Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Synthetic Biologics, Inc. | v345690_8k.htm |
EXHIBIT 99.1

May 20, 2013 NYSE MKT: SYN

Forward - Looking Statements This presentation includes forward - looking statements on Synthetic Biologics’ current expectations and projections about future events . In some cases forward - looking statements can be identified by terminology such as "may," "should," "potential," "continue," "expects," "anticipates," "intends," "plans," "believes,“ "estimates,” “indicates,” and similar expressions . These statements are based upon current beliefs, expectations and assumptions and are subject to a number of risks and uncertainties, many of which are difficult to predict and include statements regarding our clinical trials, our establishment of collaborations and our execution of our growth strategy . The forward - looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those set forth or implied by any forward - looking statements . Important factors that could cause actual results to differ materially from those reflected in Synthetic Biologics’ forward - looking statements include, among others, a failure of our product candidates to be demonstrably safe and effective, a failure to initiate clinical trials and if initiated, a failure to achieve the desired results, a failure to obtain regulatory approval for our product candidates or to comply with ongoing regulatory requirements, regulatory limitations relating to our ability to promote or commercialize our product candidates for the specific indications, a lack of acceptance of our product candidates in the marketplace, a failure of us to become or remain profitable, a failure to establish collaborations, our inability to obtain or maintain the capital or grants necessary to fund our research and development activities, a loss of any of our key scientists or management personnel, and other factors described in Synthetic Biologics’ report on Form 10 - K for the year ended December 31 , 2012 , subsequent Form 10 - Qs and any other filings with the SEC . The information in this presentation is provided only as of the date presented, and Synthetic Biologics undertakes no obligation to update any forward - looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law . 2 NYSE MKT: SYN
Investment Proposition • Building portfolio of target - specific biologics for the prevention and treatment of serious infectious diseases ̶ Innovative, first - in - class product candidates ̶ Large markets • Clinical - stage ̶ Multiple sclerosis (MS) – Phase II (potential partnering opportunity) ̶ Prevention of Clostridium difficile (C. difficile ) infections • Discovery/preclinical stage ̶ Pertussis infections (whooping cough) ̶ Acinetobacter infections (potentially lethal infection increasing in ICUs and military injuries) • Strategic collaborations with Intrexon Corporation ̶ Private biotechnology company led by CEO, RJ Kirk ̶ Option on an additional infectious disease target • Experienced management team and advisors 3 NYSE MKT: SYN NYSE MKT: SYN

Management Team 4 • Jeffrey Riley, CEO Pfizer, Nichols Institute (Quest), SmithKline Beecham, QIC • C. Evan Ballantyne , CFO Clinical Data, Inc., Avedro , ZymeQuest , ACNielsen, IMS • John Monahan, Ph.D., EVP R&D Avigen , Somatix , Triton Biosciences, Hoffman - LaRoche • Carol Reed, M.D., SVP Clinical/Regulatory Clinical Data, Inc., Genaissance Pharmaceuticals, Inc., Bayer Pharmaceuticals, Inc. • Michael Kaleko , M.D., Ph.D ., SVP R&D Genetic Therapy, Inc. (Novartis), Advanced Vision Therapies (currently known as Wellstat Ophthalmics ) NYSE MKT: SYN

Product Pipeline 5 NYSE MKT: SYN * - SYN - 004, 2 nd generation oral enzyme candidate under development based on 1 st generation candidate (P1A) Phase II results I - Intrexon collaboration – design, engineering and optimization of lead candidates T - The University of Texas at Austin – antibody research Therapeutic Area Product Candidate Biologic Agent/ Drug Compound Discovery Preclinical Phase I Phase II Phase III Relapsing - remitting m ultiple sclerosis Trimesta TM Oral estriol Cognitive dysfunction in multiple sclerosis Trimesta TM Oral estriol C. difficile infection prevention SYN - 004* Oral enzyme Pertussis (whooping cough) SYN - 005 I,T Monoclonal antibody Acinetobacter infections SYN - 001 I Monoclonal antibody

MS: Market • 400,000 MS patients in U.S. (~2.1 million worldwide) 1 • $14.1 billion current estimated worldwide sales 2 Trimesta TM Opportunity – Oral add - on MS therapy 6 NYSE MKT: SYN Teva/Copaxone $4.178 30% Biogen Idec/Avonex $2.929 21% Merck KGaA/Rebif $2.425 17% Biogen Idec/Tysabri $1.703 12% Bayer/Betaseron $1.512 11% Novartis/Extavia $0.159 1% Novartis/Gilenya $1.195 8%

MS: Historical Efficacy of Estriol “Pregnancy Hormone” – Linked to decreased MS relapse rates • Estriol − Hormone produced by placenta during pregnancy with highest level in 3 rd trimester − Solid safety profile (approved in Europe/Asia for 40+ years) • Landmark study published in New England Journal of Medicine 3 − Study of 254 women diagnosed with MS prior to pregnancy − Relapse rates: ▪ Significantly reduced in 3 rd trimester of pregnancy (p<0.001) * ▪ Significantly increased three months post - partum (p<0.001) * 7 * C ompared to pre - pregnancy levels NYSE MKT: SYN

MS: Relapsing - Remitting MS Phase II Clinical Trial of Trimesta TM (oral estriol) • Multi - center, investigator - initiated, randomized, double - blind, placebo - controlled trial 4 ̶ Relapse rates at 2 years of oral Trimesta TM / Copaxone ® vs. placebo/ Copaxone ® • Trial status ̶ Enrollment complete: 164 female patients at 15 U.S. centers ̶ Final patient, final visit expected January 2014 ̶ Topline results expected 1H 2014 • $ 8 million + in grant funding supporting trial • Principal investigator: Rhonda Voskuhl , M.D., Professor of Neurology, Director of the UCLA MS Program 8 Copaxone ® is a registered trademark of Teva Pharmaceutical Industries Ltd. NYSE MKT: SYN

Emerging Worldwide Crisis 9 SYN building portfolio of biologics for serious infectious diseases “Infections were the second leading cause of death worldwide in 2002, killing nearly 15 million people – almost 1 in 3 deaths across the globe ” “Multidrug - resistant microbes infect more than 2 million Americans each year” Brad Spellberg, M.D., Rising Plague 5 NYSE MKT: SYN

Current Market Limited Solutions Significant Unmet Medical Needs Limited Big Pharma Investment SYN Products Targeted Biologics Novel Antibacterial R&D GAIN Act Revolutionize Infectious Disease Treatment 10 NYSE MKT: SYN SYN infectious disease strategy • Large and growing markets • Infectious disease outbreaks increasing • Widespread multidrug - resistant pathogens • Additional market exclusivity • Fast track • Accelerated NDA reviews • Limited patient population opportunities
11 • 24 million patients administered IV antibiotics annually in the U.S. 6 – 1.1 million patients infected with C. diff annually 7 – Patients with C. diff hospitalized approximately 4 - 7 extra days 8 – $ 8.2 billion in costs associated with C. diff - related stays in hospital 9 – 30,000 C. diff - related deaths annually 10 • IV antibiotics may be excreted into gastrointestinal (GI) tract where they can upset the natural balance of the microbiome • This imbalance can result in the overgrowth of disease - causing bacteria such as C . diff • Toxigenic C . diff causes diarrhea , colitis and may result in death C. d ifficile (C. diff) Infections: Market Leading cause of hospital - acquired infections NYSE MKT: SYN There are currently no approved products for the prevention of C. difficile infections

12 SYN - 004: Co - Administered with Antibiotics Prophylactic treatment for prevention of C. diff infections • Oral enzyme to be co - administered with penicillins or most cephalosporins ( β - lactam antibiotics) ̶ Remain within the intestine to degrade certain IV β - lactam antibiotics ̶ Preventative treatment expected to protect the healthy microflora from the overgrowth of C. diff • Phase I and II studies of 1 st generation oral candidate* demonstrated safety, tolerability and preservation of GI microflora when co - administered with certain penicillins • SYN - 004, a 2 nd generation candidate intended to expand activity to include penicillins plus most cephalosporins • SYN - 004 patent pending on compositions of matter and methods of use through 2031 * P1A is 1 st generation candidate NYSE MKT: SYN 13 million Americans administered IV β - lactam antibiotics in 2012 11 that may be covered by SYN - 004

SYN - 004: Co - Administered with Antibiotics Intended to neutralize effects of β - lactam antibiotics in GI tract 13 Patient hospitalized with PRIMARY infection ( eg . pneumonia) Administer IV β - lactam antibiotic for primary infection SYN - 004 eliminates the antibiotic, protecting native microflora and prevent C. diff infection Co - administer oral SYN - 004 with IV β - lactam antibiotic Antibiotic enters the GI tract leading to C. diff infection Unprotected GI tract Protected GI tract NYSE MKT: SYN

Pertussis Infections Millions of whooping cough cases globally per year • Despite aggressive vaccination strategies, incidence of Pertussis is increasing 12,13 ̶ Less effective vaccine introduced in the 1990s ̶ Non - compliance with standard vaccinations ̶ ~41,000 cases per year in the U.S. 14 • Unvaccinated infants most vulnerable • 50 million worldwide cases of whooping cough each year 15 ̶ 300,000 deaths worldwide 15 (primarily infants) • Antibiotics are not effective in treating disease symptoms • SYN - 005 mAb in development ̶ mAb intended to neutralize pertussis toxin to diminish morbidity and mortality ̶ Collaborations with Intrexon and The University of Texas at Austin 14 NYSE MKT: SYN

Acinetobacter Infections Increasingly pandrug - resistant gram - negative bacteria • M ortality rates as high as 43% reported 16 • Billion dollar market opportunity 17 • Survives on dry surfaces for up to 36 days ̶ Survives twice as long as non - biofilm - forming pathogens 18 • Acinetobacter infection profile ̶ 2.6% of hospital acquired infections 19 ̶ 7 % of ICU respiratory tract infections 19 ̶ Key infection sites include: lungs, heart, blood, urinary tract, CNS, skin and soft tissues • Increasing cause of trauma - related infections in wounded military personnel and victims of natural disasters 20 • mAb development collaboration with Intrexon 15 NYSE MKT: SYN

Corporate Snapshot • Current Price : $1.44 (as of 5/16/13) • 52 Week Range: $1.34 - $ 2.55 • Average Volume (3 months): 60,950 • Shares Outstanding: ~44.7 million (as of 5/14/13) • Market Capitalization: ~$64 million • Cap Structure: Common, no debt, no preferred • Cash: ~$8.5 million (as of 3/31/13) • Offices in Rockville, Maryland 16 NYSE MKT: SYN NYSE MKT: SYN
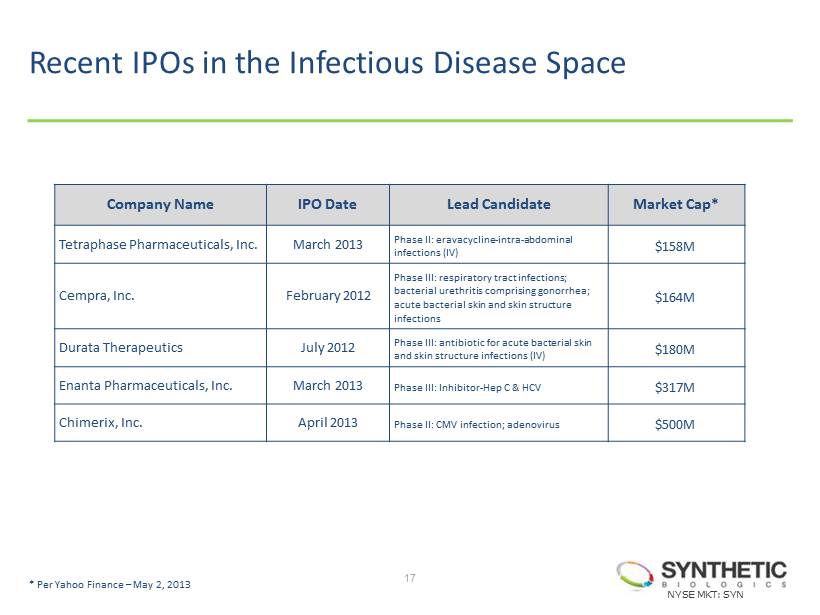
Recent IPOs in the Infectious Disease Space 17 Company Name IPO Date Lead Candidate Market Cap* Tetraphase Pharmaceuticals, Inc. March 2013 Phase II: eravacycline - intra - abdominal infections (IV) $158M Cempra, Inc. February 2012 Phase III: respiratory tract infections; bacterial urethritis comprising gonorrhea; acute bacterial skin and skin structure infections $164M Durata Therapeutics July 2012 Phase III: antibiotic for acute bacterial skin and skin structure infections (IV) $180M Enanta Pharmaceuticals, Inc. March 2013 Phase III: Inhibitor - Hep C & HCV $317M Chimerix, Inc. April 2013 Phase II: CMV infection; adenovirus $500M * Per Yahoo Finance – May 2, 2013 NYSE MKT: SYN

18 Upcoming Milestones NYSE MKT: SYN • Phase II relapsing - remitting MS clinical trial ̶ Final patient, final visit (January 2014) ̶ Topline results ( 1H 2014) • SYN - 004 oral enzyme preventative treatment for C. diff ̶ Initiate cGMP manufacturing (3Q 2013) ̶ Initiate clinical trials (2H 2014) • mAb for Pertussis infections ̶ Initiate small animal study (3Q 2013) ̶ Initiate large animal study (4Q 2013) [IND - enabling study] • mAbs for Acinetobacter infections ̶ Generate panel of antibodies (ongoing)

May 2013 – 5.20.2013 – FINAL May 20, 2013 NYSE MKT: SYN

References Slide 6: 1 National Multiple Sclerosis Society. http://www.nationalmssociety.org 2 Credit Suisse. Multiple Sclerosis - Evolution or Revolution Report. March 18, 2013. Slide 7 : 3 Pr egnancy I n M ultiple S clerosis (PRIMS) Study; Confavreux , C., Hutchinson, M., Hours, M.M., Cortinvis - Tourniaire , P., Moreau, T., and the Pregnancy in MS Group (1998). Rate of Pregnancy - Related Relapse in Multiple Sclerosis. New England Journal of Medicine , 339, 285 - 291. Slide 8 : 4 www.clinicaltrials.gov/ct2/show/NCT00451204 Slide 9 : 5 Spellberg, B. Rising Plague: The Global Threat from Deadly Bacteria and Our Dwindling Arsenal to Fight Them. Copyright 2009. Slide 11 : 6 - 7 This information is an estimate derived from the use of information under license from the following IMS Health Incorporated inf ormation service: CDM Hospital database for full year 2012. IMS expressly reserves all rights, including rights of copying, distribution and republic ation. 8 (APIC) National Prevalence Study of Clostridium difficile in U.S. Healthcare Facilities. November 11, 2008. http:// hospitalacquiredinfections.blogspot.com/2008/12/november - 11 - 2008 - association - for.html . 9 Agency for Healthcare Research and Quality. Healthcare and Cost Utilization Project. Statistical Brief #124. Clostridium difficile Infections (CDI) in Hospital Stays, 2009. January 2012. Available at http://www.hcup - us.ahrq.gov/reports/statbriefs/sb124.pdf . 10 U.S . Department of Health & Human Services. Agency for Healthcare Research and Quality. January 25, 2012. Available at http://www.ahrq.gov/news/nn/nn012512.htm . Accessed November 5, 2012 . Slide 12 : 11 This information is an estimate derived from the use of information under license from the following IMS Health Incorporated inf ormation service: CDM Hospital database for full year 2012. IMS expressly reserves all rights, including rights of copying, distribution and republic ation. 20 NYSE MKT: SYN

References 21 Slide 14 : 12 Misegades LK, Winter K, Harriman K, Talarico J, Messonnier NE, Clark TA, Martin SW, Association of childhood pertussis with receipt of 5 doses of pertussis vaccine by time since last vaccine dose, California, 2010. JAMA, 2012 Nov 28;308(20): 2126 - 32. 13 Centers for Disease Control and Prevention. Pertussis Epidemic – Washington, 2012. Morbidity and Mortality Weekly Report. July 20, 2012. 14 Centers for Disease Control and Prevention . 2012 Provisional Pertussis Surveillance Report. January 4, 2013. 15 World Health Organization. Pertussis: Immunization surveillance, assessment and monitoring. http ://www.who.int/immunization_monitoring/diseases/pertussis_surveillance/en/ Slide 15 : 16 Falagas , ME, Bliziotis , LA, and Siempos , II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case - control studies. Critical Care 2006, 10:R48. 17 Barrett, L. Former VP of US Marketing and Global Business Manager Infectious Diseases at Wyeth. Guest Blog, Antibiotic Market s a nd SPLU. http://antibiotics - theperfectstorm.blogspot.com/2012/03/antibiotic - markets - and - splu - guest.html . March 20, 2012. 18 Espinal P, Martí S, Vila J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J Hosp Infect. 2012 Jan; 80(1):56 - 60. Epub 2011 Oct 4. 19 Jones, M, et al. Emerging resistance among bacterial pathogens in the intensive care unit – a European and North American Survei llance study (2000 - 2002). Ann Clin Microbiol Antimicrob ; 3(14).; Wisplinghoff , H, et al. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin Infect Dis 2004; 39(3): 309 - 17.; Wachter , K. Step Aside, MRSA, Here Comes Acinetobacter . OB. GYN. News, January 15, 2006. 20 Camp, C and Tatum, OL. A Review of Acinetobacter baumannii as a Highly Successful Pathogen in Times of War. LABMEDICINE. November 2010, Vol. 41, Number 11. NYSE MKT: SYN
