Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - MEDICINOVA INC | d375185d8k.htm |
 the global development
and commercialization of
innovative pharmaceuticals
Exhibit 99.1
Accelerating |
 Statements in this presentation that are not historical in nature constitute
forward-looking statements within the meaning of the safe harbor
provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include
statements regarding MediciNova’s clinical trials supporting the safety and
efficacy of its product candidates and the potential novelty of such product
candidates as treatments for disease, plans and objectives for clinical trials and product
development, strategies, future performance, expectations, assumptions, financial
condition, liquidity and capital resources. These forward-looking
statements may be preceded by, followed by or otherwise include the words "believes," "expects,"
"anticipates,"
"intends,"
"estimates,"
"projects,"
"can,"
"could,"
"may,"
“will,”
"would,"
or
similar
expressions.
Actual results
or events may differ materially from those expressed or implied in any
forward-looking statements due to various factors, including the risks and
uncertainties inherent in clinical trials and product development and commercialization, such as the
uncertainty
in
results
of
clinical
trials
for
product
candidates,
the
uncertainty
of
whether
the
results
of
clinical
trials
will
be
predictive of results in later stages of product development, the risk of delays or
failure to obtain or maintain regulatory approval, the risk of failure of the
third parties upon whom MediciNova relies to conduct its clinical trials and manufacture its
product
candidates
to
perform
as
expected,
the
risk
of
increased
cost
and
delays
due
to
delays
in
the
commencement,
enrollment, completion or analysis of clinical trials or significant issues regarding
the adequacy of clinical trial designs or the execution of clinical trials and
the timing, cost and design of future clinical trials and research activities; the timing of
expected
filings
with
the
FDA;
MediciNova’s
failure
to
execute
strategic
plans
or
strategies
successfully;
MediciNova’s
collaborations with third parties; MediciNova’s ability to realize the
anticipated strategic and financial benefits from its acquisition of Avigen,
Inc., to integrate the two ibudilast development programs and to pursue discussions with potential
partners to secure a strategic collaboration to advance the clinical development of
the combined development program; the availability of funds to complete
product development plans and MediciNova’s ability to raise sufficient capital when needed,
or at all; MediciNova’s ability to comply with the covenants in its financing
agreements; intellectual property or contract rights; and the other risks and
uncertainties described in MediciNova’s filings with the Securities and Exchange Commission,
including MediciNova’s annual report on Form 10-K for the year ended
December 31, 2011 and its subsequent periodic reports on Forms 10-Q and
8-K. You are cautioned not to place undue reliance on these forward-looking statements, which
speak only as of July 3, 2012. MediciNova disclaims any intent or obligation to
revise or update these forward-looking statements.
Forward-Looking Statements
©
MediciNova, Inc. 2012 |
 MediciNova Overview:
•
Founded in September 2000
•
Headquartered in San Diego, CA, with an office in Tokyo, Japan
•
Dual listing on NasdaqGM as MNOV
and Osaka Securities Exchange as 4875
•
$37.5 million market cap (NasdaqGM) as of 6/26/2012 (aggregate value of 18.3 million
shares outstanding of common
+ preferred on an as converted
basis) In-Licensed Clinical Stage Compounds:
•
Unique access to differentiated, potentially high-value assets primarily from
Japanese alliances (Kissei, Kyorin, Mitsubishi Tanabe Pharma, Meiji)
New Approaches to Treat Serious Medical Conditions:
•
Bedoradrine Sulfate (MN-221): Intravenous (IV)
treatment for acute exacerbations of
asthma and chronic obstructive pulmonary disease (COPD)
•
Ibudilast (MN-166): Oral treatment for progressive multiple
sclerosis, neuropathic pain, and drug addiction
3
Corporate Overview:
MediciNova, Inc.
©
MediciNova, Inc. 2012 |
 4
In-License:
•
Novel, small-molecule product candidates with significant
clinical or preclinical data packages
and attractive market opportunities Conduct Proof-of-Concept Clinical
Trials: •
Conduct Phase 1 and Phase 2 clinical trials to
demonstrate safety and efficacy of compound
Two Pathways After Phase 2 (Proof-of-Concept):
1.
Internal development of compound towards commercialization in
North America
2.
Seek partnership for further development of compound
Business
Model:
Return On Investment
©
MediciNova, Inc. 2012 |
 Product
Candidates Preclinical
Phase 1
Phase 2
Phase 3
Bedoradrine Sulfate (MN-221) Program
Acute Exacerbations of Asthma
Exacerbations of COPD
Preterm Labor
Ibudilast (MN-166) Program
Progressive Multiple Sclerosis
Neuropathic Pain
Drug Addiction
Non-Core
Programs
(Various
stage
of
development
–
available
for
out-licensing)
Asthma, IC, Cancer, GAD, OAB, Thrombosis
Commercially-Attractive
Diversified Portfolio
5
©
MediciNova, Inc. 2012 |
 ©
MediciNova, Inc. 2012
6
Significant Milestones
*Anticipated completion dates based on current projections
**Tentative based on availability of non-dilutive financing
Milestone:
Receive Use Patent for Ibudilast in Progressive MS Patients
Results from Phase 2b MN-221-CL-007 Acute Asthma Trial
Top-line Results from Phase 1b Multi-Dose Trial in COPD
Plan to Announce Phase 2 Clinical Program for Ibudilast (Addiction)
Plan to Announce Phase 2 Clinical Program for Ibudilast (MS/Pain)
End of Phase 2 Meeting with FDA for MN-221 Development
Commence Pivotal MN-221 Trial
©
MediciNova, Inc. 2012
1Q, 2012
2Q, 2012
4Q, 2012
3Q, 2012
3Q, 2012**
4Q, 2012**
1H, 2013
Timeline*: |
 MN-221:
•
Acute Exacerbations of Asthma
•
Exacerbations of COPD |
 8
Acute Exacerbations of Asthma (AEA)
Definition:
•
Long-lasting and severe asthma episode that is not responsive to initial
bronchodilator or corticosteroid therapy
Market Opportunity*:
•
Approximately 1.9 million annual emergency room visits in the U.S.
•
~500,000 annual hospitalizations in U.S. (~560,000 in
UK/Spain/Germany/France/Italy) •
Average length of stay for asthma hospitalization is 3.3 days (U.S.)
•
Average cost for asthma hospitalization is $6,477
•
Roughly 50% of subjects do not initially respond to standard care
Current Standard of Care (SOC):
•
Inhaled beta agonists, inhaled anticholinergics, and IV or oral corticosteroids
•
Current
Treatments
are
limited
by
Bronchoconstriction
(Insufficient
air
flow
due
to
inflammation and airway constriction prevents inhaled drug uptake in the lungs) and
Mucus Plug
Formation
(Persistent airflow limitation due to mucus secretion
©
MediciNova, Inc. 2012
*Source:
National
Center
for
Health
Statistics /
CDC,
WHO
website, “Core
Health
indicators”,
2007
National
Hospital
Discharge
Survey,
IMS
Health’s
Disease
and
Condition
Benchmarks
–
PharMetrics
Integrated
Database, 1/2007
–
12/2008 |
 9
A COPD exacerbation
is a sustained worsening of the
patient's condition, from the stable state and beyond
normal day-to-day variations, that is acute in onset.
COPD exacerbations are associated with a significant
increase in mortality, hospitalization and healthcare
utilization.
1.5 million hospital emergency department visits
765,000 hospitalizations
119,000 deaths
COPD Exacerbations
COPD patients are generally more ill than asthmatics with
overall higher hospitalizations and mortality.
COPD
Discharged
Hospitalized
72%
28%
1,900
Asthma
52%
48%
1,500
Hospitalization rates amongst
Asthma and COPD patients
Thousands
Source: CDC, CDC COPD surveillance in U.S.; US Census; American Lung Association
website *For
COPD
pts. with
anemia;
for
pts. w/o anemia the stay is ~5.8 days at a cost of ~$25K
©
MediciNova, Inc. 2012
Average length of stay 7.4 days*
Average cost ~$32,000* |
 MN-221:
A
novel,
highly
Potential advantages over current therapy:
1.
Improved Efficacy
•
Route of administration (IV vs. inhalation)
•
Facilitates bronchodilation and onset clinical improvement
2.
Improved Safety
•
High
selectivity
for receptor versus
•
Partial
agonist
for
receptor*
3.
Reduced Health Care Expenses
•
Reduction in hospitalizations; Return ER visits
10
MN-221: A New Approach to Treating
Acute Exacerbations (Asthma & COPD)
* receptors are primarily responsible for cardiovascular
stimulation ©
MediciNova, Inc. 2012
adrenergic
receptor
agonist
selective |
 *Source: Weber, Silverman et al,
American Journal of Medicine,
Volume 113; pp 371
11
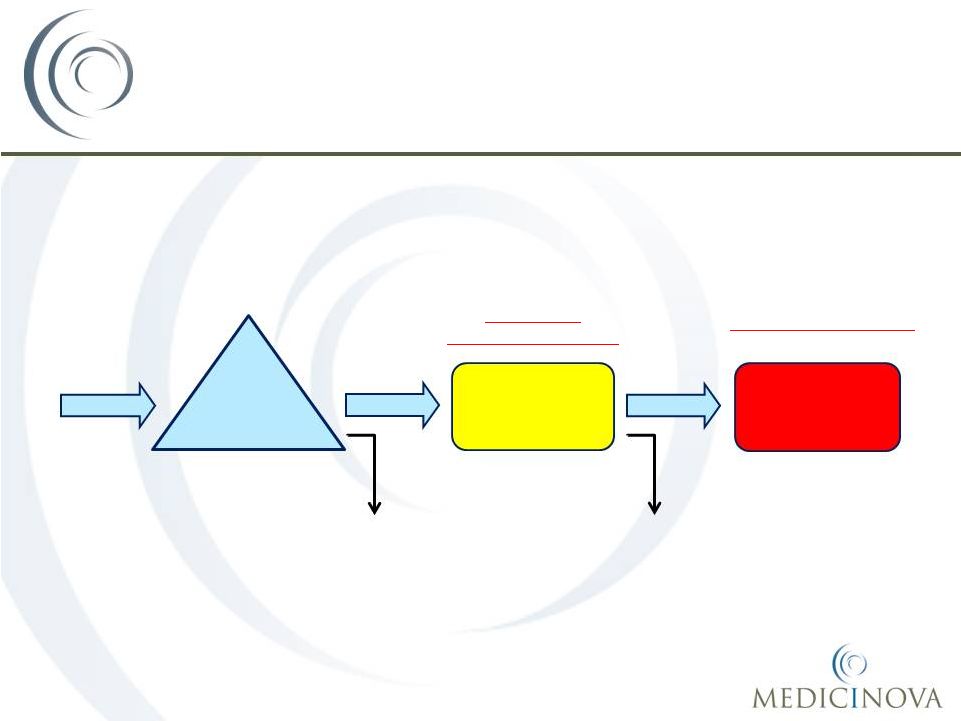
Acute Asthma Treatment Flow in
Emergency Departments (EDs) in the U.S.
965,000
935,000
935,000
410,000
525,000
525,000
Input:
1,900,000 patients
with acute
exacerbations of
asthma present at
U.S. EDs annually
1st
line therapy in ED:
Patients receive SOC,
many while in the
waiting room
2nd
line therapy in ED:
Patients who do not
initially respond continue
receiving SOC
Large Market
Opportunity for MN-221
1,900,000
1,900,000
Hospitalization:
Patients who do not
respond to SOC are
eventually hospitalized
COST ~ $6,477/patient
Patients who respond
to initial therapy and
are discharged
Patients who
eventually respond to
standard therapy and
are discharged
©
MediciNova, Inc. 2012
st
nd |
 ©
MediciNova, Inc. 2012
End-of-Phase 2 Meeting with the FDA
Scheduled for Monday, October 22nd, 2012
Pivotal Trial Design Modifications from Phase 2 Clinical Trial
Based on these Core Principles*:
1.
Primary
Endpoint
should
be
FEV
1
improvement
at
Hour
1
(delta)
or
AUC through Hour 2
2.
Reduced
variability
(FEV
1
methodology,
control
for
standard-of-care
medications between study arms)
3.
Larger sample size
4.
Simpler Protocol for ease of enrollment
5.
Include standardized clinical assessment at end of treatment period
as secondary endpoint
MN-221 for Treatment of AEA:
Pivotal Trial Development Strategy
12
*Tentative based on outcome of End-of-Phase 2 meeting with the FDA
©
MediciNova, Inc. 2012
nd |
 Goals of
the Phase 2b Clinical Trial: 1.
Assess Efficacy adjunctive to SOC treatment
2.
Establish Safety
3.
Validate Proof-of-concept (POC) and Determine Pivotal Trial Design
4.
Develop a basis for a successful End-of-Phase 2 Meeting with the FDA
MN-221-CL-007: Phase 2 Trial
Goals
13
©
MediciNova, Inc. 2012 |
 •
Randomized, placebo-controlled, double-blind, multi-center Phase 2 clinical
trial •
175 patients enrolled with acute exacerbations of asthma at multiple US ED sites
•
164 patients in the Efficacy Evaluable (EE) population; some patients early-
terminated from the study
•
Two treatment groups (1:1 randomization)
•
1,200µg
infusion
of
MN-221
over
1hr
+
Standard-of-Care
(SOC)
•
Placebo infusion + Standard-of-Care
•
Primary
Efficacy
Endpoint
was
AUC
of
change
in
FEV
1
hours
0
-
3
•
Important Secondary Endpoints include:
•
Improvements in FEV
1
at other time points
•
Clinical improvement outcomes: Dyspnea score, Respiratory Rate
•
Pharmacoeconomic
benefits:
Hospitalization
admissions
and
Return
ER
visits*
MN-221-CL-007: Phase 2 Trial
Study Design
14
*As captured in the seven-day patient follow-up; not official secondary
endpoint per protocol. ©
MediciNova, Inc. 2012 |
 Time
(Hours) 0
1
2
3
4
5
6+
Current Model
“Wait & See”
New Model
“Early Intervention
Of MN-221”
MN-221:
Potential New Model for Treating AEA in the ER
SOC
Repeat
SOC
Repeat
SOC
Repeat
SOC
Discharge
SOC
MN-221
+ SOC
Admit
Discharge
Admit
Repeat
SOC
Patient Arrives
Patient Arrives
ER cost per hour per patient is expensive
The longer the patient is in the ER the greater the probability
of hospital admission
©
MediciNova, Inc. 2012 |
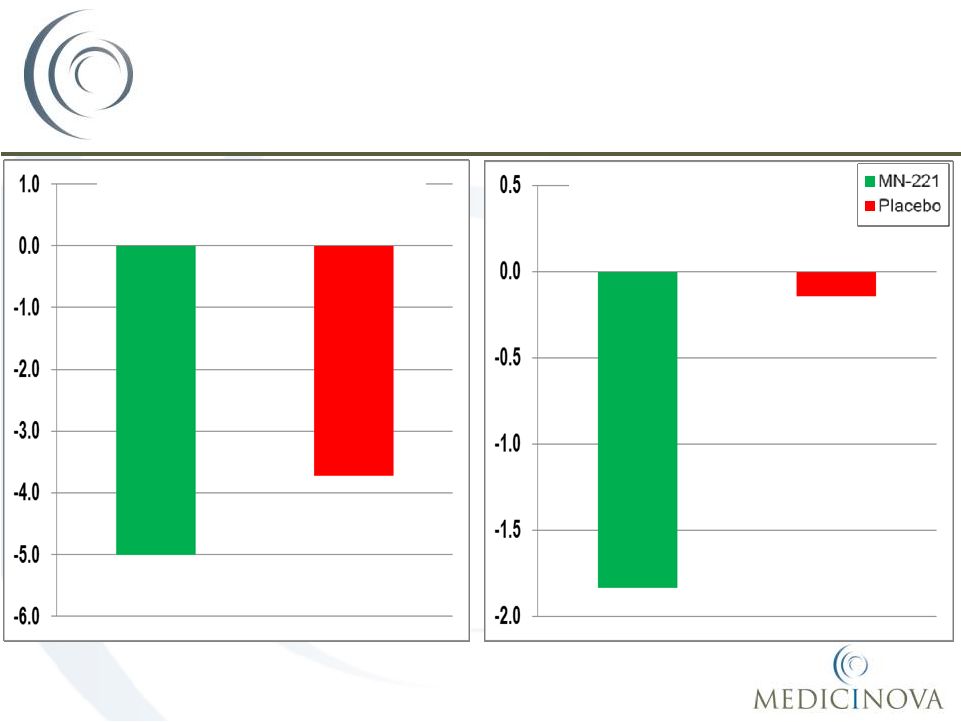
 MN-221-CL-007:
Efficacy Endpoints for FEV
1
16
AUC of Change in FEV
1
(mL)
EE Population, MM*
56%
Improvement
100%
Improvement
p=0.066
33%
Improvement
54%
Improvement
250%
Improvement
100%
Improvement
p=0.065
p=0.046
*EE = Efficacy Evaluable Population; MM= Mixed Model was used for statistical
analysis of efficacy parameters ©
MediciNova, Inc. 2012
MN-221
Placebo |
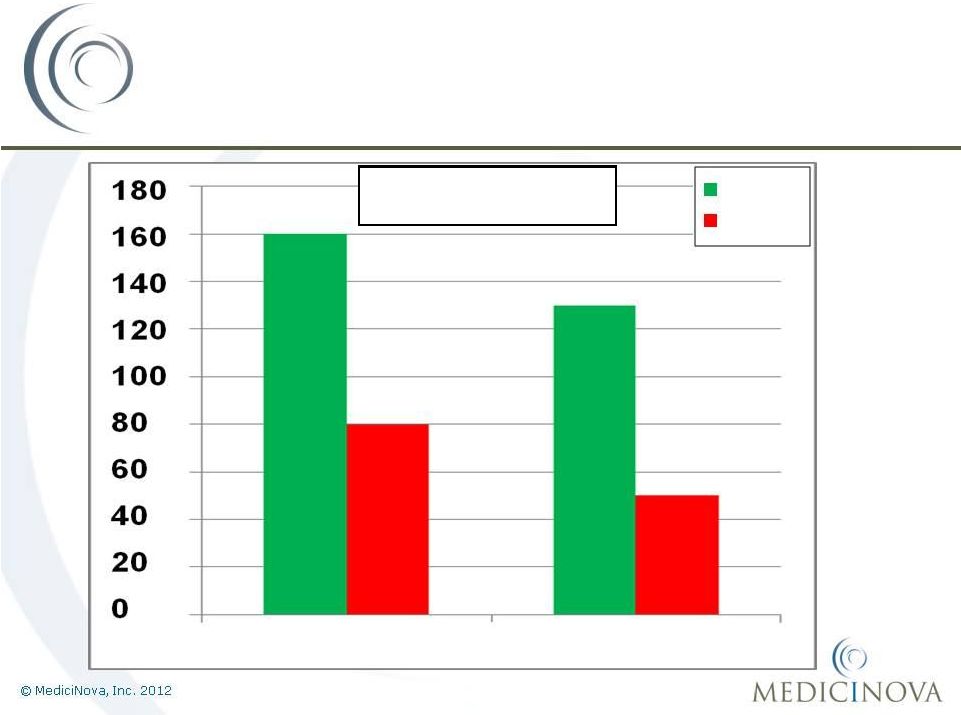 MN-221-CL-007:
Change in FEV
1
at Hour 1
17
100%
Improvement
160%
Improvement
Change in FEV
1
(mL)
Population: EE
MN-221
Placebo
MN-221-CL-007:
Change in FEV
1
at Hour 1
100%
Improvement
160%
Improvement
Change in FEV
1
(mL)
Population: EE
Hour 1 Mean
Hour 1 Median |
 18
Baseline:
64.57%
Baseline:
68.64%
Change in Dyspnea Index Score
Mean AUC(0-3)
Population: EE*
Change in Respiratory Rate
Mean AUC (0-3)
Population: EE*
MN-221-CL-007:
Improvements in Clinical Symptoms
Units are dyspnea index score
(0-10 scale; 5=severe, 3=moderate, 2=slight)
Units are breaths/min.
*EE = Efficacy Evaluable Population
p=0.039
©
MediciNova, Inc. 2012 |
 19
FEV
1
Improvement
Placebo (%)
MN-221 (%)
P-value
>
200mL
37 / 77 (48%)
48 / 78 (62%)
0.09
>
10% pred.
25 / 77 (32%)
34 / 78 (44%)
0.15
Analysis of High Responders:
FEV
1
Improvement at Any Time Point during the Treatment Period
EE population*
MN-221-CL-007:
Analysis of High Responders
*EE = Efficacy Evaluable Population
©
MediciNova, Inc. 2012 |
 20
The addition of MN-221 resulted in a 17%
reduction in hospital admissions*
*EE = Efficacy Evaluable Population; Performing Sites analysis includes patients
from sites completing more than 2 patients during the trial and for which
all efficacy measurements were available. A higher percentage of patients in
the placebo group returned to the ER within 7 days
MN-221-CL-007:
Phase
2
Trial
Pharmacoeconomic Benefits
©
MediciNova, Inc. 2012 |
 1.
Assess Efficacy adjunctive to SOC treatment
MN-221 group showed improvement over placebo group in measurements of
lung function
MN-221 showed a notable reduction in hospital admissions in performing sites
and a notable reduction in ER return visits
2.
Establish Safety
No Serious Adverse Events related to MN-221; >400 subjects exposed
3.
Affirm POC and guides Trial Design for Pivotal Trials
Endpoint modifications, Larger ‘n’, reduced variability with protocol and
standard-of-care treatments, and clinical assessment of improvement at
end of treatment
4.
Develop a basis for a successful End-of-Phase 2 Meeting with the FDA
Meeting Scheduled for October 22, 2012
MN-221-CL-007: Phase 2 Trial
MN-221 Met its Goals of the Phase 2 Program
21
©
MediciNova, Inc. 2012 |
 •
Randomized, placebo-controlled, double-blind, Phase 1b/2a clinical trial
•
20 stable moderate-to-severe COPD patients (30%
FEV
1
80%)
•
Two treatment groups
•
infusions
of
MN-221
every
12/24
hours
(15
pts.)
over
4
days
•
Placebo (5 pts.)
•
Primary objective is to determine the safety and tolerability of
MN-221
administered multiple times over several days in COPD patients who may also
have co-morbidities
and concomitant medications common in this population.
•
Secondary objectives include pharmacokinetics, preliminary efficacy of repeated
administration of MN-221 in COPD patients, and testing of a simple, hand-held
digital FEV
1
& Peak Flow monitoring device.
Top-line results expected 3Q, 2012
MN-221-CL-012:
Ongoing Phase 1b/2a COPD Trial
22
~
©
MediciNova, Inc. 2012
1,200µg |
 MN-221:
•
Market Opportunity
•
Patent Summary
•
Next Steps |
 24
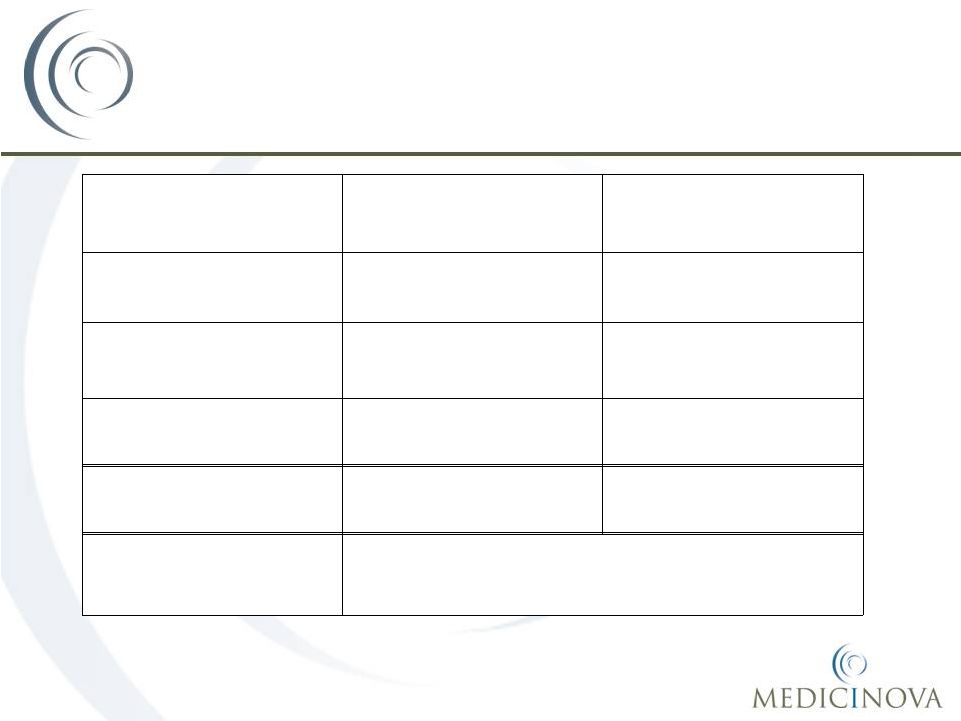
MN-221 Market Opportunity*
Market
Acute Asthma
COPD
Exacerbations
US
$375-400 million
$380-420 million
Europe
$200-300 million
$200-300 million
Rest of World
$150-250 million
$150-250 million
Worldwide
MN-221 Sales Potential
$725-950 million
$730-970 million
Combined Worldwide
MN-221 Sales Potential
$1.5-1.9 Billion
Source: Physician interviews, team analysis
*Prices in today’s dollars, do not reflect any price increases which may be implemented;
Assumes a conservative price per dose target ~$550/dose
©
MediciNova, Inc. 2012 |
 MN-221
Patent Summary •
The U.S. patent for MN-221 has composition of matter and method of use
claims and is set to expire no earlier than February
2017
•
Corresponding composition of matter patents in various other countries
•
U.S. patent expiration does not include Waxman-Hatch patent term
restoration (industry average = 4.5 years)
•
Waxman-Hatch grants 5 years of exclusivity from approval in the U.S. (We •
Exclusivity in Europe is 10 years for first approval of new chemical entities
•
In addition, MediciNova has filed multiple patent applications related to
MN-221 which if granted, could protect MN-221 until at least 2030
25
©
MediciNova, Inc. 2012
anticipate
this
along
with
pediatric
exclusivity
and
ANDA
review
time
will
give
us at
least
7.0
years
of
exclusivity) |
 ©
MediciNova, Inc. 2012
1.
Complete COPD Phase 1b/2a Clinical Trial
Important Safety data of multiple infusions
Validation of hand-held spirometry device
2.
Market Analysis
Collaborate with leading market research firm to quantify the value
of reduced hospital admissions
Quantify the value of reduced ER visits
3.
End-of-Phase 2 Meeting with the FDA
Scheduled
for
Monday,
October
22
,
2012
Confirm endpoints and trial design for pivotal program; Review
overall development plan
4.
Strategic Partnership Discussions Ongoing
MN-221 Next Steps
26
nd |
 Ibudilast
Ibudilast
(MN-166/AV-411):
(MN-166/AV-411):
•
•
Progressive
Progressive
Multiple
Multiple
Sclerosis
Sclerosis
•
•
Neuropathic
Neuropathic
Pain
Pain
•
•
Addiction
Addiction |
 ©
MediciNova, Inc. 2012
28
Ibudilast (MN-166)
•
Oral administration
•
Safe and well tolerated
•
Approved in Japan/Korea over 3.2M patient exposures
•
>420 subjects treated with ibudilast
•
Dosing up to 100 mg daily doses
•
Mechanism(s) of action primarily:
•
Inhibition of macrophage Migration Inhibitor Factor (MIF)
•
PDE-4,10 inhibition
•
Neurotrophic action and attenuation of glial cell activation
Clinical Safety & Preliminary Efficacy Established
•
Phase 2 multiple sclerosis proof-of-concept study
•
Indicators of dose-related neuroprotective efficacy validated
•
Phase 1 dosing to 100 mg/d
•
Phase 1b/2a trial in diabetic neuropathic pain completed
•
Phase 1b/2a clinical trial in opioid withdrawal & analgesia completed
Ibudilast (MN-166) Overview |
 ©
MediciNova, Inc. 2012
Ibudilast (MN-166) Program:
Ongoing and Future Development
29
*Pending Grant /
*Pending Grant /
Partner Funding
Partner Funding
Progressive MS* / Neuropathic Pain*
Phase 2b Trial (POC)
Opioid Dependence
Columbia University –
Phase 2a Trial
Medication Overuse Headache Pain
Univ. Adelaide –
Phase 2a Trial
Methamphetamine Addiction
UCLA –
Phase 1b Trial
Ibudilast (MN-166) Program
Ibudilast (MN-166) Program
1Q 2012
2Q 2012
3Q 2012
4Q 2012 |
 ©
MediciNova, Inc. 2012
•
Sustain NIDA-sponsored Drug Addiction Development
•
Potential for Phase 2 POC Trial support
•
Potential for Gov’t and MS Society consortium funding of Phase 2 Progressive
MS POC Trial
•
Collaboration with Development Partner:
1.
Advance to Phase 2b Proof-of-Concept in MS and/or Pain
2.
Provide competitive economics for first in class therapy
3.
Could be collaboration through:
i.
Pharma partner
ii.
Project financing
iii.
Shared-risk with competitive CRO agreement
•
Consider Investigator-sponsored Neurological Trials in Focus Areas
30
Strategy for Ibudilast’s
Development |
 ©
MediciNova, Inc. 2012
31
Patent/Commercial Overview
Exp. 2018
Exp. 2025
Exp. 2027
Exp. 2027
Exp. 2026
Exp. 2029
Exp. 2027
Exp. 2028
Key:
Method of Use
Composition of Matter
MS
Neuropathic
Pain
MIF Inh.
screen
Addiction
Progressive MS
AV1013
2
Generation
Analogs
Acute & Sub-
chronic Pain
MOH Pain
Anxiety -
Traumatic
Brain Injury/PTSD
Ibudilast +
Immunomodulator
for MS
AV1013
Enantiomer
Issued or
Allowed
Pending
nd |
          ©
MediciNova, Inc. 2012
32
Leadership
Years
Experience
Background
Yuichi Iwaki, MD, PhD
Yuichi Iwaki, MD, PhD
CEO & President
35
Professor at USC, formerly Professor at University
of Pittsburgh; Advisor to JAFCO, Tanabe
Michael Coffee
Michael Coffee
Chief Business Officer
26
Avigen, Amarin Corp., Elan Pharmaceuticals, N.A.,
Athena Neurosciences
Kirk Johnson, Ph.D.
Kirk Johnson, Ph.D.
Chief Scientific Officer
21
Avigen, Genesoft Pharmaceuticals, Chiron
Corporation (Novartis -
San Francisco)
Masatsune Okajima, CMA
Masatsune Okajima, CMA
VP, Head of Japanese Office
19
Daiwa Securities SMBC, Sumitomo Capital
Securities, Sumitomo Bank
Kazuko Matsuda, M.D., Ph.D., MPH
Kazuko Matsuda, M.D., Ph.D., MPH
Chief Medical Officer
20
Assistant Professor USC, Keck School of Medicine;
Children’s Hospital Los Angeles.
Michael Gennaro, CPA, MBA
Michael Gennaro, CPA, MBA
Chief Financial Officer
37
Partner at FLG Partners, Sylantro Systems, Inverse
Network Technology, Novell, Piiceon, Verticom
Management Team with
Global Experience |
 ©
MediciNova, Inc. 2012
33
Financial Resources:
•
$11.0
million
in
cash
&
cash
equivalents
as
of
3/31/2012
•
Including $10 million raised through equity sale and non-dilutive funding by
Kissei
•
$7.5 million raised in private stock sale to Kissei Pharmaceutical Co., Ltd.
•
$2.5 million additional non-dilutive R&D funding from Kissei
•
~18.3 million shares outstanding on an as converted basis
•
Cash Runway into 2013
Financial Overview |
 ©
MediciNova, Inc. 2012
34
MediciNova Corporate Summary
*Anticipated completion dates based on current projections
1.
Announced Results from CL-007 Trial -
Q2
2.
Announce Results from CL-012 Trial -
Q3
3.
Announce Phase 2 Trial(s) for Ibudilast -
Q4
4.
End-of-Phase 2 Meeting with FDA -
Q4
Raised ~$18M in 2011
Cash Runway into Q2, 2013
Translational Medicine & Clinical development expertise
Strong international presence, especially Japan
Large and small pharma/biotech experience |
 Addendum
Addendum
Data from Completed Trials
Data from Completed Trials
MN-221
MN-221
Ibudilast (MN-166/AV411)
Ibudilast (MN-166/AV411) |
 MN-221:
MN-221:
Data from Completed Trials
Data from Completed Trials
Asthma Program:
Asthma Program:
CL-004, CL-005, CL-006, CL-007
CL-004, CL-005, CL-006, CL-007
COPD Program:
COPD Program:
CL-010
CL-010
Safety Review
Safety Review |
 ©
MediciNova, Inc. 2012
MN-221 Clinical Development
Acute Asthma Program:
•
Multiple doses tested at infusion length of
15min, 1hr, and 2hr
•
Completed 2 trials in asthmatics with
stable disease
•
CL-004
(23
patients)
•
CL-005
(17
patients)
•
Completed Phase 2a trial in patients with
AEA in the ED
•
CL-006
(29
patients)
•
Completed Phase 2b study in patients
with AEA in ED
•
CL-007
(175
pts.)
•
End of Phase 2 Meeting with FDA
•
Scheduled for October 22
COPD Program:
•
Multiple doses tested at 1 hour infusion
•
Completed 1 trial in COPD patients with
stable disease
•
CL-010
(48
patients)
•
Preparing to initiate multi-dose trial in
patients with stable COPD
•
CL-012
(20
patients)
•
Efficacy and Safety data will
also be very useful in further
development of MN-221 for
acute asthma
37
nd |
 ©
MediciNova, Inc. 2012
MN-221 Clinical Results
Improved Lung Function at
Different Dosing Levels: Stable Asthmatics
38
CL-004: Stable Mild/Moderate Asthmatics
CL-005: Stable Moderate/Severe Asthmatics |
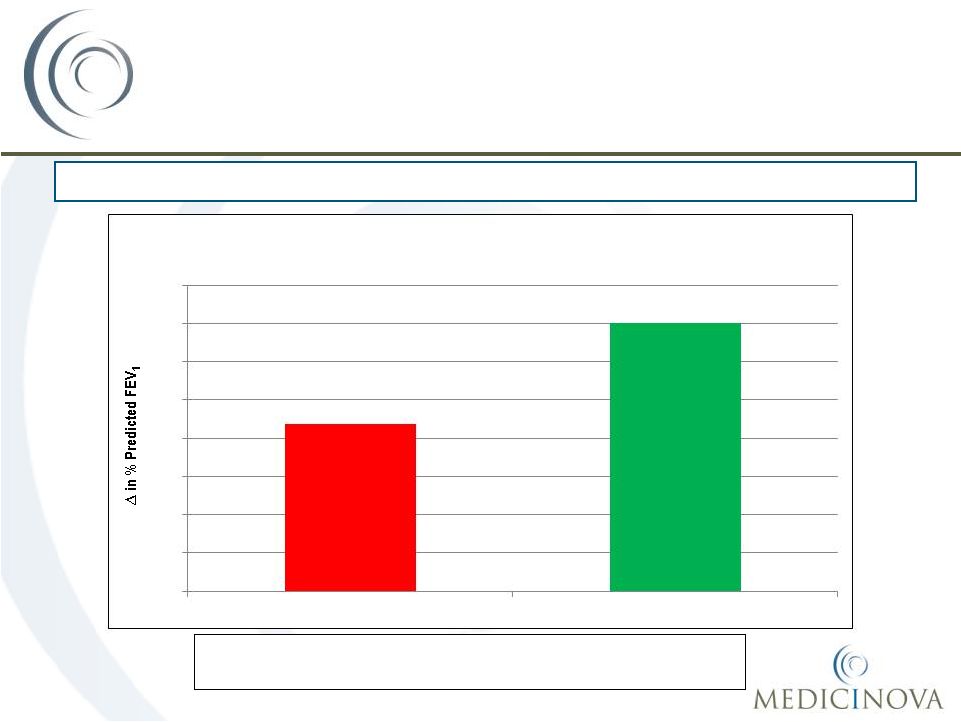 ©
MediciNova, Inc. 2012
MN-221 Clinical Results
Improved Lung Function and Clinical Outcome
Above and Beyond Standard of Care (SOC)
Mean
change
in
FEV
1
from
baseline
was
5.3%
higher
in
the
MN-221 dose groups versus the placebo group
39
CL-006: Patients Suffering from Acute Exacerbation of Asthma in Emergency
Department 8.7%
14.0%
0%
2%
4%
6%
8%
10%
12%
14%
16%
SOC + Placebo
MN-221 + SOC
Mean FEV
1
Change from Baseline (Hour 5) MN-221
(all dose groups) + SOC vs. SOC + placebo |
 ©
MediciNova, Inc. 2012
MN-221-CL-007: Phase 2 Trial
Subject Populations
Placebo
MN-221
Enrolled subjects
86
89
Safety population
84
83
EE population
83
81
Performing Sites population
70
72
Definitions
of
Note:
EE = Efficacy Evaluable Population
Performing Sites Population: analysis includes patients from sites completing more than
2 patients during the trial and for which all efficacy measurements were
available. 40 |
 ©
MediciNova, Inc. 2012
*EE = Efficacy Evaluable Population; MM= Mixed Model was used for statistical
analysis of efficacy parameters; Performing Sites analysis includes
patients from sites completing more than 2 patients during the trial and
for which all efficacy measurements were available. MN-221-CL-007: Phase 2
Trial Efficacy
Endpoints for FEV 1
119%
Improvement
225%
Improvement
68%
Improvement
172%
Improvement
81%
Improvement
39%
Improvement
AUC of Change in FEV
1
(mL)
EE Population, MM; Performing Sites*
41
p=0.065
p=0.043
p=0.050
p=0.066 |
 ©
MediciNova, Inc. 2012
42
Baseline:
69.35%
MN-221-CL-007: Phase 2
Trial
Improvements in Clinical Symptoms
Change in Dyspnea Index Score
AUC (0-3) Mean
Population: EE; Performing Sites*
p=0.055
*EE = Efficacy Evaluable Population; Performing Sites analysis includes patients
from sites completing more than 2 patients during the trial and for which
all efficacy measurements were available. Change in Respiratory Rate
Mean AUC (0-3)
Population: EE; Performing Sites* |
 ©
MediciNova, Inc. 2012
43
MN-221 Clinical Results
Improved Lung Function at
Different Dosing Levels: COPD Patients |
 ©
MediciNova, Inc. 2012
•
MediciNova has preclinical data and clinical data which demonstrates the
safety of MN-221.
•
In summary, we have not seen clinically significant safety concerns with
MN-221 and it has been tested in over 400 subjects to date.
•
According to interviews of emergency room physicians, less-selective
injectable
beta
agonists
such
as
epinephrine
and
terbutaline
are
not
commonly used to treat acute asthma.
The main reason they are not used
more often is due to safety concerns, particularly cardiovascular side
effects.
MN-221 Safety Review
44 |
 ©
MediciNova, Inc. 2012
45
Human
-Adrenergic Receptor
Selectivity
Test Drug
1
IC
50
(M)
2
IC
50
(M)
2
-Adrenoceptor Selectivity
(IC
50
for
1
/
IC
50
for
2
)
Levalbuterol
7.40E-06
1.40E-06
5.3
Albuterol
9.40E-06
1.60E-06
5.9
Terbutaline
6.00E-05
6.50E-06
9.2
MN-221
5.90E-06
1.40E-07
42.4 |
 ©
MediciNova, Inc. 2012
•
MN-221-CL-004:
Evaluation
of
MN-221
(bedoradrine),
a
Novel,
Highly
Selective
Beta2-Adrenergic
Receptor Agonist in Mild to Moderate Asthma via Intravenous Infusion (Poster
#145) •
MN-221-CL-005:
Comparison
of
Administration
Rates
of
MN-221
(bedoradrine),
a
Novel,
Highly
Selective Beta2 Receptor Agonist in Patients with Stable Moderate to Severe Asthma
(Poster #143) •
MN-221-CL-006:
Reduced
Hospital
Admission
and
Improved
Pulmonary
Function
Following
Intravenous MN-221 (bedoradrine), a Novel Highly Selective Beta2-Adrenergic
Receptor Agonist, Adjunctive to Standard of Care in Severe Acute Exacerbation
of Asthma (Poster #144) •
Pharmacokinetic
(PK)
and
Pharmacodynamic
(PD)
Modeling
and
Simulation
Support
the
Novelty
of
MN-221, a Highly-Selective Beta2-Adrenergic Agonist for Treatment of Acute
Asthma (Poster #146) •
MN-221
FY08-065:
Cardiovascular
Effects
of
i.v.
MN-221
(bedoradrine)
Administered
with
Nebulized
Albuterol in Dogs (Poster #147)
•
Pharmacokinetics
and
Pharmacodynamics
of
MN-221,
a
Novel
Highly-Selective
Beta2-Adrenergic
Agonist for Treatment of Acute Chronic Obstructive Pulmonary Disease (Poster
#685) •
MN-221-CL-010:
Intravenous
MN-221,
a
Novel,
Highly
Selective
Beta2
Adrenergic
Receptor
Agonist,
Improves Lung Function in Stable Moderate to Severe Chronic Obstructive Pulmonary
Disease (COPD) Patients (Poster #686)
*Posters available on MediciNova website at
www.medicinova.com MN-221: CHEST Posters (Nov.
2010)* 46 |
 Ibudilast
(MN-166): Ibudilast (MN-166):
Data from Completed Trials
Data from Completed Trials
Multiple Sclerosis Program
Multiple Sclerosis Program
Neuropathic Pain Program
Neuropathic Pain Program
Drug Abuse/Addiction Program
Drug Abuse/Addiction Program
Safety Review
Safety Review |
 ©
MediciNova, Inc. 2012
Placebo-controlled, Randomized, Double-blind Phase II Study:
•
Year 1 -
Placebo, 30 mg/day, 60mg/day
•
Year 2 -
30 mg/day, 60mg/day
•
297 patients (~100 patients/group) @ 25 sites in Serbia, Ukraine, Belarus,
Bulgaria and Romania
Key Inclusion Criteria:
•
Males or females aged 18 to 55 years, with relapsing remitting (RR) and/or
secondary progressive (SP) Multiple Sclerosis with continued relapses;
•
One MRI scan taken two weeks prior to treatment start using a standardized MRI
protocol with at least one Gd-enhancing lesion;
•
An Expanded Disability Status Scale (EDSS) score of 5.5 or less at the screening
and baseline visits.
Safety Profile:
•
89% (264 of 297) of subjects completed the first 12 months of the study
•
82.5% (245 of 297) of subjects completed the full 24 months of the study
•
Adverse effects reported more frequently in MN-166-treated than
placebo-treated subjects: GI effects & depression
Multiple Sclerosis Clinical Study:
MN-166-CL-001
48 |
 ©
MediciNova, Inc. 2012
49
P-Value: 0.04
P-Value: 0.035
MN-166-CL-001 Study Results
P-Value:
0.026
P-Value: 0.004
P-Value: 0.09
P-Value: 0.08
Note: P-values listed on this slide compare placebo group to 60mg/day group of
MN-166 Indicative of Potential Neuroprotective Effect:
•
Reduced brain volume loss
•
Reduced conversion of acute lesions to persistent black holes
•
Sustained disability progression was significantly less likely (~50%)
Acute Clinical Benefit:
•
Prolong time to relapse (by 127 days.)
•
Annualized relapse rate
Protocol-Defined Primary Endpoint (Surrogate Endpoint):
•
No significant reduction in the cumulative number of active (gadolinium-enhancing
(T1) and non-enhancing new/enlarging (T2)) lesions on cranial MRI scans
over 12 months of treatment was observed
•
Positive trends were observed in volume of
gadolinium-enhancing (T1) lesions |
 50
MN-166-CL-001:
Efficacy Review (One Year)
Endpoints Indicative of Disease Modifying Effect (Chronic aspects of MS):
Endpoints Relating to Acute Clinical Benefit:
:
Statistically
significant
Brain Volume Loss
Dose (mg/d)
Dose (mg/d)
Dose (mg/d)
Dose (mg/d)
Dose (mg/d)
Progression to PBH
EDSS progression
Time to 1st Relapse
% Relapse Free After One Year
©
MediciNova, Inc. 2012 |
 ©
MediciNova, Inc. 2012
51
Secondary Progressive MS:
Subset Analysis
Treatment Group (patient n)
Subset of
MS Patients:
Placebo
30 mg/day
Low Dose Group
60 mg/day
High Dose Group
% Brain
Volume
Change
% Brain
Volume
Change
Magnitude of
Effect
% Brain
Volume
Change
Magnitude of
Effect
RRMS
-1.2 (81)
-1.1 (69)
8% less
-0.8 (75)
33% less
SPMS
-1.0 (3)
-0.7 (4)
30% less
-0.4 (2)
60% less
Next
Steps
for
Progressive
MS:
Two-year
Phase
2
in
Progressive
MS
-
month
12
data.
Potential
first-in-class,
once-
or
twice-daily
oral
well-tolerated
drugs
with
established
endpoints.
Draft
protocols,
costs,
and
trial
operations
completed. |
 ©
MediciNova, Inc. 2012
Design: Two-center (Australian), Phase 1b/2a, randomized, double-blind,
placebo-controlled, parallel-group study.
Subjects:
•
Patients, aged 18 to 75
years, with painful diabetic peripheral neuropathy (DPN) or complex
regional
pain
syndrome
(CRPS)
of
6
months
duration
and
screening
VAS
score
4
cm
on
a
10 cm scale
•
29 subjects: 19 active, 10 placebo
Dosing:
•
20 mg BID (n=4), 20 mg TID (n=4), 40 mg BID (n=11)
•
AV411 (ibudilast) added
to patients’
standard medication regimen for DM and pain
Study objectives:
•
Establish safety/tolerability & PK in intended patient population
•
Explore potential efficacy endpoints
52
Diabetic Peripheral Neuropathic
Pain Study: AV411-010 |
 ©
MediciNova, Inc. 2012
Reduction Observed in Opioid Usage
53
-20
-15
-10
-5
0
5
10
15
20
-5
0
5
10
15
20
25
Mean
Median
Placebo
AV411 |
 ©
MediciNova, Inc. 2012
54
Greater % of “Responders”
Above
Ibudilast Plasma Thresholds
Plasma Ibudilast Parameter
VAS ‘Responder’
%
AUC
0-24h
> 1000 ng*hr/mL
60%
< 1000 ng*hr/mL
25%
C
max
> 60 ng/mL
64%
< 60 ng/mL
14%
C
min
> 27 ng/mL
55%
< 27 ng/mL
29%
Next Steps for Neuropathic Pain:
Twelve week Phase 2 DPN trial. Potential first-in-class, once- or
twice-daily oral well-tolerated drug with established endpoints.
Draft protocols, costs and trial operations completed.
|
 ©
MediciNova, Inc. 2012
•
Recent validation of CNS action in opioid withdrawal & analgesia
•
Ongoing Methamphetamine interaction Phase 1b
•
Opioid self-administration Phase 2a initiating
•
Ongoing Phase 2a Medication Overuse Headache Pain trial
•
Randomized, double-blind, placebo-controlled, investigator-initiated (Dr.
Pail Rolan at Univ. of Adelaide, Australia; reduced headache index, acute
medication (codeine) use and headache impact on Quality of Life (QOL);
8-week trial + follow-up; n = 20 patients each at placebo vs. 80 mg/day
of MN-166 •
Acquired rights to treatment of
post-traumatic brain injury (TBI)
•
Led by the research of Daniel Barth, Ph.D., Professor of Neuroscience and
Psychology at CU-Boulder, ibudilast demonstrated significant efficacy in a model
of post-TBI anxiety, one of the most common disorders caused by TBI.
Investigator-Led Development
55 |
 ©
MediciNova, Inc. 2012
Ibudilast (MN-166):
Neurological Indications and Translational Record
Indication
Preclinical
Validation
Clinical Validation
Comment
MS
+
(EAE)
+
* MN-166-Cl-001
Progressive MS Phase
2b indicated
Neuropathic Pain
+
(multiple models)
+
AV411-010
Phase 2b enabled
Opioid Dependence
(and Tolerance)
+
(multiple models)
+
*
AV411-OWA
(SOWS, Miosis)
Enhanced Opioid
Analgesia
+
(2 rat models)
+
*
AV411-OWA
(McGill PQ)
Methamphetamine
Relapse
+
(rat models)
in progress
Traumatic Brain Injury
+ (rat models)
TBD
56
* = p < 0.05, dose-related & certain endpoints
|
 ©
MediciNova, Inc. 2012
•
Ibudilast -
References
57
Barkhof, F. et al. Ibudilast in Relapsing-Remitting
Multiple Sclerosis: a Neuroprotectant? Neurology, Mar 30 2010. Fox, R. Primary Neuroprotection: the Holy Grail of Multiple Sclerosis
Therapy. Neurology, Mar 30 2010. Kagitani-Shimono K. and Mohri I. J Neuroinflammation.
Anti-inflammatory Therapy by Ibudilast, a Phosphodiesterase
Inhibitor, in Demyelination of Twitcher, a Genetic Demyelination Model.
Kreutzberg G. W. Microglia: A Sensor for Pathological Events in the CNS.
Trends Neurosci. 1996; 19(8): 312-8. Ledeboer A., Hutchinson M. R., Watkins L. R., and Johnson K. W. Ibudilast (AV411):A
New Class Therapeutic Candidate for Neuropathic Pain and Opioid
Withdrawal Syndromes.
Mizuno, T et al. Neuroprotective role of phosphodiesterase inhibitor
ibudilast on neuronal cell death induced by activated microglia.
Muzio L., Martino G., et al. (2007). Multifaceted Aspects of Inflammation in
Multiple Sclerosis: The Role of Microglia. J Neuroimmunol 2007;
191(1-2): 39-44. Rolan, P., Hutchinson, M., and Johnson, K. Ibudilast: A Review of its Safety,
Efficacy, and Pharmacology in Respiratory and Neurologic Diseases.
Wang, F. et al. Spinal Macrophage Migration Inhibitory Factor Is a
Major Contributor to Rodent Neuropathic Pain-like
•
•
•
•
•
•
•
•
Hypersensitivity.
Anesthesiology.
2011
Feb
2.
Neuropharmacology 46:404, 2004.
Expert Opinion Pharmacotherapy 2009. Expert
Opin.
Investig.
Drugs
2007;
16:
935-950.
J Neuroinflammation. 2005; 2(1): 10. |
