Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PALISADE BIO, INC. | v319862_8-k.htm |
Exhibit 99.01

Neural Stem Cell Technology Platform Cell Therapy and Pharmaceuticals August 2012 NYSE MKT: CUR

Safe Harbor statements under the Private Securities Litigation Reform Act of 1995: This presentation contains forward - looking statements as defined in Section 27A of the Securities Act of 1933 as amended, and section 21E of the Securities Exchange Act of 1934, as amended. Such forward - looking statements are based upon Neuralstem , Inc.’s management’s current expectations, estimates, beliefs, assumptions, and projections about Neuralstem’s business and industry. Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “may,” “will,” “should,” “would,” “potential,” “continue,” and variations of these words (or negatives of these words) or similar expressions, are intended to identify forward - looking statements. In addition, any statements that refer to expectations, projections, or other characterizations of future events or circumstances, including any underlying assumptions, are forward - looking statements. These forward - looking statements are not guarantees of future performance and are subject to certain risks, uncertainties, and assumptions that are difficult to predict. Therefore, our actual results could differ materially and adversely from those expressed in any forward - looking statements as a result of various risk factors. These risks and uncertainties include the risks associated with the effect of changing economic conditions, trends in the products markets, variations in Neuralstem’s cash flow, market acceptance risks, technical development risks and other risk factors detailed in Neuralstem’s Securities and Exchange Commission filings. For links to SEC documents please visit the company’s Web site: neuralstem.com. 2 Neuralstem , Inc. Safe Harbor Statement

Neuralstem , Inc. Investment Highlights □ Proprietary Patented Neural Stem Cell Platform Foundation for Cell Therapy and Small Molecule Programs □ NIH - discovered platform; F ully characterized, regionally specific neural stem cells □ Positive Human Data for Neural Stem Cell Therapy in ALS Patients; Cervical Phase □ 17 ALS patients treated with NSI - 566, final cohort; Reported data from initial 12 ALS patients showing the procedure and therapy were safe and well - tolerated with promising early indications of treatment effect; cervical transplantation phase commenced Nov. 2011 □ First - in - class Neurogenic Small Molecule Drug Program NSI - 189 Advances to Phase Ib □ Ib dosing of 24 depressed patients commenced June 2012, testing safety of escalating doses for 28 days of patented oral compound which stimulates new neuron growth in the hippocampus, in ongoing MDD trial □ World - class Clinical Research Team □ Leaders in their fields include Karl Johe , Ph.D., discoverer and developer of neural stem cell technology; independent ALS researchers University of Michigan’s Eva L. Feldman, M.D., Ph.D. and Emory University’s Jonathan D. Glass, M.D. and Nicolas M. Boulis , M.D.; and Director of the Harvard Clinical T rial Network and Neuralstem’s depression trial consultant Maurizio Fava, M.D. □ Capital Efficient Business Model 3

Neuralstem , Inc. Collaborators World - Class Clinical P artners □ ALS Trial Clinical Investigators : □ Principal Investigator: Eva L. Feldman, M.D., Ph.D. , Professor of Neurology & Director A. Alfred Taubman Medical Research Inst. of the University of Michigan Medical School; President of American Neurological Assn. □ Site Principal Investigator: Jonathan D. Glass, M.D. , Professor of Neurology & Director Emory ALS Center Emory University □ Co - Investigator & Neurosurgeon: Nicholas M. Boulis M.D., Assist. Professor Neurosurgery Emory University □ ALS Trial Advisory Board: □ Zachary Simmons M.D., Chairman, SMB , Professor of Neurology Penn State University Hershey Medical Center and Director Neuromuscular Program and ALS Center □ Mark Bromberg M.D., Ph.D. Professor of Neurology & Director of the Motor Neuron Disease/ALS Clinic University of Utah School of Medicine □ Lucie Bruijn , Ph.D., Chief Scientist & Sr. VP of Research and Development ALS Association □ Thomas Freeman M.D., Professor, Dept of Neurosurgery & Medical Director, Center for Aging and Brain Repair University of South Florida College of Medicine □ Clifton L. Gooch, M.D., Professor & Chairman Department of Neurology & Director Division of Neuromuscular Disease University of South Florida College of Medicine □ Hiroshi Mitsumoto M.D., D.Sc., Professor of Neurology Columbia University & Director of the Eleanor and Lou Gehrig MDA/ALS Research Center and Neuromuscular Division at the Neurological Institute of New York □ Paul Park M.D., Assistant Professor & Neurosurgeon University of Michigan School of Medicine □ Mike Vogelbaum M.D., Ph.D. , Associate Director, Brain Tumor and Neuro - Oncology Center Cleveland Clinic □ Glioblastoma (Brain Cancer) Principal Investigator John Zhang, M.D., Ph.D., Professor of Neurosurgery, Loma Linda University, CA □ Spinal Cord Injury Lead Collaborator Martin Marsala , M.D., Ph.D., University of California San Diego □ Psychiatric/Small Molecule Trial Consultant Maurizio Fava, M.D., Slater Family Professor of Psychiatry at Harvard Medical School and Executive Vice Chair of the Department of Psychiatry at Massachusetts General Hospital 4

Neuralstem , Inc. Management □ Karl Johe , PhD Co - founder, Chairman of the Board, and Chief Scientific Officer, 1997 - present □ Discoverer and developer of Human Neural Stem Cell Technology □ Staff Scientist at the NIH Laboratory of Molecular Biology of the National Institute of Neurological Disorders and Stroke in Bethesda, 1993 - 1997 □ Post Doctoral, Molecular Genetics, University of California San Francisco; PhD, Biochemistry, Albert Einstein College of Medicine □ Richard Garr, JD Co - founder, Director, President, CEO, and General Counsel, 1996 - present; Interim CFO eff. April 2012 □ Focused on the business of science for 16 years; corporate legal, regulatory and patent experience □ Previous corporate and commercial law practice: Beli , Weil & Jacobs, the B&G Companies, and Circle Management Companies □ JD, Columbus School of Law, The Catholic University of America; BA, Psychology, Drew University; Co - founder and Director, First Star Foundation; Mid - Atlantic Chapter Co - founder, The Starlight Foundation; Former Honorary Chairman, Brain Tumor Society □ Thomas G. Hazel, PhD Senior Vice President, Research, 2008 - present □ Served as Neuralstem’s Stem Cell Discovery Program Director , 2000 - 2004; Sr. Scientist, 1998 - 2000 □ Staff Scientist at the NIH Laboratory of Molecular Biology of the National Institute of Neurological Disorders and Stroke in Bethesda, 1996 - 1998; IRTA fellow, 1993 - 1996 □ PhD, Genetics, University of Illinois College of Medicine 5

Neuralstem Technology Stem Cells of the Brain and the Spinal Cord □ Platform technology □ Numerous cell therapy products □ Regionally specific CNS stem cells □ cGMP manufacturing □ Patented □ Issued and re - affirmed worldwide □ Fully characterized □ Expanded under defined conditions: no animal - derived reagents, serum or feeder cells □ Reproducible differentiation: constitutive behavior of cells □ Physiologically relevant neurons: 50% Midbrain Spinal Cord GABAergic Dopaminergic Cholingergic Neuronal Differentiation Neuronal Subtypes Hippocampus 6

Neuralstem Technology U.S. Patents Issued and Re - affirmed Worldwide □ U. S. Pat. No. 8,236,299 (August 2012) □ Transplantation of human n eural c ells for treatment of neurodegenerative c onditions □ U.S. Pat. No. 8,058,434 (November 2011) □ Compositions to effect neuronal growth □ U.S . Pat. No. 8,030,492 (October 2011 ) □ Compositions to effect neuronal growth □ U.S. Pat. No. 7,691,629 (April 2010) □ Transplantation of human neural cells for treatment of neurodegenerative conditions □ U.S. Pat. Nos. 7,560,553 and 7,858,628 (July 2009, December 2010) □ Use of fused nicotinamides to promote neurogenesis (div) □ U.S. Pat. No. 7,544,511 (June 2009) □ Stable neural stem cell line methods □ U.S. Pat. No. 6,284,539 (September 2001) □ Method for generating dopaminergic cells derived from neural precursors □ U.S. Pat. No. 6,040,180 (March 2000) □ In vitro generation of differentiated neurons from cultures of mammalian multipotential CNS stem cells □ U.S. Pat. No. 5,753,506 (May 1998) □ Isolation propagation and directed differentiation of stem cells from embryonic and adult central nervous system of mammals ( div ) 7 Neuralstem.com Live Map Includes Worldwide I.P.
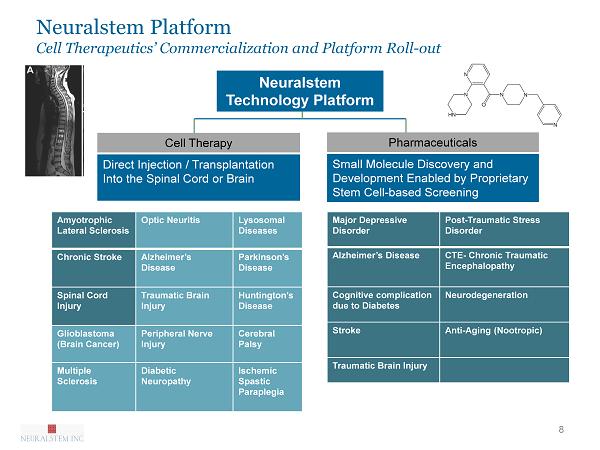
Neuralstem Platform Cell Therapeutics’ Commercialization and Platform Roll - out 8 Neuralstem Technology Platform Direct Injection / Transplantation Into the Spinal Cord or Brain Cell Therapy Small Molecule Discovery and Development Enabled by Proprietary Stem Cell - based Screening Pharmaceuticals Ischemic Spastic Paraplegia Amyotrophic Lateral Sclerosis Optic Neuritis Lysosomal Diseases Chronic Stroke Alzheimer’s Disease Parkinson’s Disease Spinal Cord Injury Traumatic Brain Injury Huntington’s Disease Glioblastoma (Brain Cancer) Peripheral Nerve Injury Cerebral Palsy Multiple Sclerosis Diabetic Neuropathy Ischemic Spastic Paraplegia Major Depressive Disorder Post - Traumatic Stress Disorder Alzheimer’s Disease CTE - Chronic Traumatic Encephalopathy Cognitive complication due to Diabetes Neurodegeneration Stroke Anti - Aging ( Nootropic ) Traumatic Brain Injury

Neuralstem ALS Trial ALS Phase I Safety Data Published and Presented: First 12 Patients □ “Lumbar intraspinal injection of neural stem cells in patients with ALS: Interim results of a Phase I trial,” poster presented at American Neurological Association Annual Meeting, September 2011 □ “Lumbar Intraspinal Injection of Neural Stem Cells in Patients with ALS: Results of a Phase I Trial in 12 Patients,” Stem Cells. 2012 Jun;30(6):1144 - 51 - Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman, EL , Department of Neurology, Emory University School of Medicine, Atlanta 9 PATIENT GROUP GENDER AGE AT SURGERY DISEASE DURATION AT SURGERY (Yrs) IMPLANT DATE 1 A1 Male 61.7 5.2 (trach) 1/20/2010 2 A1 Male 43.4 12.7 (trach) 3/12/2010 3 A1 Male 51.1 2.1 4/14/2010 4 A2 Male 37.5 2.0 (trach) 5/26/2010 5 A2 Male 66.2 2.2 6/23/2010 6 A2 Male 55.0 2.2 8/18/2010 7 B Male 59.0 1.6 10/20/2010 8 B Male 41.1 5.5 11/17/2010 9 B Male 54.5 3.5 12/29/2010 10 C Male 48.9 11 2/9/2011 11 C Male 39.4 1.5 3/9/2011 12 C Male 65 3 4/13/2011

Neuralstem ALS Trial ALS Phase I Safety Data: First 17 Patients 10 □ Phase I Data Key Findings □ Successful, safe injection of NSI - 566 neural stem cells into 17 ALS patients in lumbar and/or cervical regions of spinal cords □ No complications related to cell delivery □ No toxicity related to cells □ No evidence of acceleration of disease due to surgery or cells, following injections □ Three patients from initial nonambulatory cohorts died due to advanced ALS progression; another by unrelated heart failure □ A utopsy evidence of graft survival in each case □ Several patients showed slowed ALS progression after injections □ One patient of initial 12 showed reversal of symptoms (patient #11 on following charts)

11 Glass, et. al. STEM CELLS 2012;30:1144 – 1151 Neuralstem ALS Trial ALS Phase I Disease Progression: ALSFR - R - First 12 Patients □ The Amyotrophic Lateral Sclerosis Functional Rating Scale - Revised ( ALSFRS - R) is an instrument used by the physician for evaluating the functional status of patients with ALS □ ALSFRS - R can be used to monitor changes in a patient over time with ten measures including speech , swallowing, walking and breathing, total score of 48 ALSFRS - R Nonambulatory Cohorts Ambulatory Cohorts

12 Glass , et. al. STEM CELLS 2012;30:1144 – 1151 Neuralstem ALS Trial ALS Phase I Disease Progression: FVC - First 12 Patients □ Forced Vital Capacity (FVC) is a lung function test that measures total lung capacity by the amount of gas contained in the lung at the end of a maximum inhalation FVC (% predicted) Nonambulatory Cohorts Ambulatory Cohorts
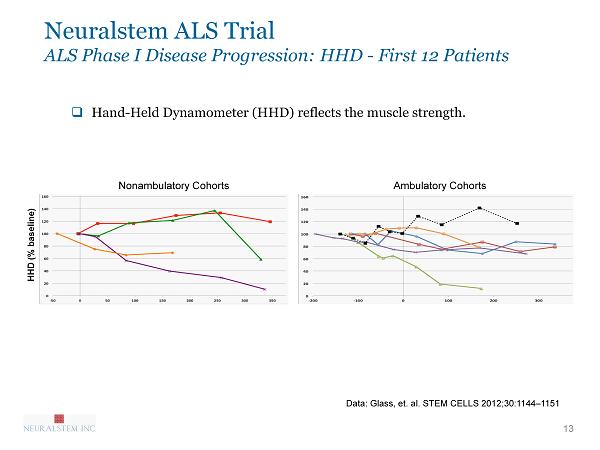
13 Data: Glass, et. al. STEM CELLS 2012;30:1144 – 1151 Neuralstem ALS Trial ALS Phase I Disease Progression: HHD - First 12 Patients □ Hand - Held Dynamometer ( HHD) reflects the muscle strength. HHD (% baseline) Nonambulatory Cohorts Ambulatory Cohorts

Neuralstem Cell Therapy Program (U.S.) ALS Phase I Clinical Trial: Cervical Phase, Return Patients □ Continuation of Phase I Clinical Trial □ Patient 17 is fifth of s i x patients scheduled to demonstrate the safety of cervical injections □ FDA approves return of three lumbar patients for second treatments, provided they meet inclusion requirements at the scheduled time □ Group E receives world’s first FDA - approved cervical and lumbar transplantations □ Single center ( Emory University Hospital) 14 Patients #1 – 3: Five injections at Lumbar Cord (Unilateral) Patients #4 – 6: Ten Injections at Lumbar Cord (Bilateral) Group A: Non - ambulatory January 2010 – August 2010 Patients #7 – 9 (Group B): Five injections at Lumbar Cord (Unilateral) Patients #10 – 12 (Group C): Ten Injections at Lumbar Cord (Bilateral) Group B/C: Ambulatory - Lumbar October 2010 – April 2011 Patients #13 – 15 (Group D): Five injections at Cervical Cord Patients #16 – 18 (Group E): Ten Lumbar and Five Cervical Injections Group D/E: Ambulatory - Cervical November 2011 – Late 2012

Neuralstem Cell Therapy Program (U.S.) ALS Phase I Clinical Trial: Cervical Phase Timing □ Cervical cohort transplantations completed (5 of 6): □ First Cervical Patient #13: Nov. 2011 □ Patient #14: Feb. 2012 □ Patient #15 : April 2012 □ First Returning Patient #16: June 2012 □ Patient #17: July 2012 □ Patient #18: scheduled August 2012 □ Entire 18 - patient trial, as it currently stands, concludes six months after final surgery □ Trial amendment submitted to FDA to: □ Add three more cervical patients □ Double the cell dose to 200,000 cells per injections 15 Patients #1 – 3: Five injections at Lumbar Cord (Unilateral) Patients #4 – 6: Ten Injections at Lumbar Cord (Bilateral) Group A: Non - ambulatory January 2010 – August 2010 Patients #7 – 9 (Group B): Five injections at Lumbar Cord (Unilateral) Patients #10 – 12 (Group C): Ten Injections at Lumbar Cord (Bilateral) Group B/C: Ambulatory - Lumbar October 2010 – April 2011 Patients #13 – 15 (Group D): Five injections at Cervical Cord Patients #16 – 18 (Group E): Ten Lumbar and Five Cervical Injections Group D/E: Ambulatory - Cervical November 2011 – Late 2012

Neuralstem Cell Therapy Program (U.S.) Additional Indications □ Spinal Cord Injury (SCI) □ IND filed August 2010, on hold pending submission of rodent tumorigenicity data to FDA □ Same cells and injection procedure as ALS trial □ Chronic Stroke □ Positive proof - of - principle data from rat stroke model (University of South Florida) □ Intracerebral injections at peri - infarct sites through method previously shown to be safe □ Study planned in stroke patients with stable paralysis for at least four months □ Glioblastoma (Brain Cancer) □ Pre - clinical research program to engineer Neuralstem's cells to express various anti - cancer agents □ DOD funded program to develop and engineer neural stem cell technology through $1.6 M award □ Goal is to submit IND to treat glioblastoma (brain cancer) the FDA by 2015 16

Neuralstem Cell Therapy Program (International) Cell Therapy Product Status □ Neuralstem China : Stroke □ Phase I / II / III Trial expected to commence in January 2013 □ Wholly owned subsidiary: Neuralstem China 神脑生物医药公司 (Suzhou Neuralstem Biopharmaceutical Company, Ltd.) □ BaYi Brain Hospital in Beijing, collaborator □ Mexico City: ALS □ Phase I / II Trial expected to commence in Sept. 2012 and complete in 2Q2013 □ Pivotal Phase II / III Trial expected to commence in 4Q13 □ South Korea, Indonesia, Malaysia, Philippines, Singapore and Vietnam □ Exclusive option agreement with South Korean partner CJ CheilJedang for cell therapy products in South Asian countries: South Korea, Indonesia, Malaysia, Philippines, Singapore and Vietnam 17

Neuralstem Small Molecule Drug Program First - in - class Neurogenic Small Molecule Drugs □ Initially funded by U.S. DOD □ Patented neurogenic small molecule compounds: the ability to recruit endogenous neural stem cells to generate more new neurons in the adult brain □ Lead Drug in Clinical Development: NSI - 189 □ First novel neurogenic compound □ Mechanism of Action: To Be Determined □ Route of Administration: Oral □ Key biological activity: □ Enhanced neurogenesis and □ Increased hippocampal volume □ Neuralstem owns 100% of the commercialization rights for the compounds 18

19 Latency to eat (sec) 0 100 200 300 400 500 600 Water Imipramine 60 mg/kg Vehicle NSI-144 NSI-150 NSI-158 NSI-189 Latency to eat (sec) 0 100 200 300 400 500 600 * * * Mean BrdU + Cells/2DG/Brain □ Behavior Assay Validated for Antidepressants □ Increased Neurogenesis in Hippocampus NSI - 189 Compelling Data from Animal Studies

20 Latency to eat (sec) 0 150 300 450 600 750 900 1050 1200 Water Imipramine 60 mg/kg NSI-189 3mg/kg NSI-189 10mg/kg NSI-189 30mg/kg NSI-189 100mg/kg * * * * NSI - 189 Pre - clinical Animal Data Dose - dependent Behavior Effect and Increased Hippocampal Volume □ NSI - 189 shown to be as effective as antidepressant Imipramine

21 Cohort Statistics C MAX ( ng /mL) T MAX ( hr ) AUC INF ( hr * ng /mL) t 1/2 ( hr ) 40mg (n=9) Mean 300 0.7 874 18 60mg (n=8) Mean 275 0.8 730 19 80mg (n=8) Mean 387 0.8 1149 17 40mg Fasting (n=10) Mean 250 0.8 1056 19.3 40mg Fed (n=10) Mean 156 2.6 1100 18.0 NSI - 189 Clinical Data Phase Ia Single Ascending Dose □ No Adverse Events (AE) or Serious Adverse Events (SAE) in all cohorts

Neuralstem Small Molecule Drug Program NSI - 189/MDD Phase Ib Clinical Trial: Depressed Patients □ Major Depressive Disorder (MDD) – leading indication □ Phase Ib , 28 - day multiple ascending dose study, commenced June 2012 □ Other potential NSI - 189 applications □ Alzheimer’s Disease □ Cognitive complication due to Diabetes □ Stroke □ Traumatic Brain Injury □ Post - Traumatic Stress Disorder □ CTE – Chronic Traumatic Encephalopathy □ Neurodegeneration □ Anti - Aging ( Nootropic ) 22 41 Volunteers Escalating doses of single oral administration of NSI - 189 Phase Ia : Healthy Normal Volunteers 24 Patients (8 patients per dose X 3 doses) Escalating doses of daily administration for 28 days Phase Ib : Depression Patients June 2012 – est. 1Q 2013 February 2011 – October 2011

Neuralstem , Inc. Clinical Programs Plan for 2012 - 2013 23 2011 2012 2013 Cell Therapy Programs ALS (NSI - 566) Spinal Cord Injury (NSI - 566) Stroke (NSI - 566) Small Molecule Programs Major Depressive Disorder (NSI - 189) Completed Trials Core U.S. Trials Ex - U.S. Expansion Trials Phase I / Lumbar Cervical Phase Ia Phase Ib China Phase I/II/III Phase I Mexico City Phase I/II

Neuralstem , Inc. Catalysts: Next 12 Months □ Ongoing Clinical Trials □ ALS □ Phase I cervical - transplantations completion □ Phase I potential expansion, pending FDA amendment approval, to increase both number of patients treated and NSI - 566 dose □ Phase II trial application □ NSI - 189 □ Phase Ib trial data and completion □ Phase IIa trial application in U.S. □ New Clinical Trials □ NSI - 189 Phase II approval □ SCI IND approval □ Stroke Phase I / II / III trial in China □ ALS Phase I / II in Mexico City □ Business Milestones □ License of NSI - 189 with a Japanese pharmaceutical company for Japanese market □ First license(s) of cell therapy surgical device to industry and academia □ Partnerships expected on cell therapy in selected markets 24

Neuralstem , Inc. Investment Highlights □ Proprietary Patented Neural Stem Cell Platform Serves as Foundation for Cell Therapy and Small Molecule Programs □ Positive Human Data for Neural Stem Cell Therapy in ALS Patients □ Cell Therapy procedure and NSI - 566 treatment shown to be safe and well - tolerated, with promising early indications of treatment effect □ Patented First - in - class Neurogenic Small Molecule Drug Program NSI - 189 □ NSI - 189 shown to be safe in human clinical trials □ World - class Team Including Discoverer and Developer of Neural Stem Cell Technology □ Capital Efficient Business Model with Modest Burn Relative to Clinical Progress 25

Neuralstem , Inc. Appendix □ ALS Phase I Trial Design □ NSI - 189/MDD Phase I Trial Design □ Cell Therapy Surgical Device □ Published Papers 26

Neuralstem ALS Trial ALS Phase I Trial Design □ Primary Endpoint □ Safety □ Secondary Endpoints □ Attenuation of motor function loss □ Maintenance of respiratory capacity □ Stabilization of ALS functional rating scale □ Reduction of spasticity/rigidity if present □ Graft survival at autopsy upon mortality 27 Patients #1 – 3: Five injections at Lumbar Cord (Unilateral) Patients #4 – 6: Ten Injections at Lumbar Cord (Bilateral) Group A: Non - ambulatory January 2010 – August 2010 Patients #7 – 9 (Group B): Five injections at Lumbar Cord (Unilateral) Patients #10 – 12 (Group C): Ten Injections at Lumbar Cord (Bilateral) Group B/C: Ambulatory - Lumbar October 2010 – April 2011 Patients #13 – 15 (Group D): Five injections at Cervical Cord Patients #16 – 18 (Group E): Five Injections at Cervical Cord Group D/E: Ambulatory - Cervical November 2011 – Late 2012 Patients #13 – 15 (Group D): Five injections at Cervical Cord Patients #16 – 18 (Group E): Ten Lumbar and Five Cervical Injections Group D/E: Ambulatory - Cervical November 2011 – Late 2012

Neuralstem NSI - 189/MDD Trial Small Molecule Phase I Trial Design □ Primary Endpoint □ Safety □ Secondary Endpoints □ Tolerability □ Pharmacokinetics □ Pharmacodynamic effects (hippocampal volume by MRI, BDNF/CRH/other factors in blood, urine biomarkers, cortisol in saliva, qEEG , MADRS, NGH Depression Questionnaire, Columbia Suicidal Rating) □ Design: randomized, double - blind, placebo - controlled, multiple - dose escalating trial. Subject: diagnosis of recurrent major depressive disorder. Eight subjects per dose (six treated, two placebo). Three doses: 40 mg. q.d ., 40 mg. b.i.d ., 40 mg. t.i.d . Administration: daily for 28 days. Two - month recovery follow - up. 28 41 Volunteers Escalating doses of single oral administration of NSI - 189 Phase Ia : Healthy Normal Volunteers February 2011 – October 2011 24 Patients ( 8 patients per dose X 3 doses) Escalating doses of daily administration for 28 days Phase Ib : Depression Patients June 2012 – est. 1Q 2013

Neuralstem Cell Therapy Surgical Device Exclusive Worldwide License □ Spinal Platform and Floating Cannula □ Proprietary breakthrough medical device □ Expected to be the standard in industry and research community for intraspinal procedures □ Neuralstem holds exclusive worldwide license □ Designed by ALS trial neurosurgeon, Nicholas M. Boulis , MD, specifically for the world's first intraspinal delivery of neural stem cells □ To be utilized to deliver Neuralstem cells in the spinal cord safely and effectively for myriad diseases and injuries □ Patent - protected □ U.S. Patent No. 8,092,495 (January 2012) □ Spinal Platform and Method for Delivering a Therapeutic Agent to a Spinal Cord Target □ Issued & Pending Patents: U.S. Patent No. 7,833,217 (November 2010); U.S. Application No. 12/913,527 □ Floating Spinal Cannula and Method of Use 29

Neuralstem Technology Published Papers □ Lumbar Intraspinal Injection of Neural Stem Cells in Patients with ALS: Results of a Phase I Trial in 12 Patients. □ Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman, EL, Department of Neurology, Emory University School of Medicine, Atlanta □ Stem Cells, 2012 Jun;30(6):1144 - 51 . □ Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. □ Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE , Department of Pathology, Division of Neuropathology, The Johns Hopkins Medical Institutions, Baltimore □ The Journal of Comparative Neurology , 2009 Jun 1;514(4):297 - 309. □ Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. □ Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, Koliatsos VE , Department of Pathology, Division of Neuropathology, The Johns Hopkins Medical Institutions, Baltimore □ PLoS Medicine , 2007 Feb;4(2):e39. □ Functional recovery in rats with ischemic paraplegia after spinal grafting of human spinal stem cells. □ Cizkova D, Kakinohana O, Kucharova K, Marsala S, Johe K, Hazel T, Hefferan MP, Marsala M , Institute of Neurobiology, Centrum of Excellence, Slovak Academy of Science, Kosice, Soltesovej 4, Slovakia. □ Neuroscience , 2007 Jun 29;147(2):546 - 60. Epub 2007 May 23. □ Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. □ Yan J, Xu L, Welsh AM, Chen D, Hazel T, Johe K, Koliatsos VE, Department of Pathology, Neuropathology Division, The Johns Hopkins University School of Medicine, Baltimore □ Stem cells (Dayton, Ohio) , 2006 Aug;24(8):1976 - 85. Epub 2006 Apr 27. 30
