Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ACURA PHARMACEUTICALS, INC | v322926_8-k.htm |

September 5, 2012 Stifel Nicolaus Conference Boston, MA Copyright © 2012 Acura Pharmaceuticals. All rights reserved.

Caution Regarding Forward Looking Statements Certain statements in this presentation constitute "forward - looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 . Such forward - looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements expressed or implied by such forward - looking statements . Forward - looking statements may include, but are not limited to, our and our licensee’s ability to successfully launch and commercialize our products and technologies including Oxecta ® Tablets and Nexafed ® Tablets, the price discounting that may be offered by Pfizer for Oxecta ®, the ability of us or our licensee’s to obtain necessary regulatory approvals and commercialize products utilizing our technologies and the market acceptance of and competitive environment for any of our products, expectations regarding potential market share for our products and the timing of first sales, our ability to enter into additional license agreements for our other product candidates, the ability to avoid infringement of patents, trademarks and other proprietary rights of third parties, and the ability of our patents to protect our products from generic competition, and the ability to fulfill the U . S . Food and Drug Administration’s, or FDA, requirements for approving our product candidates for commercial manufacturing and distribution in the United States, including, without limitation, the adequacy of the results of the laboratory and clinical studies completed to date, the results of laboratory and clinical studies we may complete in the future to support FDA approval of our product candidates and the sufficiency of our development to meet over - the - counter, or OTC, Monograph standards as applicable, the adequacy of the development program for our product candidates, including whether additional clinical studies will be required to support FDA approval of our product candidates, changes in regulatory requirements, adverse safety findings relating to our product candidates, whether the FDA will agree with our analysis of our clinical and laboratory studies and how it may evaluate the results of these studies or whether further studies of our product candidates will be required to support FDA approval, whether or when we are able to obtain FDA approval of labeling for our product candidates for the proposed indications and will be able to promote the features of our abuse discouraging technologies, whether our product candidates will ultimately deter abuse in commercial settings and whether our Impede™ technology will disrupt the processing of pseudoephedrine into methamphetamine . In some cases, you can identify forward - looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions intended to identify forward - looking statements . These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties . Given these uncertainties, you should not place undue reliance on these forward - looking statements . We discuss many of these risks in greater detail in our filings with the Securities and Exchange Commission . 2

Business Highlights • FDA approved technology • 1 st Aversion® opioid product launched by Pfizer Feb. 2012 • 1 Aversion® opioid in active development • 6 Aversion® opioids formulations developed • Aversion® (Tamper Resistant) Technology • Impede™ (Meth Resistant) Technology • Targeting Nexafed ® commercial availability late 2012 • Follow - on products under evaluation • Technology enhancements being evaluated • Opioids are the largest U.S. prescription drug category • Sizeable non - prescription cough/cold/allergy market • $30.5 million of cash at 7/31/12 • Sufficient cash to execute current business plan through at least the next two years 3
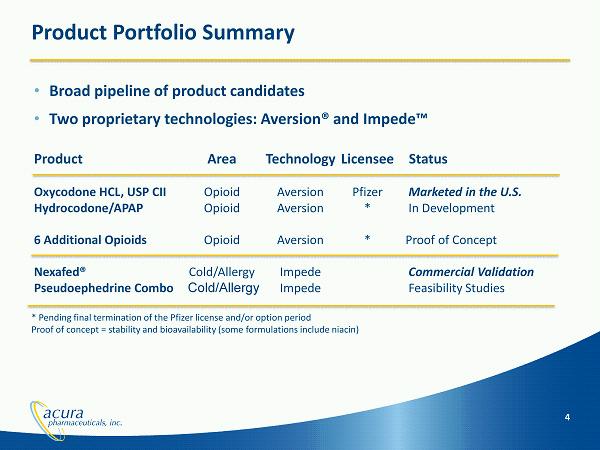
Product Portfolio Summary • Broad pipeline of product candidates • Two proprietary technologies: Aversion® and Impede™ Product Area Technology Licensee Status Oxycodone HCL, USP CII Opioid Aversion Pfizer Marketed in the U.S. Hydrocodone/APAP Opioid Aversion * In Development 6 Additional Opioids Opioid Aversion * Proof of Concept Nexafed ® Cold/Allergy Impede Commercial Validation Pseudoephedrine Combo Cold/Allergy Impede Feasibility Studies 4 * Pending final termination of the Pfizer license and/or option period Proof of concept = stability and bioavailability (some formulations include niacin)

Impede™ Technology Unique mixture of inactive ingredients added to pseudoephedrine HCl tablets designed to disrupt conversion into methamphetamine 5

6 (Pseudoephedrine HCl ) 30 mg Tablets • Pseudoephedrine HCl 30 mg Tablets • With Impede™ Technology • Efficacy Demonstrated • Pilot formulation bioequivalent to existing tablets • Meth Resistance Testing • no extraction of pseudoephedrine for further processing in two common Meth extraction methods • 50% reduced Meth yield in direct conversion method compared to existing tablets

7 Nexafed ® Development Status • Commercial scale - up to 700,000 tablet batches complete • Formulation adjustments do not impact meth resistance profile • Commencing Commercial Process Validation • If successful, expected to be the first commercial product • Bioequivalence testing on validated product • Stability testing to establish shelf life • Market under the FDA’s OTC Monograph (21 C.F.R. Parts 330 - 358) • No prior approval application required • Development/product must adhere to Monograph standards

• Expect Nexafed® price to be comparable to existing branded products • Initial distribution expected to be to drug wholesalers • Nationwide coverage targeted • Independent pharmacies in high meth impact areas are anticipated to be early adopters • Chain pharmacists may purchase from drug wholesalers • Support distribution with pharmacist targeted advertising/promotion • Publication support for our Meth resistance testing • Requesting 2013 pseudoephedrine quota from the DEA • Arranging 3 rd party distribution logistics 8 Nexafed ® Marketing Status

9 • $1 billion U.S. non - prescription nasal decongestant market in 2009 1 • Market fragmented by combination and extended - release products incorporating pseudoephedrine HCl and other decongestants • 53% of sales are from phenylephrine containing products • Sold by chain and independent pharmacies • All pseudoephedrine containing products are held behind - the - counter • Requires a physicians prescription in some jurisdictions • Consumer monthly purchases are limited and require registration • Pharmacist - Consumer interaction required for each sale Nexafed ® Target Market 1 AC Nielsen

Aversion ® Technology Proprietary mixture of inactive ingredients added to immediate release tablets intended to discourage tampering associated with misuse and abuse 10

11 Aversion® Opioid Product Portfolio • Focus on rapid development of Hydrocodone/APAP • Demonstrated application to multiple opioid formulations Product Area Technology Licensee Status Oxycodone HCL, USP CII Opioid Aversion Pfizer Marketed in the U.S. Hydrocodone/APAP Opioid Aversion * In Development 6 Additional Opioids Opioid Aversion * Proof of Concept Complete * Pending final termination of the Pfizer license and/or option period Proof of concept = stability and bioavailability (some include niacin)

• 35 million people in the United States have used Rx opioids non - medically in their lifetime • Immediate Release (IR) opioids are more frequently abused than Extended Release (ER) opioids Abuse of Opioids is Prevalent Source: SAMHSA, Office of Applied Studies, 2009 National Survey on Drug Use and Health Immediate - Release Only Extended - Release and Immediate - Release 12 Lifetime Abuse of Selected Pain Relievers Age 12 or Older 0 5 10 15 20 25 Ultram® Methadone Demerol® Morphine OxyContin® Codeine Hydrocodone Percocet®, Percodan®, or Tylox® Darvocet®, Darvon®, or Tylenol® with Codeine Vicodin®, Lortab®, or Lorcet® Number in Millions

Physician Perception of Opioid Abuse Source: Company Research, 400 Primary Care, Surgeon, Pain Specialists & Emergency Room Physicians, December 2011 • Snorting and Injecting are the methods with the highest concern for serious adverse health consequences with immediate release opioids 13

Aversion IR Product Markets Oxycodone/APAP Hydrocodone/APAP Oxycodone Only Source: IMS NPA, MAT Dec. 2011 • 188 million dispensed prescriptions in 2011 • $1.7 billion in sales (mostly generic prices) 14

• FDA Approval : June 17, 2011 • Marketed By : Pfizer, Inc. • First Commercial Sale : February 2, 2012 • Brand Name: Oxecta ® • Direct Market Size : 14.8 million annual IR oxycodone Rx’s Aversion® Oxycodone HCl Tablets CII Source: IMS NPA, MAT Dec. 2011 Oxecta is a registered trademark of Pfizer Inc. 15 • Legislative activities are supportive of tamper resistant technologies • We believe Pfizer’s list price remains a barrier to utilization until appropriate managed care contracts are executed • We anticipate Pfizer to be enabled to promote to physicians in 2013

Aversion® Hydrocodone/APAP • Development Status (completed by Pfizer) • Bioequivalence demonstrated to the Reference Listed Drug • Pre - IND meeting held with the FDA • Assessment of formulation stability ongoing • Expected Acura Clinical Development Program • 505(b)(2) New Drug Application - Priority Review will be requested • Nasal snorting abuse liability liking study (~40 subjects) • Dose proportionality and food effect PK study (~40 subjects) • PK Bridging study for safety and manufacturing (~40 subjects) • Prevalence of hydrocodone snorting analysis (non - clinical) • <$5 million estimated in remaining external development expenses • US submission only • Excludes PDUFA filing fee • NDA submission anticipated 12 - 18 month after return from Pfizer 16

• Aversion® Technology patents expire in 2025 • 3 U.S. patents issued covering compositions of opioids and certain other abused drugs with functional inactive ingredients • 1 U.S. Patent issued covering an extended release opioid dosage form • Impede™ Technology patents pending • Additional U.S. and foreign patents issued • Multiple patent applications pending in the United States and internationally Intellectual Property Position 17

Financial Information 18

19 Selected Financial Information $ Millions Cash and Cash Equivalents (6/30/12) $31.2 Debt (6/30/12) None Shares Outstanding at 6/30/12 Millions Total Common Shares Outstanding 45.864 Shares Held by GCE Holdings (~72%) 33.064 • Sufficient cash to execute current business plan through at least the next two years • ~ $4.5 million of Net Cash Used (Six Months Ended June 30, 2012) Financial Summary and Share Data

Acura Pharmaceuticals, Inc. 616 N. North Court, Suite 120 Palatine, IL 60067 (847) 705 - 7709 NASDAQ: ACUR www.AcuraPharm.com Copyright © 2012 Acura Pharmaceuticals. All rights reserved.
