Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Fibrocell Science, Inc. | d533468d8k.htm |
Exhibit 99.1

| May 7, 2013 |
| 2 This presentation includes statements that are "forward-looking statements." Forward-looking statements include, without limitation, our ability (i) to timely commence our clinical programs in 2013 to treat patients with restrictive burn scars and vocal cord scars, and a timely completion of these trial (ii) to successfully genetically modify the fibroblast cell to treat patients who have collagen deficient diseases, (iii) to successfully leverage our relationships with UCLA and MIT to develop new applications, and (iv) to adopt technologies that will dramatically reduce manufacturing time, complexity, and labor costs for our cell therapies. While management has based any forward- looking statements contained in the presentation on its current expectations, the information on which such expectations were based may change. These forward-looking statements rely on a number of assumptions concerning future events and are subject to a number of risks, uncertainties, and other factors, many of which are outside of Fibrocell Science's control, that could cause actual results to materially differ from such statements. Such risks, uncertainties, and other factors include, but are not necessarily limited to, those set forth under the caption "Item 1A. Risk Factors" in Fibrocell Science's most recent Form 10-K filing, as updated in "Item 1A. Risk Factors" in Fibrocell Science's most recent Form 10-Q filing. In addition, Fibrocell Science operates in a highly competitive and rapidly changing environment, and new risks may arise. Accordingly, you should not place any reliance on forward-looking statements as a prediction of actual results. Fibrocell Science disclaims any intention to, and undertakes no obligation to, update or revise any forward-looking statement. You are also urged to carefully review and consider the various disclosures in Fibrocell Science's most recent annual report on Form 10-K, our most recent Form 10-Q as well as other public filings with the SEC since the filing of Fibrocell Science's most recent annual report. Forward Looking Statement |

| Investor Highlights Autologous cell therapy company on cutting edge of developing first-in-class medical applications using fibroblasts LAVIV(r) - lead product Indicated for improvement of the appearance of moderate to severe nasolabial fold wrinkles in adults One of only three FDA-approved cell based products Completing cosmetic trial for personalized cream LAVIV(r) is positioned as a premium aesthetic Strategic focus on a robust pipeline for medical applications of LAVIV: Restrictive burn scarring, vocal cord scarring and acne scarring Intrexon collaboration to develop therapeutics for rare collagen deficient diseases Strong cash position, US $31.3 million on hand end of December 2012 No debt 3 |
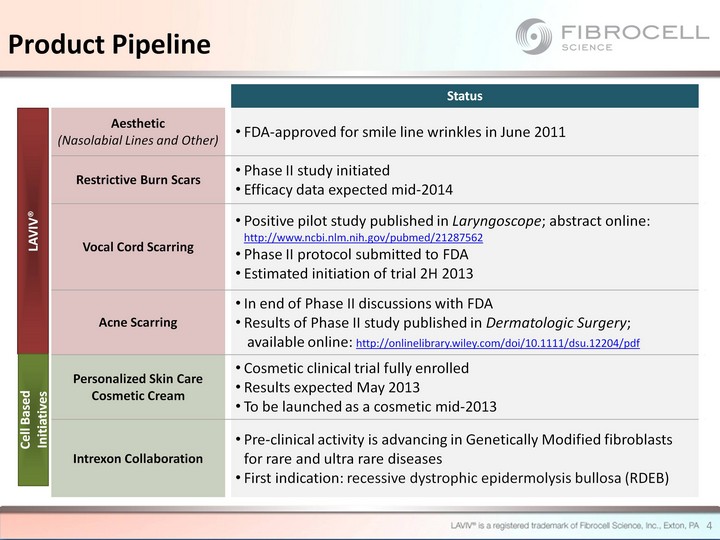
| Status Aesthetic (Nasolabial Lines and Other) FDA-approved for smile line wrinkles in June 2011 Restrictive Burn Scars Phase II study initiated Efficacy data expected mid-2014 Vocal Cord Scarring Positive pilot study published in Laryngoscope; abstract online: http://www.ncbi.nlm.nih.gov/pubmed/21287562 Phase II protocol submitted to FDA Estimated initiation of trial 2H 2013 Acne Scarring In end of Phase II discussions with FDA Results of Phase II study published in Dermatologic Surgery; available online: http://onlinelibrary.wiley.com/doi/10.1111/dsu.12204/pdf Personalized Skin Care Cosmetic Cream Cosmetic clinical trial fully enrolled Results expected May 2013 To be launched as a cosmetic mid-2013 Intrexon Collaboration Pre-clinical activity is advancing in Genetically Modified fibroblasts for rare and ultra rare diseases First indication: recessive dystrophic epidermolysis bullosa (RDEB) LAVIV(r) Cell Based Initiatives Product Pipeline 4 |
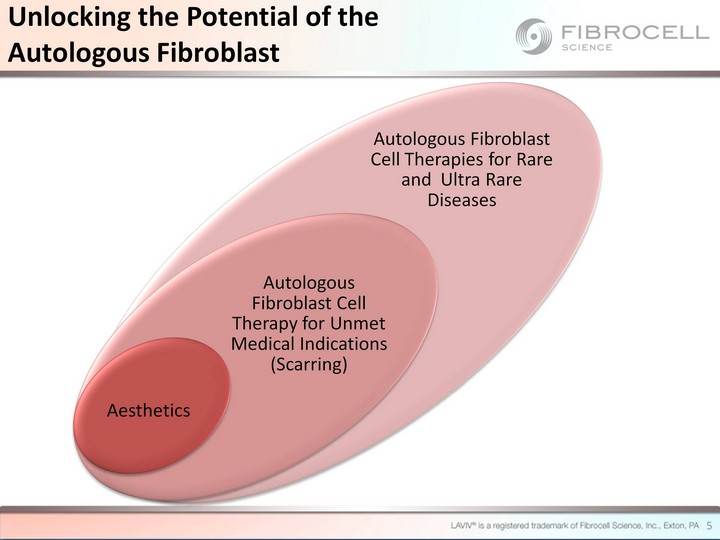
| Aesthetics Autologous Fibroblast Cell Therapy for Unmet Medical Indications (Scarring) Autologous Fibroblast Cell Therapies for Rare and Ultra Rare Diseases 5 Unlocking the Potential of the Autologous Fibroblast |

| 6 American Society for Aesthetic Plastic Surgery. www.ameriburn.org; Goodis. J and E.d. Schraga. Burns, thermal. eMedicine Journal Personal communication with several expert burn clinicians Cohen, (2010); Dailey et al. (2007); Roy et al. (2005); Poels et al. (2003); Koufman and Isaacson (1991); Painter (1990); Bouchayer et al. (1985); Laguaite (1972) Acne Resource Center. www.acne-resource.org; Herper, Matthew. "How A $440,000 Drug Is Turning Alexion Into Biotech's New Innovation Powerhouse." Forbes. 5 September 2012. http://www.forbes.com/sites/matthewherper/2012/09/05/how- a-440000-drug-is-turningalexion-into-biotechs-new-innovation-powerhouse Significant Addressable Markets Aesthetics: 8.4 million non-surgical cosmetic procedures in 2011(1) Scarring: 45,000 people hospitalized each year with severe burns, not including previous burn victims and military personnel (2) About half of hospitalized burn patients will develop some restrictive scarring (3) 200,000 - 700,000 patients with vocal cord scarring (4) 20 million patients in U.S. have acne badly enough to cause scars (5) No approved drugs for RBS, VCS, or acne scars Rare and Ultra Rare Disease: Currently at least 12 products for other rare diseases have a wholesale cost of $200,000+ (6) Currently there are no drugs approved for treatment of RDEB |

| The Autologous Fibroblast |
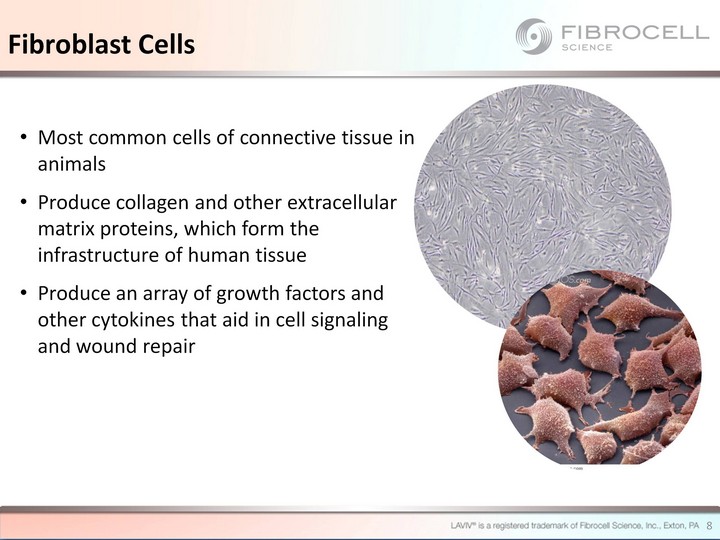
| Most common cells of connective tissue in animals Produce collagen and other extracellular matrix proteins, which form the infrastructure of human tissue Produce an array of growth factors and other cytokines that aid in cell signaling and wound repair Fibroblast Cells 8 |
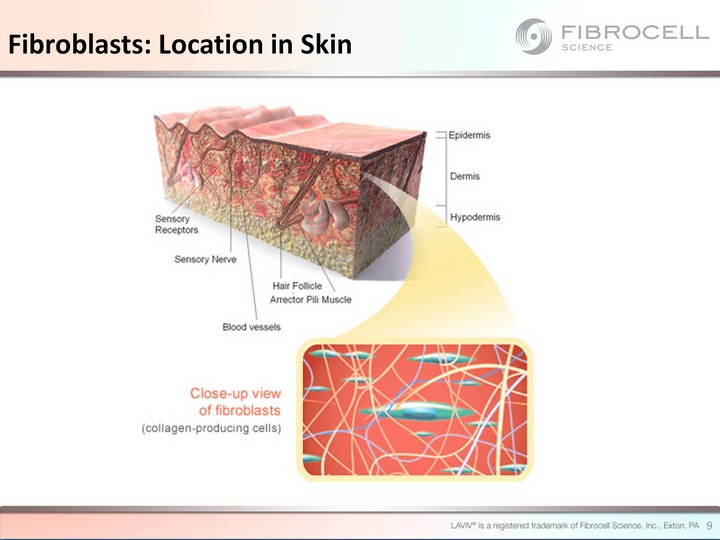
| Fibroblasts: Location in Skin 9 |
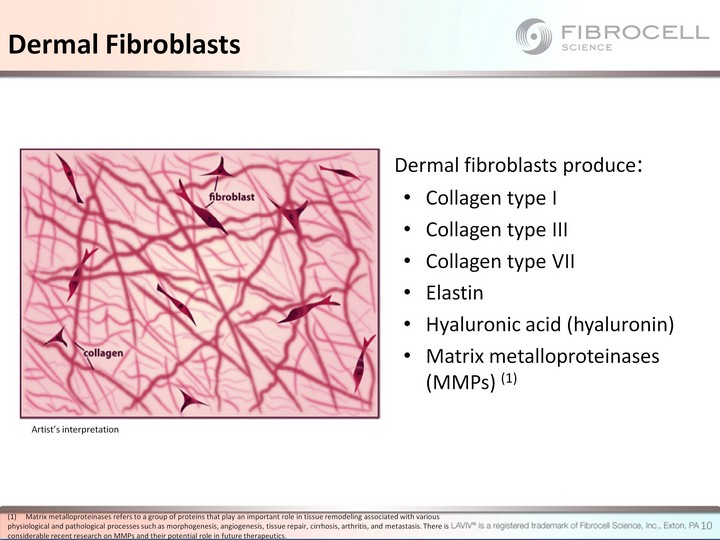
| Dermal fibroblasts produce: Collagen type I Collagen type III Collagen type VII Elastin Hyaluronic acid (hyaluronin) Matrix metalloproteinases (MMPs) (1) Artist's interpretation Dermal Fibroblasts Matrix metalloproteinases refers to a group of proteins that play an important role in tissue remodeling associated with various physiological and pathological processes such as morphogenesis, angiogenesis, tissue repair, cirrhosis, arthritis, and metastasis. There is considerable recent research on MMPs and their potential role in future therapeutics. 10 |

| Aesthetics |

| 12 Positioned as premium aesthetic First in New Class - Personalized Aesthetics Replaces a person's own fibroblast cells LAVIV has received recognition in national beauty and fashion magazines Allure magazine 'Breakthrough Award' Wall Street Journal Technology Innovation Awards Edison Awards Silver Winner in Aesthetics of Science/Medical Category LAVIV(r) |

| Restrictive Burn Scarring 13 |
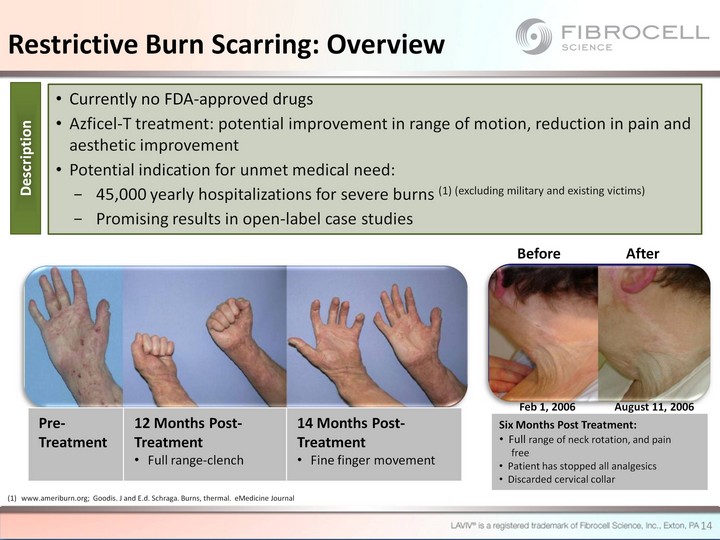
| Currently no FDA-approved drugs Azficel-T treatment: potential improvement in range of motion, reduction in pain and aesthetic improvement Potential indication for unmet medical need: 45,000 yearly hospitalizations for severe burns (1) (excluding military and existing victims) Promising results in open-label case studies Pre-Treatment 12 Months Post- Treatment Full range-clench 14 Months Post- Treatment Fine finger movement www.ameriburn.org; Goodis. J and E.d. Schraga. Burns, thermal. eMedicine Journal Description Restrictive Burn Scarring: Overview Before After Six Months Post Treatment: Full range of neck rotation, and pain free Patient has stopped all analgesics Discarded cervical collar 14 Feb 1, 2006 August 11, 2006 |

| Burn Patient Case Studies * *Note: Photos from LAVIV prescribers. Not from clinical studies. These are not restrictive burn scar patients. Case 1 Case 2 15 |
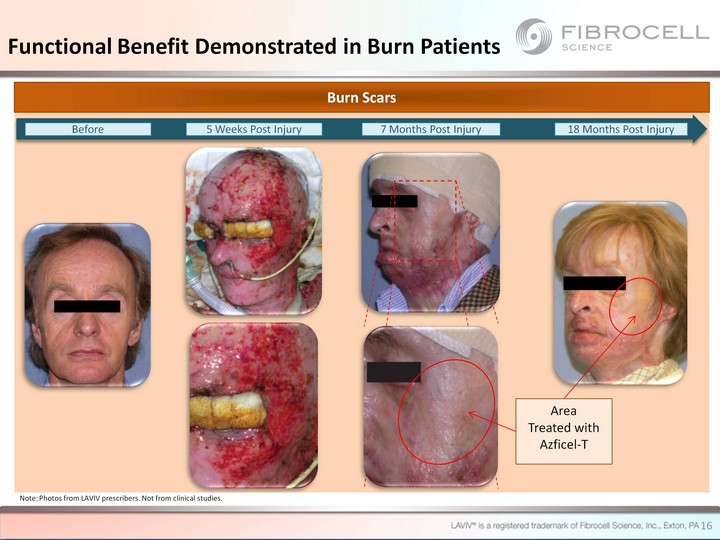
| 16 Burn Scars Before 5 Weeks Post Injury 7 Months Post Injury 18 Months Post Injury Area Treated with Azficel-T Note: Photos from LAVIV prescribers. Not from clinical studies. Functional Benefit Demonstrated in Burn Patients |
| Phase II Trial: Single site, double-blind, randomized, placebo- controlled, 21 subjects Subjects: Unilateral restrictive burn scars of a jointed area 20-50 percent restriction in normal range of motion; no improvement after physical therapy Measurement Scales: Range of Motion Measurement Brief Pain Index Scar Appearance Phase II Clinical Study initiated in 2Q 2013; efficacy data expected mid-2014 Restrictive Burn Scarring: Status 17 |

| Vocal Cord Scarring |
| No currently approved drugs Most commonly encountered finding is loss of voice Results from surgery, radiation therapy, vocal cord trauma and idiopathic causes Positive pilot study results published in peer-reviewed journal The Laryngoscope, 2011. (1) Azficel-T well-tolerated when injected into vocal folds Improvements lasted up to one year Findings supported by mucosal wave grade, voice handicap index, and voice quality questionnaire No similar current treatment exists Patient's own cells recreate the extracellular matrix of vocal cord tissue, potentially re-establishing normal pliability "Cellular-based approaches using tissue engineering and regenerative medicine techniques like Fibrocell's autologous fibroblasts are promising for the treatment of vocal scars"(2) Chhetri, Dinesh, Injection of Cultured Autologous Fibroblasts for Human Vocal Fold Scars. The Laryngoscope 121(4) : 785-792, 2011. Chhetri, Dinesh, MD, Associate Professor, Division of Head and Neck Surgery, David Geffen School of Medicine at UCLA. Description Vocal Cord Scarring: Overview 19 |
| Phase II protocol submitted to FDA Estimated initiation of trial 2H 2013 Efficacy data expected in 2H 2014 Proposed Endpoints: Impression of improvement in voice quality (VAS) Change from baseline in the noise-to-harmonic ratio (dB) Absolute and percentage change from baseline in the Voice Handicap Index (VHI) Proposed trial design: Two-site, double-blind, randomized, placebo-controlled, 21 subjects Vocal Cord Scarring: Status 20 |

| Acne Scarring |
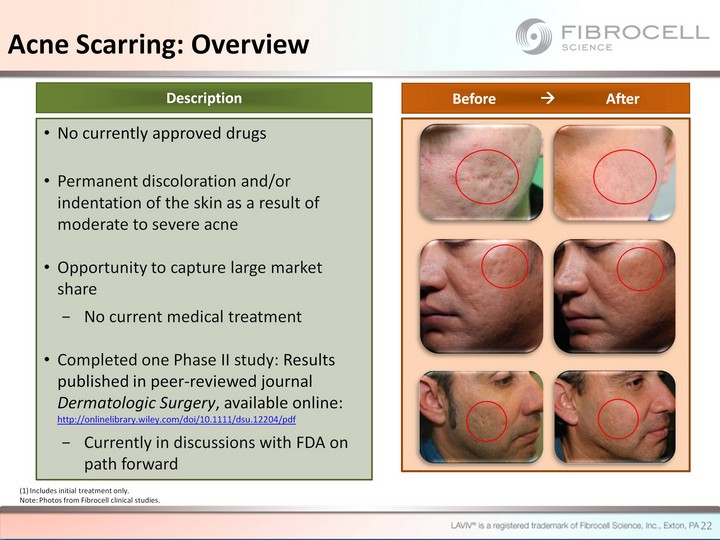
| 22 No currently approved drugs Permanent discoloration and/or indentation of the skin as a result of moderate to severe acne Opportunity to capture large market share No current medical treatment Completed one Phase II study: Results published in peer-reviewed journal Dermatologic Surgery, available online: http://onlinelibrary.wiley.com/doi/10.1111/dsu.12204/pdf Currently in discussions with FDA on path forward Description Before ? After (1) Includes initial treatment only. Note: Photos from Fibrocell clinical studies. Acne Scarring: Overview |
| 23 Efficacy: - Fibroblast treatment demonstrated statistically significant number of responders in subject and evaluator assessments at four months - 98% of patients who completed the study indicated interest in receiving additional treatment Safety: - Most common adverse events: erythema (11.1%), swelling (10.1%); all were mild or moderate in severity Potential Long-Lasting Effect: - Reports anecdotally suggest >75% of patients demonstrate treatment benefit 9 and 12 months following treatment -Photos anecdotally demonstrate sustained improvement 25 months following last treatment Acne Scarring: Phase II Study Results |
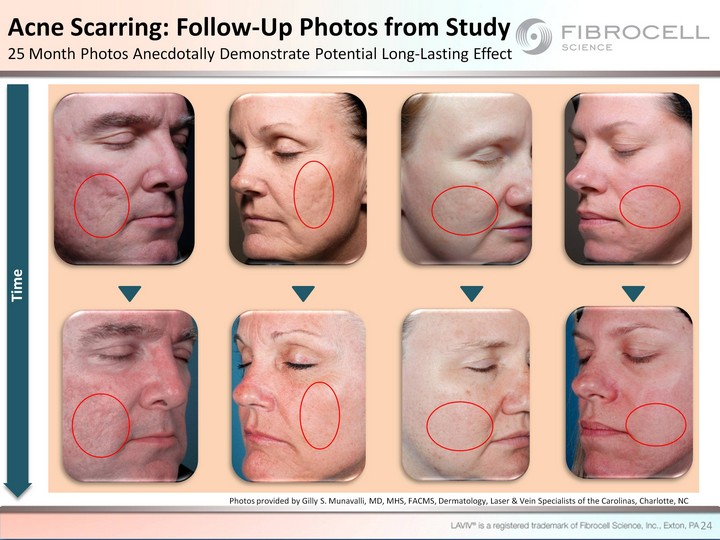
| 24 Acne Scarring: Follow-Up Photos from Study 25 Month Photos Anecdotally Demonstrate Potential Long-Lasting Effect Time Photos provided by Gilly S. Munavalli, MD, MHS, FACMS, Dermatology, Laser & Vein Specialists of the Carolinas, Charlotte, NC |

| Intrexon Collaboration: Genetically Modified Fibroblasts for Rare and Ultra Rare Diseases |
| Proposed new therapeutic product: Genetically modified (GM) fibroblast Up-regulated to produce Collagen VII Employ Intrexon's synthetic biology platforms for optimal gene expression from GM fibroblasts Pre-clinical activity underway in GM fibroblasts for rare and ultra rare diseases i.e. recessive dystrophic epidermolysis bullosa (RDEB) Intrexon Collaboration 26 |
| Initial target: recessive dystrophic epidermolysis bullosa (RDEB) Epidermolysis bullosa: genetic conditions that cause blistering and fragile skin Mutations in the COL7A1 gene cause disease This gene helps create type VII collagen Type VII collagen key in strengthening and stabilizing the skin It is the main component of structures called anchoring fibrils, which anchor the epidermis to the dermis Twelve existing rare diseases with a wholesale cost $200,000 plus (1) RDEB: Overview 27 Herper, Matthew. "How A $440,000 Drug Is Turning Alexion Into Biotech's New Innovation Powerhouse." Forbes. 5 September 2012 . http://www.forbes.com/sites/matthewherper/2012/09/05/how-a-440000-drug-is-turningalexion-into-biotechs-new-innovation-powerhouse |

| UCLA Collaboration: Stem Cell Program |

| Identified a rare mesenchymal stem cell (MSC) population from adult human skin The data was published in the article, "Vega-Crespo et al 2013, BioResearch Open Access, 1:25" (http://online.liebertpub.com/doi/pdf/10.1089/biores.2012.0204) Goal to purify MSCs using biomarkers to identify a number of personalized cellular therapeutics using these multipotent cells Also identified a small molecule BAY11 that can stabilize the expression of the OCT4 synthetic mRNA, the key programming factor for generating induced pluripotent stem cells The results are published in the article, "Awe et al 2013, Stem Cell Res Ther; 4:15" (http://stemcellres.com/content/pdf/scrt163.pdf) Technology could be useful for producing patient-specific iPSCs using the mRNA platform Exclusive license to both patent applications Overview Stem Cell Collaboration 29 |

| The Only Creme of Its Kind |

| Personalized autologous cream Conditioned complete growth media from the azficel-T production process is collected, concentrated and used to formulate a personalized topical cosmetic 19 subject cosmetic clinical study to be completed in May 2013 Autologous Creme(tm) 31 |

| Financials |

| Strong cash position; US $31.3 million on hand at end of December 2012 Large private capital raise completed in October 2012, investor syndicate led by Third Security, an investment firm led by RJ Kirk No debt on the balance sheet Company's capital structure greatly simplified All outstanding preferred stock was converted into common stock All outstanding convertible debt was extinguished Approximately 99% of warrants conceded anti-dilution provisions Financial Information 33 |
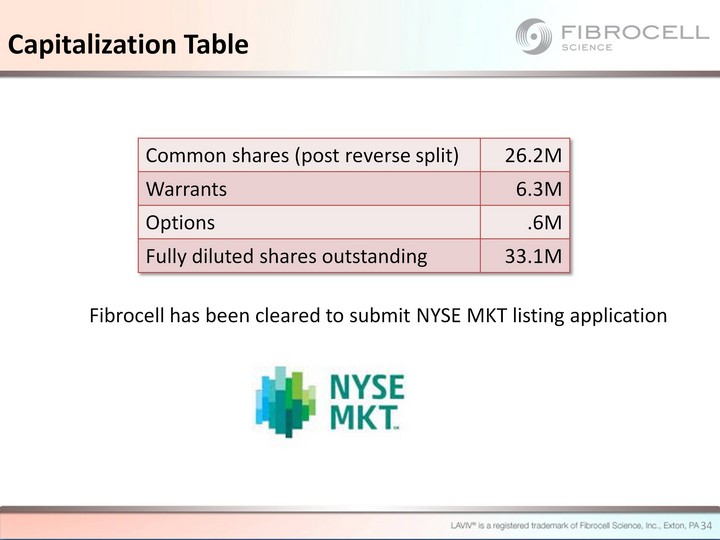
| Common shares (post reverse split) 26.2M Warrants 6.3M Options .6M Fully diluted shares outstanding 33.1M Fibrocell has been cleared to submit NYSE MKT listing application Capitalization Table 34 |

| Summary |

| Autologous cell therapy company on cutting edge of developing first in class medical applications Strategic focus on a robust pipeline for LAVIV medical applications: Restrictive burn scarring, vocal cord scarring and acne scarring LAVIV(r) - lead product Indicated for improvement of the appearance of moderate to severe nasolabial fold wrinkles in adults One of only three cell based FDA-approved cell based products LAVIV(r) is positioned as a premium aesthetic Completing cosmetic trial for personalized cream Intrexon collaboration to develop therapeutics for rare collagen deficient diseases Strong cash position, US $31.3 million on hand end of December 2012 No debt Investor Highlights 36 |
