Attached files
| file | filename |
|---|---|
| 8-K - SUCAMPO PHARMACEUTICALS, INC. 8-K - Sucampo Pharmaceuticals, Inc. | a50399863.htm |
Exhibit 99.1

BioCentury Thomson Reuters NewsMakers 2012 in the BioTech Industry Ryuji Ueno, MD, PhD, PhD, Chair, CEO, CSO Cary J. Claiborne, CFO Stanley G. Miele, SVP, Sales & Marketing Silvia Taylor, SVP, IR, PR & Corporate Communications September 7, 2012

Forward-Looking Statements This presentation contains “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements are based on management’s current expectations and involve risks and uncertainties which may cause results to differ materially from uncertainties, those set forth in the statements. The forward-looking statements may include statements regarding product development, product potential, future financial and operating results, and other statements that are not historical facts. The following factors, among others, could cause actual results to differ from those set forth in the forward-looking statements: the impact of pharmaceutical industry regulation and health care legislation; Sucampo’s ability to accurately predict future market conditions; dependence on the effectiveness of Sucampo’s patents and other protections for innovative products; the risk of new and changing regulation and health policies in the US and internationally and the exposure to litigation and/or regulatory actions. No forward-looking statement can be guaranteed and actual results may differ materially from those projected. Sucampo undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise. Forward-looking statements in this presentation should be evaluated together with the many uncertainties that affect Sucampo’s business, particularly those mentioned in the risk factors and cautionary statements in Sucampo’s Form 10-K for the year ended Dec. 31, 2011, which the Company incorporates by reference. 2
Sucampo Snapshot A commercial-stage, global biopharmaceutical company since 1996 2 FDA-approved drugs based on our proprietary prostone technology AMITIZA ® (lubiprostone) in Gastroenterology market RESCULA® (unoprostone isopropyl) in Ophthalmology market The therapeutic potential of prostones was first identified by Ryuji Ueno, M.D., Ph.D., Ph.D., Sucampo Pharmaceuticals' chairman and chief executive officer. Dr. Ueno and Sachiko Kuno, Ph.D., founded Sucampo Pharmaceuticals in 1996 AMITIZA has helped 6 million patients over 6 years combat the effects of chronic idiopathic constipation and irritable bowel syndrome with constipation New indications for current areas New global markets and diseases Deep and promising pipeline 3

Second Quarter 2012 and Recent Highlights AMITIZA® Approved in Japan June 2012 Filed OIC sNDA July 2012 RESCULA® US: Anticipate approval of sNDA for glaucoma indication in US (new label/updated MOA) EUROPE: Re-approval filings in EU and Switzerland Research and Development Pipeline prioritized Financial/Corporate Cash, cash equivalents, and investments Ended Q2 2012 with $89M Revenue Q2 $16.7M up 19%, 1H $31.1M up 19% 1H 2012 - Net Operating Cash Flow Positive Dual class of Common Stock Eliminated 4
Proprietary Prostone Technology Prostones are naturally occurring compounds that act locally to restore injured cells and tissues Prostones possess a unique mechanism of action as potent and specific activators of ion channels. Ion channels regulate the flow of specific ions into and out of cells. This regulation is key to the functioning of cells, such as metabolic processes and cell survival There is evidence that prostones have anti-inflammatory properties, prevent cell death, and restore cells and organs Ion channels are ubiquitous in the body, and targeted dosing of prostones may have broad applicability in many degenerative diseases Sucampo has pioneered the field of prostones Broad patent estate (>580 issued patents) Developed synthetic analogs of naturally occurring prostones, which are more potent, selective and stable than naturally occurring prostones, enabling their use as drugs The safety profile of prostone compounds is excellent, as demonstrated by the clinical safety record of AMITIZA and RESCULA 5
Sucampo Is a Leader in Gastroenterology: AMITIZA Gastroenterology Areas of Focus: Chronic Idiopathic Constipation (CIC) and Irritable Bowel Syndrome (IBS) CIC Constipation is characterized by infrequent stools, difficult stool passage, or both1 CIC affects an estimated 14% of the US adult population (33 million)2 Constipation accounts for 92,000 hospitalizations in the US each year3 Studies have found that severe constipation is associated with increased cardiovascular risk in women4,5 IBS IBS is defined as abdominal pain or discomfort that occurs in association with altered bowel habits over a period of at least 3 months1 IBS affects an estimated 15% of the adult population (45 million)6 IBS with constipation (IBS-C) affects approx. 5% of the adult population (12 million)6 Direct and indirect costs of IBS care in the US are estimated at about $20 billion per year6 Studies have documented that patients with IBS consume more than 50% more health care resources than those without IBS7 Source: See References 1-7 6
Sucampo Is an Emerging Player in Ophthalmology: RESCULA Ophthalmology Area of Focus: Glaucoma Glaucoma is a group of ocular diseases with various causes that ultimately are associated with a progressive optic neuropathy leading to loss of vision function8 Glaucoma is an age-related disease Glaucoma is the second leading cause of bilateral blindness worldwide9 It will affect an estimated 79.6 million people worldwide by 20209 As of 2009, 120,000 people in the United States were blind because of glaucoma10 An estimated 2.2 million people had the most common form of glaucoma – OAG – expected to increase to 3.36 million in 202011 Source: See References 8-11 7
Sucampo’s Prostone Technology Has Resulted in Two FDA-Approved Products AMITIZA® (lubiprostone) for Chronic Idiopathic Constipation (CIC) in adults and Irritable Bowel Syndrome with Constipation (IBS-C) in adult women Product Overview AMITIZA is a chloride ion channel activator FDA approved and marketed for CIC in adults and IBS-C in adult women; sNDA for OIC filed July 2012 Activation of this channel in cells within the intestinal tract results in fluid secretion and repair of epithelial cell barriers which are essential to the normal function of the digestive track RESCULA® (unoprostone isopropyl) for the lowering of intraocular pressure (IOP) in open-angle glaucoma and ocular hypertension in patients who are intolerant of or insufficiently responsive to other IOP-lowering medications Product Overview RESCULA is a potassium ion channel activator FDA approved for the lowering of intraocular pressure (IOP) in primary open-angle glaucoma and ocular hypertension Activation of this channel relaxes contractile cells, resulting in decreased intraocular pressure which is a known risk factor for glaucoma-associated vision loss 8
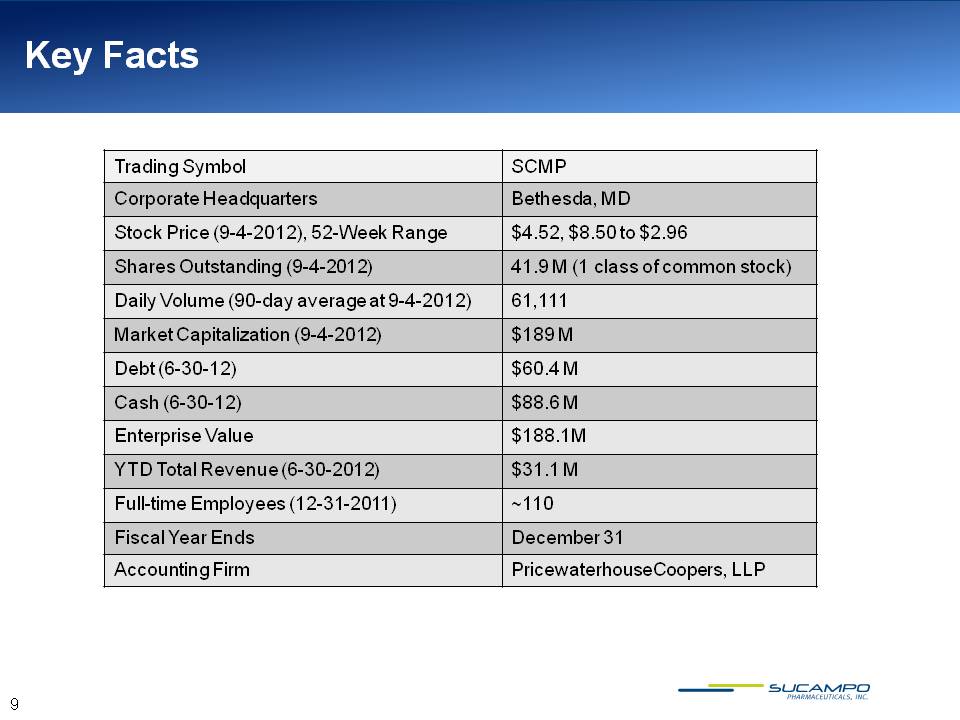
Key Facts Trading Symbol SCMP Corporate Headquarters Bethesda, MD Stock Price (9-4-2012), 52-Week Range $4.52, $8.50 to $2.96 Shares Outstanding (9-4-2012) 41.9 M (1 class of common stock) Daily Volume (90-day average at 9-4-2012) 61,111 Market Capitalization (9-4-2012) $189 M Debt (6-30-12) $60.4 M Cash (6-30-12) $88.6 M Enterprise Value $188.1M YTD Total Revenue (6-30-2012) $31.1 M Full-time Employees (12-31-2011) ~110 Fiscal Year Ends December 31 Accounting Firm PricewaterhouseCoopers, LLP 9

Management Ryuji Ueno, M.D., Ph.D., Ph.D., Chairman, Chief Executive Officer, Chief Scientific Officer, and Co-Founder R-Tech Ueno, LTD, Co-Founder MD and Ph.D. (Medicinal Chemistry) from Keio University; Ph.D. (Pharmacology) from Osaka University Cary J. Claiborne, Chief Financial Officer New Generation Biofuels, CEO, CFO, Director Osiris Therapeutics, CFO Constellation Energy Group, VP Financial Planning Senior leadership positions with General Electric (15 years), MCI and Home Depot Stanley G. Miele, President, Sucampo Pharma Americas, LLC and Senior Vice President, Sales and Marketing, Sucampo Pharmaceuticals, Inc. Abbott Laboratories Millennium Pharmaceuticals (COR Therapeutics) Greg Deener, Senior Vice President, Marketing Strategy and Implementation GTx, Inc. GlaxoSmithKline Thomas J. Knapp, Executive Vice President, Chief Legal Officer and Secretary NorthWestern Corporation, General Counsel and Corporate Secretary Boeing Other executive experience includes FDA/Center for Drug Evaluation and Research, Procter & Gamble, Pfizer, MedImmune, Allergan, and GlaxoSmithKline 10
Amitiza® (lubiprostone) AMITIZA® (lubiprostone) Approved in US for CIC in adults (2006) and IBS-C in adult women (2008) Partnered with Takeda Pharmaceuticals in US Royalty revenue of $41.5 M on net sales of $226.4M in 2011 Switzerland for CIC (2009) Limited marketing Japan for chronic constipation (2012) 4Q 2012 launch Partnered with Abbott Japan Filed in US for OIC (July 2012) UK for CIC (August 2011) Expect UK approval 3Q 2012 Sucampo plans to commercialize AMITIZA in Europe 11
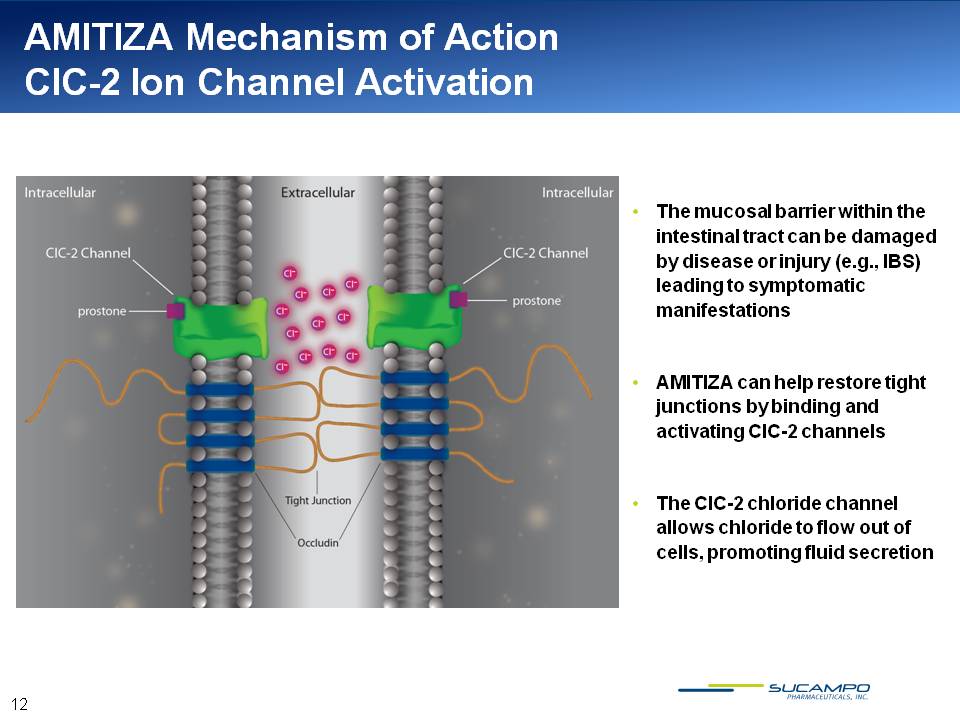
AMITIZA Mechanism of Action ClC-2 Ion Channel Activation The mucosal barrier within the intestinal tract can be damaged by disease or injury (e.g., IBS) leading to symptomatic manifestations AMITIZA can help restore tight junctions by binding and activating ClC-2 channels The ClC-2 chloride channel allows chloride to flow out of cells, promoting fluid secretion 12
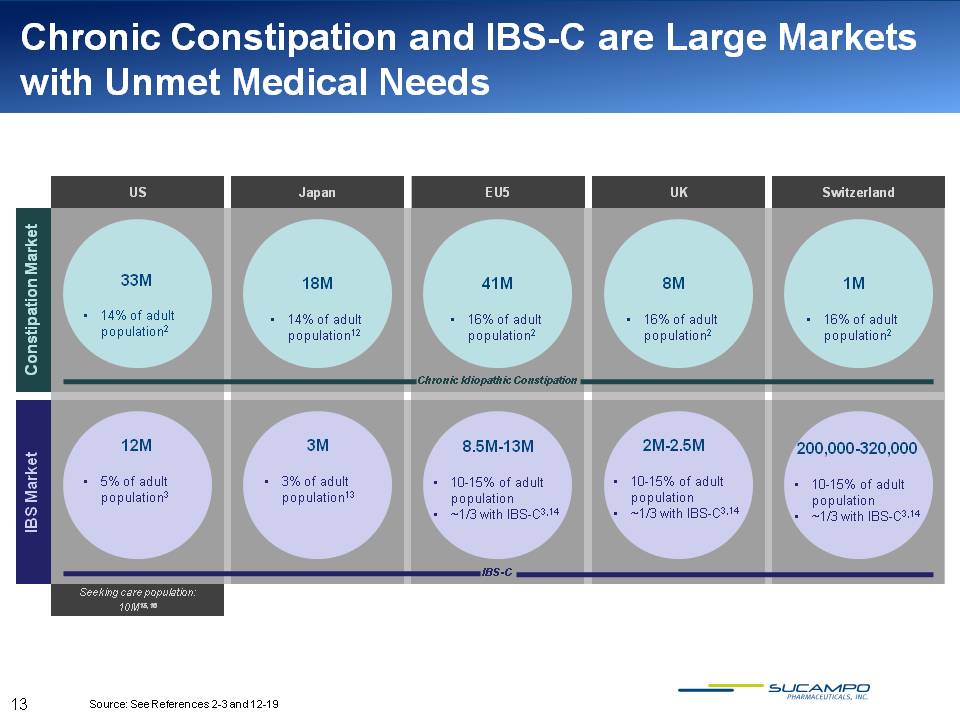
Chronic Constipation and IBS-C are Large Markets with Unmet Medical Needs US Japan EU5 UK Switzerland Constipation Market 33M 18M 41M 8M 1M 14% of adult population2 14% of adult population12 16% of adult population2 16% of adult population2 16% of adult population2 Chronic Idiopathic Constipation IBS Market 12M 3M 8.5M-13M 2M-2.5M 200,000-320,000 5% of adult population3 3% of adult population13 10-15% of adult population ~1/3 with IBS C3,14 10-15% of adult population ~1/3 with IBS C3,14 10-15% of adult population ~1/3 with IBS-C3,14 Seeking care population: 10M15,16 Source: See References 2-3 and 12-19 13

New Indication in OIC Will Expand the AMITIZA Franchise Sucampo and Takeda announced filing of sNDA for OIC on July 26, 2012 Seeking approval for a new indication for AMITIZA® (lubiprostone) for the treatment of opioid-induced constipation (OIC) in patients with chronic, non-cancer pain Filing is based on results from three Phase 3, well-controlled studies of 12 weeks’ duration in patients taking opioids chronically for non-cancer pain, as well as a long-term, open-label safety study, which provide additional support for use in this population Global trials: 250 sites, 1,500 patients Takeda funded the first $50M and 50% of trial costs in excess of $50 million FDA decision expected 1H12 14
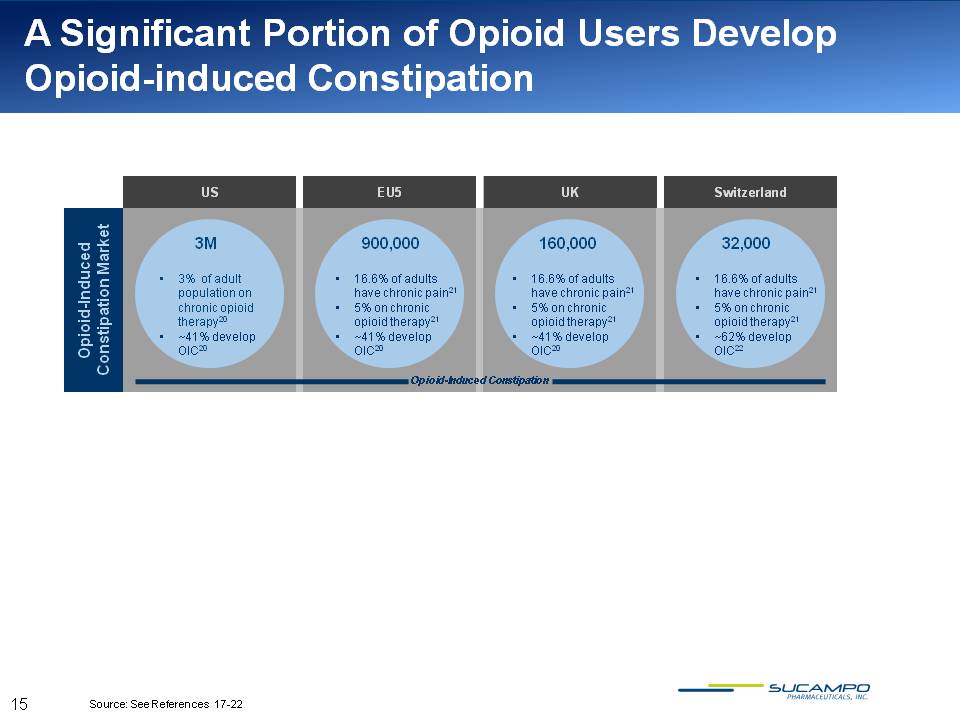
A Significant Portion of Opioid Users Develop Opioid-induced Constipation US EU5 UK Switzerland Opioid-Induced Constipation Market 3M 3% of adult population on chronic opioid therapy20 ~41% develop OIC20 900,000 16.6% of adults have chronic pain21 5% on chronic opioid therapy21 ~41% develop OIC20 160,000 16.6% of adults have chronic pain21 5% on chronic opioid therapy21 ~41% develop OIC20 32,000 16.6% of adults have chronic pain21 5% on chronic opioid therapy21 ~62% develop OIC22 Opioid-Induced Constipation Source: See References 17-22 15

Amitiza Growth Opportunities Expansion of the AMITIZA franchise New geographic markets New indications Growth in United States Increase CIC and IBS-C sales Patient awareness Customer targeting Managed care access Sales force efforts Approval of sNDA for OIC Growth in Japan and Europe Recent approval in Japan, partnered with Abbott Japan Expect approval of an MAA for CIC in the UK Anticipate filing for OIC in the UK with other European countries to follow 16
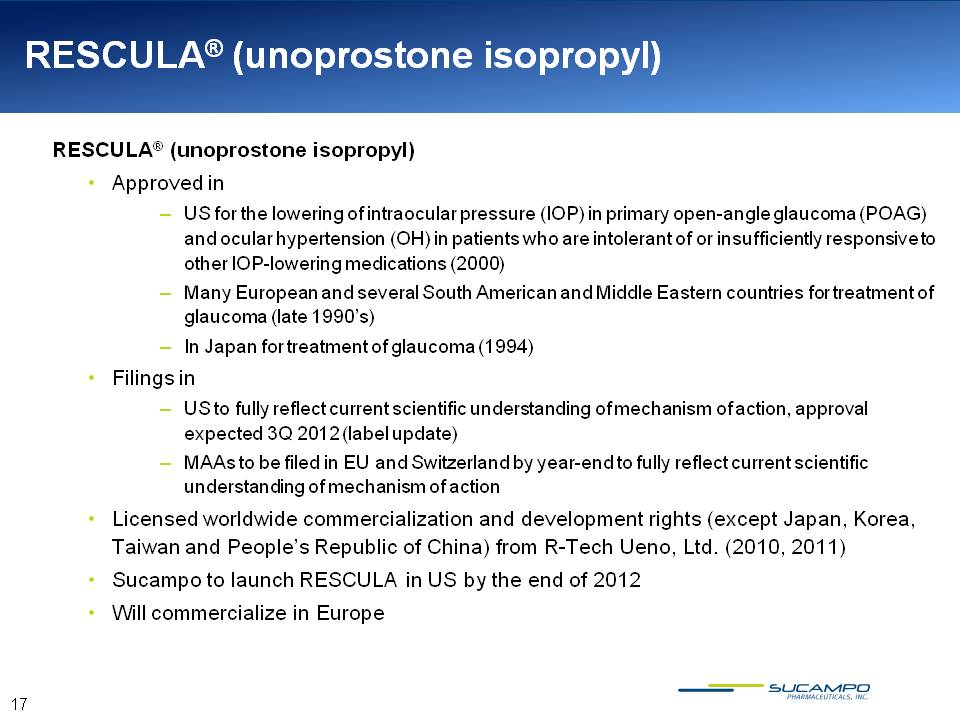
RESCULA® (unoprostone isopropyl) RESCULA® (unoprostone isopropyl) Approved in US for the lowering of intraocular pressure (IOP) in primary open-angle glaucoma (POAG) and ocular hypertension (OH) in patients who are intolerant of or insufficiently responsive to other IOP-lowering medications (2000) Many European and several South American and Middle Eastern countries for treatment of glaucoma (late 1990’s) In Japan for treatment of glaucoma (1994) Filings in US to fully reflect current scientific understanding of mechanism of action, approval expected 3Q 2012 (label update) MAAs to be filed in EU and Switzerland by year-end to fully reflect current scientific understanding of mechanism of action Licensed worldwide commercialization and development rights (except Japan, Korea, Taiwan and People’s Republic of China) from R-Tech Ueno, Ltd. (2010, 2011) Sucampo to launch RESCULA in US by the end of 2012 Will commercialize in Europe 17
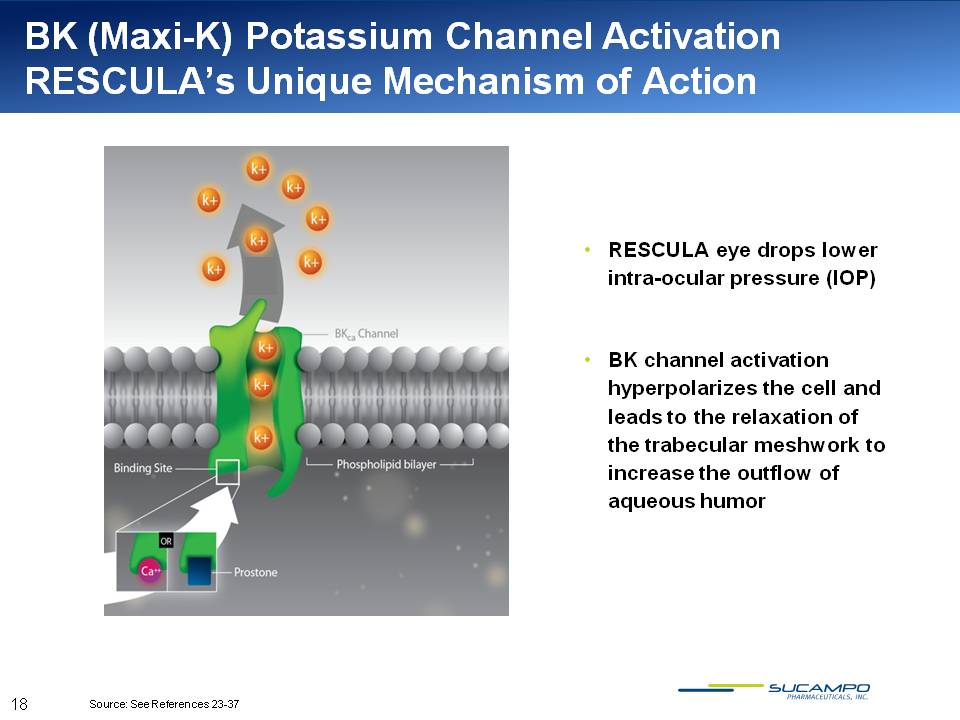
BK (Maxi-K) Potassium Channel Activation RESCULA’s Unique Mechanism of Action RESCULA eye drops lower intra-ocular pressure (IOP) BK channel activation hyperpolarizes the cell and leads to the relaxation of the trabecular meshwork to increase the outflow of aqueous humor Source: See References 23-37 18

US Glaucoma Market Overview The US glaucoma market is 29.2M TRx’s41 4-5M potential patients40,41,43 67% of the market is generic42 80% of TRx’s are by eye specialists42 Limited new products vs. reformulations Compliance and adherence are unmet needs 50% of new patients drop off therapy within one year of initiation Prostaglandins are inflammatory agents which depolarize cell membranes #1 reason for discontinuation of prostaglandins is hyperemia39,43,44 There is opportunity in this market for a differentiated product with a novel mechanism of action, such as Rescula Glaucoma sales volume (market opportunity): ~$3B in US (2012) ~$1B in Japan (2011) Source: See References 38-43 19

RESCULA Growth Opportunities Sucampo is seeking revisions to the label to more accurately reflect current scientific understanding We anticipate agreement on the final RESCULA label during 3Q 2012 Advantages of RESCULA Reduces IOP throughout the day alone or in combination Avoids side effects seen with other agents Novel MOA: ion channel activator promotes aqueous humor outflow through the trabecular meshwork Sucampo plans to launch RESCULA by the end of 2012 20
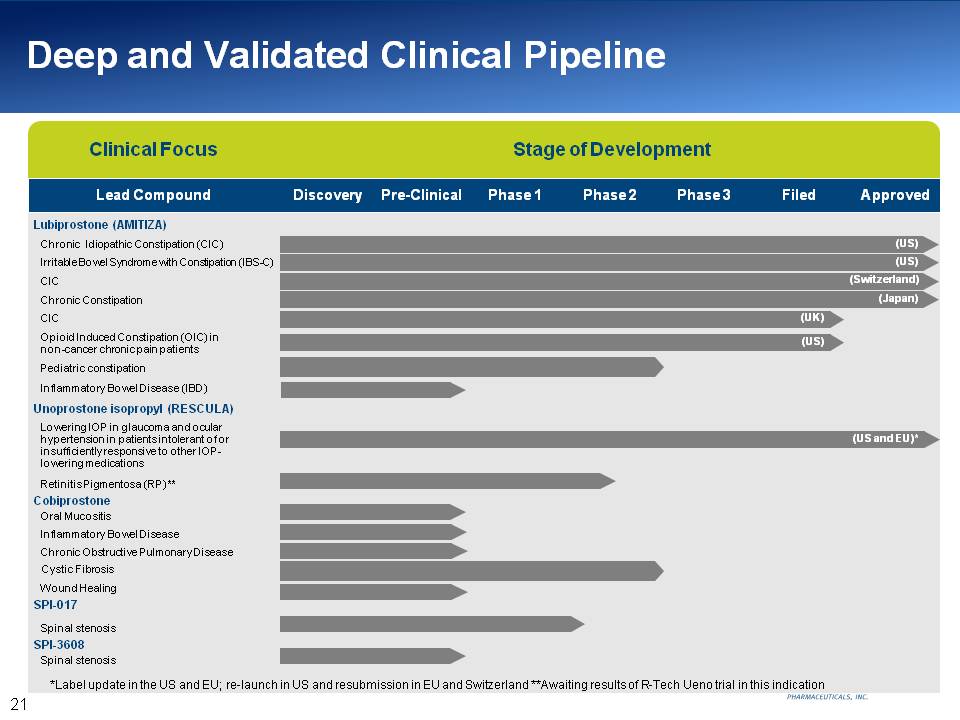
Deep and Validated Clinical Pipeline Clinical Focus Stage of Development Lead Compound Discovery Pre-Clinical Phase 1 Phase 2 Phase 3 Filed Approved Lubiprostone (AMITIZA) Chronic Idiopathic Constipation (CIC) (US) Irritable Bowel Syndrome with Constipation (IBS-C) (US) CIC (Switzerland) Chronic Constipation (Japan) CIC (UK) Opioid Induced Constipation (OIC) in non-cancer chronic pain patients (US) Pediatric constipation Inflammatory Bowel Disease (IBD) Unoprostone isopropyl (RESCULA) Lowering IOP in glaucoma and ocular hypertension in patients intolerant of or insufficiently responsive to other IOP-lowering medications (US and EU)* Retinitis Pigmentosa (RP) ** Cobiprostone Oral Mucositis Inflammatory Bowel Disease Chronic Obstructive Pulmonary Disease Cystic Fibrosis Wound Healing SPI-017 Spinal stenosis SPI-3608 Spinal stenosis *Label update in the US and EU; re-launch in US and resubmission in EU and Switzerland **Awaiting results of R-Tech Ueno trial in this indication 21
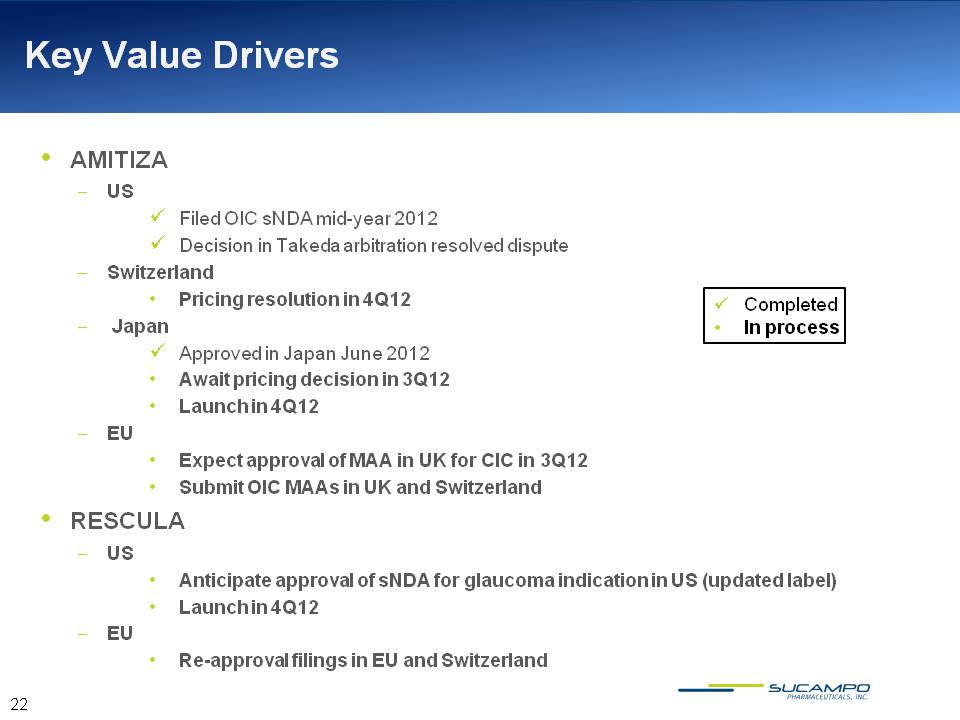
Key Value Drivers Completed In process AMITIZA US Filed OIC sNDA mid-year 2012 Decision in Takeda arbitration resolved dispute Switzerland Pricing resolution in 4Q12 Japan Approved in Japan June 2012 Await pricing decision in 3Q12 Launch in 4Q12 EU Expect approval of MAA in UK for CIC in 3Q12 Submit OIC MAAs in UK and Switzerland RESCULA US Anticipate approval of sNDA for glaucoma indication in US (updated label) Launch in 4Q12 EU Re-approval filings in EU and Switzerland 22

Appendix 23
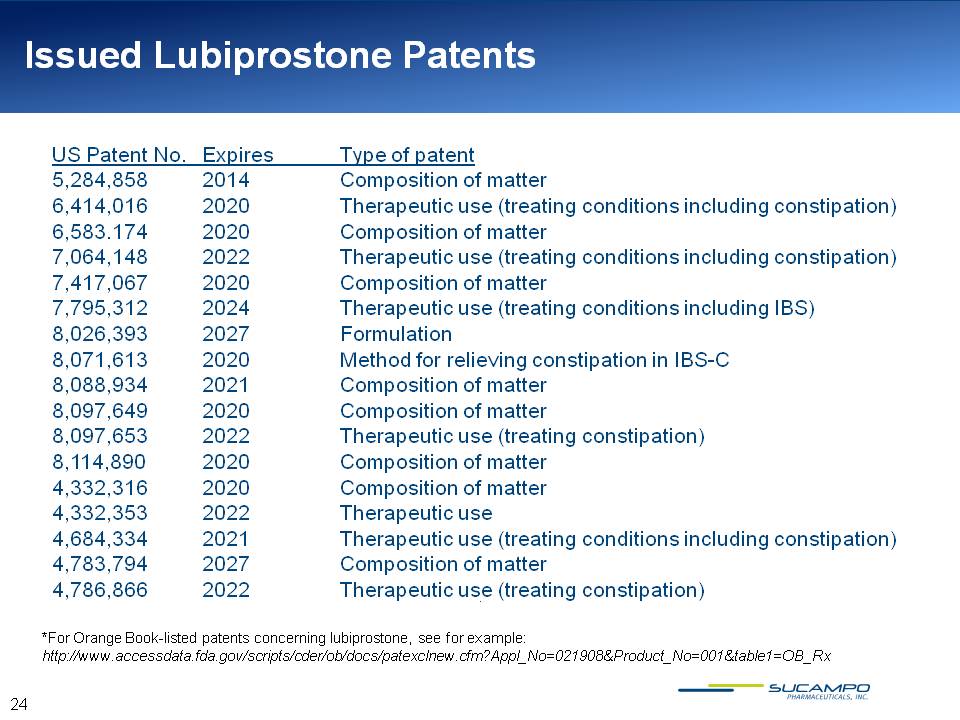
Issued Lubiprostone Patents US Patent No. Expires Type of patent 5,284,858 2014 Composition of matter 6,414,016 2020 Therapeutic use (treating conditions including constipation) 6,583.174 2020 Composition of matter 7,064,148 2022 Therapeutic use (treating conditions including constipation) 7,417,067 2020 Composition of matter 7,795,312 2024 Therapeutic use (treating conditions including IBS) 8,026,393 2027 Formulation 8,071,613 2020 Method for relieving constipation in IBS-C 8,088,934 2021 Composition of matter 8,097,649 2020 Composition of matter 8,097,653 2022 Therapeutic use (treating constipation) 8,114,890 2020 Composition of matter 4,332,316 2020 Composition of matter 4,332,353 2022 Therapeutic use 4,684,334 2021 Therapeutic use (treating conditions including constipation) 4,783,794 2027 Composition of matter 4,786,866 2022 Therapeutic use (treating constipation) *For Orange Book-listed patents concerning lubiprostone, see for example: http://www.accessdata.fda.gov/scripts/cder/ob/docs/patexclnew.cfm?Appl_No=021908&Product_No=001&table1=OB_Rx 24
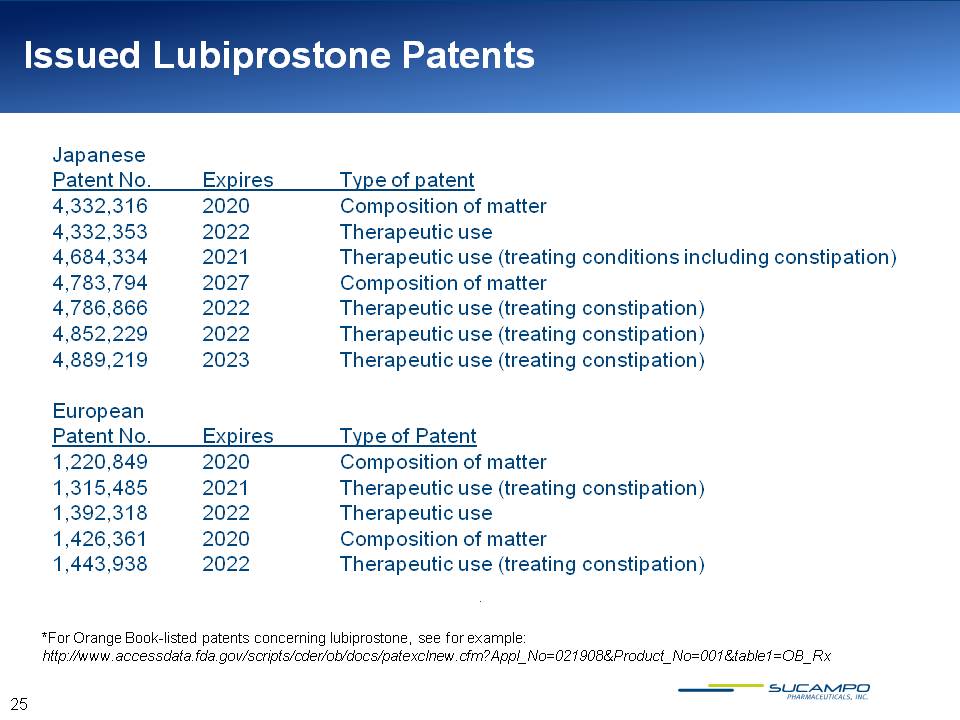
Issued Lubiprostone Patents Japanese Patent No. Expires Type of patent 4,332,316 2020 Composition of matter 4,332,353 2022 Therapeutic use 4,684,334 2021 Therapeutic use (treating conditions including constipation) 4,783,794 2027 Composition of matter 4,786,866 2022 Therapeutic use (treating constipation) 4,852,229 2022 Therapeutic use (treating constipation) 4,889,219 2023 Therapeutic use (treating constipation) European Patent No. Expires Type of Patent 1,220,849 2020 Composition of matter 1,315,485 2021 Therapeutic use (treating constipation) 1,392,318 2022 Therapeutic use 1,426,361 2020 Composition of matter 1,443,938 2022 Therapeutic use (treating constipation) *For Orange Book-listed patents concerning lubiprostone, see for example: http://www.accessdata.fda.gov/scripts/cder/ob/docs/patexclnew.cfm?Appl_No=021908&Product_No=001&table1=OB_Rx 25

References 1. American College of Gastroenterology 2. Suares et al. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and metaanalysis. Am J Gastroenterol. 2011; 106:1582-1591. 3 Lembo et al. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9th ed. Philadelphia, PA: Elsevier Saunders. 2010; 259-284. 4. Salmoirago-Blotcher et al. Constipation and risk of cardiovascular disease among postmenopausal women. Am J Med. 2011; 124:714-723. 5. Talley NJ, et al. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71-76. 6. Saito YA, et al. The epide-miology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910-1915. 7. Hulisz D. The burden of illness of irritable bowel syndrome: current challenges and hope for the future. J Manag Care Pharm. 2004 Jul-Aug;10(4):299-309. 8. Fingeret, AOA Optometric Clin Pract Guideline, 2011 9. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Opthalmol. 2006; 90: 262-267 10. Glaucoma Research Foundation. Glaucoma facts and stats. http://www.glaucoma.org. Updated January 12, 2009 11. Friedman DS. Prevalence of open angle glaucoma among adults in the United States. Arch Ophthalmol. 2004; 122: 532-538 12. Kantar Health Epi database http://epidb.khapps.jp/ (per Abbott Japan). 13. Kubo. et al. (2010) Differences between risk factors among irritable bowel syndrome subtypes in Japanese adults 23, 249-254. 14. Muller-Lissner, S. et al. (2001) Epidemiological Aspects of Irritable Bowel Syndrome in Europe and North America. Digestion, 64, 200-204. 15. Chey, W. et al “Frequency and Bothersomeness of Symptoms, Health Care Seeking Behavior and Satisfaction with Therapy in IBS-C Patients Meeting ROME II Criteria: Results of a Population Based Survey.” 26

References Cont. 16. Schoenfield, P. “System Frequency, Health Care Seeking Behavior, and Satisfaction with Therapy among Chronic Constipation Patients: Results of a Population-Based Survey”. 17. US population statistics based on data from http://www.census.gov/compendia/statab/cats/population.html and http://quickfacts.census.gov/qfd/states/00000.html accessed July 2012. 18. Japanese population statistics based on data from http://www.indexmundi.com/g/g.aspx?c=ja&v=21 and http://www.ipss.go.jp/webj-ad/WebJournal.files/population/2012_Vol.10/Web%20Journal_Vol.10_02.pdf accessed July 2012. 19. European population statistics based on data from http://epp.eurostat.ec.europa.eu/portal/page/portal/statistics/search_database accessed July 2012. 20. Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Clinical and Systematic Reviews. 2011; 106: 835-842. 21. Breivik et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006 May;10(4):287-333 22. Hess et al. Attitude of Swiss physicians towards opioid-induced constipation: a national survey. Eur J Intern Med. 2011 Oct;22(5):527-31. 23. Yu DY et al. Invest Ophthalmol Vis Sci. 1994;35:4087-4099. 24. Kern TS. Exp Diabetes Res. 2007;2007:95013. 25. Hardy P et al. Prostaglandins Leukot Essent Fatty Acids. 2005;72(5):301-325. 26. Alm A et al. Exp Eye Res. 2009;88:760-768. 27. Toris CB et al. Arch Ophthalmol. 2004;122:1782-1787. 28. Llobet A et al. News Physiol Sci. 2003;18:205-209. 29. Kojima S al. Nippon Ganka Bakkai Zasshi. 1997:101;605-610. 30. Makimoto Y et al. Jpn J Ophthalmol. 2002;46:31-35. 31. Kimura I et al. Jpn J Ophthalmol. 2005;49:287-293. 27

References Cont. 32. Sugiyama T et al. Arch Ophthalmol. 2009;127:454-459. 33. Inoue K et al. Clinical Ophthalmology 2011:5 1003-1005. 34. Hayami K et al. Ophthalmic Res. 2001 Jul-Aug;33(4):203-9. 35. Melamed S. Drugs Exp Clin Res 2002;28(2-3):63-73. 36. Ishida T al. Topical Monotherapy for Normal Tension Glaucoma-Comparison of Long-term Monotherapies in Maintaining Visual Field. Ophthalmology 47:1107-1112,2005. 37. ARVO 2011, Poster#4992,A416. 38. American Academy of Ophthalmology. 39. Friedman et al. Prevalence of Open-Angle Glaucoma Among Adults in the United States. Arch Ophthalmol. 2004 Apr;122(4):532-8. 40. July 2011-June 2012 MATTY IMS NPS data. 41. July 2011-June 2012 MATTY IMS NPA data. 42. Catalina presentation 2011. 43. Input from KOLs. 28

BioCentury Thomson Reuters NewsMakers 2012 in the BioTech Industry Ryuji Ueno, MD, PhD, PhD, Chair and CEO Cary J. Claiborne, CFO Stanley G. Miele, SVP, Sales & Marketing Silvia Taylor, SVP, IR, PR & Corporate Communications September 7, 2012
