Attached files
| file | filename |
|---|---|
| 8-K - THERMOGENESIS CORP 8-K 7-16-2013 - ThermoGenesis Holdings, Inc. | form8k.htm |
| EX-99.1 - EXHIBIT 99.1 - ThermoGenesis Holdings, Inc. | ex99_1.htm |
| EX-99.2 - EXHIBIT 99.2 - ThermoGenesis Holdings, Inc. | ex99_2.htm |

Corporate Presentation
The Merger of ThermoGenesis & TotipotentRX
July 2013
Exhibit 99.3
Filed by: ThermoGenesis Corp.
Pursuant to Rule 425 under the Securities Act of 1933
Subject Company: ThermoGenesis Corp.
Commission File No.: 333-82900

CescaTM Therapeutics
Forward Looking Statement
Forward Looking Statement
ThermoGenesis Corp.
Web site: http://www.thermogenesis.com
Contact: Investor Relations
+1-916-858-5107, or
ir@thermogenesis.com

Cesca Therapeutics
Launches only integrated regenerative medicine company
Launches only integrated regenerative medicine company

4 | Page
Cesca Therapeutics
TotipotentRX snapshot
TotipotentRX snapshot
• Developing cellular therapies for vascular, orthopedic and neurological diseases
• Core capabilities in development and clinical testing of cellular therapies
• Pipeline of 10 clinical stage therapies
THERAPEUTICS
DEVICES
CLINICAL SERVICES

5 | Page
Cesca Therapeutics
ThermoGenesis snapshot (Nasdaq: KOOL)
• Leading designer and supplier of clinical technologies for processing, storage
and administration of stem cells
and administration of stem cells
• Core capabilities include quality innovation, design IP, commercialization,
regulatory, and global distribution
regulatory, and global distribution
• ~$18M in annual revenues
Automated
Point-of-Care/
Lab-based
Lab-based
cGMP
Closed Systems
Scalable

6 | Page
Regenerative Medicine’s Future
Macro drivers
Macro drivers
• Global Trends in Healthcare
− Aging population, rapidly rising costs
− Medical reimbursement; pay for performance
− Pharma:
Ø Patent expirations, empty pipelines
Ø Not curative, treats symptoms
• Regenerative Medicine
− Curative; targets root cause of disease
− Creating value:
Ø Will save $250B/year1
Uniquely able to control key variables to minimize regulatory risk and
maximize clinical effectiveness
maximize clinical effectiveness
1 Alliance for Regenerative Medicine, Annual Report 2013
7 | Page
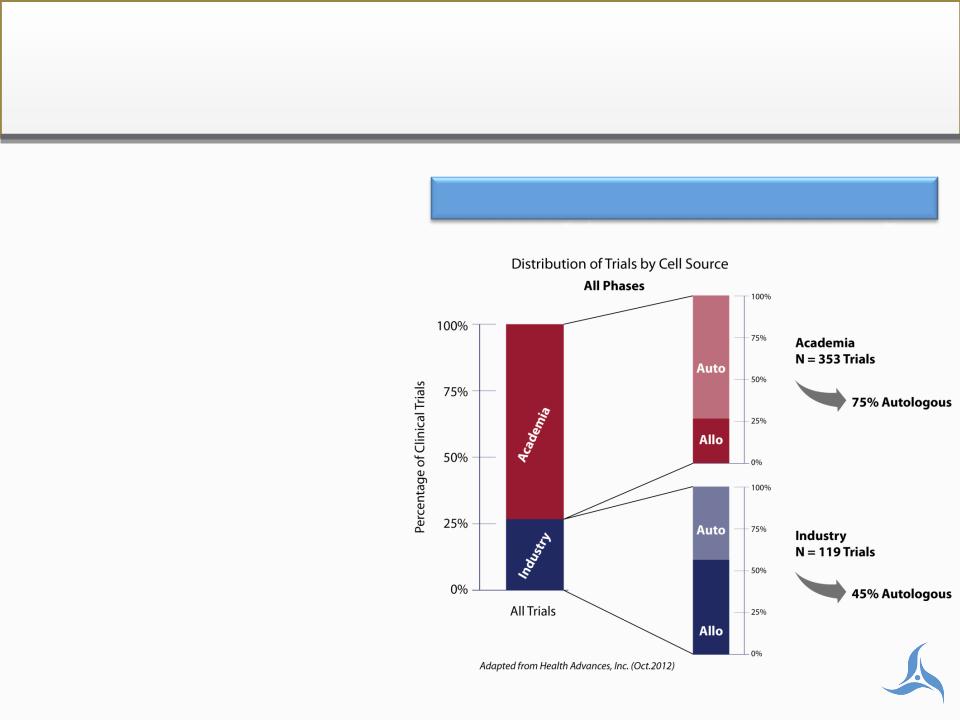
Healthcare Market
Macro drivers
Macro drivers
• Regenerative medicine gains
industry’s eye
industry’s eye
− Industry clinical trial sponsors:
ü 2007: <1%
ü 2010: 5%
ü 2012: 20%
• Academic cell evolution; From
embryonic, to allogeneic, to
autologous:
embryonic, to allogeneic, to
autologous:
− CIRM has begun funding
autologous trials again
autologous trials again
− Strong NIH funding for autologous
studies continues
studies continues
Industry presence accelerating

8 | Page
Autologous Therapy Today
Current regenerative medicine POC practice
Current regenerative medicine POC practice

9 | Page
Cesca Therapeutics
The autologous cell therapy solution
The autologous cell therapy solution
• Patient friendly
• Physician friendly
• Fast, at-the-bedside
• Effective
• In the regulatory “sweet spot”
• Commercially viable
Consistent cell compositions
cGMP compliant protocols
Clinical data
10 | Page
Cesca Commercialization Advantage
The first commercially viable autologous cell therapy
The first commercially viable autologous cell therapy

11 | Page
Cesca CellwerksSM
Our Unique Development Process
Our Unique Development Process
• Uniquely integrated development
- Chemistry, cellularity and quality
- Controlled, consistent delivery
- 550 patients treated across eight clinical
indications (Toti)
indications (Toti)

12 | Page
Cesca SurgwerksTM
Indication specific, proprietary product suite
Indication specific, proprietary product suite

13 | Page
Cesca SurgwerksTM
Sustainable differentiation
Sustainable differentiation
• Developed at the point-of-care, over 20,000 patients treated through
ThermoGenesis
ThermoGenesis
• Clinically validated, proprietary clinical protocols & method patents
• Proprietary, smart platforms (device and algorithm patents)
• Proprietary cell formulations addressing multiple disease indications
• Pioneering with regulatory strategy to be first FDA combination product
approved - 21CFR 3.2 (e)
approved - 21CFR 3.2 (e)
• IP Suite
− 43 Design and device patents
− Three protocol provisional patents (6 indications)
− 10 pilot & phase 1b clinical trials
− 7 clinical algorithms

14 | Page
In the Regulatory “Sweet Spot”
Lower risk = speed to market
Lower risk = speed to market
15 | Page
Proprietary Cell Formulations
Unique algorithms for treatment of multiple indications
Unique algorithms for treatment of multiple indications
|
Source
Material |
Clinical
Indication |
Harvest Hct
(%) |
MNC recovery
(%) |
Monocyte
recovery
|
Platelet
recovery (%) |
|
PB
|
Chronic Dermal
Wound |
≤ 25%
|
> 80%
|
> 85%
|
> 85%
|
|
PB
|
Chronic Dermal
Wound |
≤ 10%
|
> 80%
|
> 85%
|
> 85%
|
|
PB
|
Soft Tissue
|
≤ 3%
|
> 50%
|
> 80%
|
> 85%
|
|
PB
|
Soft Tissue
|
≤ 2%
|
> 50%
|
> 80%
|
> 85%
|
|
BM
|
Cardiac & CLI
|
≤ 25%
|
> 85%
|
> 85%
|
> 85%
|
|
BM
|
Non-Union
|
≤ 15%
|
> 85%
|
> 85%
|
> 85%
|
|
BM
|
Neurological
|
< 3%
|
> 60%
|
> 85%
|
> 85%
|
PB = Peripheral Blood
BM = Bone Marrow
VXP® Algorithmic Control

16 | Page
Cesca Pilot Trial Experience
Developing the CellWerks model
Developing the CellWerks model
|
Specialty
|
Indication
|
Trial Completion
|
|
Orthopedics
|
OA
|
50 Patients Pilot Completed
|
|
Non Union
|
30 Patients Pilot Completed
19 Patients Phase 1 Completed
|
|
|
Avascular
Necrosis |
10 Patients Pilot Completed
|
|
|
Cardiovascular
|
AMI
|
30 Patient Phase 1b Underway
|
|
Stroke
|
15 Patient Phase 1a Reg Review
|
|
|
CLI
|
15 Patient Phase 1b Completed
20 Patient Phase 1 Completed
|
|
|
Non Healing
Ulcers |
10 Patient Pilot
|
Market for our target therapies totals $16.6B
More than 300 patients treated in other trials and applications

17 | Page
High Impact Clinical Results
Compelling early vascular results
Compelling early vascular results
• N=17 patients
• All patients “no option” and 24
hours from leg amputation
hours from leg amputation
• 88.9% of gangrene sores
improved
improved
• Major Amputation Free Rate post
SurgWerks™ Therapy = 85.7%
SurgWerks™ Therapy = 85.7%
• Minor Amputation Free Rate Post
SurgWerks™ = 55.5% to date
SurgWerks™ = 55.5% to date
• 31% Reduction in VAS Pain
Score at 3 Mo. F/U
Score at 3 Mo. F/U
Major Revascularization

18 | Page
• N=19 patients
• All patients failed traditional
surgical fixation
surgical fixation
• 71% union rate in 18 weeks post
SurgWerks™ treatment
SurgWerks™ treatment
– No SAEs/AES
– 2 patients lost to F/U
Long Bone Fusion
Non-Union Fractures
High Impact Clinical Results
Compelling early orthopedic results
Accepted for publication: 2012
International Society of Cell Therapy,
Rotterdam
International Society of Cell Therapy,
Rotterdam

19 | Page
Clinical Pipeline
Blockbuster drug candidates
Blockbuster drug candidates
KEY CESCA CRO ADVANTAGES
− US FDA accepts foreign
trials
trials
− Better control of trial
management
management
− 1/5th the cost of US/EU
Patient related Clinical Trials
costs
Patient related Clinical Trials
costs
− Speed to completion

20 | Page
Our Fortis Partnership
CRO embedded in New Delhi facility
CRO embedded in New Delhi facility
• Cesca is exclusive regenerative medicine
provider to Fortis
provider to Fortis
– 72 hospitals (6 countries)
– 10,000 inpatient beds
– 15,000 outpatients per day
– Competent clinical research staff
– 2x as many sites as Kaiser
• Physician/patient access
• World class clinical facilities and
equipment
equipment
• Lobby partner with government
• Embedded CRO
– Only global cell therapy CRO
– US FDA registered
– Over 500 patients treated
By leveraging our Fortis
partnership and in-house CRO,
Cesca has the potential to get
new cell treatments approved
faster and cheaper than our
competitors
partnership and in-house CRO,
Cesca has the potential to get
new cell treatments approved
faster and cheaper than our
competitors

21 | Page
Co-Development and Marketing Prospects
Prospective partnerships
Prospective partnerships
|
|
VASCULAR CONDITIONS
|
ORTHOPEDIC CONDITIONS
|
||||||
|
|
AMI
|
CLI
|
STROKE
|
NON
HEALING ULCERS |
SPINE
|
OA
|
NON UNION
FRACTURES |
AVN
|
|
Cook
|
X
|
X
|
X
|
|
|
|
|
|
|
Sanofi
|
|
|
|
X
|
|
X
|
|
|
|
Shire
|
|
|
|
X
|
|
X
|
X
|
|
|
Pfizer
|
X
|
X
|
|
X
|
|
|
|
|
|
Medtronic
|
X
|
X
|
|
|
X
|
|
X
|
X
|
|
Abbott
|
X
|
X
|
|
|
|
|
|
|
|
J&J
|
X
|
X
|
X
|
X
|
X
|
|
X
|
X
|
|
Terumo
|
X
|
X
|
X
|
X
|
|
|
|
|
|
Baxter
|
X
|
|
X
|
|
|
|
|
|
|
Amgen
|
|
|
|
X
|
|
|
|
|
|
Stryker
|
|
|
|
|
X
|
X
|
X
|
X
|
|
Biomet
|
|
|
|
|
X
|
X
|
X
|
X
|
|
Arthrex
|
|
|
|
|
X
|
X
|
X
|
X
|
|
TOTAL
PROSPECTS |
7
|
6
|
4
|
6
|
5
|
5
|
6
|
5
|
|
2012 Market
Size ($B) |
$2.40
|
$2.00
|
$5.20
|
$0.80
|
$0.80
|
$5.00
|
$1.10
|
$0.08
|

22 | Page
Unlocking Intrinsic Value of Base Business
Expanding clinical uses for cord blood
Expanding clinical uses for cord blood
• Accelerate Cord Blood Organic Revenue
− Leverage first mover position in Asia
− Take competitive share in Europe and the Americas
− Introduce advanced AXP upgrades, new products, and support
• Expand Practice of Medicine Usage of Cord Blood
− Leverage Cesca CRO for rapid, cost effective introduction of 5 new therapies
− Leverage Cesca research cord blood bank for clinical and engineering innovation
− Expanding indications beyond hematopoietic, making cord blood a neo-natal, point-of-
care therapy
care therapy
− Establish presence in US for direct sales to “Clinical Commercial Zones”
− Leverage Fortis Hospital Relationship for POM in Wound Care & Orthopedics

23 | Page
Leveraging Cord blood
Global automated processing leader
Global automated processing leader
Exclusive automated cord
blood processing device
provider to CCBC
Exclusive AXP distributor
in China and several
Southeast Asian
countries
in China and several
Southeast Asian
countries
Strong foundation in Golden Meditech and Cesca Research Bank
allows us to capitalize on the Asia growth opportunity
allows us to capitalize on the Asia growth opportunity

24 | Page
Overview of Key Milestones
Substantial near term clinical value drivers
Substantial near term clinical value drivers
• 2013
− Cardiac Patient Ph1b Enrollment Commences
• 2014
− Clinical Trial Approval (NHU IRB)
− Clinical Trial Approval (OA IRB)
− US Investigational New Drug Granted (CLI Ph1/2)
− Cardiac Patient Ph1b Enrollment Completed
− Ph1 Trial Concluded (NHU)
− Ph1 Trial Concluded (OA)
• 2015
− Cardiac Patient Ph1b Results
− US Investigational New Drug Granted Ph2 (OA)
− Cell Diagnostic Analyzer Product Launch
− US Investigational New Drug Granted Cardiac Ph2
− Pivotal Trial Initiated (NHU)

25 | Page
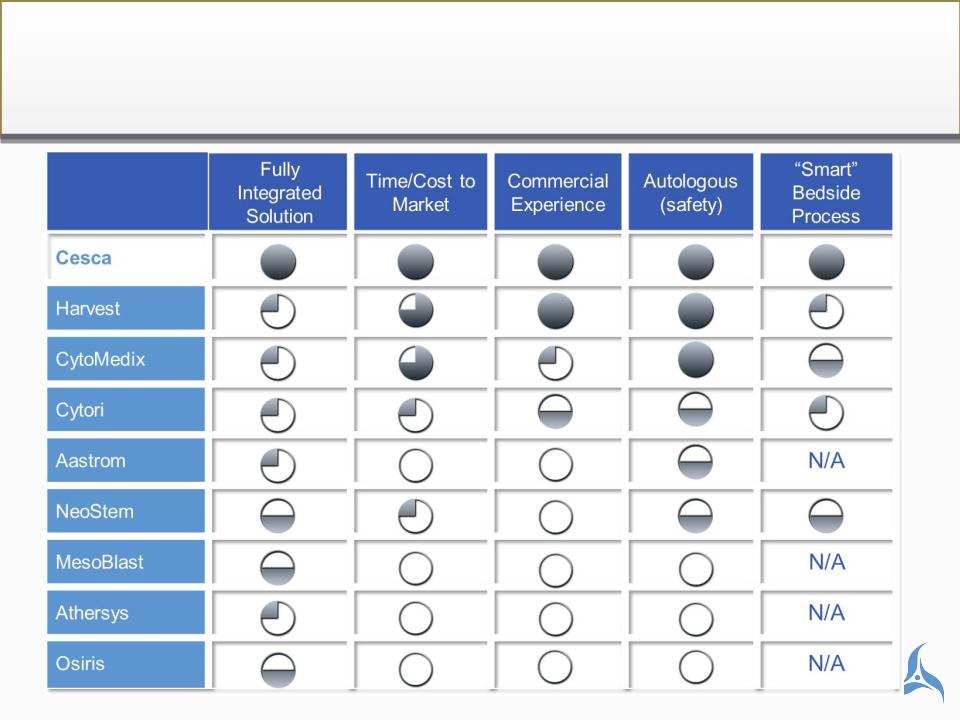
Value Capture of Cesca Transformation
Revenue multiple expansion catalyst
Revenue multiple expansion catalyst
CESCA THERAPEUTICS PEER GROUP
Cytori, Aastrom, Athersys, Neostem, Cytomedix, Mesoblast,
BioTime, Osiris, Harvest
BioTime, Osiris, Harvest

26 | Page
Cesca Growth Capital Investment
36 month horizon
36 month horizon
|
Category
|
Growth Capital
|
|
• Clinical Development & Regulatory Initiatives
- AMI, Stroke, OA, NHU
- CLI, Spine, NUF, AVN
|
$15M
|
|
• Product Commercialization
- Cord Blood (Distribution, Launches)
- Clinical Commercial Zones
|
$2M
|
|
• Device Development & General Capital Req’s
- Diagnostics Devices
|
$3M
|
|
Total
|
$20M
|
NOTE: To maximize the value of our clinical trials and accelerate our ability to commercialize
our products in the growing market place we expect to invest approximately $15 to $20
million in our clinical and product platform initiatives in the next 36 months. We anticipate
that the timing and amount of our investment will be staged consistent with our major clinical
milestones. We will rely on potential cash flows from our combined operations and explore all
available funding alternatives, including grant monies and strategic partnerships, to pursue a
strategy that is in the best interest of our stockholders.
our products in the growing market place we expect to invest approximately $15 to $20
million in our clinical and product platform initiatives in the next 36 months. We anticipate
that the timing and amount of our investment will be staged consistent with our major clinical
milestones. We will rely on potential cash flows from our combined operations and explore all
available funding alternatives, including grant monies and strategic partnerships, to pursue a
strategy that is in the best interest of our stockholders.

27 | Page
Cesca Senior Leadership
Seasoned industry executives:
Significant Insider ownership
Seasoned industry executives:
Significant Insider ownership
Matthew T. Plavan
CHIEF EXECUTIVE OFFICER, BOD
§ COO ’08-’10 (3yrs), CFO ‘05-’13 (8yrs), & CFO for two venture-backed start-ups (5 yrs)
§ Vice-President of Finance & Gen. Mgr McKesson (7yrs) & Ernst & Young (6yrs)
Kenneth L. Harris, M.S.
PRESIDENT, BOD
§ 25 yrs exp. in healthcare & biotech. Previously Chairman, CEO, & Co-
Founder of MK Alliance, Inc. a position (5 yrs)
Founder of MK Alliance, Inc. a position (5 yrs)
§ President of Pall Corp’s BioSciences Div. & Cell Therapy Div., ‘01- ‘08
Dan T. Bessey
CHIEF FINANCIAL OFFICER
§ Served from ‘08-’12 as Vice-President & CFO of SureWest Communications
(Telecom)
(Telecom)
§ VP Finance, Controller & Dir. of Corporate Finance (various roles) during 13yr
tenure at SureWest (previous to CFO)
tenure at SureWest (previous to CFO)
Mitchel Sivilotti, M.Sc.
SVP, BIOLOGICS
§ 10 yrs exp. In biotech. Previously Vice-Chairman, President & Co-Founder of MK
Alliance, Inc. a position held for 5yrs
Alliance, Inc. a position held for 5yrs
§ Prior to MKA, Mitch Managed the Global Cell Therapy Business Unit at Pall Corp.
Hal Baker
SVP, COMMERCIAL OPERATIONS
§ Various roles as head of sales for ThermoGenesis ‘09- ‘13 (4yrs)
§ VP, Global Sales for Hygenic Corporation ‘06- ’09 (3yrs) & managed sales
and marketing for Pall Corporation ‘01- ’05 (4yrs)
and marketing for Pall Corporation ‘01- ’05 (4yrs)
Ken Pappa
VP, OPERATIONS
§ VP Engineering, Manufacturing & various operations management roles ‘06 -
’13 (7yrs) ThermoGenesis.
’13 (7yrs) ThermoGenesis.
§ Various positions with Hewlett Packard-Agilent Technologies including Mfg.
Controller & Sr. Operations Manager
Controller & Sr. Operations Manager
Kevin Cooksy
VP, BUSINESS DEVELOPMENT
§ VP, Business Development ‘11- ‘13 (2yrs) ThermoGenesis
§ VP, Business Development ‘07 ‘10 (3yrs) for Perlegen Sciences & held
various management positions in Corporate Development and Global
Marketing ‘05- ‘10 (5yrs) Agilent Technologies
various management positions in Corporate Development and Global
Marketing ‘05- ‘10 (5yrs) Agilent Technologies

28 | Page
Cesca Investment Highlights
Uniquely positioned to lead in regenerative medicine
Uniquely positioned to lead in regenerative medicine
• Regenerative medicine is a major element of our future
healthcare system
healthcare system
• Our integrated device, point-of-care services and Indian
CRO business is a capital efficient, regulatory compliant
model able to expedite new cell therapies to market
CRO business is a capital efficient, regulatory compliant
model able to expedite new cell therapies to market
• Strong growth in ThermoGenesis’ base business in Asia
provides working capital leverage for our therapeutic
plans
provides working capital leverage for our therapeutic
plans
• TotiPotent’s global therapeutic competence and Asian
infrastructure is our primary growth engine
infrastructure is our primary growth engine
• Cesca assembles the management talent and
experience to execute our plan
experience to execute our plan

29 | Page
Cesca Therapeutics
Additional Information
Additional Information
• Non-Solicitation
− This presentation and the information contained herein shall not constitute an offer to sell,
buy or exchange or the solicitation of an offer to sell, buy or exchange any securities, nor
shall there be any sale, purchase or exchange of securities in any jurisdiction in which such
offer, solicitation, sale, purchase or exchange would be unlawful prior to registration or
qualification under the securities laws of any such jurisdiction. No offer of securities shall
be made except by means of a prospectus meeting the requirements of Section 10 of the
Securities Act of 1933, as amended.
buy or exchange or the solicitation of an offer to sell, buy or exchange any securities, nor
shall there be any sale, purchase or exchange of securities in any jurisdiction in which such
offer, solicitation, sale, purchase or exchange would be unlawful prior to registration or
qualification under the securities laws of any such jurisdiction. No offer of securities shall
be made except by means of a prospectus meeting the requirements of Section 10 of the
Securities Act of 1933, as amended.
• Additional Information
− In connection with the merger, ThermoGenesis intends to file a registration statement
(including a prospectus) on Form S-4 with the Securities and Exchange Commission.
Holders of ThermoGenesis Common Stock and TotipotentRx Corporation common stock
are urged to read the proxy statement/prospectus and any other relevant documents when
filed because they contain important information about ThermoGenesis, TotipotentRx and
the merger. A proxy statement will be sent to holders of our Common Stock and a proxy
statement/prospectus will be sent to holders of TotipotentRx Corporation common stock.
When filed, the proxy statement/prospectus and other documents relating to the proposed
merger can be obtained free of charge from the SEC’s website at www.sec.gov. These
documents can also be obtained free of charge from ThermoGenesis upon written request
to ThermoGenesis, Investor Relations, 2711 Citrus Road Rancho Cordova, CA 95742.
(including a prospectus) on Form S-4 with the Securities and Exchange Commission.
Holders of ThermoGenesis Common Stock and TotipotentRx Corporation common stock
are urged to read the proxy statement/prospectus and any other relevant documents when
filed because they contain important information about ThermoGenesis, TotipotentRx and
the merger. A proxy statement will be sent to holders of our Common Stock and a proxy
statement/prospectus will be sent to holders of TotipotentRx Corporation common stock.
When filed, the proxy statement/prospectus and other documents relating to the proposed
merger can be obtained free of charge from the SEC’s website at www.sec.gov. These
documents can also be obtained free of charge from ThermoGenesis upon written request
to ThermoGenesis, Investor Relations, 2711 Citrus Road Rancho Cordova, CA 95742.

Thank you
