Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - BIOCRYST PHARMACEUTICALS INC | d426793d8k.htm |
| EX-99.1 - PRESS RELEASE - BIOCRYST PHARMACEUTICALS INC | d426793dex991.htm |
 BioCryst &
Presidio Merger Overview
October 18, 2012
Exhibit
99.2 |
 2
Disclaimer
Important Additional Information and Where to Find It BioCryst Pharmaceuticals,
Inc. (“BioCryst”) intends to file with the SEC a registration statement on Form S-4, which will also include a proxy statement and prospectus with respect to
the proposed acquisition of Presidio. The final proxy statement/prospectus will be mailed
to the stockholders of BioCryst. Investors and security holders are urged to read the proxy
statement/prospectus regarding the proposed transaction carefully and in its entirety when it
becomes available because it will contain important information regarding BioCryst,
Presidio and the proposed merger. Investors will be able to obtain a free copy of the
proxy statement/prospectus, as well as other filings containing information about BioCryst,
without charge, at the SEC’s website (http://www.sec.gov/).
Investors may also obtain these documents, without charge, from BioCryst’s website at
http://investor.shareholder.com/biocryst/sec.cfm.
This communication shall not constitute an offer to sell or the solicitation of an offer to
buy any securities in the equity financing.
Participants in the Merger Solicitation BioCryst and its
directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies from shareholders
with respect to the transactions contemplated by the merger agreement. Information
regarding BioCryst’s directors and executive officers is contained in BioCryst’s 2011 Annual
Report on Form 10-K filed with the SEC on March 6, 2012 and its definitive proxy statement
filed with the SEC on April 9, 2012 in connection with its 2012 meeting of stockholders.
Other information regarding the participants in the proxy solicitation and a description of
their direct and indirect interests, by security holdings or otherwise, will be contained in the
proxy statement/prospectus and other relevant materials to be filed with the SEC when they
become available.
BioCryst Forward-Looking Statements This press release
contains forward-looking statements, including statements regarding future results, performance or achievements. These statements involve known and unknown
risks, uncertainties and other factors which may cause BioCryst’s actual results,
performance or achievements to be materially different from any future results, performances or
achievements expressed or implied by the forward-looking statements. These
statements reflect our current views with respect to future events and are based on assumptions and
subject to risks and uncertainties. Given these uncertainties, you should not place
undue reliance on these forward-looking statements. Some of the factors that could affect the
forward-looking statements contained herein include: that the merger might not be
completed for any number of reasons, most of which are outside of the control of BioCryst; that
BioCryst may not be able to obtain the requisite financing on commercially reasonable terms or
that or that the financing may be raised at prices below the currently prevailing price
for BioCryst common stock; that integration of BioCryst and Presidio may prove more
challenging than anticipated or that anticipated benefits of the merger may not be achieved, or
may be achieved less rapidly than anticipated; the outcome of any legal proceedings that may
be instituted against BioCryst or Presidio; risks relating to any unforeseen liabilities,
future capital expenditures, revenues, expenses, earnings, economic performance, indebtedness,
financial condition, losses and future prospects, business and management strategies or
the expansion and growth of Presidio’s operations; BioCryst’s ability to integrate Presidio’s business successfully after the closing of the merger agreement; and the risk
that disruptions from the merger agreement will harm BioCryst’s or Presidio’s
businesses. There can be no assurance that the proposed merger and financing will in fact be
consummated. Other important factors include: that there can be no assurance that
BioCryst’s or Presidio’s compounds will prove effective in clinical trials; that development and
commercialization of BioCryst’s or Presidio’s compounds may not be successful; that
BARDA/HHS may further condition, reduce or eliminate future funding of the peramivir program;
that BioCryst, Presidio or licensees may not be able to enroll the required number of subjects
in planned clinical trials of its product candidates and that such clinical trials may not be
successfully completed; that the companies or licensees may not commence as expected
additional human clinical trials with product candidates; that the FDA may require additional
studies beyond the studies planned for product candidates or may not provide regulatory
clearances which may result in delay of planned clinical trials, clinical hold with respect to
such product candidate or the lack of market approval for such product candidate; that ongoing
and future preclinical and clinical development may not have positive results; that the
companies or licensees may not be able to continue future development of current and future
development programs; that such development programs may never result in future
product, license or royalty payments being received; that the companies may not be able to
retain their current pharmaceutical and biotechnology partners for further development
of its product candidates or may not reach favorable agreements with potential pharmaceutical
and biotechnology partners for further development of product candidates; that their
actual cash burn rate may not be consistent with its expectations; that BioCryst or Presidio
may not have sufficient cash to continue funding the development, manufacturing,
marketing or distribution of products and that additional funding, if necessary, may not be
available at all or on terms acceptable to BioCryst or Presidio. Please refer to the
documents BioCryst files periodically with the Securities and Exchange Commission,
specifically BioCryst's most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q,
and current reports on Form 8-K, all of which identify important factors that could cause
the actual results to differ materially from those contained in BioCryst’s projections and
forward-looking statements. |
 Experienced
leadership
Near-term
milestones
Two pronged focus: high-value antivirals & orphan indications
Resourced to reach value
creating events
Three oral, pan-genotypic HCV molecules with distinct MOAs
First oral prophylactic would revolutionize HAE treatment
Peramivir & ulodesine programs have potential to contribute non-dilutive capital
Planned $60 million financing to reach potential value creating events for HCV & HAE
HCV
Unique
portfolio
HAE
Revolutionary
treatment
3 |
 4
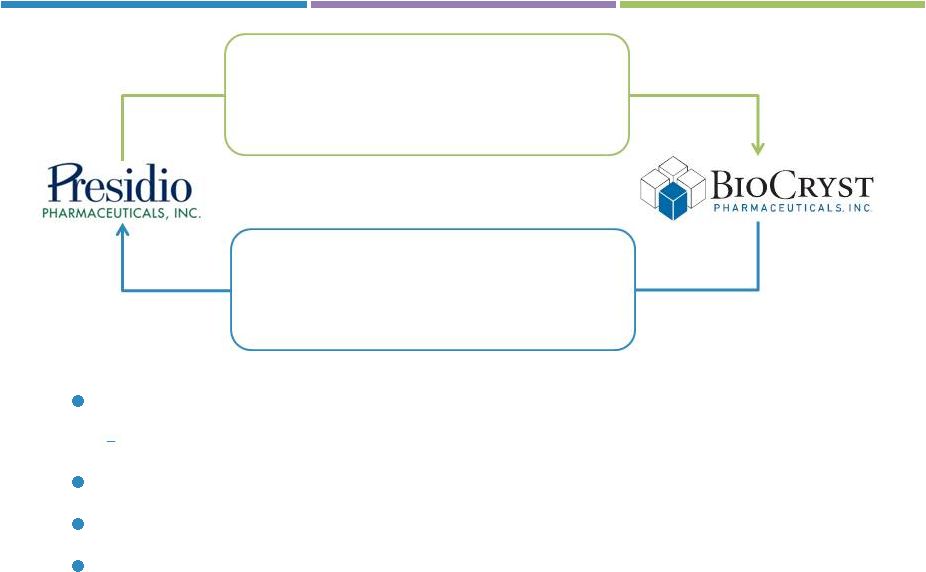
Transaction overview & capitalization plan
•
All Presidio assets
•
$25M cash committed from Presidio
shareholders
•
24.5M BCRX shares valued at $101M
Minimum $60 million equity financing required as a closing condition
Includes $25 million committed by Presidio shareholders
Transaction & financing close expected 1Q13
Headquarters in Durham, NC with sites in San Francisco, CA & Birmingham, AL
NewCo to launch with a new name and ticker at closing |
 Hepatitis industry
leaders joining an established leadership team Nathaniel
Brown,
MD,
Chief
Medical
Officer,
Presidio
-
Former EVP Clinical Development & CMO, Idenix Pharmaceuticals;
Head of Hepatitis Section, Infectious Disease, GlaxoWellcome/GSK
-
23 years of experience in antiviral/antiinfective development U.S. & global
-
Clinical development leader for globally registered antivirals/antiinfectives:
HCV -
Wellferon (interferon alfa-n1)
Pneumocystis pneumonia -
Mepron (atovaquone)
HBV -
Epivir-HBV (lamivudine)
Varicella -
Zovirax (acyclovir)
HBV -
Tyzeka (telbivudine)
HIV -
Retrovir (zidovudine) for children
HIV -
Reyataz (atazanavir),
protease inhibitor
HBV -
Baraclude (entecavir),
polymerase inhibitor
Richard
Colonno,
PhD,
Chief
Scientific
Officer,
Presidio
-
Former VP Infectious Diseases Drug Discovery, Bristol-Myers Squibb;
Senior Director, Merck Research Labs
-
Internationally
recognized
expert
in
the
areas
of
antiviral
drug
discovery
&
viral
resistance, with over 30 years of pharmaceutical industry experience
-
Key leadership role in advancement & global approval of important antivirals:
|
 Experienced
governance & HCV investors: Board nominees Presidio nominees to Board
-
Srinivas Akkaraju, MD, PhD
Managing Director, New Leaf Venture Partners
-
Felix
J.
Baker,
PhD
Managing
Partner,
Baker
Bros.
Advisors
-
Kenneth
Galbraith
General
Partner,
Ventures
West
Capital
BioCryst nominees to Board
-
George Abercrombie
Former President & CEO, Hoffmann-La Roche
-
Fred Cohen, MD, D.Phil
Partner & Managing Director, TPG Biotech
-
Nancy Hutson, PhD
Former Senior Vice President of Global R&D, Pfizer
-
Peder
Jensen,
MD
Former
SVP/GM,
R&D
Japan/Asia/Pacific,
Schering-Plough
-
Kenneth
B.
Lee,
Jr.
General
Partner,
Hatteras
Venture
Partners
-
Jon
Stonehouse
President
&
CEO,
BioCryst
Pharmaceuticals
Jon Stonehouse will be CEO & Kenneth Galbraith will be Board Chairman
6 |
 Hepatitis C
Portfolio Overview |
 Opportunity for
unique, all-oral, pan-genotypic combinations HCV Mechanism of Action
Preclinical
Phase 1
Status
NS5A inhibitor
Ph 2 ready
Nucleoside NS5B inhibitor
Ph 1 ready 4Q12
Non-nucleoside NS5B inhibitor
Ph 1 ready 1H13
PPI-668
+
BCX5191
PPI-668
+
PPI-383
PPI-668
+
BCX5191
+
PPI-383
PPI-668
PPI-383
BCX5191
Strategy:
Build
HCV
combination
therapies
around
lead
NS5A
compound,
PPI-668
8 |
 Aggressively
pursue external collaborations to identify optimal regimens Opportunity to address
significant HCV market segments: Pan-genotypic & genotype-specific
combination therapies Regional opportunities
Patient subpopulations
+
NS5A inhibitor: Potential foundation for curative combinations
BCX5191
and/or
PPI-383
Additional
DAAs from
external
sources
Internal
combinations
Internal/external
combinations
9
PPI-668 |
 PPI-668 is an
“optimized” NS5A inhibitor
Oral, QD dosing in humans
Pan-genotypic coverage of all
major HCV genotypes
–
Equivalent to daclatasvir (BMS-052)
Additive to synergistic with other
classes of HCV antivirals, no
antagonism noted
Excellent safety profile in 3-month
animal studies & well tolerated in
human trials
PK profile results in strong clinical
potency & coverage of pre-existing
resistant variants
HCV Genotype
0.01
0.1
1
10
1a
1b
2a
3a
4a
5a
6a
7a
PPI-668
BMS-052
10 |
 Completed
single & multi-dose administration (40-320 mg once daily)
All doses generally safe & well
tolerated
PK results support once-daily dosing
Rapid (2 hr) attainment of high plasma
levels (C
max
2-7 µM), with first dose
Excellent trough coverage (60-415 nM),
exceeds the EC for all HCV genotypes
Dose-proportional systemic exposures
No significant food effect
Efficacy implications
Rapid efficacy starting with first dose
against WT & resistant variants harboring
single amino acid substitutions
Hours Post Dosing
Rapid tissue distribution
(liver and other tissues)
Prolonged
-phase half-life,
maintains inhibitory levels
Favorable PPI-668 pharmacokinetic (PK) profile –
Phase 1a results
Micromolar blood levels within 2 hr,
for rapid HCV inhibition
0
1000
2000
3000
4000
5000
6000
0
6
12
18
24
30
80 mg
160 mg
320 mg
11
90 |
 PPI-668:
Optimal PK profile with once-daily dosing Time Post Dosing (hr)
Only C
24 hr
PK time points examined
5-day once-daily oral
dosing @ 320 mg
Intensive PK sampling
after 1st & 5th doses;
trough levels (C
24hr
) on
other days
Steady
state
achieved
by
Day 2, no subsequent
accumulation or
induced elimination
No need for loading
dose
to
achieve
maximal
efficacy
10
100
1000
10000
0
10
20
30
40
50
60
70
80
90
100
110
120
12
Steady state
achieved
Dose 1
Dose 2
Dose 3
Dose 4
Dose 5 |
 PPI-668 Phase
1b: POC & antiviral activity demonstrated Phase 1b design
10 patients per dose cohort, randomized 8:2 (active : placebo)
40, 80, 160 or 240 mg QD x 3 days, with 14 days follow up
3-day dosing consistent with draft FDA HCV guidance
Dose group
Dose group
Mean maximal HCV RNA
Mean maximal HCV RNA
reduction,
reduction,
3 days of treatment
3 days of treatment
40 mg/day
3.2 log
10
IU/mL
80 mg/day
3.5 log
10
IU/mL
160 mg/day
3.5 log
10
IU/mL
240 mg/day
3.7 log
10
IU/mL
Detailed results to be presented at AASLD
meeting November 9-13
Results
Well tolerated at all doses
Unsurpassed viral load reduction in
first 24-30 hr, indicating potent
antiviral activity
HCV RNA reductions typically
exceeded 3 log
10
during first day of
dosing
Coverage of resistant variants
demonstrated
Four patients with high levels of
pre-existing resistant variants (single
substitutions) responded well
13 |
 Two complementary
inhibitors of HCV NS5B polymerase NS5B protein serves as the viral polymerase & is
essential for HCV replication •
Nuc Catalytic Site (A)
•
NNuc Binding Sites
–
Palm I (B)
–
Palm II (C)
–
Thumb I (D)
–
Thumb II (E)
•
Opportunity to
develop a superior
NNuc as a 3rd class of
oral, potent, pan-
genotypic agents
•
Opportunity to
develop highly
potent Nuc-based
combinations with a
high barrier to
resistance
Allosteric
non-nucleoside
inhibitor
PPI-383
Adenosine
nucleoside
inhibitor
BCX5191
14 |
 PPI-383
(NNuc) exhibits pan-genotypic activity in replicon assays HCV Genotype
NNucs currently in advanced
development are HCV GT-1 specific
PPI-383 distinct feature: near
equivalent coverage of HCV
genotypes tested
Additive to synergistic with other
classes of HCV antivirals
Favorable pharmacological profile:
Metabolic stability
No CYP inhibition
Low protein binding
Animal PK profile predictive of QD-
BID dosing in humans
PPI-383 is undergoing GLP toxicology studies to enable Phase 1 studies to initiate 1H13
10000
1000
100
10
1
1a
1b
2a
3a
4a
PPI-383
VX-222
15 |
 NS5B
Enzyme *Data from Lam et al 2010 AAC 54:3187-3196
BCX5191 (Nuc): Pan-genotypic at sub-micromolar concentrations
Adenosine nucleoside analog
Pan-genotypic coverage
Sub-micromolar potency on NS5B
Compares favorably with GS-7977
Favorable pharmacological profile:
Metabolic stability
No CYP inhibition
Parent drug plasma concentrations
track liver concentrations
Once-daily dosing
Preclinical PK predicts antiviral efficacy
at low daily doses
Initiation of Phase 1 trial program
planned for 4Q12
BCX5191
GS-7977
10000
1000
100
10
1
1a
1b
2a
3a
4a
16
* |
 Hereditary
Angioedema Overview |
 BCX4161 could be
the first oral prophylactic HAE therapy
Problems with current parenteral KK inhibitors:
IV infusions create a significant treatment burden
IV access maintenance
Risk of infection
SC injection reactions
Goals of BCX4161 development program:
Oral administration
Highly effective attack prevention
Safe
18
A safe, effective ORAL kallikrein inhibitor could revolutionize the lives of patients
with hereditary angioedema
Image
obtained
from
www.haeimages.com
&
used
with
permission |
 BCX4161 targets
kallikrein, which is fully validated clinically in HAE Factor XIIa
Plasmin
High-Molecular-Weight
Kininogen
Prekallikrein
Kallikrein
Bradykinin
Stop vasodilatation, nonvascular
smooth muscle contraction & edema
Trauma
BK receptor
1
2
Inhibit kallikrein activity
Prevent BK from binding receptors
2
Cinryze —
IV prophylaxis
Berinert —
IV acute therapy
Kalbitor —
SC acute therapy
BCX4161 —
Oral prophylaxis
Firazyr —
SC acute therapy
19
1 |
 BCX4161 has
demonstrated preclinical POC for oral dosing Inhibition of kallikrein activity through 24
hours post-dose PK of BCX4161 in rats –
30, 100 & 300 mg/kg oral dosing
Pharmacodynamics of BCX4161 in rats –
100 mg/kg oral dosing
50%
Kl
100000
10000
1000
100
10
0
4
8
12
16
20
24
Rat Kl
EC
50
0.1
1
10
100
30 mg/kg
100 mg/kg
300 mg/kg
100
80
60
40
20
0
0
4
8
12
16
20
24
Time, hr
Time, hr
20 |
 BCX4161 inhibits kallikrein
in human plasma
Median EC
50
~ 6 nM
BCX4161 at 50-100 nM maximally
inhibits kallikrein
Phase 1 trial will deliver:
Preliminary safety
PK from oral dosing
Degree of kallikrein inhibition
Dose selection for HAE patient trials
BCX4161 inhibits Kallikrein at very low doses
21
Inhibition of kallikrein in plasma from 51
normal subjects: EC
50
< 12 nM in all cases
Kallikrein inhibition assay will be used as a
PD biomarker in the clinical program to select effective doses |
 Biomarker
assay developed to support clinical program First clinical studies will determine
pharmacokinetics & pharmacodynamics, as well as BCX4161 profile for HAE: Entering Phase
1 4Q12 Profile
Results
Attractive preclinical pharmacology
profile
BCX4161 inhibits kallikrein in human plasma
Median EC50 ~ 6 nM
Preclinical POC for oral dosing
Nonclinical safety
IND-enabling program complete
Therapeutic window assessed
Doses selected for first clinical studies
22
likelihood of success |
 Pipeline with
development focus on HCV & HAE Disease
Program
Pre-IND
Phase 1
Phase 2
Pivotal
HCV
PPI-668
NS5A inhibitor
BCX5191
Nucleoside NS5B
PPI-383
Non-nucleoside NS5B
HAE
BCX4161
Oral kallikrein inhibitor
Other
Peramivir, i.v.
Outpatient, seasonal influenza
Peramivir, i.v.
Inpatient, influenza
Ulodesine, BCX4208
Gout
1. Peramivir is approved in Japan & Korea
23
1
Approved |
 Important
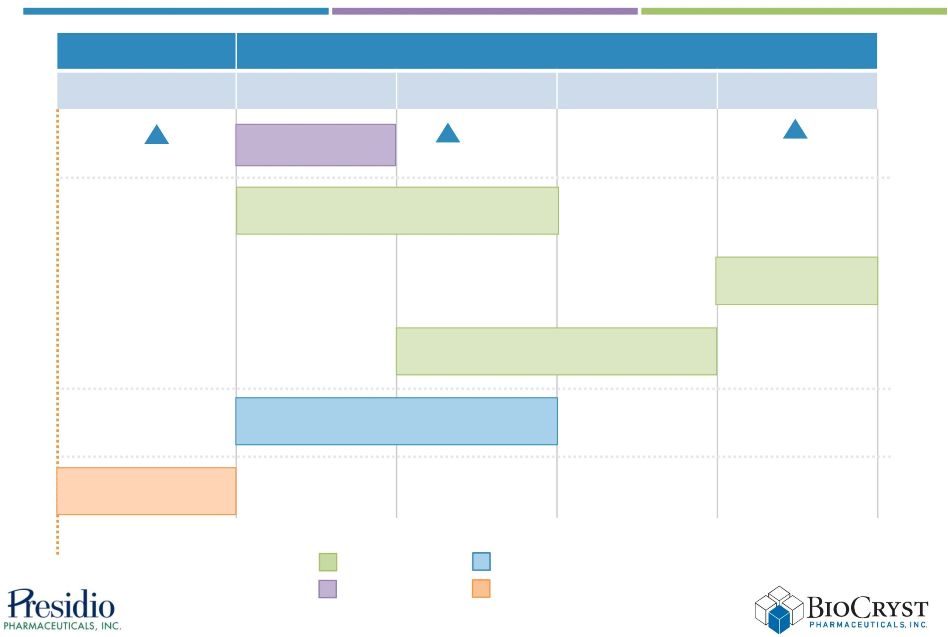
near-term events support value creation 2012
2013
4Q
1Q
2Q
3Q
4Q
BCX5191 Phase 1 results
AASLD
BCX4161 Phase 1 results
Peramivir Phase 3
interim analysis
Complete merger
PPI-383 Phase 1 results
PPI-668 Phase 2a
combo results
EASL
AASLD
HAE events
Influenza events
HCV events
Business events
24 |
 A
well-capitalized HCV player with numerous value creating events Experienced
leadership
Near-term
milestones
Attractive assets
Resourced to reach value
creating events
HCV
Unique
portfolio
HAE
Revolutionary
treatment
NewCo
25
Shareholder value |
