Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Cardiff Oncology, Inc. | a13-4950_18k.htm |
| EX-99.1 - EX-99.1 - Cardiff Oncology, Inc. | a13-4950_1ex99d1.htm |
Exhibit 99.2
|
|
Introduction to Trovagene, Inc. Antonius Schuh, PhD Chief Executive Officer 13 February 2013 |
|
|
Forward-Looking Statements DISCLAIMER Statements in this presentation about the Company's expectations, applications of its technology, markets, launch of tests and other statements that are not historical facts are "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on management's current beliefs, assumptions, estimates and projections. Actual results may differ materially from those projected in the forward-looking statements for various reasons, including risks associated with product and test development, test transfer to contracting labs, government regulation, market acceptance, limited commercial experience, dependence on key personnel, obtaining financing and other factors discussed in the Company's periodic reports filed with the Securities and Exchange Commission. |
|
|
Trovagene’s Mission Trovagene is committed to developing urine-based molecular diagnostics as an enabling technology that can transform the way physicians diagnose, monitor and treat disease. By analyzing genomic markers in urine rather than in blood, Trovagene introduces a systemic liquid biopsy for truly non-invasive assessment of surgical intervention, therapeutic response, and monitoring for disease recurrence. Our diagnostic tests are designed to achieve meaningful improvements in both outcomes and cost of care. INTRODUCTION |
|
|
Experienced Management Team INTRODUCTION Executive Management Team Antonius Schuh, PhD, CEO Stephen Zaniboni, CFO Charlie Rodi, PhD, VP and CTO Keith McCormick, VP Comm. Ops Michael Terry, VP Corp. Devel. Scientific Advisory Board Paul Billings, MD, PhD Carlo M. Croce, MD Riccardo Dalla-Favera, MD Mark Erlander, PhD Brunangelo Falini, MD Sinuhe Hahn, MD Kunwar Shailubhai, PhD Corporate Board Thomas Adams, PhD - Chairman John P. Brancaccio, CPA Gabriele M. Cerrone, MBA Gary S. Jacob, PhD Antonius Schuh, PhD Stanley Tennant, MD Chris McGuigan, PhD |
|
|
Urine-based Detection of Molecular Markers VALUE PROPOSITION Billions of cells die naturally each day, releasing DNA/RNA Nucleic acids are broken into packets of genetic material which are carried away by the blood stream DNA/RNA fragments are filtered through the kidneys and appears in urine as Transrenal DNA or RNA TESTING by Trovagene What are Transrenal Nucleic Acids (TrNAs) |
|
|
Perfect Timing for Next Generation Urine-based Testing Enables Unique Opportunity Next generation sequencing technologies Next generation digital PCR platforms Breadth of novel molecular diagnostic markers Urine-based Molecular Tests Broad range of clinical applications Replace complex and less robust earlier technologies based on circulating cells and nucleic acids in blood IINTRODUCTION |
|
|
Transplantation In recipient urine for acute allograft rejection Prenatal Diagnostics In maternal urine for aneuploidy screening Infectious Disease Viruses: HPV, HIV, EBV, JC Bacteria: Mycobacterium tuberculosis, Helicobacter pylori, Bacillus anthracis Parasites: Leishmania, Plasmodium Oncology KRAS: Colorectal, Pancreatic, others BRAF: Melanoma, Thyroid, Colorectal, Ovarian, others PIK3CA: Breast, Colorectal, Bladder, others Patient-specific TECHNOLOGY & IP Broad Clinical Utility |
|
|
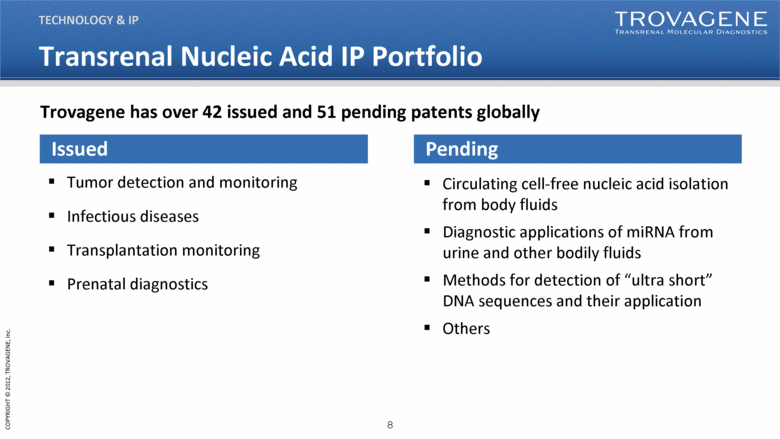
Transrenal Nucleic Acid IP Portfolio Issued Tumor detection and monitoring Infectious diseases Transplantation monitoring Prenatal diagnostics Pending Circulating cell-free nucleic acid isolation from body fluids Diagnostic applications of miRNA from urine and other bodily fluids Methods for detection of “ultra short” DNA sequences and their application Others TECHNOLOGY & IP Trovagene has over 42 issued and 51 pending patents globally |
|
|
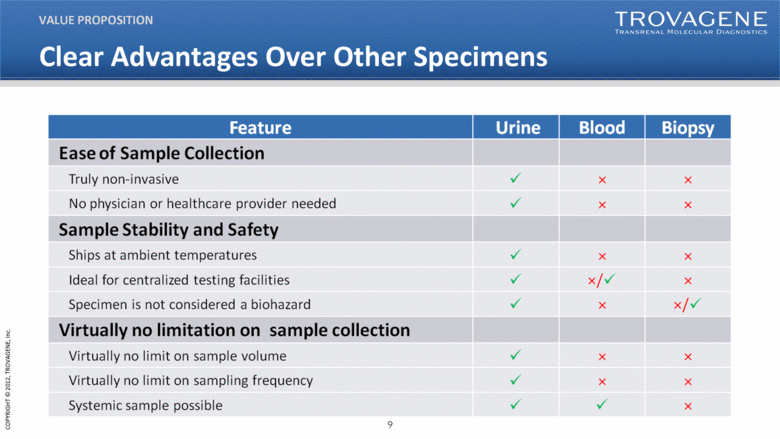
Clear Advantages Over Other Specimens VALUE PROPOSITION Feature Urine Blood Biopsy EAse of Sample Collection Truly non-invasive No physician or healthcare provider needed Sample Stability and Safety Ships at ambient temperatures Ideal for centralized testing facilities Specimen is not considered a biohazard Virtually no limitation on sample collection Virtually no limit on sample volume Virtually no limit on sampling frequency Systemic sample possible |
|
|
Commercialization Through CLIA Lab and Out-licensing Q1 2013: HPV carrier test Q2 2013: KRAS Q3 2013: BRAF Q4 2013: PIK3CA, HBV, p53 (Hepatocellular Carcinoma) Commercialize urine based diagnostic tests through CLIA lab Transplant medicine Prenatal medicine Infectious disease Export markets Portfolio of proprietary markers for hematology NPM1 for AML BRAF in HCL SF3B1 for CLL Partnerships with Laboratories and IVD makers COMMERCIALIZATION STRATEGY |
|
|
Trovagene’s Proprietary High Risk HPV Test |
|
|
Trovagene HPV DNA Test Value Proposition No Pap Smear or other tissue sample required Unique primer pair amplifying the E1 region provides Freedom-To-Operate (FTO) for molecular HPV testing E1 region offers novel, proprietary approach to high risk HPV identification Trovagene’s patented method for DNA isolation Simplicity of testing: only 1 PCR reaction identifies all high-risk HPV subtypes |
|
|
HPV in Urine: US Market Dynamics Estimated 12-15M HPV tests run annually (US) 1 Represents only about 35% market penetration 8.7 – 14.2% of cases come back as positive2 Repeat HPV testing often performed at 6 and 12 months No convenient test for male partner to check for carrier status Potentially >2 million male partners eligible for testing Risk of re-infection by male partner exists and should be addressed Potential US Market Women needing repeat HPV Testing (1.04M – 2.13M) Male partners for screening (1.04M – 2.13M) Internal estimate based on 10M HPV tests performed in 2007-2008, with up to 25% CAGR through 2011, as published in CAP Today, August 2008 Castle et al. Clinical Human Papilloma Virus Detection Forecasts Cervical Cancer Risk in Women Over 18 Years of Follow Up. J Clin Oncol, 30 Jul 2012 1., 2. |
|
|
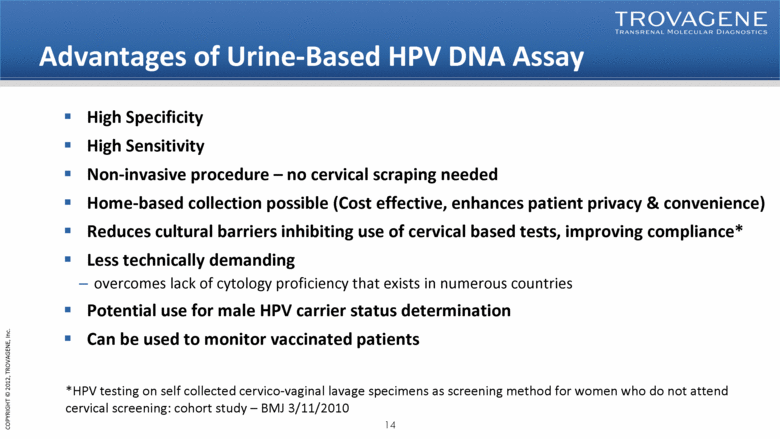
Advantages of Urine-Based HPV DNA Assay High Specificity High Sensitivity Non-invasive procedure – no cervical scraping needed Home-based collection possible (Cost effective, enhances patient privacy & convenience) Reduces cultural barriers inhibiting use of cervical based tests, improving compliance* Less technically demanding overcomes lack of cytology proficiency that exists in numerous countries Potential use for male HPV carrier status determination Can be used to monitor vaccinated patients *HPV testing on self collected cervico-vaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study – BMJ 3/11/2010 |
|
|
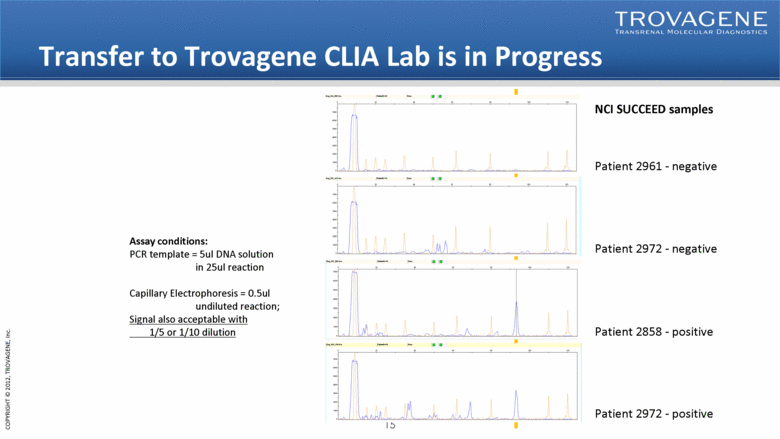
Transfer to Trovagene CLIA Lab is in Progress NCI SUCCEED samples Patient 2961 - negative Patient 2972 - negative Patient 2858 - positive Patient 2972 - positive Assay conditions: PCR template = 5ul DNA solution in 25ul reaction Capillary Electrophoresis = 0.5ul undiluted reaction; Signal also acceptable with 1/5 or 1/10 dilution |
|
|
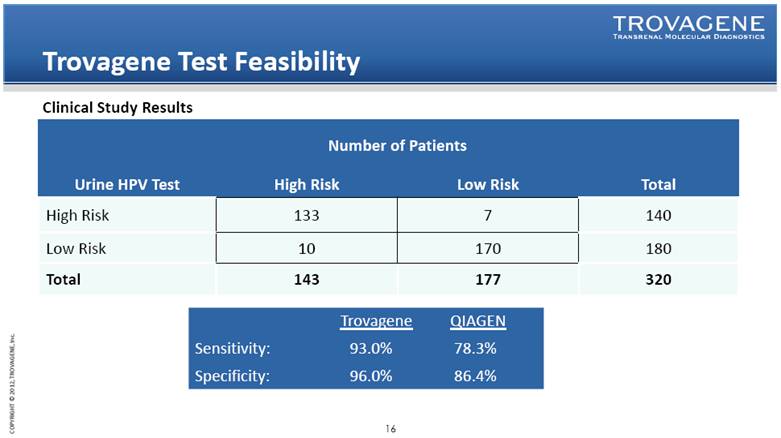
Trovagene Test Feasibility Urine HPV Test Number of Patients Total High Risk Low Risk High Risk 133 7 140 Low Risk 10 170 180 Total 143 177 320 Trovagene QIAGEN Sensitivity: 93.0% 78.3% Specificity: 96.0% 86.4% Clinical Study Results |
|
|
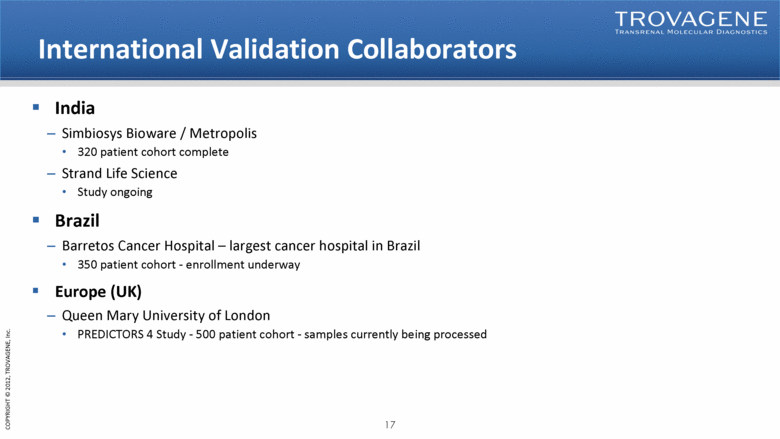
International Validation Collaborators India Simbiosys Bioware / Metropolis 320 patient cohort complete Strand Life Science Study ongoing Brazil Barretos Cancer Hospital – largest cancer hospital in Brazil 350 patient cohort - enrollment underway Europe (UK) Queen Mary University of London PREDICTORS 4 Study - 500 patient cohort - samples currently being processed |
|
|
HPV: US Launch March 2013 Southern California Pilot Launch Target 600 Ob-Gyns in Orange, Riverside and San Diego Counties Use of direct mail to promote awareness and trial Three waves of direct mail planned Web-based promotion and awareness building Internal Client Services to follow up on lead generation Regional sales consultant to generate leads, follow up on accounts |
|
|
Oncogene Mutation Detection |
|
|
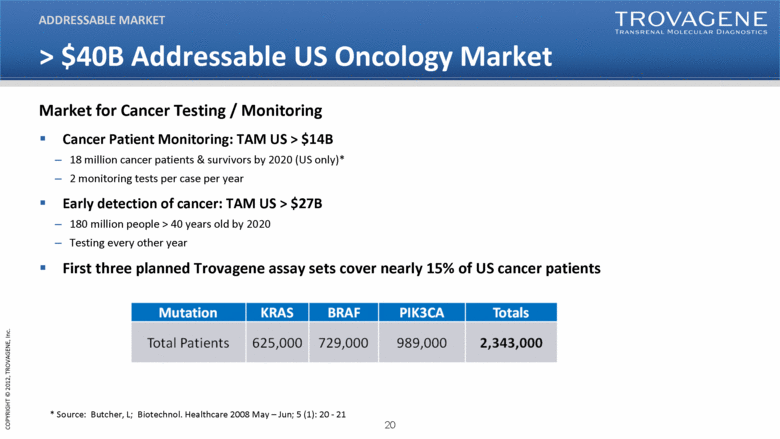
> $40B Addressable US Oncology Market Cancer Patient Monitoring: TAM US > $14B 18 million cancer patients & survivors by 2020 (US only)* 2 monitoring tests per case per year Early detection of cancer: TAM US > $27B 180 million people > 40 years old by 2020 Testing every other year First three planned Trovagene assay sets cover nearly 15% of US cancer patients ADDRESSABLE MARKET * Source: Butcher, L; Biotechnol. Healthcare 2008 May – Jun; 5 (1): 20 - 21 Market for Cancer Testing / Monitoring Mutation KRAS BRAF PIK3CA Totals Total Patients 625,000 729,000 989,000 2,343,000 |
|
|
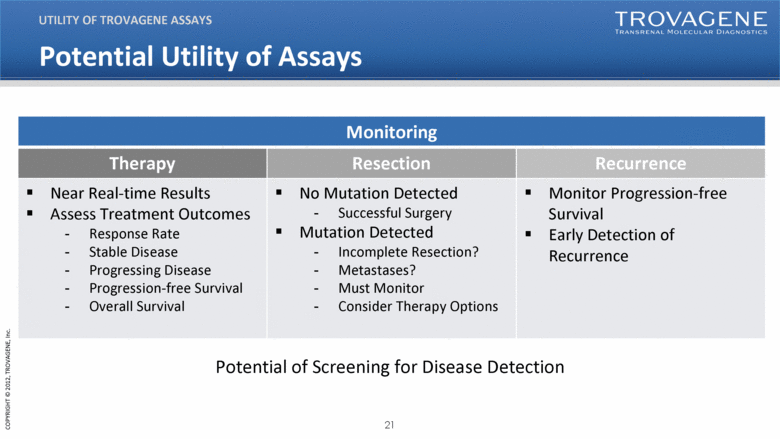
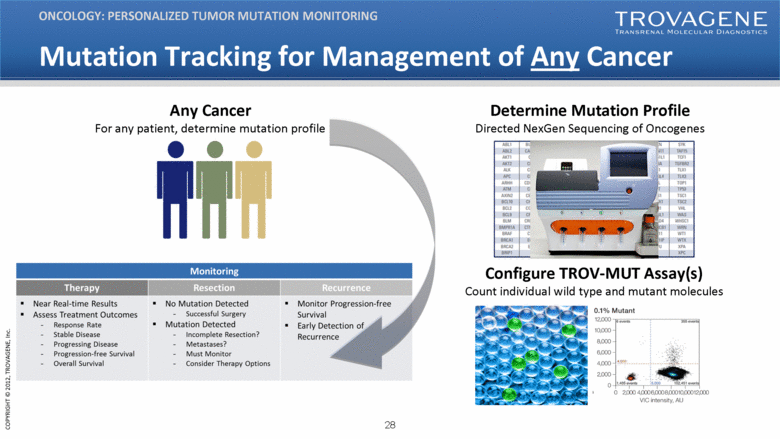
Potential Utility of Assays UTILITY OF TROVAGENE ASSAYS Monitoring Therapy Resection Recurrence Near Real-time Results Assess Treatment Outcomes Response Rate Stable Disease Progressing Disease Progression-free Survival Overall Survival No Mutation Detected Successful Surgery Mutation Detected Incomplete Resection? Metastases? Must Monitor Consider Therapy Options Monitor Progression-free Survival Early Detection of Recurrence Potential of Screening for Disease Detection |
|
|
Detection of KRAS Mutations in Both Urine and Blood ONCOLOGY: TUMOR MUTATION MONITORING Blood Lecomte T, et al. 2010. Circulating free tumor DNA and colorectal cancer. Gastroenterol Clin Biol. 34(12):662-81. Diehl F, et al. 2008. Circulating mutant DNA to assess tumor dynamics. Nat Med. 14(9):985-90. Lecomte T, et al. 2002. Detection of free-circulating tumor-associated DNA in plasma of colorectal cancer patients and its association with prognosis. Int J Cancer. Aug 10;100(5):542-8. Diehl F, et al. 2008. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology. 135(2):489-98. Urine Botezatu I, et al. 2000. Genetic analysis of DNA excreted in urine: a new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin Chem. 46(8 Pt 1):1078-84. Serdyuk OI, et al. 2001. Detection of mutant k-ras sequences in the urine of cancer patients. Bull Exp Biol Med. 131(3):283-4. Su YH, et al. 2004. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J Mol Diagn. 6(2):101-7. Su YH, et al. 2005. Detection of a K-ras mutation in urine of patients with colorectal cancer. Cancer Biomark. 1(2-3):177-82. Su YH, et al. 2008. Detection of mutated K-ras DNA in urine, plasma, and serum of patients with colorectal carcinoma or adenomatous polyps. Ann N Y Acad Sci. 1137:197-206. Feature comparison of blood vs. urine: Specimen volume 50 mL of urine yields 150,000 GE* A typical 5 mL blood draw yields approximately 7,500 GE* Mutant KRAS detected using equivalent technologies would be 20-fold more for urine (at least 20-fold, since larger volumes of urine are easily possible) * With a concentration of cell-free DNA of 10 ng/mL for both urine and plasma (one-half of the blood volume) |
|
|
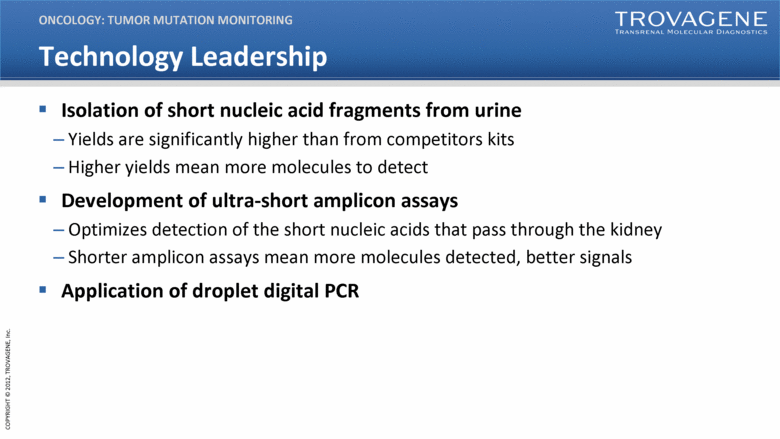
Technology Leadership Isolation of short nucleic acid fragments from urine Yields are significantly higher than from competitors kits Higher yields mean more molecules to detect Development of ultra-short amplicon assays Optimizes detection of the short nucleic acids that pass through the kidney Shorter amplicon assays mean more molecules detected, better signals Application of droplet digital PCR ONCOLOGY: TUMOR MUTATION MONITORING |
|
|
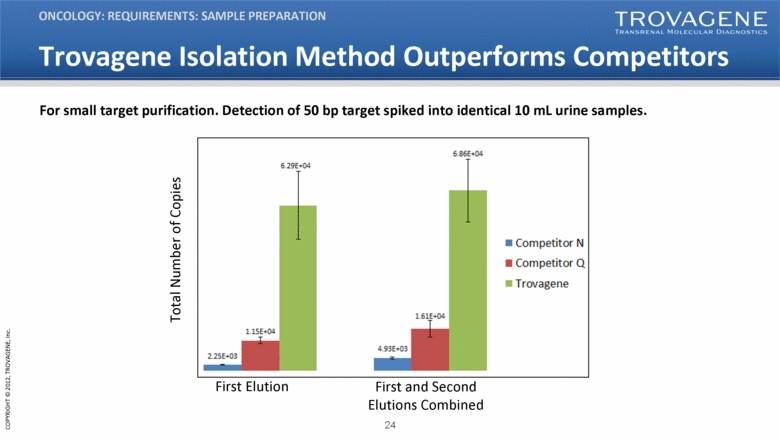
Trovagene Isolation Method Outperforms Competitors ONCOLOGY: REQUIREMENTS: SAMPLE PREPARATION For small target purification. Detection of 50 bp target spiked into identical 10 mL urine samples. First Elution First and Second Elutions Combined Total Number of Copies |
|
|
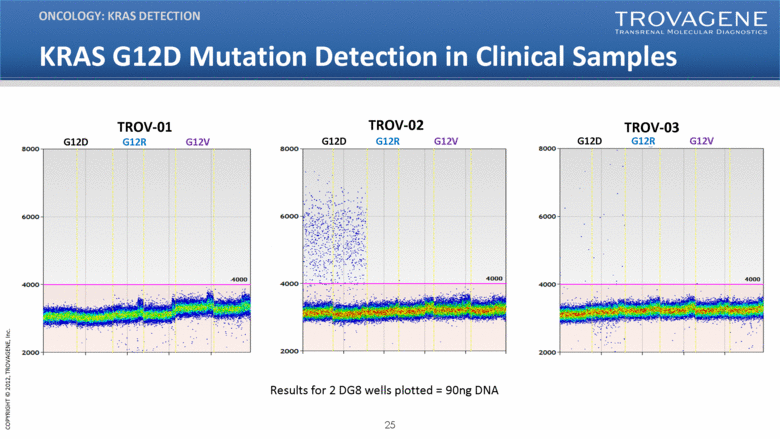
KRAS G12D Mutation Detection in Clinical Samples ONCOLOGY: KRAS DETECTION TROV-02 G12D G12R G12V TROV-01 G12D G12R G12V TROV-03 G12D G12R G12V Results for 2 DG8 wells plotted = 90ng DNA |
|
|
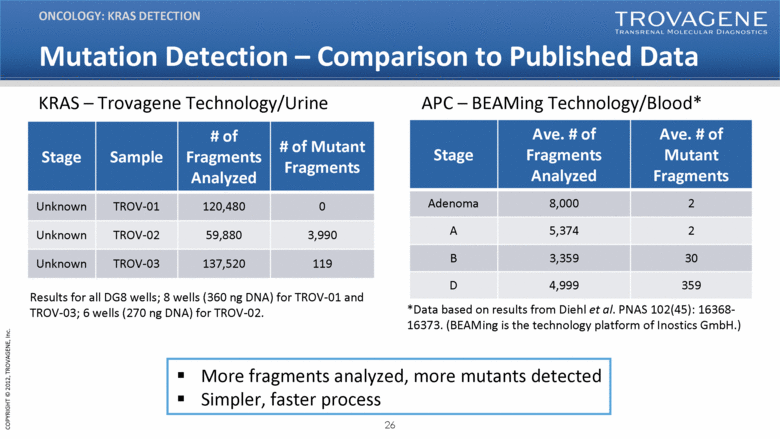
Mutation Detection – Comparison to Published Data ONCOLOGY: KRAS DETECTION Stage Ave. # of Fragments Analyzed Ave. # of Mutant Fragments Adenoma 8,000 2 A 5,374 2 B 3,359 30 D 4,999 359 Results for all DG8 wells; 8 wells (360 ng DNA) for TROV-01 and TROV-03; 6 wells (270 ng DNA) for TROV-02. Stage Sample # of Fragments Analyzed # of Mutant Fragments Unknown TROV-01 120,480 0 Unknown TROV-02 59,880 3,990 Unknown TROV-03 137,520 119 KRAS – Trovagene Technology/Urine APC – BEAMing Technology/Blood* *Data based on results from Diehl et al. PNAS 102(45): 16368-16373. (BEAMing is the technology platform of Inostics GmbH.) More fragments analyzed, more mutants detected Simpler, faster process |
|
|
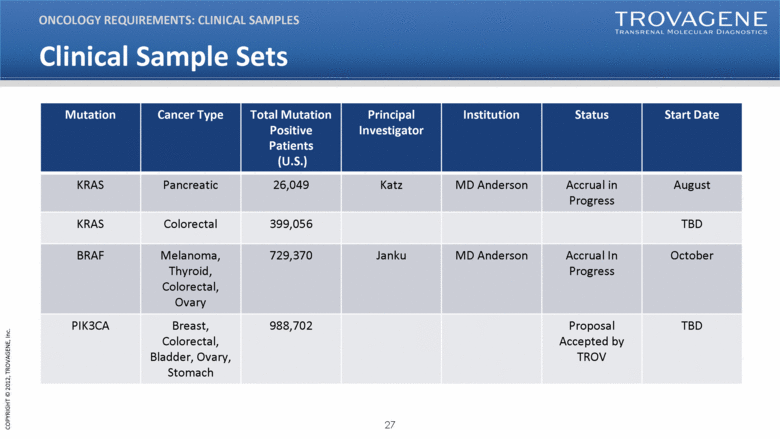
Clinical Sample Sets Mutation Cancer Type Total Mutation Positive Patients (U.S.) Principal Investigator Institution Status Start Date KRAS Pancreatic 26,049 Katz MD Anderson Accrual in Progress August KRAS Colorectal 399,056 TBD BRAF Melanoma, Thyroid, Colorectal, Ovary 729,370 Janku MD Anderson Accrual In Progress October PIK3CA Breast, Colorectal, Bladder, Ovary, Stomach 988,702 Proposal Accepted by TROV TBD ONCOLOGY REQUIREMENTS: CLINICAL SAMPLES |
|
|
Mutation Tracking for Management of Any Cancer ONCOLOGY: PERSONALIZED TUMOR MUTATION MONITORING Configure TROV-MUT Assay(s) Count individual wild type and mutant molecules Any Cancer For any patient, determine mutation profile Determine Mutation Profile Directed NexGen Sequencing of Oncogenes |
|
|
Summary and Financials |
|
|
2012/2013 Achievements CONCLUSION Acquired CLIA Laboratory CAP certified, high complexity molecular diagnostic lab Strengthened Patent Portfolio Issued U.S. Patent for NPM Mutants to Diagnose and Monitor Acute Myeloid Leukemia Issued European Patent for Detection of Pathogenic Infections Improved Liquidity and Access to Capital Completed Public Offering of $10 million and began trading on NASDAQ Added to the Russell Microcap and the MSCI Microcap Index Closed Private Placement of $4.4 million Expansion of KRAS Mutation Detection Development Program Began Validation Program and Commenced Study at MD Anderson Cancer Center for Transrenal KRAS Mutation Detection in Pancreatic Cancer Commenced Second Study at MD Anderson Cancer Center focusing on BRAF Mutations in 2013 Expansion of High Risk HPV Carrier Screening Development Program Partnered with Strand Life Sciences to Validate and Offer Urine-based HPV Screening Test in India Partnered with Barretos Cancer Hospital to Evaluate Urine-based HPV Assay in Brazil |
|
|
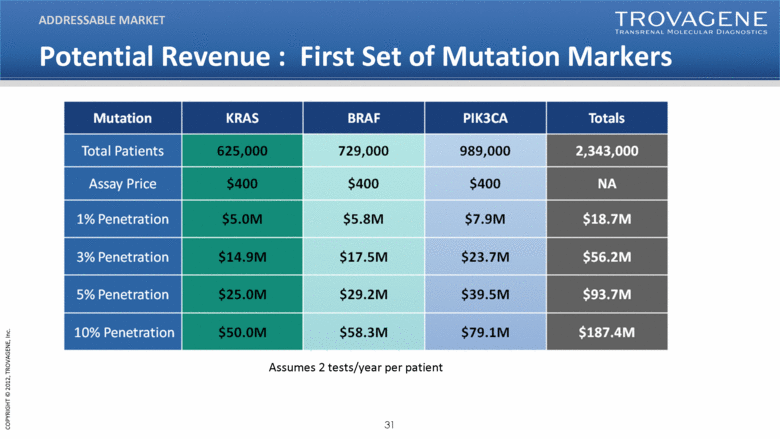
Potential Revenue : First Set of Mutation Markers ADDRESSABLE MARKET Assumes 2 tests/year per patient |
|
|
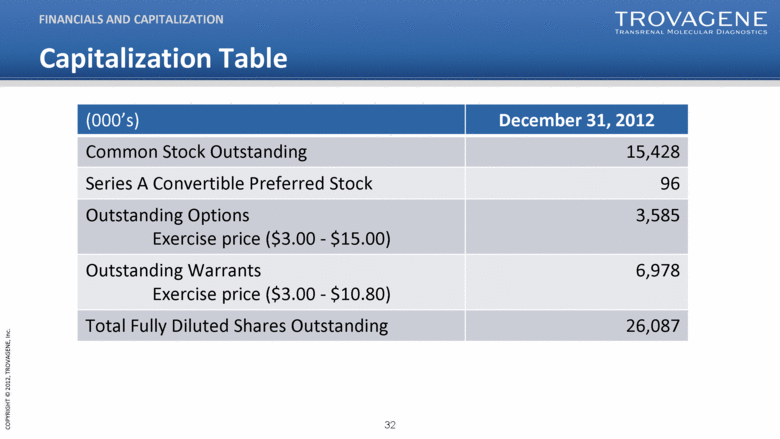
Capitalization Table FINANCIALS AND CAPITALIZATION (000’s) December 31, 2012 Common Stock Outstanding 15,428 Series A Convertible Preferred Stock 96 Outstanding Options Exercise price ($3.00 - $15.00) 3,585 Outstanding Warrants Exercise price ($3.00 - $10.80) 6,978 Total Fully Diluted Shares Outstanding 26,087 |
|
|
Please contact: For further information Antonius Schuh, CEO aschuh@trovagene.com Stephen Zaniboni, CFO szaniboni@trovagene.com |