the listing of our ordinary shares on The NASDAQ Global Market and general economic and industry conditions affecting the availability and cost of capital.
Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration, strategic alliance and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates beyond the rights we have already relinquished, or grant licenses on terms that are not favorable to us.
20
Table of Contents
Failure in our information technology or storage systems could significantly disrupt our operations and our research and development efforts, which could adversely impact our revenue, as well as our research, development and commercialization efforts.
Our ability to execute our business strategy depends, in part, on the continued and uninterrupted performance of our information technology, or IT, systems, which support our operations, including our laboratory information system, or LIS, our billing system, and our customer interfaces. Due to the sophisticated nature of the technology we use in our laboratories and our complex billing procedures, we are substantially dependent on our IT systems. IT systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptive problems. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our IT systems, sustained or repeated system failures that interrupt our ability to generate and maintain data, and in particular to operate our LIS and billing system, could adversely affect our ability to operate our business. Any interruption in the operation of our LIS or billing system, due to IT system failures, part failures or potential disruptions in the event we are required to relocate our IT systems within our facility or to another facility could have an adverse effect on our operations.
We rely on courier delivery services to transport samples to our facilities for testing. If these delivery services are disrupted, our business and customer satisfaction could be negatively impacted.
Customers in the United States and the United Kingdom ship samples to us by air and ground express courier delivery service for testing in our Memphis, Tennessee and Abingdon, England facilities. If we suffer from disruptions in delivery service, whether due to bad weather, natural disaster, terrorist acts or threats, or for other reasons, we may be unable to provide timely services to customers or at all. As a result, such disruptions could materially and adversely affect our financial results and our reputation.
Because our business relies heavily on international operations and revenue, changes in currency exchange rates and our need to convert currencies may negatively affect our financial condition and results of operations.
Our business relies heavily on our operations outside the United States. For the year ended December 31, 2012, 50% of our total revenue was derived from sales outside the United States and for the nine months ended September 30, 2013, 56% of our total revenue was derived from sales outside the United States. Because we currently operate in three major regions of the world (the United States, Europe and rest of world, or Europe & ROW, and Asia), our revenue is denominated in multiple currencies. Sales in the United States are denominated in U.S. Dollars. Sales in China are denominated in U.S. Dollars and sales in Japan are denominated in Yen but, in each case, these sales are made by our U.K.-based legal entity where the Pound Sterling is the functional currency. As a result, these sales are subject to remeasurement into Pounds Sterling and then translation into U.S. Dollars when we consolidate our financial statements. Sales in Europe are denominated primarily in the Pound Sterling and Euro. As we grow Europe & ROW sales outside the United Kingdom and the European Union countries whose national currency is the Euro, or the Euro Zone, we will be subject to exchange rate risk from additional currencies. As a result, our exchange rate exposure may change over time as our business practices evolve and could result in increased costs or reduced revenue and could affect our actual cash flow.
21
Table of Contents
Changes in the relative values of currencies occur regularly and, in some instances, may have a significant impact on our operating results. We cannot predict with any certainty changes in currency exchange rates or the degree to which we can effectively mitigate these risks.
Our future success depends on our ability to successfully develop, obtain clearance or approval for and commercialize new products.
Our future success partially depends on our ability to successfully develop and market new products. Our ability to develop any of these products is dependent on a number of factors, including funding availability to complete development efforts, our ability to develop products that adequately detect or measure the targeted function, condition or disease, our ability to secure required FDA or other regulatory clearance or approval, our ability to obtain licenses to necessary third-party intellectual property and our ability to commercialize our products, thereby generating revenue once development efforts prove successful. We may encounter problems in the development phase for our products, which can result in substantial setbacks and delays or abandonment of further work on the potential product. There can be no assurance that we will not encounter such setbacks with the products in our pipeline, or that funding from outside sources and our revenue will be sufficient to bring any future product to the point of commercialization. There can be no assurance that the products we seek to develop will work effectively in the marketplace, or that we will be able to produce them on an economical basis. As with our current T-SPOT.TB test, the success of any future products will also depend upon the degree of market acceptance by physicians, hospitals, third-party payors and others in the medical community.
We may seek to grow our business through acquisitions of or investments in new or complementary businesses, products or technologies, and the failure to manage acquisitions or investments, or the failure to integrate them with our existing business, could have a material adverse effect on us.
From time to time we expect to consider opportunities to acquire or make investments in other technologies, products and businesses that may enhance our capabilities, complement our current products or expand the breadth of our product offerings, markets or customer base. Potential and completed acquisitions and strategic investments involve numerous risks, including:
| • | problems assimilating the purchased technologies, products or business operations; |
| • | issues maintaining uniform standards, procedures, controls and policies; |
| • | unanticipated costs associated with acquisitions; |
| • | diversion of management’s attention from our core business; |
| • | adverse effects on existing business relationships with suppliers and customers; |
| • | risks associated with entering new markets in which we have limited or no experience; |
| • | potential loss of key employees of acquired businesses; and |
| • | increased legal and accounting compliance costs. |
We have no current commitments with respect to any acquisition or investment. Any acquisitions we undertake could be expensive and time consuming, and may disrupt our ongoing business and prevent management from focusing on our operations. If we are unable to manage acquisitions or investments, or integrate any acquired businesses, products or technologies effectively, our business, results of operations and financial condition may be materially adversely affected.
22
Table of Contents
Our business could suffer if we lose the services of, or are unable to attract and retain, key members of our senior management, key advisors or other personnel.
We are dependent upon the continued services of key members of our senior management and a limited number of key advisors and personnel. In particular, we are highly dependent on the skills and leadership of our Chief Executive Officer, Dr. Peter Wrighton-Smith, and the other members of management named in the “Management” section elsewhere in this prospectus. The loss of any one of these individuals could disrupt our operations or our strategic plans. Additionally, our future success will depend on, among other things, our ability to continue to hire and retain the necessary qualified scientific, technical, sales, marketing and managerial personnel, for whom we compete with numerous other companies, academic institutions and organizations. The loss of members of our management team, key advisors or personnel, or our inability to attract or retain other qualified personnel or advisors, could have a material adverse effect on our business, results of operations and financial condition. Although all members of our senior management team have entered into agreements that restrict their ability to compete with us for a period of time after the end of their employment, we may be unable to enforce such restrictive covenants at all or for a sufficient duration of time to prevent members of our management team from competing with us.
The outcome of any future claims and litigation could have a material adverse impact on our business, financial condition and results of operations.
We may, from time to time, be party to litigation in the normal course of business, including class action lawsuits. Due to the inherent uncertainties of litigation, it is not possible to predict the final outcome of these lawsuits or determine the amount of any potential losses we may incur. In the event we are required or determine to pay amounts in connection with any such lawsuits, such amounts could be significant and could have a material adverse impact on our liquidity, business, financial condition and results of operations.
Our ability to use net operating losses to offset future taxable income may be subject to substantial limitations.
As of December 31, 2012, our available U.S. federal net operating losses, or NOLs, totaled $38.1 million and U.S. state loss carryforwards totaled $32.9 million. The amount of these NOLs remains subject to review and possible adjustment by the Internal Revenue Service and state revenue authorities, as applicable. NOLs may become subject to an annual limitation if there is a cumulative change in the ownership interest of significant shareholders (or certain shareholder groups) over a three-year period in excess of 50%, in accordance with rules established under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, and similar state rules (we refer to each as an ownership change). Such an ownership change could limit the amount of historic NOLs that can be utilized annually to offset future taxable income. The amount of this annual limitation is determined based on the value of the Company immediately prior to the ownership change. We have completed several financings since its inception that may have resulted in one or more ownership changes or could result in an ownership change in the future. Future changes in our share ownership, some of which are outside of our control, could result in additional ownership changes for purposes of these rules. We are unable to predict future ownership changes or the way an ownership change could limit the use of our NOLs. If we undergo an ownership change in connection with or after this public offering, we may not be able to utilize a material portion of our NOLs even if we attain profitability.
23
Table of Contents
Risks related to regulatory and other legal issues.
If we fail to comply with extensive regulations of domestic and international regulatory authorities, sales of our T-SPOT.TB test in new markets and the development and commercialization of any new product candidates could be delayed or prevented.
Our T-SPOT.TB test is, and any new product candidates will be, subject to extensive government regulations related to development, testing, manufacturing and commercialization in the United States and other countries before we can sell in these markets. The process of obtaining and complying with FDA and other governmental regulatory approvals and regulations is costly, time consuming, uncertain and subject to unanticipated delays. Securing regulatory approval for a new product, in the United States and many other countries, typically requires several years. Despite the time and expense exerted, regulatory approval is never guaranteed. We may not be able to obtain FDA or other required regulatory approval and market any further products we may develop during the time we anticipate, or at all. We also are subject to the following risks and obligations, among others:
| • | regulators may refuse to approve an application if they believe that applicable regulatory criteria are not satisfied. |
| • | regulators may require additional testing for safety and effectiveness. |
| • | regulators may interpret data from clinical studies in different ways than we interpret them. |
| • | if regulatory approval of a product is granted, the approval may be limited to specific indications or limited with respect to its distribution. |
| • | regulators may change their approval policies and/or adopt new regulations that affect our ability to secure approvals for new products, which would decrease the chance we would be able to commercialize new diagnostic tests. |
In addition, some international jurisdictions, such as China, require periodic recertification. Even if we obtain initial certifications from regulatory bodies, we may lose certification after a periodic review. Failure to maintain requisite certifications from regulatory bodies would adversely affect our ability to generate future revenue and operating income.
If we or our suppliers fail to comply with ongoing regulatory requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Any product for which we obtain marketing approval in the United States or in international jurisdictions, along with the manufacturing processes, post-approval clinical data and promotional activities for such product, will be subject to continual review and periodic inspections by the FDA and other regulatory bodies. Furthermore, our suppliers may be subject to similar regulatory oversight, and may not currently be or may not continue to be in compliance with applicable regulatory requirements. Failure by us or one of our suppliers to comply with statutes and regulations administered by the FDA and other regulatory bodies, or failure to take adequate action in response to any observations, could result in, among other things, any of the following enforcement actions:
| • | warning letters or untitled letters; |
| • | fines and civil penalties; |
24
Table of Contents
| • | unanticipated expenditures for corrective actions; |
| • | delays in approving, or refusal to approve, our products; |
| • | withdrawal or suspension of approval by the FDA or other regulatory bodies; |
| • | product recall or seizures; |
| • | interruption of production; |
| • | operating restrictions; |
| • | injunctions; and |
| • | criminal penalties. |
If any of these actions were to occur, it could harm our reputation and could cause our product sales and profitability to suffer.
Any regulatory approval of a product may also be subject to limitations on the indicated uses for which the product may be marketed. If the FDA or another regulatory body determines that our promotional materials, training or other activities constitute promotion of an unapproved use, it could request that we cease or modify our training or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our training or promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under applicable statutory authorities, such as laws prohibiting false claims for reimbursement.
Additionally, we may be required to conduct costly post-market testing, and we will be required to report adverse events and malfunctions related to our products. Later discovery of previously unknown problems with our products, including unanticipated adverse events or adverse events of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements may result in restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties.
Furthermore, the FDA and various other authorities will inspect our facilities and those of our suppliers from time to time to determine whether we are in compliance with regulations relating to the manufacture of diagnostic products, including regulations concerning design, manufacture, testing, quality control, product labeling, distribution, promotion and record-keeping practices. A determination that we are in material violation of such regulations could lead to the imposition of civil penalties, including fines, product recalls, product seizures or, in extreme cases, criminal sanctions.
If we are unable to comply with the requirements of the U.S. Clinical Laboratories Improvement Amendments of 1988 and state laws governing clinical laboratories or if we are required to expend significant additional resources to comply with these requirements, the success of our business could be threatened.
The United States Department of Health and Human Services, or HHS, has classified our T-SPOT.TB test as a high-complexity test under the Clinical Laboratories Improvement Amendments of 1988, commonly referred to as CLIA. Under CLIA, personnel requirements for laboratories conducting high-complexity tests are more stringent than those applicable to
25
Table of Contents
laboratories performing less complex tests. As a result of these personnel requirements, we must employ more experienced or more highly educated personnel and additional categories of employees, which increases our operating costs. If we fail to meet CLIA requirements, HHS or state agencies could require us to cease our T-SPOT.TB testing or other testing subject to CLIA that we may develop in the future. Continued compliance with CLIA requirements may cause us to incur significant expenses and potentially lose revenue in doing so. Moreover, new interpretations of current regulations or future changes in regulations under CLIA may make it difficult or impossible for us to comply with our CLIA classification, which would significantly harm our business.
Many states in which our physician and laboratory clients are located, such as New York, have laws and regulations governing clinical laboratories that are more stringent than federal law and may apply to us even if we are not located, and do not perform our T-SPOT.TB test, in that state. We may also be subject to additional licensing requirements as we expand our sales and operations into new geographic areas, which could impair our ability to pursue our growth strategy.
We may potentially be subject to product liability claims.
The testing, manufacturing and marketing of medical diagnostic tests such as our T-SPOT.TB test entail an inherent risk of product liability claims. Further, providing clinical testing services entails a risk of claims for errors or omissions made by our laboratory staff. Potential liability claims may exceed the amount of our insurance coverage or may be excluded from coverage under the terms of the policy. As of September 30, 2013, we had product liability insurance of $15.1 million. Our existing insurance will have to be increased in the future if we are successful at introducing new diagnostic products and this will increase our costs. Under certain of our customer and license agreements, we have agreed to provide indemnification for product liability claims arising out of the use of our T-SPOT.TB test. In the event that we are held liable for a claim or for damages exceeding the limits of our insurance coverage, we may be required to make substantial payments.
Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for our product and product candidates; |
| • | injury to our reputation; |
| • | costs of related litigation; |
| • | substantial monetary awards to patients and others; |
| • | loss of revenue; and |
| • | the inability to commercialize our products and product candidates. |
Any of these outcomes may have an adverse effect on our consolidated results of operations, financial condition and cash flows, and may increase the volatility of our share price.
Our inadvertent or unintentional failure to comply with the complex government regulations concerning privacy of medical records could subject us to fines and adversely affect our reputation.
The U.S. federal privacy regulations limit use or disclosure of protected health information, without written patient authorization, to purposes of payment, treatment or healthcare operations (as defined under the U.S. Health Insurance Portability and Accountability Act, or
26
Table of Contents
HIPAA) except for disclosures for various public policy purposes and other permitted purposes outlined in the privacy regulations. The privacy regulations provide for significant fines and other penalties for wrongful use or disclosure of protected health information, including potential civil and criminal fines and penalties.
We have policies and practices that we believe make us compliant with the privacy regulations. Nevertheless, the documentation and process requirements of the privacy regulations are complex and subject to interpretation. Failure to comply with the privacy regulations could subject us to sanctions or penalties, loss of business and negative publicity.
The HIPAA privacy regulations establish a “floor” of minimum protection for patients as to their medical information and do not supersede state laws that are more stringent. Therefore, we are required to comply with both HIPAA privacy regulations and various state privacy laws. Although the HIPAA statute and regulations do not expressly provide for a private right of action, we could incur damages under state laws to private parties for the wrongful use or disclosure of confidential health information or other private personal information. Internationally, virtually every jurisdiction in which we operate has established its own data security and privacy legal framework with which we or our customers must comply, including the Data Protection Directive established in the European Union. We may also need to comply with varying and possibly conflicting privacy laws and regulations in other jurisdictions. As a result, we could face regulatory actions, including significant fines or penalties, adverse publicity and possible loss of business.
We maintain sensitive data on our computer networks, including certain personal information regarding our customers. We may face threats to our networks from unauthorized access, security breaches and other system disruptions. Despite our security measures, our infrastructure may be vulnerable to attacks by hackers or other disruptive problems. Any such security breach may compromise information stored on our networks and may result in significant data losses or theft of our customers’ personally identifiable information. A cybersecurity breach could hurt our reputation by adversely affecting the perception of customers and potential customers of the security of their orders and personal information. In addition, a cybersecurity attack could result in other negative consequences, including disruption of our internal operations, increased cyber security protection costs, lost revenue, regulatory actions or litigation.
Our use of biological and hazardous materials and wastes requires us to comply with regulatory requirements, including environmental, health and safety laws, regulations and permitting requirements and subjects us to significant costs and exposes us to potential liabilities.
The handling of materials used in the diagnostic testing process involves the controlled use of biological and hazardous materials and wastes. The primary hazardous materials we handle or use include human blood samples and solvents. Our business and facilities and those of our suppliers are subject to federal, state, local and foreign laws and regulations relating to the protection of human health and the environment, including those governing the use, manufacture, storage, handling and disposal of, and exposure to, such materials and wastes. In addition, under some environmental laws and regulations, we could be held responsible for costs relating to any contamination at our past or present facilities and at third-party waste disposal sites even if such contamination was not caused by us. A failure to comply with current or future environmental laws and regulations, including the failure to obtain, maintain or comply with any required permits, could result in severe fines or penalties. Any such expenses or liability could
27
Table of Contents
have a significant negative impact on our business, results of operations and financial condition. In addition, we may be required to incur significant costs to comply with regulatory requirements in the future.
Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors play a primary role in the recommendation and ordering of any product candidates, including our T-SPOT.TB test, for which we obtain marketing approval. Our arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our product. Restrictions under applicable federal and state healthcare laws and regulations include the following:
| • | The U.S. federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federally funded healthcare programs such as Medicare and Medicaid. This statute has been broadly interpreted to apply to manufacturer arrangements with prescribers, purchasers and formulary managers, among others. Several other countries, including the United Kingdom, have enacted similar anti-kickback, fraud and abuse, and healthcare laws and regulations. |
| • | The U.S. federal False Claims Act imposes criminal and civil penalties against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. |
| • | HIPAA imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. HIPAA also imposes criminal liability for knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services. |
| • | The federal Physician Payment Sunshine Act requirements under the PPACA (as defined below) require manufacturers of drugs, devices, biologics and medical supplies to report to HHS information related to payments and other transfers of value made to or at the request of covered recipients, such as physicians and teaching hospitals, and physician ownership and investment interests in such manufacturers. Payments made to physicians and research institutions for clinical trials are included within the ambit of this law. Certain state laws and regulations also require the reporting of certain items of value provided to health care professionals. |
| • | Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. |
28
Table of Contents
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations involve substantial costs. We may be subject to qui tam litigation brought by private individuals on behalf of the government under the U.S. False Claims Act, which would include claims for up to treble damages. Additionally, it is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. Exclusion, suspension and debarment from government funded healthcare programs would significantly impact our ability to commercialize, sell or distribute any product. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
We are subject to the U.K. Bribery Act, the U.S. Foreign Corrupt Practices Act and other anti-corruption laws, as well as export control laws, customs laws, sanctions laws and other laws governing our operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, other remedial measures, and legal expenses, which could adversely affect our business, results of operations and financial condition.
Our operations are subject to anti-corruption laws, including the U.K. Bribery Act 2010, or Bribery Act, the U.S. Foreign Corrupt Practices Act, or FCPA, and other anti-corruption laws that apply in countries where we do business. The Bribery Act, FCPA and these other laws generally prohibit us and our employees and intermediaries from bribing, being bribed or making other prohibited payments to government officials or other persons to obtain or retain business or gain some other business advantage. We and our commercial partners operate in a number of jurisdictions that pose a high risk of potential Bribery Act or FCPA violations, and we participate in collaborations and relationships with third parties whose actions could potentially subject us to liability under the Bribery Act, FCPA or local anti-corruption laws. In addition, we cannot predict the nature, scope or effect of future regulatory requirements to which our international operations might be subject or the manner in which existing laws might be administered or interpreted.
We are also subject to other laws and regulations governing our international operations, including regulations administered by the governments of the United Kingdom and the United States, and authorities in the European Union, including applicable export control regulations, economic sanctions on countries and persons, customs requirements and currency exchange regulations, collectively referred to as the Trade Control laws.
There is no assurance that we will be completely effective in ensuring our compliance with all applicable anti-corruption laws, including the Bribery Act, the FCPA or other legal requirements, including Trade Control laws. If we are not in compliance with the Bribery Act, the FCPA and other anti-corruption laws or Trade Control laws, we may be subject to criminal and civil penalties, disgorgement and other sanctions and remedial measures, and legal expenses, which could have an adverse impact on our business, financial condition, results of operations and liquidity. Likewise, any investigation of any potential violations of the Bribery Act, the FCPA,
29
Table of Contents
other anti-corruption laws or Trade Control laws by U.K., U.S. or other authorities could also have an adverse impact on our reputation, our business, results of operations and financial condition.
Healthcare reform measures could hinder or prevent the commercial success of our diagnostic tests.
In March 2010, President Obama signed into law a legislative overhaul of the U.S. healthcare system, known as the Patient Protection and Affordable Care Act of 2010, as amended by the Healthcare and Education Affordability Reconciliation Act of 2010, or the PPACA, which may have far-reaching consequences for most healthcare companies, including diagnostic companies like us. For example, if reimbursement for our diagnostic tests is substantially less than we or our clinical laboratory customers expect, our business could be materially and adversely impacted.
Regardless of the impact of the PPACA on us, the U.S. government and other governments have shown significant interest in pursuing healthcare reform and reducing healthcare costs. Any government-adopted reform measures could cause significant pressure on the pricing of healthcare products and services, including our T-SPOT.TB test, in the United States and internationally, as well as the amount of reimbursement available from governmental agencies and other third-party payors.
Risks related to our intellectual property.
We may be unable to protect or obtain proprietary rights that we utilize or intend to utilize.
In developing, manufacturing and using our T-SPOT.TB test, we employ a variety of proprietary and patented technologies, including technologies we license from third parties. We have licensed, and expect to continue to license, various other technologies and methods. We cannot provide any assurance that the intellectual property rights that we own or license provide protection from competitive threats or that we would prevail in any challenge mounted to our intellectual property rights. In addition, we cannot provide any assurances that we will be successful in obtaining and retaining licenses or proprietary or patented technologies in the future.
We cannot assure investors that any of our currently pending or future patent applications will result in issued patents, and we cannot predict how long it will take for such patents to be issued. Further, we cannot assure investors that other parties will not challenge any patents issued or licensed to us or that courts or administrative agencies will hold our patents or the patents we license to be valid and enforceable. We cannot guarantee investors that we will be successful in defending challenges made against our patents and patent applications. Any successful third-party challenge to our patents could result in the unenforceability or invalidity of such patents. Both the patent application process and the process of managing patent disputes can be time consuming and expensive.
The patent positions of life sciences companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in the United States. Furthermore, in the biotechnology field, courts frequently render opinions that may affect the patentability of certain inventions or discoveries and the patent positions of companies engaged in development and commercialization of certain diagnostic tests. Various
30
Table of Contents
courts, including the U.S. Supreme Court, have recently rendered decisions that impact the scope of patentability of certain inventions or discoveries relating to genomic diagnostics. These decisions generally stand for the proposition that inventions that recite laws of nature are not themselves patentable unless they have sufficient additional features that provide practical assurance that the processes are genuine inventive applications of those laws rather than patent drafting efforts designed to monopolize the law of nature itself. What constitutes a “sufficient” additional feature is uncertain. While we do not generally rely on gene sequence patents, this evolving case law in the United States may adversely impact our ability to obtain new patents and may facilitate third-party challenges to our existing owned and licensed patents.
Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property rights. We cannot predict the breadth of claims that may be allowed or enforced in patents we own or in those to which we have license rights. For example:
| • | the inventor might not have been the first to make the inventions covered by patents we rely on; |
| • | the inventor or his assignee might not have been the first to file patent applications for the claimed inventions; |
| • | others may independently develop similar or alternative products and technologies or duplicate our product and technologies; |
| • | it is possible that the patents we own or license may not provide us with any competitive advantages, or may be challenged and invalidated by third parties; |
| • | any patents we obtain or license may expire before, or shortly after, the products and services relating to such patents are commercialized; |
| • | we may not develop additional proprietary products and technologies that are patentable; and |
| • | the patents of others may have an adverse effect on our business. |
In particular, in September 2011, the U.S. Congress passed the Leahy-Smith America Invents Act, or the AIA, which became effective in March 2013. The AIA reforms U.S. patent law in part by changing the standard for patent approval for certain patents from a “first to invent” standard to a “first to file” standard and developing a post-grant review system. It is too early to determine what the effect or impact the AIA will have on the operation of our business and the protection and enforcement of our intellectual property. However, the AIA and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
Some patent applications in the United States may be maintained in secrecy until the patents are issued, other patent applications in the United States and many foreign jurisdictions are not published until eighteen months after filing, and publications in the scientific literature often lag behind actual discoveries. We therefore cannot be certain that others have not filed patent applications for technology covered by issued patents or pending applications that we own or license or that we or our licensors, as applicable, were the first to invent the technology (pre-AIA) or first to file (post-AIA). Our competitors may have filed, and may in the future file, patent
31
Table of Contents
applications covering technology similar or the same as ours. Any such patent application may have priority over patent applications that we own or license and could further require us to obtain rights to such technologies in order to carry on our business. If another party has filed a U.S. patent application on inventions similar or the same as those that we own or license, we or our licensors may have to participate in an interference or other proceeding in the U.S. Patent and Trademark Office, or PTO, or a court to determine priority of invention in the United States, for pre-AIA applications and patents. For post-AIA applications and patents, we or our licensors may have to participate in a derivation proceeding to resolve disputes relating to inventorship. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in a loss of our U.S. patent position with respect to such inventions.
Some of our competitors may be able to sustain the costs of complex patent disputes and litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any disputes or litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
In addition to pursuing patents on our technology, we seek to protect our intellectual property and proprietary technology by entering into intellectual property assignment agreements with our employees, consultants and third party collaborators. See “—We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.”
Our intellectual property rights may not be sufficient to protect our competitive position and to prevent others from manufacturing, using or selling competing products.
The scope of our owned and licensed intellectual property rights may not be sufficient to prevent others from manufacturing, using or selling competing tests. For example, our intellectual property position depends in part on intellectual property that we license from third parties. However, many of the key patents we license are expected to expire by 2020. In addition, while many of the licenses we have been granted are exclusive, such rights may be limited to a narrowly defined field of use. As a result, our competitors may have obtained or be able to obtain a license to the same intellectual property in a closely related field of use. Finally, we have also granted sublicenses to third parties under certain of the intellectual property that we license. Such sublicenses may allow third parties or their licensees to market a TB test that would otherwise infringe upon such intellectual property.
Moreover, competitors could purchase our product and attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights. If our intellectual property is not adequately protected so as to protect our market against competitors’ products and methods, our competitive position could be adversely affected, as could our business.
We depend on certain technologies that are licensed or sublicensed to us. We do not control these technologies and any loss of our rights to them could prevent us from selling our product.
We rely on licenses in order to be able to use various proprietary technologies that are material to our business. For example, we license technology relating to the use of the ELISPOT technique, which forms part of the core platform of our T.SPOT technology, from Isis Innovation Limited, and we license the use of other patents to protect our T-SPOT.TB product from the Statens Serum
32
Table of Contents
Institut and Rutgers, The State University of New Jersey. We expect the patents that we license from Isis Innovation Limited to be assigned to us in connection with this offering. See “Business—Intellectual property—Our license agreements—Isis Innovation Limited (Isis).” Otherwise, we do not own the patents that underlie these licenses. Our rights to use these technologies and employ the inventions claimed in the licensed patents are subject to the continuation of and our compliance with the terms of those licenses.
In some cases, we do not control the prosecution, maintenance or filing of the patents to which we hold licenses. Enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents is often subject to the control or cooperation of our licensors. We cannot be certain that our licensors will prosecute, maintain, enforce and defend the licensed patent rights in a manner consistent with the best interests of our business. We also cannot be certain that drafting or prosecution of the licensed patents and patent applications by the licensors have been or will be conducted in compliance with applicable laws and regulations, will result in valid and enforceable patents and other intellectual property rights, or that any issued patents or patents that may issue in the future will provide any competitive advantage.
Certain of our licenses contain provisions that allow the licensor to terminate the license upon specific conditions. Our rights under each of the licenses are subject to our continued compliance with the terms of the license, including certain diligence, disclosure and confidentiality obligations and the payment of royalties and other fees. If we were found to be in breach of any of our license agreements, in certain circumstances our licensors may take action against us, including termination of the applicable license. Because of the complexity of our product and the patents we have licensed, determining the scope of the license and related obligations can be difficult and can lead to disputes between us and the licensor. An unfavorable resolution of such a dispute could lead to an increase in the royalties payable pursuant to the license or termination of the license. If a licensor believed we were not paying the royalties due under the license or were otherwise not in compliance with the terms of the license, the licensor may have the right to terminate the license or, in certain circumstances, to convert an exclusive license to a non-exclusive one. If such an event were to occur, the value of our product or product candidates could be materially adversely affected, we might be barred from producing and selling some or all of our products and may be subject to other liabilities.
In addition to the above risks, certain of our licensors do not own certain intellectual property included in the license, but instead have licensed such intellectual property from a third party, and have granted us a sub-license. As a result, the actions of our licensors or of the ultimate owners of the intellectual property may affect our rights to use our sublicensed intellectual property, even if we are in compliance with all of the obligations under our license agreements. For example, one of our licenses comprises a sublicense to us of certain patent rights owned by a third party that is not our direct licensor. If our licensors were to fail to comply with their obligations under the agreements pursuant to which they obtain the rights that are sublicensed to us, or should such agreements be terminated or amended, our ability to produce and sell our product and product candidates may be materially harmed. Finally, the legal issues surrounding the treatment of intellectual property licenses in bankruptcy proceedings are complex and may vary from jurisdiction to jurisdiction. We therefore cannot provide assurance that we would not lose some or all of our rights under a license if the applicable licensor was involved in such proceedings.
33
Table of Contents
We may become involved in disputes relating to our intellectual property rights, and may need to resort to litigation in order to defend and enforce our intellectual property rights. In addition, we could face claims that our activities or the manufacture, use or sale of our products infringe the intellectual property rights of others, which could cause us to pay substantial damages or licensing fees and limit our ability to sell some or all of our products and services.
Extensive litigation regarding patents and other intellectual property rights has been common in the medical diagnostics industry. Litigation may be necessary to assert infringement claims, enforce patent rights, protect trade secrets or know-how and determine the enforceability, scope and validity of certain proprietary rights. Litigation may even be necessary to resolve disputes of inventorship or ownership of proprietary rights. The defense and prosecution of intellectual property lawsuits, PTO interference or derivation proceedings, and related legal and administrative proceedings (e.g., a reexamination) in the U.S. and internationally involve complex legal and factual questions. As a result, such proceedings are costly and time consuming to pursue, and their outcome is uncertain.
Even if we prevail in such a proceeding, the remedy we obtain may not be commercially meaningful or adequately compensate us for any damages we may have suffered. If we do not prevail in such a proceeding, our patents could potentially be declared to be invalid, unenforceable or narrowed in scope, or we could otherwise lose valuable intellectual property rights. Similar proceedings involving the intellectual property we license could also have an impact on our business. For example, the scope of one of the European patents that we license from Rutgers, The State University of New Jersey, was recently narrowed as a result of a third party opposition proceeding before the European Patent Office. The decision is currently under appeal and the outcome of that appeal may adversely affect our competitive position. Further, if any of our other owned or licensed patents are declared invalid, unenforceable or narrowed in scope, our competitive position could be adversely affected.
In addition, our research, development and commercialization activities, including our T-SPOT.TB test, may infringe or be claimed to infringe patents or other intellectual property rights owned by other parties. Certain of our competitors and other companies have substantial patent portfolios, and may attempt to use patent litigation as a means to obtain a competitive advantage or to extract licensing revenue. The risks of being involved in such litigation may also increase as we gain greater visibility as a public company and as we gain commercial acceptance of our products and move into new markets and applications for our products. For example, we are aware of an issued U.S. patent owned by a third party which claims technology that may be relevant to our T-SPOT.TB test. We believe this patent is invalid and/or unenforceable, and we therefore challenged the validity of the patent through an ex parte reexamination proceeding before the PTO. Although the validity of the patent was upheld in that proceeding, we continue to believe that the patent is invalid and/or unenforceable based in part on information we discovered after the PTO’s decision in the reexamination proceeding. Nevertheless, if the patent holder were to pursue an infringement claim against us and we were unable to either negotiate acceptable license terms or otherwise resolve the matter, we could incur substantial expense to defend a claim, we could be ordered to pay substantial damages for infringement, and we could be enjoined from future conduct that would infringe the patent, which may include the making, using and selling of our T-SPOT.TB test in the United States. There may also be patents and patent applications that are relevant to our technologies or tests that we are not aware of. For example, certain relevant patent applications may have been filed but not published. If such patents exist, or if a patent issues on any of such patent applications, that patent could be
34
Table of Contents
asserted against us. In addition to patent infringement claims, we may also be subject to other claims relating to the violation of intellectual property rights, such as claims that we have misappropriated trade secrets or infringed third party trademarks.
Regardless of merit or outcome, our involvement in any litigation, interference or other administrative proceedings could cause us to incur substantial expense and could significantly divert the efforts of our technical and management personnel. Any public announcements related to litigation or interference proceedings initiated or threatened against us could cause our share price to decline. An adverse determination, or any actions we take or agreements we enter into in order to resolve or avoid disputes, may subject us to the loss of our proprietary position or to significant liabilities, or require us to seek licenses that may include substantial cost and ongoing royalties. Licenses may not be available from third parties, or may not be obtainable on satisfactory terms. An adverse determination or a failure to obtain necessary licenses may restrict or prevent us from manufacturing and selling our products and offering our services. These outcomes could materially harm our business, financial condition and results of operations.
We may not be able to adequately protect our intellectual property outside of the United States.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology, which could make it difficult for us to stop the infringement of our licensed and owned patents. For example, we are aware that third parties, particularly in China, are currently selling TB diagnostic products that we believe are covered by certain patents we license. We do not know whether our licensor will take the necessary steps to enforce its patent rights in China or whether it is likely to be successful in any such action. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business. Additionally, prosecuting and maintaining intellectual property (particularly patent) rights are very costly endeavors, and for these and other reasons we may not pursue or obtain patent protection in all major markets. We do not know whether legal and government fees will increase substantially and therefore are unable to predict whether cost may factor into our global intellectual property strategy.
In addition to the risks associated with patent rights, the laws in some foreign jurisdictions may not provide protection for our trade secrets and other intellectual property. If our trade secrets or other intellectual property are misappropriated in foreign jurisdictions, we may be without adequate remedies to address these issues. Additionally, we also rely on confidentiality and assignment of invention agreements to protect our intellectual property in foreign jurisdictions. These agreements may provide for contractual remedies in the event of misappropriation, but we do not know to what extent, if any, these agreements and any remedies for their breach, will be enforced by a foreign court. In the event our intellectual property is misappropriated or infringed upon and an adequate remedy is not available, our future prospects will likely diminish. The sale of products that infringe our intellectual property rights, particularly if such products are offered at a lower cost, could negatively impact our ability to achieve commercial success and may materially and adversely harm our business.
35
Table of Contents
Our failure to secure trademark registrations could adversely affect our business and our ability to market our product and product candidates.
Our trademark applications in the United States and any other jurisdictions where we may file may not be allowed for registration, and our registered trademarks may not be maintained or enforced. During trademark registration proceedings, we may receive rejections. Although we are given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the PTO and in corresponding foreign agencies, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our applications and/or registrations, and our applications and/or registrations may not survive such proceedings. Failure to secure such trademark registrations in the United States and in foreign jurisdictions could adversely affect our business and our ability to market our product and product candidates.
Obtaining and maintaining our patent protection depends upon compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The PTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent prosecution process and following the issuance of a patent. There are situations in which noncompliance with these requirements can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case if our patent were in force.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information, or the misappropriation of the intellectual property we regard as our own.
We rely on trade secrets to protect our proprietary know-how and technological advances, particularly where we do not believe patent protection is appropriate or obtainable. Nevertheless, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, third party collaborators and other advisors to protect our trade secrets and other proprietary information. These agreements generally require that the other party to the agreement keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to seek to pursue a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, and the outcome would be unpredictable. Further, courts outside the United States may be less willing to protect trade secrets. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights. In addition, our trade secrets and proprietary information may be misappropriated as a result of breaches of our electronic or physical security systems in which case we may have no legal recourse. Failure to obtain, or maintain, trade secret protection could enable competitors to use our proprietary information to develop products that compete with our product or cause additional, material adverse effects upon our competitive business position.
36
Table of Contents
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the medical diagnostics industry, we employ individuals who were previously employed at other medical diagnostics companies, including our competitors or potential competitors. We may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks related to our ordinary shares and this offering.
We are eligible to be treated as an emerging growth company and we cannot be certain that the reduced disclosure requirements applicable to emerging growth companies will not make our ordinary shares less attractive to investors.
We are an emerging growth company, as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (1) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, (2) reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements and (3) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In addition, as an emerging growth company, we are only required to provide two years of audited financial statements and two years of selected financial data in this prospectus. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our ordinary shares held by non-affiliates exceeds $700.0 million as of any June 30 in any fiscal year before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31 or, if we issue more than $1.0 billion in non-convertible debt during any three-year period before that time, we would cease to be an emerging growth company immediately. We cannot predict if investors will find our ordinary shares less attractive because we may rely on these exemptions. If some investors find our ordinary shares less attractive as a result, there may be a less active trading market for our ordinary shares and our share price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
There is no established trading market for our ordinary shares.
This offering constitutes our initial public offering of our ordinary shares, and no public market for our ordinary shares currently exists. Our ordinary shares will be listed and quoted on The NASDAQ Global Market. There can be no assurance that an active trading market for our ordinary shares will develop or be sustained after this offering is completed. The initial offering
37
Table of Contents
price has been determined by negotiations among the lead underwriters and us. Among the factors considered in determining the initial offering price were our future prospects and the prospects of our industry in general, our revenue, net income and certain other financial and operating information in recent periods, and the financial ratios, market prices of securities and certain financial and operating information of companies engaged in activities similar to ours. Nevertheless, there can be no assurance that following this offering our ordinary shares will trade at a price equal to or greater than the offering price.
Our share price may be volatile.
In addition, like other early-stage medical diagnostic companies, the market price of our ordinary shares may be volatile. The factors below may also have a material adverse effect on the market price of our ordinary shares:
| • | fluctuations in our results of operations; |
| • | our ability to enter new markets; |
| • | negative publicity; |
| • | changes in securities or industry analyst recommendations regarding our company, the sectors in which we operate, the securities market generally and conditions in the financial markets; |
| • | regulatory developments affecting our industry; |
| • | announcements of studies and reports relating to our products or those of our competitors; |
| • | changes in economic performance or market valuations of our competitors; |
| • | actual or anticipated fluctuations in our quarterly results; |
| • | conditions in the industries in which we operate; |
| • | announcements by us or our competitors of new products, acquisitions, strategic relations, joint ventures or capital commitments; |
| • | additions to or departures of our key executives and employees; |
| • | fluctuations of exchange rates; |
| • | release or expiry of lock-up or other transfer restrictions on our outstanding ordinary shares; and |
| • | sales or perceived sales of additional shares of our ordinary shares. |
In addition, the equity markets have recently experienced significant volatility, particularly with respect to the securities of life sciences companies. The volatility of the securities of life sciences companies often does not relate to the operating performance of those companies. As we operate in a single industry, we are especially vulnerable to these factors to the extent that they affect our industry or our products, or to a lesser extent our markets. In the past, securities class action litigation has often been initiated against companies following periods of volatility in their stock price. This type of litigation could result in substantial costs and divert our management’s attention and resources, and could also require us to make substantial payments to satisfy judgments or to settle litigation.
38
Table of Contents
If securities or industry analysts do not publish research or reports about our business or publish negative reports about our business, our share price and trading volume could decline.
The trading market for our ordinary shares will depend on the research and reports that securities or industry analysts publish about us or our business. Currently, we do not have any analyst coverage and we may not obtain analyst coverage in the future. In the event we obtain analyst coverage, we will not have any control over such analysts. If one or more of the analysts who cover us downgrade our shares or change their opinion of our shares, our share price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Substantial future sales of our ordinary shares in the public market, or the perception that these sales could occur, could cause the price of our ordinary shares to decline.
Additional sales of our ordinary shares in the public market after this initial public offering, or the perception that these sales could occur, could cause the market price of our ordinary shares to decline. Upon completion of this offering, we will have ordinary shares outstanding, assuming no exercise of the underwriters’ overallotment option. All ordinary shares sold in this offering will be freely transferable without restriction or additional registration under the Securities Act. A limited number of ordinary shares may be available for sale shortly after this offering since they are not subject to existing contractual and legal restrictions on resale. The remaining ordinary shares outstanding after this offering will be available for sale upon the expiration of a lock-up period, which we expect will expire 180 days after the date of this prospectus, subject to volume and other restrictions as applicable under Rule 144 under the Securities Act, or Rule 144. Any or all of these shares may be released prior to expiration of the lock-up period at the discretion of the lead underwriters for this offering. To the extent any of these shares are sold into the market, particularly in substantial quantities, the market price of our ordinary shares could decline. See “Shares eligible for future sale.”
We do not intend to pay cash dividends on our ordinary shares in the foreseeable future.
We have never paid dividends on ordinary shares and do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. Under English law, any payment of dividends would be subject to relevant legislation and our articles of association, which provide that all dividends must be approved by our Board of Directors and, in some cases, our shareholders, and may only be paid from our distributable profits available for the purpose, determined on an unconsolidated basis.
Our institutional investors and management own a significant percentage of our ordinary shares and will be able to exercise significant influence over matters subject to shareholder approval.
As of September 30, 2013, our executive officers, directors and each of the investment funds identified in the section under the heading “Principal shareholders,” whom we refer to as our institutional investors, together with their respective affiliates, held approximately 77% of our outstanding ordinary shares, and we expect that upon completion of this offering, that same group will continue to hold at least % of our outstanding ordinary shares. Accordingly, even after this offering, these shareholders will be able to exert a significant degree of influence over our management and affairs and over matters requiring shareholder approval, including the election of our Board of Directors and approval of significant corporate transactions. This concentration of ownership could have the effect of entrenching our management and/or our
39
Table of Contents
Board of Directors, delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material and adverse effect on the fair market value of our ordinary shares.
Management will have broad discretion as to the use of the proceeds from this offering, and we may not use the proceeds effectively.
Our management will have broad discretion as to the application of the net proceeds of this offering and could use them for purposes other than those currently contemplated. Our shareholders may not agree with the manner in which our management chooses to allocate and spend the net proceeds. Moreover, our management may use the net proceeds for corporate purposes that may not increase our profitability or our market value. See “Use of proceeds” for a description of our management’s intended use of the proceeds from this offering.
You will incur immediate and substantial dilution as a result of this offering.
If you purchase ordinary shares in this offering, assuming a public offering price of $ , the midpoint of the range set forth on the cover of this prospectus, you will incur immediate and substantial dilution of $ per share, representing the difference between the assumed initial public offering price of $ per share and our pro forma net tangible book value per share after giving effect to this offering. Moreover, we have previously issued warrants and options to acquire ordinary shares at prices significantly below the assumed initial public offering price. As of September 30, 2013, there were shares subject to outstanding warrants and shares subject to outstanding options. To the extent that these outstanding warrants or options are ultimately exercised, you will incur further dilution.
We will incur increased costs as a result of being a public company whose ordinary shares are publicly traded in the United States and our management expects to devote substantial time to public company compliance programs.
As a public company, we will incur significant legal, insurance, accounting and other expenses that we did not incur as a private company. In addition, our administrative staff will be required to perform additional tasks. For example, in anticipation of becoming a public company, we will need to adopt additional internal controls and disclosure controls and procedures, retain a transfer agent, adopt an insider trading policy and bear all of the internal and external costs of preparing and distributing periodic public reports in compliance with our obligations under the securities laws. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment will result in increased general and administrative expenses and may divert management’s time and attention. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed. In connection with this offering, we are increasing our directors’ and officers’ insurance coverage, which will increase our insurance costs. In the future, it will be more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our Board of Directors, particularly to serve on our audit committee and remuneration committee, and qualified executive officers.
In addition, in order to comply with the requirements of being a public company, we may need to undertake various actions, including implementing new internal controls and procedures and
40
Table of Contents
hiring new accounting or internal audit staff. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are continuing to develop and refine our disclosure controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file with the Securities and Exchange Commission, or the SEC, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that information required to be disclosed in reports under the Securities Exchange Act of 1934, as amended, or the Exchange Act, is accumulated and communicated to our principal executive and financial officers. Any failure to develop or maintain effective controls could adversely affect the results of periodic management evaluations. In the event that we are not able to demonstrate compliance with the Sarbanes-Oxley Act, that our internal control over financial reporting is perceived as inadequate, or that we are unable to produce timely or accurate financial statements, investors may lose confidence in our operating results and the price of our ordinary shares could decline. In addition, if we are unable to continue to meet these requirements, we may not be able to remain listed on The NASDAQ Global Market.
We are not currently required to comply with the SEC’s rules that implement Section 404 of the Sarbanes-Oxley Act, and are therefore not yet required to make a formal assessment of the effectiveness of our internal control over financial reporting for that purpose. Upon becoming a public company, we will be required to comply with certain of these rules, which will require management to certify financial and other information in our quarterly and annual reports and provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our second annual report. This assessment will need to include the disclosure of any material weaknesses in our internal control over financial reporting identified by our management or our independent registered public accounting firm. We are just beginning the costly and challenging process of implementing the system and processing documentation needed to comply with such requirements. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion.
Our independent registered public accounting firm will not be required to attest formally to the effectiveness of our internal control over financial reporting until the later of our second annual report or the first annual report required to be filed with the SEC following the date we are no longer an emerging growth company. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal controls in the future.
Our ordinary shares will be listed on The NASDAQ Global Market, but we cannot guarantee that we will be able to satisfy the continued listing standards going forward.
Although our ordinary shares will be initially listed on The NASDAQ Global Market, we cannot ensure that we will be able to satisfy the continued listing standards of The NASDAQ Global Market going forward. If we cannot satisfy the continued listing standards going forward, The NASDAQ Stock Market may commence delisting procedures against us, which could result in our ordinary shares being removed from listing on The NASDAQ Global Market. If our ordinary shares were to be delisted, the liquidity of our ordinary shares could be adversely affected and the market price of our ordinary shares could decrease. Delisting could also adversely affect our shareholders’ ability to trade or obtain quotations on our shares because of lower trading volumes and transaction delays. These factors could contribute to lower prices and larger spreads in the bid and ask price for our ordinary shares. You may also not be able to resell your shares at or above the price you paid for such shares or at all.
41
Table of Contents
English law and provisions in our articles of association may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our shareholders, and may prevent attempts by our shareholders to replace or remove our current management.
Certain provisions of English law and our articles of association may have the effect of delaying or preventing a change in control of us or changes in our management. For example, English law and our articles of association include provisions that:
| • | create a classified Board of Directors whose members serve staggered three-year terms; |
| • | prohibit shareholder action by written resolution; |
| • | establish an advance notice procedure for shareholder approvals to be brought before an annual meeting of our shareholders, including proposed nominations of persons for election to our Board of Directors; and |
| • | provide that vacancies on our Board of Directors may be filled only by a majority of directors then in office, even though less than a quorum. |
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management. See also “—Provisions in the U.K. City Code on Takeovers and Mergers may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our shareholders.”
Our holding company structure makes us dependent on the operations of our subsidiaries to meet our financial obligations.
We are a public limited company organized under the laws of England and Wales and have no significant assets other than our interest in Oxford Immunotec Limited. As a result, we rely exclusively upon payments, dividends and distributions from our direct and indirect subsidiaries for our cash flows. Our ability to pay dividends to our shareholders is dependent on the ability of our subsidiaries to generate sufficient net income and cash flows to pay upstream dividends and make loans or loan repayments.
Risks related to being an English company listing ordinary shares.
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation organized in Delaware.
We are incorporated under English law. The rights of holders of our ordinary shares are governed by English law, including the provisions of the Companies Act 2006, and by our articles of association. These rights differ in certain respects from the rights of shareholders in typical U.S. corporations organized in Delaware. The principal differences are set forth in “Description of our share capital—Differences in corporate law.”
U.S. investors may have difficulty enforcing civil liabilities against our company, our directors or members of senior management and the experts named in this prospectus.
We are incorporated under the laws of England and Wales. Many of our directors and officers reside outside the United States, and a substantial portion of our assets and all or a substantial portion of the assets of such persons are located outside the United States. As a result, it may be difficult for you to serve legal process on us or our directors and executive officers or have any of
42
Table of Contents
them appear in a U.S. court. The United States and the United Kingdom do not currently have a treaty providing for the recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. The enforceability of any judgment of a U.S. federal or state court in the United Kingdom will depend on the laws and any treaties in effect at the time, including conflicts of laws principles (such as those bearing on the question of whether a U.K. court would recognize the basis on which a U.S. court had purported to exercise jurisdiction over a defendant). In this context, there is doubt as to the enforceability in the United Kingdom of civil liabilities based solely on the federal securities laws of the United States. In addition, awards for punitive damages in actions brought in the United States or elsewhere may be unenforceable in the United Kingdom. An award for monetary damages under the U.S. securities laws would likely be considered punitive if it did not seek to compensate the claimant for loss or damage suffered and was intended to punish the defendant.
Provisions in the U.K. City Code on Takeovers and Mergers may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our shareholders.
The U.K. City Code on Takeovers and Mergers, or the Takeover Code, applies, among other things, to an offer for a public company whose registered office is in the United Kingdom (or the Channel Islands or the Isle of Man) and whose securities are not admitted to trading on a regulated market in the United Kingdom (or the Channel Islands or the Isle of Man) if the company is considered by the Panel on Takeovers and Mergers, or the Takeover Panel, to have its place of central management and control in the United Kingdom (or the Channel Islands or the Isle of Man). This is known as the “residency test.” The test for central management and control under the Takeover Code is different from that used by the U.K. tax authorities. Under the Takeover Code, the Takeover Panel will determine whether we have our place of central management and control in the United Kingdom by looking at various factors, including the structure of our Board of Directors, the functions of the directors and where they are resident.
If at the time of a takeover offer the Takeover Panel determines that we have our place of central management and control in the United Kingdom, we would be subject to a number of rules and restrictions, including but not limited to the following: (1) our ability to enter into deal protection arrangements with a bidder would be extremely limited; (2) we might not, without the approval of our shareholders, be able to perform certain actions that could have the effect of frustrating an offer, such as issuing shares or carrying out acquisitions or disposals; and (3) we would be obliged to provide equality of information to all bona fide competing bidders.
If we are a passive foreign investment company, U.S. investors in our ordinary shares could be subject to adverse U.S. federal income tax consequences.
The rules governing passive foreign investment companies, or PFICs, can have adverse effects for U.S. federal income tax purposes. The tests for determining PFIC status for a taxable year depend upon the relative values of certain categories of assets and the relative amounts of certain kinds of income. As discussed in “—Material tax considerations—Material U.S. federal income tax considerations,” we do not believe that we are currently a PFIC, and we do not anticipate becoming a PFIC in the foreseeable future. Notwithstanding the foregoing, the determination of whether we are a PFIC depends on the particular facts and circumstances (such as the valuation of our assets, including goodwill and other intangible assets) and may also be affected by the application of the PFIC rules, which are subject to differing interpretations. The fair market value
43
Table of Contents
of our assets is expected to depend, in part, upon (a) the market price of our ordinary shares and (b) the composition of our income and assets, which will be affected by how, and how quickly, we spend any cash that is raised in any financing transaction, including this offering. In light of the foregoing, no assurance can be provided that we are not currently a PFIC or that we will not become a PFIC in any future taxable year.
If we are a PFIC, U.S. holders of our ordinary shares would be subject to adverse U.S. federal income tax consequences, such as ineligibility for any preferred tax rates on capital gains or on actual or deemed dividends, interest charges on certain taxes treated as deferred, and additional reporting requirements under U.S. federal income tax laws and regulations. Whether or not U.S. holders of our ordinary shares make a timely qualified electing fund, or QEF, election or mark-to-market election may affect the U.S. federal income tax consequences to U.S. holders with respect to the acquisition, ownership and disposition of our ordinary shares and any distributions such U.S. holders may receive. Investors should consult their own tax advisors regarding all aspects of the application of the PFIC rules to our ordinary shares.
U.S. holders of 10% or more of the voting power of our ordinary shares may be subject to U.S. federal income taxation at ordinary income tax rates on undistributed earnings and profits.
There is a risk that we will be classified as a controlled foreign corporation, or CFC, for U.S. federal income tax purposes. We will generally be classified as a CFC if more than 50% of our outstanding shares, measured by reference to voting power or value, are owned (directly, indirectly or by attribution) by “U.S. Shareholders.” For this purpose, a “U.S. Shareholder” is any U.S. person that owns directly, indirectly or by attribution, 10% or more of the voting power of our outstanding shares. If we are classified as a CFC, a U.S. Shareholder may be subject to U.S. income taxation at ordinary income tax rates on all or a portion of our undistributed earnings and profits attributable to “subpart F income” and may also be subject to tax at ordinary income tax rates on any gain realized on a sale of ordinary shares, to the extent of our current and accumulated earnings and profits attributable to such shares. The CFC rules are complex and U.S. Shareholders of the ordinary shares are urged to consult their own tax advisors regarding the possible application of the CFC rules to them in their particular circumstances.
44
Table of Contents
Cautionary note regarding forward-looking statements
This prospectus contains forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, and other future conditions. Forward-looking statements can be identified by words such as “strategy, “objective,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “contemplate” and other similar expressions, although not all forward-looking statements contain these identifying words.
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place significant reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. Important factors that could cause actual results and events to differ materially from those indicated in the forward-looking statements include those identified under the heading “Risk factors.”
The forward-looking statements in this prospectus represent our views as of the date of this prospectus. We undertake no obligation to publicly update any forward-looking statements whether as a result of new information, future developments or otherwise.
45
Table of Contents
We estimate that the net proceeds of the sale of ordinary shares in this offering will be approximately $ million at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their option to purchase additional shares in full, we estimate that the net proceeds will be approximately $ million after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each $1.00 increase or decrease in the assumed initial public offering price of $ per share would increase or decrease our net proceeds by $ million, assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use the net proceeds of this offering as follows:
| • | approximately $ to hire additional sales, marketing and customer service personnel and expand marketing programs both in the United States and outside the United States; |
| • | approximately $ to fund research and development programs dedicated to development of new diagnostic tests in the field of immunology; |
| • | approximately $ to repay indebtedness outstanding under our senior secured term debt and |
| • | approximately $ for working capital and other general corporate purposes. |
As of September 30, 2013, we had $6.0 million outstanding under our senior secured term debt. This indebtedness matures in May 2017 and bears an interest rate equal to the greater of 2.75% above the prime rate or 6.0%. The use of proceeds of such indebtedness was to repay prior indebtedness and for working capital.
We may also use a portion of the net proceeds to opportunistically acquire, license and invest in complementary products, technologies or businesses; however, we currently have no agreements or commitments to complete any such transaction.
We cannot specify with certainty all of the particular uses for the net proceeds to be received upon the closing of this offering. In addition, the amount, allocation and timing of our actual expenditures will depend upon numerous factors, including the revenue generated from the sale of our products and our ability to satisfy ourselves that each investment is likely to have a good return. Accordingly, we will have broad discretion in using these proceeds. Pending their uses, we plan to invest the net proceeds of this offering in short-term, interest-bearing, investment-grade instruments or other securities.
46
Table of Contents
We have never declared or paid cash dividends on our ordinary shares. We currently intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination as to the declaration and payment of dividends, if any, will be made at the discretion of our Board of Directors and will depend on then existing conditions, including our results of operations, financial conditions, contractual restrictions, capital requirements, business prospects and other factors our Board of Directors may deem relevant. Under English law, we may pay dividends only out of our accumulated, realized profits, so far as not previously utilized by distribution or capitalization, less our accumulated, realized losses, so far as not previously written off in a reduction or reorganization of capital duly made. Because we are a holding company and have no direct operations, we will only be able to pay dividends from our available cash on hand and any funds we receive from our subsidiaries, including Oxford Immunotec Limited.
47
Table of Contents
The following table sets forth our cash, cash equivalents and capitalization as of September 30, 2013:
| • | on an actual basis; |
| • | on a pro forma basis to reflect (1) the Scheme of Arrangement completed on October 2, 2013, (2) a 1-for- reverse share split of our ordinary shares to be effected on , 2013, (3) the conversion of all outstanding preferred ordinary shares and A ordinary shares (4) the repayment of indebtedness outstanding under our senior secured debt and (5) our issuance and sale of ordinary shares at an assumed initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us as if they had occurred on September 30, 2013; |
You should read this information together with our consolidated financial statements and related notes appearing elsewhere in this prospectus and the information set forth under the heading “Selected consolidated financial data” and “Management’s discussion and analysis of financial condition and results of operations.”
| As of September 30, 2013 | ||||||||
| (in thousands, except share and per share data) | Actual | Pro forma | ||||||
| Cash and cash equivalents(1) |
$ | 13,035 | $ | |||||
|
|
|
|
|
|||||
| Interest-bearing loans and borrowings, short-term |
667 | |||||||
| Interest-bearing loans and borrowings, long-term |
5,333 | |||||||
|
|
|
|
|
|||||
| 6,000 | ||||||||
| A preferred ordinary shares, £0.001 par value; 903,220 shares authorized, 903,220 shares issued and outstanding at December 31, 2012 and September 30, 2013. |
2 | |||||||
| B preferred ordinary shares, £0.001 par value; 362,020 shares authorized, 362,020 shares issued and outstanding at December 31, 2012 and September 30, 2013. |
1 | |||||||
| D preferred ordinary shares, £0.001 par value; 3,488,448 shares authorized, 3,266,885 shares issued and outstanding at December 31, 2012 and September 30, 2013. |
5 | |||||||
| E preferred ordinary shares, £0.001 par value; 32,000,000 shares authorized, 17,081,014 shares issued and outstanding at December 31, 2012 and September 30, 2013. |
33 | |||||||
| F preferred ordinary shares, £0.001 par value; 20,000,000 shares authorized, 17,262,618 shares issued and outstanding at December 31, 2012 and September 30, 2013. |
26 | |||||||
| G preferred ordinary shares, £0.001 par value; 25,000,000 shares authorized, 10,079,933 and 16,559,756 shares issued and outstanding at December 31, 2012 and September 30, 2013 respectively. |
27 | |||||||
| Ordinary shares, £0.001 par value; 110,179,750 shares authorized, 14,442,575 and 15,677,098 shares issued and outstanding at December 31, 2012 and September 30, 2013, respectively. |
25 | |||||||
| Subscription of G preferred ordinary shares |
— | |||||||
| Additional paid-in capital |
114,480 | |||||||
| Accumulated deficit |
(96,327 | ) | ||||||
| Accumulated other comprehensive loss |
(4,069 | ) | ||||||
|
|
|
|
|
|||||
| Total shareholders’ equity |
14,203 | |||||||
|
|
|
|
|
|||||
| Total capitalization |
$ | 20,203 | $ | |||||
|
|
|
|
|
|||||
(1) A $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the range set forth on the cover of this prospectus) would increase (decrease)
48
Table of Contents
our actual cash and cash equivalents by $ , after deducting the estimated underwriting discounts and commissions and estimated expenses payable by us.
The table above does not include:
| • | ordinary shares issuable upon exercise of options outstanding as of , 2013 at a weighted-average exercise price of $ per share; |
| • | ordinary shares issuable upon the exercise of warrants outstanding as of , 2013 at a weighted-average exercise price of $ per share; and |
| • | ordinary shares reserved for future issuance under our equity incentive plans as of , 2013. |
49
Table of Contents
If you invest in our ordinary shares in this offering, your interest will be diluted to the extent of the difference between the initial public offering price per share of our ordinary shares in this offering and the pro forma as adjusted net tangible book value per share of our ordinary shares after this offering.
As of September 30, 2013, we had a historical net tangible book value of $14.0 million, or $ per ordinary share, taking into account the expected conversion of our outstanding preferred ordinary shares and our outstanding A ordinary shares into ordinary shares prior to the completion of this offering. Without giving effect to the conversion of our outstanding preferred ordinary shares and A ordinary shares into ordinary shares, we had a historical net tangible book value of $14.0 million, or $ per ordinary share, as of September 30, 2013. Historical net tangible book value per share is equal to our total tangible assets, less total liabilities, divided by the number of outstanding ordinary shares.
Investors participating in this offering will incur immediate and substantial dilution. After giving effect to (1) the conversion of all of our preferred ordinary shares and A ordinary shares into ordinary shares prior to the completion of this offering and (2) the sale of ordinary shares in this offering, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, our pro forma as adjusted net tangible book value as of , 2013 would have been $ million, or $ per ordinary share. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to our existing shareholders and an immediate dilution of $ per share to investors participating in this offering. The following table illustrates this per share dilution:
| Assumed initial public offering price per share |
$ | |||||||
| Historical net tangible book value per share as of September 30, 2013 |
$ | |||||||
| Pro forma net tangible book value per share as of September 30, 2013 |
||||||||
| Increase in net tangible book value per share attributable to new investors |
||||||||
| Pro forma net tangible book value per share after this offering |
||||||||
| Dilution per share to new investors |
$ | |||||||
|
|
||||||||
If the underwriters exercise their overallotment option in full, the pro forma as adjusted net tangible book value would be $ per share, representing an immediate dilution of $ per share to new investors, based on the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus.
Each $1.00 increase or decrease in the assumed initial public offering price of $ per share would increase or decrease our pro forma as adjusted net tangible book value by $ million, the pro forma as adjusted net tangible book value per share by $ per share and the dilution to investors purchasing shares in this offering by $ per share, assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
50
Table of Contents
The following table summarizes, on a pro forma as adjusted basis as of , 2013, the differences between the number of ordinary shares purchased from us, the total consideration and the average price per share paid by existing shareholders (giving effect to the conversion of all of our preferred ordinary shares and A ordinary shares into ordinary shares prior to the completion of this offering) and by investors participating in this offering, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus.
| Shares purchased | Total consideration |
Average price per share |
||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||
|
|
||||||||||||||||||
| Existing shareholders |
% | $ | % | $ | ||||||||||||||
| New investors |
||||||||||||||||||
| Total |
100% | $ | 100% | |||||||||||||||
|
|
||||||||||||||||||
The number of ordinary shares to be outstanding after this offering is based on ordinary shares outstanding as of , 2013 and excludes the following:
| • | ordinary shares issuable upon exercise of options outstanding as of , 2013 at a weighted-average exercise price of $ per share; |
| • | ordinary shares issuable upon the exercise of warrants outstanding as of , 2013 at a weighted-average exercise price of $ per share; and |
| • | ordinary shares reserved for future issuance under our equity incentive plans as of , 2013. |
To the extent that new options are issued under our equity incentive plans or we issue additional ordinary shares in the future, there will be further dilution to investors participating in this offering. See “Risk factors—You will incur immediate and substantial dilution as a result of this offering.”
51
Table of Contents
Selected consolidated financial data
The following tables summarize our consolidated financial and other data. The consolidated statement of operations data for the years ended December 31, 2011 and 2012 and the consolidated balance sheet data as of December 31, 2011 and 2012 have been derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statement of operations data for the nine months ended September 30, 2012 and 2013 and the consolidated balance sheet data as of September 30, 2013 have been derived from our unaudited consolidated financial statements appearing elsewhere in this prospectus. We derived the consolidated statement of operations data for the years ended December 31, 2008, 2009 and 2010 and the consolidated balance sheet data as of December 31, 2008, 2009 and 2010 from our unaudited consolidated financial statements not included in this prospectus.
On October 2, 2013, we completed the Scheme of Arrangement. Prior to the Scheme of Arrangement, our business was conducted by Oxford Immunotec Limited and its consolidated subsidiaries. Following the Scheme of Arrangement, our business has been conducted by Oxford Immunotec Global PLC and its consolidated subsidiaries, including Oxford Immunotec Limited.
We have prepared the unaudited consolidated financial information presented below on the same basis as our audited consolidated financial statements. The unaudited consolidated financial information includes all adjustments, consisting only of normal recurring adjustments that are necessary for a fair presentation of our financial position and results of operations for these periods. Our historical results are not necessarily indicative of the results that may be expected in the future and our results for any interim period are not necessarily indicative of the results that may be expected for a full fiscal year. You should read the following selected financial data together with “Management’s discussion and analysis of financial condition and results of operations” and our financial statements and accompanying notes included elsewhere in this prospectus. The selected financial data in this section are not intended to replace our financial statements and the accompanying notes.
52
Table of Contents
| Year ended December 31, | Nine months |
|||||||||||||||||||||||||||
| (in thousands, except share and per share data) (unaudited) |
2008 | 2009 | 2010 | 2011 | 2012 | 2012 | 2013 | |||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
| Consolidated statement of operations data: |
||||||||||||||||||||||||||||
| Revenue |
$ | 3,706 | $ | 4,308 | $ | 7,741 | $ | 12,641 | $ | 20,685 | $ | 15,406 | $ | 28,559 | ||||||||||||||
| Cost of revenue |
2,179 | 2,310 | 4,871 | 8,417 | 12,424 | 9,123 | 14,165 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Gross profit |
1,527 | 1,998 | 2,870 | 4,224 | 8,261 | 6,283 | 14,394 | |||||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||
| Research and development |
4,142 | 2,596 | 1,938 | 1,780 | 1,947 | 1,232 | 1,583 | |||||||||||||||||||||
| Sales and marketing |
7,646 | 6,507 | 9,375 | 10,536 | 11,177 | 7,895 | 9,557 | |||||||||||||||||||||
| General and administrative |
4,254 | 5,679 | 5,050 | 5,232 | 8,068 | 5,784 | 8,457 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Total operating costs |
16,042 | 14,782 | 16,363 | 17,548 | 21,192 | 14,911 | 19,597 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Loss from operations |
(14,515 | ) | (12,784 | ) | (13,493 | ) | (13,324 | ) | (12,931 | ) | (8,628 | ) | (5,203 | ) | ||||||||||||||
| Other (expense) income |
2,946 | (565 | ) | 1,500 | 101 | (2,103 | ) | (1,944 | ) | (98 | ) | |||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Loss before income taxes |
(11,569 | ) | (13,349 | ) | (11,993 | ) | (13,223 | ) | (15,034 | ) | (10,572 | ) | (5,301 | ) | ||||||||||||||
| Income tax provision (benefit) |
(365 | ) | (256 | ) | (147 | ) | (119 | ) | (151 | ) | 21 | 35 | ||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Net loss |
$ | (11,204 | ) | $ | (13,093 | ) | $ | (11,846 | ) | $ | (13,104 | ) | $ | (14,883 | ) | $ | (10,593 | ) | $ | (5,336 | ) | |||||||
|
|
|
|||||||||||||||||||||||||||
| Net loss per share attributable to ordinary shareholders, basic and diluted |
$ | (9.18 | ) | $ | (6.71 | ) | $ | (2.20 | ) | $ | (1.61 | ) | $ | (1.26 | ) | $ | (1.02 | ) | $ | (0.35 | ) | |||||||
|
|
|
|||||||||||||||||||||||||||
| Weighted-average shares used to compute net loss attributable to ordinary shareholders, basic and diluted |
1,220,145 | 1,951,633 | 5,381,173 | 8,150,146 | 11,825,803 | 10,338,893 | 15,129,791 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Pro forma (loss) per share, basic and diluted(1) |
$ | $ | ||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||
| Pro forma weighted-average number of shares, basic and diluted |
||||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||
| Supplemental financial metric: |
||||||||||||||||||||||||||||
| Adjusted EBITDA(2) |
$ | (8,904 | ) | $ | (12,083 | ) | $ | (11,019 | ) | $ | (12,519 | ) | $ | (12,131 | ) | $ | (8,057 | ) | $ | (4,296 | ) | |||||||
|
|
||||||||||||||||||||||||||||
53
Table of Contents
| As of December 31, | As of September 30, 2013 | |||||||||||||||||||||||||||
| 2008 | 2009 | 2010 | 2011 | 2012 | Actual | Pro forma(1) | ||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||||||||||
| Cash and cash equivalents |
$ | 15,197 | $ | 9,056 | $ | 6,644 | $ | 2,334 | $ | 12,578 | $ | 13,035 | $ | |||||||||||||||
| Total assets |
18,444 | 13,527 | 11,547 | 9,639 | 25,483 | 31,538 | ||||||||||||||||||||||
| Total liabilities |
2,449 | 2,796 | 3,371 | 4,413 | 8,534 | 17,335 | ||||||||||||||||||||||
| Total shareholders’ equity |
15,995 | 10,731 | 8,176 | 5,226 | 16,949 | 14,203 | ||||||||||||||||||||||
| Shares outstanding: |
||||||||||||||||||||||||||||
| Preferred ordinary shares |
21,613,139 | 25,928,795 | 31,477,499 | 37,642,730 | 48,955,690 | 55,435,513 | ||||||||||||||||||||||
| Ordinary shares |
1,374,212 | 2,817,847 | 6,406,358 | 8,492,175 | 14,442,575 | 15,677,098 | ||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
| (1) | The pro forma data gives effect to the Scheme of Arrangement, which was completed on October 2, 2013, the 1-for- reverse share split of our ordinary shares to be effected on , 2013, the conversion of all outstanding preferred ordinary shares and A ordinary shares into an aggregate of ordinary shares upon closing of this offering, the repayment of indebtedness outstanding under our senior secured term debt and our issuance and sale of ordinary shares at an assumed initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| (2) | Adjusted EBITDA is a non-GAAP financial measure that we calculate as profit (loss), adjusted for tax benefit and impairment (expense), unrealized exchange fluctuations, interest expense, interest income, depreciation and amortization and share-based compensation. We believe that Adjusted EBITDA provides useful information to investors and analysts in understanding and evaluating our operating results in the same manner as our management and Board of Directors. Our presentation of Adjusted EBITDA is not made in accordance with U.S. GAAP, and our computation of Adjusted EBITDA may vary from others in the industry. Our use of Adjusted EBITDA has limitations as an analytical tool, and you should not consider it in isolation or as a substitute for analysis of our results as reported under U.S. GAAP. For example, Adjusted EBITDA does not reflect the impact of earnings or charges resulting from matters that we consider not to be indicative of our ongoing operations. |
The following table presents a reconciliation of net income (loss), the most comparable U.S. GAAP financial measure, to Adjusted EBITDA for each of the periods indicated:
| Year ended December 31, | Nine months ended September 30, |
|||||||||||||||||||||||||||
| (in thousands) | 2008 | 2009 | 2010 | 2011 | 2012 | 2012 | 2013 | |||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
| Reconciliation of (loss) profit to Adjusted EBITDA |
||||||||||||||||||||||||||||
| Net loss |
$ | (11,204 | ) | $ | (13,093 | ) | $ | (11,846 | ) | $ | (13,104 | ) | $ | (14,883 | ) | $ | (10,593 | ) | $ | (5,336 | ) | |||||||
|
|
|
|||||||||||||||||||||||||||
| Income tax provision (benefit) |
(365 | ) | (256 | ) | (147 | ) | (119 | ) | (151 | ) | 21 | 35 | ||||||||||||||||
| Interest income |
(211 | ) | (8 | ) | (5 | ) | (1 | ) | (1 | ) | — | — | ||||||||||||||||
| Interest expense |
263 | 40 | 23 | 4 | 1,478 | 1,452 | 256 | |||||||||||||||||||||
| Depreciation and amortization |
535 | 569 | 586 | 630 | 801 | 580 | 863 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| EBITDA |
(10,982 | ) | (12,748 | ) | (11,389 | ) | (12,590 | ) | (12,576 | ) | (8,540 | ) | (4,182 | ) | ||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Reconciling items: |
||||||||||||||||||||||||||||
| Share-based compensation expense |
278 | 171 | 261 | 125 | 79 | 59 | 77 | |||||||||||||||||||||
| Unrealized exchange gains (losses) |
1,800 | 494 | 109 | (54 | ) | 546 | 424 | (191 | ) | |||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Adjusted EBITDA |
$ | (8,904 | ) | $ | (12,083 | ) | $ | (11,019 | ) | $ | (12,519 | ) | $ | (12,131 | ) | $ | (8,057 | ) | $ | (4,296 | ) | |||||||
|
|
||||||||||||||||||||||||||||
54
Table of Contents
Management’s discussion and analysis of
financial condition and results of operations
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the financial statements and the related notes to those statements included later in this prospectus. In addition to historical financial information, the following discussion contains forward-looking statements that reflect our plans, estimates, beliefs and expectations that involve risks and uncertainties. Our actual results and the timing of events could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this prospectus, particularly in “Risk factors.”
Overview
We are a global, commercial-stage diagnostics company committed to improving patient care by providing advanced, innovative tests in the field of immunology. Our proprietary T-SPOT technology platform allows us to measure the responses of specific immune cells, known as T cells, to inform the diagnosis, prognosis and monitoring of patients with immunologically controlled diseases. T cells are a central component of the human body’s immune system, and are implicated in the control and progression of many medical conditions, including certain types of infectious diseases, cancers and autoimmune diseases.
The initial product we have developed using our T-SPOT technology platform is our T-SPOT.TB test, which is used to test for LTBI. Our T-SPOT.TB test has been approved for sale in over 50 countries, including the United States, where we have received PMA from the FDA, in Europe, where we have obtained a CE mark, as well as Japan and China. Our T-SPOT.TB test has been included in clinical guidelines for TB screening in 17 countries. In addition, we have established reimbursement for our test in the United States, as well as a CPT® code2 that is used only for our test. We believe that many payors rely upon CPT codes to determine the amount they pay providers. Outside the United States, we have established reimbursement in several countries where reimbursement applies, including Japan, Switzerland and Germany. Our customers benefit from the existence of reimbursement mechanisms as it provides more certainty of the amount they will be paid for performing our test, as described in the section under the heading “Business—Funding and reimbursement.” We believe the annual global market opportunity for our T-SPOT.TB test is well in excess of $1 billion, assuming we can largely displace the TST in the developed world.
We are a global business with 151 employees, including sales and marketing teams, on three continents, and laboratories in the United States and the United Kingdom. We sell to customers in over 40 countries and derived 50% of our revenue from outside the United States for the year ended December 31, 2012. Our current customer base is comprised of over 1,000 active customers, consisting of hospitals, public health departments, commercial testing laboratories, importers and distributors.
We have incurred significant losses from inception and as of September 30, 2013 had an accumulated deficit of $96.3 million. We anticipate that our operating losses will continue for the next few years as we continue to invest to grow our customer base. Our revenue for the year ended December 31, 2011 was $12.6 million, for the year ended December 31, 2012 was $20.7 million, and for the nine months ended September 30, 2013 was $28.6 million. Our net loss for the year ended December 31, 2011 was $13.1 million, for the year ended December 31, 2012 was $14.9 million and for the nine months ended September 30, 2013 was $5.3 million.
| 2 | CPT is a registered trademark of the American Medical Association. |
55
Table of Contents
On October 2, 2013, pursuant to the Scheme of Arrangement, the holders of equity interests in Oxford Immunotec Limited, including holders of ordinary shares, preferred ordinary shares, options and warrants, exchanged their interests in Oxford Immunotec Limited for identical interests in Oxford Immunotec Global PLC, which became the parent company of Oxford Immunotec Limited.
Financial operations overview
Revenue
We generate revenue from sales associated with our T-SPOT technology platform via our direct sales force and also through distributors. Our T-SPOT.TB test is our first commercialized product based on this platform. For a description of our revenue recognition policies, see “—Critical accounting policies and significant judgments and estimates—Revenue recognition and accounts receivable.”
Revenue mix
We currently offer our T-SPOT.TB test in either an in vitro diagnostic kit or a service format. In the former, we sell test kits and associated accessories to distributors for resale and directly to institutions and laboratories that perform TB testing. In the latter, we have established clinical testing laboratories in the United States and the United Kingdom, where we perform our T-SPOT.TB test on samples sent to us by customers. In these markets, we have found that many customers prefer to send samples to us rather than perform their own analysis on-site.
Our U.S. business derived 94%, 95% and 96% of revenue from our service offering for the years ended December 31, 2011 and 2012 and for the nine months ended September 30, 2013, respectively, which reflects our experience that U.S. customers prefer to send IGRA tests out for processing and analysis rather than run them in-house. For the majority of our U.S. customers in the hospital and public health segments, TB testing programs are funded primarily from institutional budgets. We receive payment from these customers according to our pre-negotiated prices. For other segments of the U.S. market (notably, for example, the physicians’ office segment) third-party reimbursement is often available to cover the cost of our T-SPOT.TB test.
Outside the United States, we derived 84%, 83% and 89% of our revenue from the sale of our in vitro diagnostic kits and associated accessories for the years ended December 31, 2011 and 2012 and for the nine months ended September 30, 2013, respectively. For the majority of our customers outside the United States, we primarily negotiate pricing directly with our customers, and our prices are influenced to some degree by the mechanism and level of funding our customers receive for performing testing.
|
Year ended December 31, |
Nine months ended September 30, |
|||||||||||||||
| (in thousands) | 2011 | 2012 | 2012 | 2013(1) | ||||||||||||
|
|
||||||||||||||||
| Revenue |
||||||||||||||||
| Product |
$ | 6,281 | $ | 9,080 | $ | 6,807 | $ | 14,888 | ||||||||
| Service |
6,360 | 11,605 | 8,599 | 13,671 | ||||||||||||
|
|
|
|||||||||||||||
| Total revenue |
$ | 12,641 | $ | 20,685 | $ | 15,406 | $ | 28,559 | ||||||||
|
|
||||||||||||||||
| (1) | This revenue mix is not necessarily indicative of year-end results due to seasonality and ordering patterns. |
56
Table of Contents
Revenue by geography
We sell our T-SPOT.TB test through our own sales force in the United States, certain European countries and Japan. We sell through distributors in other parts of the world. In the future, we intend to expand our sales force globally and establish additional distributor relationships outside of our direct markets to better access international markets.
The following table reflects product revenue by geography (United States, Europe & ROW and Asia) and as a percentage of total product revenue, based on the billing address of our customers.
| Year ended December 31, | Nine months ended September 30, |
|||||||||||||||||||||||||||||||
| (in thousands, except percentages) | 2011 | 2012 | 2012 | 2013 | ||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
| Revenue |
||||||||||||||||||||||||||||||||
| United States |
$ | 5,604 | 44 | % | $ | 10,366 | 50 | % | $ | 7,826 | 51 | % | $ | 12,597 | 44 | % | ||||||||||||||||
| Europe & ROW |
5,587 | 44 | % | 6,530 | 32 | % | $ | 4,724 | 31 | % | 5,110 | 18 | % | |||||||||||||||||||
| Asia |
1,450 | 12 | % | 3,789 | 18 | % | $ | 2,856 | 19 | % | 10,852 | 38 | % | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total revenue |
$ | 12,641 | 100 | % | $ | 20,685 | 100 | % | $ | 15,406 | 100 | % | $ | 28,559 | 100 | % | ||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
Our revenue is denominated in multiple currencies. Sales in the United States and China are denominated in U.S. Dollars. Sales in Europe & ROW are denominated primarily in the Pound Sterling and the Euro. Sales in Japan are denominated in Yen. Our expenses are generally denominated in the currencies in which our operations are located, which is primarily in the United States, the United Kingdom and Japan. We operate globally and therefore changes in foreign currency exchange rates may become material to us in the future due to factors beyond our control. See “—Quantitative and qualitative disclosure about market risk—Foreign currency exchange risk.”
Cost of revenue and operating expenses
Cost of revenue and gross margin
Cost of revenue consists of direct labor expenses, including employee benefits and share-based compensation expenses, overhead expenses, material costs, cost of laboratory supplies, freight costs, royalties paid under license agreements, U.S. medical device excise tax and depreciation of laboratory equipment and leasehold improvements. During the years ended December 31, 2011 and 2012 and the nine months ended September 30, 2013, our cost of revenue represented 67%, 60% and 50%, respectively, of our total revenue.
|
Year ended December 31, |
Nine months ended September 30 |
|||||||||||||||
| (in thousands) | 2011 | 2012 | 2012 | 2013 | ||||||||||||
|
|
||||||||||||||||
| Cost of Revenue |
||||||||||||||||
| Product |
$ | 2,955 | $ | 4,329 | 3,116 | 6,767 | ||||||||||
| Service |
5,462 | 8,095 | 6,007 | 7,398 | ||||||||||||
|
|
|
|||||||||||||||
| Total cost of revenue |
$ | 8,417 | $ | 12,424 | 9,123 | 14,165 | ||||||||||
|
|
||||||||||||||||
Our gross profit represents total revenue less the cost of revenue, and gross margin is gross profit expressed as a percentage of total revenue. Our gross margins were 33%, 40% and 50%, respectively, for the years ended December 31, 2011 and 2012 and the nine months ended September 30, 2013. We expect our overall cost of revenue to increase in absolute U.S. Dollars as we continue to increase our volume of kits manufactured and tests performed. However, we also
57
Table of Contents
believe that we can achieve certain efficiencies in our manufacturing and laboratory operations, through these increased volumes, that could help maintain or improve our overall margins.
Research and development expenses
Our research and development efforts are focused on developing multiple new diagnostic tests that use our quantitative T cell measurement technology, including assays that would help transplant physicians better manage patients at risk of rejection and infection.
Our research and development expenses include those costs associated with performing research, development, clinical and regulatory activities and validating improvements to our technology and processes for the purposes of enhancing product performance. Research and development expenses include personnel-related expenses, including share-based compensation, fees for contractual and consulting services, travel costs, laboratory supplies, amortization, depreciation, rent, insurance, repairs and maintenance. We expense all research and development costs as incurred.
Given the relatively small size of our research and development staff and the limited number of active projects at any given time, we have found that, to date, it has been effective for us to manage our research and development activities on a departmental basis. Accordingly, we do not require employees to report their time by project nor do we allocate our research and development costs to individual projects.
During the years ended December 31, 2011 and 2012 and the nine months ended September 30, 2013, our research and development expenses represented 14%, 9% and 6%, respectively, of our total revenue. We expect that our overall research and development expenses will continue to increase in absolute U.S. Dollars as we focus on developing new diagnostic tests that utilize our T-SPOT technology platform.
Sales and marketing expenses
Our sales and marketing expenses include costs associated with our sales organization, including our direct sales force and sales management, and our marketing and customer service personnel. These expenses consist principally of salaries, commissions, bonuses and employee benefits for these personnel, including share-based compensation, as well as travel costs related to sales, marketing and customer service activities, marketing and medical education activities and overhead expenses. We expense all sales and marketing costs as incurred.
During the years ended December 31, 2011 and 2012 and the nine months ended September 30, 2013, our sales and marketing expenses represented 83%, 54% and 33%, respectively, of our total revenue. We expect our sales and marketing costs to increase in absolute U.S. Dollars, as we expand our sales force, increase our geographic presence, and increase marketing and medical education to drive awareness and adoption of our current T-SPOT.TB test and future products.
General and administrative expenses
Our general and administrative expenses include costs for our executive, accounting and finance, legal, corporate development, IT and human resources functions. These expenses consist principally of salaries, bonuses and employee benefits for the personnel included in these functions, including share-based compensation and travel costs, professional services fees, such as
58
Table of Contents
consulting, audit, tax and legal fees, costs related to our Board of Directors, general corporate costs, overhead expenses, and bad debt expense. We expense all general and administrative expenses as incurred.
During the years ended December 31, 2011 and 2012 and the nine months ended September 30, 2013, our general and administrative expenses represented 41%, 39% and 30%, respectively, of our total revenue. We expect that our general and administrative expenses will increase after this offering, primarily due to the costs of operating as a public company, such as additional legal, accounting and corporate governance expenses, including expenses related to compliance with the Sarbanes-Oxley Act, directors’ and officers’ insurance premiums and investor relations expenses.
Other income (expense)
Other income (expense) primarily consists of interest income, interest expense and exchange fluctuations. Interest income consists of interest earned on our cash and cash equivalents. During the years ended December 31, 2011 and 2012 and the nine months ended September 30, 2013, this income has not been material, although we expect our interest income to increase following this offering as we invest the net proceeds from the offering. Pending application of the net proceeds from the offering, we plan to invest the net proceeds of the offering in short-term, interest-bearing, investment-grade instruments or other securities.
Interest expense consists primarily of interest expense on our loan balances and the amortization of debt discounts and debt issuance costs. We amortize debt issuance costs over the life of the loan and report them as interest expense in our statements of operations.
Monetary assets and liabilities that are denominated in foreign currencies are remeasured at the period-end closing rate with resulting unrealized exchange fluctuations. Realized exchange fluctuations result from the settlement of transactions in currencies other than the functional currencies of our businesses. The functional currencies of our businesses are U.S. Dollars, Pounds Sterling and Yen, depending on the entity.
59
Table of Contents
Results of operations
Comparison of nine months ended September 30, 2012 and 2013
The following table sets forth, for the periods indicated, the amounts of certain components of our statements of operations and the percentage of total revenue represented by these items, showing period-to-period changes.
| Nine months ended September 30, | ||||||||||||||||||||||||
| 2012 | 2013 | Change | ||||||||||||||||||||||
| (in thousands, except percentages) | Amount | % of revenue |
Amount | % of revenue |
Amount | % | ||||||||||||||||||
|
|
||||||||||||||||||||||||
| Revenue: |
||||||||||||||||||||||||
| Product |
$ | 6,807 | 44 % | $ | 14,888 | 52 % | $ | 8,081 | 119 % | |||||||||||||||
| Service |
8,599 | 56 % | 13,671 | 48 % | 5,072 | 59 % | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total revenue |
15,406 | 100 % | $ | 28,559 | 100 % | $ | 13,153 | 85 % | ||||||||||||||||
| Cost of revenue: |
||||||||||||||||||||||||
| Product |
3,116 | 20 % | 6,767 | 24 % | 3,651 | 117 % | ||||||||||||||||||
| Service |
6,007 | 39 % | 7,398 | 26 % | 1,391 | 23 % | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total cost of revenue |
9,123 | 59 % | 14,165 | 50 % | 5,042 | 55 % | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Gross profit |
6,283 | 41 % | 14,394 | 50 % | 8,111 | 129 % | ||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Research and development |
1,232 | 8 % | 1,583 | 6 % | 351 | 28 % | ||||||||||||||||||
| Sales and marketing |
7,895 | 51 % | 9,557 | 33 % | 1,662 | 21 % | ||||||||||||||||||
| General and administrative |
5,784 | 38 % | 8,457 | 30 % | 2,673 | 46 % | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total operating expenses |
14,911 | 97 % | 19,597 | 69 % | 4,686 | 31 % | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss from operations |
(8,628 | ) | -56 % | (5,203 | ) | -18 % | 3,425 | -40 % | ||||||||||||||||
| Interest income (expense) |
(1,452 | ) | -9 % | (256 | ) | -1 % | 1,196 | -82 % | ||||||||||||||||
| Foreign exchange gains (losses) |
(492 | ) | -3 % | 44 | 0 % | 536 | -109 % | |||||||||||||||||
| Other (expense) income |
— | 0 % | 114 | 0 % | 114 | — | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Loss before income taxes |
(10,572 | ) | -69 % | (5,301 | ) | -19 % | 5,271 | -50 % | ||||||||||||||||
| Income tax provision (benefit) |
21 | 0 % | 35 | 0 % | 14 | 67 % | ||||||||||||||||||
| Net loss |
$ | (10,593 | ) | -69 % | $ | (5,336 | ) | -19 % | $ | 5,257 | -50 % | |||||||||||||
|
|
||||||||||||||||||||||||
60
Table of Contents
Revenue
Revenue increased by 85% to $28.6 million for the nine months ended September 30, 2013 compared to $15.4 million for the same period in 2012. This increase in revenue was due to an increase in volumes across all the regions where we sell our test. U.S. revenue grew by 61% driven by growth of $1.3 million from existing customers and $3.4 million from the addition of new customers as a result of an increased focus on selling to larger institutional accounts. Asia revenue grew by $8.0 million due to $2.4 million higher revenue in China and $5.7 million higher revenue in Japan where our T-SPOT.TB test was launched in the fourth quarter of 2012. We have seen significant demand for the test since its launch in Asia. Europe & ROW revenue growth was 8% over the same period in 2012.
| Nine months ended September 30, | Change | |||||||||||||||
| (in thousands, except percentages) | 2012 | 2013 | Amount | % | ||||||||||||
|
|
||||||||||||||||
| Revenue |
||||||||||||||||
| Product |
$ | 6,807 | $ | 14,888 | $ | 8,081 | 119% | |||||||||
| Service |
8,599 | 13,671 | 5,072 | 59% | ||||||||||||
|
|
|
|||||||||||||||
| Total revenue |
$ | 15,406 | $ | 28,559 | $ | 13,153 | 85% | |||||||||
|
|
||||||||||||||||
| Nine months ended September 30, | Change | |||||||||||||||
| (in thousands, except percentages) | 2012 | 2013 | Amount | % | ||||||||||||
|
|
||||||||||||||||
| Revenue |
||||||||||||||||
| United States |
$ | 7,826 | $ | 12,597 | $ | 4,771 | 61% | |||||||||
| Europe & ROW |
4,724 | 5,110 | 386 | 8% | ||||||||||||
| Asia |
2,856 | 10,852 | 7,996 | 280% | ||||||||||||
|
|
|
|||||||||||||||
| Total revenue |
$ | 15,406 | $ | 28,559 | $ | 13,153 | 85% | |||||||||
|
|
||||||||||||||||
61
Table of Contents
Cost of revenue and gross margin
Cost of revenue increased by 55% to $14.1 million for the nine months ended September 30, 2013 from $9.1 million in the same period in 2012. This increase in cost of revenue was due to the increased volume of kits sold and an increase in volume of tests sold through our laboratories in the United States and the United Kingdom. Gross margin increased to 50% in 2013 from 40% in 2012. The gross margin percent improvement was attributable to a reduction in material costs per test and efficiency from increased volume in our manufacturing operations. We have incurred costs related to the start-up of our new laboratory in Memphis, Tennessee and incurred extra costs related to running two labs. In the first quarter of 2013, we consolidated our U.S. laboratory operations in Memphis, Tennessee and closed our Marlborough, Massachusetts laboratory. Operating a single lab in the United States has begun to yield significant operating leverage that has also led to improving margins.
| Nine months ended September 30, |
Change | |||||||||||||||
| (in thousands, except percentages) | 2012 | 2013 | Amount | % | ||||||||||||
|
|
||||||||||||||||
| Cost of revenue |
||||||||||||||||
| Product |
$ | 3,116 | $ | 6,767 | $ | 3,651 | 117% | |||||||||
| Service |
6,007 | 7,398 | 1,391 | 23% | ||||||||||||
|
|
|
|||||||||||||||
| Total cost of revenue |
$ | 9,123 | $ | 14,165 | $ | 5,042 | 55% | |||||||||
|
|
||||||||||||||||
Sales and marketing expenses
Sales and marketing expenses increased 21% to $9.6 million for the nine months ended September 30, 2013 from $7.9 million for the same period in 2012. The increase was primarily due to an increase in personnel-related costs associated with the expansion of the U.S. sales and marketing team and as a result of hiring sales, marketing, administrative and technical support personnel in our newly opened office in Japan. As a percentage of total revenue, sales and marketing expenses decreased to 33% for the nine months ended September 30, 2013 from 51% for the same period in 2012.
General and administrative expenses
General and administrative expenses increased by 46% to $8.5 million for the nine months ended September 30, 2013 from $5.8 million for the same period in 2012. The increase was due to accounting and auditing costs related to this registration statement and increases in personnel-related costs associated with increases in our legal, accounting and finance, IT and human resources headcount and corporate development and consulting costs to support our growth. As a percentage of total revenue, general and administrative expenses decreased to 30% for the nine months ended September 30, 2013 from 38% for the same period in 2012.
Research and development expenses
Research and development expenses increased by 28% to $1.6 million for the nine months ended September 30, 2013 from $1.2 million for the same period in 2012. This increase was primarily related to development project expenses and to the establishment of a technical team in the United States to improve processes efficiency and reduce costs in our U.S. laboratory operations. As a percentage of total revenue, research and development expenses decreased to 6% for the nine months ended September 30, 2013 from 8% for the same period in 2012.
62
Table of Contents
Interest expense, net
Interest expense, net was $0.3 million for the nine months ended September 30, 2013 as compared to $1.5 million in the same period in 2012. The 2013 expense consists primarily of interest expense on our term debt and revolving credit facilities. The 2012 expense also included interest on a revolving line of credit and a $1.3 million loan discount that was recorded as interest expense, related to a 2012 convertible bridge loan agreement with our existing investors.
Comparison of years ended December 31, 2011 and 2012
The following table sets forth, for the periods indicated, the amounts of certain components of our statements of operations and the percentage of total revenue represented by these items, showing period-to-period changes.
| Year ended December 31, | ||||||||||||||||||||||||
| 2011 | 2012 | Change | ||||||||||||||||||||||
| (in thousands, except percentages) | Amount | % of revenue |
Amount | % of revenue |
Amount | % | ||||||||||||||||||
|
|
||||||||||||||||||||||||
| Revenue: |
||||||||||||||||||||||||
| Product |
$ | 6,281 | 50 % | $ | 9,080 | 44 % | $ | 2,799 | 45 % | |||||||||||||||
| Service |
6,360 | 50 % | 11,605 | 56 % | 5,245 | 82 % | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total revenue |
12,641 | 100 % | 20,685 | 100 % | 8,044 | 64 % | ||||||||||||||||||
| Cost of revenue: |
||||||||||||||||||||||||
| Product |
2,955 | 23 % | 4,329 | 21 % | 1,374 | 47 % | ||||||||||||||||||
| Service |
5,462 | 43 % | 8,095 | 39 % | 2,633 | 48 % | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total cost of revenue |
8,417 | 67 % | 12,424 | 60 % | 4,007 | 48 % | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Gross profit |
4,224 | 33 % | 8,261 | 40 % | 4,037 | 96 % | ||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Research and development |
1,780 | 14 % | 1,947 | 9 % | 167 | 9 % | ||||||||||||||||||
| Sales and marketing |
10,536 | 83 % | 11,177 | 54 % | 641 | 6 % | ||||||||||||||||||
| General and administrative |
5,232 | 41 % | 8,068 | 39 % | 2,836 | 54 % | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total operating expenses |
17,548 | 139 % | 21,192 | 102 % | 3,644 | 21 % | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss from operations |
(13,324 | ) | (105)% | (12,931 | ) | (63)% | 393 | (3)% | ||||||||||||||||
| Interest income (expense) |
(3 | ) | 0 % | (1,477 | ) | (7)% | (1,474 | ) | — | |||||||||||||||
| Foreign exchange gains (losses) |
28 | 0 % | (626 | ) | (3)% | (654 | ) | — | ||||||||||||||||
| Other income (expense) |
76 | 1 % | — | 0 % | (76 | ) | (100)% | |||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Loss before income taxes |
(13,223 | ) | (105)% | (15,034 | ) | (73)% | (1,811 | ) | 14 % | |||||||||||||||
| Income tax provision (benefit) |
(119 | ) | (1)% | (151 | ) | (1)% | (32 | ) | 27 % | |||||||||||||||
| Net loss |
$ | (13,104 | ) | (104)% | $ | (14,883 | ) | (72)% | $ | (1,779 | ) | 14 % | ||||||||||||
|
|
||||||||||||||||||||||||
Revenue
Revenue increased by 64% to $20.7 million for the year ended December 31, 2012 compared to $12.6 million for the year ended December 31, 2011. This increase in revenue was due to an increase in volume across all the regions where we sell our test. U.S. revenue grew by 85%, driven by growth of $2.7 million from existing customers and $1.6 million from the addition of
63
Table of Contents
new customers. Asia revenue grew by $2.3 million or 161% due to increased uptake of our T-SPOT.TB test in China since its launch in 2010. Europe & ROW revenue growth was 17% led by strong growth in our U.K. laboratory service volume.
| Year ended December 31, |
Change | |||||||||||||||
| (in thousands, except percentages) | 2011 | 2012 | Amount | % | ||||||||||||
|
|
||||||||||||||||
| Revenue |
||||||||||||||||
| Product |
$ | 6,281 | $ | 9,080 | $ | 2,799 | 45% | |||||||||
| Service |
6,360 | 11,605 | 5,245 | 82% | ||||||||||||
|
|
|
|||||||||||||||
| Total revenue |
$ | 12,641 | $ | 20,685 | $ | 8,044 | 64% | |||||||||
|
|
||||||||||||||||
| Year ended December 31, |
Change | |||||||||||||||
| (in thousands, except percentages) | 2011 | 2012 | Amount | % | ||||||||||||
|
|
||||||||||||||||
| Revenue |
||||||||||||||||
| United States |
$ | 5,604 | $ | 10,366 | $ | 4,762 | 85% | |||||||||
| Europe & ROW |
5,587 | 6,530 | 943 | 17% | ||||||||||||
| Asia |
1,450 | 3,789 | 2,339 | 161% | ||||||||||||
|
|
|
|||||||||||||||
| Total revenue |
$ | 12,641 | $ | 20,685 | $ | 8,044 | 64% | |||||||||
|
|
||||||||||||||||
Cost of revenue and gross margin
Cost of revenue increased by 48% to $12.4 million for the year ended December 31, 2012 from $8.4 million for the same period in 2011. This increase in cost of revenue was due to an increase in volume of tests ordered by our customers. In addition, we incurred costs related to the start-up of our new laboratory in Memphis, Tennessee and incurred extra costs related to running two labs until our Marlborough, Massachusetts lab was closed in the first quarter of 2013. Gross margin increased to 40% for the year ended December 31, 2012 from 33% for the same period in 2011. The gross margin percentage improvement resulted from an increase in volume of kits manufactured, reduction in material costs and leverage related to the significant increase in test volume processed through our U.S. lab operations.
| Year ended December 31, |
Change | |||||||||||||||
| (in thousands, except percentages) | 2011 | 2012 | Amount | % | ||||||||||||
|
|
||||||||||||||||
| Cost of Revenue |
||||||||||||||||
| Product |
$ | 2,955 | $ | 4,329 | $ | 1,374 | 46% | |||||||||
| Service |
5,462 | 8,095 | 2,633 | 48% | ||||||||||||
|
|
|
|||||||||||||||
| Total cost of revenue |
$ | 8,417 | $ | 12,424 | $ | 4,007 | 48% | |||||||||
|
|
||||||||||||||||
Sales and marketing expenses
Sales and marketing expenses increased 6% to $11.2 million for the year ended December 31, 2012 from $10.5 million for the same period in 2011. The increase was primarily due to an increase in personnel-related costs associated with the opening our sales office in Japan and the addition of sales representatives in the United States. As a percentage of total revenue, sales and marketing expenses decreased to 54% for the year ended December 31, 2012 from 83% for the same period in 2011.
64
Table of Contents
General and administrative expenses
General and administrative expenses increased by 54% to $8.1 million for the year ended December 31, 2012 from $5.2 million in the same period last year. The increase was primarily due to the addition of 12 key management and professional positions in our accounting and finance, legal, IT and human resources departments, and other related costs to support our growth. As a percentage of total revenue, general and administrative expenses decreased to 39% for the year ended December 31, 2012 from 41% for the same period in 2011.
Research and development expenses
Research and development expenses increased by 9% to $1.9 million for the year ended December 31, 2012 from $1.8 million in the same period in 2011. The increase was primarily related to cost improvement activities. As a percentage of total revenue, research and development expenses decreased to 9% for the year ended December 31, 2012 from 14% for the same period in 2011.
Interest expense, net
Interest expense, net was $1.5 million for the year ended December 31, 2012 as compared to $3,000 for the same period in 2011. The 2012 expense included interest on a revolving line of credit and a $1.3 million loan discount that was recorded as interest expense, related to a 2012 convertible bridge loan agreement with then-existing investors. The 2011 expense was for interest on capitalized leases.
Quarterly results of operations
The following table sets forth selected unaudited consolidated quarterly statements of operations data for the eleven most recent fiscal quarters. We have prepared the consolidated quarterly operations data on a consistent basis with the audited consolidated financial statements included elsewhere in this prospectus. In the opinion of management, the quarterly consolidated operations data reflects all necessary adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of these data. Historical results are not necessarily indicative of the results to be expected in future periods, and the results for a quarterly period are not necessarily indicative of the operating results for a full year. This information should be read in conjunction with the consolidated financial statements included elsewhere in this prospectus.
| Three Months Ended | ||||||||||||||||||||||||||||||||||||||||||||
| (in thousands) |
March 31, 2011 |
June 30, 2011 |
September 30, 2011 |
December 31, 2011 |
March 31, 2012 |
June 30, 2012 |
September 30, 2012 |
December 31, 2012 |
March 31, 2013 |
June 30, 2013 |
September 30, 2013(1) |
|||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
| Revenue: |
||||||||||||||||||||||||||||||||||||||||||||
| Product |
$ | 1,231 | $ | 1,788 | $ | 1,367 | $ | 1,894 | $ | 2,225 | $ | 2,199 | $ | 2,383 | $ | 2,273 | $ | 4,121 | $ | 5,790 | $ | 4,977 | ||||||||||||||||||||||
| Service |
1,042 | 1,449 | 2,166 | 1,703 | 2,449 | 2,712 | 3,438 | 3,006 | 3,558 | 4,364 | 5,749 | |||||||||||||||||||||||||||||||||
| Total revenue |
$ | 2,273 | $ | 3,237 | $ | 3,533 | $ | 3,597 | $ | 4,674 | $ | 4,911 | $ | 5,821 | $ | 5,279 | $ | 7,679 | $ | 10,154 | $ | 10,726 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Net loss |
$ | (4,101 | ) | $ | (2,478 | ) | $ | (2,994 | ) | $ | (3,531 | ) | $ | (2,927 | ) | $ | (4,034 | ) | $ | (3,633 | ) | $ | (4,289 | ) | $ | (1,201 | ) | $ | (954 | ) | $ | (3,181 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
| (1) | Net loss includes $1.6 million of accounting and auditing costs related to this registration statement, as described in Note 1, “Initial Public Offering (IPO) Costs,” to the Interim Condensed Consolidated Financial Statements. |
Our revenue fluctuates from quarter to quarter as a result of a number of factors, many of which are outside our control. Our service revenue has historically been strong in the third quarter as a result of a concentration of testing in the United States related to college students returning to school, while the fourth quarter has historically been weaker due to the holiday periods and
65
Table of Contents
decreased screening activity in hospitals as they focus on other priorities. Additionally, we see fluctuation in our product revenue from quarter to quarter, due to ordering patterns, particularly relating to our large distributor customers. As a result of such factors, we expect to continue to see seasonality and quarter-to-quarter variations in our revenue.
Liquidity and capital resources
Sources of funds
Since our inception, we have incurred significant net losses and negative cash flows from operations. We incurred a net loss of $14.9 million and used $14.4 million of cash for operating activities for the year ended December 31, 2012. For the nine months ended September 30, 2013 we had a net loss of $5.3 million and used $13.4 million of cash for operating activities. As of September 30, 2013, we had an accumulated deficit of $96.3 million.
As of September 30, 2013, we had cash and cash equivalents of $13.0 million. To date we have financed our operations principally through private placements of our convertible preferred ordinary shares and convertible debt, borrowings from our credit facilities and revenue from the sale of our tests. Through September 30, 2013, we have raised $110 million gross proceeds through private placements of our convertible preferred ordinary shares and convertible debt.
Credit facilities
In February 2012 we entered into a loan and security agreement with Comerica Bank that provided for borrowings of up to $3.0 million initially through February 2013 and extended through May 2013. In February 2012, we borrowed $1.5 million under the credit facility. Interest accrues daily on the outstanding balance at the prime rate plus 1.5%, with a minimum of the Daily Adjusting LIBOR rate plus 2.5% per annum. The loan was secured by substantially all of our assets. This loan was repaid in May 2013.
In May 2013, we entered into a new loan and security agreement with Square 1 Bank consisting of a term loan and a revolving line of credit, and repaid the loan from Comerica Bank. The Square 1 loan is secured by substantially all of our assets. Tranche A of the term loan, which was borrowed at closing, is for $6.0 million. For the first year, interest only is payable. After the first year, the outstanding balance plus all accrued interest is payable in 36 equal monthly installments through the maturity date of May 24, 2017. Tranche B of the term loan, subject to achievement of certain revenue milestones, allows us to borrow $1.0 million from January 1, 2014 and January 31, 2014. Tranche B matures 36 months from the funding date. For the first 12 months interest only is due. After one year, Tranche B is payable in 24 equal installments. The term loan may be prepaid without penalty or premium and once prepaid, may not be reborrowed. The interest rate for Tranche A of the term loan is the greater of 2.75% above the prime rate or 6.0% per annum. If we achieve certain revenue milestones and borrow $1.0 million in Tranche B then the rates for Tranches A and B will be reduced to the greater of 2.5% above prime or 5.75% per annum. The revolving line of credit allows us to borrow up to $5.0 million, has a maturity date of May 24, 2015 and bears interest at 1.75% above the prime rate or 5.0% per annum, whichever is greater. The total amount outstanding as of September 30, 2013 was $6.0 million. We intend to use a portion of the net proceeds from this offering to repay this indebtedness. See “Use of proceeds.”
66
Table of Contents
Convertible promissory note
In October 2013, we issued a convertible promissory note in the amount of $5.0 million to Fosun Industrial Co., Ltd., which will convert at the time of this offering into our ordinary shares at a price per share which reflects a 10% discount to the offering price. The shares issuable upon conversion will be subject to restrictions prohibiting sale or transfer of more than one-third of the shares each year for the first three years following this offering. In the event that the note is not converted into equity, the note bears interest at the rate of 8%, which is calculated and payable at the same time the principal is repaid. Unless otherwise converted into equity, the principal and interest must be repaid by July 1, 2016, although we may choose to repay the note earlier with no pre-payment penalties.
Summary of cash flows
Cash flows for the nine months ended September 30, 2012 and 2013
Operating activities
Net cash used in operating activities was $5.7 million during the nine months ended September 30, 2013, which included net loss of $5.3 million and non-cash items of $1.1 million. The non-cash items consisted of depreciation and amortization expense of $0.9 million, share-based compensation expense of $0.1 million and $0.1 million loss on change in fair value of warrants. We also had a net cash outflow of $1.5 million from changes in operating assets and liabilities during the period. The significant items in the changes in operating assets and liabilities included an increase in accounts receivable of $1.8 million, an increase in inventory of $1.4 million, an increase in prepaid expenses of $2.4 million and an increase in current liabilities of $4.2 million. The increase in accounts receivable and inventory was due primarily to the growth in our revenue. The increase in current liabilities was primarily related to deferred income and higher operating expenses due to growth in our business.
Net cash used in operating activities was $10.0 million during the nine months ended September 30, 2012, which included a net loss of $10.6 million and non-cash items of $2.1 million, offset by $1.5 million of net cash outflow from changes in operating assets and liabilities during the period. The non-cash items consists primarily of $0.6 million in depreciation and amortization and a $1.4 million loan discount that was recorded as interest expense, related to the 2012 convertible bridge note agreement with then existing investors. The change in operating assets and liabilities was primarily driven by an increase in our accounts receivable of $1.6 million and an increase in inventory of $0.4 million resulting from the growth in our revenue.
Investing activities
Net cash used in investing activities was $1.0 million and $1.3 million for the nine months ended September 30, 2012 and 2013, respectively. These amounts related primarily to purchases of property and equipment and a change in restricted cash pledged as security in connection with our facilities leases.
Financing activities
Net cash provided by financing activities was $7.4 million during the nine months ended September 30, 2013, consisting primarily of $2.9 million in proceeds from the issuance of G preferred ordinary shares and $6.0 million from borrowings under the Square 1 Bank loan, offset by Comerica Bank loan repayments of $1.5 million.
67
Table of Contents
Net cash provided by financing activities was $18.2 million during the nine months ended September 30, 2012, consisting primarily of $12.7 million in proceeds from the issuance of G preferred ordinary shares, $4.0 million in proceeds from the 2012 convertible bridge note agreement and $1.5 million from borrowings under the Comerica Bank loan.
Cash flows for the years ended December 31, 2011 and 2012
Operating activities
Net cash used in operating activities was $14.4 million during the year ended December 31, 2012, which included net loss of $14.9 million and non-cash items of $2.4 million. The non-cash items consisted primarily of a $1.4 million loan discount that was recorded as interest expense related to the 2012 convertible bridge note agreement, depreciation and amortization expense of $0.8 million and share-based compensation expense of $0.1 million. We also had a net cash outflow of $1.9 million from changes in operating assets and liabilities during the period. The significant items in the changes in operating assets and liabilities included an increase in accounts receivable of $3.1 million and an increase in inventory of $1.0 million offset by an increase in accrued expenses of $1.6 million and an increase in deferred revenue of $0.8 million. The increase in accounts receivable and inventory was due primarily to the growth in our revenue. The increase in accrued expenses was primarily related to increases in accrued employee related expenses and accrued royalties. The increase in deferred revenue relates to the growth in sales to our Japanese importer.
Net cash used in operating activities was $12.7 million during the year ended December 31, 2011, which included a net loss of $13.1 million, non-cash items of $0.9 million and $0.4 million of net cash outflow from changes in operating assets and liabilities during the period. The non-cash items consist primarily of $0.6 million in depreciation and amortization. The change in operating assets and liabilities was primarily driven by an increase in our accounts receivable of $1.0 million as a result of the growth in our revenue, and an increase in inventory of $0.3 million offset by increases in accounts payable and accrued expenses of $0.5 million and $0.4 million, respectively, to support our growth.
Investing activities
Net cash used in investing activities was $1.6 million and $1.9 million for the year ended December 31, 2011 and 2012, respectively. These amounts related primarily to purchases of property and equipment of $1.2 million and $1.5 million for the years ended December 31, 2011 and 2012, respectively, related to the expansion of our manufacturing and laboratory facilities in the United States and the United Kingdom. In addition, restricted cash pledged as security related to our facilities leases increased by $0.4 million and $0.3 million for the years ended December 31, 2011 and 2012, respectively.
Financing activities
Net cash provided by financing activities was $26.2 million during the year ended December 31, 2012, consisting primarily of $12.7 million in proceeds from the first tranche of the G preferred ordinary share financing, $8.1 million of proceeds received in advance related to the second tranche of the G preferred ordinary share financing, $4.0 million in proceeds from the 2012 convertible bridge notes and $1.5 million from borrowings under the Comerica Bank loan.
68
Table of Contents
Net cash provided by financing activities was $9.9 million during the year ended December 31, 2011, consisting primarily of $10.0 million in proceeds from the third tranche of the F preferred ordinary share financing.
Operating and capital expenditure requirements
We have not achieved profitability on a quarterly or annual basis since our inception and we expect to incur net losses in the future. We expect that our operating expenses will increase as we continue to invest to grow our customer base, expand our marketing and distribution channels, hire additional employees and increase product development expenditures.
Additionally, as a public company, we will incur significant audit, legal and other expenses that we did not incur as a private company. We believe that our existing capital resources, including funds available through our credit facility, together with the net proceeds from this offering, will be sufficient to fund our operations for the next few years.
Our future capital requirements will depend on many factors, including:
| • | Our ability to continue to penetrate our existing market and new markets in the United States; |
| • | The costs and timing of further expansion of our sales and marketing efforts; |
| • | Our ability to penetrate existing markets outside the United States and enter and develop new geographies; |
| • | The progress that we make in developing new products based on our platform technology; |
| • | The percentage of sales that are reimbursed by payors and our ability to collect our accounts receivable; |
| • | Our ability to generate cash from operations; and |
| • | The acquisition of businesses or technologies that we may undertake. |
Contractual obligations
We have contractual obligations for non-cancelable facilities leases, our credit facilities, equipment leases and purchase commitments. Purchase commitments include future minimum royalty, license, and exclusivity payments to be paid under our license agreements with third parties for access to certain technologies. The following table reflects a summary of our contractual obligations as of December 31, 2012.
| Payments due by period | ||||||||||||||||||||
| (in thousands) | Total | Less than 1 year |
1-3 Years |
3-5 Years |
More than 5 years |
|||||||||||||||
|
|
||||||||||||||||||||
| Operating lease obligations |
$ | 5,119 | $ | 627 | $ | 1,815 | $ | 1,698 | $ | 979 | ||||||||||
| Credit facility obligations |
1,548 | 1,548 | — | — | — | |||||||||||||||
| Purchase commitments |
12,477 | 1,423 | 3,082 | 3,196 | 4,776 | |||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
$ | 19,144 | $ | 3,598 | $ | 4,897 | $ | 4,894 | $ | 5,755 | ||||||||||
|
|
||||||||||||||||||||
Critical accounting policies and significant judgments and estimates
We have prepared our consolidated financial statements in accordance with U.S. GAAP. Our preparation of these consolidated financial statements requires us to make estimates,
69
Table of Contents
assumptions and judgments that affect the reported amounts of assets, liabilities, expenses and related disclosures at the date of the consolidated financial statements, as well as revenue and expenses during the reporting periods. We evaluate our estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could therefore differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 1 to our consolidated financial statements included in this prospectus, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our financial statements.
Revenue recognition and accounts receivable
We derive revenue from the sale of our T-SPOT.TB diagnostic test to a broad range of customers including hospitals, public health departments, commercial testing laboratories, importers and distributors. We offer our T-SPOT.TB test in either an in vitro diagnostic kit or a service format.
Revenue from tests is generally paid directly by the customer and is recognized on the accrual basis when the following revenue recognition criteria are met: (1) persuasive evidence that an arrangement exists; (2) the kit has been shipped or delivered or, in the case of tests performed in our laboratory, when final results have been reported to the customer; (3) the price is fixed or determinable; and (4) collectability is reasonably assured.
In the United States, we also generate revenue from payments that are received from a variety of third-party payors, including government programs (Medicare and Medicaid) and commercial insurance companies, each with different billing requirements. Revenue from tests paid by third-party payors is recognized on an accrual basis based on our historical collection experience.
For kits sold in Japan, we recognize revenue after delivery to the wholesaler and when the wholesaler receives a firm order from its customer at which point our price becomes determinable.
Accounts receivable are primarily amounts due from hospitals, public health departments, commercial testing laboratories, distributors and universities in addition to third party payors such as commercial insurance companies (including managed care organizations), government programs (Medicare and Medicaid in the United States) and individual patients.
Accounts receivable are reported net of an allowance for uncollectible accounts. The process of estimating the collection of accounts receivable involves significant assumptions and judgments. Specifically, the accounts receivable allowance is based on management’s analysis of current and past due accounts, collection experience in relation to amounts billed, channel mix, any specific customer collection issues that have been identified and other relevant information. Our provision for uncollectible accounts is recorded as bad debt expense and included in general and administrative expenses. Although we believe amounts provided are adequate, the ultimate amounts of uncollectible accounts receivable could be in excess of the amounts provided.
Income taxes
We account for income taxes under the asset and liability method, which requires, among other things, that deferred income taxes be provided for temporary differences between the tax basis
70
Table of Contents
of our assets and liabilities and their financial statement reported amounts. In addition, deferred tax assets are recorded for the future benefit of utilizing NOLs and research and development credit carryforwards. A valuation allowance is established when necessary to reduce deferred tax assets to the amount expected to be realized.
We follow the accounting guidance for uncertainties in income taxes, which prescribes a recognition threshold and measurement process for recording uncertain tax positions taken, or expected to be taken, in a tax return in the financial statements. Additionally, the guidance also prescribes the derecognition, classification, accounting in interim periods and disclosure requirements for uncertain tax positions. We accrue for the estimated amount of taxes for uncertain tax positions if it is more likely than not that we would be required to pay such additional taxes. An uncertain tax position will not be recognized if it has less than a 50% likelihood of being sustained. We did not have any accrued interest or penalties associated with any unrecognized tax positions, and there were no such interest or penalties recognized during the years ended December 31, 2011 or 2012.
Share-based compensation
Share-based compensation includes grants of options to purchase ordinary shares. Currently, we maintain one share incentive plan pursuant to which we may grant options to purchase our ordinary shares, restricted stock units, or RSUs, and other share-based awards to our employees, directors and officers. This incentive plan is called the Amended and Restated 2008 Stock Incentive Plan, or the 2008 Plan.
We measure the cost of equity-settled transactions with employees by reference to the fair value of the equity instruments at the date on which they are granted. Estimating fair value for share-based payment transactions requires determining the most appropriate valuation model, which is dependent on the terms and conditions of the grant. This estimate also requires determining the most appropriate inputs to the valuation model, including the expected life of the award, volatility and dividend yield, and making certain assumptions about the award. We describe the assumptions and models that we use to estimate the fair value for share-based payment transactions in our financial statements included with this prospectus.
Our share-based compensation expense is as follows:
| Year ended December 31, |
Nine months
ended September 30, |
|||||||||||||||
| 2011 | 2012 | 2012 | 2013 | |||||||||||||
| Cost of revenue |
$ | 4 | $ | 2 | $ | 2 | $ | 3 | ||||||||
| General and administrative |
85 | 55 | 38 | 68 | ||||||||||||
| Research and development |
4 | 4 | 4 | 0 | ||||||||||||
| Sales and marketing |
32 | 18 | 15 | 6 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total share-based compensation expense |
$ | 125 | $ | 79 | $ | 59 | $ | 77 | ||||||||
Share-based compensation expense decreased by $46,000, or 37%, to $79,000 in 2012 from $125,000 in 2011. The decrease was due to the cessation of approximately $51,000 of expense associated with a large number of 2008 option grants with a four year vesting schedule and a 2007 vesting commencement date becoming fully vested during 2011, $46,000 of which was associated with options becoming fully vested during the fourth quarter of 2011. The decrease was partially offset by $9,000 of increased expense associated with options granted in 2012,
71
Table of Contents
approximately two-thirds of which were granted during the fourth quarter of 2012, therefore lessening the impact on the full year operating results.
Share-based compensation expense increased by $18,000, or 31%, to $77,000 in the nine months ended September 30, 2013 from $59,000 in the nine months ended September 30, 2012. This increase was primarily due to $42,000 in expense associated with options granted in the third quarter of 2013 at a fair value per share of $1.379, of which certain options were granted with a portion fully vested, as well as $19,000 in increased expense associated with options granted in the fourth quarter of 2012 and the first six months of 2013. This increase was partially offset by a $49,000 decrease resulting from the cessation of expense associated with a large number of 2009 option grants with a four year vesting schedule and a 2008 vesting commencement date becoming fully vested in 2012. In addition, of the options granted during the first nine months of 2013, 875,376 represented the reissuance of options previously granted and then subsequently cancelled. The reissuance of the options was accounted for as a cancellation and concurrent replacement resulting in a modification to the original award. As the options were reissued at a higher exercise price, this modification resulted in no incremental expense.
We use the Black-Scholes option pricing model to value the share option awards. The Black-Scholes option pricing model requires the input of subjective assumptions, including assumptions about the expected life of share-based payment awards and share price volatility. In addition, as a private company, one of the most subjective inputs into the Black-Scholes option pricing model is the estimated fair value of our ordinary shares. As a private company, the share price does not have sufficient historical volatility for us to adequately assess the fair value of the share option grants. Therefore, for all share option grants, we use the same comparable public companies that are used in our market approach valuation model, which is discussed further below, as a basis for determining our expected volatility. We intend to continue to consistently apply this methodology of using comparable companies until a sufficient amount of historical information regarding the volatility of our own share price becomes available.
We determine the expected term for share option grants to employees based on the “simplified” method prescribed under Staff Accounting Bulletin Topic 14: Share-based Payments. Under this approach, the weighted-average expected life is presumed to be the average of the vesting term and the contractual term of the option. The risk-free interest rate is a weighted-average assumption equivalent to the expected term based on the United States Treasury yield curve in effect as of the date of grant. The assumptions used in calculating the fair value of the share-based payment awards represent our best estimate and involve inherent uncertainties and the application of our judgment. As a result, if factors change and we use different assumptions, share-based compensation expense could be materially different in the future.
For the years ended December 31, 2011 and 2012 and the nine months ended September 30, 2012 and 2013, we calculated the fair value of share options granted under the Plan using the Black-Scholes option pricing model with the following assumptions:
| Year ended December 31, |
Nine
months ended September 30, |
|||||||||||||||
| 2011 | 2012 | 2012 | 2013 | |||||||||||||
| Volatility |
40.01 | % | 39.58 | % | 39.66 | % | 39.28 | % | ||||||||
| Expected term, in years |
6.25 | 6.25 | 6.25 | 6.24 | ||||||||||||
| Dividend yield |
— | — | — | — | ||||||||||||
| Risk-free interest rate |
1.75 | % | 1.03 | % | 1.21 | % | 1.38 | % | ||||||||
72
Table of Contents
In accordance with ASC 718, Compensation—Stock Compensation, or ASC 718, we recognize expense based on the share option grant’s pre-defined vesting schedule over the requisite service period using the straight-line method for all employee share options. In addition to the assumptions used to calculate the fair value of the share options, we are required to estimate the expected forfeiture rate of all share-based awards and only recognize expense for those awards expected to vest. The estimation of the number of share awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from our current estimates, such amounts will be recorded as a cumulative adjustment in the period in which estimates are revised. We consider multiple factors when estimating expected forfeitures, including employee position and historical employee turnover data. During the period in which the share options vest, we will record additional expense if the actual forfeiture rate is lower than the estimated forfeiture rate and a recovery of expense if the actual forfeiture rate is higher than estimated.
Based upon an assumed initial public offering price of $ per share, which is the estimated midpoint of the range listed on the cover page of this prospectus, the aggregate intrinsic value of our share options outstanding as of , 2013 was $ million, of which $ million related to vested share options and $ million related to unvested share options.
Valuation of share options
For all share option grants during the years ended December 31, 2011 and 2012 and the period beginning January 1, 2013 through September 30, 2013, the fair value of the ordinary shares underlying the share option grants was determined by our Board of Directors, with the assistance of an unrelated third-party valuation firm. When establishing the fair value of ordinary shares at each grant date, we relied upon the guidance provided by the American Institute of Certified Public Accountants, or the AICPA, in the AICPA Technical Practice Aid: Valuation of Privately-Held-Company Equity Securities Issued as Compensation, which we refer to as the “AICPA Practice Aid.”
Based on the guidance provided in the AICPA Practice Aid and ASC 820, Fair Value Measurements, or ASC 820, the recent transactions in our securities completed by independent investors represent the best indication of fair value of such security. In addition, new rounds of venture capital financing, which reflect the expectations of independent investors with respect to our future performance, usually provide a good indication of the fair value of the common equity securities. In this case, the fair value of the ordinary equity securities is derived based on the price paid by the venture capital investors for the preferred equity securities, taking into account the differences in various rights and liquidation preferences between ordinary and preferred equity securities. This method is also known as a back-solve approach. In cases where there are no transactions and/or new financings, the use of a discounted cash flow, or DCF, analysis and guideline public firm multiples, adjusted for unique characteristics of the issuing private firm, are accepted methodologies. All three methodologies have been used to establish the fair value of our ordinary shares.
For valuations performed and discussed below, our Board of Directors, with the assistance of an unrelated third-party valuation firm, applied three methods: a back-solve approach based on the recent rounds of venture capital financings, an income approach using a DCF analysis and a market approach based on market multiples of selected guideline firms. These approaches were employed in order to estimate the fair value of ordinary shares underlying the share option grants under the Plan during this period. These approaches are consistent with the guidance provided in the AICPA Practice Aid and ASC 820 and represent appropriate methodologies given our stage of development at the time of each valuation analysis.
73
Table of Contents
The market approach is a general way of estimating the value of a business using one or more methods that compare the subject company to similar businesses, business ownership interests, securities or assets that have been sold. The market approach arrives at an indication of value by comparing the entity being appraised to guideline publicly traded entities or comparable entities which have been recently acquired in arm’s-length transactions as well as prior company transactions. The guideline public companies method results in an indication of value by comparing the business being appraised to guideline publicly traded companies. After guideline companies are identified, various market-based valuation multiples are developed—e.g., enterprise value-to-revenue, enterprise value-to-cash flow, etc. The market-based valuation multiples are then applied to the subject company’s financial characteristics, resulting in an indication of value for the operations of the business enterprise and/or equity interest. Valuation discounts or premiums may be applied to reflect the unique characteristics of the business and/or equity interest being appraised. The market approach considered in our valuation model, as mentioned above, utilized the guideline company method by analyzing a population of comparable public companies. We selected those companies that we considered to be the most comparable to us based on their business model, strategic focus on the business and the degree to which the firms were serving the same customer base as we have. The selection of benchmarked companies requires us to make judgments as to the comparability of these companies to us and may change over time based on whether we believe the selected companies remain comparable to us. Based on these considerations, we believe that the companies we selected are a representative group for purposes of performing valuations. Under the market approach, we use these guideline companies to develop relevant market multiples and ratios, which are then applied to our corresponding financial metrics to estimate our equity value.
The DCF analysis, a variation of the income approach, is a general way of estimating the value of a business, business ownership interest, security or intangible asset using one or more methods that convert anticipated economic benefits into a present single amount. This approach considers future income levels for the entity or asset under analysis usually based on historical or current income that, to an independent investor, is reasonably reflective of the sustainable/recurring level of income the entity may hope to obtain in future periods. The DCF method rests on the assumptions that: (i) a business is worth today what it can generate in future cash to its owners; (ii) cash today is worth more than an equal amount of cash in the future; (iii) future cash flows can be predicted; and (iv) the cost of capital and investors’ required returns can be estimated. This method assumes that the income derived from a business will, to a large extent, control the value of that business. In our analysis, the DCF model included the present value of invested capital net cash flow associated with the projected period, plus the present value of stabilized cash flow into perpetuity, referred to as the “terminal value.” We discounted the cash flows using an appropriate discount rate, which was derived using market-based cost of capital of the comparable public companies selected in our guideline company method under the market approach.
In our analysis, we considered an application of the discount for lack of marketability, or DLOM. Since a holder of a minority interest in the equity of a privately held company has no ready market for his or her interest other than by a private sale to another partner or a willing buyer, these equity securities lack marketability. Marketability refers to an owner’s ability to sell his interest, with nominal transaction costs, and to realize the cash proceeds of the sale in three to five business days. Accordingly, a DLOM should be applied to estimate the fair value of the shareholders’ equity in a privately held enterprise. The DLOM represents a discount necessary to generate a sufficient incremental return to the purchaser of a minority interest of an entity’s
74
Table of Contents
closely held shares to induce the purchaser to make this particular investment rather than an alternative investment identical in all respects, except marketability.
Based on the guidance provided in the AICPA Practice Aid and general SEC guidance, a put option model may be appropriate for determining the DLOM since it takes into account the expected life of the restriction and expected volatility. Typically, the Black-Scholes option-pricing model is used to estimate the DLOM by calculating a theoretical put option price for closely held shares. The put option price is then compared with the closely held company’s share price to derive an estimated DLOM. The basic inputs for the Black-Scholes model include: (i) share price, (ii) strike price, (iii) risk-free interest rate, (iv) expected option life, (v) interest rate, and (vi) expected volatility. The strike price is typically set to be equal to the market value of the underlying shares as of the date of the valuation analysis. The expected option life is equal to the assumed holding period, the interest rate is equal to the expected dividend rate, and volatility refers to the rate at which a share price moves up and down. Since a closely held company’s shares do not trade it is necessary to estimate the subject share price volatility by using comparable publicly traded company data.
In allocating the total equity value between our preferred ordinary shares and ordinary shares, we considered the liquidation preferences allocable to the preferred ordinary shares in determining valuations performed prior to the elimination of the preferences on these shares. Additionally, each valuation during this period utilizes the option-pricing method, or OPM, for allocating the total equity value between our preferred ordinary shares and ordinary shares.
The OPM (also known as Contingent Claims Analysis, or CCA) estimates the fair value of each class of security using call options. Similar to call options for publicly-traded stock, call options used in a CCA assign value to each class of security based on the potential to profit from the upside of the business while taking into account the unique characteristics of each class of security.
Each call option gives its owner the right, but not the obligation, to buy the underlying asset at a predetermined price, or exercise price. The exercise price is based on an equity value, rather than, in the case of a “regular” call option, the per-share price.
The equity value is represented by “breakpoints,” which are the points at which there is a change in the proportion of the claims of the various equity securities on the equity value change. Each more junior security is considered to represent a call option with a claim on the equity at an exercise price which settles all of the more senior claims and takes into account the unique characteristics of each class of security. Accordingly, the following steps were performed in our analysis using the OPM:
| • | Step 1—Determined breakpoints based on the economic rights and preferences of the various classes of our securities; |
| • | Step 2—Selected inputs for the Black-Scholes option pricing model to determine the value of the call options; and |
| • | Step 3—Allocated the call option values among the different classes of equity securities based on their rights and individual characteristics. |
The primary inputs and components of the OPM include the following:
| • | Current price—The value of the underlying asset, which is represented by our enterprise or equity value. |
75
Table of Contents
| • | Exercise price or breakpoints—Breakpoints are enterprise values in which different classes of our equity securities participate in the value of the business. Breakpoints are determined by the rights and individual characteristics of the different securities, including the conversion ratio and any liquidation preference of the preferred shares and represent the amount at which an investor is indifferent between exercising the option or not. |
| • | Dividend yield—The dividend yield assumption generally reflects a company’s historical dividend yield. |
| • | Expiration date—The term of the option or the estimated time until a liquidation event. |
| • | Volatility—The amount of uncertainty or risk inherent in the size of changes in the underlying asset’s value. A higher volatility indicates that a security’s value can potentially be dispersed over a larger range of values, which means that the price of the security can change significantly, in either direction, over a short period of time. On the other hand, a lower volatility indicates that a security’s value does not fluctuate significantly in the short term, but, rather, changes in value at a relatively steady pace over a longer period of time. When used as a variable in option-pricing formulas, volatility represents the extent to which the return of the underlying asset will fluctuate between the valuation date and the option’s expiration. Volatility, as expressed as a percentage within option-pricing formulas, arises from daily trading activities. Volatility is generally measured based on historical results of guideline companies, or it can be implied based on market activity. |
| • | Risk-free rate—The nominal risk-free rate of return for the period commensurate with the expected term to the exit event. |
The significant input assumptions used in our valuation models are based on subjective future expectations combined with the judgment of our Board of Directors.
76
Table of Contents
Valuations under the Plan
Below is a summary of option grants issued under the 2008 Plan during the period beginning January 1, 2011 through October 28, 2013:
| Grant date | Options granted |
Exercise price per share(1) |
Fair value per share(2) |
|||||||||
| February 17, 2011 |
10,000 | $ | 0.038 | $ | 0.038 | |||||||
| June 8, 2011 |
85,000 | 0.038 | 0.038 | |||||||||
| July 28, 2011 |
152,500 | 0.038 | 0.038 | |||||||||
| August 12, 2011 |
10,000 | 0.038 | 0.038 | |||||||||
| October 20, 2011 |
6,000 | 0.038 | 0.038 | |||||||||
| December 31, 2011 |
97,000 | 0.038 | 0.038 | |||||||||
| February 29, 2012 |
249,895 | 0.038 | 0.026 | |||||||||
| March 8, 2012 |
1,297,224 | 0.026 | 0.026 | |||||||||
| April 17, 2012 |
15,000 | 0.026 | 0.026 | |||||||||
| October 24, 2012 |
1,000 | 0.120 | 0.120 | |||||||||
| October 29, 2012 |
530,000 | 0.120 | 0.120 | |||||||||
| November 7, 2012 |
2,350,647 | 0.120 | 0.120 | |||||||||
| November 16, 2012 |
26,000 | 0.120 | 0.120 | |||||||||
| December 7, 2012 |
128,000 | 0.120 | 0.120 | |||||||||
| December 31, 2012 |
3,000 | 0.120 | 0.120 | |||||||||
| January 4, 2013 |
4,000 | 0.120 | 0.120 | |||||||||
| January 31, 2013 |
36,000 | 0.120 | 0.120 | |||||||||
| February 18, 2013 |
145,000 | 0.120 | 0.120 | |||||||||
| February 28, 2013 |
1,028,876 | (3) | 0.120 | 0.120 | ||||||||
| March 25, 2013 |
1,000 | 0.120 | 0.120 | |||||||||
| April 8, 2013 |
5,000 | 0.120 | 0.240 | |||||||||
| April 15, 2013 |
2,000 | 0.120 | 0.240 | |||||||||
| April 22, 2013 |
3,000 | 0.120 | 0.240 | |||||||||
| May 1, 2013 |
35,000 | 0.120 | 0.240 | |||||||||
| May 6, 2013 |
5,000 | 0.120 | 0.240 | |||||||||
| May 20, 2013 |
1,000 | 0.120 | 0.240 | |||||||||
| May 28, 2013 |
8,000 | 0.120 | 0.240 | |||||||||
| June 1, 2013 |
7,000 | 0.120 | 0.240 | |||||||||
| June 3, 2013 |
3,000 | 0.120 | 0.240 | |||||||||
| June 11, 2013 |
1,000 | 0.120 | 0.240 | |||||||||
| June 17, 2013 |
3,000 | 0.120 | 0.240 | |||||||||
| June 18, 2013 |
1,000 | 0.120 | 0.240 | |||||||||
| June 19, 2013 |
28,151 | 0.120 | 0.240 | |||||||||
| June 24, 2013 |
2,000 | 0.120 | 0.240 | |||||||||
| June 29, 2013 |
1,000 | 0.120 | 0.240 | |||||||||
| August 5, 2013 |
200,000 | 0.240 | 1.379 | |||||||||
| August 6, 2013 |
31,000 | 0.240 | 1.379 | |||||||||
|
|
||||||||||||
| (1) | The exercise price per share was the estimated fair value of an ordinary share on the date of each grant, as determined by our Board of Directors, taking into account various objective and subjective factors as described below, and using the assistance of an unrelated third-party valuation firm. In certain instances, the third-party valuation firm delivered its report, which was retroactive to a certain specified date, after the date our Board of Directors determined the fair value for an option grant. |
| (2) | During 2011, 2012 and the first and third quarters of 2013, we performed contemporaneous valuations to estimate the fair value of our ordinary shares with the assistance of an unrelated |
77
Table of Contents
| third-party valuation firm. These valuations were used to determine the fair value of the options granted at the various grant dates set forth in the table above. In certain instances, as described in footnote (1) above, the valuation report was delivered after the date the options were granted, but was retrospective to an earlier date specified in the report. The significant input assumptions used in our valuation models during 2011, 2012 and 2013 are based on subjective future expectations combined with the judgment of our Board of Directors. |
| (3) | 875,376 of these options represent the replacement of voluntarily cancelled non-statutory options granted on March 19, 2010, with the new award being tax-qualified but at a higher exercise price of $0.12, reflecting the most recent fair value per share at the time of reissuance. |
The estimated fair value of our ordinary shares in April 2010 was $0.040. This valuation was based on the issuance of second tranche of units (consisting of one-third of an ordinary share and one F preferred ordinary share) which took place on April 30, 2010 and were issued at a price of $1.62 per share. By using the price paid for these units and calculating the breakpoints using the OPM (as described above), the value of our total equity and the fair value of ordinary shares implied by the F financing was simultaneously determined using a back solve technique. As preferred shareholders have rights that give them effective control of us, any equity value based on a preferred share financing reflects an incremental value associated with the right to control how our assets are deployed. Since this incremental value is allocated to all components of the capital structure by the OPM, we have removed the portion allocated to ordinary shares to reflect the fair value of a minority interest. The ordinary share control adjustment applied in this valuation analysis was 13.76%.
The estimated fair value of our ordinary shares in February 2011 was $0.038, which represents a decrease of $0.002 per share from the April 2010 valuation. This valuation was based on the issuance of a third tranche of units (consisting of one-third of an ordinary share and one F preferred ordinary share) which took place on February 28, 2011 and was also issued at a price of $1.62 per share. The valuation methodology was similar to that described in the April 2010 valuation noted above. The ordinary share control adjustment applied in this valuation analysis was 15.67%.
The estimated fair value of our ordinary shares in February 2012 was $0.026, which represents a decrease of $0.012 per share from the February 2011 valuation. This decrease was primarily due to the dilution that occurred because of the additional issuance of ordinary shares associated with the debt conversion. The value implied by the convertible debt financing using the OPM back solve methodology was considered as part of this valuation. However, due to the fact that the debt was issued to then-existing investors with the option to convert at the original F unit issue price of $1.62, we determined that the value implied by the financing may not fully meet the fair value standard and therefore other accepted methods should be used to establish fair value. As a result, an equity value was also calculated using a binomial method as well as the method of multiples. We then applied a DLOM of 9.87% to the equity value to ensure comparability with the other methods employed. The DLOM was calculated as the value of an at-the-money put option (as described above). This value reflects what a market participant would pay at the measurement date to ensure that the security can be sold at a point in the future at its liquid fair value. These three methods were then equally-weighted to determine the value of equity to be allocated to the various securities in our capital structure. The ordinary share control adjustment applied in this valuation analysis was 13.98%.
The estimated fair value of our ordinary shares in June 2012 was $0.120, which represents an increase of $0.094 per share from the February 2012 valuation. This increase was primarily supported by the issuance of G preferred ordinary shares which took place on June 15, 2012. Such
78
Table of Contents
shares had an issue price of $1.70 per share which represented an increase from the last fair value financing. As the financing was deemed to be at fair value, the ordinary share price was established by back solving the OPM such that the G preferred ordinary share price is equal to its issue price. The ordinary share control adjustment applied in this valuation analysis was 14.99%.
The estimated fair value of our ordinary shares as of March 31, 2013 was $0.240, which represents an increase of $0.12 per share from the June 2012 valuation. This increase was primarily due to an improvement in forecasted revenues and cash flows resulting from increased sales in Asia, particularly in China, and approval in the fourth quarter of 2012 by Japan’s Ministry of Health, Labour and Welfare, or the MHLW, as well as improving market conditions. To determine the estimated fair value of the Company as of March 2013, we calculated a value of $108,019,912, or $0.24 per ordinary share, using two equally weighted valuation method outcomes: the equity value based on the DCF analysis and the value that emerges when the equity value underlying the June 2012 financing is moved forward based on a Monte Carlo analysis. The value based on the DCF analysis was then adjusted for a DLOM of 10.44% using a put option methodology (as described above) as this value is based on market inputs that reflect a liquid market place. This methodology was selected to ensure that our projections underlying the DCF analysis were consistent with the assumptions underlying the June 2012 financing. The average returns as well as volatility of our guideline publicly traded entities were used to derive the assumptions applied in the Monte Carlo analysis. Such assumptions were calculated from the June 2012 financing date to the measurement date, resulting in an average return of 2.96% and a volatility of 7.23%. This methodology was adopted for three reasons: 1) the earlier financing was concluded to be at fair value, 2) the investors were informed market participants, and 3) less than one year had passed between the current measurement date and the earlier financing which means that many of the assumptions underlying the financing are still likely to be relevant and drivers of value at the measurement date. The ordinary share control adjustment applied in this valuation analysis was 14.82%.
As our Board of Directors did not determine the occurrence of an initial public offering to be likely until the third quarter of 2013, a probability factor for such event was not assigned as part of any of the valuation methodologies described above.
The estimated fair value of our ordinary shares as of August 19, 2013 was $1.379, which represents an increase of $1.139 per share from the March 2013 valuation. This increase was primarily due to the decision made by the Board of Directors during the third quarter of 2013 to begin preparations for an offering, which required a third valuation methodology to be incorporated into our estimate of the total fair value of our ordinary shares, as further described below. The increase in the estimated fair value of the ordinary shares primarily arises from the inclusion of this third valuation methodology under which the liquidation preferences allocable to the preferred ordinary shares were eliminated. The consideration of the liquidation preferences under the first and second valuation methodologies caused a reduction in the estimated fair value of the ordinary shares under those methodologies.
As with prior valuations, to determine the estimated fair value of our ordinary shares as of August 19, 2013, we first calculated a value of $120,156,184, or $0.26 per ordinary share, using two equally weighted valuation method outcomes: the equity value based on the DCF analysis and the value that emerges when the equity value underlying the June 2012 financing is moved forward based on a Monte Carlo analysis. The value based on the DCF analysis was then adjusted for a DLOM of 9.24% using a put option methodology (as described above) as this value is based on market inputs that reflect a liquid marketplace. The average returns as well as volatility of our
79
Table of Contents
guideline publicly traded entities were used to derive the assumptions applied in the Monte Carlo analysis. Such assumptions were calculated from the June 2012 financing date to the measurement date, resulting in an average return of 2.43% and a volatility of 7.86%. The ordinary share control adjustment applied in this valuation analysis was 13.12%. The small increase from the March 2013 valuation is directly related to our improved operating performance between the valuation dates.
In addition and as described above, due to our intent to prepare for an offering, a third valuation methodology was applied utilizing an offering scenario and resulted in a value of $191,460,475, or $2.50 per ordinary share. To calculate the offering scenario outcome, we evaluated the ratios of equity value to revenue for the guideline publicly traded entities used in the Monte Carlo analysis as well as entities identified by two investment banking firms. An average revenue multiple of 4.14 and a standard deviation of revenue multiples of 2.14 was then calculated at the measurement date. The weighted-average revenue multiple, calculated as the weighted-average of various multiples expected to emerge at the offering date plus/minus one standard deviation, was 4.99. The weighted-average revenue multiple was then multiplied by the expected trailing twelve month revenue as of February 2014 to obtain the expected equity value at the offering date. The resulting value was much higher than the value derived from the combined outcome of the first and second valuation methodologies, which did not take into account the offering, primarily due to the fact that the capital raising activity provided by an offering will allow the Company to take advantage of the growth opportunities that would not have been possible or highly unlikely without the infusion of new capital, as well as the fact that a public entity’s shares are more liquid and therefore have additional value.
A probability factor of 50% was then applied to both the combined outcome of the first and second valuation methods of $0.26 per ordinary share and to the outcome of the third valuation method using the offering scenario of $2.50 per ordinary share. The resulting values of $0.13 and $1.25 were then added together resulting in a combined fair value per ordinary share of $1.379.
The midpoint of the estimated price range reflected on the cover page of this prospectus, $ , is an increase of $ , or approximately %, as compared to the estimated fair market value of our common shares as of the date of the latest valuation performed in August 2013 of $1.379. The $ low end of the estimated price range reflected on the cover page of this prospectus is an increase of $ , or approximately %. The increase is primarily due to the increased probability of the offering scenario (i.e., the occurrence of this offering) and the elimination of the preferred share preferences upon the conversion of preferred shares to ordinary shares in connection with this offering.
The assumptions used in determining the fair value of our ordinary shares represent the best estimate of our Board of Directors but are highly subjective and inherently uncertain. If our Board of Directors had made different assumptions, our calculation of the options’ fair values and the resulting share-based compensation expense could differ materially from the amounts recognized in our financial statements.
Off-balance sheet arrangements
We do not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or for any other contractually narrow or limited purpose.
80
Table of Contents
Quantitative and qualitative disclosure about market risk
We are exposed to market risks in the ordinary course of our business. These market risks are principally limited to interest rate fluctuations and foreign currency exchange rate fluctuations.
Interest rate sensitivity
We are exposed to market risk related to changes in interest rates as it impacts our interest income and expense.
Cash and cash equivalents. As of September 30, 2013, we had cash and cash equivalents of $13.0 million, and restricted cash of $449,000. Restricted cash primarily consists of amounts pledged as security for our facility leases in the United States. Our exposure to market risk includes interest income sensitivity, which is impacted by changes in the general level of U.S. and European interest rates. Our cash and cash equivalents are invested in interest-bearing savings and money market accounts. Our cash and cash equivalents are invested in interest-bearing savings and money market accounts. We do not enter into investments for trading or speculative purposes. Due to the conservative nature of our investment portfolio, which is predicated on capital preservation of investments with short term maturities, we do not believe an immediate one percentage point change in interest rates would have a material effect on the fair market value of our portfolio, and therefore we do not expect our operating results or cash flows to be significantly affected by changes in market interest rates.
Term loan and line of credit. As of September 30, 2013, we had debt obligations of $6.0 million under our credit facility with Square 1 Bank. The term loan carries a variable interest rate of 2.75% above the prime rate, with a floor of 6.0%. The revolving line of credit carries a variable interest rate of 1.75% above the prime rate, with a floor of 5.0%. If there is a rise in interest rates, our debt service obligations under our credit facility would increase even though the amount borrowed remained the same, which would affect our results of operations, financial condition and liquidity. Assuming no change in our debt obligations from the amount outstanding at September 30, 2013, a hypothetical one percentage point change in underlying variable rates would change our annual interest expense and cash flow from operations by approximately $60,000 without taking into account the effect of any hedging instruments. We have not entered into, and do not expect to enter into any interest-rate hedging arrangements. We intend to use a portion of the net proceeds from this offering to repay this indebtedness. See “Use of proceeds.”
Foreign currency exchange risk
We are exposed to foreign exchange rate risk. Because we currently operate in three major regions of the world: the United States, Europe & ROW and Asia, our revenue is denominated in multiple currencies. About half our sales are in the United States, which are denominated in U.S. Dollars. Sales in China are denominated in U.S. Dollars but these sales are made by our United Kingdom-based legal entity where the Pound Sterling is the functional currency. As a result, these sales are subject to remeasurement into Pounds Sterling and then translation into U.S. Dollars when we consolidate our financial statements. Sales in Europe are denominated primarily in the Pound Sterling and Euro. As we grow Europe & ROW sales outside the United Kingdom and the Euro Zone, we will be subject to risk from additional currencies. Sales in Japan are denominated in Yen, and our sales in Japan, which started in late 2012, have grown significantly in the first nine months of 2013.
81
Table of Contents
Our expenses are generally denominated in the currencies in which our operations are located, which is primarily in the United States, the United Kingdom and Japan.
As we continue to grow our business outside the United States, our results of operations and cash flows will be subject to fluctuations due to changes in foreign currency exchange rates, which could harm our business in the future. To date, we have not entered into any foreign currency hedging contracts although we may do so in the future.
Recent accounting pronouncements
We have considered recent accounting pronouncements and determined that they are either not applicable to our business or that no material effect is expected on the consolidated financial statements as a result of future adoption.
JOBS Act
Under the JOBS Act, emerging growth companies that become public can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards following the completion of this offering and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
82
Table of Contents
Overview
We are a global, commercial-stage diagnostics company committed to improving patient care by providing advanced, innovative tests in the field of immunology. Our proprietary T-SPOT technology platform allows us to measure the responses of specific immune cells, known as T cells, to inform the diagnosis, prognosis and monitoring of patients with immunologically controlled diseases. T cells are a central component of the human body’s immune system, and are implicated in the control and progression of many medical conditions, including certain types of infectious diseases, cancers and autoimmune diseases.
The initial product we have developed using our T-SPOT technology platform is our T-SPOT.TB test, which is used to test for LTBI. Our T-SPOT.TB test has been approved for sale in over 50 countries, including the United States, where we have received PMA from the FDA, in Europe, where we have obtained a CE mark, as well as Japan and China. Our T-SPOT.TB test has been included in clinical guidelines for TB screening in 17 countries, including the United States, several European countries and Japan. In addition, we have established reimbursement for our test in the United States, as well as a CPT code that is used only for our test. We believe that many payors rely upon CPT codes to determine the amount they pay providers. Outside the United States, we have established reimbursement in several countries where reimbursement applies, including Japan, Switzerland and Germany. Our customers benefit from the existence of reimbursement mechanisms as it provides more certainty of the amount they will be paid for performing our test, as described in the section under the heading “– Funding and reimbursement.” We believe the annual global market opportunity for our T-SPOT.TB test is well in excess of $1 billion, assuming we can largely displace the TST in the developed world.
Tuberculosis remains a significant global public health problem. According to the WHO, approximately two billion people globally have LTBI, and on average each carries a 10% lifetime risk of progressing to active TB disease. In 2011, approximately 9 million people contracted active TB disease, of which approximately 1.5 million people died.
A central component of TB control strategies worldwide, particularly in developed markets, is to screen large numbers of people in high-risk groups for LTBI. These screening programs seek to identify infected people so that treatment can be administered to prevent these individuals from subsequently progressing to active TB disease and infecting others. According to the WHO, at least 50 million such screening tests are performed worldwide each year.
The vast majority of these tests are performed using the 100-year-old TST. Our T-SPOT.TB test is designed to replace the TST and has several important advantages over the TST.
Sales of our T-SPOT.TB test are growing rapidly. As of September 30, 2013, we had cumulatively sold over two million T-SPOT.TB tests, with approximately one million tests sold over the 12 months ended September 30, 2013. Over the last three years we have grown our revenue from $4.3 million in 2009 to $20.7 million in 2012, a compound annual growth rate of 69%. We attribute the growing commercial success of our T-SPOT.TB test to the following factors:
| • | Compelling advantages over the TST. Our T-SPOT.TB test enables better TB control due to its clinical, logistical and health-economic advantages. The cost-effectiveness of our T-SPOT.TB test versus the TST has been demonstrated in multiple studies and has been persuasive in adoption of the test. |
83
Table of Contents
| • | Broad regulatory approval and scientific validation. Our T-SPOT.TB test is approved for sale in over 50 countries, giving us a substantial accessible market. The performance of our T-SPOT.TB test has been validated in over 300 peer-reviewed publications in scientific journals. |
| • | Supportive guidelines. Our T-SPOT.TB test has been included in clinical guidelines for TB screening in 17 countries, including the United States, several European countries and Japan. |
| • | Established payment mechanisms. We have established reimbursement in several key countries, including the United States, Japan, Switzerland and Germany. |
| • | Large underpenetrated market. Our T-SPOT.TB test addresses the estimated global market of 50 million tests per annum, which we believe represents a market opportunity for us of well in excess of $1 billion. Our penetration of this market is in its early stages. We estimate that over 90% of testing is still performed with the TST, giving us a significant opportunity for long-term growth through displacement of the TST. |
| • | Flexible business model. We offer our T-SPOT.TB test in two formats to accommodate customer preference and maximize sales. Our in vitro diagnostic kit format, which is available globally, allows customers to perform the test in their own institutions. In our service format, which we offer in the United States and the United Kingdom, we perform our T-SPOT.TB test on samples sent by customers to our laboratory facilities. Our service offering provides us with direct customer contact and, therefore, unique market insights. |
| • | Recurring revenue. Once a customer begins using our T-SPOT.TB test instead of the TST, our experience is that the customer rarely goes back to using the TST. This purchasing pattern allows us to continually leverage our sales force to generate new business, rather than to maintain existing customers. |
We are a global business with 151 employees, including sales and marketing teams, on three continents, and laboratories in the United States and the United Kingdom. We sell to customers in over 40 countries and derived 50% of our revenue from outside the United States for the year ended December 31, 2012. Our current customer base is comprised of over 1,000 active customers, consisting of hospitals, public health departments, commercial testing laboratories, importers and distributors. Our revenue for the year ended December 31, 2011 was $12.6 million, for the year ended December 31, 2012 was $20.7 million, and for the nine months ended September 30, 2013 was $28.6 million. Our net loss for the year ended December 31, 2011 was $13.1 million, for the year ended December 31, 2012 was $14.9 million and for the nine months ended September 30, 2013 was $5.3 million.
TB overview
Tuberculosis is a common and, if not properly treated, potentially lethal infectious disease caused by a bacterium called Mycobacterium tuberculosis. When an individual with active TB disease of the respiratory tract coughs, sneezes, yells or spits, respiratory fluid droplets that contain M. tuberculosis are expelled into the air and can infect others. TB typically infects the lungs, but the lymph nodes, kidneys, brain and bones may also be infected. Within two to ten weeks of the original infection, a specific T cell immune response usually develops. This immune response prevents further multiplication and spread of the TB bacteria. Individuals who have a successful T cell immune response will still have live bacteria in their body and are considered to have LTBI. Those with LTBI are asymptomatic and are not infectious; however, each person with LTBI has on average a 10% chance of progressing to active TB disease over his or her lifetime.
84
Table of Contents
TB is considered to have progressed to active TB disease when the body is unable to effectively control the replication of the TB bacteria and their growth causes damage to the body. This risk of progression to active TB disease is significantly elevated among individuals with weakened immune systems, such as smokers, those with HIV or diabetes or those on drugs that suppress the immune system (e.g., those taking biologic therapies for autoimmune disease or those undergoing immune-suppression post-transplantation). When a person develops active TB disease, the symptoms, including coughing, chest pains, weakness, weight loss, fever and night sweats, may be mild for many months. This can lead to delays in seeking care, which can result in transmission of the bacteria to others. As the disease progresses, the person may develop symptoms that can become increasingly worse. Without proper treatment, up to two thirds of people with active TB disease will die.
According to the WHO, approximately one-third of the world’s population, over two billion people, is infected with M. tuberculosis. This represents an enormous population of infected persons at risk of progressing to active TB disease. Despite the availability of an effective treatment, TB is one of the leading causes of infectious disease death worldwide. In 2011, the WHO estimated that approximately 9 million people contracted active TB disease, of which approximately 1.5 million people died. TB is a leading killer of people living with HIV, causing one quarter of all deaths in that population. Although TB rates are declining slowly across the world, even in the developed world, current screening and management tools have failed to eliminate the disease. For example, in the United States an estimated 11 million people have LTBI, which acts as a constant source of new infections. In addition, new cases of TB commonly arise from immigration and from travel to and from countries with higher incidence of TB.
There are three broad strategies to control TB: vaccination, finding and treating active TB disease, and finding and treating LTBI to prevent the development of new cases.
| • | Vaccination. The traditional means of seeking to protect individuals who may be exposed to infectious diseases is vaccination. The only vaccine available for TB is the BCG vaccine, which was first used in the 1920s. The vaccine is widely used around the world outside the United States; however, BCG’s efficacy is highly variable and it does not provide adequate protection against TB disease in adults. Therefore, the vaccine alone is insufficient to control TB. |
| • | Finding and treating active TB disease. Although TB is typically a curable disease when treated with the standard multi-month regimen of potent antibiotics, diagnosing active TB can be problematic. For instance, TB symptoms are often non-specific and/or confused with other diseases, causing delays in seeking and receiving appropriate medical diagnosis. In addition, traditional diagnostic tests for active TB disease are imperfect. Delays in diagnosis result in increased morbidity and mortality and worsen the spread of TB infection, as people with active TB disease can infect as many as 10 to 15 people per year. The emergence of drug resistant TB strains is a growing problem, as they make treatment with standard anti-TB drugs more difficult and in some instances, where resistance is present to all front-line drugs, the mortality rate exceeds 50%. |
| • | Finding and treating LTBI. The identification of individuals with LTBI by screening high-risk groups is an essential component of TB control in developed markets. In the United States, for example, screening high-risk groups has been an important practice for more than four |
85
Table of Contents
| decades. In the United States and other countries with a low incidence of TB, most new, active TB disease cases have occurred among persons who were once infected, contained the initial infection, and then later progressed from LTBI to active TB disease. The identification and treatment of individuals with LTBI prevents any further risk of these individuals progressing to active TB disease and prevents the further spread of TB. |
The United States has one of the most comprehensive LTBI screening programs in the world. Several high-risk groups have been identified by the U.S. Centers for Disease Control and Prevention, or the CDC, for screening and subsequent treatment of LTBI, including:
| • | healthcare workers; |
| • | those with immunosuppressive conditions, such as diabetes, certain carcinomas, organ transplantation and persons receiving immunosuppressive agents; |
| • | those with HIV and those working at HIV clinics; |
| • | refugees and immigrants from countries with high incidence of TB; |
| • | close contacts of active TB cases; |
| • | prisoners and jail detainees, as well as staff employed in prisons and jails; |
| • | intravenous drug users and staff employed at substance abuse centers; |
| • | homeless persons and staff employed at homeless facilities; and |
| • | those living in congregate living facilities, such as nursing homes or assisted living facilities. |
In addition to the screening of high-risk groups recommended by the CDC, TB screening is also mandated by many states to include additional populations, such as day care staff, school teachers and pupils, and police officer candidates. Additionally, the screening of healthcare workers is recommended as part of the accreditation standards for U.S. hospitals and screening of certain U.S. military personnel for LTBI is included in military guidelines.
Generally, other developed markets have similar practices to screen high-risk groups for LTBI, although the populations screened may differ from those in the United States.
In total, we estimate that there are 22 million LTBI tests performed each year in the United States, the majority of which are performed within the healthcare system in a variety of settings, including hospitals, public health offices, physicians’ offices and clinics. Outside the United States, we estimate the total number of tests to be 28 million each year, for a combined market size of 50 million LTBI tests annually.
Current TB skin test and its limitations
The primary test currently used for TB screening is the 100-year-old TST. The TST is administered by injecting an extract from cultured M. tuberculosis, called Tuberculin or PPD, into the skin of a subject’s forearm using a needle and syringe. The injection of the PPD into the skin of a subject previously infected with TB stimulates the immune response, including T cells, causing the formation of a hard lump at the site of the injection. Because it takes time for this reaction to occur, the subject must return 48 to 72 hours after the PPD injection to have the result read. The test result is graded by feeling for the boundaries of the swelling, marking these with a pen and then measuring the diameter with a ruler.
86
Table of Contents
The TST suffers from several limitations, including the following:
| • | Antiquated technique results in substantial test variability. The technique of administering the PPD injection and reading the TST is inherently variable. Too little of the PPD may be injected to stimulate the appropriate response, the injection may be too shallow, allowing the PPD to leak out of the skin, or the PPD may be injected too deeply to stimulate the appropriate response. Because this technique is inherently operator dependent, healthcare workers who administer the PPD injections and read TST tests should undergo specialist training. However, even with such training, test results vary with the training levels, responsibility, and conscious and unconscious bias of the healthcare workers administering the injections and reading the tests. Variability in the size of the swelling due to administration of the injection averages approximately 15%. Similarly, variation in reading test results among experienced healthcare workers is also estimated at approximately 15%. |
| • | Multiple patient visits required. The TST requires that the patient return 48 to 72 hours from the time of injection. This requirement presents a significant logistical challenge. Additionally, non-return rates can be as high as 30%, resulting in considerable time and money being wasted to persuade the subjects to be rescreened as well as the duplicated materials costs and time associated with retesting. |
| • | False negatives. False-negative results to the TST are common due to a number of factors relating to the quality of the PPD used and the patient receiving the injection. Specifically, the PPD may be improperly stored, improperly diluted or contaminated. In addition, a fungal, viral or bacterial infection (including active TB disease) can suppress the TST response, leading to a false-negative. False negatives are also prominent among newborns and elderly subjects. Other conditions can also cause false-negative TST results, including HIV, certain live-virus vaccinations (e.g., measles, mumps and polio), chronic renal failure, nutritional factors, diseases affecting the lymphoid organs (e.g., Hodgkin’s disease, lymphoma, chronic lymphocytic leukemia and sarcoidosis), drugs (e.g., corticosteroids, tumor necrosis factor (TNF) biologics and many other immunosuppressive agents) and stress. |
| • | False positives. False-positive results to the TST are common and are attributed to the presence in the PPD of antigens that are shared with other mycobacteria. As a result, the TST can cross-react in those patients who are infected with non-tuberculous mycobacteria as well as those patients who have received the BCG vaccine, which is the most widely administered vaccine in the world. |
| • | “Boosting” of results. The TST result can also be “boosted,” which occurs when an infected subject’s reaction to an initially false-negative skin test causes increased sensitivity in a subsequent test such that it tests positive. The misinterpretation of a boosted reaction as a new infection with M. tuberculosis can result in unnecessary additional testing for the subject, unnecessary treatment and unnecessary testing of other personnel. As a result of this “boosting” effect, when the TST is used, the CDC recommends two-step testing for newly employed healthcare workers in order to ensure that an initial negative test is not a false negative. This recommendation effectively requires four patient visits when using the TST (two administrations of the PPD and two reads), a process that can lead to significant and costly delays in the hiring of new personnel at U.S. healthcare institutions. |
87
Table of Contents
Our solution
Our T-SPOT.TB test is a highly sensitive and specific, single-cell based method for identifying LTBI. It is a single-tube blood test that directly measures antigen-specific T cells that indicate LTBI. Our T-SPOT.TB test has been approved for sale in over 50 countries, including the United States, in Europe, Japan and China. In addition, our T-SPOT.TB test is included in clinical guidelines for TB screening in 17 countries, including the United States, several European countries and Japan.
Our T-SPOT.TB test takes advantage of the T cell response that results from infection with M. tuberculosis. Our T-SPOT.TB test quantifies individual M. tuberculosis-sensitized T cells by challenging them with M. tuberculosis antigens that are recognized by the immune system. We employ two antigens, ESAT-6 and CFP10, to stimulate T cells that have previously been exposed to M. tuberculosis, which causes them to release a cytokine called interferon-gamma. Interferon-gamma is one of the dominant cytokines released by activated T cells when encountering M. tuberculosis. In contrast to the PPD reagent used in the TST, these two antigens are not shared with the BCG vaccine or with non-tuberculous mycobacteria. Because our test detects individual T cells via their release of interferon-gamma, our test is sometimes referred to generically as an IGRA.
We believe our T-SPOT.TB test has a number of compelling advantages that make it a superior alternative to the 100-year-old TST, including:
| • | In head-to-head studies, our T-SPOT.TB test is frequently found to have higher sensitivity than the TST. In regulatory clinical trials (see “—Regulatory approvals and clinical validation”), we have demonstrated a sensitivity for the T-SPOT.TB test that exceeds 95%. In comparison, the TST is reported to have a sensitivity between 75-90% in similar populations. In addition, and unlike the TST, our T-SPOT.TB test is not significantly affected by immune-suppression. |
| • | Our T-SPOT.TB test is more specific than the TST, primarily because the antigens in our T-SPOT.TB test do not cross-react in individuals who have had the BCG vaccination or who have been infected with most other non-tuberculous mycobacteria. |
| • | Our T-SPOT.TB test requires a simple blood draw, which does not require specifically trained healthcare workers to administer the test. |
| • | There is no requirement for a return visit in 48 to 72 hours to obtain our T-SPOT.TB test result. This makes the testing process more convenient for patients and avoids the costs and inconvenience of readministering the test to those who fail to return to have the TST read. |
| • | Our T-SPOT.TB test does not suffer from the “boosting” phenomenon that can affect the TST, as there is no injection of immunogenic substances into the body. Consequently, with our T-SPOT.TB test, two-step testing for new hires, which entails four visits, is not required and pre-hire screening can be condensed to a single visit. |
| • | The combination of our T-SPOT.TB test’s greater accuracy and its logistical benefits means that the adoption of our T-SPOT.TB test can improve patient care while reducing costs for institutions. |
The TST is often considered to be “cheap,” as the PPD reagent and other materials used in the test typically cost less than $5 per test. However, the cost of the TST itself is only one element of the total cost involved when conducting a TB screening program or TB control strategy. Substantial costs beyond the materials cost of the TST test include additional costs associated with: (i) false-negatives and false-positives to the TST; (ii) individuals who fail to return within the
88
Table of Contents
prescribed period; and (iii) implementing and maintaining training programs for healthcare workers who administer and read TST tests.
Several studies have been published investigating the costs or cost-effectiveness of a TB screening program using the TST and in comparison to our T-SPOT.TB test. We believe the following two studies are informative in demonstrating how expensive the TST actually is to implement and how deploying our T-SPOT.TB test in preference to the TST can be a more cost-effective solution when implementing TB screening programs.
| • | Infection Control and Hospital Epidemiology (Lambert et al., 2003). This CDC-led study sought to determine the annual costs of implementing and maintaining TST screening programs for healthcare workers at hospitals and health departments. The authors concluded that compliance with the CDC guidelines regarding TB infection control may require a substantial investment in personnel time, effort and commitment. The costs of running a TST program were found to be between $41 and $362 per healthcare worker for hospitals and between $172 and $264 per healthcare worker for health departments. The materials cost of the TST itself amounted to less than 1.5% of the total cost of the screening program in all the studied institutions. |
| • | Journal of Occupational and Environmental Medicine: The SWITCH study (Wrighton-Smith et al., 2012). The SWITCH study, conducted at The Johns Hopkins Healthcare System and Medical School, was conceived to systematically identify and then measure all the costs of screening healthcare workers using either a TST or an IGRA (specifically, our T-SPOT.TB test). The key study findings were that administering a TST testing program costs $73.20 per person screened, $90.80 per new hire, and $63.42 per annual screen. Use of an IGRA for employee health testing was found to be cost saving, with an IGRA test cost of $54.83 or less per test, and to result in higher screening completion rates due to the elimination of the need for a second visit to interpret the TST. Dr. Peter Wrighton-Smith, our Chief Executive Officer, contributed as an author and scientific collaborator in this study. |
Although primarily designed for use in detecting LTBI, our test can also be used to assist in the diagnosis of active TB disease, particularly in suspected cases where conventional diagnostic methods such as chest x-ray or sputum smear are inconclusive. Because infection is a pre-requisite for disease, ruling out LTBI can aid physicians in diagnosing a different disease or condition. Our test has been included in guidelines in several countries for this purpose, such as those from the Netherlands, France, Ireland and Italy.
Our strategy
Our near-term objective is to increase adoption of our T-SPOT.TB test for screening and detecting persons infected with LTBI. Our longer-term objective is to leverage our proprietary T-SPOT technology platform, immunology domain expertise and regulatory experience to cost-effectively introduce other high-value immunology-based diagnostic tests. To achieve these objectives, our strategy is to:
| • | Accelerate our penetration into proven market segments in the United States. We intend to selectively invest in our direct sales and customer service teams to increase our capacity to fully cover the hospital and public health segments, which have primarily supported our success to date. In addition, we expect to build upon our marketing and medical education programs to |
89
Table of Contents
| increase awareness and understanding of the advantages of our T-SPOT.TB test over the TST by leveraging scientific publications, including the SWITCH study results. |
| • | Expand into other market segments in the United States. We intend to increase our presence in other market segments where feasible, including physicians’ offices, universities, chronic care facilities and the military. |
| • | Expand our sales presence outside the United States. We intend to make investments to expand our direct sales presence, particularly in Europe and Japan. In addition, we intend to establish a presence in select additional geographies to accelerate test adoption in countries where we already have regulatory approval. |
| • | Expand our addressable market outside the United States. We intend to continue to invest in opening up new markets by gaining additional regulatory approvals. In addition, we intend to continue to invest to develop markets in which we already have regulatory approval through generating the data to yield supportive guidelines and reimbursement. |
| • | Launch new diagnostic tests. We plan to leverage our proprietary T-SPOT technology platform, domain expertise in immunology, lab and commercial infrastructure, regulatory experience and customer relationships to launch new immunology-based diagnostic tests. |
Regulatory approvals and clinical validation
Our T-SPOT.TB test is approved for commercial sale in over 50 countries. Key geographies where we have regulatory approval include:
| • | The United States. We obtained PMA for our T-SPOT.TB test from the FDA in 2008. Since 2008, an additional ten PMA supplements have been approved, including supplements relating to manufacturing improvements and label extensions, such as those that enable overnight shipment of blood samples. |
| • | Europe. We obtained a CE mark in 2004, which allows us to sell our T-SPOT.TB test in Europe as well as other countries that accept the CE mark. |
| • | China. We obtained approval for our T-SPOT.TB test from China’s State Food and Drug Administration, or the SFDA, in 2010. |
| • | Japan. We obtained approval for our T-SPOT.TB test from the MHLW in 2012. |
90
Table of Contents
Two key metrics measured by the regulatory bodies responsible for approving our T-SPOT.TB test are sensitivity, a measure of how many test positives there are in a population known to be infected, and specificity, a measure of how many test negatives there are in a population known to be uninfected. The following is a chart showing the performance of our T-SPOT.TB test in studies conducted in certain key geographies:
| Country/Region (trial size) | Sensitivity (%) | Specificity (%) | ||
|
| ||||
| United States (2,355 subjects) |
95.6% | 97.1% | ||
|
|
|
| ||
| Europe (180 subjects) |
98.8% | 100% | ||
|
|
|
| ||
| China (1,333 subjects) |
95.3% | Not applicable* | ||
|
|
|
| ||
| Japan (212 subjects) |
97.5% | 99.1% | ||
|
| ||||
| * | Specificity data are not available in the Chinese study because the design of the studies focused on active TB disease, for which specificity is not a relevant metric. In China, the positive and negative predictive values for the diagnosis of active TB disease were 95.4% and 93.9%, respectively. |
These data, which were generated in controlled studies under strict regulatory standards, demonstrate that our T-SPOT.TB test is able to detect TB infection with high accuracy. In addition, our T-SPOT.TB test has also been validated in over 300 peer-reviewed publications in scientific journals.
Guidelines
We believe that clinical guidelines, which are recommendations issued by national medical societies or public health bodies, are a driving factor in a clinician’s decision to use a specific diagnostic test. Our T-SPOT.TB test is included in clinical guidelines for TB screening in 17 countries, including the United States, several European countries, and Japan.
Guidelines typically refer to our T-SPOT.TB test generically as an IGRA. Guidelines generally incorporate one of four common approaches: (1) a two-step approach in which TST is administered and subsequently followed by an IGRA, either when the TST is negative (to increase sensitivity, mainly in immunocompromised individuals) or when the TST is positive (to increase specificity, mainly in BCG-vaccinated individuals); (2) either TST or IGRA, but not both; (3) IGRA and TST together (to increase sensitivity); and (4) IGRA only, replacing the TST.
In recent years, the use of IGRAs has been increasingly recommended. For example, key recommendations contained in the CDC’s 2010 guidelines are as follows:
| • | An IGRA may be used in place of a TST in all situations in which the CDC recommends TST as an aid in diagnosing TB. |
| • | An IGRA is preferred for testing persons from groups that historically have low rates of returning to have TSTs read. |
| • | An IGRA is preferred for testing persons who have received BCG (as a vaccine or for cancer therapy). |
| • | A TST is preferred for testing children under the age of five, though use of an IGRA in conjunction with a TST has been advocated by some experts to increase diagnostic sensitivity in this age group. |
91
Table of Contents
| • | An IGRA or a TST may be used without preference to test recent contacts of persons known or suspected to have active TB disease, with special considerations for follow-up testing. IGRAs offer the possibility of detecting M. tuberculosis infection with greater specificity than with a TST. Also, unlike TSTs, IGRAs do not boost subsequent test results and can be completed following a single patient visit. |
We believe that these guidelines (and similar national guidelines outside the United States) allow us to access the vast majority of the current TST market and assert the superiority of an IGRA in significant segments of the market.
Market segments and revenue mix
We have a geographically diversified business. In 2012, our revenue was derived half in the United States and half outside the United States.
Our U.S. business derived 94%, 95% and 96% of revenue from our service offering (as opposed to kit sales) for the years ended December 31, 2011 and 2012 and for the nine months ended September 30, 2013, respectively. The growth in our service offering reflects our experience that U.S. customers prefer to send out for IGRA tests than run them in-house. We categorize the U.S. market into four main areas:
| • | Hospital based-testing. We estimate that there are 7.0 million tests performed in hospitals in the United States each year. This test volume is made up primarily of testing of hospital employees, although there is also some in-patient and out-patient testing of high-risk patient groups. Testing in this segment is primarily non-reimbursed, with the test costs borne by institutional budgets. Consequently, test pricing results from direct negotiation with each institution. Our current average selling price is approximately $50 per test for this segment. We therefore believe that this segment has a total value of approximately $350 million. |
| • | Public health departments. We estimate that there are 1.1 million tests performed by public health departments across the United States each year. This test volume is made up of testing contacts of infectious TB patients, testing of refugees and other immigrants and testing conducted in public health clinics, which covers testing for a wide variety of purposes. Testing in this segment is primarily non-reimbursed and thus subject to negotiated prices, although there are some testing populations in this segment that are covered by government payors. We currently collect approximately $45 per test for this segment. We therefore believe that this segment has a total value of approximately $50 million. |
| • | Physicians’ offices and clinics. We estimate that there are 7.3 million tests performed in physicians’ offices and clinics across the United States each year. This test volume is made up of testing of various high-risk groups, including HIV patients, rheumatology patients and those undergoing immunosuppressive treatment regimens. Testing for these patients is typically reimbursed by Medicare, Medicaid and third-party commercial payors. We have limited experience with billing these payors, but based on our Medicare national limitation amount of $103 per test, we believe that we may be able to collect as much as $75 to $95 per test performed in this segment. Taking the mid-point of this estimate, this segment could have a potential value of approximately $620 million. |
| • | Other. We estimate that each year there are 6.4 million tests performed in various other settings, including military installations, correctional facilities and universities and schools. This test volume is made up of testing various groups, including military personnel, prisoners and prison workers, |
92
Table of Contents
| foreign-born students and residents and workers in long-term care homes. Reimbursement coverage and mechanisms vary based on the tested population. Because of our limited experience in this segment to date, we cannot currently estimate the potential value of this segment. |
Currently, we are primarily focused on, and derive the majority of our U.S. revenue from, the hospital and public health segments.
Our business outside the United States consists of sales in over 40 countries and represents a total potential market of over 28 million tests annually. Eighty-four percent, 83% and 89% of our revenue from outside the United States came from sales of kits and associated accessories, as opposed to service offering revenue, for the years ended December 31, 2011 and 2012 and for the nine months ended September 30, 2013, respectively. We, either directly or through our distributors, sell our testing kits primarily to hospital laboratories and commercial testing laboratories that perform the tests and provide test results to the ordering clinicians. Test prices are negotiated with each of our customers.
Funding and reimbursement
The funding and reimbursement structures for LTBI testing vary among countries, as discussed in more detail below.
United States
In the hospital and public health segments, TB testing programs are funded primarily from institutional budgets. We receive payment from these institutions according to our pre-negotiated prices. For other segments of the U.S. market (notably, for example, the physicians’ office segment) third-party reimbursement from governmental payors and/or private insurers is often available to cover the cost of our T-SPOT.TB test.
CPT codes are used by payors to identify services provided to patients and determine the appropriate level of reimbursement for such services. As such, obtaining a CPT code for a particular service facilitates payment to the provider. We applied for and were successful in obtaining a unique CPT code to cover our T-SPOT.TB test (code 86481), which became effective in January 2011. The reimbursement amount of this code was initially linked to CPT code 86480. We appealed this decision on the basis that our T-SPOT.TB test uses a different methodology and that this leads to differentiated clinical outcomes to the test covered under code 86480. Our appeal was successful and in January 2012 the reimbursement amount for code 86481 was increased by 22%. The current CMS national limitation amount for 86481 is $103. We have a national coverage determination for our CPT code 86481 from Medicare, which means we are able to obtain Medicare reimbursement nationally. Individual state agencies establish reimbursement levels for Medicaid. Our T-SPOT.TB test is currently reimbursed by Medicaid in 42 states and the District of Columbia, and our Oxford Diagnostic Laboratories®, or ODL®, facility is an enrolled provider with Medicaid in 30 states. We submit claims to these federal insurance programs and also to private insurers as an out of network laboratory. Based on our limited experience to date, we believe that our code is covered by most private insurers.
There are a number of other segments of the U.S. TB screening market, such as correctional facilities, military personnel, university students and chronic care facility residents. We believe that funding varies within and among these segments, encompassing both funding from institutional budgets and from third-party payors.
93
Table of Contents
Outside the United States
Although outside the United States we primarily negotiate pricing directly with our customers, our prices are influenced to some degree by the mechanism and level of funding our customers receive for performing LTBI testing. The funding mechanisms for selected countries are explained below.
Japan. IGRAs are listed on the clinical lab fee schedule in Japan (code D015-25), which attracts a reimbursement level of ¥6,300 (approximately $63.14) per test. We believe that this reimbursement code covers all testing done in hospitals and clinics. There also exists a mechanism to partially reimburse public health entities for IGRA testing from central government funds.
China. In China, test pricing is regulated by provincial government bodies. These bodies determine the price at which a test can be charged to the test recipient. To date, pricing approval has been granted for our T-SPOT.TB test in five provinces with test pricing in the range of RMB600-800 (approximately $114.39) per test. We believe that certain hospitals (e.g., military hospitals) fall outside of this formal pricing approval, in which case the test is funded from hospital budgets. Similarly, in provinces where no pricing approval exists, hospitals may still purchase and perform our T-SPOT.TB test, but testing must be funded using the hospitals’ pre-existing resources.
United Kingdom. No formal centralized reimbursement mechanism for diagnostic tests exists in the United Kingdom. Instead, the testing is funded from institutional budgets whether we sell kits or our service offering.
Germany. Outpatient testing is covered in Germany under the “EBM” reimbursement system. A code for IGRAs was established in January 2011 (Code 32670), which qualifies for reimbursement at €58 (approximately $76.54) per test. In addition, the cell-purification step inherent in our T-SPOT.TB test methodology can also attract an additional €10.40 (approximately $13.72) per test in reimbursement. Testing that is not eligible for EBM reimbursement (e.g., inpatient testing and public health testing) is typically funded from institutional budgets.
Sales, marketing and distribution
We currently market our T-SPOT.TB test directly in the United States, Northern Europe and Japan. Outside of these territories, we have contracted with third-party distributors. In countries where we have a direct presence, we use a combination of sales managers, sales representatives, customer service staff and technical experts to interact with clinicians, nurses, administrative staff, laboratories and other groups who are involved in the implementation of TB screening programs. Our goal is to educate these groups about the medical, logistical and economic benefits of switching from the TST to our T-SPOT.TB test. Our customer service staff and technical experts are also involved in the practical training of customers to perform and order our T-SPOT.TB test as well as answering customer questions. These teams are supported by marketing activities, which include advertising, medical education, attendance at scientific meetings and other awareness-raising activities. As of September 30, 2013, we had 56 employees engaged worldwide in sales, marketing and customer service functions.
Under our flexible business model, we currently offer our T-SPOT.TB test in either an in vitro diagnostic kit or a service format. In the former, we sell test kits and associated accessories to
94
Table of Contents
laboratories for them to perform the testing themselves. In the latter, we have established clinical testing laboratories in the United States and the United Kingdom, where we perform our T-SPOT.TB test on samples sent to us by customers. In these markets, we have found that many customers prefer to send samples to us rather than perform their own analysis on-site. We market our service offering under the name Oxford Diagnostic Laboratories, or ODL.
Our ODL service is typically comprised of the following steps:
| • | We provide our customers with pre-paid sample packaging for shipping samples back to our laboratories for analysis. |
| • | The customer draws a blood sample and places it in our sample packs, along with a completed test requisition form. |
| • | The sample is picked up by our designated courier (although customers can also drop off samples themselves to courier locations) and shipped overnight. |
| • | When the package arrives at our ODL facilities, we unpack and enter the sample data into our LIS. The LIS assists us in sample processing and tracking and provides various automation options for result delivery and invoicing. |
| • | We process the sample and, once the test is complete, we report the results back to the customer and submit an invoice to the customer or, in certain cases, to a patient’s insurance provider. We have various mechanisms for customers to order and receive their results according to their preference, including fax, encrypted e-mail, web-portal or an interface with their electronic medical records system. |
Our approximately 35,000 square foot U.S. ODL facility is located in Memphis, Tennessee, approximately ten miles from the FedEx global headquarters and sorting facility. We use FedEx as our courier for samples in the United States and have negotiated discounted shipment rates that our customers are able to take advantage of via our pre-paid sample shipment packs. We believe that our location gives our laboratory the competitive advantage of being able to access almost all parts of the continental United States with a patient-to-lab time of typically less than 20 hours. In addition, we believe it gives us market access and convenience advantages because customers can use our service wherever there is a FedEx pick-up or drop-off location. Further, as we typically receive the majority of our packages from FedEx’s sort facility at 4 a.m., Memphis time, each morning we are able to achieve turnaround times that we believe are substantially quicker than other competing laboratories. Our U.S. ODL facility is College of American Pathologists accredited and has obtained the necessary Clinical Laboratory Improvement Amendments, or CLIA, registrations to accept samples from all 50 states.
Our U.K. ODL facility is located within our manufacturing facility in Abingdon, England. We use the U.K. National Health Service courier, called DX, as our primary courier in the United Kingdom. Our U.K. lab is accredited to the ISO17025 quality standard.
Our technology platform
T cells are a central component of the human body’s immune system, and are involved in the control and progression of many medical conditions, including certain types of infectious diseases, cancers and autoimmune diseases. Our proprietary T-SPOT technology platform allows us to efficiently measure marker-specific T cell responses at a single-cell level and thereby inform
95
Table of Contents
the diagnosis, prognosis and monitoring of patients with immunologically controlled diseases. By measuring T cells, we can provide additional data to clinicians that are not available through existing methods, such as molecular diagnostics. For example, LTBI cannot be diagnosed by a molecular test.
We employ a proprietary quantitative method to detect antigen-specific effector T cells releasing interferon-gamma. Interferon-gamma is a principal immune messenger molecule, called a cytokine, released by effector T cells. Our technology is designed to selectively measure responses from this subtype of T cells because they are primarily present when active, replicating pathogens are inside the body, as opposed to other T cell subtypes that may be present long after an infection has been cleared from the body. For diagnosis and monitoring applications, it is more relevant to be able to measure the immune response associated with the current disease rather than the immune response associated only with past, cleared exposure.
Additionally, we have developed a patented method for enabling the processing of blood samples after they have been shipped overnight. This method involves the removal of contaminating granulocytes from the shipped sample to rejuvenate it prior to processing. Granulocytes are a normal component of whole blood. However, once blood is removed from the body, granulocytes start to progressively decay, which can cause contamination of the T cell containing white blood cell, or WBC, components used in T cell assays. In addition, decaying granulocytes release chemicals that can suppress cytokine secretion by T cells, further reducing test sensitivity. By removing granulocytes prior to starting an assay, we restore the sample to the same composition and function as a fresh sample. To further the commercialization of this technology, we use our T-Cell Xtend reagent in conjunction with our assay methodology. The T-Cell Xtend reagent is an antibody complex that binds granulocyte cells to red blood cells, thereby ensuring that they do not contaminate the WBC components used in our assay. By using the T-Cell Xtend reagent, we can test blood samples that have been shipped and/or stored for up to 32 hours before processing commences.
Our T-Cell Xtend reagent addresses the significant process limitation inherent in some laboratory tests that require a fresh blood sample for the assay. When this requirement exists, the diagnostic test may not be accessible for many subjects unless a local laboratory is available and able to quickly process the sample. An alternative approach is sometimes employed in which blood samples are carefully frozen before shipment to a laboratory. We believe this approach is impractical in regular clinical use, particularly when a large volume of samples is involved, and reduces sample quality. Our solution, the T-Cell Xtend reagent, addresses this problem without the need for freezing the blood. Specifically, our solution does not require the customer to do anything to process blood samples prior to shipment as the T-Cell Xtend reagent is added to the sample when it arrives in the laboratory. This approach is practical for routine clinical use and has the ability to significantly broaden the potential market for certain diagnostic tests.
We also employ proprietary manufacturing processes and protocols designed to cost-effectively and reliably produce key elements of our T-SPOT technology, including the process for coating microtiter plates with interferon-gamma antibodies and our quality control testing procedures. Further, we have developed proprietary methods designed to achieve rapid throughput in assay performance. These methods involve harvesting WBC components from whole blood and specific protocols related to the use of automation in the assay process.
The principles of our T-SPOT assay system, incorporating our T-Cell Xtend reagent, are shown in Figure 1.1 below, using blood as the body fluid in the example. The process starts with a blood
96
Table of Contents
sample, from which WBC components (specifically Peripheral Blood Mononuclear Cells, or PBMCs) containing T cells are separated, washed and counted. A pre-determined number of WBCs and antigens specific to the disease or condition of interest are then added to the wells of a microtiter plate to which antibodies to interferon-gamma, or IFN-g, are bound. The test is based on the principle that the T cells of an individual who carries an active infection will respond to the antigens and secrete interferon-gamma. The secretion of interferon-gamma by the T cells of the subject is captured by the anti-interferon-gamma antibodies coated to the floor of each well. The numbers of individual reacting T cells are enumerated through visualizing the footprint of each T cell by this secretion of interferon-gamma.
Figure 1.1: Principles of our T-SPOT assay system
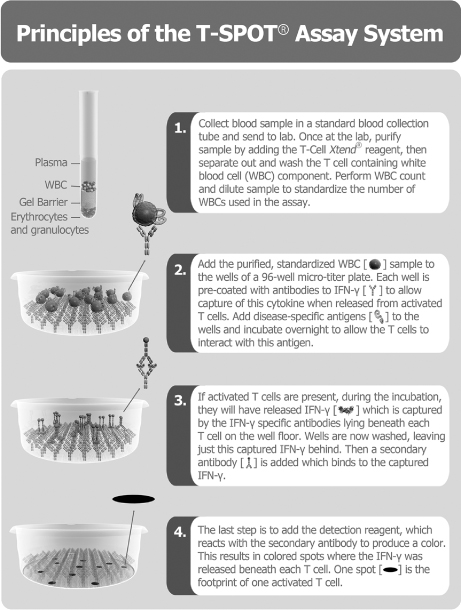
97
Table of Contents
Key features of this assay method that give it technical advantages over other platforms for measuring antigen-specific T cells include:
| • | Ability to ship blood samples overnight. We believe our intellectual property position and our T-Cell Xtend reagent give us an advantage in that we can ship blood overnight for T cell measurement assays. We believe this allows for much easier adoption of our technology, as customers do not need to go through labor-intensive freezing protocols prior to shipping samples and widespread access to the test can be accomplished without the need for a suitable local lab to run the test. In addition, overnight shipment allows the centralization of samples in a single testing facility, such as our ODL facilities, which can yield cost savings through economies of scale and gives us the advantage of direct relationships with the individuals and organizations who order the tests. |
| • | Low background noise. The use of our T-Cell Xtend reagent, plus the separation and washing of WBCs prior to performing the test, ensure that the subsequent steps of the process start with a purified sample. Our assay system therefore has a low background noise that is essential for the detection of weaker responses, which is critical in many applications, including screening for TB. |
| • | Standardization. We standardize the number of WBCs added to each well, which ensures that variations in WBC numbers, such as those caused by disease or immunosuppression, are eliminated prior to starting the assay. This is particularly relevant in populations with lower numbers of WBCs, such as HIV patients and other immunosuppressed groups. In addition, standardization of the number of WBCs is important to establish a stable baseline against which to validly compare longitudinal measurements within an individual. This standardization is thus important for disease monitoring indications. |
| • | High analytical sensitivity. Our analytical method measures responses at a single-cell level, which, in combination with the two steps above, provides high analytical sensitivity. As a result, we are able to reliably detect specific T cells at frequencies of 1 per 50,000 WBCs or less. |
| • | Designed to be incorporated in standard clinical practice. The sample collection process is designed to be easy to perform in a wide variety of clinical settings using a standard blood draw. By using industry-standard sample collection procedures, we believe our T-SPOT.TB test and subsequent assays we develop using our T-SPOT platform will be accessible to a wide variety of customers. |
In addition to its technical advantages, our T-SPOT technology can be applied to diagnose and monitor a variety of diseases and conditions. First, by altering the target-specific antigen used in our T-SPOT assay, we can direct our technology platform to detection of different diseases or conditions where T cell function is involved. Second, our proprietary methods can be used to visualize cytokines other than interferon-gamma. Third, our methodology can be and has been successfully applied to other body fluids that contain T cells. This provides us the ability to detect T cell responses not just in the bloodstream, but also from T cells that have migrated to sites of disease.
As scientific knowledge increases regarding the potential utility of measuring T cell function to inform disease diagnosis and outcomes, we expect to have further opportunities to develop tests for diseases and conditions that are governed by an immunological response. We believe our technology platform will provide us with significant competitive advantages in this effort and enable us to become a leader in the field of immunology diagnostics.
98
Table of Contents
Research and development
Our research and development efforts are focused on developing new diagnostic tests that use our quantitative T cell measurement technology.
We believe that we have assembled experienced research and development teams across our sites with the scientific talent needed to develop new products that leverage our technology platform and immunology expertise. We believe that our experience in developing assays based on our T-SPOT method will allow us to conceive and develop assays and validate multiple tests on our platform. Our initial product, our T-SPOT.TB test, was developed, validated and commercialized within 18 months. Initially, we intend to focus our research and development efforts on potential tests for which the antigens are known, which eliminates the lead time required for antigen discovery.
We are currently investigating multiple opportunities to develop additional diagnostic tests, including assays that would help transplant physicians better manage patients at risk of rejection and infection. Because the antigens in this context are largely known, reducing the lead time required for antigen discovery, we believe that we may be able to develop a test for use in the transplant market more quickly and with less development risk. In addition, because we already have sales penetration in hospitals where such centers are generally located, we believe that we may be able to efficiently build upon our existing sales and marketing infrastructure in order to introduce a test in this market. Given that intensive patient monitoring is required in the first few years post-surgery, we believe that this can be a significant market for our tests. We believe our market opportunity in the transplant segment could be as high as $500 million annually.
As of September 30, 2013, we had 8 employees engaged in research and development functions. Our research and development expenses were $1.8 million and $1.9 million for the years ended December 31, 2011 and December 2012, respectively, and $1.2 million and $1.6 million for the nine months ended September 30, 2012 and September 30, 2013, respectively.
Intellectual property
We seek to secure and maintain protection of the proprietary aspects of our technology platform and of our existing and planned products. We rely on a combination of patents, trademarks, trade secret and other intellectual property laws, and confidentiality, license and invention assignment agreements and other contracts to protect our intellectual property rights. In addition, we have developed substantial knowledge in the field of immunology diagnostics including proprietary methods that we believe provides us with a significant advantage relative to potential competitors.
The intellectual property relating to our T-SPOT.TB test that we own or license includes 12 issued U.S. patents, more than 20 issued patents in other jurisdictions, 3 pending U.S. patent applications and 4 pending patent applications in other jurisdictions, as well as registered trademarks, proprietary manufacturing processes and protocols, and proprietary methods directed towards achieving rapid throughput in assay performance.
99
Table of Contents
Our owned and licensed patents
The table below identifies the patents and pending patent applications we own or to which we have license rights that relate to our T-SPOT.TB test.
| Patent and patent application numbers(1) |
Form of rights(2) | Expected expiration date |
General description of subject matter | |||
|
|
|
|
| |||
| US 7,575,870, US 12/510,594*, EP 941478, JP 4094674, AU 728357, CA 2,272,881 |
In-licensed from Isis Innovation Limited | November 2017 | Methods, including use of ELISPOT technique, to detect and quantify in vitro effector T cells that respond to pathogen specific antigen stimulation with the release of interferon-gamma | |||
| EP 2084508, CN 10105293224, US 13/253,598*, JP 2009-530943*, AU 2007303994*, CA 2,665,205*, IN 2165/DELNP/2009* |
Owned | October 2027 | Methods of improving stored blood sample stability by removing granulocytes | |||
| US 7,115,361 |
In-licensed from Isis Innovation Limited | December 2019 | Method and kit for detecting TB specific T cells following stimulation with antigen peptides | |||
| US 7,632,646, US 7,901,898, US 8,216,795, US 8,507,211 US 13/940,758*, EP 1144447, JP 4633931, ZA 2001-3356 |
In-licensed from Isis Innovation Limited | November 2019 | Composition, method and kit for diagnosis of TB using peptides from ESAT-6 | |||
| US 6,290,969, US 6,338,852, US 8,084,042, EP 1203817, JP 4324597, CN 1200147, AU 727602, CA 2,653,566, ZA 9607394, and a number of other countries |
In-licensed from Statens Serum Institut | September 2015 (US) August 2016 (other jurisdictions) |
Composition and method of making an isolated polynucleotide of TB specific protein CFP10 | |||
| US 5,955,077, EP 706571, AU 682879, CA 2,165,949, NZ 267984 |
In-licensed from Statens Serum Institut | September 2016 (US) July 2014 (other jurisdictions) |
Composition and sequences of TB polypeptide antigen ESAT-6 and uses in diagnosis of TB |
100
Table of Contents
| Patent and patent application numbers(1) |
Form of rights(2) | Expected expiration date |
General description of subject matter | |||
| US 7,579,141, US 8,021,832, EP 1214088, JP 4820489, AU 773268, CA 2,372,583 |
In-licensed from Rutgers, The State University of New Jersey | May 2020 | Methods of in vitro diagnosis utilizing the T cell response to CFP10 to distinguish between exposure to TB and BCG vaccination | |||
|
|
|
|
| |||
| * | Reflects pending patent applications |
| (1) | Where we have rights to patents granted by the European Patent Office, or the EPO, the patents have been validated in numerous countries in Europe, which vary by specific patent but typically include at least the United Kingdom, Germany and France. |
| (2) | For a discussion of the terms of the licenses referenced in this table, please see “—Our license agreements” below. |
Many of the patent rights we in-license have claims directed to the use of ESAT-6 and/or CFP10 to detect Mycobacterium tuberculosis. We believe that these are the most important TB-specific antigens and we include peptides from both of these in our T-SPOT.TB test. We also believe that using an ELISPOT technique for an IGRA enhances its accuracy and suitability for use in testing individuals with compromised immune systems. Our T-SPOT.TB test employs this technique.
The first two patent groups listed in the table above also have potential applications beyond the TB field. Specifically, the core technology patents we have licensed from Isis Innovation Limited, or Isis, contain claims to methods of measuring marker-specific effector T cell responses at a single-cell level. These methods cover the measurement of intracellular pathogens by detecting, through a quantitative method using an ELISPOT technique, the in vitro release of cytokines by antigen-specific effector T cells. These measurements can inform the diagnosis, prognosis and monitoring of patients with immunologically controlled diseases or conditions, such as infectious diseases, cancers and autoimmune diseases.
The inventions claimed in our patents and patent applications relating to removal of granulocytes from stored blood samples may also have applications in relation to other diseases, conditions or situations where blood samples cannot be tested soon after the blood draw. This proprietary method to improve the stability of stored blood enables our service offering as it allows for overnight shipment of blood samples.
We have also licensed certain patent rights from three parties that we believe may assist us to develop future diagnostic tests, particularly in the transplant field. The expected expiration dates of these patents range from March 2018 for three issued U.S. patents to which we have non-exclusive rights to May 2027 for pending patent applications to which we have exclusive rights for in vitro diagnostics measuring immune status in humans related to organ transplantation, graft versus host disease and autoimmune disease. We can give no assurance that any of our current or future research and development programs will result in the development and validation of any diagnostic test.
Our license agreements
We have relied upon three license agreements, referenced in the table above, to obtain rights under certain patents that we believe may be necessary to make, use and sell our T-SPOT.TB test. We may in the future rely, at least in part, upon licensing agreements with third parties to obtain patent rights and transfers of technology, information and know-how to enable us to take
101
Table of Contents
advantage of research work already completed, including potentially the identification of antigens useful for measuring disease conditions. We believe such licensing arrangements have enabled us, and may in the future enable us, to reduce the amount of time we need to develop and validate new diagnostic tests.
We have royalty obligations under each of our license agreements regardless of the format in which we offer our test (i.e., a kit format or a service format). Our royalty obligations are calculated on our net sales, the definition of which varies by agreement and typically results in a lower effective royalty rate on our service revenue than on sales of our kits. Currently, our aggregate royalty burden under all license agreements, as a percentage of gross product and service revenue, is in the low double digits. Under some of our license agreements, we are responsible for paying, or contributing to, patent prosecution and maintenance costs. All of the license agreements related to our T-SPOT.TB test impose diligence obligations on us. These obligations include certain requirements relating to the pursuit of clinical development and commercialization of licensed products in various markets worldwide. We believe we are in compliance with such obligations.
Isis Innovation Limited (Isis)
We entered into our current license agreement with Isis in 2005, replacing an amended license agreement from 2003, which had replaced an original agreement from 2002. Our current license agreement was amended by a deed of variance in 2006 and by a letter agreement in 2012.
Pursuant to the agreement, Isis granted us an exclusive, worldwide, royalty-bearing license to certain patents to manufacture and commercialize any products and services. Our license extends to all applications and fields of use, except for research use only applications. The licensor has reserved the right to grant to the University of Oxford, its employees, students, agents and appointees, the right to use the technology for academic and research purposes. Our license includes the right to sublicense, either with or without the licensor’s consent, depending on the proposed sublicensee and the terms of the sublicense. We have granted one non-exclusive sublicense under these patents with a field of two infectious diseases and we have granted a second non-exclusive sublicense under these patents limited to the sublicensee’s internal use to monitor vaccine response. We have granted a third party a sublicense under some of the patents covered by this license, with the right to further sublicense, limited to use with ESAT-6 and CFP10 antigens, and excluding use of the ELISPOT format for diagnosis and monitoring of TB infection, disease or therapy. We do not believe this third party has granted any sublicense rights as of September 30, 2013.
Effective immediately prior to this offering, we expect to become the assignee of the patents that are subject to this license, with ongoing obligations to pay annual royalties to Isis until the patents expire and to continue to extend license rights to the University of Oxford as described above. Upon such an assignment, we will also be required to grant a license back to Isis to enable it to maintain any other existing license, such as for research use only.
Prior to the assignment to us of the patents, our rights and obligations are covered solely by the license agreement. Under the agreement, our rights and obligations terminate, territory by territory, when we no longer engage in any commercial practice covered by a valid claim of an unexpired licensed patent. The license agreement provides that either party may terminate the agreement for the other party’s uncured material breach, we may terminate by providing a
102
Table of Contents
required period of advance written notice and Isis may terminate if we undergo certain events relating to a winding up or similar actions or if we raise certain challenges to the licensed technology, including challenges to the validity or necessity of any licensed patent.
Although the agreement contains minimum royalty obligations, the amount of royalties due based on our actual sales has exceeded the minimum for a number of years and we expect our obligations will continue to exceed the minimum for the duration of our royalty obligations. We pay a royalty rate in the low single digits and we expect this rate to be reduced for certain of our sales after the expiration of certain specified patents, which we believe will be in late 2017. Our aggregate payments to Isis through September 30, 2013 for signing fees, milestones and royalties, including minimum royalties, have been $0.8 million. Our royalty obligations to Isis will cease when there are no valid patent claims still in force.
Statens Serum Institut (SSI)
We entered into our current license agreement with SSI in 2009, replacing an original license agreement from 2003. The current license agreement has been amended by one supplement entered into in 2010.
Pursuant to the agreement, SSI granted us an exclusive, worldwide, royalty-bearing license with the right to sublicense, to certain patents to use certain antigens in a diagnostic kit for in vitro diagnosis of TB in humans using an ELISPOT-based detection of interferon-gamma producing T cells using any fluid sample other than whole blood in the diagnostic assay. We have not granted any sublicenses under this license.
Previously, we made a number of milestone payments due under the license to SSI, although no future milestone payments are required. We pay royalties at a rate between 10-20% of net sales, as defined in the agreement, subject to minimum annual royalty payments, which vary by territory. The license agreement provides that royalty obligations continue after the expiration date of licensed patents for a period of four years at a single digit royalty rate. Our aggregate payments to SSI through September 30, 2013 for milestones and royalties, including minimum royalties, have been $7.8 million.
Our license agreement expires, unless earlier terminated, five years after the expiration of the last to expire of individual licensed patents listed as part of the agreement at the effective date in 2003. SSI may terminate the agreement if we, or any future sublicensees, challenge the licensed patents or other SSI intellectual property covered by the agreement. The agreement provides that either party may terminate for material uncured breach by the other party or for certain bankruptcy or insolvency events involving the other party. SSI may also terminate the exclusivity of the license and cease licensing improvements to us if we engage in certain activities related to the development or commercialization of a diagnostic test for latent tuberculosis that does not incorporate any of the licensed diagnostic antigens and which competes with research into, development of or commercialization of the intellectual property rights licensed to us.
Rutgers, The State University of New Jersey (Rutgers)
We entered into our license agreement with Rutgers in 2006 and it has been amended four times, in 2009, 2011, 2012 and 2013.
Pursuant to the agreement, Rutgers granted us an exclusive license to certain patents to manufacture and commercialize kits for in vitro diagnostic assays relating to TB other than in the ELISA format. Our license is royalty-bearing, worldwide, with the right to sublicense. We have
103
Table of Contents
not granted any sublicenses under this license. Rutgers has reserved the right to grant one additional license to this technology, limited to an ELISA format. To date, we do not believe Rutgers has entered into any such license.
We must make semi-annual royalty payments to Rutgers. Although the agreement contains minimum royalty obligations, the amount of royalties due based on our actual sales has exceeded the minimum for a number of years and we expect our obligations will continue to exceed the minimum for the duration of our royalty obligations. We pay a royalty rate in the low single digits. Our aggregate payments to Rutgers through September 30, 2013 for signing fees, annual fees, milestones and royalties, including minimum royalties, have been $1.7 million. Our royalty rate may be reduced, depending on the outcome of an EPO opposition appeal and could also be reduced if Rutgers grants another license to the technology covering an ELISA format. See “Risk factors—Risks related to our intellectual property.” Our royalty obligations to Rutgers will cease when there are no valid patent claims still in force covering licensed products or assays. Previously, we made a number of other payments to Rutgers for license issue fees, annual license fees and milestone payments. No such future payments are required under the license.
Rights under the agreement expire on the last to expire of the licensed patents or the abandonment of all patent applications related to the licensed patent rights. We may terminate the license by advance written notice. Either party may terminate the license for material uncured breach by the other party. Rutgers may terminate the license if a court or administrative body finds it liable or culpable due to our performance, or the performance of any future sublicensee, unless we agree to indemnify it from damages resulting from the decision. Our license rights terminate automatically if any bankruptcy, insolvency or similar proceedings are instituted by or against us (subject to reinstatement if the matter is removed within a specified time frame).
Trademarks and other protection
The trademarks we employ in our TB screening business include T-SPOT, T-Cell Xtend, Oxford Diagnostic Laboratories, ODL, the Oxford Immunotec logo and our laboratory logo. We have obtained registrations in the United States for T-SPOT, T-Cell Xtend, Oxford Diagnostic Laboratories and the Oxford Immunotec logo. We have also obtained or are seeking registrations for certain of these trademarks in other jurisdictions, including the United Kingdom, the European Community, Japan and China. We have also secured numerous domain name registrations.
We have a policy of requiring all our employees to sign agreements that obligate them to maintain in confidence all confidential information they receive during the course of their employment, except in certain circumstances. Substantially all of our employees are also bound by invention assignment obligations, which provide that rights to all inventions and other types of intellectual property, whether or not patentable, conceived by them during the course of employment are assigned to us. We seek to enter into similar confidentiality and invention assignment agreements with our consultants.
Our proprietary processes
There are several areas in which we have developed proprietary approaches to manufacturing that we believe provide a competitive advantage not only with respect to our T-SPOT.TB test, but also for future tests we may develop on our T-SPOT technology platform. It is essential to the
104
Table of Contents
performance of ELISPOT tests used to detect the release of interferon-gamma from stimulated T cells that the microtiter plates used in the test be smoothly coated with the proper amount of interferon-gamma antibodies. For volume manufacturing, these coated plates must also meet stringent shelf life requirements. Our plate-coating process meets these criteria and cost-effectively provides reliable results. We have also developed a proprietary approach to conducting conformance testing and validation as part of our quality control processes. We believe this approach results in significant cost savings for us without sacrificing our compliance with either good manufacturing practices or our own high standards.
As part of our T-SPOT.TB test, we use a proprietary formulation of peptides which we believe is important to the accuracy of our test. Further, we have devoted substantial time and resources to the development of processes and techniques that have resulted in cost reductions in our test manufacture and in assay performance in our service laboratories. In our ODL facilities, we have streamlined the workflow process in our laboratories to allow for rapid throughput, which reduces labor costs and reduces the time we take to provide test results to our customers. In addition, we have developed and validated automated solutions to portions of the assay process, including proprietary protocols for maximizing efficiencies garnered from the automation equipment. These methods are useful in our T-SPOT.TB test, and will be applicable to future blood-based tests we may develop using our T-SPOT platform. We believe the manufacturing process and assay performance efficiencies we have developed and employ could not easily or quickly be developed by others.
Key supplier relationships
Our T-SPOT.TB test is generally manufactured by us from materials we obtain from a limited number of suppliers. Generally, we believe our relationships with our key suppliers to be good.
Mabtech AB. We entered into a manufacturing agreement with Mabtech AB, or Mabtech, in 2003 and the agreement was amended in 2010 and 2011. We entered into a separate purchase agreement with Mabtech in 2010, which was amended in September 2013.
Pursuant to the manufacturing agreement, Mabtech supplies us with antibody-coated membrane plates, using plates we purchase from another supplier and provide to Mabtech. These antibody-coated membrane plates are a component of our T-SPOT.TB test. We provide rolling forecasts of our anticipated purchases and portions of those forecasts become binding orders. We receive pricing discounts based on the volume of our purchases.
The manufacturing agreement expires, unless earlier terminated, on December 31, 2016. Either party may terminate by providing written notice to the other in the event of a material uncured breach by the other party, a liquidation, insolvency or bankruptcy proceeding involving the other party or cessation in trading by the other party.
Pursuant to the purchase agreement, Mabtech supplies the antibodies used to coat the membrane plates in our T-SPOT.TB test. We provide rolling forecasts of our anticipated purchases and portions of those forecasts become binding orders. We receive pricing discounts based on the volume of our purchases. We have agreed to purchase these antibodies exclusively from Mabtech, although our exclusivity obligations may cease in the event Mabtech raises prices by more than a certain percentage over a defined period of time and declines to match a competitive third-party quotation for the antibodies.
105
Table of Contents
The purchase agreement expires, unless earlier terminated, on December 31, 2018. Either party may terminate by providing written notice to the other in the event of a material uncured breach by the other party, a liquidation, insolvency, or bankruptcy proceeding involving the other party or cessation in trading by the other party.
EMD Millipore Corporation. We entered into a supply agreement with EMD Millipore Corporation, or Millipore, in 2009, which was amended in September 2013. Pursuant to this agreement, Millipore supplies us with the membrane plates used in our T-SPOT.TB test. We provide rolling forecasts of our anticipated purchases and portions of those forecasts become binding orders. The agreement expires, unless earlier terminated, on December 31, 2018. Each party has the right to terminate in the event of a material uncured default by the other party.
MicroCoat Biotechnologie GmbH. Pursuant to our 2010 supply agreement with MicroCoat Biotechnologie GmbH, or MicroCoat, MicroCoat performs antibody coating on membrane plates using plates and antibodies we supply. Under the supply agreement, we provide rolling forecasts of our anticipated purchases and portions of those forecasts become binding orders. We receive pricing discounts based on the size of our orders. These antibody-coated plates are a component of our T-SPOT.TB test.
The agreement expires, unless earlier terminated, on December 31, 2015, subject to automatic renewals for additional one-year periods in the absence of specified notice by either party. Each party has the right to terminate in the event of a material uncured breach by the other party, or in the event of a bankruptcy or insolvency proceeding involving the other party.
StemCell Technologies, Inc. We entered into a supply agreement with StemCell Technologies, Inc., or StemCell, in 2008, which was amended in 2011. Pursuant to this agreement, StemCell supplies us with a product that can be used in performing an assay with our T-SPOT.TB test.
We have the exclusive right to market this product for use in association with ELISPOT tests to detect and/or quantify T-cells for use in the in vitro diagnosis, prognosis and/or clinical monitoring of infectious diseases (including tuberculosis) and non-infectious diseases and medical conditions, except our rights in China and India are non-exclusive. StemCell retains the right to sell this product for use in other applications and in our non-exclusive territories. We are obligated to use commercially reasonable efforts to promote sales of the product for the applications to which we have exclusive rights.
We paid a signing fee in the amount of $0.1 million and milestone payments in the aggregate amount of $0.2 million. We are not obligated to make additional milestone payments. We are obligated to pay an annual exclusivity fee during the term of the agreement, creditable against certain future purchases. The aggregate amount of exclusivity fees due under the agreement is, absent early termination, $1.8 million. Our product purchases exceeded the amount of the exclusivity fee in 2012 and we expect we will continue to exceed this minimum. We receive pricing discounts based on our quarterly orders. We have also agreed to make StemCell our supplier of choice for certain types of products, subject to performance obligations of StemCell, and we are generally obligated to acquire all of our requirements for such products from StemCell.
The agreement expires, unless earlier terminated, on January 31, 2018, but will continue indefinitely thereafter in the absence of specified notice by either party. Each party may terminate for material uncured breach, the insolvency or bankruptcy of the other party or the cessation of trading by or dissolution of the other party. If we terminate the agreement for other reasons prior to January 31, 2018, we may be obligated to pay a termination fee of up to
106
Table of Contents
$0.5 million to the extent that we have not previously made other payments for the signing fee, milestone payments and actual product purchases in excess of this amount. Based on our payments to date, we do not expect to incur any termination fee if we terminate the agreement.
Life Technologies Corporation. We entered into a supply and reseller agreement with Life Technologies Corporation, or Life Tech, in August 2013, pursuant to which we purchase and resell a product that can be used in performing an assay with our T-SPOT.TB test. We have minimum annual purchase obligations under this agreement, as well as obligations to purchase certain amounts based on our forecasts.
The agreement expires, unless earlier terminated, on January 1, 2017. Either party may terminate for a material uncured breach, the insolvency or bankruptcy of the other party, if one of our twelve-month forecasts does not reflect any anticipated purchases of product or if we purchase no product during a consecutive twelve-month period.
Key customer relationships
Shanghai Fosun Long March Medical Science Co. Ltd. We have a distribution agreement with Shanghai Fosun Long March Medical Science Co. Ltd., or Fosun, pursuant to which Fosun distributes our products in China. In October 2013, we revised our distribution arrangement and this new agreement will become effective on January 1, 2014, replacing the current agreement in its entirety.
Under both the existing and new distribution agreement, Fosun serves as our exclusive distributor in a territory consisting of the People’s Republic of China, including Macau Special Administrative Regions, and also serves as our non-exclusive distributor in Hong Kong. Fosun commits to using its best efforts to promote, sell and distribute our products in the territory in compliance with our policies and procedures and applicable law. The agreement imposes certain annual minimum purchase obligations at agreed upon pricing and covers our products, as well as other accessories which may be used in conjunction with our products. Fosun is obligated to refrain from dealing in any products in the territory which would be competitive with ours through a period extending 12 months after the termination of the agreement.
The new agreement expires on January 1, 2021. Either party may terminate the agreement for a material uncured breach or in the event of bankruptcy or an equivalent winding up of the other party’s business. We may terminate the agreement if Fosun does not meet the minimum purchase requirements, for late payment, if Fosun undergoes a change in control, or in certain circumstances based on Fosun’s elections under the note. See “Liquidity and capital resources—Sources of funds—Convertible promissory note.”
Riken Genesis Co., Ltd. We sell our products to a Japanese importer, Riken Genesis Co., Ltd., or Riken, which also serves as our marketing authorization holder in Japan, a position required by Japanese regulatory authorities. We entered into a marketing authorization holder agreement with Riken in July 2011 and it was amended in September 2013. Pursuant to this agreement, Riken provides services for importation into Japan. We paid an initiation fee to Riken in the amount of ¥200,000, or approximately $2,000. We pay Riken a flat monthly fee in the amount of ¥150,000, or approximately $1,500, and also pay a single-digit commission based on the prices at which end users purchase our products.
The initial agreement with Riken had a one-year term and automatically renews for additional one-year periods in the absence of specified notice by either party. Either party may terminate for a material uncured breach or in the event of bankruptcy, insolvency or similar proceedings of the other party.
107
Table of Contents
Competitive tests and our advantages
Our T-SPOT.TB test competes primarily with the TST. In the United States, there are two brands of PPD for the TST: Aplisol® (manufactured by JHP Pharmaceuticals, Inc.)3 and Tubersol® (manufactured by Sanofi Pasteur Limited)4. Outside the United States, we believe the dominant brand worldwide is Tuberculin PPD RT 23 SSI (manufactured by the Statens Serum Institut, Denmark).
Other than the TST, our principal competitor is QFN. As this test also measures interferon-gamma release, QFN is, like our own test, sometimes referred to generically as an IGRA.
We have been competing with QFN, or prior versions of this test, since the inception of our company. Based on our experience, we believe that we have several performance advantages over QFN, including:
| • | In our pivotal clinical trials conducted in the United States, our T-SPOT.TB test was shown to be unaffected by immune-suppression. The U.S. package insert for the QFN test notes that QFN has not been extensively evaluated in immunosuppressed populations and that indeterminate results may be related to immunosuppressed status of the patient. We believe this is an important differentiating factor in patient populations with weakened immune systems, such as those on biologic therapies, corticosteroid or other immunosuppressive treatments, those with HIV and those undergoing dialysis or organ transplantation. |
| • | In the FDA pivotal trials in the United States, our T-SPOT.TB test was shown to have clinical sensitivity exceeding 95%. In the clinical trials for QFN reported in its U.S. package insert, that test was shown to have overall sensitivity of 89%. We believe this allows us to differentiate our test based on accuracy. |
| • | Our test requires only a single tube of blood collected in a ubiquitous heparin blood tube. In contrast, QFN requires the use of three specialist antigen-coated blood collection tubes, either at the time of the blood draw or later. We believe this gives us a reliability and simplicity advantage. |
| • | By using the T-Cell Xtend reagent with our T-SPOT.TB test, we have up to 32 hours to get a blood sample to a processing laboratory, as compared with QFN where blood must be incubated within 16 hours. We believe this significant time advantage gives our test greater flexibility over blood collection windows, as the processing of the blood is less time critical. Our test also allows for the overnight shipment of blood samples without imposing additional processing steps on the customer. |
In addition to the performance advantages we believe we have over QFN, we have developed and implemented the ability to offer our test as a service in the United States and the United Kingdom. The advantages of offering our test as a service include:
| • | We are in direct contact with the individuals and organizations ordering our test via our ODL service offering. We believe this level of direct engagement provides us market insights and marketing opportunities not available to the manufacturer of QFN. |
| • | Our test’s availability is not limited by whether or not a suitable laboratory exists local to the customer. As a result, we believe we can offer testing with more convenient access times to a more diverse set of customers. |
| 3 | Aplisol is a registered trademark of JHP Pharmaceuticals, Inc. |
| 4 | Tubersol is a registered trademark of Sanofi Pasteur Limited. |
108
Table of Contents
| • | We offer our customers access to our laboratory service seven days a week. |
| • | We offer a rapid and consistent test turnaround time, which we believe is typically faster than that offered by other laboratories running QFN. |
| • | Our ODL customers require minimal set-up and training in order to access the test because our service allows our customers to package the test samples in our pre-paid shipper boxes. This development reduces on-boarding time and maximizes simplicity for the customer. |
| • | We are well positioned to offer additional services to our customers over and above providing a test result. We believe that this advantage allows us the ability to generate additional revenue and increase switching costs for customers. |
As a result of clinical and service advantages, we have been able to negotiate a higher average selling price for our service than when selling test kits. We believe this advantage provides us a larger addressable market than is available through selling QFN kits, as our revenue potential from the same testing volume is higher. We believe that this will also enable us to achieve a higher absolute profit per test. However, to the extent that national and regional laboratories offer QFN as a service, we may also face competition from them in our service offering.
The benchmark reimbursement for our T-SPOT.TB test is higher than for QFN in the United States, with CMS reimbursement of $103 per test for CPT code 86481 as opposed to CMS reimbursement of $85 per test for CPT code 86480. We believe that this higher reimbursement provides us with pricing and access advantages in certain segments of the U.S. market. In addition, to our knowledge, as of August 1, 2013, QFN is not yet approved for sale in China.
Government regulation
Federal Food, Drug, and Cosmetic Act
In the United States, in vitro diagnostics are regulated by the FDA as medical devices under the Federal Food, Drug, and Cosmetic Act, or FDCA.
Marketing pathways
There are two regulatory pathways to receive authorization to market in vitro diagnostic devices, or IVDs: a 510(k) premarket notification and a PMA. The FDCA establishes a risk-based standards for determination the pathway for which a particular IVD device is eligible.
The information that must be submitted to the FDA in order to obtain clearance or approval to market a new medical device varies depending on how the medical device is classified by the FDA. Medical devices are classified into one of three classes on the basis of the controls necessary to reasonably ensure their safety and effectiveness. Class I devices are subject to general controls, including labeling and adherence to the FDA’s quality system regulation, which establishes device-specific good manufacturing practices. Class II devices are subject to general controls and special controls, including performance standards and post-market surveillance. Class III devices are subject to these requirements as well as to premarket approval. Most Class I devices are exempt from premarket submissions to the FDA; most Class II devices require the submission of a 510(k) premarket notification to the FDA; and Class III devices require submission of a PMA application. Our T-SPOT.TB test is a Class III device.
Premarket approval. The PMA process, by which we received marketing authorization for our T-SPOT.TB test in 2008, is complex, costly and time consuming. A PMA application must be
109
Table of Contents
supported by detailed and comprehensive scientific evidence, including clinical data, to demonstrate the safety and efficacy of the medical device for its intended purpose. If the device is determined to present a “significant risk,” the sponsor may not begin a clinical trial until it submits an investigational device exemption, or IDE, to the FDA and obtains approval from the FDA to begin the trial.
After the PMA application is submitted, the FDA has 45 days to make a threshold determination that the application is sufficiently complete to permit a substantive review. If the application is complete, the FDA will accept it for filing. The FDA is subject to a non-binding performance goal review time for a PMA application of 180 days from the date of filing, although in practice this review time is often longer. Questions from the FDA, requests for additional data and referrals to advisory committees may delay the process considerably. Indeed, the total process may take several years and there is no guarantee that the PMA application will ever be approved. Even if approved, the FDA may limit the indications for which the device may be marketed. The FDA may also request additional clinical data as a condition of approval or after the PMA is issued. Any changes to the medical device may require a supplemental PMA application to be submitted and approved. Since we received initial PMA application approval of our T-SPOT.TB test in 2008, the FDA has granted approval for ten supplemental PMA applications for our T-SPOT.TB test, including supplements relating to the use of our T-Cell Xtend reagent with our T-SPOT.TB test.
Post-marketing regulations and controls
Under the medical device regulations, the FDA regulates quality control and manufacturing procedures by requiring us to demonstrate and maintain compliance with the quality system regulation, which sets forth the FDA’s current good manufacturing practices requirements for medical devices. The FDA monitors compliance with the quality system regulation and current good manufacturing practices requirements by conducting periodic inspections of manufacturing facilities. FDA inspections are typically unannounced. Violations of applicable regulations noted by the FDA during inspections of our manufacturing facilities could adversely affect the continued marketing of our tests.
The FDA also enforces post-marketing controls that include the requirement to submit medical device reports to the agency when a manufacturer becomes aware of information suggesting that any of its marketed products may have caused or contributed to a death, serious injury or serious illness or any of its products has malfunctioned and that a recurrence of a malfunction would likely cause or contribute to a death or serious injury or illness. The FDA relies on medical device reports to identify product problems and utilizes these reports to determine, among other things, whether it should exercise its enforcement powers. The FDA also enforces the requirement that manufacturers submit reports of recalls and field actions to the FDA if the actions are initiated to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health. The FDA may also require post-market surveillance studies for specified devices.
FDA regulations also govern, among other things, the preclinical and clinical testing, manufacture, distribution, labeling and promotion of medical devices. In addition to compliance with good manufacturing practices and medical device reporting requirements, we are required to comply with the FDCA’s general controls, including establishment registration, device listing and labeling requirements. If we fail to comply with any requirements under the FDCA, we could be subject to, among other things, fines, injunctions, civil penalties, recalls or product corrections, total or partial suspension of production, denial of premarket notification clearance or approval
110
Table of Contents
of products, rescission or withdrawal of clearances and approvals, and criminal prosecution. We cannot assure you that any final FDA policy, once issued, or future laws and regulations concerning the manufacture or marketing of medical devices will not increase the cost and time to market of new or existing tests. If we fail to comply with these FDA regulations or guidelines, we may be subject to warnings from, or enforcement action by, the FDA.
International medical device regulations
International marketing of medical devices is subject to foreign government regulations, which vary substantially from country to country. The European Commission is the legislative body responsible for directives with which manufacturers selling medical products in the European Union and the European Economic Area, or EEA, must comply. The European Union includes most of the major countries in Europe, while other countries, such as Switzerland, are part of the EEA and have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. The European Union has adopted directives that address regulation of the design, manufacture, labeling, clinical studies and post-market vigilance for medical devices, including IVDs. Devices that comply with the requirements of a relevant directive, including the IVD Directive (Directive 98/79 EC), will be entitled to bear the CE conformity marking, indicating that the device conforms to the essential requirements of the applicable directives and, accordingly, can be marketed throughout the European Union and EEA.
Outside of the European Union, regulatory pathways for the marketing of medical devices vary greatly from country to country. In many countries, local regulatory agencies conduct an independent review of IVD medical devices prior to granting marketing approval. For example, in China, approval by the SFDA, must be obtained prior to marketing an IVD medical device. In Japan, approval by the MHLW following review by the Pharmaceuticals and Medical Devices Agency, or the PMDA is required prior to marketing an IVD medical device. The process in such countries may be lengthy and require the expenditure of significant resources, including the conduct of clinical trials. In other countries, the regulatory pathway may be shorter and/or less costly. The timeline for the introduction of new IVD medical devices is heavily impacted by these various regulations on a country-by-country basis, which may become more lengthy and costly over time.
Our T-SPOT.TB test has been approved for sale in over 50 countries, including in Europe, China, and Japan. Our T-SPOT.TB test obtained a CE mark in 2004, SFDA approval in China in 2010 and MHLW approval in Japan in 2012.
Laboratory certification, accreditation and licensing
As a company engaged in the diagnostic testing business, we are required to maintain certain federal and state licenses, certificates and permits.
United States. In the United States, CLIA imposes requirements relating to test processes, personnel qualifications, facilities and equipment, record keeping, quality assurance and participation in proficiency testing, which involves comparing the results of tests on specimens that have been specifically prepared for our laboratory to the known results of the specimens. The CLIA requirements also apply as a condition for participation by clinical laboratories under the Medicare program. Under the CLIA regulations, the complexity of the tests performed determines the level of regulatory control. HHS classifies our T-SPOT.TB test as a high-complexity test. As a result, we must employ more experienced and highly educated personnel, as well as additional categories of employees.
111
Table of Contents
HHS, or an organization to which HHS delegates authority, verifies compliance with CLIA standards through periodic on-site inspections. Sanctions for failure to meet these certification, accreditation and licensure requirements include suspension or revocation of the certification, accreditation or license, as well as imposition of plans to correct deficiencies, injunctive actions and civil and criminal penalties. If HHS should remove or suspend our CLIA certificate, we would be forced to cease performing testing at our laboratory in Memphis, Tennessee.
We are also accredited by the College of American Pathologists, or CAP. The CAP Laboratory Accreditation Program is an internationally recognized program that utilizes teams of practicing laboratory professionals as inspectors, and accreditation by CAP can often be used to meet CLIA and state certification requirements.
United Kingdom. Our laboratory located in the United Kingdom operates under accreditation by the United Kingdom Accreditation Service, or UKAS, for the International Standard: ISO 17025:2005 (General requirements for the competence of testing and calibration laboratories). Compliance with this standard is required to maintain accreditation and the continued use of the UKAS logo on our laboratory documentation. National Health Service (NHS)-based customers require that the testing services they procure operate to an accredited quality management system, which is evidenced by the UKAS accreditation. Therefore, a failure to maintain this accreditation could cause us to lose a substantial majority of our U.K. service business.
HIPAA and other privacy laws
HIPAA established for the first time in the United States comprehensive protection for the privacy and security of health information. The HIPAA standards apply to three types of organizations, or Covered Entities: health plans, healthcare clearing houses, and healthcare providers that conduct certain healthcare transactions electronically. Covered Entities and their Business Associates, as defined in HIPAA, must have in place administrative, physical, and technical standards to guard against the misuse of individually identifiable health information. Because we are a healthcare provider and we conduct certain healthcare transactions electronically, we are currently a Covered Entity, and we must have in place the administrative, physical, and technical safeguards required by HIPAA, the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations. Additionally, some state laws impose privacy protections more stringent than HIPAA. We may conduct other activities that may implicate HIPAA, such as conducting clinical studies or entering into specific kinds of relationships with a Covered Entity or a Business Associate of a Covered Entity.
If we or our operations are found to be in violation of HIPAA, HITECH or their implementing regulations, we may be subject to penalties, including civil and criminal penalties, fines, and exclusion from participation in U.S. federal or state health care programs, and the curtailment or restructuring of our operations. HITECH increased the civil and criminal penalties that may be imposed against Covered Entities, their Business Associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions.
Our activities must also comply with other applicable privacy laws. For example, there are also international privacy laws that impose restrictions on the access, use, and disclosure of health information. All of these laws may impact our business. Our failure to comply with these privacy laws or significant changes in the laws could significantly impact our business and our future plans.
112
Table of Contents
U.S. federal and state billing and fraud and abuse laws
Although only a small portion of our U.S. diagnostics business currently involves payment by third-party payors, including government payors, we are subject to numerous laws governing billing for health care services.
Antifraud laws / overpayments. As participants in federal and state healthcare programs, we are subject to numerous federal and state anti-fraud and abuse laws. Prohibitions under some of these laws include:
| • | the submission of false claims or false information to government programs; |
| • | deceptive or fraudulent conduct; |
| • | excessive or unnecessary services or services at excessive prices; and |
| • | defrauding private sector health insurers. |
We could be subject to substantial penalties for violations of these laws, including denial of payment, obligation to issue refunds, suspension of payments from Medicare, Medicaid or other federal healthcare programs and exclusion from participation in the federal healthcare programs, as well as civil and criminal penalties and imprisonment. One of these statutes, the False Claims Act, is a key enforcement tool used by the government to combat healthcare fraud.
Numerous federal and state agencies enforce anti-fraud and abuse laws. In addition, private insurers may bring private actions. In some circumstances, private whistleblowers are authorized to bring fraud suits on behalf of the government against providers and are entitled to receive a portion of any final recovery.
U.S. federal and state “anti-kickback” and “self-referral” restrictions
Anti-kickback statute. The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” is not defined in the federal Anti-Kickback Statute and has been broadly interpreted to include anything of value, including for example, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payment, ownership interests and providing anything at less than its fair market value.
Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs, and do not contain identical safe harbors.
If we or our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in U.S. federal or state health care programs, and the curtailment or restructuring of our operations. We may also be subject to similar foreign laws and regulations.
Self-referral law. We are subject to a federal “self-referral” law, commonly referred to as the “Stark” law, which provides, unless a specific exception applies, that physicians who, personally or through a family member, have ownership interests in or compensation arrangements with a
113
Table of Contents
laboratory are prohibited from making a referral to that laboratory for laboratory tests reimbursable by Medicare, and also prohibits laboratories from submitting a claim for Medicare payments for laboratory tests referred by physicians who, personally or through a family member, have ownership interests in or compensation arrangements with the testing laboratory.
We are subject to comparable state laws, some of which apply to all payors regardless of source of payment, and do not contain identical exceptions to the Stark law. The self-referral laws may cause some physicians who would otherwise use our laboratory to use other laboratories for their testing. Providers are subject to sanctions for claims submitted for each service that is furnished based on a referral prohibited under the federal self-referral laws. These sanctions include denial of payment, obligation to issue refunds, civil monetary payments and exclusion from participation in federal healthcare programs and civil monetary penalties. They may also include penalties for applicable violations of the False Claims Act, which may require payment of up to three times the actual damages sustained by the government, plus civil penalties of up to $5,500 to $11,000 for each separate false claim.
U.S. health care reform
In March 2010, the PPACA was enacted, which includes measures that have or will significantly change the way healthcare is financed by both governmental and private insurers. Beginning in August 2013, the Physician Payment Sunshine Act, enacted as part of PPACA, and its implementing regulations will require medical device manufacturers to track certain financial arrangements with physicians and teaching hospitals, including any “transfer of value” made or distributed to such entities, as well as any investment interests held by physicians and their immediate family members. Manufacturers will be required to report this information to CMS beginning in 2014. Various states have also implemented regulations prohibiting certain financial interactions with healthcare professionals and/or mandating public disclosure of such financial interactions. We may incur significant costs to comply with such laws and regulations now or in the future.
Other laws
We are also subject to numerous U.S. federal, state and local laws as well as international laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and transportation and disposal of blood and hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
Employees
As of September 30, 2013, we had 151 employees. None of our employees is represented by a labor union or covered under a collective bargaining agreement, nor have we experienced any work stoppages. We believe our employee relations are good.
Facilities
Our U.K. corporate headquarters and operations, including our laboratory facility, are located in Abingdon, England, where we currently lease approximately 5,963 square feet of office space and 8,566 square feet of lab space. The leases on these facilities expire in 2019. Our current rent under these leases is $322,000 annually for the office and $248,000 annually for the lab, which are subject to change.
114
Table of Contents
Our U.S. corporate headquarters is located in Marlborough, Massachusetts, where we currently lease approximately 14,541 square feet of office space. The lease on this facility expires in 2018. Our current rent under this lease is $417,500 annually, subject to annual increases. Our U.S. laboratory facility is located in Memphis, Tennessee, where we currently lease approximately 34,560 square feet of lab space. The lease on this facility expires in 2016. Our current rent under this lease is $109,000 annually, subject to annual increases.
We believe that our current facilities are suitable and adequate to meet our current needs and that suitable additional or substitute space will be available to accommodate future growth of our business.
Environmental matters
Our operations require the use of hazardous materials, which, among other matters, subjects us to a variety of federal, state, local and foreign environmental, health and safety laws, regulations and permitting requirements, including those relating to the handling, storage, transportation and disposal of biological and hazardous materials and wastes. The primary hazardous materials we handle or use include human blood samples and solvents. Some of the regulations under the current regulatory structure provide for strict liability, holding a party liable for contamination at currently and formerly owned, leased and operated sites and at third-party sites without regard to fault or negligence. We could be held liable for damages and fines as a result of our, or others’, operations or activities should contamination of the environment or individual exposure to hazardous substances occur. We could also be subject to significant fines for failure to comply with applicable environmental, health and safety requirements. We cannot predict how changes in laws or development of new regulations will affect our business operations or the cost of compliance.
Legal proceedings
We are not currently a party to any pending legal proceedings that we believe will have a material adverse effect on our business or financial condition. However, we may be subject to various claims and legal actions arising in the ordinary course of business from time to time.
115
Table of Contents
Executive officers and directors
Below is a list of the names, ages as of October 1, 2013 and positions, and a brief account of the business experience of the individuals who serve as our executive officers and directors as of the date of this prospectus. Charles Jonathan Gee, Ph.D. and Rainer Strohmenger, M.D. previously served as Directors, but each has submitted a letter of resignation, which will be effective immediately prior to the effectiveness of this registration statement.
| Name | Age | Position | ||
|
| ||||
| Peter Wrighton-Smith, Ph.D. |
39 | Chief Executive Officer and Director (Class III) | ||
| Richard M. Altieri |
53 | Chief Financial Officer | ||
| Jeff R. Schroeder |
52 | Chief Commercial Officer | ||
| Peter Edwardson, Ph.D. |
51 | Chief Operations Officer | ||
| Patricia Randall |
63 | Vice President and General Counsel | ||
| Richard A. Sandberg |
71 | Chairman of the Board of Directors (Class III) | ||
| Stephen L. Spotts |
58 | Director (Class II) | ||
| Nigel A. Pitchford, Ph.D. |
43 | Director (Class II) | ||
| Michael Steinmetz, Ph.D. |
66 | Director (Class I) | ||
| Vijay Lathi |
41 | Director (Class I) | ||
| Herman Rosenman |
66 | Director (Class I) | ||
|
| ||||
Peter Wrighton-Smith, Ph.D., Chief Executive Officer and Director
Dr. Wrighton-Smith is our Chief Executive Officer and a Director. He has held both positions since founding Oxford Immunotec in 2002. Before founding Oxford Immunotec, Dr. Wrighton-Smith held senior positions with PowderJect Pharmaceuticals PLC, now part of Chiron Corporation, including senior projects director, a business development role, and managing director of PowderJect Diagnostics Ltd., a subsidiary of PowderJect engaged in the development of cellular immune based diagnostics. During his tenure with PowderJect, the company became a global vaccines business with more than 1,000 employees. Dr. Wrighton-Smith has a Masters in Engineering, Economics & Management, and a Doctorate in Medical Engineering, both from Oxford University. We believe that Dr. Wrighton-Smith’s experience and extensive knowledge of the diagnostics industry and our company qualifies him to serve as a member of our Board of Directors.
Richard M. Altieri, Chief Financial Officer
Mr. Altieri is our Chief Financial Officer, a position he has held since joining our company in 2012. Before joining Oxford Immunotec, from 2007 through 2011, Mr. Altieri was vice president and chief financial officer of Salient Surgical Technologies, Inc., a medical technology company that developed and marketed surgical advanced energy products. From 2004 to 2007, Mr. Altieri was senior vice president of finance and chief financial officer of Straumann USA, a global leader in
116
Table of Contents
the field of implant dentistry and dental tissue regeneration. Earlier in his career, beginning in 1992, Mr. Altieri was vice president and chief financial officer of Mitek Surgical Products, Inc., which was acquired by Johnson & Johnson in 1995. Following the acquisition, Mr. Altieri continued to oversee the financial operations of DePuy Mitek as vice president – finance through 2004. Mr. Altieri earned his Bachelors degree in Accounting from Northeastern University and his Masters in Business Administration from Babson College.
Jeff R. Schroeder, Chief Commercial Officer
Mr. Schroeder has been our Chief Commercial Officer since 2013, overseeing worldwide sales and marketing operations. Prior to becoming our Chief Commercial Officer, Mr. Schroeder served as our President, North America from 2007 to 2013, overseeing sales and marketing operations in the United States. Before joining Oxford Immunotec, from 2002 to 2007, he served as vice president of sales, breast health and vice president of marketing at Cytyc Corporation, which manufactured and sold tests used in the diagnosis of cervical cancer and other women’s health-related illnesses and merged with Hologic Corporation in 2007. Before working at Cytyc, from 2000 to 2002, Mr. Schroeder was vice president, sales and marketing for CytoLogix Corporation, a cellular diagnostics company. Earlier in his career, from 1984 to 2000, Mr. Schroeder held various sales positions with increasing levels of responsibility for the diagnostics division of Abbott Laboratories, ultimately becoming director of worldwide marketing for the division. Mr. Schroeder earned his Bachelors degree from Southeast Missouri State University.
Peter Edwardson, Ph.D., Chief Operations Officer
Dr. Edwardson has been our Chief Operations Officer since 2013, overseeing our operations worldwide, including all manufacturing, research and development activities, quality assurance and regulatory affairs and laboratory operations. Prior to becoming our Chief Operations Officer, Dr. Edwardson served as General Manager of our operations in the United Kingdom, with primary responsibility for all manufacturing and research and development activities. Dr. Edwardson joined Oxford Immunotec in 2008 and has over 20 years of experience in the medical device industry in both small start-up companies and large corporations. Immediately before joining Oxford Immunotec, from 2003 to 2008, Dr. Edwardson was vice president of medical technologies for Prometic Biosciences Ltd., a subsidiary of Prometic Life Sciences Inc., which designed, developed, manufactured and commercialized affinity adsorbents sold to biopharmaceutical companies for use in their drug manufacturing processes. From 1999 to 2003, he served as technical director at Tayside Flow Technologies and Tissuemed Ltd, two start-up companies focused on development and commercialization of implantable devices. Dr. Edwardson earned his Bachelors degree in Biochemistry and Physiology from Leeds University and his Doctorate from Cambridge University.
Patricia Randall, Vice President and General Counsel
Ms. Randall is Vice President and General Counsel. Ms. Randall joined Oxford Immunotec in 2008 and has responsibility for our legal affairs. Ms. Randall has more than 35 years of experience as a lawyer and advisor to both public and private companies in a wide range of industries. Before joining Oxford Immunotec, from 2003 to 2008, Ms. Randall served as vice president, general counsel and secretary of Matritech, Inc., a biotechnology company specializing in proteomic diagnostic products for the early detection of a variety of cancers. Before joining Matritech, she served as vice president and general counsel of Hadco Corporation, a global manufacturer of
117
Table of Contents
printed circuit boards, and as vice president and general counsel of Robotic Vision Systems, Inc., a leading supplier of machine vision technologies. In November 2004, more than twenty months after Ms. Randall’s departure from Robotic Vision Systems, Inc., that company filed a petition under the federal bankruptcy laws. Ms. Randall earned her Bachelors degree from American University in Philosophy and her Juris Doctor from Northeastern University School of Law.
Richard A. Sandberg, Chairman of our Board of Directors
Mr. Sandberg is Chairman of our Board of Directors, a position he has held since 2008. He has played a principal role in starting and building a number of medical diagnostic companies including that of founder, chairman, chief executive officer, and chief financial officer of DIANON Systems Inc., a publicly traded oncology diagnostics company that was a pioneer in marketing new diagnostic technologies as a service rather than a product offering. He also served as chairman and chief financial officer of Lifecodes Corporation, a pioneer in DNA testing technology; chief financial officer and director of Matritech Inc., a publicly traded biotechnology company specializing in proteomic diagnostic products for the early detection of a variety of cancers; and as chief financial officer of Critical Diagnostics, Inc., a company specializing in developing new diagnostic tests for cardiology. He has also served as a director of North American Scientific Inc., a publicly held developer and marketer of radiation therapy products and systems for treating breast and prostate cancer, from 2005 to 2009; as a director of Ethan Allen Interiors Inc., an international manufacturer and retailer of fine home furnishings, from 2003 to 2009; and as chairman of two privately held diagnostic companies. Mr. Sandberg earned his Bachelors degree in Business from Northwestern University and a Masters in Business Administration from Harvard University. We believe that Mr. Sandberg’s demonstrated leadership, his understanding of the diagnostics industry and his senior management experience in several companies in our industry qualify him to serve as the Chairman of our Board of Directors.
Stephen L. Spotts, Director
Mr. Spotts is a Director, appointed in 2010. Mr. Spotts is chief executive officer, president, director and founder of ProTom International, Inc., a medical technology company focused on proton therapy for cancer patients founded in 2007. Before founding ProTom, from 2000 to 2006, Mr. Spotts was president, chief executive officer, and a member of the board of directors of Pathology Partners, Inc. (now known as Miraca Life Sciences, Inc.). From 2005 through mid-2008, he served on the board of directors of Genoptix, Inc., a California-based, publicly traded specialized laboratory service provider focused on delivering personalized and comprehensive diagnostic services to community-based hematologists and oncologists (Genoptix, Inc. was acquired by Novartis AG in 2011). Mr. Spotts earned his Bachelors degree in Business Administration from the University of Mississippi. We believe that Mr. Spotts’s experience in the diagnostics industry and his senior management experience in other companies in our industry qualify him to serve on our Board of Directors.
Nigel A. Pitchford, Ph.D., Director
Dr. Pitchford is a Director, a position he has held since 2012. Dr. Pitchford is currently the Chief Investment Officer and a director of Imperial Innovations Group plc (AIM), or Innovations, and leads Innovations’ investment activities. From 2011 until October 2013, Dr. Pitchford was the
118
Table of Contents
Managing Director of Healthcare at Innovations, where he led the firm’s activities in the healthcare sector. From 2009 to 2011, he was a partner at DFJ Esprit and in that capacity served as a member of our Board of Directors from 2010 to 2011. Before joining DFJ Esprit, Dr. Pitchford spent twelve years, from 1997 to 2009, at 3i Group plc, an international investment company focused on mid-market private equity, infrastructure and debt management, where he led the venture team’s healthcare activities across Europe and the United States. Dr. Pitchford earned his Bachelors degree in Chemistry at Oxford University and his Doctorate in Chemistry at the University of Durham. He also has a Masters degree in Business Administration from the Warwick Business School of University of Warwick. Based on his extensive experience as a venture capitalist in the healthcare sector and his knowledge of our company, we believe that Dr. Pitchford is qualified to serve on our Board of Directors.
Michael Steinmetz, Ph.D., Director
Dr. Steinmetz is a Director, a position he has held since 2007. He has been a Managing Director of Clarus Ventures since the firm’s inception in 2005. He has over 24 years of direct industry and investment experience within the healthcare sector, including being a general partner in a healthcare venture capital firm since 1997. From 1986 to 1997, Dr. Steinmetz was an executive at Hoffmann-LaRoche where he held various positions, including vice president of preclinical research and development and global head of biotechnology. From 2001 through 2011, Dr. Steinmetz was a director and member of the remuneration committee of the board of directors of SOBI, a publicly traded, international healthcare company focused on the development of innovative therapies for the treatment of rare diseases. Dr. Steinmetz earned his Bachelors degree in Chemistry from University of Hamburg and his Doctorate in Biochemistry from the University of Munich. Based on his extensive experience as a venture capitalist in the healthcare sector and his knowledge of our company, we believe that Dr. Steinmetz is qualified to serve on our Board of Directors.
Vijay Lathi, Director
Mr. Lathi is a Director, having been appointed in 2009. Mr. Lathi is a Managing Director of New Leaf Venture Partners, L.L.C., or NLV Partners, a company that he helped found in 2005. As Managing Director, Mr. Lathi focuses primarily on the firm’s diagnostics and healthcare information technology investments. Before founding NLV Partners, Mr. Lathi spent several years with the Sprout Group, a venture capitalist affiliate of Credit Suisse, a leading technology investment bank. Before joining the Sprout Group, Mr. Lathi was an analyst in the Healthcare Venture Capital Group at Robertson Stephens & Co. and at Cornerstone Research, a consulting firm focused on financial and economic analysis for business litigation. Mr. Lathi has a Masters degree in Chemical Engineering from Stanford University. Based on his extensive experience as a venture capitalist focused on diagnostics and healthcare information technology investments and his knowledge of our company, we believe that Mr. Lathi is qualified to serve on our Board of Directors.
Herman Rosenman, Director
Mr. Rosenman is a Director, having been appointed in 2013. Mr. Rosenman served as senior vice president, finance and chief financial officer of Gen-Probe Incorporated, a publicly traded molecular diagnostics company, from 2001 through the sale of that company to Hologic, Inc. in
119
Table of Contents
August 2012. From 1997 to 2000, Mr. Rosenman served as president and chief executive officer of Ultra Acquisition Corp., a manufacturer and parts retailer. From 1994 to 1997, he served as president and chief executive officer of RadNet Management, Inc., a healthcare provider. He has also served as chief financial officer of Rexene Corp. and was an audit partner at Coopers & Lybrand (now PricewaterhouseCoopers LLP).
Mr. Rosenman currently serves as a member of the board of directors and audit committee chairman of Vivus, Inc., a publicly traded biopharmaceutical company, Medistem, Inc., a stem cell therapy company, and BioFire Diagnostics, Inc., a molecular diagnostics company, as well as an advisory board member of Scripps Clinics/Green Hospital, a large healthcare provider in San Diego, CA. Previously, he has served on numerous public and private company boards of directors, frequently as a member of the company’s audit committee, including ARYx Therapeutics, Infinity Pharmaceuticals, Inc., Emphasys Medical, Inc. and Discovery Partners International, Inc. Mr. Rosenman is a certified public accountant who received a B.B.A. in finance and accounting from Pace University and a Masters in Business Administration in finance from the Wharton School of the University of Pennsylvania. Based on his extensive experience as a senior executive and member of the board of directors of numerous companies in the diagnostics and healthcare sectors, as well as his substantial background as a public company chief financial officer and as an auditor and certified public accountant, we believe that Mr. Rosenman is qualified to serve on our Board of Directors.
Composition of our Board of Directors and director independence
Our business and affairs are managed under the direction of our Board of Directors. Our Board of Directors is currently composed of eight directors. Our articles of association will provide that our Board of Directors will be divided into three classes of directors, with the classes as nearly equal in number as possible. Subject to any earlier resignation or removal in accordance with the terms of our articles of association, our Class I directors will serve until the first annual meeting of shareholders following the completion of this offering, our Class II directors will serve until the second annual meeting of shareholders following the completion of this offering and our Class III directors will serve until the third annual meeting of shareholders following the completion of this offering. Upon completion of this offering, we expect that each of our directors will serve in the classes indicated above.
The listing standards of The NASDAQ Stock Market require that, subject to specified exceptions, each member of a listed company’s audit, remuneration and nominating and governance committees be independent. In addition, the listing standards of The NASDAQ Stock Market require that audit committee members satisfy independence criteria set forth in Rule 10A-3 under the Exchange Act and that the remuneration committee members satisfy independence criteria set forth in Rule 5605(d) of The NASDAQ Stock Market rules. The listing standards of The NASDAQ Stock Market further provide that a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Our Board of Directors has determined that all of our directors, except Dr. Wrighton-Smith, are independent directors. In making this determination, our Board of Directors considered the relationships that each such non-employee director has with our company and all other facts and circumstances that our Board of Directors deemed relevant in determining their independence, including beneficial ownership of our ordinary shares. As a result, in accordance with listing standards of The NASDAQ Global Market, a majority of our directors are independent.
120
Table of Contents
Committees of our Board of Directors
Upon the completion of this offering, our Board of Directors will have three standing committees: the audit committee; the remuneration committee; and the nominating and corporate governance committee.
Audit committee
Following this offering, our audit committee will be composed of Messrs. Sandberg, Rosenman and Spotts, with Mr. Rosenman serving as chairman of the committee. Our Board of Directors has determined that each member of the audit committee meets the independence requirements of Rule 10A-3 under the Exchange Act and the applicable listing standards of The NASDAQ Global Market. Our Board of Directors has determined that Mr. Rosenman is an “audit committee financial expert” within the meaning of SEC regulations and applicable listing standards of The NASDAQ Global Market. The audit committee’s responsibilities upon completion of this offering will include:
| • | appointing, approving the compensation of, and assessing the qualifications, performance and independence of our independent registered public accounting firm; |
| • | pre-approving audit and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm; |
| • | reviewing the internal audit plan with the independent registered public accounting firm and members of management responsible for preparing our financial statements; |
| • | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures as well as critical accounting policies and practices used by us; |
| • | reviewing the adequacy of our internal control over financial reporting; |
| • | establishing policies and procedures for the receipt and retention of accounting-related complaints and concerns; |
| • | reviewing and discussing with management and our independent registered public accounting firm our audited financial statements to be included in our Annual Report on Form 10-K; |
| • | monitoring our compliance with legal and regulatory requirements as they relate to our financial statements and accounting matters; |
| • | preparing the audit committee report required by the rules of the SEC to be included in our annual proxy statement; |
| • | reviewing and assessing the adequacy of the committee charter and submitting any changes to our Board of Directors for approval; |
| • | viewing all related party transactions for potential conflict of interest situations and approving all such transactions; and |
| • | reviewing and discussing with management and our independent registered public accounting firm our earnings releases and scripts. |
121
Table of Contents
Remuneration committee
Following this offering, our remuneration committee will be composed of Messrs. Sandberg and Lathi and Drs. Steinmetz and Pitchford, with Mr. Sandberg serving as chairman of the committee. Our Board of Directors has determined that each member of the remuneration committee is “independent” as defined under the applicable listing standards of The NASDAQ Global Market. The remuneration committee’s responsibilities upon completion of this offering will include:
| • | reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer; |
| • | evaluating the performance of our chief executive officer in light of such corporate goals and objectives and determining and approving the compensation of our chief executive officer; |
| • | reviewing and approving the compensation of our other executive officers; |
| • | appointing, compensating and overseeing the work of any compensation consultant, legal counsel or other advisor retained by the remuneration committee; |
| • | conducting the independence assessment outlined in the rules of The NASDAQ Stock Market with respect to any compensation consultant, legal counsel or other advisor retained by the remuneration committee; |
| • | producing a remuneration committee report on executive compensation as required by the rules of the SEC to be included in our annual proxy statement; |
| • | annually reviewing and reassessing the adequacy of the committee charter in its compliance with the listing requirements of The NASDAQ Global Market; |
| • | reviewing and establishing our overall management compensation philosophy and policy; |
| • | overseeing and administering our compensation and equity-based plans; |
| • | reviewing and approving our policies and procedures for the grant of equity-based awards; and |
| • | reviewing and making recommendations to our Board of Directors with respect to director compensation. |
Nominating and corporate governance committee
Following this offering, our nominating and corporate governance committee will be composed of Messrs. Sandberg, Rosenman and Spotts, with Mr. Sandberg serving as chairman of the committee. Our Board of Directors has determined that each member of the nominating and corporate governance committee is “independent” as defined under the applicable listing standards of The NASDAQ Global Market. The nominating and corporate governance committee’s responsibilities upon completion of this offering will include:
| • | establishing a policy under which shareholders of the Company may recommend a candidate to the nominating and corporate governance committee for consideration for nomination as a Director; |
| • | identifying individuals qualified to become members of our Board of Directors, consistent with criteria approved by our Board of Directors; |
122
Table of Contents
| • | recommending to our Board of Directors the persons to be nominated for election as directors and to each of the committees of our Board of Directors; |
| • | developing and recommending to our Board of Directors a set of corporate governance principles; |
| • | articulating to each director what is expected, including reference to the corporate governance principles and directors’ duties and responsibilities; |
| • | reviewing and recommending to our Board of Directors practices and policies with respect to directors; |
| • | recommending to our Board of Directors qualified individuals to serve as members of the committees of our Board of Directors; |
| • | reviewing and assessing the adequacy of the committee charter and submitting any changes to our Board of Directors for approval; |
| • | overseeing the systems and processes established by the Company to ensure compliance with the Company’s Code of Business Conduct and Ethics; and |
| • | performing an evaluation of the performance of the committee. |
Remuneration committee interlocks and insider participation
None of the members of our remuneration committee has at any time during the prior three years been one of our officers or employees. None of our executive officers currently serves, or in the past fiscal year has served, as a member of our Board of Directors or remuneration committee of any entity that has one or more executive officers serving on our Board of Directors or remuneration committee. For a description of transactions between us and members of our remuneration committee and affiliates of such members, please see “Certain relationships and related party transactions.”
Code of business conduct and ethics
We intend to adopt a written code of business conduct and ethics that will apply to our directors, officers and employees, including our principal executive officer and principal financial officer, prior to the completion of this offering. Following this offering, a current copy of the code will be posted on the investor section of our website, www.oxfordimmunotec.com. We intend to disclose any amendment to the code, or any waivers of its requirements, on our website.
123
Table of Contents
Summary compensation table
The following table summarizes information regarding the compensation awarded to, earned by or paid to Dr. Peter Wrighton-Smith, our Chief Executive Officer, as well as Jeff R. Schroeder, our Chief Commercial Officer, and Richard M. Altieri, our Chief Financial Officer, who are our two most highly compensated other executive officers, during 2012. We refer to these individuals in this prospectus as our named executive officers.
| Name and Principal Position | Year | Salary ($) | Option awards ($)(1) |
Non-equity incentive plan compensation ($)(2) |
All other compensation ($) |
Total ($) | ||||||||||||||||||
|
|
||||||||||||||||||||||||
| Peter Wrighton-Smith, |
2012 | $ | 303,440 | $ | 62,187 | $ | 121,150 | $ | 15,780 | (4) | $ | 502,557 | ||||||||||||
| Chief Executive Officer(3) |
||||||||||||||||||||||||
| Jeff R. Schroeder, |
2012 | 272,726 | 22,262 | 89,999 | — | 384,987 | ||||||||||||||||||
| Chief Commercial Officer |
||||||||||||||||||||||||
| Richard M. Altieri, |
2012 | 225,543 | 30,634 | 97,600 | — | 353,777 | ||||||||||||||||||
| Chief Financial Officer(5) |
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||
| (1) | Amounts shown reflect the grant date fair value of options awarded in 2012 determined in accordance with FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 11, “Share option and equity incentive plans,” to our audited consolidated financial statements included elsewhere in this prospectus. |
| (2) | Includes amounts paid in March or April 2013 under our annual cash bonus program and earned with respect to 2012 performance. See “—2012 incentive compensation” below for more information on these payments. |
| (3) | Compensation paid to Dr. Wrighton-Smith is denominated in Pounds Sterling. For purposes of this table, all amounts have been converted based on the U.S. Dollar/Pound Sterling exchange rate in effect as of December 31, 2012 ($1.61533/£1). |
| (4) | Represents (i) a Company match of voluntary retirement plan contributions made by Dr. Wrighton-Smith in the amount of $15,173 and (ii) Company contributions made to private health care insurance on behalf of Dr. Wrighton-Smith in the amount of $607. |
| (5) | Mr. Altieri commenced employment with us on January 30, 2012. |
2012 incentive compensation
In 2012, our management incentive compensation plan provided our named executive officers with an annual incentive compensation opportunity equal, in each case, to 50% of the executive’s base salary, subject to our achievement of corporate performance goals established by the remuneration committee at the beginning of each year and, for officers other than our Chief Executive Officer, the achievement of qualitative and quantitative individual performance goals established by Dr. Wrighton-Smith at the beginning of each year. The remuneration committee annually establishes the weightings to be given to corporate versus individual goals. Actual incentive payments to our named executive officers in 2012 could have exceeded 50% of the executive’s base salary to the extent that corporate and individual performance were determined to have exceeded 100% of the established goals.
For 2012, our corporate goals included the achievement of certain revenue levels for the United States and outside the United States, gross margin targets, product development milestones and deadlines for full migration of our U.S. laboratory operations to Memphis, Tennessee. For 2012, our corporate goals were achieved at 80%. The following was our determination of individual goal attainment in 2012: Mr. Schroeder, 62.5%; and Mr. Altieri, 80%. The actual award amounts were calculated by weighing corporate goal attainment and individual goal attainment for each named executive officer as follows: Dr. Wrighton-Smith, 100% corporate goals; Mr. Schroeder, 20% corporate goals/80% individual goals; and Mr. Altieri, 20% corporate goals/80% individual goals.
124
Table of Contents
For a discussion of the program under which these bonuses were granted, see “—Equity and incentive plans—Management Incentive Compensation Plan” below.
Outstanding equity awards at fiscal year-end
The following table sets forth information regarding equity awards held by our named executive officers as of December 31, 2012. All options are options to purchase ordinary shares.
OPTION AWARDS
| Name | Vesting start date |
Number of securities underlying exercisable options (#) |
Equity incentive plan awards: number of securities underlying unexercisable options (#) (1) |
Option exercise price ($)(2) |
Option expiration date |
|||||||||||||||
|
|
||||||||||||||||||||
| Peter Wrighton-Smith |
|
3-19-10 1-1-13 |
|
|
764,158 — |
(3)
|
|
611,218 1,292,963 |
(3) (4) |
$
|
0.013 0.12 |
|
|
3-18-20 11-6-22 |
| |||||
| Jeff R. Schroeder |
|
3-19-10 3-19-10 3-19-10 4-1-10 1-1-12 1-1-13 |
|
|
92,416 150,000 150,000 419,239 19,182 — |
(5) (6) (7) (4) (4)
|
|
— — — 209,721 64,535 448,884 |
(4) (4) (4) |
|
0.013 0.013 0.013 0.013 0.026 0.12 |
|
|
2-11-17 10-23-17 5-11-18 2-17-20 12-31-21 11-6-22 |
| |||||
| Richard M. Altieri |
|
4-1-12 1-1-13 |
|
|
— — |
|
|
1,021,376 418,098 |
(8) (8) |
|
0.026 0.12 |
|
|
2-2-22 11-6-22 |
| |||||
|
|
||||||||||||||||||||
| (1) | Vesting of all options is subject to continued service through the applicable vesting date. |
| (2) | The exercise price of the options is not less than the fair market value of our ordinary shares, as determined by our Board of Directors using an independent third-party valuation. See “Management’s discussion and analysis of financial condition and results of operations—Valuation of share options.” |
| (3) | This option was partially vested on the date of grant and continued to vest as to the balance in 36 equal monthly installments commencing on April 1, 2011. |
| (4) | This option vests in 48 equal monthly installments for each full month of service after the vesting start date. |
| (5) | This option was issued in connection with an option exchange offer that occurred on March 19, 2010. The option was partially vested on the date of grant and continued to vest as to the balance in equal monthly installments based on a 48-month vesting schedule that originally commenced, prior to the exchange offer, on February 12, 2007. |
| (6) | This option was issued in connection with an option exchange offer that occurred on March 19, 2010. The option was partially vested on the date of grant and continued to vest as to the balance in equal monthly installments based on a 48-month vesting schedule that originally commenced, prior to the exchange offer, on October 24, 2007. |
| (7) | This option was issued in connection with an option exchange offer that occurred on March 19, 2010. The option was fully vested on the date of grant. |
| (8) | Mr. Altieri’s outstanding options are subject to a service requirement before any portion of the option will vest. Assuming Mr. Altieri remains employed, this service requirement will be satisfied on April 1, 2014, at which time the option will vest in 48 equal monthly installments, with vesting retroactive to the vesting start date. |
125
Table of Contents
Agreements with our named executive officers
Peter Wrighton-Smith, Ph.D.
We entered into a service agreement with Dr. Wrighton-Smith dated October 21, 2002 that, together with an amendment dated October 17, 2007, sets forth the terms and conditions under which Dr. Wrighton-Smith serves as our Chief Executive Officer. The agreement has no specific term. Dr. Wrighton-Smith’s current annual base salary is £200,000, or approximately $309,360.
On November 7, 2012, Dr. Wrighton-Smith received an option award covering 1,292,963 of our shares with an exercise price of $0.12 per share, the then fair market value of our shares. These options vest ratably over four years beginning February 1, 2013, subject to continued employment.
Dr. Wrighton-Smith’s option awards provide that all unvested options will accelerate and fully vest immediately prior to a change of control event. For purposes of these option awards, a change of control event will be deemed to occur if upon the purchase of substantially all of our outstanding shares by, or the sale of substantially all of our assets to, a third party.
Both we and Dr. Wrighton-Smith must give a minimum of 12 months’ prior notice to terminate his employment, other than for cause (as defined in his service agreement). We have the right to place Dr. Wrighton-Smith on paid leave rather than allowing him to continue to provide services during this notice period. If we elect to place him on leave, the period of leave would be counted as part of the post-employment non-competition period. Dr. Wrighton-Smith is obligated to refrain from competition with us for 12 months after his termination, unless that period is shortened by a period of leave.
Although not specified in his service agreement, as discussed above, Dr. Wrighton-Smith is eligible for an annual cash incentive opportunity equal to 50% of his base salary, subject to achievement of corporate and individual performance goals established by the remuneration committee at the beginning of each year.
Jeff R. Schroeder
We entered into an amended and restated employment agreement with Mr. Schroeder dated October 1, 2013 that sets forth the terms and conditions of Mr. Schroeder’s employment as our Chief Commercial Officer. Mr. Schroeder’s employment was previously governed by an employment agreement dated February 12, 2007. The agreement has no specific term and establishes an at-will employment relationship. Mr. Schroeder’s current annual base salary is $276,816.
On February 29, 2012, Mr. Schroeder received an option award covering 83,717 of our shares with an exercise price of $0.026 per share, the then fair market value of our shares. These options vest ratably over four years beginning February 1, 2012, subject to continued employment. On November 7, 2012, Mr. Schroeder received an option covering 448,884 of our shares with an exercise price of $0.12 per share, the then fair market value of our shares. These options vest ratably over four years beginning February 1, 2013, subject to continued employment.
Mr. Schroeder’s option awards provide that, upon a change of control event, the portion of the option that would have vested in the succeeding twelve months will accelerate and vest immediately prior to the change of control event. For purposes of these option awards, a change
126
Table of Contents
of control event will generally be deemed to occur upon the purchase of substantially all of our outstanding shares by, or the sale of substantially all of our assets to, a third party.
We may terminate Mr. Schroeder’s employment with or without cause and without notice, but Mr. Schroeder is required to provide at least one month’s advance notice to us if he is terminating his employment. If we terminate Mr. Schroeder’s employment other than for cause (as defined in his employment agreement) or Mr. Schroeder terminates his employment for good reason (as defined in his employment agreement), he will be entitled to receive, subject to certain conditions, severance equal to nine months of his then current base salary, payable as salary continuation. The severance amount will be reduced by any other compensation Mr. Schroeder earns from employment or self-employment during the severance period. Mr. Schroeder’s right to receive any severance payment is conditioned on, among other things, providing us with a signed release, complying with all post-employment obligations and restrictions, including non-competition restrictions, and diligently pursuing alternate employment. Mr. Schroeder is obligated to refrain from engaging in competition with us for a period of one year after any termination.
Although not specified in his employment agreement, as discussed above, Mr. Schroeder is eligible for an annual cash incentive opportunity equal to 50% of his base salary, subject to achievement of corporate performance goals established by the remuneration committee as well as individual performance goals established by Dr. Wrighton-Smith at the beginning of each year.
Richard M. Altieri
We entered into an amended and restated employment agreement with Mr. Altieri dated October 1, 2013 that sets forth the terms and conditions under which Mr. Altieri serves as our Chief Financial Officer. Mr. Altieri’s employment was previously governed by an employment agreement dated January 30, 2012. The agreement has no specific term and establishes an at-will employment. Mr. Altieri’s current annual base salary is $255,004.
On February 3, 2012, Mr. Altieri received an option award covering 1,021,376 of our shares with an exercise price of $0.026 per share, the then fair market value of our shares. Except as set forth below, these options vest ratably over four years beginning May 1, 2012. On November 7, 2012, Mr. Altieri received an option award covering 418,098 of our shares with an exercise price of $0.12 per share, the then fair market value of our shares. Except as set forth below, these options vest ratably over four years beginning February 1, 2013.
No options granted to Mr. Altieri will vest and become exercisable until he has completed 24 months of service with us. As a result, his options will become partially vested, based on the above-described vesting schedule, as of April 1, 2014, if Mr. Altieri remains employed by us through that date, and thereafter will continue to further vest monthly over the balance of the four years from the vesting start dates set forth above, subject to continued employment.
Mr. Altieri’s option awards provide that, upon a change of control event, the portion of the option that would have vested in the succeeding twelve months will accelerate and vest immediately prior to the change of control event. Also, if a change of control event occurs prior to April 1, 2014, the service requirement described above will be automatically waived. For purposes of these option awards, a change of control event will generally be deemed to occur upon the purchase of substantially all of our outstanding shares by, or the sale of substantially all of our assets to, a third party.
127
Table of Contents
We may terminate Mr. Altieri’s employment with or without cause and without notice, but Mr. Altieri is required to provide at least one month’s advance notice to us if he is terminating his employment. If we terminate Mr. Altieri’s employment other than for cause (as defined in his employment agreement), or Mr. Altieri terminates his employment for good reason (as defined in his employment agreement), he will be entitled to receive, subject to certain conditions, severance equal to nine months of his then current base salary, payable as salary continuation. The severance amount will be reduced by any other compensation Mr. Altieri earns from employment or self-employment during the severance period. Mr. Altieri’s right to receive any severance payment is conditioned on, among other things, providing us with a signed release, complying with all post-employment obligations and restrictions, including non-competition restrictions, and diligently pursuing alternate employment. Mr. Altieri is obligated to refrain from engaging in competition with us for a period of one year after any termination.
Although not specified in his employment agreement, as discussed above, Mr. Altieri is eligible for an annual cash incentive opportunity equal to 50% of his base salary, subject to achievement of corporate performance goals established by the remuneration committee as well as individual performance goals established by Dr. Wrighton-Smith at the beginning of each year.
Each of the named executive officers is within the class of persons who may receive benefits under specific circumstances under our Incentive Bonus Plan for Holders of Company Share Options, or the Incentive Bonus Plan. See “—Equity and incentive plans—Incentive Bonus Plan for Holders of Company Share Options.” In addition, we maintain a directors’ and officers’ liability insurance policy for the benefit of our named executive officers and our directors.
Director compensation
We do not pay any director compensation to Dr. Wrighton-Smith, as he is compensated as an employee of our company. We also pay no director compensation to directors who are affiliated with one or more of the investment funds that hold significant share ownership positions in our company, including Mr. Lathi and Drs. Pitchford and Steinmetz, as well as Drs. Gee and Strohmenger, whose resignations will become effective immediately prior to the effectiveness of this registration statement, and Mr. Alan Duncan, who resigned from our Board of Directors in June 2013.
We have three unaffiliated directors: Mr. Sandberg, who serves as Chairman of our Board of Directors, and Messrs. Spotts and Rosenman. As further described below, each of Messrs. Sandberg and Spotts currently has a service agreement with us, providing for quarterly payments of cash compensation. In addition, each of them has received option awards. No cash compensation or option awards have been or will be provided to Mr. Rosenman except pursuant to our newly adopted non-executive director compensation policy. We reimburse all directors for out-of-pocket costs incurred in their performance of service as directors in accordance with our standard expense reimbursement policies and procedures.
In October 2013, our Board of Directors established a policy with respect to the compensation of our non-employee directors who are not affiliated with significant shareholders, or unaffiliated directors. For purposes of the director compensation policy, a director is considered affiliated with a significant shareholder if he or she is an employee, officer, director, manager, managing member or general partner of a shareholder of our company that holds, at the time of this offering or at the time of initial election or appointment in the future, 5% or more of our outstanding capital stock or an employee, officer, director, manager, managing member or general partner of an entity that is an affiliate of such a shareholder.
128
Table of Contents
Our compensation policy for unaffiliated directors will become effective immediately prior to the consummation of this offering. The cancellation of existing service agreements with Messrs. Sandberg and Spotts will become effective at that same time and all amounts accrued as of the date of termination will be paid promptly thereafter. Our new unaffiliated director compensation policy provides for cash payments to be made quarterly on or before the last day of each calendar quarter for service during that quarter, with payments prorated for any partial service quarter. The policy also provides for awards of share options pursuant to our Share Incentive Plan.
Cash compensation for unaffiliated directors is divided into several components: (1) an annual cash retainer in the amount of $35,000, (2) an additional annual retainer for service on any committee of our Board of Directors in the amount of $7,500 for audit committee service, $6,250 for remuneration committee service and $5,000 for nominating and corporate governance committee service (other than for service as chair of any such committee), (3) an additional annual retainer for service as chair of any committee of our Board of Directors in the amount of $15,000 for each of the audit and remuneration committees and $10,000 for the nominating and corporate governance committee and (4) an additional annual retainer for service as chairman of our Board of Directors in the amount of $65,000. The chairman of our Board of Directors is not eligible for any additional cash compensation for service on any committee or for service as chair of any committee.
Equity compensation for unaffiliated directors is divided into two components: (1) an initial option award granted upon first appointment or election to our Board of Directors and covering of our ordinary shares, with the right to exercise vesting in three equal installments on the date of the three succeeding annual shareholders meetings and (2) an annual option award granted to each newly elected or appointed unaffiliated director upon the date of first election or appointment and to each continuing unaffiliated director at the time of our annual shareholders’ meeting. The annual option award will cover of our ordinary shares, except if initial election or appointment is not at a regular annual shareholders meeting and is made less than six months before such meeting will be held, in which case the award will cover shares. In each case, the right to exercise will vest on the date of the next succeeding annual meeting of shareholders; provided that both the initial option and the annual option will accelerate and become immediately exercisable upon a change of control of our company. The exercise price of all options granted to unaffiliated directors will be the fair market value of the shares on the date of grant. Although Mr. Rosenman has already been appointed as a director, for purposes of equity compensation awards to be made under this policy, he will be treated as if his appointment as a director occurred on the day this offering becomes effective.
The following table sets forth information concerning the compensation paid or accrued for service rendered by Messrs. Sandberg and Spotts for the year ended December 31, 2012. For compensation paid or accrued for services rendered to us by Dr. Wrighton-Smith in his role as Chief Executive Officer, please refer to “—Summary compensation table.”
| Name | Fees earned or paid in cash ($) |
Option awards ($) |
Total ($) |
|||||||||
|
|
||||||||||||
| Richard A. Sandberg |
$ | 100,000 | (1) | $ | 4,471 | (2)(3)(4) | $ | 104,471 | ||||
| Stephen L. Spotts |
25,000 | (1) | — | 25,000 | ||||||||
|
|
||||||||||||
| (1) | Represents quarterly cash payments paid to the director pursuant to his service agreement with the Company, as described below. |
129
Table of Contents
| (2) | Amounts shown reflect the grant date fair value of options awarded in during 2012 determined in accordance with FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 11, “Share option and equity incentive plans,” to our audited consolidated financial statements included elsewhere in this prospectus. |
| (3) | In November 2012, we granted Mr. Sandberg an option to acquire 93,853 shares at an exercise price of $0.12 per share, the then fair market value of our shares. The option vests ratably over four years beginning February 1, 2013, subject to continued service. In June 2013, Mr. Sandberg voluntarily surrendered this option with respect to 28,151 shares. |
| (4) | As of December 31, 2012, Mr. Sandberg held options to acquire 421,118 shares and Mr. Spotts held options to acquire 170,048 shares. |
For more information regarding the equity compensation of our non-executive directors, please refer to “—Equity and incentive plans—Amended and Restated 2008 Stock Incentive Plan.”
Director service agreements
Richard A. Sandberg
We entered into an agreement with Mr. Sandberg dated April 1, 2009 that sets forth the terms and conditions under which Mr. Sandberg serves as the non-executive Chairman of our Board of Directors. We pay Mr. Sandberg a quarterly fee of $25,000, payable in arrears, for his service. Neither we nor Mr. Sandberg may terminate the agreement on less than 90 days’ notice, other than for cause (as defined in the agreement) or in circumstances where, as a result of a corporate transaction, our company ceases to exist. Under the agreement, Mr. Sandberg has agreed to certain post-termination restrictive covenants.
Stephen L. Spotts
We entered into an agreement with Mr. Spotts dated November 30, 2010 that sets forth the terms and conditions under which Mr. Spotts serves as a non-executive director. We pay Mr. Spotts a quarterly fee of $6,250, payable in arrears, for his service. Neither we nor Mr. Spotts may terminate the agreement on less than 30 days’ notice, other than for cause (as defined in the agreement) or in circumstances where, as a result of a corporate transaction, our company ceases to exist. Under the agreement, Mr. Spotts has agreed to certain post-termination restrictive covenants.
Prior to this offering, we expect to terminate these service agreements and to compensate Messrs. Sandberg and Spotts for their service in accordance with the director compensation policy described above. Messrs. Sandberg and Spotts are within the class of persons who may receive benefits under specific circumstances under our Incentive Bonus Plan. This plan will terminate upon completion of this offering. See “Equity and incentive plans—Incentive Bonus Plan for Holders of Company Share Options.”
Equity and incentive plans
Amended and Restated 2008 Stock Incentive Plan
We have adopted the 2008 Plan to enhance our ability to attract, retain and motivate persons expected to make important contributions to our company by providing such persons with equity ownership opportunities and performance-based incentives. All of our employees, officers, directors, consultants and advisors are eligible to be granted options, restricted stock, RSUs and other stock-based awards under the 2008 Plan. The 2008 Plan is administered by our Board of Directors. Option grants are subject to approval of the remuneration committee. Subject to adjustments, as described below, the 2008 Plan limits the number of awards that may be granted to 14.6% of the fully diluted share capital of our company.
130
Table of Contents
The Chief Executive Officer has discretion within pre-agreed limits to recommend and, subject to confirmation from our Board of Directors, approve new hire and performance option grants. All option grants are reviewed regularly by the remuneration committee to ensure consistency with our policies and objectives.
The 2008 Plan permits grants of incentive stock options, as defined in Section 422 of the Code, for U.S.-based employees and enterprise management incentive options, or EMI options, under the terms of Schedule 5 to the U.K. Income Tax (Earnings and Pensions) Act 2003 for U.K.-based employees. No more than 7,000,000 shares under the 2008 Plan may be used for ISOs, subject to certain adjustments as a result of changes in our capitalization, as described below. Although the 2008 Plan allows for grants of RSUs and other stock-based awards, no such awards have been granted to date under the 2008 Plan.
Each option grant is documented through an option agreement. The exercise price per share of all options must be equal to at least 100% of the fair market value per share of our ordinary shares on the date of grant. Generally, employee option awards vest ratably over the course of four years; however, the vesting percentage remains 0% until the second anniversary of the vesting start date of the employee’s first option award. Except for certain nonstatutory options to executive directors, options must be exercised during the term of employment or service to our company or within 40 days of termination of employment or service to our company (or within one year in the case of termination on account of a participant’s death). For officers, upon a change of control (as described below), the portion of the option that would have vested in the succeeding twelve months will accelerate and vest immediately prior to the change of control. The maximum term of an option award is ten years. Upon exercise, an optionholder must provide notice of the exercise, pay the amount due upon exercise, and execute a deed of adherence to our amended and restated subscription and shareholders’ agreement and voting undertaking.
Awards are non-transferable and our Board of Directors retains discretion to amend, modify or terminate any outstanding award. Awards may be accelerated to become immediately exercisable in full or in part upon approval of our Board of Directors.
In the event of certain changes in our capitalization, the number of shares available for issuance under the 2008 Plan, as well as the number of ordinary shares covered by each outstanding option and the exercise price per share of each outstanding option may be appropriately adjusted by our Board of Directors. In the event of a change of control, our Board of Directors may terminate unexercised awards prior to the change of control upon advance written notice to a participant, provide substitute awards, make all outstanding awards immediately exercisable in whole or in part, provide for a “cash-out” payment to a participant in respect of an award, convert the award into a right to receive liquidation proceeds or any combination of the foregoing. For this purpose, a change of control generally includes a merger, consolidation or acquisition of all of our shares or share capital, or the sale of substantially all of our assets. The formation of a holding company with substantially the same shareholders and proportionate shareholdings is not considered a change of control.
Following this offering, share-based awards will be granted under the 2013 Share Incentive Plan, the material terms of which are described below. Existing awards, however, will remain outstanding in accordance with their terms and the terms of the 2008 Plan. As of September 30, 2013, options to purchase 8,759,155 ordinary shares were outstanding under the 2008 Plan.
131
Table of Contents
Management Incentive Compensation Plan
We have adopted an incentive compensation plan that applies to our named executive officers to incentivize performance in support of corporate and individual objectives. In 2012, for our named executive officers, other than our Chief Executive Officer, performance goals included both an individual component and a corporate component. For 2013, for each of our named executive officers, including our Chief Executive Officer, performance goals include both an individual component and a corporate component. Each named executive officer is assigned individual goals and objectives on an annual basis by our Chief Executive Officer or, in the case of our Chief Executive Officer, the remuneration committee, against which his performance is measured.
We annually establish corporate goals and objectives that apply to each of our named executive officers, including our Chief Executive Officer, which include revenue and margin targets as well as other significant objectives relating to product and business development activities. The corporate goals for each year are reviewed and approved by the remuneration committee. In connection with an annual performance review, our Chief Executive Officer reviews our corporate performance against the established goals and objectives and recommends a level of attainment of these goals, expressed as a percentage. The Chief Executive Officer’s recommendation is reviewed by the remuneration committee and, if it is approved by the committee, is used as the level of achievement of corporate performance in calculating each executive officer’s incentive compensation payout.
The weightings of the individual and corporate components of incentive compensation for executive officers is annually approved by the remuneration committee. In 2012, 80% of the incentive compensation opportunity under the plan depended upon individual performance; the remaining 20% depended upon corporate performance, except for the incentive compensation opportunity for our Chief Executive Officer, 100% of which depended upon corporate performance. For 2013, 65% of the incentive compensation opportunity under the plan depends on individual performance; the remaining 35% depends upon corporate performance.
Following this offering, incentive bonuses will be granted under the Incentive Plan, the material terms of which are described below. This new bonus plan will replace our current incentive compensation plan for executive officers, as described above.
Incentive Bonus Plan for Holders of Company Share Options
We have established the Incentive Bonus Plan to establish a retention bonus pool to provide incentives for employees, including each of our named executive officers and, if selected, certain non-employee directors, to continue in our service through the closing of certain major corporate transactions. Generally, participants in the Incentive Bonus Plan will receive an amount equal to 11% of the value of the “total consideration” in a corporate transaction (defined as the amount available to shareholders and participants after payment of all expenses relating to the corporate transaction) as a result of (a) their holdings of ordinary shares acquired as a result of the exercise of their options or otherwise pursuant to one of our equity incentive plans taken together with (b) the payments to be made pursuant to the Incentive Bonus Plan. The 11% value is subject to downward adjustment for additional equity capital raises following the completion of the G preferred ordinary share financing. By its terms, the Incentive Bonus Plan will automatically terminate upon completion of this offering.
132
Table of Contents
2013 Share Incentive Plan
In connection with this offering, our Board of Directors adopted the Oxford Immunotec Global PLC 2013 Share Incentive Plan, or the Share Incentive Plan, and, following this offering, all equity-based awards will be granted under the Share Incentive Plan. As of the date of this prospectus, no awards have been made under the Share Incentive Plan. The following summary describes the material terms of the Share Incentive Plan. This summary of the Share Incentive Plan is not a complete description of all provisions of the Share Incentive Plan and is qualified in its entirety by reference to the Share Incentive Plan, which is filed as an exhibit to the registration statement of which this prospectus is a part.
The Share Incentive Plan is administered by our remuneration committee. Our remuneration committee has the authority to, among other things, interpret the Share Incentive Plan, determine eligibility for, grant and determine the terms of awards under the Share Incentive Plan, and do all things necessary or appropriate to carry out the purposes of the Share Incentive Plan. Our remuneration committee’s determinations under the Share Incentive Plan are conclusive and binding. Our key employees, directors, consultants and advisors are eligible to participate in the Share Incentive Plan.
Subject to adjustment, as described below, the maximum number of our shares that may be delivered in satisfaction of awards under the Share Incentive Plan is . The number of our shares available for issuance under the Share Incentive Plan will be automatically increased annually on each January 1st, from January 1, 2015 through January 1, 2023, in an amount equal to 4.0% of our outstanding shares as of the close of business on the immediately preceding December 31st, unless our Board of Directors acts to stop any increase or to substitute a smaller increase.
Each of our shares subject to an option award or share appreciation right, or SAR, will be counted against the maximum number of our shares available under the Share Incentive Plan as one share and each of our shares subject to an award other than an option or SAR will be counted against such maximum number as 1.5 shares. The shares to be issued under the Share Incentive Plan may be our authorized but unissued shares or our treasury shares. Any of our shares underlying awards that are settled in cash, forfeited, repurchased by us, cancelled or terminated, or that otherwise lapse or expire, will again be available for issuance under the Share Incentive Plan.
The maximum number of our shares that may be issued in satisfaction of incentive stock options, or ISOs, under the Share Incentive Plan is . The maximum number of our shares subject to options and the maximum number of our shares subject to SARs that may be granted to any U.S. participant in the Share Incentive Plan in any calendar year is . The maximum number of our shares subject to other awards that may be granted to any U.S. participant in the Share Incentive Plan in any calendar year is shares.
The Share Incentive Plan provides for awards of options, SARs, restricted shares, unrestricted shares, share units, performance awards and other awards convertible into or otherwise based on our shares. Certain U.K. participants may be granted approved options under the terms of Schedule 4 to the U.K. Income Tax (Earnings and Pensions) Act 2003. Eligibility for options intended to be ISOs is limited to our U.S. employees. Dividend equivalents may also be provided in connection with an award under the Share Incentive Plan.
| • | Options and SARs. The exercise price of an option, and the base price against which a SAR is to be measured, may not be less than the fair market value (or, in the case of an ISO granted to |
133
Table of Contents
| a ten percent shareholder, 110% of the fair market value) of our shares on the date of grant. Our remuneration committee will determine the time or times at which options or SARs become exercisable and the terms on which such awards remain exercisable. |
| • | Restricted and unrestricted shares. A restricted share award is an award of our shares subject to forfeiture restrictions, while an unrestricted share award is not subject to restrictions. |
| • | Share units. A share unit award is an award denominated in our shares that entitles the participant to receive our shares or cash measured by the value of the shares in the future. The delivery of shares or cash under a share unit may be subject to the satisfaction of performance conditions or other vesting conditions. |
| • | Performance awards. A performance award is an award the vesting, settlement or exercisability of which is subject to specified performance criteria. |
| • | Other awards. Other awards are awards that are convertible into or otherwise based on our shares. |
The Share Incentive Plan provides for the grant of performance awards that are made based upon, and subject to achieving, performance targets. Performance targets with respect to those awards that are intended to qualify as “performance-based compensation” for purposes of Section 162(m) of the Code, or Section 162(m), to the extent applicable, are limited to an objectively determinable measure or measures of performance relating to any or any combination of the following (measured either absolutely or by reference to an index or indices and determined either on a consolidated basis or, as the context permits, on a divisional, subsidiary, line of business, project or geographical basis or in combinations thereof): sales; revenues; assets; expenses; earnings before or after deduction for all or any portion of interest, taxes, depreciation, amortization or equity expense, whether or not on a continuing operations or an aggregate or per share basis; return on equity, investment, capital, capital employed or assets; one or more operating ratios; operating income or profit, including on an after-tax basis; net income; borrowing levels, leverage ratios or credit rating; market share; capital expenditures; cash flow; share price; shareholder return; sales of particular products or services; customer acquisition or retention; acquisitions and divestitures (in whole or in part); joint ventures, strategic alliances, licenses or collaborations; spin-offs, split-ups and the like; reorganizations; recapitalizations, restructurings, financings (issuance of debt or equity) or refinancings; manufacturing or process development; or achievement of clinical trial or research objectives, regulatory or other filings or approvals or other product development milestones.
To the extent consistent with the requirements for satisfying the performance-based compensation exception under Section 162(m), to the extent applicable, our remuneration committee may provide in the case of any award intended to qualify for such exception that one or more of the performance targets applicable to an award will be adjusted in an objectively determinable manner to reflect events (for example, the impact of charges for restructurings, discontinued operations, mergers, acquisitions, extraordinary items, and other unusual or non-recurring items, and the cumulative effects of tax or accounting changes, each as defined by U.S. generally accepted accounting principles) occurring during the performance period that affect the applicable performance target or targets.
Our remuneration committee has the authority to determine the vesting schedule applicable to each award, and to accelerate the vesting or exercisability of any award.
134
Table of Contents
Our remuneration committee will determine the effect of termination of employment or service on an award. Unless otherwise provided by our remuneration committee, upon a termination of a participant’s employment or service, all unvested options and SARs then held by the participant will terminate and all other unvested awards will be forfeited and all vested options and SARs then held by the participant will remain outstanding for three months following such termination, or one year in the case of death, or, in each case, until the applicable expiration date, if earlier. All options and SARs held by a participant immediately prior to the participant’s termination of employment or service will immediately terminate if such termination is for cause, as defined in the Share Incentive Plan, or occurs in circumstances that would have constituted grounds for the participant’s employment or service to be terminated for cause, in the determination of the remuneration committee.
Awards under the Share Incentive Plan may not be transferred except to personal representatives upon death, unless, for awards other than ISOs and approved options, otherwise provided by our remuneration committee.
Our remuneration committee may cancel, rescind, withhold or otherwise limit or restrict any award at any time under the Share Incentive Plan if the participant is not in compliance with the provisions of the Share Incentive Plan or any award thereunder or if the participant breaches any agreement with our company with respect to non-competition, non-solicitation or confidentiality. Our remuneration committee also may recover any award or payments or gain in respect of any award under the Share Incentive Plan in accordance with any applicable company recoupment policy or as otherwise required by applicable law or applicable stock exchange listing standards.
In the event of a consolidation, merger or similar transaction, a sale or transfer of all or substantially all of our assets or our dissolution or liquidation, our remuneration committee may, among other things, provide for continuation or assumption of outstanding awards, for new grants in substitution of outstanding awards, for the accelerated vesting or delivery of shares under awards or for a cash-out of outstanding awards, in each case on such terms and with such restrictions as it deems appropriate. Except as our remuneration committee may otherwise determine, awards not assumed in connection with such a transaction will terminate automatically and, in the case of outstanding restricted shares, will be forfeited automatically upon the consummation of such covered transaction.
In the event of a share dividend, share split or combination of shares, including a reverse share split, recapitalization or other change in our capital structure, our remuneration committee will make appropriate adjustments to the maximum number of shares that may be delivered under, and the individual share limits included in, the Share Incentive Plan, and will also make appropriate adjustments to the number and kind of shares or securities subject to awards, the exercise prices of such awards or any other terms of awards affected by such change. Our remuneration committee will also make the types of adjustments described above to take into account distributions and other events other than those listed above if it determines that such adjustments are appropriate to avoid distortion in the operation of the Share Incentive Plan.
Our remuneration committee will be able to amend the Share Incentive Plan or outstanding awards, or terminate the Share Incentive Plan as to future grants of awards, except that our remuneration committee will not be able to alter the terms of an award if it would affect materially and adversely a participant’s rights under the award without the participant’s consent (unless expressly provided in the Share Incentive Plan or the right to alter the terms of an award
135
Table of Contents
was expressly reserved by our remuneration committee at the time the award was granted). Shareholder approval will be required for any amendment to the Share Incentive Plan to the extent such approval is required by law, including applicable stock exchange requirements.
Oxford Immunotec Global PLC Incentive Plan
In connection with this offering, our Board of Directors has adopted the Oxford Immunotec Global PLC Incentive Plan, or the Incentive Plan. Starting with our 2014 fiscal year, annual award opportunities for executive officers, including our named executive officers, and other key employees will be granted under the Incentive Plan. The following summary describes the material terms of the Incentive Plan. This summary is not a complete description of all provisions of the Incentive Plan and is qualified in its entirety by reference to the Incentive Plan, which is filed as an exhibit to the registration statement of which this prospectus forms a part.
The Incentive Plan will be administered by our remuneration committee. Our remuneration committee has authority to interpret the Incentive Plan and awards granted under it, to determine eligibility for awards and to do all things necessary to administer the Incentive Plan. Any interpretation or decision by the remuneration committee will be final and conclusive on all participants.
Our executive officers and other key employees will be selected from time to time by the remuneration committee to participate in the Incentive Plan.
With respect to each award granted under the Incentive Plan, the remuneration committee will establish the performance criteria applicable to the award, the amount or amounts payable if the performance criteria are achieved, and such other terms and conditions as the remuneration committee deems appropriate. The Incentive Plan permits the grant of awards that are intended to qualify as exempt performance-based compensation under Section 162(m), to the extent applicable, as well as awards that are not intended to so qualify. Any awards that are intended to qualify as performance-based compensation will be administered in accordance with the requirements of Section 162(m).
Awards under the Incentive Plan will be made based on, and subject to achieving, performance criteria established by our remuneration committee. Performance criteria for awards intended to qualify as performance-based compensation for purposes of Section 162(m), to the extent applicable, are limited to the objectively determinable measures of performance relating to any or any combination of the following (measured either absolutely or by reference to an index or indices or the performance of one or more companies and determined either on a consolidated basis or, as the context permits, on a divisional, subsidiary, line of business, project or geographical basis or in combinations thereof): sales; revenues; assets; expenses; earnings before or after deduction for all or any portion of interest, taxes, depreciation, amortization or equity expense, whether or not on a continuing operations or an aggregate or per share basis; return on equity, investment, capital, capital employed or assets; one or more operating ratios; operating income or profit, including on an after-tax basis; net income; borrowing levels, leverage ratios or credit rating; market share; capital expenditures; cash flow; share price; shareholder return; sales of particular products or services; customer acquisition or retention; acquisitions and divestitures (in whole or in part); joint ventures, strategic alliances, licenses or collaborations; spin-offs, split-ups and the like; reorganizations; recapitalizations, restructurings, financings (issuance of debt or equity) or refinancings; manufacturing or process development; or achievement of clinical trial or research objectives, regulatory or other filings or approvals or other product development milestones.
136
Table of Contents
To the extent consistent with the requirements of Section 162(m), to the extent applicable, the remuneration committee may establish that in the case of any award intended to qualify as exempt performance-based compensation under Section 162(m), that one or more of the performance criteria applicable to such award be adjusted in an objectively determinable manner to reflect events (for example, the impact of charges for restructurings, discontinued operations, mergers, acquisitions, extraordinary items, and other unusual or non-recurring items, and the cumulative effects of tax or accounting changes, each as defined by U.S. generally accepted accounting principles) occurring during the performance period of such award that affect the applicable performance criteria.
A participant will be entitled to payment under an award only if all conditions to payment have been satisfied in accordance with the Incentive Plan and the terms of the award. Following the close of the performance period, our remuneration committee will determine (and, to the extent required by Section 162(m), will certify) whether and to what extent the applicable performance criteria have been satisfied. Our remuneration committee will then determine the actual payment, if any, under each award. Our remuneration committee has the sole and absolute discretion to reduce the actual payment to be made under any award. Our remuneration committee will determine the payment dates for awards under the Incentive Plan. Our remuneration committee may provide that all or a portion of an award granted under the Incentive Plan will be paid or settled in our shares or in equity awards based upon our shares, in each case, on terms and conditions specified by our remuneration committee. Any such shares will be delivered from shares reserved for issuance under our equity incentive plan, as it may exist from time to time. Our remuneration committee may permit a participant to defer payment of an award.
The maximum payment to any participant under the Incentive Plan in any fiscal year will in no event exceed $2.5 million.
Awards under the Incentive Plan will be subject to forfeiture, termination and rescission, and a participant who receives a payment pursuant to the Incentive Plan will be obligated to return to us such payment to the extent provided by our remuneration committee in an award agreement, pursuant to our policy relating to the recovery of erroneously-paid incentive compensation or any applicable clawback or recoupment policy, or as otherwise required by law or applicable stock exchange listing standards.
Our remuneration committee may amend the Incentive Plan at any time, provided that any amendment will be approved by our shareholders if required by Section 162(m). Our remuneration committee may terminate the Incentive Plan at any time.
Retirement plans
401(k) plan
We maintain a tax-qualified retirement plan for our U.S.-based employees that provides eligible employees with an opportunity to save for retirement on a tax-advantaged basis. All participants’ interests in their deferrals are 100% vested when contributed. We do not make matching contributions into the 401(k) plan. Pre-tax contributions are allocated to each participant’s individual account and are then invested in selected investment alternatives according to the participant’s directions. The 401(k) plan is intended to qualify under Sections 401(a) and 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions are not taxable to the employees until distributed from the 401(k) plan.
137
Table of Contents
U.K. defined contribution plan
In the United Kingdom, we maintain a defined contribution plan that provides employees with an opportunity to contribute a portion of their monthly salary into the plan. There is a minimum contribution of 5% of their monthly salary and no maximum limit is set. The employee contribution to this plan is matched by us up to a maximum of 5% of monthly salary. All U.K. employees are eligible to contribute to this program. We also offer all our U.K. employees the opportunity to participate in a tax-efficient so-called “salary exchange” program pursuant to which employees may agree to a voluntary reduction in monthly salary in an amount equal to the defined contribution plan election and we make a contribution to the plan in that amount in addition to the 5% matching contribution described above.
138
Table of Contents
Certain relationships and related party transactions
The following is a description of transactions, since January 1, 2010, in which (a) we were a participant, (b) the amount involved exceeded $120,000 and (c) one or more of our executive officers, directors, director nominees or 5% shareholders, or their immediate family members, each of whom we refer to as a “related person,” had a direct or indirect material interest. We refer to these as “related person transactions.”
G preferred ordinary share financing
As of September 30, 2013, we had issued 16,559,756 Series G preferred ordinary shares at a price of $1.70 per share for aggregate consideration of $28 million to certain of our shareholders, including each of the investment funds identified in the section under the heading “Principal shareholders,” whom we refer to collectively as our “institutional investors,” and Mr. Sandberg. The issuance occurred in two tranches; in June 2012, we issued 10,079,933 G preferred ordinary shares and in January 2013, we issued 6,479,823 G preferred ordinary shares.
2012 convertible bridge loan
In February 2012, we entered into a convertible loan facility, pursuant to which we borrowed an aggregate of $4 million from certain of our shareholders, including Mr. Sandberg and each of our institutional investors other than the investment funds associated with Invesco Asset Management Limited, or Invesco, and Imperial Innovations Businesses LLP, or Imperial. In connection with this loan facility, we issued 410,994 ordinary shares and 1,233,027 F preferred ordinary shares to the lenders as payment for a facility fee associated with the loan. As of September 30, 2013, all lenders converted their notes into G preferred ordinary shares in connection with the financing described above.
Amended and restated subscription and shareholders’ agreement
In connection with our G preferred ordinary share financing, in June 2012 we entered into an amended and restated subscription and shareholders’ agreement with certain of our shareholders, including each of our institutional investors as well as Dr. Wrighton-Smith and Mr. Sandberg, with respect to the election of directors, registration rights and certain other matters. This agreement, with the exception of certain lock-up provisions, will terminate in connection with this offering and we will enter into a new registration rights agreement with certain of our shareholders. For more information regarding the new registration rights agreement, please refer to “Description of share capital—General—Registration rights.”
Amended and restated irrevocable voting undertaking
In connection with our G preferred ordinary share financing, in June 2012 we entered into an amended and restated irrevocable voting undertaking with certain of our shareholders, including each of our institutional investors as well as Dr. Wrighton-Smith and Mr. Sandberg, pursuant to which the parties thereto agreed to vote their shares in favor of certain transactions. This agreement will terminate in connection with this offering.
Tranches 2 and 3 of the F preferred ordinary share financing
Beginning in 2009, we consummated a series of financing transactions that involved the issuance of ordinary shares and F preferred ordinary shares for a total of $26 million. The financing occurred in three tranches, with the first tranche closing in July 2009. The second tranche occurred in April 2010 and, in connection therewith, we issued a total of 1,849,568 ordinary
139
Table of Contents
shares and 5,548,704 F preferred ordinary shares at a price per unit (consisting of one-third of an ordinary share and one F preferred ordinary share) of $1.622, for aggregate consideration of $9 million to certain of our shareholders, including each of our institutional investors other than the investment funds associated with Invesco and Imperial. The third tranche occurred in February 2011 and, in connection therewith, we issued 2,055,077 ordinary shares and 6,165,231 F preferred ordinary shares at a price per unit (consisting of one-third of an ordinary share and one F preferred ordinary share) of $1.622, for aggregate consideration of $10 million. In connection with this financing, we entered into a subscription and shareholders’ agreement and an irrevocable voting undertaking with certain of our shareholders, including our institutional investors other than the investment funds associated with Invesco and Imperial. The June 2012 amended and restated subscription and shareholders’ agreement referenced above superseded and replaced in its entirety the 2009 subscription and shareholders’ agreement. The June 2012 irrevocable voting undertaking referenced above superseded and replaced in its entirety the 2009 irrevocable voting undertaking.
Deeds of indemnification
In connection with this offering, we have entered into deeds of indemnification with each of our directors and executive officers. Pursuant to these agreements, we agree to indemnify these individuals to the fullest extent permissible under English law against liabilities arising out of, or in connection with, the actual or purported exercise of, or failure to exercise, any of his or her powers, duties or responsibilities as a director or officer, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. We also agree to use all reasonable endeavors to provide and maintain appropriate directors’ and officers’ liability insurance (including ensuring that premiums are properly paid) for their benefit for so long as any claims may lawfully be brought against them.
Non-executive director appointment letters
In connection with this offering, we have entered into letters of appointment with each of our non-executive directors. These letters set forth the main terms on which each of our non-executive directors serve on our Board of Directors. Continued appointment under the letter is contingent on continued satisfactory performance, renomination by the nominating and corporate governance committee and approval of the Board of Directors, re-election by the shareholders and any relevant statutory provisions and provisions of our articles of association relating to removal of a director.
Related person transactions policy
In connection with this offering, we have adopted a policy with respect to the review, approval and ratification of related party transactions. Under the policy, our audit committee will be responsible for reviewing and approving related person transactions. In the course of its review and approval of related person transactions, our audit committee will consider the relevant facts and circumstances to decide whether to approve such transactions. In particular, our policy will require our audit committee to consider, among other factors it deems appropriate:
| • | the related person’s relationship to us and interest in the transaction; |
| • | the material facts of the proposed transaction, including the proposed aggregate value of the transaction; |
140
Table of Contents
| • | the impact on a director’s independence in the event the related person is a director or an immediate family member of the director; |
| • | the benefits to us of the proposed transaction; |
| • | if applicable, the availability of other sources of comparable products or services; and |
| • | an assessment of whether the proposed transaction is on terms that are comparable to the terms available to an unrelated third party or to employees generally. |
The audit committee may only approve those transactions that are in, or are not inconsistent with, our best interests and those of our shareholders, as the audit committee determines in good faith.
We did not have a written policy regarding the review and approval of related person transactions prior to this offering. Nevertheless, with respect to such transactions, it was our policy for our Board of Directors to consider the nature of and business reason for such transactions, how the terms of such transactions compared to those which might be obtained from unaffiliated third parties and whether such transactions were otherwise fair to and in the best interests of, or not contrary to, our best interests.
141
Table of Contents
The following table sets forth information relating to the beneficial ownership of our ordinary shares as of October 1, 2013, by:
| • | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our outstanding ordinary shares; |
| • | each of our directors; |
| • | each of our named executive officers; and |
| • | all directors and executive officers as a group. |
Beneficial ownership is determined in accordance with SEC rules. In general, under these rules a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise has or shares voting power or investment power with respect to such security. A person is also deemed to be a beneficial owner of a security if that person has the right to acquire beneficial ownership of such security within 60 days. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all ordinary shares held by that person.
The percentage of shares beneficially owned prior to the offering is computed on the basis of 71,112,611 ordinary shares outstanding as of October 1, 2013, which reflects the assumed conversion of all of our outstanding preferred ordinary shares and A ordinary shares into an aggregate of 55,515,263 ordinary shares. The percentage of shares beneficially owned after the offering is computed on the basis of ordinary shares outstanding, which assumes no exercise of the underwriters’ option to purchase additional shares. Ordinary shares that a person has the right to acquire within 60 days of October 1, 2013 are deemed outstanding for purposes of computing the percentage ownership of such person’s holdings, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all directors and executive officers as a group. Unless otherwise indicated below, the address for each beneficial owner listed is c/o Oxford Immunotec Global PLC, 94C Innovation Drive, Milton Park, Abingdon, OX14 4RZ, United Kingdom.
142
Table of Contents
| Name and address of beneficial owner | Number of shares beneficially owned |
Percentage of shares beneficially owned | ||||||||
| Before offering | After offering | |||||||||
|
| ||||||||||
| 5% shareholders: |
||||||||||
| Clarus Lifesciences I, L.P.(1) |
17,292,507 | 24.3% | ||||||||
| New Leaf Ventures II L.P. (2) |
9,236,067 | 13.0% | ||||||||
| Esprit Nominees Limited(3) |
7,184,662 | 10.1% | ||||||||
| Imperial Innovations Businesses LLP(4) |
5,588,235 | 7.9% | ||||||||
| Invesco Asset Management Limited(5) |
5,588,235 | 7.9% | ||||||||
| Wellington Partners Management Limited(6) |
5,440,201 | 7.7% | ||||||||
| Quester Venture Partnership and related funds(7) |
4,125,658 | 5.8% | ||||||||
| Named executive officers and directors: |
||||||||||
| Peter Wrighton-Smith, Ph.D.(8) |
3,338,905 | 4.6% | ||||||||
| Richard M. Altieri |
— | — | ||||||||
| Jeff R. Schroeder(9) |
1,087,635 | 1.5% | ||||||||
| Vijay Lathi(10) |
— | — | ||||||||
| Nigel A. Pitchford, Ph.D.(11) |
— | — | ||||||||
| Herman Rosenman |
— | — | ||||||||
| Richard A. Sandberg(12) |
393,084 | * | ||||||||
| Stephen L. Spotts(13) |
126,294 | * | ||||||||
| Michael Steinmetz, Ph.D.(14) |
— | — | ||||||||
| All executive officers and directors as a group (11 persons) (15) |
5,464,482 | 7.6% | ||||||||
|
| ||||||||||
| * | Represents beneficial ownership of less than one percent of our outstanding ordinary shares. |
| (1) | Consists of 17,292,507 ordinary shares issuable upon conversion of preferred ordinary shares held by Clarus Lifesciences I, L.P., or Clarus. Clarus Ventures I GP, L.P., or the GPLP, as the sole general partner of Clarus, may be deemed to beneficially own certain of the shares held of record by Clarus. The GPLP disclaims beneficial ownership of all shares held of record by Clarus in which the GPLP does not have an actual pecuniary interest. Clarus Ventures I, LLC, or the GPLLC, as the sole general partner of the GPLP, may be deemed to beneficially own certain of the shares held of record by Clarus. The GPLLC disclaims beneficial ownership of all shares held of record by Clarus in which it does not have an actual pecuniary interest. Each of Dr. Steinmetz, a member of our Board of Directors, and Nicholas Galakatos, Dennis Henner, Robert W. Liptak, Nicholas J. Simon and Kurt C. Wheeler, as individual Managing Directors of the GPLLC, may be deemed to beneficially own certain of the shares held of record by Clarus. Each of Messrs. Galakatos, Henner, Liptak, Simon and Wheeler and Dr. Steinmetz disclaims beneficial ownership of all shares held of record by Clarus in which he does not have an actual pecuniary interest. The address of Clarus is 101 Main Street, Suite 1210, Cambridge, MA 02142. |
| (2) | Consists of 9,236,067 ordinary shares issuable upon conversion of preferred ordinary shares held by New Leaf Ventures II, L.P., or NLV-II. New Leaf Venture Management II L.L.C. is the general partner of New Leaf Venture Associates II L.P., which in turn is the general partner of NLV-II. Philippe O. Chambon, Jeani Delagardelle, Ronald M. Hunt, James Niedel, Vijay Lathi, and Liam Ratcliffe, the individual managers of New Leaf Venture Management L.L.C., have the power to vote or dispose of these shares and therefore each of the foregoing individual managers may be deemed to have voting and investment power with respect to such shares. Each of the foregoing individual managers disclaims beneficial ownership of such shares except to the extent of his pecuniary interest therein, if any. Mr. Lathi is a member of our Board of Directors. The address of NLV-II is 7 Times Square, Suite 3502, New York, NY 10036. |
| (3) | Consists of 7,184,662 ordinary shares issuable upon conversion of preferred ordinary shares held by Esprit Nominees Ltd, or the Esprit fund. DFJ Esprit LLP is the Manager of the Esprit fund. Simon Cook, Stuart Chapman, Krishna Visvanathan, Richard Marsh, Brian Caulfield, Scott Sage, Jonathan Freuchet-Sibilia and Graham Redman control DFJ Esprit LLP and therefore may be deemed to beneficially own certain of the shares held of record by the Esprit fund. Each of Messrs. Cook, Chapman, |
143
Table of Contents
| Visvanathan, Marsh, Caulfield, Sage, Freuchet-Sibilia and Redman disclaims beneficial ownership of all shares held of record by the Esprit fund, except to the extent of his pecuniary interest. The address of the Esprit fund is 14 Buckingham Gate, London SW1E 6LB. |
| (4) | Consists of 5,588,235 ordinary shares issuable upon conversion of preferred ordinary shares held by Imperial. Imperial is controlled by its designated members, Imperial Innovations Limited and Imperial Innovations Investments Limited, each of which is a wholly owned subsidiary of Imperial Innovations Group plc, which is publicly traded on the Alternative Investment Market of the London Stock Exchange in the United Kingdom. Imperial Innovations Group plc is controlled by its board of directors, which consists of Russ Cummings, Martin Knight, David Allen, Mark Rowan, Paul Atherton, Stephen Richardson and David Begg. None of Messrs. Cummings, Knight, Allen, Rowan, Atherton, Richardson and Begg has any beneficial ownership of the shares held by Imperial except to the extent of his pecuniary interest (if any) in Imperial. The address of Imperial is 52 Princes Gate, Exhibition Road, London SW7 2PG. |
| (5) | Consists of 5,588,235 ordinary shares issuable upon conversion of preferred ordinary shares held by Invesco. Voting and investment determinations regarding shares held by Invesco are made by its directors. As a result, each of Gregory Armour, Paul Joubert, Martin McLoughlin, Nicholas Mustoe, Graeme Proudfoot, John Rowland, and Ian Trevers may be deemed to beneficially own the shares held by Invesco. Each of Messrs. Armour, Joubert, McLoughlin, Mustoe, Proudfoot, Rowland, and Trevers disclaims beneficial ownership of such shares except to the extent of his pecuniary interest. The address of Invesco is 30 Finsbury Square, London EC2A 1AG. |
| (6) | Consists of (i) 4,580,863 ordinary shares issuable upon conversion of preferred ordinary shares held by Wellington Partners Management Limited, or WPML, on behalf of Wellington Partners Ventures III Life Science Fund L.P., or the Life Science fund, (ii) 355,174 ordinary shares issuable upon conversion of preferred ordinary shares held by WPML on behalf of Wellington Partners Ventures III Life Science Network Fund L.P., or the Life Science Network fund, (iii) 235,294 ordinary shares issuable upon conversion of preferred ordinary shares held by WPML on behalf of RM Beteiligungs-verwaltung GmbH, or the RM investor, and (iv) 268,870 ordinary shares issuable upon conversion of preferred ordinary shares held by WPML on behalf of HR Alpha GmbH, or the HR investor. WPML is the general partner of the Life Science fund and the Life Science Network fund and acts as trustee for the RM investor and the HR investor. The directors of WPML are John Bothman, Graeme Millar, Rolf Christof Dienst, Harald Keller and Ernst Mannheimer. As a result, each of Messrs. Bothman, Millar, Dienst, Keller and Mannheimer may be deemed to beneficially own the shares held by WPML. Each of Messrs. Bothman, Millar, Dienst, Keller and Mannheimer disclaims beneficial ownership of such shares except to the extent of his pecuniary interest. The address of WPML is 11-15 Seaton Place, St. Helier JE4 0QH, British Channel Islands. |
| (7) | Consists of (i) 2,304,905 ordinary shares issuable upon conversion of preferred ordinary shares held by Quester Venture Partnership, or the Quester fund, (ii) 548,580 ordinary shares issuable upon conversion of preferred ordinary shares held by The Isis College Fund No. 1 Limited Partnership, or Isis Fund 1, (iii) 556,324 ordinary shares issuable upon conversion of preferred ordinary shares held by The Isis College Fund No. 2 Limited Partnership, or Isis Fund 2, and (iv) 715,849 ordinary shares issuable upon conversion of preferred ordinary shares held by The Second Isis College Fund Limited Partnership, or Second Isis fund. We refer to these funds collectively as the “Spark funds.” Quester Venture GP Partnership acting by its general partner Quester Venture GP Limited is the general partner of the Quester Fund and SPARK Venture Management Limited is the manager of the Quester Fund, and as such they control the voting and investment decisions of the Quester Fund. Quester Academic GP Limited is the general partner of Isis Fund 1 and SPARK Venture Management Limited is the manager of Isis Fund 1, and as such they control the voting and investment decisions of Isis Fund 1. Quester Academic GP Limited is the general partner of Isis Fund 2 and SPARK Venture Management Limited is the manager of Isis Fund 2, and as such they control the voting and investment decisions of Isis Fund 2. Quester Academic GP Limited is the general partner of Second Isis Fund and SPARK Venture Management Limited is the manager of Second Isis Fund, and as such they control the voting and investment decisions of Second Isis Fund. The directors of Quester Venture GP Limited and Quester Academic GP Limited are Andrew Carruthers, Andrew Betton, Thomas Teichman, Jayesh Patel and Jonathan Gee. The directors of Spark Venture Management Limited are Andrew Carruthers, Andrew Betton, Thomas Teichman, Jayesh Patel, Anthony Duffy and Jonathan Gee. As a result, each of Messrs. Carruthers, Betton, Teichman, Patel and Duffy and Dr. Gee may be deemed to beneficially own the shares held by the Spark funds. Each of Messrs. Carruthers, Betton, Teichman, Patel and Duffy and Dr. Gee disclaims beneficial ownership of such shares except to the extent of his pecuniary interest. Dr. Gee is a member of our Board of Directors. The address of the Spark funds is 33 Glasshouse Street, London W1B 5DG. |
| (8) | Includes 953,675 ordinary shares underlying options exercisable within 60 days. |
| (9) | Includes 1,087,635 ordinary shares underlying options exercisable within 60 days. |
| (10) | Does not include shares held by NLV-II. Mr. Lathi is a manager of New Leaf Venture Management L.L.C. As a result, Mr. Lathi may be deemed to beneficially own the shares held by NLV-II. Mr. Lathi disclaims beneficial ownership of such shares except to the extent of his pecuniary interest. |
| (11) | Does not include shares held by Imperial. Dr. Pitchford is Managing Director, Healthcare Ventures of Imperial Innovations Group plc. Dr. Pitchford disclaims beneficial ownership of such shares except to the extent of his pecuniary interest. |
| (12) | Includes 33,196 ordinary shares underlying options exercisable within 60 days. |
| (13) | Includes 126,294 ordinary shares underlying options exercisable within 60 days. |
| (14) | Does not include shares held by Clarus. Dr. Steinmetz is a Managing Director of GPLLC. As a result, Dr. Steinmetz may be deemed to beneficially own the shares held by Clarus. Dr. Steinmetz disclaims beneficial ownership of such shares except to the extent of his pecuniary interest. |
| (15) | Includes 2,901,364 ordinary shares underlying options exercisable within 60 days. |
144
Table of Contents
General
The following description of our share capital is intended as a summary only and is qualified in its entirety by reference to our articles of association to be in effect at the closing of this offering, which is filed as an exhibit to the registration statement of which this prospectus forms a part, and to the applicable provisions of the U.K. Companies Act 2006.
As of September 30, 2013, we had issued and outstanding:
| • | 15,597,348 of our ordinary shares held by 67 shareholders of record; |
| • | 79,750 of our A ordinary shares that are convertible into 79,750 of our ordinary shares; |
| • | 903,220 of our A preferred ordinary shares that are convertible into 903,220 of our ordinary shares; |
| • | 362,020 of our B preferred ordinary shares that are convertible into 362,020 of our ordinary shares; |
| • | 3,266,885 of our D preferred ordinary shares that are convertible into 3,266,885 of our ordinary shares; |
| • | 17,081,014 of our E preferred ordinary shares that are convertible into 17,081,014 of our ordinary shares; |
| • | 17,262,618 of our F preferred ordinary shares that are convertible into 17,262,618 of our ordinary shares; and |
| • | 16,559,756 of our G preferred ordinary shares that are convertible into 16,559,756 of our ordinary shares. |
As of September 30, 2013, we also had outstanding:
| • | options to purchase 8,759,155 ordinary shares, at a weighted average exercise price of $0.07 per share; and |
| • | warrants to purchase 130,573 of our ordinary shares, at a weighted average exercise price of $0.10 per share. |
Upon consummation of this offering, all of our outstanding preferred ordinary shares and A ordinary shares will automatically convert into an aggregate of 55,515,263 ordinary shares, nominal value £ per share.
Ordinary shares
Dividend rights. Subject to preferences that may apply to preferred ordinary shares outstanding at the time, holders of outstanding ordinary shares will be entitled to receive dividends out of assets legally available at the times and in the amounts as our Board of Directors may determine from time to time. All dividends are declared and paid according to the amounts paid up on the shares in respect of which the dividend is paid. Any dividend unclaimed after a period of 12 years from the date of declaration of such dividend shall be forfeited and shall revert to us. In addition, the payment by our Board of Directors of any unclaimed dividend, interest or other sum payable on or in respect of an ordinary share into a separate account shall not constitute us as a trustee in respect thereof.
145
Table of Contents
Voting rights. Each outstanding ordinary share will be entitled to one vote on all matters submitted to a vote of shareholders. Holders of ordinary shares shall have no cumulative voting rights. None of our shareholders will be entitled to vote at any general meeting or at any separate class meeting in respect of any share unless all calls or other sums payable in respect of that share have been paid. The directors may from time to time make calls on shareholders in respect of any amounts unpaid on their shares, whether in respect of nominal value of the shares or by way of premium. Shareholders are required to pay called amounts on shares subject to receiving at least 14 clear days’ notice specifying the time and place for payment. If a shareholder fails to pay any part of a call, the directors may serve further notice naming another day not being less than 14 clear days from the date of the further notice requiring payment and stating that in the event of non-payment the shares in respect of which the call was made will be liable to be forfeited. Subsequent forfeiture requires a resolution by the directors.
Preemptive rights. There are no rights of preemption under our articles of association in respect of transfers of issued ordinary shares. In certain circumstances, our shareholders may have statutory preemption rights under the Companies Act 2006 in respect of the allotment of new shares as described in “—Differences in corporate law—Preemptive rights.” These statutory pre-emption rights would require us to offer new shares for allotment to existing shareholders on a pro rata basis before allotting them to other persons. In such circumstances, the procedure for the exercise of such statutory pre-emption rights would be set out in the documentation by which such ordinary shares would be offered to our shareholders.
Conversion or redemption rights. Our ordinary shares will be neither convertible nor redeemable.
Liquidation rights. Holders of ordinary shares are entitled to participate in any distribution of assets upon a liquidation after payment of all debts and other liabilities and subject to the prior rights of any holders of preferred ordinary shares then outstanding.
Variation of rights. The rights or privileges attached to any class of shares may (unless otherwise provided by the terms of the issue of the shares of that class) be varied or abrogated by a special resolution passed at a general meeting of the shareholders of that class.
Capital calls. Our Board of Directors has the authority to make calls upon the shareholders in respect of any money unpaid on their shares and each shareholder shall pay to us as required by such notice the amount called on its shares. If a call remains unpaid after it has become due and payable, and the 14 days’ notice provided by our Board of Directors has not been complied with, any share in respect of which such notice was given may be forfeited by a resolution of our Board of Directors. None of our ordinary shares to be sold in this offering will be subject to a capital call.
Transfer of shares. Our share register is maintained by our transfer agent, Computershare Investor Services, Inc. Registration in this share register is determinative of share ownership. A shareholder who holds our shares through The Depository Trust Company, or DTC, is not the holder of record of such shares. Instead, the depositary (for example, Cede & Co., as nominee for DTC) or other nominee is the holder of record of such shares. Accordingly, a transfer of shares from a person who holds such shares through DTC to a person who also holds such shares through DTC will not be registered in our official share register, as the depositary or other nominee will remain the record holder of such shares. The directors may decline to register a transfer of a share that is:
| • | not fully paid or on which we have a lien; |
146
Table of Contents
| • | not lodged duly stamped at our registered office or at such other place as the directors may appoint, except where uncertificated shares are transferred without a written instrument; |
| • | not accompanied by the certificate of the share to which it relates or such other evidence reasonably required by the directors to show the right of the transferor to make the transfer, except where a certificate has not been issued; |
| • | in respect of more than one class of share; or |
| • | in the case of a transfer to joint holders of a share, the number of joint holders to whom the share is to be transferred exceeds four. |
Limitations on ownership. Under U.K. law and our articles of association, there are no limitations on the right of nonresidents of the United Kingdom or owners who are not citizens of the United Kingdom to hold or vote our ordinary shares.
Listing. We have applied for listing of our ordinary shares on The NASDAQ Global Market under the symbol “OXFD.”
Preferred ordinary shares
Our Board of Directors may, from time to time, following an ordinary resolution of the ordinary shareholders granting authority to the directors to allot shares and special resolution of the ordinary shareholders to amend the articles of association (and disapply pre-emption rights, if not already disapplied), direct the issuance of preferred ordinary shares in series and may, at the time of issuance, determine the designations, powers, preferences, privileges, and relative participating, optional or special rights as well as the qualifications, limitations or restrictions thereof, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights of the ordinary shares. Satisfaction of any dividend preferences of outstanding preferred ordinary shares would reduce the amount of funds available for the payment of dividends on ordinary shares. Holders of preferred ordinary shares may be entitled to receive a preference payment in the event of our liquidation before any payment is made to the holders of ordinary shares. Upon consummation of this offering, there will be no preferred ordinary shares outstanding, and we have no present intention to issue any preferred ordinary shares.
Warrants
As of September 30, 2013, we had the following warrants outstanding:
| • | A warrant issued to Square 1 Bank exercisable for 105,882 ordinary shares at an exercise price of $0.12 per share. The warrant contains anti-dilution provisions that will be triggered in the event we issue securities at a price per share less than the exercise price of the warrants. This warrant will expire in May 2023. |
| • | A warrant issued to Comerica Bank exercisable for 24,691 ordinary shares at an exercise price of $0.01 per share. The warrant contains anti-dilution provisions that will be triggered in the event we issue securities at a price per share less than the exercise price of the warrants. This warrant will expire in February 2019. |
Pursuant to each of these warrants, the holders are entitled to registration rights with respect to the underlying ordinary shares under our registration rights agreement. See “—Registration rights.”
147
Table of Contents
Registration rights
In connection with this offering, we have entered into a new registration rights agreement with certain of our shareholders. Under this agreement, following the closing of this offering, the holders of approximately ordinary shares and warrants convertible into ordinary shares have the right to require us to register the offer and sale of their shares, or to include their shares in registration statements we file, in each case as described below.
Demand registration rights. At any time beginning six months after the date of this prospectus, the holders of at least a majority of the registrable shares have the right to demand that we use our best efforts to file a registration statement covering at least $10.0 million of registrable shares. We are only obligated to file up to two registration statements in connection with the exercise of demand registration rights and we are not obligated to effect any demand registration if we have effected another demand registration within the preceding 12 months. These registration rights are subject to specified conditions and limitations, including the right of the underwriters to limit the number of shares included in any such registration under certain circumstances and our ability to defer the filing of a registration statement with respect to an exercise of such demand registration rights for up to 60 days under certain circumstances.
Form S-3 registration rights. At any time after we qualify to file a registration statement on Form S-3, any holder of registrable shares has the right to demand that we use our commercially reasonable efforts to file a registration statement on Form S-3 covering at least $5.0 million of registrable shares. We are not obligated to file more than two such registration statements in any 12-month period. These registration rights are subject to specified conditions and limitations, including our ability to defer the filing of a registration statement with respect to an exercise of such Form S-3 registration rights for up to 60 days under certain circumstances.
Piggyback registration rights. Following this offering, if we propose to register the offer and sale of any of our securities under the Securities Act either for our own account or for the account of other shareholders, a holder of registrable shares will have the right, subject to certain exceptions, to require us to use our best efforts to include its ordinary shares in the registration statement.
Expenses of registration. We will pay all expenses relating to any demand registrations and Form S-3 registrations, other than underwriting discounts and commissions.
Termination. The registration rights terminate upon the earlier of (1) the date that is seven years after the closing of this offering and (2) as to a particular holder, when such holder can sell all of its shares pursuant to Rule 144 or another similar exemption.
Articles of association and U.K. law considerations
Directors
Number. Unless and until we, in a general meeting of our shareholders, otherwise determine, the number of directors shall not be more than ten and shall not be less than two.
Borrowing powers. Under our directors’ general power to manage our business, our directors may exercise all the powers of the Company to borrow money and to mortgage or charge our undertaking, property and uncalled capital or parts thereof and to issue debentures and other securities whether outright or as collateral security for any debt, liability or obligation of the Company or of any third party.
148
Table of Contents
Directors’ interests and restrictions. The following discussion should be read in conjunction with the “Certain relationships and related party transactions” section located elsewhere in this prospectus.
(a) Our Board of Directors may, in accordance with our articles of association and the requirements of the Companies Act 2006, authorize a matter proposed to us that would, if not authorized, involve a breach by a director of his duty under section 175 of the Companies Act 2006 to avoid a situation in which he or she has, or can have, a direct or indirect interest that conflicts, or possibly may conflict, with our interests. A director is not required, by reason of being a director, to account to the Company for any remuneration or other benefit that he or she derives from a relationship involving a conflict of interest or possible conflict of interest which has been authorized by our Board of Directors.
(b) Provided that he or she has disclosed to the directors the nature and extent of any material interest, a director may be a party to, or otherwise interested in, any transaction, contract or arrangement with us and he or she may be a director or other officer of, or employed by, or a party to any transaction or arrangement with, or otherwise interested in any body corporate promoted by the Company or in which the Company is otherwise interested and that director shall not, by reason of his or her office, be accountable to the Company for any benefit that he or she derives from any such office or employment or from any such transaction or arrangement or from any interest in any such body corporate; and no such transaction or arrangement shall be required to be avoided because of any such interest or benefit.
(c) A director shall not vote at a meeting of the directors in respect of any contract or arrangement or any other proposal whatsoever in which he or she has an interest that (together with any person connected with him or her within the meaning of section 252 of the Companies Act 2006) is to his or her knowledge a material interest, other than (i) an interest in shares or debentures or other securities of the Company, (ii) where permitted by the terms of any authorization of a conflict of interest or by an ordinary resolution, or (iii) in the circumstances set out in paragraph (d) below, and shall not be counted in the quorum at a meeting with respect to any resolution on which he or she is not entitled to vote.
(d) A director shall (in the absence of some material interest other than those indicated below) be entitled to vote (and be counted in the quorum) in respect of any resolution concerning any of the following matters:
(i) the giving of any guarantee, security or indemnity in respect of money lent or obligations incurred by him or her at the request of or for the benefit of us or any of our subsidiaries;
(ii) the giving of any guarantee, security or indemnity in respect of a debt or obligation of ours or any of our subsidiaries for which he or she has assumed responsibility in whole or in part under a guarantee or indemnity or by the giving of security;
(iii) any proposal concerning an offer of shares or debentures or other securities of or by us or any of our subsidiaries for subscription or purchase or exchange in which offer he or she is or will be interested as a participant in the underwriting or sub-underwriting of such offer;
(iv) any proposal concerning any other company in which he or she is interested, directly or indirectly and whether as an officer or shareholder or otherwise, provided that he or she (together with persons connected with him or her) does not to his or her knowledge hold an interest in shares representing one percent or more of the issued shares of any class of such
149
Table of Contents
company (or of any third company through which his or her interest is derived) or of the voting rights available to shareholders of the relevant company;
(v) any proposal concerning the adoption, modification or operation of a pension, superannuation fund or retirement, death or disability benefits scheme or an employees’ share scheme under which he or she may benefit and which relates to our employees and/or directors and does not accord to such director any privilege or benefit not generally accorded to the persons to whom such scheme relates;
(vi) any proposal under which he or she may benefit concerning the giving of indemnities to our directors or other officers which the directors are empowered to give under our articles of association;
(vii) any proposal under which he or she may benefit concerning the purchase, funding and/or maintenance of insurance for any of our directors or other officers that the directors are empowered to purchase, fund or maintain under our articles of association; and
(viii) any proposal under which he or she may benefit concerning the provision to directors of funds to meet expenditures in defending proceedings.
(e) Where proposals are under consideration to appoint two or more directors to offices or employments with us or with any company in which we are interested or to fix or vary the terms of such appointments, such proposals may be divided and considered in relation to each director separately and in such case each of the directors concerned (if not prohibited from voting under paragraph (d)(iv) above) shall be entitled to vote (and be counted in the quorum) in respect of each resolution except that concerning his or her own appointment.
(f) If any question shall arise at any meeting as to the materiality of a director’s interest or as to the entitlement of any director to vote and such question is not resolved by his agreeing voluntarily to abstain from voting, such question shall be referred to the chairman of the meeting (or, where the interest concerns the chairman, to the deputy chairman of the meeting) and his or her ruling in relation to any director shall be final and conclusive except in a case where the nature or extent of the interests of the director concerned have not been disclosed fairly.
Remuneration. The following discussion should be read in conjunction with “Executive compensation—Director compensation” elsewhere in this prospectus.
(a) Each of the directors may (in addition to any amounts payable under paragraph (b) and (c) below or under any other provision of our articles of association) be paid out of the funds of our company such fees as the directors may from time to time determine.
(b) Any director who is appointed to hold any employment or executive office with us or who, at our request, goes or resides abroad for any of our purposes or who otherwise performs services that in the opinion of the directors are outside the scope of his or her ordinary duties may be paid such additional remuneration (whether by way of salary, commission, participation in profits or otherwise) as the directors (or any duly authorized committee of the directors) may determine either in addition to or in lieu of any other remuneration.
(c) Each director may be paid his or her reasonable travelling expenses (including hotel and incidental expenses) of attending and returning from meetings of the directors or committees of the directors or general meetings or any separate meeting of the holders of any class of our
150
Table of Contents
shares or any other meeting that as a director he or she is entitled to attend and shall be paid all expenses properly and reasonably incurred by him or her in the conduct of our company’s business or in the discharge of his or her duties as a director.
Pensions and other benefits. The directors may exercise all the powers of our company to provide benefits, either by the payment of gratuities or pensions or by insurance or in any other manner whether similar to the foregoing or not, for any director or former director, or any person who is or was at any time employed by, or held an executive or other office or place of profit in, our company or any body corporate that is or has been a subsidiary of our company or a predecessor of the business of our company or of any such subsidiary and for the families and persons who are or was a dependent of any such persons and for the purpose of providing any such benefits contribute to any scheme trust or fund or pay any premiums.
Appointment and retirement of directors
(a) The directors shall have power to appoint any person who is willing to act to be a director, either to fill a vacancy or as an additional director so long as the total number of directors shall not exceed ten. Any director so appointed shall retire from office at our annual general meeting following such appointment. Any director so retiring shall be eligible for re-election.
(b) We may by ordinary resolution elect any person who is willing to act as a director either to fill a vacancy or as an addition to the existing directors or to replace a director removed from office under our articles of association so long as the total number of directors does not at any time exceed ten.
(c) At each annual general meeting a minimum number equal to one-third of the number of those directors who are not due to retire at the annual general meeting under sub-paragraph (a) above (referred to for the purposes of this section as “relevant directors”) (or, if their number is not a multiple of three, the number nearest to but not greater than one-third) shall retire from office. Directors retiring under paragraph (e) below shall be counted as part of this minimum number.
(d) The directors to retire by rotation pursuant to paragraph (c) above shall include (so far as necessary to obtain the minimum number required and after taking into account the directors to retire under paragraph (e) below) any relevant director who wishes to retire and not be re-elected. Any further directors to retire shall be those of the other relevant directors who have been longest in office since their last re-election or appointment and so that as between persons who became or were last re-elected directors on the same day, those to retire shall (unless they otherwise agree among themselves) be determined by lot.
(e) In any event, each director shall retire and shall (unless his or her terms of appointment with our company specify otherwise) be eligible for re-election at the annual general meeting held in the third calendar year (or such earlier calendar year as may be specified for this purpose in his terms of appointment with our company) following his last appointment, election or re-election at any general meeting of our company.
151
Table of Contents
(f) At the meeting at which a director retires under any provision of our articles of association, we may by ordinary resolution fill the vacated office by appointing a person to it, and in default the retiring director shall be deemed to have been re-appointed except where:
(i) that director has given notice to us that he or she is unwilling to be elected; or
(ii) at such meeting it is expressly resolved not to fill such vacated office or a resolution for the reappointment of such director shall have been put to the meeting and not passed.
(g) In the event of the vacancy not being filled at such meeting, it may be filled by the directors as a vacancy in accordance with sub-paragraph (a) above.
(h) The retirement of a director pursuant to paragraphs (c), (d) and (e) shall not have effect until the conclusion of the relevant meeting except where a resolution is passed to elect some other person in the place of the retiring director or a resolution for his re-election is put to the meeting and not passed and accordingly a retiring director who is re-elected or deemed to have been re-elected will continue in office without break.
Indemnity of directors. Under our articles of association, each of our directors is entitled to be indemnified by us against all costs, charges, losses, expenses and liabilities incurred by such director or officer in the execution and discharge of his or her duties or in relation to those duties. The Companies Act 2006 renders void an indemnity for a director against any liability attaching to him or her in connection with any negligence, default, breach of duty or breach of trust in relation to the company of which he or she is a director, as described in “—Differences in corporate law—Liability of directors and officers.”
Shareholders meetings
Annual general meetings. Each year, we will hold a general meeting of our shareholders in addition to any other meetings in that year, and will specify the meeting as such in the notice convening it. The annual general meeting will be held at such time and place as the directors may appoint.
Calling of general meetings. The arrangements for the calling of general meetings are described in “—Differences in corporate law—Notice of general meetings.”
Quorum of meetings. No business shall be transacted at any general meeting unless a quorum is present when the meeting proceeds to business, but the absence of a quorum shall not preclude the appointment of a chairman, which appointment shall not be treated as part of the business of a meeting. Two persons present and entitled to vote upon the business to be transacted, each being either a shareholder or a proxy for a shareholder or a duly authorized representative of a corporation which is a shareholder shall be a quorum for all purposes.
Other U.K. law considerations
Mandatory purchases and acquisitions. Pursuant to sections 979 to 991 of the Companies Act 2006, where a takeover offer has been made for us and the offeror has acquired or unconditionally contracted to acquire not less than 90% of the voting rights carried by the shares to which the offer relates, the offeror may give notice to the holder of any shares to which the offer relates that the offeror has not acquired or unconditionally contracted to acquire that it desires to acquire those shares on the same terms as the general offer.
152
Table of Contents
Disclosure of interest in shares. Pursuant to Part 22 of the Companies Act 2006 and our articles of association, we are empowered by notice in writing to require any person whom we know to be, or have reasonable cause to believe to be, interested in our shares, or at any time during the three years immediately preceding the date on which the notice is issued has been so interested, within a reasonable time to disclose to us the details of that person’s interest and (so far as is within such person’s knowledge) details of any other interest that subsists or subsisted in those shares. Under our articles of association, if a person defaults in supplying us with the required details in relation to the shares in question, or Default Shares, a court may order that:
| • | in respect of the Default Shares, the relevant member shall not be entitled to vote or exercise any other right conferred by membership in relation to general meetings; and/or |
| • | where the Default Shares represent at least 0.25% of their class, (a) any dividend or other money payable in respect of the Default Shares shall be retained by us without liability to pay interest, and/or (b) no transfers by the relevant person of shares other than approved transfers may be registered (unless such person is not in default and the transfer does not relate to Default Shares), and/or (c) any shares held by the relevant person in uncertificated form shall be converted into certificated form. |
Purchase of own shares. Under English law, a public limited company may purchase its own shares only out of the distributable profits of the company or the proceeds of a new issue of shares made for the purpose of financing the purchase. A limited company may not purchase its own shares if as a result of the purchase there would no longer be any issued shares of the company other than redeemable shares or shares held as treasury shares. Subject to the foregoing, because The NASDAQ Global Market is not a “recognized investment exchange” under the Companies Act 2006, we may purchase our own fully paid shares only pursuant to a purchase contract authorized by ordinary resolution of the holders of our ordinary shares before the purchase takes place. Any authority will not be effective if any shareholder from whom we propose to purchase shares votes on the resolution and the resolution would not have been passed if such shareholder had not done so. The resolution authorizing the purchase must specify a date, not being later than five years after the passing of the resolution, on which the authority to purchase is to expire.
A share buy-back by us of our ordinary shares will give rise to U.K. stamp duty at the rate of 0.5% of the amount or value of the consideration payable by us, and such stamp duty will be paid by us.
153
Table of Contents
Differences in corporate law
Certain provisions of the Companies Act 2006 differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of certain differences between the provisions of the Companies Act 2006 applicable to us and the Delaware General Corporation Law relating to shareholders’ rights and protections. This summary is not intended to be a complete discussion of the respective rights and it is qualified in its entirety by reference to Delaware law and English law.
| England and Wales | Delaware | |
|
| ||
| Number of directors | ||
| Under the Companies Act 2006, a public limited company must have at least two directors and the number of directors may be fixed by or in the manner provided in a company’s articles of association. Our articles of association provide that the maximum number of directors is ten. |
Under Delaware law, a corporation must have at least one director and the number of directors shall be fixed by or in the manner provided in the bylaws. | |
| Removal of directors |
||
| Under the Companies Act 2006, shareholders may remove a director without cause by an ordinary resolution (which is passed by a simple majority of those voting in person or by proxy at a general meeting) irrespective of any provisions of any service contract the director has with the company, provided that 28 clear days’ notice of the resolution is given to the company and its shareholders and certain other procedural requirements under the Companies Act 2006 are followed (such as allowing the director to make representations against his or her removal either at the meeting or in writing). |
Under Delaware law, unless otherwise provided in the certificate of incorporation, directors may be removed from office, with or without cause, by a majority shareholder vote, though in the case of a corporation whose board is classified, shareholders may effect such removal only for cause. | |
| Vacancies on the Board of Directors |
||
| Under English law, the procedure by which directors (other than a company’s initial directors) are appointed is generally set out in a company’s articles of association, provided that where two or more persons are appointed as directors of a public limited company by resolution of the shareholders, resolutions appointing each director must be voted on individually. |
Under Delaware law, vacancies on a corporation’s board of directors, including those caused by an increase in the number of directors, may be filled by a majority of the remaining directors. | |
| Annual general meeting |
||
| Under the Companies Act 2006, a public limited company must hold an annual general meeting in each six-month period following the company’s annual accounting reference date. |
Under Delaware law, the annual meeting of shareholders shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as provided in the certificate of incorporation or by the bylaws. | |
|
| ||
154
Table of Contents
| England and Wales | Delaware | |
|
|
| |
| General meeting |
||
| Under the Companies Act 2006, a general meeting of the shareholders of a public limited company may be called by the directors.
Shareholders holding at least 5% of the paid-up capital of the company carrying voting rights at general meetings can require the directors to call a general meeting. |
Under Delaware law, special meetings of the shareholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. | |
| Notice of general meetings | ||
| Under the Companies Act 2006, 21 clear days’ notice must be given for an annual general meeting and any resolutions to be proposed at the meeting. Subject to a company’s articles of association providing for a longer period, at least 14 clear days’ notice is required for any other general meeting. In addition, certain matters (such as the removal of directors or auditors) require special notice, which is 28 clear days’ notice. The shareholders of a company may in all cases consent to a shorter notice period, the proportion of shareholders’ consent required being 100% of those entitled to attend and vote in the case of an annual general meeting and, in the case of any other general meeting, a majority in number of the members having a right to attend and vote at the meeting, being a majority who together hold not less than 95% in nominal value of the shares giving a right to attend and vote at the meeting. |
Under Delaware law, unless otherwise provided in the certificate of incorporation or bylaws, written notice of any meeting of the shareholders must be given to each shareholder entitled to vote at the meeting not less than ten nor more than 60 days before the date of the meeting and shall specify the place, date, hour and purpose or purposes of the meeting. | |
| Proxy | ||
| Under the Companies Act 2006, at any meeting of shareholders, a shareholder may designate another person to attend, speak and vote at the meeting on their behalf by proxy. |
Under Delaware law, at any meeting of shareholders, a shareholder may designate another person to act for such shareholder by proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period. | |
| Preemptive rights | ||
| Under the Companies Act 2006, equity securities proposed to be allotted for cash must be offered first to the existing equity shareholders in the company in proportion to the respective nominal value of their holdings, unless an exception applies or a special resolution to the contrary has been passed by shareholders in a general meeting or the articles of association provide otherwise, in each case in accordance with the provisions of the Companies Act 2006. |
Under Delaware law, unless otherwise provided in a corporation’s certificate of incorporation, a shareholder does not, by operation of law, possess preemptive rights to subscribe to additional issuances of the corporation’s stock. | |
|
| ||
155
Table of Contents
| England and Wales | Delaware | |
|
| ||
| Liability of directors and officers | ||
| Under the Companies Act 2006, any provision (whether contained in a company’s articles of association or any contract or otherwise) that purports to exempt a director of a company (to any extent) from any liability that would otherwise attach to him in connection with any negligence, default, breach of duty or breach of trust in relation to the company is void. Any provision by which a company directly or indirectly provides an indemnity (to any extent) for a director of the company or of an associated company against any liability attaching to him in connection with any negligence, default, breach of duty or breach of trust in relation to the company of which he is a director is also void except as permitted by the Companies Act 2006, which provides exceptions for a company to (a) purchase and maintain insurance against such liability; (b) provide a “qualifying third party indemnity” (being an indemnity against liability incurred by the director to a person other than the company or an associated company as long as he or she is successful in defending the claim or criminal proceedings); and (c) provide a “qualifying pension scheme indemnity” (being an indemnity against liability incurred in connection with the company’s activities as trustee of an occupational pension plan). |
Under Delaware law, a corporation’s certificate of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its shareholders for damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of a director for:
• any breach of the director’s duty of loyalty to the corporation or its shareholders;
• acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law;
• intentional or negligent payment of unlawful dividends or stock purchases or redemptions; or
• any transaction from which the director derives an improper personal benefit. | |
| Voting rights | ||
| Under English law, unless a poll is demanded by the shareholders of a company or is required by the chairman of the meeting or the company’s articles of association, shareholders shall vote on all resolutions on a show of hands. Under the Companies Act 2006, a poll may be demanded by (a) not fewer than five shareholders having the right to vote on the resolution; (b) any shareholder(s) representing at least 10% of the total voting rights of all the shareholders having the right to vote on the resolution; or (c) any shareholder(s) holding shares in the company conferring a right to vote on the resolution (being shares on which an aggregate sum has been paid up equal to not less than 10% of the total sum paid up on all the shares conferring that right). A company’s articles of association may provide more extensive rights for shareholders to call a poll. Under English law, an ordinary resolution is passed on a show of hands if it is approved by a simple majority (more than 50%) of the votes cast by shareholders present (in person or by proxy) and entitled to vote. If a poll is demanded, an |
Delaware law provides that, unless otherwise provided in the certificate of incorporation, each shareholder is entitled to one vote for each share of capital stock held by such shareholder. | |
|
| ||
156
Table of Contents
| England and Wales | Delaware | |
|
| ||
| ordinary resolution is passed if it is approved by holders representing a simple majority of the total voting rights of shareholders present (in person or by proxy) who (being entitled to vote) vote on the resolution. Special resolutions require the affirmative vote of not less than 75% of the votes cast by shareholders present (in person or by proxy) at the meeting. If a poll is demanded, a special resolution is passed if it is approved by holders representing not less than 75% of the total voting rights of shareholders present (in person or by proxy) who (being entitled to vote) vote on the resolution. | ||
| Shareholder vote on certain transactions | ||
| The Companies Act 2006 provides for schemes of arrangement, which are arrangements or compromises between a company and any class of shareholders or creditors and used in certain types of restructurings, amalgamations, capital reorganizations or takeovers.
These arrangements require: • the approval at a shareholders’ or creditors’ meeting convened by order of the court, of a majority in number of shareholders or creditors representing 75% in value of the capital held by, or debt owed to, the class of shareholders or creditors, or class thereof present and voting, either in person or by proxy; and
• the approval of the court. |
Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or substantially all of a corporation’s assets or dissolution requires:
• the approval of the board of directors;
• and approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per share, a majority of the votes of the outstanding stock of a corporation entitled to vote on the matter. | |
| Standard of conduct for directors | ||
| Under English law, a director owes various statutory and fiduciary duties to the company, including:
• to act in the way he or she considers, in good faith, would be most likely to promote the success of the company for the benefit of its members as a whole;
• to avoid a situation in which he or she has, or can have, a direct or indirect interest that conflicts, or possibly conflicts, with the interests of the company;
• to act in accordance with the company’s constitution and only exercise his or her powers for the purposes for which they are conferred;
|
Delaware law does not contain specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined by the courts of the State of Delaware. In general, directors have a duty to act without self-interest, on a well-informed basis and in a manner they reasonably believe to be in the best interest of the shareholders. | |
|
| ||
157
Table of Contents
| England and Wales | Delaware | |
|
| ||
| • to exercise independent judgment;
• to exercise reasonable care, skill and diligence;
• not to accept benefits from a third party conferred by reason of his or her being a director or doing (or not doing) anything as a director; and
• a duty to declare any interest that he or she has, whether directly or indirectly, in a proposed or existing transaction or arrangement with the company. |
||
| Shareholder litigation | ||
| Under English law, generally, the company, rather than its shareholders, is the proper claimant in an action in respect of a wrong done to the company or where there is an irregularity in the company’s internal management.
Notwithstanding this general position, the Companies Act 2006 provides that (i) a court may allow a shareholder to bring a derivative claim (that is, an action in respect of and on behalf of the company) in respect of a cause of action arising from a director’s negligence, default, breach of duty or breach of trust and (ii) a shareholder may bring a claim for a court order where the company’s affairs have been or are being conducted in a manner that is unfairly prejudicial to some of its shareholders.
|
Under Delaware law, a shareholder may initiate a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must:
• state that the plaintiff was a shareholder at the time of the transaction of which the plaintiff complains or that the plaintiffs shares thereafter devolved on the plaintiff by operation of law; and
• allege with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff’s failure to obtain the action; or
• state the reasons for not making the effort.
Additionally, the plaintiff must remain a shareholder through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware Court of Chancery. | |
|
| ||
158
Table of Contents
U.K. City Code on Takeovers and Mergers
If at the time of a takeover offer the Takeover Panel determines that we have our place of central management and control in the United Kingdom, we would be subject to the Takeover Code, which is issued and administered by the Takeover Panel. The Takeover Code provides a framework within which takeovers of companies subject to it are conducted. In particular, the Takeover Code contains certain rules in respect of mandatory offers. Under Rule 9 of the Takeover Code, if a person:
(a) acquires an interest in our shares which, when taken together with shares in which such person or persons acting in concert with such person are interested, carries 30% or more of the voting rights of our shares; or
(b) who, together with persons acting in concert with such person, is interested in shares that in the aggregate carry not less than 30% and not more than 50% of the voting rights in the company, acquires additional interests in shares that increase the percentage of shares carrying voting rights in which that person is interested,
the acquirer and, depending on the circumstances, its concert parties, would be required (except with the consent of the Takeover Panel) to make a cash offer for our outstanding shares at a price not less than the highest price paid for any interests in the shares by the acquirer or its concert parties during the previous 12 months.
Exchange controls
There are no laws, decrees, regulations or other legislation in the United Kingdom that may affect the import or export of capital, including the availability of cash and cash equivalents for use by us, or that may affect the remittance of dividends, interest, or other payments by us to non-resident holders of our ordinary shares, other than withholding tax requirements. There is no limitation imposed by English law or our articles of association on the right of non-residents to hold or vote shares.
159
Table of Contents
Shares eligible for future sale
Before this offering, there has been no public market for our ordinary shares. As described below, only a limited number of shares currently outstanding will be available for sale immediately after this offering due to contractual and legal restrictions on resale. Nevertheless, future sales of substantial amounts of our ordinary shares, including shares issued upon the exercise of outstanding options or warrants, in the public market after this offering, or the perception that those sales may occur, could cause the prevailing market price for our ordinary shares to fall or impair our ability to raise capital through sales of our equity securities.
Upon the closing of this offering, we will have outstanding ordinary shares, after giving effect to the issuance of ordinary shares in this offering, assuming no exercise by the underwriters of their option to purchase additional shares and no exercise of options or warrants outstanding as of September 30, 2013.
Of the shares that will be outstanding immediately after the closing of this offering, we expect that the shares to be sold in this offering will be freely tradable without restriction under the Securities Act unless purchased by our “affiliates,” as that term is defined in Rule 144. Shares purchased by our affiliates may not be resold except pursuant to an effective registration statement or an exemption from registration, including the safe harbor under Rule 144 described below. In addition, following this offering, ordinary shares issuable pursuant to awards granted under certain of our equity plans that are covered by a registration statement on Form S-8 will be freely tradable in the public market, subject to certain contractual and legal restrictions described below. The remaining ordinary shares outstanding after this offering will be “restricted securities,” as that term is defined in Rule 144, and we expect that substantially all of these restricted securities will be subject to the lock-up agreements described below. These restricted securities may be sold in the public market only if the sale is registered or pursuant to an exemption from registration, such as Rule 144.
Lock-up agreements
We and each of our directors and executive officers and certain shareholders, who collectively own ordinary shares following this offering, have agreed that, without the prior written consent of J.P. Morgan Securities LLC and Piper Jaffray & Co., on behalf of the underwriters, we and they will not, subject to limited exceptions, directly or indirectly sell or dispose of any ordinary shares or any securities convertible into or exchangeable or exercisable for ordinary shares for a period of 180 days after the date of this prospectus, unless extended pursuant to its terms. The lock-up restrictions and specified exceptions are described in more detail in the section under the heading “Underwriting.”
Rule 144
In general, under Rule 144, immediately upon completion of this offering, any person who is not our affiliate and has held its shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell shares without restriction, subject to the availability of current public information about us. In addition, under Rule 144, any person who is not our affiliate and has not been our affiliate at any time during the preceding three months and has held its shares for at least one year, including the holding period of any prior owner
160
Table of Contents
other than one of our affiliates, would be entitled to sell an unlimited number of shares immediately upon the closing of this offering without regard to whether current public information about us is available.
A person who is our affiliate or who was our affiliate at any time during the preceding three months and who has held its shares for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of shares within any three-month period that does not exceed the greater of: (i) 1% of the number of ordinary shares outstanding, which will equal approximately shares immediately after this offering; and (ii) the average weekly trading volume of our ordinary shares on The NASDAQ Global Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
Sales under Rule 144 by our affiliates are also subject to certain manner of sale provisions, notice requirements and to the availability of current public information about us.
Rule 701
In general, under Rule 701 under the Securities Act, beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act, any of our employees, directors, officers, consultants or advisors who acquired ordinary shares from us in connection with a written compensatory stock or option plan or other written agreement in compliance with Rule 701 is entitled to sell such shares in reliance on Rule 144 but without compliance with certain of the requirements contained in Rule 144. Accordingly, subject to any applicable lock-up agreements, beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act, under Rule 701 persons who are not our affiliates may resell those shares without complying with the minimum holding period or public information requirements of Rule 144, and persons who are our affiliates may resell those shares without compliance with Rule 144’s minimum holding period requirements.
Equity incentive plans
Following this offering, we intend to file with the SEC a registration statement on Form S-8 under the Securities Act covering the ordinary shares that are subject to outstanding options and other awards issuable pursuant to our equity incentive plans. Shares covered by such registration statement will be available for sale in the open market following its effective date, subject to certain Rule 144 limitations applicable to affiliates and the terms of lock-up agreements applicable to those shares.
Registration rights
Subject to the lock-up agreements described above, certain holders of our ordinary shares or holders of our warrants convertible into ordinary shares may demand that we register the offer and sale of their shares under the Securities Act or, if we propose to register the offer and sale of any of our securities under the Securities Act either for our own account or for the account of other shareholders, may elect to include their ordinary shares in such registration. Following such registered sales, the shares will be freely tradable without restriction under the Securities Act, unless held by our affiliates. See “Description of share capital—Registration rights.”
161
Table of Contents
Material U.S. federal income tax considerations
The following is a description of material U.S. federal income tax considerations of the acquisition, ownership and disposition of ordinary shares acquired pursuant to this offering by a U.S. Holder, as defined below. This description only applies to ordinary shares held as “capital assets” (generally, property held for investment) and does not address, except as explicitly set forth below, aspects of U.S. federal income taxation that may be applicable to U.S. Holders that are subject to special tax rules, such as:
| • | banks or other financial institutions; |
| • | insurance companies; |
| • | real estate investment trusts; |
| • | regulated investment companies; |
| • | grantor trusts; |
| • | tax-exempt organizations; |
| • | persons that will own ordinary shares through partnerships or other pass-through entities; |
| • | dealers or traders in securities or currencies; |
| • | U.S. Holders that have a functional currency other than the U.S. Dollar; |
| • | certain former citizens and former long-term residents of the United States; |
| • | U.S. Holders that use a mark-to-market method of accounting; |
| • | U.S. Holders that will hold ordinary shares as part of a position in a straddle or as part of a hedging, conversion or integrated transaction for U.S. federal income tax purposes; or |
| • | direct, indirect or constructive owners of 10% or more of our total combined voting power. |
Moreover, this description does not address the 3.8% Medicare contribution tax on net investment income, the U.S. federal estate and gift tax or the alternative minimum tax consequences of the acquisition, ownership, and disposition of ordinary shares. We have not received nor do we expect to seek a ruling from the IRS regarding any matter discussed herein. No assurance can be given that the IRS would not assert, or that a court would not sustain, a position contrary to any of those set forth below. Each prospective investor should consult its own tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of acquiring, owning and disposing of ordinary shares.
This description is based on the Code, U.S. Treasury Regulations promulgated thereunder and administrative and judicial interpretations thereof, each as available and in effect on the date hereof, all of which are subject to change or differing interpretations, possibly with retroactive effect, which could affect the tax considerations described herein.
162
Table of Contents
For purposes of this description, a U.S. Holder is a beneficial owner of ordinary shares who for U.S. federal income tax purposes is:
| • | a citizen or individual resident of the United States; |
| • | a corporation (or any other entity treated as a corporation for U.S. federal income tax purposes) organized in or under the laws of the United States or any State thereof, including the District of Columbia; |
| • | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust (1) that has a valid election in effect to be treated as a U.S. person for U.S. federal income tax purposes or (2)(a) the administration over which a U.S. court can exercise primary supervision and (b) all of the substantial decisions of which one or more U.S. persons have the authority to control. |
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds ordinary shares, the tax treatment of the partnership and a partner in such partnership generally will depend on the status of the partner and the activities of the partnership. Such partner or partnership should consult its own tax advisors as to the U.S. federal income tax consequences of acquiring, owning and disposing of the ordinary shares.
PROSPECTIVE INVESTORS SHOULD CONSULT THEIR OWN TAX ADVISORS WITH REGARD TO THE PARTICULAR TAX CONSEQUENCES APPLICABLE TO THEIR SITUATIONS AS WELL AS THE APPLICATION OF ANY STATE, LOCAL, NON-U.S. OR OTHER TAX LAWS, INCLUDING GIFT AND ESTATE TAX LAWS.
Distributions on ordinary shares
As described in “Dividend policy,” above, we have never paid any distributions on our ordinary shares, and we do not anticipate paying any distributions on our ordinary shares in the foreseeable future. If we were to pay any distributions on our ordinary shares, subject to the considerations in “— Passive foreign investment company considerations,” discussed below, such distributions generally would be taxable to a U.S. Holder as foreign-source dividend income, and would not be eligible for the dividends received deduction allowed to certain corporations. Dividend income generally is taxed as ordinary income. A preferential rate may apply to dividend income paid to U.S. Holders that are individuals (or certain trusts and estates) if we and the ordinary shares meet certain requirements.
Distributions, if any, in excess of our current or accumulated earnings and profits would be treated as a non-taxable return of capital to the extent of a U.S. Holder’s adjusted basis in its ordinary shares and thereafter as capital gain. However, we have not maintained calculations of our earnings and profits in accordance with U.S. federal income tax accounting principles. U.S. Holders should therefore assume that any distribution paid with respect to ordinary shares would constitute ordinary dividend income. U.S. Holders should consult their own tax advisors with respect to the appropriate U.S. federal income tax treatment of any distribution received.
Sale, exchange, or other taxable disposition of ordinary shares
Subject to the considerations in “— Passive foreign investment company considerations,” discussed below, upon the sale, exchange, or other taxable disposition of ordinary shares, a U.S.
163
Table of Contents
Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized on such disposition and the U.S. Holder’s adjusted tax basis in its ordinary shares. Such gain or loss generally will be U.S. source and generally will be treated as long-term capital gain or loss if the U.S. Holder’s holding period in such ordinary shares exceeds one year at the time of such disposition. Long-term capital gains may be taxed at lower rates than ordinary income for certain non-corporate taxpayers. Prospective investors should consult their own tax advisors regarding the U.S. federal income tax treatment of capital gains and capital losses (the deductibility of which is subject to limitations).
Passive foreign investment company considerations
Status as a PFIC
The rules governing PFICs can have adverse tax effects on U.S. Holders. We generally will be classified as a PFIC for U.S. federal income tax purposes if, for any taxable year, either:
| • | 75% or more of our gross income consists of certain types of passive income, or |
| • | the average value (determined on a quarterly basis), of our assets that produce, or are held for the production of, passive income is 50% or more of the value of all of our assets. |
Passive income generally includes dividends, interest, rents and royalties (other than certain rents and royalties derived in the active conduct of a trade or business), annuities and gains from assets that produce passive income. If a non-U.S. corporation owns at least 25% by value of the stock of another corporation, the non-U.S. corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation and as receiving directly its proportionate share of the other corporation’s income.
Additionally, if we are classified as a PFIC in any taxable year with respect to which a U.S. Holder owns ordinary shares, we generally will continue to be treated as a PFIC with respect to such U.S. Holder in all succeeding taxable years, regardless of whether we continue to meet the tests described above, unless the U.S. Holder makes the “deemed sale election” described below.
We do not believe that we are currently a PFIC, and we do not anticipate becoming a PFIC in the foreseeable future. Notwithstanding the foregoing, the determination of whether we are a PFIC depends on the particular facts and circumstances (such as the valuation of our assets, including goodwill and other intangible assets) and also may be affected by the application of the PFIC rules, which are subject to differing interpretations. The fair market value of our assets is expected to depend, in part, upon (a) the market price of our ordinary shares, which is likely to fluctuate, and (b) the composition of our income and assets, which will be affected by how, and how quickly, we spend any cash that is raised in any financing transaction, including this offering. In light of the foregoing, no assurance can be provided that we are not currently a PFIC or that we will not become a PFIC in any future taxable year. Prospective investors should consult their own tax advisors regarding our PFIC status.
U.S. federal income tax treatment of a shareholder of a PFIC
If we are classified as a PFIC for any taxable year during which a U.S. Holder owns ordinary shares, the U.S. Holder, absent certain elections (including the mark-to-market and QEF elections described below), generally will be subject to adverse rules (regardless of whether we continue to be classified as a PFIC) with respect to (i) any “excess distributions” (generally, any
164
Table of Contents
distributions received by the U.S. Holder on its ordinary shares in a taxable year that are greater than 125% of the average annual distributions received by the U.S. Holder in the three preceding taxable years or, if shorter, the U.S. Holder’s holding period for its ordinary shares) and (ii) any gain realized on the sale or other disposition of its ordinary shares.
Under these adverse rules (a) the excess distribution or gain will be allocated ratably over the U.S. Holder’s holding period, (b) the amount allocated to the current taxable year and any taxable year prior to the first taxable year in which we are classified as a PFIC will be taxed as ordinary income and (c) the amount allocated to each other taxable year during the U.S. Holder’s holding period in which we were classified as a PFIC (i) will be subject to tax at the highest rate of tax in effect for the applicable category of taxpayer for that year and (ii) will be subject to an interest charge at a statutory rate with respect to the resulting tax attributable to each such other taxable year.
If we are classified as a PFIC, a U.S. Holder will generally be treated as owning stock or shares owned by us in any direct or indirect subsidiaries that are also PFICs and will be subject to similar adverse rules with respect to any distributions we receive from, and dispositions we make of, the stock or shares of such subsidiaries.
If we are classified as a PFIC and then cease to be so classified, a U.S. Holder may make an election (a “deemed sale election”) to be treated for U.S. federal income tax purposes as having sold such U.S. Holder’s ordinary shares on the last day our taxable year during which we were a PFIC. A U.S. Holder that makes a deemed sale election would then cease to be treated as owning stock in a PFIC by reason of ownership of our ordinary shares. However, gain recognized as a result of making the deemed sale election would be subject to the adverse rules described above and loss would not be recognized.
PFIC “mark-to-market” election
A U.S. Holder can avoid certain of the adverse rules described above by making a mark-to-market election with respect to its ordinary shares, provided that the ordinary shares are “marketable.” Ordinary shares will be marketable if they are “regularly traded” on a “qualified exchange” or other market within the meaning of applicable U.S. Treasury Regulations.
A U.S. Holder that makes a mark-to-market election must include in gross income, as ordinary income, for each taxable year an amount equal to the excess, if any, of the fair market value of the U.S. Holder’s ordinary shares at the close of the taxable year over the U.S. Holder’s adjusted tax basis in its ordinary shares. An electing U.S. Holder may also claim an ordinary loss deduction for the excess, if any, of the U.S. Holder’s adjusted tax basis in its ordinary shares over the fair market value of its ordinary shares at the close of the taxable year, but this deduction is allowable only to the extent of any net mark-to-market gains previously included in income. A U.S. Holder that makes a mark-to-market election generally will adjust such U.S. Holder’s tax basis in its ordinary shares to reflect the amount included in gross income or allowed as a deduction because of such mark-to-market election. Gains from an actual sale or other disposition of ordinary shares will be treated as ordinary income, and any losses incurred on a sale or other disposition of ordinary shares will be treated as ordinary losses to the extent of any net mark-to-market gains previously included in income.
If we are classified as a PFIC for any taxable year in which a U.S. Holder owns ordinary shares but before a mark-to-market election is made, the adverse PFIC rules described above will apply to any mark-to-market gain recognized in the year the election is made. Otherwise, a mark-to-
165
Table of Contents
market election will be effective for the taxable year for which the election is made and all subsequent taxable years. The election cannot be revoked without the consent of the IRS unless the ordinary shares cease to be marketable, in which case the election is automatically terminated.
A mark-to-market election is not permitted for the shares of any of our subsidiaries that are also classified as PFICs. Prospective investors should consult their own tax advisors regarding the availability of, and the procedure for making, a mark-to-market election.
PFIC “QEF” election
In some cases, a shareholder of a PFIC can avoid the interest charge and the other adverse PFIC consequences described above by obtaining certain information from such PFIC and by making a QEF election to be taxed currently on its share of the PFIC’s undistributed income. We do not, however, expect to provide the information regarding our income that would be necessary in order for a U.S. Holder to make a QEF election with respect to ordinary shares if we are classified as a PFIC.
PFIC information reporting requirements
If we are a PFIC in any year, a U.S. Holder of ordinary shares in such year will be required to file an annual information return on IRS Form 8621 regarding any distributions received on such ordinary shares and any gain realized on disposition of such ordinary shares. In addition, under U.S. tax legislation and subject to future guidance, if we are a PFIC, a U.S. Holder will be required to file an additional annual information return with the IRS on a revised IRS Form 8621 with such U.S. Holder’s U.S. federal income tax or information return. Pursuant to IRS to Notice 2011-55, the IRS has suspended this new filing requirement for U.S. Holders that are not otherwise required to file the current version of the IRS Form 8621 until the IRS releases a subsequent revision of IRS Form 8621 modified to reflect the additional reporting requirement. Guidance has not yet been issued regarding the information required to be included on such revised form. This new filing requirement is in addition to the pre-existing reporting requirements described above that apply to a U.S. Holder’s interest in a PFIC (which the recently enacted tax legislation and IRS Notice 2011-55 do not affect).
NO ASSURANCE CAN BE GIVEN THAT WE ARE NOT CURRENTLY A PFIC OR THAT WE WILL NOT BECOME A PFIC IN THE FUTURE. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS WITH RESPECT TO THE OPERATION OF THE PFIC RULES AND RELATED REPORTING REQUIREMENTS IN LIGHT OF THEIR PARTICULAR CIRCUMSTANCES, INCLUDING THE ADVISABILITY OF MAKING ANY ELECTION THAT MAY BE AVAILABLE.
U.S. backup withholding tax and information reporting
Backup withholding and information reporting requirements may apply to distributions on, and to proceeds from the sale or disposition of ordinary shares that are held by U.S. Holders. The payor will be required to backup withhold tax on payments made within the United States, or by a U.S. payor or U.S. middleman (and certain subsidiaries thereof), on an ordinary share to a U.S. Holder, other than an exempt recipient, if the U.S. Holder fails to furnish its correct taxpayer identification number or otherwise fails to comply with, or establish an exemption from, the backup withholding requirements.
166
Table of Contents
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s U.S. federal income tax liability. A U.S. Holder may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for a refund with the IRS and furnishing any required information in a timely manner.
In addition to the reporting requirements described elsewhere in this discussion, certain U.S. Holders are required to report information relating to an interest in ordinary shares, subject to certain exceptions, on their tax returns. U.S. Holders are urged to consult their own tax advisors regarding the effect, if any, of these requirements on their acquisition, ownership and disposition of ordinary shares.
Material U.K. tax considerations
The following is a general summary of material U.K. tax considerations relating to the ownership and disposal of our ordinary shares. It is based on current U.K. tax law and published HM Revenue & Customs, or HMRC, practice as at the date of this prospectus, both of which are subject to change, possibly with retrospective effect. Except as otherwise provided, this summary applies only to persons who are resident (and, in the case of individuals, domiciled) in the United Kingdom for tax purposes and who are not resident for tax purposes in any other jurisdiction and do not have a permanent establishment or fixed base in any other jurisdiction with which the holding of the ordinary shares is connected, or U.K. Holders.
This summary is for general information only and is not intended to be, nor should it be considered to be, legal or tax advice to any particular investor. It does not address all of the tax considerations that may be relevant to specific investors in light of their particular circumstances or to investors subject to special treatment under U.K. tax law. In particular:
| • | this summary only applies to the absolute beneficial owners of the ordinary shares, U.K. Holders who hold ordinary shares through DTC, and any dividends paid in respect of the ordinary shares where the dividends are regarded for U.K. tax purposes as that person’s own income (and not the income of some other person); |
| • | this summary: (a) only addresses the principal U.K. tax consequences for investors who hold the ordinary share as capital assets, (b) does not address the tax consequences which may be relevant to certain special classes of investors such as dealers, brokers or traders in shares or securities and other persons who hold the ordinary shares otherwise than as an investment, (c) does not address the tax consequences for holders that are financial institutions, insurance companies, collective investment schemes, pension schemes, charities and tax-exempt organizations, (d) assumes that the holder is not an officer or employee of our company (or of any related company) and has not (and is not deemed to have) acquired the ordinary shares by virtue of an office or employment (whether current, historic or prospective), and (e) assumes that the holder does not control or hold (and is not deemed to control or hold), either alone or together with one or more associated or connected persons, directly or indirectly an interest of 10% or more in the issued share capital (or in any class thereof), voting power, rights to profits or capital of our company, and is not otherwise connected with our company; and |
| • | this summary does not address any inheritance tax considerations. |
167
Table of Contents
POTENTIAL INVESTORS SHOULD SATISFY THEMSELVES PRIOR TO INVESTING AS TO THE OVERALL TAX CONSEQUENCES, INCLUDING, SPECIFICALLY, THE CONSEQUENCES UNDER U.K. TAX LAW AND HMRC PRACTICE OF THE ACQUISITION, OWNERSHIP AND DISPOSAL OF THE ORDINARY SHARES IN THEIR OWN PARTICULAR CIRCUMSTANCES BY CONSULTING THEIR OWN TAX ADVISERS.
Taxation of dividends
Withholding tax. Dividend payments in respect of the ordinary shares may be made without withholding or deduction for or on account of U.K. tax.
Income tax. Dividends received by individual U.K. Holders will be subject to U.K. income tax on the full amount of the dividend paid, grossed up for the amount of the non-refundable U.K. dividend tax credit referred to below.
An individual holder of ordinary shares who is not a U.K. Holder will not be chargeable to U.K. income tax on dividends paid by our company, unless such holder carries on (whether solely or in partnership) a trade, profession or vocation in the United Kingdom through a branch or agency in the United Kingdom to which the ordinary shares are attributable. In these circumstances, such holder may, depending on his or her individual circumstances, be chargeable to U.K. income tax on dividends received from our company.
The rate of U.K. income tax which is chargeable on dividends received in the tax year 2013/2014 by (i) additional rate taxpayers is 37.5%, (ii) higher rate taxpayers is 32.5%, and (iii) basic rate taxpayers is 10%. Individual U.K. Holders will be entitled to a non-refundable tax credit equal to one-ninth of the full amount of the dividend received from our company, which will be taken into account in computing the gross amount of the dividend which is chargeable to U.K. income tax. The tax credit will be credited against such holder’s liability (if any) to U.K. income tax on the gross amount of the dividend. After taking into account the tax credit, the effective rate of tax (i) for additional rate taxpayers will be approximately 31% of the dividend paid, (ii) for higher rate taxpayers will be 25% of the dividend paid, and (iii) for basic rate taxpayers will be nil. An individual holder who is not subject to U.K. income tax on dividends received from our company will not generally be entitled to claim repayment of the tax credit in respect of such dividends. An individual’s dividend income is treated as the top slice of their total income which is chargeable to U.K. income tax.
Corporation tax. A U.K. Holder within the charge to U.K. corporation tax may be entitled to exemption from U.K. corporation tax in respect of dividend payments. If the conditions for the exemption are not satisfied, or such U.K. Holder elects for an otherwise exempt dividend to be taxable, U.K. corporation tax will be chargeable on the amount of any dividends. U.K. Holders that make an election for an otherwise exempt dividend to be taxable, must do so within two years of the end of the accounting period in which the dividend is received.
A corporate holder of ordinary shares that is not a U.K. Holder will not be subject to U.K. corporation tax on dividends received from our company, unless it carries on a trade in the United Kingdom through a permanent establishment to which the ordinary shares are attributable. In these circumstances, such holder may, depending on its individual circumstances and if the exemption from U.K. corporation tax discussed above does not apply, be chargeable to U.K. corporation tax on dividends received from our company.
168
Table of Contents
Taxation of disposals
U.K. Holders
A disposal or deemed disposal of ordinary shares by an individual U.K. Holder may, depending on his or her individual circumstances, give rise to a chargeable gain or to an allowable loss for the purpose of U.K. capital gains tax. The principal factors that will determine the capital gains tax position on a disposal of ordinary shares are the extent to which the holder realizes any other capital gains in the tax year in which the disposal is made, the extent to which the holder has incurred capital losses in that or any earlier tax year and the level of the annual allowance of tax-free gains in that tax year, or the annual exemption. The annual exemption for the 2013/2014 tax year is £10,900. If, after all allowable deductions, an individual U.K. Holder’s total taxable income for the year exceeds the basic rate income tax limit, a taxable capital gain accruing on a disposal of ordinary shares will be taxed at 28%. In other cases, a taxable capital gain accruing on a disposal of ordinary shares may be taxed at 18% or 28% or at a combination of both rates.
An individual U.K. Holder who ceases to be resident in the United Kingdom for a period of less than five years and who disposes of his or her ordinary shares during that period of temporary non-residence may be liable for U.K. capital gains tax on a chargeable gain accruing on such disposal on his or her return to the United Kingdom (subject to available exemptions or reliefs).
A disposal of ordinary shares by a corporate U.K. Holder may give rise to a chargeable gain or an allowable loss for the purpose of U.K. corporation tax. Such a holder should be entitled to an indexation allowance, which applies to reduce capital gains to the extent that such gains arise due to inflation. The allowance may reduce a chargeable gain but will not create an allowable loss.
Non-U.K. Holders
An individual holder who is not a U.K. Holder will not be liable to U.K. capital gains tax on capital gains realized on the disposal of his or her ordinary shares unless such holder carries on (whether solely or in partnership) a trade, profession or vocation in the United Kingdom through a branch or agency in the United Kingdom to which the ordinary shares are attributable. In these circumstances, such holder may, depending on his or her individual circumstances, be chargeable to U.K. capital gains tax on chargeable gains arising from a disposal of his or her ordinary shares.
A corporate holder of ordinary shares that is not a U.K. Holder will not be liable for U.K. corporation tax on chargeable gains realized on the disposal of its ordinary shares unless it carries on a trade in the United Kingdom through a permanent establishment to which the ordinary shares are attributable. In these circumstances, a disposal of ordinary shares by such holder may give rise to a chargeable gain or an allowable loss for the purposes of U.K. corporation tax.
Stamp duty and stamp duty reserve tax
Issue and transfer of ordinary shares
No U.K. stamp duty or stamp duty reserve tax, or SDRT, is payable on the issue of the ordinary shares.
169
Table of Contents
Based on current published HMRC practice and recent case law, there should be no U.K. SDRT payable on the issue of ordinary shares to a depositary receipt system or a clearance service (for example DTC). An agreement to transfer ordinary shares through a depositary receipt system or clearance service should not give rise to a liability for U.K. SDRT.
Transfers of ordinary shares to, or to a nominee or agent for, a person whose business is or includes issuing depositary receipts or to, or to a nominee or agent for, a person whose business is or includes the provision of clearance services, will generally be regarded by HMRC as subject to stamp duty or SDRT at 1.5% of the amount or value of the consideration or, in certain circumstances, the value of the ordinary shares transferred. In practice, this liability for stamp duty or SDRT is in general borne by such person depositing the relevant shares in the depositary receipt system or clearance service.
The transfer on sale of ordinary shares by a written instrument of transfer will generally be liable to U.K. stamp duty at the rate of 0.5% of the amount or value of the consideration for the transfer. The purchaser normally pays the stamp duty.
An agreement to transfer ordinary shares will generally give rise to a liability on the purchaser to SDRT at the rate of 0.5% of the amount or value of the consideration. Such SDRT is payable on the seventh day of the month following the month in which the charge arises, but where an instrument of transfer is executed and duly stamped before the expiry of a period of six years beginning with the date of that agreement, (i) any SDRT that has not been paid ceases to be payable, and (ii) any SDRT that has been paid may be recovered from HMRC, generally with interest.
The Proposed Financial Transactions Tax
The European Commission has published a proposal for a directive for a common Financial Transactions Tax, or FTT, in Belgium, Germany, Estonia, Greece, Spain, France, Italy, Austria, Portugal, Slovenia and Slovakia, or the participating member states.
The proposed FTT has very broad scope and could, if introduced in its current form, apply to financial transactions (as defined in the proposed directive) relating to the ordinary shares (including secondary market transactions) in certain circumstances.
Under current proposals the FTT could apply in certain circumstances to persons both within and outside of the participating member states. Generally, it would apply to financial transactions relating to the ordinary shares where at least one party is a financial institution (as defined), and at least one party is established in a participating member state. A party may be deemed to be “established” in a participating member state in a broad range of circumstances, including (a) by transacting with a person established in a participating member state or (b) where the financial instrument which is the subject of the transaction is issued in a participating member state. Prospective holders of the ordinary shares should therefore note, in particular, that financial transactions relating to the ordinary shares may be subject to the FTT at a minimum rate of 0.1% provided certain conditions are met.
The FTT proposal remains subject to negotiation between the participating member states and is the subject of legal challenge. It may therefore be altered prior to any implementation, the timing of which remains unclear. Additional EU member states may decide to participate. Prospective holders of ordinary shares are advised to seek their own professional advice in relation to the FTT.
170
Table of Contents
We are offering the ordinary shares described in this prospectus through a number of underwriters. J.P. Morgan Securities LLC and Piper Jaffray & Co. are acting as joint book-running managers of the offering and as representatives of the underwriters. We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of ordinary shares listed next to its name in the following table:
| Underwriter | Number of shares | |
|
| ||
| J.P. Morgan Securities LLC |
||
| Piper Jaffray & Co. |
||
| Cowen and Company, LLC. |
||
| Robert W. Baird & Co. Incorporated |
||
|
| ||
| Total |
||
|
| ||
The underwriters are committed to purchase all the ordinary shares offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The underwriters propose to offer the ordinary shares directly to the public at the initial public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. After the initial public offering of the shares, the offering price and other selling terms may be changed by the underwriters. Sales of shares made outside of the United States may be made by affiliates of the underwriters. The representatives have advised us that the underwriters do not intend to confirm discretionary sales in excess of % of the ordinary shares offered in this offering.
The underwriters have an option to buy up to additional ordinary shares from us to cover sales of shares by the underwriters which exceed the number of shares specified in the table above. The underwriters have 30 days from the date of this prospectus to exercise this overallotment option. If any shares are purchased with this overallotment option, the underwriters will purchase shares in approximately the same proportion as shown in the table above. If any additional ordinary shares are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
The underwriting fee is equal to the public offering price per share less the amount paid by the underwriters to us per ordinary share. The underwriting fee is $ per share. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Without overallotment exercise |
With full overallotment exercise |
|||||||
|
|
||||||||
| Per share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
|
|
||||||||
171
Table of Contents
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately $ . We have agreed to reimburse the underwriters for certain expenses and application fees incurred in connection with any filing with, and clearance of the offering by, the Financial Industry Regulatory Authority, Inc.
A prospectus in electronic format may be made available on the web sites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
We, all of our directors and executive officers and holders of substantially all of our ordinary shares and securities exercisable for or convertible into our ordinary shares outstanding immediately prior to this offering have agreed not to (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any of our ordinary shares or securities convertible into or exchangeable or exercisable for any of our ordinary shares (including, without limitation, ordinary shares or such other securities which may be deemed to be beneficially owned by such directors, executive officers and security holders in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a share option or warrant), or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, (2) enter into any swap or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of any our ordinary shares or any such other securities (whether any such transactions described in clause (1) or (2) above is to be settled by the delivery of ordinary shares or such other securities, in cash or otherwise) or (3) in the case of our directors, executive officers and holders of ordinary shares and securities exercisable for or convertible into our ordinary shares outstanding immediately prior to this offering, make any demand for or exercise any right with respect to the registration of any our ordinary shares or any security convertible into or exercisable or exchangeable for our ordinary shares, in each case without the prior written consent of J.P. Morgan Securities LLC and Piper Jaffray & Co. for a period of 180 days after the date of this prospectus.
In our case, we and the underwriters have agreed in the underwriting agreement to certain exceptions to these restrictions.
In the case of our directors, executive officers and shareholders, and subject to certain conditions, such restrictions shall not apply to:
| • | ordinary shares to be sold by the undersigned pursuant to the underwriting agreement; |
| • | transfers of ordinary shares as a bona fide gift or gifts; |
| • | transfers or distributions of ordinary shares to limited or general partners, members, shareholders or direct or indirect affiliates of the undersigned, including funds or other entities under common control or management with the undersigned; |
| • | transfers of ordinary shares to any immediate family member, any trust for the direct or indirect benefit of the undersigned or the immediate family of the undersigned or any of their |
172
Table of Contents
| successors upon death or any partnership or limited liability company the partners or members of which consist of the undersigned and one or more members of the undersigned’s immediately family, provided that such transfers shall not involve a disposition of value; |
| • | transfers of ordinary shares to any beneficiary of the undersigned pursuant to a will, other testamentary document or applicable laws of descent; |
| • | transfers of ordinary shares to us for the primary purpose of satisfying any tax or other governmental withholding obligation with respect to ordinary shares issued upon the exercise of an option or warrant (or upon the exchange of another security or securities) outstanding on or prior to the date of this prospectus, or issued under an employee equity or benefit plan in existence on or prior to the date of this prospectus; |
| • | dispositions of ordinary shares or any security convertible into or exercisable or exchangeable for ordinary shares acquired in open market transactions after the completion of this offering; |
| • | the establishment of a trading plan pursuant to Rule 10b5-1 under the Exchange Act for the transfer of ordinary shares, provided that such plan does not provide for the transfer of ordinary shares during the restricted period specified in this letter and no filing or other public announcement regarding such plan shall be required or voluntarily made during the restricted period specified in this letter; and |
| • | transfers of ordinary shares in connection with the Scheme of Arrangement and related transactions that we completed on October 2, 2013, |
provided, in certain cases, the donee, transferee or distributee, as applicable, must execute and deliver to the representatives a lock-up letter, and provided, further, in certain cases, that no filing by any party under the Exchange Act or other public announcement shall be required or shall be made voluntarily in connection with such transfer.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act.
We have applied for listing of our ordinary shares on The NASDAQ Global Market under the symbol “OXFD.”
In connection with this offering, the underwriters may engage in stabilizing transactions, which involve making bids for, purchasing and selling ordinary shares in the open market for the purpose of preventing or retarding a decline in the market price of the ordinary shares while this offering is in progress. These stabilizing transactions may include making short sales of the ordinary shares, which involves the sale by the underwriters of a greater number of ordinary shares than they are required to purchase in this offering, and purchasing ordinary shares on the open market to cover positions created by short sales. Short sales may be “covered” shorts, which are short positions in an amount not greater than the underwriters’ overallotment option referred to above, or may be “naked” shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their overallotment option, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market compared to the price at which the underwriters may purchase shares through the overallotment option. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of
173
Table of Contents
the ordinary shares in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the ordinary shares, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase ordinary shares in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
These activities may have the effect of raising or maintaining the market price of the ordinary shares or preventing or retarding a decline in the market price of the ordinary shares, and, as a result, the price of the ordinary shares may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry out these transactions on The NASDAQ Global Market, in the over-the-counter market or otherwise.
Prior to this offering, there has been no public market for our ordinary shares. The initial public offering price will be determined by negotiations between us and the representatives of the underwriters. In determining the initial public offering price, we and the representatives of the underwriters expect to consider a number of factors including:
| • | the information set forth in this prospectus and otherwise available to the representatives; |
| • | our prospects and the history and prospects for the industry in which we compete; |
| • | an assessment of our management; |
| • | our prospects for future earnings; |
| • | the general condition of the securities markets at the time of this offering; |
| • | the recent market prices of, and demand for, publicly traded shares of generally comparable companies; and |
| • | other factors deemed relevant by the underwriters and us. |
Neither we nor the underwriters can assure investors that an active trading market will develop for our ordinary shares, or that the shares will trade in the public market at or above the initial public offering price.
Selling restrictions
General
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The ordinary shares offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
174
Table of Contents
European Economic Area
In relation to each member state of the EEA, or a Relevant Member State which has implemented the EU Prospectus Directive, as defined below, from and including the date on which the EU Prospectus Directive was implemented in that Relevant Member State, or the Relevant Implementation Date, an offer of securities described in this prospectus may not be made to the public in that Relevant Member State prior to the publication of a prospectus in relation to the securities which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the EU Prospectus Directive, except that, with effect from and including the Relevant Implementation Date, an offer of securities described in this prospectus may be made to the public in that Relevant Member State at any time:
| • | to any legal entity which is a qualified investor as defined under the EU Prospectus Directive; |
| • | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150 natural or legal persons (other than qualified investors as defined in the EU Prospectus Directive); or |
| • | in any other circumstances falling within Article 3(2) of the EU Prospectus Directive, provided that no such offer of securities described in this prospectus shall result in a requirement for the publication by us of a prospectus pursuant to Article 3 of the EU Prospectus Directive. |
For the purposes of this provision, the expression an ‘‘offer of securities to the public’’ in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that member state by any measure implementing the EU Prospectus Directive in that member state. The expression “EU Prospectus Directive” means Directive 2003/71/EC (and any amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Germany
Any offer or solicitation of ordinary shares within or into Germany must be in full compliance with the German Securities Prospectus Act (Wertpapierprospektgesetz, or WpPG). The offer and solicitation of securities to the public in Germany requires the approval of the prospectus by the German Federal Financial Services Supervisory Authority (Bundesanstalt fur Finanzdienstleistungsaufsicht, or BaFin). This prospectus has not been and will not be submitted for approval to the BaFin. This prospectus does not constitute a public offer under the WpPG. This prospectus and any other document relating to the ordinary shares, as well as any information contained therein, must not be supplied to the public in Germany or used in connection with any offer for subscription of the ordinary shares to the public in Germany, any public marketing of the ordinary shares or any public solicitation for offers to subscribe for or otherwise acquire the ordinary shares. The prospectus and other offering materials relating to the offer of the ordinary shares are strictly confidential and may not be distributed to any person or entity other than the designated recipients hereof.
175
Table of Contents
Switzerland
The ordinary shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange or on any other stock exchange or regulated trading facility in Switzerland.
This document has been prepared without regard to the disclosure standards for issuance prospectuses under article 652a or article 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under article 27 et seq. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the ordinary shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company or the ordinary shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of ordinary shares will not be supervised by, the Swiss Financial Market Supervisory Authority, and the offer of ordinary shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of our ordinary shares.
United Kingdom
This document is only being distributed to and is only directed at (i) persons who are outside the United Kingdom or (ii) to investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, or the Order, or (iii) high net worth entities, and other persons to whom it may lawfully be communicated, falling with Article 49(2)(a) to (e) of the Order (all such persons together being referred to as “relevant persons”). The securities are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such securities will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
Other activities
Certain of the underwriters and their affiliates have provided in the past to us and our affiliates and may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
The validity of the issuance of our ordinary shares offered hereby will be passed upon for us by Ropes & Gray International LLP, our English counsel, and certain other matters will be passed upon for us by Ropes & Gray LLP, Boston, Massachusetts. The underwriters are being represented by Davis Polk & Wardwell LLP, New York, New York.
176
Table of Contents
The financial statements of Oxford Immunotec Limited at December 31, 2011 and 2012, and for the years then ended, appearing in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The balance sheet of Oxford Immunotec Global PLC as of August 16, 2013, appearing in this Prospectus and Registration Statement has been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
We are incorporated under the laws of England and Wales. Many of our directors and officers reside outside the United States, and a substantial portion of our assets and all or a substantial portion of the assets of such persons are located outside the United States. As a result, it may be difficult for you to serve legal process on us or our directors and executive officers or have any of them appear in a U.S. court.
The United States and the United Kingdom do not currently have a treaty providing for the recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. The enforceability of any judgment of a United States federal or state court in the United Kingdom will depend on the laws and any treaties in effect at the time, including conflicts of laws principles (such as those bearing on the question of whether a United Kingdom court would recognize the basis on which a United States court had purported to exercise jurisdiction over a defendant). In this context, there is doubt as to the enforceability in the United Kingdom of civil liabilities based solely on the federal securities laws of the United States. In addition, awards for punitive damages in actions brought in the United States or elsewhere may be unenforceable in the United Kingdom. An award for monetary damages under the United States securities laws would likely be considered punitive if it did not seek to compensate the claimant for loss or damage suffered and was intended to punish the defendant.
Where you can find more information
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the ordinary shares offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information with respect to us and the ordinary shares offered hereby, please refer to the registration statement and the exhibits and schedules filed therewith. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. A copy of the registration statement and the exhibits and schedules filed therewith may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street N.E., Washington, D.C. 20549, and copies of all or any part of the
177
Table of Contents
registration statement may be obtained from such offices upon the payment of the fees prescribed by the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address is www.sec.gov.
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act and, in accordance therewith, we will file periodic reports, proxy statements and other information with the SEC. Such periodic reports, proxy statements and other information will be available for inspection and copying at the public reference room and website of the SEC referred to above.
178
Table of Contents
Oxford Immunotec Limited
Audited consolidated financial statements
Interim condensed consolidated financial statements
| Page | ||||
| F-36 | ||||
| F-37 | ||||
| F-38 | ||||
| F-39 | ||||
| F-40 | ||||
| Notes to the interim condensed consolidated financial statements (unaudited) |
F-41 | |||
F-1
Table of Contents
Oxford Immunotec Global PLC
Audited balance sheet
| Page | ||||
| Report of independent registered public accounting firm |
F-46 | |||
| Balance sheet as of August 16, 2013 (Date of inception) |
F-47 | |||
| Notes to the balance sheet |
F-48 | |||
F-2
Table of Contents
Report of independent registered public accounting firm
The Board of Directors and Shareholders of Oxford Immunotec Limited:
We have audited the accompanying consolidated balance sheets of Oxford Immunotec Limited as of December 31, 2012 and 2011, and the related consolidated statements of operations, other comprehensive loss, shareholders’ equity, and cash flows for each of the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Oxford Immunotec Limited at December 31, 2012 and 2011, and the consolidated results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Reading, United Kingdom
October 15, 2013
F-3
Table of Contents
Consolidated balance sheets
(in thousands, except share and per share data)
| December 31, | ||||||||
| 2011 | 2012 | |||||||
|
|
||||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 2,334 | $ | 12,578 | ||||
| Restricted cash |
— | 387 | ||||||
| Accounts receivable, net |
2,211 | 5,400 | ||||||
| Inventory |
1,975 | 3,073 | ||||||
| Prepaid expenses and other |
1,029 | 1,342 | ||||||
|
|
|
|||||||
| Total current assets |
7,549 | 22,780 | ||||||
| Restricted cash, non-current |
359 | 287 | ||||||
| Property and equipment, net |
1,677 | 2,249 | ||||||
| Intangible assets, net |
54 | 107 | ||||||
| Other assets |
— | 60 | ||||||
|
|
|
|||||||
| Total assets |
$ | 9,639 | $ | 25,483 | ||||
|
|
|
|||||||
| Liabilities and shareholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 1,691 | $ | 1,754 | ||||
| Accrued expenses |
2,333 | 3,962 | ||||||
| Deferred income |
71 | 908 | ||||||
| Revolving line of credit |
— | 1,548 | ||||||
| Current portion of loans payable |
31 | 79 | ||||||
| Taxes payable |
147 | 140 | ||||||
|
|
|
|||||||
| Total current liabilities |
4,273 | 8,391 | ||||||
| Long-term portion of loans payable |
140 | 143 | ||||||
|
|
|
|||||||
| Total liabilities |
$ | 4,413 | $ | 8,534 | ||||
|
|
|
|||||||
| Shareholders’ equity: |
||||||||
| Convertible preferred ordinary shares: |
||||||||
| A preferred ordinary shares, £0.001 par value; 903,220 shares authorized, 903,220 shares issued and outstanding at December 31, 2011 and 2012. |
$ | 2 | $ | 2 | ||||
| B preferred ordinary shares, £0.001 par value; 362,020 shares authorized, 362,020 shares issued and outstanding at December 31, 2011 and 2012. |
1 | 1 | ||||||
| D preferred ordinary shares, £0.001 par value; 3,488,448 shares authorized, 3,266,885 shares issued and outstanding at December 31, 2011 and 2012. |
5 | 5 | ||||||
| E preferred ordinary shares, £0.001 par value; 32,000,000 shares authorized, 17,081,014 shares issued and outstanding at December 31, 2011 and 2012. |
33 | 33 | ||||||
| F preferred ordinary shares, £0.001 par value; 20,000,000 shares authorized, 16,029,591 and 17,262,618 shares issued and outstanding at December 31, 2011 and 2012, respectively. |
25 | 26 | ||||||
| G preferred ordinary shares, £0.001 par value; 25,000,000 shares authorized, 10,079,933 shares issued and outstanding at December 31, 2012. |
— | 16 | ||||||
| Ordinary shares, £0.001 par value; 60,179,750 and 110,179,750 shares authorized at December 31, 2011 and 2012, respectively, 8,492,175 and 14,442,575, shares issued and outstanding at December 31, 2011 and 2012, respectively. |
14 | 24 | ||||||
| Subscription of G preferred ordinary shares |
— | 8,075 | ||||||
| Additional paid-in capital |
85,221 | 103,380 | ||||||
| Accumulated deficit |
(76,108 | ) | (90,991 | ) | ||||
| Accumulated other comprehensive loss |
(3,967 | ) | (3,622 | ) | ||||
|
|
|
|||||||
| Total shareholders’ equity |
5,226 | 16,949 | ||||||
|
|
|
|||||||
| Total liabilities and shareholders’ equity |
$ | 9,639 | $ | 25,483 | ||||
|
|
||||||||
See accompanying notes to these consolidated financial statements.
F-4
Table of Contents
Consolidated statements of operations
(in thousands, except share and per share data)
| Year ended December 31, | ||||||||
| 2011 | 2012 | |||||||
|
|
||||||||
| Revenue |
||||||||
| Product |
$ | 6,281 | $ | 9,080 | ||||
| Service |
6,360 | 11,605 | ||||||
|
|
|
|||||||
| Total revenue |
12,641 | 20,685 | ||||||
| Cost of revenue |
||||||||
| Product |
2,955 | 4,329 | ||||||
| Service |
5,462 | 8,095 | ||||||
|
|
|
|||||||
| Total cost of revenue |
8,417 | 12,424 | ||||||
|
|
|
|
|
|||||
| Gross profit |
4,224 | 8,261 | ||||||
| Operating expenses: |
||||||||
| Research and development |
1,780 | 1,947 | ||||||
| Sales and marketing |
10,536 | 11,177 | ||||||
| General and administrative |
5,232 | 8,068 | ||||||
|
|
|
|||||||
| Total operating expenses |
17,548 | 21,192 | ||||||
|
|
|
|||||||
| Loss from operations |
(13,324 | ) | (12,931 | ) | ||||
| Other (expense) income: |
||||||||
| Interest income (expense) |
(3 | ) | (1,477 | ) | ||||
| Foreign exchange gains (losses) |
28 | (626 | ) | |||||
| Other income (expense) |
76 | — | ||||||
|
|
|
|||||||
| Loss before income taxes |
(13,223 | ) | (15,034 | ) | ||||
| Income tax benefit |
(119 | ) | (151 | ) | ||||
|
|
|
|||||||
| Net loss |
$ | (13,104 | ) | $ | (14,883 | ) | ||
|
|
|
|||||||
| Net loss per share attributable to ordinary shareholders—basic and diluted |
$ | (1.61 | ) | $ | (1.26 | ) | ||
|
|
|
|||||||
| Weighted-average shares used to compute net loss attributable to ordinary shareholders—basic and diluted |
8,150,146 | 11,825,803 | ||||||
|
|
||||||||
See accompanying notes to these consolidated financial statements.
F-5
Table of Contents
Consolidated statements of other comprehensive loss
(in thousands)
| Year ended December 31, |
||||||||
| 2011 | 2012 | |||||||
|
|
||||||||
| Net loss |
$ | (13,104 | ) | $ | (14,883 | ) | ||
| Other comprehensive income: |
||||||||
| Foreign currency translation adjustment |
47 | 345 | ||||||
|
|
|
|||||||
| Other comprehensive income, net of taxes |
47 | 345 | ||||||
|
|
|
|||||||
| Total comprehensive income (loss) |
$ | (13,057 | ) | $ | (14,538 | ) | ||
|
|
||||||||
See accompanying notes to these consolidated financial statements.
F-6
Table of Contents
Consolidated statements of shareholders’ equity
(in thousands, except share and per share data)
| Convertible preferred ordinary shares | ||||||||||||||||||||||||||||||||||||||||||||||||
| A preferred ordinary |
B preferred ordinary |
D preferred ordinary |
E preferred ordinary |
F preferred ordinary |
G preferred ordinary |
Ordinary | Subscription G preferred ordinary |
Additional paid-in capital |
Accumulated deficit |
Accumulated other |
Total shareholders’ equity |
|||||||||||||||||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2010 |
$ | 2 | $ | 1 | $ | 5 | $ | 33 | $ | 15 | $ | — | $ | 11 | $ | — | $ | 75,126 | $ | (63,004 | ) | $ | (4,014 | ) | $ | 8,175 | ||||||||||||||||||||||
| Exercise of share options |
— | — | — | — | — | — | — | — | 2 | — | — | 2 | ||||||||||||||||||||||||||||||||||||
| Issuance of F preferred ordinary shares |
— | — | — | — | 10 | — | — | — | 9,968 | — | — | 9,978 | ||||||||||||||||||||||||||||||||||||
| Issuance of ordinary shares |
— | — | — | — | — | — | 3 | — | — | — | — | 3 | ||||||||||||||||||||||||||||||||||||
| Share-based compensation expense |
— | — | — | — | — | — | — | — | 125 | — | — | 125 | ||||||||||||||||||||||||||||||||||||
| Other comprehensive income |
— | — | — | — | — | — | — | — | — | — | 47 | 47 | ||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (13,104 | ) | — | (13,104 | ) | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2011 |
$ | 2 | $ | 1 | $ | 5 | $ | 33 | $ | 25 | $ | — | $ | 14 | $ | — | $ | 85,221 | $ | (76,108 | ) | $ | (3,967 | ) | $ | 5,226 | ||||||||||||||||||||||
| Issuance of ordinary shares—anti-dilution |
— | — | — | — | — | — | 9 | — | (9 | ) | — | — | — | |||||||||||||||||||||||||||||||||||
| Issuance of F preferred ordinary shares in connection with debt financing |
— | — | — | — | 1 | — | 1 | — | 1,314 | — | — | 1,316 | ||||||||||||||||||||||||||||||||||||
| Subscription of G preferred ordinary shares |
— | — | — | — | — | — | — | 8,075 | — | — | — | 8,075 | ||||||||||||||||||||||||||||||||||||
| Issuance of G preferred ordinary shares, net of financing costs |
— | — | — | — | — | 16 | — | — | 16,685 | — | — | 16,701 | ||||||||||||||||||||||||||||||||||||
| Interest on convertible notes settled with G preferred ordinary shares |
— | — | — | — | — | — | — | — | 90 | — | — | 90 | ||||||||||||||||||||||||||||||||||||
| Share-based compensation expense |
— | — | — | — | — | — | — | — | 79 | — | — | 79 | ||||||||||||||||||||||||||||||||||||
| Other comprehensive income |
— | — | — | — | — | — | — | — | — | — | 345 | 345 | ||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (14,883 | ) | — | (14,883 | ) | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2012 |
$ | 2 | $ | 1 | $ | 5 | $ | 33 | $ | 26 | $ | 16 | $ | 24 | $ | 8,075 | $ | 103,380 | $ | (90,991 | ) | $ | (3,622 | ) | $ | 16,949 | ||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||
See accompanying notes to these consolidated financial statements.
F-7
Table of Contents
Consolidated statements of cash flows
(in thousands)
| Years ended December 31, |
||||||||
| 2011 | 2012 | |||||||
|
|
||||||||
| Cash flows from operating activities |
||||||||
| Net loss |
$ | (13,104 | ) | $ | (14,883 | ) | ||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
||||||||
| Depreciation and amortization |
630 | 801 | ||||||
| Share-based compensation expense |
125 | 79 | ||||||
| Loss on disposal of property and equipment |
128 | 71 | ||||||
| Noncash interest expense |
4 | 1,425 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable, net |
(1,042 | ) | (3,103 | ) | ||||
| Inventory |
(316 | ) | (1,045 | ) | ||||
| Prepaid expenses and other |
66 | (333 | ) | |||||
| Accounts payable |
455 | 196 | ||||||
| Accrued expenses |
445 | 1,608 | ||||||
| Deferred income |
(48 | ) | 814 | |||||
|
|
|
|||||||
| Net cash provided by (used in) operating activities, |
(12,657 | ) | (14,370 | ) | ||||
|
|
|
|||||||
| Cash flows from investing activities |
||||||||
| Purchases of property and equipment |
(1,238 | ) | (1,482 | ) | ||||
| Purchases of intangible assets |
(1 | ) | (79 | ) | ||||
| Change in restricted cash |
(359 | ) | (315 | ) | ||||
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
(1,598 | ) | (1,876 | ) | ||||
|
|
|
|||||||
| Cash flows from financing activities |
||||||||
| Proceeds from revolving line of credit |
— | 1,500 | ||||||
| Proceeds from convertible note |
— | 4,000 | ||||||
| Proceeds received in advance of share issuance |
— | 8,075 | ||||||
| Proceeds from issuance of ordinary shares |
3 | — | ||||||
| Proceeds from issuance of preferred ordinary shares |
9,978 | 12,701 | ||||||
| Proceeds from exercise of share options |
2 | 1 | ||||||
| Payments on loan |
(102 | ) | (60 | ) | ||||
|
|
|
|||||||
| Net cash provided by (used in) financing activities |
9,881 | 26,217 | ||||||
|
|
|
|||||||
| Effect of exchange rate changes on cash and cash equivalents |
64 | 273 | ||||||
| Net increase (decrease) in cash and cash equivalents, excluding restricted cash |
(4,374 | ) | 9,971 | |||||
| Cash and cash equivalents at beginning of year |
6,644 | 2,334 | ||||||
|
|
|
|||||||
| Cash and cash equivalents at end of year |
$ | 2,334 | $ | 12,578 | ||||
|
|
|
|||||||
| Supplemental disclosures |
||||||||
| Cash paid for interest |
— | 81 | ||||||
| Cash paid (received) for taxes |
(184 | ) | (281 | ) | ||||
| Noncash investing and financing activities |
||||||||
| Interest on convertible notes settled with G preferred ordinary shares |
— | 90 | ||||||
| F preferred ordinary shares issued with convertible notes |
— | 1,316 | ||||||
| Convertible notes converted into G preferred ordinary shares |
— | 4,000 | ||||||
|
|
||||||||
See accompanying notes to these consolidated financial statements.
F-8
Table of Contents
1. Description of business and significant accounting policies
Description of business
The Company is a global, commercial-stage diagnostics company committed to improving patient care by providing advanced, innovative tests in the field of immunology. The Company’s proprietary T-SPOT technology platform allows it to measure the responses of specific immune cells (T cells) to inform the diagnosis, prognosis and monitoring of patients with immunologically controlled diseases. Substantially all of the Company’s revenue is currently derived from the sale of its T-SPOT.TB test, which is sold in two formats: an in vitro diagnostic kit format (allowing customers to perform the test at their own institutions), and a service format (in which the Company performs the test on samples sent by customers to the Company’s own laboratory facilities). The Company sells its T-SPOT.TB test through a direct sales force in the United States, certain European countries and Japan. The Company sells through distributors in other parts of the world.
Reorganization, reverse share split and conversion
On October 2, 2013, the Scheme of Arrangement was approved by the High Court of Justice in England and Wales. All holders of ordinary shares, preferred ordinary shares, options and warrants exchanged their interests in Oxford Immunotec Limited for identical interests in Oxford Immunotec Global PLC. As a result of this exchange, Oxford Immunotec Global PLC is now the parent company of Oxford Immunotec Limited.
Prior to closing the IPO of Oxford Immunotec Global PLC, the Company will undertake a reverse share split of Oxford Immunotec Global PLC’s outstanding ordinary shares, which will result in a proportional decrease in the number of ordinary shares outstanding as well as appropriate adjustments to outstanding A ordinary shares, preferred ordinary shares, warrants and options. After the reverse share split and immediately prior to the IPO, all outstanding preferred ordinary shares will convert into ordinary shares. These consolidated financial statements will be revised to give effect to these share changes for the most recent annual and interim periods presented.
Basis of presentation, accounting principles and principles of consolidation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (U.S. GAAP), and include the financial statements of Oxford Immunotec Limited, a company incorporated in England and Wales and its wholly-owned subsidiaries, collectively referred to as the Company. All intercompany accounts and transactions have been eliminated upon consolidation.
Use of estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements and that affect the reported amounts of revenue and expenditures during the reporting periods. Actual results could differ from those estimates and assumptions used.
F-9
Table of Contents
Foreign currency translation
The functional currency for each of the Company’s foreign operations is the local currency. Revenue and expenses of foreign operations are translated into U.S. Dollars at the average rates of exchange during the year. Assets and liabilities of foreign operations are translated into U.S. Dollars at year-end rates. The Company reflects resulting translation gains or losses in accumulated other comprehensive income, which is a component of shareholders’ equity. The Company does not record tax provisions or benefits for the net changes in foreign currency translation adjustments, as the Company intends to permanently reinvest undistributed earnings in its foreign subsidiaries.
Realized and unrealized foreign currency transaction gains or losses, arising from exchange rate fluctuations on balances denominated in currencies other than the functional currencies, are included in “Other income (expense)” in the consolidated statements of operations.
Revenue recognition
The Company derives product revenue from the sale of its T-SPOT.TB diagnostic test kits and related accessories to a broad range of customers including: hospitals, public health departments, commercial testing laboratories, importers and distributors.
Product revenue is generally paid directly by the customer and is recognized on an accrual basis when the following revenue recognition criteria are met: (1) persuasive evidence that an arrangement exists; (2) the product has been shipped or delivered in accordance with the shipping terms of the arrangement; (3) the price is fixed or determinable and, known at time of shipment; and (4) collectability is reasonably assured.
For products sold in Japan, the price only becomes determinable upon the wholesaler receiving a firm order from its customer and as a result this is when the Company recognizes revenue for such sales.
No product return rights are extended to customers of the Company.
The Company derives service revenue from its diagnostic laboratories in the United States and in the United Kingdom where the Company performs its T-SPOT.TB test on samples sent by customers to its laboratory facilities.
Service revenue in the United Kingdom and revenue from direct bill customers in the United States are recognized on an accrual basis when the following revenue recognition criteria are met: (1) persuasive evidence that an arrangement exists; (2) when the diagnostic result has been delivered; (3) the price is fixed or determinable; and (4) collectability is reasonably assured. This service revenue is referred to as “direct-bill” sales because the Company receives payment directly from the ordering entity.
In the United States, the Company also generates revenue from payments that are received from a variety of third-party payors, including government programs (Medicare and Medicaid) and commercial insurance companies, each with different billing requirements. Revenue from tests paid by third-party payors is recognized on an accrual basis based on the Company’s historical collection experience.
Taxes assessed by governmental authorities on revenue, including sales and value added taxes, are recorded on a net basis (excluded from revenue) in the consolidated statement of operations.
F-10
Table of Contents
Cost of revenue—cost of product and cost of service
Cost of product revenue consists primarily of costs incurred in the production process, including costs of raw materials and components, assembly labor and overhead, quality costs, royalties paid under licensing agreements, the U.S. medical device excise tax and packaging and delivery costs.
Cost of service revenue consists primarily of costs incurred in the operation of the Company’s diagnostic laboratories including labor and overhead, kit costs, quality costs, consumables used in the testing process and packaging and delivery costs.
Shipping and handling
The Company does not bill its service customers for shipping and handling charges. Charges relating to inbound and outbound freight costs are incurred by the Company and recorded within cost of service.
The Company generally bills product customers for shipping and handling and records the customer payments as product revenue. The associated costs are recorded as cost of product sold.
Cash and cash equivalents
The Company maintains its available cash balances in cash and bank savings accounts in the United States, United Kingdom, Germany and Japan. The Company maintains deposits in government insured financial institutions in excess of government insured limits. Management believes that the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held.
Restricted cash
As of December 31, 2011 and 2012, bank balances totaling $0.4 million were pledged as security for the Company’s office and laboratory space operating leases.
As of December 31, 2012, $0.3 million was held in an escrow account; these funds were released in February 2013, as explained in Note 20, “Subsequent events” to these consolidated financial statements.
Accounts receivable
Accounts receivable are primarily amounts due from hospitals, public health departments, commercial testing laboratories, distributors and universities in addition to third-party payors such as commercial insurance companies and government programs (Medicare and Medicaid).
Accounts receivable are reported net of an allowance for uncollectible accounts. The process of estimating the collection of accounts receivable involves significant assumptions and judgments. Specifically, the accounts receivable allowance is based on management’s analysis of current and past due accounts, collection experience and other relevant information. The Company’s provision for uncollectible accounts is recorded as a bad debt expense and included in general and administrative expenses. Although the Company believes amounts provided are adequate, the ultimate amounts of uncollectible accounts receivable could be in excess of the amounts provided.
F-11
Table of Contents
Inventory
Inventory consists of finished goods and raw materials. The Company does not maintain work in progress balances as the nature of the manufacturing process does not allow for test kits to be left in a partially manufactured state. Inventory is removed at cost. Inventory is stated at the lower of cost or market. Cost is determined by the actual cost of components by batch plus estimated labor and overhead costs per unit. Market value is based on an estimated selling price less any costs expected to be incurred to completion and sale. The Company reviews the components of its inventory on a periodic basis for excess, obsolete or impaired inventory, and records a reserve for the identified items. At December 31, 2011 and 2012, the Company determined no inventory reserve was required.
Property and equipment
Property and equipment are stated at cost. Property and equipment includes specialized shipping containers provided to customers, primarily in the United States, for transporting samples to its laboratory for testing. Property and equipment financed under capital leases are initially recorded at the present value of minimum lease payments at the inception of the lease.
Depreciation is calculated using the straight-line method over the estimated useful lives of the assets. Property and equipment under capital leases and leasehold improvements are amortized using the straight-line method over the shorter of the lease term or estimated useful life of the asset. Depreciable lives range from three to ten years for laboratory equipment, office equipment and furniture and fixtures and three years for software and specialized shipping containers.
Impairment of long-lived assets
The Company evaluates its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may be impaired, and assesses their recoverability based upon anticipated future cash flows. If changes in circumstances lead the Company to believe that any of its long-lived assets may be impaired, the Company will (a) evaluate the extent to which the remaining book value of the asset is recoverable by comparing the future undiscounted cash flows estimated to be associated with the asset to the asset’s carrying amount and (b) write-down the carrying amount to market value to the extent necessary. There has been no impairment of long-lived assets to date.
Intangible assets
Intangible assets include technology licenses which are capitalized and amortized over estimated useful lives (generally five to ten years) using the straight-line method. The weighted-average useful life is 9.7 years. On an ongoing basis, the Company assesses the recoverability of its intangible assets by determining its ability to generate undiscounted future cash flows sufficient to recover the unamortized balances over the remaining useful lives. Intangible assets determined to be unrecoverable are expensed in the period in which the determination is made.
Derivative financial instruments
The Company does not use derivative instruments to hedge exposures to cash flow, market, interest rate or foreign currency risks.
F-12
Table of Contents
The Company reviews the terms of its shares, warrants and convertible debt it issues to determine whether there are embedded derivative instruments, including embedded conversion options, which are required to be bifurcated and accounted for separately as derivative financial instruments. In circumstances where the host instrument contains more than one embedded derivative instrument, including the conversion option, that is required to be bifurcated, the bifurcated derivative instruments are accounted for as a single, compound derivative instrument.
Bifurcated embedded derivatives are initially recorded at fair value and are then revalued at each reporting date with changes in the fair value reported as other income or expense. When the equity or convertible debt instruments contain embedded derivative instruments that are to be bifurcated and accounted for as liabilities, the total proceeds received are first allocated to the fair value of all the bifurcated derivative instruments. The remaining proceeds, if any, are then allocated to the host instruments themselves, usually resulting in those instruments being recorded at a discount from their face value.
Fair value of financial instruments
The Company measures certain financial assets and liabilities at fair value based on the price that would be received for an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. As of December 31, 2011 and 2012, the Company’s financial instruments consist of cash, accounts receivable, prepaid expenses, and other accounts payable, accrued expenses, a revolving line of credit, loans payable and ordinary share warrant liability. See Note 2, “Fair value measurement,” to these consolidated financial statements for further information on the fair value of the Company’s financial instruments.
Research and development expenses
Research and development expenses include all costs associated with the development of the Company’s T-SPOT technology platform and potential future products including new diagnostic tests that utilize the T-SPOT technology platform and are charged to expense as incurred. Research and development expenses include direct costs and an allocation of indirect costs, including amortization, depreciation, rent, supplies, insurance and repairs and maintenance.
Income taxes
The Company accounts for income taxes under the asset and liability method, which requires, among other things, that deferred income taxes be provided for temporary differences between the tax basis of the Company’s assets and liabilities and its financial statement reported amounts. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating losses and research and development credit carryforwards. A valuation allowance is established when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company adheres to the accounting guidance for uncertainties in income taxes, which prescribes a recognition threshold and measurement process for recording in the financial statements uncertain tax positions taken, or expected to be taken, in a tax return. The Company accrues for the estimated amount of taxes for uncertain tax positions if it is more likely than not that the Company would be required to pay such additional taxes. An uncertain tax position will
F-13
Table of Contents
not be recognized if it has less than a 50% likelihood of being sustained. The Company does not have any accrued interest or penalties associated with any unrecognized tax positions for the years ended December 31, 2011 and 2012.
Advertising
Advertising costs, which are included in sales and marketing expenses, are expensed as incurred. Advertising expense was $0.5 million and $0.4 million for the years ended December 31, 2011 and 2012, respectively.
Share-based compensation
The Company accounts for share-based compensation arrangements with employees, officers and directors by recognizing compensation expense based on the grant date fair value of share-based transactions in the consolidated financial statements.
Share-based compensation costs are based on the fair value of the underlying option calculated using the Black-Scholes option-pricing model on the date of grant for share options and recognized as expense on a straight-line basis over the requisite service period. Determining the appropriate fair value model and related assumptions requires judgment, including estimating share price volatility, expected term and forfeiture rates. The expected volatility rates are estimated based on the actual volatility of comparable public companies over the expected term. The expected terms represent the average time that options are expected to be outstanding based on the midpoint between the vesting date and the end of the contractual term of the award. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company has not paid dividends and does not anticipate paying cash dividends in the foreseeable future and, accordingly, uses an expected dividend yield of zero. The risk-free interest rate is based on the rate of U.S. Treasury securities with maturities consistent with the estimated expected term of the awards.
The cumulative expense recognized for share-based transactions at each reporting date until the vesting date reflects the extent to which the vesting period has expired and the Company’s best estimate of the number of equity instruments that will ultimately vest. The charge or credit for a period represents the movement in cumulative expense recognized as at the beginning and end of that period. No expense is recognized for awards that do not ultimately vest.
Where the terms of an equity award are modified, the minimum expense recognized is the expense as if the terms had not been modified if the original terms of the award are met. An additional expense is recognized for any modification that increases the total fair value of the share-based compensation, or is otherwise beneficial to the employee as measured at the date of modification.
Where a share-based compensation award is cancelled, it is treated as if it had vested on the date of cancellation, and any expense not yet recognized for the award is recognized immediately. However, if a new award is substituted for the cancelled award, and designated as a replacement award on the date it is granted, the cancelled and new awards are treated as if they were a modification of the original award, as described in the previous paragraph.
Upon exercise share options are redeemed for newly issued ordinary shares.
F-14
Table of Contents
Segment reporting
The Company operates in one operating segment. The Company’s chief operating decision maker (the CODM), its chief executive officer, manages the Company’s operations on an integrated basis for the purposes of allocating resources. When evaluating the Company’s financial performance, the CODM reviews separate revenue information for the Company’s product and service offerings and for each country, while all other financial information is on a combined basis. While the Company’s principal operations and decision-making functions are located in both the United States and United Kingdom, the CODM makes decisions on a global basis. Accordingly, the Company has determined that it operates in a single reporting segment.
Basic and diluted net loss per ordinary share
Earnings or net loss attributable to ordinary shareholders for the period, after deduction of preferred ordinary share preferences, are allocated between the ordinary shareholders and preferred ordinary shareholders based on their respective rights to receive dividends. Basic and diluted net loss per ordinary share is determined by dividing net loss applicable to ordinary shareholders by the weighted-average number of ordinary shares outstanding during the period. As the Company reports net losses, outstanding share options, warrants and preferred ordinary shares, have not been included in the calculation of diluted net loss attributable to ordinary shareholders per share because to do so would be anti-dilutive. Accordingly, the numerator and the denominator used in computing both basic and diluted net loss per share for each period are the same. Since the Company’s participating preferred ordinary shares are not contractually required to share in the Company’s losses, in applying the two-class method to compute basic net loss per share, no allocation was made to preferred ordinary shares if a net loss existed.
Ordinary share warrant policy
Warrants to purchase the Company’s ordinary shares are classified as equity unless otherwise required. Warrants issued with a down round provision, whereby the exercise price would be adjusted downward in the event that additional ordinary shares of the Company or securities exercisable, convertible or exchangeable for the Company’s ordinary shares are issued at a price less than the exercise price, and are recorded as a liability and marked to market each reporting period until they are exercised, expire or otherwise extinguished.
Recent accounting pronouncements
The Company has considered recent accounting pronouncements and concluded that they are either not applicable to the business, or that no material effect is expected on the consolidated financial statements as a result of future adoption.
Concentration of risks
The Company derives product revenue from the sale of its T-SPOT.TB diagnostic test kits and related accessories to a broad range of customers including: hospitals, public health departments, commercial testing laboratories, importers and distributors. Importers and distributors sell to third parties including end-user customers in specific territories.
F-15
Table of Contents
In the year ended December 31, 2011 the Company did not have any product customers that individually represented more than 10% of revenue. In the year ended December 31, 2012 the Company had one customer, its Chinese distributor, Shanghai Fosun Long March Medical Science Co. Ltd., that represented 15% of annual revenue, the loss of which could have a material impact on the Company’s operating results.
The Company derives service revenue from its diagnostic laboratories in the United States and in the United Kingdom where the Company performs its T-SPOT.TB test on samples sent by customers to its laboratory facilities. In the years ended December 31, 2011 and 2012, the Company did not have any service customers that represented more than 10% of revenue.
The Company depends on a limited number of suppliers, including single-source suppliers, of various critical components used to assemble its products. The loss of these suppliers, or their failure to supply the Company with necessary components on a timely basis or in accordance with the Company’s quality specifications could cause an interruption in the supply of products and delivery of test services to the Company’s customers and adversely affect the Company.
2. Fair value measurement
As a basis for determining the fair value of certain of the Company’s financial instruments, the Company utilizes a three-tier value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level I—Observable inputs such as quoted prices in active markets for identical assets or liabilities.
Level II—Observable inputs, other than Level I prices, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level III—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
This hierarchy requires the Company to use observable market data, when available, and to minimize the use of unobservable inputs when determining fair value. The carrying amount of certain of the Company’s financial instruments, including cash, accounts receivable, prepaid expenses and other assets, accounts payable and accrued expenses approximate fair value due to their short maturities.
Recurring fair value measurements
The Company’s financial instruments that are measured at fair value on a recurring basis consist only of the ordinary share warrant liability. Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the entire fair value measurement requires management to make judgments and consider factors specific to the asset or liability.
F-16
Table of Contents
3. Accounts receivable
Accounts receivable, net, consists of the following as of:
| December 31, | ||||||||
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Accounts receivable |
$ | 2,411 | $ | 5,544 | ||||
| Less allowance for uncollectible accounts receivable |
(200 | ) | (144 | ) | ||||
|
|
|
|||||||
| Accounts receivable, net |
$ | 2,211 | $ | 5,400 | ||||
|
|
||||||||
Activity for the allowance for uncollectible accounts receivable is as follows:
| Year ended December 31, | ||||||||
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Balance at beginning of period |
$ | (46 | ) | $ | (200 | ) | ||
| Provision for bad debt expense |
(154 | ) | — | |||||
| Write-off, net of recoveries |
— | 56 | ||||||
|
|
|
|||||||
| Balance at end of period |
$ | (200 | ) | $ | (144 | ) | ||
|
|
||||||||
4. Inventory
Inventory consisted of the following as of:
| December 31, | ||||||||
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Raw materials |
$ | 1,485 | $ | 857 | ||||
| Finished goods |
490 | 2,216 | ||||||
|
|
|
|||||||
| Inventory, net |
$ | 1,975 | $ | 3,073 | ||||
|
|
||||||||
5. Property and equipment, net
Property and equipment, net consists of the following as of:
| December 31, | ||||||||
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Laboratory equipment |
$ | 1,535 | $ | 1,926 | ||||
| Office equipment, furniture and fixtures |
851 | 1,319 | ||||||
| Leasehold improvements |
1,075 | 1,184 | ||||||
| Software |
313 | 493 | ||||||
| Construction in progress |
— | 149 | ||||||
| Specialized shipping containers |
343 | 338 | ||||||
|
|
|
|||||||
| Property and equipment |
4,117 | 5,409 | ||||||
| Less accumulated depreciation |
(2,440 | ) | (3,160 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 1,677 | $ | 2,249 | ||||
|
|
||||||||
For the years ended December 31, 2011 and December 31, 2012, the Company recorded depreciation expense of $0.6 million and $0.7 million, respectively. Depreciation expense includes amortization of capital leases.
F-17
Table of Contents
Depreciable lives range from three to ten years for laboratory equipment, office equipment and furniture and fixtures and three years for software and specialized shipping containers.
For the years ended December 31, 2011 and 2012, there were no material capital leases, disposals or retirements.
6. Intangible assets
The Company’s intangible assets consist of in-licensed intellectual property, principally technology licenses. During the year ended December 31, 2012, the Company entered into a new license agreement under which it capitalized $0.1 million. There were no amounts capitalized during the year ended December 31, 2011. The licenses are being amortized over the estimated remaining useful lives of the underlying license agreements, which range from 4 to 10 years.
Intangible assets as of December 31, 2011 and 2012 consist of the following:
| December 31, | ||||||||
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Gross carrying value |
$ | 521 | $ | 626 | ||||
| Accumulated amortization |
(467 | ) | (519 | ) | ||||
|
|
|
|||||||
| Intangible assets, net |
$ | 54 | $ | 107 | ||||
|
|
||||||||
7. Prepaid expenses
Prepaid expenses consist of the following as of:
| December 31, | ||||||||
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Prepaid insurance |
$ | 196 | $ | 309 | ||||
| Expense reimbursement, potential acquisition |
— | 250 | ||||||
| Deferred cost of revenue |
87 | 247 | ||||||
| Payroll and other taxes |
353 | 228 | ||||||
| Property costs |
193 | 151 | ||||||
| Other prepaid expenses |
200 | 157 | ||||||
|
|
|
|||||||
| Total prepaid expenses |
$ | 1,029 | $ | 1,342 | ||||
|
|
||||||||
8. Accrued expenses
Accrued expenses consist of the following as of:
| December 31, | ||||||||
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Employee related expenses |
$ | 1,240 | $ | 1,863 | ||||
| Professional services |
129 | 250 | ||||||
| Royalties |
381 | 1,090 | ||||||
| Rent |
247 | 296 | ||||||
| Inventory |
132 | 87 | ||||||
| Other accrued expenses |
204 | 376 | ||||||
|
|
|
|||||||
| Total accrued expenses |
$ | 2,333 | $ | 3,962 | ||||
|
|
|
|||||||
|
|
||||||||
F-18
Table of Contents
9. Borrowings
In February 2012 the Company entered into a secured credit facility with a commercial bank that provided for borrowings of up to $3.0 million originally through February 2013 and extended through May 2013. In February 2012 the Company borrowed $1.5 million under the credit facility. Interest accrued daily on the outstanding balance at the prime rate plus 1.5% per annum, with a minimum of the Daily Adjusting LIBOR rate plus 2.5% per annum. The credit facility was secured by substantially all assets of the Company. The total amount outstanding on the facility as of December 31, 2012 was $1.5 million. As of December 31, 2012 the Company was in compliance with all financial and non-financial covenants under this credit facility. The loan was re-paid in full on May 24, 2013.
In connection with this credit facility, the Company issued a warrant to purchase up to 24,691 ordinary shares of the Company at an exercise price of $0.01 per ordinary share. The warrant became exercisable immediately upon entering the secured credit facility and expires in February 2019. The fair value of the warrant was $3,000 at the date of grant and was determined by applying the Black-Scholes option pricing model, using the following assumptions:
| Fair value at date of grant |
Valuation technique | Assumption | Input value |
|||||||||
|
|
|
|
|
|||||||||
| Warrant liability |
$ | 3,000 | Black-Scholes option pricing model | Expected volatility | 37.6% | |||||||
| Estimated fair value of ordinary share |
$ |
0.14 |
| |||||||||
| Exercise price |
$ | 0.01 | ||||||||||
| Expected term |
3 yrs | |||||||||||
| Dividend yield |
0.0% | |||||||||||
| Risk-free interest rate |
0.37% | |||||||||||
|
|
|
|
|
|||||||||
In February 2012, the Company entered into an unsecured convertible note agreement with existing investors allowing the Company to borrow a total of $4.0 million. The Company issued unsecured convertible notes (the 2012 Notes) in two separate tranches of $3.0 million and $1.0 million in March 2012 and April 2012, respectively. The 2012 Notes matured four months after funding. The 2012 Notes bore interest at 10% per annum and interest was payable upon redemption or conversion.
Concurrently with the issuance of each tranche of the 2012 Notes the Company was obligated to pay the holders of the 2012 Notes a facility fee, payable in F preferred units, which consists of one F preferred ordinary share and one third of an ordinary share per unit. The number of F Preferred Units issued was equal to 50% of the nominal amount of each tranche of the 2012 Notes, divided by $1.622, with no fractional shares issued. 924,770 F preferred ordinary shares and 308,246 ordinary shares were issued in connection with the March 2012 tranche and 308,257 F preferred ordinary shares and 102,748 ordinary shares were issued in connection with the April 2012 tranche. The fair value of the F Preferred Units on the dates of issuance totaled approximately $2.0 million. The proceeds from the 2012 Notes were allocated to the 2012 Notes and F Preferred Units based on the relative fair value of each on the issuance dates. The F Preferred Units were recorded as a discount to the 2012 Notes carrying value of $1.3 million that will be amortized to interest expense over the term of the 2012 Notes.
F-19
Table of Contents
The 2012 Notes were (1) automatically convertible upon the consummation of a qualifying equity fundraising into the class of shares to be issued to investors participating in the fundraising at the price per share at which such shares would be offered, (2) automatically convertible upon the consummation of a non-qualifying equity fundraising into either the class of shares to be issued to investors participating in the fundraising at the price per share at which such shares would be offered or F Preferred Units at a price of $1.622 per unit, as elected by holders of at least 65% of the nominal amount of outstanding notes, or (3) convertible into F Preferred Units at a price of $1.622 per unit, at the option of the holders of at least 65% of the nominal amount of outstanding notes upon the consummation of a debt fundraising.
The feature which required automatic conversion upon a qualifying or non-qualifying equity fundraising is a redemption feature that meets the definition of an embedded derivative and requires bifurcation from the 2012 Notes. The derivative was recorded as a liability with a corresponding discount to the 2012 Notes’ carrying value at its fair value of $0.1 million. The discount was amortized to interest expense over the term of the 2012 Notes.
The feature which provided for the optional conversion upon the consummation of a debt fundraising represents a beneficial conversion feature. Because the beneficial conversion feature was contingent upon a future debt fundraising that was not certain to occur, the beneficial conversion feature was not recognized in these financial statements until the contingency was resolved. In June 2012 the Company closed the first tranche of the G preferred ordinary share financing round. Both tranches of the 2012 Notes were converted into a total of 2,352,941 shares of G preferred ordinary shares. In a conversion of a convertible bond pursuant to the original conversion terms the debt was settled in exchange for equity and no gain or loss was recognized on conversion. At the conversion date the discount on the borrowing was fully amortized. The redemption feature was adjusted to its fair value of zero upon conversion and the liability was reduced to this amount with an offsetting adjustment to interest expense.
As of December 31, 2012 the Company was in compliance with all financial and non-financial covenants relating to the 2012 Notes, as the 2012 Notes converted in June 2012.
10. Share capital
a. Preferred ordinary shares
The following is a summary of the Company’s preferred ordinary shares.
Preferred ordinary shares consisted of the following as of December 31, 2012:
| Preferred shares authorized |
Preferred ordinary shares issued and outstanding |
|||||||
|
|
||||||||
| A preferred ordinary |
903,220 | 903,220 | ||||||
| B preferred ordinary |
362,020 | 362,020 | ||||||
| D preferred ordinary |
3,488,448 | 3,266,885 | ||||||
| E preferred ordinary |
32,000,000 | 17,081,014 | ||||||
| F preferred ordinary |
20,000,000 | 17,262,618 | ||||||
| G preferred ordinary |
25,000,000 | 10,079,933 | ||||||
|
|
|
|||||||
| 81,753,688 | 48,955,690 | |||||||
|
|
||||||||
F-20
Table of Contents
Preferred ordinary shares consisted of the following as of December 31, 2011:
| Preferred shares authorized |
Preferred ordinary shares issued and outstanding |
|||||||
|
|
||||||||
| A preferred ordinary |
903,220 | 903,220 | ||||||
| B preferred ordinary |
362,020 | 362,020 | ||||||
| D preferred ordinary |
3,488,448 | 3,266,885 | ||||||
| E preferred ordinary |
32,000,000 | 17,081,014 | ||||||
| F preferred ordinary |
20,000,000 | 16,029,591 | ||||||
|
|
|
|||||||
| 56,753,688 | 37,642,730 | |||||||
|
|
||||||||
In February 2011 the Company issued 2,055,077 ordinary shares and 6,165,231 F preferred ordinary shares for consideration of $10 million cash in the third and final tranche of the F preferred ordinary share financing round.
The Company issued 924,770 F preferred ordinary shares and 308,246 ordinary shares in March 2012 and 308,257 F preferred ordinary shares and 102,748 ordinary shares in April 2012 in the form of F Preferred Units as a financing fee for the Company’s $4 million issuance of the 2012 Notes.
In June 2012, the Company issued 10,027,084 G preferred ordinary shares for consideration of $17 million in the first tranche of the G preferred ordinary share financing round. The Company issued an additional 52,849 G preferred ordinary shares in June 2012 as payment of interest on the 2012 Notes.
In January 2013, the Company issued 6,479,823 G preferred ordinary shares for consideration of $11 million cash in the second and final tranche of the G preferred ordinary share financing round.
The rights, preferences and privileges of the Company’s A preferred ordinary shares, B preferred ordinary shares, D preferred ordinary shares, E preferred ordinary shares, F preferred ordinary shares and G preferred ordinary shares (collectively, the preferred ordinary shares) are as follows:
Voting and consent rights—The preferred ordinary shares in issue rank pari passu with regards to voting rights. Holders of preferred ordinary shares are entitled to vote on all matters and are entitled to the number of votes equal to the number of ordinary shares into which each preferred ordinary share is then convertible. The consent of the holders of at least 60% of the E, F, and G preferred ordinary shares outstanding (taken together as a single class) is required for certain corporate actions including a deemed liquidation event, sale of all or a substantial portion of the Company’s assets or the creation of any debt of the Company in excess of $2,000,000. The approval of the holders of G preferred ordinary shares is required for any amendment or change to the Company’s articles of association that would be disproportionately adverse to the holders of G preferred ordinary shares and not similarly adverse to the rights of the holders of the other preferred ordinary shares and for the creation of any security convertible into a security having rights, preferences or privileges senior to the G preferred ordinary shares.
Liquidation rights—Upon the liquidation of the Company, including certain transactions deemed to be a liquidation, the holders of G preferred ordinary shares and, as a separate class, the holders of F preferred ordinary shares have a liquidation preference to all other holders of preferred ordinary shares and ordinary shares, in an amount equal to, in the case of the G preferred ordinary shares, 1.25 times the original issue price of $1.70 per share, and, in the case
F-21
Table of Contents
of the F preferred ordinary shares, 1.50 times the original issue price of $1.216 per share. The liquidation preference for each of the holders of the G preferred ordinary shares and the F preferred ordinary shares is limited to 50% of the assets or sale amount available for distribution. In the event that the assets or sale amount is insufficient to make such distributions to the holders of G preferred ordinary shares and F preferred ordinary shares separately, then the holders of G preferred ordinary shares and F preferred ordinary shares will participate, within their own classes, pro rata to their respective shareholdings of G preferred ordinary shares and F preferred ordinary shares, respectively. In the event that 50% of the assets or sale amount available for distribution is sufficient to satisfy one but not the other of the G preferred ordinary share preference and the F preferred ordinary share preference, separately, then any undistributed amount of assets or sale amount would be distributed to either the holders of G preferred ordinary shares or F preferred ordinary shares, as the case may be.
Subsequent to the payments of the liquidation preferences of the G preferred ordinary shares and the F preferred ordinary shares, each holder of E preferred ordinary shares would receive an amount equal to the aggregate amount paid by such holder for such shares, which is $2.616 for the shares acquired in the first tranche in October 2007, $2.616 for the shares acquired in the second tranche in August 2008 and £0.001 for the shares acquired pursuant to cashless exercise of warrants issued in October 2007.
After the payments of the liquidation preferences to holders of G preferred ordinary shares, F preferred ordinary shares and E preferred ordinary shares in full, the remaining assets or sale amount would generally be paid, depending on the amount available for distribution, to holders of all shares based on their respective preferences or, if no preferences are applicable, to all holders on an as-converted basis.
Transfer restrictions—The preferred ordinary shares may be transferred to any person with the prior consent in writing of holders of shares entitled to cast 90% of the votes exercisable at a general meeting of the Company. The preferred ordinary shares may be transferred at any time, without prior consent, to certain parties including, where the shares are held by individual members, to certain privileged relations and family trusts; where the shares are held by a company, to a member of the same group as such company; where the shares are held by an investment manager, to a participant or partner in or member of an investment fund which is managed by such investment manager, an investment fund whose business is managed by the investment manager, any other investment manager who manages the business of the investment fund in respect of which the shares are held, or any other person if required by a regulatory authority; where the shares are held by an investment fund, to a participant or partner in or member of such investment fund, any other investment fund whose business is managed by the same investment manager, or the investment manager who manages the business of the investment fund; where the shares are held by trustees under an employee trust, to the new trustees of that employee trust on any change of trustees or to any beneficiary of that employee trust.
Anti-dilution rights—In the event of a relevant issue of securities at a price which, in the case of the G preferred ordinary shares, is less than the original issue price of $1.70 per share, the Company shall issue to each holder of G preferred ordinary shares such number of ordinary shares as would result in such holder of G preferred ordinary shares holding such number of shares as would be held if the aggregate original issue price of $1.70 per share in respect of all G preferred ordinary shares then held by such holder was applied wholly in subscribing for the new shares at the weighted-average subscription price in respect of the relevant issue. In the event of
F-22
Table of Contents
a relevant issuance of securities at a price which, in the case of the F preferred ordinary shares and E preferred ordinary shares, is less than the original issue price of $1.216 per share, the Company shall issue to each holder of E preferred ordinary shares and each holder of F preferred ordinary shares, with the exception of holders of F preferred ordinary shares who received such shares pursuant to the issuance of F preferred units, such number of ordinary shares as would result in such holders holding such number of shares as would be held if the aggregate original issue price of $1.216 per share in respect of all shares then held by such holder was applied wholly in subscribing for the new shares at the weighted-average subscription price in respect of the relevant issue. In June 2012, the anti-dilution rights resulted in the issuance of 5,483,085 ordinary shares to the holders of the E preferred ordinary shares. The transaction did not result in the recognition of a beneficial conversion feature as the effective conversion price of the shares exceeded the fair value of the ordinary shares at the date of issuance. Other than the issuance of share capital the transaction did not have accounting implications. In the event that additional shares are issued as a result of the anti-dilution rights the Company will record the issuance of share capital and assess whether a beneficial conversion feature or other accounting implications are present. The anti-dilution rights do not preclude the classification of the shares as permanent equity.
Conversion—Upon a conversion, each preferred ordinary share would automatically be converted to, re-designated as, and ranked pari passu with the ordinary shares then in issue immediately prior to and conditional upon a qualified listing on a one-for-one basis. Each preferred ordinary share is convertible into one ordinary share at any time at the holder’s request.
The Company classifies its convertible preferred ordinary shares as permanent equity, as they do not contain redemption rights or other terms that would require classification outside of permanent equity.
b. Ordinary shares
The Company has three classes of ordinary shares authorized, which have identical rights.
Ordinary shares consisted of the following as of December 31, 2012:
| Ordinary authorized |
Ordinary shares issued and |
|||||||
|
|
||||||||
| Ordinary shares |
110,000,000 | 14,362,825 | ||||||
| A ordinary shares |
79,750 | 79,750 | ||||||
| D ordinary shares |
100,000 | — | ||||||
|
|
|
|||||||
| 110,179,750 | 14,442,575 | |||||||
|
|
||||||||
Ordinary shares consisted of the following as of December 31, 2011:
| Ordinary authorized |
Ordinary shares |
|||||||
|
|
||||||||
| Ordinary shares |
60,000,000 | 8,412,425 | ||||||
| A ordinary shares |
79,750 | 79,750 | ||||||
| D ordinary shares |
100,000 | — | ||||||
|
|
|
|||||||
| 60,179,750 | 8,492,175 | |||||||
|
|
||||||||
F-23
Table of Contents
11. Share option and equity incentive plans
Since 2003, the Company has issued share options to incentivize employees and directors providing services to the Company. Prior to 2010, the Company issued options out of multiple plans.
In February 2010, the Company adopted an Amended and Restated 2008 Stock Incentive Plan (the Plan) which provides for the grant of share options, restricted shares, restricted share units (RSUs) and other share-based awards to employees, officers, directors and consultants of the Company. The Plan reserves ordinary shares equal to 14.6% of the fully diluted share capital of the Company on an as converted basis.
In February 2010, in order to further incentivize employees, the Company offered all option holders the opportunity to reduce the exercise price of their existing options through a tender offer. As part of this offer, the option holder had to agree to the cancellation of their existing share options and the reissuance of new share options under the Plan. Additional compensation cost resulting from the tender offer was approximately $14,000, of which approximately $8,000 was recognized during 2010 and approximately $6,000 was recognized over a maximum remaining vesting period of three years. As of March 2010, with the exception of an immaterial number of share options, all of the Company’s share options outstanding are governed by the Plan.
Under the Plan, share options have been granted to employees, officers and directors who provide services to the Company. Options generally vest based on the grantee’s continued service with the Company during a specified period following grant or, in rare instances, based on the achievement of performance or other conditions as determined by the Board of Directors, and expire after ten years. Awards to employees generally vest monthly over a four year period; however, the vesting percentage remains 0% until the second anniversary of the vesting start date of the employee’s first option award. The expense recognized during the year related to share-based compensation transactions was as follows:
| Year ended December 31, | ||||||||
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Cost of revenue |
$ | 4 | $ | 2 | ||||
| General and administrative |
85 | 55 | ||||||
| Research and development |
4 | 4 | ||||||
| Sales and marketing |
32 | 18 | ||||||
|
|
|
|||||||
| Total share-based compensation |
$ | 125 | $ | 79 | ||||
|
|
||||||||
The Company engages a third-party consultant to assist the Board of Directors in the determination of the estimated fair market value of the Company’s ordinary shares. The share price is determined by the Board of Directors using contemporaneous valuations. In certain instances, the valuation was delivered after the date the options were granted, but was retrospective to an earlier date specified in the valuation report.
Recent transactions in the Company’s shares completed by independent investors represented the best indication of fair value of the securities. In addition, new rounds of venture capital financing, which reflect the expectations of independent investors with respect to the Company’s future performance, usually provide a good indication of the fair value of the ordinary shares. In this case, the fair value of the ordinary shares, was derived based on the price paid by the
F-24
Table of Contents
venture capital investors for the preferred ordinary shares, taking into account the differences in various rights and liquidation preferences between ordinary shares and the preferred ordinary shares. This is also known as the back-solve approach. In cases where there were no transactions or new financings, the use of a discounted cash flow analysis and guideline public firm multiples, adjusted for unique characteristics of the Company were used, as accepted methodologies.
The fair value of the options is estimated at the grant date using the Black-Scholes option pricing model, taking into account the terms and conditions upon which options are granted. The fair value of the options is amortized on a straight-line basis over the requisite service period of the awards. The weighted-average grant date fair value per share relating to share options granted under the Plan during the years ended December 31, 2011 and 2012 was $0.02 and $0.04, respectively.
The fair value of each option granted under the Plan has been calculated on the date of grant using the following weighted-average assumptions:
| 2011 | 2012 | |||||||
|
|
||||||||
| Expected dividend yield (%) |
— | — | ||||||
| Expected volatility (%) |
40.01 | 39.58 | ||||||
| Risk-free interest rate (%) |
1.75 | 1.03 | ||||||
| Expected life of option (years) |
6.25 | 6.25 | ||||||
| Weighted-average share price (USD) |
0.04 | 0.09 | ||||||
| Weighted-average exercise price (USD) |
0.04 | 0.09 | ||||||
| Model used |
Black-Scholes Model | Black-Scholes Model | ||||||
|
|
||||||||
Expected dividend yield: The Company has not paid and does not anticipate paying any dividends in the foreseeable future.
Risk-free interest rate: The Company determined the risk-free interest rate by using a weighted-average equivalent to the expected term based on the U.S. Treasury yield curve in effect as of the date of grant.
Expected volatility: As the Company has been operating as a private company, there is not sufficient historical volatility for the expected term of the options. Therefore, the Company used an average historical share price volatility based on an analysis of reported data for a peer group of comparable companies which were selected based upon industry similarities.
Expected term (in years): Expected term represents the period that the Company’s share option grants are expected to be outstanding. As the Company has been operating as a private company, there is not sufficient historical share data to calculate the expected term of the options. Therefore, the Company elected to utilize the “simplified” method to value share option grants. Under this approach, the weighted-average expected life is presumed to be the average of the vesting term and the contractual term of the option.
Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from estimates. The Company estimates forfeitures based on historical termination behavior. For the years ended December 31, 2011 and 2012, a forfeiture rate of 5% was applied.
F-25
Table of Contents
The following table illustrates the number of ordinary shares and weighted-average exercise prices (WAEP) of, and movements in, share options during the year:
| Number of ordinary shares | WAEP in $ | |||||||
|
|
||||||||
| Outstanding as of January 1, 2011 |
5,424,510 | $ | 0.02 | |||||
| Granted |
360,500 | 0.04 | ||||||
| Exercised |
(29,917 | ) | 0.01 | |||||
| Forfeited |
(500,046 | ) | 0.02 | |||||
|
|
|
|||||||
| Outstanding as of December 31, 2011 |
5,255,047 | $ | 0.02 | |||||
|
|
|
|||||||
| Exercisable as of December 31, 2011 |
2,305,898 | $ | 0.03 | |||||
|
|
|
|||||||
| Outstanding as of January 1, 2012 |
5,255,047 | $ | 0.02 | |||||
| Granted |
4,600,766 | 0.09 | ||||||
| Exercised |
(62,922 | ) | 0.02 | |||||
| Forfeited |
(91,229 | ) | 0.04 | |||||
|
|
|
|||||||
| Outstanding as of December 31, 2012 |
9,701,662 | $ | 0.05 | |||||
|
|
|
|||||||
| Vested or expected to vest as of December 31, 2012 |
9,396,331 | $ | 0.05 | |||||
|
|
|
|||||||
| Exercisable as of December 31, 2012 |
3,595,002 | $ | 0.02 | |||||
|
|
||||||||
As of December 31, 2012, there was $0.1 million of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the share option plan. That cost is expected to be recognized over a weighted-average period of 2.3 years.
A summary of options outstanding and exercisable as of December 31, 2012, follows:
| Total options outstanding | Total options exercisable | |||||||||||||||
| Exercise prices $ |
Number of options | Weighted-average remaining life in years |
Number of options | Weighted-average remaining life in years |
||||||||||||
|
|
||||||||||||||||
| 0.013 |
4,392,805 | 3,178,731 | ||||||||||||||
| 0.026 |
1,305,224 | 53,589 | ||||||||||||||
| 0.038 |
128,000 | 37,564 | ||||||||||||||
| 0.040 |
782,853 | 268,984 | ||||||||||||||
| 0.120 |
3,036,647 | — | ||||||||||||||
| 0.163 |
3,158 | 3,158 | ||||||||||||||
| 0.470 |
52,976 | 52,976 | ||||||||||||||
|
|
|
|||||||||||||||
| 9,701,663 | 8.16 | 3,595,002 | 6.71 | |||||||||||||
|
|
||||||||||||||||
The aggregate intrinsic value of all share options outstanding under the Plan as of December 31, 2011 and 2012 is $107,000 and $503,000, respectively. The aggregate intrinsic value of share options that were fully vested under the Plan as of December 31, 2012 is $96,000.
During the years ended December 31, 2011 and 2012, current and former employees of the Company exercised a total of 29,917 and 62,922 share options, respectively, resulting in total proceeds of less than $1,000 and $1,000, respectively. The intrinsic value of share options exercised during the years ended December 31, 2011 and 2012 was less than $1,000 and $6,000, respectively. In accordance with Company policy, the shares were issued from a pool of shares reserved for issuance under the Plan described above.
F-26
Table of Contents
A summary of the activity of the Company’s unvested share options is as follows:
| Number of shares | Weighted-average grant date fair value |
|||||||
|
|
||||||||
| Balance as of December 31, 2011 |
2,949,150 | $ | 0.01 | |||||
| Granted |
4,600,766 | 0.04 | ||||||
| Vested |
(1,356,175 | ) | 0.01 | |||||
| Forfeited |
(87,080 | ) | 0.02 | |||||
|
|
|
|||||||
| Balance as of December 31, 2012 |
6,106,661 | $ | 0.03 | |||||
|
|
||||||||
The total fair value of shares vested for the years ended December 31, 2011 and 2012 was $7,000 and $12,000, respectively.
Shares available for grant under the Plan were 1,795,616 and 1,068,415 as of December 31, 2011 and 2012, respectively.
In June 2012, the Company established the Incentive Bonus Plan for Holders of Company Share Options, or the Incentive Bonus Plan, to establish a retention bonus pool to provide incentives for employees to continue service through the closing of certain major corporate transactions. Generally, participants in the Incentive Bonus Plan will receive an amount equal to 11% of the value of the “total consideration” in a corporate transaction (defined as the amount available to shareholders and participants after payment of all expenses relating to the corporate transaction) as a result of (a) their holdings of ordinary shares acquired as a result of the exercise of their options or otherwise pursuant to one of our equity incentive plans taken together with (b) the payment to be made pursuant to the Incentive Bonus Plan. The 11% value is subject to downward adjustment for additional equity capital raises following the completion of the G preferred ordinary share financing. By its terms, the Incentive Bonus Plan will automatically terminate upon completion of an IPO.
12. Net (loss) per share
The following table provides a reconciliation of the numerator and denominator used in computing basic and diluted net loss per share:
| Year ended December 31, | ||||||||
| 2011 | 2012 | |||||||
|
|
||||||||
| (In thousands, except share and per share data) |
||||||||
| Numerator: |
||||||||
| Net loss attributable to ordinary shareholders |
$ | (13,104 | ) | $ | (14,883 | ) | ||
| Denominator: |
||||||||
| Weighted-average ordinary shares outstanding-basic |
8,150,146 | 11,825,803 | ||||||
| Dilutive effect of ordinary share equivalents resulting from ordinary share options, ordinary share warrants and preferred ordinary shares (as converted) |
— | — | ||||||
|
|
|
|||||||
| Weighted-average ordinary shares outstanding-diluted |
8,150,146 | 11,825,803 | ||||||
|
|
||||||||
F-27
Table of Contents
The following numbers of outstanding ordinary share options, ordinary share warrants and preferred ordinary shares (on an “as converted to ordinary shares” basis) were excluded from the computation of diluted net loss per share for the periods presented because their effect would have been anti-dilutive:
| Year ended December 31, | ||||||||
| 2011 | 2012 | |||||||
|
|
||||||||
| Options to purchase ordinary shares |
1,916,835 | 7,574,352 | ||||||
| Ordinary share warrant |
— | 24,691 | ||||||
| Convertible preferred ordinary shares (as converted) |
37,642,730 | 48,955,690 | ||||||
|
|
||||||||
13. Related party transactions
The Company licenses certain intellectual property from Isis Innovation Limited (Isis), a wholly owned subsidiary of the University of Oxford. The Chancellor, Master and Scholars of the University of Oxford owned 0.4 million ordinary shares and 0.6 million preferred ordinary shares of the Company at December 31, 2011 and 0.5 million ordinary shares and 0.9 million preferred ordinary shares of the Company at December 31, 2012. In the years ended December 31, 2011 and 2012, the Company made payments in respect of intellectual property and patent maintenance related to the patents licensed from Isis in the amounts of $0.2 million and $0.3 million, respectively. Accounts payable in respect of these arrangements at December 31, 2011 and 2012 were $0.2 million and $0.3 million, respectively.
The Company purchases inventory from Mabtech AB, a shareholder that owned 0.3 million preferred ordinary shares of the Company at December 31, 2011 and 0.1 million ordinary shares and 0.4 million preferred ordinary shares of the Company at December 31, 2012. In the years ended December 31, 2011 and 2012 the Company made payments to Mabtech AB in the amount of $1.2 million and $1.7 million, respectively. Accounts payable in respect of these arrangements at December 31, 2011 and 2012 were immaterial.
14. Income taxes
The components of loss before income taxes are as follows for the years ended December 31:
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Domestic (United Kingdom) |
$ | (3,776 | ) | $ | (2,754 | ) | ||
| Foreign (United States) |
(9,447 | ) | (12,280 | ) | ||||
|
|
|
|||||||
| Loss before income taxes |
$ | (13,223 | ) | $ | (15,034 | ) | ||
|
|
|
|
|
|
||||
F-28
Table of Contents
The components for the income tax expense (benefit) are as follows for the years ended December 31:
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Current: |
||||||||
| Federal |
— | — | ||||||
| UK |
$ | (145 | ) | $ | (173 | ) | ||
| State |
26 | 22 | ||||||
|
|
|
|||||||
| Total current provision |
(119 | ) | (151 | ) | ||||
| Deferred: |
||||||||
| Federal |
— | — | ||||||
| UK |
— | — | ||||||
| State |
— | — | ||||||
|
|
|
|||||||
| Total deferred benefit |
— | — | ||||||
|
|
|
|||||||
| $ | (119 | ) | $ | (151 | ) | |||
|
|
||||||||
Deferred income taxes reflect the net tax effect of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
Significant components of the Company’s deferred tax assets are as follows for the years ended December 31:
| (in thousands) | 2011 | 2012 | ||||||
|
|
||||||||
| Deferred tax assets: |
||||||||
| Long term deferred tax assets: |
||||||||
| U.S. federal net operating losses |
$ | 8,868 | $ | 12,939 | ||||
| State net operating loss (net of federal) |
1,178 | 1,700 | ||||||
| U.K. net operating loss |
6,727 | 7,733 | ||||||
| Share options |
105 | 120 | ||||||
| Accrued expenses |
95 | 160 | ||||||
| Other |
120 | 97 | ||||||
| Short term deferred tax assets: |
||||||||
| Accrued expenses |
168 | 250 | ||||||
| Other assets |
295 | — | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
17,556 | 22,999 | ||||||
| Valuation allowance |
(16,263 | ) | (21,979 | ) | ||||
|
|
|
|||||||
| Total deferred tax assets |
1,293 | 1,020 | ||||||
| Deferred tax liabilities: |
||||||||
| Long term deferred tax liabilities: |
||||||||
| Other assets |
— | (70 | ) | |||||
| Cumulative translation adjustment |
(1,293 | ) | (950 | ) | ||||
|
|
|
|||||||
| Total deferred tax liabilities |
$ | (1,293 | ) | $ | (1,020 | ) | ||
|
|
||||||||
For the years ended December 31, 2011 and 2012, the Company had United Kingdom Net Operating Losses (U.K. NOLs) of $33.6 million and $38.7 million, respectively. U.S. federal net operating loss carryforwards for the years ended December 31, 2011 and 2012 were $26.1 million
F-29
Table of Contents
and $38.1 million, respectively. U.S. State net economic loss carryforwards for the years ended December 31, 2011 and 2012 were $22.9 million and $32.9 million, respectively.
The federal and state net operating loss carryforwards begin to expire in 2027 and 2021, respectively and the U.K. NOLs can be carried forward indefinitely.
For financial reporting purposes, a valuation allowance has been recognized to offset the deferred tax assets related to the carryforwards. The utilization of the loss carryforwards to reduce future income taxes will depend on the Company’s ability to generate sufficient taxable income prior to the expiration of the loss carryforwards. To date the Company has incurred significant operating losses. In addition, the maximum annual use of net operating losses and research credit carryforwards is limited in certain situations where changes occur in stock ownership.
The following table reflects the rollforward of the Company’s valuation allowance:
| (in thousands) | ||||
|
|
||||
| December 31, 2011 |
$ | 16,263 | ||
| Increase in valuation allowance |
5,716 | |||
|
|
|
|||
| December 31, 2012 |
$ | 21,979 | ||
|
|
||||
The Company is headquartered in the United Kingdom and qualifies as a Small Company in accordance with HM Revenue and Customs regulations; the effective U.K. corporate tax rate for the years ended December 31, 2011 and 2012 is 20%. The U.S. federal corporate tax rate is 34% for the years ended December 31, 2011 and 2012.
The Company reviewed its historical tax filings and tax positions and has determined no material uncertain tax positions exist at December 31, 2011 and 2012. The Company continues to monitor its tax filings and positions.
The Company generates research and development credits in the United Kingdom which are refundable. In the United Kingdom for each of the years ended December 31, 2011 and 2012 the Company has been reimbursed $0.2 million, for research and development tax credits. These are recorded as a reduction against income tax expense.
15. Intellectual property—license agreements
The Company has entered into three license agreements by which it has secured certain patent rights that are necessary to make, use and sell the T-SPOT.TB test. These license agreements are generally exclusive in the stated field, cover a worldwide territory, are royalty-bearing and give the Company the right to grant sublicenses. The Company has minimum royalty obligations under each license agreement, which continue so long as patents licensed under the agreement remain unexpired. The minimum contractual royalty payments after December 31, 2011 and 2012 are set forth in the commitments and contingencies table in Note 17, “Commitments and contingencies” to these consolidated financial statements.
The Company incurs royalties under each agreement based on its product and service revenue. The aggregate royalty expense relating to the three license agreements amounted to $1.5 million and $2.4 million for the years ended December 31, 2011 and 2012, respectively. The Company paid other license-related expenses, including patent prosecution expenses and the final milestone payments due under the agreements, amounting to $0.1 million and $0.1 million for the years ended December 31, 2011 and 2012, respectively. The aggregate royalty rate paid by
F-30
Table of Contents
the Company in the years ended December 31, 2011 and 2012 as a percentage of the gross product and service revenue of the Company, was 12% and 11%, respectively.
16. Employee benefit plans
In the United States, the Company has adopted a defined contribution plan (the U.S. Plan) which qualifies under Section 401(k) of the Internal Revenue Code. All U.S. employees of the Company who have attained 21 years of age are eligible for participation in the U.S. Plan upon employment. The effective date of the U.S. Plan is January 1, 2008. Under the U.S. Plan, participating employees may defer up to the Internal Revenue Service annual contribution limit. The Company does not match employee contributions.
In the United Kingdom, the Company has adopted a defined contribution plan (the U.K. Plan) which qualifies under the rules established by HM Revenue & Customs. The U.K. Plan allows all U.K. employees to contribute a minimum of 5% of salary with no maximum limit. The contribution is matched by the Company, up to a maximum of 5% of salary. The Company paid $0.4 million in matching contributions in each of the years ended December 31, 2011 and 2012 to the U.K. Plan.
17. Commitments and contingencies
Operating leases
The Company leases facilities under four non-cancelable operating leases, with terms that expire between 2013 and 2019. The Company leases office, laboratory and manufacturing space in Abingdon, U.K., which leases are due to expire on June 11, 2019. The Company leases office and laboratory space in Marlborough, Massachusetts, which lease expired on June 30, 2013. The Company leases laboratory space in Memphis, Tennessee, which lease is due to expire on December 31, 2016. For property in the United States, the Company has bank balances pledged as security as described in Note 1, “Description of business and significant accounting policies—Restricted cash” to these consolidated financial statements.
Future minimum lease payments required under the non-cancelable operating leases in effect as of December 31, 2012 are as follows:
| (in thousands) | December 31, 2011 |
December 31, 2012 |
||||||
|
|
||||||||
| Year 1 |
$ | 596 | $ | 627 | ||||
| Year 2 |
610 | 904 | ||||||
| Year 3 |
882 | 911 | ||||||
| Year 4 |
890 | 918 | ||||||
| Year 5 |
897 | 780 | ||||||
| Thereafter |
1,705 | 979 | ||||||
|
|
|
|||||||
| Total minimum lease payments |
$ | 5,580 | $ | 5,119 | ||||
|
|
||||||||
Rent expense is calculated on a straight-line basis over the term of the lease. Rent expense recognized under operating leases totaled $0.6 million and $0.7 million for the years ended December 31, 2011 and 2012, respectively.
As of December 31, 2012, the Company has an outstanding letter of credit in the amount of $0.3 million that serves as security with the landlord of its Memphis, Tennessee location and expires on December 31, 2015. This letter of credit is securitized by restricted cash.
F-31
Table of Contents
Purchase commitments
The Company has license agreements with third parties that provide for minimum royalty, license, and exclusivity payments to be paid by the Company for access to certain technologies. In addition, the Company pays royalties as a percent of revenue as described in Note 15, “Intellectual property—License agreements” to these consolidated financial statements.
Future minimum payments required under the license agreements in effect as of December 31, 2012 are as follows:
| (in thousands) | December 31, 2011 |
December 31, 2012 |
||||||
|
|
||||||||
| Year 1 |
$ | 1,220 | $ | 1,423 | ||||
| Year 2 |
1,390 | 1,484 | ||||||
| Year 3 |
1,440 | 1,598 | ||||||
| Year 4 |
1,560 | 1,598 | ||||||
| Year 5 |
1,560 | 1,598 | ||||||
| Thereafter |
6,210 | 4,776 | ||||||
|
|
|
|||||||
| Total minimum license payments |
$ | 13,380 | $ | 12,477 | ||||
|
|
||||||||
The Company has outstanding purchase obligations from its suppliers in the amount of $0.9 million as of December 31, 2012.
Legal contingencies
The Company is subject to claims and assessments from time to time in the ordinary course of business. The Company does not believe that any such matters, individually or in the aggregate, will have a material adverse effect on the Company’s business, financial condition, results of operations or cash flows.
Indemnification
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnification. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but that have not yet been made. To date, the Company has not paid any claims or been required to defend any action related to its indemnification obligations. However, the Company may record charges in the future as a result of these indemnification obligations.
In accordance with its articles of association, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving at the Company’s request in such capacity. There have been no claims to date, and the Company has director and officer insurance that may enable it to recover a portion of any amounts paid for future potential claims.
F-32
Table of Contents
18. Geographic revenue and long-lived assets distribution
The Company was domiciled in the United Kingdom and operated in three geographies: the United States, Europe and the Rest of the World (ROW) and Asia. Revenue and long-lived assets for the years ended December 31, 2011 and 2012 are shown in the following table:
| Revenue | Long-lived assets | |||||||||||||||
| Year ended December 31, |
Year ended December 31, |
|||||||||||||||
| (in thousands) | 2011 | 2012 | 2011 | 2012 | ||||||||||||
|
|
||||||||||||||||
| United States |
$ | 5,604 | $ | 10,366 | $ | 1,394 | $ | 1,742 | ||||||||
| United Kingdom |
1,800 | 2,466 | 283 | 314 | ||||||||||||
| Europe & ROW (excluding United Kingdom) |
3,787 | 4,064 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Europe & ROW |
5,587 | 6,530 | 283 | 314 | ||||||||||||
| Asia |
1,450 | 3,789 | — | 193 | ||||||||||||
|
|
|
|||||||||||||||
| Total |
$ | 12,641 | $ | 20,685 | $ | 1,677 | $ | 2,249 | ||||||||
|
|
||||||||||||||||
19. Subscription of G preferred ordinary shares
On December 31, 2012, the Company held $8.1 million in cash received from investors related to the closing of the second and final tranche of the G preferred ordinary share financing round, which was recorded in shareholders’ equity. As described in Note 20, “Subsequent events” to these consolidated financial statements, this cash was exchanged for G preferred ordinary shares on January 4, 2013.
20. Subsequent events
G Preferred ordinary shares Tranche 2
On January 4, 2013, the Company completed the second and final tranche of the G preferred ordinary share financing, raising $11.0 million.
Potential acquisition activity
During November 2012 the Company entered into an agreement to acquire the assets of another corporation. As part of the process, in December 2012 the Company deposited $0.3 million in an escrow account with an escrow agent that was recorded as restricted cash on the balance sheet as of December 31, 2012.
In January 2013 the Company’s agreement to purchase the assets was terminated and, in connection therewith the Company received the $0.3 million cash held in escrow. In February 2013 the Company received a break up fee in the amount of $0.2 million, which was recorded in other income in the first quarter of 2013, and authorized expense reimbursements of $0.3 million, recorded as an offset to the related general and administrative expenses.
New U.S. headquarters lease
On March 1, 2013, the Company signed a five year lease for its new U.S. corporate headquarters in Marlborough, Massachusetts. During June 2013, the Company moved into this facility and vacated the old facility prior to lease expiration on June 30, 2013. The new lease term runs from June 2013 to October 2018. Annual lease costs are approximately $0.3 million.
F-33
Table of Contents
Memphis, Tennessee grant
During May 2013, the Economic Development Growth Engine (EDGE) Industrial Development Board of the city of Memphis and county of Shelby, Tennessee paid $100,000 to the Company for the reimbursement of business and project expenses related to the establishment of its laboratory facility in Memphis, Tennessee. The proceeds from the grant were recorded as deferred income and are being recognized as a component of other income on a straight-line basis over the estimated useful life of the underlying assets.
Bank financing
On May 24, 2013, the Company refinanced its existing secured credit facility with a new borrowing arrangement with a commercial bank, comprised of a term loan and a revolving line of credit. The loan is secured by substantially all the Company’s assets. The Company concurrently issued a warrant to the bank to purchase up to 105,882 ordinary shares of the Company at an exercise price of $0.12 per share. Tranche A of the term loan, which was received at signing, is $6.0 million. For the first year, only interest is payable on the loan. After the first year, the outstanding balance plus all accrued interest is payable in 36 equal monthly installments through the maturity date of May 24, 2017.
Tranche B of the term loan allows the Company to borrow $1.0 million between January 1, 2014 and January 31, 2014, subject to the achievement of certain revenue milestones. Tranche B matures 36 months from the funding date. For the first 12 months only interest is due. After the first year, the loan is payable in 24 equal monthly installments. The term loan may be prepaid without penalty or premium and once prepaid, may not be reborrowed. The loan agreement contains certain restrictions, including restrictions on additional indebtedness, dispositions, dividend payments and future loans.
Bank interest rates for the Tranche A term loan are the greater of 2.75% above the prime rate or 6.0% per annum. Upon the achievement of certain revenue milestones and the Tranche B funding, the rate for Tranche A and B will be reduced to the greater of 2.5% above prime or 5.75% per annum. The revolving line of credit provides the Company funding of up to $5.0 million, has a maturity date of May 24, 2015 and bears interest at 1.75% above the prime rate or 5% per annum, whichever is greater.
The Company has restricted cash in the amount of less than $0.1 million pledged as collateral for procurement cards issued by the commercial bank.
Warrant liability
In connection with the May 2013 term loan, the Company issued a warrant to purchase up to 105,882 ordinary shares of the Company. The warrant was exercisable upon issuance and expires in May 2023. The exercise price was $0.12 per share. The warrant was issued with a down-round provision whereby the exercise price would be adjusted downward in the event that additional ordinary shares of the Company or securities exercisable, convertible or exchangeable for ordinary shares of the Company were issued at a price less than the exercise price. The fair value estimate of the Company’s warrant liability was determined by applying the Black-Scholes model. The Company believes that the Black-Scholes model is a reasonable valuation model for valuing these warrants considering the facts and circumstances pertaining to the terms of these warrants,
F-34
Table of Contents
and the Company does not believe that using an alternative or more complex valuation model (including, for example, a lattice pricing model) would have a material effect on, or otherwise materially impact, the consolidated financial statements. The fair values were derived by applying the following assumptions as of May 24, 2013 (unaudited):
| Fair value at June 30, 2013 |
Valuation technique | Assumption | Input range/ value | |||||||
|
| ||||||||||
| (unaudited) | ||||||||||
| Warrant liability |
17,100 | Black-Scholes option pricing model |
Expected volatility | 37.6% | ||||||
| Estimated fair value of ordinary share |
$0.24 | |||||||||
| Exercise price | $0.12 | |||||||||
| Expected term | 10 yrs | |||||||||
| Dividend yield | 0% | |||||||||
| Risk-free interest rate | 2.01% | |||||||||
|
| ||||||||||
Convertible promissory note
In October 2013, the Company issued and received the proceeds from a convertible promissory note in the amount of $5.0 million to Fosun Industrial Co., Ltd., which will convert at the time of this offering into the Company’s ordinary shares at a price per share which reflects a 10% discount to the offering price. The shares issuable upon conversion will be subject to restrictions prohibiting sale or transfer of more than one-third of the shares each year for the first three years following this offering. In the event that the note is not converted into equity, the note bears interest at the rate of 8% per annum, which is calculated and payable at the same time the principal is repaid. Unless otherwise converted into equity, the principal and interest must be repaid by July 1, 2016, although the Company may choose to repay the note earlier with no pre-payment penalties. The Company is in the process of addressing the accounting and related disclosures for this arrangement which will be recognized in the fourth quarter of 2013.
21. Pro forma net (loss) per share (unaudited)
The pro forma net (loss) per share is presented below to reflect the effect of the reorganization via a scheme of arrangement resulting in the exchange of equity interests in Oxford Immunotec Limited for identical equity interests in Oxford Immunotec Global PLC.
| (in thousands, except share and per share data) | Year ended December 31, 2012 |
|||
|
|
||||
| Net (loss) |
$ | |||
| Pro forma (loss) per share, basic and diluted |
||||
| Pro forma weighted-average number of shares, basic and diluted |
||||
|
|
||||
Outstanding options and warrants were excluded from the computation of pro forma net loss per share because their effect would have been anti-dilutive.
F-35
Table of Contents
Condensed consolidated balance sheets
(in thousands, except share and per share data)
(unaudited)
| December 31, | September 30, | |||||||
| 2012 | 2013 | |||||||
|
|
||||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 12,578 | $ | 13,035 | ||||
| Restricted cash |
387 | 87 | ||||||
| Accounts receivable, net |
5,400 | 7,181 | ||||||
| Inventory |
3,073 | 3,957 | ||||||
| Prepaid expenses and other |
1,342 | 3,886 | ||||||
|
|
|
|||||||
| Total current assets |
22,780 | 28,146 | ||||||
| Restricted cash, non-current |
287 | 362 | ||||||
| Property and equipment, net |
2,249 | 2,896 | ||||||
| Intangible assets, net |
107 | 74 | ||||||
| Other assets |
60 | 60 | ||||||
|
|
|
|||||||
| Total assets |
$ | 25,483 | $ | 31,538 | ||||
|
|
|
|||||||
| Liabilities and shareholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 1,754 | $ | 3,519 | ||||
| Accrued expenses |
3,962 | 5,250 | ||||||
| Deferred income |
908 | 2,148 | ||||||
| Revolving line of credit |
1,548 | — | ||||||
| Current maturities of long-term debt |
— | 667 | ||||||
| Current portion of loans payable |
79 | 78 | ||||||
| Taxes payable |
140 | 135 | ||||||
|
|
|
|||||||
| Total current liabilities |
8,391 | 11,797 | ||||||
| Warrant liability |
— | |
136 |
| ||||
| Long-term debt |
— | 5,318 | ||||||
| Long-term portion of loans payable |
143 | 84 | ||||||
|
|
|
|||||||
| Total liabilities |
$ | 8,534 | $ | 17,335 | ||||
|
|
|
|||||||
| Shareholders’ equity: |
||||||||
| Convertible preferred ordinary shares: |
||||||||
| A preferred ordinary shares, £0.001 par value; 903,220 shares authorized, 903,220 shares issued and outstanding at December 31, 2012 and September 30, 2013. |
$ | 2 | $ | 2 | ||||
| B preferred ordinary shares, £0.001 par value; 362,020 shares authorized, 362,020 shares issued and outstanding at December 31, 2012 and September 30, 2013. |
1 | 1 | ||||||
| D preferred ordinary shares, £0.001 par value; 3,488,448 shares authorized, 3,266,885 shares issued and outstanding at December 31, 2012 and September 30, 2013. |
5 | 5 | ||||||
| E preferred ordinary shares, £0.001 par value; 32,000,000 shares authorized, 17,081,014 shares issued and outstanding at December 31, 2012 and September 30, 2013. |
33 | 33 | ||||||
| F preferred ordinary shares, £0.001 par value; 20,000,000 shares authorized, 17,262,618 shares issued and outstanding at December 31, 2012 and September 30, 2013. |
26 | 26 | ||||||
| G preferred ordinary shares, £0.001 par value; 25,000,000 shares authorized, 10,079,933 and 16,559,756 shares issued and outstanding at December 31, 2012 and September 30, 2013 respectively. |
16 | 27 | ||||||
| Ordinary shares, £0.001 par value; 110,179,750 shares authorized, 14,442,575 and 15,677,098 shares issued and outstanding at December 31, 2012 and September 30, 2013, respectively. |
24 | 25 | ||||||
| Subscription of G preferred ordinary shares |
8,075 | — | ||||||
| Additional paid-in capital |
103,380 | 114,480 | ||||||
| Accumulated deficit |
(90,991 | ) | (96,327 | ) | ||||
| Accumulated other comprehensive loss |
(3,622 | ) | (4,069 | ) | ||||
|
|
|
|||||||
| Total shareholders’ equity |
16,949 | 14,203 | ||||||
|
|
|
|||||||
| Total liabilities and shareholders’ equity |
$ | 25,483 | $ | 31,538 | ||||
|
|
||||||||
See accompanying notes to these interim condensed consolidated financial statements.
F-36
Table of Contents
Condensed consolidated statements of operations
(in thousands, except share and per share data)
(unaudited)
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| 2012 | 2013 | 2012 | 2013 | |||||||||||||
|
|
||||||||||||||||
| Revenue |
||||||||||||||||
| Product |
$ | 2,383 | $ | 4,977 | $ | 6,807 | $ | 14,888 | ||||||||
| Service |
3,438 | 5,749 | 8,599 | 13,671 | ||||||||||||
|
|
|
|||||||||||||||
| Total revenue |
5,821 | 10,726 | 15,406 | 28,559 | ||||||||||||
| Cost of revenue |
||||||||||||||||
| Product |
1,045 | 2,158 | 3,116 | 6,767 | ||||||||||||
| Service |
2,342 | 2,773 | 6,007 | 7,398 | ||||||||||||
|
|
|
|||||||||||||||
| Total cost of revenue |
3,387 | 4,931 | 9,123 | 14,165 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
2,434 | 5,795 | 6,283 | 14,394 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
490 | 579 | 1,232 | 1,583 | ||||||||||||
| Sales and marketing |
2,942 | 3,325 | 7,895 | 9,557 | ||||||||||||
| General and administrative |
2,279 | 4,084 | 5,784 | 8,457 | ||||||||||||
|
|
|
|||||||||||||||
| Total operating expenses |
5,711 | 7,988 | 14,911 | 19,597 | ||||||||||||
|
|
|
|||||||||||||||
| Loss from operations |
(3,277 | ) | (2,193 | ) | (8,628 | ) | (5,203 | ) | ||||||||
| Other (expense) income: |
||||||||||||||||
| Interest income (expense) |
(19 | ) | (139 | ) | (1,452 | ) | (256 | ) | ||||||||
| Foreign exchange gains (losses) |
(327 | ) | (721 | ) | (492 | ) | 44 | |||||||||
| Other (expense) income |
— | (113 | ) | — | 114 | |||||||||||
|
|
|
|||||||||||||||
| Loss before income taxes |
(3,623 | ) | (3,166 | ) | (10,572 | ) | (5,301 | ) | ||||||||
| Income tax provision |
10 | 15 | 21 | 35 | ||||||||||||
|
|
|
|||||||||||||||
| Net loss |
$ | (3,633 | ) | $ | (3,181 | ) | $ | (10,593 | ) | $ | (5,336 | ) | ||||
|
|
|
|||||||||||||||
| Net loss per share attributable to ordinary shareholders—basic and diluted |
$ | (0.27 | ) | $ | (0.20 | ) | $ | (1.02 | ) | $ | (0.35 | ) | ||||
|
|
|
|||||||||||||||
| Weighted average shares used to compute net loss attributable to ordinary shareholders—basic and diluted |
13,416,765 | 15,635,995 | 10,338,893 |
|
15,129,791 |
| ||||||||||
|
|
||||||||||||||||
See accompanying notes to these interim condensed consolidated financial statements.
F-37
Table of Contents
Condensed consolidated statements of other comprehensive loss
(in thousands)
(unaudited)
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| 2012 | 2013 | 2012 | 2013 | |||||||||||||
|
|
||||||||||||||||
| Net loss |
$ | (3,633 | ) | $ | (3,181 | ) | $ | (10,593 | ) | $ | (5,336 | ) | ||||
| Other comprehensive income (loss): |
||||||||||||||||
| Foreign currency translation adjustment |
132 | 646 | 322 | (447 | ) | |||||||||||
|
|
|
|||||||||||||||
| Other comprehensive income (loss), net of taxes |
132 | 646 | 322 | (447 | ) | |||||||||||
|
|
|
|||||||||||||||
| Total comprehensive income (loss) |
$ | (3,501 | ) | $ | (2,535 | ) | $ | (10,271 | ) | $ | (5,783 | ) | ||||
|
|
||||||||||||||||
See accompanying notes to these interim condensed consolidated financial statements.
F-38
Table of Contents
Condensed consolidated statement of shareholder’s equity
(in thousands, except share and per share data)
(unaudited)
| Convertible preferred ordinary shares | ||||||||||||||||||||||||||||||||||||||||||||||||
| A preferred |
B preferred |
D preferred |
E preferred |
F preferred |
G preferred |
Ordinary | Subscription G preferred ordinary |
Additional paid-in capital |
Accumulated deficit |
Accumulated other comprehensive loss |
Total shareholders’ equity |
|||||||||||||||||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2012 |
$ | 2 | $ | 1 | $ | 5 | $ | 33 | $ | 26 | $ | 16 | $ | 24 | $ | 8,075 | $ | 103,380 | $ | (90,991 | ) | $ | (3,622 | ) | $ | 16,949 | ||||||||||||||||||||||
| Exercise of share options |
— | — | — | — | — | — | 1 | — | 17 | — | — | 18 | ||||||||||||||||||||||||||||||||||||
| Issuance of G preferred ordinary shares |
— | — | — | — | — | 11 | — | (8,075 | ) | 11,006 | — | — | 2,942 | |||||||||||||||||||||||||||||||||||
| Share-based compensation expense |
— | — | — | — | — | — | — | — | 77 | — | — | 77 | ||||||||||||||||||||||||||||||||||||
| Other comprehensive loss |
— | — | — | — | — | — | — | — | — | — | (447 | ) | (447 | ) | ||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (5,336 | ) | — | (5,336 | ) | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||
| Balance at September 30, 2013 |
$ | 2 | $ | 1 | $ | 5 | $ | 33 | $ | 26 | $ | 27 | $ | 25 | $ | — | $ | 114,480 | $ | (96,327 | ) | $ | (4,069 | ) | $ | 14,203 | ||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||
See accompanying notes to these interim condensed consolidated financial statements.
F-39
Table of Contents
Condensed consolidated statement of cash flows
(in thousands)
(unaudited)
| Nine months ended September 30, | ||||||||
| 2012 | 2013 | |||||||
|
|
||||||||
| Cash flows from operating activities |
||||||||
| Net loss |
$ | (10,593 | ) | $ | (5,336 | ) | ||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
||||||||
| Depreciation and amortization |
580 | 863 | ||||||
| Share-based compensation expense |
59 | 77 | ||||||
| Loss on change in fair value of warrants |
— | 119 | ||||||
| Loss on disposal of property and equipment |
30 | (1 | ) | |||||
| Noncash interest expense |
1,418 | — | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable, net |
(1,599 | ) | (1,788 | ) | ||||
| Inventory |
(447 | ) | (1,389 | ) | ||||
| Prepaid expenses and other |
226 | (2,445 | ) | |||||
| Accounts payable |
74 | 1,732 | ||||||
| Accrued expenses |
258 | 1,242 | ||||||
| Deferred income |
(28 | ) | 1,196 | |||||
|
|
|
|||||||
| Net cash provided by (used in) operating activities |
(10,022 | ) | (5,730 | ) | ||||
|
|
|
|||||||
| Cash flows from investing activities |
||||||||
| Purchases of property and equipment |
(1,048 | ) | (1,542 | ) | ||||
| Proceeds on sales of property and equipment |
— | 22 | ||||||
| Change in restricted cash |
— | 225 | ||||||
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
(1,048 | ) | (1,295 | ) | ||||
|
|
|
|||||||
| Cash flows from financing activities |
||||||||
| Proceeds from revolving line of credit |
1,500 | — | ||||||
| Proceeds from convertible note |
4,000 | — | ||||||
| Proceeds from term loan |
— | 6,000 | ||||||
| Proceeds from issuance of ordinary shares |
— | — | ||||||
| Proceeds from issuance of preferred ordinary shares |
12,702 | 2,942 | ||||||
| Proceeds from exercise of share options |
1 | 17 | ||||||
| Payments on loan |
(2 | ) | (60 | ) | ||||
| Payments on revolving line of credit |
— | (1,500 | ) | |||||
|
|
|
|||||||
| Net cash provided by (used in) financing activities |
18,201 | 7,399 | ||||||
|
|
|
|||||||
| Effect of exchange rate changes on cash and cash equivalents |
254 | 83 | ||||||
| Net increase (decrease) in cash and cash equivalents, excluding restricted cash |
7,131 | 374 | ||||||
| Cash and cash equivalents at beginning of period |
2,334 | 12,578 | ||||||
|
|
|
|||||||
| Cash and cash equivalents at end of period |
$ | 9,719 | $ | 13,035 | ||||
|
|
|
|||||||
| Supplemental disclosures |
||||||||
| Cash paid for interest |
56 | 127 | ||||||
| Cash paid (received) for taxes |
(148 | ) | 37 | |||||
| Noncash investing and financing activities |
||||||||
| Interest on convertible notes settled with G preferred ordinary shares |
90 | — | ||||||
| F preferred ordinary shares issued with convertible notes |
1,314 | — | ||||||
| Convertible notes converted into G preferred ordinary shares |
4,000 | — | ||||||
| Fair value of warrant issued with convertible notes |
— | 136 | ||||||
|
|
||||||||
See accompanying notes to these interim condensed consolidated financial statements.
F-40
Table of Contents
Notes to the interim condensed consolidated financial statements (unaudited)
Unaudited interim financial information
The accompanying balance sheet as of September 30, 2013, the statements of operations for the three and nine months ended September 30, 2012 and 2013, the statements of other comprehensive loss for the three and nine months ended September 30, 2012 and 2013, the statement of shareholders’ equity for the three and nine months ended September 30, 2013 and the statements of cash flows for the nine months ended September 30, 2012 and 2013 are unaudited. The unaudited interim condensed consolidated financial statements include all adjustments (consisting of normal recurring adjustments) which are necessary for a fair presentation of results for such interim periods. The information disclosed in the notes to the interim condensed consolidated financial statements for these periods is unaudited. The statements of operations for the three and nine months ended September 30, 2013 are not necessarily indicative of the results to be expected for the entire fiscal year or any future period.
1. Description of business and significant accounting policies
Reorganization, reverse share split and conversion
On October 2, 2013, the Scheme of Arrangement was approved by the High Court of Justice in England and Wales. All holders of ordinary shares, preferred ordinary shares, options and warrants exchanged their interests in Oxford Immunotec Limited for identical interests in Oxford Immunotec Global PLC. As a result of this exchange, Oxford Immunotec Global PLC is now the parent company of Oxford Immunotec Limited.
Prior to closing the IPO of Oxford Immunotec Global PLC, the Company will undertake a reverse share split of Oxford Immunotec Global PLC’s outstanding ordinary shares, which will result in a proportional decrease in the number of ordinary shares outstanding as well as appropriate adjustments to outstanding A ordinary shares, preferred ordinary shares, warrants and options. After the reverse share split and immediately prior to the IPO, all outstanding preferred ordinary shares will convert into ordinary shares. These interim condensed consolidated financial statements will be revised to give effect to these share changes for the most recent annual and interim periods presented.
Basis of presentation, accounting principles and principles of consolidation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (U.S. GAAP), and include the financial statements of Oxford Immunotec Limited, a company incorporated in England and Wales and its wholly-owned subsidiaries, collectively referred to as the Company. All intercompany accounts and transactions have been eliminated upon consolidation.
Concentration of risks
The Company derives product revenue from the sale of its T-SPOT.TB diagnostic test kits and related accessories to a broad range of customers including: hospitals, public health departments,
F-41
Table of Contents
commercial testing laboratories, importers and distributors. Importers and distributors sell to third parties including end-user customers in specific territories.
In the nine months ended September 30, 2013 the Company had two customers that individually represented more than 10% of total revenue. Collectively these customers contributed 34% of revenue in the nine months ended September 30, 2013. Shanghai Fosun Long March Medical Science Co. Ltd., the Company’s Chinese distributor, represented 17% of revenue, the loss of which could have a material impact on the Company’s operating results. One importer of record in Japan also contributed 17% of revenue in the nine months ended September 30, 2013.
The Company derives service revenue from its diagnostic laboratories in the United States and in the United Kingdom where the Company performs its T-SPOT.TB test on samples sent by customers to its laboratory facilities. In the nine months ended September 30, 2013 and 2012, the Company did not have any service customers that represented more than 10% of revenue.
Initial Public Offering (IPO) Costs
Incremental costs incurred that are directly attributable to a proposed or actual offering of securities are deferred and deducted from the related proceeds of the offering, and the net amount recorded as contributed shareholders’ equity in the period when such shares are issued. As at September 30, 2013, the Company had deferred initial offering costs of $1.8 million that are included in prepaid expenses and other in the Company’s unaudited interim condensed consolidated balance sheet. Other costs incurred in the offering of $1.6 million (which are principally related to audit expenses) in the three months ended September 30, 2013, are expensed as incurred and are included in general and administrative expenses.
2. Borrowings
In May 2013, the Company entered a loan and security agreement with a commercial bank that provided for an initial borrowing of $6.0 million and, subject to the achievement of certain revenue milestones, the ability to borrow an additional $1.0 million in January 2014. The Company also received access to a $5.0 million revolving line of credit. The Company concurrently issued a warrant to purchase up to 105,882 ordinary shares of the Company at an exercise price of $0.12 per share. The loan is secured by the substantially all assets of the Company. Interest accrues daily on the outstanding balance at the prime rate plus 2.75%, with a minimum 6.0% per annum. The loan agreement contains certain restrictions on the Company, including restrictions on additional indebtedness, dispositions, dividend payments and future loans. The warrant is exercisable upon issuance and expires in May 2023. The proceeds from the loan were first allocated to the warrant based upon the estimated fair value as of the issuance date, with the residual proceeds allocated to the term loan. This warrant was issued with a down-round provision whereby the exercise price would be adjusted downward in the event that additional ordinary shares or securities exercisable, convertible or exchangeable for the Company’s existing ordinary shares were issued at a price less than the exercise price. Therefore, the fair value of this warrant was recorded as a liability in the consolidated balance sheet and is marked to market at each reporting period end until it is exercised or expires or is otherwise extinguished. The fair value of the warrant was recorded as a liability upon issuance with a corresponding discount on the borrowing of $17,000 that will be amortized to interest expense over the term of the loan. The estimated fair value of the warrants at September 30, 2013 was $136,000. The change in the estimated fair value of the warrants during the three months ended September 30, 2013 was
F-42
Table of Contents
recorded as a component of other (expense) income in the condensed consolidated statement of operations. The fair value was derived by applying the following assumptions:
| Fair value at September 30, 2013 |
Valuation technique | Assumption | Input range/ value |
|||||||||
|
|
||||||||||||
| (unaudited) | ||||||||||||
| Warrant liability |
136,000 | Black-Scholes option pricing model |
Expected volatility | 32.9% | ||||||||
| Estimated fair value of ordinary share |
$ | 1.3794 | ||||||||||
| Exercise price | $ | 0.12 | ||||||||||
| Expected term | 10 yrs | |||||||||||
| Dividend yield | 0% | |||||||||||
| Risk-free interest rate | 2.64% | |||||||||||
|
|
||||||||||||
The total amount outstanding as of September 30, 2013 was $6.0 million.
3. Net (loss) income per share
Net loss attributable to ordinary shareholders per share is computed by dividing the net loss allocable to ordinary shareholders by the weighted-average number of ordinary shares outstanding. Outstanding share options, warrants and preferred ordinary shares, have not been included in the calculation of diluted net loss attributable to ordinary shareholders per share for the three and nine month periods ended September 30, 2012 and 2013 because to do so would be anti-dilutive. Accordingly, both the numerator and the denominator used in computing both basic and diluted net loss per share for these periods are the same.
The following table provides a reconciliation of the numerator and denominator used in computing basic and diluted net (loss) per share:
| Three months ended September 30, |
Nine months ended September 30, |
|||||||||||||||
| 2012 | 2013 | 2012 | 2013 | |||||||||||||
|
|
||||||||||||||||
| (In thousands, except share and per share data) | ||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| Numerator: |
||||||||||||||||
| Net loss attributable to ordinary shareholders |
$ | (3,633 | ) | $ | (3,181 | ) | $ | (10,593 | ) | $ | (5,336 | ) | ||||
| Denominator: |
||||||||||||||||
| Weighted-average ordinary shares outstanding-basic |
13,416,765 | |
15,635,995 |
|
10,338,893 | 15,129,791 | ||||||||||
| Dilutive effect of ordinary share equivalents resulting from ordinary share options, warrants and preferred ordinary shares (as converted) |
— | — | — | — | ||||||||||||
|
|
|
|||||||||||||||
| Weighted-average ordinary shares outstanding-diluted |
|
13,416,765 |
|
15,635,995 | 10,338,893 | 15,129,791 | ||||||||||
|
|
||||||||||||||||
F-43
Table of Contents
The following numbers of outstanding ordinary share options, ordinary share warrants and preferred ordinary shares (on an “as converted to ordinary shares” basis) were excluded from the computation of diluted net loss per share for the periods presented because their effect would have been anti-dilutive:
| Three months ended September 30, |
Nine months ended September 30, |
|||||||||||||||
| 2012 | 2013 | 2012 | 2013 | |||||||||||||
|
|
||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| Options to purchase ordinary shares |
6,689,017 | 8,759,821 | 6,689,017 | 8,759,821 | ||||||||||||
| Ordinary share warrant |
24,691 | 1,479,089 | 24,691 | 1,479,089 | ||||||||||||
| Convertible preferred ordinary shares (as converted) |
45,036,563 | 55,435,513 | 45,036,563 | 55,435,513 | ||||||||||||
|
|
||||||||||||||||
4. Related-party transactions
The Company licenses certain intellectual property from Isis Innovation Limited (Isis), a wholly owned subsidiary of the University of Oxford. The Chancellor, Master and Scholars of the University of Oxford owned 0.5 million ordinary shares and 1.0 million preferred ordinary shares of the Company as of September 30, 2013. In the nine months ended September 30, 2013 the Company made payments in respect of intellectual property and patent maintenance related to the patents licensed from Isis of $0.4 million. Accounts payable in respect of these arrangements were $0.3 million as of September 30, 2013.
The Company purchased inventory from Mabtech AB, a shareholder that owned 0.1 million ordinary shares of the Company and 0.3 million preferred ordinary shares of the Company, as of September 30, 2013. In the nine months ended September 30, 2013, the Company made payments to Mabtech AB of $1.7 million. Accounts payable in respect of these arrangements were $0.7 million as of September 30, 2013.
5. Subsequent events
Scheme of arrangement
On October 2, 2013, Oxford Immunotec Global PLC completed the scheme of arrangement described in Note 1, “Description of business and significant accounting policies—Reorganization, reverse share split and conversion” to these interim condensed consolidated financial statements.
Convertible promissory note
In October 2013, the Company issued and received the proceeds from a convertible promissory note in the amount of $5.0 million to Fosun Industrial Co., Ltd., which will convert at the time of this offering into the Company’s ordinary shares at a price per share which reflects a 10% discount to the offering price. The shares issuable upon conversion will be subject to restrictions prohibiting sale or transfer of more than one-third of the shares each year for the first three years following this offering. In the event that the note is not converted into equity, the note bears interest at the rate of 8% per annum, which is calculated and payable at the same time the principal is repaid. Unless otherwise converted into equity, the principal and interest must be repaid by July 1, 2016, although the Company may choose to repay the note earlier with no pre-payment penalties. The Company is in the process of addressing the accounting and related disclosures for this arrangement which will be recognized in the fourth quarter of 2013.
F-44
Table of Contents
6. Pro forma net (loss) per share (unaudited)
As a result of the material impact on net (loss) per share, the pro forma net (loss) per share is presented below to reflect:
| • | the 1-for- reverse share split |
| • | the conversion of all outstanding preferred ordinary shares and A ordinary shares into ordinary shares |
| • | the issuance of ordinary shares in connection with a proposed Initial Public Offering (IPO) |
| • | repayment of indebtedness outstanding under senior secured term debt |
| (in thousands, except share and per share data) | Nine months ended September 30, 2013 |
|||
|
|
||||
| Net (loss) |
$ | |||
| Pro forma (loss) per share, basic and diluted |
||||
| Pro forma weighted-average number of shares, basic and diluted |
||||
|
|
||||
Adjusted pro forma (loss) per share reflects the use of proceeds of the proposed IPO for the repayment of outstanding debt. These adjustments are reflected as if the IPO occurred on January 1, 2012. Adjusted pro forma net (loss) per share has been reflected assuming no debt outstanding under the Company’s credit facilities for the nine months ended September 30, 2013.
| (in thousands, except share and per share data) | Nine months ended September 30, 2013 |
|||
|
|
||||
| Net (loss) as reported |
$ | |||
| Adjustments to net (loss) for interest expense |
||||
| Adjusted net (loss) |
||||
| Adjusted pro forma (loss) per share, basic and diluted |
||||
| Adjusted pro forma weighted-average number of shares, basic and diluted |
||||
| Adjusted pro forma weighted-average number of shares is calculated below: |
||||
| Weighted-average shares as reported |
||||
| Due to reorganization |
||||
| Due to reverse share spilt |
||||
| Due to conversion of preferred ordinary shares |
||||
| Due to repayment of indebtedness |
||||
| Due to shares issued in connection with the IPO |
||||
| Adjusted pro forma weighted-average number of shares, basic and diluted |
||||
|
|
||||
Outstanding options and warrants were excluded from the computation of pro forma net loss per share because their effect would have been anti-dilutive.
F-45
Table of Contents
The Board of Directors and Shareholders of Oxford Immunotec Global PLC
We have audited the accompanying balance sheet of Oxford Immunotec Global PLC as of August 16, 2013. This balance sheet is the responsibility of the Company’s management. Our responsibility is to express an opinion on this balance sheet based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the balance sheet referred to above presents fairly, in all material respects, the financial position of Oxford Immunotec Global PLC at August 16, 2013, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Reading, United Kingdom
October 15, 2013
F-46
Table of Contents
Oxford Immunotec Global PLC
Balance sheet as of August 16, 2013
(Date of inception)
| August 16, | ||||
| 2013 | ||||
|
|
||||
| Total assets |
$ | — | ||
|
|
|
|||
| Liabilities |
— | |||
| Equity |
||||
| A preferred ordinary shares, £0.001 par value; 903,220 shares authorized, 0 shares issued and outstanding |
— | |||
| B preferred ordinary shares, £0.001 par value; 362,020 shares authorized, 0 shares issued and outstanding |
— | |||
| D preferred ordinary shares, £0.001 par value; 3,266,885 shares authorized, 0 shares issued and outstanding |
— | |||
| E preferred ordinary shares, £0.001 par value; 32,000,000 shares authorized, 0 shares issued and outstanding |
— | |||
| F preferred ordinary shares, £0.001 par value; 20,000,000 shares authorized, 0 shares issued and outstanding |
— | |||
| G preferred ordinary shares, £0.001 par value; 25,000,000 shares authorized, 0 shares issued and outstanding |
— | |||
| Ordinary shares, £0.001 par value; 110,179,750 shares authorized, 1 share issued and outstanding |
— | |||
| Additional paid-in capital |
— | |||
| Receivable from shareholder |
— | |||
|
|
|
|||
| Total equity |
— | |||
|
|
|
|||
| Total liabilities and equity |
$ | — | ||
|
|
|
|||
| See accompanying notes to this balance sheet. |
||||
F-47
Table of Contents
Oxford Immunotec Global PLC
Notes to the balance sheet as of August 16, 2013
(Date of inception)
1. Description of business and significant accounting policies
Description of business
Oxford Immunotec Global PLC (the Company) was incorporated on August 16, 2013. The Company completed a scheme of arrangement under the laws of England and Wales, or the Scheme of Arrangement, which was approved by the High Court of Justice in England and Wales, whereby holders of equity interests in Oxford Immunotec Limited, a private limited company incorporated in England and Wales, including holders of ordinary shares, preferred ordinary shares, options and warrants, exchanged their interests in Oxford Immunotec Limited for identical interests and rights in Oxford Immunotec Global PLC, a public limited company incorporated in England and Wales, which is the parent company of Oxford Immunotec Limited. Oxford Immunotec Global PLC currently has no activity, operations or financing. One ordinary share with par value £0.001 was issued at par value and recorded as a receivable from its shareholder.
Basis of presentation
The accompanying balance sheet has been prepared in conformity with accounting principles generally accepted in the United States of America (U.S. GAAP). Separate Statements of Operations, Statements of Other Comprehensive Loss, Statements of Shareholders’ Equity and Statements of Cash Flows have not been presented in the financial statement because there have been no activities of the company.
2. Subsequent events
On October 2, 2013, the Scheme of Arrangement was completed.
F-48
Table of Contents
Shares
Oxford Immunotec Global PLC

Ordinary shares
Prospectus
| J.P. Morgan | Piper Jaffray | |||
| Cowen and Company |
Baird | |||
, 2013
Table of Contents
Part II
Information not required in prospectus
Item 13. Other expenses of issuance and distribution
The following table sets forth the costs and expenses, other than the underwriting discounts and commissions, payable by us in connection with the sale of ordinary shares being registered. All amounts are estimates except for the SEC registration fee, the FINRA filing fee and The NASDAQ Global Market listing fee.
| Item | Amount to be paid |
|||
|
|
||||
| SEC registration fee |
$ | 11,109 | ||
| FINRA filing fee |
$ | 13,438 | ||
| The NASDAQ Global Market listing fee |
$ | 25,000 | ||
| Blue sky fees and expenses |
* | |||
| Printing and engraving expenses |
* | |||
| Legal fees and expenses |
* | |||
| Accounting fees and expenses |
* | |||
| Transfer agent fees and expenses |
* | |||
| Miscellaneous expenses |
* | |||
| Total |
$ | * | ||
|
|
||||
| * | To be completed by amendment. |
Item 14. Indemnification of directors and officers
Our articles of association provide that, subject to the Companies Act 2006, we shall indemnify, out of our assets, any director of the Company or any associated company against all losses, liabilities and expenditures which he or she may sustain or incur in the execution of the duties of his or her office or otherwise in relation thereto.
The relevant provisions under the Companies Act 2006 are Sections 205, 206, 232, 233, 234, 235, 236, 237, 238 and 1157.
Section 205 provides that a company can provide a director with the funds to meet expenditures incurred or to be incurred in defending any criminal or civil proceedings or in connection with any application under sections 661(3) and 661(4) (acquisition of shares by innocent nominee) or section 1157 (described below). Such financial assistance must be repaid if the director is convicted, judgment is found against such director or the court refuses to grant the relief on the application.
Section 206 provides that a company can provide a director with the funds to meet expenditures incurred or to be incurred by him or her in defending in an investigation by a regulatory authority, or against action proposed to be taken by a regulatory authority, in connection with any alleged negligence, default, breach of duty or breach of trust by him or her in relation to the company or an associated company.
II-1
Table of Contents
Section 232 provides that any provision to exempt to any extent a director from liability from negligence, default, breach of duty or trust by him or her in relation to the company is void. Any provision by which a company directly or indirectly provides (to any extent) an indemnity for a director of the company or an associated company against any such liability is also void unless it is a qualifying third party indemnity provision.
Section 233 permits liability insurance, commonly known as directors’ and officers’ liability insurance, purchased and maintained by a company against liability for negligence, default, breach of duty or breach of trust in relation to the company.
Pursuant to Section 234, an indemnity is a qualifying third party indemnity as long as it does not provide: (i) any indemnity against any liability incurred by the director to the company or to any associated company; (ii) any indemnity against any liability incurred by the director to pay a fine imposed in criminal proceedings or a sum payable to a regulatory authority by way of a penalty in respect of non-compliance with any requirement of a regulatory nature; and (iii) any indemnity against any liability incurred by the director in defending criminal proceedings in which he or she is convicted, civil proceedings brought by the company or an associated company in which judgment is given against such director or where the court refuses to grant such director relief under an application under sections 661(3) and 661(4) (acquisition of shares by innocent nominee) or its power under section 1157 (described below).
Section 235 allows a company to provide an indemnity to a director if the company is a trustee of an occupational pension scheme, with such indemnity to protect against liability incurred in connection with the company’s activities as trustee of the scheme.
Any indemnity provided under Section 234 or Section 235 in force for the benefit of one or more directors of the company or an associated company must be disclosed in the directors’ annual report in accordance with Section 236 and copies of such indemnification provisions made available for inspection in accordance with Section 237 (and every shareholder has a right to inspect and request such copies under Section 238).
Section 1157 provides that in proceedings against an officer of a company for negligence, default, breach of duty or breach of trust, the court may relieve such officer from liability if it appears to the court that such officer may be liable but acted honestly and reasonably and that having regard to all the circumstances of the case, such officer ought fairly to be excused. Further, an officer who has reason to apprehend that a claim of negligence, default, breach of duty or breach of trust will or might be made against him or her, such officer may apply to the court for relief, and the court will have the same power to relieve such officer as it would if the proceedings had actually been brought.
A court has wide discretion in granting relief, and may authorize civil proceedings to be brought in the name of the company by a shareholder on terms that the court directs. Except in these limited circumstances, English law does not generally permit class action lawsuits by shareholders on behalf of the company or on behalf of other shareholders.
In connection with this offering, we have also entered into deeds of indemnification with each of our directors and executive officers. Pursuant to these agreements, we agree to indemnify these individuals to the fullest extent permissible under English law against liabilities arising out of, or in connection with, the actual or purported exercise of, or failure to exercise, any of his or her powers, duties or responsibilities as a director or officer, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. We also agree to
II-2
Table of Contents
use all reasonable endeavors to provide and maintain appropriate directors’ and officers’ liability insurance (including ensuring that premiums are properly paid) for their benefit for so long as any claims may lawfully be brought against them.
We have obtained and expect to continue to maintain insurance policies under which our directors and officers are insured, within the limits and subject to the limitations of those policies, against certain expenses in connection with the defense of, and certain liabilities that might be imposed as a result of, actions, suits or proceedings to which they are parties by reason of being or having been directors or officers. The coverage provided by these policies may apply whether or not we would have the power to indemnify such person against such liability under the provisions of English law.
In the underwriting agreement, the underwriters will agree to indemnify, under certain conditions, us, members of our Board of Directors, members of management and persons who control us within the meaning of the Securities Act, against certain liabilities.
Item 15. Recent sales of unregistered securities
In the three years preceding the filing of this registration statement, we have issued the following securities that were not registered under the Securities Act.
Financing transactions.
On October 8, 2013 we issued a convertible promissory note with a principal amount of $5 million to Fosun Industrial Co., Limited. We issued this note in connection with the execution of a distribution agreement with certain of Fosun Industrial Co., Limited’s affiliates. The note will automatically convert into ordinary shares immediately prior to this offering at a price per share that reflects a 10% discount to the per share price at which we sell ordinary shares to the public in this offering. The note bears interest at the rate of 8%.
As of September 30, 2013, we had issued 16,559,756 Series G preferred ordinary shares at a price of $1.70 per share for aggregate consideration of approximately $28 million to certain of our shareholders, including each of the investment funds identified in the section under the heading “Principal shareholders,” whom we refer to collectively as our “institutional investors,” and Mr. Sandberg. The issuance occurred in two tranches; in June 2012, we issued 10,079,933 G preferred ordinary shares and in January 2013, we issued 6,479,823 G preferred ordinary shares.
In February 2012, we entered into a convertible loan facility, pursuant to which we borrowed an aggregate of $4 million from certain of our shareholders. In connection with this loan facility, we issued 410,994 ordinary shares and 1,233,027 F preferred ordinary shares to the lenders as payment for a facility fee associated with the loan. As of September 30, 2013, all lenders had converted their notes into G preferred ordinary shares in connection with the financing described above.
Beginning in 2009, we consummated a series of financing transactions that involved the issuance of ordinary shares and F preferred ordinary shares for a total of $26 million. The financing occurred in three tranches, with the first tranche closing in July 2009. The second tranche occurred in April 2010 and, in connection therewith, we issued a total of 1,849,568 ordinary shares and 5,548,704 F preferred ordinary shares at a price per unit (consisting of one-third of an ordinary share and one F preferred ordinary share) of $1.622, for aggregate consideration of $9 million to certain of our shareholders. The third tranche occurred in February 2011 and, in connection therewith, we issued 2,055,077 ordinary shares and 6,165,231 F preferred ordinary
II-3
Table of Contents
shares at a price per unit (consisting of one-third of an ordinary share and one F preferred ordinary share) of $1.622, for aggregate consideration of $10 million.
These issuances were exempt from registration under the Securities Act pursuant to Rule 506 or Section 4(a)(2).
Warrants.
In 2013, we issued a warrant to purchase 105,882 shares at $0.12 per share to a lender in connection with a credit facility. That warrant expires May 25, 2023. In 2012, we issued a warrant to purchase 24,691 shares at $0.01 per share to a lender in connection with a credit facility. That warrant expires on February 2, 2019.
These issuances were exempt from registration under the Securities Act pursuant to Rule 506 or Section 4(a)(2).
Option grants and exercises.
From January 1, 2013 through October 15, 2013, we granted options to purchase a total of 1,551,027 ordinary shares to employees at a weighted-average price of $0.14 per share. During the same period, we issued 1,231,523 ordinary shares upon the exercise of options to purchase such shares at a weighted-average price of $0.03 per share.
In 2012, we granted options to purchase a total of 4,600,766 ordinary shares to employees at a weighted-average price of $0.09 per share. During the same period, we issued 57,505 ordinary shares upon the exercise of options to purchase such shares at a weighted-average price of $0.02 per share.
In 2011, we granted options to purchase a total of 360,500 ordinary shares to employees at a weighted-average price of $0.04 per share. During the same period, we issued 31,636 ordinary shares upon the exercise of options to purchase such shares at a weighted-average price of $0.02 per share.
From August 1, 2010 to December 31, 2010, we granted options to purchase a total of 97,000 ordinary shares to employees at a weighted-average price of $0.04 per share. During the same period, we issued 3,000 ordinary shares upon the exercise of options to purchase such ordinary shares at a weighted-average price of $0.02 per share.
These option grants and the issuances of ordinary shares upon exercise of such options were exempt from registration under the Securities Act pursuant to Rule 701 and Section 4(a)(2).
Scheme of arrangement.
On October 2, 2013, our equity holders exchanged their equity interests in Oxford Immunotec Limited for equity interests in Oxford Immunotec Global PLC pursuant to a scheme of arrangement under the laws of England and Wales. The issuance was exempt from registration under the Securities Act pursuant to Section 3(a)(10).
Item 16. Exhibits and financial statement schedules
(a) Exhibits
See Exhibit Index following the signature page.
II-4
Table of Contents
(b) Financial statement schedules
Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
Item 17. Undertakings
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes:
(1) That for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933 shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) That for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
Table of Contents
Signatures
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Abingdon, England, on November 7, 2013.
| OXFORD IMMUNOTEC GLOBAL PLC | ||
| By: |
/s/ Patricia Randall | |
| Name: |
Patricia Randall | |
| Title: |
General Counsel | |
***
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
|
| ||||
| * Peter Wrighton-Smith, Ph.D. |
Chief Executive Officer (Principal Executive Officer) |
November 7, 2013 | ||
| * Richard M. Altieri |
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
November 7, 2013 | ||
| * Richard A. Sandberg |
Chairman of the Board of Directors |
November 7, 2013 | ||
| * Stephen L. Spotts |
Director |
November 7, 2013 | ||
| * Nigel A. Pitchford, Ph.D. |
Director |
November 7, 2013 | ||
| * Michael Steinmetz, Ph.D. |
Director |
November 7, 2013 | ||
| * Vijay Lathi |
Director |
November 7, 2013 | ||
II-6
Table of Contents
| Signature | Title | Date | ||
| * Charles Jonathan Gee, Ph.D. |
Director |
November 7, 2013 | ||
| * Rainer Strohmenger, M.D. |
Director |
November 7, 2013 | ||
| * Herman Rosenman |
Director |
November 7, 2013 | ||
| * Richard M. Altieri |
Authorized Representative in the United States |
November 7, 2013 | ||
|
| ||||
| *By: |
/s/ Patricia Randall | |
| Name: Title: |
Patricia Randall Attorney-in-fact | |
II-7
Table of Contents
| Exhibit number |
Description of exhibit | |||
|
|
| |||
| 1.1* | Form of Underwriting Agreement | |||
| 3.1* | Memorandum and Articles of Association of the Registrant | |||
| 4.1* | Form of Ordinary Shares Certificate | |||
| 4.2* | Registration Rights Agreement | |||
| 4.3** | Warrant to Purchase Ordinary Shares, issued to Comerica Bank | |||
| 4.4** | Warrant to Purchase Ordinary Shares, issued to Square 1 Bank | |||
| 5.1* | Opinion of Ropes & Gray International LLP | |||
| 10.1+** | License Agreement dated July 27, 2005 between Isis Innovation Limited and Oxford Immunotec Limited | |||
| 10.2+** | 2006 Deed of Variation to License Agreement between Isis Innovation Limited and Oxford Immunotec Limited | |||
| 10.3+** | Letter Agreement Amendment dated December 13, 2012 to License Agreement between Isis Innovation Limited and Oxford Immunotec Limited | |||
| 10.4+** | License and Supply Agreement dated December 22, 2009 between Statens Serum Institut and Oxford Immunotec Limited | |||
| 10.5** | Supplement dated November 9, 2010 to License and Supply Agreement between Statens Serum Institut and Oxford Immunotec Limited | |||
| 10.6+** | License Agreement dated June 30, 2006 between Rutgers, The State University of New Jersey and Oxford Immunotec Limited | |||
| 10.7+** | First Amendment dated July 1, 2009 to License Agreement between Rutgers, The State University of New Jersey and Oxford Immunotec Limited | |||
| 10.8+** | Second Amendment dated January 6, 2011 to License Agreement between Rutgers, The State University of New Jersey and Oxford Immunotec Limited | |||
| 10.9** | Third Amendment dated December 20, 2012 to License Agreement between Rutgers, The State University of New Jersey and Oxford Immunotec Limited | |||
| 10.10+** | Fourth Amendment dated April 24, 2013 to License Agreement between Rutgers, The State University of New Jersey and Oxford Immunotec Limited | |||
| 10.11+** | Supply Agreement dated December 17, 2010 between MicroCoat Biotechnologie GmbH and Oxford Immunotec Limited | |||
| 10.12+** | Purchase Agreement dated February 6, 2010 between Mabtech AB and Oxford Immunotec Limited | |||
| 10.13+** | Amendment to Purchase Agreement dated September 10, 2013 between Mabtech AB and Oxford Immunotec Limited | |||
| 10.14+** | Manufacturing Agreement dated August 26, 2003 between Mabtech AB and Oxford Immunotec Limited | |||
| 10.15** | First Amendment dated January 1, 2010 to Manufacturing Agreement between Mabtech AB and Oxford Immunotec Limited | |||
| 10.16** | Second Amendment dated May 24, 2011 to Manufacturing Agreement between Mabtech AB and Oxford Immunotec Limited | |||
|
|
| |||
II-8
Table of Contents
| Exhibit number |
Description of exhibit | |||
|
|
| |||
| 10.17+** | Supply Agreement dated January 1, 2009 between EMD Millipore Corporation and Oxford Immunotec Ltd | |||
| 10.18+** | First Amendment to Supply Agreement dated September 27, 2013 between EMD Millipore Corporation and Oxford Immunotec Limited | |||
| 10.19+** | Supply Agreement dated January 31, 2008 between StemCell Technologies, Inc. and Oxford Immunotec Limited | |||
| 10.20+** | Amendment dated October 26, 2011 to Supply Agreement between StemCell Technologies, Inc. and Oxford Immunotec Limited | |||
| 10.21+** | Supply and Reseller Agreement dated August 12, 2013 between Life Technologies Corporation and Oxford Immunotec Limited | |||
| 10.22 +** | Distributorship Agreement dated June 5, 2009 among Shanghai Fosun Long March Medical Science Co. Ltd., Shanghai Xin Chang Medical Device Co. Ltd. and Oxford Immunotec Limited | |||
| 10.23+** | Amendment dated December 22, 2011 to Distributorship Agreement among Shanghai Fosun Long March Medical Science Co. Ltd., Shanghai Xin Chang Medical Device Co. Ltd. and Oxford Immunotec Limited | |||
| 10.24+** | Distributorship Agreement dated October 8, 2013 among Shanghai Fosun Long March Medical Science Co. Ltd., Shanghai Xin Chang Medical Device Co. Ltd. and Oxford Immunotec Limited | |||
| 10.25+** | Marketing Authorization Holder Agreement dated July 29, 2011 between Riken Genesis Co., Ltd. and Oxford Immunotec Limited | |||
| 10.26+** | Amendment to Marketing Authorization Holder Agreement dated September 1, 2013 between Riken Genesis Co., Ltd. and Oxford Immunotec Limited | |||
| 10.27** | Lease Agreement dated September 30, 2008 among MEPC Milton Park No. 1 Limited, MEPC Milton Park No. 2 Limited and Oxford Immunotec Limited, relating to Milton Park Units 115B and D | |||
| 10.28** | Lease Agreement dated August 8, 2007 among MEPC Milton Park No. 1 Limited, MEPC Milton Park No. 2 Limited and Oxford Immunotec Limited, relating to Milton Park Unit 94C | |||
| 10.29** | Deed of Variation dated December 23, 2009 to Lease Agreement among MEPC Milton Park No. 1 Limited, MEPC Milton Park No. 2 Limited and Oxford Immunotec Limited, relating to Milton Park Unit 94C and Units 115B and D | |||
| 10.30** | Deed of Variation dated May 4, 2012 to Lease Agreement among MEPC Milton Park No. 1 Limited, MEPC Milton Park No. 2 Limited and Oxford Immunotec Limited, relating to Milton Park Units 115B and D | |||
| 10.31** | Deed of Variation dated May 4, 2012 to Lease Agreement among MEPC Milton Park No. 1 Limited, MEPC Milton Park No. 2 Limited and Oxford Immunotec Limited, relating to Milton Park Unit 94C | |||
| 10.32** | Office Lease Agreement dated March 1, 2013 between Normandy Nickerson Road, LLC and Oxford Immunotec, Inc. | |||
| 10.33** | Lease Agreement dated October 17, 2011 between OMC Investment Venture and Oxford Immunotec, Inc. | |||
|
|
| |||
II-9
Table of Contents
| Exhibit number |
Description of exhibit | |||
|
|
|
| ||
| 10.34** | Loan and Security Agreement dated May 24, 2013 between Square 1 Bank and Oxford Immunotec, Inc. | |||
| 10.35** | Amended and Restated 2008 Stock Incentive Plan | |||
| 10.36** | Form of Incentive Stock Option Award for executive officers under the Amended and Restated 2008 Stock Incentive Plan | |||
| 10.37** | Form of Non-Statutory Stock Option Award for Non-Executive Directors under the Amended and Restated 2008 Stock Incentive Plan | |||
| 10.38** | Form of Enterprise Management Incentive Stock Option Agreement for Chief Executive Officer under the Amended and Restated 2008 Stock Incentive Plan | |||
| 10.39* | Oxford Immunotec Global PLC 2013 Share Incentive Plan | |||
| 10.40** | Oxford Immunotec Global PLC Incentive Plan | |||
| 10.41** | Service Agreement dated October 21, 2002 between Oxford Immunotec Limited and Peter Wrighton-Smith, as amended | |||
| 10.42** | Amended and Restated Employment Agreement dated October 1, 2013 between Oxford Immunotec, Inc. and Jeff R. Schroeder | |||
| 10.43** | Amended and Restated Employment Agreement dated October 1, 2013 between Oxford Immunotec, Inc. and Richard M. Altieri | |||
| 10.44* | Form of Deed of Indemnity for Directors | |||
| 10.45* | Form of Deed of Indemnity for Officers | |||
| 10.46** | Form of Non-Executive Director Appointment Letter | |||
| 10.47** | Oxford Immunotec Global PLC Management Incentive Compensation Plan for Fiscal Years 2012 and 2013 | |||
| 21.1** | List of Subsidiaries | |||
| 23.1** | Consent of Ernst & Young LLP | |||
| 23.2* | Consent of Ropes & Gray International LLP (included in Exhibit 5.1) | |||
| 24.1** | Power of Attorney executed by Directors and Officers other than Herman Rosenman | |||
| 24.2** | Power of Attorney executed by Herman Rosenman | |||
|
|
| |||
| * | To be filed by amendment. |
| ** | Previously filed. |
| + | Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been submitted separately to the Securities and Exchange Commission. |
II-10
