Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ROCKWELL MEDICAL, INC. | a13-11891_28k.htm |
| EX-99.1 - EX-99.1 - ROCKWELL MEDICAL, INC. | a13-11891_2ex99d1.htm |
Exhibit 99.2
|
|
Targeting End-Stage Renal Disease (ESRD) and Chronic Kidney Disease (CKD) with Innovative Products for the Treatment of Iron Deficiency, Secondary Hyperparathyroidism and Hemodialysis May 2013 |
|
|
Safe Harbor Statement This presentation contains forward-looking statements. All statements, other than statements of historical facts, including, among others, statements regarding the Company’s future plans, products (investigational or otherwise), financial position, business strategy, projected levels of growth, projected costs and projected financing needs, projected roll-out or approval dates, are forward-looking statements. Those statements include statements regarding the intent, belief or current expectations of Rockwell Medical and members of the Company’s management team, as well as the assumptions on which such statements are based, and generally are identified by the use of words such as “may,” “will,“ “seeks,” “anticipates,” “forecast,” “upcoming,” “believes,” “estimates,” “expects,” “plans,” “intends,” “should”, “potential” or similar expressions. Forward looking statements are not guarantees of future performance and involve risks and uncertainties, including those set forth in the Company’s annual and quarterly reports filed with the SEC. Actual results may differ materially from those contemplated by such forward-looking statements. The Company believes these forward-looking statements are reasonable. However, undue reliance should not be placed on any forward-looking statements, which are based on current expectations. All written and oral forward-looking statements attributable to the Company or persons acting on its behalf are qualified in their entirety by these cautionary statements. Further, forward-looking statements speak only as of the date they are made, and the Company undertakes no obligation to update or revise forward-looking statements to reflect changed assumptions, the occurrence of unanticipated events or changes to future operating results over time unless required by law. 2 |
|
|
3 Key Investment Drivers Proprietary, disruptive lead drug SFP Novel delivery for iron maintenance therapy Established safety profile plus substantial cost saving benefits Est. $600mm spent on IV iron delivery in the U.S.; $1bn+ globally SFP clinical studies nearing completion PRIME study demonstrated 35% ESA-sparing data Feb. 2013 CRUISE-1 / CRUISE-2 efficacy study data due Q3/Q4 2013 FDA approved generic drug nearing commercial launch Calcitriol (vitamin D injection); lowest-cost therapy, clinically equivalent Addresses estimated $350mm+ U.S. market Established operating business $50mm ttm; cash flow positive; recent long-term supply contract with DVA Ready-made distribution channel for fast, seamless market penetration New CMS reimbursement ideal for RMTI products “Bundled-payment” effective January 2011 Drugs move from “profit-center” to “cost-center” 3 |
|
|
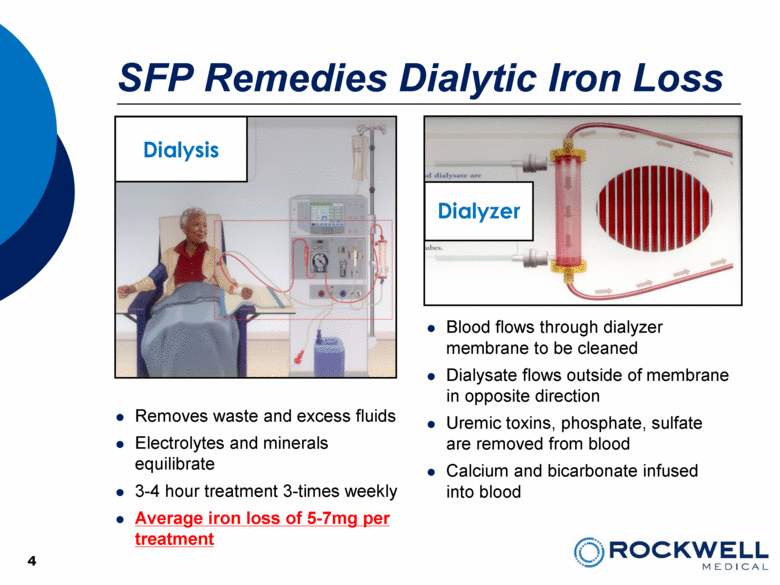
SFP Remedies Dialytic Iron Loss 4 Removes waste and excess fluids Electrolytes and minerals equilibrate 3-4 hour treatment 3-times weekly Average iron loss of 5-7mg per treatment Dialysis Dialyzer Blood flows through dialyzer membrane to be cleaned Dialysate flows outside of membrane in opposite direction Uremic toxins, phosphate, sulfate are removed from blood Calcium and bicarbonate infused into blood 4 |
|
|
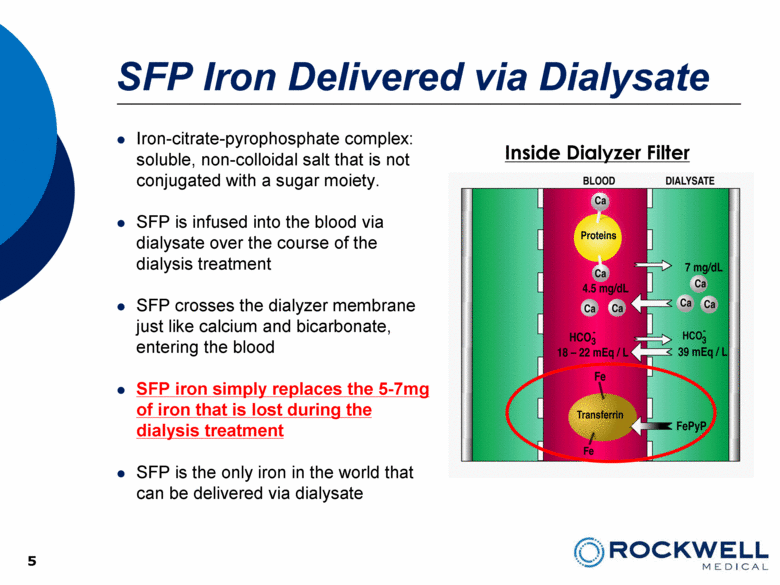
5 SFP Iron Delivered via Dialysate Iron-citrate-pyrophosphate complex: soluble, non-colloidal salt that is not conjugated with a sugar moiety. SFP is infused into the blood via dialysate over the course of the dialysis treatment SFP crosses the dialyzer membrane just like calcium and bicarbonate, entering the blood SFP iron simply replaces the 5-7mg of iron that is lost during the dialysis treatment SFP is the only iron in the world that can be delivered via dialysate Inside Dialyzer Filter 5 |
|
|
6 SFP Clinical Benefits SFP iron delivery is “physiologic” – it mimics the way dietary iron is processed in the human body SFP enters bloodstream, binds immediately to apo-transferrin and travels to bone marrow SFP efficiently matches the erythropoietin stimulating agent (ESA) and healthy red blood cell generation occurs SFP delivers iron effectively, while not increasing iron stores (ferritin) Excellent safety demonstrated SFP is a water-soluble iron salt and therefore avoids patient inflammation and reticuloendothelial (RE) iron block, bypassing liver storage and associated organ toxicity Anaphylactoid reactions have not been seen in nearly 60,000 human doses; safety confirmed by four DSMB reviews In contrast, IV iron very different; encased in carbohydrate shell Iron delivery causes inflammation, hepcidin release, reticuloendothelial (RE) block, iron trapped in the liver, potential organ toxicity Hypersensitivity and anaphylactoid reactions occur, due to carbohydrate moiety and labile/free iron release 6 |
|
|
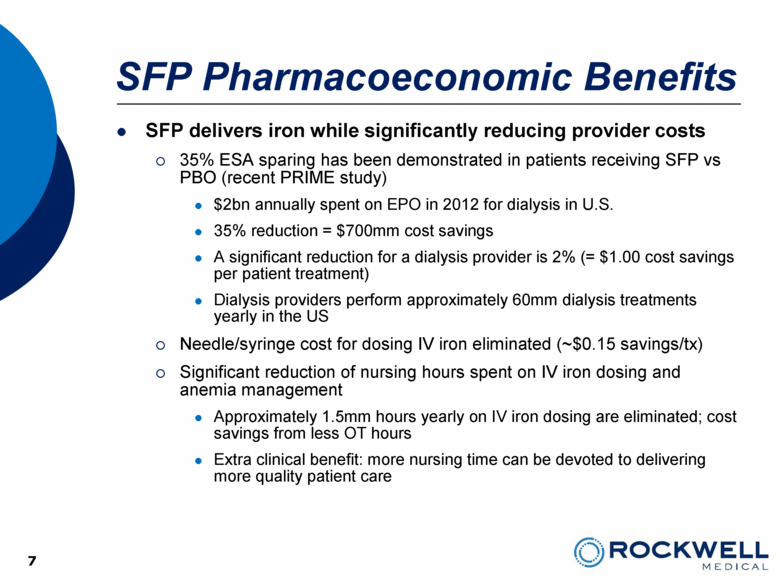
7 SFP Pharmacoeconomic Benefits SFP delivers iron while significantly reducing provider costs 35% ESA sparing has been demonstrated in patients receiving SFP vs PBO (recent PRIME study) $2bn annually spent on EPO in 2012 for dialysis in U.S. 35% reduction = $700mm cost savings A significant reduction for a dialysis provider is 2% (= $1.00 cost savings per patient treatment) Dialysis providers perform approximately 60mm dialysis treatments yearly in the US Needle/syringe cost for dosing IV iron eliminated (~$0.15 savings/tx) Significant reduction of nursing hours spent on IV iron dosing and anemia management Approximately 1.5mm hours yearly on IV iron dosing are eliminated; cost savings from less OT hours Extra clinical benefit: more nursing time can be devoted to delivering more quality patient care 7 |
|
|
Easy, Convenient Delivery of SFP via Bicarbonate Concentrate 8 Current Bicarbonate Mixing Process SFP Simply Added into Liquid Bicarbonate Add to Mixer / Water Add to Liquid Bicarbonate SFP 50ml vial Bicarbonate Powder |
|
|
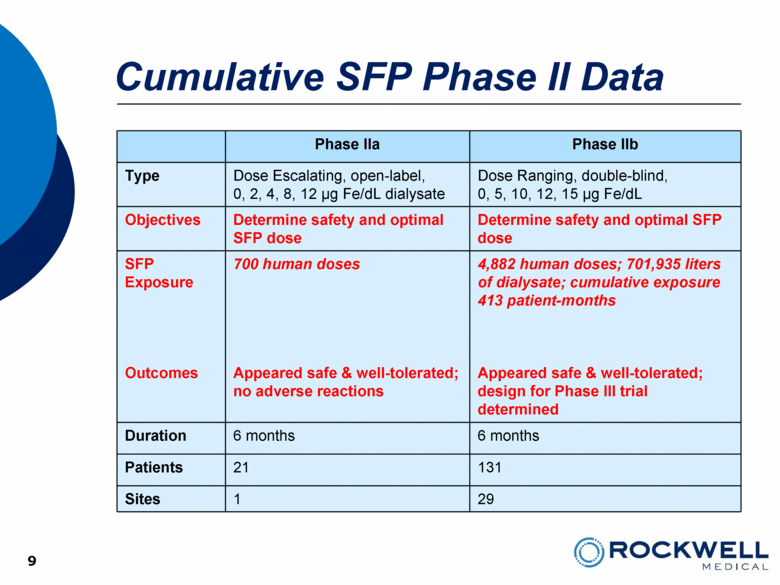
Cumulative SFP Phase II Data Phase IIa Phase IIb Type Dose Escalating, open-label, 0, 2, 4, 8, 12 µg Fe/dL dialysate Dose Ranging, double-blind, 0, 5, 10, 12, 15 µg Fe/dL Objectives Determine safety and optimal SFP dose Determine safety and optimal SFP dose SFP Exposure Outcomes 700 human doses Appeared safe & well-tolerated; no adverse reactions 4,882 human doses; 701,935 liters of dialysate; cumulative exposure 413 patient-months Appeared safe & well-tolerated; design for Phase III trial determined Duration 6 months 6 months Patients 21 131 Sites 1 29 9 9 |
|
|
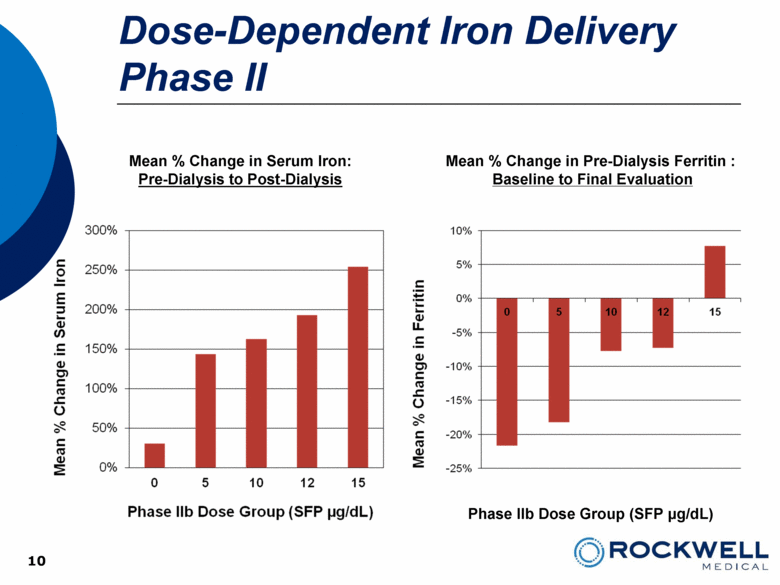
10 Mean % Change in Serum Iron: Pre-Dialysis to Post-Dialysis Phase IIb Dose Group (SFP µg/dL) Dose-Dependent Iron Delivery Phase II Mean % Change in Pre-Dialysis Ferritin : Baseline to Final Evaluation |
|
|
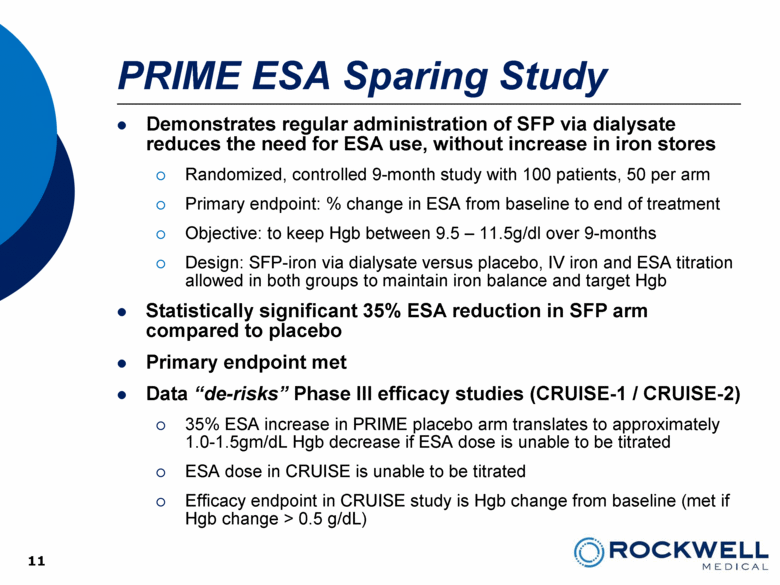
PRIME ESA Sparing Study Demonstrates regular administration of SFP via dialysate reduces the need for ESA use, without increase in iron stores Randomized, controlled 9-month study with 100 patients, 50 per arm Primary endpoint: % change in ESA from baseline to end of treatment Objective: to keep Hgb between 9.5 – 11.5g/dl over 9-months Design: SFP-iron via dialysate versus placebo, IV iron and ESA titration allowed in both groups to maintain iron balance and target Hgb Statistically significant 35% ESA reduction in SFP arm compared to placebo Primary endpoint met Data “de-risks” Phase III efficacy studies (CRUISE-1 / CRUISE-2) 35% ESA increase in PRIME placebo arm translates to approximately 1.0-1.5gm/dL Hgb decrease if ESA dose is unable to be titrated ESA dose in CRUISE is unable to be titrated Efficacy endpoint in CRUISE study is Hgb change from baseline (met if Hgb change > 0.5 g/dL) 11 11 |
|
|
SFP Phase III Efficacy Studies CRUISE-1 and CRUISE-2 Phase III efficacy studies Two identical studies, fully enrolled, 600 total patients, 12 months Design: 300 patients per trial; 150 patients per arm; one-to-one randomization between SFP-iron dialysate vs control standard dialysate ESA dose clamped at baseline dose and no oral or IV iron allowed Primary efficacy endpoint: Hgb change from baseline to end of treatment FDA-preferred endpoint; met statistical significance in Phase 2b study Safety: 4 DSMB safety reviews completed with no issues Largest Dialysis Organizations participating Studies complete in May and August 2013 Top-line data expected Q3/Q4 2013 12 12 |
|
|
SFP Intellectual Property Exclusive worldwide license for SFP delivery via dialysate in hemodialysis and peritoneal dialysis patients Composition of matter and method of delivery patent Patent expires 2021 (with addition of five year Hatch-Waxman) Patents issued in several countries, including U.S., Europe and Japan – the three largest ESRD markets in the world U.S. and E.U. patent issued on GMP-grade SFP formulation Provides protection through mid-2029 Covers HD, PD, TPN, Oral OTC/Rx, IV for Oncology and GI markets U.S. patent issued on SFP packaging and method of use SFP dissolved in bicarbonate solution held in HDPE container Provides protection through 2027 Multiple patents pending, including ESA sparing patent Rights to patent issued for SFP iron delivered intravenously Oncology and GI markets Patent expires in 2019 13 13 |
|
|
Calcitriol Vitamin D Injection Preparing drug for commercial launch FDA approved generic vitamin D injection to treat secondary hyperparathyroidism in dialysis patients New manufacturing facility required product stability review Stability data has been submitted to FDA Entry into commercial market anticipated 2013 High-margin drug with clinically equivalent safety and efficacy compared to the two branded drugs (Hectorol® and Zemplar®) Lowest cost per treatment vitamin D injection available Addresses an est. $350mm+ U.S. market Significant sales expected from existing Rockwell customer base High-margin product expected to increase GP considerably Simply added to existing sales and marketing infrastructure with minimal expense 14 |
|
|
Growing Operating Business to Leverage Drug Sales Manufacturer and supplier of hemodialysis concentrates (dialysate) in U.S. and abroad $50mm operating business; cash flow positive; 40% market share (ex-FMC) 3 manufacturing facilities; company-owned distribution; 50+ tractor-trailers Industry innovator with four successful new product launches: Dri-Sate® Dry Acid Mix System SteriLyte® Liquid Bicarbonate RenalPure® 100-Dex Acid Concentrate CitraPure® Citric Acid Concentrate (commands premium price) Newly signed 5-year supply contract with DaVita Healthcare Partners Significant sales increase; CitraPure® conversion; future sale of new products Infrastructure in place for fast SFP / Calcitriol market penetration 18 years of proven sales and marketing in the renal space Solid purchasing relationships & distribution channel in place with customers Seamless transition of SFP / Calcitriol into market at minimal cost Minimal SG&A expense required to penetrate market Consolidated customer base (dialysis chains) does not require traditional pharma sales force 15 15 |
|
|
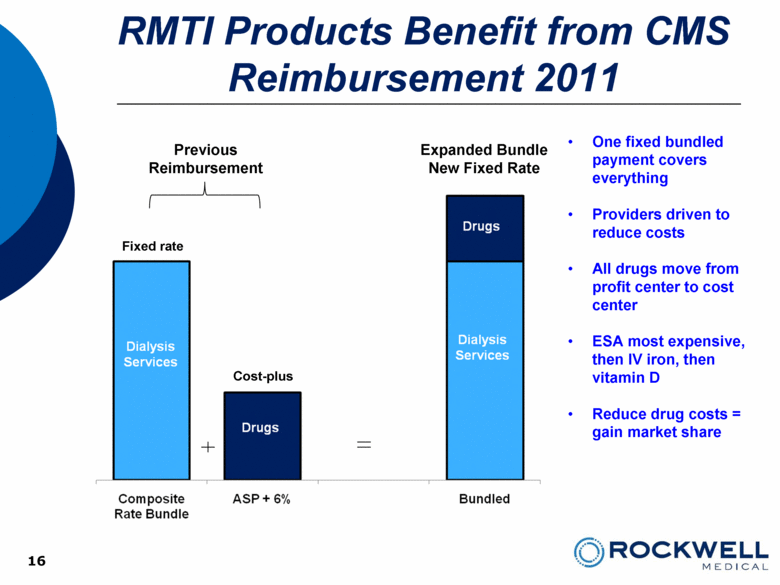
RMTI Products Benefit from CMS Reimbursement 2011 16 Expanded Bundle New Fixed Rate Fixed rate Previous Reimbursement One fixed bundled payment covers everything Providers driven to reduce costs All drugs move from profit center to cost center ESA most expensive, then IV iron, then vitamin D Reduce drug costs = gain market share Cost-plus |
|
|
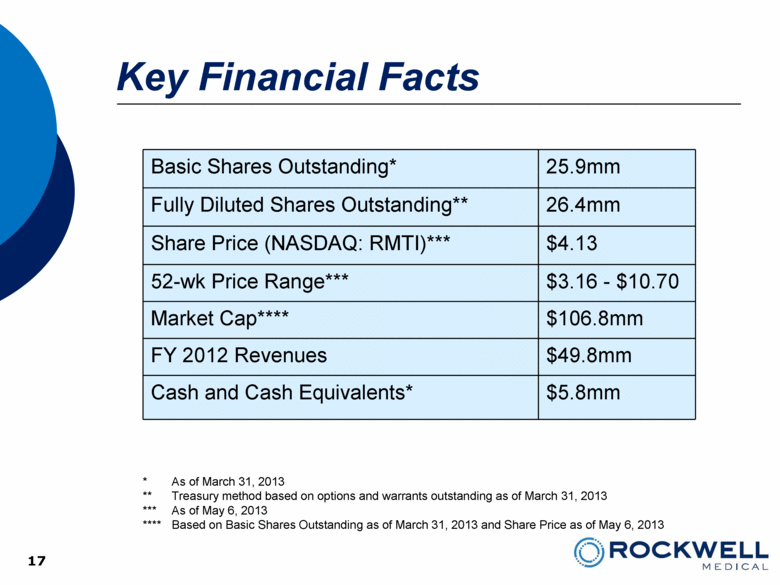
Key Financial Facts Basic Shares Outstanding* 25.9mm Fully Diluted Shares Outstanding** 26.4mm Share Price (NASDAQ: RMTI)*** $4.13 52-wk Price Range*** $3.16 - $10.70 Market Cap**** $106.8mm FY 2012 Revenues $49.8mm Cash and Cash Equivalents* $5.8mm 17 * As of March 31, 2013 ** Treasury method based on options and warrants outstanding as of March 31, 2013 *** As of May 6, 2013 **** Based on Basic Shares Outstanding as of March 31, 2013 and Share Price as of May 6, 2013 |
|
|
Opportunity Summary Lead drug candidate SFP has “blockbuster” potential Novel delivery provides safe, cost-effective and convenient iron delivery Estimated $600mm U.S. market opportunity; $1bn+ globally Excellent PRIME ESA sparing data, “de-risks” CRUISE Phase III studies CRUISE Phase 3 efficacy studies readout in Q3 and in Q4 FDA approved Calcitriol vitamin D nearing commercial launch Generic active vitamin D; clinically equivalent, lowest-cost therapy New high-margin accretive opportunity addresses $350mm+ U.S. market Established $50mm ttm operating business Ready-made distribution channel for fast market penetration Strong relationships; captive customer base already purchasing product Bundled reimbursement ideal for RMTI current/new products New CMS reimbursement puts premium on lowering cost SFP and Calcitriol will deliver substantial cost savings $1bn market potential in U.S. alone IV iron est. $600mm; Calcitriol est. $350mm; Dialysate est. $180mm 18 |
|
|
Appendix |
|
|
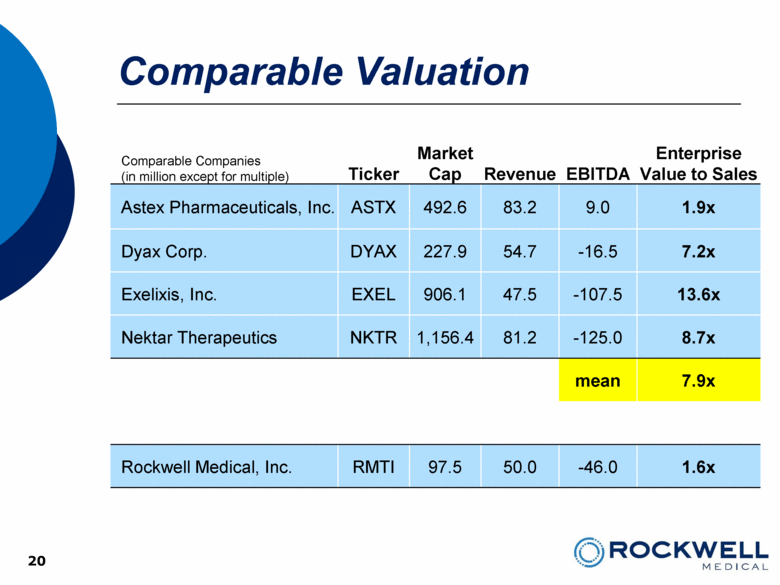
20 Comparable Valuation 20 Comparable Companies (in million except for multiple) Ticker Market Cap Revenue EBITDA Enterprise Value to Sales Astex Pharmaceuticals, Inc. ASTX 492.6 83.2 9.0 1.9x Dyax Corp. DYAX 227.9 54.7 -16.5 7.2x Exelixis, Inc. EXEL 906.1 47.5 -107.5 13.6x Nektar Therapeutics NKTR 1,156.4 81.2 -125.0 8.7x mean 7.9x Rockwell Medical, Inc. RMTI 97.5 50.0 -46.0 1.6x |
|
|
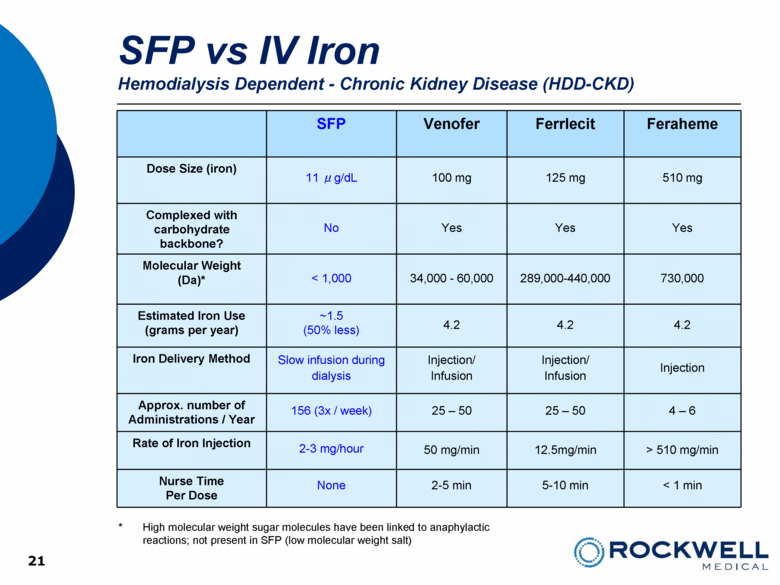
SFP Venofer Ferrlecit Feraheme Dose Size (iron) 11 μg/dL 100 mg 125 mg 510 mg Complexed with carbohydrate backbone? No Yes Yes Yes Molecular Weight (Da)* < 1,000 34,000 - 60,000 289,000-440,000 730,000 Estimated Iron Use (grams per year) ~1.5 (50% less) 4.2 4.2 4.2 Iron Delivery Method Slow infusion during dialysis Injection/ Infusion Injection/ Infusion Injection Approx. number of Administrations / Year 156 (3x / week) 25 – 50 25 – 50 4 – 6 Rate of Iron Injection 2-3 mg/hour 50 mg/min 12.5mg/min > 510 mg/min Nurse Time Per Dose None 2-5 min 5-10 min < 1 min 21 * High molecular weight sugar molecules have been linked to anaphylactic reactions; not present in SFP (low molecular weight salt) SFP vs IV Iron Hemodialysis Dependent - Chronic Kidney Disease (HDD-CKD) 21 |