Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GALECTIN THERAPEUTICS INC | d552936d8k.htm |
Exhibit 99.1

Tumor Immunotherapy Efficacy Increased in Both Breast and Prostate
Cancer Preclinical Models with Addition of Galectin Inhibitor
— Galectin Therapeutics and Earle A. Chiles Research Institute —
Norcross, GA, June 12, 2013 – Galectin Therapeutics (NASDAQ: GALT), the leading developer of therapeutics that target galectin proteins to treat fibrosis and cancer, today announced that preclinical studies have shown that combining the galectin inhibitor GR-MD-02 with monoclonal antibodies that function as immune checkpoint blockade inhibitors enhance shrinkage of prostate and breast cancer tumors. These data were generated by the laboratory of Dr. William Redmond of the Robert W. Franz Cancer Research Center in the Earle A. Chiles Research Institute (EACRI) at Providence Cancer Center (Portland, OR), an expert in tumor immunology and part of a comprehensive pre-clinical and clinical program in cancer immunotherapy. The addition of GR-MD-02, a drug that inhibits galectin proteins, was found to increase tumor shrinkage and enhance survival in immune competent mice with prostate and breast cancers when combined with one of the immune checkpoint blockade inhibitors, anti-CTLA-4 or anti-PD-1.
“Immunotherapy with checkpoint blockade inhibitors, such as anti-CTLA-4 monoclonal antibodies (marketed as YERVOYtm by Bristol-Myers Squibb (BMS)) and anti-PD-1 monoclonal antibodies (in development at BMS and Merck), is an extremely exciting area for advancing human cancer immunotherapy.” said Dr. Peter G. Traber, President, Chief Executive Officer and Chief Medical Officer, Galectin Therapeutics. “However, these agents are currently only effective in a limited percentage of patients. The ability of GR-MD-02 to increase the effectiveness of these important drugs is a potentially important approach to enhance cancer immunotherapy.”
“The findings of these experiments show that GR-MD-02 had a robust effect on augmenting the immune response and tumor efficacy when combined with well characterized agents for cancer immunotherapy,” said Dr. Redmond, Assistant Member at the EACRI. “The experimental tumor models used are relatively resistant to therapy to checkpoint blockade inhibitors alone, indicating that the addition of GR-MD-02 may be a potentially important agent in combination therapy.”
“We have determined that these pre-clinical results are compelling for clinical translation and we are planning to seek FDA agreement to open a trial using YERVOYtm (ipilimumab, anti-CTLA-4 mAb, BMS) in combination with GR-MD-02 in patients with advanced melanoma” said Brendan Curti, MD, Medical Oncologist and Director of the Providence Biotherapy Program at EACRI. “We are in the process of designing such a trial with Galectin Therapeutics”.
Initial data was reported at the Tumor Immunology Keystone Conference in March of 2013 which showed that treatment with GR-MD-02 augmented CD8+ T cell function in mice (poster available on Galectin web site: http://bit.ly/19gmjHX) These data show that in response to vaccination with an antigenic protein, inhibition of extracellular galectin-3 with GR-MD-02 increases the T-cell response to vaccination. Data was then obtained in several syngeneic tumor models in mice to evaluate the efficacy of anti-CTLA-4 and anti-PD1 monoclonal antibodies alone and in combination with GR-MD-02. As shown below, the addition of GR-MD-02 resulted in augmented tumor shrinkage in both prostate and breast cancer models.

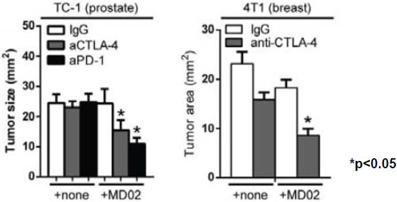
These data were presented as part of the annual stockholder meeting on May 23, 2013 which can be viewed on the Galectin Therapeutics website (http://bit.ly/11QKp2P).
Notes to the reader: Checkpoint Blockade involves molecules on the cell surface of CD4 and CD8 T cells. These molecules serve as “brakes” to down-modulate or inhibit an anti-tumor immune response. Checkpoint Blockade Inhibitors release the “brakes” and thereby increases the immune response. Syngeneic tumor models in mice use mouse tumors in immunologically normal mice and therefore allow evaluation of the effect of the immune system on the tumors.
YERVOYtm is a registered trademark of Bristol-Myers Squibb Company
About Galectin Therapeutics
Galectin Therapeutics (NASDAQ: GALT) is developing promising carbohydrate-based therapies for the treatment of fibrotic liver disease and cancer based on the Company’s unique understanding of galectin proteins, key mediators of biologic function. We are leveraging extensive scientific and development expertise as well as established relationships with external sources to achieve cost effective and efficient development. We are pursuing a clear development pathway to clinical enhancement and commercialization for our lead compounds in liver fibrosis and cancer. Additional information is available at www.galectintherapeutics.com.
About Robert W. Franz Cancer Research Center, Earle A. Chiles Research Institute (EACRI), Providence Cancer Center, Portland Oregon
Providence Cancer Center, a part of Providence Health & Services, offers the latest in cancer services, including diagnostic, treatment, prevention, education, support and internationally renowned research. The Earle A. Chiles Research Institute at Providence Cancer Center is one of 10 research institutions selected to form the International Immuno-Oncology Network. This global collaboration will focus on helping the body’s own immune system fight cancer and bring more clinical trials to more patients in our community than ever before. Visit www.providence.org/cancer.

Forward Looking Statements
This press release contains, in addition to historical information, forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to future events or future financial performance, and use words such as “may,” “estimate,” “could,” “expect” and others. They are based on our current expectations and are subject to factors and uncertainties which could cause actual results to differ materially from those described in the statements. These statements include those regarding the potential benefits of using galectin inhibitors in combination tumor immunotherapy and the consideration of the initiation of a related clinical trial. Factors that could cause our actual performance to differ materially from those discussed in the forward-looking statements include, among others, that our plans, expectations and goals regarding any potential benefits of our drugs and any future clinical trials are subject to factors beyond our control. Future clinical trials may not begin or produce positive results in a timely fashion, if at all, and could prove time consuming and costly. Plans regarding development, approval and marketing of any of our drugs, including GR-MD-02, are subject to change at any time based on the changing needs of our company as determined by management and regulatory agencies. To date, we have incurred operating losses since our inception, and our ability to successfully develop and market drugs may be impacted by our ability to manage costs and finance our continuing operations For a discussion of additional factors impacting our business, see our Annual Report on Form 10-K for the year ended December 31, 2012, and our subsequent filings with the SEC. You should not place undue reliance on forward-looking statements. Although subsequent events may cause our views to change, we disclaim any obligation to update forward-looking statements.
Contact
Galectin Therapeutics Inc.
Peter G. Traber
Chief Executive Officer
(678) 620-3186
ir@galectintherapeutics.com
