Attached files
| file | filename |
|---|---|
| 8-K - SCIENTELLE FORM 8K - JUNIPER PHARMACEUTICALS INC | scientelle8k.htm |
LICENSE AGREEMENT
This LICENSE AGREEMENT (the “Agreement”), dated as of the 24 day of July 2012 (the “Effective Date”), is entered into by and between Columbia Laboratories, Inc. (“Columbia”), a Delaware corporation; Columbia Laboratories (Bermuda) Ltd. (“Bermuda”), a Bermuda corporation (Columbia and Bermuda collectively “Licensor”); and Scientelle LLC, a New Jersey limited liability company (“Licensee”). Licensor and Licensee are each sometimes referred to herein as a “Party”, and collectively, as the “Parties.”
RECITALS
WHEREAS, Licensor owns certain patents, patent applications and know how relating to the use of peroxides for the treatment or prevention of bacterial vaginosis and wishes to grant a license to such intellectual property to Licensee; and
WHEREAS, Licensee wishes to accept such license, on the terms and conditions set forth herein.
NOW, THEREFORE, in consideration of the foregoing and the mutual covenants contained herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
ARTICLE I.
DEFINITIONS
The following terms when used in this Agreement, with initial capital letters shall have the respective meanings ascribed to them in this Article 1.
“Affiliate” means, with respect to any Person, any other Person that directly or indirectly Controls, is Controlled by or is under common Control with such first Person. A Person will be deemed to “Control” another Person if such first Person has the power to direct or cause the direction of the management and policies of such other Person, whether through ownership of securities, by contract or otherwise.
“Applicable Law” means all laws, rules, regulations and guidances applicable to this Agreement or the activities contemplated hereunder, including, without limitation, the United States Food, Drug, and Cosmetic Act or 1938, as amended, and applicable regulations, FDA-issued guidances, federal and state anti-kickback laws, privacy laws, consumer protection statutes, and any rules, regulations, guidelines, guidances, ordinances or other requirements of the United States, the European Union, any state, county, city or other political subdivision or of any Governmental Entity that may be in effect from time to time in the Territory and that are applicable to this Agreement or the activities contemplated hereunder.
“Confidential Information” shall mean all information and materials disclosed by one Party, the disclosing Party, to the other Party, the receiving Party, pursuant to this Agreement, including but not limited to, all information relating to the disclosing Party's technology, know-how, Inventions, data, materials, products, processes, potential or actual customers, business strategies, suppliers or business. Confidential Information does not include information that: (i) is in the receiving Party's lawful possession prior to disclosure by the disclosing Party, as evidenced by receiving Party's written records; (ii) has become generally available to the public through no wrongful act or omission of the receiving Party; or (iii) has been provided to the receiving Party by any third party having no obligation of confidentiality, direct or indirect, to the disclosing Party.
“Field of Use” means any and all uses of the Inventions and Licensed Intellectual Property.
“GAAP” means generally accepted accounting principles (i) in the United States; or (ii) consistent with International Financial Reporting Standards (IFRS), each as in effect from time to time, applied on a consistent basis.
“Governmental Entity” means any court, administrative agency or commission or other governmental authority or instrumentality of applicable jurisdiction, whether domestic or foreign.
“Gross Sales” means the gross amount billed or invoiced by Licensee, and its subsidiaries and other Affiliates and any third party to whom Licensee sublicenses the Licensed Intellectual Property with respect to sales of the Product, for their sales of the Product to independent, unaffiliated third party customers, not including any sales that take place in connection with the further development, testing or clinical trials of the Product
“Inventions” mean any findings, developments, discoveries, inventions, additions, modifications, formulations, or changes which are necessary for or used in the development, manufacture or commercialization of the Products, including without limitation, new or improved methods, processes, manufacturing techniques, specifications, ingredients, formulations, compositions, preparations, presentations, means of delivery, dosages, packaging or labeling of the Products, whether or not protected or protectable as a trade secret, patent, trademark or copyright, but expressly excluding any tangible or intellectual property or any derivatives thereof or improvements thereon, which do not relate to Products, and which are assigned to Licensee.
“Knowledge of Licensor” means the actual knowledge after due inquiry under the circumstances of the following individuals: Frank Condella (Chief Executive Officer), Michael McGrane (Senior Vice President, General Counsel and Secretary), George Creasy (Vice President of Clinical Research), and Lawrence A. Gyenes (Senior Vice President, Chief Financial Officer and Treasurer).
“Licensed Know How” means all the know how, trade secrets, expertise, inventions, discoveries, technical information and other unpatented information related to the Product that is owned or controlled by, or licensed to, Licensor or its Affiliates, including, but not limited to, all information reasonably necessary for Licensee and its Affiliates to make, have made, use or sell the Product.
“Licensed Intellectual Property” means: (a) the Licensed Patents; and (b) the Licensed Know How.
“Licensed Patents” means all: (a) patents and patent applications set forth on Exhibit A attached hereto; (b) patents and patent applications claiming priority thereto or to which the patents and patent applications set forth on Exhibit A claim priority, whether or not filed before or after the Effective Date, including provisionals, continuations, continuations-in-part, and divisions; (c) all patents and registrations issuing therefrom; (d) re-examinations, renewals, reissues, and extensions allowed on any of the foregoing; and (e) all other patents and patent applications owned or controlled by, or licensed to, Licensor or its Affiliates as of the Effective Date that cover the Product or that the practice of which is reasonably necessary for Licensee to make, have made, use, sell, offer to sell or import the Product. For purposes of this definition, any Licensed Patents that meet the criteria in subsection (e) above at any time during the Term shall not be excluded from this definition simply because a particular Affiliate of Licensor is no longer an Affiliate.
“Net Sales” means Gross Sales less deductions for (a) quantity, trade or cash discounts or allowances (including customer rebates) actually allowed and taken, (b) amounts deducted by reason of rejections or returns of goods, rebates, coupons or chargebacks or retroactive price reductions and (c) third party wholesaler
fees, and specialty pharmacy charges, in each case, to the extent specifically attributable to the Product. To the extent applicable, components of Net Sales shall be determined in the ordinary course of business in accordance with GAAP.
“Partnering Consideration” means the fair market value of all consideration received by Licensee from unrelated third parties relating to a grant of rights to such Third Parties to the Product. For avoidance of doubt, Partnering Consideration (See Section 4.2(c)) does not include the value of in kind services to be performed by a third party for the purpose of obtaining regulatory approvals or marketing authorizations.
“Person” means any individual, corporation, partnership, limited liability company, joint venture, trust, business association, organization, Governmental Entity or other entity.
“Product” means the product developed by Licensee or its Affiliates covered by one or more valid claims of the Licensed Patents.
“Royalty Period” means on a country-by-country basis, the period between the Effective Date and the last to expire valid Licensed Patent covering the Product.
“Snap Back Period” means the period from the Effective Date through the eighteen (18) months anniversary of the Effective Date.
“Territory” means the world.
Definitions of the defined terms listed below are contained in the Section set forth opposite the defined term in the table below.
Defined Term | Section of Agreement |
Agreement | Preamble |
Commercially Reasonable Efforts | 4.1 |
Disclosure Schedule | 7.1(c) |
Effective Date | Preamble |
Infringement Notice | 3.4(a) |
Licensee | Preamble |
Licensee Invention | 3.2(a) |
Licensor | Preamble |
Partnering Consideration Fee | 4.2(c) |
Party/Parties | Preamble |
Proceeding | 3.5 |
Qualified Development Expenses | 6.4 |
Royalty Fee | 4.2(b) |
Snap Back Exercise Amount | 6.2 |
Snap Back Option | 6.1 |
Snap Back Option Effective Date | 6.1 |
Snap Back Option Notice | 6.1 |
The following attached Exhibits are incorporated into this Agreement and form a part hereof:
Exhibit A: Licensed Patents
Exhibit B: Proof of Concept Plan
Exhibit C: Royalty Fee Calculation Example
ARTICLE II.
LICENSE GRANT
2.1 Grant of Exclusive License. Subject to the terms and conditions of this Agreement, Licensor hereby grants to Licensee on behalf of itself and its Affiliates, an exclusive (even as to Licensor and its Affiliates), and transferable license under the Licensed Intellectual Property to develop, have developed, manufacture, have manufactured, seek regulatory approval, use, market, sell, have sold, offer to sell, import or otherwise exploit the Product in the Field of Use in the Territory, with the right to grant sublicenses subject to Section 2.2.
2.2 Sublicenses. Licensee shall have the right to grant sublicenses (with rights to further sublicense) under the licenses set forth in Section 2.1 to sublicensees, provided that Licensee shall ensure that its sublicensees shall be subject to a written agreement with terms and conditions that are consistent with, and no less protective of Licensor than, the terms and conditions hereunder, including the conditions set forth in Section 8.1. Notwithstanding the foregoing, Licensor's written consent shall be required for any sublicenses in the United States or European Union granted prior to the expiration of the Snap Back Period.
2.3 No Implied Licenses. Any intellectual property rights of a Party not expressly granted to the other Party under the provisions of this Agreement shall be retained by such Party. Except as expressly provided in this Agreement, a Party does not grant to the other Party any right or license in any intellectual property belonging to such Party, whether by implication, estoppel or otherwise.
2.4 Licensee's Retained Rights. The licensed rights granted herein shall be deemed licenses of “intellectual property” for purposes of the United States Code, Title 11, Section 365(n). In the event of Licensor's bankruptcy and a subsequent rejection or disclaimer of this Agreement by a bankruptcy trustee or by Licensor as a debtor-in-possession, whether under the law of the United States or elsewhere, or in the event of a similar action under Applicable Law, the Licensee and its respective Affiliates may elect to retain their licensed rights, subject to and in accordance with the provisions of the United States Code, Title 11, Section 365(n) or other Applicable Law.
2.5 Technology Transfer. Upon the Effective Date, Licensor shall deliver to Licensee or its designee all Licensed Know How in its or its Affiliates' possession, if any. Licensed Know How developed after the date of this Agreement by Licensor or its Affiliates, if any, shall be disclosed promptly to Licensee and delivered within thirty (30) days following such disclosure.
ARTICLE III.
INVENTIONS; INTELLECTUAL PROPERTY
3.1 Invention Disclosures. Licensee shall promptly disclose to Licensor any and all Inventions arising on or after the Effective Date under the Licensee's or its Affiliate's activities conducted pursuant to this Agreement. Such disclosures shall be provided in writing and in sufficient detail for the Licensor to understand the scope and nature of such Inventions. Any such disclosure of Inventions shall be treated as Confidential Information of the disclosing Party, subject to the terms of this Article III.
3.2 Ownership of Inventions.
Subject to the provisions of the Snap Back Option, Licensee shall solely own any and all Inventions solely made, conceived, or reduced to practice by Licensee or its Affiliates on or after the Effective Date (the “Licensee Invention”).
3.3 Patent Management and Assignment.
(a)Licensed Patents. During the Term, and subject to Sections 3.3(b) and (c) below, Licensor will have the responsibility for, and shall diligently carry out, the filing, prosecuting and maintaining of the Licensed Patents, at Licensor's sole cost and expense. Licensor shall provide Licensee with an
opportunity to consult with Licensor regarding the content of any amendment, response or correspondence with any applicable patent agency within a reasonable time period prior to the submission of the same, and Licensor will incorporate reasonable requests by Licensee regarding the prosecution of such patent applications.
(b)During the Snap Back Period, Licensor shall have the obligation to continue filing, prosecution and maintenance of Licensed Patents, as well as patent applications not issued as of the Effective Date, provided that in the case of pending applications, Licensor's obligations under this Section 3.3(b) shall be limited to applications relating to the following countries: Japan, Canada, China, Norway, Brazil, Argentina, Venezuela, Hungary, and Poland (the “Patent Pending Countries”), and Licensor shall have no obligation to file new patent applications in the Territory.
(c)Following the expiration of the Snap Back Period, Licensor may elect, at its sole discretion, to discontinue the filing, prosecution and maintenance of any issued License Patent or any applications related to Patent Pending Countries, provided however, that Licensor shall (i) provide Licensee notice of its intent to abandon such Licensed Patent or patent application no less than ninety (90) days in advance of any USPTO or equivalent foreign patent office filing deadlines; (ii) promptly assign such Licensed Patent or patent application to the Licensee should Licensee elect to accept such assignment (at Licensee's sole discretion); and (iii) forego any Royalty Fee or Partnering Consideration Fee in the country related to such abandoned or assigned patent.
(d)Sole Inventions. Except as contemplated in Section 3.3(a), (b) and (c) above, Licensee shall be solely responsible for the filing, prosecution, and maintenance of its respective Licensee Invention at its sole cost and expense and in its sole discretion. Neither Party shall be permitted to disclose Confidential Information of the other Party in any patent application for its Invention without the other Party's express written consent.
(e)Assistance. Each Party will fully cooperate with the other Party in connection with the filing, prosecution and maintenance of the patents and patent applications for which such Party is responsible, including by providing reasonable access to relevant Persons and executing all documentation reasonably requested at the sole cost and expense of the Party requesting such assistance.
(f)Assumption of Responsibility. In the event that either Party declines or fails to prepare, file, prosecute or maintain patent applications or patents or take such other actions relating to the patents and patent applications for which it is primarily responsible in Sections 3.3 (a) through (e) above, then such Party shall promptly and in no event later than ninety (90) days prior to any USPTO or equivalent foreign patent office filing deadline, provide written notice to the other Party. The other Party shall have the right upon such notice or, if no notice is received, at any time within ninety (90) days prior to any such filing deadline, to assume such responsibilities at its own expense, using counsel of its choice, but the ownership shall remain unchanged unless otherwise agreed by the Parties in writing.
3.4 Enforcement.
(a) Notice. Each Party will promptly notify the other of any suspected or actual infringement, misuse or other violation by a third party of any of the Licensed Intellectual Property or Licensee Inventions (the “Infringement Notice”), including any “patent certification” filed in the United States under 21 U.S.C. §355(b)(2) or 21 U.S.C. §355(j)(2) or similar provisions in the Territory and of any declaratory judgment, opposition, or similar action alleging the invalidity, unenforceability or non-infringement of the same.
(b) Licensor's First Right of Enforcement. With the exception of Licensed Patents and patent applications transferred to Licensee pursuant to Section 3.3(c), during the Term, Licensor will have the first right to bring and control any legal action in connection with Licensed Patents and Licensed Know How at Licensor's own expense as it reasonably determines appropriate. If Licensor fails to bring an action, then Licensee shall have the right to bring and control any such action by counsel of its own choice, at Licensee's cost and expense.
(c) Licensee's First Right of Enforcement. Licensee will have the first right to bring and
control any legal action in connection with the Licensee Inventions and any Licensed Patents and patent applications transferred to Licensee pursuant to Section 3.3(c), at Licensee's own expense as it reasonably determines appropriate. If Licensee fails to bring an action or proceeding with respect to, or to terminate, infringement described in this Section 3.4(c) within ninety (90) days following the Infringement Notice, then Licensor shall have the right to bring and control any such action by counsel of its own choice, at Licensor's cost and expense.
(d) Cooperation. At the request of the Party prosecuting any enforcement action or proceeding hereunder, the other Party, at the prosecuting Party's cost and expense, subject to the foregoing Sections 3.4(b) through (c) shall provide reasonable assistance in connection therewith, including by executing reasonably appropriate documents, cooperating in discovery and joining as a party to the action if required.
(e) Allocation of Damages. In the event that either Party recovers any amounts from any litigation or settlement resulting from an enforcement action in this Section 3.4, then such amounts shall be applied: (i) first, to reimburse Licensor and Licensee for their respective actual out-of-pocket expenses; and (ii) second, any remaining amount shall be the sole property of the enforcing Party.
3.5 Infringement of Third Party Patents. If Licensee, or any of its Affiliates or sublicensees, is subject to an action, suit or other proceeding (“Proceeding”) by a third party for infringement of a third party's patent, trademark or other intellectual property rights in the Territory because of the manufacture, use, sale or other exploitation of the Product or the Licensed Intellectual Property, Licensee shall promptly notify Licensor in writing of such Proceeding, and the Parties shall consult with each other to agree upon the course of action to be taken. During the Snap Back Period, unless otherwise agreed in writing by the Parties, Licensor shall undertake the defense of such Proceeding with counsel of its choice (which shall be reasonably acceptable to Licensee), at its own expense, in which event Licensee shall have the right to be represented by advisory counsel of its own selection at its own expense. Licensor and Licensee shall reasonably cooperate and coordinate with each other in the defense of such Proceeding and furnish all pertinent evidence and reasonable assistance in their control. Each Party shall keep the other Party reasonably informed of all material developments in connection therewith. In the event Licensor fails to assume the defense of such Proceeding during the Snap Back Period, Licensee may assume such defense at the expense of Licensor; provided, however, that Licensee will not consent to the entry of any judgment or enter into any settlement with respect to the Proceeding without the prior written consent of Licensor. In connection with the foregoing, Licensor agrees to indemnify Licensee and its Affiliates and their respective officers, directors, managers, members, employees, successors and assigns against, and agrees to hold them harmless from, any loss arising or resulting from or relating to infringement claims of the type described in this Section 3.5.
3.6 No Encumbrances/Covenant Not to Sue. The Parties agree not to undertake any action to render the Licensed Patents invalid or otherwise seek to encumber the Licensed Patents. Licensee covenants not to sue Licensor, its Affiliates or licensees, or seek other forms of legal remedy from Licensor, its Affiliates or licensees, for infringement of any Licensee Invention so long as such Licensee Invention arises out of or is related to the development or commercialization by Licensor of a Product after the Snap Back Option Effective Date. Licensor agrees not to undertake any action to render Licensee Inventions invalid or otherwise seek to encumber Licensee Inventions that are not subject to the provisions of Section 6.2(iv).
3.7 Confidential Information.
(a) Non-Disclosure. The Parties agree that during the Term, and for a period of five (5) years after the expiration thereof, with respect to Confidential Information of the disclosing Party, the receiving Party will (i) maintain such Confidential Information in confidence to the same extent the receiving Party maintains its own confidential or proprietary information or trade secrets of similar kind and value, but in no event less than a reasonable degree of care; (ii) subject to the provisions of this Section 3.7, not disclose such Confidential Information to any third party without the prior written consent of the disclosing Party, except for disclosures to its Affiliates, sublicensees, partners, and potential sublicensees or potential partners as necessary to execute the Proof of Concept Plan and who agree to be bound by obligations of
non‑disclosure and non‑use at least as stringent as those contained in this Section 3.7(a); and (iii) not use Confidential Information for any purpose except those purposes permitted by this Agreement.
(b) Authorized Disclosure. Notwithstanding the foregoing Section 3.7(a), a receiving Party may disclose Confidential Information of the disclosing Party if the receiving Party or any of its Affiliates or their respective representatives is compelled to disclose any such information by a valid court or administrative order or by other requirements of law, provided that the receiving Party: (i) shall promptly notify the disclosing Party in writing and shall disclose only that portion of such information that is legally required to be disclosed; (ii) shall cooperate with the disclosing Party at the disclosing Party's expense in the disclosing Party's efforts to obtain an appropriate protective order or other remedy of the disclosing Party's election; and (iii) shall obtain reasonable assurances for the confidential treatment of the Confidential Information, if available.
ARTICLE IV.
DEVELOPMENT; ROYALTY FEE; PARTNERING CONSIDERATION FEE; AUDITS;
NO IMPLIED LICENSES
4.1 Development. During the Snap Back Period, Licensee shall use Commercially Reasonable Efforts to execute the Proof of Concept Plan as outlined in Exhibit B. During the Royalty Period, Licensee shall use its Commercially Reasonable Efforts to formulate, develop, seek regulatory approval of Product, and promote and sell the Product in the United States and the European Union. For purposes of this Section 4.1, “Commercially Reasonable Efforts” means such efforts that are commensurate with the efforts a reasonable Person would extend to a product at a similar stage taking into account its safety and efficacy, the cost to develop and commercialize the Product, the establishment of the Product in the marketplace, the competitiveness of the marketplace, the proprietary position of the Product, the regulatory structure involved, the profitability of the Product and all other relevant factors.
4.2 Royalty Fee and Partnering Consideration Fee.
(a) General. During the Royalty Period, Licensee shall pay to Licensor the Royalty Fee or Partnering Consideration Fee described in Section 4.2(b), below, with respect to Net Sales of the Product or Partnering Consideration received by Licensee. Upon the expiration of the Royalty Period, the license granted pursuant to Section 2.1 shall be fully-paid up and no further royalties or other payments shall be due to Licensor from Licensee.
(b) Royalty Fee. Subject to Section 4.2(a), during the Royalty Period, Licensee shall pay to Licensor, within sixty (60) days following each calendar quarter or portion thereof during the Term as related to U.S. and within one-hundred-twenty (120) days as related to rest-of-world, the following royalty in countries in which the Licensee commercializes the Products (“Royalty Fee”):
(i)three percent (3.0%) of Net Sales in the U.S.; and
(ii)a tiered royalty in countries outside of the U.S., as calculated on a country-by-country basis of:
Of the portion of Net Sales greater than or equal to: | But less than: | Royalty on such portion |
$0 million | $10 million | 2% |
$10 million | $50 million | 2.5% |
$50 million | 3% | |
(c) Partnering Consideration Fee. In countries where the Licensee sublicenses rights to the Product or otherwise receives Partnering Consideration, Licensee shall pay to Licensor ten percent (10%) of all Partnering Consideration (the “Partnering Consideration Fee”) received in the U.S. and the following percentages, on a country-by-country basis outside the U.S.
Of the portion of Partnering Consideration greater than or equal to: | But less than: | Percent of such Partnering Consideration |
$0 million | $5 million | 6% |
$5 million | $15 million | 8% |
$15 million | 10% | |
A Royalty Fee and Partnering Consideration Fee calculation example is attached hereto as Exhibit C.
(d) For the avoidance of doubt, Licensee shall owe no Royalty Fee or Partnering Consideration Fee to Licensor relating to Licensed Patents or patent applications transferred to Licensee pursuant to Section 3.3(c), or otherwise abandoned by Licensor.
(e) Reporting. At the time of Licensee's remittance of each earned Royalty Fee or Partnering Consideration Fee, Licensee shall furnish to Licensor a report showing the Gross Sales, detailed deductions to Gross Sales, and Net Sales of the Products or Partnering Consideration received for the corresponding calendar quarter. Licensee shall also provide a report on or before February 28 of each year during the Royalty Period stating the Gross Sales, Net Sales and Partnering Consideration received for the immediately preceding year. Licensee shall maintain all records in accordance with GAAP.
(f) Late Payments. If Licensor does not receive the total applicable payment when due under this Section 4.2, Licensee shall pay interest with respect to any payments past due to Licensor at the lower of: (i) the maximum rate allowed by law; or (ii) the rate of one (1.0%) percent per month, computed from the original due date until paid.
(g) Audits. Upon thirty (30) days prior written notice to Licensee, Licensor shall have the right to have an independent certified public accountant, selected by Licensor and acceptable to Licensee, which acceptance may not be unreasonably withheld, audit Licensee's records pertaining to calculation of Net Sales and Partnering Consideration subject to this Agreement, during normal business hours, to verify the amounts payable hereunder; provided, however, that (a) such audit shall not take place more frequently than once per year; (b) such audit shall not cover such records for more than the preceding three (3) years; (c) any such audit shall take place at the location Licensee maintains such records, or at such other location mutually agreed; and (d) all information obtained during the audit shall constitute Confidential Information of Licensee pursuant to the terms of this Agreement. Any such audit shall be solely for the purpose of determining, and to report, compliance or non-compliance with the Royalty Fee and Partnering Consideration Fee requirements of this Agreement. The expenses of such audit shall be paid by Licensor unless the audit conclusively demonstrates that Licensee has paid Licensor less than ninety-five (95%) percent of the amount determined to be due for a given time period, in which case the expense of the audit shall be paid by Licensee. Licensee shall preserve and maintain all such records and accounts required for such audits for a period of five (5) years after the quarter to which such records and accounts apply. Any independent accounting firm that is hired by Licensor to perform such audit shall agree in writing to be bound by the confidentiality and non-disclosure terms and conditions of this Agreement with respect to any information, data, records and accounts audited.
ARTICLE V.
TERM AND TERMINATION; LIMITED LICENSE
5.1 Term. Unless otherwise terminated pursuant to the provisions of the Snap Back Option, the term of this Agreement shall commence on the Effective Date and shall continue until the expiration of the last to expire Licensed Patents.
ARTICLE VI.
SNAP BACK OPTION
6.1 Snap Back Option. During the Snap Back Period, the Licensor shall have an option (the “Snap Back Option”), at its sole discretion, to terminate the licenses granted to Licensee hereunder by providing consideration to Licensee, as further outlined in this Article VI. At any time during the Snap Back Period, Licensor may provide written notice to Licensee (the “Snap Back Option Notice”) of Licensor's intent to exercise its rights under the Snap Back Option, with the Snap Back Option to be effective thirty (30) days from delivery of such notice (the “Snap Back Option Effective Date”).
6.2 Effect of Snap Back Option Exercise. Upon receipt of the Snap Back Option Notice, Licensee shall (i) have no further obligation to continue to execute against the Proof of Concept Plan; (ii) shall cease incurring any new Qualified Development Expenses, as defined below; (iii) take such actions to reasonably wind down its development activities no later than the Snap Back Option Effective Date; (iv) take such actions to reasonably transfer all Licensee Inventions solely related to the Product, (including all formulations, specifications, methodologies, in-vitro and in-vivo data, and any other thing developed by Licensee) to Licensor no later than the Snap Back Option Effective Date. For avoidance of doubt, Licensee shall not be required to surrender to Licensor any patents, know how, or inventions that are not solely related to the Product; and (v) submit a final report to Licensor no later than thirty (30) days following the Snap Back Option Effective Date summarizing in sufficient detail all Qualified Development Expenses and Licensee's calculation of the Snap Back Exercise Amount (the “Snap Back Exercise Amount”). As of the Snap Back Option Effective Date, all licenses granted to Licensee under this Agreement shall terminate subject only to the provision that if the Snap Back Option is exercised the Licensor agrees that any sublicenses to the Licensed Intellectual Property, which are in force with Licensee at the time of the Snap Back Option Effective Date, shall be transferred to Licensor, and Licensor agrees to the terms and conditions set forth therein, which shall remain in force, provided however, that a sublicense for any country that joins the European Union after the Effective Date may be terminated by Licensor as to that country on 90 days written notice.
6.3 Snap Back Exercise Amount. In consideration for termination of this Agreement during the Snap Back Period, Licensor shall pay to Licensee an amount calculated pursuant to the following table:
Snap Back Option Effective Date | Snap Back Exercise Amount |
On or before the six (6) month anniversary of the Effective Date | An amount equal to 1.5 multiplied by the lessor of (x) the sum of (i) $115,000 and (ii) Qualified Development Expenses; and (y) $450,000. |
After the six (6) month anniversary of the Effective Date, but on or before the twelve (12) month anniversaries of the Effective Date | An amount equal to 2.0 multiplied by the lessor of (x) the sum of (i) $230,000 and (ii) Qualified Development Expenses; and (y) $950,000. |
After the twelve (12) month anniversary of the Effective Date, but on or before the eighteen (18) month anniversaries of the Effective Date | An amount equal to 3.0 multiplied by the lessor of (x) the sum of (i) $345,000 and (ii) Qualified Development Expenses; and (y) $2,000,000. |
6.4. Qualified Development Expenses. For purposes of the calculation of the Snap Back Exercise Amount, “Qualified Development Expenses” shall include Licensee's expenses incurred from the Effective Date through the Snap Back Option Effective Date related to the activities in research, development, manufacturing, commercialization, use, import and export, funding, partnering, and development of Products and Licensee Inventions under the Licensed Patents. Qualified Development Expenses shall include: direct out of pocket cash expenses; the fair market value of in-kind contributions of research and development partners and collaborators. Qualified Development Expenses shall explicitly exclude any salary or other personal remuneration for Dr. Konstantin Zubovskiy.
6.5 During the Snap Back Period, Licensee shall make good faith effort to execute the Proof of
Concept Plan and shall keep good and accurate accounting of the Qualified Development Expenses, consistent with GAAP. At the 5, 11, and 16-month anniversaries of the Effective Date, Licensor shall provide Licensee a report of its good faith estimate of the Qualified Development Expenses incurred since the Effective Date in order for the Licensor to evaluate whether it would like to exercise the Snap Back Option.
6.6 Licensor shall pay Licensee the Snap Back Exercise Amount within ten (10) days of receipt of the Snap Back Exercise Amount Calculation.
ARTICLE VII.
REPRESENTATIONS AND WARRANTIES
7.1 Licensor's Representations and Warranties. Licensor hereby represents and warrants as of the Effective Date that:
(a) it has the right, power and corporate authority to enter into this Agreement including without limitation to grant the license in Section 2.1 on its own behalf and on behalf of its Affiliates and to make the covenants and agreements set forth in this Agreement;
(b) the execution, delivery and performance of this Agreement do not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it is bound, nor to the Knowledge of Licensor, violate any law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it;
(c) the Licensed Intellectual Property is (i) owned by Licensor or its Affiliates, (ii) freely licensable by Licensor to Licensee without the payment of any royalties, license fees or other amounts to any other Person and (iii) free and clear of any rights or claims of any other Person;
(d) the Licensed Patents set forth on Exhibit A, and the Licensed Know How constitutes all of the intellectual property of Licensor related to the manufacture, marketing, distribution and sale of the Product in the Territory;
(e) Licensor has not received any written notice of a claim, and, to the Knowledge of Licensor, there has not been any threatened claim, made by any third party of infringement or misappropriation, or contesting the validity, enforceability, use or ownership of the assets, properties or rights subject to the Licensed Intellectual Property and, to the Knowledge of Licensor, there is no basis therefore;
(f) the Licensed Intellectual Property is not subject to any contractual obligation (i) restricting Licensor's use or rights thereof, (ii) entitling third parties to use the same or (iii) in any way obligating Licensor to make royalty or similar payments to others;
(g) all of the Licensed Patents are currently in compliance with formal legal requirements (including payment of filing, examination and maintenance fees);
(h) to the Knowledge of Licensor, as of the date hereof, there are no outstanding claims asserted against Licensor alleging that the manufacture, marketing or sale of the Product by Licensor infringes, misappropriates or otherwise violates any intellectual property of any other Person and, to the Knowledge of Licensor, there is no basis therefore; and
(i) to the Knowledge of Licensor, the Licensed Intellectual Property is valid, subsisting and enforceable. None of the Licensed Intellectual Property is the subject of any cancellation, abandonment or similar action or proceeding.
7.2 Licensee's Representations and Warranties. Licensee hereby represents and warrants as of the Effective Date that:
(a) it has the right, power and corporate authority to enter into this Agreement and to make the covenants and agreements set forth in this Agreement; and
(b) the execution, delivery and performance of this Agreement do not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it is bound, nor
to its knowledge, violate any law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
ARTICLE VIII.
MISCELLANEOUS
8.1 Disclaimer of Warranty.
(a) EXCEPT FOR THE EXPRESS WARRANTIES SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATIONS NOR GRANTS ANY WARRANTIES, EXPRESS OR IMPLIED, EITHER IN FACT OR BY OPERATION OF LAW, BY STATUTE OR OTHERWISE RELATED TO ANY AND ALL OF THE INTELLECTUAL PROPERTY LICENSED HEREUNDER, AND EACH PARTY SPECIFICALLY DISCLAIMS ANY OTHER REPRESENTATIONS AND WARRANTIES, WHETHER WRITTEN OR ORAL, EXPRESS, STATUTORY OR IMPLIED, INCLUDING ANY WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR USE OR PURPOSE.
(b) To the maximum extent permitted by applicable law, Licensee shall defend, indemnify and hold harmless Licensor, its directors, officers, agents and employees (individually, an "Indemnified Party", and collectively, the "Indemnified Parties"), from and against any and all liability, loss, damage, action, claim or expense suffered or incurred by the Indemnified Parties (including attorney's fees) (individually, a "Liability", and collectively, the "Liabilities") that results from or arises out of: (i) the development, use, manufacture, promotion, sale or other disposition, of any Invention, Licensed Intellectual Property, or Product by Licensee or its Affiliate(s) or their respective assignees, sublicensees, vendors or other third parties; (ii) breach by Licensee or its Affiliate(s) of any covenant or agreement contained in this Agreement; and (iii) the enforcement by an Indemnified Party of its rights under this Section. Without limiting the foregoing, Licensee will defend, indemnify and hold harmless the Indemnified Parties from and against any Liabilities resulting from:
any product liability or other claim of any kind related to the use by a third party of a Product that was manufactured, sold or otherwise disposed by Licensee, its Affiliate(s) or their assignees, sublicensees, vendors or other third parties; and/or
a claim by a third party that the Invention, Licensed Intellectual Property, Product or the design, composition, manufacture, use, sale or other disposition of any Product(s) infringes or violates any patent, copyright, trademark or other intellectual property rights of such third party.
8.2 Governing Law. This Agreement (including any claim or controversy arising out of or relating to this Agreement) shall be governed by and construed in accordance with the laws of the State of New Jersey without regard to conflict of law principles that would result in the application of any law other than the laws of the State of New Jersey.
8.3 Waiver. Any term or condition of this Agreement may be waived at any time by the Party that is entitled to the benefit thereof, but no such waiver shall be effective unless set forth in a written instrument duly executed by or on behalf of the Party waiving such term or condition. The failure of any Party to enforce any condition or part of this Agreement at any time shall not be construed as a waiver of that condition or part, nor shall it forfeit any rights to future enforcement thereof.
8.4 Notices. All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be deemed to have been duly given (a) when received if delivered personally, (b) when transmitted by facsimile (which is confirmed), (c) upon receipt, if sent by registered or certified mail (postage prepaid, return receipt requested) and (d) the day after it is sent, if sent for next-day delivery to a domestic address by overnight mail or courier, to the Parties at the following addresses:
If to Licensor, to: Columbia Laboratories, Inc. 354 Eisenhower Parkway Plaza One, Second Floor Livingston, NJ 07039 Attn: General Counsel Facsimile: 973-994-3001 |
If to Licensee, to: Scientelle, LLC 65 Skyline Drive Morristown, New Jersey 07960 Facsimile: Attn: Konstantin Zubovskiy President, Scientelle LLC |
with copies (which shall not constitute notice) sent concurrently to: |
Facsimile: Attn: |
provided, however, that if any Party shall have designated a different address by notice to the others, then to the last address so designated.
8.5 Relationship of the Parties; No Third Party Beneficiaries.
(a) The Parties are independent contractors. Nothing herein is intended, or shall be deemed, to constitute a partnership, agency, joint venture or employment relationship between the Parties. No Party shall be responsible for the other Party's acts or omissions; and neither Party shall have authority to speak for, represent, obligate, or bind the other Party in any way without prior written authority from the other Party.
(b) This Agreement is solely for the benefit of the Parties hereto and their respective Affiliates and no provision of this Agreement shall be deemed to confer upon any third parties any remedy, claim, liability, reimbursement, claim of action or other right in excess of those existing without reference to this Agreement.
8.6 Amendment; Entire Agreement. This Agreement may not be amended, supplemented or otherwise modified except by an instrument in writing signed by both Parties hereto. This Agreement (together with the Exhibits attached hereto and incorporated herein by reference contains the entire agreement of the Parties hereto with respect to the subject matter hereof and thereof, superseding all negotiations, prior discussions and preliminary agreements made prior to the Effective Date with respect hereto and thereto.
8.7 Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction or other Governmental Entity to be invalid, void, unenforceable or against its regulatory policy such determination shall not affect the enforceability or validity of any other provisions or of the remainder of this Agreement.
8.8 Assignment and Transfer.
(a) Neither Party may assign its rights or obligations under this Agreement without the prior written consent of the other Party, which shall not be unreasonably withheld; provided, however, that, so long as any such successor or assign agrees in writing to be bound by this Agreement, either Party may assign this Agreement and any or all of its rights and obligations under this Agreement, without the prior written consent of the other Party, (i) to an Affiliate or to a successor to the relevant portion of the assigning Party's business by reason of merger, sale of its assets or stock or any similar transaction, and (ii) to its and its Affiliates' lenders as collateral security; provided, that prior to such assignment, the assigning Party provides to the non-assigning Party written notice of such transfer. Any permitted assignee shall assume all
obligations of its assignor under this Agreement. No assignment shall relieve either Party of its responsibility for the performance of any obligation.
(b) Except as otherwise provided herein, this Agreement shall be binding upon and inure to the benefit of the Parties hereto and their successors and permitted assigns.
8.9 Interpretation. Unless the context of this Agreement otherwise requires, (a) words of one gender include the other gender; and (b) words using the singular or plural number also include the plural or singular number, respectively. References to days are to calendar days unless specified otherwise. References to any statute, act, or regulation are to that statute, act, or regulation as amended, modified or supplemented from time to time in accordance with the terms hereof and thereof. The headings contained in this Agreement are for convenience of reference only and shall not be considered in interpreting this Agreement. The words “hereof”, “herein” and “hereunder” and words of like import used in this Agreement shall refer to this Agreement as a whole and not to any particular provision of this Agreement. Whenever the words “include”, “includes” or “including” are used in this Agreement, they shall be deemed to be followed by the words “without limitation”, whether or not they are in fact followed by those words or words of like import. The language in all parts of this Agreement shall be construed, in all cases, according to its fair meaning. The Parties acknowledge that each Party and its counsel have reviewed and revised this Agreement and that any rule of construction to the effect that any ambiguities are to be resolved against the drafting Party shall not be employed in the interpretation of this Agreement.
8.10 Counterparts. This Agreement may be executed in two or more counterparts (including by facsimile or by an electronic scan delivered by electronic mail), each of which shall be deemed to be an original, but all of which, taken together, shall constitute one and the same agreement and shall become effective when counterparts have been signed by each of the Parties hereto delivered to the other Parties, it being understood that all Parties need not sign the same counterpart.
8.11 Further Actions. Each Party will duly execute and deliver, or cause to be duly executed and delivered, such further instruments and do and cause to be done such further acts and things, as may be reasonably necessary or as the other Party may reasonably request in connection with this Agreement in order to carry out more effectively the provisions and purposes hereof.
[Remainder of Page Left Intentionally Blank]
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be duly executed as of the date first above written.
LICENSOR:
Columbia Laboratories, Inc.
By: /S/ Frank C. Condella, Jr.
Name: Frank C. Condella, Jr.
Title: President and Chief Executive Officer
Columbia Laboratories (Bermuda) Ltd.
By: /S/ Frank C. Condella, Jr.
Name: Frank C. Condella, Jr.
Title: President and Chief Executive Officer
LICENSEE:
Scientelle, LLC
By: /S/ Konstantin Zubovskiy
Name: Konstantin Zubovskiy
Title: President
Exhibit A
Licensed Patents
Application No. | Patent No. | Title | Country | Status | Filing Date | Issue Date | Exp. Date |
10/278,910 | 7,709,026 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | UNITED STATES | ISSUED | 10/24/2002 | 5/4/2010 | 6/11/2023 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | EUROPEAN PATENT CONVENT | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2003-539722 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | JAPAN | PUBLISHED | 10/28/2002 | 10/28/2022 | ||
2010-152148 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | JAPAN | PUBLISHED | 10/28/2002 | 10/28/2022 | ||
2,465,133 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | CANADA | PENDING | 10/28/2002 | 10/28/2022 | ||
91,124,849 | I337084 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | TAIWAN | ISSUED | 10/24/2002 | 2/11/2011 | 10/24/2022 |
200,610,148,523.6 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | CHINA | PUBLISHED | 10/28/2002 | 10/28/2022 | ||
2,002,346,897 | 2,002,346,897 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | AUSTRALIA | ISSUED | 10/28/2002 | 7/31/2008 | 10/28/2022 |
532,294 | 532,294 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | NEW ZEALAND | ISSUED | 10/28/2002 | 4/5/2007 | 10/28/2022 |
PA/a/2004/003977 | 252,803 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | MEXICO | ISSUED | 10/28/2002 | 12/18/2007 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | UNITED KINGDOM | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
3/8/60204584 | DE1441769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | GERMANY | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | FRANCE | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | SWITZERLAND | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | AUSTRIA | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | SPAIN | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | SWEDEN | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | DENMARK | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | NETHERLANDS | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | BELGIUM | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | ITALY | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | IRELAND | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | PORTUGAL | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | MONACO | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
20,050,402,243 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | GREECE | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2004 2199 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | NORWAY | PENDING | 10/28/2002 | 10/28/2022 | ||
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | FINLAND | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | LUXEMBOURG | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | SLOVENIA | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
PI0213584-1 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | BRAZIL | PENDING | 10/28/2002 | 10/28/2022 | ||
P02-0104078 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | ARGENTINA | PUBLISHED | 10/28/2002 | 10/28/2022 | ||
2466-2002 | 47.365 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | CHILE | ISSUED | 10/28/2002 | 4/11/2011 | 4/11/2026 |
02-002078 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | VENEZUELA | PUBLISHED | 10/28/2002 | 10/28/2022 | ||
PV/27664 | 26,289 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | MOROCCO | ISSUED | 10/28/2002 | 9/1/2004 | 10/28/2022 |
2004/2943 | 2004/2943 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | SOUTH AFRICA | ISSUED | 10/28/2002 | 3/30/2005 | 10/28/2022 |
161,512 | 161,512 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | ISRAEL | ISSUED | 10/28/2002 | 10/21/2009 | 10/28/2022 |
00982/DELNP/2004 | 227,840 | A PHARMACEUTICAL VAGINAL COMPOSITION | INDIA | ISSUED | 10/28/2002 | 1/21/2009 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | ALBANIA | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | TURKEY | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
P20040480A | P20040480 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | CROATIA | ISSUED | 10/28/2002 | 11/30/2007 | 10/28/2022 |
P-366/04 | 51,154 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | SERBIA | ISSUED | 10/28/2002 | 6/29/2010 | 10/28/2022 |
7006272/2004 | 1,009,146 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | SOUTH KOREA | ISSUED | 10/28/2002 | 1/11/2011 | 10/28/2022 |
PI 20024019 | MY-137791-A | PHARMACEUTICAL FORMULATION CONTAINING LOW CONCENTRATIONS OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | MALAYSIA | ISSUED | 10/28/2002 | 3/31/2009 | 10/28/2022 |
1-2004-500600 | 1-2004-500600 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | PHILIPPINES | ISSUED | 10/28/2002 | 11/16/2007 | 10/28/2022 |
4,106,057.3 | HK1063290 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | HONG KONG | ISSUED | 10/28/2002 | 8/12/2005 | 10/28/2022 |
200402225-7 | 104,071 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | SINGAPORE | ISSUED | 10/28/2002 | 5/31/2006 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | CYPRUS | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | MACEDONIA | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | ESTONIA | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,004,116,312 | 2,329,829 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | FEDERATION OF RUSSIA | ISSUED | 10/28/2002 | 7/27/2008 | 10/28/2022 |
P0401672 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | HUNGARY | PUBLISHED | 10/28/2002 | 10/28/2022 | ||
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | CZECH REPUBLIC | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
P-369725 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | POLAND | PENDING | 10/28/2002 | 10/28/2022 | ||
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | LITHUANIA | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
20040403209/M | 90,076 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | UKRAINE | ISSUED | 10/28/2002 | 4/12/2010 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | LATVIA | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | SLOVAK REPUBLIC | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | ROMANIA | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
2,783,026.4 | 1,441,769 | LOW CONCENTRATION OF PEROXIDE FOR TREATING OR PREVENTING VAGINAL INFECTIONS | BULGARIA | ISSUED | 10/28/2002 | 6/8/2005 | 10/28/2022 |
Exhibit B
Proof of Concept Plan
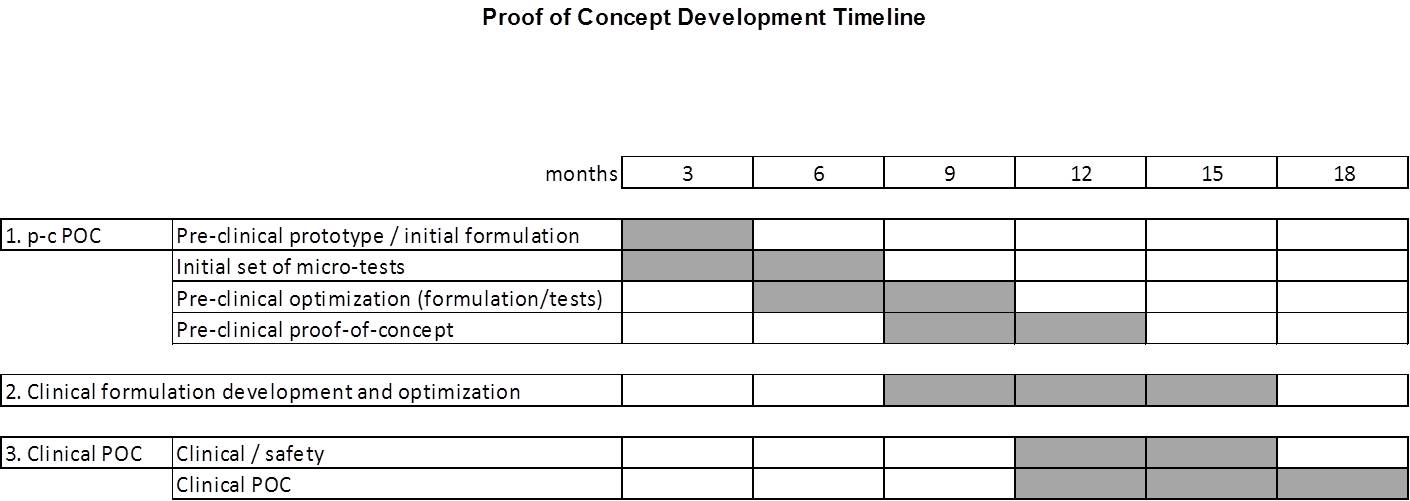
Exhibit C
Royalty Fee Calculation Example
Below is an illustrative Royalty Fee calculation pursuant to Section 4.2(b).
Example 1. Assuming annual Net Sales of $100 million in the U.S., the Royalty Fee payable to Licensor would be 3.0% x $100 million = $3.0 million
Example 2. Assuming annual Net Sales of $100 million in a country outside the U.S., the Royalty Fee payable to Licensor would be the sum of (i) 2.0% of the portion of annual Net Sales up to $10 million = $200,000; (ii) 2.5% of the next $40 million = $1,000,000; and (iii) 3.0% of the portion of annual Net Sales above $50 million, or 3.0% x ($100 million - $50 million) = $1,500,000; for a total Royalty Fee payable to Licensor of $2.7 million.
