Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Celldex Therapeutics, Inc. | a13-6660_18k.htm |
| EX-99.1 - EX-99.1 - Celldex Therapeutics, Inc. | a13-6660_1ex99d1.htm |
Exhibit 99.2
|
|
2012 Results and 2013 Strategic Outlook Conference Call March 7, 2013 |
|
|
Forward Looking Statement This communication contains "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical fact are statements that could be forward-looking statements. You can identify these forward-looking statements through our use of words such as “may,” “will,” “can,” “anticipate,” “assume,” “should,” “indicate,” “would,” “believe,” “contemplate,” “expect,” “seek,” “estimate,” “continue,” “plan,” “point to,” “project,” “predict,” “could,” “intend,” “target,” “potential” and other similar words and expressions of the future. These forward-looking statements are subject to risks and uncertainties that may cause actual future experience and results to differ materially from those discussed in these forward-looking statements. Important factors that might cause such a difference include, but are not limited to, the timing, cost and uncertainty of obtaining regulatory approvals for product candidates; our ability to develop and commercialize products before competitors that are superior to the alternatives developed by such competitors; the validity of our patents and our ability to avoid intellectual property litigation, which can be costly and divert management time and attention; and the other factors listed under “Risk Factors” in our filings with the SEC, including Forms 10-K, 10-Q and 8-K. Celldex does not undertake any obligation to release publicly any revisions to such forward-looking statement to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. |
|
|
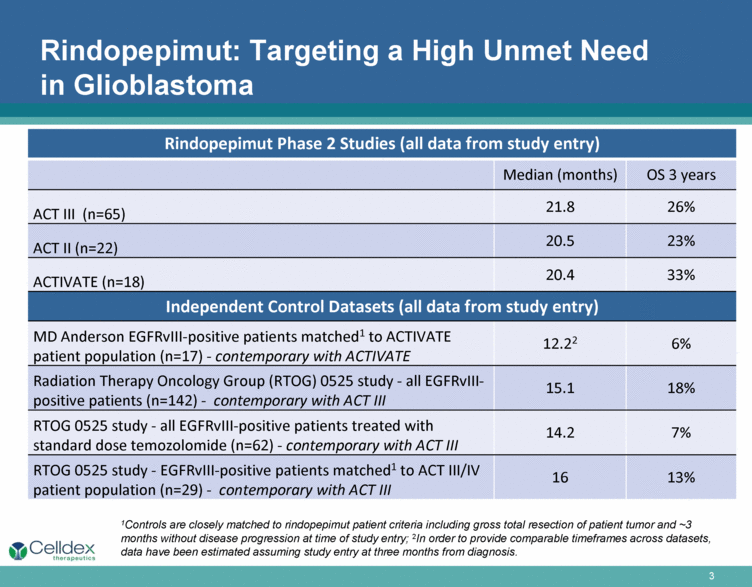
Rindopepimut: Targeting a High Unmet Need in Glioblastoma Rindopepimut Phase 2 Studies (all data from study entry) Median (months) OS 3 years ACT III (n=65) 21.8 26% ACT II (n=22) 20.5 23% ACTIVATE (n=18) 20.4 33% Independent Control Datasets (all data from study entry) MD Anderson EGFRvIII-positive patients matched1 to ACTIVATE patient population (n=17) - contemporary with ACTIVATE 12.22 6% Radiation Therapy Oncology Group (RTOG) 0525 study - all EGFRvIII-positive patients (n=142) - contemporary with ACT III 15.1 18% RTOG 0525 study - all EGFRvIII-positive patients treated with standard dose temozolomide (n=62) - contemporary with ACT III 14.2 7% RTOG 0525 study - EGFRvIII-positive patients matched1 to ACT III/IV patient population (n=29) - contemporary with ACT III 16 13% 1Controls are closely matched to rindopepimut patient criteria including gross total resection of patient tumor and ~3 months without disease progression at time of study entry; 2In order to provide comparable timeframes across datasets, data have been estimated assuming study entry at three months from diagnosis. |
|
|
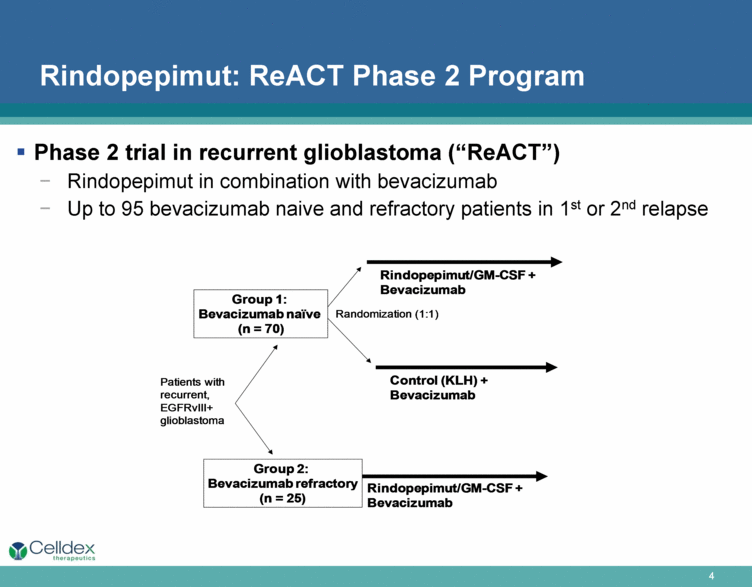
Rindopepimut: ReACT Phase 2 Program Phase 2 trial in recurrent glioblastoma (“ReACT”) Rindopepimut in combination with bevacizumab Up to 95 bevacizumab naive and refractory patients in 1st or 2nd relapse Control (KLH) + Bevacizumab Rindo pepimut /GM - CSF + Bevacizumab Randomization (1:1) Patients with recurrent, EGFRvIII + glioblastoma Rindopepimut /GM - CSF + Bevacizumab Group 1: Bevacizumab naïve (n = 70) Group 2: Bevacizumab refractory (n = 25) |
|
|
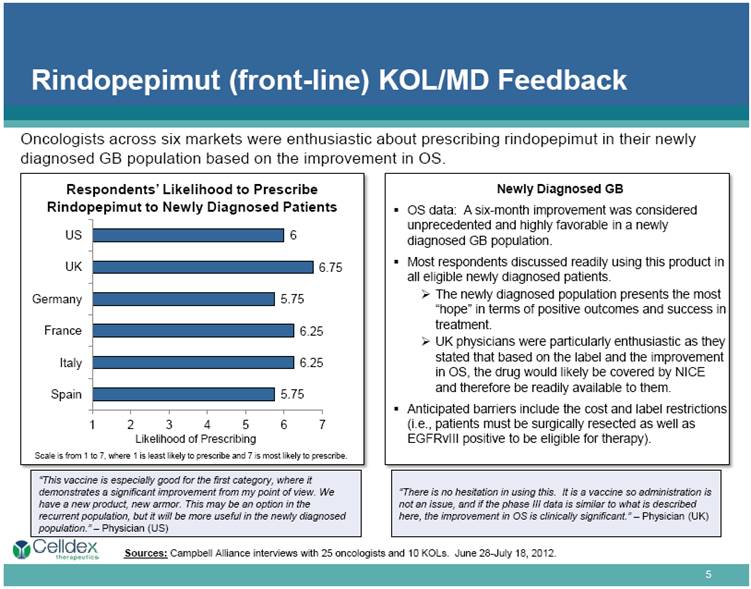
Rindopepimut (front-line) KOL/MD Feedback Respondents’ Likelihood to Prescribe Rindopepimut to Newly Diagnosed Patients Newly Diagnosed GB OS data: A six-month improvement was considered unprecedented and highly favorable in a newly diagnosed GB population. Most respondents discussed readily using this product in all eligible newly diagnosed patients. The newly diagnosed population presents the most “hope” in terms of positive outcomes and success in treatment. UK physicians were particularly enthusiastic as they stated that based on the label and the improvement in OS, the drug would likely be covered by NICE and therefore be readily available to them. Anticipated barriers include the cost and label restrictions (i.e., patients must be surgically resected as well as EGFRvIII positive to be eligible for therapy). “This vaccine is especially good for the first category, where it demonstrates a significant improvement from my point of view. We have a new product, new armor. This may be an option in the recurrent population, but it will be more useful in the newly diagnosed population.” – Physician (US) “There is no hesitation in using this. It is a vaccine so administration is not an issue, and if the phase III data is similar to what is described here, the improvement in OS is clinically significant.” – Physician (UK) Sources: Campbell Alliance interviews with 25 oncologists and 10 KOLs. June 28-July 18, 2012. Oncologists across six markets were enthusiastic about prescribing rindopepimut in their newly diagnosed GB population based on the improvement in OS. Scale is from 1 to 7, where 1 is least likely to prescribe and 7 is most likely to prescribe. Likelihood of Prescribing |
|
|
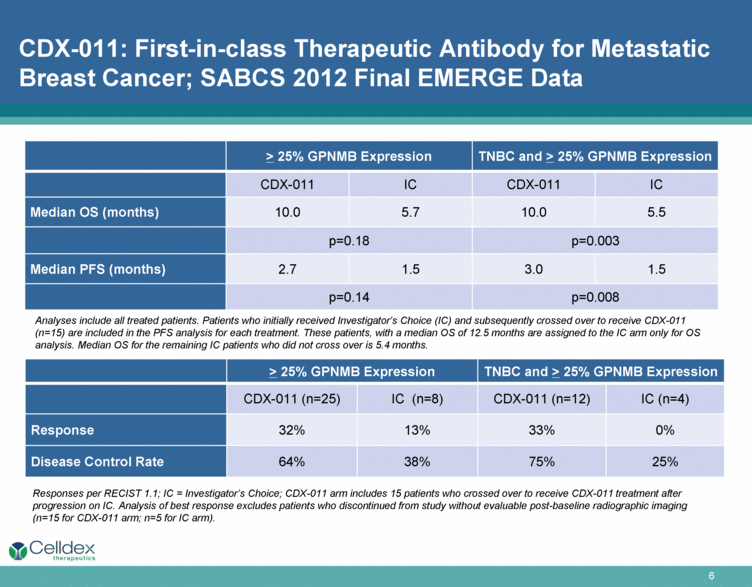
CDX-011: First-in-class Therapeutic Antibody for Metastatic Breast Cancer; SABCS 2012 Final EMERGE Data > 25% GPNMB Expression TNBC and > 25% GPNMB Expression CDX-011 IC CDX-011 IC Median OS (months) 10.0 5.7 10.0 5.5 p=0.18 p=0.003 Median PFS (months) 2.7 1.5 3.0 1.5 p=0.14 p=0.008 Analyses include all treated patients. Patients who initially received Investigator’s Choice (IC) and subsequently crossed over to receive CDX-011 (n=15) are included in the PFS analysis for each treatment. These patients, with a median OS of 12.5 months are assigned to the IC arm only for OS analysis. Median OS for the remaining IC patients who did not cross over is 5.4 months. Responses per RECIST 1.1; IC = Investigator’s Choice; CDX-011 arm includes 15 patients who crossed over to receive CDX-011 treatment after progression on IC. Analysis of best response excludes patients who discontinued from study without evaluable post-baseline radiographic imaging (n=15 for CDX-011 arm; n=5 for IC arm). > 25% GPNMB Expression TNBC and > 25% GPNMB Expression CDX-011 (n=25) IC (n=8) CDX-011 (n=12) IC (n=4) Response 32% 13% 33% 0% Disease Control Rate 64% 38% 75% 25% |
|
|
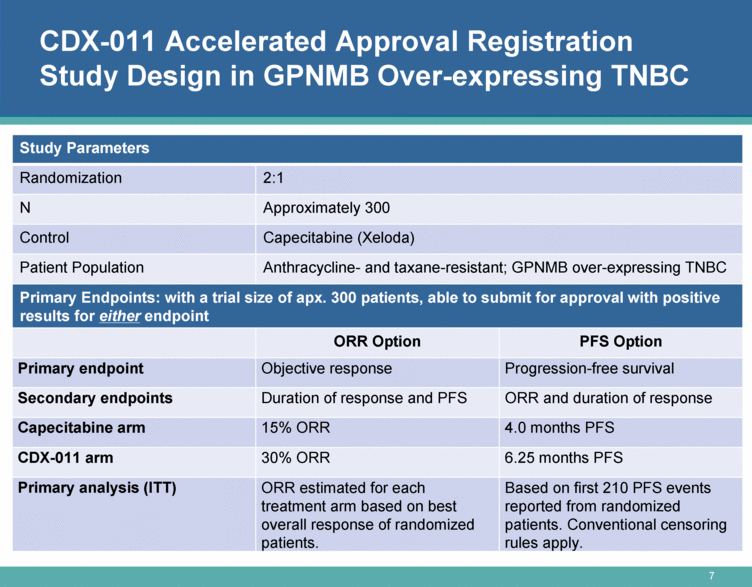
CDX-011 Accelerated Approval Registration Study Design in GPNMB Over-expressing TNBC Study Parameters Randomization 2:1 N Approximately 300 Control Capecitabine (Xeloda) Patient Population Anthracycline- and taxane-resistant; GPNMB over-expressing TNBC Primary Endpoints: with a trial size of apx. 300 patients, able to submit for approval with positive results for either endpoint ORR Option PFS Option Primary endpoint Objective response Progression-free survival Secondary endpoints Duration of response and PFS ORR and duration of response Capecitabine arm 15% ORR 4.0 months PFS CDX-011 arm 30% ORR 6.25 months PFS Primary analysis (ITT) ORR estimated for each treatment arm based on best overall response of randomized patients. Based on first 210 PFS events reported from randomized patients. Conventional censoring rules apply. |
|
|
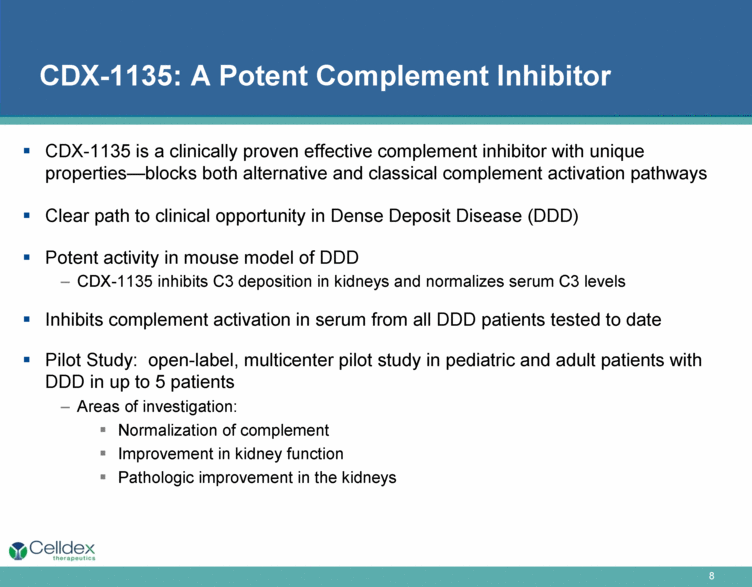
CDX-1135: A Potent Complement Inhibitor CDX-1135 is a clinically proven effective complement inhibitor with unique properties—blocks both alternative and classical complement activation pathways Clear path to clinical opportunity in Dense Deposit Disease (DDD) Potent activity in mouse model of DDD CDX-1135 inhibits C3 deposition in kidneys and normalizes serum C3 levels Inhibits complement activation in serum from all DDD patients tested to date Pilot Study: open-label, multicenter pilot study in pediatric and adult patients with DDD in up to 5 patients Areas of investigation: Normalization of complement Improvement in kidney function Pathologic improvement in the kidneys |
|
|
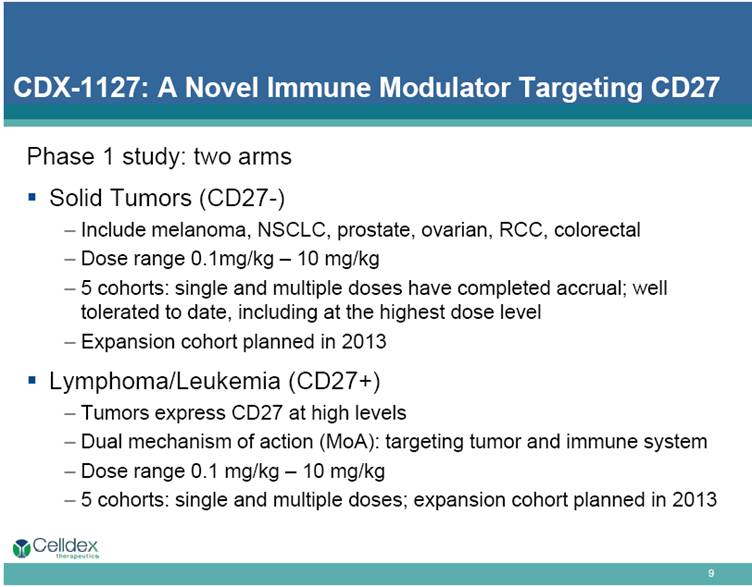
Phase 1 study: two arms Solid Tumors (CD27-) Include melanoma, NSCLC, prostate, ovarian, RCC, colorectal Dose range 0.1mg/kg – 10 mg/kg 5 cohorts: single and multiple doses have completed accrual; well tolerated to date, including at the highest dose level Expansion cohort planned in 2013 Lymphoma/Leukemia (CD27+) Tumors express CD27 at high levels Dual mechanism of action (MoA): targeting tumor and immune system Dose range 0.1 mg/kg – 10 mg/kg 5 cohorts: single and multiple doses; expansion cohort planned in 2013 CDX-1127: A Novel Immune Modulator Targeting CD27 |
|
|
CDX-301 and CDX-1401 CDX-301 FMS-like tyrosine kinase 3 Ligand (Flt3L) Completed 30 patient healthy volunteer Phase 1 study Potent inducer of stem cells and dendritic cells at various dose regimens Well tolerated and non-immunogenic Next study – Hematopoietic stem cell transplant CDX-1401 Antibody-based immunotherapy for NY-ESO-1 expressing cancers Developed from APC Targeting Technology Completed 45 patient Phase 1 study Well tolerated; robust anti-NY-ESO-1 immunity 13 patients with stable disease, 2 with significant tumor shrinkage Next study – NCI sponsored melanoma trial |
|
|
Anticipated Milestones for 2013 Rindopepimut Targeted accrual of ACT IV registration study by YE 2013 ReACT data: both Avastin naïve and refractory groups in 2H 2013 CDX-011 - Initiate accelerated approval study in metastatic breast cancer in 2H 2013 CDX-1135 - Initiate pilot study in DDD; data by YE 2013 CDX-1127 - Expansion cohorts planned; data in 2H 2013 CDX-301 - Initiate pilot study in transplant setting by YE 2013 CDX-1401 - Collaboration with NCI on Phase 2 combination study with CDX-301 |
|
|
For additional information, please visit: www.celldextherapeutics.com |