Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Pacira BioSciences, Inc. | a13-2732_28k.htm |
| EX-99.2 - EX-99.2 - Pacira BioSciences, Inc. | a13-2732_2ex99d2.htm |
Exhibit 99.1
|
|
January 2013 Corporate Presentation NASDAQ: PCRX |
|
|
2 Forward-Looking Statements This presentation contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934 (collectively, forward-looking statements), about our business, including statements related to our expectations regarding completion of the proposed notes offering, plans to manufacture and commercialize EXPAREL, the success of our commercialization of EXPAREL, the future development of product candidates and the timing thereof, the timing and results of our clinical trials, potential indications for our product candidates and the timing and likelihood of the commercialization of additional products and future financial results. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include, whether or not we will offer the notes or consummate the offering; the anticipated terms of the notes and the offering; the impact of general economic, industry or political conditions in the United States or internationally; the success of our sales and manufacturing efforts in support of the commercialization of EXPAREL; the rate and degree of market acceptance of EXPAREL; the size and growth of potential markets for EXPAREL and our ability to serve those markets; our commercialization and marketing capabilities; and other factors discussed in the "Risk Factors" section of our most recent Annual Report on Form 10-K for the fiscal year ended December 31, 2011; our Quarterly Reports on Form 10-Q for the quarters ended June 30, 2012 and September 30, 2012; and in other filings that we periodically make with the SEC. In addition, the forward-looking statements included in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. |
|
|
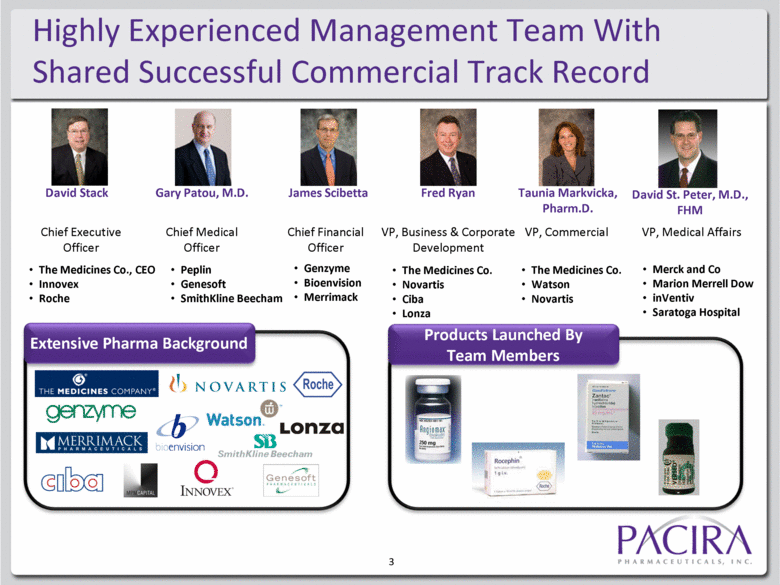
3 Highly Experienced Management Team With Shared Successful Commercial Track Record David Stack Gary Patou, M.D. James Scibetta Fred Ryan Taunia Markvicka, Pharm.D. David St. Peter, M.D., FHM Extensive Pharma Background Products Launched By Team Members The Medicines Co., CEO Innovex Roche Peplin Genesoft SmithKline Beecham Genzyme Bioenvision Merrimack The Medicines Co. Novartis Ciba Lonza The Medicines Co. Watson Novartis Merck and Co Marion Merrell Dow inVentiv Saratoga Hospital Chief Executive Officer Chief Medical Officer Chief Financial Officer VP, Business & Corporate Development VP, Commercial VP, Medical Affairs |
|
|
4 Key Investment Highlights Management Team with Shared Successful Commercial Track Record |
|
|
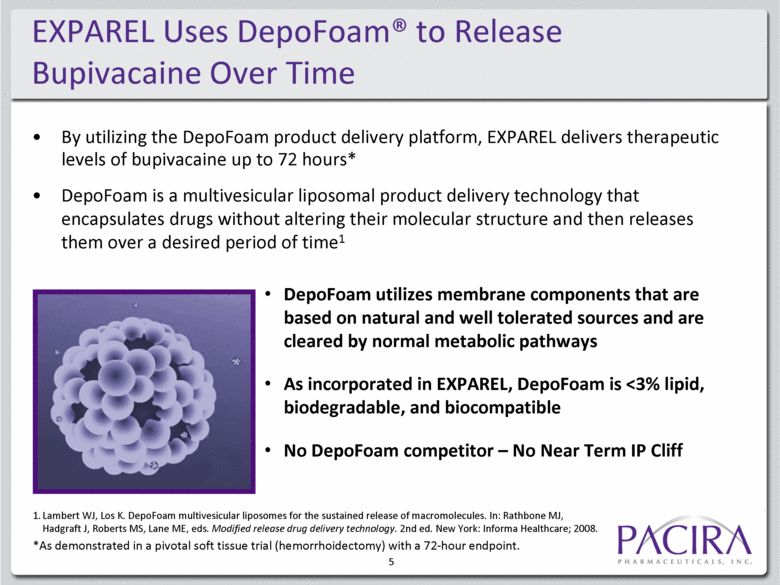
EXPAREL Uses DepoFoam® to Release Bupivacaine Over Time By utilizing the DepoFoam product delivery platform, EXPAREL delivers therapeutic levels of bupivacaine up to 72 hours* DepoFoam is a multivesicular liposomal product delivery technology that encapsulates drugs without altering their molecular structure and then releases them over a desired period of time1 1. Lambert WJ, Los K. DepoFoam multivesicular liposomes for the sustained release of macromolecules. In: Rathbone MJ, Hadgraft J, Roberts MS, Lane ME, eds. Modified release drug delivery technology. 2nd ed. New York: Informa Healthcare; 2008. DepoFoam utilizes membrane components that are based on natural and well tolerated sources and are cleared by normal metabolic pathways As incorporated in EXPAREL, DepoFoam is <3% lipid, biodegradable, and biocompatible No DepoFoam competitor – No Near Term IP Cliff 5 *As demonstrated in a pivotal soft tissue trial (hemorrhoidectomy) with a 72-hour endpoint. |
|
|
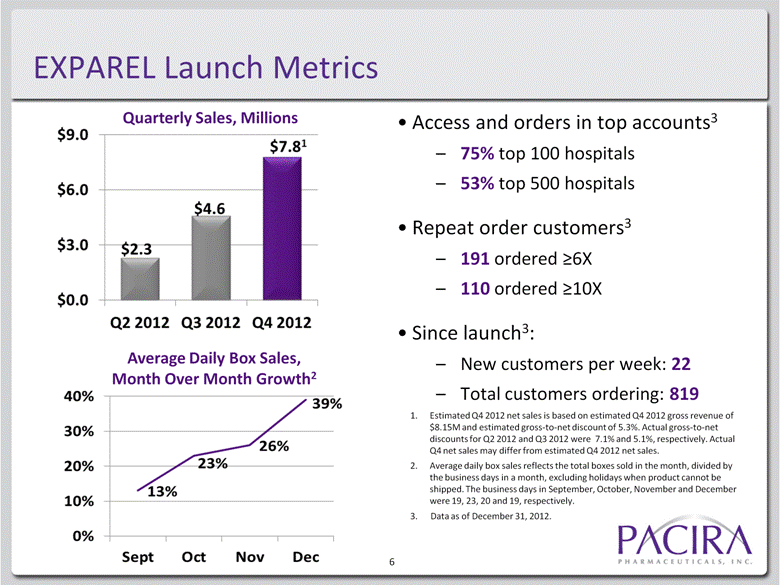
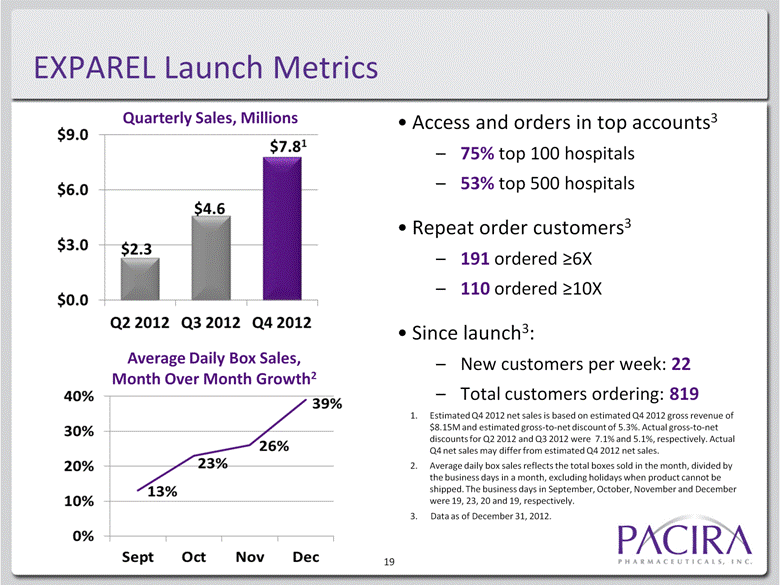
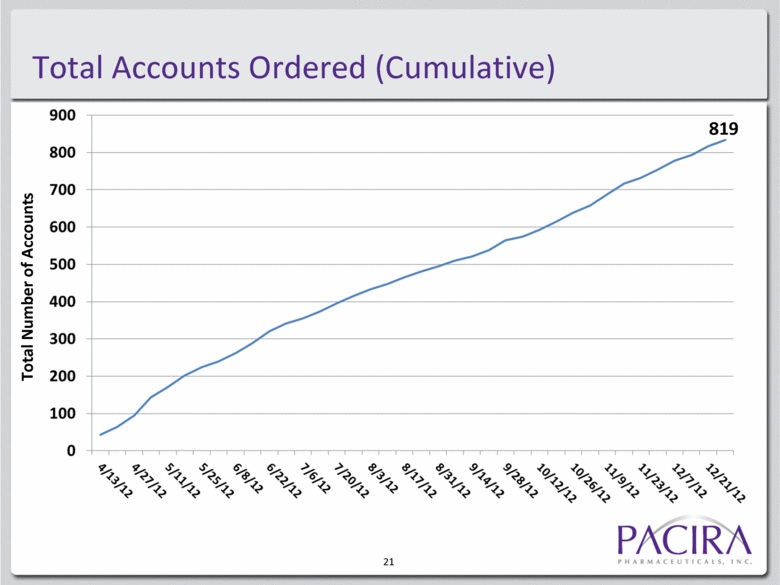
EXPAREL Launch Metrics Access and orders in top accounts3 75% top 100 hospitals 53% top 500 hospitals Repeat order customers3 191 ordered >6X 110 ordered >10X Since launch3: New customers per week: 22 Total customers ordering: 819 6 Quarterly Sales, Millions Average Daily Box Sales, Month Over Month Growth2 1. Estimated Q4 2012 net sales is based on estimated Q4 2012 gross revenue of $8.15M and estimated gross-to-net discount of 5.3%. Actual gross-to-net discounts for Q2 2012 and Q3 2012 were 7.1% and 5.1%, respectively. Actual Q4 net sales may differ from estimated Q4 2012 net sales. 2. Average daily box sales reflects the total boxes sold in the month, divided by the business days in a month, excluding holidays when product cannot be shipped. The business days in September, October, November and December were 19, 23, 20 and 19, respectively. 3. Data as of December 31, 2012. |
|
|
EXPAREL Positioning and Launch |
|
|
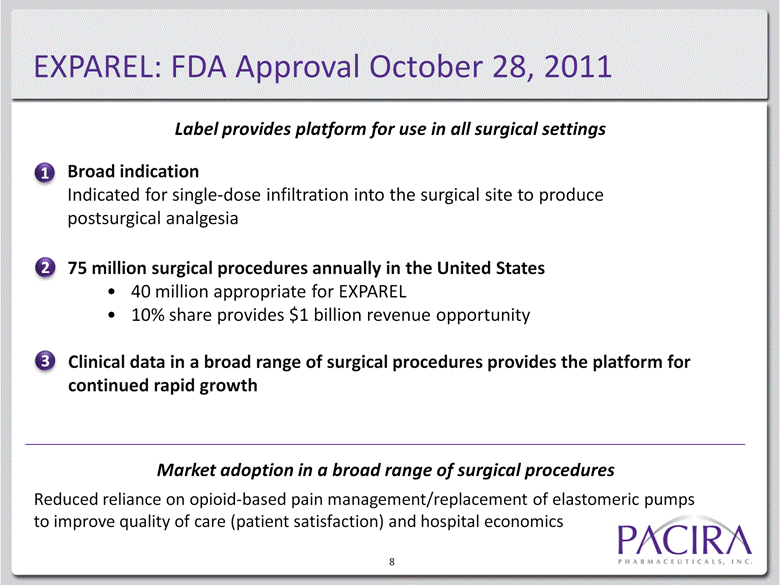
Broad indication Indicated for single-dose infiltration into the surgical site to produce postsurgical analgesia 8 1 Label provides platform for use in all surgical settings EXPAREL: FDA Approval October 28, 2011 3 2 75 million surgical procedures annually in the United States 40 million appropriate for EXPAREL 10% share provides $1 billion revenue opportunity postsurgical analgesia Clinical data in a broad range of surgical procedures provides the platform for continued rapid growth Market adoption in a broad range of surgical procedures Reduced reliance on opioid-based pain management/replacement of elastomeric pumps to improve quality of care (patient satisfaction) and hospital economics |
|
|
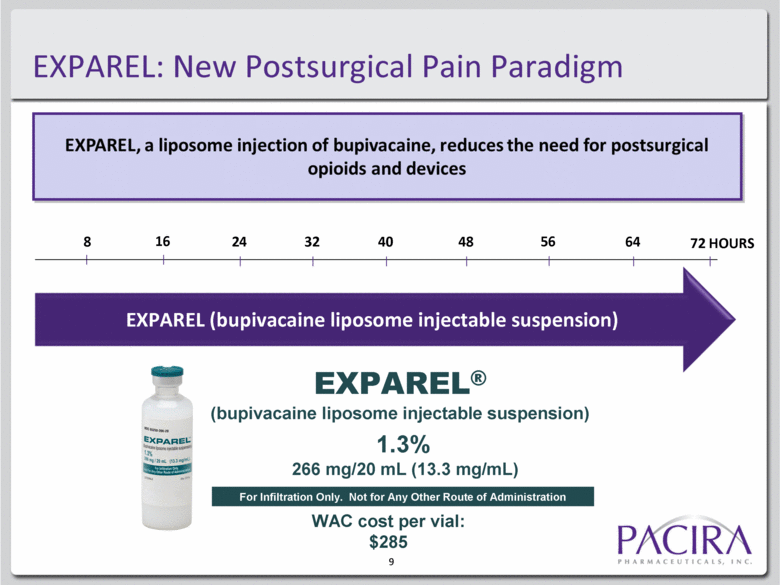
9 EXPAREL: New Postsurgical Pain Paradigm 8 16 24 32 40 48 56 64 72 HOURS EXPAREL® (bupivacaine liposome injectable suspension) 1.3% 266 mg/20 mL (13.3 mg/mL) For Infiltration Only. Not for Any Other Route of Administration EXPAREL (bupivacaine liposome injectable suspension) WAC cost per vial: $285 |
|
|
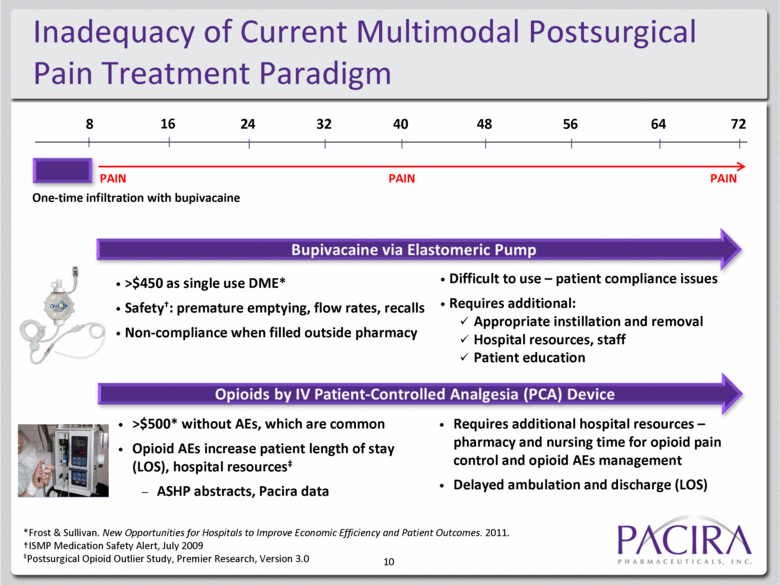
10 Inadequacy of Current Multimodal Postsurgical Pain Treatment Paradigm One-time infiltration with bupivacaine PAIN PAIN PAIN >$450 as single use DME* Safety†: premature emptying, flow rates, recalls Non-compliance when filled outside pharmacy Difficult to use – patient compliance issues Requires additional: Appropriate instillation and removal Hospital resources, staff Patient education >$500* without AEs, which are common Opioid AEs increase patient length of stay (LOS), hospital resources‡ ASHP abstracts, Pacira data Requires additional hospital resources – pharmacy and nursing time for opioid pain control and opioid AEs management Delayed ambulation and discharge (LOS) 8 16 24 32 40 48 56 64 72 *Frost & Sullivan. New Opportunities for Hospitals to Improve Economic Efficiency and Patient Outcomes. 2011. †ISMP Medication Safety Alert, July 2009 ‡Postsurgical Opioid Outlier Study, Premier Research, Version 3.0 |
|
|
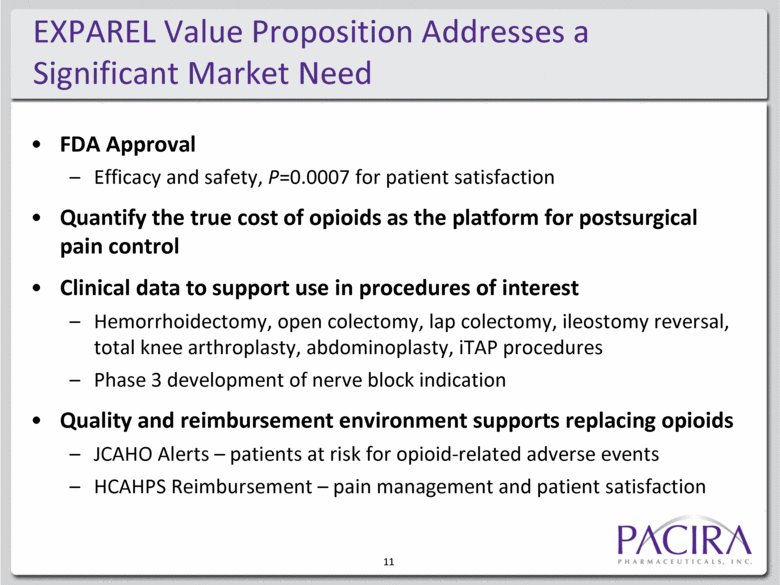
FDA Approval Efficacy and safety, P=0.0007 for patient satisfaction Quantify the true cost of opioids as the platform for postsurgical pain control Clinical data to support use in procedures of interest Hemorrhoidectomy, open colectomy, lap colectomy, ileostomy reversal, total knee arthroplasty, abdominoplasty, iTAP procedures Phase 3 development of nerve block indication Quality and reimbursement environment supports replacing opioids JCAHO Alerts – patients at risk for opioid-related adverse events HCAHPS Reimbursement – pain management and patient satisfaction EXPAREL Value Proposition Addresses a Significant Market Need 11 |
|
|
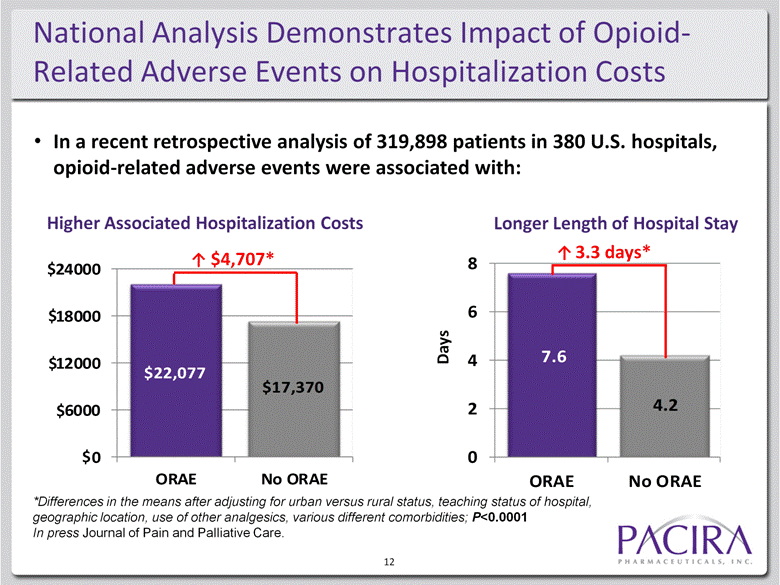
National Analysis Demonstrates Impact of Opioid-Related Adverse Events on Hospitalization Costs 12 $4,707* 3.3 days* In a recent retrospective analysis of 319,898 patients in 380 U.S. hospitals, opioid-related adverse events were associated with: $ $ $ Longer Length of Hospital Stay Days Higher Associated Hospitalization Costs $ $ *Differences in the means after adjusting for urban versus rural status, teaching status of hospital, geographic location, use of other analgesics, various different comorbidities; P<0.0001 In press Journal of Pain and Palliative Care. $22,077 $17,370 0 6000 12000 18000 24000 ORAE No ORAE 7.6 4.2 0 2 4 6 8 ORAE No ORAE |
|
|
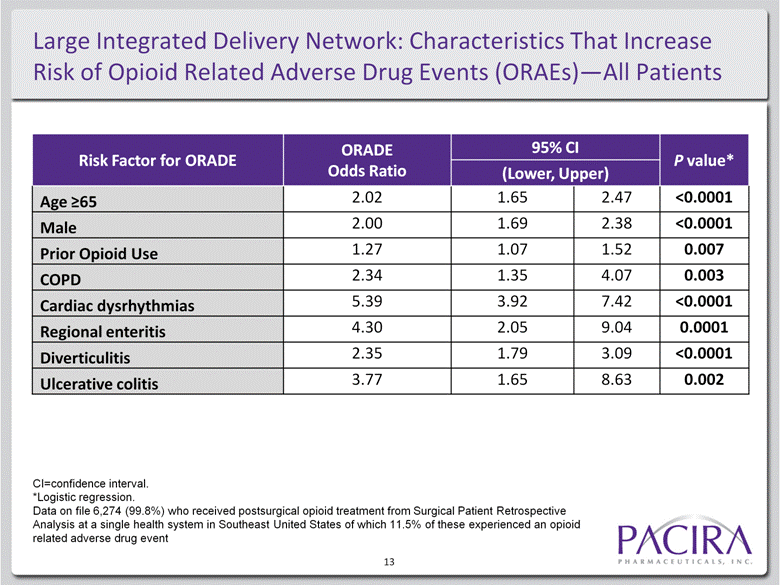
Large Integrated Delivery Network: Characteristics That Increase Risk of Opioid Related Adverse Drug Events (ORAEs)—All Patients 13 Risk Factor for ORADE ORADE Odds Ratio 95% CI P value* (Lower, Upper) Age >65 2.02 1.65 2.47 <0.0001 Male 2.00 1.69 2.38 <0.0001 Prior Opioid Use 1.27 1.07 1.52 0.007 COPD 2.34 1.35 4.07 0.003 Cardiac dysrhythmias 5.39 3.92 7.42 <0.0001 Regional enteritis 4.30 2.05 9.04 0.0001 Diverticulitis 2.35 1.79 3.09 <0.0001 Ulcerative colitis 3.77 1.65 8.63 0.002 CI=confidence interval. *Logistic regression. Data on file 6,274 (99.8%) who received postsurgical opioid treatment from Surgical Patient Retrospective Analysis at a single health system in Southeast United States of which 11.5% of these experienced an opioid related adverse drug event |
|
|
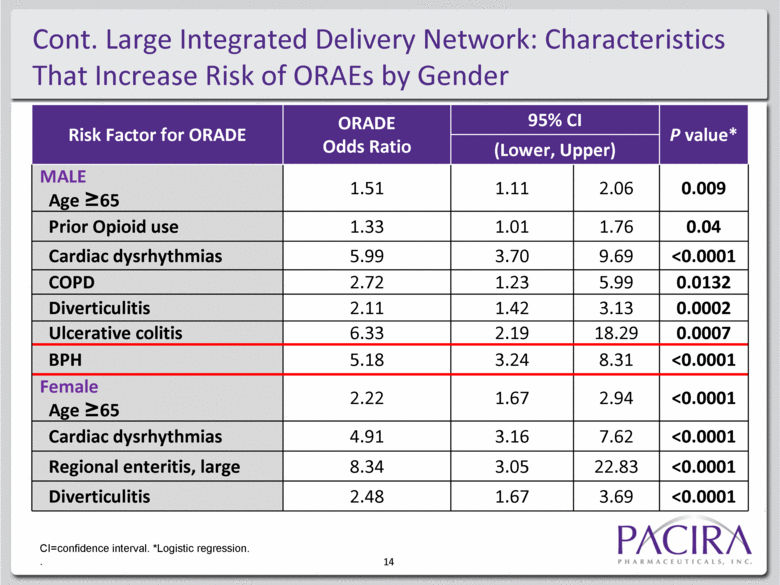
14 Risk Factor for ORADE ORADE Odds Ratio 95% CI P value* (Lower, Upper) MALE Age >65 1.51 1.11 2.06 0.009 Prior Opioid use 1.33 1.01 1.76 0.04 Cardiac dysrhythmias 5.99 3.70 9.69 <0.0001 COPD 2.72 1.23 5.99 0.0132 Diverticulitis 2.11 1.42 3.13 0.0002 Ulcerative colitis 6.33 2.19 18.29 0.0007 BPH 5.18 3.24 8.31 <0.0001 Female Age >65 2.22 1.67 2.94 <0.0001 Cardiac dysrhythmias 4.91 3.16 7.62 <0.0001 Regional enteritis, large 8.34 3.05 22.83 <0.0001 Diverticulitis 2.48 1.67 3.69 <0.0001 CI=confidence interval. *Logistic regression. Cont. Large Integrated Delivery Network: Characteristics That Increase Risk of ORAEs by Gender |
|
|
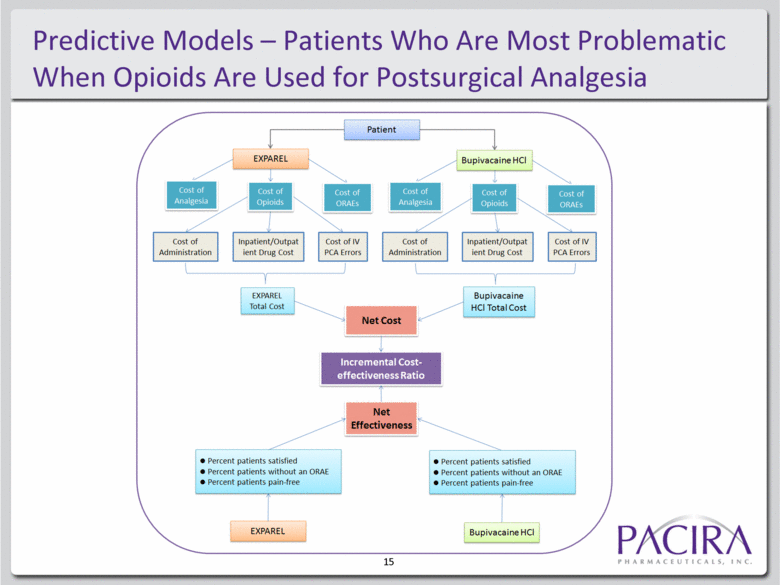
Predictive Models – Patients Who Are Most Problematic When Opioids Are Used for Postsurgical Analgesia 15 |
|
|
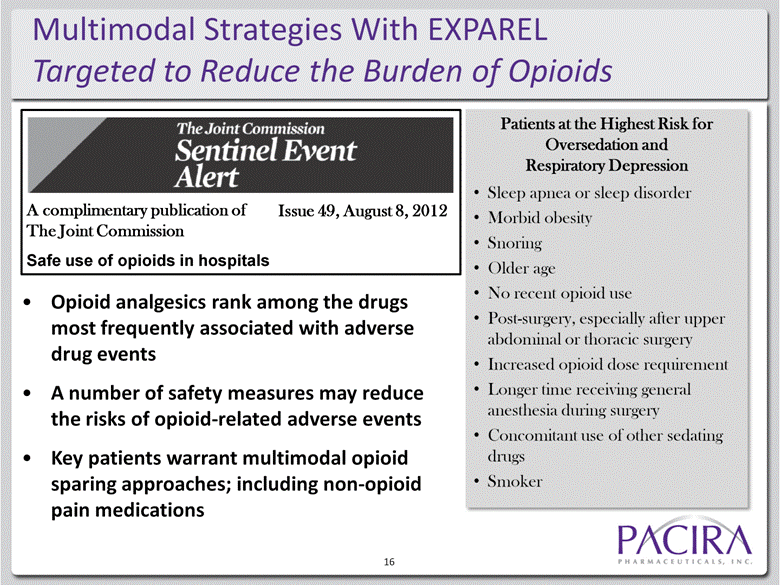
16 Patients at the Highest Risk for Oversedation and Respiratory Depression Sleep apnea or sleep disorder Morbid obesity Snoring Older age No recent opioid use Post-surgery, especially after upper abdominal or thoracic surgery Increased opioid dose requirement Longer time receiving general anesthesia during surgery Concomitant use of other sedating drugs Smoker Multimodal Strategies With EXPAREL Targeted to Reduce the Burden of Opioids Opioid analgesics rank among the drugs most frequently associated with adverse drug events A number of safety measures may reduce the risks of opioid-related adverse events Key patients warrant multimodal opioid sparing approaches; including non-opioid pain medications A complimentary publication of The Joint Commission Safe use of opioids in hospitals Issue 49, August 8, 2012 |
|
|
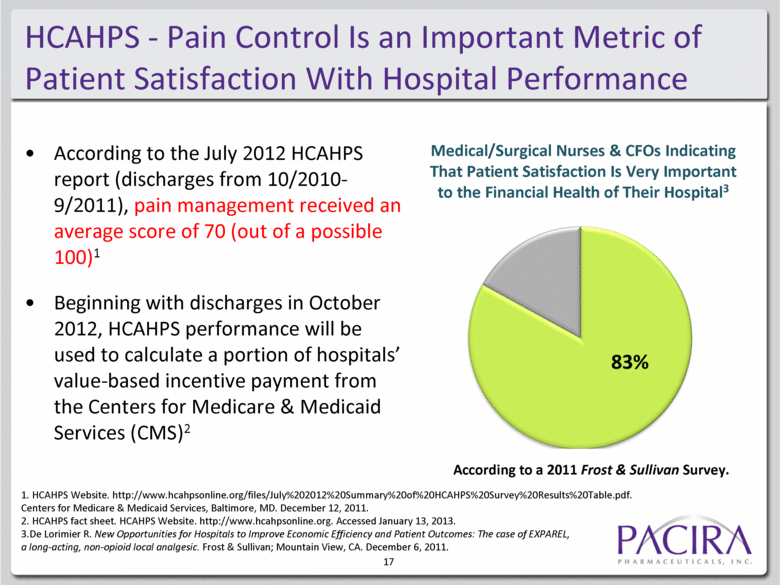
HCAHPS - Pain Control Is an Important Metric of Patient Satisfaction With Hospital Performance According to the July 2012 HCAHPS report (discharges from 10/2010-9/2011), pain management received an average score of 70 (out of a possible 100)1 Beginning with discharges in October 2012, HCAHPS performance will be used to calculate a portion of hospitals’ value-based incentive payment from the Centers for Medicare & Medicaid Services (CMS)2 1. HCAHPS Website. http://www.hcahpsonline.org/files/July%202012%20Summary%20of%20HCAHPS%20Survey%20Results%20Table.pdf. Centers for Medicare & Medicaid Services, Baltimore, MD. December 12, 2011.2. HCAHPS fact sheet. 2. HCAHPS Website. http://www.hcahpsonline.org. Accessed January 13, 2013. 3. De Lorimier R. New Opportunities for Hospitals to Improve Economic Efficiency and Patient Outcomes: The case of EXPAREL, a long-acting, non-opioid local analgesic. Frost & Sullivan; Mountain View, CA. December 6, 2011. Medical/Surgical Nurses & CFOs Indicating That Patient Satisfaction Is Very Important to the Financial Health of Their Hospital3 According to a 2011 Frost & Sullivan Survey. 17 |
|
|
Strategy and Results to Date |
|
|
EXPAREL Launch Metrics Access and orders in top accounts3 75% top 100 hospitals 53% top 500 hospitals Repeat order customers3 191 ordered >6X 110 ordered >10X Since launch3: New customers per week: 22 Total customers ordering: 819 19 Quarterly Sales, Millions 2. Average Daily Box Sales, Month Over Month Growth2 1. Estimated Q4 2012 net sales is based on estimated Q4 2012 gross revenue of $8.15M and estimated gross-to-net discount of 5.3%. Actual gross-to-net discounts for Q2 2012 and Q3 2012 were 7.1% and 5.1%, respectively. Actual Q4 net sales may differ from estimated Q4 2012 net sales. Average daily box sales reflects the total boxes sold in the month, divided by the business days in a month, excluding holidays when product cannot be shipped. The business days in September, October, November and December were 19, 23, 20 and 19, respectively. 3. Data as of December 31, 2012. |
|
|
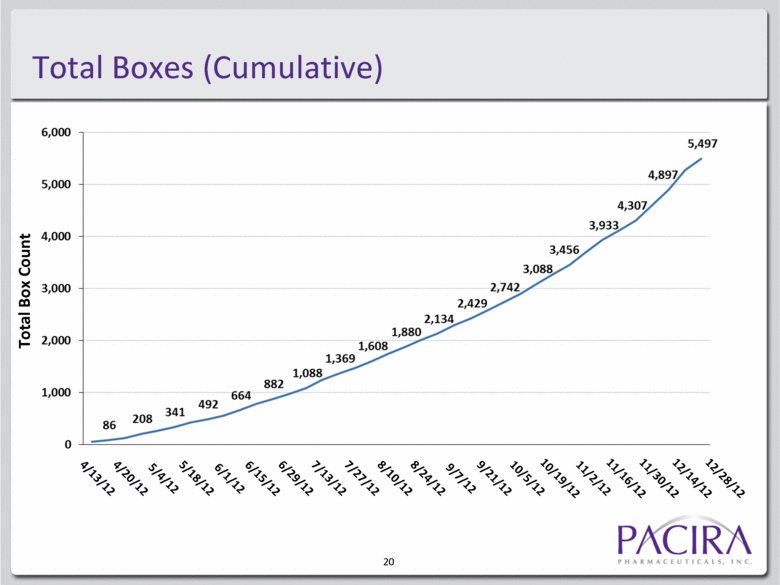
Total Boxes (Cumulative) Total Box Count 20 12/28/12 4/13/12 4/20/12 5/4/12 5/18/12 6/1/12 6/15/12 6/29/12 7/13/12 7/27/12 8/10/12 8/24/12 9/7/12 9/21/12 10/5/12 10/19/12 11/2/12 11/16/12 11/30/12 12/14/12 |
|
|
Total Accounts Ordered (Cumulative) 21 Total Number of Accounts 819 |
|
|
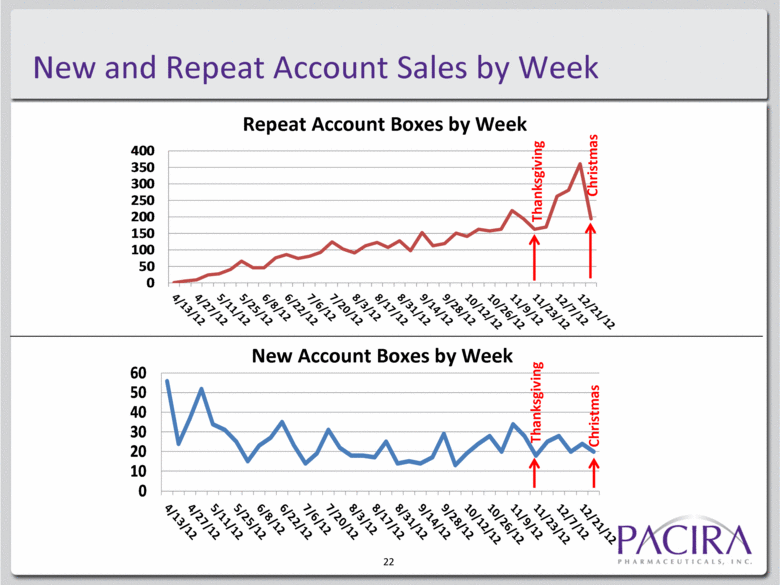
New and Repeat Account Sales by Week 22 Repeat Account Boxes by Week New Account Boxes by Week Thanksgiving Thanksgiving Christmas Christmas 0 50 100 150 200 250 300 350 400 0 10 20 30 40 50 60 |
|
|
Clinical Development |
|
|
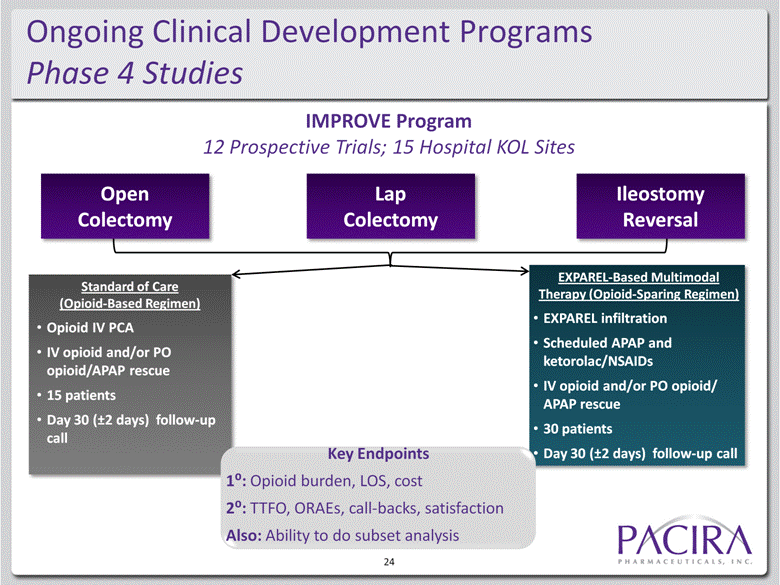
Ongoing Clinical Development Programs Phase 4 Studies 24 IMPROVE Program 12 Prospective Trials; 15 Hospital KOL Sites Open Colectomy Lap Colectomy Ileostomy Reversal Standard of Care (Opioid-Based Regimen) Opioid IV PCA IV opioid and/or PO opioid/APAP rescue 15 patients Day 30 (±2 days) follow-up call EXPAREL-Based Multimodal Therapy (Opioid-Sparing Regimen) EXPAREL infiltration Scheduled APAP and ketorolac/NSAIDs IV opioid and/or PO opioid/ APAP rescue 30 patients Day 30 (±2 days) follow-up call Key Endpoints 1: Opioid burden, LOS, cost 2: TTFO, ORAEs, call-backs, satisfaction Also: Ability to do subset analysis |
|
|
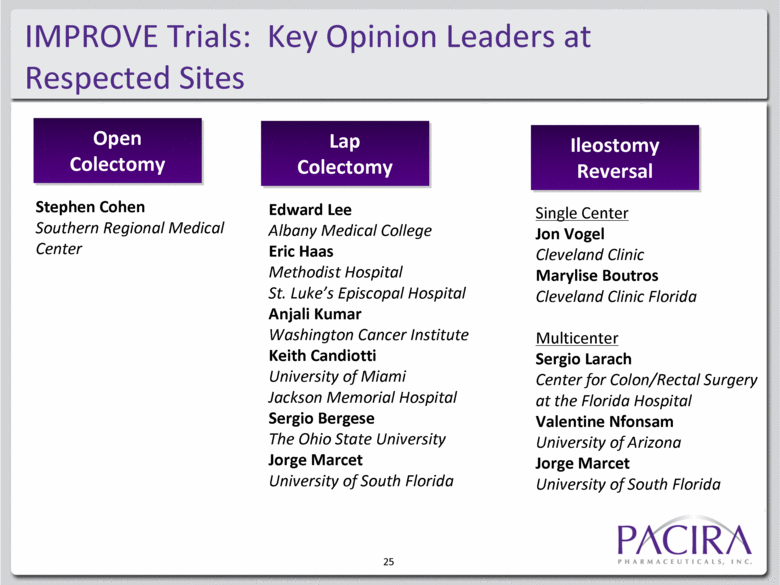
IMPROVE Trials: Key Opinion Leaders at Respected Sites Open Colectomy Lap Colectomy Ileostomy Reversal Stephen Cohen Southern Regional Medical Center Edward Lee Albany Medical College Eric Haas Methodist Hospital St. Luke’s Episcopal Hospital Anjali Kumar Washington Cancer Institute Keith Candiotti University of Miami Jackson Memorial Hospital Sergio Bergese The Ohio State University Jorge Marcet University of South Florida Single Center Jon Vogel Cleveland Clinic Marylise Boutros Cleveland Clinic Florida Multicenter Sergio Larach Center for Colon/Rectal Surgery at the Florida Hospital Valentine Nfonsam University of Arizona Jorge Marcet University of South Florida 25 |
|
|
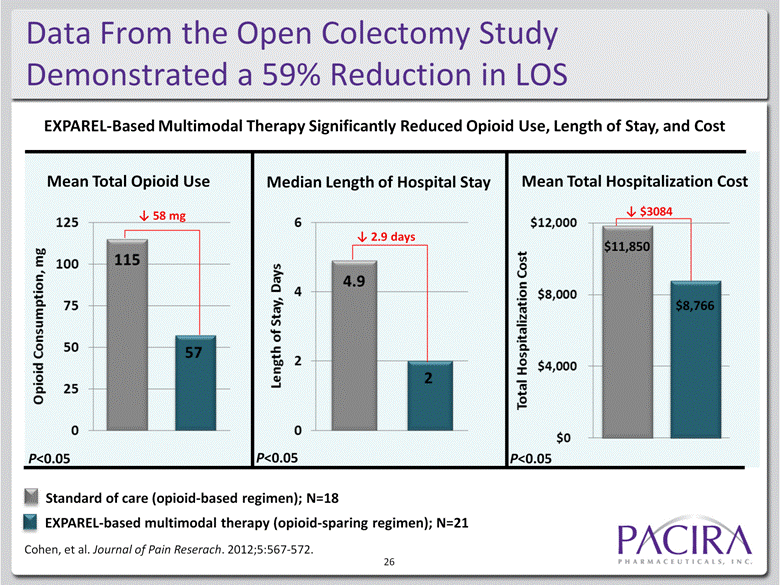
Data From the Open Colectomy Study Demonstrated a 59% Reduction in LOS Median Length of Hospital Stay Mean Total Opioid Use Mean Total Hospitalization Cost P<0.05 58 mg 2.9 days $3084 P<0.05 P<0.05 $11,850 $8,766 EXPAREL-Based Multimodal Therapy Significantly Reduced Opioid Use, Length of Stay, and Cost Standard of care (opioid-based regimen); N=18 EXPAREL-based multimodal therapy (opioid-sparing regimen); N=21 Cohen, et al. Journal of Pain Reserach. 2012;5:567-572. 26 |
|
|
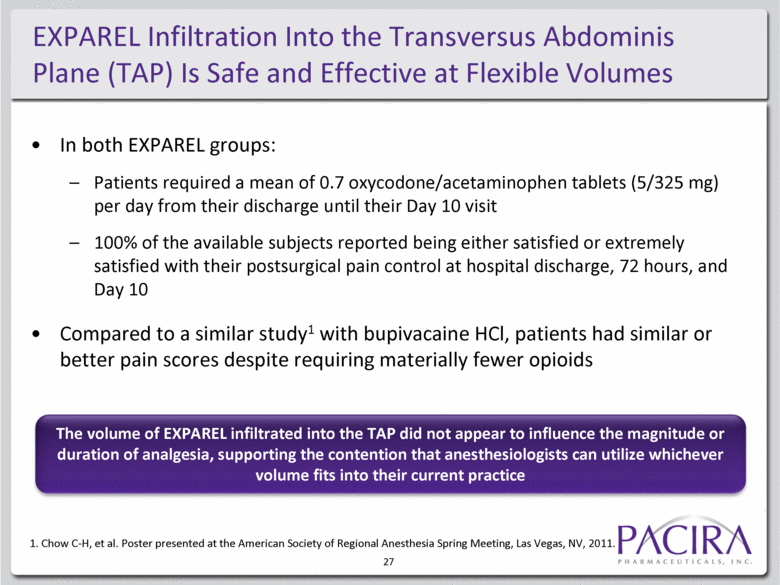
EXPAREL Infiltration Into the Transversus Abdominis Plane (TAP) Is Safe and Effective at Flexible Volumes In both EXPAREL groups: Patients required a mean of 0.7 oxycodone/acetaminophen tablets (5/325 mg) per day from their discharge until their Day 10 visit 100% of the available subjects reported being either satisfied or extremely satisfied with their postsurgical pain control at hospital discharge, 72 hours, and Day 10 Compared to a similar study1 with bupivacaine HCl, patients had similar or better pain scores despite requiring materially fewer opioids 1. Chow C-H, et al. Poster presented at the American Society of Regional Anesthesia Spring Meeting, Las Vegas, NV, 2011. The volume of EXPAREL infiltrated into the TAP did not appear to influence the magnitude or duration of analgesia, supporting the contention that anesthesiologists can utilize whichever volume fits into their current practice 27 |
|
|
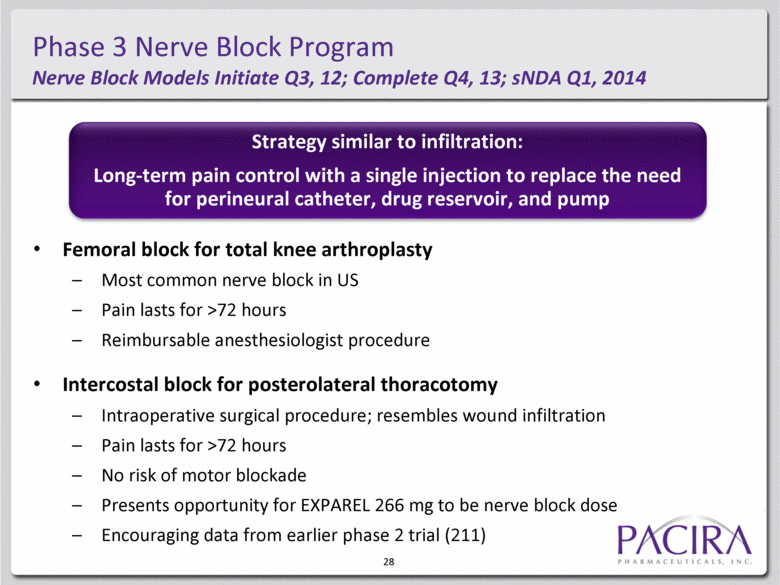
Phase 3 Nerve Block Program Nerve Block Models Initiate Q3, 12; Complete Q4, 13; sNDA Q1, 2014 Femoral block for total knee arthroplasty Most common nerve block in US Pain lasts for >72 hours Reimbursable anesthesiologist procedure Intercostal block for posterolateral thoracotomy Intraoperative surgical procedure; resembles wound infiltration Pain lasts for >72 hours No risk of motor blockade Presents opportunity for EXPAREL 266 mg to be nerve block dose Encouraging data from earlier phase 2 trial (211) Strategy similar to infiltration: Long-term pain control with a single injection to replace the need for perineural catheter, drug reservoir, and pump 28 |
|
|
EXPAREL Launch: 1H 2013 Accelerators Field Force to Pacira Employees Continued Formulary Approvals provide access with key customer accounts – hospitals, GPOs, IDNs, DoD Monthly Revenue growth, Repeat Customers, New Customers Publications and Congresses Opioid Sparing data from GPO and IDN analyses IMPROVE Lap Colectomy and Ileostomy reversal EXCLAIM data in cosmetic dermatology procedures Abstracts and Presentations at ASCRS, AORN, ASPAN, SAMBA First World Congress on Pain iTAP Phase 4 trial in abdominal soft tissue procedures Case Series Data Presentations Orthopedics (TKA, Hip, Spine), Bariatrics, Aesthetics, iTAPS Collaborative training meetings with strategic partners – ultrasound and device manufacturers 29 |
|
|
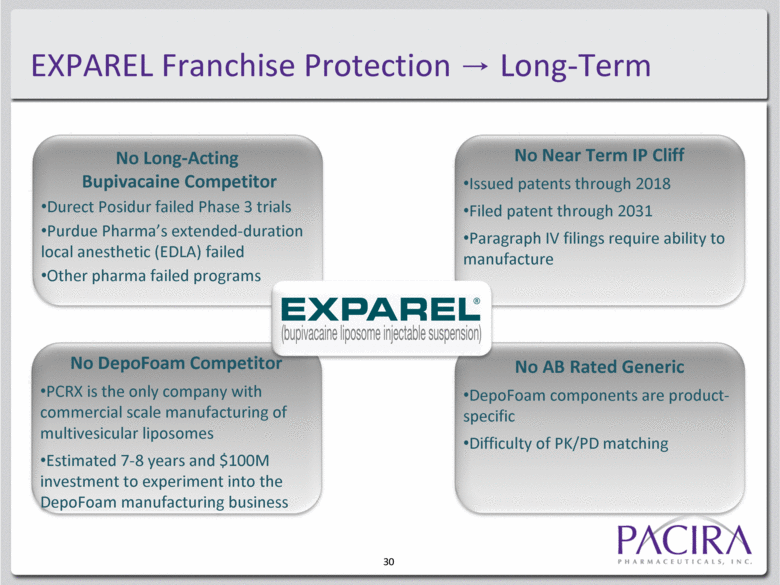
30 No Long-Acting Bupivacaine Competitor Durect Posidur failed Phase 3 trials Purdue Pharma’s extended-duration local anesthetic (EDLA) failed Other pharma failed programs No AB Rated Generic DepoFoam components are product-specific Difficulty of PK/PD matching No Near Term IP Cliff Issued patents through 2018 Filed patent through 2031 Paragraph IV filings require ability to manufacture No DepoFoam Competitor PCRX is the only company with commercial scale manufacturing of multivesicular liposomes Estimated 7-8 years and $100M investment to experiment into the DepoFoam manufacturing business EXPAREL Franchise Protection Long-Term |
|
|
Financial Summary |
|
|
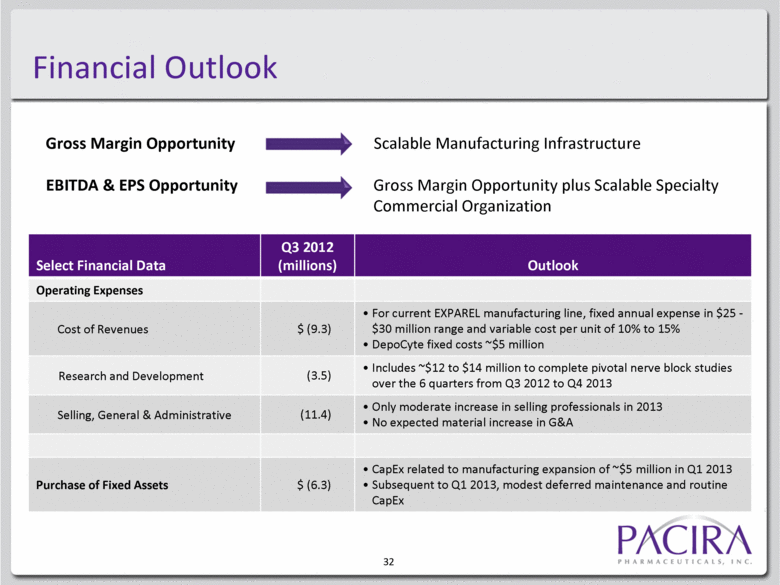
32 Financial Outlook Select Financial Data Q3 2012 (millions) Outlook Operating Expenses Cost of Revenues $ (9.3) For current EXPAREL manufacturing line, fixed annual expense in $25 - $30 million range and variable cost per unit of 10% to 15% DepoCyte fixed costs ~$5 million Research and Development (3.5) Includes ~$12 to $14 million to complete pivotal nerve block studies over the 6 quarters from Q3 2012 to Q4 2013 Selling, General & Administrative (11.4) Only moderate increase in selling professionals in 2013 No expected material increase in G&A Purchase of Fixed Assets $ (6.3) CapEx related to manufacturing expansion of ~$5 million in Q1 2013 Subsequent to Q1 2013, modest deferred maintenance and routine CapEx Gross Margin Opportunity Scalable Manufacturing Infrastructure EBITDA & EPS Opportunity Gross Margin Opportunity plus Scalable Specialty Commercial Organization |
|
|
2013: Year of Commitment to EXPAREL Commercialization Field Force Rolled Over to Pacira Employees 1.28.2013 Focus on top 500 hospitals performing >50% of US surgeries, soft tissue focus Scientific Affairs, Medical Affairs and Clinical Nursing Teams built out to address additional opportunities Orthopedic, iTaps (anesthesia), cardiothoracic, nursing Commercial manufacturing build-out, fully automated manufacturing skids installed and manufacturing for sNDA approval Clinical development for additional indications, nerve block - anesthesia audience Partnership opportunities Partner for human EXPAREL (Ex-US) No competitor in clinical development for EXPAREL or DepoFoam, no generics EXPAREL provides the basis for Pacira as a successful investment and to continue to develop DepoFoam-based products – NSAID, Methotrexate – a multi-product specialty pain company 33 |
|
|
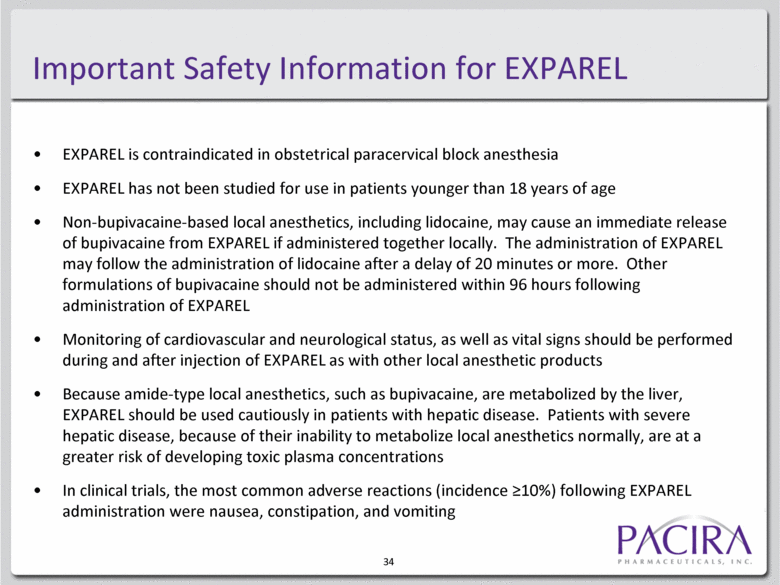
Important Safety Information for EXPAREL EXPAREL is contraindicated in obstetrical paracervical block anesthesia EXPAREL has not been studied for use in patients younger than 18 years of age Non-bupivacaine-based local anesthetics, including lidocaine, may cause an immediate release of bupivacaine from EXPAREL if administered together locally. The administration of EXPAREL may follow the administration of lidocaine after a delay of 20 minutes or more. Other formulations of bupivacaine should not be administered within 96 hours following administration of EXPAREL Monitoring of cardiovascular and neurological status, as well as vital signs should be performed during and after injection of EXPAREL as with other local anesthetic products Because amide-type local anesthetics, such as bupivacaine, are metabolized by the liver, EXPAREL should be used cautiously in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations In clinical trials, the most common adverse reactions (incidence >10%) following EXPAREL administration were nausea, constipation, and vomiting 34 |
|
|
35 Pacira January 2013 Thank You |