Attached files
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from __________ to __________.
Commission File Number: 001-33609
SUCAMPO PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 30-0520478 |
| (State or other jurisdiction of | (I.R.S. Employer |
| incorporation or organization) | Identification No.) |
| 805 King Farm Boulevard, Ste 550 | 20850 |
| Rockville, MD | (Zip Code) |
| (Address of principal executive offices, | |
| including zip code) | |
| (301) 961-3400 | |
| (Registrant’s telephone number) | |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Class A common stock, par value $0.01 | The NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ☐
Indicate by checkmark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ☐
Indicate by a check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer þ | Non-accelerated filer ☐ | Smaller reporting company ☐ |
| (Do not check if a smaller reporting company) | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No þ
The aggregate market value of the 21,721,439 shares of class A common stock held by non-affiliates of the registrant (based on the closing price of the registrant’s class A common stock on the last business day of the registrant’s most recently completed second fiscal quarter) was $238.3 million.
As of March 1, 2017, there were 46,457,221 shares of the registrant’s class A common stock outstanding, par value $0.01 per share.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the registrant’s Proxy Statement for its 2017 Annual Meeting of Stockholders, which Proxy Statement is to be filed within 120 days after the end of the registrant’s fiscal year ended December 31, 2016, are incorporated by reference in Part III of this Annual Report on Form 10-K.
| 1 |
Sucampo Pharmaceuticals, Inc.
Form 10-K
Table of Contents
| 2 |
PART I
We are including the following cautionary statement in this Annual Report on Form 10-K to make applicable and take advantage of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 for any forward-looking statements made by, or on behalf of us. Except for historical matters, the matters discussed in this Annual Report on Form 10-K are forward-looking statements (as defined in Section 21E of the Exchange Act) that involve risks and uncertainties that could cause actual results to differ materially from projected results. Accordingly, investors should not place undue reliance on forward-looking statements as a prediction of actual results. The forward-looking statements may include projections and estimates concerning the timing and success of specific projects and our future production, revenues, income and capital spending. When we use the words “believe,” “intend,” “expect,” “may,” “should,” “anticipate,” “could,” “estimate,” “plan,” “predict,” “project,” or their negatives, or other similar expressions, the statements which include those words are usually forward-looking statements. When we describe strategy that involves risks or uncertainties, we are making forward-looking statements. The forward-looking statements in this Annual Report on Form 10-K speak only as of the date of this Annual Report on Form 10-K; we disclaim any obligation to update these statements unless required by securities law, and we caution you not to rely on them unduly. We have based these forward-looking statements on our current expectations and assumptions about future events. While our management considers these expectations and assumptions to be reasonable, they are inherently subject to significant business, economic, competitive, regulatory and other risks, contingencies and uncertainties, most of which are difficult to predict and many of which are beyond our control. For a discussion of these risks, you should read this entire annual report carefully, especially the risks discussed under "Risk Factors."
| ITEM 1. | BUSINESS |
Overview
We are a global biopharmaceutical company focused on developing, identifying, acquiring and bringing to market innovative medicines that meet unmet medical needs. Our primary focus areas are gastroenterology, ophthalmology, and oncology-related disorders.
We currently generate revenue mainly from product royalties, product sales, upfront and milestone payments, and reimbursements for development activities. We expect to continue to incur significant expenses for the next several years as we continue our research and development activities, seek additional regulatory approvals and additional indications for our approved products and other compounds and seek strategic opportunities for in-licensing new products.
Our operations are conducted through subsidiaries based in the United States (U.S.), Japan and Switzerland. We operate as one segment, which focuses on the development and commercialization of pharmaceutical products.
Our Strategy
Our strategy is focused on becoming a leading biopharmaceutical company. We are built on the ongoing pursuit of scientific innovation and an unwavering passion for improving the lives of patients, their family members and their caregivers. We are committed to harnessing our past successes to maximize in-market revenues, focus our clinical development efforts, and enhance our scientific capabilities.
In 2016, we advanced our corporate strategy by further solidifying our base business, executing on business development transactions and diversifying our pipeline portfolio through the acquisition of new product candidates. We executed and accomplished the following key milestones:
| · | Entered into an option and collaboration agreement under which Cancer Prevention Pharmaceuticals, Inc. (“CPP”) has granted us the sole option to acquire an exclusive license to commercialize CPP-1X/sulindac combination product in North America. This product is currently in a phase 3 clinical trial for the treatment of familial adenomatous polyposis (FAP). |
| · | Entered into a Settlement and License Agreement to dismiss by mutual consent our lawsuit against Dr. Reddy’s Laboratories claiming infringement of seven AMITIZA-related patents listed in the FDA’s Orange Book, with the latest expiring in 2027. Under this agreement, we granted Dr. Reddy’s a non-exclusive license to market its generic version of lubiprostone 8 mcg and 24 mcg soft gelatin capsules in the U.S. for the indications approved for AMITIZA. This license does not begin until more than six years from November 9, 2016, or earlier under certain circumstances. Dr. Reddy’s will pay to us a share of net profits of generic lubiprostone products sold during the term of the agreement, which decreases over time and ends when all of our related patents have expired. |
| · | Sold $300.0 million aggregate principal amount of 3.25% convertible senior notes due 2021 (the “Convertible Notes”) in a private placement to qualified institutional buyers. pursuant to Rule 144A under the Securities Act of 1933, as amended. The proceeds from these notes were used to repay all outstanding amounts under our 2015 credit facility, as well as for general corporate purposes. |
| 3 |
Through the continued advancement of our AMITIZA lifecycle management programs, a sustainable pipeline and the acquisition and licensing of additional drug candidates with near-term launch opportunities, we will seek transformative growth by launching additional products for new therapeutic areas, strengthening an already sizable revenue base, and creating a sustainable company that is built to last.
Additionally, we continue to seek opportunities for strategic partnerships to strengthen the development of our existing pipeline and to diversify our revenue base. It is our vision to develop into a fully integrated, biopharmaceutical company centered on science and innovation and driven by the passionate and relentless efforts of our employees.
Our Competitive Strengths
Product Pipeline
The table below summarizes the development status of our marketed products and key product candidates as of March 1, 2017. The commercialization rights to lubiprostone have been licensed to Takeda Pharmaceutical Company Limited (Takeda) on a global basis other than Japan and the People’s Republic of China, to Mylan for Japan, and to Gloria for the People’s Republic of China. Commercialization of each product candidate may occur after successful completion of clinical trials and approval from appropriate governmental agencies. For CPP-1X, we have an option to acquire an exclusive license to commercialize in North America.
| Country | Program Type |
Target Indication | Development Phase | Next Milestone | |||||
| Lubiprostone (AMITIZA ®) | |||||||||
| U.S. | Commercial | Chronic idiopathic constipation (CIC) adults of all ages | Marketed | _____ | |||||
| U.S. | Commercial | Irritable bowel syndrome with constipation (adult women) (IBS-C) | Marketed | Initiate phase 4 study on higher dosage and with additional male subjects | |||||
| U.S. | Commercial | Opioid-induced constipation (OIC) in patients with chronic non-cancer pain | Marketed | _____ | |||||
| U.S. | Clinical | Alternate (Sprinkle) formulation | In development | Complete phase 3 trial | |||||
| U.S. | Clinical | Pediatric functional constipation (6 months - 6 years) |
Alternate (Sprinkle) formulation in development | Initiate phase 3 program | |||||
| U.S. | Clinical | Pediatric IBS-C (6 years - 17 years) |
Alternate (Sprinkle) formulation in development | Initiate phase 3 program | |||||
| U.S. & European Union |
Clinical | Pediatric functional constipation (6 years - 17 years) |
Open label phase 3 trials ongoing | Complete open label phase 3 trials and submit sNDA | |||||
| Japan | Commercial | Chronic constipation | Marketed | _____ | |||||
| Japan | Clinical | CIC adults, 12mcg capsule | CTN submitted | Submit sNDA | |||||
| Switzerland | Commercial | CIC-adults of all ages | Marketed | _____ | |||||
| Switzerland | Commercial | OIC in patients with chronic non-cancer pain | Marketed | _____ | |||||
| U.K. | Commercial | CIC-adults of all ages | Marketed | _____ | |||||
| Canada | Clinical | CIC-adults of all ages | Received approval from Health Canada | Market in Canada | |||||
| China | Clinical | CIC-adults of all ages | IND accepted | Initiate CIC study | |||||
| European Union | Clinical | CIC-adults of all ages | Received national marketing approvals in Ireland, Germany, Austria, Belgium, the Netherlands, Luxembourg, Italy and Spain (where product is not yet launched) | Launch feasibility and planning under evaluation | |||||
| Israel | Commercial | CIC-adults of all ages | Received national marketing approval | Develop pricing and reimbursement assessments and, based on outcome, determine launch feasibility and plans | |||||
| Mexico | Clinical | CIC-adults of all ages | CTA Approved | Complete phase 3 trial | |||||
| Mexico | Clinical | IBS-C - adult women | CTA Approved | Complete phase 3 trial | |||||
| Mexico | Clinical | OIC in patients with chronic non-cancer pain | CTA Approved | Complete phase 3 trial | |||||
| Russia | Clinical | CIC-adults of all ages | CTA Approved | Complete phase 3 trial | |||||
| Russia | Clinical | IBS-C - adult women | CTA Approved | Complete phase 3 trial | |||||
| South Korea | Clinical | CIC-adults of all ages | CTA Approved | Complete phase 3 trial | |||||
| South Korea | Clinical | IBS-C - adult women | CTA Approved | Complete phase 3 trial | |||||
| South Korea | Clinical | OIC in patients with chronic non-cancer pain | CTA Approved | Complete phase 3 trial | |||||
| Unoprostone isopropyl (RESCULA®) | |||||||||
| Japan South Korea |
Commercial | Glaucoma and ocular hypertension | Marketed | _____ | |||||
| Taiwan | |||||||||
| CPP-1X/sulindac combination product | |||||||||
| U.S. | Option | Familial adenomatous polyposis (FAP) | Phase 3 | Complete phase 3 trial | |||||
| 4 |
AMITIZA (lubiprostone)
AMITIZA is a ClC-2 chloride channel activator developed for the treatment of constipation. AMITIZA acts with a dual mechanism of action, increasing intestinal fluid secretion while also stimulating recovery of mucosal barrier function. AMITIZA has been approved for three indications that cover distinct patient types: chronic idiopathic constipation (CIC), irritable bowel syndrome with constipation (IBS-C), and opioid-induced constipation (OIC). Since 2006, AMITIZA has been dispensed over 10 million times.
Chronic Idiopathic Constipation (CIC)
Constipation is characterized by infrequent and difficult passage of stool and becomes chronic when a patient suffers specified symptoms for over 12 non-consecutive weeks within a 12-month period. Chronic constipation (CC) is idiopathic if it is not caused by other diseases or by use of medications. Symptoms of CIC include straining, hard stools, bloating and abdominal pain or discomfort. Some patients suffering from occasional constipation may be treated with lifestyle modification, dietary changes and increased fluid and fiber intake, although there is very limited well-controlled clinical trial data in support of these alternatives in CIC or IBS-C patients. For patients who fail to respond to these approaches, physicians may recommend laxatives, most of which are available over-the-counter (OTC), i.e., without a prescription, for acute use. These agents are generally not approved for long-term use by CIC or IBS-C patients nor is such use supported by long-term, well-controlled clinical trial data.
A study published in The American Journal of Gastroenterology in September 2011 estimates that approximately 14% of adults over 15 years of age, or over 30 million people, in the U.S., suffer from CIC. By the time most CIC patients seek care from a physician they have typically tried dietary and lifestyle changes, as well as a number of available OTC remedies, and remain unsatisfied. Commonly used OTC medications include laxatives, stool softeners and fiber supplements.
Irritable Bowel Syndrome with Constipation (IBS-C)
IBS is a disorder of the intestines with symptoms that include severe cramping, pain, bloating and changes of bowel habits, such as diarrhea or constipation. Patients diagnosed with IBS are commonly classified as having one of four forms: IBS-C, IBS with diarrhea, mixed-pattern IBS alternating between constipation and diarrhea, and unspecified irritable bowel syndrome. Currently, IBS in all its forms is considered to be one of the most common gastrointestinal disorders. Like CIC, some patients suffering from IBS-C may be treated with dietary measures, such as increasing fiber and fluid intake; if these measures prove ineffective, laxatives are frequently used for the management of this condition, though they are not approved for IBS-C.
Opioid-Induced Constipation (OIC)
OIC is a common adverse effect of chronic opioid use. Binding of opioids to peripheral opioid receptors in the gastrointestinal tract results in reduction of secretion of electrolytes, such as chloride, and subsequent reduction in small intestinal fluid. In addition, activation of enteric opioid receptors results in abnormal gastrointestinal motility. Together, these processes result in OIC, which is characterized by infrequent and incomplete evacuation of stool, hard stool consistency, and straining associated with bowel movements.
Current treatment options for OIC include the use of stool softeners, enemas, suppositories and peristaltic stimulants such as senna, which stimulate muscle contractions in the bowel. Additionally, the standard prescription option for OIC is osmotic laxatives. The effectiveness of these products for the treatment of OIC is limited due to the severity of the constipation caused by opioids. In addition, physicians often cannot prescribe peristaltic stimulants for the duration of narcotic treatment because of the potential for dependence upon these stimulants. Opioid drugs are known to suppress firing of secretomotor neurons in the gut which reduces intestinal fluid secretion resulting in drier, harder stools. Lubiprostone works locally in the gut to reestablish fluid secretion thus alleviating OIC. As a result, we believe that AMITIZA holds a competitive advantage over drugs that do not work through this mechanism of action. Additionally, the mechanism of action for AMITIZA is different and unique versus other products that are currently approved for OIC, which are all peripherally acting mu-opioid receptor antagonists (PAMORAs). This also allows AMITIZA to offer a unique option for treating this specific type of constipation.
There are more than 200 million prescriptions for opioid use in the U.S. annually, and a substantial number of these prescriptions are for non-cancer chronic pain. Market research indicates that there are approximately 2.5-4.5 million moderate to severe sufferers of OIC, and 40-80% of patients taking opioids chronically for non-cancer pain report constipation in the U.S.
United States and Canada
AMITIZA is marketed in the United States for the three gastrointestinal indications described above under a collaboration and license agreement, or the North America Takeda Agreement, with Takeda Pharmaceutical Company Limited, or Takeda. Under the North America Takeda Agreement, we are primarily responsible for clinical development activities, while Takeda is responsible for commercialization of AMITIZA in the United States and Canada. Takeda is required to provide a minimum annual commercial investment during the current term of the North America Takeda Agreement and may reduce the minimum annual commercial investment when a generic equivalent enters the market. In October 2015, Health Canada approved AMITIZA for CIC in adults. In October 2014, we signed an amendment, or the Takeda Amendment, to the North America Takeda Agreement, which among other things, extended the term of the North America Takeda Agreement beyond December 2020. During the extended term beginning in January 2021, we will share with Takeda the annual net sales revenue on branded AMITIZA products. More information on our collaboration with Takeda in North America is found under the heading “North America Takeda Agreement.”
| 5 |
We have also partnered with Par Pharmaceuticals, Inc., or Par, and Dr. Reddy’s Laboratories, Ltd., or Dr. Reddy’s, in connection with the settlement of patent litigation in the United States related to our AMITIZA 8 mcg and 24 mcg soft gelatin capsule products. Under our agreement with Par, we granted Par a non-exclusive license to market Par’s generic version of lubiprostone 8 mcg and 24 mcg soft gelatin capsules in the United States for the indications approved for AMITIZA beginning January 1, 2021, or earlier under certain circumstances. Beginning on January 1, 2021, Par will split with us the gross profits of the licensed products sold during the term of the agreement, which continues until each of our related patents has expired. Under our agreement with Dr. Reddy’s, we granted Dr. Reddy’s a non-exclusive license to market Dr. Reddy’s generic version of lubiprostone 8 mcg and 24 mcg soft gelatin capsules in the United States for the indications approved for AMITIZA. This license does not begin until more than six years from November 9, 2016, or earlier under certain circumstances. Dr. Reddy’s will pay to us a share of net profits of generic lubiprostone products sold during the term of the agreement, which decreases over time and ends when all of our related patents have expired. In the event that either Par or Dr. Reddy’s elect to launch an authorized generic form of lubiprostone, we have agreed to supply such product under the terms of a manufacturing and supply agreement at a negotiated price.
Japan
In Japan, AMITIZA is the only prescription medicine for chronic constipation, excluding constipation caused by organic diseases, and is marketed under a license, commercialization and supply agreement, or the Japan Mylan Agreement, originally entered into with Abbott Laboratories, Inc., or Abbott. In February 2015, Mylan purchased Abbott’s non-U.S. developed markets specialty and branded generics business, as a result of which Mylan acquired the rights to commercialize AMITIZA in Japan. We did not experience any significant changes in the commercialization of AMITIZA in Japan as a result of the transfer of the Japan Mylan Agreement from Abbott to Mylan. According to epidemiology data from Japan’s Ministry of Health. Labour and Welfare (MHLW), millions of people in Japan may live daily with the pain and discomfort of CC, yet not seek physician care. Medical attention could mean early diagnosis and effective, long-term treatment. It is estimated that approximately 14.3% of the Japanese population, or over 18 million people, suffer from CC.
People’s Republic of China
In May 2015, we entered into an exclusive license, development, commercialization and supply agreement, or the China Gloria Agreement, with Harbin Gloria Pharmaceuticals Co., Ltd., or Gloria, for AMITIZA in the People’s Republic of China. We will be the exclusive supplier of AMITIZA to Gloria at an agreed upon supply price. Under the China Gloria Agreement, Gloria is responsible for all development activities and costs, as well as commercialization and regulatory activities, for AMITIZA in the People’s Republic of China. Upon entering into the China Gloria Agreement, we received an upfront payment of $1.0 million. In June 2015, the China Food and Drug Administration accepted an Investigational New Drug, or IND, application for a pivotal trial of AMITIZA in patients with CIC, as a result of which we received an additional payment of $500,000 from Gloria. In addition to the $1.5 million in payments received and recognized as revenue through June 2015, we are eligible to receive an additional payment in the amount of $1.5 million upon the occurrence of a specified regulatory or commercial milestone event.
Other Global Markets
In October 2014, we entered into an exclusive license, development, commercialization and supply agreement, or the Global Takeda Agreement, for lubiprostone with Takeda. Under the Global Takeda Agreement, Takeda develops and markets AMITIZA globally except in the United States, Canada, Japan and the People’s Republic of China. We supply Takeda with the clinical and commercial product at a negotiated price. Takeda currently markets AMITIZA for CIC and OIC in Switzerland and currently markets AMITIZA for CIC in the United Kingdom. Takeda became the marketing authorization holder in Switzerland in April 2015, in the United Kingdom, Austria, Belgium, Germany, Netherlands, Ireland, Italy, Luxembourg and Spain during 2016. More information on our collaboration with Takeda in global markets is found under the heading “Global Takeda Agreement.”
Before the execution of the Global Takeda Agreement, we retained full rights to develop and commercialize AMITIZA for the rest of the world’s markets outside of the United States, Canada and Japan. In the United Kingdom, we received approval in September 2012 from the Medicines and Healthcare Products Regulatory Agency for the use of AMITIZA to treat CIC. We made AMITIZA available in the United Kingdom in the fourth quarter of 2013. In July 2014, National Institute of Health and Care Excellence published the technology appraisal guidance recommending the use of AMITIZA in the treatment of CIC and associated symptoms in adults who have failed laxatives. In January 2015, we successfully completed the European mutual recognition procedure for AMITIZA for the treatment of CIC in select European countries, resulting in marketing authorizations in these countries.
| 6 |
In Switzerland, AMITIZA was approved to treat CIC in 2009. In 2012, we reached an agreement with the Bundesamt fur Gesundheit (BAG), the Federal Office of Public Health in Switzerland, on a reimbursement price for AMITIZA in Switzerland, and began active marketing in the first quarter of 2013. In February 2014, the BAG revised several reimbursement limitations with which AMITIZA was first approved for reimbursement and inclusion in the Spezialitätenliste (SL) to allow all Swiss physicians to prescribe AMITIZA to patients who have failed previous treatments with at least two laxatives over a nine-month period. In July 2014, AMITIZA was approved for the treatment of OIC in chronic, non-cancer adult patients by the Swissmedic, the Swiss Agency for Therapeutic Products, and in October 2015, the BAG added this indication to the SL.
In October 2015, Takeda obtained approval of the clinical trial application, or CTA, for AMITIZA for the treatment of CIC and IBS-C in Russia that was submitted in June 2015. In December 2015, a CTA was filed for AMITIZA for the treatment of CIC, IBS-C and OIC in Mexico and South Korea. Takeda initiated phase 3 registration trials in Russia in March 2016 and in South Korea and Mexico in May 2016. Takeda submitted a new drug application, or NDA, for the treatment of CIC, IBS-C, and OIC in Israel in June 2015, which was approved in July 2016, and an NDA for the same indications in Kazakhstan in December 2015. Additional NDA submissions have been made by Takeda in Singapore in May 2016, and South Africa and Indonesia in June 2016.
A study published in The American Journal of Gastroenterology in September 2011 estimates that approximately 16% of adults over 15 years of age, or over 42 million people, in Northern Europe suffer from CIC.
In a study conducted in ten European countries, including Switzerland, the results of which were published in Alimentary Pharmacology and Therapeutics in 2012, approximately 28% of the participants suffering from constipation for at least 6 months were dissatisfied with their current treatment options using laxatives. Of that group, approximately 83% were interested in seeking alternative methods to relieve their constipation.
RESCULA (unoprostone isopropyl)
RESCULA is a Big Potassium (BK) channel activator used to lower intraocular pressure (IOP). In October 2015, we acquired R-Tech Ueno, Ltd., or R-Tech, a global biopharmaceutical company focused on the research and development of drugs for inflammatory conditions, oncology and ophthalmology. Pursuant to the acquisition, we acquired global rights to RESCULA. In the United States, we ceased marketing RESCULA in the fourth quarter of 2014 and no product was made available after the March 2015 expiration date. In May 2015, we returned all licenses for unoprostone isopropyl to R-Tech. In June 2016, we completed the withdrawal of the marketing authorization for RESCULA in the United States. RESCULA is being commercialized by Santen Pharmaceutical Co., Ltd in Japan, and Sinphar Pharmaceutical, Co., Ltd and Zuellig Pharma Inc. in Taiwan.
Our Clinical Development Programs
Lubiprostone
Alternate Formulation
We are developing an alternate formulation of lubiprostone for both adult and pediatric patients who are unable to take or do not tolerate capsules and for naso-gastric tube fed patients. It is estimated that approximately 40% of American adults have difficulty swallowing pills. Of those who have experienced difficulty swallowing pills, approximately 14% have delayed taking doses of their medication, 8% have skipped a dose and 4% have discontinued using their medication. In addition, the current formulation of pills is not amenable for administration to young children (6 months and older). Takeda has agreed to fund 100% of the costs, up to a cap, of this alternate formulation work. We initiated the phase 3 program of the alternate formulation of lubiprostone in adults in the second half of 2016 and, if the program is successful, we intend to file an NDA in the United States for the alternate formulation for adults in the second half of 2017.
Pediatric Functional Constipation
The phase 3 program required to support an application for marketing authorization of lubiprostone for pediatric functional constipation comprises four clinical trials. The first two trials, one of which was recently completed, test the soft gelatin capsule formulation of lubiprostone in patients 6 to 17 years of age. The first of these trials was a pivotal 12-week, randomized, placebo-controlled trial which was initiated in December 2013 and completed enrollment in April 2016. The second trial is a follow-on, long-term safety extension trial that was initiated in March 2014. In November 2016, we announced that the phase 3 trial of AMITIZA in pediatric functional constipation in children 6 to 17 years of age failed to achieve its primary endpoint of overall spontaneous bowel movement, or SBM, response. The trial achieved statistical significance for some secondary endpoints, notably overall SBM frequency, straining, and stool consistency. In addition, in this study lubiprostone was well tolerated. We have entered into a process with the U.S. Food and Drug Administration, or FDA, and other constituencies, and as a result of initial discussion with the FDA will submit an sNDA in the second half of 2017. Additionally, after further consultations with the FDA to better determine the doses and endpoints that should be studied, the phase 3 program for the alternate formulation of lubiprostone described above will be followed in mid-2018 with a phase 3 program in patients 6 months to 6 years of age using the alternate formulation. Takeda has agreed to fund 70% of the costs, up to a cap, of this pediatric functional constipation program.
| 7 |
Constipation in children has similar characteristics to those of constipation in adults; symptoms include infrequent bowel movements, hard stools, large diameter stools and painful passage of stools. Children may also experience fecal retention due to withholding, since there is a tendency to avoid defecation and withhold bowel movements as a result of the pain experienced from the passage of large stools. This withholding of bowel movements can result in episodes of fecal incontinence. The Rome III diagnostic criteria for childhood functional constipation dictate that such symptoms occur at least once per week for at least 2 months prior to diagnosis. Furthermore, ninety percent of pediatric constipation is functional constipation and it occurs in all age groups. An analysis of longitudinal data in the U.S. showed that over the last decade there has been a nearly 4-fold increase in rates of constipation. Nevertheless, the estimates of the prevalence rate of functional constipation in the pediatric population worldwide have varied greatly, from 4% to 37%. Regardless of this wide range of estimated prevalence, only 50-70% of children with functional constipation achieve long-term improvement with the current treatments, indicating a need for better treatments.
VAP-1 Inhibitors
In 2016, we discontinued our VAP-1 Inhibitor RTU-1096 development program and our VAP-1 Inhibitor RTU-009 program.
CPP- 1X/Sulindac Combination Product
In January 2016, we entered into an option and collaboration agreement under which CPP has granted us the sole option to acquire an exclusive license to commercialize CPP-1X/sulindac combination product in North America. This product is currently in a Phase 3 clinical trial being conducted by CPP for the treatment of familial adenomatous polyposis, or FAP. Under our agreement with CPP, we have the exclusive option to license this product for North America. There are currently no approved treatments for FAP. The ongoing Phase 3 study is a 150-patient, three-arm, double-blind, randomized trial of the combination agent and the single agent comparators. Enrollment in the study has completed and the results from a Phase 3 futility analysis are expected to be available mid-2017. The trial is expected to conclude in 2019.
AMITIZA Collaboration Agreements
We have the following collaboration agreements with our partners to supply, develop and commercialize AMITIZA:
| · | North America Takeda Agreement; |
| · | Global Takeda Agreement; |
| · | Japan Mylan Agreement; and, |
| · | China Gloria Agreement. |
The collaboration agreements are covered by geographic location.
North America Takeda Agreement
In October 2004, we entered into an agreement with Takeda to supply, develop and commercialize AMITIZA for gastrointestinal indications in the U.S. and Canada. The original agreement was amended on February 1, 2006 through a supplemental agreement and, in October 2014, we and Takeda and certain Takeda affiliates executed amendments to the agreement. Collectively, these are referred to as the North America Takeda Agreement. Payments to us under these agreements include a non-refundable upfront payment, non-refundable development and commercial milestone payments, reimbursement of certain development and co-promotion costs, product royalties and product sales.
Under the North America Takeda Agreement, which has an initial contract term through 2020, and thereafter continues until terminated by Takeda in its sole discretion:
| · | We recognize product sales revenue from the supply of AMITIZA to Takeda at a negotiated supply price. |
| · | We recognize royalty income from Takeda’s net sales of AMITIZA in the U.S. and Canada. The royalty rates consist of several tiers ranging from 18%-26% with the royalty rate resetting every year: |
| · | We recognize research and development revenue for the reimbursement of research and development costs as Takeda has agreed to fund all development costs, including regulatory-required studies, to a maximum of $50.0 million for each additional indication and $20.0 million for each additional formulation. Takeda and we have agreed to equally share all costs in excess of those amounts. With respect to any studies required to modify or expand the label for AMITIZA for the treatment of CIC, IBS-C or OIC, Takeda has agreed to fund 70% of the costs of such studies, and we have agreed to fund the remainder. Additionally, Takeda has agreed to fund 100% of the development costs for the new formulation of AMITIZA, and 70% of the development costs for the treatment of pediatric functional constipation. |
| 8 |
| · | We are eligible for additional commercial milestone payments contingent on the achievement of certain net sales revenue targets. |
| · | Takeda is required to provide a minimum annual commercial investment during the current term of the North America Takeda Agreement and may reduce it when a generic equivalent enters the market. |
| · | We retain the right to co-promote AMITIZA for gastrointestinal indications. In December 2014, as part of the amendments to the North America Takeda Agreement, we ceased our co-promoting activity. |
| · | Our collaboration with Takeda is administered in part by four committees consisting of an equal number of representatives from both companies. In the case of a deadlock within the joint steering committee, our chief executive officer has the determining vote on matters arising from the joint development and manufacturing committees, while the chief operating officer of Takeda has the determining vote on matters arising from the joint commercialization committee. |
| · | During the extended term, beginning on January 1, 2021, we will share equally with Takeda in the net annual sales revenue from branded AMITIZA sales. |
Global Takeda Agreement
In October 2014, we entered into the Global Takeda Agreement to develop and commercialize AMITIZA for gastrointestinal indications. The territories excluded from the Global Takeda Agreement are Canada, the U.S., Japan and the People’s Republic of China. Canada and the U.S. are covered by the North America Takeda Agreement, Japan is covered by the Japan Mylan Agreement, and China is covered by the China Gloria Agreement. The agreement is effective until it expires on a country-by-country basis on the fourteenth anniversary of the date of first commercial sale in that country. Under the terms of the agreement:
| · | We recognize product sales revenue from the supply of AMITIZA to Takeda at a negotiated supply price. |
| · | We received an upfront payment of $14.0 million from Takeda in October 2014. |
| · | We are eligible for up to $35.0 million in commercial milestone payments contingent upon the achievement of certain net sales revenue targets. |
| · | We are responsible for the first $6.0 million in development costs, and Takeda is responsible for all subsequent development activities and related costs. |
| · | Takeda is, or will become, the marketing authorization holder for each country upon regulatory approval and will be responsible for all commercialization and regulatory activities. |
Japan Mylan Agreement
In February 2009, we entered into a license, commercialization and supply agreement (the Japan Mylan Agreement) for AMITIZA in Japan with Mylan. Under the terms of the Japan Mylan Agreement (which continues until 2027):
| · | We recognize product sales revenues from the supply of AMITIZA to Mylan at a negotiated supply price. |
| · | Mylan has a right of first exclusive negotiation to obtain a license to develop and commercialize AMITIZA in Japan for any new indications that we may develop, such as OIC. We retain the rights to AMITIZA for all other therapeutic uses. We are required to fund and complete all the development work including any additional clinical studies required to maintain regulatory approval in Japan. We own all the rights covered under the regulatory filings. |
| · | Mylan is required to fund and undertake all commercialization efforts including pre-launch and post-launch marketing, promotion and distribution. Mylan is required to maintain the number of sales staff and the estimated level of annual net sales based on the commercialization plan approved by the committees described below. |
| · | We have retained the right to co-promote the product in Japan under certain conditions and all other development and commercialization rights to all other therapeutic areas and are responsible for the cost of co-promotion. |
| · | Our collaboration efforts under the Japan Mylan Agreement are administered by two committees consisting of an equal number of representatives from both parties. In the case of a deadlock within the committees, we have the determining vote on matters relating to development, while Mylan has the determining vote on matters relating to commercialization. |
China Gloria Agreement
In May 2015, we entered into an exclusive license, development, commercialization and supply agreement (China Gloria Agreement), for AMITIZA in the People’s Republic of China. The China Gloria Agreement is effective until the thirteenth anniversary of the effective date and will automatically renew for successive three year periods unless terminated upon one year’s prior written notice by one of the parties. Under the terms of the China Gloria Agreement:
| · | We will recognize product sales revenues as the exclusive supplier of AMITIZA to Gloria at a negotiated supply price. |
| 9 |
| · | We received an upfront payment of $1.0 million from Gloria in May 2015, and an upfront payment of $500,000 in June 2015 after the CFDA accepted the IND application for a pivotal trial of AMITIZA in patients with CIC. |
| · | We are eligible to receive an additional payment of $1.5 million upon the occurrence of a specified regulatory or commercial milestone event. |
| · | Gloria is responsible for all development activities and costs, as well as commercialization and regulatory activities for AMITIZA in the People’s Republic of China. |
RESCULA Collaboration Agreement
Japan Santen Agreement
In March 2012, we entered into an exclusive transaction agreement (Japan Santen Agreement) with Santen Pharmaceutical Co. Ltd (Santen) to commercialize RESCULA in Japan. The initial term of the Japan Santen Agreement ended on March 31, 2016, but the agreement automatically extends for successive one-year renewal terms unless either party gives an 11-month prior notice. Under the terms of the Japan Santen Agreement we recognize revenues from the product sales of RESCULA to Santen at a negotiated price.
Pipeline Agreement
CPP Agreement
In January 2016, we entered into an option and collaboration agreement (the “CPP Agreement”) under which CPP has granted us the sole option to acquire an exclusive license to commercialize CPP-1X/sulindac combination product in North America. Under the terms of the CPP Agreement:
| · | We have invested $5.0 million in CPP in the form of a convertible note, with a planned additional $5.0 million equity investment in CPP’s next qualified financing, which will be either an IPO or a private financing as defined by the agreement; |
| · | We will pay CPP an option fee of up to $7.5 million, payable in two tranches; the first tranche of $3.0 million was paid at signing; |
| · | CPP will complete the ongoing phase 3 trial off CPP-1x/sulindac for the treatment of FAP under the oversight of a joint steering committee; |
| · | Upon exercise of our exclusive option, we would acquire an exclusive license to the product, for all indications, and would be obligated to pay CPP up to an aggregate of $190.0 million in license fees and milestone payments upon the achievement of specified clinical development and sales milestones; and |
| · | We and CPP would share equally in profits from the sale of licensed products. |
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for the technology and know-how upon which our products are based, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights.
We hold ownership rights to develop and commercialize our products and product candidates covered by patents and patent applications. Our portfolio of patents includes patents or patent applications with claims directed to compositions of matter, including both compounds and pharmaceutical formulations, methods of use, or a combination of these claims, and methods of manufacturing the compounds. Depending upon the timing, duration and specifics of FDA approval of the use of a compound for a specific indication, some of our U.S. patents may be eligible for a limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. Similar extensions to patent term may be available in other countries for particular patents in Sucampo’s portfolio.
As of December 31, 2016, the patent rights relating to lubiprostone include compositions of matter, methods of use and methods of manufacturing, and several of the U.S. patents are listed in the U.S. FDA Orange Book. Patents have been granted in the U.S., Europe, Japanese and other countries relating to pharmaceutical formulations, manufacturing, dosing regimens and therapeutic uses. Many of the U.S. patents in the lubiprostone portfolio expire between 2020 and 2027. Many of the corresponding foreign patents expire between 2020 and 2026. Several other patents have later termination dates.
As of December 31, 2016, the patent rights relating to unoprostone isopropyl include compositions of matter, methods of use and methods of manufacturing. However, RESCULA is not covered by patents in Japan, the largest market for the product. Two U.S. patents cover the product, which was discontinued by Sucampo in the U.S. The U.S. patent relating to compositions of matter expires in 2018 and the patent relating to method of use expires in 2021. Corresponding foreign patents also expire in this time frame.
We are actively seeking to augment our portfolio of compounds by focusing on the development of new chemical entities, or NCEs, which have not previously received FDA approval. Upon approval by the FDA, NCEs are entitled to market and data exclusivity in the U.S. with respect to generic drug competition for a period of five years from the date of FDA approval, even if the related patents have expired. We are also engaged in patent lifecycle management strategies for our marketed products.
| 10 |
Manufacturing
Following the acquisition of R-Tech Ueno in October 2015 we obtained direct control and management of the production and supply chain of commercial quantities of AMITIZA and RESCULA as well as preclinical or clinical supplies of the other compounds that we are testing in our development programs. Our manufacturing network is a combination of owned assets and external suppliers. There are existing supply agreements between R-Tech and our external suppliers to ensure continued supply of our products.
Competition
AMITIZA (lubiprostone)
In the U.S., an estimated 40-50 million patients who suffer from constipation that is idiopathic in nature or a consequence of other conditions such as IBS or chronic opioid use. Many patients are currently treated for CIC, IBS-C or OIC with a variety of medications. Over-the-counter (OTC) medications are available and are generally intended to provide relief for occasional constipation. Prescription products are also available and are generally intended to provide relief for chronic constipation. As such, the U.S. constipation market is expansive and diverse with a multitude of products intended to treat a large heterogeneous patient population.
The prescription chronic constipation market can generally be bifurcated into two categories: 1) generic laxatives and 2) branded products. Generic laxatives make up roughly 80%-90% of the total prescription volume while branded prescriptions have grown to represent 10%-20% of the prescription market. The branded prescription products are briefly described below:
| · | AMITIZA (lubiprostone): AMITIZA, is approved by the FDA for the treatment of CIC (from an unknown cause; not constipation due to another condition or treatment), IBS-C and CIC. AMITIZA softens the stool by increasing its water content, so the stool can pass easily. AMITIZA is taken twice daily. |
| · | Linzess (linaclotide): Linzess is approved for CIC and IBS-C. This drug is a capsule taken once daily and helps relieve constipation by helping bowel movements occur more often. It is not approved for use in those age 17 years and younger. | |
| · | Trulance (Plecanatide): Trulance is approved (but not yet launched as of early March, 2017) for CIC. This drug is a capsule that can be taken once daily for use in adults, and is a similar mechanism as Linzess (guanylate cyclase type-C agonist). |
| · | Lactulose: Lactulose, a prescription laxative for chronic constipation, draws water into the bowel to soften and loosen the stool. |
| · | Polyethylene glycol (PEG): PEG is an osmotic laxative for chronic constipation and causes water to remain in the stool, which results in softer stools. |
| · | Movantik (naloxegol): Movantik is approved for OIC. This drug works by binding to mu-receptors in the brain and other parts of the central nervous system to block pains signals as well as bind to mu-receptors in the bowel which may cause OIC. |
| · | Relistor, oral and injectable (methylnaltrexone bromide): Relistor is approved for OIC. Similar to Movantik, this product works by decreasing the constipating effects of opioids by inhibiting opioids from binding to mu-receptors in GI tract. |
At this time AMITIZA is the only branded product that has a unique mechanism of action. AMITIZA is also the only branded product on the market today to be indicated in three separate indications for CIC, IBS-C and OIC.
AMITIZA Competitors
The key branded and generic products currently on the U.S. market include:
| Product | Company | Approved Indications |
|
AMITIZA (lubiprostone) |
Sucampo/Takeda | CIC, IBS-C, OIC |
|
Linzess (linaclotide) |
Allergan/Ironwood | CIC, IBS-C |
| Trulance (Plecanatide) |
Synergy Pharmaceuticals | CIC (approved, not launched) |
|
Movantik (naloxegol) |
AstraZeneca | OIC |
| Relistor (Injection and Oral) (methylnaltrexone bromide) |
Valeant/Progenics | OIC |
| Lactulose | Several | CIC, IBIS-C |
| Generic PEG | Several | Chronic Constipation |
| 11 |
Key pipeline competitors include:
| Product | Company | Status |
| Plecanatide | Synergy Pharmaceuticals Inc. |
CIC approved January 2017 IBS-C filing expected H1 2017 |
| Naldemedine | Shionogi | OIC Phase III; positive Phase III topline, potential approval expected H1 2017 |
Other agents in various stages of development include :
| Product | Company | Status |
| Tenapanor | Ardelyx | IBS-C, Phase III |
| Linaclotide | Allergan/Ironwood | Linaclotide Colonic Release, Phase IIb data announced in December 2016, Phase III-ready |
| RM-131/relamorelin | Ipsen/Rhythm | CIC, Phase II |
| TD-1211/axelopran | Theravance | OIC, Phase II |
| SP-333/dolcanatide | Synergy | OIC, IBD Phase II |
| Elobixibat | Albireo | CIC Phase III (Japan), Phase II (US) |
Additionally, there are several 5-HT Receptor agonists in various stages of development as well (Resolor/Shire Phase III in CIC, YKP-10811/SK Biopharma in CIC Phase II)
RESCULA (unoprostone isopropyl)
RESCULA (unoprostone isopropyl) is approved for Ocular Hypertension and Open-Angle Glaucoma and is currently marketed in several global regions including Japan and Taiwan. RESCULA was originally launched in Japan in 1994 and is no longer covered by patent or regulatory exclusivity in Japan. RESCULA is no longer commercialized in the U.S.
According to recent market data in Japan, the glaucoma treatment market grew 0.9% to ¥105.7 billion. Treatments for glaucoma represent the largest segment of Japan’s prescription ophthalmic pharmaceutical market, accounting for approximately 33% of the total. Increased intraocular pressure is a significant risk factor resulting in damage to the optic nerve. This can lead to visual field loss and in some cases, blindness. Glaucoma is the most common cause of blindness in people with ophthalmic disease in Japan. The glaucoma market is expected to expand in the future, mainly due to the increase in patient numbers owing to population aging.
RESCULA faces many competitors which promote products for primary-angle glaucoma (PAOG), and ocular hypertension. There are several products in the regions where RESCULA is marketed that have become generic and have therefore had an impact on the usage of prostaglandins as first line therapy. Other competitive products include latanaprost and travoprost, ophthalmic solutions and suspensions and generic beta blockers. Prostaglandin analogues continue to have strong first line market share followed by generic beta blockers. Our competitors are also developing additional pipeline products for PAOG and ocular hypertension.
Product Candidates
We face similar competition from approved therapies and potential pipeline products for the diseases and conditions potentially addressed by our product candidates, and are likely to face competition for any other product candidates we may elect to develop in the future.
Government Regulation
Government authorities in the U.S., at the federal, state and local level, and in other countries extensively regulate the research, development, testing, approval, manufacturing, labeling, post-approval monitoring and reporting, packaging, promotion, storage, advertising, distribution, marketing and export and import of pharmaceutical products such as those we are developing. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources.
| 12 |
United States Government Regulation
In the U.S., the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, as amended, and implements regulations. The FDA has jurisdiction over all of our products and administers requirements covering the safety, effectiveness, manufacturing, quality control, distribution, labeling, marketing, advertising, dissemination of information, post-marketing study, and pharmacovigilance of our pharmaceutical products. Information that must be submitted to the FDA in order to obtain approval to market a drug varies depending upon whether the drug is a new product whose safety and efficacy have not previously been demonstrated in humans or a drug whose active ingredients and certain other properties are the same as those of a previously approved drug. The results of product development, preclinical studies and clinical trials must be submitted to the FDA as part of the approval process. The FDA may deny approval if the applicable regulatory criteria are not satisfied, or it may require additional clinical data or analyses or even an additional clinical trial. Even if such data are submitted, the FDA may ultimately decide that the application does not satisfy the criteria for approval.
Obtaining FDA approval for new products and manufacturing processes can take a number of years and involve the expenditure of substantial resources. To obtain FDA approval for the commercial sale of a therapeutic agent, the potential product must undergo testing programs on animals, the data from which is used to file an investigational NDA with the FDA. In addition, there are three phases of human testing following Good Clinical Practices (GCP) guidelines:
- Phase 1 consists of safety tests with human clinical evaluations, generally in normal, healthy volunteers;
- Phase 2 programs expand safety tests and measure efficacy along with dose finding evaluations and are conducted in volunteers with a particular disease condition that the drug is designed to treat; and
- Phase 3 programs are greatly expanded clinical trials to determine the effectiveness of the drug at a particular dosage level in the affected patient population.
The data from these clinical tests are combined with data regarding chemistry, manufacturing and animal pharmacology and toxicology, and are then submitted to the FDA in the form of an NDA. The preparation of an NDA requires the expenditure of substantial funds and the commitment of substantial resources.
Failure to comply with the applicable FDA requirements at any time during the product development process, approval process or following approval may result in administrative or judicial sanctions. These sanctions could include the FDA’s imposition of a hold on clinical trials, refusal to approve pending applications, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on our business.
The FDA extensively regulates all aspects of manufacturing quality under its current good manufacturing practice (cGMP) regulations. The FDA inspects the facility or the facilities at which drug products are manufactured. The FDA will not approve the product unless cGMP compliance is satisfactory. If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the application and often will request corrective actions including additional validation or information.
The pharmaceutical testing and approval process requires substantial time, effort and financial resources. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products. The FDA may limit the indications for use or place other conditions on any approvals that could restrict the commercial application of the products. After approval, some types of changes to the approved product, such as manufacturing changes and additional labeling claims, are subject to further FDA review and approval.
Post-Approval Requirement
After regulatory approval of a product is obtained, we are obligated to comply with a number of post-approval requirements. For example, the FDA may require post marketing, or phase 4 clinical trials to assess additional elements of the product’s safety or efficacy. In addition, holders of an approved NDA are required to report certain adverse drug reactions and production problems to the FDA, to provide updated safety information and to comply with requirements concerning advertising and promotional labeling for their products. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes certain fiscal, procedural, substantive and record-keeping requirements.
| 13 |
We rely substantially on third parties for the performance of certain activities related to the production, packaging and distribution of our drug products for clinical and commercial use. Future FDA inspections may identify compliance issues at our facilities or at the facilities of our manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings, precautions and contraindications. Also, new government requirements, including those resulting from new legislation, may be established that could delay or prevent regulatory approval of our products under development.
Regulation Outside of the United States
In addition to regulations in the U.S., we are subject to a variety of regulations in other jurisdictions most notably by the Health Canada in Canada, European Medicines Agency (EMA) in the E.U., Swissmedic in Switzerland and the MHLW in Japan. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of countries outside the U.S. before we can commence clinical trials or marketing of the product in those countries. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country, and the time for approval is country dependent and may be longer or shorter than that required by the FDA.
Canada
In Canada, the new drug approval process is similar to that in the U.S. The process is divided into four phases: preclinical studies, clinical trials, new drug submission and marketing. Health Canada regulates the clinical trials and grants market authorization based on an assessment of the safety, efficacy and quality of drug products. In addition to approval of new drugs, the federal government also regulates drug pricing through the Patented Medicines Prices Review Board (PMPRB).
Europe
In Europe, medicinal products are governed by a framework of E.U. directives which apply across all E.U. member states. To obtain regulatory approval of a drug under the E.U. regulatory system, we may submit an MAA, either under a centralized, decentralized, or mutual recognition procedure (MRP). The centralized procedure, which is compulsory for medicines produced by certain biotechnological processes and optional for those which are innovative, provides for the grant of a single marketing authorization that is valid for all E.U. member states. The decentralized procedure provides for a member state, known as the reference member state, to assess an application, with one or more concerned, member states subsequently approving that assessment. The MRP provides approval in one country and then allows for a request from subsequent countries to mutually recognize the original country’s approval. The E.U. also governs among other areas, the authorization and conduct of clinical trials, the marketing authorization process for medical products, manufacturing and import activities, and post-authorization activities including pharmacovigilance. The E.U. has established regulations on pediatric medicines which impose certain obligations on pharmaceutical companies with respect to the investigation of their products in children.
Japan
In Japan, pre-marketing approval and clinical studies are required for all pharmaceutical products. The regulatory requirements for pharmaceuticals in Japan have in the past been so lengthy and costly that it has been cost-prohibitive for many pharmaceutical companies. Historically, Japan has required that pivotal clinical data submitted in support of a NDA be performed on Japanese patients. Recently, however, as a part of the global drug harmonization process, Japan has signaled a willingness to accept U.S. or E.U. patient data when submitted along with a bridging study, which demonstrates that Japanese and non-Japanese subjects react comparably to the product. This approach, which is executed on a case-by-case basis, may reduce the time required for approval and introduction of new products into the Japanese market. To obtain manufacturing/marketing approval, we must submit an application for approval to the MHLW with results of nonclinical and clinical studies to show the quality, efficacy and safety of a new drug. A data compliance review, GCP on-site inspection, cGMP audit and detailed data review are undertaken by the PMDA. The application is then discussed by the committees of the Pharmaceutical Affairs and Food Sanitation Council (PAFSC). Based on the results of these reviews, the final decision on approval is made by MHLW. After the approval, negotiations regarding the reimbursement price with MHLW will begin. The price will be determined within 60 to 90 days unless the applicant disagrees, which may result in extended pricing negotiations.
| 14 |
Regulation of the Health Care Industry
In addition to the regulatory approval requirements described above, we are or will be directly or indirectly through our customers, subject to extensive regulation of the health care industry by the federal and state government and foreign countries in which we may conduct our business. The laws that directly or indirectly affect our ability to operate our business include the following:
- The federal Medicare and Medicaid Anti-Kickback laws, which prohibit persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual, or furnishing or arranging for a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs;
- Other Medicare laws, regulations, rules, manual provisions and policies that prescribe the requirements for coverage and payment for services performed by our customers, including the amount of such payment;
- The federal False Claims Act which imposes civil and criminal liability on individuals and entities who submit, or cause to be submitted, false or fraudulent claims for payment to the government;
- The False Claims Act which prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
- The Foreign Corrupt Practices Act (FCPA), which prohibits certain payments made to foreign government officials;
- State and foreign law equivalents of the foregoing and state laws regarding pharmaceutical company marketing compliance, reporting and disclosure obligations; and
- The Patient Protection and Affordable Care Act (ACA), which among other things changes access to healthcare products and services; creates new fees for the pharmaceutical and medical device industries; changes rebates and prices for health care products and services; and requires additional reporting and disclosure.
If our operations are found to be in violation of any of these laws, regulations, rules or policies or any other law or governmental regulation, or if interpretations of the foregoing change, we may be subject to civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment or restructuring of our operations.
Pharmaceutical Pricing and Reimbursement
In the U.S. and other countries, sales of any products for which we receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third-party payers. Third-party payers include government health administrative authorities, managed care providers, pharmacy benefit managers, private health insurers and other organizations. These third-party payers are increasingly challenging the price and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare product candidates. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our products. Our products may not be considered cost-effective. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
United States
Federal, state and local governments in the U.S. continue to work towards significant legislation aimed to limit the growth of healthcare costs, including the cost of prescription drugs. Following the U.S. Supreme Court decision in June 2012 upholding the Patient Protection and Affordable Care Act there has been an increase in the pace of regulatory issuances by those U.S. government agencies designated to carry out the extensive requirements of the ACA. These regulatory actions are expected to have both positive and negative impacts on the U.S. healthcare industry, although uncertainty remains regarding the ACA’s ultimate effects. This legislation has both current and long term impacts on us. The provisions of the U.S. Healthcare Reform Act are effective on various dates over the next several years.
Medicaid is a joint federal and state program that is administered by the states for low income and disabled beneficiaries. Under the Medicaid Drug Rebate Program, we are required to pay a rebate for each unit of product reimbursed by the state Medicaid programs. The amount of the rebate for each product is set by law as the greater of 23.1% of the average manufacturer price (AMP) or the difference between AMP and the best price available from us to any customer (with limited exceptions). The rebate amount must be adjusted upward if AMP increases more than inflation (measured by the Consumer Price Index - Urban). The adjustment can cause the rebate amount to exceed the minimum 23.1% rebate amount. The rebate amount is calculated each quarter based on our report of current AMP and best price for each of our products to the Centers for Medicare & Medicaid Services. The requirements for calculating AMP and best price are complex. We are required to report any revisions to AMP or best price previously reported within a certain period, which revisions could affect our rebate liability for prior quarters. In addition, if we fail to provide information timely or we are found to have knowingly submitted false information to the government, the statute governing the Medicaid Drug Rebate Program provides for civil monetary penalties.
| 15 |
Medicare is a federal program that is administered by the federal government that covers individuals age 65 and over as well as those with certain disabilities. Medicare Part D provides coverage to enrolled Medicare patients for self-administered drugs (i.e., drugs that do not need to be injected or otherwise administered by a physician). Medicare Part D is administered by private prescription drug plans approved by the U.S. government and each drug plan establishes its own Medicare Part D formulary for prescription drug coverage and pricing, which the drug plan may modify from time-to-time. The prescription drug plans negotiate pricing with manufacturers and may condition formulary placement on the availability of manufacturer discounts. Manufacturers, including us, are required to provide a 50% discount on brand name prescription drugs utilized by Medicare Part D beneficiaries when those beneficiaries reach the coverage gap in their drug benefits.
Our products are subject to discounted pricing when purchased by federal agencies via the Federal Supply Schedule (FSS). FSS participation is required for our products to be covered and reimbursed by the Veterans Administration (VA) Department of Defense, (DoD), Coast Guard, and Public Health Service (PHS). Coverage under Medicaid, the Medicare Part B program and the PHS pharmaceutical pricing program is also conditioned upon FSS participation. FSS pricing is negotiated periodically with the Department of Veterans Affairs. FSS pricing is intended not to exceed the price that we charge our most-favored non-federal customer for a product. In addition, prices for drugs purchased by the VA, DoD (including drugs purchased by military personnel and dependents through the TriCare retail pharmacy program), Coast Guard, and PHS are subject to a cap on pricing equal to 76.0% of the non-federal average manufacturer price, or non-FAMP. An additional discount applies if non-FAMP increases more than inflation (measured by the Consumer Price Index - Urban). In addition, if we fail to provide information timely or we are found to have knowingly submitted false information to the government, the governing statute provides for civil monetary penalties in addition to other penalties available to the government.
To maintain coverage of our products under the Medicaid Drug Rebate Program, we are required to extend discounts to certain purchasers under the PHS pharmaceutical pricing program. Purchasers eligible for discounts include hospitals that serve a disproportionate share of financially needy patients, community health clinics and other entities that receive health services grants from the PHS.
Canada
The purpose of the PMPRB is to ensure that prices of patented and non-patented medicines are not excessive. Accordingly, the PMPRB monitors and reports prices of non-patented drugs and publishes annual reports on the prices using international median prices as a benchmark. The PMPRB does not set drug prices, analyze relative cost effectiveness or value of new drugs or take an active role in formulary listing and reimbursement pricing as these responsibilities are assumed either by the provinces and territories. In order to determine whether the price for a given drug is “excessive”, new drugs are labelled in one of three categories: Category 1 refers to line extensions of existing medicines. The price of a Category 1 drug is presumed excessive if it does not bear a reasonable relationship to the price of other medicines of the same strength sold by the patentee; Category 2 refers to breakthrough or substantial improvements over existing drugs. The price of a Category 2 drug is presumed excessive if it exceeds the prices of all the medicines in the same therapeutic class or the median of the prices in seven countries (France, Germany, Italy, Sweden, Switzerland, the U.K. and the U.S.). Category 3 refers to new chemical entities offering moderate, little or no therapeutic improvement. The price of a Category 3 drug is presumed excessive if it exceeds the prices of all the medicines in the same therapeutic class. The PMPRB also monitors the price of existing drugs, which is considered excessive if it exceeds the increase in the general Canadian Consumer Price Index. When manufacturers set the price of a patented medicine too high, the PMPRB first attempts to have the manufacturer reduce the price voluntarily. Barring this, it can hold a public hearing into the price following which it can order the manufacturer to reduce the price, withdraw the manufacturer’s market authorization, or impose a fine equal to or double the amount of the excessive increase in price.
Europe
Different pricing and reimbursement schemes exist in other countries. In Europe, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of such products to consumers. The approach taken varies from state to state. Some jurisdictions permit products to be marketed only after a reimbursement price has been agreed. Other states allow companies to fix their own prices for medicines, but monitor company profits. In some cases, pharmacoeconomic analyses from clinical studies and other available resources are used to establish pricing using risk-benefit comparisons with currently available products.
In the U.K., pharmaceutical companies set their own price, and then national bodies (e.g., NICE and Scottish Medicines Consortium), sub-national bodies (e.g., Greater Manchester Medicines Management Group and London Procurement Partnership), or local bodies (e.g., Clinical Commissioning Groups and Health Boards) will determine if a medicine is cost-effective. The national and sub-national bodies advise local bodies, which are greatly influenced by a NICE endorsement; however, ultimately the decision to pay for a medicine is made at a local level in the U.K.
In Switzerland, the Swiss health care system is a compulsory private system where patients pay a monthly variable fee to a registered health insurance fund. All insurers reimburse against a common national formulary, the SL. The BAG makes the decisions on reimbursement and pricing of all prescription drugs in the market with their review taking three to four months. For new drugs, it is not uncommon for there to be several rounds of review. It also conducts regular price reviews of the drugs on the formulary. The Federal Commission on drugs or Arzneimittelkommission (EAK) is a body assisting the BAG with expert advice. Once a product is approved the BAG, in consultation with EAK, decides whether or not the drug will appear on the SL. After EAK’s evaluation of a drug, BAG and EAK decide on the maximum price in the market. The criteria used are:
- Internal comparison with reimbursed and non-reimbursed therapeutic equivalents,
- External cross country comparison (reference countries: Denmark, Germany, the U.K. and the Netherlands), and
- Cost benefit analysis
| 16 |
Japan
In Japan, pricing is established utilizing various information including reference prices from other international markets. However, the MHLW biannually reviews the pharmaceutical prices of individual products. In the past, these reviews have resulted in price reductions. We expect similar price reviews in the future, in line with the government’s previously announced plan for controlling health care costs. It is not possible to predict the outcome of these reviews, and it is possible that Japanese authorities will again reduce drug reimbursement rates, which could adversely affect the reimbursement levels for our products or product candidates.
Regulation Pertaining to Sales and Marketing
We are subject to various federal and state laws pertaining to health care “fraud and abuse,” including anti-kickback laws and false claims laws. Anti-kickback laws generally prohibit a prescription drug manufacturer from soliciting, offering, receiving, or paying any remuneration to generate business, including the purchase or prescription of a particular drug. Although the specific provisions of these laws vary, their scope is generally broad and there may be no regulations, guidance or court decisions that clarify how the laws apply to particular industry practices. There is therefore a possibility that our practices might be challenged under the anti-kickback or similar laws. False claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for payment to third party payers (including Medicare and Medicaid), claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. Our activities relating to the sale and marketing of our products may be subject to scrutiny under these laws. Violations of fraud and abuse laws may be punishable by criminal or civil sanctions, including fines and civil monetary penalties, and exclusion from federal health care programs (including Medicare and Medicaid). Federal and state authorities are paying increased attention to enforcement of these laws within the pharmaceutical industry and private individuals have been active in alleging violations of the laws and bringing suits on behalf of the government under the federal False Claims Act. If we were subject to allegations concerning, or were convicted of violating, these laws, our business could be harmed.
Laws and regulations have been enacted by the federal government and various states to regulate the sales and marketing practices of pharmaceutical manufacturers. The laws and regulations generally limit financial interactions between manufacturers and health care providers or require disclosure to the government and public of such interactions. The laws include federal “sunshine” provisions enacted in 2010 as part of the comprehensive federal health care reform legislation. The sunshine provisions apply to pharmaceutical manufacturers with products reimbursed under certain government programs and require those manufacturers to disclose annually to the federal government (for re-disclosure to the public) certain payments made to physicians and certain other healthcare practitioners or to teaching hospitals. State laws may also require disclosure of pharmaceutical pricing information and marketing expenditures. Many of these laws and regulations contain ambiguous requirements. Given the lack of clarity in laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent federal and state laws and regulations. Outside the U.S., other countries have implemented requirements for disclosure of financial interactions with healthcare providers and additional countries may consider or implement such laws.
Other Regulations
Foreign Anti-Corruption
We are subject to various federal and foreign laws that govern our international business practices with respect to payments to government officials. Those laws include the U.S. Foreign Corrupt Practices Act which prohibits U.S. companies and their representatives from paying, offering to pay, promising, or authorizing the payment of anything of value to any foreign government official, government staff member, political party, or political candidate for the purpose of obtaining or retaining business or to otherwise obtain favorable treatment or influence a person working in an official capacity. In many countries, the health care professionals we regularly interact with may meet the Foreign Corrupt Practices Act (FCPA) definition of a foreign government official. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect their transactions and to devise and maintain an adequate system of internal accounting controls.
The laws to which we are subject also include the U.K. Bribery Act 2010 (Bribery Act) which proscribes giving and receiving bribes in the public and private sectors, bribing a foreign public official, and failing to have adequate procedures to prevent employees and other agents from giving bribes. U.S. companies that conduct business in the U.K. generally will be subject to the Bribery Act. Penalties under the Bribery Act include potentially unlimited fines for companies and criminal sanctions for corporate officers under certain circumstances.
Other Laws
Our present and future business has been and will continue to be subject to various other laws and regulations. Various laws, regulations and recommendations relating to safe working conditions, laboratory practices, the experimental use of animals, and the purchase, storage, movement, import and export and use and disposal of hazardous or potentially hazardous substances used in connection with our research work are or may be applicable to our activities. Certain agreements entered into by us involving exclusive license rights may be subject to national or international antitrust regulatory control, the effect of which cannot be predicted. The extent of government regulation, which might result from future legislation or administrative action, cannot accurately be predicted.
| 17 |
Employees
As of March 1, 2017, we had 139 full-time employees, including 63 with doctoral or other advanced degrees. Of our workforce, 94 employees are engaged in research, development and manufacturing, and 45 are engaged in business development, legal, finance, administration and sales and marketing. None of our employees are represented by a labor union or covered by collective bargaining agreements. We have never experienced a work stoppage and believe our relationship with our employees is good.
Research and Development
For information regarding research and development expenses incurred during 2016, 2015 and 2014, see Item 7, “Management Discussion and Analysis of Financial Condition and Results of Operations—Research and Development Expenses”.
Financial Information About Geographic Areas
Consolidated revenues by geographic area where derived were as follows:
| Years Ended December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| United States | $ | 140,240 | $ | 95,769 | $ | 74,688 | ||||||
| Japan | 83,130 | 55,371 | 32,128 | |||||||||
| Rest of the world | 6,686 | 2,040 | 8,634 | |||||||||
| Total | $ | 230,056 | $ | 153,180 | $ | 115,450 | ||||||
Total revenues generated outside the U.S. were $89.8 million, $57.4 million and $40.8 million in the years ended December 31, 2016, 2015 and 2014, respectively.
Property and equipment, net by geographic area where located was as follows:
| December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| United States | $ | 3,065 | $ | 3,105 | $ | 566 | ||||||
| Japan | 3,119 | 3,232 | 114 | |||||||||
| Rest of the world | 32 | 56 | 83 | |||||||||
| Total | $ | 6,216 | $ | 6,393 | $ | 763 | ||||||
Our Class Capital Structure
We have two classes of common stock authorized; class A common stock and class B common stock. In 2012, our then-majority stockholder and only holder of our class B common stock converted all outstanding shares of our class B common stock into shares of our class A common stock. We are not authorized to issue additional shares of class B common stock except in limited circumstances. As a result of the conversion, there is now only a single class of outstanding common stock, class A common stock, which is entitled to one vote per share.
Our Corporate Information
We were incorporated under the laws of Delaware in December 1996.
| 18 |
The following is a list of our direct and indirect subsidiaries as of December 31, 2016:
| Subsidiary | State or other jurisdiction of incorporation or organization | |
| Sucampo Pharma Americas, LLC | Delaware | |
| Sucampo LLC | Delaware | |
| Sucampo AG | Switzerland | |
| Sucampo Pharma, LLC | Japan | |
| Sucampo Pharma Europe Ltd. | United Kingdom | |
| Sucampo Acquisitions GmbH | Switzerland |
Our principal executive offices are located at 805 King Farm Boulevard, Suite 550, Rockville, Maryland 20850, and our telephone number is (301) 961-3400.
Website Access to United States Securities and Exchange Commission Reports
Our Internet address is http://www.sucampo.com. Through our website, we make available, free of charge, access to all reports filed with the U.S. Securities and Exchange Commission (SEC) including our Annual Reports on Form 10-K, our Quarterly Reports on Form 10-Q, our Current Reports on Form 8-K and amendments to these reports, as filed with or furnished to the SEC pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (Exchange Act), as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Copies of any materials we file with, or furnish to, the SEC can also be obtained free of charge through the SEC’s website at http://www.sec.gov or at the SEC’s Public Reference Room at 100 F Street, N.E., Room 1580, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
| ITEM 1A. | RISK FACTORS |
Before deciding to purchase, hold or sell our common stock, you should carefully consider the risks described below in addition to the other cautionary statements and risks described elsewhere and the other information contained in this report and in our other filings with the SEC, including subsequent Quarterly Reports on Forms 10-Q and Current Reports on Form 8-K. We operate in a rapidly changing environment that involves numerous risks. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our business. These known and unknown risks could materially and adversely affect our business, financial condition, prospects, operating results or cash flows.
Risks Related to Our Business and Industry
If we are unable to continue successful commercialization of AMITIZA for the approved indications and other indications or dosage forms for which we are developing this drug, or experience significant delays in doing so, our ability to generate royalty and product-based revenues and achieve profitability will be jeopardized.
Our business currently depends entirely on the successful commercialization of our first product, lubiprostone. Lubiprostone was launched in the U.S. in 2006 under the brand name AMITIZA. AMITIZA is currently marketed in the U.S., U.K., Switzerland and Japan for various indications. We have a limited history of generating global revenues from the sale of lubiprostone. Prior to the acquisition of R-Tech (the Acquisition), R-Tech was responsible for the manufacture and supply of all of our drug products for commercial use and clinical development. Through the Acquisition, we obtained control over the manufacturing and supply chain of AMITIZA. This increased responsibility could detract attention from operating the day-to-day components of our business prior to the Acquisition.
Our ability to meet expectations with respect to global sales of lubiprostone and revenues from such sales, and to attain profitability and maintain positive cash flow from the lubiprostone business, in the time periods we anticipate, or at all, will depend on a number of factors, including the following:
| · | our and our partners’ ability to continue to build, and to maintain, market acceptance for lubiprostone among healthcare professionals and patients in the U.S., and to gain such market acceptance in the countries where lubiprostone is approved, or may in the future receive approval; |
| · | the efforts of Takeda and Mylan to commercialize and maximize net sales revenue of AMITIZA; |
| · | the degree to which both physicians and patients determine that the safety and side effect profiles of lubiprostone are manageable, and that the benefits of lubiprostone outweigh the risks; |
| · | the current and future prevalence of CIC, IBS-C, OIC, or chronic constipation; |
| · | the willingness of insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs in the U.S. to continue to provide reimbursement for lubiprostone at the prices at which we offer lubiprostone without imposing any additional major hurdles to access or other significant restrictions or limitations, and the ability and willingness of patients to commit to any co-pay amounts for lubiprostone applicable under their insurance coverage; |
| 19 |
| · | our commercial partners’ ability to obtain pricing approval and/or reimbursement required for selling lubiprostone in the major countries of the E.U., Japan and in other countries in which we may receive approval to market lubiprostone on a timely basis and at price levels that are acceptable to us without the applicable government agencies or other payers in such countries imposing onerous caps, rebate, risk sharing or other requirements which effectively and significantly lower the reimbursement rates for lubiprostone; |
| · | the extent of the likely negative impact of the introduction of new competitive products on sales of lubiprostone; |
| · | our ability to gain regulatory approval of lubiprostone outside the countries in which we have already received approval without restrictions that are substantially more onerous or manufacturing specifications that are more difficult to consistently achieve than those imposed in the U.S. and E.U.; |
| · | our ability to accurately forecast revenues from sales of lubiprostone and the metrics that impact revenues, such as prescription rate, short-term and long-term drop-out rate, conversion rate, reimbursement and pricing; the timing and availability of named patient sales and the impact of future competition; |
| · | our ability to successfully gain approval of a dosage form of lubiprostone for pediatric functional constipation, and to generate revenues from sales of the dosage form for pediatric functional constipation, if approved; |
| · | successful completion of clinical trials of AMITIZA for the treatment of other constipation-related gastrointestinal indications beyond CIC, IBS-C and OIC as well as other dosage forms other than the 24 mcg and 8 mcg soft gelatin capsule, and successful commercialization of these indications and dosage forms within and outside the U.S.; |
| · | our ability to manufacture sufficient bulk quantities of active pharmaceutical ingredient and sufficient quantities of each dosage strength and dosage form of lubiprostone to meet demand; |
| · | our ability to hire and retain key personnel necessary to optimize the lubiprostone business; and |
| · | our and our partners’ ability to continue to execute effectively on key activities related to lubiprostone in the U.S. and to launch lubiprostone successfully in those key markets outside the U.S. in which we receive pricing and reimbursement approval, and the level of cost required to conduct such activities. |
AMITIZA faces significant competition from competitors’ products such as Linzess (marketed) and Trulance (approved, to launch in H1 2017), which, in addition to other factors, could in certain circumstances lead to a significant reduction in royalty revenues and product sales.
As a general matter, the pharmaceutical industry is highly competitive. To be successful, we must be able to, among other things, effectively discover, develop, test and obtain regulatory approvals for products. We or our partners must be able to effectively commercialize, market and promote approved products, including communicating the effectiveness, safety and value of products to actual and prospective customers and medical professionals. Many of our competitors have greater resources than we have. This enables them, among other things, to make greater investments in research and development, marketing and promotion.
Our product, AMITIZA, faces competition from competitors’ products. Specifically, AMITIZA faces competition from linaclotide which, in the U.S. and Canada, is approved for two of the three indications for which AMITIZA has been approved, and, in certain European countries, is approved for IBS-C. Its manufacturer is seeking approval in other markets for IBS-C that we currently or intend to market AMITIZA. We also face competition from naloxegol which is approved for OIC in the U.S. and E.U. Competitor products such as linaclotide and naloxegol may be more effective or more effectively marketed and sold than AMITIZA is by our partners or by us. Alternatively, in the case of generic competition, including the generic availability of competitors’ branded products, they may be equally safe and effective products that are sold at a substantially lower price than our products. As a result, if we fail to maintain our competitive position, this could have a material adverse effect on our business, cash flow, results of operations, financial position and prospects.
Developments by our competitors, the entry of new competitors into the markets in which we compete, or consolidation in the pharmaceutical industry could make our products or technologies less competitive or obsolete. Our future growth depends, in part, on our ability to develop and introduce products which are more effective than those developed by our competitors. Royalties or sales from our existing products may decline rapidly if a new product is introduced that represents a substantial improvement over our existing products.
Our future success depends upon our ability to develop new products, and new indications for existing products, that achieve regulatory approval for commercialization.
For our business model to be successful, we must continually develop, manufacture and commercialize new products or achieve approval for new indications or label extensions for the use of our existing products. Prior to commercialization, these new products and product indications must satisfy stringent regulatory standards and receive requisite approvals or clearances from regulatory authorities in the U.S. and other countries. The development, regulatory review and approval, and commercialization processes are time consuming, costly and subject to numerous factors that may delay or prevent the development, approval or clearance, and commercialization of new products, including legal actions brought by our competitors. To obtain approval or clearance of new indications or products, we must submit, among other information, the results of preclinical and clinical studies on the new indication or product candidate to the applicable regulatory authorities. The number of preclinical and clinical studies that will be required for regulatory approval varies depending on the regulatory authority, the new indication or product candidate, the disease or condition for which the new indication or product candidate is in development and the regulations applicable to that new indication or product candidate. Even if we believe that the data collected from clinical trials of new indications for our existing products or for our product candidates are promising, applicable regulatory authorities may find such data to be insufficient to support approval of the new indication or product. The regulatory authority can delay, limit or deny approval or clearance of a new indication or product candidate for many reasons, including:
| • | the product is not safe or effective either generally or for a new indication; |
| 20 |
| • | our preclinical and clinical data is interpreted in different ways than we interpret that data; | |
| • | we may be required to perform post-marketing clinical studies; or | |
| • | there may be changes in the approval policies or adoption of new regulations. |
Products that we are currently developing, other future product candidates or new indications or label extensions for our existing products, may or may not receive the regulatory approvals or clearances necessary for marketing or may receive such approvals or clearances only after delays or unanticipated costs.
We continue to rely on third parties for the successful commercialization of our drug products. The success of these third parties will affect our ability to continue to develop new drug candidates.
For most of our operating history, we have been a research and development company. As we continue to expand our management, organizational and operational capabilities, expand our global partnerships, develop our diversified product pipeline, acquire non-prostone clinical candidates, and enhance our capital structure, our operations will focus on organizing and staffing our company, building the necessary infrastructure to support these capabilities, developing the pipeline assets which we may acquire, undertaking preclinical and clinical trials of our product candidates, and pursuing the regulatory approval processes for additional indications for AMITIZA. Though we will continue to rely upon Takeda and Mylan to commercialize AMITIZA in most of the world, we may not be able to cause these third parties to effectively market and sell AMITIZA. In addition, we may encounter unforeseen expenses, difficulties, complications and delays as Takeda obtains regulatory approvals and establishes the commercial markets for AMITIZA outside of North America, Japan and China. As we continue to develop and seek regulatory approval of our product candidates, both within and outside the U.S., it could be difficult for us to access capital, to build the necessary infrastructure, to obtain and devote the resources necessary to obtain and develop product candidates, to effectively sell our products, and to provide resources to support commercialization of our products.
We are subject to on-going obligations to monitor the safety of our products and product candidates. Any failure to meet these obligations could adversely affect our ability to generate revenue.
Safety problems or signals can arise as our products are marketed and our product candidates are evaluated in clinical trials. With our collaborators, we are required to continuously collect and assess adverse events reported to us and to communicate to regulatory agencies these adverse events and safety signals regarding our products. Regulatory agencies periodically perform inspections of our pharmacovigilance processes, including our adverse event reporting. If regulatory agencies determine that we or our collaborators have not complied with the applicable reporting or other pharmacovigilance requirements, we may become subject to additional inspections, warning letters or other enforcement actions, including monetary fines, marketing authorization withdrawal and other penalties.
We face potential product liability exposure, and, if claims are brought against us, we may incur substantial liability.
The use of lubiprostone or any other product candidate in clinical trials and the sale of AMITIZA or any other product candidate for which we obtain marketing approval expose us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers or others selling or otherwise coming into contact with our product and product candidates. If we cannot successfully defend ourselves against product liability claims, we could incur substantial liabilities. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| · | decreased demand for lubiprostone or any other product candidate for which we obtain marketing approval; |
| · | impairment of our business reputation and exposure to adverse publicity; |
| · | increased warnings on product labels; |
| · | withdrawal of clinical trial participants; |
| · | costs as a result of related litigation; |
| · | distraction of management’s attention from our primary business; |
| 21 |
| · | substantial monetary awards to patients or other claimants; |
| · | loss of revenue; and |
| · | the inability to successfully commercialize lubiprostone or any other product candidate for which we obtain marketing approval. |
We have obtained product liability insurance coverage for both our clinical trials and our commercial exposures. However, our insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects or warnings found to be inadequate. The cost of any product liability litigation or other proceedings, even if resolved in our favor, could be substantial. A product liability claim or series of claims brought against us could cause our stock price to decline and, if the claim is successful and judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
Recent federal legislation, including potentially unfavorable pricing regulations or other healthcare reform initiatives, and other negative pricing trends could limit our ability to generate revenues.
In March 2010, the Patient Protection and Affordable Care Act (ACA) was enacted in the U.S. In 2012, the U.S. Supreme Court upheld the ACA. This legislation may have both immediate and long-term impacts on us. A number of the provisions of legislation require rulemaking action by governmental agencies to implement, many of which have not yet occurred. The laws change access to health care products and services and create new fees for the pharmaceutical and medical device industries. Future rulemaking could increase rebates, reduce prices or the rate of price increases for health care products and services, or require additional reporting and disclosure. Additionally, we cannot predict the impact of uncertainty regarding the potential repeal of the ACA may have on us nor can we predict the timing or impact of any legislation or future rulemaking.
The regulations that govern, among other things, regulatory approvals, coverage, pricing and reimbursement for new drug products vary widely from country to country. In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay regulatory approval of our product candidates, restrict or regulate post-approval activities and affect our ability to successfully sell any product candidates for which we obtain regulatory approval.
In the U.S., the E.U., and other potentially significant markets for our product candidates, government authorities and third-party payers are increasingly attempting to limit or regulate the price of medical products and services, particularly for new and innovative products and therapies, which has resulted in lower average selling prices. Furthermore, the increased emphasis on managed healthcare in the U.S. and on country and regional pricing and reimbursement controls in the E.U. will put additional pressure on product pricing, reimbursement and usage, which may adversely affect our future product sales and results of operations. These pressures can arise from rules and practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and healthcare reform, pharmaceutical reimbursement policies and pricing in general.
We may generate growth through acquisitions and in-licensing and such strategy may not be successful if we are not able to identify suitable acquisition or licensing candidates, to negotiate appropriate terms of any such transaction or to successfully manage the integration of any acquisition.
As part of our business strategy, we intend to continue pursuing strategic acquisitions and in-licensing opportunities with third parties for our existing products and to complement our existing product pipeline. We have limited experience in completing acquisitions with third parties as well as performing under in-licensing agreements and we may not be able to identify appropriate acquisition or licensing candidates or to successfully negotiate the terms of any such transaction. The licensing and acquisition of pharmaceutical and biological products is a competitive area. A number of more established companies are also pursuing strategies to license or acquire products in the pharmaceutical field, and they may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities. If we are unable to successfully complete acquisitions or in-licensing transactions for suitable products and product candidates, our prospects for growth could suffer.
Even if we are successful in completing one or more acquisitions, the failure to adequately address the financial, operational or legal risks of these transactions could harm our business. To finance an acquisition, we could be required to use our cash resources, issue potentially dilutive equity securities or incur or assume debt or contingent liabilities. Accounting for acquisitions can require impairment losses or restructuring charges, large write-offs of in-process research and development expense and ongoing amortization expenses related to other intangible assets. In addition, integrating acquisitions can be difficult, and could disrupt our business and divert management resources. If we are unable to manage the integration of any acquisitions successfully, our ability to develop new products and continue to expand our product pipeline may be impaired.
| 22 |
Risks Related to Our Commercial Operations
We have a relatively short history of profitability. We may not maintain operating profitability in the future, and this could force us to delay, reduce or abandon our commercialization efforts or product development programs.
We have recorded net income since 2012. However, we expect to continue to incur significant and increasing expenses for at least the next several years as we continue our research activities, conduct development of our product candidates, seek and develop new products and compounds, seek regulatory approvals for additional indications and additional territories for AMITIZA and for other drug candidates, and protect the patents of our products from generic challenges. Regulatory changes and changes in market conditions, including the generic competition, may require us to incur more expenses or change the timing of expenses such that we may incur unexpected losses. We may not be able to sustain or increase profitability on a quarterly or annual basis. If we are unable to maintain profitability, the market value of our class A common stock may decline.
We may need substantial additional funding and be unable to raise capital when needed, which could force us to delay, reduce or abandon our commercialization efforts or product development programs.
We expect our research and development expenses and selling, general and administrative expenses to increase in connection with our ongoing activities. We may need substantial additional funding and be unable to raise capital when needed or on attractive terms, which would force us to delay, reduce or abandon our development programs.
We have continued to finance much of our operations by payments received under our collaboration agreements with Takeda and Mylan. We believe that our existing cash and cash equivalents and internally generated funds that we anticipate from AMITIZA royalty revenues and product sales will be sufficient to enable us to fund our current operating expenses but not for all of our future research and development programs. Our future funding requirements, however, will depend on many factors, including:
| • | actual levels of product royalty and product sales from AMITIZA; | |
| • | the cost of commercialization activities, including product marketing, sales and distribution; | |
| • | the scope and results of our research, preclinical and clinical development activities; | |
| • | the timing of, and the costs involved in, obtaining regulatory approvals; | |
| • | the costs involved in obtaining and maintaining proprietary protection for our products, technology and know-how, including litigation costs and the results of such litigation; | |
| • | our ability to recruit and retain internal qualified human resources to conduct these activities; | |
| • | the extent to which we acquire or invest in businesses, products and technologies; | |
| • | the success of our collaboration with Takeda and Mylan; | |
| • | the success of the commercialization efforts of AMITIZA; and | |
| • | our ability to establish and maintain additional collaborations. |
If we are required to raise additional funds from external sources, we might accomplish this through at-the-market sales, public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. If we raise additional funds by at-the-market sales or issuing equity securities, current stockholders may experience dilution. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish valuable rights and related intellectual property to our technologies, research programs, products or product candidates.
In connection with the acquisition of R-Tech in October 2015, we entered into the Credit Facility and, under such facility, issued secured promissory notes in the aggregate amount of approximately $250.0 million to various lenders (the Term Notes). As of December 27, 2016 we repaid the entire outstanding balance of the Term Notes through a private offering of the Convertible Notes described below.
On December 27, 2016, we issued $300.0 million aggregate principal amount of our 3.25% Convertible Senior Notes due 2021 (the Convertible Notes) to Leerink Partners LLC, who subsequently resold the Convertible Notes to qualified institutional buyers in reliance on the exemption from registration provided by Rule 144A under the Securities Act of 1933, as amended. Regarding the Convertible Notes, if we do not generate sufficient cash flows from our operations, we may not be able to pay the obligations of the Convertible Notes upon their maturity date in December 2021, which may adversely affect our operating results. Our failure to comply with the covenants and/or obligations related to the Convertible Notes could result in an event of default or a fundamental change (as defined in the indenture relating to the Convertible Notes), which could result in an immediate acceleration of the of the maturity of the Convertible Notes or immediate repurchase for cash all or any portion of the Convertible Notes at a fundamental change repurchase price equal to 100% of the principal amount of the notes to be repurchased, plus accrued and unpaid interest. These outcomes would materially and adversely affect our operating results and our financial condition. As of December 31, 2016, we were compliant with our covenants and conditions under the Convertible Notes.
| 23 |
We are developing internationally and licensing our products globally; therefore, we have an increased exposure to foreign political conditions and regulatory requirements and fluctuations in foreign currency exchange rates.
We expect that we will continue to seek global opportunities for our products and to develop candidates internationally in the future. Such opportunities and development will inherently subject us to a number of risks and uncertainties, including:
| · | changes in international regulatory and compliance requirements that could restrict our ability to develop, market and sell our products; |
| · | political and economic instability; |
| · | diminished protection of intellectual property in some countries outside of the U.S.; |
| · | trade protection measures and import or export licensing requirements; |
| · | difficulty in staffing and managing international operations; |
| · | differing labor regulations and business practices; |
| · | potentially negative consequences from changes in or interpretations of tax laws; |
| · | changes in international medical reimbursement policies and programs; |
| · | financial risks such as longer payment cycles, difficulty collecting accounts receivable and exposure to fluctuations in foreign currency exchange rates; and |
| · | regulatory and compliance risks that relate to maintaining accurate information and control over sales and distributors’ and service providers’ activities that may fall within the purview of the FCPA or similar foreign laws such as the U.K. Bribery Act. |
Any of these factors may, individually or as a group, have a material adverse effect on our business and results of operations. These or other similar risks could adversely affect our revenue and profitability. As we develop internationally, our exposure to these factors will increase.
Risks Related to Product Pipeline
If our preclinical studies do not produce successful results or if our clinical trials do not demonstrate safety and efficacy in humans, our ability to develop and commercialize our pipeline will be impaired, which may jeopardize our business.
Before obtaining regulatory approval for the sale of our product candidates, we must conduct extensive preclinical tests and clinical trials to demonstrate the safety and efficacy in humans of our product candidates. Preclinical and clinical testing is expensive, is difficult to design and implement, can take many years to complete, is subject to varying regulatory requirements and is uncertain as to outcome. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, preclinical testing and the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates, including:
| · | regulators or institutional review boards may not authorize us to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| · | clinical research organizations we retain to conduct clinical trials may not perform according to the terms of the contract, causing delays or negative results in the clinical trials; |
| · | our preclinical tests or clinical trials may produce negative or inconclusive results, and as a result we may decide, or regulators may require us, to conduct additional preclinical testing or clinical trials or we may abandon projects altogether; |
| · | design of or enrollment in our clinical trials may be slower than we currently anticipate, resulting in significant delays, or participants may drop out of our clinical trials at rates that are higher than we had anticipated; |
| · | we might have to suspend or terminate our clinical trials, or perform additional trials, if we discover that the participating patients are being exposed to unacceptable health risks; |
| · | regulators or institutional review boards may require that we hold, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements; |
| · | the cost of our clinical trials may be greater than we currently anticipate; |
| · | we might have difficulty obtaining sufficient quantities of the product candidate being tested to complete our clinical trials; |
| · | any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the product not commercially viable; |
| 24 |
| · | many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, site selection, conducting clinical trials, obtaining regulatory approvals, and marketing approved products than we do and smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies; |
| · | the effects of our product candidates may not be the desired or anticipated effects or may include undesirable side effects, or the product candidates may have other unexpected characteristics; and |
| · | if we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete our clinical trials or other testing or if the results of these trials or tests are not positive or are only modestly positive, we may be delayed in obtaining marketing approval for our product candidates, not be able to obtain marketing approval, or obtain approval for indications that are not as broad as those for which we apply. |
Our product development costs will also increase if we experience delays in testing or approvals. We do not know whether our clinical trials will begin as planned, will need to be restructured or will be completed on schedule, if at all. Significant clinical trial delays also could allow our competitors to bring products to market before we do and impair our ability to commercialize our products or product candidates.
We may fail to select or capitalize on the most scientifically, clinically, or commercially promising or profitable product candidates.
We continue to evaluate our business strategy and, as a result, may modify our strategy in the future. In this regard, we may, from time to time, focus our product development efforts on different product candidates or may delay or halt the development of various product candidates. As a result of changes in our strategy, we may change or refocus our existing product development, commercialization and manufacturing activities. This could require changes in our facilities and our personnel. Any product development changes that we implement may not be successful. In particular, we may fail to select or capitalize on the most scientifically, clinically or commercially promising or profitable product candidates. Our decisions to allocate our research and development, management and financial resources toward particular product candidates or therapeutic areas may not lead to the development of viable commercial products and may divert resources from better opportunities. Similarly, our decisions to delay or terminate product development programs may also prove to be incorrect and could cause us to miss valuable opportunities.
We may perform additional clinical trials for other indications or in support of applications for regulatory marketing approval in jurisdictions outside the U.S. for our products. These supplemental trials could be costly and could result in findings inconsistent with or contrary to our historic U.S. clinical trials.
In the future, we may be required, or we may elect, to conduct additional clinical trials of AMITIZA to improve the current label or address regulatory authorities concerns about AMITIZA. In addition, if we seek marketing approval from regulatory authorities in jurisdictions outside the U.S., they may require us to perform additional clinical trials that would be costly and difficult to know if there will be successful outcomes and to submit data from supplemental clinical trials in addition to data from the clinical trials that supported our U.S. filings with the FDA. Any requirements to conduct supplemental trials would add to the cost of developing our product candidates. Additional or supplemental trials could also produce findings that are inconsistent with the trial results we have previously submitted to the FDA, in which case we would be obligated to report those findings to the FDA. This could result in new restrictions on the existing marketing approval for AMITIZA or could force us to stop selling AMITIZA. Inconsistent trial results could also lead to delays in obtaining marketing approval in the U.S. for other indications for AMITIZA or for other product candidates and could cause regulators to impose restrictive conditions on marketing approvals and could even make it impossible for us to obtain marketing approval. Any of these results could materially impair our ability to generate revenues and to achieve or maintain profitability.
Our agreements with makers of generic AMITIZA products are subject to government scrutiny in the U.S.
We have been involved in patent litigations that have resulted in settlement agreements. We have filed our settlement and license agreements with Par and Dr. Reddy’s Laboratories, Inc. (Dr. Reddy’s) and will file any future settlement agreements with the Federal Trade Commission (FTC) and the Antitrust Division of the Department of Justice for review. The FTC has, in the past, brought actions against some brand and generic companies that have entered into such agreements alleging violations of antitrust laws in connection therewith.
We may receive civil investigative demands from the FTC that requires us to provide the FTC information and documents relating to various settlement and other agreements with makers of generic AMITIZA products following patent infringement claims and litigation, and other efforts principally regarding AMITIZA. If the FTC believes that these or other agreements or efforts violate antitrust laws, it could challenge us through an administrative or judicial proceeding, which could result in the imposition of monetary and/or injunctive relief, including the invalidation of agreements, any of which could have a material adverse effect on our results of operations and financial condition. In addition, any such litigation could be protracted, requiring a substantial commitment of our management’s time and cash expenditures over multiple years.
| 25 |
Risks Related to Manufacturing
Following our acquisition of R-Tech, we now manufacture and supply the active ingredient for our product and product candidates. However, we have limited experience in the management of pharmaceutical manufacturing operations and still rely on third parties for encapsulation, packaging and other manufacturing activities. If we or our third party manufacturers are unable to manufacture AMITIZA or our other product candidates in sufficient quantities, at acceptable quality levels and at acceptable cost and if we are unable to identify a suitable replacement manufacturer, our sales of AMITIZA and our further clinical development and commercialization of other products could be delayed, prevented or impaired.
Although we now control the manufacture and supply of AMITIZA and our other product candidates, following our acquisition of R-Tech, we have little experience in manufacturing pharmaceutical products. In addition, we currently rely, and expect to continue to rely, on various third party suppliers to create the finished, packaged forms of AMITIZA, unoprostone, and any future compounds that we may determine to develop or commercialize. We do not currently have an alternative source of supply for AMITIZA. If we are not able to supply AMITIZA or these other compounds on a timely basis, in sufficient quantities and at acceptable levels of quality and price, and if we are unable to identify an alternate manufacturer to perform these functions on acceptable terms, sales of AMITIZA would be significantly impaired, and our development programs could be seriously jeopardized.
The risks relating to the manufacture of our products include:
| · | we rely solely on our personnel, and that of our third party vendors, for quality assurance and their continued compliance with regulations relating to the manufacture of pharmaceuticals; |
| · | our manufacturing capacity may not be sufficient to produce commercial quantities of our product, or to keep up with subsequent increases in the quantities necessary to meet potentially growing demand; |
| · | we may not have access to the capital necessary to expand our manufacturing facilities in response to our needs; |
| · | if our operations were to be interrupted, or were we to elect to contract with another manufacturer to supply us, it would be difficult and time consuming for us to find an alternate supplier and the change would need to be submitted to and approved by the FDA and/or foreign regulatory agencies; |
| · | we rely on numerous sub-contractors to fulfill its manufacturing obligations, and any difficulty or disruption at one of these sub-contractors could jeopardize our ability to produce AMITIZA or our other products; |
| · | we may experience events, such as a fire or natural disaster, that force us to stop or curtail production for an extended period; and |
| · | we could encounter significant increases in labor, capital or other costs that would make it difficult to produce our products cost-effectively. |
In addition, we currently use one supplier for a key ingredient used in the manufacture of our commercial and clinical products. We could experience delays in production should it become necessary to switch its source of supply for such ingredient to another supplier or to manufacture such ingredient itself. We have subcontracted with a single contract manufacturer to encapsulate the bulk form AMITIZA we supply into soft gelatin capsules and another manufacturer to package the final product for distribution in the U.S. If these subcontractors experience difficulties or delays in performing these services for any reason, our ability to deliver adequate supplies of finished product to physicians and patients will be impaired, which could cause us to lose revenues. In addition, any change in the party providing encapsulation of AMITIZA would need to be approved by the FDA and/or foreign regulatory agencies, and any change in the party packaging the product would need to be submitted to and reviewed by the FDA and/or foreign regulatory agencies, which could increase the time required to replace these subcontractors should that become necessary.
Our current and anticipated future dependence upon these third parties for the manufacture of our products and product candidates may adversely affect our future revenues, our cost structure, our ability to expand globally and our ability to develop product candidates and commercialize any approved products on a timely and competitive basis. In addition, if our ability to manufacture prostones for our clinical trials is impaired for any reason, we likely would experience delays in advancing these trials while we seek to identify and qualify replacement suppliers. We may be unable to obtain replacement supplies on a timely basis, on terms that are favorable to us, or at all.
We and the other third-party manufacturers of our products and product candidates are subject to significant regulations governing manufacturing facilities and procedures.
We, our subcontractors and suppliers and any other potential manufacturer of our products or product candidates may fail to comply with the FDA’s cGMP regulations or other governmental regulations. These regulations govern manufacturing processes and procedures and the implementation and operation of systems to control and assure the quality of products approved for sale. In addition, the FDA or other regulatory agencies outside the U.S. may at any time audit or inspect a manufacturing facility to ensure compliance with cGMP or similar regulations. Our failure, or the failure of our subcontractors and suppliers or any other third-party manufacturer we use, to comply with applicable manufacturing regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure of regulatory authorities to grant marketing approval of our product candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our products and product candidates. For example, in connection with an inspection by the FDA of our manufacturing facilities in Sanda, Japan, in November 2016, the FDA issued a Form 483 letter indicating certain deficiencies with respect to our compliance with current good manufacturing practices. We are remediating the deficiencies and do not expect any adverse impact on our ability to continue manufacturing our products in that facility.
| 26 |
If it were to become necessary for us to activate a second source of supply, we would compete with other companies for access to appropriate manufacturing facilities. Any such change would need to be submitted to and approved by the FDA and/or foreign regulatory agencies before commercial activities of AMITIZA or any other product could resume. Among manufacturers that operate under cGMP regulations, there are a limited number that would be both capable of manufacturing for us and willing to do so.
Risks Related to Our Dependence on Third Parties
We depend significantly on our collaborations with Takeda, Mylan, Gloria and Santen and may depend in the future on collaborations with other third parties, to develop and commercialize our product candidates.
A key element of our business strategy is to collaborate where appropriate with third parties, particularly leading pharmaceutical companies, to co-develop, commercialize and market our products and product candidates. We are currently party to the North America Takeda Agreement for the co-development and commercialization of AMITIZA for gastrointestinal indications in the U.S. and Canada, as well as to the Global License Agreement for AMITIZA whereby Takeda is responsible for all development, commercialization and regulatory activities other than in Canada, the U.S., Japan and the People’s Republic of China.
We are also party to the Japan Mylan Agreement for the development and commercialization of AMITIZA in Japan and the China Gloria Agreement under which Harbin Gloria is responsible for all development, commercialization and regulatory activities for AMITIZA in the People’s Republic of China.
We are a party to the Santen Agreement for the distribution and commercialization of RESCULA in Japan.
The success of our collaboration arrangements will depend heavily on the efforts and activities of Takeda, Mylan, Gloria and Santen. The risks that we face in connection with these collaborations and that we anticipate being subject to in any future collaborations, include the following:
| · | our existing agreements are, and any future collaboration agreements that we may enter into are likely to be, subject to termination under various circumstances; |
| · | our present and future collaborators may develop and commercialize, either alone or with others, products and services that are similar to or competitive with the products that are the subject of their collaboration with us; |
| · | our present and future collaborators may underfund or not commit sufficient resources to the testing, marketing, distribution or other development of our products or may use committed resources inefficiently; |
| · | we may become involved in disputes with our collaborators regarding operations, strategies, intellectual property or financial matters; |
| · | our present and future collaborators may not properly maintain or defend our intellectual property rights or may utilize our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential liability; and |
| · | our present and future collaborators may change the focus of their development and commercialization efforts. |
The ability of our products and product candidates to reach their potential could be limited if Takeda, Mylan, Gloria, Santen or any other future collaborators decrease or fail to increase spending relating to such products, fail to dedicate sufficient resources to developing or promoting our products or change their business focus.
We rely on third parties to conduct our clinical trials and those third parties may not perform satisfactorily or may fail to meet established deadlines for the completion of these trials.
We generally do not have the independent ability to conduct global clinical trials for our product candidates. We rely on third parties, such as contract research organizations (CROs), clinical data management organizations, medical institutions, and clinical investigators, to perform this function. We use multiple CROs to coordinate the efforts of our clinical investigators and to accumulate the results of our trials. Our reliance on these third parties for clinical development activities reduces our control over these activities. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors.
| 27 |
In addition, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. The FDA and foreign regulatory agencies require us to comply with standards, commonly referred to as cGCP, for conducting and recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements.
If tax authorities disagree with our transfer pricing policies or other tax positions, we could become subject to significant tax liabilities.
We are a member of an affiliated group of entities, and have had and will continue to have significant commercial transactions with these entities. Furthermore, we operate a number of foreign subsidiaries. We expect to operate through a consolidated organizational structure and we expect to enter into commercial transactions with some of these entities or future subsidiaries on an ongoing basis. As a result of these transactions, we will be subject to complex transfer pricing and other tax regulations in both the U.S. and the other countries in which we and our affiliates operate. Transfer pricing regulations generally require that, for tax purposes, transactions between our subsidiaries and affiliates and us be priced on a basis that would be comparable to an arm’s length transaction and that contemporaneous documentation be maintained to support the related party agreements. To the extent that U.S. or any foreign tax authorities disagree with our transfer pricing or other policies, we could become subject to significant tax liabilities and penalties related to prior, existing and future related party agreements. As of December 31, 2016, we performed updated tax analyses wherein liabilities for uncertain tax positions were recorded for certain state jurisdictions based on nexus related to the sourcing of revenues. Should the tax authorities in one or more of these states have different interpretations than us, we may be subject to additional tax liabilities.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain proprietary protection for the intellectual property relating to our technology and products, the value of our technology and products will be adversely affected and our ability to derive revenue from our products would be adversely affected. In addition, generic companies may file Abbreviated New Drug Applications (ANDA) with the FDA against our products, which would likely require us to initiate patent infringement lawsuits against those generic companies.
Our success depends in part on our ability to obtain and maintain proprietary protection for the technology and know-how upon which our products are based, to operate without infringing on the proprietary rights of others and to prevent others from infringing on our proprietary rights. The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. Our ability to maintain and solidify our proprietary position for our intellectual property will depend on our success, in obtaining effective claims and enforcing those claims once granted. The scope of protection afforded by a set of patent claims is subject to inherent uncertainty unless the patent has already been litigated and a court has ruled on the meaning of the claim language and other issues affecting how broadly a patent claim can be enforced. In some cases, we licensed patent applications from R-Tech instead of issued patents, and we do not know whether these patent applications will result in the issuance of any patents.
Our licensed patents have recently been challenged in the U.S. for AMITIZA (lubiprostone) by Par and Dr. Reddy’s and for RESCULA (unoprostone isopropyl) by Par Pharmaceutical and Apotex through the filing of ANDAs by those generic companies with the FDA. While these challenges have all been resolved, the patents at issue in those suits, as well as other patents, may be challenged, invalidated or circumvented, which could limit the term of patent protection for lubiprostone, unoprostone isopropyl or our other products, diminish our ability to stop competitors from marketing related products, and materially adversely affect our business and results of operations.
In connection with the settlement of patent litigation in the United States related to our AMITIZA 8 mcg and 24 mcg soft gelatin capsule products, we have partnered with Par Pharmaceuticals, Inc., or Par, and Dr. Reddy’s Laboratories, Ltd., or Dr. Reddy’s. Under our agreement with Par, we granted Par a non-exclusive license to market Par’s generic version of lubiprostone 8 mcg and 24 mcg soft gelatin capsules in the United States for the indications approved for AMITIZA beginning January 1, 2021, or earlier under certain circumstances. Beginning on January 1, 2021, Par will split with us the gross profits of the licensed products sold during the term of the agreement, which continues until each of our related patents has expired. Under our agreement with Dr. Reddy’s, we granted Dr. Reddy’s a non-exclusive license to market Dr. Reddy’s generic version of lubiprostone 8 mcg and 24 mcg soft gelatin capsules in the United States for the indications approved for AMITIZA. This license does not begin until more than six years from November 9, 2016, or earlier under certain circumstances. Dr. Reddy’s will pay to us a share of net profits of generic lubiprostone products sold during the term of the agreement, which decreases over time and ends when all of our related patents have expired. In the event that either Par or Dr. Reddy’s elect to launch an authorized generic form of lubiprostone, we have agreed to supply such product under the terms of a manufacturing and supply agreement at a negotiated price.
We have certain patents on our products that expire in the near future. We may not be able to use other existing patents or patent applications to successfully protect our products from generic competition. In addition, changes in either patent laws or in interpretations of patent laws in the U.S. and other countries may diminish the value of our patents and other intellectual property or narrow the scope of the protection provided by these patents. Accordingly, we cannot determine the degree of future protection for our proprietary rights in the patents and patent applications. Furthermore, because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that, before any of our product candidates can be commercialized, a related patent may expire or may remain in force for only a short period following commercialization, thereby reducing any advantage of the patent.
| 28 |
Patents may not afford us protection against competitors with similar technology. Because patent applications in the U.S. and many foreign jurisdictions are typically not published until 18 months after filing, or in some cases not at all, and because publications of discoveries in the scientific literature often lag behind actual discoveries, we cannot be certain whether a judicial court will uphold the validity of a patent.
If our patent position does not adequately protect our product and product candidates, others could compete against us more directly, which would harm our business, possibly materially.
The patent rights relating to lubiprostone consist of 16 issued U.S. patents, and various issued European and Japanese patents. Our patent rights also include various U.S., European and Japanese patent applications relating to dosing regimens, pharmaceutical formulations and other claims. The U.S. patents relating to compositions of matter expire between 2020 and 2027. The other U.S. and foreign patents expire between 2020 and 2035.
Our commercial success with respect to lubiprostone will depend significantly on our ability to protect our existing patent position with respect to lubiprostone as well as our ability to obtain and maintain adequate protection of other intellectual property for our technologies, product candidates and any future products in the U.S. and other countries.
The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, validity and enforceability cannot be predicted with certainty.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
| · | we or our licensors were the first to make the inventions covered by each of our pending patent applications; |
| · | we or our licensors were the first to file patent applications for these inventions; |
| · | others will not independently develop similar or alternative technologies or duplicate any of our technologies; |
| · | any of our pending patent applications or those we have licensed will result in issued patents; |
| · | any of our patents or those we have licensed will be valid or enforceable; |
| · | any patents issued to us or our licensors and collaborators will provide a basis for any additional commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties; |
| · | we will develop additional proprietary technologies or product candidates that are patentable; or |
| · | the patents of others will not have an adverse effect on our business. |
We may infringe the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing our product and any product candidates.
Our success will depend in part on our ability to operate without infringing the proprietary rights of third parties. There could be issued patents of which we are not aware that our products or product candidates infringe. There also could be patents that we believe we do not infringe, but that we may ultimately be found to infringe.
The pharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights. Other parties may obtain patents in the future and allege that our products or product candidates or the use of our technologies infringes these patent claims or that we are employing their proprietary technology without authorization. Likewise, third parties may challenge or infringe upon our existing or future patents.
Proceedings involving our patents or patent applications or those of others could result in adverse decisions regarding:
| · | the patentability of our inventions relating to our product or any product candidates; and |
| · | the enforceability, validity or scope of protection offered by our patents relating to our product or any product candidates. |
Even if we are successful in these proceedings, we may incur substantial costs and divert management’s time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion.
| 29 |
In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or have infringed patents declared invalid, we may:
| · | incur substantial monetary damages; |
| · | encounter significant delays in bringing our product candidates to market; and |
| · | be precluded from manufacturing or selling our product candidates. |
In such event, our business could be adversely affected, possibly materially.
Risks Related to Regulatory Approval and Oversight
If we are not able to obtain required regulatory approvals, we will not be able to commercialize our product candidates and our ability to generate revenue will be materially impaired.
Our product candidates and the activities associated with their development and commercialization, including testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory agencies in and outside the U.S. Failure to obtain regulatory approval or appropriate pricing for a product candidate will prevent us from commercializing the product candidates.
As we increase our foreign license arrangements, we or our partner are seeking and will continue to seek approval in different territories. Different regulatory agencies may reach different decisions in assessing the approval and pricing of our product candidates. Securing regulatory approval requires the submission of extensive preclinical and clinical data, information about product manufacturing processes and inspection of facilities and supporting information to the regulatory agencies for each therapeutic indication to establish the product candidate’s safety and efficacy. Our future products may not be effective, may be only moderately effective or may prove to have undesirable side effects, toxicities or other characteristics that may preclude our obtaining regulatory approval or prevent or limit commercial use.
The process of obtaining regulatory approvals is expensive, often takes many years, if approval is obtained at all, and can vary substantially based upon the type, complexity and novelty of the product candidates involved. Changes in the regulatory approval policy during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA and foreign regulatory agencies have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent regulatory approval of a product candidate. Any regulatory approval we ultimately obtain may be limited in scope or subject to restrictions or post-approval commitments that render the product not commercially viable. If any regulatory approval that we obtain is delayed or is limited, we may decide not to commercialize the product candidate after receiving the approval.
We may not be able to obtain orphan drug exclusivity for our product candidates. If our competitors are able to obtain orphan drug exclusivity for a product that is competitive with one or more of our product candidates and we cannot show that our product candidate is clinically superior, we may not be able to have competing products approved by the applicable regulatory authority for a significant period of time.
Regulatory authorities in some jurisdictions, including Europe and the U.S., may designate drugs that target relatively small patient populations as orphan drugs. Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity. The exclusivity applies only to the indication for which the drug has been designated and approved. The applicable exclusivity period is seven years in the U.S., but this period may be interrupted if a sponsor of a competitive product that is otherwise the same drug for the same use can show that its drug is clinically superior to our orphan drug candidate. The European exclusivity period is ten years, but may be reduced to six years if a drug no longer meets the criteria for orphan drug designation, including where it is shown that the drug is sufficiently profitable so that market exclusivity is no longer justified. Even if we obtain orphan drug exclusivity for specified indications, we may not be able to maintain it if a competitor with a product that is otherwise the same drug can establish that its product is clinically superior.
We must comply with federal, state and foreign laws, regulations, and other rules relating to the health care business, and, if we are unable to fully comply with such laws, regulations and other rules, we could face substantial penalties.
We are or will be directly or indirectly through our collaborators, subject to extensive regulation by the federal government, the states and foreign countries in which we may conduct our business. The laws that directly or indirectly affect our ability to operate our business include the following:
| · | the federal Medicare and Medicaid Anti-Kickback law, which prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual, or furnishing or arranging for a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid Programs; |
| 30 |
| · | other Medicare laws, regulations, rules, manual provisions and policies that prescribe the requirements for coverage and payment for services performed by our customers, including the amount of such payment; |
| · | the federal False Claims Act, which imposes civil and criminal liability on individuals and entities who submit, or cause to be submitted, false or fraudulent claims for payment to the government; |
| · | the federal False Statements Act, which prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| · | the Foreign Corrupt Practices Act, which prohibits certain payments made to foreign government officials; |
| · | state and foreign law equivalents of the foregoing and state laws regarding pharmaceutical company marketing compliance, reporting and disclosure obligations; and |
| · | the Patient Protection and Affordable Care Act, which changes access to healthcare products and services; creates new fees for the pharmaceutical and medical device industries; changes rebates and prices for health care products and services; and requires additional reporting and disclosure. |
If our operations are found to be in violation of any of the laws, regulations, rules or policies described above or any other law or governmental regulation to which we or our collaborators are or will be subject, or if the interpretation of the foregoing changes, we may be subject to civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment or restructuring of our operations. Similarly, we do not control our collaborators, including their compliance activities and if our collaborators are found non-compliant with applicable laws, they may be subject to sanctions, which could also have a negative impact on us. Any penalties, damages, fines, curtailment or restructuring of our operations would harm our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions may be open to a variety of interpretations. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert management resources from the operation of our business and damage our reputation.
We only have regulatory approval for commercial distribution and reimbursement of lubiprostone and unoprostone isopropyl in a limited number of countries, and may not receive regulatory approval in other countries.
We are currently permitted to market our approved products in only a limited number of countries on a commercial basis. To obtain marketing approval in other countries, we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy and governing, among other things, clinical trials, pricing, promotion and distribution of the product. Approval procedures vary among countries, and can involve additional product testing and additional administrative review periods. For example, we and Takeda are currently exploring the commercialization of AMITIZA in a number of countries. We may not be successful in obtaining such approval.
In addition, regulatory authorities in countries outside the U.S. and E.U. are increasingly requiring risk management plans and post-marketing commitments which may be more onerous than those required in the U.S. and E.U. The time required to obtain approval in other countries may differ from that required to obtain FDA approval or marketing authorization from the E.U. In particular, in many countries outside the U.S., including most E.U. countries and Canada, a product must receive pricing and reimbursement approval before it can be commercialized broadly. This can result in substantial delays in such countries, and the price that is ultimately approved may be lower than the price for which we expect to offer, or would be willing to offer, lubiprostone in such countries, and may impact pricing in other countries. Marketing and pricing and reimbursement approval in one country does not ensure such approvals in another. Failure to obtain the approvals necessary to commercialize lubiprostone in other countries at reimbursement levels that are acceptable to us or any delay or setback in obtaining such approvals would impair our partners’ ability to develop foreign markets for lubiprostone.
Risks Related to Our Class A Common Stock
Our largest stockholders and their affiliates maintain the ability to have significant control over matters submitted to stockholders for approval, which could result in actions of which you or other stockholders do not approve.
As of March 1, 2017, (i) our founder Dr. Ryuji Ueno, through his direct or indirect interest in RJ Fund LLC and the Ryuji Ueno Foundation Inc., held 10,537,628 shares of class A common stock, representing approximately 24.3% of our outstanding class A common stock, (ii) our founder Dr. Sachiko Kuno, through her direct or indirect interest in SK Impact Fund LLC and the Sachiko Kuno Foundation Inc., held 10,537,627 shares of class A common stock, representing approximately 24.3% of our outstanding class A common stock. Therefore, until such time that such stockholders further dispose of additional shares of class A common stock, this concentration of ownership and voting power could influence all matters requiring stockholder approval and have the effect of delaying or preventing a change in control of our company and could prevent stockholders from receiving a premium over the market price if a change in control is proposed.
| 31 |
Provisions in our corporate charter documents and under Delaware law may prevent or frustrate attempts by our stockholders to change our management and hinder efforts to acquire a controlling interest in us, and the market price of our class A common stock may be lower as a result.
There are provisions in our certificate of incorporation and by-laws that may make it difficult for a third party to acquire, or attempt to acquire, control of our company, even if a change in control was considered favorable by our stockholders. For example, our Board of Directors has the authority to issue up to 5,000,000 shares of preferred stock. The Board of Directors can fix the price, rights, preferences, privileges, and restrictions of the preferred stock without any further vote or action by our stockholders. The issuance of shares of preferred stock may delay or prevent a change in control transaction. As a result, the market price of our class A common stock and the voting and other rights of our stockholders may be adversely affected. An issuance of shares of preferred stock may result in the loss of voting control to other stockholders.
Our charter documents contain other provisions that could have an anti-takeover effect, including:
- only one of our three classes of directors will be elected each year;
- stockholders are not entitled to remove directors other than by a 75.0% vote and for cause;
- stockholders are not permitted to take actions by written consent;
- stockholders cannot call a special meeting of stockholders; and
- stockholders must give advance notice to nominate directors or submit proposals for consideration at stockholder meetings.
In addition, we are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law, which regulates corporate acquisitions. Furthermore, the indenture governing our Convertible Notes requires us to repurchase the notes for cash, plus accrued and unpaid interest to, but excluding, the fundamental change repurchase date, if we undergo certain fundamental changes. For example, an acquisition or a tender offer representing more than 50% of the voting power of our class A common stock may trigger the requirement that we repurchase our Convertible Notes, which could make it more costly for a potential acquirer to engage in a business transaction with us. These provisions could discourage potential acquisition proposals and could delay or prevent a change in control transaction. They could also have the effect of discouraging others from making tender offers for our class A common stock. These provisions may also prevent changes in our management.
The price of our class A common stock is volatile; investors in our class A common stock could incur substantial losses.
The public trading market for our class A common stock is characterized by a highly volatile stock price. The stock market in general and the market for pharmaceutical and biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. These broad market and industry factors might seriously harm the market price of our class A common stock, regardless of our operating performance. As a result of this volatility, investors may not be able to sell their class A common stock at or above the price they paid, and may have difficulty selling their shares at any price. The market price for our class A common stock may be influenced by many factors, including:
| · | failure of AMITIZA (lubiprostone) or other approved products, if any, to achieve commercial success; |
| · | results of clinical trials of our product candidates or those of our competitors; |
| · | the regulatory status of our product candidates; |
| · | the success of competitive products or technologies; |
| · | regulatory developments in the U.S. and foreign countries; |
| · | developments or disputes concerning patents or other proprietary rights; |
| · | the ability of our third-party suppliers and manufacturers to perform; |
| · | actual or anticipated fluctuations in our quarterly financial results; |
| · | variations in the financial results of companies that are perceived to be similar to us; |
| · | changes in the structure of healthcare payment systems and other regulatory developments; |
| · | market conditions in the pharmaceutical and biotechnology sectors, including those relating to the pricing of pharmaceutical products, and issuance of new or changed securities analysts’ reports or recommendations; and |
| · | general economic, industry and market conditions. |
We will not be able to control many of these factors, and we believe that period-to-period comparisons of our financial results will not necessarily be indicative of our future performance. In addition, the market price of our class A common stock could also be affected by possible sales of our class A common stock by holders of our Convertible Notes, who may view the Convertible Notes as a more attractive means of equity participation in our company and by the holders possibly engaging in hedging or arbitrage trading activity involving our class A common stock associated with the Convertible Notes.
| 32 |
We do not anticipate paying dividends on our capital stock.
We do not intend to pay dividends on our capital stock in the foreseeable future. We currently intend to retain all cash we generate to fund the growth of our business. The declaration of dividends is subject to the discretion of our board of directors and will depend on various factors, including our operating results, financial condition, future prospects and any other factors deemed relevant by our board of directors. You should not rely on an investment in our company if you require dividend income from your investment in our company. The success of your investment will likely depend entirely upon any future appreciation of the market price of our capital stock, which is uncertain and unpredictable. There is no guarantee that our capital stock will appreciate in value or even maintain the price at which you purchased your shares.
Substantial future sales of our class A common stock in the public market, or the existence or conversion of our Convertible Notes, may depress our stock price and make it difficult for you to recover the full value of your investment in our class A common stock.
As of March 1, 2017, we had 46,457,221 shares of class A common stock outstanding. Substantially all of these shares are available for public sale, subject in some cases to volume and other limitations or delivery of a prospectus. The market price of our class A common stock may decline if our class A common stockholders sell a large number of shares of our class A common stock in the public market, or the market perceives that such sales may occur. In addition, future issuances of our class A common stock upon the exercise or settlement of equity-based awards and maturity of our Convertible Notes would dilute existing stockholders’ ownership interest in our company and any sales in the public market of these class A common stock, could also adversely affect the market price of our class A common stock. In addition, as of March 1, 2017, we had 4,695,478 outstanding options to purchase an aggregate of 4,695,478 shares of our class A common stock. If these options are exercised and the shares issued upon exercise are sold, the market price of our securities may also decline. These factors also could impair our ability to raise needed capital by depressing the price at which we could sell our securities.
Pursuant to the indenture governing the Convertible Notes, holders may convert their Convertible Notes into shares of Sucampo’ s Class A common stock at any time prior to December 14, 2021. Conversions of the Convertible Senior Notes dilute the ownership interests of existing shareholders to the extent that we elect to deliver shares of our class A common stock (or a combination of cash and shares of our class A common stock) in connection therewith. In addition, the existence of the Convertible Senior Notes may encourage short selling by market participants because the conversion of the Convertible Notes could depress the price of our class A common stock.
Risks Related to Strategic Acquisitions
Our strategy of generating growth through acquisitions may not be successful.
Our business strategy includes growing our business through acquisition and in-licensing transactions. We may not be successful in identifying, effectively evaluating, acquiring or in-licensing, and developing and commercializing additional products on favorable terms, or at all. Competition for attractive product opportunities is intense and may require us to devote substantial resources, both managerial and financial, to an acquisition opportunity. A number of more established companies are also pursuing strategies to acquire or in-license products. These companies may have a competitive advantage over us due to their size, cash resources and greater development and commercialization capabilities.
Acquisition efforts can consume significant management attention and require substantial expenditures, which could detract from our other programs. In addition, we may devote significant resources to potential acquisitions that are never completed. Even if we are successful in acquiring a product or company, it may not result in a successfully developed or commercialized product or, even if an acquired product is commercialized, competing products or technologies could render a product noncompetitive, uneconomical or obsolete. Moreover, the cost of acquiring other companies or in-licensing products could be substantial, and in order to acquire companies or new products, we may need to incur substantial debt or issue dilutive securities. If we are unsuccessful in our efforts to acquire other companies or in-license and develop additional products, or if we acquire or in-license unproductive assets, it could have a material adverse effect on the growth of our business.
Our failure to successfully integrate acquired assets into our operations could adversely affect our ability to grow our business.
We may not be able to integrate any acquired business successfully or operate any acquired business profitably. In addition, cost synergies, if achieved at all, may be less than we expect, or may take greater time to achieve than we anticipate.
Issues that could delay or prevent successful integration or cost synergies of an acquired business include, among others:
| · | retaining existing customers and attracting new customers; |
| · | retaining key employees; |
| · | diversion of management attention and resources; |
| · | conforming internal controls, policies and procedures, business cultures and compensation programs; |
| · | consolidating corporate and administrative infrastructures; |
| 33 |
| · | consolidating sales and marketing operations; |
| · | identifying and eliminating redundant and underperforming operations and assets; |
| · | assumption of known and unknown liabilities; |
| · | coordinating geographically dispersed organizations; and |
| · | managing tax costs or inefficiencies associated with integrating operations. |
If we are unable to successfully integrate future acquisitions with our existing businesses, or operate any acquired business profitably, we may not obtain the advantages that the acquisitions were intended to create, which may materially adversely affect the growth of our business.
We may be unable to realize the benefits we anticipate from strategic acquisitions, or it may take longer than anticipated for us to achieve those benefits.
Our realization of the benefits anticipated as a result of our future strategic acquisitions will depend in part on the integration of the acquired company’s business with ours. However, there can be no assurance that we will be able to operate the target’s business profitably or integrate it successfully into our operations in a timely fashion, or at all. Our future success as a combined company depends, in part, upon our ability to manage this expanded business, which will pose substantial challenges for our management, including challenges related to the management and monitoring of new operations and associated increased costs and complexity. The dedication of management resources to this integration could detract attention from our current day-to-day business, and we cannot assure stockholders that there will not be substantial costs associated with the transition process or other negative consequences as a result of these integration efforts. These effects, including incurring unexpected costs or delays in connection with integration of the two businesses, or the failure of the combined company to perform as expected, could negatively affect our stock price or could harm our financial condition, results of operations or business prospects.
The loss of key personnel could hurt our business and our prospects.
The success of our strategic acquisitions will depend, in part, on our ability to retain key employees who continue employment with the combined company after each such acquisition is completed. If any of these key employees terminate their employment, our manufacturing, supply or development activities might be negatively affected and our management’s attention might be diverted from successfully integrating the acquired company’s operations. In addition, we might not be able to locate suitable replacements on reasonable terms for any such key employees who leave the combined company.
Financial Related Risks
If we fail to comply with the covenants and other obligations under our Convertible Notes, holders may be able to accelerate amounts owed under the Convertible Notes or require repurchase of all Convertible Notes.
On December 27, 2016, we issued the Convertible Notes consisting of an approximately $300.0 million aggregate principal amount with interest of 3.25% in a private placement to qualified institutional buyers. The Convertible Notes are senior unsecured obligations, and interest of 3.25% per year payable semiannually in arrears on June 15 and December 15 of each year, beginning on June 15, 2017. The notes will mature on December 15, 2021, unless earlier repurchased or converted in accordance with their terms. The Convertible Notes are not redeemable prior to the maturity date, and no sinking fund is provided for the Convertible Notes.
The Convertible Notes are convertible at an initial conversion rate of 60.2637 shares of class A common stock per $1,000 principal amount of the Convertible Notes, subject to adjustment under the indenture, which is equal to an initial conversion price of approximately $16.59 per share of class A common stock. Upon conversion, the Convertible Notes will be settled in shares of our class A common stock, together with a cash payment in lieu of delivering any fractional share. The conversion rate will be subject to adjustment in some events but will not be adjusted for any accrued and unpaid interest. In addition, following certain corporate events that occur prior to the maturity date, we will increase the conversion rate for a holder who elects to convert its Convertible Notes in connection with such a corporate event in certain circumstances.
If we undergo a fundamental change, holders may require us to repurchase for cash all or any portion of their Convertible Notes at a fundamental change repurchase price equal to 100% of the principal amount of the Convertible Notes to be repurchased, plus accrued and unpaid interest to, but excluding, the fundamental change repurchase date.
The indenture includes customary terms and covenants, including certain events of default after which the Convertible Notes may be due and payable immediately. If the maturity date of the Convertible Notes were to be accelerated, we may not have sufficient funds to repay the related indebtedness, which could have a material adverse effect on our financial condition and our business.
| 34 |
Our current indebtedness and any additional debt financing may restrict the operation of our business and limit the cash available for investment in our business operations.
In addition to our current debt, we may seek additional debt financing to support our ongoing activities or to provide additional financial flexibility. Debt financing could have significant adverse consequences for our business, including:
| · | requiring us to dedicate a substantial portion of any cash flow from operations to payment on our debt, which would reduce the amounts available to fund other corporate initiatives; |
| · | increasing the amount of interest that we have to pay on debt with variable interest rates, if market rates of interest increase; |
| · | subjecting us to restrictive covenants that may reduce our ability to take certain corporate actions, acquire companies, products or technology, or obtain further debt financing; |
| · | requiring us to pledge our assets as collateral, which could limit our ability to obtain additional debt financing; |
| · | limiting our flexibility in planning for, or reacting to, general adverse economic and industry conditions; and |
| · | placing us at a competitive disadvantage compared to our competitors that have less debt, better debt servicing options or stronger debt servicing capacity. |
We may not have sufficient funds or be able to obtain additional financing to pay the amounts due under the Convertible Notes or our indebtedness. An event of default could result in the acceleration of amounts due under a particular debt instrument and a cross default and acceleration under other debt instruments, and we may not have sufficient funds or be able to obtain additional financing to make any accelerated payments.
We may require significant additional funding and may be unable to raise capital when needed or on acceptable terms, which would harm our ability to grow our business, results of operations and financial condition.
We may require significant additional funding to grow our business, including to acquire other companies or products, in-license and develop additional products, enhance our manufacturing capacity, support commercial marketing activities or otherwise provide additional financial flexibility. We may also require additional funding to support our ongoing operations in the event that our ability to sell AMITIZA to Takeda, Mylan and Gloria or sell RESCULA to Santen is interrupted for an extended period of time, reducing our revenues and decreasing our cash balances.
As of December 31, 2016, we had $267.6 million of cash, cash equivalents, accounts receivable and product royalties receivable. Our future capital requirements will depend on many factors, including, among others:
| · | the level, timing and cost of product sales; |
| · | the extent to which we acquire or invest in companies, products or technologies; |
| · | the payment obligations under our indebtedness; |
| · | the scope, progress, results and costs of our development activities; |
| · | our ability to obtain funding from collaborative partners, government entities and non-governmental organizations for our development programs; and |
| · | the costs of commercialization activities, including product marketing, sales and distribution. |
If our capital resources are insufficient to meet our future capital requirements, we will need to finance our cash needs through public or private equity or debt offerings, bank loans or collaboration and licensing arrangements. If we raise funds by issuing equity securities, our stockholders may experience dilution. Public or bank debt financing, if available, may involve agreements that include covenants, limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, pursuing acquisition opportunities or declaring dividends. If we raise funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish valuable rights to our technologies or product candidates or grant licenses on terms that may not be favorable to us.
Current economic conditions may make it difficult to obtain financing on attractive terms, or at all. If financing is unavailable or lost, our business, results of operations and financial condition would be adversely affected and we could be forced to delay, reduce the scope of or eliminate many of our planned activities.
Our effective tax rate may fluctuate and we may incur obligations in tax jurisdictions in excess of accrued amounts.
As a global biopharmaceutical company, we are subject to taxation in numerous countries, states and other jurisdictions. As a result, our effective tax rate is derived from a combination of applicable tax rates in the various places that we operate. In preparing our financial statements, we estimate the amount of tax that will become payable in each of such places. Our effective tax rate, however, may be different than experienced in the past due to numerous factors, including changes in the mix of our profitability from country to country, currency fluctuations, the results of examinations and audits of our tax filings, adjustments to the value of our uncertain tax positions, changes in accounting for income taxes, and changes in tax laws. Any of these factors could cause us to experience an effective tax rate significantly different from previous periods or our current expectations. In addition, our inability to secure or sustain acceptable arrangements with tax authorities and future changes in the tax laws, among other things, may result in tax obligations in excess of amounts accrued in our financial statements.
| 35 |
In the U.S., there are several proposals under consideration to reform tax law, including proposals that may reduce or eliminate the deferral of U.S. income tax on our unrepatriated earnings, penalize certain transfer pricing structures, and reduce or eliminate certain foreign or domestic tax credits or deductions. Our future reported financial results may be adversely affected by tax law changes which restrict or eliminate certain foreign tax credits or our ability to deduct expenses attributable to foreign earnings, or otherwise affect the treatment of our unrepatriated earnings.
In addition to U.S. tax reform proposals, the adoption of some or all of the recommendations set forth in the Organization for Economic Co-operation and Development’s project on “Base Erosion and Profit Shifting” (BEPS) by tax authorities in the countries in which we operate, could negatively impact our effective tax rate. These recommendations focus on payments from affiliates in high tax jurisdictions to affiliates in lower tax jurisdictions and the activities that give rise to a taxable presence in a country.
| 36 |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
Our corporate headquarters, including our principal executive office and some of our administrative and research and development activities, are located in Rockville, Maryland. The lease for this facility, which comprises approximately 27,000 square feet of office space, has an initial term expiring in April 2027. The lease for our former headquarters in Bethesda, Maryland expired in February 2017.
Following our acquisition of R-Tech, we now maintain a manufacturing facility in Sanda, Japan. We own the structure at the Sanda facility and lease the land on which it is located. The current term of the land lease expires in 2031.
Our international subsidiaries lease offices in Tokyo and Kobe, Japan, as well as Zug, Switzerland to support our administrative, storage, and research and development activities. These facilities are under short-term leases.
| ITEM 3. | LEGAL PROCEEDINGS |
On December 28, 2015, in connection with our acquisition of R-Tech, three non-tendering stockholders of R-Tech submitted complaints to the Tokyo District Court alleging that the purchase price of R-Tech’s shares was unfair, and demanding an appraisal of the fair value of the shares. The number of shares subject to these proceedings is minimal. On November 11, 2016, the Court (i) dismissed the petitions with respect to all shares purchased by the complainants after the public notice of the acquisition and (ii) with respect to shares purchase prior to such public notice, determined that the tender offer price was fair. One of the petitioners appealed this ruling; however, the appellate proceeding was dismissed on February 15, 2017. The petitioner has appealed to the Supreme Court of Japan; this final appeal remains ongoing as of the date of this filing.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
| 37 |
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our class A common stock is listed on The NASDAQ Global Market under the symbol “SCMP”. The following table sets forth, for the periods indicated, the range of high and low sale prices of our class A common stock as reported on The NASDAQ Global Market.
| 2015 | High | Low | ||||||
| First quarter | $ | 18.56 | $ | 13.12 | ||||
| Second quarter | 21.75 | 14.50 | ||||||
| Third quarter | 28.96 | 16.34 | ||||||
| Fourth quarter | 21.48 | 15.56 | ||||||
| 2016 | High | Low | ||||||
| First quarter | $ | 16.35 | $ | 9.84 | ||||
| Second quarter | 12.42 | 10.09 | ||||||
| Third quarter | 13.38 | 10.82 | ||||||
| Fourth quarter | 17.15 | 10.95 | ||||||
As of March 1, 2017, we had 46,457,221 shares of class A common stock outstanding held by 10 stockholders of record. The number of holders of record of our class A common stock is not representative of the number of beneficial holders because many shares are held by depositories, brokers or nominees. On March 1, 2017, the closing price of our class A common stock was $11.75.
We have never declared or paid any cash dividends on our common stock. We currently intend to retain earnings, if any, to support our growth strategy and do not anticipate paying cash dividends in the foreseeable future.
The information regarding the securities authorized for issuance under our equity compensation plan is incorporated into this section by reference from the section captioned “Equity Compensation Plan Information” in our Proxy Statement for our 2017 Annual Meeting of Stockholders.
Stock Performance Graph
The information included under this heading “Stock Performance Graph” is “furnished” and not “filed” and shall not be deemed to be “soliciting material” or subject to Regulation 14A, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act, or the Exchange Act.
The following graph compares the cumulative total return, assuming the investment of $100 on December 31, 2011, in each of (1) our class A common stock, (2) The NASDAQ Composite Index (U.S. and Foreign) and (3) the NASDAQ Biotechnology Index, assuming reinvestment of any dividends. These comparisons are required by the SEC and are not intended to forecast or be indicative of possible future performance of our class A common stock.
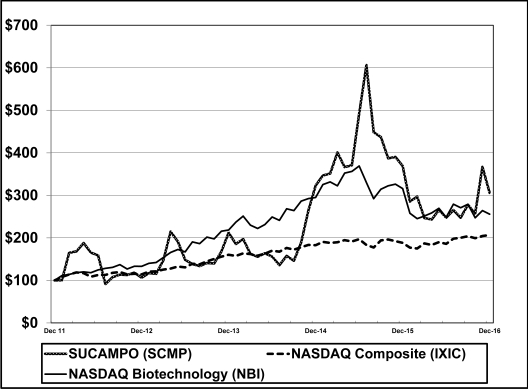
| 38 |
| ITEM 6. | SELECTED FINANCIAL DATA |
The following consolidated balance sheet data as of December 31, 2016 and 2015 and consolidated statement of operations data for the years ended December 31, 2016, 2015 and 2014 are derived from our audited Consolidated Financial Statements appearing elsewhere in this Annual Report. The following consolidated balance sheet data as of December 31, 2014, 2013 and 2012 and consolidated statement of operations data for the years ended December 31, 2013 and 2012 are derived from audited Consolidated Financial Statements not included in this Annual Report. The information set forth below is not necessarily indicative of the results of future operations and should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Consolidated Financial Statements and related footnotes appearing elsewhere in this Annual Report on Form 10-K.
| Year Ended December 31, | ||||||||||||||||||||
| (In thousands, except per share data) | 2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||
| Statement of operations data | ||||||||||||||||||||
| Revenues: | $ | 230,056 | $ | 153,180 | $ | 115,450 | $ | 89,594 | $ | 81,487 | ||||||||||
| Costs and expenses: | ||||||||||||||||||||
| Costs of goods sold | 76,003 | 36,731 | 16,269 | 12,402 | 3,030 | |||||||||||||||
| In-process research & development impairment | 7,286 | - | - | - | - | |||||||||||||||
| Intangible assets impairment | - | - | 5,631 | - | - | |||||||||||||||
| Research and development | 46,615 | 33,631 | 20,566 | 21,524 | 21,292 | |||||||||||||||
| General and administrative | 43,798 | 35,517 | 31,230 | 25,413 | 30,157 | |||||||||||||||
| Selling and marketing | 2,478 | 2,842 | 14,523 | 21,059 | 18,691 | |||||||||||||||
| Total costs and expenses | 176,180 | 108,721 | 88,219 | 80,398 | 73,170 | |||||||||||||||
| Income (loss) from operations | 53,876 | 44,459 | 27,231 | 9,196 | 8,317 | |||||||||||||||
| Total non-operating income (expense), net | (39,501 | ) | (784 | ) | (98 | ) | 1,747 | (340 | ) | |||||||||||
| Income (loss) before income taxes | 14,375 | 43,675 | 27,133 | 10,943 | 7,977 | |||||||||||||||
| Income tax benefit (provision) | 4,112 | (10,304 | ) | (14,005 | ) | (3,928 | ) | (2,916 | ) | |||||||||||
| Net income (loss) | $ | 18,487 | $ | 33,371 | $ | 13,128 | $ | 7,015 | $ | 5,061 | ||||||||||
| Basic net income (loss) per share | $ | 0.43 | $ | 0.76 | $ | 0.30 | $ | 0.17 | $ | 0.12 | ||||||||||
| Diluted net income (loss) per share | $ | 0.42 | $ | 0.73 | $ | 0.29 | $ | 0.16 | $ | 0.12 | ||||||||||
| Weighted average common shares outstanding - basic | 42,791 | 44,150 | 43,691 | 41,716 | 41,660 | |||||||||||||||
| Weighted average common shares outstanding - diluted | 43,749 | 45,680 | 44,506 | 42,544 | 41,785 | |||||||||||||||
| December 31, | ||||||||||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||
| Balance sheet data: | ||||||||||||||||||||
| Cash and cash equivalents | $ | 198,308 | $ | 108,284 | $ | 71,622 | $ | 44,102 | $ | 52,022 | ||||||||||
| Investments, current | - | - | 22,393 | 16,003 | 6,035 | |||||||||||||||
| Working capital | 274,881 | 163,233 | 88,514 | 70,741 | 52,843 | |||||||||||||||
| Total assets | 520,851 | 457,181 | 141,574 | 136,877 | 127,796 | |||||||||||||||
| Notes payable, current | - | 39,083 | 8,240 | 26,892 | 19,129 | |||||||||||||||
| Notes payable, non-current | 290,516 | 213,277 | 17,578 | 25,828 | 33,722 | |||||||||||||||
| Total liabilities | 353,752 | 370,732 | 59,262 | 77,908 | 84,541 | |||||||||||||||
| Retained earnings (Accumulated deficit) | 38,126 | 19,639 | (13,732 | ) | (26,860 | ) | (33,875 | ) | ||||||||||||
| Total stockholders' equity | 167,099 | 86,449 | 82,312 | 58,969 | 43,255 | |||||||||||||||
| 39 |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read the following discussion and analysis together with our Consolidated Financial Statements and the related notes included elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements that are based on our current expectations, estimates and projections about our business and operations. Our actual results may differ materially from those currently anticipated and expressed in such forward-looking statements as a result of a number of factors, including those we discuss under Item 1A - “Risk Factors” and elsewhere in this Annual Report.
Overview
We are a global biopharmaceutical company focused on innovative research and development of proprietary drugs to meet major unmet medical needs. In 2016, we advanced our corporate strategy by continuing to deliver outstanding financial performance, focusing our pipeline portfolio through the acquisition of new products and the termination of certain programs, and positioning Sucampo for sustained mid- to long-term growth by integrating our global operations and executing new business development transactions.
We currently generate revenue mainly from product royalties, product sales, development, upfront and milestone payments, and reimbursements for clinical development activities. We expect to continue to incur significant expenses for the next several years as we continue our research and development activities, seek additional regulatory approvals and additional indications for our approved products and other compounds and seek strategic opportunities through acquisitions or in-licensing new products.
Our operations are conducted through subsidiaries based in the United States (U.S.), Japan and Switzerland. We operate as one segment, which focuses on the development and commercialization of pharmaceutical products.
Our Products (Approved and in Clinical Development)
Our commercial-stage products are AMITIZA and RESCULA. Overviews of these products are:
AMITIZA (lubiprostone)
North America
In October 2014, we signed the Takeda Amendment which, among other things, extended the term beyond December 2020; during the extended term we will split the annual net sales revenue with Takeda on branded AMITIZA sales. In addition, we have granted Par and Dr. Reddy’s licenses to distribute either a generic or authorized generic version of AMITIZA. Par’s license begins in 2021; the Dr. Reddy’s license begins more than six years from November 9, 2016. We will split with Par and Dr. Reddy’s the gross profits for the sales of their lubiprostone products. Further, if Par or Dr. Reddy’s decides to distribute an authorized generic, we will supply the product at a negotiated supply price.
Japan
In Japan, AMITIZA was approved in November 2012 for chronic constipation (“CC”) excluding constipation caused by organic diseases. AMITIZA is Japan’s only prescription medicine for CC. In Japan, AMITIZA is marketed under a license, commercialization and supply agreement (the “Japan Mylan Agreement”) that was transferred to Mylan, Inc. (“Mylan”) from Abbott Laboratories, Inc. (Abbott), as of February 2015, as part of Mylan’s acquisition of a product portfolio from Abbott.
People’s Republic of China
In May 2015, we and Gloria entered into the China Gloria Agreement to license, develop, commercialize and supply AMITIZA in the People’s Republic of China. Upon entering into the China Gloria Agreement, we received an upfront payment of $1.0 million. In June 2015, the China Food and Drug Administration accepted an Investigational New Drug (IND) application for a pivotal trial of AMITIZA in patients with CIC; as a result, we received an additional payment of $500,000 from Gloria. In addition to the $1.5 million in payments received to date, we are eligible to receive an additional payment in the amount of $1.5 million upon the occurrence of a specified regulatory or commercial milestone event.
Other Global Markets
In October 2014, we and Takeda entered into the Global Takeda Agreement to develop and commercialize AMITIZA. The territories excluded from the Global Takeda Agreement are Canada, the U.S., Japan and the People’s Republic of China. Under the terms of the Global Takeda Agreement, we will supply Takeda the clinical and commercial product at a negotiated price. In addition, under the Global Takeda Agreement, Takeda became the marketing authorization holder in Switzerland in April 2015, in the United Kingdom, Austria, Belgium, Germany, Netherlands, Ireland, Italy, Luxembourg and Spain during 2016.
| 40 |
In October 2015, Takeda obtained approval of the clinical trial application, or CTA, for AMITIZA for the treatment of CIC and IBS-C in Russia. In December 2015, a CTA was filed for AMITIZA for the treatment of CIC, IBS-C and OIC in Mexico and South Korea. Takeda initiated phase 3 registration trials in Russia in March 2016 and in South Korea and Mexico in May 2016. Takeda submitted a new drug application, or NDA, for the treatment of CIC, IBS-C, and OIC in Israel in June 2015, which was approved in July 2016, and an NDA for the same indications in Kazakhstan in December 2015. Additional NDA submissions have been made by Takeda in Singapore in May 2016, and South Africa and Indonesia in June 2016.
RESCULA (unoprostone isopropyl)
As part of the acquisition of R-Tech in October 2015, we acquired all rights to RESCULA. RESCULA is being commercialized by Santen Pharmaceutical Co., Ltd in Japan, and Sinphar Pharmaceutical, Co., Ltd and Zuellig Pharma Inc. in Taiwan.
Our Clinical Development Programs
Lubiprostone
Alternate Formulation
We are developing an alternate formulation of lubiprostone for both adult and pediatric patients who are unable to take or do not tolerate capsules and for naso-gastric tube fed patients. Takeda has agreed to fund 100% of the costs, up to a cap, of this alternate formulation work. We have initiated the phase 3 program of the alternate formulation of lubiprostone in adults in the second half of 2016 and, if the program is successful, we expect to file an NDA in the United States for the alternate formulation for adults in the second half of 2017.
Pediatric Functional Constipation
The phase 3 program required to support an application for marketing authorization of lubiprostone for pediatric functional constipation comprises four clinical trials. The first two trials, one of which was recently completed, test the soft gelatin capsule formulation of lubiprostone in patients 6 to 17 years of age. The first of these trials was a pivotal 12-week, randomized, placebo-controlled trial which was initiated in December 2013 and completed enrollment in April 2016. The second trial was a follow-on, long-term safety extension trial that was initiated in March 2014. In November 2016, we announced that the phase 3 trial of AMITIZA in pediatric functional constipation in children 6 to 17 years of age failed to achieve its primary endpoint of overall spontaneous bowel movement, or SBM, response. The trial achieved statistical significance for some secondary endpoints, notably overall SBM frequency, straining, and stool consistency. In addition, in this study lubiprostone was well tolerated. We have entered into a process with the U.S. Food and Drug Administration, or FDA, and other constituencies, and as a result of initial discussion with the FDA will submit an sNDA in the second half of 2017. Additionally, after further consultations with the FDA to better determine the doses and endpoints that should be studied, the phase 3 program for the alternate formulation of lubiprostone described above will be followed in mid-2018 with a phase 3 program in patients 6 months to 6 years of age using the alternate formulation. Takeda has agreed to fund 70% of the costs, up to a cap, of this pediatric functional constipation program.
VAP-1 Inhibitors
In 2016, we discontinued our VAP-1 Inhibitor RTU-1096 development program and our VAP-1 Inhibitor RTU-009 program.
CPP- 1X/Sulindac Combination Product
In January 2016, we entered into an option and collaboration agreement under which CPP has granted us the sole option to acquire an exclusive license to commercialize CPP-1X/sulindac combination product in North America. This product is currently in a Phase 3 clinical trial being conducted by CPP for the treatment of familial adenomatous polyposis, or FAP. Under our agreement with CPP, we have the exclusive option to license this product for North America. There are currently no approved treatments for FAP. The ongoing Phase 3 study is a 150-patient, three-arm, double-blind, randomized trial of the combination agent and the single agent comparators. Enrollment in the study has completed and the results from a Phase 3 futility analysis are expected to be available mid-2017. The trial is expected to conclude in 2019.
| 41 |
Cobiprostone
In April 2016, we announced the discontinuation of development of cobiprostone for the treatment of proton pump inhibitor (PPI)-refractory non-erosive reflux disease (NERD) or symptomatic gastroesophageal reflux disease (sGERD), based on its analysis of top-line data from a Phase 2a study. While cobiprostone demonstrated significant benefit in some of the secondary measures of this exploratory study, the trial did not meet its primary endpoints. In July 2016, we announced the decision to discontinue the development of cobiprostone based on the results of a pre-specified futility analysis of the Phase 2a study of an oral spray formulation of cobiprostone for the prevention of oral mucositis in patients that are undergoing radio chemotherapy for head and neck cancer. The futility analysis indicated that the clinical benefit of cobiprostone was insufficient to support continuation of the study.
Collaboration Agreements
We have the following collaboration agreements with our partners to supply, develop and commercialize AMITIZA:
| · | North America Takeda Agreement; |
| · | Global Takeda Agreement; |
| · | Japan Mylan Agreement; and, |
| · | China Gloria Agreement. |
The collaboration agreements are covered by geographic location.
For RESCULA, we have a collaboration agreement with Santen to commercialize that product in Japan.
North America Takeda Agreement
Under the terms of the North America Takeda Agreement, we supply Takeda with AMITIZA at a negotiated supply price. We have recognized product sales revenue of approximately $13.2 million and $10.3 million for the years ended December 31, 2016 and 2015, respectively.
Takeda is obligated to pay us a sliding royalty rate based on a percentage of the net sales revenue from their sale of AMITIZA in the U.S. and Canada. The actual percentage depends on the level of net sales revenue attained each calendar year. The deductions from gross sales used to calculate net sales for royalty purposes are capped at 20% which protects us from deep discounting. AMITIZA is currently marketed only in the U.S. and during the years ended December 31, 2016, 2015 and 2014 we recognized a total of $82.3 million, $74.0 million and $62.8 million respectively, as product royalty revenue. We believe the year-over-year growth is primarily due to an increase in total market growth and continued performance of the AMITIZA brand in volume growth.
Takeda has agreed to fund all development costs, including regulatory-required studies, to a maximum of $50.0 million for each additional indication and $20.0 million for each additional formulation. Takeda and we have agreed to equally share all costs in excess of those amounts. With respect to any studies required to modify or expand the label for AMITIZA for the treatment of CIC, IBS-C or OIC, Takeda has agreed to fund 70% of the costs of such studies, and we have agreed to fund the remainder. Additionally, Takeda has agreed to fund 100% of the development costs for the alternate formulation of AMITIZA, and 70% of the development costs for the treatment of pediatric functional constipation.
Takeda agreed to reimburse a portion of our expenses related to our specialty sales force. We recognized zero, zero, and $3.4 million of co-promotion revenue reflecting these reimbursements for the years ended December 31, 2016, 2015 and 2014 respectively. In December 2014, as a result of the amendment to the North America Takeda Agreement, we ceased co-promoting AMITIZA.
Our agreements also require Takeda to pay us up to an additional aggregate of $50.0 million upon the achievement of specified targets for annual net sales revenue from AMITIZA in the U.S. and Canada. Sales of AMITIZA have not met these targets as of December 31, 2016, 2015 and 2014.
| 42 |
The following table summarizes the cash streams and related revenue recognized or deferred under the North America Takeda Agreement for the year ended December 31, 2016:
| Cash Received Through December 31, | Revenue Recognized for the Year Ended December 31, | Accounts Receivable for the Year Ended December 31, | Amount Deferred at December 31, | |||||||||||||||||||||
| (In thousands) | 2016 | Through 2014 | 2015 | 2016 | 2016 | 2016 | ||||||||||||||||||
| Product royalty revenue | $ | 484,205 | $ | 354,202 | $ | 74,000 | $ | 82,264 | $ | 26,261 | $ | - | ||||||||||||
| Product sales revenue | $ | 40,789 | $ | - | $ | 10,311 | $ | 45,956 | $ | 15,478 | $ | - | ||||||||||||
| Research and development revenue: | ||||||||||||||||||||||||
| Up-front payment - remainder | $ | 17,624 | $ | 17,624 | $ | - | $ | - | $ | - | $ | - | ||||||||||||
| Development milestones | 140,000 | 140,000 | - | - | - | - | ||||||||||||||||||
| Reimbursement of research and development expenses | 143,581 | 124,026 | 10,164 | 12,839 | 3,448 | - | ||||||||||||||||||
| Total | $ | 301,205 | $ | 281,650 | $ | 10,164 | $ | 12,839 | $ | 3,448 | $ | - | ||||||||||||
| Collaboration revenue: | ||||||||||||||||||||||||
| Supply agreements - Manufacturing | $ | 2,337 | $ | - | $ | 2,337 | $ | - | $ | - | $ | - | ||||||||||||
| Up-front payment associated with our obligation to participate in joint committees | $ | 2,375 | $ | 1,493 | $ | 147 | $ | 147 | $ | - | $ | 588 | ||||||||||||
| Total | $ | 4,712 | $ | 1,493 | $ | 2,484 | $ | 147 | $ | - | $ | 588 | ||||||||||||
| Co-promotion revenue | $ | 32,813 | $ | 32,813 | $ | - | $ | - | $ | - | $ | - | ||||||||||||
Global Takeda Agreement
Upon signing the Global Takeda Agreement in October 2014, we received a nonrefundable upfront payment of $14.0 million, of which $8.0 million was allocated to collaboration revenue and $6.0 million was considered a collaboration obligation to reimburse Takeda for the first $6.0 million in developmental expenses incurred by Takeda.
Takeda will be responsible for any additional development activities and costs incurred (including the supply price for any licensed product that is supplied by us to Takeda for development purposes).
Under the terms of the Global Takeda Agreement, we will supply Takeda with AMITIZA at a negotiated supply price.
Our agreement also requires Takeda to pay us up to an additional aggregate of $35.0 million upon the achievement of specified targets for annual net sales revenue from AMITIZA in global territories covered under the Global Takeda Agreement.
For the years ended December 31, 2016, 2015 and 2014, we recognized product sales revenues under the Global Takeda Agreement, of $792,000, $0 and $0, respectively.
Japan Mylan Agreement
We have recognized product sales revenue of approximately $62.7 million, $48.9 million and $29.6 million for the years ended December 31, 2016, 2015 and 2014, respectively. We believe the year-over-year growth is due to strong physician patient awareness and that AMITIZA is still the only prescription medication approved in Japan for chronic constipation.
During 2016, 2015 and 2014 we received net sales milestones of $10.0 million, $5.0 million and $2.5 million, respectively. We could receive additional milestone payments based on achieving other specified development and commercialization goals, although there can be no assurance that we will receive any such payments.
| 43 |
The following table summarizes the cash streams and related revenue recognized or deferred under the Japan Mylan Agreement for the year ended December 31, 2016:
| Cash Received Through December 31, | Revenue Recognized for the Year Ended December 31, | Accounts Receivable for the Year Ended December 31, | Foreign Currency | Amount Deferred at December 31, | ||||||||||||||||||||||||
| (In thousands) | 2016 | Through 2014 | 2015 | 2016 | 2016 | Effects | 2016 | |||||||||||||||||||||
| Collaboration revenue: | ||||||||||||||||||||||||||||
| Up-front payment associated with the Company's obligation to participate in joint committees | $ | 846 | $ | 280 | $ | 34 | $ | 29 | $ | - | $ | 110 | $ | 393 | ||||||||||||||
| Research and development revenue | ||||||||||||||||||||||||||||
| Up-front payment - remainder | $ | 9,154 | $ | 9,302 | $ | - | $ | - | $ | - | $ | (148 | ) | $ | - | |||||||||||||
| Development milestone payment | 27,500 | 27,755 | - | - | - | (255 | ) | - | ||||||||||||||||||||
| Total | $ | 36,654 | $ | 37,057 | $ | - | $ | - | $ | - | $ | (403 | ) | $ | - | |||||||||||||
| Product sales revenue | ||||||||||||||||||||||||||||
| Product sales revenue | $ | 154,236 | $ | 50,418 | $ | 48,908 | $ | 62,682 | $ | 10,845 | $ | 3,073 | $ | - | ||||||||||||||
| Sales milestone payment | 7,500 | 2,500 | 5,000 | 10,000 | 10,000 | - | - | |||||||||||||||||||||
| $ | 161,736 | $ | 52,918 | $ | 53,908 | $ | 72,682 | $ | 20,845 | $ | 3,073 | $ | - | |||||||||||||||
China Gloria Agreement
Upon signing the China Gloria Agreement in May 2015, we received an upfront payment of $1.0 million and an upfront payment of $500,000 in June 2015 after the CFDA accepted the IND application for a pivotal trial of AMITIZA in patients with CIC.
Once AMITIZA is developed in China, we will supply AMITIZA to Gloria at a negotiated supply price.
Japan Santen Agreement
We recorded product sales revenue for RESCULA of $9.9 million and $1.5 million, for the years ended December 31, 2016 and 2015, respectively.
CPP Agreement
In January 2016, we entered into the CPP Agreement under which CPP has granted us the sole option to acquire an exclusive license to commercialize CPP-1X/sulindac combination product in North America. Under the terms of the CPP Agreement:
We have invested $5.0 million in CPP in the form of a convertible note, with a planned additional $5.0 million equity investment in CPP’s next qualified financing, which will be either an IPO or a private financing as defined by the agreement;
We will pay CPP an option fee of up to $7.5 million, payable in two tranches; the first tranche of $3.0 million was paid at signing;
CPP will complete the ongoing phase 3 trial under the oversight of a joint steering committee;
Upon exercise of its exclusive option, we would acquire an exclusive license to the product and would be obligated to pay CPP up to an aggregate of $190.0 million in license fees and milestone payments upon the achievement of specified clinical development and sales milestones; and
We and CPP would share equally in profits from the sale of approved products.
R-Tech Supply Agreement
The October 2015 acquisition of R-Tech enabled us to secure a larger portion of the global economics of AMITIZA as R-Tech is the exclusive manufacturer and supplier of AMITIZA.
| 44 |
Under the exclusive global manufacturing and supply agreement with R-Tech prior to the acquisition, R-Tech had the exclusive right to manufacture and supply lubiprostone in most global markets. During the years ended December 31, 2015 and 2014, we recorded the following expenses under all of our agreements with R-Tech:
| (In thousands) | January 1 through October 20, 2015 | Year Ended December 31, 2014 | ||||||
| Clinical supplies | $ | 155 | $ | 396 | ||||
| Other research and development services | 347 | 171 | ||||||
| Commercial supplies | 21,415 | 15,776 | ||||||
| $ | 21,917 | $ | 16,343 | |||||
Critical Accounting Policies and Estimates
This discussion and analysis of our financial condition and results of operations is based upon our Consolidated Financial Statements, which have been prepared in accordance with Generally Accepted Accounting Principles (“GAAP”) in the United States. The preparation of our Consolidated Financial Statements requires us to make estimates and judgments that affect our reported assets, liabilities, revenues and expenses. Actual results may differ significantly from those estimates under different assumptions and conditions.
We regard an accounting estimate or assumptions underlying our financial statements as a critical accounting estimate if:
| · | the nature of the estimate or assumption is material due to the level of subjectivity and judgment necessary to account for highly uncertain matters or the susceptibility of such matters to change; and |
| · | the impact of the estimates and assumptions on financial condition or operating performance is material. |
Our significant accounting policies are described in more detail in note 2 of our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K.
Revenue Recognition
Our revenues are derived primarily from collaboration and license agreements which include product royalties, product sales, upfront payments, development milestone payments, reimbursements of development and co-promotion costs.
We recognize revenue when four basic criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed and determinable; (4) collectability is reasonably assured. Determination of criteria (3) and (4) could require management’s judgments regarding the fixed nature of the fee charged for services rendered and products delivered and the collectability of those fees. While the majority of our sales agreements contain standard terms and conditions, we do enter into agreements that contain multiple-element or non-standard terms and conditions. Sometimes interpretation of the sales agreement or contract for multiple-element is complex in determining whether there is more than one unit of accounting and if so, how and when revenue should be recognized for each element is subject to certain estimates or assumptions. We record revenue as the separate elements are delivered to the customer if the delivered item has value on a stand-alone basis and delivery or performance of the undelivered items is probable and substantially in our control. Revenue is allocated according to the relative selling price method. Should changes in conditions cause management to determine that these criteria are not met for certain future transactions, revenue recognized for any reporting period could be adversely affected.
Since 2011, we have entered into the following multiple-element arrangements: (1) research and development studies which are being reimbursed by Takeda and are treated as separate elements within the North America Takeda Agreement, (2) the Global Takeda Agreement and (3) the China Gloria Agreement. We evaluated the multiple deliverables under these arrangements to determine whether the delivered elements that are our obligation have value to other parties to the agreement on a stand-alone basis and whether objective, reliable evidence of fair value of the undelivered items exists. Deliverables that meet these criteria are considered a separate unit of accounting. Deliverables that do not meet these criteria are combined and accounted for as a single unit of accounting. The appropriate recognition of revenue is then applied to each separate unit of accounting.
Where agreements include contingent milestones, we evaluate whether each milestone is substantive. Milestones are considered substantive if all of the following conditions are met: (1) it is commensurate with either our performance to meet the milestone or the enhancement of the value of the delivered item or items as a result of a specific outcome resulting from the our performance to achieve the milestone, (2) it relates solely to past performance, and (3) the value of the milestone is reasonable relative to all the deliverables and payment terms (including other potential milestone consideration) within the arrangement. Where milestones are not considered substantive their treatment is based on either a time-based or proportional performance model.
We apply a time-based model of revenue recognition for cash flows associated with research and development deliverables entered into prior to January 1, 2011 under the North America Takeda Agreement. Under this model, cash flow streams related to each unit of accounting are recognized as revenue over the estimated performance period. Upon receipt of cash payments, such as development milestones, revenue is recognized to the extent the accumulated service time has occurred. The remainder is deferred and recognized as revenue ratably over the remaining estimated performance period. A change in the period of time expected to complete the deliverable is accounted for as a change in estimate on a prospective basis. In cases where milestone payments are received after the completion of the associated development period, we recognize revenue upon completion of the performance obligation. Revenue is limited to amounts that are nonrefundable and that the other party to the agreement is contractually obligated to pay to us. The research and development revenue for these obligations is limited to the lesser of the actual reimbursable costs incurred or the straight-line amount of revenue recognized over the estimated performance period. Revenues are recognized for reimbursable costs only if those costs can be reasonably determined.
| 45 |
For research and development deliverables agreed upon subsequent to January 1, 2011, which are reimbursable under the North America Takeda Agreement at contractually predetermined percentages, we recognize revenue when the underlying research and development expenses are incurred, assuming all other revenue recognition criteria are met.
Under the North America Takeda Agreement, royalties are based on net sales of licensed products and are recorded on the accrual basis when earned in accordance with contractual terms when third-party results are reliably measurable, collectability is reasonably assured and all other revenue recognition criteria are met.
Product sales consist of AMITIZA sales to Takeda under the North America Takeda Agreement and under the Global Takeda Agreement. Product sales also consist of AMITIZA sales to Mylan in Japan under the Mylan Agreement and by us directly in Europe (prior to entering into the Global Takeda Agreement). In addition, product sales of RESCULA occur in Japan under the Santen Agreement and directly by us in the U.S. We ceased marketing RESCULA in the U.S. in the fourth quarter of 2014. Revenue from product sales is recognized when persuasive evidence of an arrangement exists, delivery has occurred and title to product and associated risk of loss have passed to the customer, the price is fixed or determinable, and collection from the customer is reasonably assured. We do not record sales deductions and returns for sales of AMITIZA to Mylan and Takeda due to the absence of discounts and rebates and no right of return under the contracts with Mylan and Takeda.
We recognize contract revenue related to development and commercialization activities under the time-based method over the applicable period.
We consider our participation in the joint committees under the North America Takeda Agreement and the Mylan Agreement as separate deliverables under the contracts and recognize the fair value of such participation as revenue over the period of the participation per the terms of the contracts.
Inventories
Inventories are valued under a weighted average costing method and are stated at the lower of cost or net realizable value. Inventories consist of raw material, work-in-process and finished goods. Our inventories include the direct purchase cost of materials and supplies and manufacturing overhead costs.
Accrued Research and Development Expenses
As part of our process of preparing our Consolidated Financial Statements, we are required to estimate an accrual for research and development expenses. This process involves reviewing and identifying services which have been performed by third parties on our behalf and determining the value of these services. Examples of these services are payments to clinical investigators and CROs. In addition, we make estimates of costs incurred to date but not yet invoiced to us in relation to external CROs and clinical site costs. We analyze the progress of clinical trials, including levels of patient enrollment; invoices received and contracted costs, when evaluating the adequacy of the accrued liabilities for research and development. We must make significant judgments and estimates in determining the accrued balance in any accounting period. No material adjustments have been required for this accrual during the years ended December 31, 2016, 2015 and 2014.
Stock-Based Compensation
We estimate the fair value of option awards on the date of the grant using an option-pricing model. We estimate the fair value of restricted stock units on the date of the grant using the market price of our common stock. Stock-based compensation expense is recognized over the required service periods.
For recording our stock-based compensation expense for service based and market condition options, we have chosen to use:
| · | the straight-line method of allocating compensation cost for service based options and graded vesting for market condition options; |
| · | the Black-Scholes-Merton option pricing formula for time based options and the Monte Carlo simulation model for the market condition options as our chosen option-pricing models; |
| · | the simplified method to calculate the expected term for options as discussed under the SEC’s guidance for share-based payments for service based options; |
| 46 |
| · | an estimate of expected volatility based on the historical volatility of our share price; and |
| · | an estimate for expected forfeitures. |
The three factors which most affect stock-based compensation are the fair value of the common stock underlying the stock options, the vesting term of the options, and the volatility of such fair value of the underlying common stock. If our estimates are too high or too low, we may overstate or understate our stock-based compensation expense.
Mergers and Acquisitions
In a business combination, the acquisition method of accounting requires that the assets acquired and liabilities assumed be recorded as of the date of the merger or acquisition at their respective fair values. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Accordingly, we may be required to value assets at fair value measures that do not reflect our intended use of those assets. Any excess of the purchase price (consideration transferred) over the estimated fair values of net assets acquired is recorded as goodwill. Transaction costs and costs to restructure the acquired company are expensed as incurred. The operating results of the acquired business are reflected in our consolidated financial statements after the date of the merger or acquisition. If we determine the assets acquired do not meet the definition of a business under the acquisition method of accounting, the transaction will be accounted for as an acquisition of assets rather than a business combination and, therefore, no goodwill will be recorded. The fair values of intangible assets, including acquired in-process research and development (“IPR&D”) are determined utilizing information available near the merger or acquisition date based on expectations and assumptions of a market participant that are deemed reasonable by management. Given the considerable judgment involved in determining fair values, we typically obtain assistance from third-party valuation specialists for significant items. Amounts allocated to acquire IPR&D are capitalized and accounted for as indefinite-lived intangible assets. Upon successful completion of each project, we will make a separate determination as to the then useful life of the asset and begin amortization. The judgments made in determining estimated fair values assigned to assets acquired and liabilities assumed in a business combination, as well as asset lives, can materially affect results of operations.
The fair values of identifiable intangible assets related to currently marketed products and product rights are primarily determined by using an "income approach" through which fair value is estimated based on each asset's discounted projected net cash flows. Our estimates of market participant net cash flows consider historical and projected pricing, margins and expense levels; the performance of competing products where applicable; relevant industry and therapeutic area growth drivers and factors; current and expected trends in technology and product life cycles; the time and investment that will be required to develop products and technologies; the ability to obtain marketing and regulatory approvals; the ability to manufacture and commercialize the products; the extent and timing of potential new product introductions by competitors; and the life of each asset's underlying patent, if any. The net cash flows are then probability-adjusted where appropriate to consider the uncertainties associated with the underlying assumptions, as well as the risk profile of the net cash flows utilized in the valuation. The probability-adjusted future net cash flows of each product are then discounted to present value utilizing an appropriate discount rate.
The fair values of identifiable intangible assets related to IPR&D are determined using an income or cost approach. Under the income approach fair value is estimated based on each asset's probability-adjusted future net cash flows, which reflect the different stages of development of each product and the associated probability of successful completion. The net cash flows are then discounted to present value using appropriate discounts. Under the replacement cost approach, historical research and development spending is analyzed to derive the costs incurred to date related to the asset. An expected return of 20% is applied to the cumulative mid-year pre-tax research and development costs incurred to date, as this estimates the required rate of a return a prudent investor would require on a similarly situated asset. No tax amortization benefit has been applied given the asset is valued under the replacement cost approach, which is representative of buying the asset in the market at fair value.
Income Taxes
As part of the process of preparing our Consolidated Financial Statements, we are required to estimate our income taxes in each of the jurisdictions in which we operate. We follow the Financial Accounting Standards Board’s (“FASB”) guidance for accounting for income taxes which requires us to estimate our actual current tax exposure while assessing our temporary differences resulting from the differing treatment of items for tax and accounting purposes. These differences have resulted in deferred tax assets and liabilities, which are included in our Consolidated Balance Sheets. We record a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. We consider forecasted earnings, future taxable income, the mix of earnings in the jurisdictions in which we operate, expiration dates of net operating loss carry-forwards, and prudent and feasible tax planning strategies in determining the need for a valuation allowance. Considerable judgment is involved in developing such estimates. In the event we were to determine that we would not be able to realize all or part of our net deferred tax assets in the future, we would recognize a valuation allowance in the period in which we make that determination. Likewise, if we later determine that it is more likely than not that the net deferred tax assets would be realized, we would reverse the applicable portion of the previously provided valuation allowance. In order for us to realize our deferred tax assets we must be able to generate sufficient taxable income in the tax jurisdictions in which our deferred tax assets are located.
| 47 |
Significant judgment is required in determining the provision for income taxes and any valuation allowance recorded against our net deferred tax assets in certain jurisdictions. Significant future events, not under our control, including continued success in commercialization of products in U.S. markets or regulatory approvals for products in international markets, could affect our future earnings potential and consequently the amount of deferred tax assets that will be utilized. We followed the FASB’s guidance for uncertainty in income taxes that requires the application of a “more likely than not” threshold to the recognition and de-recognition of uncertain tax positions. If the recognition threshold is met, this guidance permits us to recognize a tax benefit measured at the largest amount of the tax benefit that, in our judgment, is more than 50.0% likely to be realized upon settlement.
We recognize interest and penalties accrued related to uncertain tax positions as a component of the income tax provision.
Non-GAAP Financial Metrics
In addition to disclosing financial results that are determined in accordance with GAAP, we also use the following non-GAAP financial metrics to understand and evaluate our operating performance:
| · | Adjusted net income, which is GAAP net income adjusted to exclude the tax-effected impact of (i) amortization of acquired intangibles, (ii) inventory step-up adjustment, (iii) impairment of in-process research and development, (iv) restructuring costs, (v) legal settlement, (vi) acquisition related expenses, (vii) amortization of debt financing costs, (viii) loss on debt extinguishment, (ix) research and development license option expense, (x) acceleration of deferred revenue, and (xi) foreign currency translation; |
| · | Adjusted EPS-diluted, which is adjusted net income as defined above expressed on a diluted per share basis; |
| · | EBITDA, which is GAAP net income adjusted to exclude (i) taxes, (ii) interest expense, (iii) interest income, (iv) depreciation, (v) impairment of in-process research and development, (vi) amortization of acquired intangibles, and (vii) inventory step-up adjustment; |
| · | Adjusted EBITDA, which is EBITDA as defined above further adjusted to exclude (i) share-based compensation expense, (ii) restructuring costs, (iii) acquisition-related expenses, (iv) loss on debt extinguishment, (v) research and development license option expense, (vi) legal settlement, (vii) foreign currency translation, and (viii) acceleration of deferred revenue. |
We believe that providing this additional information is useful to the reader to better assess and understand our operating performance, primarily because management typically monitors the business adjusted for these items in addition to GAAP results. These non-GAAP financial metrics should be considered supplemental to and not a substitute for financial information prepared in accordance with GAAP. Our definition of these non-GAAP metrics may differ from similarly titled metrics used by others. We view these non-GAAP financial metrics as a means to facilitate our financial and operational decision-making, including evaluation of our historical operating results and comparison to competitors’ operating results. These non-GAAP financial metrics reflect an additional way of viewing aspects of the company’s operations that, when viewed with GAAP results may provide a more complete understanding of factors and trends affecting the company’s business. The determination of the amounts that are excluded from these non-GAAP financial metrics is a matter of management judgment and depends upon, among other factors, the nature of the underlying expense or income amounts. Because non-GAAP financial metrics exclude the effect of items that will increase or decrease the company’s reported results of operations, we strongly encourage investors to review our consolidated financial statements and periodic reports in their entirety.
| 48 |
The following tables present reconciliations of these non-GAAP financial metrics to the most directly comparable GAAP financial measure for the years ended December 31, 2016 and 2015.
| Year Ended December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Non-GAAP adjusted net income | ||||||||
| GAAP net income | $ | 18,487 | $ | 33,371 | ||||
| Amortization of acquired intangibles | 25,655 | 3,732 | ||||||
| Inventory step-up adjustment | 15,236 | 5,645 | ||||||
| Impairment of in-process research and development | 7,286 | - | ||||||
| Restructuring costs | 2,350 | 958 | ||||||
| Legal settlement | (9,515 | ) | - | |||||
| Acquisition related expenses | 2,173 | 5,135 | ||||||
| Amortization of debt financing costs | 3,526 | 870 | ||||||
| Loss on debt extinguishment | 14,047 | - | ||||||
| Research and development license option expense | 3,000 | - | ||||||
| Acceleration of deferred revenue | - | (4,079 | ) | |||||
| Foreign currency translation | 11,280 | 178 | ||||||
| Tax effect of adjustments | (27,313 | ) | (2,119 | ) | ||||
| Non-GAAP adjusted net income | $ | 66,212 | $ | 43,691 | ||||
| Non-GAAP adjusted EPS - diluted | $ | 1.51 | $ | 0.96 | ||||
| (In thousands) | ||||||||
| Non-GAAP EBITDA | ||||||||
| GAAP net income | $ | 18,487 | $ | 33,371 | ||||
| Taxes | (4,112 | ) | 10,304 | |||||
| Interest expense | 23,761 | 6,854 | ||||||
| Interest income | (72 | ) | (181 | ) | ||||
| Depreciation | 904 | 623 | ||||||
| Impairment of in-process research and development | 7,286 | - | ||||||
| Amortization of acquired intangibles | 25,655 | 3,732 | ||||||
| Inventory step-up adjustment | 15,236 | 5,645 | ||||||
| EBITDA | $ | 87,145 | $ | 60,348 | ||||
| (In thousands) | ||||||||
| Non-GAAP adjusted EBITDA | ||||||||
| EBITDA | $ | 87,145 | $ | 60,348 | ||||
| Share-based compensation expense | 7,258 | 7,349 | ||||||
| Restructuring costs | 2,350 | 958 | ||||||
| Acquisition related expenses | 2,173 | 5,135 | ||||||
| Loss on debt extinguishment | 14,047 | - | ||||||
| Research and development license option expense | 3,000 | - | ||||||
| Legal settlement | (9,515 | ) | - | |||||
| Foreign currency translation | 11,280 | 178 | ||||||
| Acceleration of deferred revenue | - | (4,079 | ) | |||||
| Adjusted EBITDA | $ | 117,738 | $ | 69,889 | ||||
| 49 |
Results of Operations
Comparison of years ended December 31, 2016 and 2015
Revenues
The following table summarizes our revenues for the years ended December 31, 2016 and 2015:
| Year Ended December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Product royalty revenue | $ | 82,480 | $ | 74,138 | ||||
| Product sales revenue - AMITIZA | 118,638 | 65,533 | ||||||
| Product sales revenue - RESCULA | 10,158 | 743 | ||||||
| Research and development revenue | 12,839 | 10,199 | ||||||
| Contract and collaboration revenue | 5,941 | 2,567 | ||||||
| Total | $ | 230,056 | $ | 153,180 | ||||
Total revenues were $230.1 million in 2016 compared to $153.2 million in 2015, an increase of $76.9 million or 50.2%.
Product royalty revenue
Product royalty revenue primarily represents royalty revenue earned on Takeda net sales of AMITIZA in North America and was $82.5 million in 2016 compared to $74.1 million in 2015, an increase of $8.3 million or 11.3%. The increase was primarily due to higher net sales of AMITIZA as reported by Takeda for royalty calculation purposes resulting from a combination of higher sales volume and higher prices.
Product sales revenue
Product sales revenue consists of drug product sales of AMITIZA in North America, Japan and Europe, and drug product sales of RESCULA in Japan and the U.S.
AMITIZA product sales revenue was $118.6 million in 2016 compared to $65.5 million in 2015, an increase of $53.1 million or 81.0%. This increase was primarily due to a $45.2 million increase in AMITIZA sales in North America from the acquisition of R-Tech in October 2015; and a $10.0 million milestone payment recognized in 2016 compared to a $5.0 million milestone payment recognized in 2015.
RESCULA product sales revenue was $10.2 million for 2016 compared to $743,000 in 2015, an increase of $9.4 million. This increase was due to the acquisition of R-Tech in October 2015 and resulting revenue from sales of RESCULA under the Japan Santen Agreement.
Research and development revenue
Research and development revenue was $12.8 million in 2016 compared to $10.2 million in 2015, an increase of $2.6 million or 25.9%. The increase was primarily due to increased activity on the advancement of pediatric and alternative formulation studies in 2016, for which we receive reimbursement from Takeda.
Contract and collaboration revenue
Contract and collaboration revenue was $5.9 million in 2016 compared to $2.6 million in 2015, an increase of $3.4 million or 131.4%. The increase was primarily attributable to a higher release of the collaboration obligation under the Global Takeda Agreement in 2016, partially offset by upfront and milestone payments of $1.5 million recognized in 2015 under the China Gloria Agreement.
Costs of Goods Sold
Costs of goods sold were $76.0 million in 2016 compared to $36.7 million in 2015, an increase of $39.3 million or 106.9%. The increase in costs of goods sold was primarily due to the full year amortization of the acquired intangible assets of $25.7 million and full year amortization of the inventory step-up adjustment of $15.2 million related to the acquisition of R-Tech in October 2015, coupled with an increase in sales volumes.
| 50 |
Research and Development Expenses
The following table summarizes our research and development expenses for the years ended December 31, 2016 and 2015:
| Year Ended December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Direct costs: | ||||||||
| Lubiprostone | $ | 25,692 | $ | 16,306 | ||||
| Cobiprostone | 6,131 | 8,740 | ||||||
| CPP-1X | 3,000 | - | ||||||
| RTU-1096 | 2,662 | 1,131 | ||||||
| Other | 4,497 | 1,056 | ||||||
| Total | 41,982 | 27,233 | ||||||
| Indirect costs | 4,633 | 6,398 | ||||||
| Total | $ | 46,615 | $ | 33,631 | ||||
Total research and development expenses were $46.6 million in 2016 compared to $33.6 million in 2015, an increase of $13.0 million or 38.6%. The increase was primarily due to an increase in expenses related to the ongoing AMITIZA pediatric trials, costs associated with the CPP Agreement, and the inclusion of R-Tech’s research and development costs during the post-acquisition period.
Impairment of In-Process Research and Development
During 2016, we discontinued our VAP-1 Inhibitor RTU-1096 development program and our VAP-1 Inhibitor RTU-009 program. We considered the discontinuance as a potential indicator of impairment of the related IPR&D asset. Accordingly, we performed an interim assessment and recorded an impairment charge of $7.3 million in 2016, which represented the entire carrying value of the IPR&D asset. This charge is classified in our Consolidated Statements of Operations and Comprehensive Income as Impairment of In-process Research and Development.
General and Administrative Expenses
The following summarizes our general and administrative expenses for years ended December 31, 2016 and 2015:
| Year Ended December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Salaries, benefits and related costs | $ | 12,320 | $ | 10,963 | ||||
| Legal, consulting and other professional expenses | 12,676 | 6,946 | ||||||
| Stock-based compensation expense | 5,092 | 4,936 | ||||||
| Rent and facilities | 2,079 | 1,445 | ||||||
| Insurance | 940 | 792 | ||||||
| Pharmacovigilance | 1,555 | 1,406 | ||||||
| Investor relations | 816 | 838 | ||||||
| R-Tech integration and transaction costs | 2,142 | 5,115 | ||||||
| Restructuring costs | 2,350 | 286 | ||||||
| Other expenses | 3,828 | 2,790 | ||||||
| Total | $ | 43,798 | $ | 35,517 | ||||
General and administrative expenses were $43.8 million in 2016 compared to $35.5 million in 2015, an increase of $8.3 million or 23.3%. The increase is primarily due to the legal costs associated with the settlement of the Dr. Reddy’s matter, business development expenses and the inclusion of R-Tech general and administrative expenses and restructuring costs in 2016.
Selling and Marketing Expenses
Selling and marketing expenses were $2.5 million in 2016 compared to $2.8 million in 2015, a decrease of $364,000 or 12.8%. The decrease was the result of restructuring sales and marketing activities in the U.S. during 2015, partially offset by the inclusion of R-Tech RESCULA-related sales and marketing activities in 2016.
| 51 |
Non-Operating Income and Expense
The following table summarizes our non-operating income and expense for the years ended December 31, 2016 and 2015:
| Year Ended December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Interest income | $ | 72 | $ | 181 | ||||
| Interest expense | (23,761 | ) | (6,854 | ) | ||||
| Loss on debt extinguishment | (14,047 | ) | - | |||||
| Other income (expense), net | (1,765 | ) | 5,889 | |||||
| Total | $ | (39,501 | ) | $ | (784 | ) | ||
Interest expense was $23.8 million in 2016 compared to $6.9 million in 2015, an increase of $16.9 million or 246.7%. The increase was the result of interest payable on our Credit Facility that was entered into in October 2015.
Loss on debt extinguishment of $14.0 million was the result of the pay off of the amounts due under our Credit Facility in December 2016 and the write off of all of the remaining associated capitalized debt issuance costs.
Other income (expense), net was $1.8 million expense in 2016 compared to $5.9 million income in 2015, a decrease of $7.7 million or 130.0%. The 2016 amount consisted primarily of $11.3 million in unrealized and noncash foreign exchange losses partially offset by $9.3 million income recognized from the AMED grant forgiveness. The 2015 amount consisted primarily of a $3.9 million settlement of a pre-existing relationship related to the R-Tech supply agreements and a $2.0 million payment received from R-Tech in May 2015 for the transfer and assignment of RESCULA licensing rights.
Income Taxes
For the years ended December 31, 2016 and 2015, we recorded a tax benefit of $4.1 million and a tax expense of $10.3 million, respectively, and our consolidated effective tax rate was (28.6)% and 23.6%, respectively. The change in our effective tax rate in 2016 compared to 2015 was attributable primarily to the change in the mix of earnings of our foreign subsidiaries and foreign exchange gains and losses.
| 52 |
Comparison of years ended December 31, 2015 and December 31, 2014
Revenues
The following table summarizes our revenues for the years ended December 31, 2015 and 2014:
| Year Ended December 31, | ||||||||
| (In thousands) | 2015 | 2014 | ||||||
| Product royalty revenue | $ | 74,138 | $ | 62,775 | ||||
| Product sales revenue | 66,276 | 33,252 | ||||||
| Research and development revenue | 10,199 | 7,246 | ||||||
| Contract and collaboration revenue | 2,567 | 8,817 | ||||||
| Co-promotion revenue | - | 3,360 | ||||||
| Total | $ | 153,180 | $ | 115,450 | ||||
Total revenues were $153.2 million in 2015 compared to $115.5 million in 2014, an increase of $37.7 million or 32.7%.
Product royalty revenue
Product royalty revenue represents royalty revenue earned on Takeda net sales of AMITIZA in North America and was $74.1 million in 2015 compared to $62.8 million in 2014, an increase of $11.4 million or 18.1%. The increase was primarily due to higher net sales of AMITIZA as reported by Takeda for royalty calculation purposes resulting from higher sales volume.
Product sales revenue
Product sales revenue represents drug product sales of AMITIZA in North America, Japan and Europe, and drug product sales of RESCULA in Japan and the U.S. Product sales revenue was $66.3 million in 2015 compared to $33.3 million in 2014, an increase of $33.0 million or 99.3%. The increase was primarily due to a $19.2 million increase in AMITIZA sales in Japan, an increase of $9.4 million in R-Tech AMITIZA sales to Takeda, a $5.0 million milestone payment and $1.5 million of RESCULA sales in Japan due to the acquisition of R-Tech in October 2015.
Research and development revenue
Research and development revenue was $10.2 million in 2015 compared to $7.2 million in 2014, an increase of $2.9 million or 40.8%. The increase was primarily due to an increase in expenses reimbursed by Takeda in relation to the ongoing AMITIZA pediatric trial.
Contract and collaboration revenue
Contract and collaboration revenue was $2.6 million in 2015 compared to $8.8 million in 2014, a decrease of $6.3 million or 70.9%. The decrease was primarily due to the recognition of $8.0 million in upfront payment from Takeda under the Global Takeda Agreement in 2014, partially offset by the recognition of $1.5 million in upfront payments from Gloria under the China Gloria Agreement in May and June 2015.
Co-promotion revenue
Co-promotion revenue represents reimbursements by Takeda of a portion of our co-promotion costs for our specialty sales force and was $3.4 million in 2014. Beginning in 2015, we no longer engage in co-promotion activities and, as a result, we no longer receive any co-promotion reimbursements from Takeda.
Costs of Goods Sold
Costs of goods sold were $36.7 million in 2015 compared to $16.3 million in 2014, an increase of $20.5 million or 125.8%. The increase in costs of goods sold was primarily due to the increased volume of AMITIZA sales in Japan, the addition of RESCULA sales in Japan due to the acquisition of R-Tech in October 2015 and the resulting amortization of the acquired intangible assets of $3.7 million and inventory step-up adjustment of $5.6 million.
| 53 |
Research and Development Expenses
The following table summarizes our research and development expenses for the years ended December 31, 2015 and 2014:
| Year Ended December 31, | ||||||||
| (In thousands) | 2015 | 2014 | ||||||
| Direct costs: | ||||||||
| Lubiprostone | $ | 16,306 | $ | 11,002 | ||||
| Cobiprostone | 8,740 | 1,887 | ||||||
| Ion Channel Activator | 6 | 1,664 | ||||||
| Unoprostone isopropyl | 478 | 846 | ||||||
| RTU-1096 | 1,131 | - | ||||||
| RTU-009 | 139 | - | ||||||
| Other | 433 | 1,554 | ||||||
| Total | 27,233 | 16,953 | ||||||
| Indirect costs | 6,398 | 3,613 | ||||||
| Total | $ | 33,631 | $ | 20,566 | ||||
Total research and development expenses were $33.6 million in 2015 compared to $20.6 million in 2014, an increase of $13.1 million or 63.5%. The increase was primarily due to costs associated with the initiation of phase 2 clinical trials for cobiprostone, an increase in expenses related to the ongoing AMITIZA pediatric trials, the acquisition of R-Tech in October 2015 and the inclusion of the respective share of R-Tech’s research and development costs during the post-acquisition period.
General and Administrative Expenses
The following summarizes our general and administrative expenses for years ended December 31, 2015 and 2014:
| Year Ended December 31, | ||||||||
| (In thousands) | 2015 | 2014 | ||||||
| Salaries, benefits and related costs | $ | 10,963 | $ | 9,322 | ||||
| Legal, consulting and other professional expenses | 6,946 | 12,951 | ||||||
| Stock-based compensation expense | 4,936 | 1,802 | ||||||
| Pharmacovigilance | 1,406 | 1,289 | ||||||
| R-Tech transaction costs | 5,115 | - | ||||||
| Restructuring costs | 286 | - | ||||||
| Other expenses | 5,865 | 5,866 | ||||||
| Total | $ | 35,517 | $ | 31,230 | ||||
General and administrative expenses were $35.5 million in 2015 compared to $31.2 million in 2014, an increase of $4.3 million or 13.7%. The increase was primarily due to a $1.6 million increase in salaries, benefits and related costs, a $3.1 million increase in stock-based compensation expense, and $5.2 million of R-Tech transaction costs incurred in connection with the acquisition of R-Tech, partially offset by a $6.0 million decrease in legal fees due to settlement of our patent infringement lawsuit against Par Pharmaceutical, et al.
Selling and Marketing Expenses
The following summarizes our selling and marketing expenses for years ended December 31, 2015 and 2014:
| Year Ended December 31, | ||||||||
| (In thousands) | 2015 | 2014 | ||||||
| Salaries, benefits and related costs | $ | 830 | $ | 2,724 | ||||
| Consulting and other professional expenses | 188 | 5,732 | ||||||
| Samples expense | 4 | 276 | ||||||
| Contract fees | 66 | 1,816 | ||||||
| Data purchases | 201 | 913 | ||||||
| Promotional materials & programs | 71 | 982 | ||||||
| Restructuring costs | 473 | - | ||||||
| Other expenses | 1,009 | 2,080 | ||||||
| Total | $ | 2,842 | $ | 14,523 | ||||
Selling and marketing expenses were $2.8 million in 2015 compared to $14.5 million in 2014, a decrease of $11.7 million or 80.4%. The decrease was the result of eliminating our contract sales force related to AMITIZA in the fourth quarter of 2014.
| 54 |
Non-Operating Income and Expense
The following table summarizes our non-operating income and expense for the years ended December 31, 2015 and 2014:
| Year Ended December 31, | ||||||||
| (In thousands) | 2015 | 2014 | ||||||
| Interest income | $ | 181 | $ | 172 | ||||
| Interest expense | (6,854 | ) | (1,520 | ) | ||||
| Other income, net | 5,889 | 1,250 | ||||||
| Total | $ | (784 | ) | $ | (98 | ) | ||
Interest expense was $6.9 million in 2015 compared to $1.5 million in 2014, an increase of $5.3 million or 350.9%. The increase was the result of additional debt incurred in conjunction with the acquisition of R-Tech.
Other income, net was $5.9 million in 2015 compared to $1.3 million in 2014, an increase of $4.6 million or 371.1%. The increase was primarily due to a $3.9 million settlement of a pre-existing relationship related to the R-Tech supply agreements and a $2.0 million payment received from R-Tech in May 2015 for the transfer and assignment of RESCULA licensing rights, partially offset by a $1.0 million decrease in unrealized and noncash foreign exchange gains.
Income Taxes
For the years ended December 31, 2015 and 2014, we recorded a tax expense of $10.3 and $14.0 million, respectively. For the years ended December 31, 2015 and 2014, our consolidated effective tax rate was 23.6% and 51.6%, respectively. The change in our effective tax rate in 2015 compared to 2014 was attributable primarily to the change in the mix of earnings of our foreign subsidiaries.
| 55 |
Liquidity and Capital Resources
Sources of Liquidity
We finance our operations principally from cash generated from revenues, cash and cash equivalents on hand, debt and to a lesser extent, from the issuance and sale of our class A common stock and through the exercise of employee stock options. Revenues generated from operations principally consist of a combination of product sales, royalty payments, upfront and milestone payments, and research and development expense reimbursements received from Takeda, Mylan and other parties.
Our cash, cash equivalents and restricted cash consist of the following:
| As of December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Cash and cash equivalents | $ | 198,308 | $ | 108,284 | ||||
| Restricted cash, current | 213 | 55,218 | ||||||
| Total | $ | 198,521 | $ | 163,502 | ||||
Cash and cash equivalents represent deposits in operating accounts. Restricted cash at December 31, 2016 represents a certificate of deposit pledged to support an operating lease for our former office facility in Bethesda, Maryland. Restricted cash at December 31, 2015 consisted primarily of (i) $25.0 million related to the Credit Facility (see note 18 in the Notes to Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K), (ii) $17.7 million for payment of the Ueno and Kuno Trust Notes (see note 17 in the Notes to Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K), and (iii) $8.2 million related to the squeeze out of non-tendering R-Tech shareholders (see note 5 in the Notes to Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K). All such restricted cash amounts at December 31, 2015 were released from restricted cash during the year ended December 31, 2016.
Cash Flows
The following table summarizes our cash flows for the years ended December 31, 2016, 2015 and 2014:
| Year Ended December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| Cash provided by (used in): | ||||||||||||
| Operating activities | $ | 8,313 | $ | 18,585 | $ | 30,878 | ||||||
| Investing activities | (1,842 | ) | (131,151 | ) | 12,959 | |||||||
| Financing activities | 70,533 | 149,341 | (15,413 | ) | ||||||||
| Effect of exchange rates | 13,020 | (113 | ) | (904 | ) | |||||||
| Net increase (decrease) in cash and cash equivalents | $ | 90,024 | $ | 36,662 | $ | 27,520 | ||||||
Year ended December 31, 2016
Net cash provided by operating activities was $8.3 million for the year ended December 31, 2016. This was primarily due to net income of $18.5 million, adjustments to reconcile net income to net cash consisting of depreciation and amortization of $45.7 million, loss on debt extinguishment of $14.0 million, write down of in-process research and development of $7.3 million, and stock-based compensation expense of $7.3 million, offset by foreign currency re-measurement gain of $1.2 million, a deferred tax benefit decrease of $37.0 million and forgiveness of AMED deferred grant of $9.3 million. Additional cash used in operating activities consisted of increases in receivables of $22.3 million, decreases in payables of $7.1 million, decreases in prepaid and income taxes receivable and payable, net of $8.7 million, and an increase in inventory of $1.2 million.
Net cash used in investing activities was $1.8 million for the year ended December 31, 2016. This was primarily due to the payment of the squeeze-out liability for non-tendering R-Tech shareholders of $7.7 million, investment in a convertible note receivable of $5.0 million, purchases of property and equipment of $1.2 million, partially offset by a decrease in restricted cash of $12.3 million.
Net cash provided by financing activities was $70.5 million for the year ended December 31, 2016. This was primarily due to proceeds from notes payable, net of debt issuance costs of $290.5 million, decrease in restricted cash of $42.7 million, the issuance of Class A common stock upon the exercise of options and the associated windfall benefit together totaling $7.1 million, partially offset by repayments of notes payable and debt extinguishment costs of $269.9 million.
| 56 |
The effect of exchange rates on the cash balances of currencies held in foreign denominations for year ended December 31, 2016 was an increase of $13.0 million.
Year ended December 31, 2015
Net cash provided by operating activities of $18.6 million for the year ended December 31, 2015 was primarily due to a net income of $33.4 million offset by net changes in operating assets and liabilities totaling $15.0 million. Net changes in other assets and liabilities of $15.0 million consisted primarily of an increase in receivables of $8.5 million, a decrease in deferred revenue of $5.4 million, an increase in product royalties receivable of $4.2 million and a decrease in accounts payable of $1.7 million, offset by cash provided by other assets and liabilities, net of $5.7 million. Major offsetting components of non-cash expenses were: depreciation and amortization of $10.6 million, stock-based compensation of $7.3 million offset by a deferred tax benefit of $9.8 million, unrealized currency translation gains of $3.7 million, windfall benefit from stock-based compensation of $2.5 million and transfer and assignment of licensing rights of $2.0 million.
Net cash used in investing activities of $131.2 million for the year ended December 31, 2015 was primarily the result of acquisition, net of acquired cash of $161.2 million and investment purchases of $39.8 million offset by cash from matured investments of $30.6 million and proceeds from investment sales of $45.1 million.
Net cash provided by financing activities of $149.3 million for the year ended December 31, 2015 was primarily due to proceeds of notes payable of $235.9 million net of debt issuance costs offset by purchase of treasury stock of $44.0 million and payment of notes payable of $8.2 million.
The effect of exchange rates on the cash balances of currencies held in foreign denominations for year ended December 31, 2015 was a decrease of $113,000.
Year ended December 31, 2014
Net cash provided by operating activities of $30.9 million for the year ended December 31, 2014 was primarily due to a net income of $13.1 million, non-cash expenses totaling $11.7 million (which includes an intangible assets impairment of $5.6 million, an increase in deferred charges of $3.2 million, stock-based compensation expense of $2.3 million, and depreciation and amortization of $1.1 million), cash provided by an increase in collaboration obligation of $6.0 million, and net changes in other assets and liabilities of $3.7 million, offset by cash used in net changes in receivables and payables of $3.7 million.
Net cash provided by investing activities of $13.0 million for the year ended December 31, 2014 was primarily the result of a decrease in restricted cash of $25.8 million, cash from matured investments of $14.7 million, and proceeds from investment sales of $1.7 million, offset by investment purchases of $29.2 million.
Net cash used in financing activities of $15.4 million for the year ended December 31, 2014 was primarily due to the payment of notes payable of $24.9 million, offset by proceeds of $5.3 million from our “at-the-market” stock offering and proceeds of $3.8 million from the exercise of employee stock options.
The effect of exchange rates on the cash balances of currencies held in foreign denominations for year ended December 31, 2014 was a decrease of $904,000.
Commitments and Contractual Obligations
The following table summarizes our significant contractual obligations as of December 31, 2016:
| (In thousands) | Total | Less than 1 year | 1 - 3 years | 3 - 5 years | More than 5 years | |||||||||||||||
| 3.25% Convertible Senior Notes due 2021 | $ | 300,000 | $ | - | $ | - | $ | 300,000 | $ | - | ||||||||||
| Interest on convertible notes | 48,325 | 9,750 | 19,500 | 19,075 | - | |||||||||||||||
| Operating lease commitments | 13,503 | 1,980 | 3,218 | 2,091 | 6,214 | |||||||||||||||
| Uncertain tax positions (1) | 4,060 | - | - | - | - | |||||||||||||||
| $ | 365,888 | $ | 11,730 | $ | 22,718 | $ | 321,166 | $ | 6,214 | |||||||||||
(1) We do not expect to settle any of this amount within the next twelve months in cash. It is reasonably possible that $1.6 million of the liability for unrecognized tax benefits will decrease within the next 12 months and the remaining $2.5 million in an unknown future period.
| 57 |
Off-Balance Sheet Arrangements
As of December 31, 2016, we did not have any off-balance sheet arrangements, as such term is defined in Item 303(a)(4) of Regulation S-K under the Securities Act of 1933, as amended.
Funding Requirements
We may need substantial amounts of capital to continue growing our business. We may require this capital, among other things, to fund:
| · | our share of the on-going development program of AMITIZA; |
| · | research, development, manufacturing, regulatory and marketing efforts for our other products and product candidates; |
| · | the costs involved in obtaining and maintaining proprietary protection for our products, technology and know-how, including litigation costs and the results of such litigation; |
| · | activities to resolve our on-going and potential legal matters; |
| · | any option and milestone payments under general option and licensing ventures, including our exclusive option and collaboration agreement with CPP; |
| · | other business development activities, including partnerships, alliances and investments in or acquisitions of other businesses, products and technologies; |
| · | the expansion of our commercialization activities including the purchase of inventory; and |
| · | the payment of principal and interest under our Convertible Notes. |
The timing of these funding requirements is difficult to predict due to many factors, including the outcomes of our preclinical and clinical research and development programs and when those outcomes are determined, the timing of obtaining regulatory approvals and the presence and status of competing products. Our capital needs may exceed the capital available from our future operations, collaborative and licensing arrangements and existing liquid assets. Our future capital requirements and liquidity will depend on many factors, including, but not limited to:
| · | the cost and time involved to pursue our research and development programs; |
| · | our ability to establish collaborative arrangements and to enter into licensing agreements and contractual arrangements with others; and |
| · | any future change in our business strategy. |
To the extent that our capital resources may be insufficient to meet our future capital requirements, we may need to finance our future cash needs through at-the-market sales, public or private equity offerings, further debt financings or corporate collaboration and licensing arrangements.
At December 31, 2016, based upon our current business plan, we believe we have sufficient liquidity for the next 12 months.
Effects of Foreign Currency
We currently receive a portion of our revenue, incur a portion of our operating expenses, and have assets and liabilities denominated in currencies other than the U.S. Dollar, the reporting currency for our Consolidated Financial Statements. As such, the results of our operations could be adversely affected by changes in exchange rates either due to transaction losses, which are recognized in the statement of operations, or translation losses, which are recognized in comprehensive income. We currently do not hedge foreign exchange rate exposure via derivative instruments, but anticipate doing so in 2017.
Accounting Pronouncements
Refer to note 2 in the Notes to Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K.
| 58 |
| ITEM 7A. | Quantitative and Qualitative Disclosures about Market Risk |
Foreign Currency Exchange Rate Risk
We are subject to foreign exchange risk for revenues and expenses denominated in foreign currencies. Foreign currency risk arises from the fluctuation of foreign exchange rates and the degree of volatility of these rates relative to the U.S. Dollar. We do not currently hedge our foreign currency transactions via derivative instruments, but anticipate doing so in 2017.
Interest Rate Risk
Our exposure to market risks associated with changes in interest rates relates to both (i) the amount of interest income earned on our investment portfolio, and (ii) the amount of interest payable by us on the Convertible Notes. As our investment portfolio is immaterial at this time and the interest rate on our Convertible Notes is fixed at 3.25% through 2021, we believe that our exposure to market risks associated with changes in interest rates is nominal.
With respect to our investments, our goal is to ensure the safety and preservation of invested funds by limiting default risk, market risk and reinvestment risk. We attempt to mitigate default risk by investing in investment grade securities. A hypothetical one percentage point decline in interest rates would not have materially affected the fair value of our interest-sensitive financial instruments as of December 31, 2016.
We do not use derivative financial instruments for trading or speculative purposes. However, we regularly invest excess cash in overnight repurchase agreements that are subject to changes in short-term interest rates. We believe that the market risk arising from holding these financial instruments is nominal.
Credit Risk
Our exposure to credit risk generally consists of cash and cash equivalents, restricted cash, investments and receivables. We place our cash, cash equivalents and restricted cash with what we believe to be highly rated financial institutions and invest the excess cash in highly rated investments. Our investment policy limits investments to certain types of debt and money market instruments issued by institutions primarily with investment grade credit ratings and places restriction on maturities and concentrations by asset class and issuer.
Our exposure to credit risk also extends to strategic investments made as part of our ongoing business development activities, such as the investment of $5.2 million in CPP in the form of a convertible note made in January 2016. A more detailed discussion of this arrangement is set forth under the heading “CPP Agreement” located under Item 7.
As of December 31, 2016 and 2015, less than 1.0% and 3.6%, respectively, of our cash, cash equivalents and restricted cash is issued or insured by the federal government or government agencies. We have not experienced any losses on these accounts related to amounts in excess of insured limits.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The Consolidated Financial Statements and related financial statement schedules required by this item are included beginning on page F-1 of this report.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, performed an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2016. In designing and evaluating such controls, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the benefits of possible controls and procedures relative to their costs. Based upon the evaluation we carried out, our Chief Executive Officer and Chief Financial Officer have concluded that, as of December 31, 2016, our disclosure controls and procedures were effective to provide reasonable assurance that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified under the applicable rules and forms of the SEC, and that such information is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosures.
| 59 |
Changes in Internal Controls Over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2016 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (defined in Rules 13a-15(f) or 15d-15(f) under the Exchange Act) for our company. Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2016. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission, or COSO, in Internal Control-Integrated Framework (2013). Based on this evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2016.
The effectiveness of our internal control over financial reporting as of December 31, 2016 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report which appears herein.
| ITEM 9B. | OTHER INFORMATION |
On March 2, 2017, we received a Paragraph IV certification notice letter (Notice Letter) regarding an Abbreviated New Drug Application (ANDA) submitted to the FDA by Amneal Pharmaceuticals (Amneal) requesting approval to market, sell and use a generic version of the 8 mcg and 24 mcg AMITIZA® (lubiprostone) soft gelatin capsule products. We are currently reviewing the Notice Letter. By statute, if we initiate a patent infringement lawsuit against Amneal within 45 days of the notice date, the FDA would automatically stay approval of the Amneal ANDA until the earlier of 30 months from the notice date or entry of a district court decision finding the patents invalid or not infringed. AMITIZA is currently protected by 15 issued patents that are listed in the FDA’s Orange Book, with the latest expiring in 2027.
| 60 |
PART III
We will file a definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, or the 2017 Proxy Statement, with the SEC, pursuant to Regulation 14A, not later than 120 days after the end of our fiscal year. Accordingly, certain information required by Part III has been omitted under General Instruction G(3) to Form 10-K. Only those sections of the 2017 Proxy Statement that specifically address the items set forth herein are incorporated by reference.
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this item is hereby incorporated by reference to the sections of the 2017 Proxy Statement under the captions “Election of Directors,” “Executive Officers,” “Corporate Governance Principles and Board Matters, Board Structure and Committee Composition” and “Section 16(a) Beneficial Ownership Reporting Compliance.”
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this item is hereby incorporated by reference to the sections of the 2017 Proxy Statement under the captions “Executive Compensation” and “Board of Directors Compensation.”
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this item is hereby incorporated by reference to the sections of the 2017 Proxy Statement under the captions “Stock Ownership Information” and “Equity Compensation Plan Information.”
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE |
The information required by this item is hereby incorporated by reference to the sections of the 2017 Proxy Statement under the captions “Related Party Transactions” and “Corporate Governance Principles and Board Matters, Board Structure and Committee Composition – Board Determination of Independence.”
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by this item is hereby incorporated by reference to the sections of the 2017 Proxy Statement under the captions “Independent Registered Public Accounting Firm’s Fees” and “Pre-Approval Policy and Procedures.”
PART IV
| ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
| (a) | The following financial statements, financial statement schedule and exhibits are filed as part of this report or incorporated herein by reference: |
| (1) | Consolidated Financial Statements. See Index to Consolidated Financial Statements on page F-1. |
| (2) | Financial Statement Schedule: Schedule II – Valuation and Qualifying Accounts on page F-41. All other schedules are omitted because they are not applicable, not required or the information required is shown in the financial statements or notes thereto. |
| (3) | Exhibits. See subsection (b) below. |
| (b) | Exhibits. The following exhibits are filed or incorporated by reference as part of this report. |
| 61 |
| Exhibit | |||||
| Number | Description | Form | File No. | Exhibit | Filing Date |
| 3.1 | Certificate of Incorporation | 8-K | 001-33609 | 3.1 | 12/29/2008 |
| 3.2 | Certificate of Amendment | 8-K | 001-33609 | 3.2 | 12/29/2008 |
| 3.3 | Amended and Restated Bylaws | 8-K | 001-33609 | 3.3 | 8/2/2013 |
| 4.1 | Specimen Stock Certificate evidencing the shares of class A common stock | S-1/A | 333-135133 | 4.1 | 2/1/2007 |
| 4.2 | Indenture, dated as of December 27, 2016, between the Company and U.S. Bank National Association, as Trustee | 8-K | 001-33609 | 4.1 | 12/27/2016 |
| 4.3 | Form of Note representing the Company’s 3.25% Convertible Senior Notes due 2021 | 8-K | 001-33609 | 4.2 | 12/27/2016 |
| 10.1^ | Amended and Restated 2001 Stock Incentive Plan | S-1 | 333-135133 | 10.1 | 6/19/2006 |
| 10.2^ | Amended and Restated 2006 Stock Incentive Plan | 10-Q | 001-33609 | 10.2 | 11/14/2007 |
| 10.3^ | Form of Indemnification Agreement, dated July 2016, between the Company and an indemnitee | 10-Q | 001-33609 | 10.1 | 11/9/2016 |
| 10.4^ | Form Sucampo Pharmaceuticals, Inc. Duration-Based Stock Option Incentive Award Stock Option Agreement Terms and Conditions | 10-K | 001-33609 | 10.36 | 3/11/2016 |
| 10.5^ | Non-employee Director Compensation Summary | 10-K | 001-33609 | 10.35 | 3/11/2016 |
| 10.6^ | 2016 Equity Incentive Plan | 10-Q | 001-33609 | 10.1 | 8/3/2016 |
| 10.7^ | Employment Agreement, dated as of October 27, 2014, between the Company and Matthias Alder | 10-K | 001-33609 | 10.81 | 3/9/2015 |
| 10.8^ | Employment Agreement, dated as of January 30, 2015, between the Company and Andrew Smith | 10-Q | 001-33609 | 10.5 | 5/5/2016 |
| 10.9^ | Employment Agreement, dated as of August 2, 2016, between the Company and Dr. Peter Kiener | 10-Q | 001-33609 | 10.3 | 11/9/2016 |
| 10.10^ | Employment Agreement, dated as of August 2, 2016, between the Company and Andrew Smith | 10-Q | 001-33609 | 10.4 | 11/9/2016 |
| 10.11^ | Employment Agreement, dated as of August 3, 2016, between the Company and Peter Greenleaf | 10-Q | 001-33609 | 10.2 | 11/9/2016 |
| 10.12^ | Employment Agreement, dated as of August 3, 2016, between the Company and Matthew Donley | 10-Q | 001-33609 | 10.5 | 11/9/2016 |
| 10.13^ | Employment Agreement, dated as of August 23, 2016, between Sucampo AG and Dr. Peter Lichtlen | 10-Q | 001-33609 | 10.6 | 11/9/2016 |
| 10.14^ | Separation Agreement, dated as of February 29, 2016, between the Company and Stanley Miele | 10-Q | 001-33609 | 10.4 | 5/5/2016 |
| 10.15* | Collaboration and License Agreement, dated October 29, 2004, between the Company and Takeda Pharmaceutical Company Limited | S-1 | 333-135133 | 10.21 | 6/19/2006 |
| 10.16* | Agreement, dated October 29, 2004, among the Company, Takeda Pharmaceutical Company Limited and Sucampo AG | S-1 | 333-135133 | 10.22 | 6/19/2006 |
| 10.17* | Supply Agreement, dated October 29, 2004, among the Company, Takeda Pharmaceutical Company Limited and R-Tech Ueno, Ltd. | S-1 | 333-135133 | 10.23 | 6/19/2006 |
| 10.18* | Supply and Purchase Agreement, dated January 25, 2006, among the Company, Takeda Pharmaceutical Company Limited and R-Tech Ueno, Ltd. | S-1 | 333-135133 | 10.24 | 6/19/2006 |
| 10.19* | Supplemental Agreement, dated February 1, 2006, between the Company and Takeda Pharmaceutical Company Limited | S-1 | 333-135133 | 10.25 | 6/19/2006 |
| 10.20 | Letter Agreement, dated January 29, 2007, between the Company and Takeda Pharmaceutical Company Limited | S-1/A | 333-135133 | 10.36 | 5/14/2007 |
| 10.21* | License, Commercialization and Supply Agreement, dated February 19, 2009, between Sucampo Pharma Ltd. and Abbott Japan Co. Ltd. | 10-K | 001-33609 | 10.43 | 3/16/2009 |
| 62 |
| 10.22* | Settlement and License Agreement, dated September 30, 2014, among the Company, Sucampo AG, R-Tech Ueno, Ltd., Takeda Pharmaceutical Company Limited, Takeda Pharmaceuticals USA, Inc., Takeda Pharmaceuticals America, Inc., Anchen Pharmaceuticals, Inc., Par Pharmaceutical, Inc. and Par Pharmaceutical Companies, Inc. | 10-Q | 001-33609 | 10.2 | 11/7/2014 |
| 10.23* | Manufacturing and Supply Agreement, dated September 30, 2014, between Sucampo AG and Par Pharmaceutical, Inc. | 10-Q | 001-33609 | 10.3 | 11/7/2014 |
| 10.24* | Amendment No. 1, dated September 30, 2014, to Collaboration and License Agreement dated October 29, 2004 and Supplemental Agreement, dated February 1, 2006, between Sucampo Pharma Americas, LLC and Takeda Pharmaceutical Company Limited | 10-Q | 001-33609 | 10.4 | 11/7/2014 |
| 10.25 | Amendment No. 1, dated September 30, 2014, to the Agreement dated October 29, 2004, between Sucampo Pharma Americas, LLC, Takeda Pharmaceutical Company Limited and Sucampo AG | 10-Q | 001-33609 | 10.5 | 11/7/2014 |
| 10.26* | Amendment No. 1, dated September 30, 2014, to Supply Agreement dated October 29, 2004, Supply and Purchase Agreement dated January 25, 2006 and the Addendum to the Supply and Purchase Agreement dated November 6, 2013, among Sucampo Pharma Americas, LLC, Takeda Pharmaceutical Company Limited and R-Tech Ueno, Ltd. | 10-Q | 001-33609 | 10.6 | 11/7/2014 |
| 10.27* | License, Development, Commercialization and Supply Agreement For Lubiprostone, dated October 17, 2014, between Sucampo AG and Takeda Pharmaceuticals International GmbH Limited | 10-K | 001-33609 | 10.79 | 3/9/2015 |
| 10.28* | Stipulation and License Agreement, dated February 5, 2015, among the Company, Sucampo AG, R-Tech Ueno, Ltd. and Par Pharmaceutical, Inc. | 10-K | 001-33609 | 10.88 | 3/9/2015 |
| 10.29* | Manufacturing and Supply Agreement, dated as of February 5, 2015, between Sucampo AG and Par Pharmaceutical, Inc. | 10-K | 001-33609 | 10.89 | 3/9/2015 |
| 10.30* | License, Development, Commercialization And Supply Agreement For Lubiprostone for People's Republic of China, dated May 5, 2015, between Harbin Gloria Pharmaceuticals Co., Ltd. and Sucampo AG | 10-Q | 001-33609 | 10.2 | 8/5/2015 |
| 10.31* | First Amendment to office Lease Agreement, dated September 14, 2015, between Four Irvington Centre Associations, LLC and Sucampo Pharmaceuticals, Inc. | 10-Q | 001-33609 | 10.1 | 11/4/2015 |
| 10.32* | Amendment No. 1, dated November 18, 2015 to the License, Development, Commercialization and Supply Agreement for Lubiprostone dated October 17, 2014, between Sucampo AG and Takeda Pharmaceuticals International AG | 10-K | 001-33609 | 10.37 | 3/11/2016 |
| 10.33* | Manufacturing Agreement, dated March 22, 2004, between R-Tech Ueno, Ltd. and Nissan Chemical Industries, Ltd. | 10-K | 001-33609 | 10.41 | 3/11/2016 |
| 10.34 | Convertible Promissory Note, dated as of January 9, 2016, between Sucampo AG and Cancer Prevention Pharmaceuticals, Inc. | 10-Q | 001-33609 | 10.1 | 5/5/2016 |
| 10.35* | Option and Collaboration Agreement, dated as of January 9, 2016, between Sucampo AG and Cancer Prevention Pharmaceuticals, Inc. | 10-Q | 001-33609 | 10.2 | 5/5/2016 |
| 10.36 | Securities Purchase Agreement, dated as of January 9, 2016, between Sucampo AG and Cancer Prevention Pharmaceuticals, Inc. | 10-Q | 001-33609 | 10.3 | 5/5/2016 |
| 63 |
| 10.37 | Subordinated Unsecured Promissory Note, dated December 23, 2010, between Ambrent Investments S.à.r.l., as borrower, and Ryuji Ueno Revocable Trust Under Trust Agreement dated December 20, 2002, as lender | 8-K | 001-33609 | 10.1 | 12/29/2010 |
| 10.38 | Subordinated Unsecured Promissory Note, dated December 23, 2010, between Ambrent Investments S.à.r.l., as borrower, and Sachiko Kuno Revocable Trust Under Trust Agreement dated December 20, 2002, as lender | 8-K | 001-33609 | 10.2 | 12/29/2010 |
| 10.39* | Loan Guarantee and Development Agreement, dated September 8, 2011, between Numab AG and Sucampo AG | 10-K | 001-33609 | 10.58 | 3/15/2012 |
| 10.40* | Credit Agreement, dated October 16, 2015, among the Company as borrower, the financial institutions listed therein as Lenders and Jefferies Finance LLC, as administrative agent and collateral agent for the Lenders | 10-K | 001-33609 | 10.38 | 3/11/2016 |
| 10.41* | Office Lease Agreement, dated May 5, 2015, between Four Irvington Centre Associations, LLC and Sucampo Pharmaceuticals, Inc. | 10-Q | 001-33609 | 10.1 | 8/5/2015 |
| 10.42 | Lease Agreement, dated December 18, 2006, between the Company and EW Bethesda Office Investors, LLC | 10-K | 001-33609 | 10.29 | 3/27/2008 |
| 10.43* | Lease Agreement, dated April 1, 2001, between Ueno Fine Chemicals and R-Tech Ueno Ltd. | 10-K | 001-33609 | 10.39 | 3/11/2016 |
| 10.44^ | Amended and Restated 2006 Employee Stock Purchase Plan | Included herewith | |||
| 10.45^ | Form Restricted Stock Unit Ward Grant Notice | Included herewith | |||
| 12.1 | Ratio of Earnings to Fixed Charges | Included herewith | |||
| 21 | Subsidiaries of the Company | Included herewith | |||
| 23.1 | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm | Included herewith | |||
| 23.2 | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm | Included herewith | |||
| 31.1 | Certification of the Principal Executive Officer, as required by Section 302 of the Sarbanes-Oxley Act of 2002 | Included herewith | |||
| 31.2 | Certification of the Principal Financial Officer, as required by Section 302 of the Sarbanes-Oxley Act of 2002 | Included herewith | |||
| 32.1 | Certification of the Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | Included herewith | |||
| 32.2 | Certification of the Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | Included herewith | |||
| 101.[SCH] | XBRL Taxonomy Extension Schema Document | Included herewith | |||
| 101.[CAL] | XBRL Taxonomy Extension Calculation Linkbase Document | Included herewith | |||
| 101.[LAB] | XBRL Taxonomy Extension Label Linkbase Document | Included herewith | |||
| 101.[PRE] | XBRL Taxonomy Extension Presentation Linkbase Document | Included herewith | |||
| ^ Compensatory plan, contract or arrangement. | |||||
| * Confidential treatment has been granted for portions of this exhibit. | |||||
| & English summary of a foreign language document. | |||||
| # Pursuant to Item 601(b)(2) of Regulation S-K promulgated by the SEC, certain schedules to this agreement have been omitted. The registrant hereby agrees to furnish supplementally to the SEC, upon its request, any or all of such omitted schedules. | |||||
| 64 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Sucampo Pharmaceuticals, Inc. | |||
| March 8, 2017 | By: | /s/ PETER GREENLEAF | |
| Peter Greenleaf | |||
| Chief Executive Officer | |||
| (Principal Executive Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | |||
| /s/ PETER GREENLEAF | Chief Executive Officer, Chairman of the Board | March 8, 2017 | |||
| Peter Greenleaf | (Principal Executive Officer) | ||||
| /s/ ANDREW P. SMITH | Chief Financial Officer | March 8, 2017 | |||
| Andrew P. Smith | (Principal Financial and Accounting Officer) | ||||
| /s/ PAUL EDICK | March 8, 2017 | ||||
| Paul Edick | Director | ||||
| /s/ DANIEL P. GETMAN | March 8, 2017 | ||||
| Daniel P. Getman | Director | ||||
| /s/ JOHN JOHNSON | March 8, 2017 | ||||
| John Johnson | Lead Independent Director | ||||
| /s/ MAUREEN E. O'CONNELL | March 8, 2017 | ||||
| Maureen E. O'Connell | Director | ||||
| /s/ ROBERT SPIEGEL | March 8, 2017 | ||||
| Robert Spiegel | Director | ||||
| /s/ TIMOTHY WALBERT | March 8, 2017 | ||||
| Timothy Walbert | Director |
| 65 |
SUCAMPO PHARMACEUTICALS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| F-1 |
Independent Registered Public Accounting Firm,
on the Audited Consolidated Financial Statements
The Board of Directors and Stockholders of Sucampo Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheets of Sucampo Pharmaceuticals, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations and comprehensive income, changes in stockholders’ equity and cash flows for the years then ended. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Sucampo Pharmaceuticals, Inc. at December 31, 2016 and 2015, and the consolidated results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Sucampo Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated March 8, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
McLean, Virginia
March 8, 2017
| F-2 |
Report of Ernst & Young LLP,
Report of Independent Registered Public Accounting Firm,
Regarding Internal Control Over Financial Reporting
The Board of Directors and Stockholders of Sucampo Pharmaceuticals, Inc.
We have audited Sucampo Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), (the COSO criteria). Sucampo Pharmaceuticals, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Managements Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Sucampo Pharmaceuticals, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet of Sucampo Pharmaceuticals, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive income, changes in stockholders’ equity and cash flows for the years then ended of Sucampo Pharmaceuticals, Inc. and our report dated March 8, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
McLean, Virginia
March 8, 2017
| F-3 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Sucampo Pharmaceuticals, Inc.:
In our opinion, the consolidated statements of operations and comprehensive income, of changes in stockholders’ equity and of cash flows for the year ended December 31, 2014 present fairly, in all material respects, the results of operations and cash flows of Sucampo Pharmaceuticals, Inc. and its subsidiaries for the year ended December 31, 2014, in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule for the year ended December 31, 2014 presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. These financial statements and financial statement schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audit. We conducted our audit of these financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
Baltimore, Maryland
March 9, 2015, except for the change in composition of reportable segments discussed in Note 4 to the consolidated financial statements, as to which the date is May 6, 2015
| F-4 |
SUCAMPO PHARMACEUTICALS, INC.
(In thousands, except share and per share data)
| December 31, 2016 | December 31, 2015 | |||||||
| ASSETS: | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 198,308 | $ | 108,284 | ||||
| Product royalties receivable | 26,261 | 22,792 | ||||||
| Accounts receivable, net | 42,998 | 22,759 | ||||||
| Deferred charge, current | 17 | 295 | ||||||
| Restricted cash, current | 213 | 55,218 | ||||||
| Inventories, net | 23,468 | 33,121 | ||||||
| Prepaid expenses and other current assets | 15,967 | 8,891 | ||||||
| Total current assets | 307,232 | 251,360 | ||||||
| Investments, non-current | 5,495 | - | ||||||
| Property and equipment, net | 6,216 | 6,393 | ||||||
| Intangible assets | 128,134 | 130,315 | ||||||
| Goodwill | 73,022 | 60,937 | ||||||
| In-process research & development | - | 6,171 | ||||||
| Deferred charge, non-current | 62 | 1,400 | ||||||
| Other assets | 690 | 605 | ||||||
| Total assets | $ | 520,851 | $ | 457,181 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY: | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 9,190 | $ | 11,213 | ||||
| Accrued expenses | 12,389 | 10,886 | ||||||
| Deferred revenue, current | 1,315 | 676 | ||||||
| Collaboration obligation | - | 5,623 | ||||||
| Income tax payable | 7,153 | 6,507 | ||||||
| Notes payable, current | - | 39,083 | ||||||
| Other current liabilities | 2,304 | 14,139 | ||||||
| Total current liabilities | 32,351 | 88,127 | ||||||
| Notes payable, non-current | 290,516 | 213,277 | ||||||
| Deferred revenue, non-current | 805 | 1,088 | ||||||
| Deferred tax liability, net | 21,289 | 52,497 | ||||||
| Other liabilities | 8,791 | 15,743 | ||||||
| Total liabilities | 353,752 | 370,732 | ||||||
| Commitments and contingencies (note 16) | ||||||||
| Stockholders' equity: | ||||||||
| Preferred stock, $0.01 par value; 5,000,000 shares authorized at December 31, 2016 and 2015; no shares issued and outstanding at December 31, 2016 and 2015, respectively | - | - | ||||||
| Class A common stock, $0.01 par value; 270,000,000 shares authorized at December 31, 2016 and 2015; 46,415,749 and 45,509,150 shares issued and outstanding at December 31, 2016 and 2015, respectively | 464 | 455 | ||||||
| Class B common stock, $0.01 par value; 75,000,000 shares authorized at December 31, 2016 and 2015; no shares issued and outstanding at December 31, 2016 and 2015 | - | - | ||||||
| Additional paid-in capital | 120,251 | 99,212 | ||||||
| Accumulated other comprehensive income | 54,527 | 13,412 | ||||||
| Treasury stock, at cost; 3,009,942 shares at December 31, 2016 and 2015 | (46,269 | ) | (46,269 | ) | ||||
| Retained earnings | 38,126 | 19,639 | ||||||
| Total stockholders' equity | 167,099 | 86,449 | ||||||
| Total liabilities and stockholders' equity | $ | 520,851 | $ | 457,181 | ||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| F-5 |
SUCAMPO PHARMACEUTICALS, INC.
Consolidated Statements of Operations and Comprehensive Income
(In thousands, except per share data)
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Revenues: | ||||||||||||
| Product royalty revenue | $ | 82,480 | $ | 74,138 | $ | 62,775 | ||||||
| Product sales revenue | 128,796 | 66,276 | 33,252 | |||||||||
| Research and development revenue | 12,839 | 10,199 | 7,246 | |||||||||
| Contract and collaboration revenue | 5,941 | 2,567 | 8,817 | |||||||||
| Co-promotion revenue | - | - | 3,360 | |||||||||
| Total revenues | 230,056 | 153,180 | 115,450 | |||||||||
| Costs and expenses: | ||||||||||||
| Costs of goods sold | 76,003 | 36,731 | 16,269 | |||||||||
| Impairment of in-process research & development | 7,286 | - | - | |||||||||
| Intangible assets impairment | - | - | 5,631 | |||||||||
| Research and development | 46,615 | 33,631 | 20,566 | |||||||||
| General and administrative | 43,798 | 35,517 | 31,230 | |||||||||
| Selling and marketing | 2,478 | 2,842 | 14,523 | |||||||||
| Total costs and expenses | 176,180 | 108,721 | 88,219 | |||||||||
| Income from operations | 53,876 | 44,459 | 27,231 | |||||||||
| Non-operating income (expense): | ||||||||||||
| Interest income | 72 | 181 | 172 | |||||||||
| Interest expense | (23,761 | ) | (6,854 | ) | (1,520 | ) | ||||||
| Loss on debt extinguishment | (14,047 | ) | - | - | ||||||||
| Other income (expense), net | (1,765 | ) | 5,889 | 1,250 | ||||||||
| Total non-operating expense, net | (39,501 | ) | (784 | ) | (98 | ) | ||||||
| Income before income taxes | 14,375 | 43,675 | 27,133 | |||||||||
| Income tax benefit (provision) | 4,112 | (10,304 | ) | (14,005 | ) | |||||||
| Net income | $ | 18,487 | $ | 33,371 | $ | 13,128 | ||||||
| Net income per share: | ||||||||||||
| Basic | $ | 0.43 | $ | 0.76 | $ | 0.30 | ||||||
| Diluted | $ | 0.42 | $ | 0.73 | $ | 0.29 | ||||||
| Weighted average common shares outstanding: | ||||||||||||
| Basic | 42,791 | 44,150 | 43,691 | |||||||||
| Diluted | 43,749 | 45,680 | 44,506 | |||||||||
| Comprehensive income: | ||||||||||||
| Net income | $ | 18,487 | $ | 33,371 | $ | 13,128 | ||||||
| Other comprehensive income (expense): | ||||||||||||
| Unrealized gain (loss) on pension benefit obligation | 239 | 105 | (978 | ) | ||||||||
| Unrealized gain (loss) on investments, net of tax effect | - | 7 | (7 | ) | ||||||||
| Foreign currency translation gain (loss) | 40,876 | (965 | ) | (351 | ) | |||||||
| Comprehensive income | $ | 59,602 | $ | 32,518 | $ | 11,792 | ||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| F-6 |
SUCAMPO PHARMACEUTICALS, INC.
Consolidated Statements of Changes in Stockholders’ Equity
(In thousands, except share data)
| Class A Common Stock | Additional Paid-In | Accumulated Other Comprehensive | Treasury Stock | Retained Earnings (Accumulated | Total Stockholders' | |||||||||||||||||||||||||||
| Shares | Amount | Capital | Income (Loss) | Shares | Amount | Deficit) | Equity | |||||||||||||||||||||||||
| Balance at December 31, 2013 | 43,315,749 | 432 | 72,109 | 15,601 | 524,792 | (2,313 | ) | (26,860 | ) | 58,969 | ||||||||||||||||||||||
| Stock-based compensation expense | - | - | 2,287 | - | - | - | - | 2,287 | ||||||||||||||||||||||||
| Stock issued upon exercise of stock options | 742,865 | 9 | 3,780 | - | - | - | - | 3,789 | ||||||||||||||||||||||||
| Stock issued under employee stock purchase plan | 5,853 | - | 36 | - | - | - | - | 36 | ||||||||||||||||||||||||
| Stock issued under "at-the-market" offering | 538,521 | 5 | 5,321 | - | - | - | - | 5,326 | ||||||||||||||||||||||||
| Windfall tax benefit from stock-based compensation | - | - | 113 | - | - | - | - | 113 | ||||||||||||||||||||||||
| Unrealized loss on pension benefit obligation | - | - | - | (978 | ) | - | - | - | (978 | ) | ||||||||||||||||||||||
| Unrealized loss on investments, net of tax effect | - | - | - | (7 | ) | - | - | - | (7 | ) | ||||||||||||||||||||||
| Foreign currency translation | - | - | - | (351 | ) | - | - | - | (351 | ) | ||||||||||||||||||||||
| Net income | - | - | - | - | - | - | 13,128 | 13,128 | ||||||||||||||||||||||||
| Balance at December 31, 2014 | 44,602,988 | 446 | 83,646 | 14,265 | 524,792 | (2,313 | ) | (13,732 | ) | 82,312 | ||||||||||||||||||||||
| Stock-based compensation expense | - | - | 7,349 | - | - | - | - | 7,349 | ||||||||||||||||||||||||
| Stock issued upon exercise of stock options | 897,077 | 9 | 5,614 | - | - | - | - | 5,623 | ||||||||||||||||||||||||
| Stock issued under employee stock purchase plan | 9,085 | - | 128 | - | - | - | - | 128 | ||||||||||||||||||||||||
| Windfall tax benefit from stock-based compensation | - | - | 2,475 | - | - | - | - | 2,475 | ||||||||||||||||||||||||
| Unrealized gain on pension benefit obligation | - | - | - | 105 | - | - | - | 105 | ||||||||||||||||||||||||
| Unrealized gain on investments, net of tax effect | - | - | - | 7 | - | - | - | 7 | ||||||||||||||||||||||||
| Foreign currency translation | - | - | - | (965 | ) | - | - | - | (965 | ) | ||||||||||||||||||||||
| Treasury stock, at cost | - | - | - | - | 2,485,150 | (43,956 | ) | - | (43,956 | ) | ||||||||||||||||||||||
| Net income | - | - | - | - | - | - | 33,371 | 33,371 | ||||||||||||||||||||||||
| Balance at December 31, 2015 | 45,509,150 | 455 | 99,212 | 13,412 | 3,009,942 | (46,269 | ) | 19,639 | 86,449 | |||||||||||||||||||||||
| Stock-based compensation expense | - | - | 7,283 | - | - | - | - | 7,283 | ||||||||||||||||||||||||
| Stock issued upon exercise of stock options | 884,204 | 9 | 5,992 | - | - | - | - | 6,001 | ||||||||||||||||||||||||
| Stock issued under employee stock purchase plan | 22,395 | - | 213 | - | - | - | - | 213 | ||||||||||||||||||||||||
| Windfall tax benefit from stock-based compensation | - | - | 1,036 | - | - | - | - | 1,036 | ||||||||||||||||||||||||
| Unrealized gain on pension benefit obligation | - | - | - | 239 | - | - | - | 239 | ||||||||||||||||||||||||
| Foreign currency translation | - | - | - | 40,876 | - | - | - | 40,876 | ||||||||||||||||||||||||
| Settlement of transaction attributed to entities under common control | - | - | 6,515 | - | - | - | - | 6,515 | ||||||||||||||||||||||||
| Net income | - | - | - | - | - | - | 18,487 | 18,487 | ||||||||||||||||||||||||
| Balance at December 31, 2016 | 46,415,749 | 464 | 120,251 | 54,527 | 3,009,942 | (46,269 | ) | 38,126 | 167,099 | |||||||||||||||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| F-7 |
SUCAMPO PHARMACEUTICALS, INC.
Consolidated Statements of Cash Flows
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net income | $ | 18,487 | $ | 33,371 | $ | 13,128 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
| Depreciation and amortization | 45,683 | 10,580 | 1,090 | |||||||||
| Intangible assets impairment | 7,286 | - | 5,631 | |||||||||
| Deferred tax (benefit) provision | (37,038 | ) | (9,779 | ) | 770 | |||||||
| Deferred charge | 1,616 | 295 | 3,223 | |||||||||
| Stock-based compensation | 7,283 | 7,349 | 2,287 | |||||||||
| Amortization of premiums on investments | - | 121 | 82 | |||||||||
| Foreign currency remeasurement gain | (1,238 | ) | (3,687 | ) | (1,146 | ) | ||||||
| Shortfall from stock-based compensation | (25 | ) | (70 | ) | (227 | ) | ||||||
| Windfall benefit from stock-based compensation | (1,061 | ) | (2,547 | ) | - | |||||||
| Transfer and assignment of licensing rights | - | (2,000 | ) | - | ||||||||
| Loss on disposal of property and equipment | 994 | - | - | |||||||||
| Forgiveness of AMED deferred grant | (9,258 | ) | - | - | ||||||||
| Loss on debt extinguishment | 14,047 | - | - | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Product royalties receivable | (3,469 | ) | (4,216 | ) | (3,747 | ) | ||||||
| Accounts receivable | (18,788 | ) | (9,149 | ) | 67 | |||||||
| Inventory | (1,233 | ) | (1,436 | ) | 206 | |||||||
| Prepaid and income taxes receivable and payable, net | (8,747 | ) | 1,110 | 592 | ||||||||
| Accounts payable | (2,772 | ) | (1,723 | ) | (3,437 | ) | ||||||
| Accrued expenses | 1,279 | 395 | 2,868 | |||||||||
| Accrued interest payable | 59 | (25 | ) | (32 | ) | |||||||
| Deferred revenue | 356 | (5,351 | ) | (191 | ) | |||||||
| Collaboration obligation | (5,623 | ) | (377 | ) | 6,000 | |||||||
| Other assets and liabilities, net | 475 | 5,724 | 3,714 | |||||||||
| Net cash provided by operating activities | 8,313 | 18,585 | 30,878 | |||||||||
| Cash flows from investing activities: | ||||||||||||
| Purchases of investments | (5,250 | ) | (39,775 | ) | (29,153 | ) | ||||||
| Proceeds from sales of investments | - | 45,062 | 1,700 | |||||||||
| Maturities of investments | - | 30,554 | 14,650 | |||||||||
| Tenant improvement allowance | - | (1,880 | ) | - | ||||||||
| Purchases of property and equipment | (1,226 | ) | (3,557 | ) | (66 | ) | ||||||
| Transfer and assignment of licensing rights | - | 2,000 | - | |||||||||
| Changes in restricted cash | 12,302 | (2,368 | ) | 25,828 | ||||||||
| Acquisition, net of acquired cash | - | (161,187 | ) | - | ||||||||
| Payment of squeeze-out liability for non-tendering R-Tech shareholders | (7,668 | ) | - | - | ||||||||
| Net cash (used in) provided by investing activities | (1,842 | ) | (131,151 | ) | 12,959 | |||||||
| Cash flows from financing activities: | ||||||||||||
| Proceeds from convertible notes and notes payable, net of debt issuance costs | 290,495 | 235,911 | - | |||||||||
| Repayment of notes payable and debt extinguishment costs | (269,913 | ) | (8,236 | ) | (24,904 | ) | ||||||
| Changes in restricted cash | 42,676 | (42,676 | ) | - | ||||||||
| Proceeds from exercise of stock options | 6,001 | 5,623 | 3,789 | |||||||||
| Proceeds from employee stock purchase plan | 213 | 128 | 36 | |||||||||
| Proceeds from "at-the market" stock issuance | - | - | 5,326 | |||||||||
| Purchase of treasury stock | - | (43,956 | ) | - | ||||||||
| Windfall benefit from stock-based compensation | 1,061 | 2,547 | 340 | |||||||||
| Net cash provided by (used in) financing activities | 70,533 | 149,341 | (15,413 | ) | ||||||||
| Effect of exchange rates on cash and cash equivalents | 13,020 | (113 | ) | (904 | ) | |||||||
| Net increase in cash and cash equivalents | 90,024 | 36,662 | 27,520 | |||||||||
| Cash and cash equivalents at beginning of period | 108,284 | 71,622 | 44,102 | |||||||||
| Cash and cash equivalents at end of period | $ | 198,308 | $ | 108,284 | $ | 71,622 | ||||||
| Supplemental cash flow disclosures: | ||||||||||||
| Cash paid for interest | $ | 20,033 | $ | 5,983 | $ | 129 | ||||||
| Tax refunds received | $ | 442 | $ | 423 | $ | 76 | ||||||
| Tax payments made | $ | 13,553 | $ | 17,621 | $ | 9,166 | ||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| F-8 |
1. Business Organization and Basis of Presentation
Description of the Business
Sucampo Pharmaceuticals, Inc., (the “Company”) is a global biopharmaceutical company focused on developing, identifying, acquiring and bringing to market innovative medicines that meet unmet medical needs, primarily in gastroenterology, ophthalmology, and oncology-related disorders.
The Company currently generates revenue from its commercial-stage products, AMITIZA and RESCULA, mainly from product royalties, product sales, upfront and milestone payments, and reimbursements for development activities. The Company expects to continue to incur significant expenses for the next several years as the Company continues its research and development activities, seeks additional regulatory approvals and additional indications for approved products and other compounds and seeks strategic opportunities for in-licensing new products.
AMITIZA is being marketed for three gastrointestinal indications under the collaboration and license agreement (as amended in October 2014, the “North America Takeda Agreement”) with Takeda Pharmaceutical Company Limited (“Takeda”). These indications are chronic idiopathic constipation (“CIC”) in adults, irritable bowel syndrome with constipation (“IBS-C”) in adult women and opioid-induced constipation (“OIC”) in adults suffering from chronic non-cancer related pain. Under the North America Takeda Agreement, the Company is primarily responsible for clinical development activities, while Takeda is responsible for commercialization of AMITIZA in the U.S. and Canada . The Company and Takeda initiated commercial sales of AMITIZA in the U.S. for the treatment of CIC in April 2006, for the treatment of IBS-C in May 2008 and for the treatment of OIC in May 2013. Takeda is required to provide a minimum annual commercial investment during the current term of the North America Takeda Agreement and may reduce the minimum annual commercial investment when a generic equivalent enters the market. In October 2015, Health Canada approved AMITIZA for CIC in adults. In October 2014, the Company and Takeda executed amendments (“Takeda Amendment”) to the North America Takeda Agreement which, among other things, extended the term of the North America Takeda Agreement beyond December 2020. During the extended term, Takeda and the Company will split the annual net sales revenue of the branded AMITIZA products. In addition, beginning April 2015, the North America Takeda Agreement was amended to terminate the Company’s right to perform commercialization activities with respect to AMITIZA and Takeda’s obligation to reimburse the Company for such commercialization activities.
In Japan, AMITIZA is marketed under a license, commercialization and supply agreement (the Japan Mylan Agreement) that was transferred to Mylan, Inc. (“Mylan”) from Abbott Laboratories, Inc. (“Abbott”), as of February 2015, as part of Mylan’s acquisition of a product portfolio from Abbott. The Company received approval of its new drug application (“NDA”) for AMITIZA for the treatment of chronic constipation (“CC”), excluding constipation caused by organic diseases, from the Ministry of Health, Labour and Welfare in June 2012 and pricing approval in November 2012. AMITIZA is Japan’s only prescription medicine for CC. The Company did not experience any significant changes in the commercialization of AMITIZA in Japan as a result of the transfer of the Japan Mylan Agreement from Abbott to Mylan.
In the People’s Republic of China, the Company entered into an exclusive license, development, commercialization and supply agreement (the “China Gloria Agreement”) with Harbin Gloria Pharmaceuticals Co., Ltd. (“Gloria”), for AMITIZA in May 2015. Under the China Gloria Agreement, Gloria is responsible for all development activities and costs, as well as commercialization and regulatory activities, for AMITIZA in the People’s Republic of China. The Company will be the exclusive supplier of AMITIZA to Gloria at an agreed upon supply price. Upon entering into the China Gloria Agreement, the Company received an upfront payment of $1.0 million. In June 2015, the China Food and Drug Administration accepted an Investigational New Drug (“IND”) application for a pivotal trial of AMITIZA in patients with CIC; as a result the Company received an additional payment of $500,000 from Gloria. In addition to the $1.5 million in payments received and recognized as revenue through June 2015, the Company is eligible to receive an additional payment in the amount of $1.5 million upon the occurrence of a specified regulatory or commercial milestone event.
In October 2014, the Company entered into an exclusive license, development, commercialization and supply agreement (the “Global Takeda Agreement”) for lubiprostone with Takeda, through which Takeda has the exclusive rights to further develop and commercialize AMITIZA in all global markets, except the U.S., Canada, Japan and the People’s Republic of China. Takeda became the marketing authorization holder in Switzerland in April 2015, in the United Kingdom, Austria, Belgium, Germany, Netherlands, Ireland, Italy, Luxembourg and Spain during 2016.
Before the execution of the Global Takeda Agreement, the Company retained full rights to develop and commercialize AMITIZA for the rest of the world’s markets outside of the U.S., Canada and Japan. In the U.K., the Company received approval in September 2012 from the Medicines and Healthcare Products Regulatory Agency (“MHRA”) for the use of AMITIZA to treat CIC. The Company made AMITIZA available in the U.K. in the fourth quarter of 2013. In Switzerland, AMITIZA was approved to treat CIC in 2009. In 2012, the Company reached an agreement with the Bundesamt fur Gesundheit, (“BAG”), the Federal Office of Public Health in Switzerland, on a reimbursement price for AMITIZA in Switzerland, and began active marketing in the first quarter of 2013. Since February 2012, AMITIZA has also been available through a Named Patient Program throughout the European Union, Iceland and Norway. In February 2014, the Company announced that the BAG revised several reimbursement limitations with which AMITIZA was first approved for reimbursement and inclusion in the Spezialitätenliste (“SL”) to allow all Swiss physicians to prescribe AMITIZA to patients who have failed previous treatments with at least two laxatives over a nine-month period. In July 2014, AMITIZA was approved for the treatment of OIC in chronic, non-cancer adult patients by the Swissmedic, the Swiss Agency for Therapeutic Products.
| F-9 |
In October 2015, Takeda obtained approval of the clinical trial application, or CTA, for AMITIZA for the treatment of CIC and IBS-C in Russia that was submitted in June 2015. In December 2015, a CTA was filed for AMITIZA for the treatment of CIC, IBS-C and OIC in Mexico and South Korea. Takeda initiated phase 3 registration trials in Russia in March 2016 and in South Korea and Mexico in May 2016. Takeda submitted a new drug application, or NDA, for the treatment of CIC, IBS-C, and OIC in Israel in June 2015, which was approved in July 2016, and an NDA for the same indications in Kazakhstan in December 2015. Additional NDA submissions have been made by Takeda in Singapore in May 2016, and South Africa and Indonesia in June 2016.
In the U.S., the Company ceased marketing RESCULA in the fourth quarter of 2014 and no product was made available after the March 2015 expiration date. In May 2015, the Company returned all licenses for unoprostone isopropyl to R-Tech. As part of the acquisition of R-Tech in October 2015, the Company acquired all rights to RESCULA. RESCULA is being commercialized by Santen Pharmaceutical Co., Ltd in Japan, and Sinphar Pharmaceutical, Co., Ltd and Zuellig Pharma Inc. in Taiwan.
The Company’s other clinical development programs include the following:
Lubiprostone Alternate Formulation
The Company has been developing an alternate formulation of lubiprostone for both adult and pediatric patients who are unable to take or do not tolerate capsules and for naso-gastric tube fed patients. Takeda has agreed to fund 100% of the costs, up to a cap, of this alternate formulation work. The Company initiated the phase 3 program of the alternate formulation of lubiprostone in adults in the second half of 2016, and, if the program is successful, the Company expects to file an NDA in the U.S. for the alternate formulation for adults in the second half of 2017.
Lubiprostone for Pediatric Functional Constipation
The phase 3 program required to support an application for marketing authorization of lubiprostone for pediatric functional constipation comprises four clinical trials. The first two trials, one of which was recently completed, test the soft gelatin capsule formulation of lubiprostone in patients 6 to 17 years of age. The first of these trials is a pivotal 12-week, randomized, placebo-controlled trial which was initiated in December 2013 and completed enrollment in April 2016. The second trial is a follow-on, long-term safety extension trial that was initiated in March 2014. In early November, the Company announced that the phase 3 trial of AMITIZA in pediatric functional constipation in children 6 to 17 years of age failed to achieve its primary endpoint of overall spontaneous bowel movement, or SBM, response. The trial achieved statistical significance for some secondary endpoints, notably overall SBM frequency, straining, and stool consistency. In addition, in this study lubiprostone was well tolerated. The Company has entered into a process with the U.S. Food and Drug Administration, or FDA, and other constituencies, and as a result of initial discussion with the FDA will submit an sNDA in the second half of 2017. Additionally, after further consultations with the FDA to better determine the doses and endpoints that should be studied, the phase 3 program for the alternate formulation of lubiprostone described above will be followed in mid-2018 with a phase 3 program in patients 6 months to 6 years of age using the alternate formulation. Takeda has agreed to fund 70% of the costs , up to a cap, of this pediatric functional constipation program.
VAP-1 Inhibitors
In 2016, the Company discontinued its VAP-1 Inhibitor RTU-1096 development program and its VAP-1 Inhibitor RTU-009 program.
CPP- 1X/Sulindac Combination Product
In January 2016, the Company entered into an option and collaboration agreement under which CPP has granted the Company the sole option to acquire an exclusive license to commercialize CPP-1X/sulindac combination product in North America. This product is currently in a Phase 3 clinical trial being conducted by CPP for the treatment of familial adenomatous polyposis, or FAP. Under the Company’s agreement with CPP, the Company has the exclusive option to license this product for North America. There are currently no approved treatments for FAP. The ongoing Phase 3 study is a 150-patient, three-arm, double-blind, randomized trial of the combination agent and the single agent comparators. Enrollment in the study has completed and the results from a Phase 3 futility analysis are expected to be available mid-2017. The trial is expected to conclude in 2019.
| F-10 |
Basis of Presentation
The accompanying Consolidated Financial Statements of the Company have been prepared in accordance with generally accepted accounting principles in the United States of America, (“GAAP”) and the rules and regulations of the Securities and Exchange Commission, (“SEC”). The Consolidated Financial Statements include the accounts of the Company and its wholly owned subsidiaries: Sucampo AG and Sucampo Acquisitions GmbH, each based in Zug, Switzerland, through which the Company conducts certain worldwide and European operations; Sucampo Pharma, LLC, based in Tokyo, Japan, through which the Company conducts its Asian operations; Sucampo Pharma Americas LLC, based in Rockville, Maryland, through which the Company conducts operations in North and South America and Sucampo Pharma Europe, Ltd., based in Oxford, U.K.. All inter-company balances and transactions have been eliminated.
The preparation of financial statements in conformity with GAAP requires management to make estimates that affect the reported amounts of assets and liabilities at the date of the financial statements, disclosure of contingent assets and liabilities, and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of Sucampo Pharmaceuticals, Inc. and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities and disclosure of contingencies at the date of the financial statements as well as the reported amounts of revenues and expense during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company maintains cash balances with financial institutions in excess of insured limits. The Company does not anticipate any losses with such cash balances. Cash equivalents include highly liquid investments with a maturity of 90 days or less at the time of purchase.
Non-Current Investments
Non-current investments consist primarily of non-marketable equity securities of companies whose securities are not publicly traded and where fair value is not readily available. These investments are measured at fair value on a recurring basis and are monitored by the Company to evaluate whether any decline in their value has occurred that would be other-than temporary, based on the implied value of recent company financings, public market prices of comparable companies and general market conditions.
Accounts Receivable
Accounts receivable primarily represents amounts due under the North America Takeda Agreement and Japan Mylan Agreement. The Company recorded an immaterial allowance for doubtful accounts at December 31, 2016 and 2015. Accounts receivable of zero, zero and $779,000 were charged off against the allowance for doubtful accounts during the years ended December 31, 2016, 2015 and 2014.
Inventories
Inventories are valued under a weighted average costing method and are stated at the lower of cost or net realizable value. Inventories consist of raw material, work-in-process and finished goods. The Company’s inventories include the direct purchase cost of materials and supplies and manufacturing overhead costs.
Restricted Cash
Restricted cash at December 31, 2016 consisted of a certificate of deposit pledged to support an operating lease for the Company’s former office facility in Bethesda, Maryland. Restricted cash at December 31, 2015 consisted primarily of $25.0 million related to the Credit Facility that required the Company to maintain $25.0 million in a restricted cash account until at least $35 million of the Term Loans have been repaid or prepaid (see note 17). The Term Loans were paid in full in December 2016 and the restricted cash was subsequently released. Further, as part of the R-Tech acquisition, $17.7 million was held in a restricted cash account for payment of the Ueno and Kuno Trust Notes, which were settled on February 1, 2016 (see note 17), and $8.2 million was held in restricted cash related to the squeeze out of non-tendering R-Tech shareholders, which was settled in January 2016.
| F-11 |
Property and Equipment
Property and equipment are recorded at cost and consist of computer and office equipment, furniture and fixtures, leasehold improvements, buildings, machinery and equipment and construction in progress. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Expenditures for maintenance and repairs are charged to earnings as incurred. When assets are sold or retired, the related cost and accumulated depreciation are removed from the respective accounts and any resulting gain or loss is included in earnings.
Certain Risks, Concentrations and Uncertainties
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist of cash and cash equivalents, restricted cash, and receivables. The Company places its cash, cash equivalents and restricted cash with highly rated financial institutions. As of December 31, 2016 and 2015, approximately $1.2 million, or less than 1.0%, and $5.9 million, or 3.6%, respectively, of the Company’s cash, cash equivalents and restricted cash were issued or insured by the U.S. government or other government agencies. The Company has not experienced any losses on these accounts related to amounts in excess of insured limits.
Revenues from Takeda, an unrelated party, accounted for 63.9%, 63.3% and 71.3% of the Company’s total revenues for the years ended December 31, 2016, 2015 and 2014, respectively. Accounts receivable and product royalties receivable from Takeda accounted for 69.6% and 78.1% of the Company’s total accounts receivable and product royalties receivable at December 31, 2016 and 2015. Revenues from another unrelated party, Mylan, accounted for 31.6%, 35.2% and 27.8% of the Company’s total revenues for the years ended December 31, 2016, 2015 and 2014 . Accounts receivable from Mylan accounted for 30.1% and 21.9% of the Company’s total accounts receivable and product royalties receivable at December 31, 2016 and 2015. The Company depends significantly upon collaborations with Takeda and Mylan, and its activities may be impacted if these relationships are disrupted (see note 19).
Fair Value of Financial Instruments
The carrying values of the Company’s financial instruments approximate their fair values due to their short maturities, independent valuations or internal assessments. The Company’s financial instruments include cash and cash equivalents, restricted cash, receivables, accounts payable and other accrued liabilities. The Company’s investment in CPP is measured at fair value on a recurring basis, and the Company estimates the fair value of its long term debt based on similar types of borrowings.
Variable Interest Entities
The Company performs initial and on-going evaluations of the entities with which it has variable interests, such as equity ownership, in order to identify entities (i) that do not have sufficient equity investment at risk to permit the entity to finance its activities without additional subordinated financial support or (ii) in which the equity investors lack an essential characteristic of a controlling financial interest. Such entities are classified as variable interest entities (VIEs). If an entity is identified as a VIE, the Company performs an assessment to determine whether the Company has both (i) the power to direct activities that most significantly impact the VIE’s economic performance and (ii) have the obligation to absorb losses from or the right to receive benefits of the VIE that could potentially be significant to the VIE. If both of these criteria are satisfied, the Company is identified as the primary beneficiary of the VIE. As of December 31, 2016, Cancer Prevention Pharmaceuticals, Inc. (“CPP”), in which the Company held a variable interest, was determined to be a VIE. See note 7 for additional information.
Revenue Recognition
The Company’s revenues are derived primarily from product royalties, product sales, development milestone payments, clinical development activities, and contract and collaboration activities.
Multiple-Element Arrangements
The Company evaluated the multiple deliverables within the AMITIZA agreements in accordance with the guidance of multiple deliverables under ASC 605-25 “Revenue Recognition — Multiple-Element Arrangements” to determine whether the deliverables can be separated for revenue recognition purposes. The separation criteria include whether the deliverables have standalone value and whether objective reliable evidence of fair value exists. Deliverables that meet these criteria are considered a separate unit of accounting. Deliverables that do not meet these criteria are combined and accounted for as a single unit of accounting. The appropriate recognition of revenue is then applied to each separate unit of accounting. The Company’s deliverables under AMITIZA agreements are more fully described in note 19 below.
| F-12 |
Where agreements include contingent milestones, the Company evaluates whether each milestone is substantive. Milestones are considered substantive if all of the following conditions are met: (1) it is commensurate with either our performance to meet the milestone or the enhancement of the value of the delivered item or items as a result of a specific outcome resulting from the our performance to achieve the milestone, (2) it relates solely to past performance, and (3) the value of the milestone is reasonable relative to all the deliverables and payment terms (including other potential milestone consideration) within the arrangement. Where milestones are not considered substantive their treatment is based on either a time-based or proportional performance model.
Research and Development Revenue
The Company applied a time-based model of revenue recognition for cash flows associated with research and development deliverables agreed upon prior to January 1, 2011 under the North America Takeda Agreement. Under this model, cash flow streams related to each unit of accounting are recognized as revenue over the estimated performance period. Upon receipt of cash payments, such as development milestones, revenue is recognized to the extent the accumulated service time has occurred. The remainder is deferred and recognized as revenue ratably over the remaining estimated performance period.
For research and development deliverables agreed upon subsequent to January 1, 2011 under the North America Takeda agreement, which are reimbursable by Takeda at contractually predetermined percentages, the Company recognizes revenue when the underlying research and development expenses are incurred, assuming all other revenue recognition criteria are met.
Product Royalty Revenue
Product royalty revenue represents royalty revenue earned on Takeda’s net sales of AMITIZA under the North America Takeda Agreement, and is recorded when earned in accordance with the contractual terms, collectability is reasonably assured and all other revenue recognition criteria are met.
Product Sales Revenue
Product sales revenue consists of AMITIZA sales under the Japan Mylan Agreement, the North America Takeda Agreement, Global Takeda Agreement, and prior to the Global Takeda Agreement, by the Company in Europe, and RESCULA sales to Santen in Japan and by the Company in the U.S. Revenue from product sales is recognized when persuasive evidence of an arrangement exists, delivery has occurred, title to product and associated risk of loss have passed to the customer, the price is fixed or determinable, and collection from the customer is reasonably assured. The Company did not record sales deductions and returns for product sales due to the absence of discounts and rebates and the lack of right of return.
Co-promotion Revenue
Takeda reimbursements of co-promotion costs under the North America Takeda Agreement, including costs associated with the Company’s specialty sales force and miscellaneous marketing activities, are recognized as co-promotion revenue as the related costs are incurred and Takeda becomes contractually obligated to pay the amounts. The Company has determined that it is acting as a principal under this agreement, and as such, records reimbursements of these amounts on a gross basis as co-promotion revenue. In December 2014, the Company ceased co-promoting AMITIZA as a result of the amendment to the North America Takeda Agreement.
Contract and Collaboration Revenue
Contract and Collaboration revenue relates to development and consulting activities and includes the recognition of the $14.0 million upfront payment received from Takeda in 2014 under the Global Takeda Agreement, of which the Company was obligated to reimburse Takeda for the first $6.0 million in developmental expenses incurred by Takeda. Of the $14.0 million upfront payment, the Company recognized $5.6 million in 2016, $400,000 in 2015, and $8.0 million in 2014.
The Company considers its participation in joint committees under the Japan Mylan Agreement and North America Takeda Agreement as separate deliverables under the contracts and recognizes the best estimate of selling price of such participation as collaboration revenue over the period of the participation per the terms of the contracts.
Deferred Revenue
Deferred revenue represents payments received for licensing fees, option fees, consulting, research and development contracts and related cost sharing and supply agreements, mainly with Takeda and Mylan, which are deferred until revenue can be recognized under the Company’s revenue recognition policy. At December 31, 2016 and 2015, total deferred revenue was approximately $2.1 million and $1.8 million, respectively.
| F-13 |
Total deferred revenue consisted of the following as of:
| December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Deferred revenue, current | $ | 1,315 | $ | 676 | ||||
| Deferred revenue, non-current | 805 | 1,088 | ||||||
| Total deferred revenue | $ | 2,120 | $ | 1,764 | ||||
Stock-Based Compensation
The Company estimates the fair value of option awards on the date of the grant using an option-pricing model. The Company estimates the fair value of restricted stock units on the date of the grant using the market price of the Company’s common stock. Stock-based compensation expense is recognized over the required service periods.
For recording the stock-based compensation expense for service based and market condition options, the Company has chosen to use:
| · | the straight-line method of allocating compensation cost for service based options and graded vesting for market condition options; |
| · | the Black-Scholes-Merton option pricing formula for time based options and the Monte Carlo simulation model for the market condition options as the Company’s chosen option-pricing models; |
| · | the simplified method to calculate the expected term for options as discussed under the SEC’s guidance for share-based payments for service based options; |
| · | an estimate of expected volatility based on the historical volatility of the Company’s share price; and |
| · | an estimate for expected forfeitures. |
The three factors which most affect stock-based compensation are the fair value of the common stock underlying the stock options, the vesting term of the options, and the volatility of such fair value of the underlying common stock. If the Company’s estimates are too high or too low, the Company may overstate or understate its stock-based compensation expense.
Mergers and Acquisitions
In a business combination, the acquisition method of accounting requires that the assets acquired and liabilities assumed be recorded as of the date of the merger or acquisition at their respective fair values. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Accordingly, the Company may be required to value assets at fair value measures that do not reflect our intended use of those assets. Any excess of the purchase price (consideration transferred) over the estimated fair values of net assets acquired is recorded as goodwill. Transaction costs and costs to restructure the acquired company are expensed as incurred. The operating results of the acquired business are reflected in our consolidated financial statements after the date of the merger or acquisition. If the Company determines the assets acquired do not meet the definition of a business under the acquisition method of accounting, the transaction will be accounted for as an acquisition of assets rather than a business combination and, therefore, no goodwill will be recorded. The fair values of intangible assets, including acquired in-process research and development (“IPR&D”) are determined utilizing information available near the merger or acquisition date based on expectations and assumptions of a market participant that are deemed reasonable by management. Given the considerable judgment involved in determining fair values, the Company typically obtains assistance from third-party valuation specialists for significant items. Amounts allocated to acquire IPR&D are capitalized and accounted for as indefinite-lived intangible assets. Upon successful completion of each project, the Company will make a separate determination as to the then useful life of the asset and begin amortization. The judgments made in determining estimated fair values assigned to assets acquired and liabilities assumed in a business combination, as well as asset lives, can materially affect the Company's results of operations.
The fair values of identifiable intangible assets related to currently marketed products and product rights are primarily determined by using an "income approach" through which fair value is estimated based on each asset's discounted projected net cash flows. Our estimates of market participant net cash flows consider historical and projected pricing, margins and expense levels; the performance of competing products where applicable; relevant industry growth drivers and factors; current and expected trends in technology and product life cycles; the time and investment that will be required to develop products and technologies; the ability to obtain marketing and regulatory approvals; the ability to manufacture and commercialize the products; the extent and timing of potential new product introductions by the Company's competitors; and the life of each asset's underlying patent, if any. The net cash flows are then probability-adjusted where appropriate to consider the uncertainties associated with the underlying assumptions, as well as the risk profile of the net cash flows utilized in the valuation. The probability-adjusted future net cash flows of each product are then discounted to present value utilizing an appropriate discount rate.
| F-14 |
The fair values of identifiable intangible assets related to IPR&D are determined using an income or cost approach. Under the income approach fair value is estimated based on each asset's probability-adjusted future net cash flows, which reflect the different stages of development of each product and the associated probability of successful completion. The net cash flows are then discounted to present value using appropriate discounts. Under the replacement cost approach, historical research and development spending is analyzed to derive the costs incurred to date related to the asset. An expected return of 20% was applied to the cumulative mid-year pre-tax research and development costs incurred to date, as this estimates the required rate of a return a prudent investor would require on a similarly situated asset. An adjustment was not made for economic obsolescence as the costs considered are historical and the method applied is considering the estimate of fair value to buy the asset in the market at its current stage of development. No tax amortization benefit has been applied given the asset is valued under the replacement cost approach, which is representative of buying the asset in the market at fair value.
Goodwill
The Company assesses the carrying value of goodwill on an annual basis, or whenever events or changes in circumstances indicate the carrying value of goodwill may not be recoverable to determine whether any impairment in this asset may exist and, if so, the extent of such impairment. The provisions of the relevant accounting guidance require that the Company perform a two-step impairment test. In the first step, the Company compares the fair value of its reporting unit to the carrying value. If the carrying value of the reporting unit exceeds the fair value of the reporting unit, then the second step of the impairment test is performed in order to determine the implied fair value of the reporting unit's goodwill. If the carrying value of the reporting unit's goodwill exceeds its implied fair value, an impairment loss equal to the difference is recognized. The Company calculates the fair value of the reporting unit utilizing the income approach. The income approach utilizes a discounted cash flow model, using a discount rate based on the Company's estimated weighted average cost of capital. The Company also evaluates goodwill using the qualitative assessment method, which permits companies to qualitatively assess whether it is more-likely-than-not that the fair value of a reporting unit is less than its carrying amount. The Company considers developments in its operations, the industry in which it operates and overall macroeconomic factors that could have affected the fair value of the reporting unit since the date of the most recent quantitative analysis of a reporting unit's fair value. As described in note 4 to these consolidated financial statements, the Company operates as one operating segment which is considered our only reporting unit.
The determination of the fair value of a reporting unit is judgmental in nature and involves the use of significant estimates and assumptions. The estimates and assumptions used in calculating fair value include identifying future cash flows, which requires that the Company makes a number of critical legal, economic, market and business assumptions that reflect best estimates as of the testing date. The Company's assumptions and estimates may differ significantly from actual results, or circumstances could change that would cause the Company to conclude that an impairment now exists or that it previously understated the extent of impairment. The Company selected October 1 as its annual impairment test date.
Research and Development Expenses
Research and development costs are expensed in the period in which they are incurred and include the expenses from third parties who conduct research and development activities pursuant to development and consulting agreements on behalf of the Company. Costs related to the acquisition of intellectual property are expensed as incurred in research and development expenses since the underlying technology associated with such acquisitions is unproven, has not received regulatory approval at its early stage of development and does not have alternative future uses. Milestone payments due under agreements with third party contract research organizations (CROs) are accrued when it is considered probable that the milestone event will be achieved.
Accrued Research and Development Expenses
As part of the process of preparing Consolidated Financial Statements, the Company is required to estimate accruals for research and development expenses. This process involves reviewing and identifying services which have been performed by third parties on the Company’s behalf and determining the value of these services. In addition, the Company makes estimates of costs incurred to date but not yet invoiced, in relation to external CROs and clinical site costs. The Company analyzes the progress of clinical trials, including levels of patient enrollment; invoices received and contracted costs, when evaluating the adequacy of the accrued liabilities for research and development. The Company makes significant judgments and estimates in determining the accrued balance in any accounting period. No material adjustments have been required for this accrual during the years ended December 31, 2016, 2015 and 2014.
Income Taxes
The Company accounts for income taxes under the asset and liability method in accordance with the relevant accounting guidance for income taxes. Under the asset and liability method, the current income tax provision or benefit is the amount of income taxes expected to be payable or refundable for the current year. A deferred income tax asset or liability is recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and tax credits and loss carry-forwards. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Tax rate changes are reflected in the income tax provision during the period such changes are enacted. Changes in ownership may limit the amount of net operating loss (NOL) carry-forwards that can be utilized in the future to offset taxable income. The Company’s discussion of income tax is described more fully under note 21 found below.
| F-15 |
In September 2011, the Company internally transferred certain intellectual property and licenses from the Company’s subsidiaries, including the U.S. based subsidiary, to SAG. Since the transfer of these assets was to a related party, the recognition of a deferred tax asset by SAG is prohibited and the net tax effect of the transaction is deferred in consolidation. The tax liability generated from this transaction is offset by a deferred charge that is being amortized over ten years. As of December 31, 2016, 2015 and 2014, the total deferred charge is $0.1 million, $1.7 million and $2.0 million, respectively, after a net current year amortization and impairment expense of $1.6 million, $0.3 million and $3.2 million, respectively. Impairment expense in 2016 was primarily a result of the cessation of commercialization activities for the use of cobiprostone for Oral Mucositis. Impairment expense included in the $3.2 million for 2014 totaled $1.8 million and resulted from the cessation of direct commercialization activities for RESCULA in 2014.
For all significant intercompany transactions, the Company’s management has evaluated the terms of the transactions using significant estimates and judgments to ensure that each significant transaction would be on similar terms if the Company completed the transaction with an unrelated party. Although the Company believes there will be no material tax liabilities to the Company as a result of multi-jurisdictional transactions, there can be no assurance that taxing authorities will not assert that transactions were entered into at monetary values other than fair values. If such assertions were made, the Company’s intention would be to vigorously defend its positions; however, there can be no assurance that additional liabilities may not occur as a result of any such assertions.
The Company considers certain undistributed earnings of foreign subsidiaries to be indefinitely reinvested outside of the U.S. and, accordingly, no U.S. deferred taxes have been recorded under the applicable accounting standard with respect to such earnings. Should the earnings be remitted to the U.S., the Company may be subject to additional U.S. taxes, net of allowable foreign tax credits. It is not practicable to estimate the amount of any additional taxes which may be payable on the undistributed earnings.
Uncertain Tax Positions
The Company applies the accounting guidance for uncertain tax positions that requires the application of a more likely than not threshold to the recognition and de-recognition of uncertain tax positions. If the recognition threshold is met, the Company recognizes a tax benefit measured at the largest amount of the tax benefit that, in its judgment, is more than 50% likely to be realized upon settlement.
The Company has recorded an income tax liability of approximately $4.1 million and $2.9 million, including interest, for uncertain tax positions as of December 31, 2016 and 2015, respectively. As of December 31, 2016 and 2015, the entire balance was reflected as other liabilities in the accompanying Consolidated Balance Sheets. These amounts represent the aggregate tax effect of differences between tax return positions and the amounts otherwise recognized in the Company’s Consolidated Financial Statements.
The Company recognizes interest and penalties related to uncertain tax positions as a component of the income tax provision. It is reasonably possible that the $1.6 million of the liability for unrecognized tax benefits will decrease within the next 12 months. In addition, future changes in the unrecognized tax benefits would have an effect on the effective tax rate when recognized.
Currently, tax years 2012 to 2015 remain open and subject to examination in the major tax jurisdictions in which tax returns are filed.
Foreign Currency
The functional currency for most of the Company’s non-U.S. subsidiaries is the U.S. Dollar. For those non-U.S. subsidiaries that transact in a functional currency other than the U.S. dollar, assets and liabilities are translated at current exchange rates as of the balance sheet date, and income and expense items are translated at average exchange rates for the period. Adjustments resulting from the translation of the financial statements of our foreign operations into U.S. dollars are excluded from the determination of net income and are recorded in accumulated other comprehensive income, a separate component of equity. For subsidiaries where the functional currency is the U.S. dollar, non-monetary assets and liabilities are translated at the exchange rates in effect on the date the non-monetary assets and liabilities were acquired, while monetary assets and liabilities are translated at current exchange rates as of the balance sheet date. Income and expense items are translated at the average exchange rates for the period. Translation adjustments of these subsidiaries are included in net income.
For the year ended December 31, 2016, the Company recorded a foreign currency exchange loss of $11.3 million, which is included in other income (expense), net on the Consolidated Statements of Operations and Comprehensive Income.
| F-16 |
Realized and unrealized foreign currency gains or losses on assets and liabilities denominated in a currency other than the functional currency are included in net income.
Other Comprehensive Income
Comprehensive income consists of net income plus certain other items that are recorded directly to stockholders’ equity. The Company has reported comprehensive income in the Consolidated Statements of Operations and Comprehensive Income.
The Company has outstanding intercompany loans and investments between its subsidiaries which are eliminated for purposes of the Consolidated Financial Statements. These intercompany loans are not expected to be repaid or settled in the foreseeable future. Accordingly, the currency transaction gains or losses on these intercompany loans are recorded as part of other comprehensive income in the Consolidated Financial Statements. In addition, the actuarial gains and losses of the Swiss Pension plan are recorded in comprehensive income.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, “Revenue from Contracts with Customers”, which will replace numerous requirements in U.S. GAAP, including industry-specific requirements. This guidance provides a five-step model to be applied to all contracts with customers, with an underlying principle that an entity will recognize revenue to depict the transfer of goods or services to customers at an amount that the entity expects to be entitled to in exchange for those goods or services. ASU No. 2014-09 requires extensive quantitative and qualitative disclosures covering the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including disclosures on significant judgments made when applying the guidance. This guidance is effective for annual reporting periods beginning after December 15, 2017 and interim periods therein. Early adoption is permitted for reporting periods and interim periods therein, beginning after December 15, 2016. An entity can elect to apply the guidance under one of the following two methods: (i) retrospectively to each prior reporting period presented – referred to as the full retrospective method or (ii) retrospectively with the cumulative effect of initially applying the standard recognized at the date of initial application in retained earning – referred to as the modified retrospective method.
While the Company has not yet completed its final review of the impact of the new standard, the adoption of ASU 2014-09 could potentially have the following impacts to our contracts:
| (i) | Variable consideration including milestone payments, escalating royalty payments based on volume, and product sales price adjustments may be recognized at an earlier point in time under the new guidance, when it is probable that the variable consideration will be achieved without a significant future reversal of cumulative revenue expected. |
| (ii) | Expense reimbursement revenue of certain R&D projects may result in a change in presentation. |
The Company has not yet completed its final review of the impact of this guidance and continues to evaluate the impact of adoption and the implementation approach to be used. The Company plans to adopt the new standard effective January 1, 2018, and will continue to monitor additional changes, modifications, clarifications or interpretations by the FASB, which may impact the Company’s current conclusions.
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements—Going Concern,” to provide guidance on management’s responsibility in evaluating whether there is substantial doubt about a company’s ability to continue as a going concern and about related footnote disclosures. For each reporting period, management will be required to evaluate whether there are conditions or events that raise substantial doubt about our ability to continue as a going concern within one year from the date the financial statements are issued. This guidance is effective for the annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. Early application is permitted. The Company has adopted ASU No. 2014-15, and the adoption had no impact on our consolidated financial statements.
In February 2015, the FASB issued ASU No. 2015-02, “Amendments to the Consolidation Analysis,” which affects reporting entities that are required to evaluate whether they should consolidate certain legal entities. The amendments place more emphasis in the consolidation evaluation on variable interests other than fee arrangements such as principal investment risk (including debt or equity interests), guarantees of the value of the assets or liabilities of the variable interest entity (“VIE”), written put options on the assets of the VIE, or similar obligations. Additionally, the amendments reduce the extent to which related party arrangements cause an entity to be considered a primary beneficiary. This guidance is to be applied using a modified retrospective approach by recording a cumulative-effect adjustment to equity as of the beginning of the fiscal year of adoption. The amendments are effective for fiscal years beginning after December 15, 2015, and interim periods therein. The Company has adopted ASU No. 2015-02 and the adoption had no impact on our consolidated financial statements.
| F-17 |
In February 2016, the FASB issued ASU No. 2016-02, “Leases,” that requires lessees to recognize assets and liabilities on the balance sheet for most leases including operating leases. Lessees now classify leases as either finance or operating leases and lessors classify all leases as sales-type, direct financing or operating leases. The statement of operations presentation and expense recognition for lessees for finance leases is similar to that of capital leases under Accounting Standards Codification (“ASC”) 840 with separate interest and amortization expense with higher periodic expense in the earlier periods of a lease. For operating leases, the statement of operations presentation and expense recognition is similar to that of operating leases under ASC 840 with single lease cost recognized on a straight-line basis. This guidance is to be applied using a modified retrospective approach at the beginning of the earliest comparative period presented in the financial statements and is effective for annual periods beginning after December 15, 2018 and interim periods therein. Early adoption is permitted. The Company is currently analyzing the impact of ASU No. 2016-02, and is currently unable to determine the impact of the new standard, if any, on the Company’s consolidated financial statements.
In March 2016, the FASB issued ASU No. 2016-09, “Improvements to Employee Share-Based Payment Accounting,” which changes the accounting for certain aspects of share-based payments to employees. The new guidance requires excess tax benefits and tax deficiencies to be recorded in the statement of operations when the awards vest or are settled. In addition, cash flows related to excess tax benefits will no longer be separately classified as a financing activity apart from other income tax cash flows. The standard also clarifies that all cash payments made on an employee’s behalf for withheld shares should be presented as a financing activity on the statement of cash flows, and provides an accounting policy election to account for forfeitures as they occur. The new standard is effective for the Company’s calendar year beginning January 1, 2017. The Company is currently analyzing the impact of ASU No. 2016-09, and is currently unable to determine the impact of the new standard, if any, on the Company’s consolidated financial statements.
In August 2016, the FASB issued ASU No. 2016-15, “Classification of Certain Cash Receipts and Cash Payments,” which clarifies how entities should classify certain cash receipts and cash payments on the statement of cash flows to eliminate diversity in practice. ASU 2015-15 provides that cash payments for debt prepayment or debt extinguishment costs should be classified as cash outflows for financing activities. The new standard is effective for fiscal years beginning after December 15, 2017 and interim periods therein. Early adoption is permitted. The Company elected to early adopt ASU No. 2017-01 for the annual period ending December 31, 2016. The adoption required the Company to classify debt extinguishment costs as financing as opposed to operating in the consolidated statement of cash flows.
In October 2016, the FASB issued ASU No. 2016-16, “Intra-Entity Transfers of Assets Other Than Inventory,” which requires companies to account for the income tax effects of intercompany sales and transfers of assets other than inventory in the period in which the transfer occurs. The new standard is effective for public business entities for annual periods beginning after December 15, 2017. Early adoption is permitted for all entities as of the beginning of an annual period. The guidance is to be applied using a modified retrospective approach with a cumulative catch-up adjustment to opening retained earnings in the period of adoption. The Company is currently analyzing the impact of ASU No. 2016-16 on its consolidated financial statements.
In November 2016, the FASB issued ASU No. 2016-18, “Restricted Cash,” which requires entities to show the changes in total of cash, cash equivalents, restricted cash and restricted cash equivalents in the statement of cash flows. As a result, entities will no longer present transfers between cash and cash equivalents and restricted cash in the statement of cash flows. When cash, cash equivalents, restricted cash and restricted cash equivalents are presented in more than one line item on the balance sheet, the new guidance requires a reconciliation of the totals in the statement of cash flows to the related captions on the balance sheet. The reconciliation can either be presented either on the face of the statement of cash flows or in the notes to the financial statements. The new standard is effective for public business entities for fiscal years beginning after December 15, 2017 and interim periods therein and is to be applied retrospectively. Early adoption is permitted. The Company is currently analyzing the impact of ASU No. 2016-18 on its consolidated financial statements.
In January 2017, the FASB issued ASU No. 2017-01, “Business Combinations,” which requires entities to evaluate if substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or a group of similar identifiable assets; if so, the set of transferred assets and activities is not a business. The new standard is to be applied prospectively to any transactions occurring within the period of adoption and is effective for public business entities for fiscal years beginning after December 15, 2017. Early adoption is permitted, including annual periods in which the financial statements have not been issued. The Company elected to early adopt ASU No. 2017-01 for the annual period ending December 31, 2016. The adoption had no impact on the Company’s consolidated financial statements.
3. Net Income per Share
Basic net income per share is computed by dividing net income by the weighted average common shares outstanding. Diluted net income per share is computed by dividing net income by the sum of the weighted average common shares and potential dilutive common shares outstanding. The treasury-stock method is used to determine the dilutive effect of the Company’s stock option grants, and the if-converted method is used to determine the dilutive effect of the Company’s Convertible Notes.
| F-18 |
The computation of net income per share for the years ended December 31, 2016, 2015 and 2014 is shown below:
| Year Ended December 31, | ||||||||||||
| (In thousands, except per share data) | 2016 | 2015 | 2014 | |||||||||
| Basic net income per share: | ||||||||||||
| Net income | $ | 18,487 | $ | 33,371 | $ | 13,128 | ||||||
| Weighted-average number of common shares-basic | 42,791 | 44,150 | 43,691 | |||||||||
| Basic net income per share | $ | 0.43 | $ | 0.76 | $ | 0.30 | ||||||
| Diluted net income per share: | ||||||||||||
| Net income | $ | 18,487 | $ | 33,371 | $ | 13,128 | ||||||
| Interest expense applicable to convertible debt, net of tax | 64 | - | - | |||||||||
| Amortization of debt issuance costs, net of tax | 12 | - | - | |||||||||
| Net income for calculation of diluted net income per share | 18,563 | 33,371 | 13,128 | |||||||||
| Weighted-average number of common shares-basic | 42,791 | 44,150 | 43,691 | |||||||||
| Assumed exercise of stock options under the treasury-stock method | 711 | 1,530 | 815 | |||||||||
| Assumed shares under if-converted method | 247 | - | - | |||||||||
| Weighted-average number of common shares-diluted | 43,749 | 45,680 | 44,506 | |||||||||
| Diluted net income per share | $ | 0.42 | $ | 0.73 | $ | 0.29 | ||||||
The potentially dilutive securities used in the calculations of diluted net income per share at December 31, 2016, 2015 and 2014 were as follows:
| December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| Employee stock options | 2,400 | 3,290 | 1,124 | |||||||||
| Non-employee stock options | - | - | 255 | |||||||||
The following securities were excluded from the computation of diluted net income per share as their effect would be anti-dilutive during the years ended December 31, 2016, 2015 and 2014:
| December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| Employee stock options | 2,387 | 1,188 | 3,012 | |||||||||
4. Segment Information
In the first quarter of 2015, the Company made several strategic and operational changes to its business, including re-evaluating and accelerating its pipeline to focus on clinical programs that it believes hold the most promise for patients, the highest likelihood for regulatory approval, and the strongest potential for commercial return. Because of such changes, the Company combined its reportable geographic segments of Asia, the Americas and Europe into one operating segment: the development and commercialization of pharmaceutical products. This change reflects the manner in which information is now being presented internally and used by the Company’s chief operating decision maker, the Company’s Chief Executive Officer, to allocate resources and assess performance.
Summarized product category and geographic information is shown in the tables below.
Product Category Information
Revenues for product categories are attributed based on the following categories.
| F-19 |
Product royalty revenue represents royalty revenue earned on the net sales of AMITIZA in North America. Product sales revenue represents drug product net sales of AMITIZA in North America, Japan and Europe and drug product net sales of RESCULA in Japan. Research and development revenue represents funded development work primarily related to AMITIZA. Contract and collaboration revenue represents the amortization of up-front payments under the North America Takeda Agreement and release of the collaboration obligation under the Global Takeda agreement. Co-promotion revenue represents reimbursements by Takeda of a portion of the Company’s co-promotion costs for its specialty sales force.
Company revenues by product category for the years ended December 31, 2016, 2015 and 2014 were as follows:
| Years Ended December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| Product royalty revenue | $ | 82,480 | $ | 74,138 | $ | 62,775 | ||||||
| Product sales revenue | 128,796 | 66,276 | 33,252 | |||||||||
| Research and development revenue | 12,839 | 10,199 | 7,246 | |||||||||
| Contract and collaboration revenue | 5,941 | 2,567 | 8,817 | |||||||||
| Co-promotion revenue | - | - | 3,360 | |||||||||
| Total | $ | 230,056 | $ | 153,180 | $ | 115,450 | ||||||
Geographical Information
Revenues are attributable to countries based on the location of the customer. The Company operates a manufacturing facility in Japan that supplies products to customers as well as the Company’s subsidiaries in other countries. The sales from the manufacturing operations to other countries are included in the net sales of the country in which the manufacturing location is based. The intersegment portions of such sales are excluded to derive consolidated revenues. The Company’s country of domicile is the United States.
Company revenues by geographic location for the years ended December 31, 2016, 2015 and 2014 were as follows:
| Years Ended December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| United States | $ | 140,240 | $ | 95,769 | $ | 74,688 | ||||||
| Japan | 83,130 | 55,371 | 32,128 | |||||||||
| Rest of the world | 6,686 | 2,040 | 8,634 | |||||||||
| Total | $ | 230,056 | $ | 153,180 | $ | 115,450 | ||||||
The Company’s long-lived assets by geographic location where located on December 31, 2016, 2015 and 2014 were as follows:
| December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| United States | $ | 3,065 | $ | 3,105 | $ | 566 | ||||||
| Japan | 3,119 | 3,232 | 114 | |||||||||
| Rest of the world | 32 | 56 | 83 | |||||||||
| Total | $ | 6,216 | $ | 6,393 | $ | 763 | ||||||
5. Acquisition of R-Tech
In August 2015, the Company entered into a share purchase agreement with Drs. Ryuji Ueno and Sachiko Kuno and S&R Technology Holdings, LLC, to acquire 44% of outstanding R-Tech shares. The total purchase price for these shares was 1,400 Japanese Yen (“JPY”) per share, or 12 billion JPY in the aggregate, or approximately $100.0 million.
In August 2015, the Company launched, through its wholly-owned Japanese subsidiary (the “Offeror”), an all-cash tender offer in Japan to acquire the remaining 56% of the outstanding shares and stock acquisition rights of R-Tech for 1,900 JPY per share, resulting in a total consideration of up to 21 billion JPY, or approximately $175.0 million. The price offered in the tender offer reflected that R-Tech held approximately $62.1 million in cash and 2.5 million shares of the Company’s common stock as of the commencement of the tender offer.
| F-20 |
On October 20, 2015, the transactions contemplated by the share purchase agreement were completed, and the tender offer was concluded. As a result of these transactions, the Company acquired approximately 98% of R-Tech’s outstanding shares. The Company acquired the remaining 2% of outstanding shares of R-Tech through a squeeze-out process under Japanese law on December 8, 2015 for total consideration of 926 JPY million, or approximately $7.7 million. This acquisition diversified the product portfolio, expanded the Company’s development pipeline and integrated the manufacturing of the Company’s main product, AMITIZA.
This transaction was accounted for under the acquisition method of accounting, with the Company as the acquirer. Under the acquisition method of accounting, the assets and liabilities of R-Tech were recorded as of the acquisition date at their respective fair values, and combined with those of the Company.
The final allocation of the purchase price based upon estimated fair value of assets acquired and liabilities assumed on October 20, 2015 is as follows:
| (In thousands) | ||||
| Cash | $ | 62,097 | ||
| Accounts receivable | 8,299 | |||
| Inventory (i) | 37,563 | |||
| Prepaid expenses | 3,792 | |||
| Property, plant and equipment | 2,796 | |||
| Other long term assets | 449 | |||
| Accounts payable and accrued liabilities | (11,598 | ) | ||
| Income tax payable | (5,025 | ) | ||
| Other liabilities, current | (3,282 | ) | ||
| Deferred tax liability, net | (63,365 | ) | ||
| Other liabilities, long termW | (9,347 | ) | ||
| R-Tech shares of Sucampo stock (treasury stock) | 43,956 | |||
| Total fair value of tangible assets acquired and liabilities assumed | 66,335 | |||
| Acquired in-process research and development | 6,200 | |||
| Acquired intangible assets | 134,600 | |||
| Goodwill | 61,618 | |||
| Total purchase price | $ | 268,753 | ||
| Total purchase price | $ | 268,753 | ||
| Settlement of net receivable from pre-existing relationship | 6,364 | |||
| Total consideration | $ | 275,117 | ||
| Acquisition, net of acquired cash | $ | 161,187 | ||
| Acquired cash | 62,097 | |||
| Purchase of treasury stock | 43,956 | |||
| Squeeze out liability for non-tendering R-Tech shareholders | 7,668 | |||
| Other | 209 | |||
| Total consideration | $ | 275,117 | ||
(i) Acquired inventory includes a $20.1 million adjustment to record inventory at fair value, referred to as a step-up adjustment. The $20.1 million step-up adjustment was recognized through costs of goods sold in alignment with inventory turnover. The recognition of the inventory step-up adjustment increased costs of goods sold during those periods. For the years ended December 31, 2016 and 2015, the Company recognized $15.2 million and $5.8 million, respectively, in additional costs of goods sold related to the recognition of the inventory step-up adjustment.
| F-21 |
The estimated fair value of intangible assets acquired and related estimated amortization periods in years is as follows:
| (In thousands) | As of October 20, 2015 | Amortization period in years | ||||||
| Acquired in-process research and development | $ | 6,200 | Indefinite | |||||
| Acquired intangible assets: | ||||||||
| AMITIZA - manufacturing know-how | 120,200 | 14 | ||||||
| RESCULA - manufacturing know-how | 14,400 | 10 | ||||||
| $ | 134,600 | |||||||
IPR&D acquired from R-Tech is related to two product candidates, RTU-009 and RTU-1096. Management estimated the fair value of IPR&D at the acquisition date to be $6.2 million. The estimated fair value was determined using the replacement cost approach. Under the replacement cost approach, historical research and development spending is analyzed to derive the costs incurred to date related to the asset. An expected return of 20% was applied to the cumulative mid-year pre-tax research and development costs incurred to date, as this estimates the required rate of a return a prudent investor would require on a similarly situated asset. An adjustment was not made for economic obsolescence as the costs considered are historical and the method applied is considering the estimate of fair value to buy the asset in the market at its current stage of development. No tax amortization benefit has been applied given the asset is valued under the replacement cost approach, which is representative of buying the asset in the market at fair value.
The Company estimated the fair values of the AMITIZA manufacturing know-how intangible asset and RESCULA manufacturing know-how intangible asset using the income approach with a present value discount rate of 18%, which is based on the estimated weighted-average cost of capital for companies with profiles substantially similar to that of R-Tech and the Company. This is comparable to the estimated internal rate of return for the acquisition and presents the rate that market participants would use to value these intangible assets. The projected cash flows from the AMITIZA manufacturing know-how intangible asset and RESCULA manufacturing know-how intangible asset were based on key assumptions including estimates of revenues, operating profits, the life of the potential commercialized product, associated risks, and the risks related to the viability of and potential alternative use in any future markets.
The weighted average amortization period of the intangible assets from the R-Tech acquisition is 78 months which is reflective of expected cash flows. The manufacturing know-how intangible assets are based on the manufacturing rights related to the Company’s AMITIZA and RESCULA agreements. Under these agreements, the maximum contractual cash flow periods for RESCULA and AMITIZA were up to 10 years and 14 years, respectively. The agreement with the largest projected cash flows had a period of five years, which reduced the weighted average amortization period to 78 months. For the years ended December 31, 2016 and 2015, the Company recorded amortization expense of $25.7 million and $3.7 million, respectively, all of which has been recorded in costs of goods sold in the Consolidated Statements of Operations and Comprehensive Income.
The Company recorded approximately $61.6 million in goodwill related to the acquisition of R-Tech, representing the purchase price paid in the acquisition that was in excess of the fair value of the tangible and intangible assets acquired. None of the goodwill generated from R-Tech acquisition is expected to be deductible for tax purposes.
The Company incurred transaction costs related to the R-Tech acquisition of $5.2 million for the year ended December 31, 2015, all of which was recorded in general and administrative expenses in the Consolidated Statements of Operations and Comprehensive Income.
For the year ended December 31, 2015, R-Tech contributed revenues and net loss to the Company’s consolidated results of $11.8 million and $4.7 million, respectively.
| F-22 |
6. Restructuring
In December 2015, the Company adopted a plan to restructure certain of its operations and to consolidate certain functions in the Company’s corporate headquarters located in Rockville, Maryland and in the Company’s Japanese subsidiaries. The restructuring plan primarily included headcount reductions due to the ongoing integration of R-Tech (see note 5). In connection with these restructuring activities, the Company recorded restructuring charges of $2.4 million and $953,000 for the years ended December 31, 2016 and 2015, respectively. These costs are reflected within operating expenses and are detailed below:
| Years ended December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Termination benefits | $ | 1,802 | $ | 953 | ||||
| Asset impairments | 183 | - | ||||||
| Contract and other costs | 365 | - | ||||||
| Total | $ | 2,350 | $ | 953 | ||||
At December 31, 2016 and 2015, a restructuring accrual of $163,000 and $851,000, respectively, was included in accrued liabilities. The following table summarizes the accrued restructuring costs at December 31, 2016 and 2015:
| (In thousands) | Accrued Restructuring | |||
| Balance at December 31, 2014 | $ | - | ||
| Expenses incurred | 953 | |||
| Amounts paid | (102 | ) | ||
| Balance at December 31, 2015 | $ | 851 | ||
| Expenses incurred | $ | 2,167 | ||
| Amounts paid | (2,869 | ) | ||
| Foreign currency translation adjustment | 14 | |||
| Balance at December 31, 2016 | $ | 163 | ||
7. Non-Current Investments
Investment in CPP
On January 9, 2016, the Company entered into a Securities Purchase Agreement (“CPP Securities Agreement”) and an Option and Collaboration Agreement (“CPP Agreement”) with CPP for the development and commercialization of CPP-1X/sulindac combination.
Under the terms of the CPP Securities Agreement, the Company provided $5.0 million to CPP in exchange for a convertible note. The convertible note bears interest at the rate of 5% per annum and matures on January 31, 2019 unless earlier converted or prepaid. The convertible note is automatically convertible into securities of CPP, subject to certain limitations, in the event CPP consummates a future financing with aggregate proceeds of at least $10.0 million, exclusive of any investment by the Company, whether through a public offering or a private offering (a “Qualified Financing”). Depending on the timing of the Qualified Financing, the convertible note will automatically convert into the same securities issued in the Qualified Financing at a 10% to 20% discount to the lowest issuance price of the securities in the Qualified Financing. The Company has also agreed to purchase up to $5.0 million of CPP’s securities in any such Qualified Financing.
Under the terms of the CPP Agreement, CPP granted the Company the sole option to acquire an exclusive license to commercialize CPP-1X/Sulindac combination product in North America. This product is currently in a Phase 3 clinical trial for the treatment of familial adenomatous polyposis (FAP). Target enrollment in the study was achieved in April 2016 and the trial is expected to conclude in 2018. The Company will pay CPP an option fee of $7.5 million, payable in two tranches. The first tranche of $3.0 million was paid in January 2016 and was recorded as research and development expense. The second tranche of $4.5 million is due upon achievement of certain results of the ongoing feasibility study. CPP will complete the ongoing Phase 3 trial under the oversight of a joint steering committee between CPP and the Company. Upon exercise of its exclusive option, the Company would negotiate an exclusive license to develop and commercialize the product in North America for all indications. In connection with the exercise of the option right, the subsequent execution of a license agreement and the development and commercialization of the product, the Company would be obligated to pay CPP up to an aggregate of $190.0 million of specified clinical development and sales milestones. Under the terms of the license, the Company and CPP would share equally in net profits from the sale of licensed products.
CPP is considered to be a VIE with respect to the Company. It has been determined that the power to direct the activities that most significantly impact CPP’s economic performance is held by the board of directors of CPP. The Company does not have a representative on CPP’s board and does not have the right to appoint or elect such a representative. Therefore, the Company is not the primary beneficiary of CPP, and the entity is not consolidated with the financial statements of the Company.
The Company’s maximum exposure to loss as a result of its involvement with CPP is $5.2 million as of December 31, 2016, which, is the investment in the convertible note of $5.2 million. As of September 30, 2016, CPP had total assets of $4.7 million and total liabilities of $20.6 million.
The Company has elected the fair value option on the convertible note received from CPP due to the nature of the financial characteristics of the investment. As of December 31, 2016, the fair value of the convertible note is $5.2 million.
| F-23 |
8. Fair Value Measurements
The Company performs fair value measurements in accordance with the FASB’s guidance for fair value measurements and disclosures, which defines fair value as the exchange price that would be received for selling an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. A fair value hierarchy is established which requires the Company to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The Company classifies its investments into the following categories based on the three levels of inputs used to measure fair value:
Level 1: Observable inputs, such as quoted prices in active markets for identical assets or liabilities;
Level 2: Inputs, other than the quoted price in active markets, that are observable, either directly or indirectly, such as quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; or
Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
The carrying values of cash and cash equivalents, restricted cash, accounts receivable, product royalties receivable, accounts payable and other accrued liabilities, approximate their fair values due to their short maturities.
The Company has elected the fair value option on its investment in CPP (see note 7); as such, it is measured at fair value on a recurring basis. At December 31, 2016, the fair value of the investment in CPP was $5.2 million. For the year ended December 31, 2016, the Company recorded $0.2 million in other income due to the increase in fair value of the investment in CPP.
The estimated fair value of long term debt at December 31, 2016 was $319.5 million, as disclosed in note 18 and was based on similar types of borrowings.
The estimated fair values may not represent actual values of the financial instruments that could be realized as of the balance sheet date or that will be realized in the future. As of December 31, 2016, there were no financial instruments measured at fair value on a non-recurring basis. The measurement of impairment attributed to RTU 1096 and RTU 009 IPR&D was a non-recurring fair value measurement that utilized level 3 inputs. These level 3 inputs consisted of future cash flows for development, the probabilities of successful clinical outcomes and discount rate. As of December 31, 2015, there were no financial instruments measured at fair value on a non-recurring basis. The acquired assets and assumed liabilities of R-Tech were measured at fair value on a non-recurring basis (see note 5) in connection with the application of purchase accounting.
There were no transfers between levels during the years ended December 31, 2016 and 2015.
9. Inventories
Inventories are valued under a weighted average costing method and are stated at the lower of cost or net realizable value. Inventories consist of raw materials, work-in-process and finished goods. The Company’s inventories include the direct purchase cost of materials and supplies and manufacturing overhead costs. In connection with the acquisition of R-Tech, all inventory held by R-Tech was stepped-up in value to $37.6 million as of the acquisition date. As of December 31, 2016 and 2015, the remaining balance of inventory step-up was zero and $14.3 million, respectively.
Inventories consisted of the following as of December 31, 2016 and 2015:
| December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Raw materials | $ | 1,414 | $ | 5,554 | ||||
| Work in process | 18,045 | 26,926 | ||||||
| Finished goods | 4,009 | 641 | ||||||
| Total | $ | 23,468 | $ | 33,121 | ||||
| F-24 |
10. Property and Equipment
Property and equipment consisted of the following at December 31, 2016 and 2015:
| December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Computer and office equipment | $ | 2,346 | $ | 2,647 | ||||
| Furniture and fixtures | 1,068 | 1,023 | ||||||
| Leasehold improvements | 3,346 | 3,066 | ||||||
| Buildings | 888 | 1,022 | ||||||
| Machinery and equipment | 2,176 | 2,074 | ||||||
| Construction in progress | 119 | 140 | ||||||
| Total cost | 9,943 | 9,972 | ||||||
| Less: accumulated depreciation | (3,727 | ) | (3,579 | ) | ||||
| Total | $ | 6,216 | $ | 6,393 | ||||
Depreciation expense for the years ended December 31, 2016, 2015 and 2014 was $904,000, $637,000 and $422,000, respectively.
11. Intangible Assets, In-Process Research and Development and Goodwill
Intangible assets by major class as of December 31, 2016 and 2015 were as follows:
| December 31, 2016 | December 31, 2015 | |||||||||||||||
| (In thousands) | Weighted average life (in months) | Carrying amount | Weighted average life (in months) | Carrying amount | ||||||||||||
| Amortized intangible assets | ||||||||||||||||
| Patent and license rights | 60 | $ | 10,513 | 72 | $ | 10,513 | ||||||||||
| Manufacturing know how | 65 | 134,600 | 76 | 134,600 | ||||||||||||
| Accumulated amortization | (34,142 | ) | (8,463 | ) | ||||||||||||
| Impairment losses | (5,651 | ) | (5,651 | ) | ||||||||||||
| Foreign currency translation adjustments | 22,814 | (684 | ) | |||||||||||||
| Total amortized intangible assets | $ | 128,134 | $ | 130,315 | ||||||||||||
| Unamortized intangible assets | ||||||||||||||||
| In-process research and development | $ | - | $ | 6,171 | ||||||||||||
| Goodwill | 73,022 | 60,937 | ||||||||||||||
| Total unamortized intangible assets | $ | 73,022 | $ | 67,108 | ||||||||||||
| Total intangible assets | $ | 201,156 | $ | 197,423 | ||||||||||||
| F-25 |
The changes in intangible assets for the years ended December 31, 2016 and 2015 were as follows:
| (In thousands) | Intangibles | Goodwill | In-process research & development | |||||||||
| Balance at December 31, 2014 | $ | 151 | $ | - | $ | - | ||||||
| Additions | 134,600 | 61,228 | 6,200 | |||||||||
| Amortization | (3,752 | ) | - | - | ||||||||
| Foreign currency translation adjustment | (684 | ) | (291 | ) | (29 | ) | ||||||
| Balance at December 31, 2015 | $ | 130,315 | $ | 60,937 | $ | 6,171 | ||||||
| Additions | - | 462 | - | |||||||||
| Amortization | (25,679 | ) | - | - | ||||||||
| Foreign currency translation adjustment | 23,498 | 11,623 | 1,115 | |||||||||
| Impairment of in-process research & development | - | - | (7,286 | ) | ||||||||
| Balance at December 31, 2016 | $ | 128,134 | $ | 73,022 | $ | - | ||||||
Amortization expense on intangible assets totaled $25.7 million, $3.7 million and $636,000, for the years ended December 31, 2016, 2015 and 2014, respectively. The manufacturing know-how intangible assets are based on the manufacturing rights related to AMITIZA and RESCULA agreements. Under these agreements, the maximum contractual cash flow periods for RESCULA and AMITIZA were up to 10 years and 14 years, respectively. The agreement with the largest projected cash flows had a period of five years, which reduced the weighted average amortization period to 65 and 76 months as of December 31 2016 and December 31, 2015, respectively.
In 2016, the Company discontinued its VAP-1 Inhibitor RTU-1096 development program and its VAP-1 Inhibitor RTU-009 program. The Company considered the discontinuance as a potential indicator of impairment of the related IPR&D asset. Accordingly, the Company performed an interim assessment, and as a result, recorded an impairment charge of $7.3 million during the year ended December 31, 2016, which represented the entire carrying value of the IPR&D asset.
Amortization of intangibles for the next five years is expected to be as follows:
| (In thousands) | Amortization | |||
| Years ended December 31, | ||||
| 2017 | $ | 26,990 | ||
| 2018 | 26,990 | |||
| 2019 | 26,990 | |||
| 2020 | 26,990 | |||
| 2021 | 4,009 | |||
12. Accrued Expenses
Accrued expenses consisted of the following at December 31, 2016 and 2015:
| December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Research and development costs | $ | 3,030 | $ | 3,843 | ||||
| Employee compensation | 7,513 | 4,860 | ||||||
| Restructuring | 163 | 851 | ||||||
| Selling and marketing costs | 40 | 1 | ||||||
| Legal and accounting fees | 622 | 428 | ||||||
| Other accrued expenses | 1,021 | 903 | ||||||
| Total | $ | 12,389 | $ | 10,886 | ||||
13. Collaboration Obligation
Under the Global Takeda Agreement (see note 19), the Company received an upfront payment from Takeda of $14.0 million in 2014, of which the Company was obligated to reimburse Takeda for the first $6.0 million in developmental expenses incurred by Takeda. At December 31, 2016 and 2015, the collaboration obligation was $0 and $5.6 million, respectively.
| F-26 |
14. Other Current Liabilities
Other current liabilities consisted of the following at December 31, 2016 and 2015:
| December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Indirect taxes payable | $ | 1,756 | $ | 5,963 | ||||
| Squeeze out liability for non-tendering R-Tech shareholders | 155 | 7,668 | ||||||
| Other current liabilities | 393 | 508 | ||||||
| Total | $ | 2,304 | $ | 14,139 | ||||
15. Other Liabilities
Other liabilities consisted of the following at December 31, 2016 and 2015:
| December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Deferred grants | $ | 750 | $ | 9,604 | ||||
| Unrecognized tax benefits | 4,060 | 3,061 | ||||||
| Deferred leasehold incentive | 1,582 | 1,715 | ||||||
| Defined benefit obligation | 818 | 949 | ||||||
| Other liabilities | 1,581 | 414 | ||||||
| Total | $ | 8,791 | $ | 15,743 | ||||
At December 31, 2016, deferred grants consisted of government grants from Montgomery County, Maryland and the state of Maryland related to the move of the Company’s headquarters. At December 31, 2015, deferred grants consisted of a $9.3 million grant from the Japan Agency for Medical Research & Development (“AMED”), for use in developing unoprostone-related medicine for Retinitis Pigmentosa (see below), and a $300,000 government grant from Montgomery County, Maryland related to the move of the Company’s headquarters. Both grants may have to be repaid if certain conditions are not met.
Under its arrangement with AMED, R-Tech received ¥1.05 billion to support the development of unoprostone. This grant would be repayable in full if R-Tech terminated the development of unoprostone for commercial or other reasons, but only in part if such termination was due to scientific failure of the compound. R-Tech discontinued the development of unoprostone in early 2016. At the time of the Company’s acquisition of R-Tech, AMED’s position, on which we relied when accounting for this liability at the time of the R-Tech acquisition, was that the full amount of the grant would be repayable by R-Tech because such termination was for convenience, rather than due to a scientific failure of the compound. In September 2016, however, AMED agreed to waive the repayment of all but approximately ¥105 million (approximately $1.0 million) of the grant, representing 10% of the funds received by R-Tech from AMED through the end of March 2014. The Company recognized approximately $9.3 million in other income for the year ended December 31, 2016 because of this forgiveness of the Company’s repayment obligation.
Defined benefit obligation relates to defined benefit pension plans for employees in the Company’s subsidiary in Switzerland (Swiss Plan). The Swiss Plan is a government-mandated retirement fund that provides employees with a minimum investment return. The minimum investment return is determined annually by the Swiss government and was 1.25% in 2016 and 1.75% in 2015. Under the Swiss Plan, the Company and certain of its employees with annual earnings in excess of government determined amounts are required to make contributions into a fund managed by an independent investment fiduciary. Employer contributions must be in an amount at least equal to the employee’s contribution. Minimum employee contributions are based on the respective employee’s age, salary, and gender. As of December 31, 2016 and 2015, the Swiss Plan had an unfunded net pension obligation of $818,000 and $949,000, respectively, plan assets of $1.7 million and $1.6 million, respectively, and projected benefit obligation of $2.6 million and $2.5 million, respectively. The entire liability is listed as non-current because plan assets are more than enough to pay expected benefit payments over the next year. The Company recognized pension expense of $260,000, $226,000 and $221,000 for the years ended December 31, 2016, 2015 and 2014, respectively, related to the Swiss Plan.
While the Swiss Plan originated in 2011, the Company only accounted for the Swiss Plan in accordance with ASC 715-30 Defined Benefit Plans - Pensions starting in 2014. The Company evaluated the impact of not recording the net pension obligation in the Consolidated Balance Sheet and corresponding charges in Net income and Total comprehensive income in the Statement of Operations and Comprehensive Income, and the omission of the required pension disclosures in prior years, and concluded that the effect was immaterial. The Company corrected the immaterial error in 2014 by recording an out of period adjustment to the net pension obligation liability of $366,000, with an offsetting amount in Net Income of $11,000 and total comprehensive income of $355,000.
| F-27 |
16. Commitments and Contingencies
Operating Leases
The Company leases office space in the U.S., Switzerland and Japan. In Japan, the Company also leases a research and development facility and the land for its manufacturing facility. At December 31, 2016, total future minimum non-cancelable lease payments under operating leases are as follows:
| (In thousands) | ||||
| 2017 | $ | 1,980 | ||
| 2018 | 1,727 | |||
| 2019 | 1,491 | |||
| 2020 | 1,096 | |||
| 2021 | 995 | |||
| Total minimum lease payments | $ | 7,289 | ||
Rent expense for all operating leases was $2.4 million, $1.8 million and $1.4 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Numab Commitment
In July 2016, Numab repaid all outstanding amounts under its loan from Zurcher Kantonalbank, which was guaranteed by the Company under the Numab Agreement (see note 17). As a result, the Company’s liability associated with the Numab Agreement guarantee has been released
CPP
Under the terms of the CPP Securities Agreement (see note 7), the Company provided $5.0 million to CPP in exchange for a convertible note. The convertible note is automatically convertible into securities of CPP, subject to certain limitations, in the event CPP consummates a future financing with aggregate proceeds of at least $10.0 million, exclusive of any investment by the Company, whether through a public offering or a private offering (a “Qualified Financing”). Depending on the timing of the Qualified Financing, the convertible note will automatically convert into the same securities issued in the Qualified Financing at a 10% to 20% discount to the lowest issuance price of the securities in the Qualified Financing. The Company has also agreed to purchase up to $5.0 million of CPP’s securities in any such Qualified Financing.
Research and Development Costs
The Company routinely enters into agreements with third-party CROs to oversee clinical research and development studies provided on an outsourced basis and assist in other research and development activities. The Company generally is not contractually obligated to pay the third party if the service or reports are not provided.
17. Related Party Transactions
R-Tech
Before the R-Tech acquisition, R-Tech was considered a related party. Drs. Ryuji Ueno and Sachiko Kuno were married to each other during such time, and they controlled, directly or indirectly, the majority of the stock of R-Tech. At such time, Drs. Ueno and Kuno also controlled, directly or indirectly, approximately 47% of the Company’s common stock. Dr. Ueno was the Company’s Chief Executive Officer and Chairman of the Company’s Board of Directors through March 3, 2014 and was our Chief Scientific Officer through March 18, 2014.
Prior to the R-Tech acquisition on October 20, 2015 (see note 5), the Company did not own manufacturing facilities. Instead, the Company contracted with R-Tech as the sole manufacturer of the Company’s products to produce AMITIZA and RESCULA. The Company had entered into multiple exclusive supply arrangements with R-Tech and had granted to R-Tech the exclusive right to manufacture and supply AMITIZA and other products and compounds to the Company to meet its commercial and clinical requirements. Since 2003, the Company had received upfront, development and milestone payments under these agreements totaling $9.0 million through October 20, 2015.
| F-28 |
The Company recorded the following expenses under all of its agreements with R-Tech for the period January 1, 2015 through October 20, 2015, and for the year ended December 31, 2014:
| (In thousands) | January 1 through October 20, 2015 | Year Ended December 31, 2014 | ||||||
| Clinical supplies | $ | 155 | $ | 396 | ||||
| Other research and development services | 347 | 171 | ||||||
| Commercial supplies | 21,415 | 15,776 | ||||||
| $ | 21,917 | $ | 16,343 | |||||
Numab AG
In September 2011, the Company entered into a loan guarantee and development agreement (the Numab Agreement) with Numab. Numab is a related party of the Company as a result of the Company hiring as an executive officer an individual who holds an ownership interest in Numab. Under the terms of the Numab Agreement, the Company provided Numab with up to CHF 5.0 million as collateral and served as guarantor for a loan to Numab from a third party, Zurcher Kantonalbank. Following the payment of the first success fee during the first quarter of 2013, this amount was reduced to CHF 2.2 million, or approximately $2.2 million, as of December 31, 2015. As of December 31, 2015, collateral of CHF 2.2 million had been deposited by the Company and Numab had utilized CHF 1.5 million of its loan facility, or approximately $1.5 million. At December 31, 2015, the Company had recorded a guarantee liability of $202,000 in collateral callable to meet a potential loan default by Numab.
In July 2016, Numab repaid all outstanding amounts under its loan from Zurcher Kantonalbank, which was guaranteed by the Company under the Numab Agreement. As a result, the Company’s liability associated with the Numab Agreement guarantee has been released, and all deposited collateral returned.
Subordinated Unsecured Promissory Notes
In connection with the SAG acquisition in 2010, the Company issued a subordinated unsecured promissory note (Notes) to the Ueno Trust and Kuno Trust, former shareholders of SAG. The Ueno Trust and Kuno Trust are considered related parties. Each of the Notes was issued with an initial principal balance of approximately $25.9 million, or approximately $51.9 million in the aggregate. The interest rate for the Notes was the sum of the London Interbank Offered Rate, or LIBOR, plus 4.0%, and was reset on December 1st and June 1st each year. The interest rate beginning December 1, 2015 was 4.7%. On February 1, 2016, these Notes were paid in full.
18. Credit Facility and Convertible Notes Payable
Credit Facility
On October 16, 2015, the Company entered into a Credit Agreement (the “Credit Facility”) with Jefferies Financing LLC. Term Loans under the Credit Facility bore interest, at the Company’s option, at the Adjusted Eurodollar Rate plus 7.25% or the Adjustable Base Rate plus 6.25%. Prior to the repayment in full, the average interest rate on the Term Loans for the period ended December 27, 2016 was 8.54%. The Company was in compliance with all covenants under the Credit Facility in 2016.
On December 27, 2016, concurrent with the Company issuing $300.0 million aggregate principal amount of its 3.25% Convertible Senior Notes due 2021 (see below), this Credit Facility was paid in full, including all accrued but unpaid interest and a prepayment premium of $2.3 million.
Convertible Notes
On December 27, 2016, the Company issued $300.0 million aggregate principal amount of its 3.25% Convertible Senior Notes due 2021 (the “Convertible Notes”) to Leerink Partners LLC (the “Initial Purchaser”), who subsequently resold the Convertible Notes to qualified institutional buyers (the “Note Offering”) in reliance on the exemption from registration provided by Rule 144A under the Securities Act of 1933, as amended (the “Securities Act”). The Company received net proceeds of approximately $290.5 million from the sale of the Convertible Notes, after deducting fees and expenses of approximately $9.5 million. Concurrent with the closing of the Note Offering, the Company used the net proceeds from the Note Offering to repay in full approximately $238.2 million due under the Company’s Credit Facility (see above), including all accrued but unpaid interest and a prepayment premium of $2.3 million. The Company plans to use the remaining net proceeds for general corporate purposes.
The Convertible Notes were issued pursuant to an Indenture, dated as of December 27, 2016 (the “Indenture”), between the Company and U.S. Bank National Association, as trustee. Interest on the Convertible Notes is payable semi-annually in cash in arrears on June 15 and December 15 of each year, beginning on June 15, 2017, at a rate of 3.25% per year. The Convertible Notes mature on December 15, 2021 unless earlier converted or repurchased, are not redeemable prior to the maturity date and no sinking fund is provided for the Convertible Notes.
| F-29 |
The Convertible Notes are convertible at an initial conversion rate of 60.2637 shares of the Company’s Class A common stock (the “Common Stock”) per $1,000 principal amount of the Convertible Notes, subject to adjustment under the Indenture, which is equal to an initial conversion price of approximately $16.59 per share of Common Stock. Upon conversion, the Convertible Notes will be settled in shares of the Company’s Common Stock, together with a cash payment in lieu of delivering any fractional share. The conversion rate will be subject to adjustment in some events but will not be adjusted for any accrued and unpaid interest. In addition, following certain corporate events that occur prior to the maturity date, the Company will increase the conversion rate for a holder who elects to convert its Convertible Notes in connection with such a corporate event in certain circumstances.
If the Company undergoes a “fundamental change” (as defined in the Indenture), holders may require the Company to repurchase for cash all or any portion of their Convertible Notes at a fundamental change repurchase price equal to 100% of the principal amount of the Convertible Notes to be repurchased, plus accrued and unpaid interest to, but excluding, the fundamental change repurchase date.
The Indenture includes customary terms and covenants, including certain events of default after which the Convertible Notes may be due and payable immediately. The following events are considered “events of default,” which may result in acceleration of the maturity of the Convertible Notes:
| · | default in any payment of interest on any Convertible Notes when due and payable and the default continues for a period of 30 days; | |
| · | default in the payment of principal of any Convertible Notes when due and payable at its stated maturity, upon any required repurchase, upon declaration of acceleration or otherwise; | |
| · | the Company’s failure to comply with its obligation to convert the Convertible Notes in accordance with the Indenture upon exercise of a holder’s conversion right and such failure continues for a period of five business days; | |
| · | the Company’s failure to give a fundamental change notice when due; | |
| · | the Company’s failure to comply with its obligations under the Indenture in connection with a consolidation, merger or sale of assets; | |
| · | the Company’s failure for 60 days after written notice from the trustee or the holders of at least 25% in principal amount of the Convertible Notes then outstanding has been received to comply with any of the Company’s other agreements contained in the Convertible Notes or the Indenture | |
| · | default by the Company or any of its significant subsidiaries (as defined in the Indenture) with respect to any mortgage, agreement or other instrument under which there may be outstanding, or by which there may be secured or evidenced, any indebtedness for money borrowed in excess of $10.0 million (or its foreign currency equivalent) in the aggregate of the Company and/or any such subsidiary, whether such indebtedness now exists or shall hereafter be created (i) resulting in such indebtedness becoming or being declared due and payable prior to its maturity date, or (ii) constituting a failure to pay the principal or interest of any such debt when due and payable at its stated maturity, upon required repurchase, upon declaration of acceleration or otherwise, and, in the cases of clauses (i) and (ii), such acceleration shall not have been rescinded or annulled or such failure to pay or default shall not have been cured or waived, or such indebtedness is not paid or discharged, as the case may be, within 30 days after written notice to us by the trustee or to us and the trustee by holders of at least 25% in aggregate principal amount of Convertible Notes then outstanding in accordance with the Indenture | |
| · | certain events of bankruptcy, insolvency, or reorganization of the Company or any of the Company’s significant subsidiaries; or | |
| · | a final judgment or judgments for the payment of $10.0 million (or its foreign currency equivalent) or more (excluding any amounts covered by insurance) in the aggregate rendered against the Company or any of its subsidiaries, which judgment is not discharged, bonded, paid, waived or stayed within 60 days after (i) the date on which the right to appeal thereof has expired if no such appeal has commenced, or (ii) the date on which all rights to appeal have been extinguished. |
As of December 31, 2016, the Company was compliant with all covenants and conditions under the Convertible Notes.
The Company analyzed the terms of the Convertible Notes and determined that under current accounting guidance the Convertible Notes would be entirely accounted for as debt and none of the terms of the Convertible Notes require separate accounting. As part of the issuance of the Convertible Notes, the Company incurred $9.5 million of transaction costs, which are capitalized in the accompanying Consolidated Balance Sheet as deferred financing costs and will be amortized to interest expense ratably over the term of the Convertible Notes.
The Company’s debt is subject to the fair value disclosure requirements as discussed in note 8 and is classified as a Level 2 instrument. The estimated fair value of the notes payable at December 31, 2016 was $319.5 million.
| F-30 |
Notes payable consisted of the following as of December 31, 2016 and 2015:
| December 31, | ||||||||
| (In thousands) | 2016 | 2015 | ||||||
| Credit facility notes payable | $ | - | $ | 252,360 | ||||
| Convertible notes payable | 290,516 | - | ||||||
| $ | 290,516 | $ | 252,360 | |||||
| Notes payable, current | $ | - | $ | 39,083 | ||||
| Notes payable, non-current | 290,516 | 213,277 | ||||||
| $ | 290,516 | $ | 252,360 | |||||
19. Collaboration and License Agreements
North America Takeda Agreement
In October 2004, the Company entered into an agreement with Takeda to supply, develop and commercialize AMITIZA for gastrointestinal indications in the U.S. and Canada. The original agreement was amended on February 1, 2006 through a supplemental agreement, and in October 2014 the Company and Takeda and certain Takeda affiliates executed amendments to the agreement. Collectively, these are referred to as the North America Takeda Agreement. Payments to the Company under these agreements include a non-refundable upfront payment, non-refundable development and commercial milestone payments, reimbursement of certain development and co-promotion costs, and product royalties.
The Company has received a total of $160.0 million in upfront and development milestone payments through December 31, 2016 under the North America Takeda Agreement. Subject to development and acceptance of future indications, the Company is potentially entitled to receive additional development milestone and commercial milestone payments under the North America Takeda Agreement, although there can be no assurance that the Company will receive any such payments.
Upon execution of the North America Takeda Agreement, the Company was required to complete several deliverables, which Takeda was responsible to fund. The following are the required deliverables of the Company, along with the related contractual cash flows from Takeda and the associated obligations and performance period of the Company relating to research and development revenue:
| · | Upon receipt of the $20.0 million upfront payment, the Company deferred $2.4 million to be recognized using the time-based model over the performance period of the participation in various joint committee meetings. The Company expects its participation on all committees to continue throughout the term of the North America Takeda Agreement. During each of the years ended December 31, 2016, 2015 and 2014, the Company recognized $147,000 of this deferred amount as collaboration revenue on the Consolidated Statements of Operations and Comprehensive Income. | |
| · | The Company granted Takeda an exclusive license for lubiprostone to co-develop, commercialize, and sell products for gastroenterology indications in the U.S. and Canada. The Company has recorded product royalty revenue of $82.3 million, $74.0 million and $62.8 million the years ended December 31, 2016, 2015 and 2014, respectively. This revenue is recorded as product royalty revenue in the Consolidated Statements of Operations and Comprehensive Income. |
| · | The Company has provided development work necessary for an NDA submission to the FDA for the treatment of CIC and IBS-C indications. Takeda funded the initial $30.0 million of development costs, the Company was obligated to fund the first $20.0 million in excess of the initial $30.0 million funded by Takeda and the two parties are to equally share any required development costs in excess of $50.0 million. Although there was no defined performance period for this development work, the period to perform the work would not exceed the term of the North America Takeda Agreement. In January 2006, the Company received approval for its NDA for AMITIZA to treat CIC and completed and submitted the supplemental NDA for IBS-C to the FDA in June 2007. |
| · | In conjunction with the R-Tech acquisition in October 2015, the Company now recognizes product sales for the supply of product under the North America Takeda Agreement. The Company recorded product sales revenue of $45.2 million and $10.3 million for the years ended December 31, 2016 and 2015. |
The Company initially deferred the residual amount of the $20.0 million upfront payment totaling $17.6 million, development milestone payments received totaling $50.0 million, and reimbursement of the initial $30.0 million of research and development costs for the development of AMITIZA for CIC and IBS-C indications. These deferred amounts were applied towards the unit of accounting that combines the participation in the joint development committee and the development of CIC and IBS-C and was recognized over the performance period of developing the CIC and IBS-C NDA submissions. The Company completed the development of the CIC and IBS-C in June 2007 and filed a sNDA for IBS-C. This was the culmination of the performance period. In June 2007, the Company also recognized as revenue, in full, $30.0 million from Takeda upon the filing of the sNDA for AMITIZA to treat IBS-C. The Company received a $50.0 million development milestone payment from Takeda as a result of the FDA’s approval on April 29, 2008 of the sNDA for IBS-C in women aged 18 years and older and recognized the payment as research and development revenue during the year ended December 31, 2008.
| F-31 |
During 2006, the joint commercialization committee granted approval for the Company and Takeda to begin three new studies. The following are the three additional deliverables of the Company, along with the related contractual cash flows from Takeda and the associated obligations and performance period of the Company, when the three studies were agreed upon:
| · | The Company is obligated to perform studies in connection with changes to labeling for CIC. Takeda is obligated to fund 70% of the labeling studies and the Company is obligated to fund the remaining 30%. There is no defined performance period, but the performance period will not exceed the term of the North America Takeda Agreement. |
| · | The Company is obligated to perform studies for the development of an additional indication for OIC. Takeda is obligated to fund all development work up to a maximum aggregate of $50.0 million for each additional indication and $20.0 million for each new formulation. If development costs exceed these amounts, Takeda and the Company shall equally share such excess costs. There is no defined performance period, but the performance period will not exceed the term of the North America Takeda Agreement. The Company decided to conduct one additional phase 3 efficacy studies in order to submit a sNDA for the OBD indication. In February 2012, the Company announced that lubiprostone met the primary endpoint in a phase 3 clinical trial for the treatment of OBD in patients with chronic, non-cancer pain, excluding those taking methadone. |
| · | The Company is obligated to perform all development work necessary for phase 4 studies, for which Takeda is obligated to fund all development work. There is no defined performance period, but the performance period will not exceed the term of the North America Takeda Agreement. |
The Company has assessed these required deliverables to determine which deliverables are considered separate units of accounting. As a result of the Company and Takeda agreeing to perform and fund these studies simultaneously, the Company determined that there is no objective and reliable evidence to determine the fair value for each of the studies. Accordingly, the Company has combined these three required deliverables as a single unit of accounting. All cash payments from Takeda related to these three deliverables are deferred upon receipt and recognized over the estimated performance period to complete the three studies using the time-based model.
In 2011, the Joint Commercialization Committee (JCC) granted approval to begin studies for a liquid formulation. In addition, in 2012, the JCC granted approval for studies for a pediatric dosage. These additional deliverables are considered separate units of accounting and the Company recognizes revenue from Takeda reimbursements for these deliverables when earned.
Co-promotion costs after May 31, 2011 were reimbursed under the Takeda Agreement through 2014. The Company recognized $3.4 million of revenues for the year ended December 31, 2014, which is recorded as co-promotion revenue in the Consolidated Statements of Operations and Comprehensive Income.
The Company has assessed these required deliverables to determine which deliverables are considered separate units of accounting. The Company determined that its sales force and miscellaneous marketing activities are treated as separate units of accounting. The Company is recognizing the cost reimbursements received for these deliverables as co-promotion revenues when services are performed and the reimbursement payments are due under the Supplemental Takeda Agreement.
Global Takeda Agreement
In October 2014, the Company and Takeda entered the Global Takeda Agreement to develop and commercialize AMITIZA. The territories excluded from the Global Takeda Agreement are Canada, the U.S., Japan and the People’s Republic of China. Canada and the U.S. are covered by the North America Takeda Agreement, and Japan is covered by the Japan Mylan Agreement. Switzerland and the U.K. have already received regulatory approval for AMITIZA to be marketed and sold. All other territories covered under the Global Takeda Agreement will need to have regulatory approval before AMITIZA can be sold.
Under the terms of the Global Takeda Agreement, the Company supplies Takeda with AMITIZA at a negotiated supply price. The Company also received a nonrefundable upfront payment of $14.0 million from Takeda for exclusive rights to develop and commercialize AMITIZA in the global markets covered by the Global Takeda Agreement. In addition, the Company is also eligible for up to $35.0 million in additional commercial milestone payments contingent on the achievement of certain net sales revenue targets. Takeda is responsible for all development activities and costs, except that the Company assumed responsibility for the first $6.0 million of those development expenses incurred by Takeda. The Company’s financial obligations under this agreement were fulfilled in 2016.
The Global Takeda Agreement is considered a multiple-element arrangement for accounting purposes. The Company identified the rights to use the Company’s license to develop and commercialize AMITIZA and the sale of AMITIZA product at a negotiated price as the deliverables. During the fourth quarter of 2014, the Company received a $14.0 million milestone payment and allocated $8.0 million to the right to use the license and $6.0 million to a collaboration obligation to reimburse Takeda for the first $6.0 million in developmental expenses incurred. The Company’s financial obligations under this agreement were fulfilled in 2016.
| F-32 |
The Company recognized product sales to Takeda under the Global Takeda Agreement of $792,000, $0 and $0 during 2016, 2015 and 2014, respectively.
Japan Mylan Agreement
In February 2015, Abbott and Mylan closed Mylan’s purchase of Abbott’s non-U.S. developed markets specialty and branded generics business, which included the Company’s February 2009 Japan Mylan Agreement to develop and commercialize lubiprostone for the treatment of CIC in Japan. The Company did not experience any significant changes in the commercialization of AMITIZA in Japan as a result of the transfer of the Japan Mylan Agreement from Abbott to Mylan.
The Japan Mylan Agreement grants Mylan the right of exclusive negotiation to any additional indications for which lubiprostone is developed in Japan under all relevant patents, know-how and trademarks. Under the terms of the Japan Mylan Agreement, payments to the Company include sales of product at a negotiated sales price, a non-refundable upfront payment and non-refundable development and commercial milestone payments based on achieving specified development, regulatory and sales goals.
The collaboration efforts under the agreement are governed by two committees consisting of an equal number of representatives from both parties. The joint commercialization and steering committee oversees commercialization-related activities and resolves any conflicts arising from a joint development committee, which oversees the development-related activities in Japan.
The Company is required to fund and complete all the development work including additional clinical studies required to obtain regulatory approval for the treatment of CIC in Japan. The Company completed all development activities in the fourth quarter of 2012. The Company owns all the rights covered under the regulatory filings.
Mylan is required to fund and undertake all commercialization efforts including pre-launch and post-launch marketing, promotion and distribution. Mylan is required to maintain the number of sales staff and the estimated level of annual net sales based on the commercialization plan to be developed and approved by the joint commercialization and steering committee described above.
The Company recorded product sales revenue under the Japan Mylan Agreement of $72.7 million, $53.9 million and $32.1 million for the years ended December 31, 2016, 2015 and 2014, which includes $10.0 million, $5.0 million and $2.5 million in net sales milestone payments in 2016, 2015 and 2014, respectively. As of December 31, 2016, the Company had received a total of $47.5 million in up-front and development milestone payments under the Japan Mylan Agreement. Under the Japan Mylan Agreement, the Company could receive additional milestone payments based on achieving other specified development and commercialization goals although there can be no assurance that the Company will receive any such payments.
China Gloria Agreement
In May 2015, the Company entered into an exclusive license, development, commercialization and supply agreement (China Gloria Agreement), for AMITIZA in the People’s Republic of China. The China Gloria Agreement is effective until the thirteenth anniversary of the effective date and will automatically renew for successive three year periods unless terminated upon one year’s prior written notice by one of the parties. Under the terms of the China Gloria Agreement:
| · | The Company received an upfront payment of $1.0 million from Gloria during May 2015 and an upfront payment of $500,000 in June 2015 after the CFDA accepted the IND application for a pivotal trial of AMITIZA in patients with CIC. |
| · | The Company is eligible to receive an additional payment in the amount of $1.5 million upon the occurrence of a specified regulatory or commercial milestone event. |
| · | Gloria is responsible for all development activities and costs, as well as commercialization and regulatory activities, for AMITIZA in the People’s Republic of China. |
| · | The Company will be the exclusive supplier of AMITIZA to Gloria at an agreed upon supply price. |
| · | The Company has no recorded product sales revenue under the China Gloria Agreement for 2016 and 2015. |
RESCULA Agreement
Japan Santen Agreement
In March 2012, R-Tech entered into an exclusive transaction agreement (“Japan Santen Agreement”) with Santen Pharmaceutical Co. Ltd (“Santen”) to commercialize RESCULA in Japan. The initial term of the Japan Santen Agreement ended on March 31, 2016 but was automatically extended through March 31, 2017. Under the terms of the agreement the contract is automatically extended for successive one-year renewal terms unless either party gives the other party an 11-month notice, which to date has not occurred. Under the terms of the Japan Santen Agreement the Company recognizes revenue from the product sales of RESCULA to Santen at a negotiated price. For the years ended December 31, 2016 and 2015, the Company recorded RESCULA product sales revenue under the Japan Santen Agreement of $9.9 million and $1.5 million, respectively.
| F-33 |
20. Stockholders’ Equity
Capital Structure
The Company has two classes of common stock authorized: class A common stock and class B common stock. In 2012, the company’s majority stockholder and only holder of the Company’s class B common stock converted all its outstanding shares of class B common stock into shares of the Company’s class A common stock. The Company is not authorized to issue additional shares of class B common stock except in limited circumstances. Because of the conversion, there is now only a single class of outstanding common stock, class A common stock, which is entitled to one vote per share.
Treasury Stock
Between 2008 and 2013, the Company implemented a stock repurchase program under which the Company purchased a total of 524,792 shares of its class A common stock in open-market transactions. These shares were added to Treasury Stock on the Company’s consolidated balance sheet
On October 20, 2015, as part of the R-Tech acquisition, the Company acquired 2,485,150 shares of the Company’s class A common stock, which shares were added to Treasury Stock on the Company’s consolidated balance sheet. As part of the R-Tech purchase price allocation, $44.0 million was allocated to these shares based on the October 20, 2015 closing price of $17.68 per share.
Employee Stock-Based Compensation
The Company accounts for stock-based compensation using the fair value method. The fair value of awards granted is estimated at the date of grant and recognized as expense on a straight-line basis over the requisite service period with the offsetting credit to additional paid-in capital. For awards with performance conditions, the Company recognizes the compensation expense if and when the Company concludes that it is probable that the performance condition will be achieved. The Company reassesses the probability of achieving the performance condition at each reporting date. The assumptions used to estimate the fair value of stock options granted for the years ended December 31, 2016, 2015 and 2014 were as follows:
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Expected volatility | 54% | - | 56% | 54% | - | 70% | 70% | - | 72% | |||
| Risk-free interest rate | 1.28% | - | 1.57% | 1.34% | - | 1.93% | 1.63% | - | 2.01% | |||
| Expected term (in years) | 6.08 | - | 6.25 | 5.28 | - | 6.25 | 5.28 | - | 6.25 | |||
| Expected dividend yield | 0% | 0% | 0% | |||||||||
Employee stock-based compensation expense for the years ended December 31, 2016, 2015 and 2014 has been reduced for estimated forfeitures as such expense is based upon awards expected to ultimately vest. Estimated forfeiture rates used during the years ended December 31, 2016, 2015 and 2014 ranged from 10.2% to 30.9%.
Employee stock-based compensation expense recorded in the Company’s Consolidated Statements of Operations and Comprehensive Income for the years ended December 31, 2016, 2015 and 2014 was as follows:
| Year Ended December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| Research and development expense | $ | 2,136 | $ | 2,165 | $ | 349 | ||||||
| General and administrative expense | 5,028 | 5,053 | 1,816 | |||||||||
| Selling and marketing expense | 119 | 131 | 122 | |||||||||
| Total | $ | 7,283 | $ | 7,349 | $ | 2,287 | ||||||
| F-34 |
Stock Option Plans
Amended and Restated 2001 Stock Incentive Plan
In 2001, the Company adopted the 2001 Stock Incentive Plan (“2001 Plan”) to provide common stock incentives to certain eligible employees, officers, directors, consultants and advisors of the Company. In 2003, the Board amended the 2001 Plan (“Amended 2001 Plan”) to allow for a maximum of 8,500,000 shares of class A common stock to be issued under all awards. At December 31, 2016, there were no shares available for future grants under this plan.
Employee options outstanding and exercisable under the Amended 2001 Plan at December 31, 2016 and 2015 totaled zero and 37,400, respectively, with a remaining contractual life of zero and 0.34 years, respectively. During 2016 and 2015, 20,400 and 76,500 options were exercised, respectively, with an aggregate intrinsic value of $90,000 and $0.6 million, respectively. The Company received $0.2 million and $0.8 million upon the exercise of these options in 2016 and 2015, respectively.
A summary of the employee stock option activity for the year ended December 31, 2016 under the Amended 2001 Plan is presented below:
| 2001 Stock Incentive Plan | Shares | Weighted Average Exercise Price Per Share | Weighted Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | ||||||||||||
| Options outstanding, December 31, 2015 | 37,400 | $ | 10.00 | |||||||||||||
| Options exercised | (20,400 | ) | $ | 10.00 | ||||||||||||
| Options expired | (17,000 | ) | $ | 10.00 | ||||||||||||
| Options outstanding, December 31, 2016 | - | - | ||||||||||||||
| Options exercisable, December 31, 2016 | - | - | ||||||||||||||
| Options vested and expected to vest, December 31, 2016 | - | - | ||||||||||||||
2006 Stock Incentive Plan
In 2006, the Board approved the 2006 Stock Incentive Plan, which has been amended and restated (as amended and restated, “2006 Plan”), and reserved 8,500,000 shares of class A common stock for issuance under the 2006 Plan. Option awards under the 2006 Plan are granted with an exercise price equal to the closing market price of the Company’s stock on the date of grant. The options generally vest over four years and have a ten-year contractual term. The 2006 Plan expired in 2016 and was succeeded by the 2016 Equity Incentive Plan (see below). At December 31, 2016, there were no shares available for future grants under this plan.
The 2006 Plan included an “evergreen” provision by which the number of shares of the Company’s class A common stock available for issuance increased automatically on the first day of each calendar year by 5.0% of the aggregate number of shares of the Company’s class A common stock outstanding on such date, or such lesser number as the Board may determine. The Board determined that the amount of the increase in the shares available for issuance under the 2006 Plan as of January 1, 2016, and 2015 pursuant to the “evergreen” provision was 2,275,458 and zero, respectively.
A summary of the employee stock option activity for the year ended December 31, 2016 under the 2006 Plan is presented below:
| 2006 Stock Incentive Plan | Shares | Weighted Average Exercise Price Per Share | Weighted Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | ||||||||||||
| Options outstanding, December 31, 2015 | 4,440,608 | $ | 9.37 | |||||||||||||
| Options granted | 1,266,050 | $ | 13.60 | |||||||||||||
| Options exercised | (862,740 | ) | $ | 6.71 | ||||||||||||
| Options forfeited | (134,379 | ) | $ | 13.05 | ||||||||||||
| Options expired | (60,990 | ) | $ | 14.49 | ||||||||||||
| Options outstanding, December 31, 2016 | 4,648,549 | $ | 10.86 | 8.0 | $ | 14,770,043 | ||||||||||
| Options exercisable, December 31, 2016 | 1,729,000 | $ | 8.73 | 7.0 | $ | 9,017,049 | ||||||||||
| Options vested and expected to vest, December 31, 2016 | 3,643,698 | $ | 10.50 | 7.8 | $ | 12,774,846 | ||||||||||
| F-35 |
Time-based stock options granted in 2016 totaled 1,266,050. These options vest in equal annual installments over four years from date of grant, and expire ten years from date of grant.
The weighted average grant date fair value of options granted during the years ended December 31, 2016, 2015 and 2014 were $7.10, $9.45 and $4.86, respectively. As of December 31, 2016, $9.8 million of total unrecognized compensation costs, net of estimated forfeitures, related to non-vested awards are expected to be recognized over a weighted average period of 2.4 years. When an option is exercised, the Company issues a new share of class A common stock. During 2016 and 2015, 862,740 options and 693,077 options were exercised, respectively, with an aggregate intrinsic value of $7.1 million and $8.6 million, respectively. The Company received $5.8 million and $4.1 million upon the exercise of these options in 2016 and 2015, respectively.
2016 Equity Incentive Plan
In 2016, the Board approved the 2016 Equity Incentive Plan (“2016 Plan”), and reserved up to 10,740,125 shares of class A common stock for issuance under the 2016 Plan. The 2016 Plan is intended to be the successor to the 2006 Plan, which expired in 2016. Option awards under the 2016 Plan are granted with an exercise price equal to the closing market price of the Company’s stock on the date of grant. The options generally vest over four years and have a ten-year contractual term. At December 31, 2016, there were 5,170,312 shares available for future grants under this plan.
A summary of the employee stock option activity for the year ended December 31, 2016 under the 2016 Plan is presented below:
| 2016 Equity Incentive Plan | Shares | Weighted Average Exercise Price Per Share | Weighted Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | ||||||||||||
| Options outstanding, December 31, 2015 | - | |||||||||||||||
| Options granted | 75,814 | $ | 12.58 | |||||||||||||
| Options exercised | (1,064 | ) | $ | 6.75 | ||||||||||||
| Options expired | - | |||||||||||||||
| Options outstanding, December 31, 2016 | 74,750 | $ | 12.66 | 5.0 | $ | 81,741 | ||||||||||
| Options exercisable, December 31, 2016 | 36,700 | $ | 13.97 | 0.1 | $ | 0 | ||||||||||
| Options vested and expected to vest, December 31, 2016 | 55,956 | $ | 13.09 | 3.4 | $ | 41,367 | ||||||||||
Time-based stock options granted in 2016 totaled 75,814. These options primarily vest ratably over four years from date of grant, and expire ten years from date of grant.
The weighted average grant date fair value of options granted during the year ended December 31, 2016 was $3.51. As of December 31, 2016, $108,000 of total unrecognized compensation costs, net of estimated forfeitures, related to non-vested awards are expected to be recognized over a weighted average period of 3.8 years. When an option is exercised, the Company issues a new share of class A common stock. During 2016, 1,064 options were exercised. The aggregate intrinsic value and amount received by the Company upon the exercise of these options were insignificant.
A summary of restricted stock unit activity for the year ended December 31, 2016 under the Company’s 2016 Plan is presented below:
| 2016 Equity Incentive Plan | Shares | Weighted Average Grant Date Fair Value | ||||||
| Nonvested Restricted Stock Units, December 31, 2015 | - | - | ||||||
| Restricted Stock Units granted | 63,700 | $ | 12.29 | |||||
| Restricted Stock Units vested | - | - | ||||||
| Restricted Stock Units forfeited | - | - | ||||||
| Nonvested Restricted Stock Units, December 31, 2016 | 63,700 | $ | 12.29 | |||||
Restricted stock units awarded to members of our board of directors vest ratably over one to three years from date of grant.
| F-36 |
As of December 31, 2016, $348,000 of total unrecognized compensation costs, net of estimated forfeitures, related to non-vested restricted stock units are expected to be recognized over a weighted average period of 1.2 years. When a restricted stock unit vests, the Company issues a new share of class A common stock. During 2016, no restricted stock units vested.
Employee Stock Purchase Plan
In 2006, the Board approved the 2006 Employee Stock Purchase Plan. On December 8, 2016, the Board amended and restated the 2006 Employee Stock Purchase Plan (as amended and restated, “ESPP”) and reserved 500,000 shares of class A common stock for issuance under the ESPP. The ESPP is non-compensatory and is intended to qualify as an Employee Stock Purchase Plan as defined in Section 423 of the Internal Revenue Code of 1986, as amended. Under the ESPP, eligible employees may purchase common stock through payroll deductions up to 15% of compensation during the plan period, which is generally one year with four, three-month purchase periods. In January 2015, the Board approved a purchase price of 85% of the lower of market price on the offering date or the purchase date. The following table summarizes the ESPP activity:
| Years Ended December 31, | ||||||||||||
| (In thousands, except share amounts) | 2016 | 2015 | 2014 | |||||||||
| Shares issued under the ESPP | 22,395 | 9,085 | 5,853 | |||||||||
| Cash received under the ESPP | $ | 213 | $ | 128 | $ | 35 | ||||||
Tax Benefits
As of December 31, 2016, the balance of the Company's additional paid-in capital pool related to tax windfall benefits from the stock option exercises was $3.8 million.
The Company applies a with-and-without approach in determining its intra-period allocation of tax expense or benefit attributable to stock based compensation deductions. Since the Company does not have any net operating loss carry-forwards in the U.S., the tax benefit reduces income taxes payable in the current year and is therefore recorded to additional paid-in-capital.
Accumulated Other Comprehensive Income (Loss)
The following table details the accumulated other comprehensive income (loss) activity for the years ended December 31, 2016, 2015, and 2014:
| (In thousands) | Foreign currency translation adjustments | Unrealized income (loss) on investments, net of tax effect | Unrealized income (loss) on pension benefit obligation | Accumulated other comprehensive income (loss) | ||||||||||||
| Balance at December 31, 2013 | $ | 15,559 | $ | 42 | $ | - | $ | 15,601 | ||||||||
| Other comprehensive loss before reclassifications | (351 | ) | (7 | ) | (978 | ) | (1,336 | ) | ||||||||
| Amounts reclassified from accumulated other comprehensive income (loss) | - | - | - | - | ||||||||||||
| Balance at December 31, 2014 | $ | 15,208 | $ | 35 | $ | (978 | ) | $ | 14,265 | |||||||
| Other comprehensive income (loss) before reclassifications | (965 | ) | 7 | 105 | (853 | ) | ||||||||||
| Amounts reclassified from accumulated other comprehensive income (loss) | - | - | - | - | ||||||||||||
| Balance at December 31, 2015 | $ | 14,243 | $ | 42 | $ | (873 | ) | $ | 13,412 | |||||||
| Other comprehensive income before reclassifications | 40,876 | - | 239 | 41,115 | ||||||||||||
| Amounts reclassified from accumulated other comprehensive income (loss) | - | - | - | - | ||||||||||||
| Balance at December 31, 2016 | $ | 55,119 | $ | 42 | $ | (634 | ) | $ | 54,527 | |||||||
21. Income Taxes
Income before income taxes for the years ended December 31, 2016, 2015 and 2014 was as follows:
| Year Ending December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| U.S. | $ | 1,153 | $ | 14,685 | $ | 18,005 | ||||||
| Foreign | 13,222 | 28,990 | 9,128 | |||||||||
| Total income before income taxes | $ | 14,375 | $ | 43,675 | $ | 27,133 | ||||||
| F-37 |
The provision for income taxes consisted of the following for the years ended December 31, 2016, 2015 and 2014:
| Year Ended December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| Current tax provision | ||||||||||||
| U.S. Federal | $ | 9,749 | $ | 15,678 | $ | 11,319 | ||||||
| U.S. State | 334 | 1,810 | 1,351 | |||||||||
| Foreign | 21,123 | 2,709 | 793 | |||||||||
| Total current tax provision | 31,206 | 20,197 | 13,463 | |||||||||
| Deferred provision (benefit): | ||||||||||||
| U.S. Federal | (5,062 | ) | (6,375 | ) | (172 | ) | ||||||
| U.S. State | (340 | ) | (899 | ) | (16 | ) | ||||||
| Foreign | (29,916 | ) | (2,619 | ) | 730 | |||||||
| Total deferred provision (benefit) | (35,318 | ) | (9,893 | ) | 542 | |||||||
| Total income tax provision (benefit) | $ | (4,112 | ) | $ | 10,304 | $ | 14,005 | |||||
Deferred tax assets (liabilities), net, consisted of the following as of December 31, 2016 and 2015:
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| (In thousands) | ||||||||
| Deferred tax assets: | ||||||||
| Foreign net operating loss carry-forwards | $ | 309 | $ | 716 | ||||
| State net operating loss carry-forwards | 279 | 2 | ||||||
| Tax credit carry-forwards | 4,694 | 976 | ||||||
| Deferred revenue | 370 | 446 | ||||||
| Accrued expenses | 2,517 | 1,450 | ||||||
| Tax benefits on stock options | 3,870 | 3,288 | ||||||
| Research and development credits | 469 | 1,759 | ||||||
| Deductible state taxes | 1,351 | - | ||||||
| Other | 601 | 3,775 | ||||||
| Gross deferred tax assets | 14,460 | 12,412 | ||||||
| Deferred tax liabilities: | ||||||||
| Property and equipment | (1,000 | ) | (654 | ) | ||||
| Inventory | - | (3,990 | ) | |||||
| Investments | - | (13,963 | ) | |||||
| Intangibles | (34,046 | ) | (44,025 | ) | ||||
| Gross deferred tax liabilities | (35,046 | ) | (62,632 | ) | ||||
| Less: valuation allowance | (704 | ) | (2,277 | ) | ||||
| Net deferred tax assets (liabilities) | $ | (21,290 | ) | $ | (52,497 | ) | ||
| F-38 |
The provision for income taxes varied from the income taxes provided based on the federal statutory rate as follows for the years ended December 31, 2016, 2015 and 2014:
| Year Ended December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| Federal tax at statutory rates | 35.0 | % | 35.0 | % | 35.0 | % | ||||||
| State taxes, net of federal tax benefit | -0.4 | % | 0.9 | % | 2.1 | % | ||||||
| Foreign tax rate differential | -83.2 | % | -16.8 | % | -3.7 | % | ||||||
| Changes in valuation allowance | -0.4 | % | 0.5 | % | 1.4 | % | ||||||
| Nondeductible expenses | 0.0 | % | 2.3 | % | 1.7 | % | ||||||
| Stock based compensation | 2.3 | % | 0.6 | % | -0.1 | % | ||||||
| Impact of intangible transfer | 11.2 | % | 0.7 | % | 5.8 | % | ||||||
| Impact of uncertain tax positions | 3.0 | % | 1.8 | % | -0.2 | % | ||||||
| Adjustment to deferred tax asset | 3.1 | % | -1.6 | % | 0.5 | % | ||||||
| Exchange gain/(loss) | -13.5 | % | 0.0 | % | 0.0 | % | ||||||
| Subpart F income, net of FTC | 11.5 | % | 2.3 | % | 9.1 | % | ||||||
| Loan forgiveness | 0.0 | % | -2.3 | % | 0.0 | % | ||||||
| Japanese R&D credit | -4.6 | % | -1.6 | % | 0.0 | % | ||||||
| Impact of subsidiary wind up | -11.2 | % | 0.0 | % | 0.0 | % | ||||||
| Deductible and withholding taxes | 3.2 | % | 0.0 | % | 0.0 | % | ||||||
| Nondeductible interest | 13.4 | % | 0.0 | % | 0.0 | % | ||||||
| Changes in other tax matters | 2.0 | % | 1.8 | % | 0.0 | % | ||||||
| Effective tax rate | -28.6 | % | 23.6 | % | 51.6 | % | ||||||
The significant decrease in the net deferred tax liability shown above was primarily a result of the amortization of intangibles and the repurchase of intercompany treasury stock.
At December 31, 2016 and 2015, the Company had foreign net operating loss carry-forwards of $1.8 million and $2.8 million, respectively. The foreign NOLs in existence at December 31, 2016 do not expire. As of December 31, 2016 and 2015, the Company had no material NOLs in the U.S. At December 31, 2016 and 2015, the Company had tax credit carry-forwards of $4.7 million and $1.0 million, respectively. These credit carry-forwards are scheduled to expire in 2025 and 2026.
As of December 31, 2016 and 2015, the Company had a valuation allowance on its deferred tax assets of $0.7 million and $2.3 million, respectively. The change in the valuation allowance was primarily due to adjustments to tax attributes in the U.S. and enacted statutory tax rate changes in the United Kingdom.
The remaining valuation allowance at December 31, 2016 and 2015 relates to deferred tax assets in the foreign jurisdictions. The Company will continue to evaluate its valuation allowance position in each jurisdiction on a regular basis. To the extent the Company determines that all or a portion of its valuation allowance is no longer necessary, the Company will recognize an income tax benefit in the period such determination is made for the reversal of the valuation allowance. Once the valuation allowance is eliminated in whole or in part, it will not be available to offset the Company’s future tax provision.
The Company has recorded a total income tax liability for uncertain tax positions (including interest) of approximately $4.1 million and $3.1 million, as of December 31, 2016 and 2015, respectively. The Company presently does not expect to settle any of this amount within the next twelve months in cash and as such, has reflected the entire balance as other liabilities in the accompanying Consolidated Balance Sheets. The amount represents the aggregate tax effect of differences between tax return positions and the amounts otherwise recognized in the Company’s Consolidated Financial Statements. The liability for uncertain tax positions as of December 31, 2016 and 2015 mainly pertains to the Company’s interpretation of nexus in certain states related to revenue sourcing for state income tax purposes, as well as uncertain tax positions related to accrued expense items in the U.S. and related party interest in foreign jurisdictions.
A reconciliation of the beginning and ending amount of unrecognized tax benefits, excluding interest and penalties, for the years ended December 31, 2016, 2015 and 2014 is as follows:
| Year Ended December 31, | ||||||||||||
| (In thousands) | 2016 | 2015 | 2014 | |||||||||
| Balance at January 1 | $ | 2,992 | $ | 712 | $ | 550 | ||||||
| Increases for tax positions taken during prior periods | 38 | 452 | (91 | ) | ||||||||
| Decreases in unrecognized tax benefits related to taxing authority correspondence | - | (280 | ) | - | ||||||||
| Increases for tax positions taken during current period | 195 | 2,108 | 253 | |||||||||
| Balance at December 31 | $ | 3,225 | $ | 2,992 | $ | 712 | ||||||
| F-39 |
The Company recognizes interest and penalties related to uncertain tax positions as a component of the income tax provision. During 2016, the Company recorded $0.5 million of interest and penalties related to uncertain tax positions. During 2015 and 2014, the Company recorded an immaterial amount of interest and penalties related to uncertain tax positions. Of the unrecognized tax benefits noted above, approximately $1.7 million would impact the effective tax rate if a future change were to occur. It is reasonably possible that the $1.6 million of the liability for unrecognized tax benefits will decrease within the next 12 months.
Currently tax years 2012 to 2015 remain open and subject to examination in the major tax jurisdictions in which tax returns are filed. The tax years 2009-2011 were examined by the U.S. tax authorities and resulted in no tax adjustments.
22. Quarterly Financial Data (unaudited)
| 2016 Quarters Ended | ||||||||||||||||
| (In thousands, except per share data) | December 31 | September 30 | June 30 | March 31 | ||||||||||||
| Total revenues | $ | 73,024 | $ | 57,873 | $ | 51,951 | $ | 47,208 | ||||||||
| Income (loss) from operations | 33,493 | 13,268 | 7,618 | (503 | ) | |||||||||||
| Net income (loss) | 15,283 | 8,092 | (832 | ) | (4,057 | ) | ||||||||||
| Net income (loss) per share: | ||||||||||||||||
| Basic | $ | 0.36 | $ | 0.19 | $ | (0.02 | ) | $ | (0.10 | ) | ||||||
| Diluted | 0.34 | 0.19 | (0.02 | ) | (0.10 | ) | ||||||||||
| 2015 Quarters Ended | ||||||||||||||||
| (In thousands, except per share data) | December 31 | September 30 | June 30 | March 31 | ||||||||||||
| Total revenues | $ | 55,368 | $ | 33,448 | $ | 34,884 | $ | 29,480 | ||||||||
| Income from operations | 11,568 | 11,657 | 11,580 | 9,654 | ||||||||||||
| Net income | 10,151 | 7,236 | 9,576 | 6,408 | ||||||||||||
| Net income per share: | ||||||||||||||||
| Basic | $ | 0.24 | $ | 0.16 | $ | 0.21 | $ | 0.14 | ||||||||
| Diluted | 0.23 | 0.16 | 0.21 | 0.14 | ||||||||||||
Net income per share is computed independently for each of the quarters presented. Therefore, the sum of the quarterly net income per share information may not equal annual net income per share.
23. Subsequent Event
On March 2, 2017, the Company received a Paragraph IV certification notice letter (Notice Letter) regarding an Abbreviated New Drug Application (ANDA) submitted to the FDA by Amneal Pharmaceuticals (Amneal) requesting approval to market, sell and use a generic version of the 8 mcg and 24 mcg AMITIZA® (lubiprostone) soft gelatin capsule products. The Company is currently reviewing the Notice Letter. By statute, if the Company initiates a patent infringement lawsuit against Amneal within 45 days of the notice date, the FDA would automatically stay approval of the Amneal ANDA until the earlier of 30 months from the notice date or entry of a district court decision finding the patents invalid or not infringed. AMITIZA is currently protected by 15 issued patents that are listed in the FDA’s Orange Book, with the latest expiring in 2027.
| F-40 |
Schedule II – Valuation and Qualifying Accounts
| Additions | ||||||||||||||||
| Balance at | Charged to | |||||||||||||||
| Beginning | Costs and | Balance at | ||||||||||||||
| (In thousands) | of Year | Expenses | Deductions | End of Year | ||||||||||||
| Allowance for doubtful accounts: | ||||||||||||||||
| 2014 | $ | 440 | $ | 364 | (a) | $ | (779 | )(b) | $ | 25 | ||||||
| 2015 | 25 | 29 | - | 54 | ||||||||||||
| 2016 | 54 | - | (54 | ) | - | |||||||||||
| Valuation allowance for deferred tax assets: | ||||||||||||||||
| 2014 | $ | 1,751 | $ | 381 | (d) | $ | - | $ | 2,132 | |||||||
| 2015 | 2,132 | 1,177 | (e) | (1,032 | )(c) | 2,277 | ||||||||||
| 2016 | 2,277 | - | (1,573 | )(f) | 704 | |||||||||||
| (a) | In 2014, the increase in allowance for doubtful accounts is associated with certain disputed Takeda invoices. |
| (b) | In 2014 the deduction from allowance for doubtful accounts resulted from a charge-off of certain disputed Takeda invoices. |
| (c) | In 2015, the net decrease in valuation n allowance for deferred tax assets of $1.0 million was due primarily to the release of the valuation allowance in certain jurisdictions that management believes the deferred tax assets are more likely than not to be utilized |
| (d) | The net increase of $381,000 was primarily due to the additional NOL’s in foreign jurisdictions where management believes it is more likely than not a portion of the NOL balance will expire prior to utilization. |
| (e) | In 2015, the net increase in the valuation allowance of $1.2 million was a result of the accrual of tax credit carryforwards that are expected to expire prior to utilization. |
| (f) | In 2016, the net decrease is primarily a result of the ability to utilize more of the U.S. tax credit carryforwards and changes in the enacted statutory tax rates in the United Kingdom. |
| F-41 |
Sucampo Pharmaceuticals, Inc.
Exhibit Index
| Exhibit | |||||
| Number | Description | Form | File No. | Exhibit | Filing Date |
| 3.1 | Certificate of Incorporation | 8-K | 001-33609 | 3.1 | 12/29/2008 |
| 3.2 | Certificate of Amendment | 8-K | 001-33609 | 3.2 | 12/29/2008 |
| 3.3 | Amended and Restated Bylaws | 8-K | 001-33609 | 3.3 | 8/2/2013 |
| 4.1 | Specimen Stock Certificate evidencing the shares of class A common stock | S-1/A | 333-135133 | 4.1 | 2/1/2007 |
| 4.2 | Indenture, dated as of December 27, 2016, between the Company and U.S. Bank National Association, as Trustee | 8-K | 001-33609 | 4.1 | 12/27/2016 |
| 4.3 | Form of Note representing the Company’s 3.25% Convertible Senior Notes due 2021 | 8-K | 001-33609 | 4.2 | 12/27/2016 |
| 10.1^ | Amended and Restated 2001 Stock Incentive Plan | S-1 | 333-135133 | 10.1 | 6/19/2006 |
| 10.2^ | Amended and Restated 2006 Stock Incentive Plan | 10-Q | 001-33609 | 10.2 | 11/14/2007 |
| 10.3^ | Form of Indemnification Agreement, dated July 2016, between the Company and an indemnitee | 10-Q | 001-33609 | 10.1 | 11/9/2016 |
| 10.4^ | Form Sucampo Pharmaceuticals, Inc. Duration-Based Stock Option Incentive Award Stock Option Agreement Terms and Conditions | 10-K | 001-33609 | 10.36 | 3/11/2016 |
| 10.5^ | Non-employee Director Compensation Summary | 10-K | 001-33609 | 10.35 | 3/11/2016 |
| 10.6^ | 2016 Equity Incentive Plan | 10-Q | 001-33609 | 10.1 | 8/3/2016 |
| 10.7^ | Employment Agreement, dated as of October 27, 2014, between the Company and Matthias Alder | 10-K | 001-33609 | 10.81 | 3/9/2015 |
| 10.8^ | Employment Agreement, dated as of January 30, 2015, between the Company and Andrew Smith | 10-Q | 001-33609 | 10.5 | 5/5/2016 |
| 10.9^ | Employment Agreement, dated as of August 2, 2016, between the Company and Dr. Peter Kiener |
10-Q | 001-33609 | 10.3 | 11/9/2016 |
| 10.10^ | Employment Agreement, dated as of August 2, 2016, between the Company and Andrew Smith |
10-Q | 001-33609 | 10.4 | 11/9/2016 |
| 10.11^ | Employment Agreement, dated as of August 3, 2016, between the Company and Peter Greenleaf |
10-Q | 001-33609 | 10.2 | 11/9/2016 |
| 10.12^ | Employment Agreement, dated as of August 3, 2016, between the Company and Matthew Donley |
10-Q | 001-33609 | 10.5 | 11/9/2016 |
| 10.13^ | Employment Agreement, dated as of August 23, 2016, between Sucampo AG and Dr. Peter Lichtlen | 10-Q | 001-33609 | 10.6 | 11/9/2016 |
| 10.14^ | Separation Agreement, dated as of February 29, 2016, between the Company and Stanley Miele | 10-Q | 001-33609 | 10.4 | 5/5/2016 |
| 10.15* | Collaboration and License Agreement, dated October 29, 2004, between the Company and Takeda Pharmaceutical Company Limited | S-1 | 333-135133 | 10.21 | 6/19/2006 |
| 10.16* | Agreement, dated October 29, 2004, among the Company, Takeda Pharmaceutical Company Limited and Sucampo AG | S-1 | 333-135133 | 10.22 | 6/19/2006 |
| 10.17* | Supply Agreement, dated October 29, 2004, among the Company, Takeda Pharmaceutical Company Limited and R-Tech Ueno, Ltd. | S-1 | 333-135133 | 10.23 | 6/19/2006 |
| 10.18* | Supply and Purchase Agreement, dated January 25, 2006, among the Company, Takeda Pharmaceutical Company Limited and R-Tech Ueno, Ltd. | S-1 | 333-135133 | 10.24 | 6/19/2006 |
| 10.19* | Supplemental Agreement, dated February 1, 2006, between the Company and Takeda Pharmaceutical Company Limited | S-1 | 333-135133 | 10.25 | 6/19/2006 |
| 10.20 | Letter Agreement, dated January 29, 2007, between the Company and Takeda Pharmaceutical Company Limited | S-1/A | 333-135133 | 10.36 | 5/14/2007 |
| II-1 |
| 10.21* | License, Commercialization and Supply Agreement, dated February 19, 2009, between Sucampo Pharma Ltd. and Abbott Japan Co. Ltd. | 10-K | 001-33609 | 10.43 | 3/16/2009 |
| 10.22* | Settlement and License Agreement, dated September 30, 2014, among the Company, Sucampo AG, R-Tech Ueno, Ltd., Takeda Pharmaceutical Company Limited, Takeda Pharmaceuticals USA, Inc., Takeda Pharmaceuticals America, Inc., Anchen Pharmaceuticals, Inc., Par Pharmaceutical, Inc. and Par Pharmaceutical Companies, Inc. | 10-Q | 001-33609 | 10.2 | 11/7/2014 |
| 10.23* | Manufacturing and Supply Agreement, dated September 30, 2014, between Sucampo AG and Par Pharmaceutical, Inc. | 10-Q | 001-33609 | 10.3 | 11/7/2014 |
| 10.24* | Amendment No. 1, dated September 30, 2014, to Collaboration and License Agreement dated October 29, 2004 and Supplemental Agreement, dated February 1, 2006, between Sucampo Pharma Americas, LLC and Takeda Pharmaceutical Company Limited | 10-Q | 001-33609 | 10.4 | 11/7/2014 |
| 10.25 | Amendment No. 1, dated September 30, 2014, to the Agreement dated October 29, 2004, between Sucampo Pharma Americas, LLC, Takeda Pharmaceutical Company Limited and Sucampo AG | 10-Q | 001-33609 | 10.5 | 11/7/2014 |
| 10.26* | Amendment No. 1, dated September 30, 2014, to Supply Agreement dated October 29, 2004, Supply and Purchase Agreement dated January 25, 2006 and the Addendum to the Supply and Purchase Agreement dated November 6, 2013, among Sucampo Pharma Americas, LLC, Takeda Pharmaceutical Company Limited and R-Tech Ueno, Ltd. | 10-Q | 001-33609 | 10.6 | 11/7/2014 |
| 10.27* | License, Development, Commercialization and Supply Agreement For Lubiprostone, dated October 17, 2014, between Sucampo AG and Takeda Pharmaceuticals International GmbH Limited | 10-K | 001-33609 | 10.79 | 3/9/2015 |
| 10.28* | Stipulation and License Agreement, dated February 5, 2015, among the Company, Sucampo AG, R-Tech Ueno, Ltd. and Par Pharmaceutical, Inc. | 10-K | 001-33609 | 10.88 | 3/9/2015 |
| 10.29* | Manufacturing and Supply Agreement, dated as of February 5, 2015, between Sucampo AG and Par Pharmaceutical, Inc. | 10-K | 001-33609 | 10.89 | 3/9/2015 |
| 10.30* | License, Development, Commercialization And Supply Agreement For Lubiprostone for People's Republic of China, dated May 5, 2015, between Harbin Gloria Pharmaceuticals Co., Ltd. and Sucampo AG | 10-Q | 001-33609 | 10.2 | 8/5/2015 |
| 10.31* | First Amendment to office Lease Agreement, dated September 14, 2015, between Four Irvington Centre Associations, LLC and Sucampo Pharmaceuticals, Inc. | 10-Q | 001-33609 | 10.1 | 11/4/2015 |
| 10.32* | Amendment No. 1, dated November 18, 2015 to the License, Development, Commercialization and Supply Agreement for Lubiprostone dated October 17, 2014, between Sucampo AG and Takeda Pharmaceuticals International AG | 10-K | 001-33609 | 10.37 | 3/11/2016 |
| 10.33* | Manufacturing Agreement, dated March 22, 2004, between R-Tech Ueno, Ltd. and Nissan Chemical Industries, Ltd. | 10-K | 001-33609 | 10.41 | 3/11/2016 |
| 10.34 | Convertible Promissory Note, dated as of January 9, 2016, between Sucampo AG and Cancer Prevention Pharmaceuticals, Inc. | 10-Q | 001-33609 | 10.1 | 5/5/2016 |
| II-2 |
| 10.35* | Option and Collaboration Agreement, dated as of January 9, 2016, between Sucampo AG and Cancer Prevention Pharmaceuticals, Inc. | 10-Q | 001-33609 | 10.2 | 5/5/2016 |
| 10.36 | Securities Purchase Agreement, dated as of January 9, 2016, between Sucampo AG and Cancer Prevention Pharmaceuticals, Inc. | 10-Q | 001-33609 | 10.3 | 5/5/2016 |
| 10.37 | Subordinated Unsecured Promissory Note, dated December 23, 2010, between Ambrent Investments S.à.r.l., as borrower, and Ryuji Ueno Revocable Trust Under Trust Agreement dated December 20, 2002, as lender | 8-K | 001-33609 | 10.1 | 12/29/2010 |
| 10.38 | Subordinated Unsecured Promissory Note, dated December 23, 2010, between Ambrent Investments S.à.r.l., as borrower, and Sachiko Kuno Revocable Trust Under Trust Agreement dated December 20, 2002, as lender | 8-K | 001-33609 | 10.2 | 12/29/2010 |
| 10.39* | Loan Guarantee and Development Agreement, dated September 8, 2011, between Numab AG and Sucampo AG | 10-K | 001-33609 | 10.58 | 3/15/2012 |
| 10.40* | Credit Agreement, dated October 16, 2015, among the Company as borrower, the financial institutions listed therein as Lenders and Jefferies Finance LLC, as administrative agent and collateral agent for the Lenders | 10-K | 001-33609 | 10.38 | 3/11/2016 |
| 10.41* | Office Lease Agreement, dated May 5, 2015, between Four Irvington Centre Associations, LLC and Sucampo Pharmaceuticals, Inc. | 10-Q | 001-33609 | 10.1 | 8/5/2015 |
| 10.42 | Lease Agreement, dated December 18, 2006, between the Company and EW Bethesda Office Investors, LLC | 10-K | 001-33609 | 10.29 | 3/27/2008 |
| 10.43* | Lease Agreement, dated April 1, 2001, between Ueno Fine Chemicals and R-Tech Ueno Ltd. | 10-K | 001-33609 | 10.39 | 3/11/2016 |
| 10.44^ | Amended and Restated 2006 Employee Stock Purchase Plan | Included herewith | |||
| 10.45^ | Form Restricted Stock Unit Ward Grant Notice | Included herewith | |||
| 12.1 | Ratio of Earnings to Fixed Charges | Included herewith | |||
| 21 | Subsidiaries of the Company | Included herewith | |||
| 23.1 | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm | Included herewith | |||
| 23.2 | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm | Included herewith | |||
| 31.1 | Certification of the Principal Executive Officer, as required by Section 302 of the Sarbanes-Oxley Act of 2002 | Included herewith | |||
| 31.2 | Certification of the Principal Financial Officer, as required by Section 302 of the Sarbanes-Oxley Act of 2002 | Included herewith | |||
| 32.1 | Certification of the Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | Included herewith | |||
| 32.2 | Certification of the Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | Included herewith | |||
| II-3 |
| 101.[SCH] | XBRL Taxonomy Extension Schema Document | Included herewith | |||
| 101.[CAL] | XBRL Taxonomy Extension Calculation Linkbase Document | Included herewith | |||
| 101.[LAB] | XBRL Taxonomy Extension Label Linkbase Document | Included herewith | |||
| 101.[PRE] | XBRL Taxonomy Extension Presentation Linkbase Document | Included herewith | |||
| ^ Compensatory plan, contract or arrangement. | |||||
| * Confidential treatment has been granted for portions of this exhibit. | |||||
| & English summary of a foreign language document. | |||||
| # Pursuant to Item 601(b)(2) of Regulation S-K promulgated by the SEC, certain schedules to this agreement have been omitted. The registrant hereby agrees to furnish supplementally to the SEC, upon its request, any or all of such omitted schedules. | |||||
II-4
