Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - GenMark Diagnostics, Inc. | d370165d8k.htm |
 GenMark Diagnostics
Multiplex tests.
Personalized results.
Exhibit 99.1
Management Presentation |
 2
Forward-Looking Statements
This presentation contains forward-looking statements about GenMark Diagnostics, Inc.
These statements involve known and unknown risks that relate to the Company’s
future events or future financial performance and the actual results could differ materially from those discussed in this
presentation. Factors that may cause the Company's actual results to differ materially from
those discussed in the presentation, include: •
failure to obtain sufficient funding for the continued development and commercialization of
the Company's products;
•
failure to expand the Company's menu of diagnostic tests, including the failure to obtain
licenses to additional biomarkers on commercially reasonable terms; •
increases in the Company's projected expenditures on sales and marketing, research and
development and administrative activities;
•
less than anticipated growth in the market for diagnostic testing generally and for the
tests the Company is developing or may develop in the future; •
failure of the Company's products to gain market acceptance domestically or
internationally;
•
inability to obtain regulatory clearance or approval for any of the Company's products; •
changes in the regulatory environment which may adversely impact the commercialization of
the Company's new products and result in significant additional capital
expenditures;
•
failure to enter into or maintain successful strategic alliances, which may delay the
development or commercialization of the Company's products or may result in
significant additional expenditures;
•
inability to attract or retain skilled personnel for the Company's product development and
commercialization efforts;
•
inability to protect the Company's intellectual property and operate the Company's business
without infringing upon the intellectual rights of others, which could result in
litigation and significant expenditures;
•
refusal of third-party payors to reimburse the Company's customers for use of
diagnostic systems and tests; and
•
failure to develop the Company's NexGen System with the capabilities the Company intends to
offer.
Additional risks and uncertainties relating to the Company and its business can be found in
the "Risk Factors" section of GenMark's Form 10-K and other filings with
the United States Securities and Exchange Commission. The forward-looking statements contained in this presentation
represent the Company’s estimates and assumptions only as of the date of this
presentation and the Company undertakes no duty or obligation to update or revise
publicly any forward-looking statements contained in this presentation as a result of new information, future events or changes in
the Company’s expectations.
|
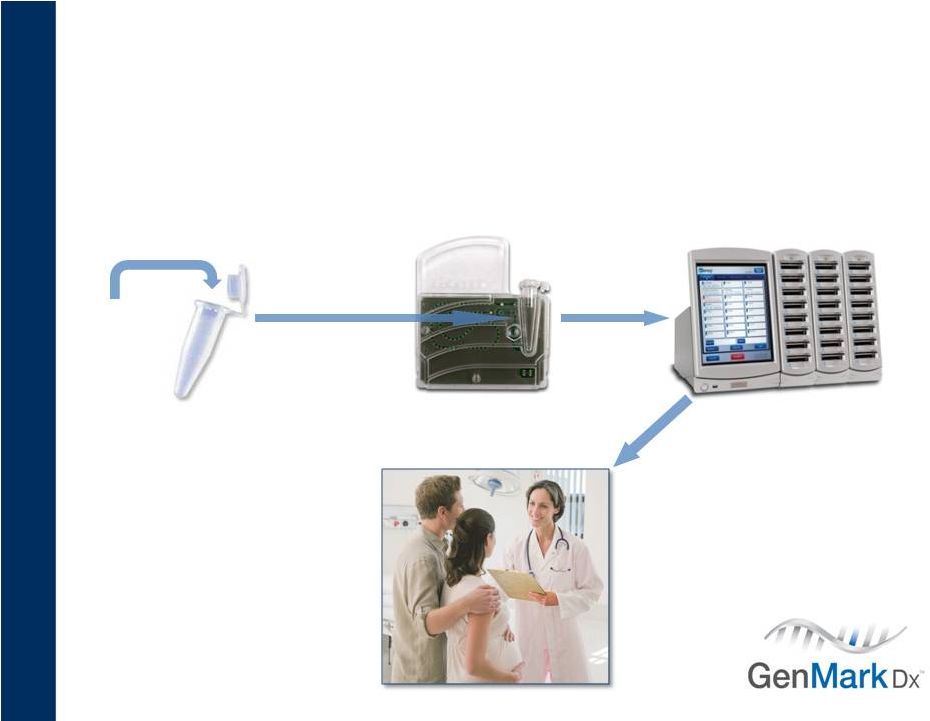 GenMark’s Solution
Standardized, Random Access, Scalable
3
Amplified
patient sample
Add
proprietary
label
Load mixture
Patient results
Insert cartridge |
 Growth
Drivers •
Clinical need
•
Decentralization
•
Reimbursement
Highest Growth
•
PGx ~30%
•
Oncology ~17%
•
ID ~14%
4
Source: Decisive Bio-Insights 2012, LEK 2009, Management estimates
Multiplex MDx Opportunity
Current Global MDx market approximately $4.5B growing 15%
GenMark Addressable Market
$900M
Global Market
$2.3B+
Global Market
growing 20% |
 Clinical Importance of Multiplex Testing
Improving diagnosis of complex medical problems
Viral diversity impacts therapy regimens
Hepatitis C virus genotyping
5
Multiple pathogens present similar symptoms
Respiratory virus panel |
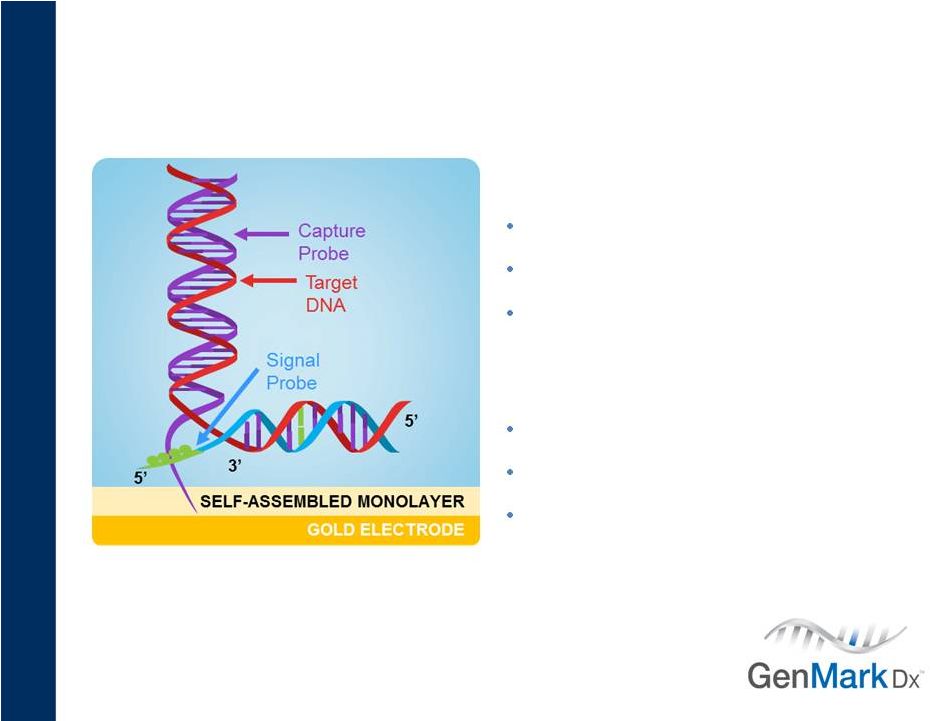 6
Core Technology
Electronic DNA detection
Differentiating specificity & sensitivity
$120M invested, 100+ U.S. patents
Competitive Advantages
True multiplexing
Supports decentralization
Provides cost advantages
GenMark’s Technology |
 7
Testing Standardization
•
Single detection system for broad menu
•
Uniform assay procedures
Laboratory Efficiency
•
Random & continuous access
•
No calibration or routine maintenance
Accuracy & Performance
•
100% accuracy & reproducibility
•
30 minute processing
Customer Value Proposition
Sources: Package Inserts, LEK Market Research, Scientific Conference Posters.
|
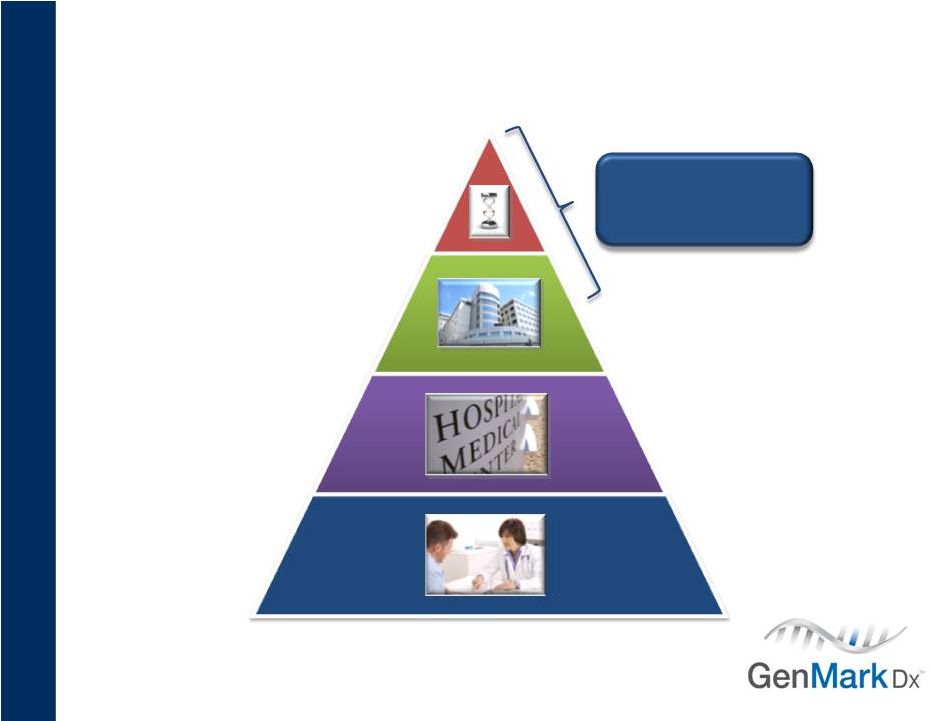 8
Target Customers
Management estimates subject to change.
~1,000 labs
Limited MDx menu
500 full service
MDx labs
35,000+
POLs
~5,000 labs
No MDx menu
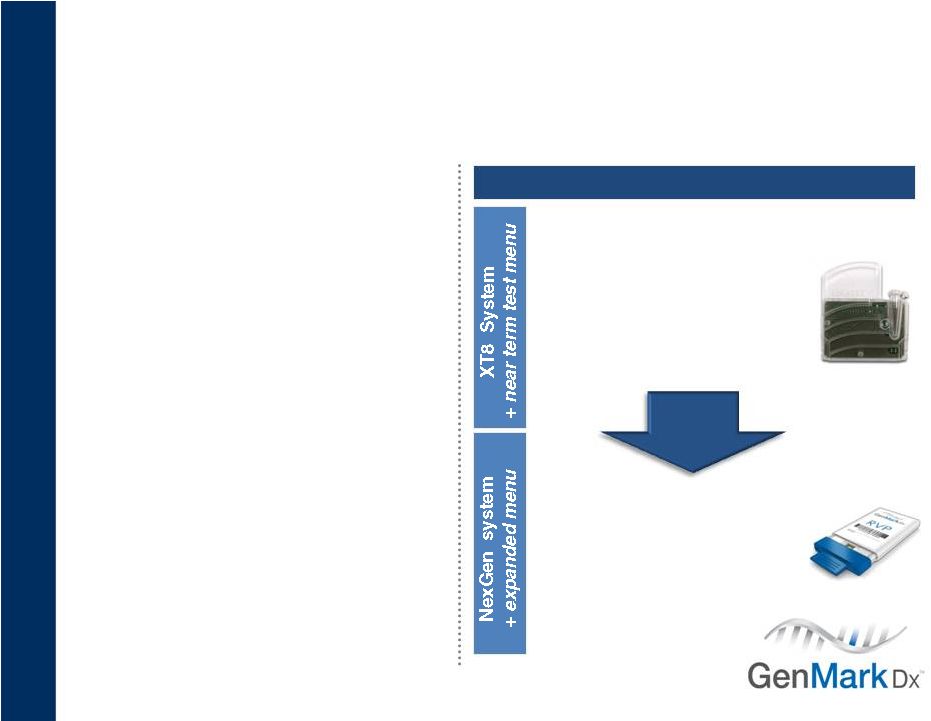
XT-8 System
~1000 Post-PCR Labs |
 Development
Design
Clinical trials /
RUO product
FDA cleared
IVD product
Thrombophilia
Risk Test
Warfarin
Cystic
Fibrosis
Respiratory
Viral Panel
Plavix
(2C19)
HCV
Genotyping
9
Menu & Pipeline
Pending FDA 510(k) clearance
HCVg
Direct
KRAS
Infectious Disease
Pharmacogenetics
Inherited Disease
Oncology |
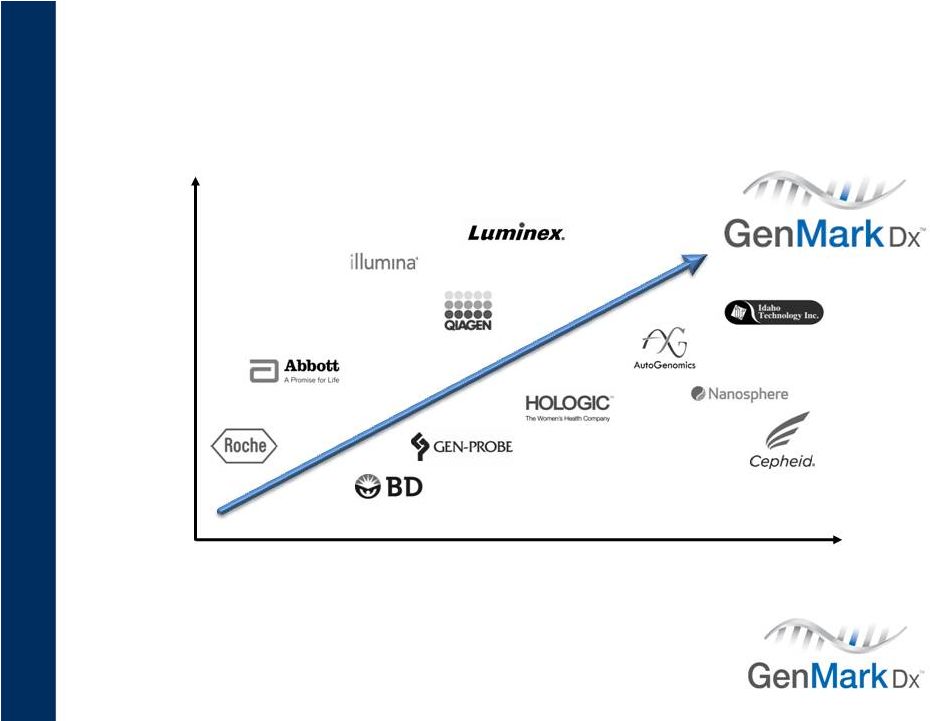 Low-plex
Multiplex
Centralized
Decentralized
10
Fragmented Competition
Increasing Emphasis on Multiplex IVD MDx
Companies listed are representative.
Positioning reflects management’s current opinion and is subject to change.
|
 11
NexGen System
The all-digital, sample-to-answer system
•
Fully integrated
Extraction to result
•
“Electrowetting”
fluidics
No channels, valves or pumps
•
Rapid configuration
Software vs hardware
•
Low cost
High profit margins
Management estimates subject to change. |
 12
NexGen System (cont’d)
The all-digital, sample-to-answer system
•
Core detection technology designed to
ensure accuracy & quality of results
•
Fully-integrated cartridge delivers
superior ease of use
•
Provides flexibility with menu and
sample types
Decentralization today
POC testing tomorrow
Management estimates subject to change. |
 NexGen Test Menu Opportunities
Addressing a significant unmet need
13
•
>$1B global market with
established test volumes
•
Tests include MRSA, C.
Diff, CT/NG
•
Limited platform and menu
options today for
decentralized market
•
>$1B emerging global
market
•
Tests include RVP, GI,
HCVg, Pneumonia, Blood
Culture ID
•
No sample-to-answer
system with appropriate
testing capacity and
menu
Low-plex Tests
(<5 targets)
Multiplex Tests
(10+ targets)
Market
Opportunity
Customer
Issue |
 XT-8
NexGen
Menu Opportunities
Technology enabled consolidation of testing
Infectious Disease
Pharmacogenetics
Inherited Disease
Oncology
Management estimates subject to change.
Gastrointestinal Infections (Bacterial, Viral…)
Oncology (KRAS, EGFR…)
Blood Culture ID
PGx (Warfarin, 2C19…)
HAI (MRSA, C Diff, VRE…)
STI (CT/NG, Vaginosis)
Viral Genotyping (HCV…)
Inherited Disease (CF, TRT…)
Respiratory Tract Infections (RVP, CAP, TB…) |
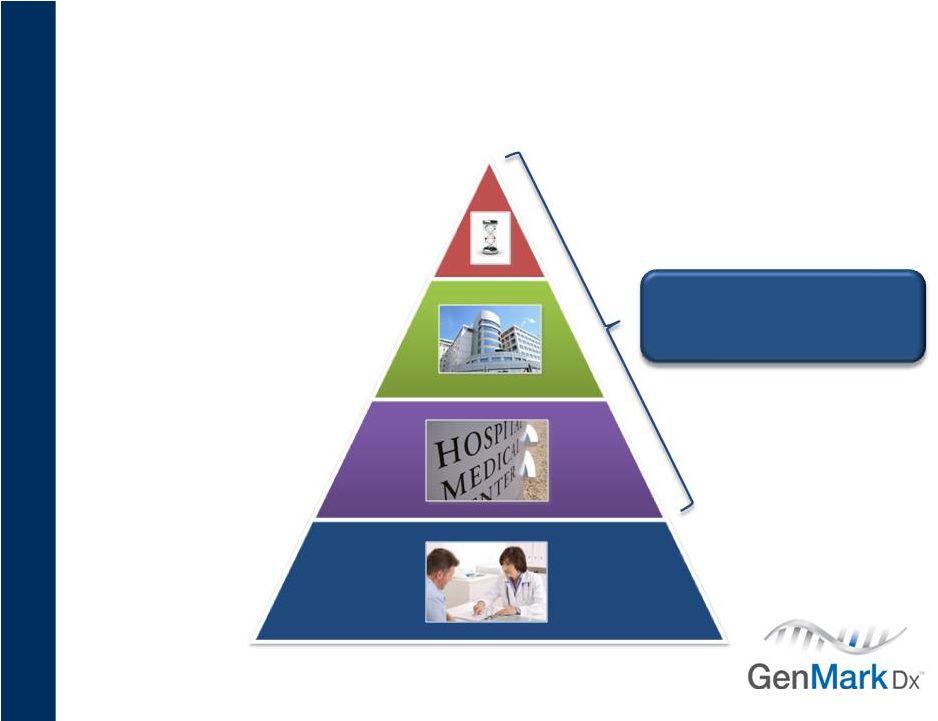 ~1,000
labs Limited MDx menu
500 full service
MDx labs
35,000+
POLs
~5,000 labs
No MDx menu
15
Target Customers
NexGen dramatically expands customer base
Management estimates subject to change.
NexGen System
Sample-to-Answer
5,000+ labs |
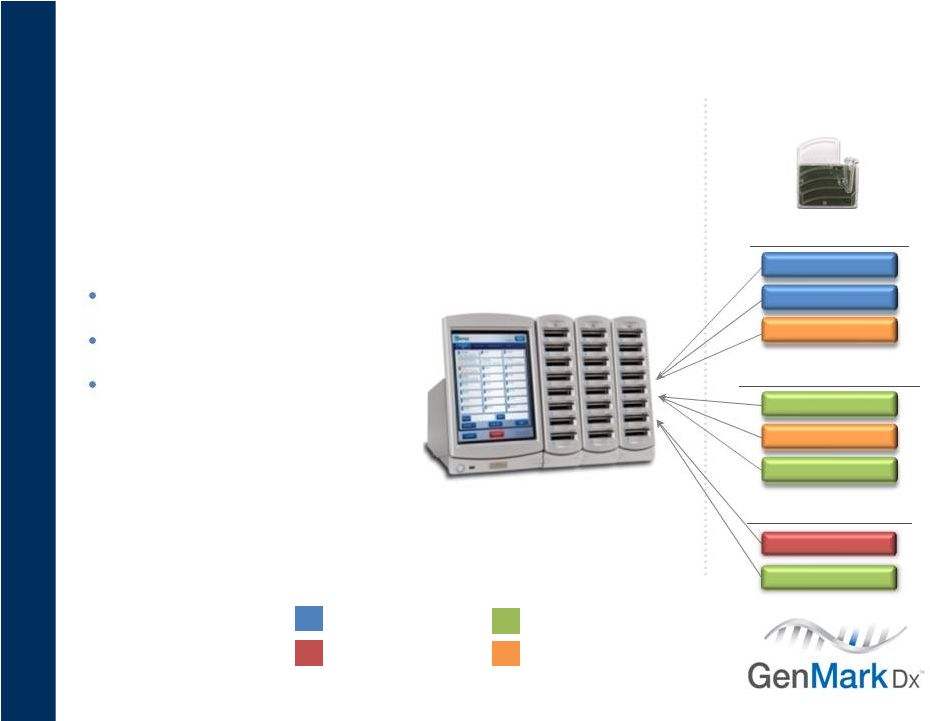 16
Attractive Business Model
Razor/Razor Blade
“Razor”
“Razor Blades”
FDA Approved Tests
CF
TRT
Warfarin
RUO/Clinical Trial Tests
Plavix (2C19)
RVP
Tests In Development
HCVg
KRAS
Increased test utilization
will be key revenue driver
HCVg Direct
Infectious Disease
Pharmacogenetics
Inherited Disease
Oncology
Grow installed base
Expand test menu
Increase test volume &
revenue per system |
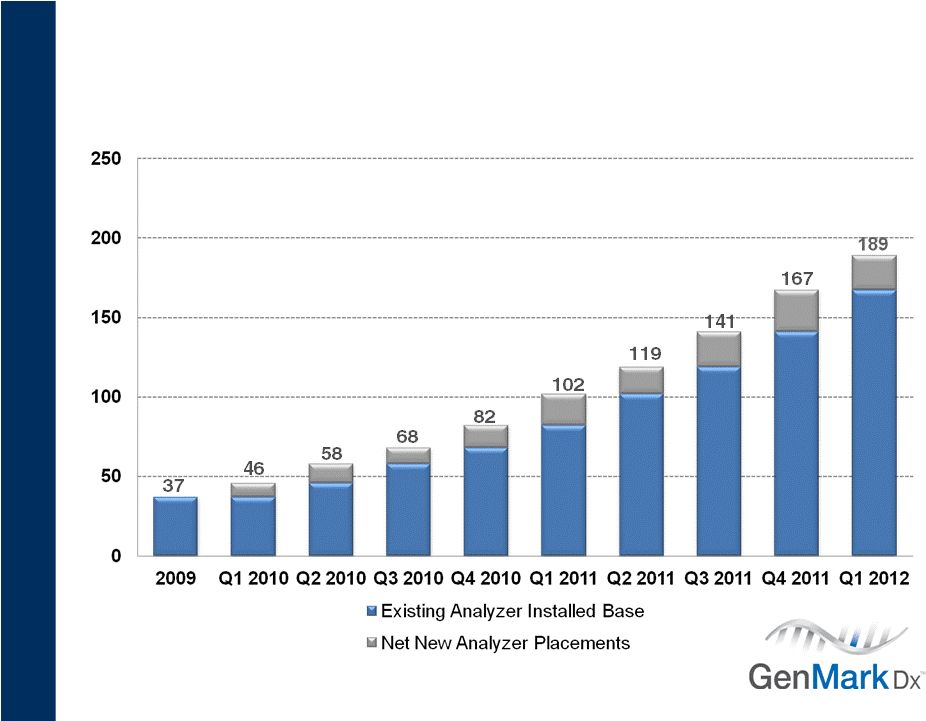 17
Growing Installed Base |
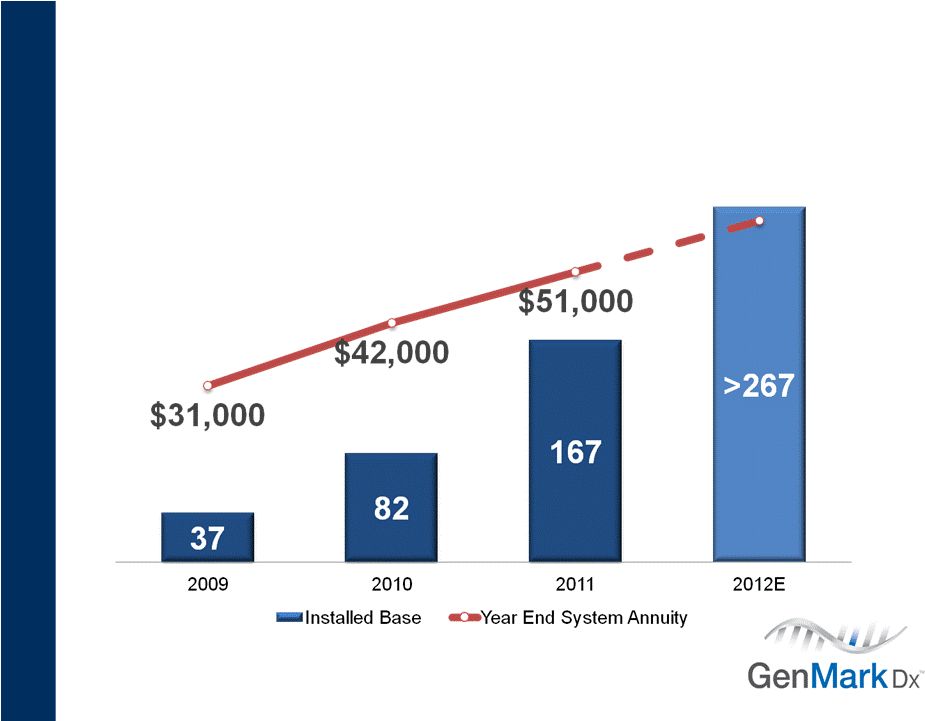 18
Executing on Business Model
Delivering value to customers & shareholders
Management estimates subject to change. |
 19
Key Milestones
Strong execution history
Management estimates subject to change.
2010
2011
•
Nasdaq IPO
•
Established direct sales force
•
Launched TRT as IVD
•
Launched RVP as RUO
•
Launched 2C19 as RUO
•
Initiated RVP clinical studies
•
Relocated to Carlsbad
•
Submitted RVP to FDA
•
Launched HCVg as RUO
•
Completed secondary raise
•
Expanded sales force to 15+
•
Initiated NexGen development |
 20
Key Milestones
2012 priorities
Management estimates subject to change.
Product Development
Commercialization
•
Launch RVP IVD
•
Launch HCVg Direct RUO
•
Initiate 2C19 clinical trial
•
Expand test menu
•
NexGen development
•
Place 100+ analyzers
•
Drive annuity per analyzer
•
Expand sales force
•
Initiate international activity
•
“Show”
NexGen at AMP |
