Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Eloxx Pharmaceuticals, Inc. | v340153_8k.htm |

Senesco Technologies, Inc. Helping to Cure Cancer and Feed the World Annual Meeting March 28 th , 2013

Certain statements included in this presentation are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 . Actual results could differ materially from such statements expressed or implied herein as a result of a variety of factors, including, but not limited to : the Company’s ability to recruit patients for its clinical trial, the ability of the Company to consummate additional financings ; the development of the Company’s gene technology ; the approval of the Company’s patent applications ; the successful implementation of the Company’s research and development programs and collaborations ; the success of the Company's license agreements ; the acceptance by the market of the Company’s products ; the timing and success of the Company’s preliminary studies, preclinical research and clinical trials ; competition and the timing of projects and trends in future operating performance, the Company’s ability to comply with the continued listing standards of the NYSE/MKT, as well as other factors expressed from time to time in the Company’s periodic filings with the Securities and Exchange Commission (the "SEC") . As a result, this press release should be read in conjunction with the Company’s periodic filings with the SEC . The forward - looking statements contained herein are made only as of the date of this press release, and the Company undertakes no obligation to publicly update such forward - looking statements to reflect subsequent events or circumstances . . 2 Safe Harbor Statement

Senesco’s Mission To Help Cure Disease and Feed the World » Senesco has a patent - protected technology to modulate inappropriate cell growth and cell death » Senesco develops products to treat disease by re - programming cell death and survival mechanisms » Senesco’s collaborators develop crop products with improved growth and yield properties 3

Senesco Technology Validated First in Agricultural Applications » eIF5A biology demonstrated by licensees ▪ Increased yields ▪ Enhanced shelf life ▪ Disease resistance » Future product opportunities ▪ Collaborators running field trials ▪ Royalty revenue if new plant products reach market
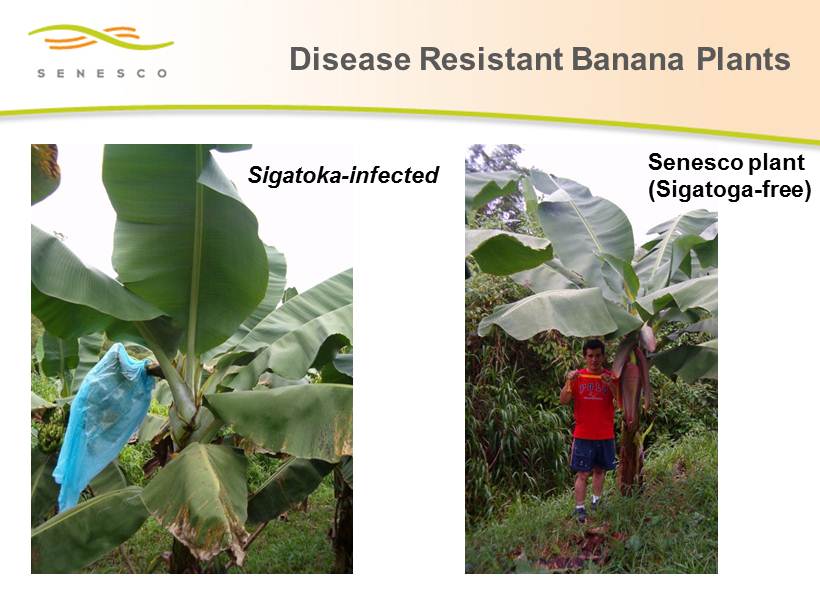
Senesco plant (Sigatoga - free) Sigatoka - infected Disease Resistant Banana Plants

Senesco Agricultural Licenses Licensee Field Status Milestones Royalties Monsanto Corn Field Trials Yes Yes Monsanto Soy Field Trials Yes Yes Bayer Canola Field Trials Yes Yes Bayer Cotton Greenhouse Yes Yes Bayer Rice Greenhouse Yes Yes Rahan Meristem Banana Field Trials No Yes Arborgen Trees Field Trials No Yes Scotts Turf Grass Greenhouse No Yes CalWest Alfalfa Field Trials Yes Yes

Specialty Therapeutics Company with Oncology Focus 7 TODAY » Novel technology platform with the potential to change the way cancer is treated » Senesco technology (>90 issued patents) can generate multiple novel therapeutic candidates in cancer and inflammation » Sponsoring Phase 1b/2a study of SNS01 - T in multiple myeloma, mantle cell and diffuse large B - cell lymphomas

SNS01 - T A novel therapeutic based on eIF5A modulation 8
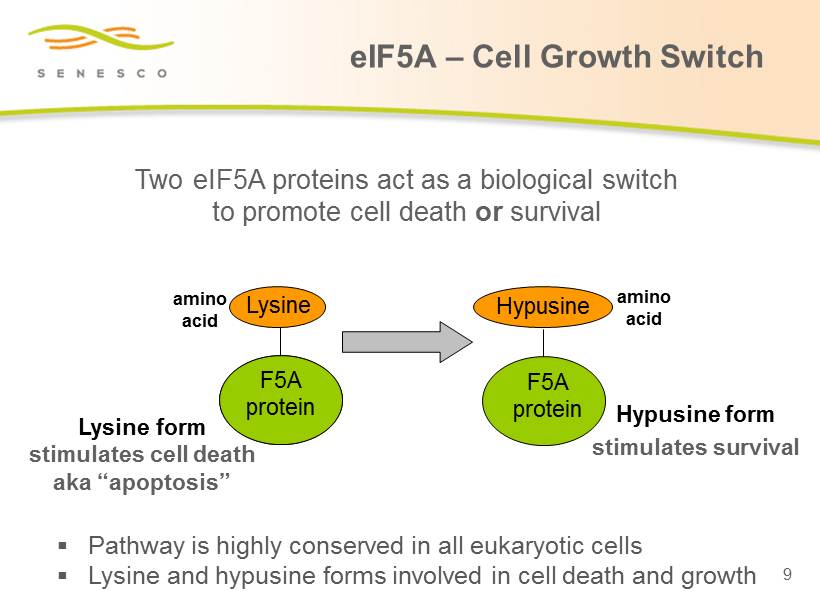
Two eIF5A proteins act as a biological switch to promote cell death or survival Lysine F5A protein Lysine form stimulates cell death aka “apoptosis” amino acid Hypusine F5A protein Hypusine form stimulates survival amino acid F5A protein ▪ Pathway is highly conserved in all eukaryotic cells ▪ L ysine and hypusine forms involved in cell death and growth 9 eIF5A – Cell Growth Switch
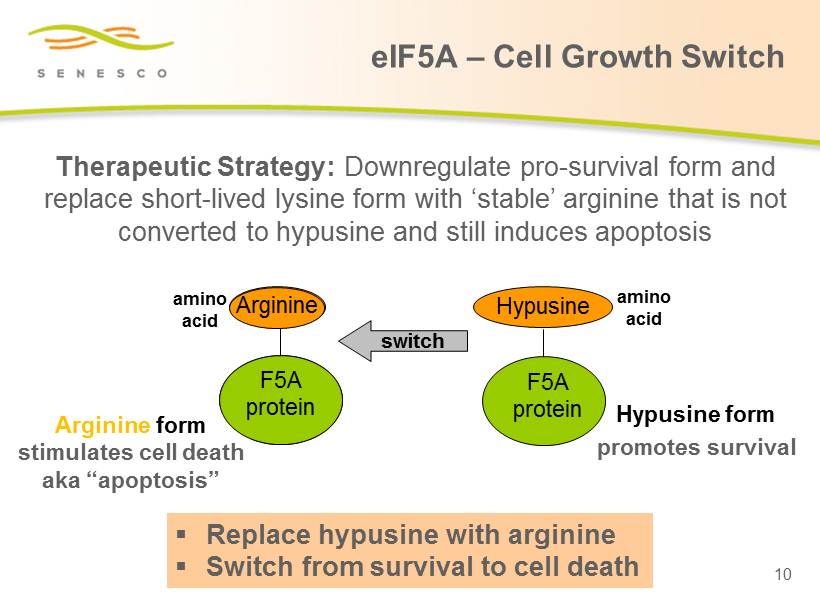
Lysine F5A protein Lysine form stimulates cell death aka “apoptosis” switch amino acid Hypusine F5A protein Hypusine form promotes survival amino acid Arginine Arginine form stimulates cell death aka “apoptosis” F5A protein ▪ Replace hypusine with arginine ▪ Switch from survival to cell death Therapeutic Strategy: Downregulate pro - survival form and replace short - lived lysine form with ‘stable’ arginine that is not converted to hypusine and still induces apoptosis 10 eIF5A – Cell Growth Switch
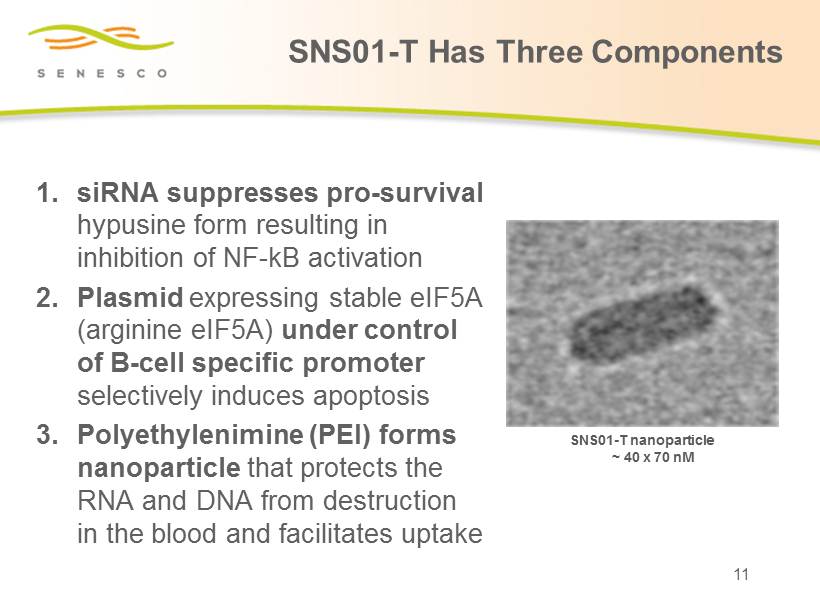
1. siRNA suppresses pro - survival hypusine form resulting in inhibition of NF - kB activation 2. Plasmid expressing stable eIF5A (arginine eIF5A) under control of B - cell specific promoter selectively induces apoptosis 3. Polyethylenimine (PEI) forms nanoparticle that protects the RNA and DNA from destruction in the blood and facilitates uptake SNS01 - T nanoparticle ~ 40 x 70 nM 11 SNS01 - T Has Three Components
Summary ▪ SNS01 - T is first - in - class agent to target eIF5A − Selectively triggers apoptosis in B - cell cancers − Both single agent activity, and, synergy with lenalidomide or bortezomib in mouse models ▪ Phase 1b/2a is ongoing: safety and early efficacy signal demonstrated at lowest dose ▪ Development plan − B - cell cancers with current focus on MM, MCL, DLBCL − Can be extended in current form to other B - cell NHL − Readily modified to target many other cancers by substituting promoter with desired tissue specificity 12 SNS01 - T modulates the eIF5A pathway

Pre - Clinical Results

» eIF5A modulation has broad activity in most cancer cell lines tested in vitro » Efficacy in multiple in vivo disease models including melanoma ( B16 - F0) and lung (A549) cancer* » Efficacy in blood cancer models in mice ▪ 85 - 95% growth inhibition in MM, MCL and DLBCL mouse xenograft models ▪ Synergy with bortezomib and lenalidomide * Gene Ther Mol Biol 12 , 207 - 218 (2008 ) 14 Efficacy in Cancer Cell Lines and In Vivo Models
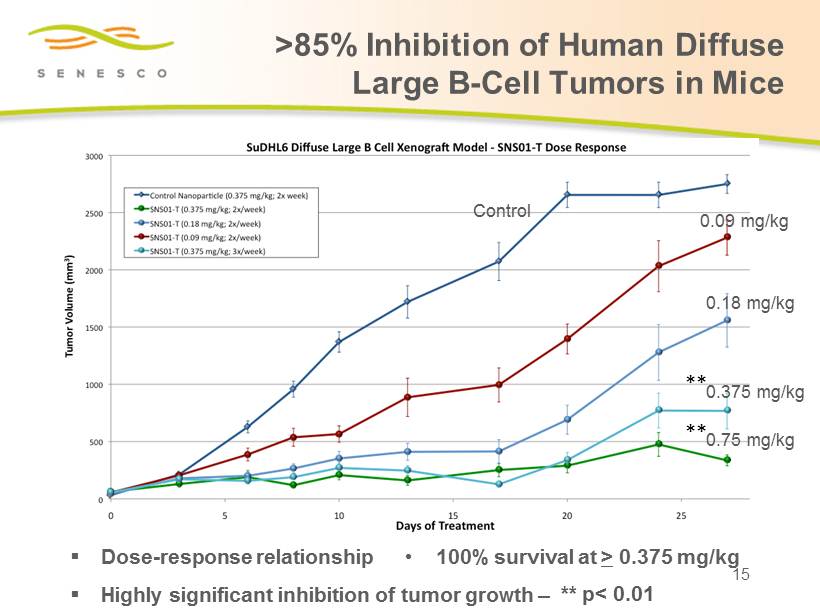
>85% Inhibition of Human Diffuse Large B - Cell Tumors in Mice 15 ▪ Dose - response relationship ▪ Highly significant inhibition of tumor growth – • 100% survival at > 0.375 mg/kg 0.09 mg/kg Control 0.18 mg/kg 0.375 mg/kg 0.75 mg/kg ** p< 0.01 ** **
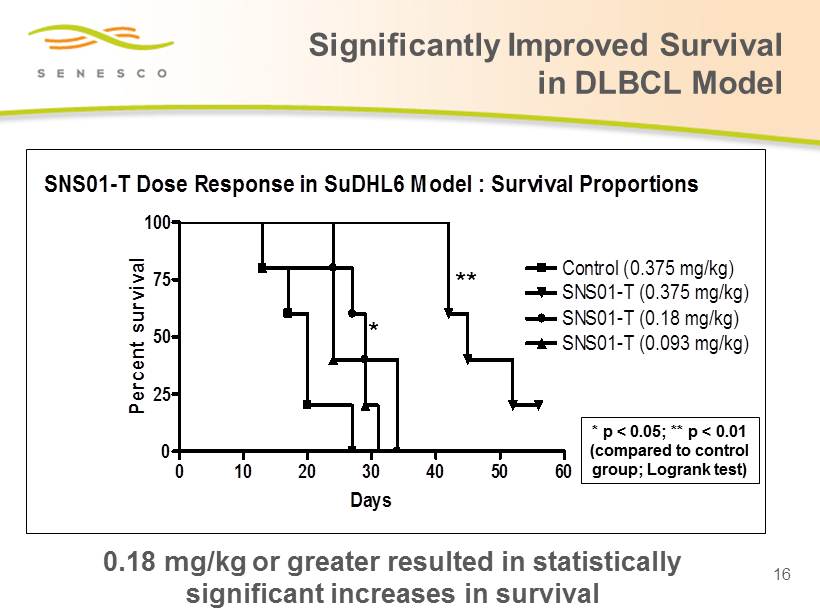
SNS01-T Dose Response in SuDHL6 Model : Survival Proportions 0 10 20 30 40 50 60 0 25 50 75 100 Control (0.375 mg/kg) SNS01-T (0.375 mg/kg) SNS01-T (0.18 mg/kg) SNS01-T (0.093 mg/kg) Days Percent survival ** * Significantly Improved Survival in DLBCL Model 16 * p < 0.05; ** p < 0.01 (compared to control group; Logrank test) 0.18 mg/kg or greater resulted in statistically significant increases in survival

No Effect of SNS01 - T on Weight Gain in Mice
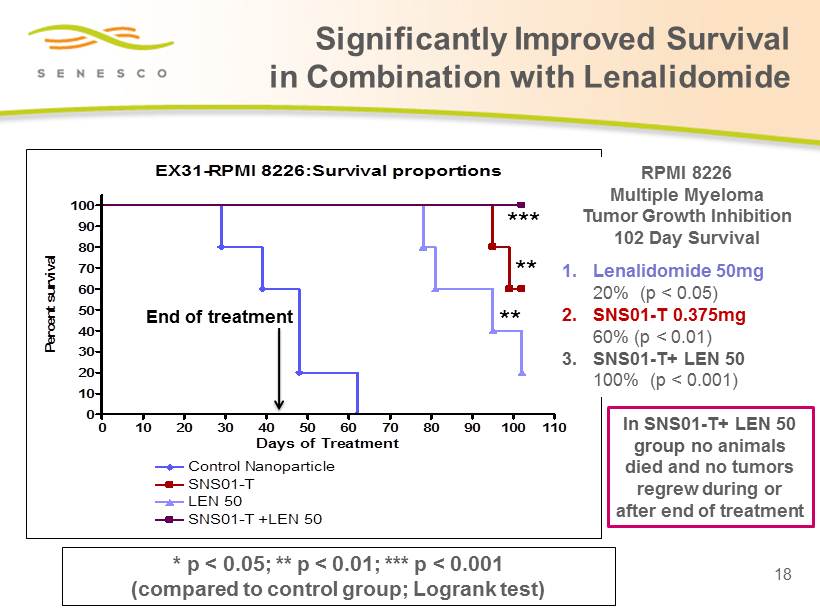
Significantly Improved Survival in Combination with Lenalidomide 18 * p < 0.05; ** p < 0.01; *** p < 0.001 (compared to control group; Logrank test) EX31-RPMI 8226:Survival proportions 0 10 20 30 40 50 60 70 80 90 100 110 0 10 20 30 40 50 60 70 80 90 100 Control Nanoparticle SNS01-T LEN 50 SNS01-T +LEN 50 Days of Treatment Percent survival *** ** ** RPMI 8226 Multiple Myeloma Tumor Growth Inhibition 102 D ay Survival 1. Lenalidomide 50mg 20% (p < 0.05) 2. SNS01 - T 0.375mg 60% (p < 0.01) 3. SNS01 - T+ LEN 50 100% (p < 0.001) End of treatment In SNS01 - T + LEN 50 group n o animals died and no tumors regrew during or after end of treatment

Clinical Trial Status
» Good tolerability and stable disease at lowest dose » 3 patients completed at lowest dose ( 0 . 0125 mg/kg) − 2 patients had stable disease at weeks 3 & 6 with M - protein levels within 25 % of baseline ( 3 . 6 , 3 . 0 g/ dL respectively) at weeks 3 ( 3 . 9 , 2 . 8 g/ dL ) and 6 ( 4 . 2 , 2 . 8 g/ dL ) − 1 responder remained stable 4 weeks post dosing − baseline plasma cell percent declined from 70 % to 50 % ( 29 % reduction), and, 50 % to 15 % ( 70 % reduction ) in patients with stable disease » No drug - related severe adverse events or dose - limiting toxicities Phase 1b/2a Interim Results from Cohort 1 20
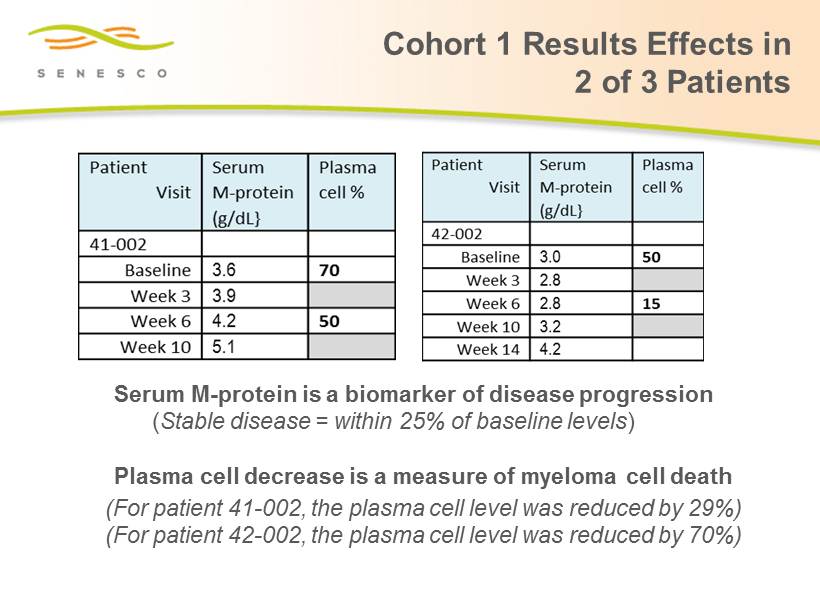
Cohort 1 Results Effects in 2 of 3 Patients Serum M - protein is a biomarker of disease progression ( Stable disease = within 25% of baseline levels ) Plasma cell decrease is a measure of myeloma cell death ( For patient 41 - 002, the plasma cell level was reduced by 29%) (For patient 42 - 002 , the plasma cell level was reduced by 70%)
Cohort 2 Update » Cohort 2 Update ▪ Patients enrolled in Cohort 2 i. Two at WVU completed (1 MM & 1 DLBCL) ii. Hackensack patient withdrew ▪ No drug - related severe adverse events (SAEs) ▪ No dose l imiting t oxicities (DLTs)

Multiple Myeloma Future Plans 23 ▪ Currently completing cohort 2 ▪ Cohort 2 results in 2Q - 2013 ▪ Topline results potentially in 2H - 2013 ▪ Potential Phase 2b design + / - Revlimid ® ▪ Phase 2b initiation planning underway

Innovation – targeting cell death and survival » Large product opportunity in hematology » Multiple additional applications in solid tumors » And other diseases including inflammation & diabetes 24 Potential Cancer Breakthrough
