Attached files
| file | filename |
|---|---|
| 8-K - DELCATH SYSTEMS, INC. FORM 8-K - DELCATH SYSTEMS, INC. | form8k.htm |
Exhibit 99.1
Investor Presentation
(NASDAQ: DCTH)
(NASDAQ: DCTH)
March 2013

2 DELCATH SYSTEMS, INC
Forward-looking Statements
Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on
its behalf. This presentation contains forward-looking statements, which are subject to certain risks and uncertainties that can cause
actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to,
uncertainties relating to: the outcome of the ODAC meeting, and the impact, if any, of the advisory panel’s recommendation on the
FDA’s decision regarding the Company’s new drug application (NDA), uncertainties relating to: timing of completion of the FDA’s
review of our NDA, the extent to which the FDA may request additional information or data and our ability to provide the same in a
timely manner, acceptability of the Phase 1, 2 and 3 clinical trial data by the FDA, FDA approval of the Company's NDA for the
treatment of metastatic ocular melanoma to the liver, adoption, use and resulting sales, if any, for the Delcath Hepatic Delivery
System in the United States, adoption, use and resulting sales, if any, for the Hepatic CHEMOSAT Delivery System in the EEA, our
ability to successfully commercialize the Delivery System in various markets and the potential of the system as a treatment for
patients with cancers in the liver, the timing and our ability to successfully enter into strategic partnership and distribution
arrangements in foreign markets including Australia and key Asian markets and resulting sales, if any, from the same, patient
outcomes using the Generation 2 system, approval of the current or future system for other indications and/or for use with various
chemotherapeutic agents, actions by the FDA or other foreign regulatory agencies, our ability to obtain reimbursement for the
CHEMOSAT system in various markets, the number of cancer centers in Germany and Italy able to successfully negotiate and
receive reimbursement for the CHEMOSAT procedure and the amount of reimbursement to be provided, submission and publication
of the Phase II and III clinical trial data, the timing and results of research and development projects, the timing and results of future
clinical trials including the initiation of clinical trials in key Asian markets with the CHEMOSAT Hepatic Delivery System device for
intra-hepatic arterial delivery and extracorporeal filtration of doxorubicin, approval of the CHEMOSAT Hepatic Delivery System to
delver and filter doxorubicin in key Asian markets and adoption, sales, if any, and patient outcomes using the same, the timing, price
and use, if any, of the committee equity financing facility with Terrapin, the timing and use, if any, of the line of credit from SVB and
our ability to access this facility and uncertainties regarding our ability to obtain financial and other resources for any research,
development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the
Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only
as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect
events or circumstances after the date they are made.
its behalf. This presentation contains forward-looking statements, which are subject to certain risks and uncertainties that can cause
actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to,
uncertainties relating to: the outcome of the ODAC meeting, and the impact, if any, of the advisory panel’s recommendation on the
FDA’s decision regarding the Company’s new drug application (NDA), uncertainties relating to: timing of completion of the FDA’s
review of our NDA, the extent to which the FDA may request additional information or data and our ability to provide the same in a
timely manner, acceptability of the Phase 1, 2 and 3 clinical trial data by the FDA, FDA approval of the Company's NDA for the
treatment of metastatic ocular melanoma to the liver, adoption, use and resulting sales, if any, for the Delcath Hepatic Delivery
System in the United States, adoption, use and resulting sales, if any, for the Hepatic CHEMOSAT Delivery System in the EEA, our
ability to successfully commercialize the Delivery System in various markets and the potential of the system as a treatment for
patients with cancers in the liver, the timing and our ability to successfully enter into strategic partnership and distribution
arrangements in foreign markets including Australia and key Asian markets and resulting sales, if any, from the same, patient
outcomes using the Generation 2 system, approval of the current or future system for other indications and/or for use with various
chemotherapeutic agents, actions by the FDA or other foreign regulatory agencies, our ability to obtain reimbursement for the
CHEMOSAT system in various markets, the number of cancer centers in Germany and Italy able to successfully negotiate and
receive reimbursement for the CHEMOSAT procedure and the amount of reimbursement to be provided, submission and publication
of the Phase II and III clinical trial data, the timing and results of research and development projects, the timing and results of future
clinical trials including the initiation of clinical trials in key Asian markets with the CHEMOSAT Hepatic Delivery System device for
intra-hepatic arterial delivery and extracorporeal filtration of doxorubicin, approval of the CHEMOSAT Hepatic Delivery System to
delver and filter doxorubicin in key Asian markets and adoption, sales, if any, and patient outcomes using the same, the timing, price
and use, if any, of the committee equity financing facility with Terrapin, the timing and use, if any, of the line of credit from SVB and
our ability to access this facility and uncertainties regarding our ability to obtain financial and other resources for any research,
development and commercialization activities. These factors, and others, are discussed from time to time in our filings with the
Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only
as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect
events or circumstances after the date they are made.

3 DELCATH SYSTEMS, INC
Investment Considerations
Concentrating the Power of Chemotherapy
• Commercial stage company focused on oncology
• Proprietary CHEMOSAT® Hepatic Delivery System allows unique whole
organ therapy for the liver
organ therapy for the liver
• CHEMOSAT system has demonstrated extension of progression free
survival
survival
• Addressing large unmet market need for cancer patients who usually die
of liver failure
of liver failure
• Estimated initial market opportunity of ~$2.3 billion in U.S. & EU
• Expanding clinical data expected to broaden clinical use and indications
• On the cusp of realizing the potential:
o EU - early commercial launch underway; reimbursement in key EU markets expected
in Q1/Q2
in Q1/Q2
o U.S. - NDA under review ; ODAC May 2, PDUFA date June 15, 2013
• Attractive financial model, multiple capital resources available and
experienced management team to execute plan
experienced management team to execute plan
4 DELCATH SYSTEMS, INC
US Market
• Proposed Trade Name
Melblez KitTM (Melblez (melphalan) for
Injection for use with the Delcath
Hepatic Delivery System)
Injection for use with the Delcath
Hepatic Delivery System)
• Proprietary Drug/Device Combination
Product Regulated as a drug 505(b)(2)
NDA by U.S. FDA
NDA by U.S. FDA
• Proposed indication for the treatment of
patients with unresectable ocular
melanoma metastatic to the liver
• Melblez Kit comprised of MelblezTM
(melphalan hydrochloride for injection)
and the Delcath Hepatic Delivery
System
Our Product
Ex US Markets
• Marketed under the trade name
CHEMOSAT® Hepatic Delivery
System
System
• Regulated as a Class IIb Medical
Device
• Indicated for the intra-hepatic of
administration of melphalan
hydrochloride and subsequent
filtration of the venous blood return.
• CHEMOSAT Kit supplied without
melphalan
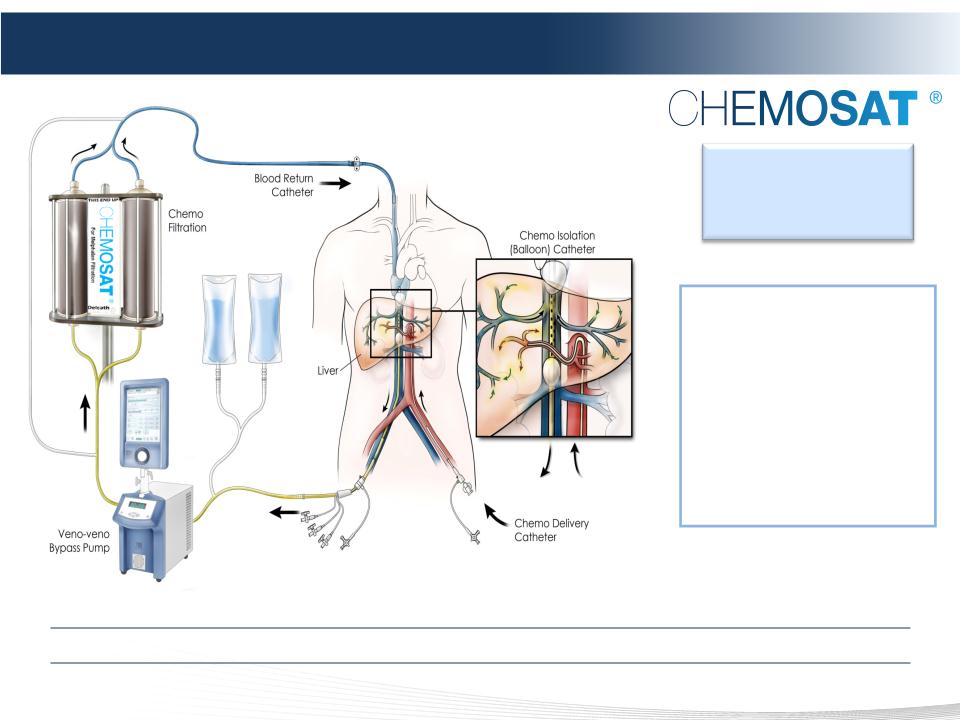
5 DELCATH SYSTEMS, INC
1. ISOLATE
2. SATURATE
3. FILTRATE
The Delcath Hepatic Delivery System
Minimally Invasive, Repeatable Procedure That Could Complement Systemic Therapy
• Improves disease
control in the liver
control in the liver
• Treats macro and micro
tumors
tumors
• Controls systemic
toxicities
toxicities
• Allows for over 100x
dose escalation at
tumor site
dose escalation at
tumor site

6 DELCATH SYSTEMS, INC
Melanoma Liver Metastases
A Great Demonstration of CHEMOSAT’s Potential
• A challenging histology
• Notoriously insensitive to
systemic chemotherapy and
focal interventions
systemic chemotherapy and
focal interventions
• CHEMOSAT has
demonstrated ability to extend
progression free survival
demonstrated ability to extend
progression free survival
Our Opportunity
• Ability to achieve ultra-high concentrations of chemotherapy provides
potential treatment options for a wide variety of cancers in the liver
potential treatment options for a wide variety of cancers in the liver

7 DELCATH SYSTEMS, INC
Clinically Differentiated Results
• Phase 1, 2 and 3 trials with percutaneous hepatic perfusion (PHP) produced positive
results in multiple histologies
results in multiple histologies
• Melanoma Liver Mets
o Positive Phase 3 results in hepatic metastatic melanoma
o n=93 (90% ocular melanoma, 10% cutaneous melanoma)
• Neuroendocrine Tumor (NET) Liver Mets
o mNET cohort in Phase 2 trial showed encouraging 42% objective response rate (ORR) vs ~10%
for approved targeted therapy
for approved targeted therapy
o median overall survival of ~32 months on ITT basis
• Hepatocellular Carcinoma (HCC)
o Positive signal with high-dose melphalan in HCC cohort of Phase 2 trial (5/8 patients) is
encouraging when approved systemic therapies have modest efficacy and challenges with
tolerability
encouraging when approved systemic therapies have modest efficacy and challenges with
tolerability
• Colorectal Cancer (CRC) Liver Mets
o Data from surgical Isolated Hepatic Perfusion (IHP) with melphalan indicates strong potential in
well-defined patient population with earlier stage CRC yielding ~50-60% median response rate
and median OS of 17.4-24.8 mos
well-defined patient population with earlier stage CRC yielding ~50-60% median response rate
and median OS of 17.4-24.8 mos
• Safety profiles consistent with pivotal US Phase 3 melanoma trial
Encouraging Initial Results on a Broad Range of Histologies
8 DELCATH SYSTEMS, INC
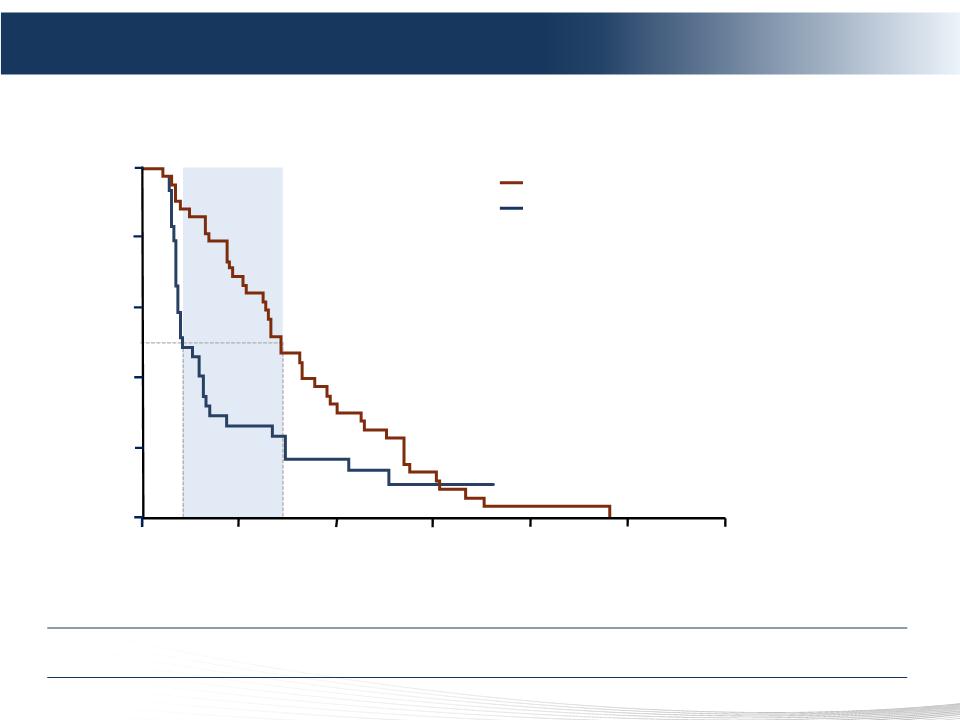
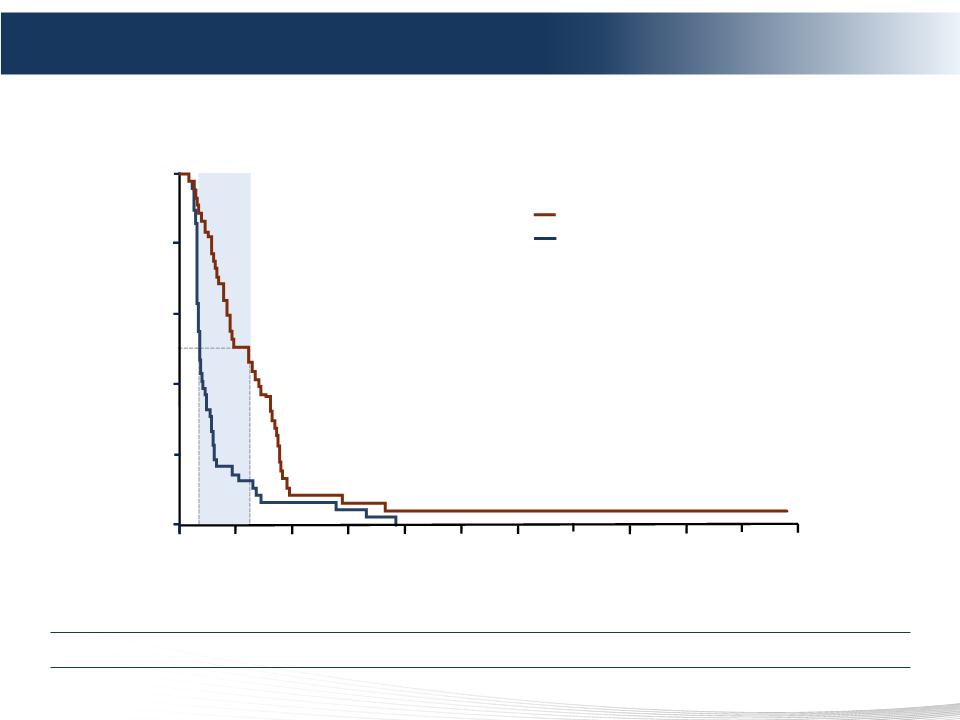
INDEPENDENT REVIEW COMMITTEE (IRC) ASSESSMENT - UPDATED ANALYSIS (4 June 2012)
Hepatic progression-free survival (IRC)
Hazard ratio = 0.50
(95% CI 0.31-0.80)
P=0.0029
0 5 10 15 20 25 30
Months
7.0
1.7
1.0
0.8
0.6
0.4
0.2
0.0
Proportion of patients surviving
5.3 mo
Intent-to-treat population
Percutaneous Hepatic Perfusion (PHP)
Best alternative care (BAC)
Best alternative care (BAC)
Positive Phase 3 Results – Primary Endpoint hPFS
PHP Demonstrated 4x or 5.3 months Improvement in Primary Endpoint of hPFS
9 DELCATH SYSTEMS, INC
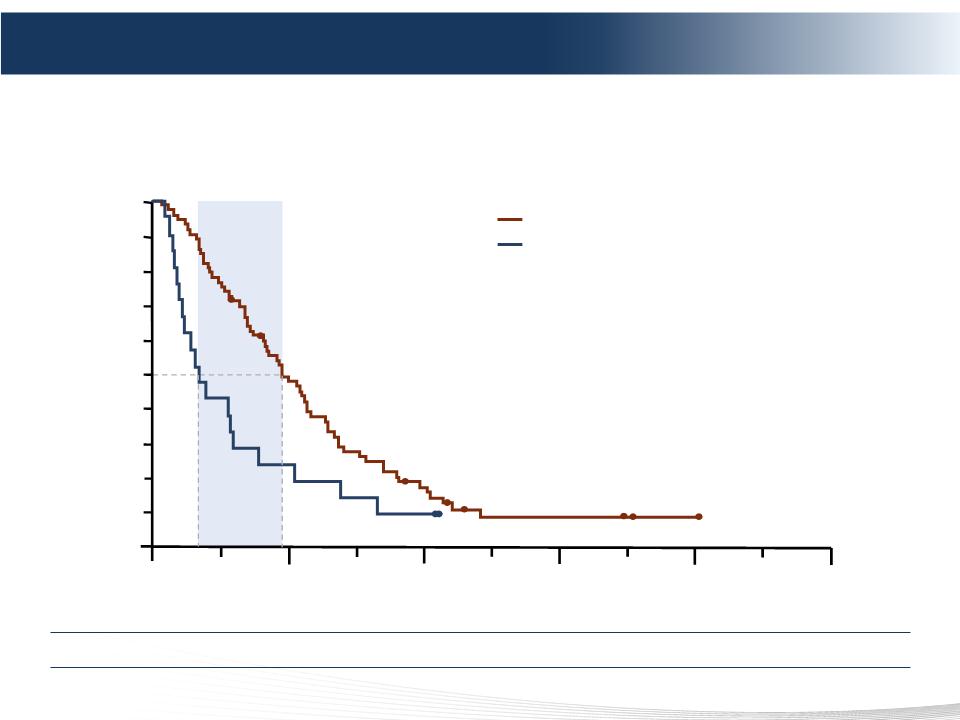
INVESTIGATOR ASSESSMENT - UPDATED ANALYSIS (4 June 2012)
Overall progression-free survival (investigator)
Months
5.4
1.6
1.0
0.8
0.6
0.4
0.2
0.0
Proportion of patients surviving
Hazard ratio = 0.42
(95% CI 0.27-0.64)
P<0.0001
0 5 10 15 20 25 30 35 40 45 50 55
3.8 mo
Intent-to-treat population
Positive Phase 3 Results – Overall PFS
PHP also Demonstrated a Highly Statistically Significant Improvement in Overall PFS
Percutaneous Hepatic Perfusion (PHP)
Best alternative care (BAC)
Best alternative care (BAC)
10 DELCATH SYSTEMS, INC
TOTAL PHP vs BAC ONLY
Proportion of subjects surviving
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
12
36
0
24
48
60
11.4
Total PHP incl. crossover
BAC only
Months
4.1
Intent-to-treat population
7.3 mo
Overall Survival – Exploratory Subset Analysis
Overall Survival Tail For PHP Treated Patients

11 DELCATH SYSTEMS, INC
Phase 2 Multi-Histology NCI Trial – Summary
• Strong efficacy signals in mNET
o 42% objective Response Rate (ORR) vs ~10%
for approved targeted therapy
for approved targeted therapy
o 66% patients had hepatic tumor shrinkage and
durable disease stabilization
durable disease stabilization
• Positive Signal in primary hepatic malignancies
(HCC and Cholangiocarcinoma) in 5 of 8 patients
(HCC and Cholangiocarcinoma) in 5 of 8 patients
• Similar safety profiles across tumor types
Positive Efficacy Signals In Additional Types of Cancer
12 DELCATH SYSTEMS, INC
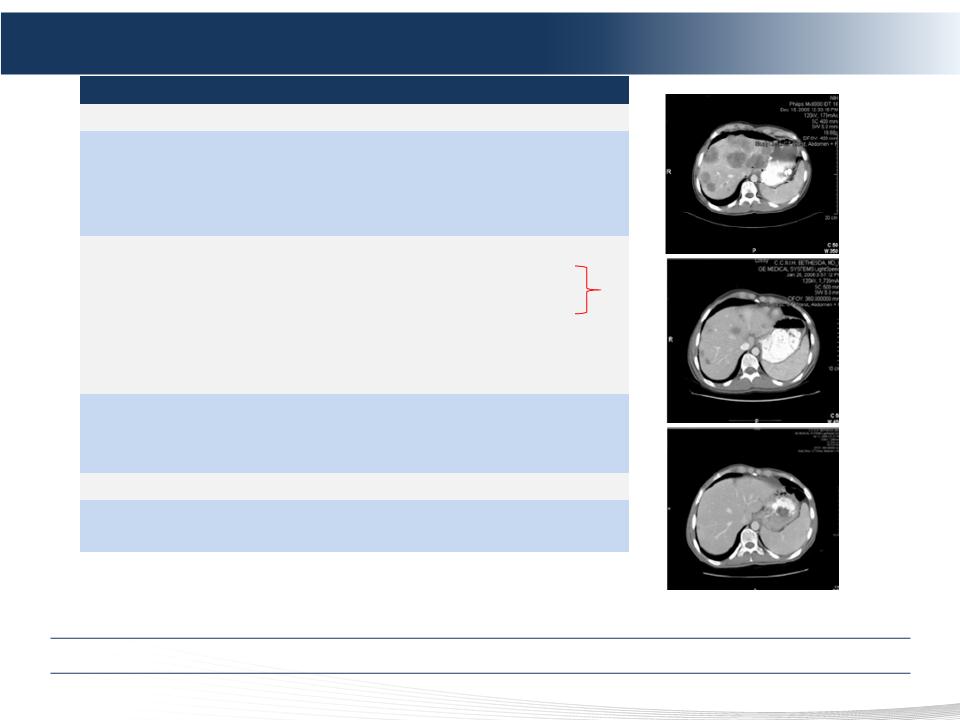
Phase 2 NCI Trial – Metastatic Neuroendocrine Cohort
Pre-PHP
(Baseline)
Post-PHP #2
(+4 Months)
Post-PHP #1
(+6 Weeks)
Compelling Clinical Data in Attractive mNET Market
|
Phase 2 mNET Tumor Cohort (n=24)*
|
|
|
|
Number (n)
|
|
Tumor Types
|
|
|
Pancreatic NET
|
13
|
|
Carcinoid tumor
|
3
|
|
Other NET
|
8
|
|
Response
|
|
|
Partial Response (PR)
|
10
|
|
Stable disease (SD)
|
6
|
|
Progressive disease
|
3
|
|
Not assessed or evaluable
|
5
|
|
Objective Response Rate
|
42%
|
|
Median Duration of Hepatic Response
|
|
|
Partial Response (n-10)
|
23.5 months
|
|
Partial Response/Stable Disease (n=16)
|
16.8 months
|
|
Hepatic Progression Free Survival (IIT n=24)
|
|
|
Median Hepatic PFS
|
16.8
|
|
Min/Max
|
2.1, 64.1
|
|
Overall Survival After CS
|
|
|
Median
|
31.9 months
|
|
Min/Max
|
2.4, 81.1
|
66%
disease
control
disease
control

13 DELCATH SYSTEMS, INC
Phase 2 NCI Trial – Hepatobiliary Carcinoma Cohort
• Best hepatic tumor response by modified RECIST assessed by investigators
o Partial response (PR) 1 patient
o Stable disease (SD) 4 patients
o Progressive disease 1 patient
o Not assessed or evaluable 2 patients
• Median duration of response
o hPR (N=1) 6.42 months
o hPR/SD (N=5) 8.12 months
• Hepatic progression free survival (ITT N=8)
o Median 5.60 months
o Minimum, Maximum 2.7, 12.2 months
• Overall survival (ITT N=8)
o Median 9.12 months
o Minimum, Maximum 3.4, 20.5 months
• HCC is the most common primary cancer of the liver, with approximately 750,000* new
cases diagnosed worldwide annually
cases diagnosed worldwide annually
• Intend to initiate new HCC trials with CHEMOSAT
Encouraging Positive Signal for Primary Liver Cancer
*Source: GLOBOCAN

14 DELCATH SYSTEMS, INC
• Substantial clinical evidence of benefit of using ultra-high dose
melphalan to treat mCRC via isolated hepatic perfusion (IHP)
procedure
melphalan to treat mCRC via isolated hepatic perfusion (IHP)
procedure
o Over 800 patients treated in 15 studies since 1998
o Patients treated only once
o Median response rate of ~50-60% and median OS of 17.4 – 24.8 mos1,2
• Delcath Phase 2 NCI Trial – mCRC Cohort
o Challenges enrolling at NCI due to competing FOLFOX & FOLFIRI trials
o 17 patients treated since 2004
o Safety profile – expected and consistent with pivotal FDA Phase III melanoma
trial
trial
• Intend to invest in new mCRC trials with CHEMOSAT Melphalan
1) van Iersel LB, Gelderblom H, et al. Ann Oncol. 2008;19:1127-34
2) Alexander, HR, Barlett DL, et al. Ann Surg Oncol, 16:1852-9, 2009
Phase 2 NCI Trial – mCRC Cohort

15 DELCATH SYSTEMS, INC
Additional Clinical Data Generation
• Goals:
§ Expand US (PHP: MEL) label indications beyond the initial
indication we are seeking
indication we are seeking
§ Generate robust clinical data to support commercialization
• FDA has accepted IND Amendment to include Gen 2 device in
Expanded Access Program (EAP), compassionate use (CU),
and all future clinical trials
Expanded Access Program (EAP), compassionate use (CU),
and all future clinical trials
• Initiated EAP to treat first patient in January, 2013
• Activate EU Registry to systematically collect data from
commercial experience
commercial experience
Establish CHEMOSAT as the Standard of Care (SOC) for Disease Control in the Liver

16 DELCATH SYSTEMS, INC
2013 Clinical Development Plan
• Planned company sponsored trials, subject to agreement with FDA
q Hepatocellular carcinoma (HCC)
o Global Phase 3 Randomized CHEMOSAT Melphalan vs. best supportive care
(BSC) for patients where Sorafenib is inappropriate
(BSC) for patients where Sorafenib is inappropriate
− Primary endpoint: Overall Survival
q Advanced colorectal cancer (CRC) with liver dominant metastasis
o Global Phase 3 Randomized CHEMOSAT Melphalan vs. best alternative care
(BAC)
(BAC)
− Primary endpoint: Overall Survival
q Metastatic Neuroendocrine tumor (NET) with liver dominant disease
o Global Phase 3 Randomized CHEMOSAT Melphalan vs. Best Alternative Care
(BAC)
(BAC)
− Primary endpoint: Hepatic PFS
• Planned phase 2 studies including global Investigator-initiated trials (IITs) in multiple
indications: HCC, NET, CRC, melanoma
indications: HCC, NET, CRC, melanoma
Establish CHEMOSAT as the Standard of Care (SOC) for Disease Control in the Liver
17 DELCATH SYSTEMS, INC
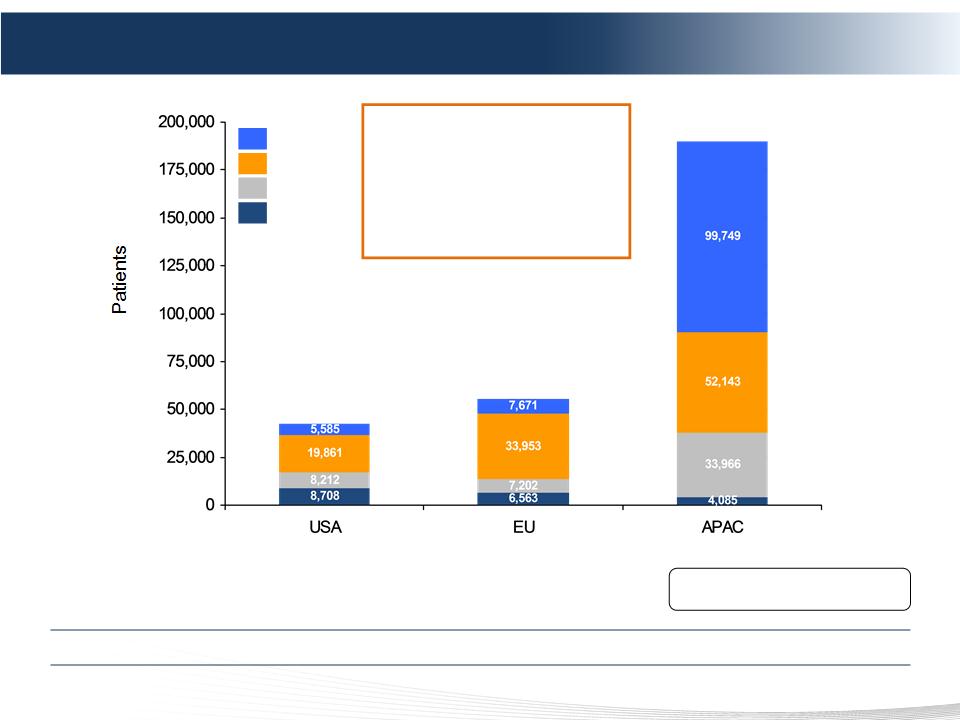
$2.3 Billion Initial Market Opportunity with Pharmaceutical-Like Gross Margins
Sources: LEK Consulting, GLOBOCAN, Company estimates.
EU: Initial target countries of Germany, UK, Italy, France, Spain, Netherlands, Ireland.
APAC: Initial target countries of China, Japan, S. Korea, Taiwan, Australia.
Assumes 2.5 treatments per patient.
Assumes EU ASP of $15K; US ASP of $25K; APAC ASP of $5K.
55,389
42,367
189,943
HCC
CRC
Melanoma
NET
$2.3B Initial Opportunity
• $100M Initial on-label
opportunity in Ocular Melanoma
in US*
opportunity in Ocular Melanoma
in US*
• $2.2B multi-histology
opportunity in EU
opportunity in EU
* Assumes FDA approval for ocular
melanoma metastatic to the liver
melanoma metastatic to the liver
CHEMOSAT – Potential Multi-Billion Dollar Global Market
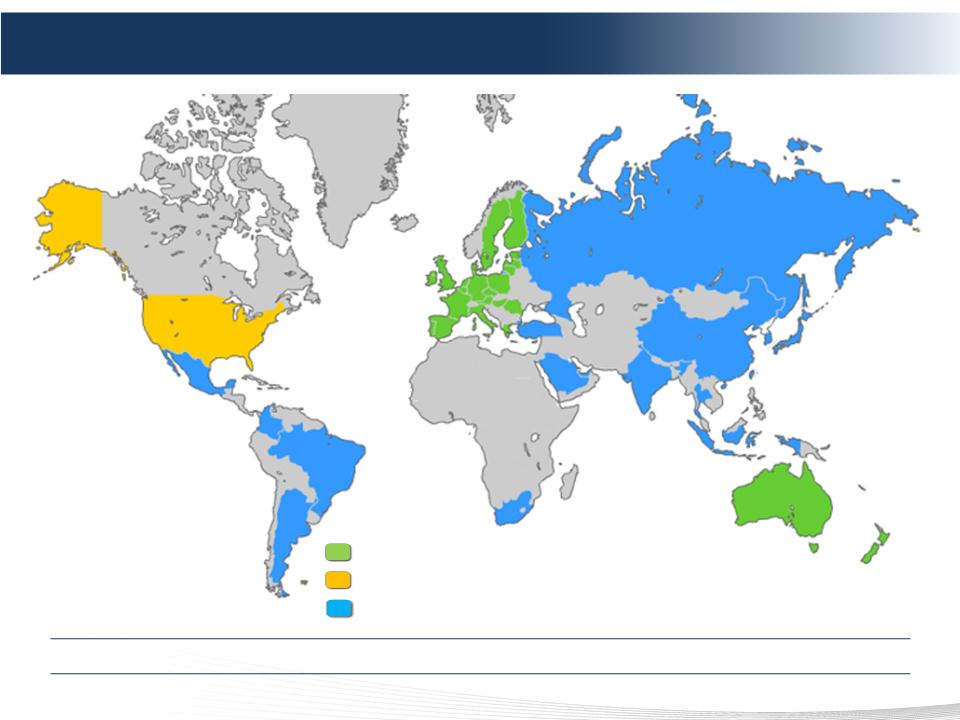
18 DELCATH SYSTEMS, INC
Approved (CE Mark Device)
NDA Filing Accepted by the FDA with PDUFA goal date of June 15, 2013
Mutual Recognition of European CE Mark – Applications Planned or
Submitted
Submitted
Global Commercialization Status
Addressing A Multi-Billion Dollar Global Market

19 DELCATH SYSTEMS, INC
CHEMOSAT: EU Launch Underway
• Marketing in target EU countries - Italy, Germany, France,
UK, Ireland, NL, Spain
UK, Ireland, NL, Spain
• Training completed in key centers
o Eight EU Clinical Sites activated in 2012
• EU clinicians using CHEMOSAT for a broad range of liver
metastases
metastases
o Use includes: cutaneous melanoma, ocular melanoma, colorectal cancer
(CRC), gastric cancer, breast cancer, neuroendocrine tumor (NET),
hepatocellular carcinoma (HCC) and Cholangiocarcinoma
(CRC), gastric cancer, breast cancer, neuroendocrine tumor (NET),
hepatocellular carcinoma (HCC) and Cholangiocarcinoma
• EU reimbursement in progress
o Italy – Existing DRG for partial reimbursement identified; supplemental
reimbursement applications submitted
reimbursement applications submitted
o Germany – Value 4 NUB interim reimbursement granted February 2013
o UK – Reimbursement anticipated Q2 2013
Expansion of EU Clinical and Commercial Footprint Expected in 2013
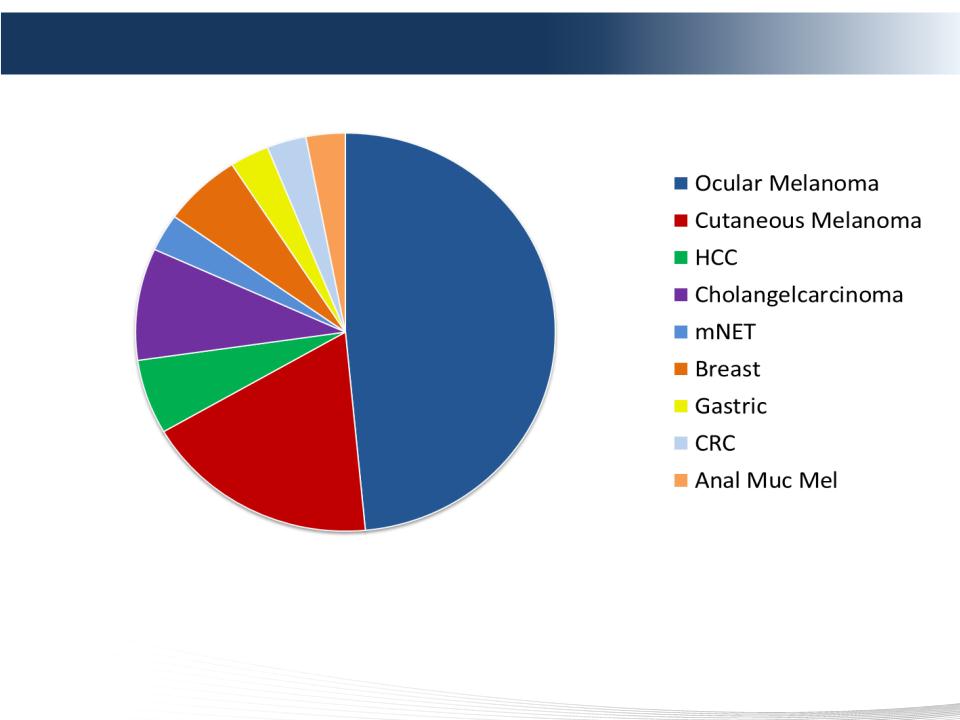
20 DELCATH SYSTEMS, INC
CHEMOSAT: Multiple Tumor Types Treated in Europe
• Physicians are recognizing the potential of CHEMOSAT in various tumor types
• CHEMOSAT utilized in Germany, Italy, UK, France, Ireland
• EU Registry To Be Initiated Q2

21 DELCATH SYSTEMS, INC
U.S. NDA Under Review
• Oncology Drug Advisory Committee (ODAC) panel
scheduled for May 2, 2013
scheduled for May 2, 2013
• PDUFA date: June 15, 2013
• Initial indication: unresectable metastatic ocular melanoma
in the liver
in the liver
o Provides lowest risk pathway to FDA approval and fastest access
• NDA filing included:
o Comprehensive set of additional data in a new FDA compliant
CDISC database
CDISC database
o Gen 2 filter as part of the Chemistry, Manufacturing and Control
(CMC) module
(CMC) module
• Three meetings scheduled with FDA to discuss clinical
programs for planned label expansions in each of NET,
HCC,CRC
programs for planned label expansions in each of NET,
HCC,CRC
FDA Decision Expected in June

22 DELCATH SYSTEMS, INC
U.S. Commercialization Strategy
• Launch in Q4 2013 assuming approval on PDUFA date of June
15, 2013
15, 2013
• Initial commercial focus on centers that are active in the EAP or
participated in the Phase 3 clinical trial
participated in the Phase 3 clinical trial
• Utilize active EAP hospitals as Centers of Excellence for training
and support of new centers
and support of new centers
• Intend to seek specific CPT reimbursement code for the Melblez
Kit procedure, based upon value proposition relative to other
cancer therapies
Kit procedure, based upon value proposition relative to other
cancer therapies
• Educate Medical Oncologists via Medical Science Liaison (MSL)
• Direct strategy to sell to hospital based Interventional Radiologists
and Surgeons
and Surgeons
Participating EAP Centers Provide Immediate Commercial Footprint

23 DELCATH SYSTEMS, INC
Barriers to Entry
• Patent Protection
o 6 U.S. patents in force and 6 U.S. patent applications pending
o 9 foreign patents in force (with patent validity in 25 countries) and 14 foreign patent
applications pending
applications pending
o Primary US device patent set to expire August 2016
o Up to 5 years of patent extension post FDA approval
• Trade Secret Protection
o Developed improved filter media via proprietary manufacturing processes
• FDA Protection
o Orphan Drug Designation granted for melphalan in the treatment of ocular
melanoma, cutaneous melanoma and metastatic neuroendocrine tumors, as well as
for doxorubicin in the treatment of HCC
melanoma, cutaneous melanoma and metastatic neuroendocrine tumors, as well as
for doxorubicin in the treatment of HCC
§ Provides 7 years of marketing exclusivity post FDA approval
o Additional Orphan Drug applications to be filed for other drugs and indications,
including melphalan for HCC and CRC
including melphalan for HCC and CRC
Multiple Levels of Protection
24 DELCATH SYSTEMS, INC
Financial Summary
|
Cash & Cash Equivalents:
|
$38.0 million at February 28, 2013 (unaudited)
|
|
ATM Program
|
up to $50.0 million available upon registration
statement being declared effective by the SEC |
|
Committed Equity Financing
Facility (CEFF) |
Up to $32.8 million as of February 28, 2013
|
|
Working Capital Line of Credit:
|
$20 million credit facility
|
|
Debt:
|
None
|
|
Cash Spend:
|
Approx. $10 million in 4Q 2012 (unaudited)
Projected 2013 quarterly cash spend $9-$12 million
|
|
Shares Outstanding:
|
90.2 million (100.5 million fully diluted1) as of
February 28, 2013 |
1) Fully diluted includes an additional 4.7 million options and 5.6 million warrants
Multiple Capital Resources Available to Execute Plan

25 DELCATH SYSTEMS, INC
Management: A Track Record of Success
|
Executive
|
Title
|
Prior Affiliation(s)
|
Years of
Experience |
|
Eamonn Hobbs
|
President and CEO
|
AngioDynamics, E-Z-EM
|
32
|
|
Graham Miao, Ph.D.
|
EVP & CFO
|
D&B, Pagoda Pharma, Schering-Plough,
Pharmacia, JP Morgan |
23
|
|
Krishna Kandarpa, M.D.,
Ph.D. |
CSO and EVP, R&D
|
Harvard, MIT(HST), Cornell, UMass
|
33
|
|
Agustin Gago
|
EVP, Global Sales
|
AngioDynamics, E-Z-EM
|
31
|
|
Jennifer Simpson, Ph.D.
|
EVP, Global Marketing
|
Eli Lilly (ImClone), Johnson & Johnson
(Ortho Biotech) |
23
|
|
Peter Graham, J.D.
|
EVP, General Counsel &
Global Human Resources
|
Bracco, E-Z-EM
|
18
|
|
David McDonald
|
EVP, Business Development
|
AngioDynamics, RBC Capital Markets
|
30
|
|
John Purpura
|
EVP, Regulatory Affairs & Quality
Assurance |
E-Z-EM, Sanofi-Aventis
|
29
|
|
Harold Mapes
|
EVP, Global Operations
|
AngioDynamics, Mallinckrodt
|
27
|
|
Gloria Lee, M.D., PH.D.
|
EVP, Clinical & Medical Affairs
|
Hoffmann-La Roche, Syndax
Pharmaceuticals, Inc. |
21
|
|
Bill Appling
|
SVP Medical Device R&D
|
AngioDynamics
|
27
|
|
Dan Johnston, Ph.D.
|
VP, Pharmaceutical R&D
|
Pfizer, Wyeth
|
12
|

26 DELCATH SYSTEMS, INC
2012 Accomplishments
• First patients treated with CHEMOSAT Melphalan in Europe in
January
January
• Obtained CE Mark for Gen 2 CHEMOSAT Melphalan filter in
April
April
• Executed contract for MSL services in EU in 1Q 2012 (Quintiles
was selected to support EU launch of CHEMOSAT)
was selected to support EU launch of CHEMOSAT)
• Secured agreements with 14 leading cancer centers in EU
• 8 EU Clinical Sites Activated for commercial use
• US NDA submitted in August 2012
• US NDA accepted with PDUFA date of June 15, 2013
• Obtained CE Mark for CHEMOSAT Doxorubicin in October
• Interim reimbursement established in Italy in December
Considerable Achievements Built the Foundation For Commercial Success

27 DELCATH SYSTEMS, INC
2013 Anticipated Milestones
ü First patient enrolled in EAP – Q1 2013
ü Obtained NUB Value 4 interim reimbursement in Germany – Q12013
• Obtain interim reimbursement in UK – Q2 2013
• Submission for publications of Phase 3 data and mNET arm of Phase 2 data in
Q1 2013
Q1 2013
• Initiate EU Registry – Q1 2013
• ODAC Panel Meeting May 2, 2013
• Receive NDA approval for Melblez Kit by PDUFA date of June 15, 2013
• First commercial sale in APLA – Q2 2013
• Commence Company’s first investigator initiated trial (IIT) – Q2 2013
• First patient enrolled in Company sponsored trial (CST) to expand indications –
Q4 2013
Q4 2013
• US commercial launch of Melblez Kit – Q4 2013
• First patient enrolled in Taiwan HCC pivotal trial – Q4 2013
• Execute strategic partnership for China
A Busy Year Focused on US Approval, Clinical Data and Commercial Adoption

28 DELCATH SYSTEMS, INC
A Compelling Investment Opportunity
Concentrating the Power of Chemotherapy
• Commercial stage company focused on oncology
• Proprietary CHEMOSAT Hepatic Delivery System allows unique whole
organ therapy for the liver
organ therapy for the liver
• CHEMOSAT system has demonstrated extension of progression free
survival
survival
• Addressing large unmet market need for cancer patients who usually die
of liver failure
of liver failure
• Estimated initial market opportunity of ~$2.3 billion in U.S. & EU
• Expanding clinical data expected to broaden clinical use and indications
• On the cusp of realizing the potential:
o EU - early commercial launch underway; reimbursement in key EU markets expected in
Q1/Q2
Q1/Q2
o U.S. - NDA under review; ODAC May 2, PDUFA date June 15, 2013
• Attractive financial model, multiple capital resources available and
experienced management team to execute plan
experienced management team to execute plan

© 2011 DELCATH SYSTEMS, INC. ALL RIGHTS RESERVED

30 DELCATH SYSTEMS, INC
Appendices

31 DELCATH SYSTEMS, INC
LIVER CANCER TREATMENT
OPTIONS
OPTIONS
Appendix 1

32 DELCATH SYSTEMS, INC
Existing Liver Cancer Treatments Have Significant Limitations
The Problem
• Metastatic disease to the liver, brain or lungs is often the life-
limiting location of solid tumors
limiting location of solid tumors
o Often life-limiting or leads to withdrawal of systemic treatments in
favor of palliative care
favor of palliative care
• Effective treatment for patients with liver-limited or dominant
cancers remains a clinical challenge
cancers remains a clinical challenge
o Can be diffuse
o Often not responsive to chemotherapy and radiation therapy
• Whole organ therapy creates a new option for patients in the
management of liver dominant disease
management of liver dominant disease
33 DELCATH SYSTEMS, INC
Existing Liver Cancer Treatments Have Limitations
Unmet Medical Need Exists for More Effective Liver Cancer Treatments
|
Treatment
|
Advantages
|
Disadvantages
|
|
Systemic
|
– Non-invasive
– Repeatable
|
– Systemic toxicities
– Limited efficacy in liver
|
|
Regional
(e.g., Isolated Hepatic Perfusion)
|
– Therapeutic effect
– Targeted
|
– Invasive/limited repeatability
– Multiple treatments are
required but not possible |
|
Focal
(e.g. surgery, radioembolization,
chemoembolization, radio frequency ablation) |
– Partial removal or
treatment of tumors |
– Only 10% to 20% resectable
– Invasive and/or limited
repeatability – Treatment is limited by tumor
size, number of lesions and location – Tumor revascularization
– Cannot treat diffuse disease
|
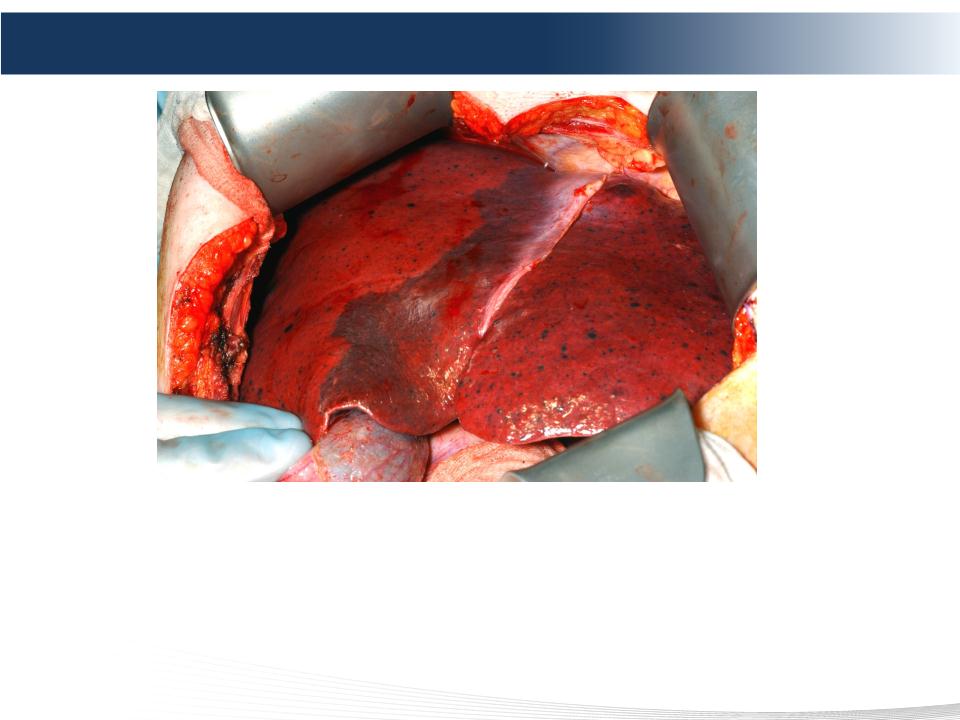
34 DELCATH SYSTEMS, INC
Diffuse Hepatic Metastases from Melanoma
• Diffuse disease in the liver is prevalent
• Effective treatment for patients with liver-limited or dominant cancers
remains a clinical challenge
remains a clinical challenge
• Whole organ therapy creates a new option for patients in the management
of liver dominant disease
of liver dominant disease

35 DELCATH SYSTEMS, INC
Concentrating the Power of Chemotherapy for Disease Control in the Liver
Our Solution – Whole Organ-Focus Disease Control
• Our proprietary CHEMOSAT System isolates the liver
circulation, delivers an ultra-high concentration of
chemotherapy (melphalan) to the liver and filters most of the
chemotherapy out of the blood prior to returning it to the patient
circulation, delivers an ultra-high concentration of
chemotherapy (melphalan) to the liver and filters most of the
chemotherapy out of the blood prior to returning it to the patient
• The procedure typically takes approximately two hours to
complete and involves a team including the interventional
radiologist and perfusionist
complete and involves a team including the interventional
radiologist and perfusionist
• CHEMOSAT (Gen 2) has demonstrated minimal systemic
toxicities and impact to blood components in initial commercial
use and may complement systemic therapy
toxicities and impact to blood components in initial commercial
use and may complement systemic therapy
• CHEMOSAT has been used on approximately 200 patients to
date through clinical development and early commercial launch
date through clinical development and early commercial launch

36 DELCATH SYSTEMS, INC
MARKET OPPORTUNITY BY DISEASE
& TARGET COUNTRIES
& TARGET COUNTRIES
Appendix 2
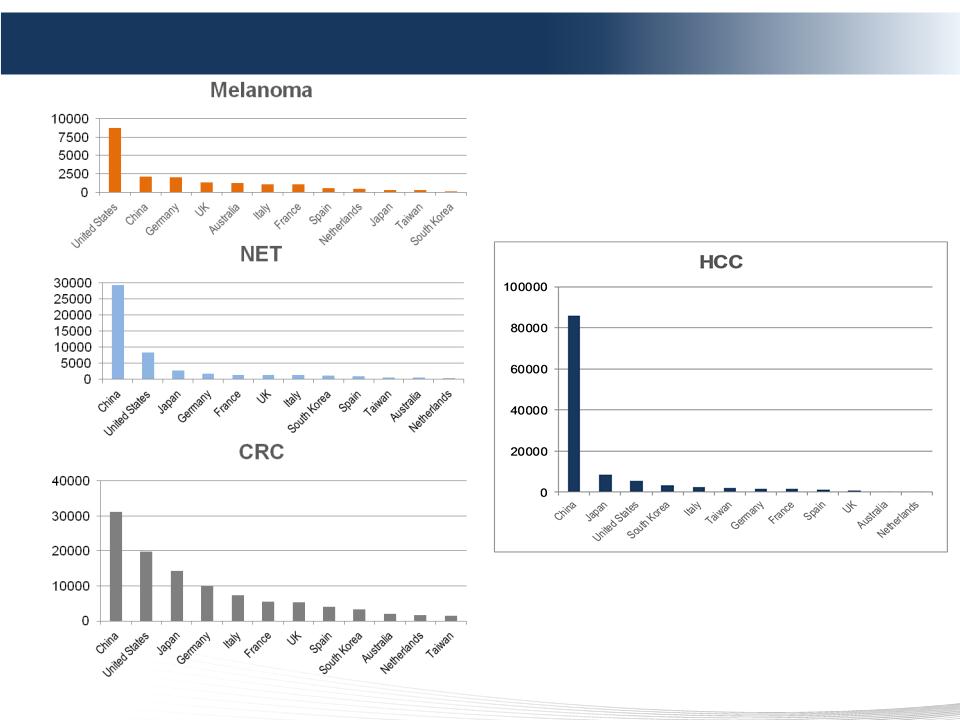
37 DELCATH SYSTEMS, INC
• Europe - Largest near-term opportunity
• CRC - Largest opportunity worldwide
• Melanoma - Largest opportunity is in the US
• China - Largest opportunity for HCC
Market Opportunity by Disease (patients)
Market Opportunity defined as Total Potential Market
(TPM) for Melblez Kit/CHEMOSAT
(TPM) for Melblez Kit/CHEMOSAT
1. Primary cancer incidence
2. Adjusted for predominant disease in the liver (primary or
metastatic cancer)
metastatic cancer)
3. Adjusted for addressable patients via Melblez Kit/CHEMOSAT

38 DELCATH SYSTEMS, INC
Europe Market by Disease – Device Only
|
|
Germany
(Direct)
|
UK
(Direct)
|
France
(Indirect)
|
Italy
(Indirect)
|
Spain
(Indirect)
|
Netherlands
(Direct)
|
Ireland
(Direct) |
Total
Potential (patients)
|
Potential
Market ($ MM)1,2,3
|
|
|
|||||||||
|
Total Potential Market #Patients
|
|||||||||
|
Ocular
Melanoma |
404
|
297
|
295
|
285
|
197
|
79
|
19
|
1,576
|
$ 62
|
|
Cutaneous
Melanoma |
1,625
|
994
|
753
|
801
|
360
|
379
|
73
|
4,987
|
$ 206
|
|
CRC
|
9,902
|
5,300
|
5,475
|
7,281
|
4,016
|
1,644
|
335
|
33,953
|
$1,339
|
|
HCC
(Primary) |
1,637
|
720
|
1,514
|
2,597
|
1,087
|
82
|
35
|
7,671
|
$277
|
|
NET
|
1,783
|
1,336
|
1,353
|
1,299
|
974
|
360
|
98
|
7,202
|
$ 281
|
|
TOTAL
|
15,351
|
8,647
|
9,389
|
12,263
|
6,634
|
2,545
|
560
|
55,389
|
$ 2,166
|
Europe Presents Significant Potential Market Opportunity
Sources: LEK Consulting, GLOBOCAN, Company estimates.
1) Assumes 2.5 treatments per patient.
2) Assumes ASP of ~$15K USD.
3) Assumes mix of direct sales and distributors.

39 DELCATH SYSTEMS, INC
US Market by Disease – Device and Drug Combination
|
Liver Metastasis
|
Potential Market
# Patients
|
Potential Market
# Procedures
|
Potential Market
($MM)1,2 |
|
Ocular
Melanoma
|
1,685
|
4,213
|
$ 105
|
|
Cutaneous
Melanoma
|
7,023
|
17,557
|
$ 439
|
|
CRC
|
19,861
|
49,653
|
$ 1,241
|
|
HCC (Primary)
|
5,586
|
13,964
|
$ 349
|
|
NET
|
8,212
|
20,530
|
$ 513
|
|
TOTAL
|
42,367
|
105,917
|
$ 2,648
|
Sources: LEK Consulting, GLOBOCAN, Company estimates.
1) Assume 2.5 treatments per patient.
2) Estimated ASP of $25K.

40 DELCATH SYSTEMS, INC
APAC Market by Disease
|
|
China
(Device)
|
S. Korea
(Device)
|
Japan
(Device)
|
Taiwan
(Device)
|
Australia
(Device)
|
Total
Potential (patients)
|
Potential
Market ($MM)1,2
|
|
|
|||||||
|
Total Potential Market #Patients
|
|||||||
|
HCC
(Primary) |
85,780
|
3,258
|
8,296
|
2,152
|
263
|
99,749
|
$ 1,156
|
|
Other
|
|||||||
|
CRC
|
31,127
|
3,245
|
14,298
|
1,441
|
2,031
|
52,143
|
$ 642
|
|
NET
|
29,197
|
1,048
|
2,759
|
500
|
462
|
33,966
|
$ 393
|
|
Ocular
Melanoma |
1,765
|
66
|
175
|
31
|
96
|
2,134
|
$ 25
|
|
Cutaneous
Melanoma |
382
|
43
|
136
|
246
|
1,144
|
1,951
|
$ 23
|
|
OTHER
TOTAL |
62,472
|
4,403
|
17,368
|
2,218
|
3,733
|
90,194
|
$ 1,083
|
|
TOTAL
|
148,104
|
7,661
|
25,665
|
4,370
|
3,996
|
189,943
|
$ 2,239
|
APAC Target Markets Represent Over $2 Billion Potential Market Opportunity
Sources: LEK Consulting, GLOBOCAN, Company estimates.
1) Assume 2.5 treatments per patient.
2) Estimated ASP of ~$5K.

41 DELCATH SYSTEMS, INC
HIGH-DOSE MELPHALAN
HISTORY AND RATIONALE
HISTORY AND RATIONALE
Appendix 3
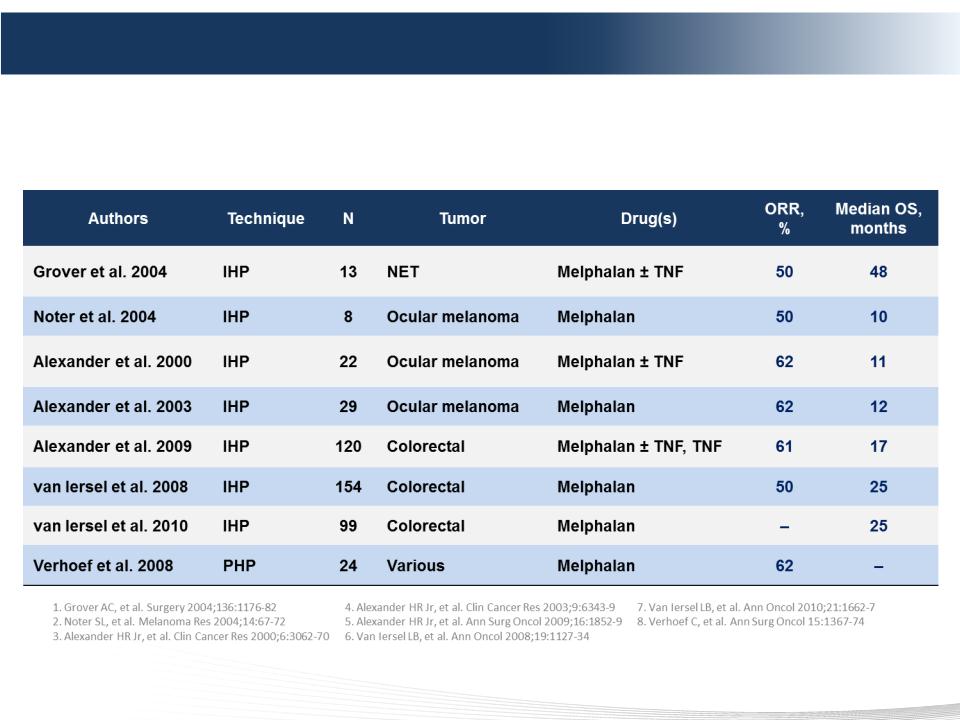
42 DELCATH SYSTEMS, INC
The Evidence for Melphalan
§ Melphalan, an established chemotherapy agent, is proven active at
high doses with broad antitumor activity
high doses with broad antitumor activity
43 DELCATH SYSTEMS, INC
Melphalan Dosing & Background
• Well understood, dose dependent, tumor preferential, alkylating cytotoxic agent
that demonstrates little to no hepatic toxicity
that demonstrates little to no hepatic toxicity
• Manageable systemic toxicities associated with Neutropenia and
Thrombocytopenia
Thrombocytopenia
• Drug dosing 12x higher than FDA-approved dose via systemic IV chemotherapy
• Dose delivered to tumor is over 100x higher than that of systemic IV
chemotherapy
chemotherapy
|
Type
|
Dosing (mg/kg)
|
|
Multiple Myeloma (label)
|
0.25
|
|
Chemoembolization
|
0.62
|
|
Surgical Isolated Hepatic Perfusion (IHP)
|
1.50
|
|
Myeloablation
|
2.50-3.50
|
|
Percutaneous Hepatic Perfusion (PHP)
|
3.00
|
An Established Drug for Liver Cancer Therapy
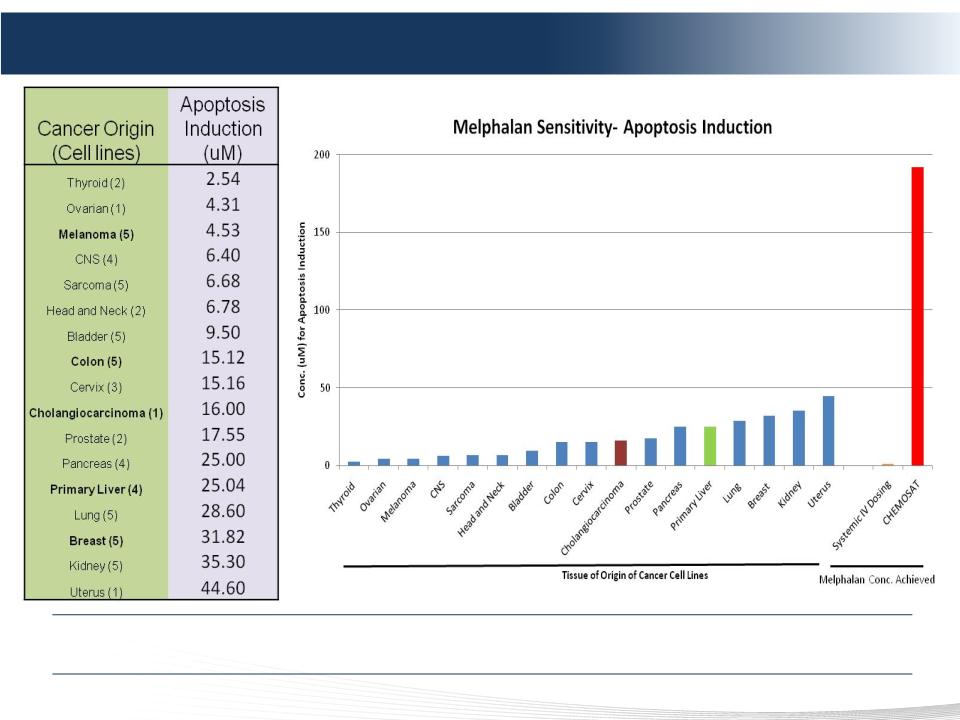
44 DELCATH SYSTEMS, INC
Melphalan Sensitivity: In Vitro Tumor Cell Lines Study
We Believe Our Technology Will Be Effective On a Wide Range of Solid Tumors
192 uM

45 DELCATH SYSTEMS, INC
PHASE 3 TRIAL
Appendix 4
46 DELCATH SYSTEMS, INC
Phase III Clinical Trial Design
Randomized to PHP
93 patients: ocular
or cutaneous melanoma
PHP/Melphalan
Treat every 4 weeks x 4 rounds
(responders can receive up to 6 rounds)
Cross-over
Primary Trial Endpoint
• Statistically significant difference in Hepatic Progression
Free Survival (“hPFS”): p < 0.05 (IRC)
Free Survival (“hPFS”): p < 0.05 (IRC)
• Over 80% of Oncologic drugs approved by FDA between
2005 - 2007 on endpoints other than overall survival
2005 - 2007 on endpoints other than overall survival
Modeled hPFS for Trial Success:
7.73 months (CS)
vs.
4 months (BAC)
Secondary Trial Endpoints
• Investigator hPFS
• Hepatic objective response rate
• Overall objective response rate
• Overall Survival - Diluted by Cross Over
• SAP calls for analysis of various patient subsets
Pre-PHP (Baseline)
Post-PHP (22+ Months)
Hepatic Response - Metastatic Melanoma
Fully Powered, 93 Patient, Randomized, Multi-Center NCI Led Study
PHP = Meblez Kit/CHEMOSAT
Best Alternative Care (BAC)
Investigator and patient decision
(any and all treatments)

47 DELCATH SYSTEMS, INC
Positive Phase 3 Results
• Primary endpoint (hPFS by IRC) exceeded, p value = 0.0029, hazard ratio of 0.50
as of June, 2012
as of June, 2012
o PHP median hepatic progression free survival (hPFS) was 4-fold of control, or 5.3 months improvement
o PHP achieved a median hPFS of 7.0 months vs 1.7 months for BAC control
o 75% overall clinical benefit (CR + PR + SD)
• Secondary endpoints consistent with primary endpoints
o CS/PHP achieved a median overall PFS of 5.4 months vs. 1.6 months for BAC
o OS – No difference demonstrated due to heavy crossover from BAC to PHP
o Median OS 10.6 months vs. 10.0 months for PHP and BAC respectively
• OS exploratory analyses supportive of key observations
o Median overall survival of 11.4 months for all patients treated with melphalan, including crossover
o BAC patients did not cross-over to PHP had a median survival of 4.1 months
o 6 PHP-treated and 2 BAC-only patients still alive as of 2/2013
• Gen 1 Safety profile – consistent with currently approved labeling for melphalan
o 30-day deaths on PHP: 3/44 patients (6.8%)
§ 1 Neutropenic Sepsis (2.3%); 1 Hepatic Failure 2.5% (95% tumor burden); 1 gastric perforation
o 30-day deaths on BAC: 3/49 patients (6.1%)
Trial Outcomes Favorable and Consistent with Special Protocol Assessment

48 DELCATH SYSTEMS, INC
PUBLISHED PHASE 1 / 2 STUDIES OF
DOXORUBICIN WITH PHP
DOXORUBICIN WITH PHP
Appendix 5
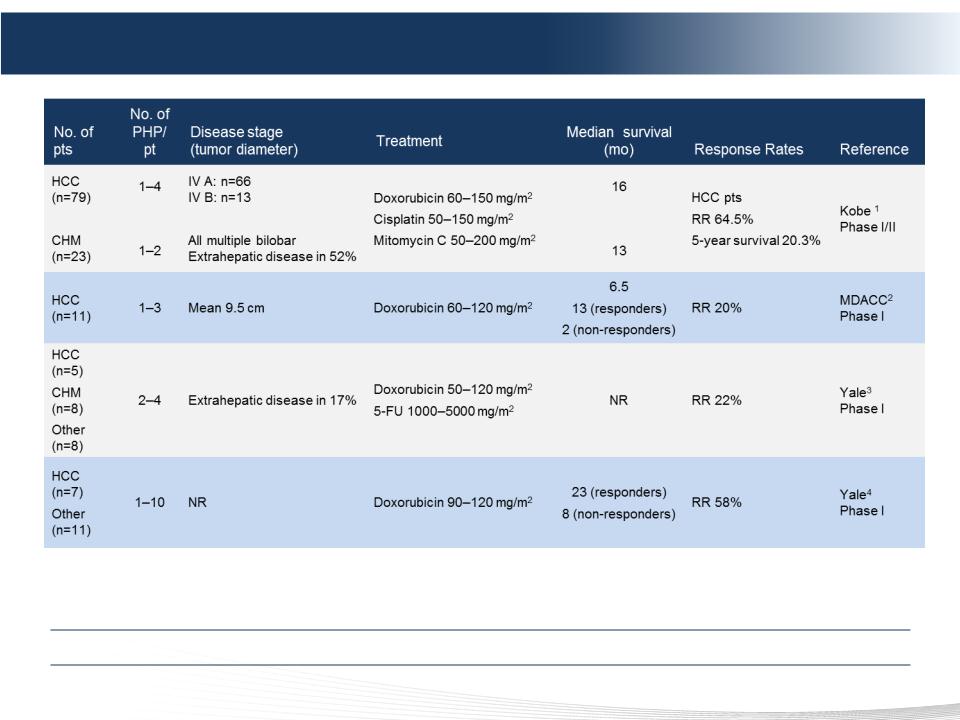
49 DELCATH SYSTEMS, INC
Phase 1 & 2 Studies of PHP-Doxorubicin For HCC
Delivered Safely in Multiple Studies with Promising Response Rates
1) Ku Y et al. Chir Gastroenterol 2003;19:370-376.
2) Curley SA et al. Ann Surg Oncol 1994;1:389-99.
3) Ravikumar TS et al. J Clin Oncol 1994;12:2723-36.
4) Hwu WJ et al. Oncol Res 1999;11:529-37.

50 DELCATH SYSTEMS, INC
PRODUCT DEVELOPMENT PIPELINE
Appendix 6
51 DELCATH SYSTEMS, INC
Product Development Pipeline
• Orphan Drug - Ocular
Melanoma liver mets
Melanoma liver mets
• Proprietary drug-melphalan &
Melblez Kit
Melblez Kit
• All liver cancers - melphalan
• Classified as Medical Device
• 3rd party melphalan
• Gen 2 melphalan CE Mark
• Doxorubicin system CE Mark
• CHEMOSAT for additional drugs
• CHEMOSAT for other organs (lung
and brain)
and brain)
• mNET, mCRC and HCC
indications
indications
Initial Opportunity
Near Term (< 5 years)
Intermediate Term (> 5 years)
• mCRC and HCC clinical trials
• Proprietary drug/delivery system for
additional drugs
additional drugs
• Proprietary drug/delivery system for
other organs (lung and brain)
other organs (lung and brain)
• CHEMOSAT Melphalan in
Taiwan and Japan
Taiwan and Japan
• CHEMOSAT Doxorubicin in
China and South Korea
China and South Korea
• 3rd party doxorubicin
• CHEMOSAT for additional drugs
• CHEMOSAT for other organs (lung
and brain)
and brain)
• CHEMOSAT Melphalan in
Australia, New Zealand, and
Hong Kong
Australia, New Zealand, and
Hong Kong
• 3rd party melphalan
Development Aligned to Address Significant Market Opportunity

52 DELCATH SYSTEMS, INC
CHEMOSAT Delivery System for Doxorubicin – CE Mark
• Satisfied all of the requirements to affix the CE Mark to Hepatic
CHEMOSAT Delivery System device for intra-hepatic arterial delivery
and extracorporeal filtration of doxorubicin in October, 2012
CHEMOSAT Delivery System device for intra-hepatic arterial delivery
and extracorporeal filtration of doxorubicin in October, 2012
o Provides a pathway for regulatory approval in China and S. Korea
• Provides basis for partnership opportunities in China and S. Korea
where doxorubicin has a broad label for multiple tumor types
where doxorubicin has a broad label for multiple tumor types
• Multiple published Phase I/II studies from MD Anderson Cancer Center
and Yale with percutaneous hepatic perfusion (PHP) and Kobe
University using doxorubicin show promising response rates for HCC*
and Yale with percutaneous hepatic perfusion (PHP) and Kobe
University using doxorubicin show promising response rates for HCC*
• Plan to use CHEMOSAT Delivery System for Doxorubicin in Asia Phase
III 2L HCC trials
III 2L HCC trials
Addressing the Large HCC Market Opportunity in China

53 DELCATH SYSTEMS, INC
NON US/EU
REGULATORY UPDATE
REGULATORY UPDATE
Appendix 7

54 DELCATH SYSTEMS, INC
International Strategy beyond EU and US
• Leverage CE Mark to obtain reciprocal regulatory approvals for CHEMOSAT
Systems in other international markets
Systems in other international markets
o Obtained approval for Gen 2 CHEMOSAT Delivery System for Melphalan in Australia
• International regulatory submissions status:
Ø Application submitted and expected approvals in
§ Hong Kong - 2013
§ Singapore - 2013
§ Argentina - 2013
§ Brazil - 2014
Ø Intend to submit applications
§ S. Korea (CHEMOSAT Doxorubicin)
§ Mexico
§ China (CHEMOSAT Doxorubicin)
§ Taiwan
§ Russia
§ India
§ Japan
§ Israel
• Utilize 3rd party melphalan and doxorubicin available to physicians
Combination of Strategic Partnerships and Specialty Distributors

55 DELCATH SYSTEMS, INC
CHEMOSAT CENTERS
Appendix 8

56 DELCATH SYSTEMS, INC
CHEMOSAT Centers in Europe
• Entered training and marketing agreements with leading cancer centers in Europe
o Milan, Italy - European Institute of Oncology (IEO)
o Frankfurt, Germany - Johann Wolfgang Goethe-Universität (JWG)
o Kiel, Germany - Universitätsklinikum Schleswig-Holstein
o Villejuif, France - Cancer Institute Gustave Roussy (IGR)
o Barcelona, Spain - El Hospital Quiron
o Naples, Italy - Instituto Nazionale Tumori Fondazione "G. Pascale"
o Amsterdam, The Netherlands - Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital
o Erlangen, Germany - University Hospital of Erlangen
o Pamplona, Spain - Clinica Universidad de Navarra
o Bordeaux, France - Hôpital Saint-André (St Andre)
o Galway, Ireland - University Hospital Galway (UHG)
o Leiden, The Netherlands - Leiden University Medical Center
o Southampton, United Kingdom - Southampton University Hospital (SUH)
o Göttingen, Germany - University Medical Center Göttingen (UMG)
o Varese, Italy - Varese University Hospital (VUH)
o Heidelberg, Germany - National Center Tumor Diseases
• Training completed and patients treated at IEO, JWG, IGR, St Andre, UHG, SUH, UMG, VUH
• Liver metastases from cutaneous melanoma, ocular melanoma, gastric cancer, breast cancer,
neuroendocrine tumor (NET), hepatocellular carcinoma (HCC) and Cholangiocarcinoma
