Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ARCA biopharma, Inc. | d524188d8k.htm |
 Pioneering
Genetically-Targeted Cardiovascular Therapies
April 23, 2013
NASDAQ: ABIO
Exhibit 99.1 |
 Safe Harbor
Statement 2
This presentation contains "forward-looking statements" for purposes of the safe
harbor provided by the Private Securities Litigation Reform Act of 1995. These
statements include, but are not limited to, statements regarding the Company’s anticipated timing for initiation or
completion of its clinical trials for any of its product candidates; the potential for Gencaro
to be an effective potential treatment for atrial fibrillation and, the Company’s
ability to fund future operations. Such statements are based on management's current expectations and
involve risks and uncertainties. Actual results and performance could differ materially from
those projected in the forward-looking statements as a result of many factors,
including, without limitation, the risks and uncertainties associated with: the Company's financial resources and
whether they will be sufficient to meet the Company's business objectives and operational
requirements; the Company’s ability to complete a strategic transaction to support
the continued development Gencaro, and/or obtain additional financing; the Company’s anticipated timing
for initiation or completion of its clinical trials for any of its product candidates; the
Company's ability to identify, develop and achieve commercial success for
products and technologies; drug discovery and the regulatory approval process; estimated timelines for regulatory
filings and the implications of interim or final results of the Company’s clinical
trials; the extent to which the Company’s issued and pending patents may protect
its products and technology; the potential of the Company’s clinical development program to lead to the approval of the
Company’s New Drug Application for Gencaro; and, the impact of competitive products and
technological changes. These and other factors are identified and described in more
detail in ARCA’s filings with the SEC, including without limitation the Company’s annual report on Form
10-K for the year ended December 31, 2012, the Company’s Registration Statement on
Form S-1 (Registration No. 333-187508), and subsequent filings. The Company
disclaims any intent or obligation to update these forward-looking statements. |
 Offering
Summary •
Ticker Symbol
ABIO
•
Offering
$20,000,000
Common stock + warrants
•
Use of proceeds
To fund Phase 2b development of Company’s
lead product candidate, Gencaro, working
capital and general corporate purposes
•
Placement agent
Dawson James Securities, Inc.
3 |
 Corporate and
Lead Product Highlights •
Late stage cardiovascular company
•
Pharmacogenomic drug development/personalized medicine approach
–
Potentially first genetically-targeted cardiovascular drug
•
Experienced, successful management team
•
Lead
product
Gencaro
TM
(bucindolol
hydrochloride)
–
4
th
generation
beta-blocker
with
extensive
patient
data
–
1
st
potential
indication
-
atrial
fibrillation
(AF)
•
Clearly defined regulatory pathway
–
Similar endpoint basis for two most recent AF approvals
–
Beta blocker profile well characterized and understood
•
Significant
market
opportunities
in
a
major
unmet
medical
need
1
–
2.7 million AF patients in U.S.; 250,000-500,000 new onset AF/year (2010)
–
Current therapies associated with adverse events
•
Robust IP portfolio
–
US and EU market exclusivity, with potential patent extension, into 2029/2030
4
1
Bristow MR, Aleong RG. JACC-Heart Fail 1:29-30, 2013 |
 ARCA biopharma
Leadership Michael
R.
Bristow,
MD,
PhD:
President
/CEO
Patrick
Wheeler,
Chief
Financial
Officer
Chris
Ozeroff:
General
Counsel
Thomas
Keuer:
EVP,
Pharmaceutical
Operations
Monique
Plamondon:
VP,
Regulatory/Quality
Board of Directors
Michael R. Bristow, MD, PhD
Jean-Francois Formela, MD
Linda Grais, MD, JD (Lead Independent)
Burton E. Sobel, MD
John Zabriskie, PhD
5 |
 Pharmacogenomics:
The science of personalized medicine
6
Personalized medicine refers to the tailoring of medical treatment to the individual
genetic characteristics of each patient in order to classify individuals into subpopulations
that differ in their susceptibility to a particular disease or their response to a
specific treatment. Preventative or therapeutic interventions can then be
concentrated on those who
will
benefit,
sparing
expense
and
side
effect
for
those
who
will
not.
1
Potential Benefits
Better diagnoses and earlier intervention
More efficient drug development
More effective therapies
Improved patient outcomes
Reduced adverse drug reactions
Reduced healthcare costs
Extension of market exclusivity
1 –
President’s Council of Advisors on Science and Technology, Priorities for Personalized
Medicine, 2008 |
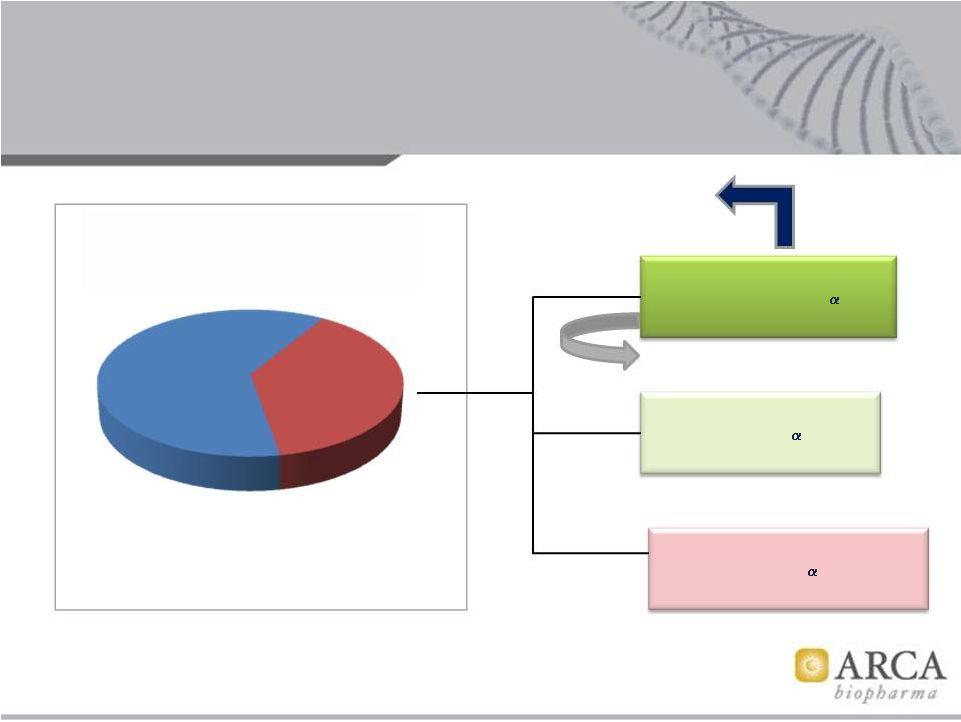 Patient
Population Genotypes Results from Phase 3 BEST trial DNA sub-study, HF
endpoints 7
Source: ARCA biopharma
‘Very Favorable’
genotype
{ß1 389
Arg/Arg + any 2C
}
~50%
‘Favorable’
genotype
{ß1
389 Gly carrier +
2C
Wt/Wt}
~40%
‘Unfavorable’
Pt. genotype
{ß1
389 Gly carrier +
2C
Del carrier}
~10%
1,040 Patients provided
DNA for sub-study
Target population
for Phase 2b/3 AF trial
Gencaro
Unique
MOA:
1)
NE
lowering
(Arg
is
NE
AR)
1
2) Inverse
agonism
2
1
Liggett et al, PNAS 2006
2
O'Connor et al, PLOS ONE 2012
BEST trial participants
–
2,708 Patients – |
 LabCorp
– Strategic Partnership
•
ARCA has exclusive rights to
Gencaro companion genetic test,
has partnered with LabCorp
•
Easy-to-administer, one-time
genetic test
that identifies genetic
markers which predict clinical
response
•
Quick turnaround time for results
8
Strategic Partner
S&P 500 Company
$5.7B in revenues in 2012
34,000 employees worldwide
220,000 clients
Conducts over 1M tests daily |
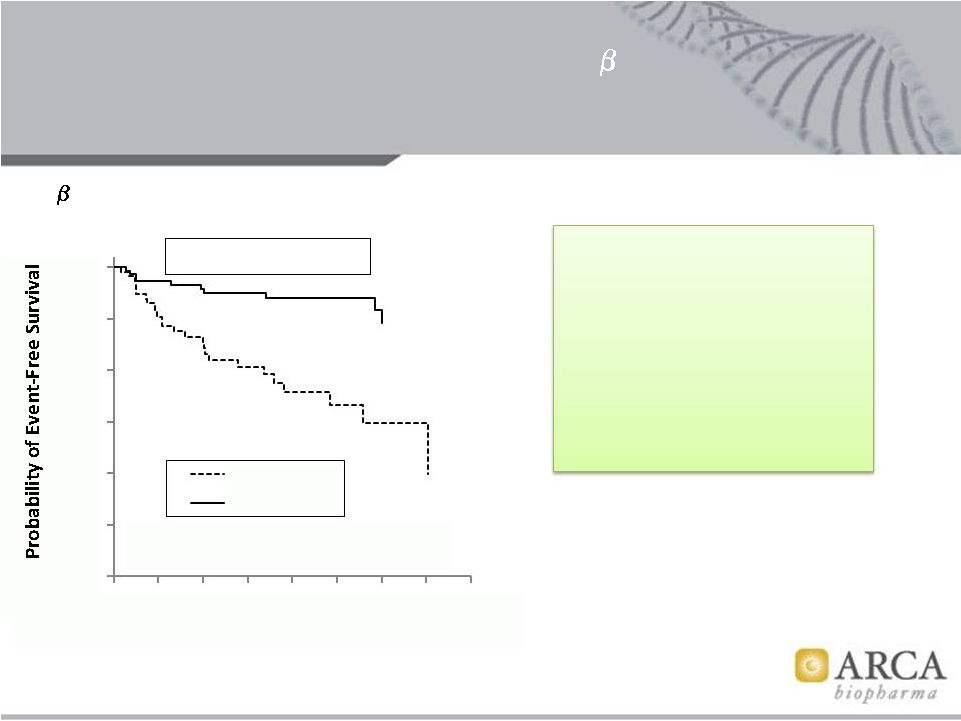 1
389 Arg/Arg (n = 441; 36 events)
Hazard Ratio = 0.26 (0.12 –
0.57)
P-value = 0.0003
Risk reduction 74%
BEST adrenergic receptor polymorphism substudy
Interaction p = 0.008
Prevention
of
new
onset
AF
in
BEST
by
1
389
Arg/Gly
genotype
Aleong et al, Circulation 124: A10438, 2011
1 -
Abi Nasr I et al, EHJ 28: 457–462, 2007
0.70
0.75
0.80
0.85
0.90
0.95
1.00
0
6
12
18
24
30
36
42
48
Months After Randomization
Placebo
Bucindolol
In a meta-analysis of Phase 3
HF trials covering ~12,000
randomized patients, there
was a
27%
average reduction
in incidence of new onset AF
in heart failure where new
onset AF was reported.¹
9 |
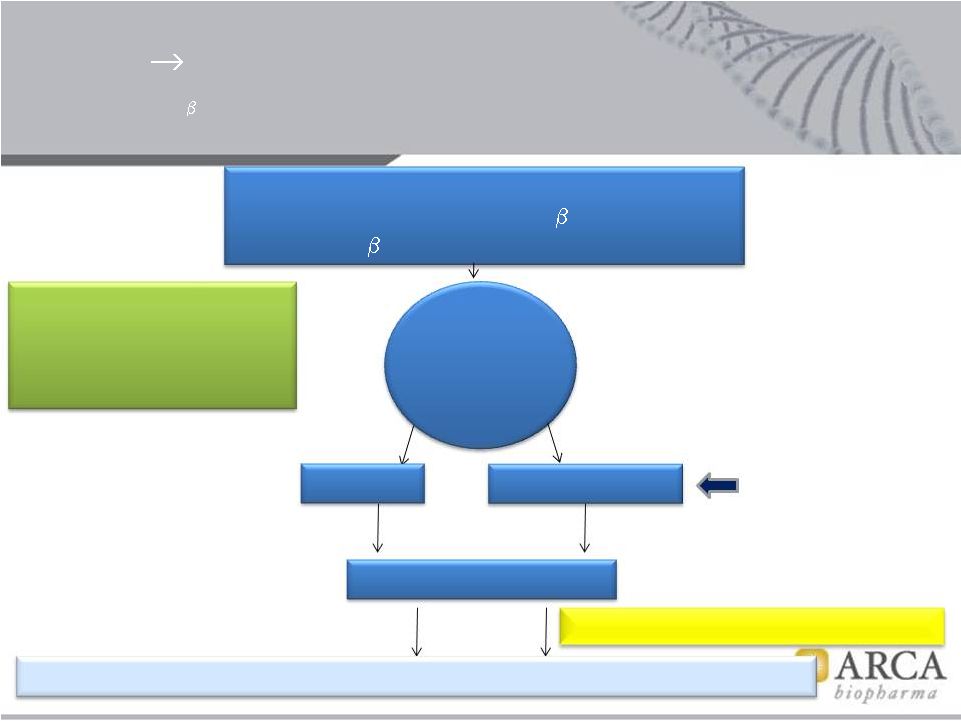 LVEF
<0.50, Class II-III HF w/in 90 days No contra-indications to
-blockers
1
389 Arg/Arg genotype
Bucindolol
Metoprolol CR/XL
ECV @ 4 wks if still in AF
Phase 2b: 1°
Endpoint = recurrent AF or ACM, 24 weeks; Co 1°
EP = AF burden
n =
100 (2b)
(Ph 3, 310)
n =
100 (2b)
(Ph 3, 310)
Phase 2b
Ph 3
Adaptive Design Superiority Trial
Bucindolol vs. Metoprolol CR/XL, Prevention of Recurrent Atrial Fibrillation in Persistent AF
HFREF Patients with the
1
389 Arg/Arg Genotype post Electrical Cardioversion (ECV), + Adaptive Design
to Phase 3
Projected timeline:
Trial Initiation –
Q4 2013
Ph 2b Trial Data –Q4 2015
Ph 3 Trial data-
Q4 2017
Time 0 (conversion to
AF counted as if ECV)
Ph 3 1°
EP: Recurrent AF or ACM , 24 wks
10
Recent
onset Sx AF,
1 wk –
90 d
Class I-III HF |
 Strategic
Partnership -
GENETIC-AF Clinical Trial Collaboration-
•
Medtronic, Inc
–
World’s largest medical technology company
–
Leader in medical technologies to improve the treatment of chronic diseases,
including cardiac rhythm disorders
–
45,000 employees
–
$16 Billion –
2012 Revenues
–
$1.5 Billion –
2012 Research & Development Investment
•
Collaboration
-
Substudy of P2b portion of GENETIC-AF
-
Measure AF burden data by means of Medtronic continuous monitoring
devices
-
Medtronic to provide support for AF burden substudy and for collection and
analysis of substudy data
11
Medtronic and ARCA have executed agreement to collaborate on GENETIC-AF trial
|
 Atrial
Fibrillation -
Regulatory Strategy -
Clearly defined Regulatory Pathway
–
Similar endpoints used for most recent AF FDA approvals
–
Safety profile –
well characterized based on BEST & prior development
–
Company has understanding of pathway, safety profile and trial design
12
Obtain an atrial fibrillation approval in a genotype specific heart failure population
via adaptive design Phase 2b/3 trial of 200/620 patients
Possibility of approval, based on GENETIC-AF (Phase3) if
p
0.01, when submitted with BEST trial data
Second trial will likely be required if GENETIC-AF p >0.01
•
•
• |
 Hatch-Waxman
[5 years from
approval]
Hatch-Waxman
Patent Extension
[7.5 years from
approval]
EU Data Exclusivity
[10 –
11 years from EU
approval]
Bucindolol
Patents
[use of drug
w/ genetic
markers]
Bucindolol Market Exclusivity /
Intellectual Property Protection
13
Potential US Approval
Patent Expires
2029*
Worldwide Rights
Bucindolol Patents Issued
US –
2010,11,12
Europe –2010
* 2029 US/2030
EU w/term extension from current terms of 2026/2025 |
 Capital
Structure 14
(as of December 31, 2012)
•
Shares outstanding:
2.66 million
•
Dilutive securities outstanding:
1.68 million
–
Stock issued post 12/31/12:
521K
–
Warrants outstanding:
930K (at $9.10 on weighted average)
–
Options outstanding:
144K (at $18.29 on weighted average)
–
Shares under Equity Plan:
80K
•
Current stock price (4/10/13):
$2.20 /share
•
Current market capitalization:
$7.01 million
•
Net cash:
$2.92 million |
 Summary
•
AF is an epidemic CV disease afflicting ~2.7 million patients & growing;
•
Significant unmet need for treatment/prevention of AF, particularly in
HFREF patients, and for rate control in HFREF (no other approved
drugs
for HFREF patients)
•
Prior clinical data have indicated Gencaro to be safe and has shown
potential treatment benefits in AF, both in the entire patient cohort, and
especially in the "very favorable" genotype (~50% of patient population)
•
Management team with extensive experience in CV disease, drug
development and corporate execution
•
Potential to be only beta-blocker indicated for AF prevention
•
Potential to be the first genetically-targeted AF treatment
15 |
