Attached files
| file | filename |
|---|---|
| 8-K - SUCAMPO PHARMACEUTICALS, INC. 8-K - Sucampo Pharmaceuticals, Inc. | a50572340.htm |
Exhibit 99.1

Commercial Update: RESCULA® Launch February 21, 2013 Stan Miele SVP, Sales and Marketing & President, Sucampo Pharma Americas

Forward-Looking Statements This presentation contains "forward-looking statements" as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements are based on management's current expectations and involve risks and uncertainties, which may cause results to differ materially from those set forth in the statements. The forward-looking statements may include statements regarding product development, product potential, future financial and operating results, and other statements that are not historical facts. The following factors, among others, could cause actual results to differ from those set forth in the forward-looking statements: the impact of pharmaceutical industry regulation and health care legislation; Sucampo's ability to accurately predict future market conditions; dependence on the effectiveness of Sucampo's patents and other protections for innovative products; the risk of new and changing regulation and health policies in the US and internationally and the exposure to litigation regulatory actions and/or actions. No forward-looking statement can be guaranteed and actual results may differ materially from those projected. Sucampo undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise. Forward-looking statements in this presentation should be evaluated together with the many uncertainties that affect Sucampo's business, particularly those mentioned in the risk factors and cautionary statements in Sucampo's most recent Form 8-K and 10-K, which the Company incorporates by reference. 2
Sucampo Has Pioneered the Field of Prostones Prostones are functional fatty acids naturally occuring in the human body Excellent clinical safety profile for AMITIZA® and RESCULA® Broad potential in various therapeutic fields With the approval of the sNDA for RESCULA, we now have two FDA approved prostone-based products marketed in the United States Sucampo is the only company developing and commercializing prostone compounds globally See Reference 1 3

History of RESCULA (Unoprostone Isopropyl Ophthalmic Solution) In 1994, RESCULA (unoprostone isopropyl 0.12%) approved in Japan In 2009, Sucampo licensed RESCULA from R-Tech Ueno, Ltd. (RTU) In 2012, Mechanism of Action in US package insert for RESCULA updated to reflect current scientific understanding Post Marketing Experience: 6.4M People Worldwide* *(Japan and some US) See Reference 1 4

RESCULA Overview Primary Open Angle Glaucoma / Ocular Hypertension market has unmet needs RESCULA offers an alternate route to IOP reduction – the strength of RESCULA is its safety and tolerability profile RESCULA will have a competitive share of voice See References 1-2 5
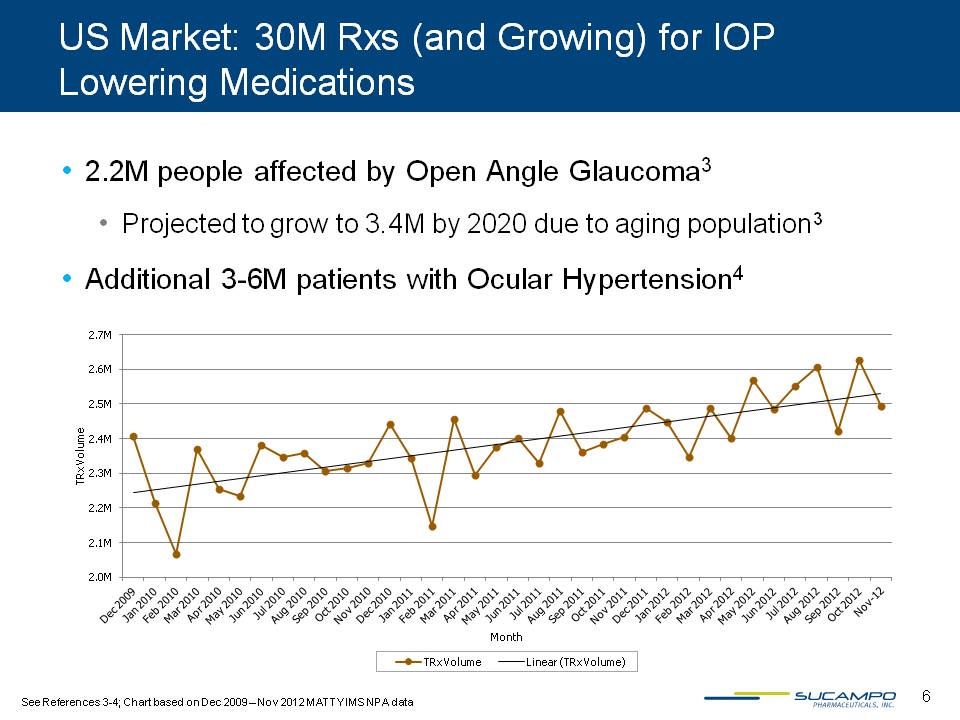
US Market: 30M Rxs (and Growing) for IOP Lowering Medications 2.2M people affected by Open Angle Glaucoma3 Projected to grow to 3.4M by 2020 due to aging population3 Additional 3-6M patients with Ocular Hypertension4 See References 3-4; Chart based on Dec 2009 – Nov 2012 MATTY IMS NPA data 6
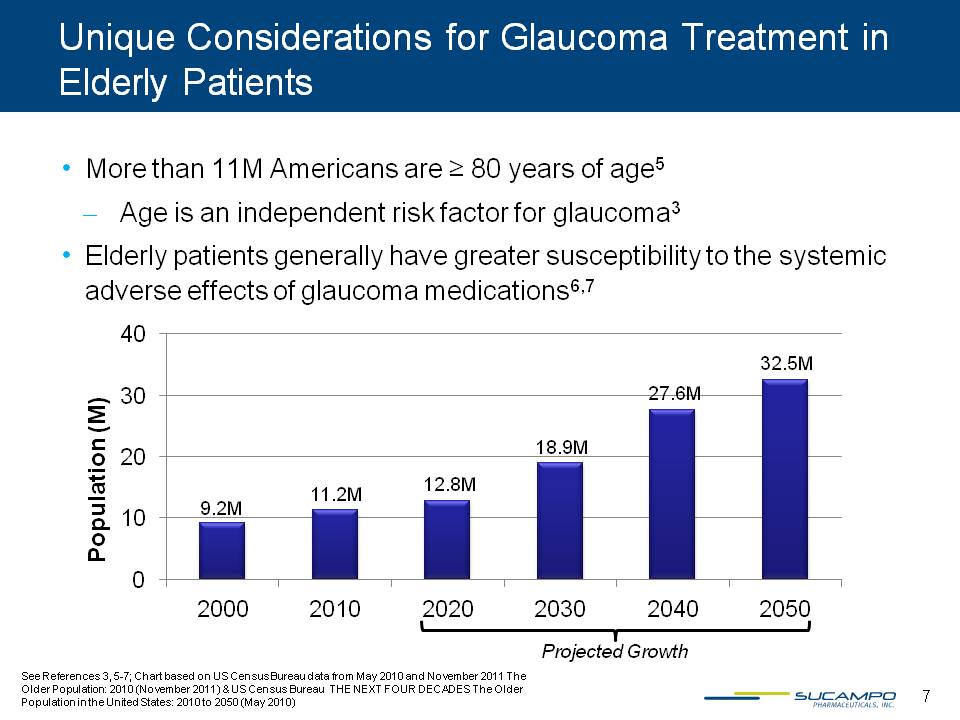
Unique Considerations for Glaucoma Treatment in Elderly Patients More than 11M Americans are ≥ 80 years of age5 Age is an independent risk factor for glaucoma3 Elderly patients generally have greater susceptibility to the systemic adverse effects of glaucoma medications6,7 See References 3, 5-7; Chart based on US Census Bureau data from May 2010 and November 2011 The Older Population: 2010 (November 2011) & US Census Bureau THE NEXT FOUR DECADES The Older Population in the United States: 2010 to 2050 (May 2010) 7
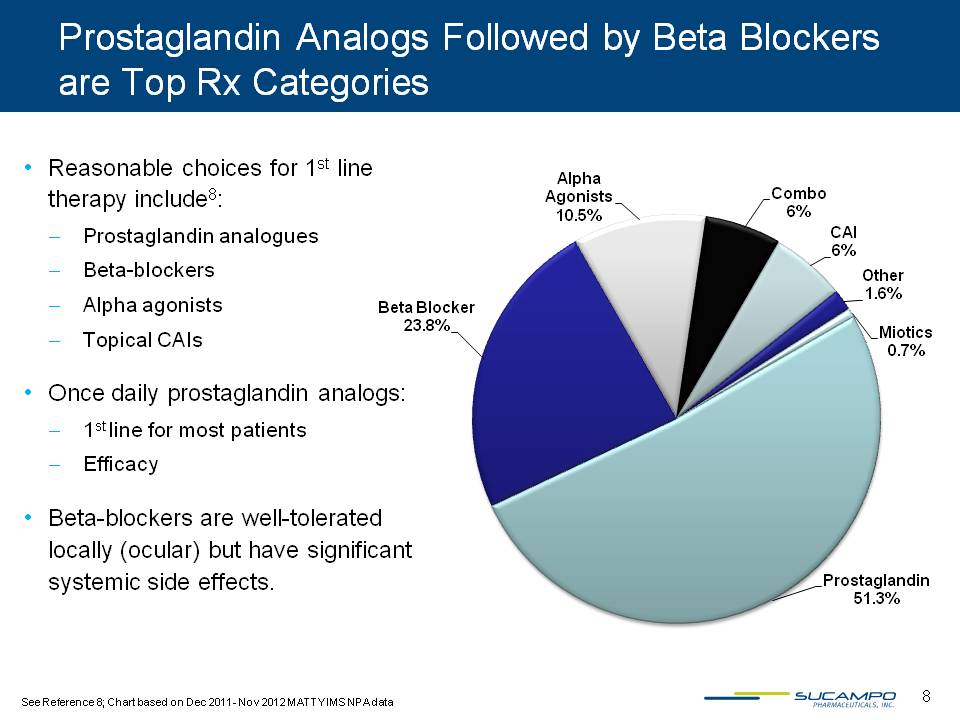
Prostaglandin Analogs Followed by Beta Blockers are Top Rx Categories Reasonable choices for 1st line therapy include8: Prostaglandin analogues Beta-blockers Alpha agonists Topical CAIs Once daily prostaglandin analogs: 1st line for most patients Efficacy Beta blockers are well-tolerated locally (ocular) but have significant systemic side effects. Prostaglandin 51.3% Beta Blocker 23.8% Alpha Agonists 10.5% Combo 6% CAI 6% Other 1.6% Miotics 0.7% See Reference 8; Chart based on Dec 2011- Nov 2012 MATTY IMS NPA data 8
RESCULA Provides an Alternate Route to IOP Reduction RESCULA Believed to reduce elevated IOP by increasing the outflow of aqueous humor through the trabecular meshwork via BK channel activation A prostone, not a prostaglandin analog Should be considered when systemic / ocular side effects are a concern: Effective at lowering IOP throughout the day and over the long-term Established ocular side effect profile: RESCULA and timolol both generally well tolerated in clinical studies with similar incidence of hyperemia Excellent systemic safety profile with no deleterious effects on CV or pulmonary function in clinical studies No labeled drug-drug interactions See Reference 2 9
Guidelines Recommend Balance Efficacy In pivotal trials at 6 months, RESCULA reduced mean IOP by ~3 to 4 mm Hg throughout the day (for 12 hours) with a flat diurnal curve (mean baseline IOP: 23 mm Hg) Reductions in IOP observed after 2 weeks and maintained long-term Safety In clinical trials, RESCULA and timolol were both generally well tolerated regarding ocular adverse events Injection (hyperemia) incidence similar to timolol maleate Excellent systemic side effect profile Extensive post marketing experience See Reference 2 10

For the reduction of IOP in patients with POAG or OHTN When it’s important to consider ocular and systemic side effects… Try Rescula See Reference 9 11
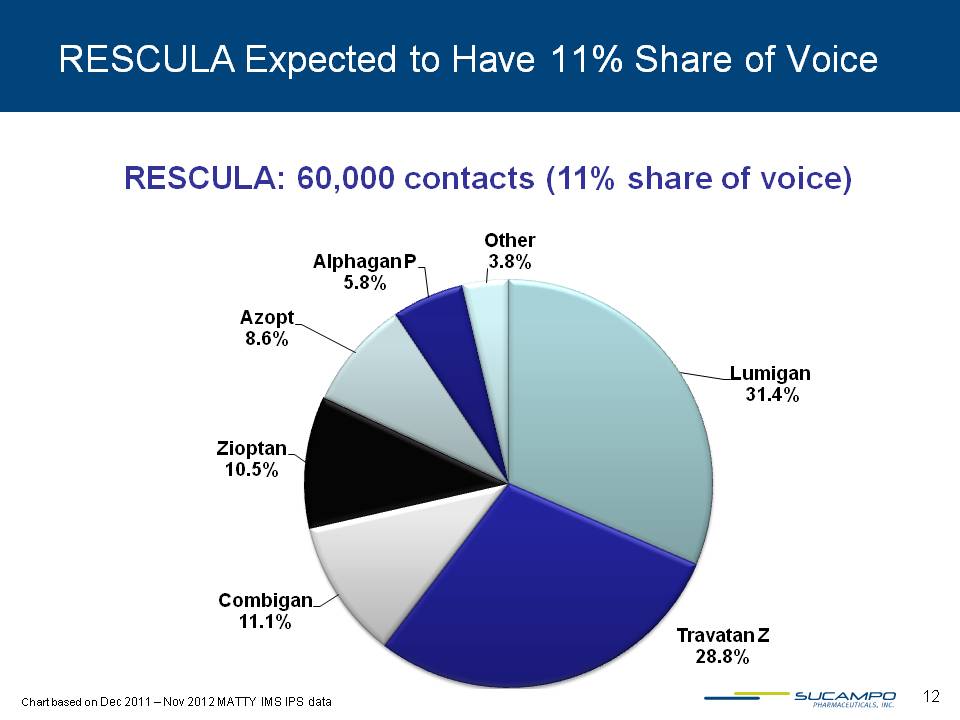
RESCULA Expected to Have 11% Share of Voice RESCULA: 60,000 contacts (11% share of voice) Lumigan 31.4% Travatan Z 28.8% Combigan 11.1% Zioptan 10.5% Azopt 8.6% Alphagan P 5.8% Other 3.8% Chart based on Dec 2011 – Nov 2012 MATTY IMS IPS data 12

Early Response to RESCULA Launch is Positive Focused on ophthalmologists and optometrists More than 2,500 calls have already been made Over 30% of leading eye specialists requested samples of RESCULA within two weeks of availability; 20% response rate to direct mail in 1 week Samples are now in doctor’s offices and RESCULA is now in pharmacies (WAC $99) 13
Managed Care Status Aggressively pursuing coverage for RESCULA Status: 41 face to face meetings with plans and PBMs Strong reception from plans No uncovered patients at launch 14

References 1. Sucampo data on file 2. RESCULA PI 3. American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern® guideline: Primary open-angle glaucoma. 2010 4. Kass MA et al. Arch Ophthalmol. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. 2002 Jun;120(6):701-13; discussion 829-30. 5. American Academy of Ophthalmology Glaucoma Basic and Clinical Science Course 2012-2013 6. US Census Bureau The Older Population: 2010 (November 2011) 7. Kaiserman I et al. Topical beta blockers in asthmatic patients-is it safe? Curr Eye Res. 2009 Jul;34(7):517-22. 8. Gottfredsdottir MS et al. Physicians' guide to interactions between glaucoma and systemic medications. J Glaucoma. 1997 Dec;6(6):377-83. 9. RESCULA CVA 15

Commercial Update: RESCULA Launch February 21, 2013 Stan Miele President, Sucampo Pharma Americas and SVP, Sales and Marketing
