Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REGENERON PHARMACEUTICALS, INC. | d608565d8k.htm |
 Safety and efficacy of dupilumab for
moderate-to-severe atopic dermatitis in
patients using topical corticosteroids
(TCS): Greater efficacy observed with
combination therapy compared to TCS
alone
Diamant
Thaçi,
1
Margitta
Worm,
2
Haobo
Ren,
3
Steven
Weinstein,
3
Neil
Graham,
3
Gianluca
Pirozzi,
4
Franck
Skobieranda,
4
Marius
Ardeleanu
3
1
Universität
zu
Lübeck,
Lübeck,
Germany;
2
Charite-Universitätsmedzin
Berlin,
Berlin,
Germany;
3
Regeneron
Pharmaceuticals,
Inc.,
Tarrytown,
USA;
4
Sanofi,
Bridgewater,
USA
Exhibit 99.1 |
 Disclosures
Disclosures
D Thaçi is a consultant for Astellas, Novartis,
D Thaçi is a consultant for Astellas, Novartis,
Regeneron, Celgene, Abbott, Pfizer, Janssen-Cilag,
Regeneron, Celgene, Abbott, Pfizer, Janssen-Cilag,
MSD, Leo-Pharma
MSD, Leo-Pharma
M Worm has nothing to disclose
M Worm has nothing to disclose
H Ren, S Weinstein, N Graham, and M Ardeleanu are
H Ren, S Weinstein, N Graham, and M Ardeleanu are
employees and shareholders of Regeneron
employees and shareholders of Regeneron
G Pirozzi is an employee and shareholder of Sanofi
G Pirozzi is an employee and shareholder of Sanofi
F Skobieranda was an employee of Sanofi when the
F Skobieranda was an employee of Sanofi when the
study was conducted
study was conducted
Study (NCT01639040) funded by Regeneron
Study (NCT01639040) funded by Regeneron
Pharmaceuticals, Inc. and Sanofi
Pharmaceuticals, Inc. and Sanofi |
 Introduction
Introduction
Moderate-to-severe atopic dermatitis (AD) is characterized by
Moderate-to-severe atopic dermatitis (AD) is characterized by
eczematous dermatitis with intractable pruritus associated with sleep
eczematous dermatitis with intractable pruritus associated with sleep
disturbance and lower quality-of-life
disturbance and lower quality-of-life
For many patients, current therapies are inadequate and can be
For many patients, current therapies are inadequate and can be
associated with unwanted side effects
associated with unwanted side effects
IL-4 and IL-13 are thought to be central to T-helper 2 (Th2)
IL-4 and IL-13 are thought to be central to T-helper 2 (Th2)
inflammation, which mediates many features of AD
inflammation, which mediates many features of AD
Dupilumab is a fully human monoclonal antibody targeting the IL-4
Dupilumab is a fully human monoclonal antibody targeting the IL-4
receptor alpha subunit (IL-4R ), thus blocking the intracellular
signaling receptor alpha subunit (IL-4R ), thus blocking the
intracellular signaling of both IL-4 and IL-13
of both IL-4 and IL-13
Earlier clinical trials indicated that dupilumab monotherapy had
Earlier clinical trials indicated that dupilumab monotherapy had
an
an
acceptable safety profile and was efficacious in patients with moderate
acceptable safety profile and was efficacious in patients with moderate
to severe AD who cannot be adequately controlled with topical
to severe AD who cannot be adequately controlled with topical
medications
medications
Since topical corticosteroids (TCS) are commonly used in AD, we
Since topical corticosteroids (TCS) are commonly used in AD, we
assessed the safety and efficacy of dupilumab co-administered with
assessed the safety and efficacy of dupilumab co-administered with
TCS
TCS |
 TGF-
IL-10
T cells in immune mediated diseases
T cells in immune mediated diseases
IFN-
IL-2
(IL-10)
TNF-
LT-a
IL-4
IL-5
IL-10
IL-13
(TNF-
)
IL-17A
IL-17F
IL-21
IL-22
(IL-10)
(TNF-
)
RORc2
Foxp3
GATA-3
IL-22
IL-13
(IL-10)
FGF´s
(TNF-
)
?
ALLERGY
INFLAMMATION,
INFECTION
EPITHELIAL
INTEGRITY
LIMITATION
INFLAMMATION
Th1
Th2
Th17
Th22
iTreg
T naive |
 Atopic dermatitis: a disease of altered
skin barrier and immune dysregulation
Boguniewicz M, Leung DM. Immunol Rev. 2011 Jul;242(1):233-46.
|
 Dupilumab blocks the
Dupilumab blocks the
IL-4/IL-13 receptor/ligand system
IL-4/IL-13 receptor/ligand system
Type I Receptor
B cells, T cells, Monocytes,
Eosinophils, Fibroblasts
Type II Receptor
Epithelial cells, Smooth muscle
cells, Fibroblasts, Monocytes,
Activated B cells |
 Study treatment
(weekly SC injection for 4 wks)
Dupilumab 300 mg + TCS (n=21)
Screening
Safety follow-up
(7 wks)
n=31
Randomized, double-blind, parallel-
group, placebo-controlled study
(NCT01639040) conducted in EU
(NCT01639040) conducted in EU
Placebo + TCS (n=10)
Adult
moderate-to-
severe AD
patients
All patients received concomitant treatment
with a potent TCS product on a standardized
regimen: daily applications to active lesions,
followed by applications two days per week
once lesions were under control
Topical treatment of any
residual active AD lesions
continues at the discretion of
the investigator
Study endpoints:
•
Primary endpoint was incidence and severity of adverse events (AEs)
•
Exploratory efficacy endpoints included EASI-50, IGA
1, SCORAD score |
 Key
inclusion/exclusion criteria Key inclusion/exclusion criteria
Inclusion
•
Male or female
18 yrs
•
Chronic AD > 2 yrs
•
IGA
3
•
SCORAD > 20
•
10% BSA of AD involvement
•
Active AD lesion(s) for which
treatment with potent TCS is
indicated
Exclusion
•
Hypersensitivity to TCS
•
50% of the cumulative lesional
surface located on face, flexural, or
genital areas (generally unsuitable
for treatment with potent TCS)
•
Acute or chronic infections
•
Recent treatment with immuno-
suppressive/immunomodulating
drugs
•
Significant co-morbidities or lab
abnormalities |
 Baseline demographics
Baseline demographics
Placebo + TCS
(n=10)
Dupilumab SC
300 mg +TCS
(n=21)
Mean age, yrs (SD)
37.8 (16.7)
36.0 (11.3)
Race, n (%)
Caucasian
10 (100%)
20 (95.2%)
Non-Caucasian
0
1 (4.8%)
Gender, n (%)
Male
5 (50.0%)
8 (38.1%)
Female
5 (50.0%)
13 (61.9%)
Mean BMI, kg/m
2
(SD)
23.92 (3.47)
25.26 (3.26) |
 Baseline disease characteristics
Baseline disease characteristics
[mean (SD)]
[mean (SD)]
Placebo + TCS
(n=10)
Dupilumab SC
300 mg +TCS
(n=21)
AD duration, yrs
32.4 (16.8)
30.9 (13.0)
EASI score (0-72)
24.10 (12.70)
23.12 (12.35)
IGA score (0-5)
3.35 (0.47)
3.43 (0.60)
SCORAD score (0-103)
58.20 (13.83)
66.31 (13.01)
% BSA of AD
38.85 (24.05)
40.43 (20.91)
Pruritus Numeric Rating
Scale (NRS) score (0-10)
5.00 (1.40)
6.43 (2.00)
EASI=Eczema Area Severity Index; IGA=Investigator’s Global Assessment;
SCORAD=scoring of atopic dermatitis; BSA = baseline body surface area;
NRS=numeric rating scale |
 Treatment emergent adverse events
Treatment emergent adverse events
Placebo + TCS
(n = 10)
Dupilumab + TCS
(n = 21)
Total number of AEs
14
41
Total number of serious AEs
1
0
Deaths
0
0
Number (%) of patients discontinued from study
due to AE
1 (10.0%)
0
Number (%) of patients with:
Any AE
7 (70.0)
12 (57.1)
Any serious AE
1 (10.0)
0
Most
common
AEs
(
5%
in
dupilumab
groups)
•
Nasopharyngitis
2 (20.0)
5 (23.8)
•
Headache
1 (10.0)
3 (14.3)
•
Oropharyngeal pain
1 (10.0)
3 (14.3)
•
Rhinitis
0
2 (9.5)
•
Cough
0
2 (9.5)
•
Influenza
0
2 (9.5)
•
Somnolence
0
2 (9.5) |
 Dupilumab+TCS significantly improved
Dupilumab+TCS significantly improved
measures of efficacy vs TCS alone
measures of efficacy vs TCS alone
Placebo+TCS
300 mg DPL+TCS
Placebo+TCS
300 mg DPL+TCS
EASI-50 Responders
(Patients achieving 50%
Reduction in EASI)
Pruritus Numeric Rating Scale
0
10
20
30
40
50
60
70
80
90
100
0
5
10
15
20
25
30
Study Day
-80
-70
-60
-50
-40
-30
-20
-10
0
10
20
0
5
10
15
20
25
30
Study Day
*p=0.0015
**p=0.0051
DPL=dupilumab
TCS=topical corticosteroids |
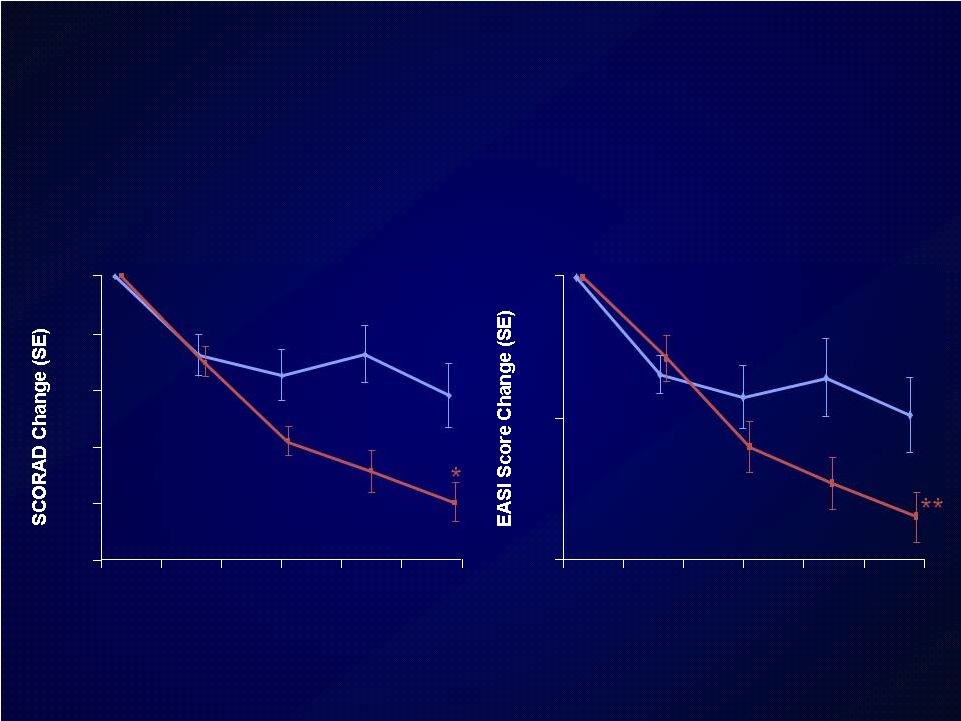 Placebo+TCS
300 mg DPL+TCS
Placebo+TCS
300 mg DPL+TCS
SCORAD
EASI
-50
-40
-30
-20
-10
0
0
5
10
15
20
25
30
Study Day
-20
-10
0
0
5
10
15
20
25
30
Study Day
Dupilumab+TCS significantly improved
Dupilumab+TCS significantly improved
measures of efficacy vs TCS alone
measures of efficacy vs TCS alone
*p=0.0191
**p=0.0042
DPL=dupilumab
TCS=topical corticosteroids |
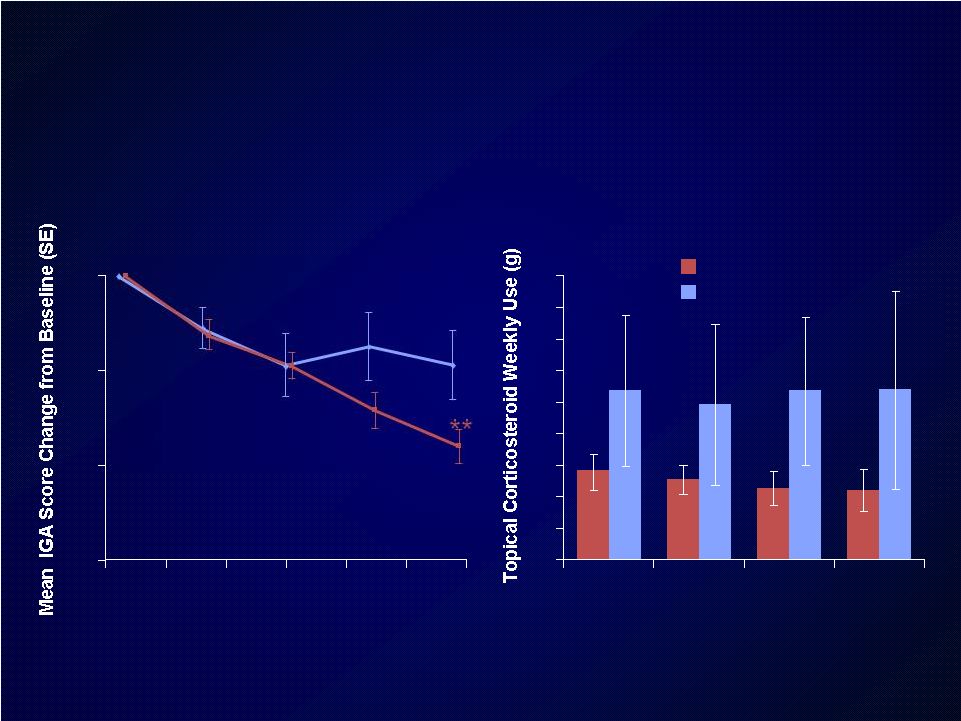 Dupilumab+TCS achieved superior
Dupilumab+TCS achieved superior
clinical outcomes vs TCS alone
clinical outcomes vs TCS alone
DPL=dupilumab
TCS=topical corticosteroids
Placebo+TCS
300 mg DPL+TCS
TCS Used Weekly (g)
0
5
15
25
35
45
Wk 1
Wk 2
Wk 3
Wk 4
10
20
30
40
*
*p=0.1413
Placebo+TCS
300 mg DPL+TCS
IGA Score
-3.0
-2.0
-1.0
0
0
5
10
15
20
25
30
Study Day
**p=0.0281 |
 Summary
Summary
In this study of adults with moderate-to-severe AD, concomitant
treatment with SC dupilumab+TCS exhibited an acceptable safety
profile (primary endpoint)
–
–
Most common treatment-emergent AEs were nasopharyngitis (23.8% vs
Most common treatment-emergent AEs were nasopharyngitis (23.8% vs
20% for placebo), headache and oropharyngeal pain (both14.3% vs 10%
20% for placebo), headache and oropharyngeal pain (both14.3% vs 10%
for placebo)
for placebo)
At 4 weeks, dupilumab+TCS group achieved superior clinical
At 4 weeks, dupilumab+TCS group achieved superior clinical
outcomes compared to TCS alone (exploratory efficacy endpoints)
outcomes compared to TCS alone (exploratory efficacy endpoints)
–
–
EASI-50: 100% responder rate for dupilumab +TCS, compared to 50% for
EASI-50: 100% responder rate for dupilumab +TCS, compared to 50% for
placebo+TCS
placebo+TCS
–
–
Significantly better improvement from baseline in EASI, SCORAD, IGA,
Significantly better improvement from baseline in EASI, SCORAD, IGA,
and pruritus NRS for dupilumab + TCS vs. Placebo + TCS
and pruritus NRS for dupilumab + TCS vs. Placebo + TCS
Patients on dupilumab + TCS used approximately 50% less TCS
Patients on dupilumab + TCS used approximately 50% less TCS
during the treatment period compared with patients on placebo + TCS
during the treatment period compared with patients on placebo + TCS
(48.7g vs 99.4g), associated with faster clearing of active AD lesions
(48.7g vs 99.4g), associated with faster clearing of active AD lesions
|
 Acknowledgements
Acknowledgements
Marius Ardeleanu
Marius Ardeleanu
Elisa Babilonia
Elisa Babilonia
Nancee Basinger
Nancee Basinger
Warren Brooks
Warren Brooks
Josh Cantor
Josh Cantor
Linda Williams
Linda Williams
Diamant Thaci
Diamant Thaci
Margitta Worm
Margitta Worm
Martin Kaatz
Martin Kaatz
Rolf Dominicus
Rolf Dominicus
Beatrice Gerlach
Beatrice Gerlach
All participating patients
All participating patients
Investigators
Investigators
Beate Schwarz
Beate Schwarz
Noemi Bakos
Noemi Bakos
Lajos Kemeny
Lajos Kemeny
Marcin Ambroziak
Marcin Ambroziak
Maria Czubek
Maria Czubek
Sanofi
Sanofi
Tara Coughlan
Tara Coughlan
Judy Cusick
Judy Cusick
Evelyn Dorsey
Evelyn Dorsey
Kristen Dougherty
Kristen Dougherty
Chad Fish
Chad Fish
Usman Chaudhry
Usman Chaudhry
Melissa Hager
Melissa Hager
Jennifer Hamilton
Jennifer Hamilton
Rebecca Indibi
Rebecca Indibi
Richard Kao
Richard Kao
Dan Kropas
Dan Kropas
Jacquie Kuritzky
Jacquie Kuritzky
Vicky Lai
Vicky Lai
Haobo Ren
Haobo Ren
Dawn Rich
Dawn Rich
Tara Seeliger
Tara Seeliger
Regeneron
Regeneron
Jennifer Cairns
Jeffrey Ming
Susan
Slaytylak-Cheeks
Maris Juszkiewicz-Borowiec
Andrzej Kaszuba
Dorota Bystrzanowska
Athanasios Tsianakas |
 BACK UP
BACK UP |
 The
march of atopic eczema The march of atopic eczema
T naive
Th2
Th1
Th17
APC
Eosinophil
(Aero)allergen
acute eczema: allergen-specific
immune
response
(Th2, IgE, eosinophils)
microbial products/
auto-antigens
Th2
B cell
IFN-
IL-13
IL-4
local lymph node
dermal vessel
chronic eczema: microorganisms,
auto-allergy,
remodelling
(Th1, Th2,
Th17/Th22)
IL-22
IL-17
IDEC
TSLP
IgE
filaggrin
filaggrin |
