Attached files
| file | filename |
|---|---|
| 8-K - 8-K - MADRIGAL PHARMACEUTICALS, INC. | a12-15567_18k.htm |
| EX-99.2 - EX-99.2 - MADRIGAL PHARMACEUTICALS, INC. | a12-15567_1ex99d2.htm |
Exhibit 99.1
|
|
Synta Pharmaceuticals (NASDAQ: SNTA) GALAXY Trial Interim Results June 27 2012 Conference Call |
|
|
Forward-looking statements This presentation may contain forward-looking statements. These statements reflect our current views with respect to future events and actual results could differ materially from those projected in the forward-looking statements. Factors that could cause actual results to differ are discussed in Synta’s 2011 Annual Report on Form 10-K and in our reports on Form 10-Q and Form 8-K. These reports are available on our website at www.syntapharma.com in the "Investors—SEC Filings" section. Synta undertakes no obligation to publicly update forward-looking statements, whether because of new information, future events or otherwise, except as required by law. Ganetespib is an investigational product and has not yet been approved for any use. |
|
|
Highlights Ganetespib: leading Hsp90 inhibitor in industry First to overcome class toxicity challenges Active as monotherapy in certain targeted patient populations Positive results from interim GALAXY analysis Ganetespib + docetaxel vs. docetaxel, 2nd line NSCLC 2.5-3x PFS improvement in pts with elevated LDH, pts with mutant KRAS PFS and OS improvements in adenocarcinoma patients No substantial increase in toxicity to docetaxel, except Gr 1/2 diarrhea Aiming for regulatory advice Q3 to discuss Phase 3 plans Synta owns 100% worldwide rights to ganetespib |
|
|
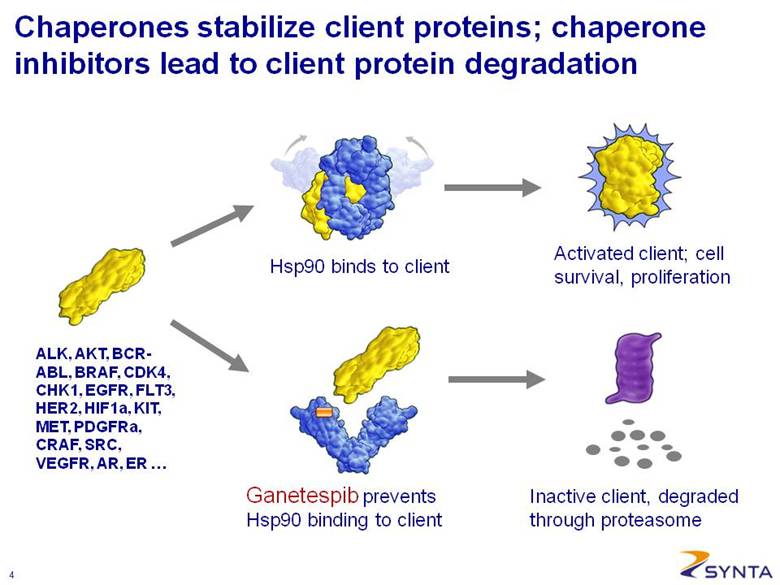
Activated client; cell survival, proliferation Hsp90 binds to client Ganetespib prevents Hsp90 binding to client Inactive client, degraded through proteasome ALK, AKT, BCR-ABL, BRAF, CDK4, CHK1, EGFR, FLT3, HER2, HIF1a, KIT, MET, PDGFRa, CRAF, SRC, VEGFR, AR, ER . . . Chaperones stabilize client proteins; chaperone inhibitors lead to client protein degradation |
|
|
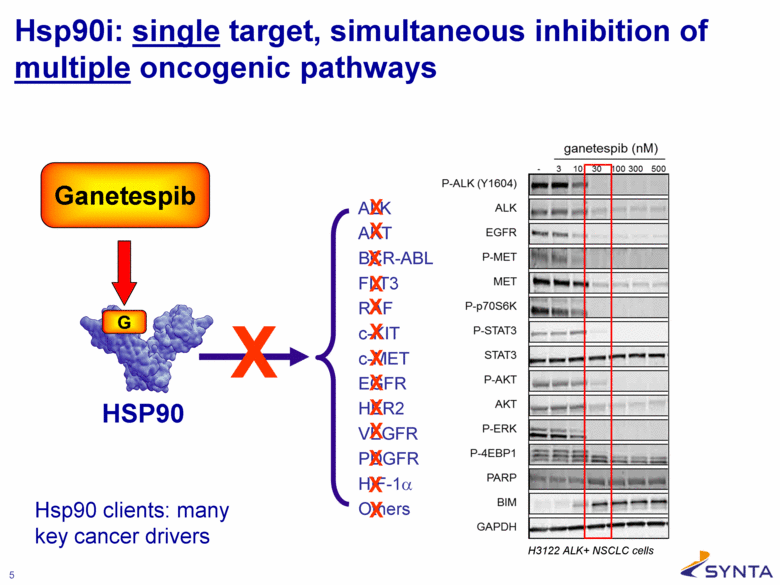
Hsp90i: single target, simultaneous inhibition of multiple oncogenic pathways HSP90 X ALK AKT BCR-ABL FLT3 RAF c-KIT c-MET EGFR HER2 VEGFR PDGFR HIF-1α Others X X X X X X X X X X X X Ganetespib X Hsp90 clients: many key cancer drivers |
|
|
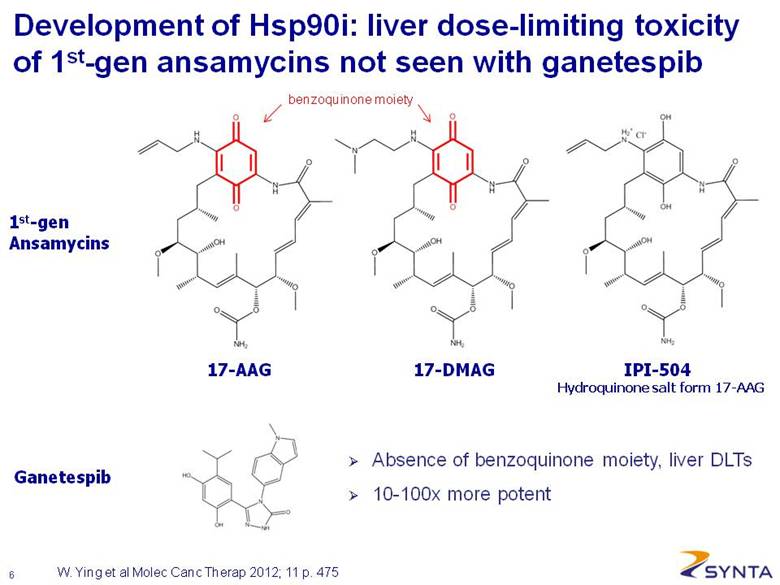
Development of Hsp90i: liver dose-limiting toxicity of 1st-gen ansamycins not seen with ganetespib W. Ying et al Molec Canc Therap 2012; 11 p. 475 17-AAG Hydroquinone salt form 17-AAG 17-DMAG IPI-504 Ganetespib 1st-gen Ansamycins Absence of benzoquinone moiety, liver DLTs 10-100x more potent benzoquinone moiety |
|
|
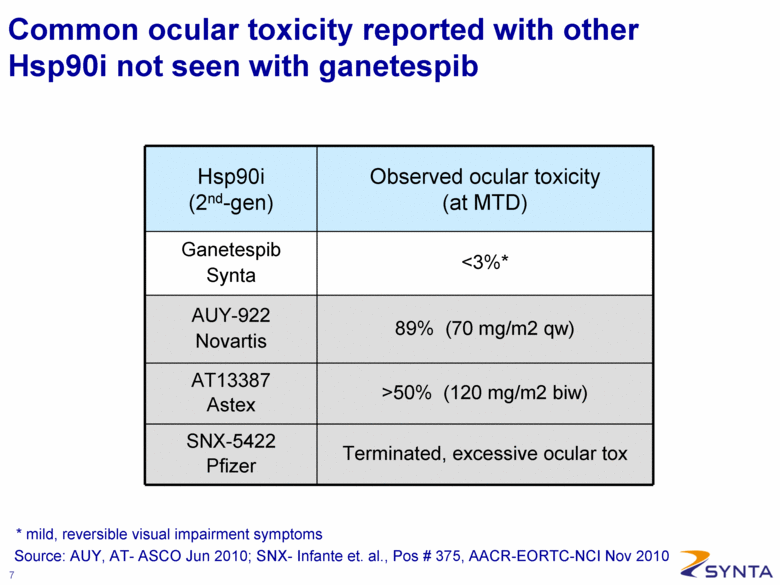
Common ocular toxicity reported with other Hsp90i not seen with ganetespib Hsp90i (2nd-gen) Observed ocular toxicity (at MTD) Ganetespib Synta <3%* AUY-922 Novartis 89% (70 mg/m2 qw) AT13387 Astex >50% (120 mg/m2 biw) SNX-5422 Pfizer Terminated, excessive ocular tox Source: AUY, AT- ASCO Jun 2010; SNX- Infante et. al., Pos # 375, AACR-EORTC-NCI Nov 2010 * mild, reversible visual impairment symptoms |
|
|
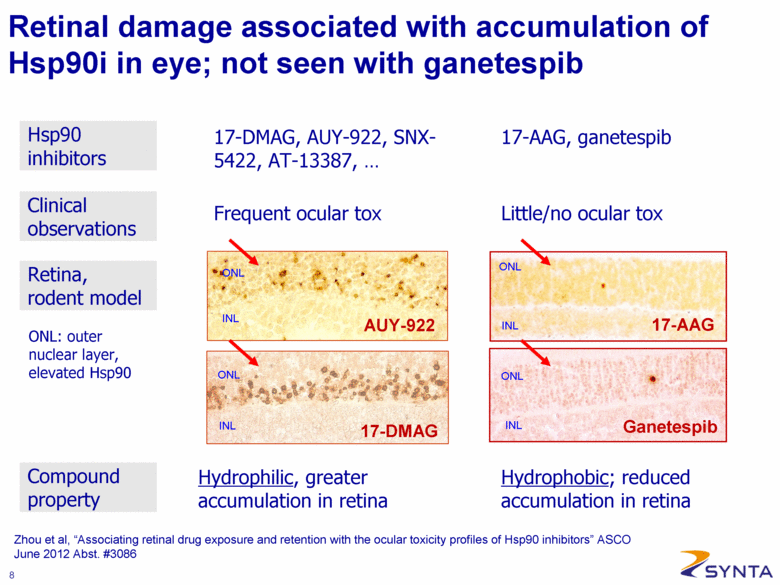
Zhou et al, “Associating retinal drug exposure and retention with the ocular toxicity profiles of Hsp90 inhibitors” ASCO June 2012 Abst. #3086 Retinal damage associated with accumulation of Hsp90i in eye; not seen with ganetespib 17-DMAG, AUY-922, SNX-5422, AT-13387, . . . Frequent ocular tox 17-AAG, ganetespib Little/no ocular tox Hydrophilic, greater accumulation in retina ONL INL ONL INL ONL INL INL ONL AUY-922 17-DMAG 17-AAG Ganetespib ONL: outer nuclear layer, elevated Hsp90 Hydrophobic; reduced accumulation in retina Clinical observations Hsp90 inhibitors Retina, rodent model Compound property |
|
|
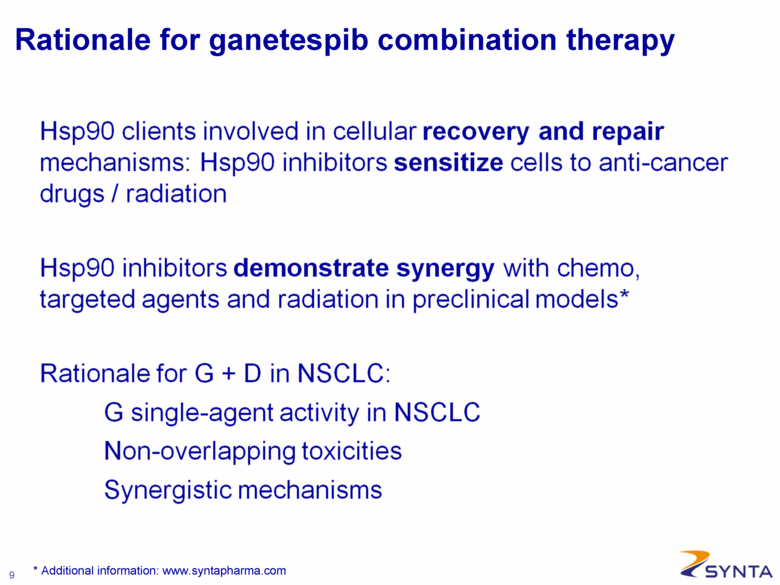
Rationale for ganetespib combination therapy Hsp90 clients involved in cellular recovery and repair mechanisms: Hsp90 inhibitors sensitize cells to anti-cancer drugs / radiation Hsp90 inhibitors demonstrate synergy with chemo, targeted agents and radiation in preclinical models* Rationale for G + D in NSCLC: G single-agent activity in NSCLC: Non-overlapping toxicities Synergistic mechanisms * Additional information: www.syntapharma.com |
|
|
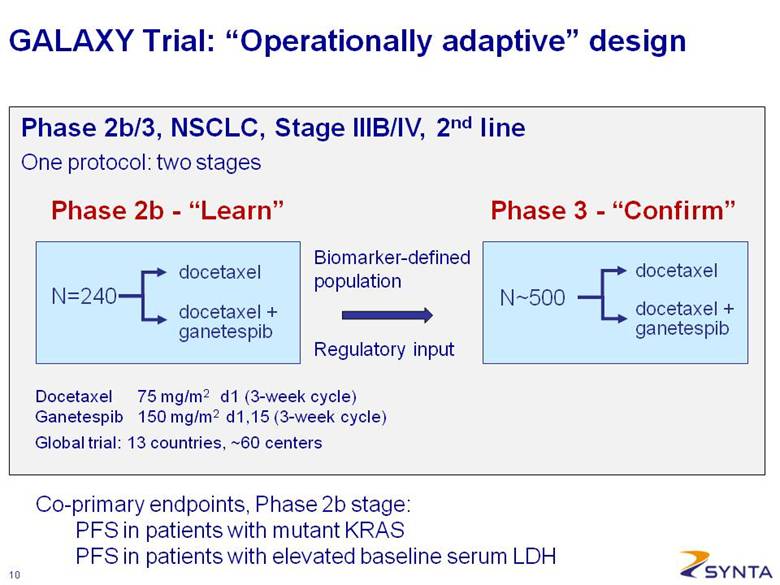
GALAXY Trial: “Operationally adaptive” design Biomarker-defined population Regulatory input Phase 2b - “Learn” N=240 docetaxel + ganetespib docetaxel Phase 3 - “Confirm” N~500 docetaxel + ganetespib docetaxel Docetaxel 75 mg/m2 d1 (3-week cycle) Ganetespib 150 mg/m2 d1,15 (3-week cycle) Global trial: 13 countries, ~60 centers Phase 2b/3, NSCLC, Stage IIIB/IV, 2nd line Co-primary endpoints, Phase 2b stage: PFS in patients with mutant KRAS PFS in patients with elevated baseline serum LDH One protocol: two stages |
|
|
Patients stratified to balance key prognostic factors between arms 1. Histology 2. ECOG Performance Status 3. Baseline serum LDH 4. Smoking status 5. Time since diagnosis of advanced NSCLC LDH measured in independent, central lab (pre-specified co-primary endpoint) |
|
|
Co-primary patient populations for Phase 2b stage: elevated LDH, mutant KRAS Elevated LDH ~30%, mutant KRAS ~15-30% of all adeno Both are prognostic variables for poor clinical outcomes1 Patients progress faster and die sooner Chemotherapy works less well Scientific and clinical rationale suggest possible enhanced ganetespib activity in these populations – i.e., predictive variables for G activity, in addition to prognostic 1. Elevated LDH: Schneider, Advan Clin Chem 42:1-41 (2006); Albain et al., J Clin Oncol 9:1618-1626 (1991); Suh and Ahn, Eur J Cancer 43:1051 (2007). Mut KRAS: Johnson, ASCO 2010 Abstr 7541; Socinski, Clinical Lung Cancer 2010 |
|
|
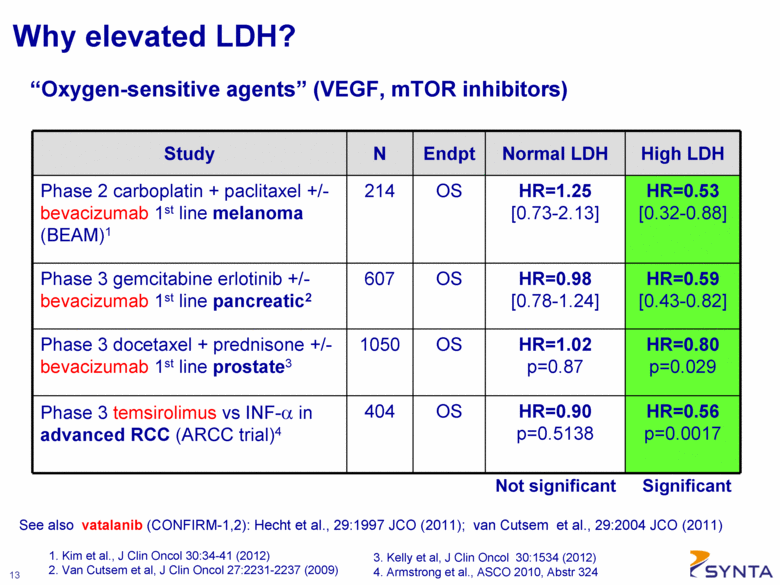
Why elevated LDH? Study N Endpt Normal LDH High LDH Phase 2 carboplatin + paclitaxel +/- bevacizumab 1st line melanoma (BEAM)1 214 OS HR=1.25 [0.73-2.13] HR=0.53 [0.32-0.88] Phase 3 gemcitabine erlotinib +/- bevacizumab 1st line pancreatic2 607 OS HR=0.98 [0.78-1.24] HR=0.59 [0.43-0.82] Phase 3 docetaxel + prednisone +/- bevacizumab 1st line prostate3 1050 OS HR=1.02 p=0.87 HR=0.80 p=0.029 Phase 3 temsirolimus vs INF-α in advanced RCC (ARCC trial)4 404 OS HR=0.90 p=0.5138 HR=0.56 p=0.0017 Not significant 1. Kim et al., J Clin Oncol 30:34-41 (2012) 2. Van Cutsem et al, J Clin Oncol 27:2231-2237 (2009) Significant “Oxygen-sensitive agents” (VEGF, mTOR inhibitors) See also vatalanib (CONFIRM-1,2): Hecht et al., 29:1997 JCO (2011); van Cutsem et al., 29:2004 JCO (2011) 3. Kelly et al, J Clin Oncol 30:1534 (2012) 4. Armstrong et al., ASCO 2010, Abstr 324 |
|
|
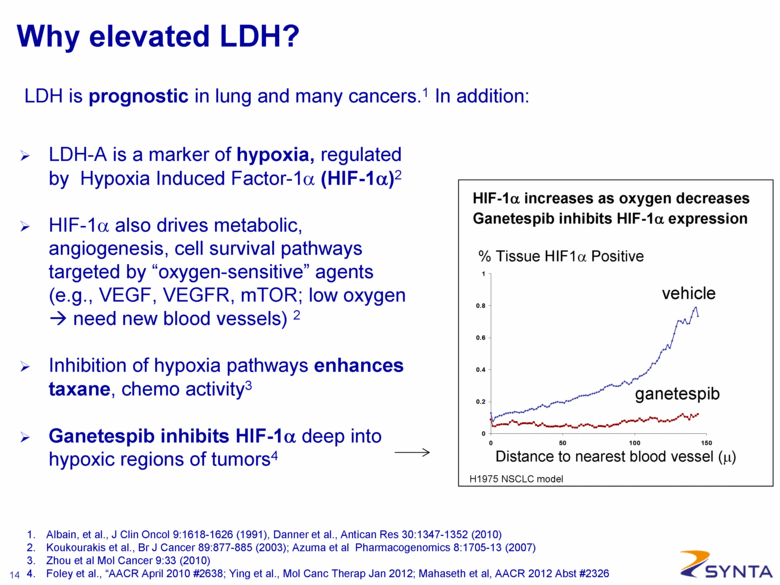
Why elevated LDH? LDH-A is a marker of hypoxia, regulated by Hypoxia Induced Factor-1α (HIF-1α)2 HIF-1α also drives metabolic, angiogenesis, cell survival pathways targeted by “oxygen-sensitive” agents (e.g., VEGF, VEGFR, mTOR; low oxygen need new blood vessels) 2 Inhibition of hypoxia pathways enhances taxane, chemo activity3 Ganetespib inhibits HIF-1α deep into hypoxic regions of tumors4 % Tissue HIF1α Positive ganetespib vehicle H1975 NSCLC model Distance to nearest blood vessel (μ) HIF-1α increases as oxygen decreases Ganetespib inhibits HIF-1α expression 1. Albain, et al., J Clin Oncol 9:1618-1626 (1991), Danner et al., Antican Res 30:1347-1352 (2010) 2. Koukourakis et al., Br J Cancer 89:877-885 (2003); Azuma et al Pharmacogenomics 8:1705-13 (2007) 3. Zhou et al Mol Cancer 9:33 (2010) 4. Foley et al., “AACR April 2010 #2638; Ying et al., Mol Canc Therap Jan 2012; Mahaseth et al, AACR 2012 Abst #2326 LDH is prognostic in lung and many cancers.1 In addition: |
|
|
“Warburg effect in chemosensitivity: Targeting LDH-A resensitizes Taxol-resistant cancer cells to Taxol” Zhou et al Mol Ca 2010; 9 p33 “Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients.” Azuma et al, Pharmacogenomics 8:1705-13 (2007) “Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis," Koukourakis et al, Br J Cancer 89:877-885 (2003) |
|
|
Why mutant KRAS? Acquaviva, Proia et al., “Targeting KRAS mutant NSCLC with the Hsp90 inhibitor ganetespib”, AACR-IASLC, San Diego Jan 2012 (manuscript submitted) Ganetespib three-way impact on RAS signaling: degrades receptor, PI3K/AKT pathway, and RAF/MEK pathway Inhibition of RAS signaling pathway proteins by ganetespib in mutant KRAS NSCLC cell lines |
|
|
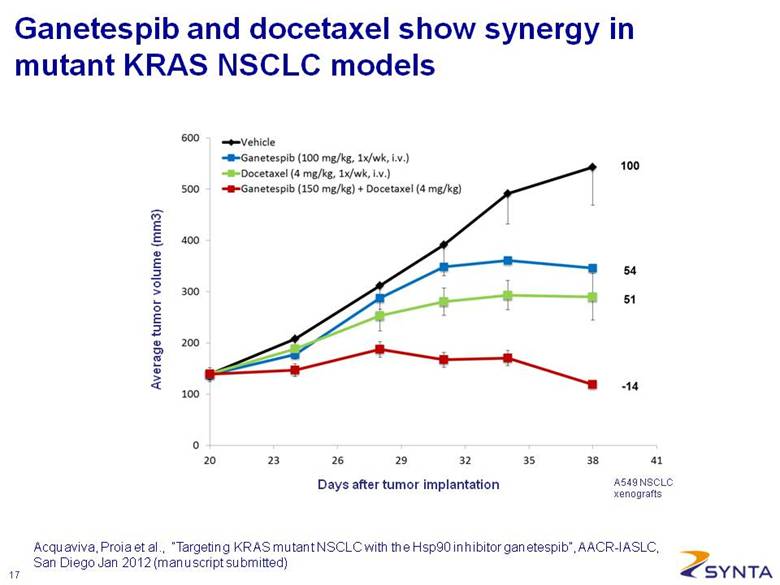
Average tumor volume (mm3) Days after tumor implantation A549 NSCLC xenografts Ganetespib and docetaxel show synergy in mutant KRAS NSCLC models Acquaviva, Proia et al., “Targeting KRAS mutant NSCLC with the Hsp90 inhibitor ganetespib”, AACR-IASLC, San Diego Jan 2012 (manuscript submitted) |
|
|
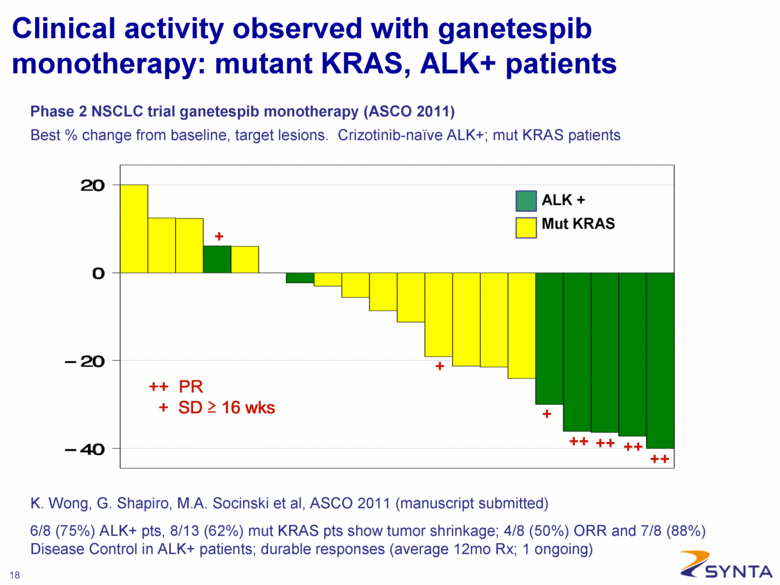
Clinical activity observed with ganetespib monotherapy: mutant KRAS, ALK+ patients Phase 2 NSCLC trial ganetespib monotherapy (ASCO 2011) Best % change from baseline, target lesions. Crizotinib-naïve ALK+; mut KRAS patients ALK + Mut KRAS ++ PR + SD > 16 wks 6/8 (75%) ALK+ pts, 8/13 (62%) mut KRAS pts show tumor shrinkage; 4/8 (50%) ORR and 7/8 (88%) Disease Control in ALK+ patients; durable responses (average 12mo Rx; 1 ongoing) K. Wong, G. Shapiro, M.A. Socinski et al, ASCO 2011 (manuscript submitted) |
|
|
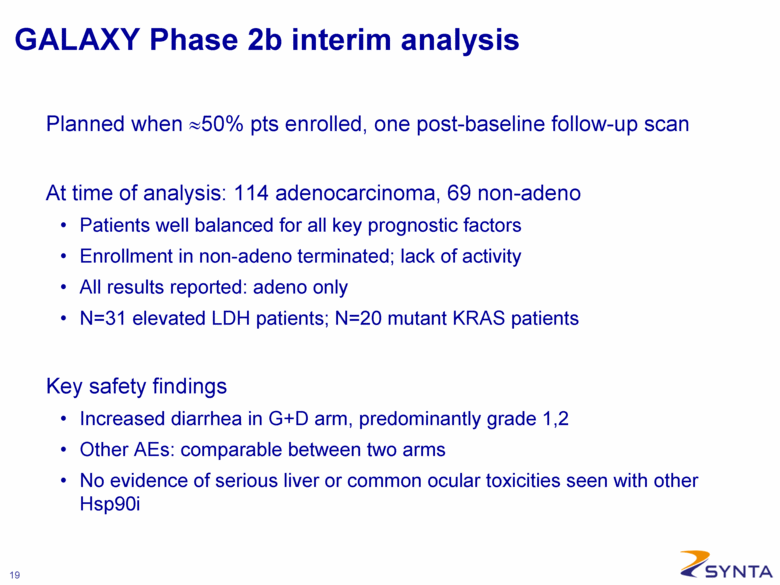
GALAXY Phase 2b interim analysis Planned when ≈50% pts enrolled, one post-baseline follow-up scan At time of analysis: 114 adenocarcinoma, 69 non-adeno Patients well balanced for all key prognostic factors Enrollment in non-adeno terminated; lack of activity All results reported: adeno only N=31 elevated LDH patients; N=20 mutant KRAS patients Key safety findings Increased diarrhea in G+D arm, predominantly grade 1,2 Other AEs: comparable between two arms No evidence of serious liver or common ocular toxicities seen with other Hsp90i |
|
|
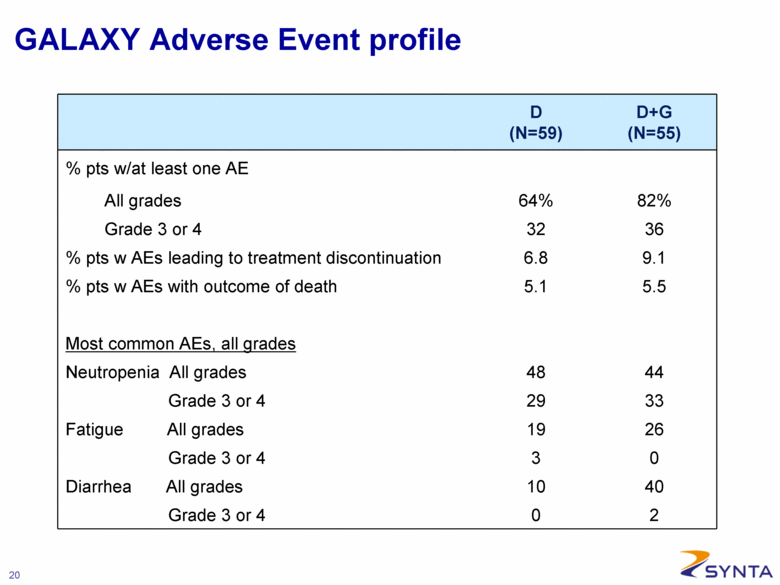
GALAXY Adverse Event profile D (N=59) D+G (N=55) % pts w/at least one AE All grades 64% 82% Grade 3 or 4 32 36 % pts w AEs leading to treatment discontinuation 6.8 9.1 % pts w AEs with outcome of death 5.1 5.5 Most common AEs, all grades Neutropenia All grades 48 44 Grade 3 or 4 29 33 Fatigue All grades 19 26 Grade 3 or 4 3 0 Diarrhea All grades 10 40 Grade 3 or 4 0 2 |
|
|
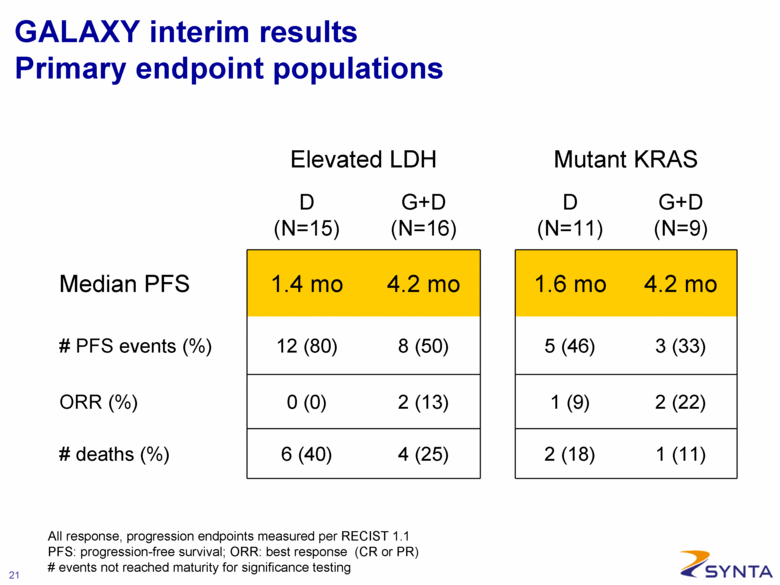
GALAXY interim results Primary endpoint populations Elevated LDH Mutant KRAS D (N=15) G+D (N=16) D (N=11) G+D (N=9) Median PFS 1.4 mo 4.2 mo 1.6 mo 4.2 mo # PFS events (%) 12 (80) 8 (50) 5 (46) 3 (33) ORR (%) 0 (0) 2 (13) 1 (9) 2 (22) # deaths (%) 6 (40) 4 (25) 2 (18) 1 (11) All response, progression endpoints measured per RECIST 1.1 PFS: progression-free survival; ORR: best response (CR or PR) # events not reached maturity for significance testing |
|
|
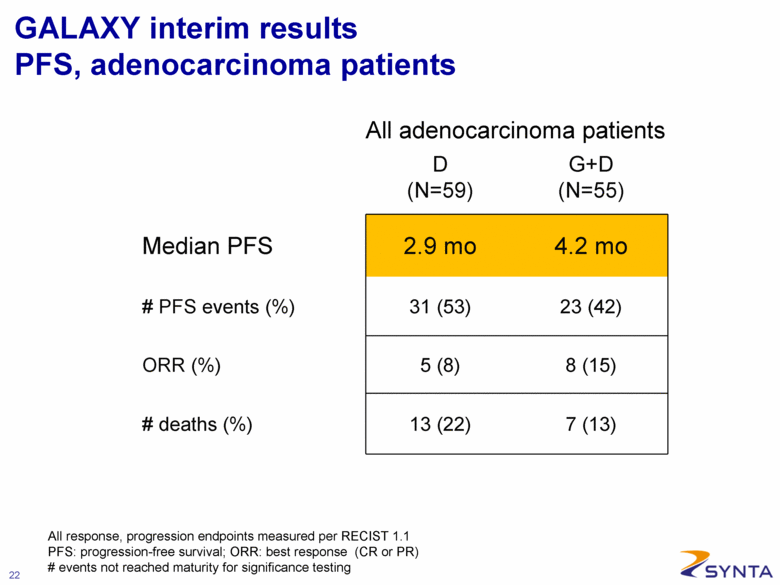
GALAXY interim results PFS, adenocarcinoma patients D (N=59) G+D (N=55) Median PFS 2.9 mo 4.2 mo # PFS events (%) 31 (53) 23 (42) ORR (%) 5 (8) 8 (15) # deaths (%) 13 (22) 7 (13) All adenocarcinoma patients All response, progression endpoints measured per RECIST 1.1 PFS: progression-free survival; ORR: best response (CR or PR) # events not reached maturity for significance testing |
|
|
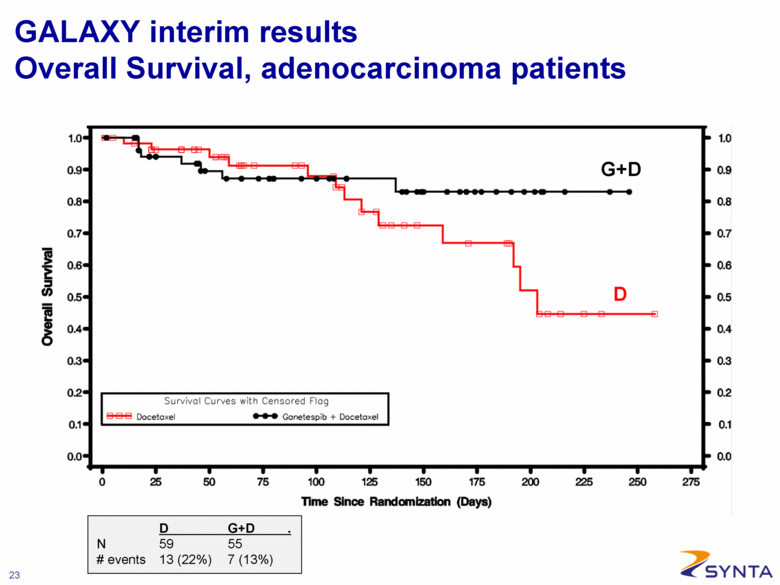
GALAXY interim results Overall Survival, adenocarcinoma patients G+D D D G+D . N 59 55 # events 13 (22%) 7 (13%) |
|
|
Next Steps Complete enrollment, N=240 adenocarcinoma patients (ongoing) Expand # sites NA, Europe, Asia in preparation for transition to Phase 3 (ongoing) Additional interim analysis, medical meeting presentation (2H 2012) Regulatory advice for Phase 3 design (Q3) Focus on GALAXY, 2nd-line NSCLC as most immediate path to registration |
|
|
References Elevated LDH in cancer Albain et al., "Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience," J Clin Oncol 9:1618-1626 (1991) Armstrong et al. "Serum lactate dehydrogenase (LDH) is a predictive biomarker for mTOR inhibition in patients with metastatic renal cell carcinoma (RCC)," ASCO 2010, Abstr 324 Azuma et al., “Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients,” Pharmacogenomics 8:1705-13 (2007) Danner et al. "Long-term survival is linked to serum LDH and partly to tumour LDH-5 in NSCLC," Antican Res 30:1347-1352 (2010) Kelly et al. "Randomized, Double-Blind, Placebo-Controlled Phase III Trial Comparing Docetaxel and Prednisone With or Without Bevacizumab in Men With Metastatic Castration-Resistant Prostate Cancer: CALGB 90401," J Clin Oncol 30:1534 (2012) Kim et al., "BEAM: A Randomized Phase II Study Evaluating the Activity of Bevacizumab in Combination With Carboplatin Plus Paclitaxel in Patients With Previously Untreated Advanced Melanoma," J Clin Oncol 30:34-41 (2012) |
|
|
References (continued) Koukourakis et al., "Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis," Br J Cancer 89:877-885 (2003) Suh and Ahn, "Lactate dehydrogenase as a prognostic factor for survival time of terminally ill cancer patients: a preliminary study," Eur J Cancer 43:1051 (2007) Van Cutsem et al., "Phase III Trial of Bevacizumab in Combination With Gemcitabine and Erlotinib in Patients With Metastatic Pancreatic Cancer, “ J Clin Oncol 27:2231-2237 (2009) Zhou et al., "Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol," Mol Cancer 9:33 (2010) Mutant KRAS in cancer Acquaviva et al., “Targeting KRAS mutant NSCLC with the Hsp90 inhibitor ganetespib,” AACR-IASLC, San Diego Jan 2012 (manuscript submitted) Douillard et al., “Molecular Predictors of Outcome With Gefitinib and Docetaxel in Previously Treated Non–Small-Cell Lung Cancer: Data From the Randomized Phase III INTEREST Trial,” J Clin Oncol 28:744-752 (2010) Janne et al. “Phase II double-blind, randomized study of selumetinib (SEL) plus docetaxel (DOC) versus DOC plus placebo as second-line treatment for advanced KRAS mutant non-small cell lung cancer (NSCLC),” ASCO abstr 7503 (2012) |
|
|
References (continued) Johnson et al., "Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinoma," ASCO Jun 2010 Abstr 7541 Schneider, "Tumor markers in detection of lung cancer," Advan Clin Chem 42:1-41 (2006) Socinski, "The emerging role of biomarkers in advanced non-small-cell lung cancer,“ Clin Lung Can 11:149-159 (2010) Hsp90 inhibition Foley et al., "Hsp90 inhibitor STA-9090 induces HIF-1α degradation in the hypoxic regions of solid tumors," AACR April 2010 Abstr 2638 Infante et al. "A Phase 1 Dose-Escalation Study of the Oral Heat Shock Protein 90 Inhibitor PF-04929113 (SNX5422) and its Associated Ocular Toxicity," AACR-EORTC-NCI Nov 2010 Pos # 375 Mahaseth et al., “Antiangiogenic Effects Associated with the Inhibition of HSP90 in Colorectal Cancer,” AACR 2012 Abstr 2326 Samuel et al., "AUY922, a novel HSP90 inhibitor: Final results of a first-in-human study in patients with advanced solid malignancies," ASCO Jun 2010 Abstr 2528 Shapiro et al., "Phase I pharmacokinetic and pharmacodynamic study of the heat shock protein 90 inhibitor AT13387 in patients with refractory solid tumors," ASCO Jun 2010 Abstr 3069 |
|
|
References (continued) Socinski et al., "Phase II study of ganetespib monotherapy in patients with advanced stage IIIB/IV NSCLC," ASCO 2011 (manuscript submitted) Ying et al., “Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy,” Molec Canc Therap 11:475 (2012) Zhou et al., “Associating retinal drug exposure and retention with the ocular toxicity profiles of Hsp90 inhibitors,” ASCO June 2012 Abstr 3086 |