Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Unilife Corp | d484615d8k.htm |
| EX-99.3 - EX-99.3 - Unilife Corp | d484615dex993.htm |
| EX-99.1 - EX-99.1 - Unilife Corp | d484615dex991.htm |
Exhibit 99.2

| NASDAQ (UNIS) and ASX (UNS) Fiscal Year 2013 2nd Quarter Earnings Call February 11, 2013 |

| This presentation contains forward looking statements under the safe harbor provisions of the US securities laws. These forward-looking statements are based on management's beliefs and assumptions and on information currently available to our management. Our management believes that these forward-looking statements are reasonable as and when made. However you should not place undue reliance on any such forward looking statements as these are subject to risks and uncertainties. Please refer to our press releases and our SEC filings for more information regarding the use of forward looking statements. Cautionary Note Regarding Forward-Looking Statements |

| Opening Statement CEO Alan Shortall NASDAQ (UNIS) and ASX (UNS) |

| Dr. Ramin Mojdeh Unilife EVP and COO NASDAQ (UNIS) and ASX (UNS) |
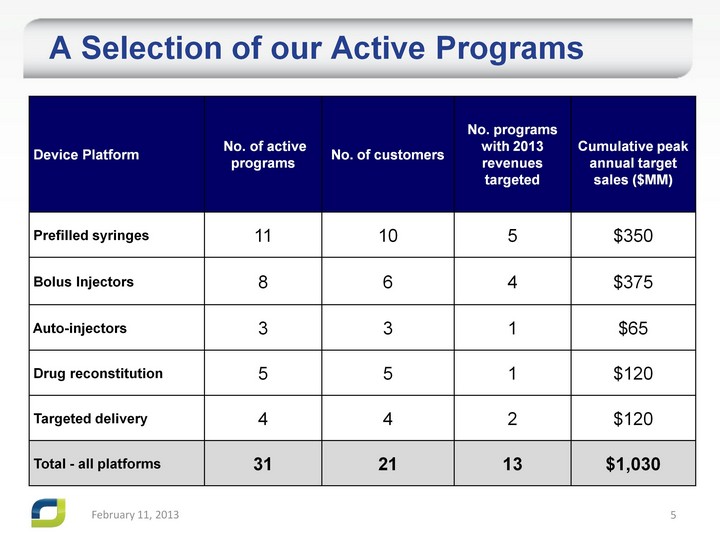
| A Selection of our Active Programs A Selection of our Active Programs February 11, 2013 |
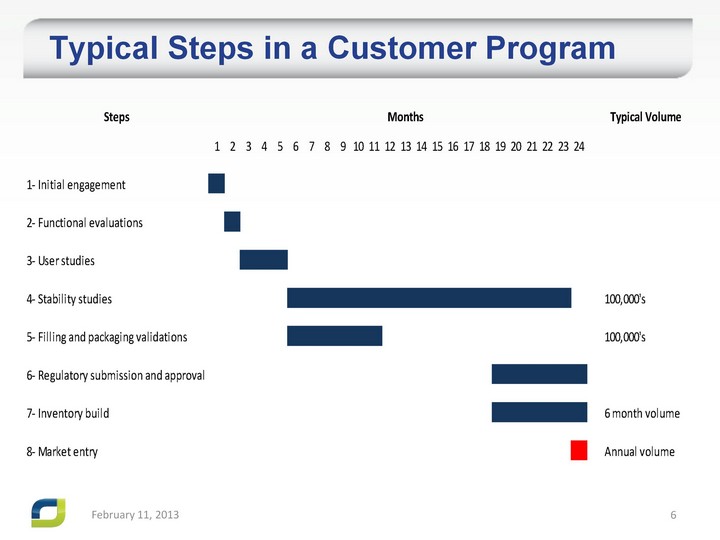
| Typical Steps in a Customer Program February 11, 2013 |

| Investing in R&D for Long-term Growth Broad commercial pipeline driving multiple programs for multiple devices with multiple customers Requires large and sustained R&D investment upfront Upfront investment generate significant returns over time Our investment in R&D covers many areas Customization of devices from existing technology platforms to address specific customer and therapy needs Development of new, value-adding device platforms Improving production efficiencies and quality processes February 11, 2013 |
| A Rapidly Expanding IP Portfolio Strong patent position covering portfolio / related technologies Backed by Freedom-To-Operate reports International coverage across U.S., EU and PCT(1) countries 80+ granted patents across 20+ countries Hundreds of additional patents filed and now in process Patents for Unifill syringe extend out to 203026 new patent families were filed in CY2012 that are now in various stages of examination. Another 24 families pending. (1) 142 countries are signatories to the Patent Cooperation Treaty (PCT) including all major industrialized countries |

| Clinical Development Programs February 11, 2013 A precursor to Commercial Supply ContractsScope can include: Customization of a device Stability studies User studies Supply for clinical trialsCan range between six to 24 months in lengthCan have fees in the range of $3MM to $10MM Can include exclusivity options with fees attached |

| Some target drugs may have their own clinical development program. i.e. customizing finger flange for easy handling by a patient populationStability studies are a regulatory requirement Clinical Development Programs A Unifill Syringe Program February 11, 2013 Announced in December 2012 that another pharmaceutical company had commenced stability studies with Unifill Multiple prospective opportunities with customers this year |

| Clinical Development Programs Global pharmaceutical company selected Unilife's bolus injectors as preferred choice in final evaluation stage for multi-drug program. Customization to specific needs of each target drugExpect to enter into program this year for the lead drug targetA number of similar prospective program opportunities with other pharmaceutical companies A Bolus Injection Device Program February 11, 2013 |
| Clinical Development Programs Developed a specialized device for target organ delivery for Global Pharmaceutical CompanySuccessfully completed $1.4MM initial stage of program last yearNext stage of program with additional revenues now being discussed with customer Specialized Device for Targeted Organ Delivery February 11, 2013 |

| Supply Contracts Scope can include: Minor customizations Stability studies Commercial supplyTerms in the range of 7 to 10 years with recurring revenuesOpportunities forUpfront fees in the range of $1MM to $5MMCustomers paying for production equipment to guarantee capacityExclusivity and royalty fees February 11, 2013 |
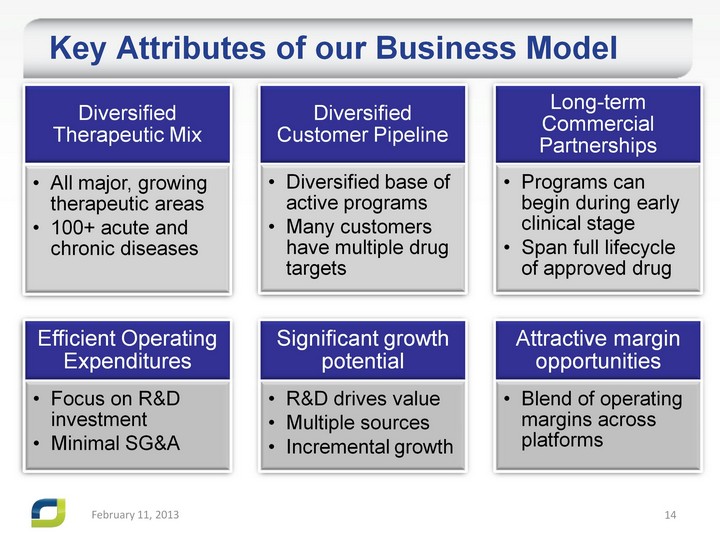
| Key Attributes of our Business Model February 11, 2013 |

| Financial Data Overview Rich Wieland Chief Financial Officer |
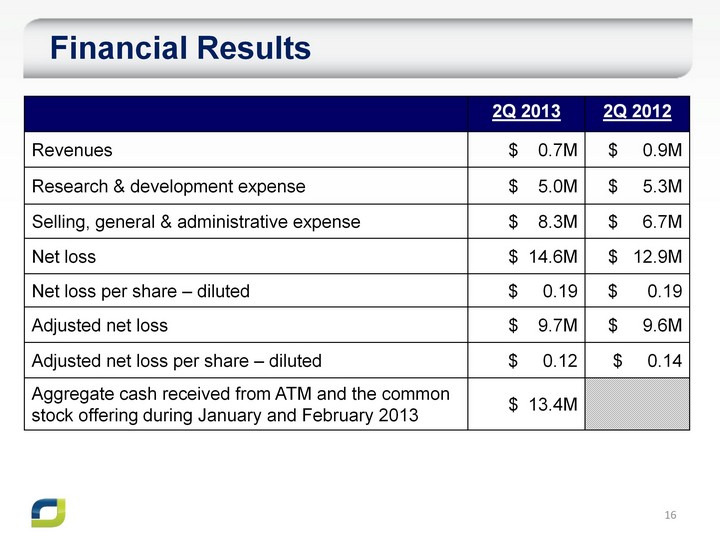
| Financial Results 2Q 2013 2Q 2012 Revenues $ 0.7M $ 0.9M Research & development expense $ 5.0M $ 5.3M Selling, general & administrative expense $ 8.3M $ 6.7M Net loss $ 14.6M $ 12.9M Net loss per share - diluted $ 0.19 $ 0.19 Adjusted net loss $ 9.7M $ 9.6M Adjusted net loss per share - diluted $ 0.12 $ 0.14 Aggregate cash received from ATM and the common stock offering during January and February 2013 $ 13.4M |

| Improving Operational Efficiencies Ongoing commitment to improve operational efficienciesContinuing to make progress towards this goal$1.6MM (13%) savings realized this quarter compared to Q420123.5% savings realized this quarter when compared against average quarterly operating expenses in fiscal year 2012 February 11, 2013 |

| Dr. Ramin Mojdeh Unilife EVP and COO NASDAQ (UNIS) and ASX (UNS) |

| Questions February 11, 2013 |
