Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PROTALEX INC | v354331_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - PROTALEX INC | v354331_ex99-1.htm |

September, 2013

Forward - Looking Statement Statements made in this presentation stating the Company ’ s beliefs, intentions, and expectations are forward - looking statements. The company ’ s actual results could differ materially from those projected. Additional information is contained in the company ’ s SEC filings such as our Form 10 - K and Form 10 - Qs filed at www.sec.gov . 2 Protalex , Inc .

Protalex , Inc. Biotechnology company focused on autoimmune diseases Market cap: recently $75 mm; 30 mm SOS Public company (OTC BB: PRTX) ▪ Current ownership and management structure established in November 2009 ▪ Niobe Ventures, LLC, has invested $14 mm since 2009 and controls 79% of the equity Lead product: PRTX - 100 ▪ Staphylococcal protein A, an immuno - modulatory protein isolated from cultured bacteria ▪ Safe and well - tolerated; three human clinical studies completed , one in progress with > 32 RA patients dosed. ▪ Scalable and optimized GMP manufacture, 4 lots of drug substance produced over past 5 years 3 Protalex , Inc.

Investment Highlights PRTX - 100 is a novel immunomodulatory biological drug candidate that may be useful in the treatment of a variety of autoimmune diseases Proof - of - concept data from ongoing phase 1b trial in rheumatoid arthritis patients expected in early 2014 Potential efficacy in a number of orphan disease indications Validated manufacturing process; low cost of goods relative to other biologics Strong and growing IP position 4 Protalex , Inc.

PRTX - 100 Background Highly purified Staphylococcus aureus protein A (SPA) 42 kDa bacterial membrane protein comprising five homologous 58 – 61 amino acid immunoglobulin binding domains ▪ Binds to Fc region of IgG ▪ Binds with high affinity to F ab framework region of Clade VH3 Igs (most autoantibodies are VH3) Forms unique complexes with IgG which down - regulate activated B - cells and monocyte/macrophages via FcR antibody receptors. PRTX - 100 binds to B cells with VH3 B - cell receptors Demonstrated activity in cellular and animal models of disease 5 Protalex , Inc.

PRTX - 100 Reduces Footpad Swelling in Murine Collagen Induced Arthritis Model Paw Thickness 0 0.2 0.4 0.6 0.8 1 1.2 28 31 35 38 42 45 49 52 56 Days After Immunization Thickness Change (mm) ▪ Female DBA/1 mice immunized with bovine collagen II, boost with CII on d21 ▪ Treat with PRTX - 100 (3x/ wk 0.01 m g), Etanercept (5x/ wk , 100 mg) or vehicle ▪ Histopathological scoring consistent with reduction in swelling Etanercept Control PRTX - 100 6 Protalex , Inc.
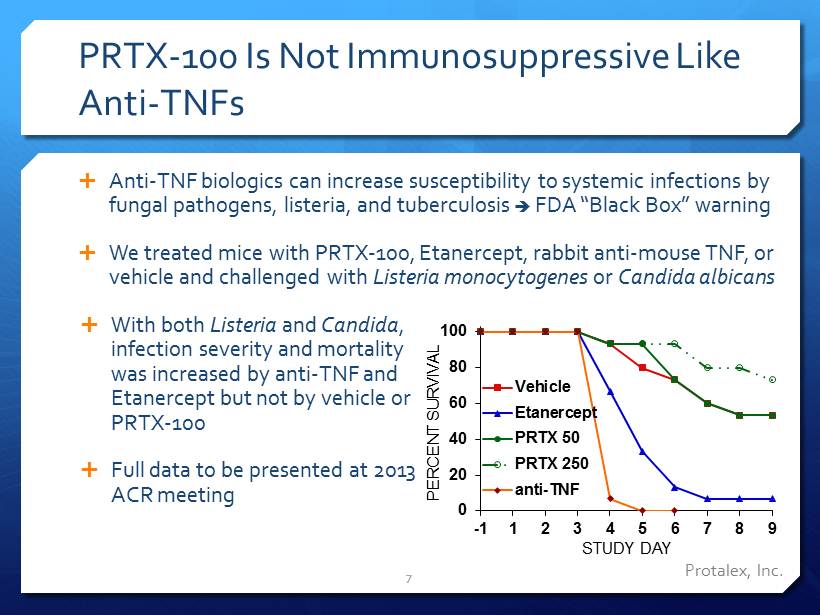
PRTX - 100 Is Not I mmunosuppressive L ike A nti - TNFs Anti - TNF biologics can increase susceptibility to systemic infections by fungal pathogens, listeria, and tuberculosis FDA “Black Box” warning We treated mice with PRTX - 100, Etanercept , rabbit anti - mouse TNF, or vehicle and challenged with Listeria monocytogenes or Candida albicans With both Listeria and Candida , infection severity and mortality was increased by anti - TNF and Etanercept but not by vehicle or PRTX - 100 Full data to be presented at 2013 ACR meeting 7 Protalex , Inc. 0 20 40 60 80 100 -1 1 2 3 4 5 6 7 8 9 PERCENT SURVIVAL STUDY DAY Vehicle Etanercept PRTX 50 PRTX 250 anti-TNF
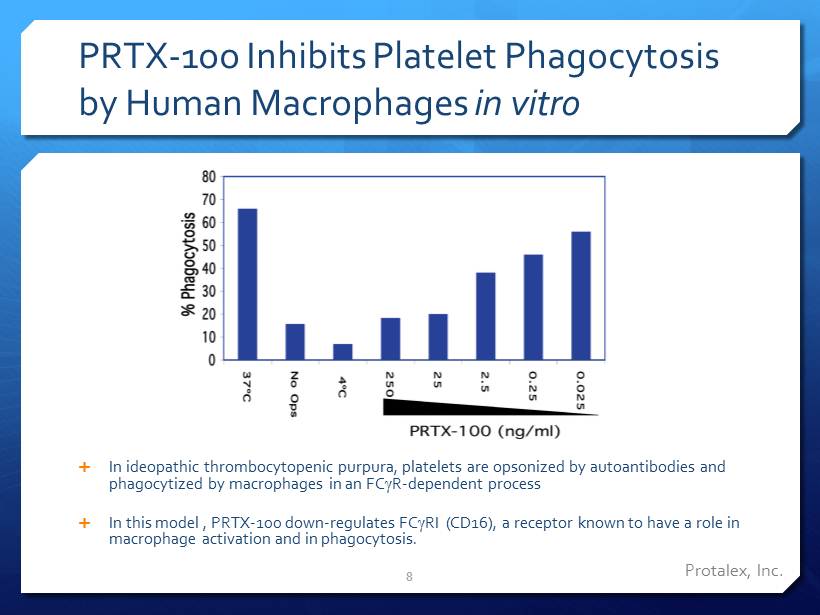
PRTX - 1oo Inhibits Platelet Phagocytosis by Human M acrophages in vitro In ideopathic thrombocytopenic purpura , platelets are opsonized by autoantibodies and phagocytized by macrophages in an FC g R - dependent process In this model , PRTX - 100 down - regulates FC g RI (CD16), a receptor known to have a role in macrophage activation and in phagocytosis. 8 Protalex , Inc.
PRTX - 100 Therapeutic Hypothesis and Key Points PRTX - 100 is an immune modulator that acts through mechanisms targeting activated macrophages and certain B - cells; it is not an immunosuppressant like TNF - inhibitors or anti - CD20s PRTX - 100 is derived from fermentation of non - recombinant bacteria; at scale, cost - of - goods will be far less than that of recombinant proteins produced in mammalian cells Dose is < 1 mg of protein, in contrast to ~ 100 - fold higher dose for mAb therapeutics. Allows rapid and safe administration. PRTX - 100 may have applications in many autoimmune diseases, including rheumatoid arthritis, idiopathic thrombocytopenic purpura , and lupus 9 Protalex , Inc.
Autoimmune Disease Market Opportunity RA biologicals current market value exceeds $18 B and is growing RA market disruptions envisioned ▪ PFE’s Xeljanz ( tofacitinib ) oral drug recently approved ▪ Bioequivalents to anti - TNFs and anti - CD20s in development High value/ lower cost modalities may expand current usage and may extend use of biologics from US, EU, and JP markets to global markets Other autoimmune diseases, e.g., ITP , CIDP, bullous pemphigus, myesthenia gravis represent highly underserved indications with potential for orphan drug designation 10 Protalex , Inc .

PRTX - 100 Potential for Orphan Drug Designation Adult idiopathic thrombocytopenic purpura (ITP) ▪ Incidence: 20 - 30 cases/million per year; patients with platelet counts > 25,000 are rarely treated, so few require aggressive treatment ▪ Treatments include steroids, splenectomy , thrombopoietin agonists ( romiplostin and eltrombopag ), high dose IVIG, and off - label, vincristine and rituximab. Chronic inflammatory demyelinating polyneuropathy (CIDP) ▪ Incidence 10 - 20 cases/million per year ▪ High - dose IVIG is first line treatment, but costs >$8000 per treatment 11 Protalex , Inc.

PRTX - 100 Clinical Experience 2005 – IND filed for RA 2006 – Phase 1 study completed 2007 – Second Phase 1 using PRTX - 100 with improved production/CMC processes 2010 - 11 – Phase 1b Study (PRTX - 100 - 103) in South Africa; presented at ACR Annual Meeting in November, 2012 November 2012 – Second Phase 1b Study (PRTX - 100 - 104 ), initiated in US August 2013 – Completed fourth cohort of PRTX - 100 - 104 12 Protalex , Inc.

PRTX - 100 - 103: Study Objectives Primary ▪ Assess safety and tolerability of iv PRTX - 100 weekly x 4 doses Secondary ▪ Assess immunogenicity after ≥3 doses ▪ Determine PK and estimate of PRTX - 100 plasma exposure after first and fourth dose ▪ Determine whether a relationship exists between immunogenicity of PRTX - 100 and safety and PK ▪ Assess effect of PRTX - 100 on measures of disease activity, e.g., DAS28 - CRP and CDAI 13 Protalex , Inc.
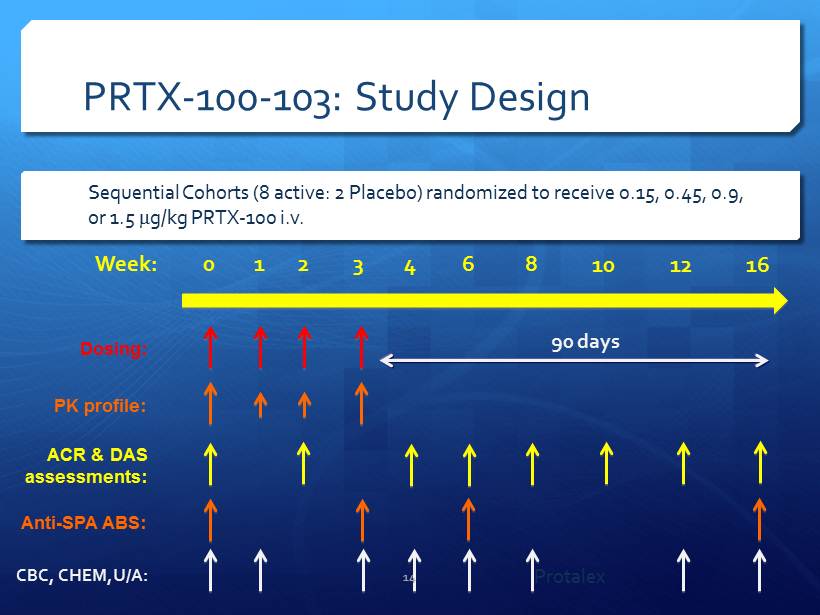
PRTX - 100 - 103: Study Design Week: 0 1 2 3 6 4 10 8 12 16 Dosing: ACR & DAS assessments: Anti - SPA ABS: 90 days PK profile: CBC, CHEM,U/ A: Sequential Cohorts (8 active: 2 Placebo) randomized to receive 0.15, 0.45, 0.9, or 1.5 m g/kg PRTX - 100 i.v. 14 Protalex

PRTX - 100 - 103: DAS28 < 3.2 0 5 10 15 20 25 30 PRTX-100 Placebo % of patients with DAS28 < 3.2 at 6 and 10 weeks Day 42 Day 70 3/29 6/29 0/8 0/8 15 Protalex , Inc.
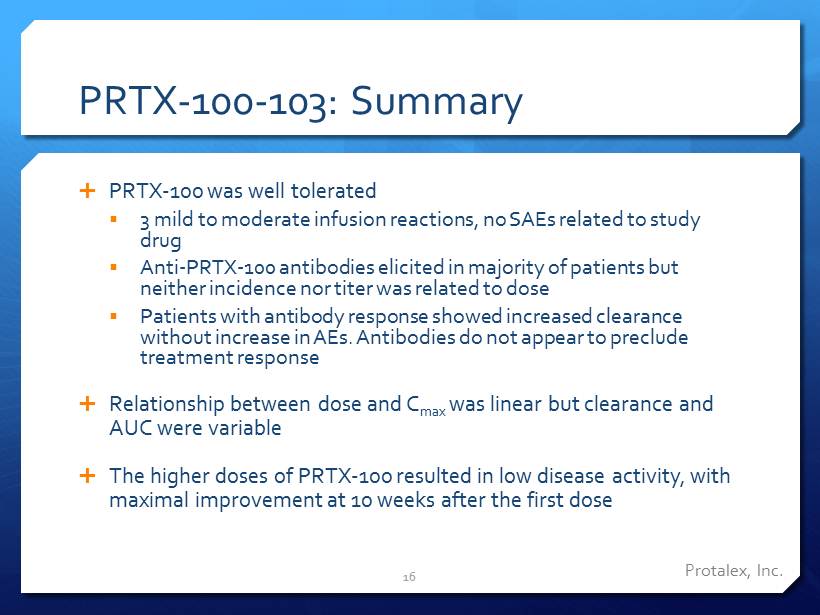
PRTX - 100 - 103: Summary PRTX - 100 was well tolerated ▪ 3 mild to moderate infusion reactions, no SAEs related to study drug ▪ Anti - PRTX - 100 antibodies elicited in majority of patients but neither incidence nor titer was related to dose ▪ Patients with antibody response showed increased clearance without increase in AEs. Antibodies do not appear to preclude treatment response Relationship between dose and C max was linear but clearance and AUC were variable The higher doses of PRTX - 100 resulted in low disease activity, with maximal improvement at 10 weeks after the first dose 16 Protalex , Inc.

PRTX - 100 - 104 : Overview New multi - center US study. Phase 1b randomized, multiple - dose, placebo - controlled, dose - escalation study of PRTX - 100 in adults with active RA on MTX Recent amendment calls for up to 44 patients in four dose - escalating cohorts, starting at 1.50 m g/kg, with fifth cohort to investigate extended dosing schedule Primary objective: safety and tolerability of PRTX - 100 administered by iv injections over five weeks Secondary objectives include determining effects on measures of disease activity, assessing immunogenicity, evaluating PK, and investigating durability of response Enrollment commenced November 2012 in the US; last dose of cohorts one through four completed July 2013; expansion cohorts initiated August 2013 Topline data to be announced 1Q14 17 Protalex , Inc .
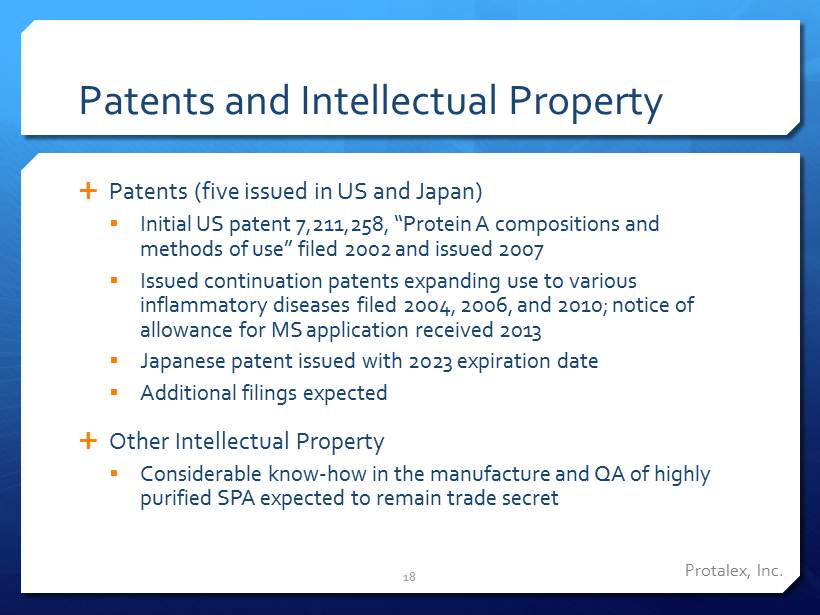
Patents and Intellectual Property Patents (five issued in US and Japan) ▪ Initial US patent 7,211,258, “Protein A compositions and methods of use” filed 2002 and issued 2007 ▪ Issued continuation patents expanding use to various inflammatory diseases filed 2004, 2006, and 2010; notice of allowance for MS application received 2013 ▪ Japanese patent issued with 2023 expiration date ▪ Additional filings expected Other Intellectual Property ▪ Considerable know - how in the manufacture and QA of highly purified SPA expected to remain trade secret 18 Protalex , Inc.
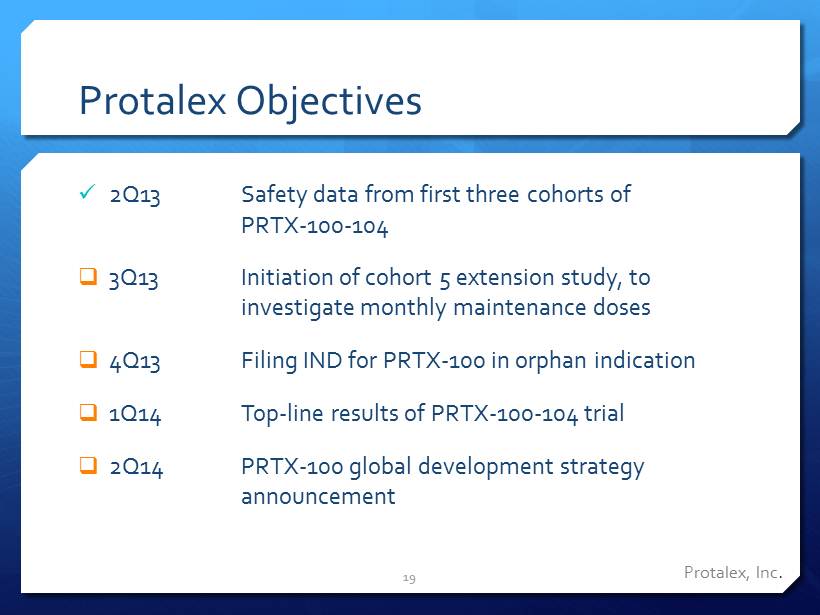
Protalex Objectives x 2Q13 Safety data from first three cohorts of PRTX - 100 - 104 □ 3Q13 Initiation of cohort 5 extension study, to investigate monthly maintenance doses □ 4Q13 Filing IND for PRTX - 100 in orphan indication □ 1Q14 Top - line results of PRTX - 100 - 104 trial □ 2Q14 PRTX - 100 global development strategy announcement 19 Protalex , Inc .

Protalex Corporate Highlights PRTX - 100 is a novel immunomodulatory biological drug candidate that may be useful in the treatment of autoimmune diseases PRTX - 100 is economical to produce relative to anti - TNFs, anti - IL - 6, and CTL4 - Ig biologicals ; validated manufacturing PRTX - 100 is safe and well - tolerated in humans Safety and initial proof - of - concept data from an ongoing phase 1b trial in RA patients are expected in 2014 20 Protalex , Inc .
