Attached files
| file | filename |
|---|---|
| EX-99.2 - PRESS RELEASE - SEQUENOM INC | d408447dex992.htm |
| 8-K - FORM 8-K - SEQUENOM INC | d408447d8k.htm |
 From
Academic
Research
Through
Translational
Applications
To
Clinical
Diagnostics
CONFIDENTIAL
Exhibit 99.1
Improving Healthcare Through Revolutionary Genetic Analysis Solutions
|
 Forward-Looking Statements
xcept
for
historical
information,
matters
set
forth
in
this
presentation,
including
statements
regarding Sequenom’s plans, potential, opportunities, financial or other
expectations, projections, goals, objectives, milestones, strategies, market
growth, timelines, product pipeline, clinical studies, product development,
and the potential benefits of its products and products under development,
are
forward-looking
statements
within
the
meaning
of
the
“safe
harbor”
provisions
of
the
Private
Securities Litigation Reform Act of 1995. These forward-looking statements are
subject to risks and uncertainties that may cause actual results to differ
materially, including the risks and uncertainties associated with
Sequenom’s operating performance and financial position, the market demand for
and acceptance of Sequenom’s and Sequenom CMM’s products and services,
research, development
and
commercialization
of
new
products,
reliance
upon
the
collaborative
efforts
of
others, competition, intellectual property rights, government regulation, obtaining
or maintaining regulatory approvals, litigation, and other risks detailed in
Sequenom’s SEC filings. These forward- looking statements are based
on current information that is likely to change, speak only as of the date
hereof, and Sequenom undertakes no obligation to revise or update such statements.
2 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
E |
 Large Potential Addressable Target Market
MaterniT21 PLUS is a Leading Prenatal, Noninvasive
Laboratory Developed Test with IP Protection
•
Clinical leader, first mover advantage, large and effective
sales force and commercial infrastructure for growth
Broad Prenatal Diagnostic Testing Services Portfolio
Genetic Analysis Segment Provides Diversified Revenue
Stream
Convertible Note Offering May Fund Sequenom to Cash Flow
Breakeven
Experienced Management Team
Investment Highlights
3 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
 Two
operating segments: •
Genetic Analysis
-
Sequenom, Inc.
-
Instrument, Reagents, Services for Genetic Analysis
-
San Diego, CA and Sales Offices US, EU and Asia
•
Molecular Diagnostics
-
Sequenom Center For Molecular Medicine (Sequenom CMM)
-
Clinical Diagnostic Service Laboratories
FY 2011 Total revenues up 18%
Y/Y to $55.9M 1H 2012
Total revenues up 24% Y/Y to $33.2M** 1H
2012 33,000 total test samples received**
YTD 2012
27,000 MaterniT21 PLUS samples received*
Sequenom, Inc.
* Sample volumes as of 8/18/12
** Partial year results are not necessarily indicative of full year or
future performance 4 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
Start-up Phase:
San Diego, CA, & Grand Rapids, MI
Raleigh, NC
Operational: |
 Genetic Analysis Business
Good penetration of target markets
•
Translational and Basic Research
•
Agricultural Genomics
•
Pharmaceutical and Biotech
Launched MassARRAY Analyzer 4
*
in 2010 with improved margins
Over 1,700 peer reviewed published articles on using Sequenom
technology
Active installed base over 330: an important source of new molecular
diagnostic opportunities
Expanding menu of research use only (RUO) panel content offering
* The MassARRAY Analyzer 4 is for Research Use Only. Not for use in
diagnostic procedures ** Partial year results are not necessarily
indicative of full year or future performance (mm)
2009
2010
2011
1H 2012
**
Revenue
$ 37.8
$ 44.9
$ 47.6
$ 20.3
GM %
63%
67%
72%
73%
Op Income
$ 6.4
$ 12.8
$ 15.5
$ 2.9
5 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
 Ophthalmology Dx
Research
Development Launch
Molecular Diagnostics Product Portfolio
RetnaGene Age-related Macular Degeneration (AMD) LDT
Laboratory-Developed Test (LDT) by Sequenom CMM
In Vitro Diagnostic (IVD) by Sequenom, Inc.
* Sequenom CMM, a wholly owned subsidiary of Sequenom, Inc. operated a
CLIA-certified, CAP accredited, molecular diagnostics laboratory that
develops and validates its laboratory-developed tests for use solely by Sequenom CMM
6 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
Fetal-Maternal Dx
SensiGene Cystic Fibrosis Carrier Screening LDT
SensiGene Fetal RHD Genotyping LDT
MaterniT21 PLUS LDT
Trisomy 21 (IVD) |
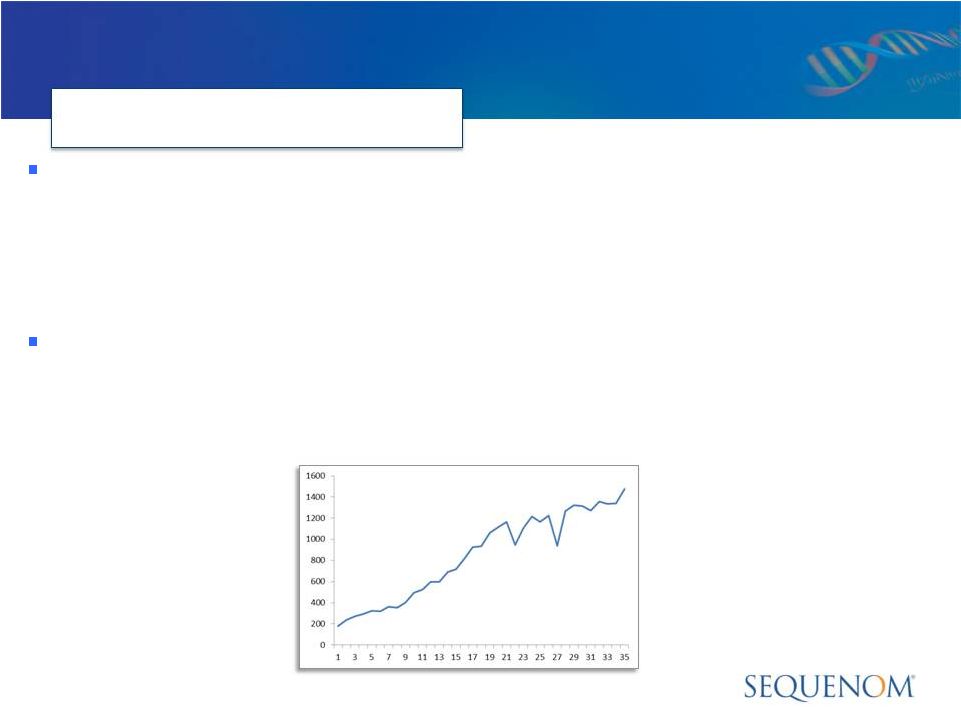 Sequenom CMM Testing Volume
FY 2011 vs YTD 2012
Samples Per Week of MaterniT21 PLUS
* Partial year results are not necessarily indicative of full year or future
performance All
Laboratory-Developed
Tests
(LDTs)
–
(CF,
RHD,
AMD,
T21+)
MaterniT21
™
PLUS LDT:
7 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
FY 2011:
21,000 total samples billed
1H 2012:
33,000 total samples received*
YTD 2012: 46,000 total samples received*
(as of 8/18/12) FY 2011:
1,000 MaterniT21 PLUS samples received (launch 10/17/11)
YTD 2012: 27,000 MaterniT21 PLUS samples
received* •
•
•
•
• |
 Sequenom, Inc.
Status of Key Goals Established for 2012
8 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
Raise additional capital to support expansion of diagnostics
business (H1)
Expand European licensing partnerships (Q3)
LifeCodexx launch
Introduction of new RUO panels for MassARRAY platform
(FY2012)
Obtain issuance of Lo random sequencing patent (FY2012)
FDA 510(k) submission for MassARRAY (Q4 2012) (mid-2013)
|
 Status of Key Goals Established for 2012
Sequenom CMM
9 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
Increase field sales force from 20 to 50+ 75+
(Q1/Q3)
Publication of MaterniT21 PLUS T18/13 clinical study results (Q1)
Complete AMD progression study (AREDS) (Q2)
Increase throughput and reduce COGS for the MaterniT21
PLUS LDT (FY2012)
Invoice a minimum of 25,000 40,000 50,000
MaterniT21
PLUS tests (FY2012)
Reimbursement agreements for MaterniT21 PLUS LDT
with two national payers (FY2012)
Complete build-out of North Carolina laboratory facility (Q4)
|
 Key
Driver for Sequenom Growth -- 10 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
 MaterniT21
™
PLUS LDT
Description:
A noninvasive laboratory developed test to identify pregnancies
at increased risk for fetal trisomies:
T21, T18 and T13 reported
Performance:
Detection
rate
for:
T21:
99.1%*
(210/212)
T18:
>99.9%
(59/59)
T13:
91.7%
(11/12)
No result rate 0.9%. Validated in large scale clinical study**
Accuracy Rate for fetal sex determination:
Market:
Pregnant women at increased risk for fetal aneuploidy
Use:
10 weeks gestation
Test Sample:
2x 10 ml blood draw in doctor’s office
Test Analyte:
Circulating cell-free DNA in a maternal blood sample
Turnaround:
Average ~7 business days (lab operates two shifts 6 days/wk)
** Palomaki et al. Genet Med. 2011; 13(11): 913-20.
* Results following application of GCRM correction
to sequencing data. 11 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
99.4% |
 Market Leadership Advantage for MaterniT21 PLUS
First to market/innovation (launch Oct 2011)
Most experienced (27,000+ tests run)
Strongest balance sheet ($98.6M cash and investments at 6/30/12)
Largest sales force (~75 field reps nationwide)
Greatest equipment capacity (>200,000 tests/year at 12 plex)
Richest content (T21/18/13/twins/Y)
Shortest turn-around time (7 business days)
Most comprehensive published clinical study (4,664 patients)
Lowest published no-call / no-result rate (0.9%)
Solid IP position (‘540 in U.S. and Europe)
Greatest market penetration (>25% MFMs)
Robust infrastructure (customer service, medical affairs, R&D)
12 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
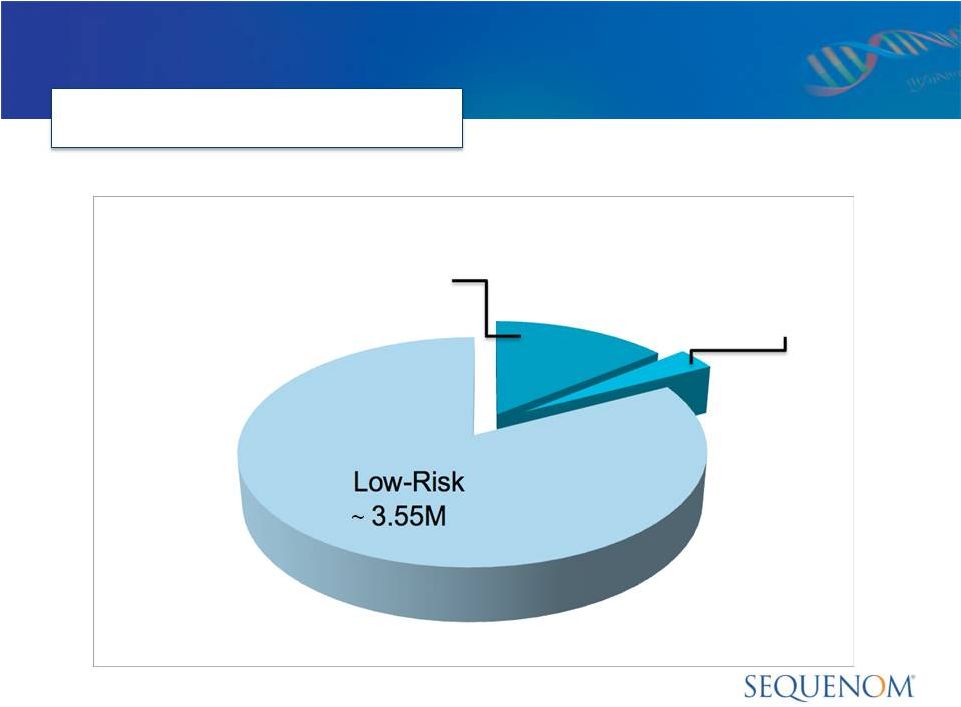 MaterniT21
™
PLUS LDT Market Opportunities
* National Center for Health Statistics, National Vital Statistics Reports;
www.cdc.gov/nchs Other High-Risk
~140,000
High-Risk
Age 35-49
~ 610,000
13 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
Potential Available Market
U.S.
2007
Total
Births:
~
4.3M
* |
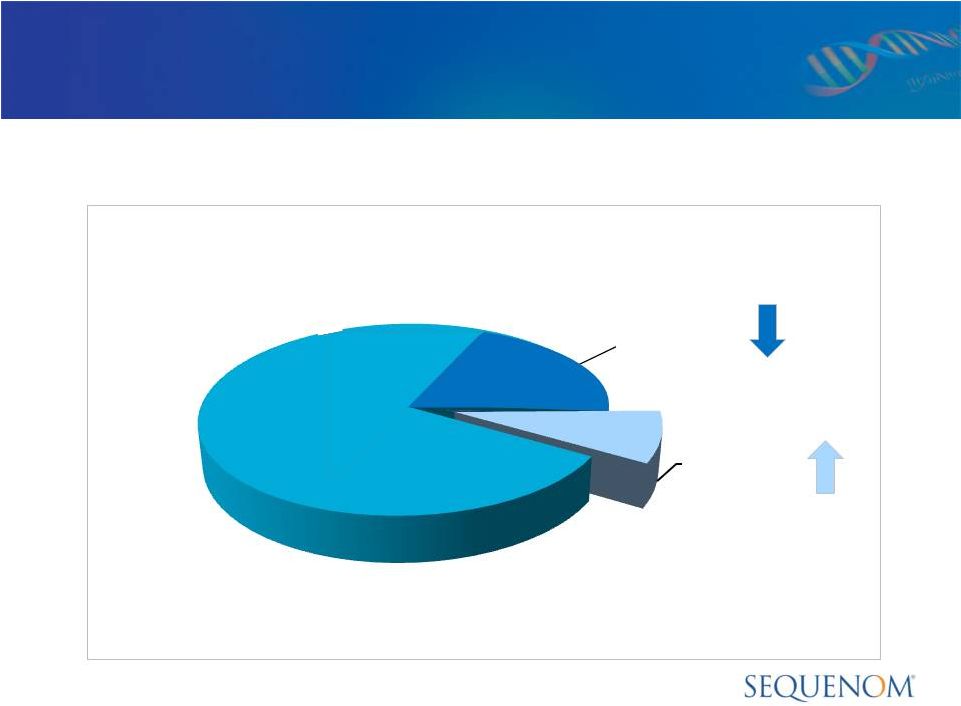 Market Penetration in High Risk U.S. Market
14 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
U.S. High Risk Births: ~750,000
Invasive
Procedures
~200,000
SQNM
Annualized
Run
Rate
~70,000 |
 MaterniT21
™
PLUS LDT: Precision Proven in Large,
Target Market Validation Studies
1.
Palomaki GE,
et al.,
DNA Sequencing of Maternal Plasma to Detect Down Syndrome: An International
Clinical Validation. Genet Med 2011; Nov;13(11):913-920.
2.
Palomaki G, et al., DNA Sequencing of Maternal Plasma Reliably Identifies Trisomy
18 and Trisomy 13, as
well
as
Down
syndrome:
An
International
Collaborative
Study.Genet
Med.
2012
Mar;14(3):296-305
3.
Canick JA, et al. DNA sequencing of maternal plasma to identify Down syndrome and other trisomies in
multiple gestations. [published online ahead of print May 14, 2012] Prenat Diagn. Dol: 10.1002/pd.3892.
15 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
WIHRI Large Validation Study
N = 4,664 Patients
T21
Genet Med
1
Nov 2011
T18, T13
Genet Med
2
Mar 2012
Multiples
Prenat Diagn
3
Online May 2012
Mosaics
In preparation
Sex
Chromosomes
In preparation |
 Peer-Reviewed Literature Basis for
Sequenom CMM Laboratory-Developed Tests (LDTs)
16 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
 MaterniT21
™
PLUS LDT: Ordered by Physician,
Reported to Physician
17 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
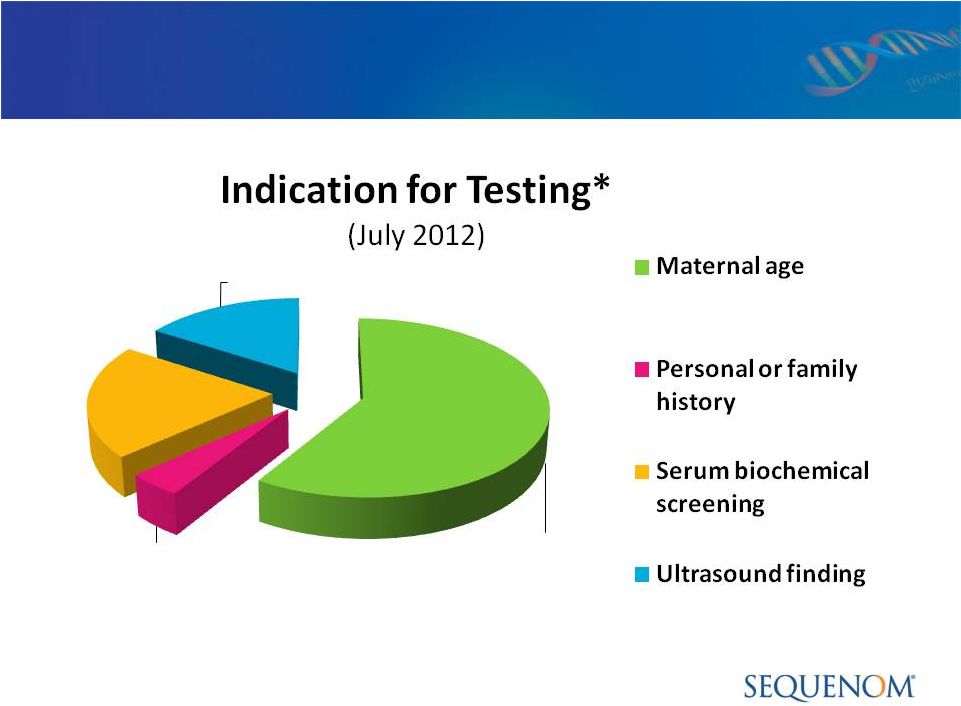 MaterniT21
™
PLUS LDT:
CLIA Quality Control & Outcome Metrics > 20,000
Patients 68%
5%
24%
19%
18 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
* Total equals greater than 100% as certain patients have multiple indications for
testing |
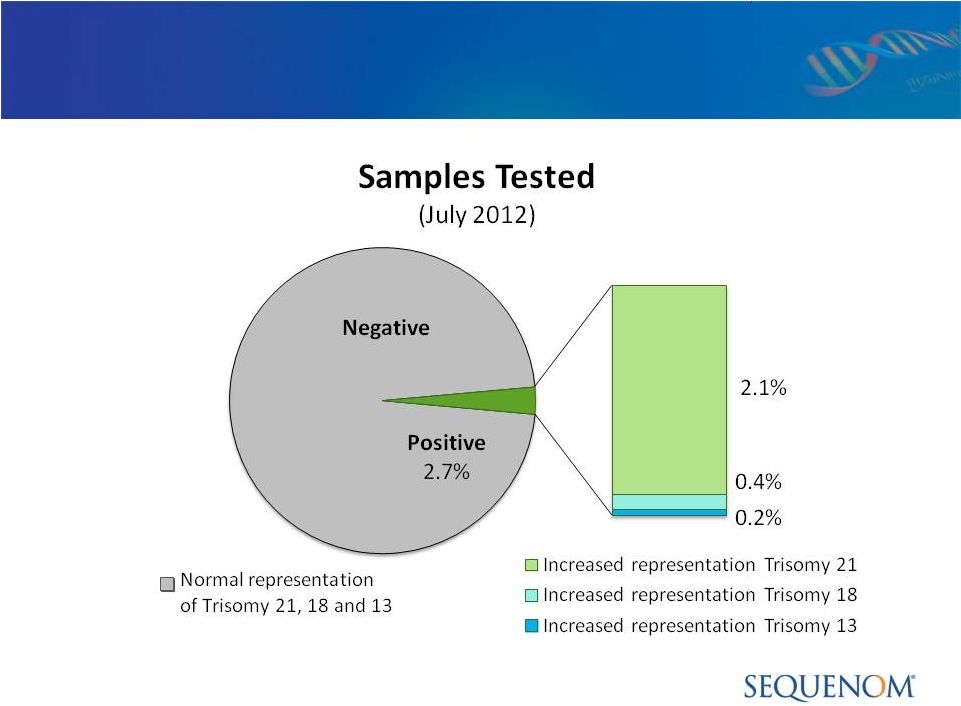 MaterniT21
™
PLUS LDT:
CLIA Laboratory Experience: > 20,000 Patients
19 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
 Intellectual Property
Pending
Issued
Over 500 issued and allowed patents
More than 330 pending patent applications
Vigorous Enforcement in Progress
Patents
‘540 Lo Patent –
U.S.
‘540 Lo Patent –
Europe
Other Relevant Prenatal Patents and Applications
‘181 Patent –
Random Sequencing (MPSS) U.S.
‘181 Patent –
Random Sequencing (MPSS) Europe
20 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
Comprehensive Strategic Patent Portfolio |
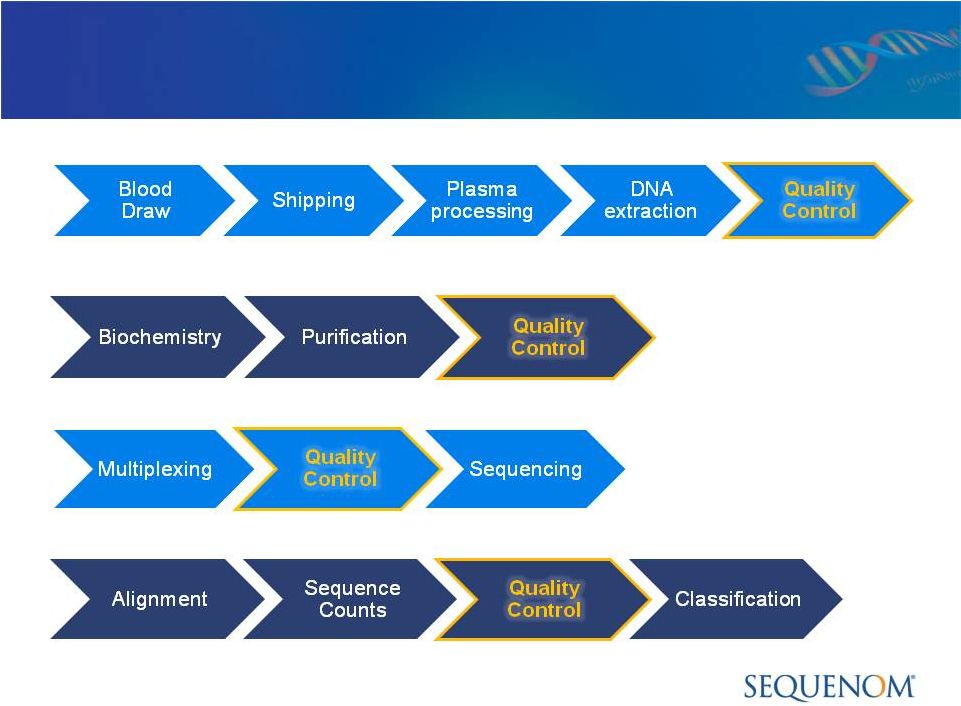 MaterniT21
™
PLUS LDT Process:
Focus on COGS Reduction in 2012/2013
Sample Processing -
Shipping and Sample Prep Automation
Data Analysis -
Enhanced Bioinformatics
Sequencing -
Increased Throughput, Faster Run Time
Library Preparation -
Automation and Simplification
21 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
 MaterniT21
™
PLUS LDT Cost of Goods Sold
Expect to reach COGS unit goal of $500-600 by Y/E 2012
* Royalty
obligation
accrued
on
revenues
recognized
*
22 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
Increased sample
throughput (plexing)
Flow cell/reagent
enhancement
Sample prep/process
automation
Collection tube
shipment at ambient
temperature
Continuing overhead
absorption with
volume growth
Anticipated
Improvements* |
 2012 Operational Updates:
27,000 tests accessioned YTD in 2012
Growing orders by Maternal-Fetal Medicine specialists (> 25%)
and the OB/GYN community
Turn-around is now ~7 business days from initial 8-10 days
Expanded prenatal sales force, now totaling ~75 active field
sales reps and managers nationwide
Solid market adoption, run rate of ~70,000 tests annually (as of
8/18/12)
Receiving reimbursement as out-of-network laboratory from
large commercial payers
Increased test content and testing capacity
Multiple international testing service agreements in place
23 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
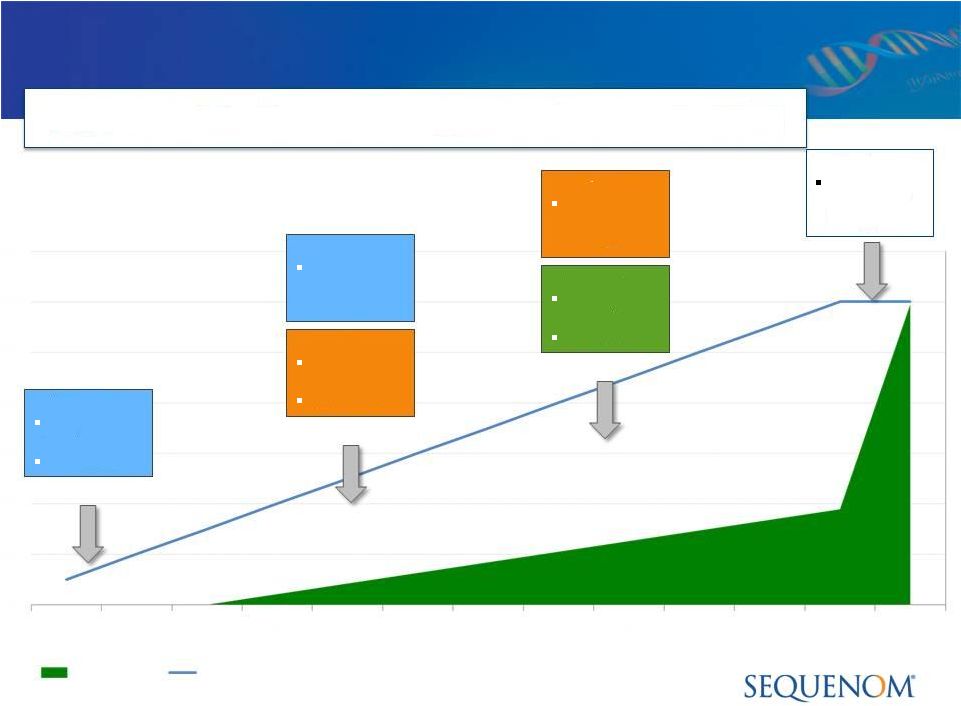 Cash
vs.
Accrual
Accounting:
MaterniT21
™
PLUS
LDT
Test #1
Costs
booked
Test billed
Test #1
Cash
payment
received
Test #2
Costs
booked
Test billed
Test #2
Cash
payment
received
Test #3
Costs
booked
Test billed
Month
Month xx
Accrual
Accounting
Implemented
Solid Test Adoption, Unpredictable Revenue in 2012
24 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
Revenues
Unit Volume |
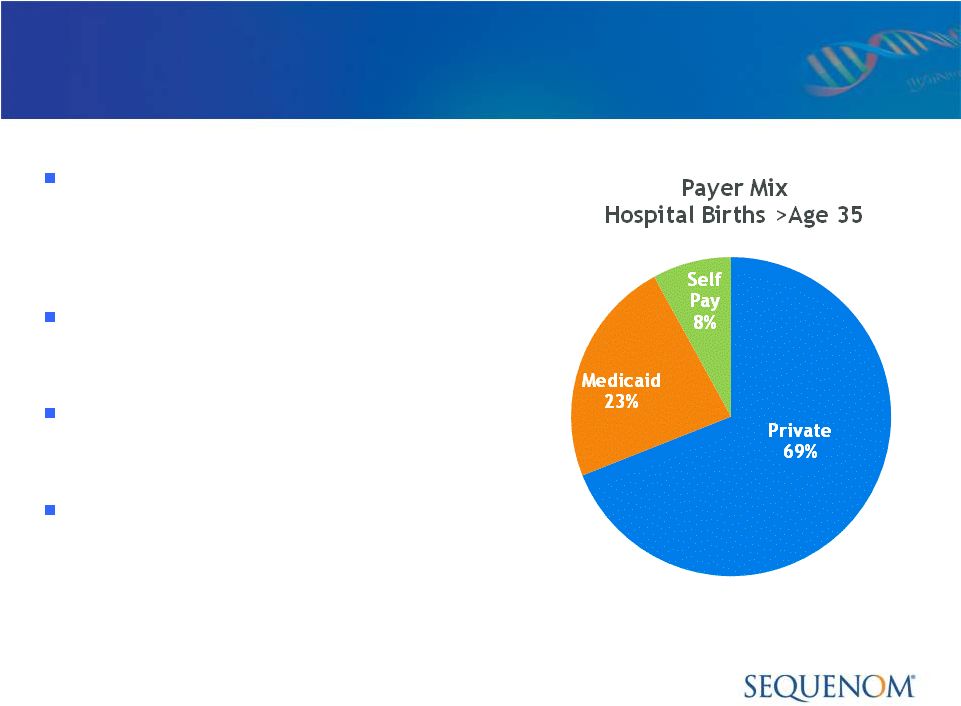 Reimbursement Environment:
MaterniT21
™
PLUS LDT
Initial emphasis with Private
Insurers, receiving payment
as out-of-network provider
First signed contract with
MultiPlan in early 2012
Additional regional contracts
in process for 2012
Covered Lives of ~26 million
(7/26/12)
Source: NHDS 2008, Other includes: Other, Other Govt. & Not stated
25 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
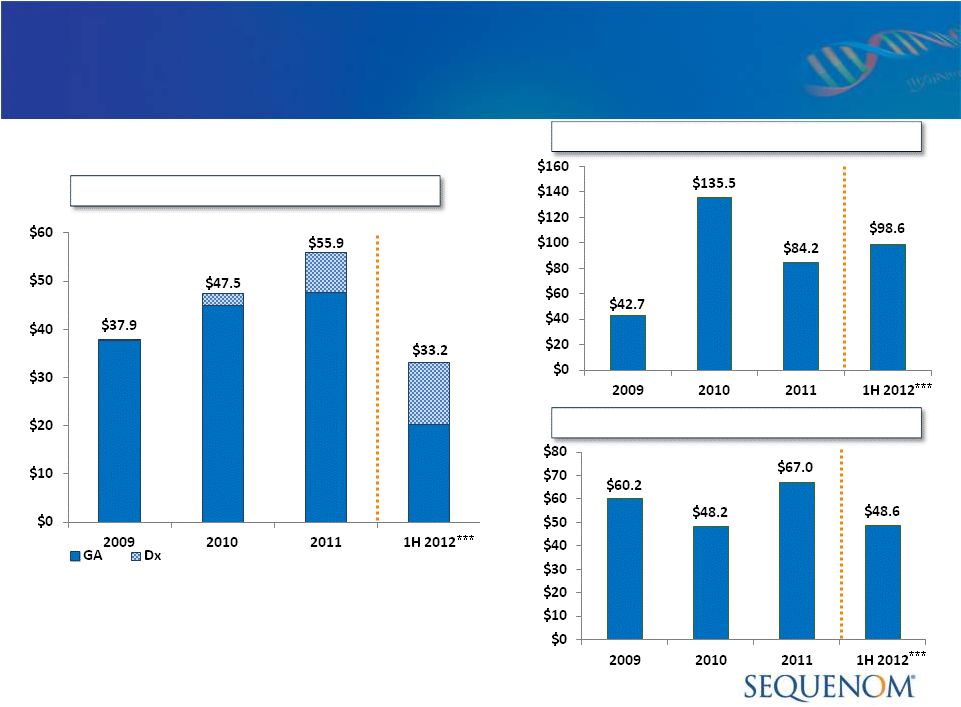 Financial Highlights
($ Millions)
Note: Principal amount of the revolving credit facility is due May 31, 2014.
The term loans mature on May 1, 2015.
*
Includes cash & marketable securities. Net proceeds from equity offering in
January of 2012 increased cash balances by $58.5M.
**
Cash burn includes the net cash used in operations of $48.7M, $42.6M , $51.3 M and
$35.7M for 2009, 2010 and 2011, 1H12. 2011/12 cash burn total
includes the use of $20M from credit facility. Revenue
Available Cash
*
Cash Burn
**
26 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL
*** Partial year results are not necessarily indicative of full year or future
performance. |
 Large Potential Addressable Target Market
MaterniT21 PLUS is a Leading Prenatal, Non-Invasive
Laboratory Developed Test with IP Protection
•
Clinical leader, first mover advantage, large and effective
sales force and commercial infrastructure for growth
Broad Prenatal Diagnostic Testing Services Portfolio
Genetic Analysis Segment Provides Diversified Revenue
Stream
Convertible Note Offering May Fund Sequenom to Cash Flow
Breakeven
Experienced Management Team
Investment Highlights
27 |
Improving healthcare through revolutionary genetic analysis solutions
CONFIDENTIAL |
 Improving healthcare through revolutionary genetic analysis solutions
Contact:
Marcy Graham
Senior Director, Investor Relations
mgraham@sequenom.com
www.sequenom.com
www.scmmlab.com
CONFIDENTIAL
©2012
Sequenom,
Inc.
All
rights
reserved.
Sequenom®,
Sequenom
CMM®,
MassARRAY®,
SensiGene®
,
MaternitT21™
PLUS
and
SEQureDx
®
are
trademarks
of
Sequenom,
Inc.
All other trademarks are the property of their respective owners. The information
herein is for informational purposes only and represents the current view of Sequenom, Inc. as of the
date of this presentation. Sequenom cannot guarantee the accuracy of any
information provided after the date of this presentation. Sequenom makes no warranties, express, implied
or statutory, as to the information in this presentation.
|
