Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Unilife Corp | d596289dex991.htm |
| 8-K - FORM 8-K - Unilife Corp | d596289d8k.htm |
 Fiscal Year 2013
4 th
Quarter and Full Year Earnings Call
September 10, 2013
1
Exhibit 99.2 |
 2
This
presentation contains forward looking statements under the safe harbor
provisions of the US securities laws. These forward-looking statements are based
on management’s beliefs and assumptions and on information currently
available to our management.
Our management believes that these forward-looking statements are reasonable
as and when made. However you should not place undue reliance on any such
forward looking statements as these are subject to risks and
uncertainties.
Please refer to our press releases and our SEC filings for more
information regarding the use of forward looking statements.
Cautionary Note Regarding Forward-Looking Statements |
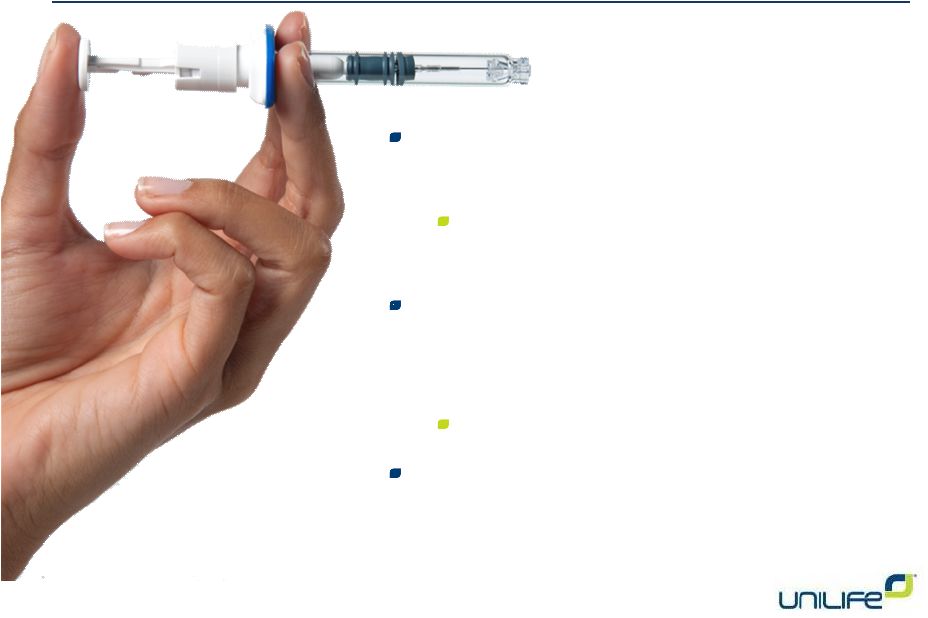 Introductory
Comments by Unilife CEO Alan Shortall Signing of commercial supply contract with
Sanofi for use of Unifill Finesse with Lovenox
Minimum purchase of 150MM units per year
after four-year ramp program
Preparing to finalize several additional contracts
with other pharmaceutical companies relating to
devices across our proprietary portfolio
Additional upfront cash payments
Re-negotiating terms received for a large non-equity
based debt financing
3 |
 Lovenox®
Supply Contract –
Key Terms
Unilife Product
Sanofi Drug
Drug Area
Geographic Territory
Contract Period
Exclusivity
Minimum Volumes
Additional Payments
Other Terms
4
Unifill Finesse (customized product from Unifill platform)
Enoxaparin Sodium (Lovenox®
/ Clexane®)
Anti-thrombotics (low molecular weight heparin)
Global
Can extend up to 2024
Use of Unifill Finesse with anti-thrombotic drugs
Minimum of 150MM units per year (after four-year ramp
period after market entry) to maintain exclusivity
Up to $15MM ($5MM expected in CY2013)
Confidential between both parties for commercial
purposes and due to confidentiality clauses
Replaces and supersedes previously signed agreements |
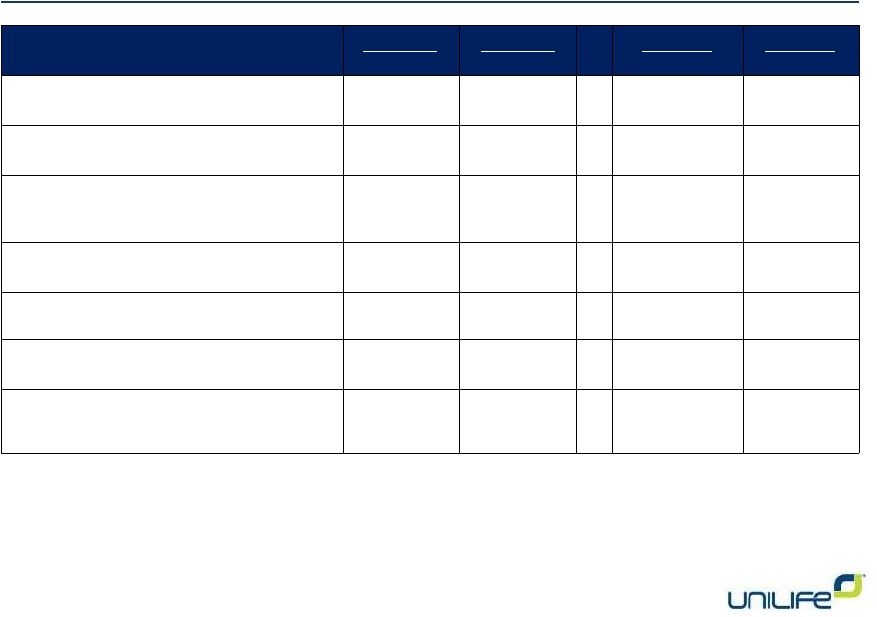 Financial
Data 5
4Q 2013
4Q 2012
FY 2013
FY 2012
Revenues
$ 0.7M
$ 1.2M
$ 2.7M
$ 5.5M
Research & development
$ 6.5M
$ 6.9M
$ 21.7M
$ 23.1M
Selling, general & administrative
$ 10.3M
$ 7.1M
$ 32.4M
$ 27.7M
Net loss
$ 22.0M
$ 14.9M
$ 63.2M
$ 52.3M
Net loss per share –
diluted
$ 0.25
$ 0.21
$ 0.78
$ 0.78
Adjusted net loss
$ 9.9M
$ 11.0M
$ 38.0M
$ 37.7M
Adjusted net loss per share -
diluted
$ 0.11
$ 0.15
$ 0.47
$ 0.56 |
 A Deep
Commercial Pipeline Driven by a Broad Product Portfolio Products are yet to be evaluated
by the FDA Unifill®
Unifill®
Select
EZMix™
EZMix™
Select
RITA™
Disposable Auto-Injector
LISA™
Reusable Auto-Injector
Precision-Therapy™
Flex-Therapy™
Depot-ject™
Ocu-ject™
Micro-ject™
Novel device for targeted organ
delivery (confidential)
Prefilled Syringes
For all prefilled biologics, drugs and vaccines
Reconstitution & Mixing
For all liquid or dry drug combination therapies
Auto-injectors
For all handheld patient self-injection therapies
Wearable Injectors
For long-duration or large-dose volume drugs that must
be worn on body during subcutaneous injection
Intraocular Delivery
For precise, accurate localized or targeted delivery of
drug depot or microliter doses to the eye
Specialized Devices
For the localized or targeted delivery of novel drugs
requiring extreme accuracy and precision
Selection of Products Within Platform
Technology Platform
6 |
 7
Questions |
