Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark | One) |
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the year ended December 31, 2011
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-34753
GenMark Diagnostics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 27-2053069 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
| 5964 La Place Court, Suite 100, Carlsbad, California | 92008-8829 | |
| (Address of principal executive offices) | (Zip code) | |
Registrant’s telephone number, including area code: 760-448-4300
Securities registered pursuant to Section 12(b) of the Act
| Title of Each Class: |
Name of Each Exchange on which Registered: | |
| Common Stock, par value $0.0001 per share | The NASDAQ Stock Market LLC (NASDAQ Global Market) |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act of 1933, as amended. YES ¨ NO x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934, as amended. YES ¨ NO x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ¨ Accelerated filer x Non-accelerated filer ¨ Smaller reporting company ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
As of June 30, 2011, the last business day of the registrant’s most recent completed second quarter, the aggregate market value of the common stock held by non-affiliates of the registrant was approximately $101,164,173 based on the closing sale price for the registrant’s common stock on the NASDAQ Global Market on that date of $5.85 per share. This number is provided only for the purpose of this report on Form 10-K and does not represent an admission by either the registrant or any such person as to the status of such person.
The number of outstanding shares of the registrant’s common stock on February 28, 2012 was 20,503,059. The common stock is listed on the NASDAQ Global Market (trading symbol “GNMK”).
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for the 2012 Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission within 120 days after the end of the year ended December 31, 2011, are incorporated by reference in Part III of this Form 10-K
Table of Contents
| Page | ||||||
| Item 1. |
2 | |||||
| Item 1A. |
23 | |||||
| Item 1B. |
53 | |||||
| Item 2. |
53 | |||||
| Item 3. |
53 | |||||
| Item 4. |
53 | |||||
| Item 5. |
54 | |||||
| Item 6. |
55 | |||||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
57 | ||||
| Item 7A. |
73 | |||||
| Item 8. |
74 | |||||
| Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
102 | ||||
| Item 9A. |
102 | |||||
| Item 9B. |
107 | |||||
| Item 10. |
108 | |||||
| Item 11. |
108 | |||||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Maters |
108 | ||||
| Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
108 | ||||
| Item 14. |
108 | |||||
| Item 15. |
109 | |||||
Table of Contents
Forward-Looking Statements
This Annual Report on Form 10-K, particularly in Item 1. “Business” and Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and the documents incorporated by reference, include forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including, but not limited to, statements regarding our future financial position, business strategy and plans and objectives of management for future operations. When used in this Annual Report, the words “believe,” “may,” “could,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “target,” “anticipate,” “aim,” “plan” and similar expressions are intended to identify forward-looking statements.
These forward-looking statements are based on current expectations, estimates, forecasts and projections about our business and the industry in which we operate and management’s beliefs and assumptions and are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this Annual Report on Form 10-K may turn out to be inaccurate. Factors that may cause such differences include, but are not limited to, the risks described under “Risk Factors,” including:
| • | failure to obtain sufficient funding for the continued development and commercialization of our products; |
| • | failure to expand our menu of diagnostic tests, including the failure to obtain licenses to additional biomarkers on commercially reasonable terms; |
| • | increases in our projected expenditures on sales and marketing, research and development and administrative activities; |
| • | less than anticipated growth in the market for diagnostic testing generally and for the tests we are developing or may develop in the future; |
| • | failure of our products to gain market acceptance domestically or internationally; |
| • | inability to obtain regulatory clearance or approval for any of our products; |
| • | changes in the regulatory environment which may adversely impact the commercialization of our new products and result in significant additional capital expenditures; |
| • | failure to enter into or maintain successful strategic alliances, which may delay the development or commercialization of our products or may result in significant additional expenditures; |
| • | inability to attract or retain skilled personnel for our product development and commercialization efforts; |
| • | inability to protect our intellectual property and operate our business without infringing upon the intellectual rights of others, which could result in litigation and significant expenditures; |
1
Table of Contents
| • | refusal of third-party payors to reimburse our customers for use of diagnostic systems and tests; and |
| • | failure to develop our NexGen System with the capabilities we intend to offer. |
Except as required by law, we do not intend to update these forward-looking statements publicly or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this report and in the documents incorporated in this report may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Accordingly, readers are cautioned not to place undue reliance on such forward-looking statements.
| Item 1. | BUSINESS |
GenMark Diagnostics, Inc., or GenMark, was formed by Osmetech plc, or Osmetech, in Delaware in February 2010, and had no operations prior to its initial public offering which was completed in June 2010. Immediately prior to the closing of the initial public offering, GenMark acquired all of the outstanding ordinary shares of Osmetech in reorganization under the applicable laws of the United Kingdom. As a result of the reorganization, all of the issued ordinary shares in Osmetech were cancelled in consideration of (i) the issuance of common stock of GenMark to the former shareholders of Osmetech and (ii) the issuance of new shares in Osmetech to GenMark. Following the reorganization, Osmetech became a subsidiary controlled by GenMark, and the former shareholders of Osmetech began to hold shares of GenMark. Any historical discussion of GenMark relates to Osmetech and its consolidated subsidiaries prior to the reorganization.
References herein to “we,” “us” or “our” refer to GenMark Diagnostics, Inc. unless the context specifically requires otherwise.
Overview
We are a molecular diagnostics company focused on developing and commercializing our proprietary eSensor® detection technology. Our proprietary electrochemical technology enables fast, accurate and highly sensitive detection of up to 72 distinct biomarkers in a single sample. Our XT-8 system received 510(k) clearance from the Food and Drug Administration, or FDA, and is designed to support a broad range of molecular diagnostic tests with a compact and easy-to-use workstation and self-contained, disposable test cartridges. Within 30 minutes of receipt of an extracted and amplified nucleic acid sample, our XT-8 system produces clear and accurate results. Our XT-8 system supports up to 24 independent test cartridges, of which each can be run independently, resulting in a highly convenient and flexible workflow for our target customers, which are hospitals and reference laboratories. As of December 31, 2011, we had an installed base of 167 analyzers, or placements, with our customers.
2
Table of Contents
Our Products
We have developed four diagnostic tests for use with our XT-8 system and expect to expand this test menu by introducing two to four new tests annually. Our Cystic Fibrosis Genotyping Test, which detects pre-conception risks of cystic fibrosis, our Warfarin Sensitivity Test, which determines an individual’s ability to metabolize the oral anticoagulant warfarin, and our Thrombophilia Risk Test, which detects an individual’s increased risk of blood clots, have received FDA clearance. Our eSensor® technology has demonstrated 100% accuracy in clinical studies in our Cystic Fibrosis Genotyping Test, our Warfarin Sensitivity Test and our Thrombophilia Risk Test as compared to DNA sequencing. We have also developed a Respiratory Viral Panel Test, currently available for Research Use Only, that has been submitted to the FDA for 510(k) clearance. We also have a pipeline of several additional potential products in different stages of development or design, including diagnostic tests for genotyping the Hepatitis C Virus, and for mutations in a gene known as KRAS, which is predictive of an individual’s response rates to certain prescribed anti-cancer therapies.
We are also developing our next-generation platform, the NexGen system. We are designing the NexGen system (formerly referred to as the AD-8 system) to integrate automated nucleic acid extraction and amplification with our eSensor® detection technology to enable technicians to place a patient sample into our test cartridge and obtain results without any additional steps. This sample-to-answer capability is enabled by the robust nature of our eSensor® detection technology, which is not impaired by sample impurities that we believe hinder competing technologies. We are designing our NexGen system to further simplify workflow and provide powerful, cost-effective molecular diagnostics solutions to a significantly expanded group of hospitals and reference laboratories.
Our XT-8 system and planned menu of tests are intended to improve patient care and physician practices by providing high value, clinically useful information that aids in the diagnosis of disease and the selection of treatments tailored to an individual’s genetic profile. We believe that these improvements in patient care are economically attractive to our customers who are generally reimbursed for these tests by third-party payors and managed care providers through established reimbursement codes. Given historically positive reimbursement levels and because the XT-8 system is designed to be flexible and easy-to-use, we believe that our customers will choose to perform a broad range of tests on our platform, in some cases providing our customers with sources of diagnostic test revenue previously unavailable to them. By focusing our product development and commercialization efforts on high value, clinically useful opportunities in genetic and infectious diseases, cancer and personalized medicine, we believe we will drive widespread clinical adoption of our products.
Our Strategy
Our goal is to become the market leading provider of automated, multiplex molecular diagnostic testing systems. We intend to expand the use of our XT-8 system and diagnostic tests targeting especially those reference laboratories and hospitals in the United States which perform a high volume of molecular diagnostic tests. To achieve this objective, we intend to:
| • | Expand our Menu of Clinical Diagnostic Products. We intend to develop a broad menu of molecular diagnostic tests that we believe satisfy important medical needs and |
3
Table of Contents
| will be attractively reimbursed by third-party payors. We are pursuing and intend to continue to pursue FDA clearance or approval for our tests. We intend to explore tests that are either already in high demand or projected to experience rapid growth. We plan to gain access to these tests by in-licensing, where required, the appropriate biomarkers that have shown correlations to diseases or therapeutic response. |
| • | Grow our Installed Base of Customers. We have identified those laboratories and hospitals in the United States that we believe will be high volume customers and who will benefit from our eSensor® technology. We intend to leverage our commercial organization to drive placements of our XT-8 system. We anticipate expansion of our installed base of customers will drive sales of our test cartridges, from which we anticipate generating the majority of our revenues. |
| • | Increase Utilization of Tests. We intend to increase the use of our diagnostic tests by developing and offering tools and support tailored to our products such as accredited physician education programs and seminars, product training for our customers and reimbursement support. These activities will aid in establishing the clinical utility of multiplex molecular diagnostic tests, which we believe will increase adoption of our products. |
| • | Develop our NexGen System. We are developing our NexGen system to provide a complete sample to answer solution for our customers. The NexGen system will retain all the customer benefits of our XT-8 system while also integrating automated nucleic acid extraction and amplification. These features will eliminate the need for time consuming and complex sample preparation steps and allow technicians to place a patient sample into our test cartridge. We have already demonstrated feasibility of direct sample to answer on a NexGen system prototype using diluted blood. We believe this advancement will make our technology attractive to a broader range of hospitals and laboratories that lack the technical or economic resources to perform molecular diagnostic testing with existing products and technology. We believe such workflow enhancements may expand our target user base from some 1,000 customers to over 5,000 potential customers in the United States. |
| • | Expand Internationally and Explore Out-Licensing Opportunities. We plan to offer our molecular diagnostic products in European and other international markets in the future. We anticipate using marketing partners and distributors as we expand internationally. We expect to supplement marketing partnerships with specialists who will train our partners’ sales forces and provide technical support. We also intend to explore opportunities to leverage our intellectual property position in detection technologies through licensing or the establishment of partnerships. |
Revenues from external customers, net loss and total assets for the past three years are contained in our consolidated financial statements in Part II of this report.
Our Products
Our XT-8 System
Our XT-8 system is an automated molecular diagnostic system that enables reference laboratories and hospitals to perform fast, accurate and easy-to-use molecular diagnostic tests. The XT-8 system, which employs our proprietary electrochemical detection technology, consists of a compact bench-top workstation with an integrated touch screen computer and an analyzer into which the self-contained, disposable test cartridges are inserted. The XT-8 system is user-friendly,
4
Table of Contents
intuitive, requires minimal maintenance and provides laboratories with the ability to perform multiplex molecular diagnostic tests in an efficient and cost-effective manner. With a footprint of approximately 16-by-16 inches in its standard configuration, the XT-8 system takes up less bench top space than most of our competitors’ systems, and its standalone design allows it to be installed and used without any required laboratory modifications. Specifically, we believe that our XT-8 system and related diagnostic tests will offer reference laboratories and hospitals the following benefits:
| • | Versatile Platform for a Broad Menu. Our XT-8 system has broad application across a number of areas in molecular diagnostic testing. In addition to our FDA-cleared Cystic Fibrosis Genotyping Test, Warfarin Sensitivity Test and Thrombophilia Risk Test, and our Respiratory Viral Panel Test, which is labeled for RUO and has been submitted to the FDA, we have a pipeline of several additional products in development or design in the fields of pharmacogenetics, genetic diseases, infectious diseases and cancer. We are currently developing a Plavix Sensitivity Test, Hepatitis C Virus genotyping test and a KRAS Mutation Test, and we have a pipeline of potential products in various stages of development or design. Laboratories using our system will be able to run the additional tests we offer without any additional capital investment or operator training. |
| • | FDA-Cleared Products. We have received FDA clearance for our Cystic Fibrosis Genotyping Test, Warfarin Sensitivity Test and Thrombophilia Risk Test. We submitted our Respiratory Viral Panel Test to the FDA for 510(k) clearance in the fourth quarter of 2011. We intend to utilize IUO-labeled products in clinical studies within the broader process of seeking FDA clearance for our diagnostic tests. |
| • | Ease of Use. Our XT-8 system eliminates the need to use complex instrumentation to generate test results. Our XT-8 system minimizes manual processing steps and streamlines data analysis, making molecular diagnostic testing available to a broad spectrum of laboratories without the need for highly skilled technicians. As a result, our XT-8 system can provide national reference laboratories with the ability to perform our menu of molecular diagnostic tests across all of their locations. We also designed our XT-8 system to require minimal maintenance. |
| • | Accuracy and Reliability. Our XT-8 system provides accurate and reliable molecular diagnostic test results. We have demonstrated 100% accuracy in clinical studies compared to DNA sequencing in our Cystic Fibrosis Genotyping Test, our Warfarin Sensitivity Test and our Thrombophilia Risk Test. Our XT-8 system limits technician contact with a patient sample, thereby reducing contamination risk. It also provides clear reports, minimizing the risk of human error and increasing the repeatability of test results. |
| • | Enhanced Laboratory Work Flow. Our unique platform allows for random access, or the ability to initiate tests while other tests are in progress, resulting in a highly convenient and flexible workflow. Our XT-8 system provides random access for up to 24 independent test cartridges. In addition, our proprietary electrochemical detection technology streamlines the sample preparation process and eliminates the need for the additional washing steps required by some other detection methods, such as optical or fluorescent detection. Laboratories using our XT-8 system can expect to obtain test |
5
Table of Contents
| results within 30 minutes of receipt of the amplified DNA sample, resulting in a total turnaround time of generally under four hours. |
| • | Multiplex Capability. Our XT-8 system can detect up to 72 separate biomarkers in a single test cartridge. This allows laboratories to run multiple tests or panels on an individual patient sample in a one-step detection process. This capability reduces the time required for a laboratory to perform a diagnostic analysis that involves multiple genetic markers or infectious disease pathogens, which otherwise would require the laboratory to run multiple, separate molecular diagnostic tests. |
Prior to performing a test, a laboratory technician takes isolated DNA from the patient sample and performs an automated nucleic acid extraction and amplification step with materials supplied with our test cartridge. In some cases, the technician also performs a routine enzymatic treatment before adding our proprietary signal probes and transferring the solution into the sample compartment in our test cartridge. The technician enters sample identification and reagent information into our XT-8 system using the supplied bar code wand or on-screen keyboard and inserts the test cartridge into an open slot on the analyzer. The on-board computer automatically assimilates input information and test cartridge information from the memory chip on the test cartridge and initiates the specified test protocol. The testing process takes under four hours to complete, and the test results can be viewed on the built-in touch screen monitor 30 minutes after the insertion of test cartridges into the XT-8. Test results can also be printed out or reported through the laboratory’s computer information system.
The key features of our XT-8 system include:
| Key Features |
Characteristics | |
| Fast Turnaround | 30 minutes to result from amplified DNA sample with minimal technician time needed | |
| Accurate Results | Our Cystic Fibrosis Genotyping Test, our Warfarin Sensitivity Test and our Thrombophilia Risk Test demonstrated 100% accuracy in clinical studies compared to DNA sequencing | |
| Ease of Use | Intuitive touch-screen interface and clear reports | |
| Small Footprint | Approximately 16 inches in width and depth in its standard configuration | |
| Random Access | Each of up to 24 test cartridge slots can be accessed independently | |
| Minimal Maintenance | No routine maintenance or calibration required | |
| Multiplex Capability | Detects up to 72 distinct biomarkers in a single sample |
Our Test Menu
We have developed six diagnostic tests for use with our XT-8 system, three of which have received clearance from the FDA and one of which has been submitted to the FDA for 510(k) clearance. During the fiscal year ended December 31, 2011, sales of our Cystic Fibrosis Genotyping Test represented approximately 25% of our revenues, and sales of our Thrombophilia Risk Test represented approximately 21% of our revenues.
Cystic Fibrosis Genotyping. Our Cystic Fibrosis Genotyping Test is a multiplex genotyping test that detects a panel of mutations associated with cystic fibrosis based on guidelines published
6
Table of Contents
by the American College of Medical Genetics and the American College of Obstetricians and Gynecologists for screening of adult couples contemplating pregnancy. Our Cystic Fibrosis Genotyping Test demonstrated 100% accuracy in clinical studies as compared to DNA sequencing and delivers results within 30 minutes of receipt of the amplified DNA sample. Test results are summarized in an easy-to-interpret report that includes a summary “carrier” or “non-carrier” determination as well as individual carrier status for each of the 23 recommended markers. Our Cystic Fibrosis Genotyping Test received FDA clearance in July 2009.
Our Cystic Fibrosis Genotyping Test addresses a market that was estimated in 2009 at over $70 million in the United States alone. More than 10 million Americans are carriers of one mutation of the cystic fibrosis gene. The American College of Obstetricians and Gynecologists suggests that all couples who are considering having a child, or those who are expecting a child, should have genetic carrier testing for cystic fibrosis. Much of current cystic fibrosis testing is performed by national reference laboratories. With the availability of highly accurate, easy to use cystic fibrosis tests, we expect that the market will continue to decentralize through regional reference laboratories and hospitals now capable of offering this test.
Warfarin Sensitivity. Our Warfarin Sensitivity Test is a multiplex pharmacogenetic test for the detection of three genetic markers that are known to play a critical role in metabolism of, and sensitivity to, warfarin. Warfarin, offered under the brand name Coumadin, is the most widely prescribed oral anticoagulant in North America and Europe and is used to prevent heart attacks, strokes, and blood clots in veins, arteries and lungs. Through detection of an individual’s sensitivity to warfarin, doctors are better able to accurately and efficiently determine the appropriate warfarin dosage level on an individual patient basis. Our Warfarin Sensitivity Test demonstrated 100% accuracy in clinical studies compared to DNA sequencing and delivers results within 30 minutes of receipt of the amplified DNA sample. Our Warfarin Sensitivity Test received FDA clearance in July 2008.
Thrombophilia Risk. Thrombophilia is a condition where a person’s blood clots easily or excessively placing them at risk of developing clots. Thrombophilia is a particular concern for high risk patients, including patients who are pregnant or undergoing certain surgeries. Our Thrombophilia Risk Test is a multiplex test for the detection of four common inherited genetic risk factors of thrombophilia: Factor V Leiden, Factor II prothrombin and two genetic markers in the methylenetetrahydrofolate reductase (MTHFR) gene. Our Thrombophilia Risk Test demonstrated 100% accuracy in clinical studies compared to DNA sequencing and delivers results within 30 minutes of receipt of the amplified DNA sample. Our Thrombophilia Risk Test received FDA clearance in April 2010.
Thrombophilia is one of the most common types of blood coagulation disorders affecting 1 in 1,000 individuals. We believe the U.S. market is approximately $55 million based on statistics provided by Kalorama Information 2009, a market research firm.
Respiratory Viral Panel (RVP). Our Respiratory Viral Panel Test, currently labeled for RUO, covers approximately 20 viruses, including influenza A (H1N1 and seasonal), influenza B, respiratory syncytial virus, or RSV, and numerous other upper respiratory viruses. We submitted it for FDA clearance in the fourth quarter of 2011. We expect to obtain FDA clearance on our Respiratory Viral Panel Test in 2012.
7
Table of Contents
Respiratory pathogens are a major source of illness and can lead to hospitalizations and death. According to the Centers for Disease Control, each year in the United States on average, 5% to 20% of the population gets the flu; more than 200,000 people are hospitalized from flu-related complications; and about 36,000 people die from flu-related causes. RSV is the most common cause of bronchitis and pneumonia in infants and young children, with up to 125,000 children hospitalized annually in the United States. The challenge to the physician assessing a patient with a respiratory illness is determining the underlying cause so that an effective treatment plan can be determined.
2C19 Genotyping Test (2C19). Our 2C19 Genotyping Test, currently labeled for RUO, is a multiplex test for the detection and genotyping of the *2, *3, *4, *5, *6, *7, *8, *9, *10, *13 and *17 alleles of the cytochrome P450 (CYP450) 2C19 gene locus. 2C19 is a member of the cytochrome P450 mixed-function oxidase system and is involved in the metabolism of several important groups of drugs including many proton pump inhibitors and anticonvulsants.
Hepatitis C Virus Genotyping (HCVg). Our HCV Genotyping Test, currently labeled for RUO, is a multiplex test for the detection and typing/subtyping of HCV 1a, 1b, 2a/c, 2b, 3, 4, 5, 6a/b. According to the Centers for Disease Control, Hepatitis C virus (HCV) infection is the most common chronic blood-borne infection in the United States with over 3.0 million persons considered chronically infected. According to the World Health Organization, it is estimated that some 130–170 million people are chronically infected with HCV globally and at risk of developing liver cirrhosis and/or liver cancer, and more than 350,000 people die from HCV-related liver diseases each year. An article recently published in the Annals of Internal Medicine found that, in the US, HCV is cited as the cause of death more than HIV. Based on the current treatment guidelines for HCV, a patient’s genotype is a component of selecting the proper treatment strategy as well as a predictor of the likelihood of treatment success.
Our Tests in Development and Design
We have a pipeline of potential products in various stages of development or design. We consider our diagnostic tests to be in the design phase once they have advanced beyond the conceptual stage. We perform market research, clinical publication reviews, customer interviews, technical feasibility and freedom to operate assessments to determine if a potential diagnostics test is a viable product candidate. We believe that all of our tests in the design stage have viable market potential and are technically feasible to develop using our eSensor® technology. While we do not currently license biomarkers for all products in the design phase, we believe we will be able to obtain such licenses, if needed, on commercially reasonable terms.
We intend to introduce two to four new tests annually. We select these tests based upon what we believe are clinically relevant products which address unmet market needs. Laboratories using our XT-8 system will be able to run the additional tests we offer without any additional capital investment or operator training. We are currently developing or designing the following diagnostic tests:
Infectious Disease Test Panels. The infectious disease diagnostics market is estimated to reach over $6 billion in the United States by 2012, with substantial growth expected in the
8
Table of Contents
molecular diagnostic segment. We are currently designing other infectious disease test panels that would align strategically with our existing respiratory viral panel test offering by leveraging our current and future XT-8 system placements in the acute care setting. The test panels we are designing fit into two categories: Genotyping tests for viruses such as hepatitis C virus (HCV) and human papillomavirus (HPV) or detection tests for panels of viruses, bacteria or fungi such as central nervous system infections or lower respiratory tract infections. Genotyping tests are run throughout the year whereas many detection tests have a seasonal component. In order to maximize the value of systems installed for infectious disease tests like our RVP product, we intend to develop a broad range of detection assays which have distinctly different seasonal peaks in prevalence to allow our customers to utilize our system for infectious disease testing throughout the year. Currently, several infectious disease panels and genotyping tests are in the design concept stage. These include: Bacterial Respiratory Tract Infections; Central Nervous System Infections (CNS); and Gastrointestinal Pathogens.
KRAS Mutation. Anti-EGFR therapy is a type of cancer treatment that interferes with the growth of cancer cells, slowing their growth and subsequent spread in the body. Anti-EGFR therapy is currently approved by the FDA to treat colorectal cancer as well as head and neck cancer. Scientific studies have demonstrated that patients whose tumors have genetic variations in the KRAS gene will not respond to anti-EGFR therapy. Currently approved anti-EGFR therapies are marketed under the brand names Erbitux and Vectibix. These therapies are approved for use in colorectal cancer and more recently head and neck cancer in the case of Erbitux.
According to the American Cancer Society’s website, there are over one million new cases of colorectal cancer globally each year with approximately 140,000 cases in the United States alone. We are currently developing a multiplex KRAS test that detects a panel of common genetic markers in the KRAS gene. The FDA requires KRAS testing on the labels of the two approved anti-EGFR antibody therapeutics, Vectibix and Erbitux, for use in colorectal cancer.
Oncology and Personalized Medicine Tests. Given the trend in oncology towards tailoring treatment to an individual’s tumor type and the emerging interest in personalized medicine, we are currently researching and evaluating the development of test panels in these areas. Expanding our product offering into these two areas would align strategically with our existing products as well as development stage products by leveraging our current and future XT-8 system placements in these laboratories.
Our NexGen System
We are developing our NexGen system to provide a complete sample to answer solution for our customers. The NexGen system will retain all the customer benefits of our XT-8 system while also integrating automated nucleic acid extraction and amplification. These features will eliminate the need for time consuming and complex sample preparation steps and allow technicians to place a patient sample into our test cartridge. We have already demonstrated feasibility of direct sample to answer on a NexGen system prototype using whole blood. We believe this advancement will make our eSensor® technology attractive to the broad range of institutions that currently lack the technical or economic resources to perform molecular diagnostic testing. We believe the NexGen system may expand our target user base from 1,000 to over 5,000 potential laboratories and hospitals in the United States.
9
Table of Contents
We believe our approach to a sample to answer system will achieve benefits over other competitive multiplex systems, which require extensive sample processing procedures in addition to other complex sample manipulations throughout their test process.
Our Technology
Our eSensor® Technology
Our proprietary eSensor® technology is based on the principles of competitive DNA hybridization and electrochemical detection. DNA naturally forms a double-stranded structure, with each strand binding with high affinity, or hybridizing, only to a complementary strand. Our technology takes advantage of this highly specific binding by first creating two types of single-stranded DNA, the capture probe and the signal probe. The capture probe and signal probe are each complementary to a different segment of the target DNA, or biomarker, that is a focus of the diagnostic test. Using our proprietary technology and processes, we attach our capture probes to a proprietary monolayer on the surface of a gold electrode within our proprietary test cartridge. We separately attach ferrocene, an electrochemically active label, to our signal probes.
Before placing the sample into our test cartridge, the technician mixes the amplified DNA sample with our signal probe. If the target biomarker is present in the prepared patient sample, a segment of the biomarker DNA will hybridize with a solution containing our signal probe. This solution is then run past an electrode, against which our capture probes have been immobilized. The as-yet unbound segment of the target biomarker binds to our capture probe, creating a target DNA, signal probe, capture probe complex at the surface of the electrode. This complex produces an electrochemical signal analyzed and interpreted by the XT-8 system. Our test cartridges currently have 72 distinct electrodes, each of which can be configured to detect a different target biomarker, enabling multiplex testing.
Our eSensor® technology is highly specific for the target biomarker, and is not based on optical or fluorescent detection. As a result, our diagnostic tests are less prone to sample contamination risk and do not require many of the time-consuming washing and preparation steps required by competing technologies. The only sample preparation step required before using our test cartridges is a polymerase chain reaction, or PCR amplification, which involves amplifying, or generating billions of copies of, the target DNA molecules, followed by transfer of the sample to our test cartridge and insertion of the test cartridge into any open slot in our XT-8 system. In some tests, amplified DNA is subject to an additional enzymatic treatment to produce a single-stranded-DNA.
Our Test Cartridges. Our test cartridges are self-contained devices specifically programmed and configured for a given diagnostic test. Each test cartridge includes a sample compartment and a plastic cover that forms a hybridization chamber. The test cartridge is fitted with a diaphragm pump and valves that circulate the hybridization solution, including the signal probe and prepared patient sample, when inserted into the XT-8 system. The test cartridge also includes a printed circuit board chip consisting of an array of 72 gold-plated working electrodes, a silver/silver chloride reference electrode, and two gold-plated auxiliary electrodes. Each electrode is customized with a proprietary monolayer that immobilizes the DNA capture probes specific for
10
Table of Contents
each target of a test panel. The test cartridge also contains an electrically erasable programmable read-only memory component that stores information related to the cartridge such as assay identifier, cartridge lot number and expiration date.
Our XT-8 Workstation. Our XT-8 system is a multiplex workstation that has a modular design consisting of an integrated touch screen workstation and up to three analyzers. Each analyzer contains eight modules into which individual test cartridges are placed. The test cartridge slots operate independently of each other allowing up to 24 independent test cartridges to be loaded at one time, with the remaining slots available for use at any future time while the system is running. Each slot contains a test cartridge connector, a precision-controlled heater, an air pump and electronics. The air pumps drive the diaphragm pump and valve system in the test cartridge, eliminating fluid contact between the system and the cartridge. The pneumatic pumping enables recirculation of the hybridization solution allowing the target DNA and the signal probes to efficiently hybridize with the complementary capture probes on the electrodes. The diaphragm pump in the test cartridge is connected to a pneumatic source from the XT-8 system and provides unidirectional pumping of the hybridization mixture through the cartridge during hybridization.
The touch screen workstation controls each analyzer, provides power and analyzes and stores data. Technicians can load patient identification numbers and reagent lot codes by the included bar code scanner, the touch screen or uploading a text file from a USB memory stick.
Advantages of Our eSensor® Electrochemical Signal Detection
We believe our proprietary electrochemical signal detection technology has several advantages over other signal detection platforms:
| • | Robust Signal. Our capture probes are highly target specific, reducing the binding of non-target DNA and, thereby, largely eliminating interference from other components in a patient’s sample, such as blood, saliva or urine. Similarly, constituents of blood that would normally interfere with fluorescence detection, such as hemoglobin or bilirubin, have no effect on the processed electronic signals produced by our eSensor® technology. This robust functionality will, we believe, facilitate the development of integrated amplification and sample to answer systems for blood and other sample types. |
| • | High Sensitivity and Accuracy. Our eSensor® technology is highly sensitive in the detection of nucleic acids. Each electrode can routinely detect approximately 1 nanomolar of target DNA, and a sensitivity of 10 picomolar of target DNA has been achieved. Such concentrations are readily produced from patient samples using several commercially-validated amplification technologies such as PCR. Our eSensor® technology has demonstrated 100% accuracy in our Cystic Fibrosis Genotyping Test, our Warfarin Sensitivity Test and our Thrombophilia Risk Test clinical studies compared to DNA sequencing. |
| • | Streamlined Sample Preparation. Our technology directly detects the target DNA sequence with highly specific signal probes and electrode-bound capture probes. As a result, our test samples do not require many of the washing steps typically required to remove unbound target DNA and labels. We believe that our eSensor® technology can |
11
Table of Contents
| minimize sample preparation requirements. We have already demonstrated direct PCR-based genotyping from diluted whole blood without the need for DNA sample preparation or washing out of interfering substances. |
| • | Efficient Multiplexing. Each of the 72 electrodes in our test cartridge configuration acts independently of the others and produces a comprehensive and informative signal. For example, a single eSensor® electrode can measure the presence or absence of control DNA, which we use for quality control, and simultaneously indicate whether a patient sample contains zero, one or two copies of a particular sequence, corresponding to mutant, heterozygous or wild type genotypes. As a result, our eSensor® technology eliminates the need for redundancy and the averaging of multiple measurements commonly required by competing technologies. |
| • | Small Footprint with Low Maintenance. Our eSensor® technology enables users to perform hybridization and detection in a low-cost system with relatively few moving parts. In contrast, conventional microarray systems require robotic instrumentation to automate multistage fluidic handling processes. As a result, these systems are often bulky, complicated and expensive and require frequent calibration and maintenance. Our XT-8 system, for example, requires no calibration and virtually no maintenance and is self-contained in a small footprint of approximately 16-by-16 inches in its standard configuration. |
| • | Cost-Effective Development. The use of electrochemical technology allows our XT-8 system to leverage third-party advances in microelectronics such as miniaturization and manufacturing efficiencies. Many electronic components associated with our core processes are produced in large volumes at low cost and size for use in numerous fields including automotive, aerospace, information technology and medical devices. By avoiding the use of fluidic handling and optical or fluorescent detection, we believe our eSensor® technology can be applied at low cost to numerous testing environments in addition to our current target markets, including field testing and point-of-care applications. |
| • | Straightforward Development of New Tests. Our eSensor® technology is highly flexible, and we believe the main design consideration in developing new diagnostic tests for our XT-8 system is our ability to access and synthesize the appropriate capture and signal probes. Our versatile platform allows us to add new diagnostic tests to our menu or to add new content to existing diagnostic tests without modifying the XT-8 system. This ease of assay development and our versatile platform allows us to focus our research and development resources on developing new commercial test products. |
| • | Functionality Outside of Molecular Diagnostics. Our eSensor® technology has broad applicability to detect a range of biomolecules. Independently, and through collaborative research with university and industry partners, we have demonstrated eSensor® detection of proteins and small molecule drugs. This versatility opens the possibility of developing mixed analyte sensors, such as tests that can detect antibodies to a certain pathogen plus the pathogen itself, or genetic variations in drug metabolism plus monitoring of the drug level itself. |
12
Table of Contents
Research and Development
As of December 31, 2011, we had 24 employees focused on research and development. Our research and development expenditures were approximately $8.7 million, $6.6 million and $5.6 million for the years ended December 31, 2011, 2010 and 2009, respectively. The increase in research and development expenses from 2010 to 2011 was primarily due to clinical trial costs incurred for potential product offerings as well as increased staffing and intellectual property-related expenses.
In addition to expanding the diagnostic test menu for our XT-8 system and developing our NexGen system, our research and development team is focused on the following initiatives:
| • | Improving the Clinical and Practical Utility of our Tests. An important role of our research and development team is to help establish the clinical utility and value of our molecular diagnostic tests. We have and intend to continue to partner with academic and reference laboratories to perform validation and clinical studies on our tests. Key aspects of our efforts are aimed at improving workflow in the laboratory setting, positively comparing our tests to historical or “gold standard” tests and demonstrating that our tests can help improve patient care and lower diagnostic and medical treatment costs. We intend to publish the results from these clinical studies in peer-reviewed or trade journals, submit them to regulatory bodies and present them at industry conferences in support of our commercialization strategy. |
| • | Developing New Test Capabilities. We are developing capabilities for utilizing our eSensor® technology in protein and small molecule detection, both independently and through research collaborations. These capabilities may enhance our future menu offerings or provide us with out-licensing opportunities. We are also exploring direct gene expression analysis opportunities through collaboration with oncology specialists in industry and academia. These opportunities may allow us to develop quantitative tests that are competitive with the “gold standard” real-time PCR tests but that are simple to perform in a multiplex manner with our XT-8 system. |
Manufacturing
We manufacture our proprietary test cartridges and ancillary reagents at our approximately 31,000 square foot headquarters in Carlsbad. Our reagent formulation, test cartridge manufacturing and packaging of final components and cartridges are performed by us in accordance with applicable guidelines for medical device manufacturing. We outsource manufacturing of our XT-8 system, as well as the oligonucleotide raw materials and much of the disposable component molding and sub-component assembly for our test cartridges. In particular, our XT-8 system is manufactured by a single source supplier that specializes in contract design and manufacturing of electronic and electromechanical devices for medical use. We believe we can secure other suppliers on commercially reasonable terms for the products and parts we outsource.
We have implemented a quality management system designed to comply with FDA regulations and ISO standards governing diagnostic medical device products. These regulations carefully control the design, manufacture, testing and release of diagnostics products as well as
13
Table of Contents
raw material receipt and control. We also have controlled methods for the consistent manufacturing of our proprietary test cartridges and reagents at our facilities. All key outsourcing partners are generally ISO-certified to help assure a continual supply of high quality components.
We plan to continue to manufacture components that we determine are highly proprietary or highly custom to produce, while outsourcing more commodity-like components. We are likely to establish additional outsourcing partnerships as we manufacture additional products. We recently signed a lease amendment to increase our office and manufacturing facilities to 53,000 square feet and believe these facilities will be adequate to meet our current and future manufacturing needs.
Sales and Marketing
Our sales and marketing strategy is to expand the installed base and utilization of the XT-8 platform and consumables. Our products are sold in the United States through a geographically dispersed direct sales and technical specialist service organization. They are supported by a centralized team of Product Managers, Marketing, Customer and Technical Support personnel.
Our sales representatives typically have experience in molecular diagnostics and a network of laboratory contacts within their respective territories. We utilize our representatives’ knowledge along with market research databases to target and qualify our customers. We execute a variety of sales campaigns and strategies to meet the buying criteria of the different customer segments we serve. To support our expanding molecular test menu, growth in our customer base and launch plans of our next generation detection platform, we continue to make investments in these customer facing organizations.
We believe the XT-8 platform competes largely on the basis of improved performance and reliability, ease of use and streamlined laboratory workflow, a high value in vitro diagnostic, or IVD, menu with multiplexing capabilities, and a superior return on investment. These and other advantages conferred by our chemistry are enabling us to provide clinicians and researchers with superior molecular solutions. Our sales cycle typically includes customer evaluations and validations of our products. Upon successful validation, a customer can acquire our XT-8 system and consumables in the following ways:
| • | Reagent Rental: The reagent rental agreement requires a customer commitment to purchase a minimum number of cartridges over the term of the agreement, and a portion of the charge for each cartridge is a rental fee for the equipment. Our reagent rental agreements do not typically provide for any cancellation rights by the customer. |
| • | Capital Purchase: The XT-8 system is paid for upfront, and in its entirety, by the customer. Customers are also eligible to receive structured pricing incentives if they enter into an optional annual minimum cartridge commitment. |
In 2012, we anticipate commencing planning for commercialization of our molecular diagnostic products in Europe and other international markets. We anticipate our sales and marketing strategy will involve a select network of partners and distributors. A distribution strategy will likely be developed for each relevant international market. It is expected that we will augment this effort with a team of our specialists who will enable our partners’ sales forces and
14
Table of Contents
provide technical support. We also intend to explore opportunities to leverage our intellectual property position in molecular diagnostics through licensing or the establishment of partnerships.
Customers
In 2011 and 2010, 33% and 28% of our revenues, respectively, were attributed to our three largest customers during the year. In 2011, one customer, Natural Molecular Testing Corporation, accounted for approximately 20% of our total revenues and in 2010 one customer accounted for 12% of our total revenues.
Placements are defined in terms of the number of analyzers sold to or placed with a customer, reflecting a direct correlation between the reagent test revenue opportunity and the number of test cartridges that can analyzed at any one time. As of December 31, 2011, there were 167 analyzers at 103 unique customer sites, or approximately 1.6 analyzers per customer. This compares with 82 analyzers at 67 unique customer sites, or approximately 1.2 analyzers per customer as of December 31, 2010.
The increase in analyzers and related revenue is due to an increase in the number of new customers buying our products and growth in the sale of consumables to existing customers. We expect our clinical molecular diagnostic revenues to continue to increase in 2012.
Competition
We primarily face competition in the molecular diagnostic testing markets with testing products and systems developed by public and private companies such as Cepheid, Gen-Probe, Inc., Siemens, Hologic, Inc., Innogenetics, Inc, Luminex Corporation, Nanosphere, Inc., Qiagen NV, Roche Diagnostics, a division of F. Hoffmann-La Roche Ltd., Idaho Technologies and Abbott Diagnostics, a division of Abbott Laboratories. Our diagnostic tests also face competition with the laboratory developed tests developed by national and regional reference laboratories and hospitals. We believe that the XT-8 system competes largely on the basis of accuracy and reliability, enhanced laboratory workflow, multiplex capability, ease-of-use and return on investment for customers.
Many of our competitors have substantially greater financial, technical, research and other resources and larger, more established marketing, sales and distribution organizations than we do. Many of our competitors also offer broader product lines and have greater brand recognition than we do. Moreover, our existing and new competitors may make rapid technological developments that may result in our technologies and products becoming obsolete before we recover the expenses incurred to develop them or before they generate significant revenue.
Intellectual Property
To establish and protect our proprietary technologies and products, we rely on a combination of our patents, copyrights, trademarks, and trade-secrets, as well as other intellectual property rights in our technology and business information. Our intellectual property portfolio for our core electrochemical technology was initially built through the combination of our acquisition of the Clinical Micro Sensors business from Motorola and licensing patents from third parties, including the California Institute of Technology and Harvard University.
15
Table of Contents
We believe that our patent portfolio, including over 100 issued U.S. and foreign patents and numerous pending applications, provides us with a robust protection of our electrochemical detection techniques, chemical insulators and attachment points on electrode surfaces and other technology that, collectively, form the staple of our eSensor® platform. We continue to pursue the issuance of new patents to protect our ongoing research, development and commercial activities. In general, patents have a term of 20 years from the application filing date or earlier claimed priority date. Our issued and exclusively licensed patents will expire between 2013 and 2021 or later, with several of our pending applications having the potential to mature into patents that might expire in 2027, 2028 and 2029. Our success depends to a significant degree upon our ability to police infringement, derive licensing revenues and continue to develop proprietary products and technologies without infringing on the intellectual property rights of others.
We also rely in part on trade-secret protection of our intellectual property. We attempt to protect our trade secrets by entering into confidentiality agreements with third parties, employees and consultants. Our employees and consultants also sign agreements requiring that they assign to us their interests in intellectual property such as patents and copyrights arising from their work for us. All employees sign an agreement not to compete unfairly with us during their employment and upon termination of their employment through the misuse of confidential information, soliciting employees and soliciting customers.
We also have filed for registration, or obtained registration, in the U.S. and other countries for marks used with our products and technology. Our trademarks registered in the U.S. include eSensor® and GenMark®. Trademark protection continues in some countries for as long as the mark is used and, in other countries, for as long as it is registered. Registrations generally are for fixed, but renewable, terms.
Government Regulation
The design, development, manufacture, testing and sale of our diagnostic products are subject to regulation by numerous governmental authorities, principally the FDA, and corresponding state and foreign regulatory agencies.
Regulation by the FDA
In the United States, the Federal Food, Drug, and Cosmetic Act, or FDCA, FDA regulations and other federal and state statutes and regulations govern, among other things, medical device design and development, preclinical and clinical testing, premarket clearance or approval, registration and listing, manufacturing, labeling, storage, advertising and promotion, sales and distribution, export and import, and post-market surveillance. The FDA regulates the design, manufacturing, servicing, sale and distribution of medical devices, including molecular diagnostic test kits and instrumentation systems. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
16
Table of Contents
Unless an exemption applies, each medical device we wish to distribute commercially in the United States will require marketing authorization from the FDA prior to distribution. The two primary types of FDA marketing authorization applicable to a device are premarket notification, also called 510(k) clearance, and premarket approval, also called PMA approval. The type of marketing authorization is generally linked to the classification of the device. The FDA classifies medical devices into one of three classes (Class I, II or III) based on the degree of risk the FDA determines to be associated with a device and the level of regulatory control deemed necessary to ensure the device’s safety and effectiveness. Devices requiring fewer controls because they are deemed to pose lower risk are placed in Class I or II. Class I devices are deemed to pose the least risk and are subject only to general controls applicable to all devices, such as requirements for device labeling, premarket notification and adherence to the FDA’s current Good Manufacturing Practices, or cGMP, and Quality System Requirements, as reflected in its QSR. Class II devices are intermediate risk devices that are subject to general controls and may also be subject to special controls such as performance standards, product-specific guidance documents, special labeling requirements, patient registries or postmarket surveillance. Class III devices are those for which insufficient information exists to assure safety and effectiveness solely through general or special controls and include life-sustaining, life-supporting or implantable devices, devices of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury.
Most Class I devices and some Class II devices are exempted by regulation from the 510(k) clearance requirement and can be marketed without prior authorization from the FDA. Some Class I devices that have not been so exempted and Class II devices are eligible for marketing through the 510(k) clearance pathway. By contrast, devices placed in Class III generally require PMA approval or 510(k) de novo clearance prior to commercial marketing. The PMA approval process is more stringent, time-consuming and expensive than the 510(k) clearance process, however, the 510(k) clearance process has also become increasingly stringent and expensive. The FDA has cleared our XT-8 system with our eSensor® Warfarin Sensitivity Test, Cystic Fibrosis Genotyping Test and Thrombophilia Risk Test as Class II devices via the 510(k) clearance process.
510(k) Clearance. To obtain 510(k) clearance for a medical device, an applicant must submit a premarket notification to the FDA demonstrating that the device is “substantially equivalent” to a device legally marketed in the United States that is not subject to PMA approval, commonly known as the “predicate device.” A device is substantially equivalent if, with respect to the predicate device, it has the same intended use and has either (i) the same technological characteristics or (ii) different technological characteristics and the information submitted demonstrates that the device is as safe and effective as a legally marketed device and does not raise different questions of safety or effectiveness. A showing of substantial equivalence sometimes, but not always, requires clinical data. Generally, the 510(k) clearance process can exceed 90 days and may extend to a year or more.
After a device has received 510(k) clearance for a specific intended use, any change or modification that significantly affects its safety or effectiveness, such as a significant change in the design, materials, method of manufacture or intended use, may require a new 510(k) clearance or PMA approval and payment of an FDA user fee. The determination as to whether or not a modification could significantly affect the device’s safety or effectiveness is initially left to the
17
Table of Contents
manufacturer using available FDA guidance; however, the FDA may review this determination to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA approval is obtained. The manufacturer may also be subject to significant regulatory fines or penalties.
Before we can submit a medical device for 510(k) clearance, we may have to perform a series of generally short studies over a period of months, including method comparison, reproducibility, interference and stability studies to ensure that users can perform the test successfully. Some of these studies may take place in clinical environments, but are not usually considered clinical trials. For PMA submissions, we would generally be required to conduct a longer clinical trial over a period of years that supports the clinical utility of the device and how the device will be used.
Although clinical investigations of most devices are subject to the investigational device exemption, or IDE, requirements, clinical investigations of molecular diagnostic tests, including our products and products under development, are generally exempt from the IDE requirements. Thus, clinical investigations by intended users for intended uses of our products generally do not require the FDA’s prior approval, provided the clinical evaluation testing is non-invasive, does not require an invasive sampling procedure that presents a significant risk, does not intentionally introduce energy into the subject and is not used as a diagnostic procedure without confirmation by another medically established test or procedure. In addition, our products must be labeled per FDA regulations “for research use only-RUO” or “for investigational use only-IUO,” and distribution controls must be established to assure that our products distributed for research, method comparisons or clinical evaluation studies are used only for those purposes.
PMA Approval. A PMA application requires the payment of significant user fees. PMA applications must be supported by valid scientific evidence, which typically requires extensive data, including technical, preclinical, clinical and manufacturing data, to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device. A PMA application must also include, among other things, a complete description of the device and its components, a detailed description of the methods, facilities and controls used to manufacture the device, and proposed labeling.
The FDA has 45 days from its receipt of a PMA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. During this review period, the FDA may request additional information or clarification of information already provided. In addition, the FDA will conduct a pre-approval inspection of the manufacturing facility or facilities to ensure compliance with the QSR, which requires manufacturers to follow design, testing, control, documentation and other quality assurance procedures.
18
Table of Contents
FDA review of an initial PMA application is required by statute to take between six to ten months, although the process typically takes significantly longer, and may require several years to complete. The FDA can delay, limit or deny approval of a PMA application for many reasons, including:
| • | it is not demonstrated that there is reasonable assurance that the device is safe or effective under the conditions of use prescribed, recommended, or suggested in the proposed labeling; |
| • | the data from preclinical studies and clinical trials may be insufficient to support approval; and |
| • | the manufacturing process, methods, controls or facilities used for the manufacture, processing, packing or installation of the device do not meet applicable requirements. |
If the FDA evaluations of both the PMA application and the manufacturing facilities are favorable, the FDA will either issue an approval letter or an approvable letter, which usually contains a number of conditions that must be met in order to secure final approval of the PMA. If the FDA’s evaluation of the PMA or manufacturing facilities is not favorable, the FDA will deny approval of the PMA or issue a not approvable letter. A not approvable letter will outline the deficiencies in the application and, where practical, will identify what is necessary to make the PMA approvable. The FDA may also determine that additional clinical trials are necessary, in which case the PMA approval may be delayed for several months or years while the trials are conducted and then the data submitted in an amendment to the PMA. Once granted, PMA approval may be withdrawn by the FDA if compliance with post approval requirements, conditions of approval or other regulatory standards is not maintained or problems are identified following initial marketing.
Approval by the FDA of new PMA applications or PMA supplements may be required for modifications to the manufacturing process, labeling, device specifications, materials or design of a device that is approved through the PMA process. PMA supplements often require submission of the same type of information as an initial PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA application and may not require as extensive clinical data or the convening of an advisory panel.
Regulation after FDA Clearance or Approval. Any devices we manufacture or distribute pursuant to clearance or approval by the FDA are subject to pervasive and continuing regulation by the FDA and certain state agencies. We are required to adhere to applicable regulations setting forth detailed cGMP requirements, as set forth in the QSR, which include, among other things, testing, control and documentation requirements. Non-compliance with these standards can result in, among other things, fines, injunctions, civil penalties, recalls or seizures of products, total or partial suspension of production, refusal of the government to grant 510(k) clearance or PMA approval of devices, withdrawal of marketing approvals and criminal prosecutions. We have designed and implemented our manufacturing facilities under the FDA’s cGMP requirements.
Because we are a manufacturer of medical devices, we must also comply with medical device reporting requirements by reviewing and reporting to the FDA whenever there is evidence that
19
Table of Contents
reasonably suggests that one of our products may have caused or contributed to a death or serious injury. We must also report any incident in which our product has malfunctioned if that malfunction would likely cause or contribute to a death or serious injury if it were to recur. Labeling and promotional activities are subject to scrutiny by the FDA and, in certain circumstances, by the Federal Trade Commission. Medical devices approved or cleared by the FDA may not be promoted for unapproved or uncleared uses, otherwise known as “off-label” promotion. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability, including substantial monetary penalties and criminal prosecution.
Environmental Regulations. We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances. Some of these laws require us to obtain licenses or permits to conduct our operations. We have numerous policies and procedures in place to ensure compliance with these laws and to minimize the risk of occupational exposure to hazardous materials. We do not expect the operations of our products to produce significant quantities of hazardous or toxic waste or radiation that would require use of extraordinary disposal practices. Although the costs to comply with these applicable laws and regulations have not been material, we cannot predict the impact on our business of new or amended laws or regulations or any changes in the way existing and future laws and regulations are interpreted or enforced, nor can we ensure we will be able to obtain or maintain any required licenses or permits.
Export of Our Products. Export of products subject to the 510(k) notification requirements, but not yet cleared to market, is permitted with FDA authorization provided certain requirements are met. Unapproved products subject to the PMA approval requirements may be exported if the exporting company and the device meet certain criteria, including, among other things, that the device complies with the laws of the receiving country and the company submits a “Simple Notification” to the FDA when the company begins to export. If the company or device does not comply with such criteria, FDA approval must be obtained for export. To obtain FDA export approval, if required, we must meet certain requirements, including, among other things and with some exceptions, documentation demonstrating that the product is approved for import into the country to which it is to be exported and, in some instances, safety data to demonstrate that export of the device will not be contrary to the public health or safety.
Clinical Laboratory Improvement Amendments of 1988. The use of our products is also affected by the Clinical Laboratory Improvement Amendments of 1988, or CLIA, and related federal and state regulations, which provide for regulation of laboratory testing. Any customers using our products for clinical use in the United States will be regulated under CLIA, which is intended to ensure the quality and reliability of laboratory testing in the United States. In particular, these regulations mandate that clinical laboratories must be certified by the federal government or a federally approved accreditation agency, or must be located in a state that has been deemed exempt from CLIA requirements because the state has in effect laws that provide for requirements equal to or more stringent than CLIA requirements. Moreover, these laboratories must meet quality assurance, quality control and personnel standards, and they must undergo
20
Table of Contents
proficiency testing and inspections. The CLIA standards applicable to clinical laboratories are based on the complexity of the method of testing performed by the laboratory, which range from “waived” to “moderate complexity” to “high complexity.” We expect that most of our products will be categorized as “high complexity,” since most molecular diagnostic tests are currently FDA-cleared as CLIA “high complexity” devices.
Other Legislation. On September 27, 2007, the President signed the Food and Drug Administration Amendments Act of 2007, or FDAAA. Among other significant changes and requirements it imposes, the new legislation expands the federal government’s clinical trial registry and results databank maintained by the National Institiute of Health, or NIH, to include all (with limited exceptions) medical device trials. In particular, it requires certain information about device trials, including a description of the trial, participation criteria, location of trial sites, and contact information, to be sent to NIH for inclusion in a publicly accessible database. In addition, the results of clinical trials that form the primary basis for efficacy claims or are conducted after a device is approved or cleared must be posted to the results databank. Under the FDAAA, companies that violate these and other provisions of the new law are subject to substantial civil monetary penalties.
Foreign Government Regulation. We intend to market our products in European and other selected international markets. Before doing so, we or our partners and distributors will need to receive regulatory approval. The regulatory review process for medical devices varies from country to country, and many countries also impose product standards, packaging requirements, labeling requirements and import restrictions on devices. Each country has its own tariff regulations, duties and tax requirements. Failure to comply with applicable foreign regulatory requirements may subject a company to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Third-Party Payor Reimbursements
Obtaining reimbursement approval for a health care product or service from a government or other third-party payor is a time consuming and costly process that could require us to provide supporting scientific, clinical and health economic data for the use of our products to the payor. We may not be able to provide data sufficient to gain acceptance with respect to reimbursement. Even when a payor determines that a product or service is eligible for reimbursement, the payor may impose coverage limitations that preclude payment for some uses that are approved by the FDA or comparable authorities. In addition, there is a risk that full reimbursement may not be available for high-priced products. Moreover, eligibility for coverage does not imply that any product or service will be reimbursed in all cases or at a rate that allows our customers to make a profit or cover their costs. Initial or interim reimbursements for products and services, if available, may also not be sufficient to cover costs and may not be made permanent.
Successful sales of our products in the United States and other countries will depend on the availability of reimbursement from third-party payors such as private insurance plans, managed care organizations, and Medicare and Medicaid. Our customers have obtained reimbursement for our Cystic Fibrosis Genotyping Test and Thrombophilia Risk Test for the XT-8 system and we believe that each of our tests in development are covered by existing current procedural
21
Table of Contents
terminology codes, or CPT codes, and will be eligible for coverage by Medicare and Medicaid and most third-party payors. However, Medicare and Medicaid generally do not reimburse providers who use our Warfarin Sensitivity Test. Outside of the United States, health care reimbursement systems vary from country to country, and to the extent we begin to sell our products outside the United States, we may not be able to obtain adequate reimbursement coverage, if any, for our products.
In addition, we may develop tests in the future that do not relate to previously established CPT codes and we may need to obtain new CPT codes in order to obtain reimbursement. Reimbursement by a third-party payor depends on a number of factors, including applicable coverage policies and limitations, the level of demand by health care providers and the payor’s determination that the use of a new product is medically necessary and represents a clinical advance. In addition, both government and non-government third-party payors routinely limit reimbursement coverage and reimbursement amounts for diagnostic tests. If our customers can not receive sufficient levels of reimbursement when using our products, our ability to sell them will be significantly constrained.
Fraud and Abuse Regulations
We are subject to numerous federal and state health care anti-fraud laws, including the federal anti-kickback statute and False Claims Act that are intended to reduce waste, fraud and abuse in the health care industry. These laws are broad and subject to evolving interpretations. They prohibit many arrangements and practices that are lawful in industries other than health care, including certain payments for consulting and other personal services, some discounting arrangements, the provision of gifts and business courtesies, the furnishing of free supplies and services, and waivers of payments. In addition, many states have enacted or are considering laws that limit arrangements between medical device manufacturers and physicians and other health care providers and require significant public disclosure concerning permitted arrangements. These laws are vigorously enforced against medical device manufacturers and have resulted in manufacturers paying significant fines and penalties and being subject to stringent corrective action plans and reporting obligations. We must operate our business within the requirements of these laws and, if we were accused of violating them, could be forced to expend significant resources on investigation, remediation and monetary penalties.
Patient Protection and Affordable Care Act
Our operations will also be impacted by the federal Patient Protection and Affordable Care Act of 2010, as modified by the Health Care and Education Reconciliation Act of 2010, which we refer to as the Health Care Act. The Health Care Act imposes a 2.3% excise tax on sales of medical devices by manufacturers. Taxable devices include any medical device defined in section 201(h) of the FDCA and intended for use by humans, with limited exclusions for devices purchased by the general public at retail for individual use. There is no exemption for small companies, and we expect to begin paying the tax in 2013. The Health Care Act also requires
22
Table of Contents
manufacturers to report to the Department of Health and Human Services detailed information about financial arrangements with physicians and teaching hospitals. These reporting provisions preempt state laws that require reporting of the same information, but not those that require reports of different or additional information. Failure to comply subjects the manufacturer to significant civil monetary penalties. We expect compliance with the Health Care Act to impose significant administrative and financial burdens on us.
Employees
As of December 31, 2011, we had 82 employees. Approximately 24 are involved in research and development, 24 in operations, manufacturing and quality assurance, 21 in sales and marketing, and 13 in finance, legal and other administrative functions. Our success will depend in large part upon our ability to attract and retain employees. We face competition in this regard from other companies, research and academic institutions, government entities and other organizations. None of our employees are covered by a collective bargaining agreement.
Corporate and Available Information
Our principal corporate offices are located at 5964 La Place Court, Suite 100, Carlsbad, California and our telephone number is (760) 448-4300. We were incorporated in Delaware in February 2010.
Our internet address is www.genmarkdx.com. There we make available, free of charge, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendments to those reports, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission, or SEC. We also make available on our Internet site public financial information for which a report is not required to be filed with or furnished to the SEC. Our SEC reports and other financial information can be accessed through the investor relations section of our Internet site. The information found on our Internet site is not part of this or any other report we file with or furnish to the SEC.
The public may read and copy any materials that we file with the SEC at the SEC’s Public Reference Room located at 100 F Street, N.E., Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at (202) 551-8090. The SEC also maintains electronic versions of our reports on its website at www.sec.gov.
| Item 1A. | RISK FACTORS |
You should consider each of the following factors as well as the other information in this Annual Report in evaluating our business and our prospects. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently consider immaterial may also impair our business operations. If any of the following risks actually occur, our business and financial results could be harmed. In that case, the trading price of our common stock could decline. You should also refer to the other information set forth in this Annual Report, including our financial statements and the related notes.
23
Table of Contents
Risks Related to Our Business
We have a history of net losses, and we may never achieve or maintain profitability.
We have a history of significant net losses and a limited history commercializing our molecular diagnostic products. We obtained FDA clearance for our first generation molecular diagnostic system in 2006, and commenced a limited marketing effort for this system. We commenced offering our XT-8 system and our Warfarin Sensitivity Test in July 2008. We commenced offering our Cystic Fibrosis Genotyping Test in July 2009 and our Thrombophilia Risk Test in April 2010. Our Respiratory Viral Panel Test is currently labeled for RUO and was submitted to the FDA for 510(k) clearance in December 2011. Our net losses were approximately $24.0 million for the year ended December 31, 2011, $18.4 million for the year ended December 31, 2010 and $20.0 million in 2009. At December 31, 2011, we had an accumulated deficit of approximately $168.5 million. We will continue to incur significant expenses for the foreseeable future in connection with our sales and marketing, research and development and regulatory activities and maintaining our existing, obtaining additional intellectual property rights and investing in corporate infrastructure. We cannot provide you any assurance that we will ever achieve profitability and, even if we achieve profitability, that we will be able to sustain or increase profitability on a quarterly or annual basis. Further, because of our limited commercialization history and because the market for molecular diagnostic products is relatively new and rapidly evolving, we have limited insight into the trends that may emerge and affect our business. We may make errors in predicting and reacting to relevant business trends, which could harm our business and financial condition.
We will need to raise additional funds in the future, and such funds may not be available on a timely basis, or at all. If additional capital is not available, we may have to curtail or cease operations.
Until such time, if ever, as we can generate substantial product revenues, we will be required to finance our operations with our cash resources. We will need to raise additional funds in the future to support our operations. We cannot be certain that additional capital will be available as needed or on acceptable terms, or at all. If we require additional capital at a time when investment in our company, in molecular diagnostics companies or the marketplace in general is limited, we may not be able to raise such funds at the time that we desire, or at all. If we do raise additional funds through the issuance of equity or convertible securities, the percentage ownership of holders of our common stock could be significantly diluted. In addition, newly issued securities may have rights, preferences or privileges senior to those of holders of our common stock. If we obtain debt financing, a substantial portion of our operating cash flow may be dedicated to the payment of principal and interest on such indebtedness, and the terms of the debt securities issued could impose significant restrictions on our operations and place encumbrances on our assets. If we raise additional funds through collaborations and licensing arrangements, we could be required to relinquish significant rights to our technologies and products, or grant licenses on terms that are not favorable to us.
24
Table of Contents
If our products do not perform as expected or the reliability of the technology on which our products are based is questioned, our operating results and business will suffer.
Our success depends on the market’s confidence that we can provide reliable, high-quality diagnostic systems and tests. We believe that customers in our target markets are likely to be particularly sensitive to product defects and errors. As a result, our reputation and the public image of our products or technologies will be significantly impaired if our products fail to perform as expected. Although our diagnostic systems are designed to be user-friendly, the functions they perform are complex, and our products may develop or contain undetected defects or errors.
If we experience a material defect or error, this could result in loss or delay of revenues, increased costs to produce our tests, delayed market acceptance, damaged reputation, diversion of development and management resources, legal claims, increased insurance costs or increased service and warranty costs, any of which could materially harm our business, financial condition and results of operations.
We also face the risk of product liability exposure related to the sale of our products. We currently carry product liability insurance that covers us against specific product liability claims. We also carry a separate general liability and umbrella policy that covers us against certain claims but excludes coverage for product liability. Any claim in excess of our insurance coverage, or for which we do not have insurance coverage, would have to be paid out of our cash reserves, which would harm our financial condition. We cannot assure you that we have obtained sufficient insurance or broad enough coverage to cover potential claims. Also, we cannot assure you that we can or will maintain our insurance policies on commercially acceptable terms, or at all. A product liability claim could significantly harm our business, financial condition and results of operations.
We may fail to successfully expand the menu of diagnostic tests for our XT-8 system or effectively predict the types of tests our existing and target customers want.
We currently market three FDA-cleared diagnostic tests and have developed one other diagnostic test currently labeled for RUO that has been submitted to the FDA for 510(k) clearance. In addition, we have several diagnostic tests in the research, development or design stage. Some hospital-based and reference laboratories may not consider adopting our XT-8 system until we offer a broader menu of diagnostic tests. Although we are developing additional tests to respond to the needs of these laboratories, we cannot guarantee that we will be able to license the appropriate technology, successfully develop, or obtain required regulatory clearances or approvals for additional tests, or do so in a manner that is cost-effective or timely. The development of new or enhanced products is a complex and uncertain process requiring the accurate anticipation of technological and market trends, as well as precise technological execution. In addition, in order to commercialize our products, we are required to undertake time consuming and costly development activities, including clinical studies for which the outcome is uncertain. Products that appear promising during early development and preclinical studies may, nonetheless, fail to demonstrate the results needed to support regulatory approval or, if approved, may not generate the demand we expect. If we are unable to successfully develop and commercialize additional diagnostic tests for use with our XT-8 system, our revenues and our ability to achieve profitability will be significantly impaired.
25
Table of Contents
We may not be able to manage our anticipated growth, and we may experience constraints or inefficiencies caused by unanticipated acceleration and deceleration of customer demand.
Demand for our Respiratory Viral Panel can be seasonal based upon influenza outbreaks. Also, unanticipated changes in customer demand for our products may result in constraints or inefficiencies related to our manufacturing, sales force, implementation resources and administrative infrastructure. These constraints or inefficiencies may adversely affect us as a result of delays, lost potential product sales or loss of current or potential customers due to their dissatisfaction. Similarly, over-expansion or investments in anticipation of growth that does not materialize, or develops more slowly than we expect, could harm our financial results and result in overcapacity.
To manage our anticipated future growth effectively, we must enhance our manufacturing capabilities and operations, information technology infrastructure, and financial and accounting systems and controls. Organizational growth and scale-up of operations could strain our existing managerial, operational, financial and other resources. Our growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of new products or enhancements of existing products. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our revenue could grow more slowly than expected and we may not be able to achieve our research and development and commercialization goals. Our failure to manage our anticipated growth effectively could have a material adverse effect on our business, operating results or financial condition.
We may not be able to correctly estimate or control our future operating expenses, which could lead to cash shortfalls.
Our operating expenses may fluctuate significantly in the future as a result of a variety of factors, many of which are outside of our control. These factors include:
| • | the time and resources required to develop, conduct clinical studies and obtain regulatory clearances for the additional diagnostic tests we develop; |
| • | the expenses we incur for research and development required to maintain and improve our technology, including developing our next-generation molecular diagnostic system; |
| • | the costs of preparing, filing, prosecuting, defending and enforcing patent claims and other patent related costs, including litigation costs and the results of such litigation. |
| • | the expenses we incur in connection with commercialization activities, including product marketing, sales and distribution; |
| • | the expenses we incur in licensing biomarkers from third parties to expand the menu of diagnostics tests we plan to offer; |
| • | our sales strategy and whether the revenues from sales of our test cartridges or XT-8 system will be sufficient to offset our expenses; |
| • | the costs to attract and retain personnel with the skills required for effective operations; and |
26
Table of Contents
| • | the costs associated with being a public company. |
Our budgeted expense levels are based in part on our expectations concerning future revenues from sales of our XT-8 system and diagnostic tests. We may be unable to reduce our expenditures in a timely manner to compensate for any unexpected shortfall in revenue. Accordingly, a shortfall in demand for our products could have an immediate and material impact on our business and financial condition.
We face intense competition from established and new companies in the molecular diagnostics field and expect to face increased competition in the future.
The markets for our technologies and products are very competitive, and we expect the intensity of competition to increase. We compete with many companies in the United States engaged in the development, commercialization and distribution of similar products intended for clinical molecular diagnostic applications. Categories of competitors include:
| • | companies developing and marketing multiplex molecular diagnostics systems, including Luminex Corporation; Nanosphere; Qiagen NV; Abbott Diagnostics, a division of Abbott Laboratories; Hologic, Inc. and Innogenetics Inc.; |
| • | large hospital-based laboratories and reference laboratories who provide large-scale testing using their own proprietary testing methods including Quest Diagnostics Incorporated and Laboratory Corporation of America; and |
| • | companies that manufacture laboratory-based tests and analyzers including Cepheid; Gen-Probe, Inc.; Siemens; Hologic, Inc.; Qiagen NV; Roche Diagnostics, a division of F. Hoffmann-La Roche Ltd.; and Abbott Diagnostics. |
Our diagnostic tests also face competition from laboratory-developed-tests, or LDTs, developed by national and regional reference laboratories and hospitals. Such laboratory-developed tests may not be subject to the same regulatory requirements, including those requiring clinical trials and FDA review and clearance or approval, that may apply to our products.
We anticipate that we will face increased competition in the future as new companies enter the market with new technologies and our competitors improve their current products and expand their menu of diagnostic tests. Many of our current competitors, as well as many of our potential competitors, have greater name recognition, more substantial intellectual property portfolios, longer operating histories, significantly greater resources to invest in new technologies, more substantial experience in new product development, greater regulatory expertise, more extensive manufacturing and distribution capabilities. The impact of these factors may result in our technologies and products becoming obsolete before we recover the expenses incurred to develop them or before they generate significant revenue.
We are reliant on the commercial success of our XT-8 system and our diagnostic tests.
We have primarily placed our XT-8 systems with customers at no initial charge through placement agreements, under which customers commit to purchasing minimum quantities of test
27
Table of Contents
cartridges over a period of generally one to three years, with a component of the reagent cartridge price allocated to recover the instrument cost. We also offer our XT-8 systems for sale. We expect sales of our diagnostic tests associated with our XT-8 system will account for the vast majority of our revenues for at least the next several years. We intend to dedicate a significant portion of our resources to the commercialization of our XT-8 system and our existing FDA-cleared diagnostic tests. Although we intend to develop a broad range of additional diagnostic tests for use with the XT-8 system, we cannot assure you when or if we will obtain FDA clearance for the tests we develop in the future, or whether the market will accept such new products. As a result, to the extent that our XT-8 system and our existing and future FDA-cleared diagnostic tests are not commercially successful or are withdrawn from the market for any reason, our revenues will be harmed and our business, operating results and financial condition will be harmed.
We may not be successful in developing our NexGen system.
We are developing a sample-to-answer platform, the NexGen system. We are designing this system to integrate automated nucleic acid extraction and amplification with our eSensor technology to allow technicians to be able to place a patient sample into our test cartridge and obtain results with significantly reduced or no technician hands-on processing time. The development of the NexGen system is a complex process, and we may not be successful in completing the development of all the currently intended features and benefits of the system, which may limit its marketability. In addition, before commercializing the NexGen system we will be required to obtain regulatory approval for the system as well as each of the diagnostic tests to be used on the system, including those tests that previously received approval for use with our XT-8 system. If we are unable to successfully develop and obtain regulatory approval for our NexGen system and related diagnostic tests, our business plan will be impaired. Additionally, prior to or upon release of our NexGen System, sales of our XT-8 system may decrease as customers migrate over to our newer technology.
Our financial results will depend on the acceptance and increased demand among reference laboratories and hospitals, third-party payors and the medical community of our molecular diagnostic technology and products.
Our future success depends on the acceptance by our target customers, third-party payors and the medical community that our molecular diagnostic products are a reliable, medically-relevant, accurate and cost-effective replacement for other molecular diagnostic testing methods.
Medical offices and many hospitals outsource their molecular diagnostic testing needs to national or regional reference laboratories. Our business success depends on our ability to convince these target laboratories and hospitals to replace their current testing platforms and/or send-out tests, with our XT-8 system and related diagnostic tests. We must also continue to increase the number of available tests, and test sell-through, on our installed systems.
Many other factors may affect the market acceptance and commercial success of our molecular diagnostic technology and products, including:
| • | the relative convenience and ease of use of our diagnostic systems over competing products; |
28
Table of Contents
| • | the introduction of new technologies and competing products that may make our technologies and products a less attractive solution for our target customers; |
| • | the breadth of our menu of available diagnostic tests relative to our competitors; |
| • | our success in training reference and hospital-based laboratories in the proper use of our products; |
| • | the acceptance in the medical community of our molecular diagnostic technology and products; |
| • | the extent and success of our marketing and sales efforts; and |
| • | general economic conditions. |
Our success depends on our ability to service and support our products.
To the extent that we fail to maintain a high quality level of service and support for our products, there is a risk that the perceived quality of our products will be diminished in the marketplace. Likewise, we may fail to provide the level, quantity or quality of service expected by the marketplace. This could result in slower adoption rates and lower than anticipated utilization of our products which could have a material adverse effect on our business, financial condition and results of operations.
Manufacturing risks and inefficiencies may adversely affect our ability to produce products; we have a sole source of supply for our XT-8 System.
We must manufacture, or engage third parties to manufacture, components of our products in sufficient quantities and on a timely basis, while maintaining product quality, acceptable manufacturing costs and complying with regulatory requirements. Our components are custom-made by only a few outside suppliers. If we are unable to satisfy our forecasted demand from existing suppliers for our kits and are unable to find alternative suppliers at reasonably comparable prices, it could have a material adverse effect on our business, financial condition, and results of operations. Additionally, we have entered into supply agreements with most of our suppliers of strategic reagents and parts to help ensure component availability and flexible purchasing terms with respect to the purchase of such components. If our suppliers discontinue production of a key component, we will be required to revalidate and may be required to resubmit a previously cleared product.
In determining the required quantities of our products and the manufacturing schedule, we must make significant judgments and estimates based on inventory levels, current market trends and other related factors. Because of the inherent nature of estimates and our limited experience in marketing our products, there could be significant differences between our estimates and the actual amounts of products we require. This can result in shortages if we fail to anticipate demand, or excess inventory and write-offs if we order more than we need.
We currently manufacture our proprietary test cartridges at our Carlsbad, California manufacturing facility. We outsource manufacturing of our XT-8 system and much of the
29
Table of Contents
disposable component molding and component assembly for our test cartridges. Our XT-8 system is manufactured by Aubrey Group Inc., our single source supplier that specializes in contract design and manufacturing of electronic and electromechanical devices for medical use. While we work closely with Aubrey Group Inc. to try to ensure continuity of supply while maintaining high quality and reliability, we cannot guarantee that these efforts will be successful. Should Aubrey Group Inc. become unable or unwilling to continue to meet our supply needs, we may experience delays in qualifying a new source or may not obtain as favorable pricing or other terms, any of which could harm our business, financial condition or results of operations.
Reliance on third-party manufacturers entails risk to which we would not be subject if we manufactured these components ourselves, including:
| • | reliance on third parties for regulatory compliance and quality assurance; |
| • | possible breaches of manufacturing agreements by the third parties because of factors beyond our control; |
| • | possible regulatory violations or manufacturing problems experienced by our suppliers; |
| • | possible termination or non-renewal of agreements by third parties, based on their own business priorities, at times that are costly or inconvenient for us; |
| • | the potential obsolescence and/or inability of our suppliers to obtain required components; |
| • | the potential delays and expenses of seeking alternate sources of supply or manufacturing services; |
| • | the inability to qualify alternate sources without impacting performance claims of our products; |
| • | reduced control over pricing, quality and timely delivery due to the difficulties in switching to alternate suppliers or assemblers; and |
| • | increases in prices of raw materials and key components. |
We may not be able to meet the demand for our products if one or more of these third-party manufacturers are not able or are unwilling to supply us with the necessary components that meet our specifications. It may be difficult to find alternate suppliers in a timely manner and on terms acceptable to us.
The manufacturing operations for our test cartridges use highly technical processes involving unique, proprietary techniques. In addition, the manufacturing equipment we use would be costly to repair or replace and could require substantial lead time to repair or replace. Any interruption in our operations or decrease in the production capacity of our manufacturing facility or the facilities of any of our suppliers because of equipment failure, natural disasters such as earthquakes, tornadoes and fires or otherwise, would limit our ability to meet customer demand for the XT-8 system and tests and would have a material adverse effect on our business, financial condition and results of operations. Other possible disruptions may include power loss and telecommunications failures. In the event of a disruption, we may lose customers and we may be unable to regain those
30
Table of Contents
customers thereafter. Our insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
We have only produced our products in limited quantities, and we may experience problems in scaling our manufacturing operations, or delays or component shortages that could limit the growth of our revenue.
To date, we have produced our products in limited quantities relative to the quantities necessary to achieve desired revenue growth. We may not be able to produce sufficient quantities or maintain consistency between differing lots of consumables. If we encounter difficulties in scaling our manufacturing operations as a result of, among other things, quality control and quality assurance issues and availability of components and raw material supplies, we will likely experience reduced sales of our products, increased repair or re-engineering costs due to product returns, and defects and increased expenses due to switching to alternate suppliers, any of which would reduce our revenues and gross margins.
Although we attempt to match our parts inventory and production capabilities to estimates of marketplace demand, to the extent system orders materially vary from our estimates, we may experience continued constraints in our systems production and delivery capacity, which could adversely impact revenue in a given fiscal period. Should our need for raw materials and components used in production continue to fluctuate, we could incur additional costs associated with either expediting or postponing delivery of those materials.
If we are unable to retain key members of our senior management and scientists or hire additional skilled employees, we may be unable to achieve our goals.
Our performance is substantially dependent on the performance of our senior management and key scientific and technical personnel. Our senior managers and other key employees can terminate their relationship with us at any time. We have a small number of senior managers, and the loss of services of any of these managers or our scientific or technical personnel could have a material adverse effect on our business, financial condition and results of operations. We do not maintain key-man life insurance on any of our employees.
In addition, our product development and marketing efforts could be delayed or curtailed if we are unable to attract, train and retain highly skilled employees and scientific advisors. To expand our research, product development and sales efforts, we will need to retain additional people skilled in areas such as electrochemical and molecular science, information technology, manufacturing, sales, marketing and technical support. Because of the complex and technical nature of our systems and the dynamic market in which we compete, any failure to attract and retain a sufficient number of qualified employees could materially harm our ability to develop and commercialize our technology. We may not be successful in hiring or retaining qualified personnel, and any failure to do so could have a material adverse effect on our business, financial condition and results of operations.
31
Table of Contents
Our success may depend upon how we and our competitors anticipate and adapt to market conditions.
The markets for our products are characterized by rapidly changing technology, evolving industry standards, changes in customer needs, emerging competition and new product introductions. New technologies, techniques or products could emerge with similar or better performance or may be perceived as providing better value than our systems and related tests and could exert pricing pressures on our products. It is critical to our success that we anticipate changes in technology and customer requirements and successfully introduce enhanced and competitive technology to meet our customers’ and prospective customers’ needs on a timely basis. We will need to respond to technological innovation in a rapidly changing industry and may not be able to maintain our technological advantages over emerging technologies in the future. If we fail to keep pace with emerging technologies, our systems and related tests will become uncompetitive and our market share will decline, which would harm our business, financial condition and results of operations.
We may be unsuccessful in our long-term goal of expanding sales of our product offerings outside the United States.
Assuming we receive the applicable regulatory approvals, we intend to market our diagnostic products outside the United States through third-party distributors. These distributors may not commit the necessary resources to market and sell our products to meet our expectations. If distributors do not perform adequately or in compliance with applicable laws and regulations in particular geographic areas, or if we are unable to locate distributors in particular geographic areas, our ability to realize revenue growth based on sales outside the United States would be harmed.
In order to market our products in the European Union and many other foreign jurisdictions, we, or our distributors or partners, must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements regarding safety and efficacy and governing, among other things, clinical studies and commercial sales and distribution of our products. The approval procedure varies among countries and can involve additional testing. The regulatory approval process outside the United States may include all of the risks associated with obtaining FDA approval, as well as additional risks. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all, which could harm our ability to expand into markets outside the United States.
If we expand sales of our products outside the United States, our business will be susceptible to risks associated with international operations.
If we execute our intent to expand our operations outside the United States, our inexperience in operating in foreign countries increases the risk that our international expansion will not be successful. Conducting international operations would subject us to new risks that, generally, we have not faced in the United States, including:
32
Table of Contents
| • | fluctuations in currency exchange rates; |
| • | unexpected complexity and changing foreign regulatory requirements; |
| • | longer accounts receivable payment cycles and difficulties in collecting accounts receivable; |
| • | difficulties in managing and staffing international operations; |
| • | potentially adverse tax consequences, including the complexities of foreign value added tax systems, tax inefficiencies related to our corporate structure and restrictions on the repatriation of earnings; |
| • | the burdens of complying with a wide variety of foreign laws and different legal standards; |
| • | increased financial accounting and reporting burdens and complexities; |
| • | hyperinflation, political, social and economic instability abroad, terrorist attacks and security concerns in general; |
| • | having to comply with a variety of U.S. laws, including the Foreign Corrupt Practices Act; and |
| • | the imposition of restrictive trade policies, including export restrictions; and |
| • | conducting business in places where business practices and customs are unfamiliar and unknown |
The occurrence of any one of these risks could harm our business, results of operations and prospects. Additionally, operating internationally requires significant management attention and financial resources. We cannot be certain that the investment and additional resources required in establishing operations in other countries will produce desired levels of revenues or profitability.
Guidelines, recommendations and studies published by various organizations can reduce the use of our products.
Professional societies, government agencies, practice management groups, private health/science foundations, and organizations involved in healthcare issues may publish guidelines, recommendations or studies to the healthcare and patient communities. Recommendations of government agencies or these other groups/organizations may relate to such matters as usage, cost-effectiveness, and use of related products. Organizations like these have in the past made recommendations about our competitors’ products, such as the need for less frequent screening tests, which could result in reduced product sales. Moreover, the perception by the investment community or stockholders that recommendations, guidelines or studies will result in decreased use of our products could adversely affect the prevailing market price for our common stock.
33
Table of Contents
Our Respiratory Viral Panel test and other menu items that we develop in the future may have sales that fluctuate on a seasonal basis and, as a result, our results of operations for any particular quarter may not accurately reflect full-year trends.
Our Respiratory Viral Panel Test and other tests that we develop in the future may have sales that fluctuate on a seasonal basis. As a result, our results of operations for any particular quarter may not accurately reflect full-year trends. For example, we expect volume of testing for our Respiratory Viral Panel Test generally will decline during the spring and summer season and accelerate during the fall and winter season. As a result, comparison of our results from quarter-to-quarter may not accurately reflect trends or results for the full year.
We have limited experience in sales and marketing and may be unable to successfully commercialize our XT-8 system and related diagnostic tests.
We have limited marketing, sales and distribution experience and capabilities. Our ability to achieve profitability depends on attracting customers for the XT-8 system, expanding the number of tests we offer, and building brand loyalty. To successfully perform sales, marketing, distribution and customer support functions ourselves, we face a number of risks, including:
| • | our ability to attract and retain the skilled support team, marketing staff and sales force necessary to commercialize and gain market acceptance for our technology and our products; |
| • | the ability of our sales and marketing team to identify and penetrate the potential customer base, including hospitals, national and regional reference laboratories; and |
| • | the difficulty of establishing brand recognition and loyalty for our products. |
In addition, we may seek to enlist one or more third parties to assist with sales, distribution and customer support globally or in certain regions of the world. If we do seek to enter into these arrangements, we may not be successful in attracting desirable sales and distribution partners, or we may not be able to enter into these arrangements on favorable terms, or at all. If our sales and marketing efforts, or those of any third-party sales and distribution partners, are not successful, our technologies and products may not gain market acceptance, which would harm our business operations.
Current economic conditions and the uncertain economic outlook may adversely impact our business, results of operations, financial condition or liquidity.
Global economic conditions may remain challenging and uncertain for the foreseeable future. The credit markets and the financial services industry have been experiencing a period of unprecedented turmoil and upheaval characterized by the bankruptcy, failure, collapse or sale of various financial institutions and an unprecedented level of intervention from the United States federal government. These conditions not only limit our access to capital but also make it extremely difficult for our customers, our vendors and us to accurately forecast and plan future business activities, and they could cause U.S. and foreign businesses and consumers to slow spending on our products and services, which would delay and lengthen sales cycles. Some of our customers rely on government research grants to fund technology purchases. If negative trends in
34
Table of Contents
the economy affect the government’s allocation of funds to research, there may be less grant funding available for certain of our customers to purchase technologies from us. Certain of our customers may face challenges gaining timely access to sufficient credit or may otherwise be faced with budget constraints, which could result in decreased purchases of, or development of products based on, our products or in an impairment of their ability to make timely payments to us. If our customers do not make timely payments to us, we may be required to assume greater credit risk relating to those customers, increase our allowance for doubtful accounts and our days sales outstanding would be negatively impacted. Although we maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments and such losses have historically been within our expectations and the provisions established, we may not continue to experience the same loss rates that we have in the past, especially given the current turmoil of the worldwide economy. Additionally, these economic conditions and market turbulence may also impact our suppliers causing them to be unable to supply in a timely manner sufficient quantities of customized components, thereby impairing our ability to manufacture on schedule and at commercially reasonable costs.
If third-party payors increasingly restrict payments for healthcare expenses or fail to adequately pay for multi-analytic testing, we may experience reduced sales which would hurt our business and our business prospects.
Third-party payors, such as government entities and healthcare programs, health maintenance organizations and private insurers, are continually seeking to reduce healthcare expenses. The federal government has also recently reduced the funding for certain government-sponsored healthcare programs which has caused these third party payors to seek further reduction in medical expenses. The U.S. federal government passed comprehensive healthcare reform in the form of the Affordable Care Act in 2010 and is considering revisions to this Act. The Affordable Care Act could further limit government reimbursement to these payors. These reductions may decrease demand for our products and the price we can charge. Increasingly, Medicaid and other third-party payors are challenging the prices charged for medical services, including clinical diagnostic tests. They are also attempting to contain costs by limiting coverage and the reimbursement level of tests and other healthcare products. In addition, cost containment initiatives by governmental or educational entities or programs may reduce funding for genetic research and development activities and retard the growth of the genetic testing market. Without adequate coverage and reimbursement, consumer demand for tests could decrease. Decreased demand could cause our customers to reduce purchases or to cancel programs or development activities and could cause sales of our products to fall. In addition, decreased demand could place pressure on us to lower prices on these products or services, resulting in lower margins. Reduced sales or margins would adversely affect our business, profitability and business prospects.
Providing XT-8 systems to our customers through reagent rental agreements may harm our liquidity.
The majority of our XT-8 systems are provided to customers via “reagent rental” agreements, under which customers are afforded the right to use the XT-8 system in return for a commitment to purchase minimum quantities of test cartridges over a period of time. Accordingly, we must incur the expense of manufacturing XT-8 systems well in advance of receiving sufficient revenues
35
Table of Contents
from test cartridges to recover our manufacturing expenses. We also offer our XT-8 systems for sale. The amount of additional capital we may need to raise depends on the amount of our revenues from sales of test cartridges sold through these reagent rental agreements. We do not currently sell enough test cartridges to recover all of our fixed manufacturing expenses associated with the production of our systems and test cartridges, and therefore we currently have a high cost of sales relative to revenue, resulting in a gross loss for 2011. If we continue not to sell a sufficient number of test cartridges to offset our expenses associated with these reagent rental agreements, our liquidity will be adversely affected.
We use hazardous chemicals, biological materials and infectious agents in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research, product development and manufacturing processes involve the controlled use of hazardous materials, including chemicals, biological materials and infectious disease agents. Our operations produce hazardous waste products. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, and our liability may exceed our insurance coverage and our total assets. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of these hazardous materials and specified waste products, as well as the discharge of pollutants into the environment and human health and safety matters. Compliance with environmental laws and regulations may be expensive and may impair our research, development and production efforts. If we fail to comply with these requirements, we could incur substantial costs, including civil or criminal fines and penalties, clean-up costs or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance. In addition, we cannot predict the impact on our business of new or amended environmental laws or regulations or any changes in the way existing and future laws and regulations are interpreted and enforced.
Our corporate structure may create tax inefficiencies.
As a result of our reorganization in 2010 and prior to the reorganization steps that took place in June 2011 (as described below), Osmetech plc was a wholly-owned subsidiary of GenMark and a controlled foreign corporation for U.S. federal income tax purposes. This organizational structure may have created inefficiencies, as certain types of income and investments of Osmetech that otherwise would not be currently taxable under general tax rules, may have become taxable. In addition, conveyance of intellectual property rights from one subsidiary to another could create taxable income. Distributions from GenMark to its operating subsidiaries or amongst the U.S. operating subsidiaries of GenMark could have been subject to additional U.S. and foreign income tax withholding and result in lower profits. During the quarter ended June 30, 2011, the Company underwent a corporate reorganization, or reorganization, intended to simplify its U.S. entity structure. As part of the reorganization, Osmetech Technologies, Inc. merged into Clinical Micro Sensors, Inc., or CMS, with CMS surviving. Additionally, Osmetech plc converted to a U.K. limited company for U.K. legal and tax purposes, and made an entity classification election to be treated as an entity disregarded from GenMark Diagnostics, Inc. for U.S. federal income tax purposes. It is anticipated that the reorganization will not trigger any material U.S. federal or U.K.
36
Table of Contents
income tax expense. Additionally, it is anticipated that the post-reorganization structure will allow GenMark Diagnostics, Inc. to elect to file a consolidated U.S. federal income tax return with its remaining U.S. subsidiaries, CMS and Osmetech, Inc. As a result of these steps, all operations will be included in a U.S. federal consolidated tax return and many of the inefficiencies described above are eliminated on a go-forward basis, however, the reorganization may result in additional tax liabilities to the Company.
Our ability to use our net operating loss carryforwards might be limited.
As of December 31, 2011, we had net operating loss carryforwards of approximately $99.5 million for U.S. federal income tax purposes. These loss carryforwards will expire in varying amounts through 2031. To the extent these net operating loss carryforwards are available, we intend to use them to reduce the corporate income tax liability associated with our operations. Section 382 of the U.S. Internal Revenue Code generally imposes an annual limitation on the amount of net operating loss carryforwards that might be used to offset taxable income when a corporation has undergone significant changes in stock ownership. As a result, prior or future changes in ownership could put limitations on the availability of our net operating loss carryforwards. In addition, our ability to use the current net operating loss carryforwards might be further limited by the issuance of common stock in the future. To the extent our use of net operating loss carryforwards is significantly limited, our income could be subject to corporate income tax earlier than it would if we were able to use net operating loss carryforwards, which could result in lower profits.
We have determined that we have experienced multiple ownership changes under Section 382 of the Internal Revenue Code, as amended, or the Code. We have estimated that approximately $42.0 million of federal net operating losses may be utilized in the future based on limitations that we have calculated under Section 382 of the Code. We are currently analyzing alternative positions and additional factual information that may increase the amount of net operating losses that could subsequently be utilized. To the extent that this additional information becomes available and could increase net operating losses available for use, we will adjust our deferred tax assets accordingly, with a corresponding adjustment to our valuation allowance. We also had non-U.S. net operating loss carryforwards of approximately $30.4 million as of December 31, 2011.
We are exposed to risks associated with long-lived and intangible assets that may become impaired and result in an impairment charge.
The carrying amounts of long-lived and intangible assets are affected whenever events or changes in circumstances indicate that the carrying amount of any asset may not be recoverable. These events or changes might include an inability to successfully deliver an instrument to the marketplace and attain customer acceptance, a change in the rights or use of licensed intellectual property or other matters. Adverse events or changes in circumstances may affect the estimated discounted future cash flows expected to be derived from long-lived and intangible assets. If at any time we determine that an impairment has occurred, we will be required to reflect the impaired value as a charge, resulting in a reduction in earnings in the quarter such impairment is identified and a corresponding reduction in our net asset value. In the past we have incurred, and
37
Table of Contents
in the future we may incur, impairment charges. A material reduction in earnings resulting from such a charge could cause us to fail meet the expectations of investors and securities analysts, which could cause the price of our stock to decline.
Failure to comply with covenants in our loan agreements could result in our inability to borrow additional funds and adversely impact our business.
We have entered into a loan and security agreements with Square 1 Bank. These loan agreements impose financial and other restrictive covenants on our operations, including covenants relating to our general profitability and our liquidity. We were in compliance with these covenants as of December 31, 2011. If we violate these or any other covenants, any outstanding amounts under these agreements could become due and payable prior to their stated maturity dates, the bank could proceed against any collateral in our operating accounts and our ability to borrow funds in the future may be restricted or eliminated. These restrictions may also limit our ability to pursue business opportunities or strategies that we would otherwise consider to be in our best interests.
Information technology systems implementation issues or security threats could disrupt our internal operations and adversely affect our financial results.
Portions of our information technology infrastructure may experience interruptions, delays or cessations of service or produce errors in connection with ongoing systems implementation work. In particular, we have implemented an enterprise resource planning software system. To more fully realize the potential of this system, we are continually reassessing and upgrading processes and this may be more expensive, time consuming and resource intensive than planned. Any disruptions that may occur in the operation of this system or any future systems or any unauthorized access to our information systems could increase our expenses and adversely affect our ability to report in an accurate and timely manner the results of our consolidated operations, our financial position and cash flows and to otherwise operate our business in a secure environment, all of which could adversely affect our financial results, stock price and reputation.
Risks Related to Regulation
The regulatory clearance or approval process is expensive, time consuming and uncertain, and the failure to obtain and maintain required clearances or approvals could prevent us from commercializing our future products.
We are investing in the research and development of new diagnostic tests to expand our menu of testing options, as well as to develop our next-generation NexGen system, which we anticipate will reduce the need for sample preparation when using our system. Our products are subject to 510(k) clearance or pre-market approval by the FDA prior to their marketing for commercial use in the United States, and to any approvals required by foreign governmental entities prior to their marketing outside the United States. In addition, any changes or modifications to a device that has received regulatory clearance or approval that could significantly affect its safety or effectiveness, or would constitute a major change in its intended use, may require the submission of a new application for 510(k) clearance, pre-market approval or foreign regulatory approvals.
38
Table of Contents
The 510(k) clearance and pre-market approval processes, as well as the process of obtaining foreign approvals, can be expensive, time consuming and uncertain. It generally takes from four to twelve months from submission to obtain 510(k) clearance, and from one to three years from submission to obtain pre-market approval; however, it may take longer, and 510(k) clearance or pre-market approval may never be obtained. Delays in receipt of, or failure to obtain, clearances or approvals for future products, including tests that are currently in design or development, would result in delayed, or no, realization of revenues from such products and in substantial additional costs which could decrease our profitability. We have limited experience in filing FDA applications for 510(k) clearance and pre-market approval. In addition, we are required to continue to comply with applicable FDA and other regulatory requirements once we have obtained clearance or approval for a product. There can be no assurance that we will obtain or maintain any required clearance or approval on a timely basis, or at all. Any failure to obtain or any material delay in obtaining FDA clearance or any failure to maintain compliance with FDA regulatory requirements could harm our business, financial condition and results of operations.
If third-party payors do not reimburse our customers for the use of our clinical diagnostic products or if reimbursement levels are set too low for us to sell our products at a profit, our ability to sell our products and our results of operations will be harmed.
We sell our products to hospital-based and reference laboratories, substantially all of which receive reimbursement for the health care services they provide to their patients from third-party payors, such as Medicare, Medicaid, other domestic and foreign government programs, private insurance plans and managed care programs. Reimbursement decisions by particular third-party payors depend upon a number of factors, including each third-party payor’s determination that use of a product is:
| • | a covered benefit under its health plan; |
| • | appropriate and medically necessary for the specific indication; |
| • | cost effective; and |
| • | neither experimental nor investigational. |
Third-party payors may deny reimbursement for covered products if they determine that a medical product was not used in accordance with cost-effective diagnosis methods, as determined by the third-party payor, or was used for an unapproved indication. Third-party payors also may refuse to reimburse for procedures and devices deemed to be experimental.
Obtaining coverage and reimbursement approval for a product from each government or third-party payor is a time consuming and costly process that could require us to provide supporting scientific, clinical and cost-effectiveness data for the use of our product to each government or third-party payor. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. For example, Medicare and Medicaid generally do not reimburse providers who use our Warfarin Sensitivity Test. In addition, eligibility for coverage does not imply that any product will be covered and reimbursed in all cases or reimbursed at a rate that allows our potential customers to make a profit or even cover their costs.
39
Table of Contents
In the United States, the American Medical Association assigns specific CPT codes, which are necessary for reimbursement of diagnostic tests. Once the CPT code is established, the Centers for Medicare and Medicaid Services establish reimbursement payment levels and coverage rules under Medicaid and Medicare, and private payors establish rates and coverage rules independently. We cannot guarantee that any of our tests are or will be covered by the CPT codes that we believe may be applied to them or that any of our tests or other products will be approved for coverage or reimbursement by Medicare and Medicaid or any third-party payor. Third-party payors may nonetheless choose to reimburse our customers on a per test basis based on individual biomarker detection, rather than on the basis of the number of results given by the test. This may result in reference laboratories, public health institutions and hospitals electing to use separate tests to screen for each disease so that they can receive reimbursement for each test they conduct. In that event, these entities may purchase separate tests for each disease, rather than products, such as ours, that can be used to return multiple test results.
Third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for medical products and services. Increasingly, Medicare, Medicaid and other third-party payors are challenging the prices charged for medical services, including clinical diagnostic tests. In addition, Medicare’s current freeze on its clinical laboratory fee schedule may harm the growth of the molecular diagnostics market for patients in the United States who are over 65 or have specific disabilities. Levels of reimbursement may decrease in the future, and future legislation, regulation or reimbursement policies of third-party payors may harm the demand for and reimbursement available for our products, which in turn, could harm pricing and sales. If our customers are not adequately reimbursed for our products, they may reduce or discontinue purchases of our products, which would cause our revenues to decline.
We are subject to evolving legislative, judicial and ethical standards on use of technology and biotechnology.
The adoption of genetic testing is occurring within the broader context of a myriad of decisions related to genetic patenting and genotyping. Issues associated with health insurance, data access, intellectual property protection, national and international legislative initiatives and other variables may have a significant impact on the wide spread adoption of genetic testing or on specific segments or tests within the genetic testing market, including the adoption of our NexGen system and other of our products that are currently in the development and design stage.
We and our suppliers, contract manufacturers and customers are subject to various governmental regulations, and we may incur significant expenses to comply with, and experience delays in our product commercialization as a result of, these regulations.
Our manufacturing processes and facilities, and those of some of our contract manufacturers, are required to comply with the federal QSR, which covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our devices. The FDA enforces the QSR through periodic announced and/or unannounced inspections of manufacturing facilities. We and our contract manufacturers have been, and anticipate in the future being, subject to such inspections, as well as to inspections by other federal and state regulatory agencies.
40
Table of Contents
We must also file reports of device corrections and removals and adhere to the FDA’s rules on labeling and promotion. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability, including substantial monetary penalties and criminal prosecution.
Failure to comply with applicable FDA requirements, or later discovery of previously unknown problems with our products or manufacturing processes, including our failure or the failure of one of our contract manufacturers to take satisfactory corrective action in response to an adverse QSR inspection, can result in, among other things:
| • | administrative or judicially imposed sanctions; |
| • | injunctions or the imposition of civil penalties; |
| • | recall or seizure of our products; |
| • | total or partial suspension of production or distribution; |
| • | the FDA’s refusal to grant pending future clearance or pre-market approval for our products; |
| • | withdrawal or suspension of marketing clearances or approvals; |
| • | clinical holds; |
| • | warning letters; |
| • | refusal to permit the import or export of our products; and |
| • | criminal prosecution. |
Any of these actions, in combination or alone, could prevent us from marketing, distributing or selling our products and would likely harm our business.
In addition, a product defect or regulatory violation could lead to a government-mandated or voluntary recall by us. We believe that the FDA would request that we initiate a voluntary recall if a product was defective or presented a risk of injury or gross deception. Regulatory agencies in other countries have similar authority to recall devices because of material deficiencies or defects in design or manufacture that could endanger health. Any recall would divert management attention and financial resources, could cause the price of our shares of common stock to decline and expose us to product liability or other claims, including contractual claims from parties to whom we sold products and harm our reputation with customers. A recall involving our XT-8 system or our FDA-cleared diagnostic tests would be particularly harmful to our business and financial results.
The use of our diagnostic products by our customers is also affected by the CLIA, and related federal and state regulations that provide for regulation of laboratory testing. CLIA is intended to ensure the quality and reliability of clinical laboratories in the United States by mandating specific standards in the areas of personnel qualifications, administration, participation in proficiency testing, patient test management, quality assurance and quality control and inspections. Current or
41
Table of Contents
future CLIA requirements or the promulgation of additional regulations affecting laboratory testing may prevent some laboratories from using some or all of our diagnostic products.
Legislative or regulatory healthcare reforms may make it more difficult and costly for us to obtain regulatory clearance or approval of our products and to produce, market and distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulatory clearance or approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. For example, in the future, the FDA may require more burdensome premarket approval of our system or diagnostic tests rather than the 510(k) clearance process we have used to date and anticipate primarily using in the future. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of our products. Delays in receipt of or failure to receive regulatory clearances or approvals for our new products would harm our business, financial condition and results of operations.
Federal and state governments in the United States are also undertaking efforts to control growing health care costs through legislation, regulation and voluntary agreements with medical care providers and third-party payors. In March 2010, Congress enacted comprehensive health care reform legislation known as the Patient Protection and Affordable Care Act of 2010, or the PPACA. While the PPACA involves expanding coverage to more individuals, it includes new regulatory mandates and other measures designed to constrain medical costs. The PPACA also imposes significant new taxes on medical device manufacturers that are expected to cost the medical device industry up to $20 billion over the next decade. There are also stringent new reporting requirements of financial relationships between device manufacturers and physicians and teaching hospitals. Complying with PPACA could significantly increase our tax liabilities and costs, which could adversely affect our business and financial condition.
Our operations will also be impacted by the PPACA, as modified by the Health Care Act. The Health Care Act imposes a 2.3% excise tax on sales of medical devices by manufacturers. Taxable devices include any medical device defined in Section 201(h) of the FDCA, and intended for use by humans, with limited exclusions for devices purchased by the general public at retail for individual use. There is no exemption for small companies, and we expect to begin paying the tax in 2013. The Health Care Act also requires manufacturers to report to the Department of Health and Human Services detailed information about financial arrangements with physicians and teaching hospitals. These reporting provisions preempt state laws that require reporting of the same information, but not those that require reports of different or additional information. Failure to comply subjects the manufacturer to significant civil monetary penalties. We expect compliance with the Health Care Act to impose significant administrative and financial burdens on us.
42
Table of Contents
We are subject to various federal and state laws pertaining to health care fraud and abuse, including anti-kickback, self-referral, false claims and fraud laws, and any violations by us of such laws could result in fines or other penalties.
Our commercial, research, and other financial relationships with healthcare providers and institutions are subject to various federal and state laws intended to prevent health care fraud and abuse. The federal anti-kickback statute prohibits the knowing offer, receipt or payment of remuneration in exchange for or to induce the referral of patients or the use of products or services that would be paid for in whole or part by Medicare, Medicaid or other federal health care programs. Remuneration has been broadly defined to include anything of value, including cash, improper discounts, and free or reduced price items and services. Many states have similar laws that apply to their state health care programs as well as private payors. Violations of the anti-kickback laws can result in exclusion from federal health care programs and substantial civil and criminal penalties.
The federal False Claims Act, or the FCA, imposes liability on persons who, among other things, present or cause to be presented false or fraudulent claims for payment by a federal health care program. The FCA has been used to prosecute persons submitting claims for payment that are inaccurate or fraudulent, that are for services not provided as claimed, or for services that are not medically necessary. The FCA includes a whistleblower provision that allows individuals to bring actions on behalf of the federal government and share a portion of the recovery of successful claims. If our marketing or other arrangements were determined to violate anti-kickback or related laws, including the FCA, then our revenues could be adversely affected, which would likely harm on our business, financial condition and results of operations.
State and federal authorities have aggressively targeted medical device companies for alleged violations of these anti-fraud statutes, based on improper research or consulting contracts with doctors, certain marketing arrangements that rely on volume-based pricing, off-label marketing schemes and other improper promotional practices. Companies targeted in such prosecutions have paid substantial fines in the hundreds of millions of dollars or more, have been forced to implement extensive corrective action plans, and have often become subject to consent decrees severely restricting the manner in which they conduct their business. If we become the target of such an investigation or prosecution based on our contractual relationships with providers or institutions, or our marketing and promotional practices, we could face similar sanctions which would materially harm our business.
To the extent we commence commercial operations overseas, we will be subject to the U.S. Foreign Corrupt Practices Act, or the FCPA, and other countries’ anti-corruption/anti-bribery regimes, such as the U.K. Bribery Act. The FCPA prohibits improper payments or offers of payments to foreign governments and their officials for the purpose of obtaining or retaining business. Safeguards we implement to discourage improper payments or offers of payments by our employees, consultants, sales agents or distributors may be ineffective, and violations of the FCPA and similar laws may result in severe criminal or civil sanctions, or other liabilities or proceedings against us, any of which would likely harm our reputation, business, financial condition and result of operations.
43
Table of Contents
Risks Related to Our Intellectual Property
We rely on third-party license agreements for patents and other technology related to our products. The termination of these agreements could delay or prevent us from being able to commercialize our products and the failure to negotiate new licenses could prevent us from expanding our menu of diagnostic products.
We depend on licenses to certain patents and patent applications that are related to electrochemical detection technology and other technology used in our molecular diagnostic systems and test cartridges. These licenses include both exclusive and non-exclusive arrangements. Many of these exclusive licenses obligate us to use commercially reasonable efforts to commercialize the subject inventions of the licensed patents, and if we fail to meet this obligation, we could lose one or more of those licenses. If, following such an event, any of our licensors were to provide a license to these patents to one or more of our competitors, our ability to compete in the market may be diminished. Furthermore, if we fail to comply with our material obligations under any of our patent license agreements, the licenses may be terminated and we could lose license rights that are important to our business.
The exclusive and non-exclusive licenses expire at various times, corresponding to the subject patents or patent applications, the expirations of which currently range from 2013 to 2028. We expect that we will need to license other technology or patents to commercialize future products, including licenses to additional biomarkers to expand our menu of diagnostic tests. These licenses may not be available to us on commercially reasonable terms, or at all, which could adversely affect our results of operations and growth prospects.
We may incur substantial costs as a result of litigation or other proceedings relating to the protection of our patents and other intellectual property rights and we may be unable to protect our rights to our technology.
If we or any of our licensors choose to go to court to stop a third party from using the inventions claimed in our owned or licensed patents, that third party may ask the court to rule that the patents are invalid and should not be enforced against that third party. These lawsuits are expensive and would consume time and other resources even if we were successful in stopping the infringement of these patents. In addition, there is a risk that the court will decide that these patents are not valid and that we do not have the right to stop others from using the inventions.
There is also the risk that, even if the validity of these patents is upheld, the court will refuse to stop the other party on the ground that such other party’s activities do not infringe our patents. In addition, the U.S. Supreme Court and the Court of Appeals for the Federal Circuit have recently changed certain tests regarding granting patents and assessing the validity of patent claims. As a consequence, issued patents may be found to contain invalid claims according to the newly revised and currently evolving standards. Some of our own or in-licensed patents may be subject to challenge and subsequent invalidation or significant narrowing of claim scope in a re-examination proceeding before the Patent and Trademark Office, or the PTO, or during litigation, under the revised criteria which make it more difficult to obtain patents.
44
Table of Contents
We may also not be able to detect infringement against our own or in-licensed patents, which may be especially difficult for methods of use. While we intend to take actions reasonably necessary to enforce our patent rights, we depend, in part, on our licensors and collaborators to protect a substantial portion of our proprietary rights.
Our products could infringe patent rights of others, which may require costly litigation and, if we are not successful, could cause us to pay substantial damages or limit our ability to commercialize our products.
Our commercial success depends on our ability to develop, manufacture and market our systems and tests and use our proprietary technology without infringing the patents and other proprietary rights of third parties. As the molecular diagnostic industry expands and more patents are issued, the risk increases that there may be patents issued to third parties that relate to our products and technology of which we are not aware or that we must challenge to continue our operations as currently contemplated. Our products may infringe or may be alleged to infringe these patents.
In addition, some patent applications in the United States may be maintained in secrecy until the patents are issued, because patent applications in the United States and many foreign jurisdictions are typically not published until eighteen months after filing and because publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered by our issued patents or our pending applications or that we were the first to invent the technology. Another party may have filed, and may in the future file, patent applications covering our products or technology similar to ours. Any such patent application may have priority over our patent applications or patents, which could further require us to obtain rights to issued patents covering such technologies. If another party has filed a U.S. patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the PTO to determine priority of invention in the United States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful if the other party had independently arrived at the same or similar invention prior to our own invention, resulting in a loss of our U.S. patent position with respect to such inventions.
There is a substantial amount of litigation involving patent and other intellectual property rights in the medical device, biotechnology and pharmaceutical industries generally. If a third party claims that we or any collaborator infringes its intellectual property rights, we may face a number of issues, including, but not limited to:
| • | infringement and other intellectual property claims which, regardless of merit, may be expensive and time-consuming to litigate and may divert our management’s attention from our core business; |
| • | substantial damages for infringement, which we may have to pay if a court decides that the product at issue infringes on or violates the third party’s rights, and if the court finds that the infringement was willful, we could be ordered to pay treble damages and the patent owner’s attorneys’ fees; |
| • | a court prohibiting us from selling or licensing our product unless the third party licenses its product rights to us, which it is not required to do; |
45
Table of Contents
| • | if a license is available from a third party, we may have to pay substantial royalties, upfront fees or grant cross-licenses to intellectual property rights for our products; and |
| • | redesigning our products or processes so they do not infringe, which may not be possible or may require substantial monetary expenditures and time. |
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
We may be infringing on the patent rights of third parties, which could prevent us from selling our current or future products.
From time to time we may become engaged in litigation with third parties having patent or other intellectual property rights alleging that our products or proprietary technologies infringe their intellectual property rights. These third parties and others who may in the future threaten us with such litigation, are or may be better capitalized and have more resources than us. In addition, in order to commercialize certain new or existing tests , we may be required to license certain biomarkers or risk that a third party may claim that the use of certain biomarkers in our tests infringes their intellectual property rights. We have received correspondence bringing to our attention certain patent rights held by third parties and offering to discuss licensing terms to the patents. Some of these letters relate to patents that are important to our products. Independently, we have also identified patents held by third parties that cover one or more of our products or planned products. Although we have taken licenses to numerous such third-party patents, we have also declined to license certain patents in instances where we do not believe our existing products infringe valid claims.
In May 2010, we received correspondence from Caliper Life Sciences, Inc., or Caliper, alleging that fluid handling technologies utilized in our test cartridges infringe certain microfluidic patents held by Caliper and demanding that we take a license to its patents or else Caliper would institute litigation against us. On November 10, 2010, we filed a complaint for declaratory judgment against Caliper in the United States District Court for the Northern District of California. In our complaint, we requested a declaration from the court that certain of Caliper’s microfluidic patents were invalid, and that we did not infringe on these patents. On February 24, 2011, we entered into an agreement with Caliper pursuant to which we agreed to dismiss our action for declaratory judgment, without prejudice, and Caliper agreed not to assert infringement by us on these patents for a period of six months. On August 24, 2011 we amended and restated our agreement with Caliper and agreed not to file or re-file a complaint, or to request reexamination of, certain Caliper patents prior to February 24, 2012, and Caliper agreed not to file any claims against GenMark asserting infringement of certain patents prior to February 24, 2012. After February 24, 2012, Caliper may again assert that we are infringing its patents and that we are required to take a license to its patents and could institute legal action. If one of Caliper’s patents or any other third-party patents were found to be valid and cover any of our products, or use of our proprietary technologies, we or any collaborator could be enjoined from using or selling our products by a court and/or required to pay damages and could be unable to commercialize our products or product candidates or use our proprietary technologies unless we or they obtained a license to the patent. A license may not be available to us or any collaborator on acceptable terms,
46
Table of Contents
or at all, which could potentially prevent us from selling our current products, using our core technologies or developing new tests. In addition, during litigation, the patent holder could obtain a preliminary injunction or other equitable relief that could prohibit us from making, using or selling our products, technologies or methods pending a trial on the merits, which could be years away. Furthermore, such litigation can be extremely costly and could significantly affect our results of operations and divert the attention of managerial and technical personnel
If we are unable to obtain, maintain and enforce intellectual property protection covering our products, others may be able to make, use, or sell products substantially the same as ours, which could adversely affect our ability to compete in the market.
Our commercial success is dependent in part on obtaining, maintaining and enforcing intellectual property rights, including patents. If we are unable to obtain, maintain and enforce intellectual property protection covering our products, others may be able to make, use or sell products that are substantially the same as ours without incurring the sizeable development and licensing costs that we have incurred, which would adversely affect our ability to compete in the market.
We seek to obtain and maintain patents and other intellectual property rights to restrict the ability of others to market products that compete with our products. Currently, our patent portfolio is comprised, on a worldwide basis, of over 100 issued U.S. and foreign patents and numerous pending applications. In general, patents have a term of 20 years from the application filing date or earlier claimed priority date. Our issued and exclusively licensed patents will expire between 2013 and 2021 or later, with several of our pending applications having the potential to mature into patents that might expire in 2027, 2028 and 2029. However, patents may not be issued based on any pending or future patent applications owned by or licensed to us and, moreover, issued patents owned or licensed to us now or in the future may be found by a court to be invalid or otherwise unenforceable. Also, even if our patents are determined by a court to be valid and enforceable, they may not be sufficiently broad to prevent others from marketing products similar to ours or designing around our patents, despite our patent rights, nor provide us with freedom to operate unimpeded by the patent rights of others.
We have also licensed certain intellectual property from third parties related to our products, and we rely on them to file and prosecute patent applications and maintain patents and otherwise protect the licensed intellectual property. We have not had and do not have primary control over these activities for certain of our patents or patent applications and other intellectual property rights. We cannot be certain that such activities by third parties have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights. Pursuant to the terms of the license agreements with some of our licensors, the licensors may have the right to control enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents and even if we are permitted to pursue such enforcement or defense, we will require the cooperation of our licensors. We cannot be certain that our licensors will allocate sufficient resources or prioritize their or our enforcement of such patents or defense of such claims to protect our interests in the licensed patents.
The patent positions of medical device companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No
47
Table of Contents
consistent policy regarding the breadth of claims allowed in patents in these fields has emerged to date in the United States or in many foreign jurisdictions. Both the U.S. Supreme Court and the Court of Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the patent laws of the U.S. are interpreted. In addition, Congress is regularly considering legislation that might change provisions of the patent law. We cannot predict future changes in the interpretation of patent laws or changes to patent laws which might be enacted into law. Those changes may materially affect our patents, our ability to obtain patents or the patents and applications of our collaborators and licensors. The patent situation in the medical device and disease diagnostic fields outside the United States is even more uncertain.
Future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| • | others may be able to make systems or devices that are similar to ours but that are not covered by the claims of our patents; |
| • | we may not be able to identify potential infringers of our technology due in part to the large number of competitors in the field; |
| • | we might not have been the first to make the inventions covered by our issued patents or pending patent applications; |
| • | we might not have been the first to file patent applications for these inventions; |
| • | our pending patent applications may not result in issued patents; |
| • | our issued patents may not provide us with any competitive advantages or may be held invalid or unenforceable as a result of legal challenges by third parties; |
| • | the claims of our issued patents or patent applications when issued may not cover our device or product candidates; |
| • | there may be dominating patents relevant to our product candidates of which we are not aware; |
| • | there may be prior public disclosures that could invalidate our inventions or parts of our inventions of which we are not aware; |
| • | the laws of foreign countries may not protect our proprietary rights to the same extent as the laws of the United States; and |
| • | we may not develop additional proprietary technologies that are patentable. |
We have a number of foreign patents and applications. However, the laws of some foreign jurisdictions do not protect intellectual property rights to the same extent as laws in the United States, and many companies have encountered significant difficulties in obtaining, protecting and defending such rights in foreign jurisdictions. If we encounter such difficulties or we are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business prospects could be substantially harmed.
48
Table of Contents
We also rely on trade-secret protection to protect our interests in proprietary know-how and for processes for which patents are difficult to obtain or enforce. We may not be able to protect our trade secrets adequately. We have limited control over the protection of trade secrets used by our licensors, collaborators and suppliers. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, outside scientific collaborators and other advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third-party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. We rely, in part, on non-disclosure and confidentiality agreements with our employees, consultants and other parties to protect our trade secrets and other proprietary technology. These agreements may be breached and we may not have adequate remedies for any breach. Moreover, others may independently develop equivalent proprietary information, and third parties may otherwise gain access to our trade secrets and proprietary knowledge. Any disclosure of confidential data into the public domain or to third parties could allow our competitors to learn our trade secrets and use the information in competition against us.
The U.S. Government has certain rights to use and disclose some of the intellectual property that we license and could exclusively license it to a third party if we fail to achieve practical application of the intellectual property.
Aspects of the technology licensed by us under agreements with third party licensors may be subject to certain government rights. Government rights in inventions conceived or reduced to practice under a government-funded program may include a non-exclusive, royalty-free worldwide license to practice such inventions for any governmental purpose. In addition, the U.S. government has the right to require us or our licensors (as applicable) to grant licenses which would be exclusive under any of such inventions to a third party if they determine that: (1) adequate steps have not been taken to commercialize such inventions in a particular field of use; (2) such action is necessary to meet public health or safety needs; or (3) such action is necessary to meet requirements for public use under federal regulations. Further, the government rights include the right to use and disclose, without limitation, technical data relating to licensed technology that was developed in whole or in part at government expense. At least one of our technology license agreements contains a provision recognizing these government rights.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in our industry, we employ individuals who were previously employed at other molecular diagnostics or medical device companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees, or we, have used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
49
Table of Contents
Risks Related to Ownership of Our Common Stock
The market price of our common stock may be volatile and fluctuate significantly, which could result in substantial losses for stockholders and subject us to litigation.
The market price of our common stock may be subject to significant fluctuations. Among the factors that may cause the market price of our common stock to fluctuate are the risks described in this “Risk Factors” section and other factors, including:
| • | fluctuations in our operating results or the operating results of our competitors; |
| • | changes in estimates of our financial results or recommendations by securities analysts; |
| • | variance in our financial performance from the expectations of securities analysts; |
| • | changes in the estimates of the future size and growth rate of our markets; |
| • | changes in accounting principles or changes in interpretations of existing principles, which could affect our financial results; |
| • | failure of our products to achieve or maintain market acceptance or commercial success; |
| • | conditions and trends in the markets we serve; |
| • | changes in general economic, industry and market conditions; |
| • | success of competitive products and services; |
| • | changes in market valuations or earnings of our competitors; |
| • | changes in our pricing policies or the pricing policies of our competitors; |
| • | announcements of significant new products, contracts, acquisitions or strategic alliances by us or our competitors; |
| • | the timing and outcome of regulatory reviews and approvals of our products; |
| • | changes in legislation or regulatory policies, practices or actions; |
| • | the commencement or outcome of litigation involving our company, our general industry or both; |
| • | recruitment or departure of key personnel; |
| • | changes in our capital structure, such as future issuances of securities or the incurrence of additional debt; |
| • | actual or expected sales of our common stock by the holders of our common stock; and |
| • | the trading volume of our common stock. |
In addition, the stock market in general, the NASDAQ Global Market and the market for diagnostics companies in particular may experience a loss of investor confidence. A loss of investor confidence may result in extreme price and volume fluctuations in our common stock that are unrelated or disproportionate to the operating performance of our business, our financial condition or results of operations. These broad market and industry factors may materially harm the market price
50
Table of Contents
of our common stock and expose us to securities class-action litigation. Class-action litigation, even if unsuccessful, could be costly to defend and divert management’s attention and resources, which could further materially harm our financial condition and results of operations.
Future sales of our common stock may depress our share price.
As of December 31, 2011, we had 20,477,761 shares of our common stock outstanding. Sales of a number of shares of common stock in the public market, or the expectation of such sales, could cause the market price of our common stock to decline. In addition, our 2010 Equity Incentive Plan provides for annual increases in the number of shares available for issuance under the plan, which may, among other things, result in dilution of the price of our common stock. We may also sell additional common stock in subsequent public offerings, which may adversely affect the market price of our common stock.
We incur costs and demands upon management as a result of complying with the laws and regulations affecting public companies in the United States, which may harm our operating results, and failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could cause investors to lose confidence in our operating results and in the accuracy of our financial reports and could harm our business and the price of our common stock.
As a public company in the United States, we are required, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. Our first report on compliance with Section 404 is in connection with our financial statements for the fiscal year ending December 31, 2011. The controls and other procedures are designed to ensure that information required to be disclosed by us in the reports that we file with the Securities and Exchange Commission, or SEC, is disclosed accurately and is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms. If we or our auditors were unable to certify that our internal control over financial reporting is effective and in compliance with Section 404, we may be subject to sanctions or investigations by regulatory authorities such as the SEC or the NASDAQ Global Market and we could lose investor confidence in the accuracy and completeness of our financial reports, which would materially harm our business and the price of our common stock and our ability to access the capital markets.
Furthermore, as a public company listed in the United States, we incur significant legal, accounting and other expenses. In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including regulations implemented by the SEC and the NASDAQ Global Market, may increase our legal and financial compliance costs and make some activities more time consuming. These laws, regulations and standards are subject to varying interpretations and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If notwithstanding our efforts to comply with new
51
Table of Contents
laws, regulations and standards, we fail to comply, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
Failure to comply with these rules might also make it more difficult or more expensive for us to obtain certain types of insurance, including director and officer liability insurance, and we might be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, on committees of our board of directors or as members of senior management.
We do not expect to declare any dividends on our common stock in the foreseeable future.
We currently intend to invest our future earnings, if any, to fund the development and growth of our business. In addition, pursuant to our Loan and Security Agreement with Square 1 Bank, we are restricted from paying any dividends. The payment of dividends will be at the discretion of our Board of Directors and will depend on our results of operations, capital requirements, financial condition, future prospects, restrictions imposed by applicable law, any limitations on payments of dividends present in any debt agreements we may enter into and other factors our Board of Directors may deem relevant. Consequently, stockholders may need to rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investment. Investors seeking cash dividends should not purchase our common stock.
Provisions of our certificate of incorporation, our bylaws and Delaware law could make an acquisition of our Company, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove the current members of our board and management.
Certain provisions of our certificate of incorporation and bylaws could discourage, delay or prevent a merger, acquisition or other change of control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove members of our Board of Directors. These provisions also could limit the price that investors might be willing to pay in the future for our common stock, thereby depressing the market price of our common stock. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions:
| • | allow the authorized number of directors to be changed only by resolution of our Board of Directors; |
| • | provide that our stockholders may only remove our directors for cause; |
| • | establish a classified board of directors, such that not all members of the board of directors may be elected at one time; |
| • | authorize our Board of Directors to issue without stockholder approval up to 100,000,000 shares of common stock, that, if issued, would dilute our stock ownership and could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our Board of Directors; |
52
Table of Contents
| • | authorize our Board of Directors to issue without stockholder approval up to 5,000,000 shares of preferred stock, the rights of which will be determined at the discretion of the Board of Directors that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our Board of Directors; |
| • | require that stockholder actions must be effected at a duly called stockholder meeting or by unanimous written consent; |
| • | establish advance notice requirements for stockholder nominations to our Board of Directors or for stockholder proposals that can be acted on at stockholder meetings; |
| • | limit who may call stockholder meetings; and |
| • | require the approval of the holders of 80% of the outstanding shares of our capital stock entitled to vote in order to amend certain provisions of our certificate of incorporation and bylaws. |
In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of the voting rights on our common stock, from merging or combining with us for a prescribed period of time.
| Item 1B. | UNRESOLVED STAFF COMMENTS |
None
| Item 2. | PROPERTIES |
We currently operate from a facility located in Carlsbad, California. We do not own any real property. On February 8, 2010, we entered into a seven-year and seven-month lease for a 31,098 square foot facility in Carlsbad, California. The facility is part of a three-building office and research and development project located at 5964 La Place Court, Carlsbad, California, and the project totals 158,733 rentable square feet. In January 2012, we signed a lease amendment to expand our executive and administrative office and manufacturing space by an additional 22,000 square feet and believe that our current and future leased facilities are adequate to meet our needs for the foreseeable future.
| Item 3. | LEGAL PROCEEDINGS |
We are from time to time subject to various claims and legal actions during the ordinary course of our business. We believe that there are currently no claims or legal actions that would reasonably be expected to have a material adverse effect on our results of operations or financial condition.
| Item 4. | MINE SAFETY DISCLOSURES |
None.
53
Table of Contents
| Item 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock has been quoted on The NASDAQ Global Market under the symbol “GNMK” since May 28, 2010. Prior to that time, our stock traded under the ticker symbol “OMH” on the London Stock Exchange. The following table sets forth, for the periods indicated, the quarterly high and low sales prices per share of our common stock as reported on The NASDAQ Global Market.
| High | Low | |||||||
| Year Ended December 31, 2011 |
||||||||
| First Quarter |
$ | 5.34 | $ | 3.62 | ||||
| Second Quarter |
$ | 6.95 | $ | 3.83 | ||||
| Third Quarter |
$ | 6.50 | $ | 4.27 | ||||
| Fourth Quarter |
$ | 5.90 | $ | 4.00 | ||||
| Period from May 28, 2010 to December 31, 2010 |
||||||||
| First Quarter |
— | — | ||||||
| Second Quarter |
$ | 6.00 | $ | 4.02 | ||||
| Third Quarter |
$ | 5.15 | $ | 3.27 | ||||
| Fourth Quarter |
$ | 5.20 | $ | 2.97 | ||||
Stock Performance Graph
The graph below compares the cumulative total stockholder returns on our common stock for the period indicated with the cumulative total stockholder returns on the NASDAQ Composite Index and the NASDAQ Biotechnology Index for the same period. The graph assumes that $100 was invested on May 28, 2010 in our common stock and in each index and that all dividends were reinvested. No cash dividends have been declared on our common stock. Stockholder returns over the indicated period should not be considered indicative of future stockholder returns.
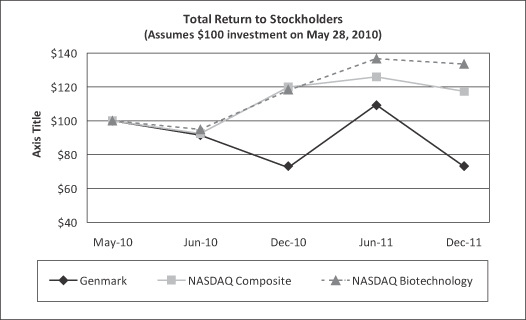
54
Table of Contents
Stockholders
The last reported sale price of common stock on March 1, 2012 as reported on the NASDAQ Global Market was $4.11. As of March 1, 2012, there were 9,113 holders of record of common stock.
Dividend Policy
We have never declared or paid any cash dividends on our common stock and do not expect to pay any dividends for the foreseeable future. We currently intend to retain any future earnings to fund the operation, development and expansion of our business. Any future determination to pay dividends will be at the sole discretion of our Board of Directors and will depend upon a number of factors, including our results of operations, capital requirements, financial condition, future prospects, contractual arrangements, restrictions imposed by applicable law, any limitations on payments of dividends present in our current and future debt arrangements, and other factors our Board of Directors may deem relevant.
| Item 6. | SELECTED CONSOLIDATED FINANCIAL DATA |
The following selected consolidated financial data relates to GenMark and its consolidated subsidiaries. The selected consolidated statement of operations data presented below of GenMark for the years ended December 31, 2011 and 2010 and Osmetech plc for the years ended December 31, 2009 and 2008 and the selected consolidated balance sheet data of GenMark as of December 31, 2011 and 2010 and Osmetech plc as of December 31, 2009 have been derived from the audited consolidated financial statements of GenMark, which have been prepared in accordance with U.S. GAAP, included elsewhere in this Form 10-K.
The selected consolidated financial statements of operations data of Osmetech plc presented below for the year ended December 31, 2007 and the selected consolidated balance sheet data of Osmetech plc as of December 31, 2008 have been derived from audited consolidated financial statements of Osmetech plc, not included in this Form 10-K, which have been prepared in accordance with U.S. GAAP.
The selected consolidated balance sheet data as of December 31, 2007 has been derived from unaudited consolidated financial information, not included in this Form 10-K, and has been prepared by GenMark in accordance with U.S. GAAP.
55
Table of Contents
The results for the periods shown below are not necessarily indicative of the results to be expected for any future periods. The selected consolidated financial data should be read together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and with the consolidated financial statements and unaudited condensed consolidated financial statements of GenMark and related notes included elsewhere in this Form 10-K.
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||
| Revenue: |
||||||||||||||||||||
| Product sales |
$ | 4,700 | $ | 2,341 | $ | 911 | $ | 560 | $ | 234 | ||||||||||
| License and other revenue |
309 | 223 | 88 | 87 | 108 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
5,009 | 2,564 | 999 | 647 | 342 | |||||||||||||||
| Cost of sales |
6,206 | 3,979 | 4,332 | 3,238 | 2,625 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross loss |
(1,197 | ) | (1,415 | ) | (3,333 | ) | (2,591 | ) | (2,283 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating expenses: |
||||||||||||||||||||
| Sales and marketing |
4,969 | 4,555 | 3,182 | 3,394 | 2,220 | |||||||||||||||
| General and administrative |
8,960 | 7,415 | 8,289 | 9,633 | 8,896 | |||||||||||||||
| Research and development |
8,737 | 6,646 | 5,634 | 13,423 | 12,554 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
22,666 | 18,616 | 17,105 | 26,450 | 23,670 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(23,863 | ) | (20,031 | ) | (20,438 | ) | (29,041 | ) | (25,953 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Other (expense) income: |
||||||||||||||||||||
| Foreign exchange (loss) gain |
6 | (1 | ) | 304 | 505 | — | ||||||||||||||
| Interest income (expense) |
(74 | ) | — | 33 | 420 | 1,715 | ||||||||||||||
| Therapeutic Discovery Credit |
— | 1,644 | — | — | — | |||||||||||||||
| Other income (expense) |
13 | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total other income |
55 | 1,643 | 337 | 925 | 1,715 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss before income taxes |
(23,918 | ) | (18,388 | ) | (20,101 | ) | (28,116 | ) | (24,238 | ) | ||||||||||
| (Provision) benefit for income taxes |
(52 | ) | (15 | ) | 138 | (247 | ) | 300 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss from continuing operations |
$ | (23,970 | ) | $ | (18,403 | ) | $ | (19,963 | ) | $ | (28,363 | ) | $ | (23,938 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per share (basic and diluted) |
$ | (1.45 | ) | $ | (1.88 | ) | $ | (4.41 | )$ | (28.13 | ) | $ | (27.13 | ) | ||||||
| Weighted average number of shares outstanding |
16,572 | 9,797 | 4,527 | 1,008 | 882 | |||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||
| Cash and cash equivalents and short-term investments (1)(2) |
$ | 30,320 | $ | 18,329 | $ | 16,483 | $ | 8,822 | $ | 27,620 | ||||||||||
| Total assets |
38,186 | 26,314 | 19,333 | 15,175 | 33,234 | |||||||||||||||
| Long-term liabilities |
1,171 | 1,307 | 795 | 769 | 720 | |||||||||||||||
| Total liabilities |
7,552 | 5,247 | 4,009 | 5,238 | 3,266 | |||||||||||||||
| Accumulated deficit |
(168,463 | ) | (144,493 | ) | (126,090 | ) | (106,127 | ) | (77,765 | ) | ||||||||||
| Total stockholders’ equity (1)(2) |
30,634 | 21,067 | 15,325 | 9,937 | 29,968 | |||||||||||||||
| (1) | In June 2010, we closed our initial public offering, in which we sold 4,600,000 shares of common stock at a price to the public of $6.00 per share. We raised approximately $22.6 million in net proceeds. |
| (2) | The Company issued 8,125,440 shares of common stock on June 22, 2011 at a price of $4.25 per share. We raised approximately $31.7 million in net proceeds. |
56
Table of Contents
| Item 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read the following in conjunction with the “Selected Consolidated Financial Information” and the consolidated financial statements of GenMark and the related notes thereto that appear elsewhere in this report. In addition to historical information, the following discussion and analysis includes forward looking information that involves risks, uncertainties and assumptions. Actual results and the timing of events could differ materially from those anticipated by these forward looking statements as a result of many factors, including those discussed under “Risk Factors” elsewhere in this prospectus. See also “Forward Looking Statements” included elsewhere in this filing.
Overview
GenMark Diagnostics, Inc., or GenMark, was formed by Osmetech plc, or Osmetech, as a Delaware corporation in February 2010 and had no operations prior to its initial public offering which was completed in June 2010. Immediately prior to the closing of the initial public offering, GenMark acquired all of the outstanding ordinary shares of Osmetech in a reorganization under the applicable laws of the United Kingdom. As a result of the reorganization, all of the issued ordinary shares in Osmetech were cancelled in consideration of (i) the issuance of common stock of GenMark to the former shareholders of Osmetech and (ii) the issuance of new shares in Osmetech to GenMark. Following the reorganization, Osmetech became a subsidiary controlled by GenMark, and the former shareholders of Osmetech began to hold shares of GenMark. Any historical discussion of GenMark relates to Osmetech and its consolidated subsidiaries prior to the reorganization.
We are a molecular diagnostics company focused on developing and commercializing our proprietary eSensor® detection technology. Our proprietary electrochemical technology enables fast, accurate and highly sensitive detection of up to 72 distinct biomarkers in a single sample. Our XT-8 system received 510(k) clearance from the Food and Drug Administration, or FDA, and is designed to support a broad range of molecular diagnostic tests with a compact and easy-to-use workstation and self-contained, disposable test cartridges. Within 30 minutes of receipt of an amplified DNA sample, our XT-8 system produces clear and accurate results. Our XT-8 system supports up to 24 independent test cartridges, which can be run independently, resulting in a highly convenient and flexible workflow for our target customers, which are hospitals and reference laboratories. As of December 31, 2011, we had an installed base of 167 analyzers, or placements, with our customers.
Our Products
We have developed six tests for use with our XT-8 system and expect to expand this test menu by introducing two to four new tests annually. Our Cystic Fibrosis Genotyping Test, which detects pre-conception risks of cystic fibrosis, our Warfarin Sensitivity Test, which determines an individual’s ability to metabolize the oral anticoagulant warfarin, and our Thrombophilia Risk Test, which detects an individual’s increased risk of blood clots, have received FDA clearance. Our eSensor® technology has demonstrated 100% accuracy in clinical studies in our Cystic Fibrosis Genotyping Test, our Warfarin Sensitivity Test and our Thrombophilia Risk Test as
57
Table of Contents
compared to DNA sequencing. We have also developed a Respiratory Viral Panel Test which detects the presence of major respiratory viruses that has been submitted to the FDA for 510(k) clearance. We also released our Hepatitis C Virus Genotyping (“HCVg”) Test which identifies the type and sub-type of the hepatitis C virus as an RUO test in 2011. We plan to conduct clinical studies with our HCVg Test with the goal of the submission to the FDA for clearance or approval. We also have a pipeline of several additional potential products in different stages of development or design, including diagnostic tests for variants in the cytochrome P450 2C19 gene and for mutations in a gene known as KRAS, which is predictive of a tumor’s response to certain prescribed anti-cancer therapies. We currently intend to initiate clinical studies in 2012 with respect to our 2C19 test with the goal of submission to the FDA for clearance or approval.
Our Technology
We are also developing our next-generation platform, the NexGen system. We are designing the NexGen system to integrate automated nucleic acid extraction and amplification with our eSensor® detection technology to enable technicians using the NexGen system to be able to place a patient sample into our test cartridge and obtain results without any additional steps. This sample to answer capability is enabled by the robust nature of our eSensor® detection technology, which is not impaired by sample impurities that we believe hinder competing technologies. We are designing our NexGen system to further simplify workflow and provide powerful, cost-effective molecular diagnostics solutions to a significantly expanded group of hospitals and reference laboratories.
Since inception, we have incurred net losses from continuing operations each year, and we expect to continue to incur losses for the foreseeable future. Our losses attributable to continuing operations for the years ended December 31, 2011, 2010 and 2009 were approximately $24.0 million, $18.4 million and $20.0 million, respectively. As of December 31, 2011, we had an accumulated deficit of $168.5 million. Our operations to date have been funded principally through sales of capital stock and sale of our previous business. We expect to incur increasing expenses over the next several years, principally to develop additional diagnostic tests, as well as to further increase our spending to manufacture, sell and market our products.
Financial Results Overview
Revenue
Revenue from continuing operations includes product sales, principally of our diagnostic tests for use with our XT-8 system. We primarily place our XT-8 system with customers through a reagent rental agreement, under which customers commit to purchasing minimum quantities of test cartridges over a period of one to three years. We also offer our XT-8 system for sale.
Revenue also includes licensing revenue from the out-licensing of our electrochemical detection technology. In addition, revenue generated from service agreements recognized using the proportional performance method of accounting is included in this category. We may enter into additional sub-licenses of our technology generating additional revenue, but do not anticipate that this will provide a significant portion of our future revenue.
58
Table of Contents
Our growth plans focus on both reagent rental agreements and system sales of our current XT-8 system and our next-generation NexGen system that is currently under development. We plan to expand our base of customers and systems as well as adding more tests for use with our systems. We believe these developments will drive accelerated use of our test cartridges, which we expect to be our primary source of revenue.
Cost of Sales
Cost of sales includes the cost of materials, direct labor and manufacturing overhead costs used in the manufacture of our consumable test kits for our XT-8 system, including royalties on product sales. Cost of sales also includes depreciation on revenue generating systems that have been placed with our customers under a reagent rental agreement, and amortization of licenses related to our products.
Our XT-8 systems are procured from a contract manufacturer and generally capitalized as fixed assets and depreciated on a straight line basis over their useful life as a charge to cost of sales. We expect our costs of sales to increase as we place additional XT-8 systems and manufacture and sell an increasing menu of accompanying diagnostic tests.
We manufacture our test cartridges in our facility and have significant capacity for expansion. This underutilized capacity results in a high cost of sales relative to revenue, resulting in a gross loss. We believe cost of sales as a percentage of revenue will decrease as our sales of test cartridges grow.
Sales and Marketing Expenses
Sales and marketing include those costs associated with our direct sales force, sales management, marketing, technical support and business development departments. These expenses primarily consist of salaries, commissions, benefits, share-based compensation, product shipment costs, travel, advertising and promotions. We expect sales and marketing costs to increase as we scale up our commercial efforts to drive an increased customer base.
Research and Development Expenses
Research and development expenses primarily include expenses related to the development of our XT-8 system, including the detection system and the test cartridges. These expenses also included clinical study expenses incurred in the process of preparing for FDA clearance for these systems and test cartridges and quality assurance costs. The expenses primarily consisted of salaries, benefits, share-based compensation costs, outside design and consulting services, laboratory supplies, contract research organizations, clinical study supplies and facility costs.
We expense all research and development costs in the periods in which they are incurred. We expect research and development costs to increase as we develop our NexGen system and increase the development of new tests for our XT-8 system.
59
Table of Contents
General and Administrative Expenses
Our general and administrative expenses include expenses related to our executive, accounting and finance, information technology, legal, business development, human resource and investor relations departments. These expenses consist primarily of salaries, benefits, share-based compensation costs, independent auditor costs, legal fees, consultants, travel, insurance, relocation, and public company expenses such as stock transfer agent fees and listing fees for NASDAQ.
Foreign Exchange Gains and Losses
Transactions in currencies other than the functional currency are translated at the prevailing rates on the dates of the transaction. Foreign exchange gains and losses arise from differences in exchange rates during the period between the date a transaction denominated in a foreign currency is consummated and the date on which it is settled or translated. Prior to the initial public offering in 2010, exchange gains and losses included those arising on cash balances held by Osmetech denominated in currencies other than its functional currency, the British pound. Since the initial public offering, the functional currency of GenMark has been the U.S. dollar. Since the initial public offering, foreign exchange gains and losses are primarily related to a contract for intellectual property licensing that is denominated in euros.
Interest Income and Interest Expense
Interest income includes interest earned on our cash and cash equivalents and investments. Interest expense represents interest incurred on our loan payable and on other liabilities.
Income Tax Provision (Benefit)
We account for income taxes in accordance with ASC Topic 740, Income Taxes. Under ASC Topic 740, deferred taxes are provided on an asset and liability method whereby deferred tax assets are recognized for deductible temporary differences and operating loss carryforwards. Deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and the tax bases. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more-likely-than-not that some portion or all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment.
Critical Accounting Polices and Significant Judgments and Estimates
Revenue
We recognize revenue from product sales and contract arrangements, net of discounts and sales related taxes. We recognize revenue from product sales when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed or determinable and collectability is reasonably assured. Where applicable, all revenue is stated net of sales taxes and trade discounts.
We offer customers the choice to either purchase a system outright or to receive a system free of charge in exchange for an annual minimum purchase commitment for diagnostic test cartridges.
60
Table of Contents
When a system is sold, revenue is generally recognized upon shipment of the unit consistent with contract terms. When a system is placed free of charge under a “reagent rental” agreement, we retain title to the equipment and the system remains capitalized on the balance sheet under property and equipment. Under our reagent rental agreements, our customers pay an additional system rental fee for each test cartridge purchased. The system rental fee varies based on the monthly volume of test cartridges purchased. The system rental fee and diagnostic test cartridges are recognized as contingent rental payments and are included in Product revenue in our consolidated financial statements.
We sell our durable systems and disposable test cartridges primarily through a direct sales force in the United States. Components are individually priced and can be purchased separately or together. The system price is not dependent upon the purchase of any amount of disposable test cartridges. Revenue on system and test cartridge sales is generally recognized upon shipment consistent with contract terms, which is when title and the risk of loss and rewards of ownership have been transferred to the customer and there are no other post-shipment obligations.
Revenue related to royalties received from licenses is generally recognized evenly over the contractual period to which the license relates. Revenue from service agreements is recognized using the proportional performance method of accounting.
Shipping and handling costs are expensed as incurred and included in sales and marketing expense. In those cases where we bill shipping and handling costs to customers, the amounts billed are classified as revenue.
Property and Equipment — net
Property, equipment and leasehold improvements are recorded at cost and depreciated using the straight-line method over the assets’ estimated useful lives, which are noted below. We generally capitalize our XT-8 systems, and provide these to customers for no charge. Each category of property and equipment is analyzed to determine its useful life. We look at the manufacturers’ estimates of useful life and adjust these for actual experience in our operating environment. Useful lives are reviewed periodically and shortened if circumstances dictate a change.
| Machinery and laboratory equipment |
- | 3 - 5 years | ||
| Instruments |
- | 3 years | ||
| Office equipment |
- | 2 - 4 years | ||
| Leasehold improvements |
- | over the shorter period of the life of the lease or the useful economic life of the asset |
During 2009, our estimate of the useful life of our systems was changed from five years to three years. This estimate was revised due to a change in our strategy to accelerate the development of our next-generation system and did not have a significant impact on the results for the period.
Maintenance and repair costs are expensed as incurred.
61
Table of Contents
Impairment of Long-Lived Assets
We assess the recoverability of long-lived assets, including intangible assets and systems at customer locations by periodically evaluating the carrying value of such assets whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. If impairment is indicated, we write down the carrying value of the asset to the estimated fair value. In the years ended December 31, 2011 and 2010, no impairment charges were recorded. In the year ended December 31, 2009, we recorded impairment against systems of $865,000, which was recorded within cost of sales ($666,000), sales and marketing ($130,000) and research and development ($70,000).
Share-Based Compensation
We have granted our options with an exercise price equal to the closing price of GenMark’s common stock on the NASDAQ Global Market on each grant date. We use the Black-Scholes option-pricing model as the method for determining the estimated fair value of stock options. The Black-Scholes model requires the use of highly subjective and complex assumptions which determine the fair value of share-based awards, including the option’s expected term and the price volatility of the underlying stock. These assumptions include:
| • | Expected Term. Our expected term represents the period that our share-based awards are expected to be outstanding and is determined by evaluating past experience and using other estimating tools. |
| • | Expected Volatility. Expected volatility represents the volatility in our stock price expected over the expected term of the option. |
| • | Expected Dividend. The Black-Scholes option-pricing model calls for a single expected dividend yield as an input. We assumed no dividends as we have never paid dividends and have no current plans to do so. |
| • | Risk-Free Interest Rate. The risk-free interest rate used in the Black-Scholes option-pricing model is based on published government rates in effect at the time of grant for periods corresponding with the expected term of option. |
Income Taxes
Our income tax expense, deferred tax assets and liabilities and reserves for unrecognized tax benefits reflect management’s best assessment of estimated future taxes to be paid. We are subject to income taxes in both the United States and the United Kingdom. Significant judgments and estimates are required in determining the consolidated income tax expense.
We believe that it is more likely than not that the benefit from our deferred tax assets will not be realized. In recognition of this risk, we have provided a full valuation allowance on the net deferred tax assets relating to our net operating loss carryforwards and other deferred tax assets. If our assumptions change and we determine we will be able to realize our deferred tax assets, the tax benefits relating to any reversal of the valuation allowance on deferred tax assets at December 31, 2011 will be accounted for as a reduction of income tax expense.
62
Table of Contents
Changes in tax laws and rates could also affect recorded deferred tax assets and liabilities in the future. Management is not aware of any such changes that would have a material effect on our results of operations, cash flows or financial position.
We recognize tax liabilities in accordance with ASC Topic 740 and we adjust these liabilities when our judgment changes as a result of the evaluation of new information not previously available. Due to the complexity of some of these uncertainties, the ultimate resolution may result in a payment that is materially different from our current estimate of the tax liabilities. These differences will be reflected as increases or decreases to income tax expense in the period in which they are determined.
Recent Accounting Pronouncements
In May 2011, the FASB issued ASU No. 2011-04, “Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs “ . This pronouncement clarifies the application of certain existing fair value measurement guidance and expands the disclosures for fair value measurements that are estimated using significant unobservable (Level 3) inputs. This pronouncement is effective on a prospective basis for annual and interim reporting periods beginning on or after December 15, 2011. This standard affects required disclosures only so is not expected to have a material impact on our consolidated financial statements.
In June 2011, the FASB issued ASU No. 2011-05, “Comprehensive Income (Topic 220): Presentation of Comprehensive Income” and in December 2011, the FASB issued ASU No. 2011-12, Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income in Accounting Standards Update No. 2011-05. These pronouncements address changes to the financial statement presentation of comprehensive income and the timing of implementation of these changes. The standards do not affect the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income. These standards are required to be applied retrospectively and are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. These standards change required financial statement presentation so are not expected to have a material impact on our consolidated financial statements.
Results of Operations—2011 compared to 2010 (in thousands):
Revenue
| December 31, | ||||||||||||||||
| 2011 | 2010 | $ Change | % Change | |||||||||||||
| Year ended |
$ | 5,009 | $ | 2,564 | $ | 2,445 | 95 | % | ||||||||
Product sales increased $2.4 million, or 101%, to $4.7 million for the year ended December 31, 2011 compared to $2.3 million for the year ended December 31, 2010 which was primarily driven by increased reagent revenues as well as system sales as our installed base of
63
Table of Contents
systems placed with customers expanded from 2010. License and other revenue increased $86,000 to $309,000, or 39%, for the year ended December 31, 2011 compared to the year ended December 31, 2010, due to increased service revenue and shipping fees of $223,000. The effects of inflation and price increases were not significant to revenues for the years ended December 31, 2011 and 2010.
Cost of Sales and Gross Loss
| December 31, | ||||||||||||||||
| 2011 | 2010 | $ Change | % Change | |||||||||||||
| Cost of Sales- year ended |
$ | 6,206 | $ | 3,979 | $ | 2,227 | 56 | % | ||||||||
| Gross Loss- year ended |
$ | 1,197 | $ | 1,415 | $ | (218 | ) | (15 | %) | |||||||
The increase in cost of sales for the twelve months ended December 31, 2011 compared to the twelve months ended December 31, 2010 was due to higher cost of goods sold expense of $910,000 directly related to the increase in reagent sales, an inventory write down of $1,002,000 recorded during the year, and higher labor costs of $358,000 related to relocating our manufacturing facilities from Pasadena to our Carlsbad location in 2011. The inventory charge reflects the write-off of several assay production lots that did not meet the Company’s quality standards and other one-time expenses related to relocating our manufacturing facility from Pasadena, California to the current Carlsbad, California location which was completed in June 2011.
The increase in gross loss resulted primarily from higher sales volumes directly related to our increase in revenues and the effect of the one-time charges to cost of sales. Gross loss improved as manufacturing efficiencies were realized due to increased production volumes in 2011 compared to 2010.
Operating Expenses
Sales and Marketing
| December 31, | ||||||||||||||||
| 2011 | 2010 | $ Change | % Change | |||||||||||||
| Year ended |
$ | 4,969 | $ | 4,555 | $ | 414 | 9 | % | ||||||||
The increase in sales and marketing expense for the year ended December 31, 2011 as compared to the year ended December 31, 2010 was primarily caused by higher compensation costs as we continue to expand our direct sales force.
General and Administrative
| December 31, | ||||||||||||||||
| 2011 | 2010 | $ Change | % Change | |||||||||||||
| Year ended |
$ | 8,960 | $ | 7,415 | $ | 1,545 | 21 | % | ||||||||
64
Table of Contents
General and administrative expense increased for the twelve months ended December 31, 2011 as compared to the twelve months ended December 31, 2010 due to increases in consulting, outside services and professional services including fees of $1,874,000 for corporate restructuring, testing required under the Sarbanes-Oxley Act and consulting related to business and process improvements, offset by reduced expenses of $400,000 related by relocating our UK and Pasadena, California operations to Carlsbad, California.
Research and Development
| December 31, | ||||||||||||||||
| 2011 | 2010 | $ Change | % Change | |||||||||||||
| Year ended |
$ | 8,737 | $ | 6,646 | $ | 2,091 | 31 | % | ||||||||
The increase in research and development expense for the year ended December 31, 2011 as compared to the year ended December 31, 2010 was due to additional costs incurred in 2011 for clinical trials for the respiratory viral panel, as well as higher payroll and patent-related legal costs incurred as we continued to develop additional product offerings.
Other (Expense) Income
| December 31, | ||||||||||||||||
| 2011 | 2010 | $ Change | % Change | |||||||||||||
| Year ended |
$ | (55 | ) | $ | 1,643 | $ | (1,698 | ) | (103 | )% | ||||||
Other (expense) income represents non-operating revenue and expenses, earnings on cash, cash equivalents and investments, interest expense related to a loan payable and foreign currency gains or losses. The decrease in other (expense) income for the year ended December 31, 2011 as compared to the year ended December 31, 2010 was due primarily to recognizing the Therapeutic Discovery Credit in 2010. There was no similar benefit for the year ended December 31, 2011.
Income Tax (Provision) Benefit
| December 31, | ||||||||||||||||
| 2011 | 2010 | $ Change | % Change | |||||||||||||
| Year ended |
$ | (52 | ) | $ | (15 | ) | $ | 37 | 247 | % | ||||||
Due to the Company’s losses it has only recorded tax provisions or benefits related to expected UK tax interest on uncertain tax positions, minimum tax payments and refunds.
65
Table of Contents
Results of Operations—2010 compared to 2009 (in thousands):
Revenue
| December 31, | ||||||||||||||||
| 2010 | 2009 | $ Change | % Change | |||||||||||||
| Year ended |
$ | 2,564 | $ | 999 | $ | 1,565 | 157 | % | ||||||||
License and other revenue increased $135,000 to $223,000, or 153%, for the year ended December 31, 2010, due to increased service revenue and shipping fees, compared to $88,000 for the year ended December 31, 2009. The increase in product revenue was primarily driven by increased reagent revenues as well as system sales and other product revenue and was due to an increase in our installed base of systems and an expanded menu of tests available for sale. License revenue increased predominantly due to a collaboration agreement executed in conjunction with a clinical trial for Warfarin.
Cost of Sales and Gross Loss
| December 31, | ||||||||||||||||
| 2010 | 2009 | $ Change | % Change | |||||||||||||
| Cost of Sales- year ended |
$ | 3,979 | $ | 4,332 | $ | (353 | ) | (8 | )% | |||||||
| Gross Loss- year ended |
$ | 1,415 | $ | 3,333 | $ | (1,918 | ) | (58 | )% | |||||||
The decrease in cost of sales in 2010 as compared to 2009 was primarily the result of higher revenue and better capacity utilization, with lower facility costs and an increased allocation of costs to research and development. Gross loss decreased $1.9 million or 58% to $1.4 million for the year ended December 31, 2010 compared to a gross loss of $3.3 million in 2009.
Sales and Marketing
| December 31, | ||||||||||||||||
| 2010 | 2009 | $ Change | % Change | |||||||||||||
| Year ended |
$ | 4,555 | $ | 3,182 | $ | 1,373 | 43 | % | ||||||||
The increase in sales and marketing expense was driven by higher payroll costs. We built our direct sales force during 2010 and expect these costs to increase during 2011 and beyond.
Research and Development
| December 31, | ||||||||||||||||
| 2010 | 2009 | $ Change | % Change | |||||||||||||
| Year ended |
$ | 6,646 | $ | 5,634 | $ | 1,012 | 18 | % | ||||||||
66
Table of Contents
The increase in research and development expense in 2010 was due to higher payroll costs, including relocation and recruiting fees and increased usage of project supplies.
General and Administrative
| December 31, | ||||||||||||||||
| 2010 | 2009 | $ Change | % Change | |||||||||||||
| Year ended |
$ | 7,415 | $ | 8,289 | $ | (874 | ) | (11 | )% | |||||||
The decline in general and administrative expense in 2010 as compared to 2009 was due to reduced facility costs and professional fees offset by relocation costs related to our move from Pasadena to Carlsbad.
Foreign Exchange
| December 31, | ||||||||||||||||
| 2010 | 2009 | $ Change | % Change | |||||||||||||
| Year ended |
$ | (1 | ) | $ | 304 | $ | (305 | ) | 100 | % | ||||||
The change in foreign exchange loss for the year ended December 31, 2010 of $1,000 as compared to a gain of $304,000 for the year ended December 31, 2009. The gain was due to the settlement of U.S. dollar liabilities during the year as the U.S. dollar weakened against the British pound combined with the benefit of maturing U.S. dollar forward contracts which were held by us during the period. There were few foreign exchange transactions during 2010.
Interest Income
| December 31, | ||||||||||||||||
| 2010 | 2009 | $ Change | % Change | |||||||||||||
| Year ended |
$ | — | $ | 33 | $ | (33 | ) | (100 | )% | |||||||
Interest income declined for the year ended December 31, 2010 compared to the year ended December 31, 2009 due to lower cash balances during 2010.
Therapeutic Discovery Credit
| December 31, | ||||||||||||||||
| 2010 | 2009 | $ Change | % Change | |||||||||||||
| Year ended |
$ | 1,644 | $ | — | $ | 1,644 | 100 | % | ||||||||
We recorded other income related to the Therapeutic Discovery Credit of $1.6 million for the year ended December 31, 2010. In July 2010, we applied for certification of qualified investments eligible for credits and grants under the qualifying therapeutic discovery project program for the years ended December 31, 2009 and December 31, 2010. The $1.6 million in grant applications
67
Table of Contents
were for expenses incurred in 2010 and 2009. The company received $561,000 for 2009 expenses and $1.1 million for 2010 expenses.
These development projects included the NexGen system, KRAS mutation cancer treatment, 2C19test, Warfarin Sensitivity Test, Thrombophilia Risk Test, Respiratory Viral Panel and Cystic Fibrosis Genotyping. In November 2010, we were notified that we were awarded a total of $1.6 million under the program. As of December 31, 2010, the Company recorded the $1.6 million tax credit as an Other Current Assets on the Balance Sheet with a corresponding credit to Other Income on the Consolidated Statement of Operations.
Benefit (Provision) for Income Taxes
| December 31, | ||||||||||||||||
| 2010 | 2009 | $ Change | % Change | |||||||||||||
| Year ended |
$ | (15 | ) | $ | 138 | $ | (153 | ) | (111 | %) | ||||||
A tax provision of $15,000 was recorded for the year ended December 31, 2010, compared to a tax benefit of $138,000 for the year ended December 31, 2009. The amount of the 2010 tax provision consists primarily of state income taxes. During 2009, a benefit was recognized relating to a carry-back of tax losses to prior years following the enactment of the Worker, Homeownership and Business Assistance Act of 2009.
Liquidity and Capital Resources
To date we have funded our operations primarily from the sale of capital stock, proceeds from sale of a business and revenues. We have incurred net losses from continuing operations each year and have not yet achieved profitability.
At December 31, 2011, we had $27.5 million of working capital, including $30.3 million in cash, cash equivalents and short- term investments. Net cash used in operations increased $300,000 to $19.2 million for the year ended December 31, 2011 compared to $18.9 million for the year ended December 31, 2010. Net cash used in investing activities increased $5.2 million to $7.1 million for the year ended December 31, 2011 compared to $1.9 million for the year ended December 31, 2010 due to the purchase of $5.0 million in short-term investments and more purchases of capital assets, primarily XT-8 systems used for reagent rental programs.
Net cash provided by financing activities increased $10.7 million for the year ended December 31, 2011 to $33.3 million, compared to $22.6 million for the year ended December 31, 2010 due to greater proceeds of our stock offering in 2011 as compared to 2010 and the proceeds of a new $2.0 million loan payable in 2011.
The Company issued 8,125,440 shares of common stock on June 22, 2011 at a price of $4.25 per share, the net proceeds of which were approximately $31.7 million after deducting underwriting discounts and commissions of $2.2 million and other offering expenses of $0.6 million.
68
Table of Contents
In March 2010, we entered into a loan and security agreement with Square 1 Bank, pursuant to which we obtained a credit facility consisting of a revolving line of credit in the amount of up to $2.0 million and an equipment term loan in the amount of up to $2.0 million. Based upon certain financial covenants, interest on the revolving line of credit will be either (i) the greater of (a) the bank’s prime rate (3.25% as of September 30, 2011) plus 2.75%, or (b) 6%; or (ii) the greater of (a) the bank’s prime rate plus 3.75%, or (b) 7%. In addition, based upon certain financial covenants, interest on the equipment term loan will be either (i) the greater of (a) the bank’s prime rate plus 3.25%, or (b) 6.50%; or (ii) the greater of (a) the bank’s prime rate plus 4.25%, or (b) 7.50%. The revolving line matures in July 2011 and the term loan matures in July 2013. In March 2011, the loan and security agreement was amended, whereby the line of credit availability was increased to $3 million and the maturity was extended to July 2012. The term loan was modified to allow invoices up to 360 days to qualify to be submitted for credit extension. There were no other changes to these two loans.
In March 2011, an additional loan was made available under the amended loan and security agreement for up to $1 million to finance equipment purchases. Based upon certain financial covenants, interest on this equipment term loan will be either (i) the greater of (a) the bank’s prime rate plus 3.25%, or (b) 6.50%; or (ii) the greater of (a) the bank’s prime rate plus 4.25%, or (b) 7.50%. This term loan matures March 2014.
As of December 31, 2011, the Company had no outstanding loans on the line of credit or the 2011 equipment loan and had a balance of $1.6 million used to finance 2010 equipment purchases and tenant improvements to its Carlsbad facility on the original 2010 equipment term loan. The loan bears an interest rate of 6.5%. Interest-only payments at the rate of 6.5% were due monthly from the date of each initial equipment advance until July 12, 2011. Initial equipment advances that were then outstanding are payable in 24 equal monthly installments of principal, plus all accrued and unpaid interest, beginning on August 12, 2011 and continuing on the same day of each month thereafter through July 12, 2013.
Pursuant to the terms of the loan and security agreement, we are required to maintain a ratio of liquidity to bank indebtedness equal to at least 1.50 to 1.00. In addition, the loan and security agreement includes several restrictive covenants, including requirements that we obtain the consent of Square 1 Bank prior to entering into any change of control event unless all debt is repaid to Square 1 Bank prior to the change of control event, incurring other indebtedness or liens with respect to our property, making distributions to our stockholders, making certain investments or entering into certain transactions with affiliates and other restrictions on storing inventory and equipment with third parties. The agreement also limits the amount we can borrow under the term loan to license genetic biomarkers to $500,000. To secure the credit facility, we granted Square 1 Bank a first priority security interest in our assets and intellectual property rights and provided a $500,000 standby letter of credit. We are currently in compliance will all ratios and covenants.
The Company’s management has prepared cash flow forecasts which indicate, based on the current cash resources available, the availability of unutilized credit facilities, and our ability to access the equity markets, that we will have sufficient resources to fund our business for at least the next 12 months. We expect capital outlays and operating expenditures to increase over the next several years as we grow our customer base and revenues, expand our research and
69
Table of Contents
development, commercialization and manufacturing activities. The amount of additional capital we may need to raise in the future depends on many factors, including:
| • | the level of revenues and the rate of revenue growth; |
| • | the level of expenses required to expand our sales and marketing activities; |
| • | the level of research and development investment required to maintain and improve our technology; |
| • | our need to acquire or license complementary technologies or acquire complementary businesses; |
| • | the costs of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; |
| • | competing technological and market developments; and |
| • | changes in regulatory policies or laws that affect our operations. |
We cannot be certain that additional capital will be available when and as needed or that our actual cash requirements will not be greater than anticipated. If we require additional capital at a time when investment in diagnostics companies or in the marketplace in general is limited due to the then prevailing market or other conditions, we may not be able to raise such funds at the time that we desire, on acceptable terms, or at all. In addition, if we raise additional funds through the issuance of equity or convertible debt securities, the percentage ownership of our stockholders could be significantly diluted, and these newly issued securities may have rights, preferences or privileges senior to those of existing stockholders. If we obtain additional debt financing, a substantial portion of our operating cash flow may be dedicated to the payment of principal and interest on such indebtedness, and the terms of the debt securities issued could impose significant restrictions on our operations and place encumbrances on our assets. If we raise additional funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or products, or grant licenses on terms that are not favorable to us.
Contractual Obligations
As of December 31, 2011, we had contractual obligations as follows:
| Payments due by period | ||||||||||||||||||||
| Contractual Obligations |
Total | Less than 1 Year |
1-3 Years |
4-5 Years |
After 5 Years |
|||||||||||||||
| Operating lease obligations (1) |
$ | 3,713 | $ | 546 | $ | 1,202 | $ | 1,254 | $ | 710 | ||||||||||
| Licensing payment obligation |
648 | 648 | — | — | — | |||||||||||||||
| Loan repayment obligations |
1,686 | 1,065 | 621 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total obligations |
$ | 6,047 | $ | 2,259 | $ | 1,823 | $ | 1,254 | $ | 710 | ||||||||||
| (1) | We enter into operating leases in the ordinary course of business with respect to facilities. Our lease agreements have fixed payment terms based on the passage of time. Certain facility leases require payment of maintenance and real estate taxes. Our future operating lease |
70
Table of Contents
| obligations could change if we exit certain contracts or if we enter into additional operating leases. |
On February 8, 2010, we entered into a seven-year and seven-month lease for a 31,098 square foot facility in Carlsbad, California. The facility is part of a three-building office and research and development project located at 5964 La Place Court, Carlsbad, California, and the project totals 158,733 rentable square feet. Monthly rental payments are $48,260 and increases 3% annually. We also pay our pro-rata share of the building and project maintenance, property tax, management and other costs subject to certain limitations. We have paid a $55,000 security deposit and provided a $500,000 standby letter of credit as security for the future rent as well as for up to $2.0 million in landlord funded tenant improvements. The lease also provides for expansion rights and rights of first refusal for expansion within our building, subject to certain limitations.
In January 2012, we entered into a lease amendment for adjoining facility space totaling an additional 22,000 square feet. We intend to utilize the additional space for storage initially and build out for additional office and warehouse space in 2013. The lease amendment requires an additional security deposit of $22,000, an increase in our standby letter of credit to $858,000, additional rental payments of $16,000 per month until the earlier of July 1, 2013 or when we commence operations in the adjoining space, at which time the rent increases approximately $35,000 per month, with annual increases of 3% to 4%. The term of the lease is also extended to ninety one months after until the earlier of July 1, 2013 or we commence operations in the adjoining space and our proportional share of common area maintenance, property management and taxes are increased under the provisions of the amendment to the lease.
On February 28, 2011, we entered into a 36 month operating lease for office equipment with total lease payments of $85,000. In conjunction with the lease, the lessor paid the Company approximately $27,000 to payoff previous contracts for similar equipment leased from a different vendor.
On October 20, 2010, we entered into a licensing agreement for intellectual property. The agreement requires minimum payments of €1.0 million in four equal installments over two years and contains provisions for additional licensing fees of €1.25 million and additional royalties based on related product sales. The license terminates upon election by us as defined or termination of every patent and application of patent right included in the agreement or other material breach as defined in the contract. The US dollar equivalent of remaining minimum payments due related to the initial agreement of €1.0 million are included in the table above.
Inventory Purchases:
In addition to the table above, the Company periodically purchases systems from a contract manufacturer. In order to guarantee delivery, we issue purchase orders each 90 day period for delivery of systems during that period. The Company had outstanding purchase orders for systems totaling $30,000 and $28,000 at December 31, 2011 and 2010, respectively.
71
Table of Contents
Tax obligations:
In addition to the table above, approximately $509,000 of unrecognized tax benefits, including accrued interest of $127,000, have been recorded as liabilities and we are uncertain as to if or when such amounts may be settled.
Impact of Inflation
The effect of inflation and changing prices on our operations was not significant during the periods presented.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements except as follows:
| • | We have unutilized credit facilities with Square 1 Bank that provide a revolving line of credit up to $2.0 million and an unutilized equipment term loan totaling $1.0 million at December 31, 2011. |
| • | We have provided a $500,000 standby letter of credit as security for future rent to our landlord in conjunction the lease of our Carlsbad facility. |
72
Table of Contents
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Quantitative and Qualitative Disclosures about Market Risk
Our exposure to market risk is limited to our cash and cash equivalents, all of which have maturities of less than three months, and short-term investments, which have maturities of less than one year. The goals of our investment policy are preservation of capital, fulfillment of liquidity needs and fiduciary control of cash and investments. We also seek to maximize income from our investments without assuming significant risk. To achieve our goals, in the future we may maintain a portfolio of cash equivalents and investments in a variety of securities that management believes to be of high credit quality. We currently do not hedge interest rate exposure. Because of the short-term maturities of our cash equivalents and short-term investments, we do not believe that an increase in market rates would have a material negative impact on the value of our portfolio.
Interest Rate Risk
We have exposure to interest rate risk related to our variable rate borrowings. In 2010, we entered into a credit facility consisting of a revolving line of credit in the amount of $2.0 million and an equipment term loan in the amount of up to $2.0 million. In 2011, we amended the credit facility to provide an additional $1.0 million of borrowings to finance equipment purchases. As of December 31, 2011, we had no outstanding loans on the line of credit or the 2011 equipment loan increase and had drawn $2.0 million against the original 2010 equipment term loan. This loan bears an interest rate of 6.5%. As of December 31, 2011, based on current interest rates and total borrowings outstanding, a hypothetical 100 basis point increase in interest rates would have an insignificant pre-tax impact on our results of operations.
Foreign Currency Exchange Risks
All of our operating facilities are located within the United States. We are a U.S. entity and our functional currency is the U.S. dollar. Virtually all of our revenues are based in the United States. In 2010, we entered into a licensing agreement for intellectual property that requires payment in Euros, and a small portion of our expenses in the first quarter of 2010, relating to our corporate office, were transacted in British pounds. We currently have no material operations outside of the United States which diminishes the extent of any foreign currency exchange risk.
73
Table of Contents
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
GenMark Diagnostics, Inc
Carlsbad, California
We have audited the accompanying consolidated balance sheets of GenMark Diagnostics, Inc. and subsidiaries (the “Company”) (formerly Osmetech plc and subsidiaries) as of December 31, 2011 and 2010, and the related consolidated statements of operations and comprehensive loss, stockholder’s equity, and cash flows for each of the two years in the period ended December 31, 2011. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2011 and 2010, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2011, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2011, based on the criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 20, 2012, expressed an adverse opinion on the effectiveness of the Company’s internal control over financial reporting because of a material weakness.
/s/ DELOITTE & TOUCHE LLP
San Diego, California
March 20, 2012
74
Table of Contents
To the Board of Directors and Stockholders of Osmetech plc
London, United Kingdom
We have audited the accompanying consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows of Osmetech plc and subsidiaries (the “Company”) for the year ended December 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the results of operations and cash flows of Osmetech plc and subsidiaries for the year ended December 31, 2009, in conformity with accounting principles generally accepted in the United States of America.
/s/ DELOITTE LLP
St. Albans, United Kingdom
March 19, 2010
75
Table of Contents
GenMark Diagnostics, Inc.
Consolidated Balance Sheets
(In thousands, except par value)
| As of December 31, | ||||||||
| 2011 | 2010 | |||||||
| Current assets |
||||||||
| Cash and cash equivalents |
$ | 25,320 | $ | 18,329 | ||||
| Short-term investments |
5,000 | — | ||||||
| Accounts receivable—net of allowance of $98 and $39 |
1,098 | 678 | ||||||
| Inventories—net |
2,168 | 897 | ||||||
| Other current assets |
322 | 2,193 | ||||||
|
|
|
|
|
|||||
| Total current assets |
33,908 | 22,097 | ||||||
| Property and equipment—net |
2,836 | 2,702 | ||||||
| Intangible assets—net |
1,362 | 1,460 | ||||||
| Other long-term assets |
80 | 55 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 38,186 | $ | 26,314 | ||||
|
|
|
|
|
|||||
| Current liabilities |
||||||||
| Accounts payable |
$ | 1,201 | $ | 823 | ||||
| Accrued compensation |
1,521 | 1,172 | ||||||
| Current portion of loan payable |
1,000 | — | ||||||
| Other current liabilities |
2,659 | 1,945 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
6,381 | 3,940 | ||||||
| Long-term liabilities |
||||||||
| Loan payable, net of current portion |
583 | — | ||||||
| Other non-current liabilities |
588 | 1,307 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
7,552 | 5,247 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies—See note 6 |
||||||||
| Stockholders’ equity |
||||||||
| Common stock, $0.0001 par value; 100,000 authorized; 20,478 and 11,728 shares issued and outstanding as of December 31, 2011 and December 31, 2010, respectively |
2 | 1 | ||||||
| Preferred stock, $0.0001 par value; 5,000 authorized, none issued |
— | — | ||||||
| Additional paid-in capital |
199,531 | 166,009 | ||||||
| Accumulated deficit |
(168,463 | ) | (144,493 | ) | ||||
| Accumulated other comprehensive loss |
(436 | ) | (450 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
30,634 | 21,067 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 38,186 | $ | 26,314 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
76
Table of Contents
GenMark Diagnostics, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except per share amounts)
| Year ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Revenue |
||||||||||||
| Product revenue |
$ | 4,700 | $ | 2,341 | $ | 911 | ||||||
| License and other revenue |
309 | 223 | 88 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
5,009 | 2,564 | 999 | |||||||||
| Cost of sales |
6,206 | 3,979 | 4,332 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross loss |
(1,197 | ) | (1,415 | ) | (3,333 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses |
||||||||||||
| Sales and marketing |
4,969 | 4,555 | 3,182 | |||||||||
| General and administrative |
8,960 | 7,415 | 8,289 | |||||||||
| Research and development |
8,737 | 6,646 | 5,634 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
22,666 | 18,616 | 17,105 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(23,863 | ) | (20,031 | ) | (20,438 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other (expense) income |
||||||||||||
| Foreign exchange gain (loss) |
6 | (1 | ) | 304 | ||||||||
| Interest income |
21 | — | 33 | |||||||||
| Interest expense |
(95 | ) | — | — | ||||||||
| Therapeutic discovery credit |
— | 1,644 | — | |||||||||
| Other income |
13 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total other (expense) income |
(55 | ) | 1,643 | 337 | ||||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(23,918 | ) | (18,388 | ) | (20,101 | ) | ||||||
| Income tax (provision) benefit |
(52 | ) | (15 | ) | 138 | |||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (23,970 | ) | $ | (18,403 | ) | $ | (19,963 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share, basic and diluted |
$ | (1.45 | ) | $ | (1.88 | ) | $ | (4.41 | ) | |||
| Weighted average number of shares outstanding |
16,572 | 9,797 | 4,527 | |||||||||
| Consolidated Statements of Comprehensive Loss For the Years ended December 31, 2011, 2010 and 2009 |
||||||||||||
| Net loss |
$ | (23,970 | ) | $ | (18,403 | ) | $ | (19,963 | ) | |||
| Foreign currency translation adjustment |
14 | (35 | ) | (93 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (23,956 | ) | $ | (18,438 | ) | $ | (20,056 | ) | |||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
77
Table of Contents
GenMark Diagnostics, Inc.
Consolidated Statements of Stockholders’ Equity
(In thousands)
| Ordinary Shares | Deferred Stock | Common Stock Shares |
Par value |
Additional paid-in capital |
Accumulated other compreh- ensive loss |
Accumulated deficit |
Total | |||||||||||||||||||||||||||||||||
| Shares | Par value |
Shares | Par value |
|||||||||||||||||||||||||||||||||||||
| Balance—January 1, 2009 |
891,607 | $ | 1,367 | 689,478 | $ | 11,781 | — | $ | — | $ | 103,238 | $ | (322 | ) | $ | (106,127 | ) | $ | 9,937 | |||||||||||||||||||||
| Share-based compensation related to share options |
— | — | — | — | — | — | 1,311 | — | — | 1,311 | ||||||||||||||||||||||||||||||
| Issuance of ordinary shares, net of offering expenses |
741,836 | 1,207 | — | — | — | — | 22,926 | — | — | 24,133 | ||||||||||||||||||||||||||||||
| Foreign currency translation adjustment |
— | — | — | — | — | — | — | (93 | ) | — | (93 | ) | ||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | (19,963 | ) | (19,963 | ) | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance—December 31, 2009 |
1,633,443 | $ | 2,574 | 689,478 | $ | 11,781 | — | $ | — | $ | 127,475 | $ | (415 | ) | $ | (126,090 | ) | $ | 15,325 | |||||||||||||||||||||
| Share-based compensation related to share options |
— | — | — | — | — | — | 1,553 | — | — | 1,553 | ||||||||||||||||||||||||||||||
| Exercise of share options |
4,965 | 7 | — | — | — | — | — | — | — | 7 | ||||||||||||||||||||||||||||||
| Reorganization |
(1,638,408 | ) | (2,581 | ) | (689,478 | ) | (11,781 | ) | 7,128 | 1 | 14,361 | — | — | — | ||||||||||||||||||||||||||
| Issuance of common stock, net of offering expenses |
— | — | — | — | 4,600 | — | 22,620 | — | — | 22,620 | ||||||||||||||||||||||||||||||
| Foreign currency translation adjustment |
— | — | — | — | — | — | — | (35 | ) | — | (35 | ) | ||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | (18,403 | ) | (18,403 | ) | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance—December 31, 2010 |
— | $ | — | — | $ | — | 11,728 | $ | 1 | $ | 166,009 | $ | (450 | ) | $ | (144,493 | ) | $ | 21,067 | |||||||||||||||||||||
| Share-based compensation expense |
— | — | — | — | — | — | 1,872 | — | — | 1,872 | ||||||||||||||||||||||||||||||
| Shares issued under stock-based compensation plans, net of cancellations |
— | — | — | — | 625 | — | (28 | ) | — | — | (28 | ) | ||||||||||||||||||||||||||||
| Issuance of common stock, net of offering expenses |
— | — | — | — | 8,125 | 1 | 31,678 | — | — | 31,679 | ||||||||||||||||||||||||||||||
| Foreign currency translation adjustment |
— | — | — | — | — | — | — | 14 | — | 14 | ||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | (23,970 | ) | (23,970 | ) | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance—December 31, 2011 |
— | $ | — | — | $ | — | 20,478 | $ | 2 | $ | 199,531 | $ | (436 | ) | $ | (168,463 | ) | $ | 30,634 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
78
Table of Contents
GenMark Diagnostics, Inc.
Consolidated Statements of Cash Flows
(In thousands)
| Year ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (23,970 | ) | $ | (18,403 | ) | $ | (19,963 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities |
||||||||||||
| Depreciation and amortization |
1,326 | 1,063 | 1,569 | |||||||||
| Loss from disposal of property and equipment |
— | — | 8 | |||||||||
| Impairment losses |
— | — | 1,506 | |||||||||
| Share-based compensation |
1,872 | 1,553 | 1,311 | |||||||||
| Non-cash inventory adjustments |
517 | — | — | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(420 | ) | (508 | ) | (51 | ) | ||||||
| Inventories |
(1,742 | ) | (651 | ) | 1,227 | |||||||
| Other assets |
1,846 | (1,404 | ) | 316 | ||||||||
| Accounts payable |
378 | (1,058 | ) | (857 | ) | |||||||
| Accrued and other liabilities |
979 | 547 | (510 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(19,214 | ) | (18,861 | ) | (15,444 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities |
||||||||||||
| Proceeds from the sale of property and equipment and intangible assets |
— | — | 10 | |||||||||
| Purchases of investments |
(5,000 | ) | — | — | ||||||||
| Purchases of intellectual property licenses |
(734 | ) | — | — | ||||||||
| Purchases of property and equipment |
(1,376 | ) | (1,860 | ) | (1,069 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(7,110 | ) | (1,860 | ) | (1,059 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from the issuance of ordinary shares and common stock |
34,533 | 27,600 | 24,133 | |||||||||
| Costs incurred in conjunction with public offerings |
(2,854 | ) | (4,991 | ) | — | |||||||
| Proceeds of borrowings |
2,000 | — | — | |||||||||
| Principal repayments of borrowings |
(417 | ) | — | — | ||||||||
| Proceeds from stock option exercises |
— | 5 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
33,262 | 22,614 | 24,133 | |||||||||
|
|
|
|
|
|
|
|||||||
| Effect of foreign exchange rate changes |
53 | (47 | ) | 30 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net increase in cash and cash equivalents |
6,991 | 1,846 | 7,660 | |||||||||
| Cash and cash equivalents—Beginning of year |
18,329 | 16,483 | 8,823 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents—End of year |
$ | 25,320 | 18,329 | $ | 16,483 | |||||||
|
|
|
|
|
|
|
|||||||
| Supplemental cash flow disclosures: |
||||||||||||
| Cash received for income taxes, net |
$ | 3 | $ | 5 | $ | 181 | ||||||
|
|
|
|
|
|
|
|||||||
| Cash received for interest |
$ | 21 | $ | 25 | $ | 33 | ||||||
|
|
|
|
|
|
|
|||||||
| Cash paid for interest |
$ | 95 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Noncash investing and financing activities: |
||||||||||||
| Intellectual property acquisition included in accrued expenses |
$ | — | $ | 1,389 | $ | — | ||||||
| Fixed asset acquisitions included in accounts payable |
$ | 76 | $ | 276 | $ | — | ||||||
| Reclassification of deposits on systems in other current assets and inventory to property and equipment |
$ | — | $ | 289 | $ | 257 | ||||||
| IPO costs incurred but not paid |
$ | — | $ | 103 | $ | — | ||||||
| Transfer of systems from property and equipment into inventory |
$ | 46 | $ | 109 | $ | — | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
79
Table of Contents
GenMark Diagnostics, Inc.
Notes to Consolidated Financial Statements
1. Organization and basis of presentation
GenMark Diagnostics, Inc., or the Company or GenMark, was formed by Osmetech plc, or Osmetech, in Delaware in February 2010, and had no operations prior to its initial public offering, or IPO, which was completed in June 2010. Immediately prior to the closing of the initial public offering, GenMark acquired all of the outstanding ordinary shares of Osmetech in a reorganization under the applicable laws of the United Kingdom. As a result of the reorganization, all of the issued ordinary shares in Osmetech were cancelled in consideration of (i) the issuance of common stock of GenMark to the former shareholders of Osmetech and (ii) the issuance of new shares in Osmetech to GenMark. Following the reorganization, Osmetech became a subsidiary controlled by GenMark, and the former shareholders of Osmetech began to hold shares of GenMark. Any historical discussion of GenMark relates to Osmetech and its consolidated subsidiaries prior to the reorganization.
As the reorganization was deemed to be a transaction under common control, GenMark accounted for the reorganization in a manner similar to a pooling-of-interests, meaning:
| (i) | assets and liabilities were carried over at their respective carrying values; |
| (ii) | common stock was carried over at the nominal value of the shares issued by GenMark; |
| (iii) | additional paid-in capital represented the difference between the nominal value of the shares issued by GenMark, and the total of the additional paid-in capital and nominal value of Osmetech’s shares cancelled pursuant to the reorganization; and |
| (iv) | the accumulated deficit represented the aggregate of the accumulated deficit of Osmetech and GenMark. |
Once the reorganization became effective, all stock options granted under the Osmetech plc 2003 U.S. Equity Compensation Plan, Long Term Incentive Awards and all warrants issued were exchanged for options and warrants exercisable for the common stock of the Company.
In these consolidated financial statements, the Company means Osmetech when referring to periods prior to the IPO.
The accompanying financial statements have been prepared on a going-concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred net losses from operations since its inception and has an accumulated deficit of $168.5 million at December 31, 2011. Cash, cash equivalents and short-term investments at December 31, 2011 were $30.3 million.
Management expects operating losses to continue through the foreseeable future until the Company has expanded its product offering and consequently increased its product revenues to an extent to cover the fixed cost base of the business. The Company’s management has prepared cash flow forecasts which indicate, based on the current cash resources available and the availability of credit facilities, that the Company has sufficient capital to fund its operations for at least the next twelve months.
80
Table of Contents
The accompanying consolidated financial statements have been prepared in accordance with United States generally accepted accounting principles and applicable regulations of the Securities and Exchange Commission (“SEC”). The Company’s operating results for the year ended December 31, 2011 are not necessarily indicative of the results that may be expected for any future periods.
The Company operates in one reportable and operating segment, which is the development and commercialization of molecular tests based on its proprietary eSensor® detection technology. Substantially all of the Company’s operations and assets are in the United States of America.
Principles of Consolidation—The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
Corporate Reorganization
During the quarter ended June 30, 2011, the Company underwent a corporate reorganization, or reorganization intended to simplify its U.S. entity structure. As part of the reorganization, Osmetech Technologies, Inc. merged into Clinical Micro Sensors, Inc., or CMS, with CMS surviving. Additionally, Osmetech plc converted to a U.K. limited company for U.K. legal and tax purposes, and made an entity classification election to be treated as an entity disregarded from GenMark Diagnostics, Inc. for U.S. federal income tax purposes. It is anticipated that the reorganization will not trigger any material U.S. federal or U.K. income tax expense. Additionally, it is anticipated that the post-reorganization structure will allow GenMark Diagnostics, Inc. to elect to file a consolidated U.S. federal income tax return with its remaining U.S. subsidiaries, CMS and Osmetech, Inc.
2. Summary of Significant Accounting Policies
Cash and cash equivalents and short-term investments
Cash and cash equivalents consist of cash on deposit with banks, money market instruments and certificates of deposit with maturities of three months or less at the date of purchase. Short-term investments consist of certificates of deposits that mature in greater than three months, but less than one year from the date of purchase. The carrying amounts reported in the balance sheets for cash, cash equivalents and short-term investments are stated at cost which approximates their fair market value.
Fair Value of Financial Instruments
Assets and liabilities are classified based upon the lowest level of input that is significant to the fair value measurement. The carrying amounts of financial instruments such as cash equivalents, accounts receivable, prepaid and other current assets, accounts payable and other current liabilities approximate the related fair values due to the short-term maturities of these instruments. The Company reviews the fair value hierarchy on a quarterly basis. Changes in the observations or valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy.
81
Table of Contents
The Company’s cash equivalents and short-term investments include money market funds and certificates of deposit. When available, the Company uses quoted market prices to determine fair value and classifies such items as Level 1. If quoted market prices are not available, prices are determined using prices for recently traded financial instruments with similar underlying terms, such as interest rates and yield curves that are observable at commonly quoted intervals. The Company classifies such items as Level 2.
Receivables
Accounts receivable consist of amounts due to the Company for sales to customers and are recorded net of an allowance for doubtful accounts. The allowance for doubtful accounts is determined based upon specific identification of accounts at risk plus a general reserve for unknown items based upon the Company’s historical experience.
Inventories
Inventories are stated at the lower of cost (first-in, first-out) or market and include direct labor, materials, and manufacturing overhead. The Company periodically reviews inventory for evidence of slow-moving or obsolete parts, and writes inventory down to market. This write down is based on management’s reviews of inventories on hand, compared to estimated future usage and sales, shelf-life assumptions, and assumptions about the likelihood of obsolescence. If actual market conditions are less favorable than those projected by the Company, additional inventory write-downs may be required. Inventory impairment charges establish a new cost basis for inventory and charges are not reversed subsequently to income, even if circumstances later suggest that increased carrying amounts are recoverable.
Property and Equipment-net
Property, equipment and leasehold improvements are recorded at cost and depreciated using the straight-line method over the assets’ estimated useful lives, which are:
| Machinery and laboratory equipment | - | 3 – 5 years | ||
| Instruments | - | 3 years | ||
| Office equipment | - | 2 – 4 years | ||
| Leasehold improvements | - | over the shorter of the remaining life of the lease or the useful economic life of the asset |
Property and equipment include diagnostic instruments used for sales demonstrations or placed with customers under several types of arrangements, including performance evaluation period programs (PEPs), and rentals. PEPs are placed with customers for evaluation periods of up to six months. The customer is required to purchase a minimum amount of reagents and at the end of the evaluation period must purchase, rent, or return the instrument. Maintenance and repair costs are expensed as incurred.
82
Table of Contents
Intangible Assets
Intangible assets are comprised of licenses or sublicenses to technology covered by patents owned by third parties, and are amortized on a straight-line basis over the expected useful lives of these assets, generally five to twenty years. Amortization of licenses typically begins upon the Company obtaining the licensed technology and is recorded in cost of sales.
Impairment of Long-Lived Assets
The Company assesses the recoverability of long-lived assets, including intangible assets, by periodically evaluating the carrying value whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. If impairment is indicated, the Company writes down the carrying value of the asset to its estimated fair value. This fair value is primarily determined based on estimated discounted cash flows. There were no impairment charges recognized during the years ended December 31, 2011 and 2010.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the amounts reported in the financial statements and the notes thereto. The Company’s significant estimates included in the preparation of the financial statements are related to accounts receivable, inventories, plant and equipment, intangible assets, certain accrued liabilities related to the Company’s former facilities, warranty liabilities, tax valuation accounts and share-based compensation. Actual results could differ from those estimates.
Revenue Recognition
The Company recognizes revenue from product sales and contract arrangements, net of discounts and sales related taxes. The Company recognizes revenue from product sales when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed or determinable and collectability is reasonably assured.
We offer customers the choice to either purchase a system outright or to receive a system free of charge in exchange for an annual minimum purchase commitment for diagnostic test cartridges. When a system is sold, revenue is generally recognized upon shipment of the unit, however, if the end user already has the instrument being purchased installed at its location, revenue is recognized when the revenue recognition terms other than delivery have been met. When a system is placed free of charge under a “reagent rental” agreement, we retain title to the equipment and the system remains capitalized on the balance sheet under property and equipment. Under our reagent rental agreements, our customers pay an additional system rental fee for each test cartridge purchased. The system rental fee varies based on the monthly volume of test cartridges purchased. The system rental fee and diagnostic test cartridges are recognized as contingent rental payments and are included in Product revenue in our consolidated financial statements.
The Company has not had significant product returns and is not contractually obligated to accept returns unless such returns are related to warranty provisions. The Company does not
83
Table of Contents
accept reagent product returns due to FDA regulations and does not offer volume rebates or provide price protection.
The Company enters into performance evaluation agreements whereby a system is installed on the premises of a pre-qualified customer for the purpose of allowing the customer to evaluate the system’s functionality over an extended trial period. The customer agrees to purchase a starter kit at the time of installation and to purchase a minimum volume of reagents over the life of the trial period.
Revenues related to royalties received from licenses are recognized evenly over the contractual period to which the license relates. Services provided are recognized using the proportional performance method of accounting.
Shipping and handling costs are expensed as incurred and included in sales and marketing expense. In those cases where the Company bills shipping and handling costs to customers, the amounts billed are classified as revenue.
Product Warranties
The Company generally offers a one-year warranty for its systems sold to customers and a sixty day warranty for reagents and provides for the estimated cost of the product warranty at the time the system sale is recognized. Factors that affect the Company’s warranty reserves include the number of units sold, historical and anticipated rates of warranty repairs and the cost per repair. The Company periodically assesses the adequacy of the warranty reserve and adjusts the amount as necessary.
Product warranty reserve activity for the years ended December 31, 2011 and 2010 is as follows (in thousands):
| 2011 | 2010 | |||||||
| Beginning balance |
$ | 25 | $ | — | ||||
| Warranty expenses incurred |
(135 | ) | — | |||||
| Provisions |
202 | 25 | ||||||
|
|
|
|
|
|||||
| Ending balance |
$ | 92 | $ | 25 | ||||
|
|
|
|
|
|||||
Research and Development Costs
Research and development expenses primarily include expenses related to the development of the Company’s XT-8 system assay menu and costs associated with the development of the Company’s NexGen platform. These expenses also included clinical study expenses incurred in the process of preparing for FDA clearance for these systems and test cartridges and quality assurance costs. The expenses primarily consisted of salaries, benefits, share-based compensation costs, outside design and consulting services, laboratory supplies, contract research organizations, clinical study supplies and facility costs.
84
Table of Contents
The Company expenses all research and development costs in the periods in which they are incurred.
Income Taxes
Current income tax expense is the amount of income taxes expected to be payable for the current year. A deferred income tax liability or asset is established for the expected future tax consequences resulting from the differences in financial reporting and tax bases of assets and liabilities. A valuation allowance is provided if it is more likely than not that some or all of the deferred tax assets will not be realized. A full valuation allowance has been recorded against the Company’s net deferred tax assets due to the uncertainty surrounding the Company’s ability to utilize these assets in the future. The Company provides for uncertain tax positions when such tax positions do not meet the recognition thresholds or measurement standards prescribed by the authoritative guidance on income taxes. Amounts for uncertain tax positions are adjusted in periods when new information becomes available or when positions are effectively settled. The Company recognizes accrued interest related to uncertain tax positions as a component of income tax expense.
A tax position that is more likely than not to be realized is measured at the largest amount of tax benefit that is greater than 50% likely of being realized upon settlement with the taxing authority that has full knowledge of all relevant information. Measurement of a tax position that meets the more likely than not threshold considers the amounts and probabilities of the outcomes that could be realized upon settlement using the facts, circumstances and information available at the reporting date.
Share-Based Compensation
The Company recognizes share-based compensation expense related to share options and warrants issued to employees and directors in exchange for services. The compensation expense is based on the fair value of the share-based compensation utilizing various assumptions regarding the underlying attributes of the options and shares. The estimated fair value of options granted, net of forfeitures expected to occur during the vesting period, is amortized as compensation expense on an accelerated basis to reflect the vesting as it occurs. The share-based compensation expense is recorded in costs of sales, sales and marketing, research and development and general and administrative expenses based on the employee’s respective function. The expense is derived from the Black-Scholes Option Pricing Model that uses several judgment based variables to calculate the expense. The inputs include the expected life of the option or warrant the expected volatility and other factors.
| • | Expected Volatility. Expected volatility represents the volatility in the Company’s stock price expected over the expected term of the option and is determined by review of the Company’s and similar Companies’ historical experience. |
| • | Expected Dividend. The Black-Scholes option pricing model calls for a single expected dividend yield as an input. The Company assumed no dividends as it has never paid dividends and has no current plans to do so. |
85
Table of Contents
| • | Risk-Free Interest Rate. The risk-free interest rate used in the Black-Scholes option pricing model is based on published government rates in effect at the time of grant for periods corresponding with the expected term of option. |
Foreign Currency Translation
During 2010, the Company changed its functional currency from the British Pound to the U.S. Dollar. Prior to this change, monetary assets and liabilities of the Company’s entities outside of the U.S. were translated into U.S. dollars based on foreign currency exchange rates in effect at the end of each period, and revenues and expenses were translated at weighted average exchange rates during the periods. Gains or losses resulting from these foreign currency translations of the Company’s assets and liabilities were recorded in accumulated other comprehensive income in the consolidated balance sheets.
Transactions in foreign currencies were recognized using the rate of exchange prevailing at the date of the transaction. Foreign exchange gain (loss), which is included in the accompanying consolidated statements of operations, totaled $6,000, $(1,000) and $304,000 for the years ended December 31, 2011, 2010, and 2009, respectively, and relate primarily to transactions denominated in U.S. dollars which were undertaken by Osmetech and to a contract for intellectual property denominated in euros.
Net Loss per Common Share
Basic net loss per share is computed by dividing loss available to shareholders of our common stock (the numerator) by the weighted average number of shares of our common stock outstanding during the period (the denominator). Shares issued during the period and shares reacquired during the period are weighted for the portion of the period that they were outstanding. Diluted loss per share is calculated in a similar way to basic loss per share except that the denominator is increased to include the number of additional shares that would have been outstanding if the dilutive potential shares had been issued unless the effect would be anti-dilutive. As the Company had a net loss in each of the periods presented, basic and diluted net loss per ordinary share are the same.
The computations of diluted net loss per share for the years ended December 31, 2011, 2010 and 2009 did not include the effects of the following options and warrants to acquire stock which were outstanding as of the end of each year as the inclusion of these securities would have been anti-dilutive (in thousands).
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Share options |
1,599 | 1,108 | 993 | |||||||||
| Warrants |
88 | 88 | 221 | |||||||||
| Restricted stock-unvested, issued and held in escrow |
403 | — | — | |||||||||
| Restricted stock-vested, not issued or outstanding |
— | 204 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| 2,090 | 1,400 | 1,214 | ||||||||||
|
|
|
|
|
|
|
|||||||
86
Table of Contents
Concentration of Risk
The Company had sales to individual customers representing greater than 10% of the total as follows:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Customer A |
— | 12 | % | — | ||||||||
| Customer B |
— | — | 15% | |||||||||
| Customer C |
— | — | 12% | |||||||||
| Customer D |
— | — | 11% | |||||||||
| Customer E |
20 | % | — | — | ||||||||
The Company’s XT-8 system is manufactured by a single source supplier that specializes in contract design and manufacturing of electronic and electromechanical devices for medical use.
Comprehensive Loss
U.S. generally accepted accounting principles require that all components of comprehensive loss, including net loss, be reported in the financial statements in the period in which they are recognized. Comprehensive loss is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources, including accumulated translation adjustments. The Company reports comprehensive loss as a separate component of stockholders’ equity.
Recent Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies that are adopted by the Company as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our financial position or results of operations upon adoption.
In May 2011, the FASB issued ASU No. 2011-04, “Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs.” This pronouncement clarifies the application of certain existing fair value measurement guidance and expands the disclosures for fair value measurements that are estimated using significant unobservable (Level 3) inputs. This pronouncement is effective on a prospective basis for annual and interim reporting periods beginning on or after December 15, 2011. This standard affects required disclosures and is not expected to have a material impact on our consolidated financial statements.
In June 2011, the FASB issued ASU No. 2011-05, “Comprehensive Income (Topic 220): Presentation of Comprehensive Income” and in December 2011, the FASB issued ASU No. 2011-12, Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income in Accounting
87
Table of Contents
Standards Update No. 2011-05. These pronouncements address changes to the financial statement presentation of comprehensive income and the timing of implementation of these changes. The standards do not affect the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income. These standards are required to be applied retrospectively and are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. These standards change required financial statement presentation and therefore are not expected to have a material impact on the Company’s consolidated financial statements.
3. Intangible assets
Intangible assets as of December 31, 2011 and 2010, respectively, comprise the following (in thousands):
| December 31, 2011 | December 31, 2010 | |||||||||||||||||||||||
| Gross carrying amount |
Accumulated amortization |
Net carrying amount |
Gross carrying amount |
Accumulated amortization |
Net carrying amount |
|||||||||||||||||||
| Licensed intellectual property |
$ | 2,474 | $ | (1,112 | ) | $ | 1,362 | $ | 3,956 | $ | (2,496 | ) | $ | 1,460 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Licenses have a weighted average remaining amortization period of 9.6 years as of December 31, 2011. Amortization expense for intangible assets amounted to $98,000, $68,000 and $165,000 for the years ended December 31, 2011, 2010, and 2009, respectively. Additionally, during 2009, licenses that were used for the manufacture of certain of the Company’s consumables were impaired due to the Company outsourcing this manufacturing process. This resulted in an impairment charge of $549,000 charged to cost of sales. In addition, an impairment of $91,000 was recorded as a general and administrative expense. Estimated future amortization expense for these licenses is as follows:
| Years Ending December 31, |
||||
| 2012 |
$ | 81 | ||
| 2013 |
81 | |||
| 2014 |
81 | |||
| 2015 |
81 | |||
| 2016 |
78 | |||
| Thereafter |
960 | |||
|
|
|
|||
| Total |
$ | 1,362 | ||
|
|
|
|||
88
Table of Contents
4. Share-based compensation
The Company recognizes share-based compensation expense related to share options, warrants and restricted stock issued to employees and directors in exchange for services. The compensation expense is based on the fair value of the awards, which are determined by utilizing various assumptions regarding the underlying attributes of the options and shares. The estimated fair value of options granted and restricted stock, net of forfeitures expected to occur during the vesting period, is amortized as compensation expense on a straight line basis over the period the vesting occurs. The share-based compensation expense is recorded in cost of sales, sales and marketing, research and development and general and administrative expenses based on the employee’s respective function. The compensation expense related to the restricted stock is calculated as the difference between the fair market value of the stock on the date of grant, less the cost to acquire the shares, which is $0.0001 per share.
On June 3, 2010, the Company exchanged all of the outstanding options under the Osmetech plc 2003 U.S. Equity Compensation Plan, or the U.S. Plan, for options under the 2010 Equity Incentive Plan, or the Plan. The options were exchanged using an exchange ratio of 230 options to purchase shares of Osmetech plc to one share of the Company and was accounted for as a modification of the share-based payment arrangement. There was no additional compensation cost recorded related to the exchange as there was no change in the economic value of the options exchanged.
Employee participation is at the discretion of the compensation committee or senior management of the Company. All options are exercisable at a price equal to the average closing quoted market price of the Company’s shares on the NASDAQ on the date of grant and generally vest between 1 and 4 years.
Options are generally exercisable for a period up to 10 years after grant and are forfeited if the employee leaves the Company before the options vest. As of December 31, 2011, 123,647 shares remained available for future grant of awards under the Plan. Restricted stock grants reduce the amount of stock options available for grant under the 2010 Plan and are excluded from the table below.
The following table summarizes stock option activity during the year ended December 31, 2011:
| Number of shares |
Weighted average exercise price (translated to dollars) |
|||||||
| Outstanding at December 31, 2010 |
1,107,920 | $ | 6.40 | |||||
| Granted |
851,500 | $ | 4.49 | |||||
| Exercised |
— | $ | — | |||||
| Cancelled |
(360,526 | ) | $ | (6.30 | ) | |||
|
|
|
|
|
|||||
| Outstanding at December 31, 2011 |
1,598,894 | $ | 5.38 | |||||
|
|
|
|
|
|||||
| Exercisable at December 31, 2011 |
531,202 | $ | 6.37 | |||||
|
|
|
|
|
|||||
89
Table of Contents
The weighted average fair value of options granted during the years ended December 31, 2011, 2010 and 2009 was $2.86, $5.29 and $3.68, respectively. The intrinsic value of options exercised during the year ended December 31, 2010 was $136,157. No options were exercised in the years ended December 31, 2011 or 2009, respectively. As of December 31, 2011, there were 1,388,231 options that were vested or expected to vest and these options have a remaining weighted average contractual term of 8.34 years, and an aggregate intrinsic value of $52,634. Options that are exercisable as of December 31, 2011 have a remaining weighted average contractual term of 7.04 years, and an aggregate intrinsic value of $0.
Valuation of Share-Based Awards—The Black-Scholes option pricing model was used for estimating the grant date fair value of stock options granted during the years ended December 31, 2011, 2010 and 2009, respectively, with the following assumptions:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Expected volatility (%) |
70.0 | 70.0 | 66.7 | |||||||||
| Expected life (years) |
6.07 | 5.91 | 0.4 | |||||||||
| Risk free rate (%) |
2.3 | 2.1 | 2.2 | |||||||||
| Expected dividend yield (%) |
0 | 0 | 0 | |||||||||
Share Warrants—During 2009, the Company issued warrants to purchase 132,475 of Osmetech’s ordinary shares with an exercise price of £4.60 per share, and warrants to purchase 88,317 of Osmetech’s ordinary shares with an exercise price of £6.90 per share to a director for services to the Company in connection with the share offering completed in 2009. Pursuant to the terms of the warrant, the warrant to purchase 132,475 was cancelled upon the closing of the IPO. At the same time, the warrant to purchase 88,317 of Osmetech’s ordinary shares was converted to a warrant to purchase 88,317 shares of the Company’s common stock at an exercise price of $9.98. These warrants were fully vested and exercisable upon issue, and shall continue to be exercisable up to and including the earlier to occur of (i) 60 days after the director leaving the Company’s board of directors (for whatever reason) and (ii) June 30, 2012.
Additionally, Osmetech’s deferred shares, which were created at the time of a 10-for-1 consolidation of ordinary shares on September 30, 2005 are excluded from basic and diluted net loss per ordinary share. Management considers these shares to be of minimal value. The deferred shares do not entitle the holder to payment of any dividend or other distribution or to receive notice or attend or vote at any general meeting of Osmetech. The deferred shares are nontransferable. In the event of a return of assets on winding up of Osmetech, the deferred shareholders receive one pence in respect of their shareholding in its entirety.
The Company’s restricted share activity for the year ended December 31, 2011 is as follows:
| Restricted Stock Awards |
Number of shares |
Weighted average Grant Date Fair Value |
||||||
| Non-vested at December 31, 2010 |
200,475 | $ | 4.33 | |||||
| Granted |
449,289 | $ | 4.20 | |||||
| Vested |
(222,602 | ) | ($ | 4.26 | ) | |||
| Cancelled or expired |
(24,100 | ) | ($ | 4.41 | ) | |||
|
|
|
|
|
|||||
| Non-vested at December 31, 2011 |
403,062 | $ | 4.22 | |||||
90
Table of Contents
As of December 31, 2011, there was $1.3 million of unrecognized compensation cost related to RSAs. That cost is expected to be recognized over a weighted average-period of 3.19 years. The total fair value of restricted shares vested during the year ended December 31, 2011 and 2010 was $948,000 and $15,000, respectively.
RSAs may be granted at the discretion of the Board of Directors under the Equity Incentive Plan in connection with the hiring or retention of personnel and are subject to certain conditions. Restrictions expire at certain dates after the grant date in accordance with specific provisions in the applicable agreement. During the year ended December 31, 2011, the Company awarded 449,289 shares of restricted stock awards, which had a fair value at the date of grant ranging from $3.95 to $5.85 per share. During the year ended December 31, 2010, the Company awarded 204,115 shares of restricted stock awards, which had a fair value at the date of grant ranging from $4.03 to $4.46 per share. Compensation under these restricted stock awards is charged to expense over the restriction period and amounted to $911,000 and $247,000 in 2011 and 2010, respectively.
There were no stock compensation costs capitalized into assets as of December 31, 2011.
Share-Based Compensation—Share-based compensation was recognized in the consolidated statements of operations as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Cost of sales |
$ | 41 | $ | 19 | $ | 19 | ||||||
| Sales and marketing |
297 | 261 | 37 | |||||||||
| Research and development |
409 | 162 | 48 | |||||||||
| General and administrative |
1,125 | 1,111 | 1,207 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 1,872 | $ | 1,553 | $ | 1,311 | |||||||
|
|
|
|
|
|
|
|||||||
No share-based compensation was capitalized during the periods presented, and there was no unrecognized tax benefit related to share-based compensation for the years ended December 31, 2011, 2010 and 2009, respectively. At December 31, 2011, the estimated total remaining unamortized compensation expense, net of forfeitures, associated with share-based awards was $2,322,000 which is expected to be recognized over a weighted-average period of 1.41 years.
5. Income Taxes
The components of loss before income taxes for the years ended December 31, 2011, 2010, and 2009, respectively, were as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Domestic (U.S. Entities) |
$ | (23,918 | ) | $ | (18,388 | ) | $ | (18,332 | ) | |||
| Foreign (Non U.S. Entities) |
— | — | (1,769 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | (23,918 | ) | $ | (18,388 | ) | $ | (20,101 | ) | ||||
|
|
|
|
|
|
|
|||||||
91
Table of Contents
The components of the income tax expense (benefit) for continuing operations are as follows for the years ended December 31, 2011, 2010, and 2009, respectively (in thousands):
| 2011 | 2010 | 2009 | ||||||||||
| Current expense (benefit): |
||||||||||||
| U.S. Provision |
$ | — | $ | — | $ | (165 | ) | |||||
| State |
20 | 15 | 3 | |||||||||
| Foreign (Non-U.S. entities) |
32 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Current |
52 | 15 | (162 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Non-current expense |
||||||||||||
| U.S. Provision |
— | — | — | |||||||||
| State |
— | — | 24 | |||||||||
| Foreign (Non-U.S. Entities) |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Non-current expense |
— | — | 24 | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred expense |
||||||||||||
| U.S. Provision |
— | — | — | |||||||||
| State |
— | — | — | |||||||||
| Foreign (Non-U.S. Entities) |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Deferred Expense |
— | — | — | |||||||||
| Total Expense |
$ | 52 | $ | 15 | $ | (138 | ) | |||||
|
|
|
|
|
|
|
|||||||
The components of net deferred income taxes consist of the following for the years ended December 31, 2011 and 2010, respectively (in thousands):
| 2011 | 2010 | |||||||
| Deferred income tax assets (liabilities): |
||||||||
| Compensation accruals |
$ | 1,055 | $ | 676 | ||||
| Accruals and reserves |
634 | 305 | ||||||
| State tax provision |
11 | 11 | ||||||
| Federal benefit of state UTP |
174 | 165 | ||||||
| UNICAP |
1,559 | — | ||||||
| Depreciation and amortization |
797 | 961 | ||||||
| Intercompany interest expense |
— | 1,980 | ||||||
| NOL and credits |
15,352 | 8,997 | ||||||
| Valuation allowance |
(19,582 | ) | (13,095 | ) | ||||
|
|
|
|
|
|||||
| Net deferred income taxes |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
92
Table of Contents
A reconciliation of income tax (expense) / benefit for continuing operations to the amount computed by applying the statutory federal income tax rate (the federal rate has been utilized as the Company’s main operation are taxed at the federal rate) to the loss from continuing operations is summarized for the years ended December 31, 2011, 2010, and 2009, respectively, as follows:
| 2011 | 2010 | 2009 | ||||||||||
| U.S. Federal statutory income tax rate |
34.0 | % | 34.0 | % | 34.0 | % | ||||||
| Permanent Differences |
(0.7 | %) | 0.4 | % | (0.1 | %) | ||||||
| State Taxes |
(0.2 | %) | (0.1 | %) | (0.1 | %) | ||||||
| Effect of non-U.S. Operations |
(0.1 | %) | (0.0 | %) | (0.5 | %) | ||||||
| Effective Rate Change non- U.S. |
(0.0 | %) | (0.0 | %) | (0.7 | %) | ||||||
| Section 382 limitation on NOLs |
(4.3 | %) | — | — | ||||||||
| Other |
(1.8 | %) | — | — | ||||||||
| Valuation allowance |
(27.1 | %) | (34.4 | %) | (31.9 | %) | ||||||
|
|
|
|
|
|
|
|||||||
| Total tax provision |
(0.2 | %) | (0.1 | %) | (0.7 | %) | ||||||
|
|
|
|
|
|
|
|||||||
The Company had federal net operating loss (NOL) carryforwards available of approximately $42.0 million and $24.7 million as of December 31, 2011 and 2010, respectively, after consideration of limitations under Section 382 as further described below. Additionally, the Company had state NOL carryforwards available of $26.4 million and $9.5 million as of December 31, 2011 and 2010, respectively. These may be used to offset future taxable income and will begin to expire in varying amounts through 2031. The Company also has non-U.S. NOL carryforwards of $30.4 million. Because the Company restructured its operations during 2011, the non-U.S. net operating losses and other deferred tax assets have been removed from the Company’s table of deferred income taxes above.
Of the $42.0 million and $26.4 million of federal and state NOL carryforwards at December 31, 2011, $0.2 million represents excess tax benefits related to the vesting of employee restricted stock which will result in an increase in equity if and when such excess benefits are ultimately realized.
The future utilization of the Company’s NOL carryforwards to offset future taxable income may be subject to a substantial annual limitation as a result of changes in ownership by our stockholders that hold 5% or more of the Company’s common stock. An assessment of such ownership changes under Section 382 of the Internal Revenue Code was completed through December 31, 2011. As a result of this assessment, the Company determined that it experienced multiple ownership changes through 2011 which will limit the future utilization of NOL carryforwards. U.S. federal NOLs of approximately $57.5 million will expire due to limitations under Section 382 and accordingly, have not been reflected in the NOL carryforward above. Additionally, future ownership changes may further impact the utilization of existing NOLs.
The Company has established a full valuation allowance for its deferred tax assets due to uncertainties that preclude it from determining that it is more likely than not that the Company will be able to generate sufficient taxable income to realize such assets. Management assesses the available positive and negative evidence to estimate if sufficient future taxable income will be
93
Table of Contents
generated to utilize the existing deferred tax assets. A significant piece of objective negative evidence evaluated was the cumulative loss incurred over the three year period ended December 31, 2011. Such objective evidence limits the ability to consider other subjective evidence such as our projections for future growth. Based on this evaluation, as of December 31, 2011, a valuation allowance of $19.6 million has been recorded in order to measure only the portion of the deferred tax asset that more likely than not will be realized. The amount of the
deferred tax asset considered realizable, however, could be adjusted if estimates of future taxable income during the carryforward period increase or if objective negative evidence in the form of cumulative losses is no longer present and additional weight may be given to subjective evidence such as our projections for growth.
The Company applies the provisions of ASC 740, “Income Taxes” (previously reported as Interpretation No. 48 “Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109), which contains a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates it is more likely than not, that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount, which is more than 50% likely of being realized upon ultimate settlement. Income tax positions must meet a more likely than not recognition threshold at the effective date to be recognized upon the adoption of ASC 740 and in subsequent periods. This interpretation also provides guidance on measurement, derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
Upon adoption of ASC 740 on January 1, 2007, the Company did not have any unrecognized tax benefits. A reconciliation of the beginning and ending amount of unrecognized tax benefits, exclusive of accrued interest, for the years ended December 31, 2011, 2010 and 2009, respectively, is as follows (in thousands):
| 2011 | 2010 | 2009 | ||||||||||
| Balance at January 1 |
$ | 382 | $ | 382 | $ | 382 | ||||||
| Additions based on tax positions related to the current year |
— | — | — | |||||||||
| Additions for tax positions of prior years |
— | — | — | |||||||||
| Reductions for tax positions of prior years |
— | — | — | |||||||||
| Lapse of statute |
— | — | — | |||||||||
| Settlements |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Balance at December 31 |
$ | 382 | $ | 382 | $ | 382 | ||||||
|
|
|
|
|
|
|
|||||||
At December 31, 2011 and December 31, 2010, the Company classified $509,000 and $487,000, respectively, of total unrecognized tax benefits, which includes accrued interest of $127,000 and $105,000 for 2011 and 2010, respectively, as a component of other long-term liabilities. This represents the amount of unrecognized tax benefits that would, if recognized, reduce the Company’s effective income tax rate in any future periods. The Company does not expect its unrecognized tax benefits to change significantly over the next 12 months. The Company recognizes interest related to uncertain tax positions in income tax expense.
94
Table of Contents
The Company is subject to taxation in the United Kingdom, U.S. and various states jurisdictions. As of December 31, 2011 the Company’s tax years after 2008 are subject to examination by the United Kingdom tax authorities. Except for net operating losses generated in prior years carrying forward to the current year, as of December 31, 2011, the Company is no longer subject to U.S. federal, state, local or foreign examinations by tax authorities for years before 2007.
6. Commitments and Contingencies
Legal Proceedings:
From time to time, the Company is party to litigation and other legal proceedings in the ordinary course, and incidental to the conduct, of its business. While the results of any litigation or other legal proceedings are uncertain, the Company does not believe the ultimate resolution of any pending legal matters is likely to have a material adverse effect on its financial position or results of operations.
Leases:
The Company has operating lease agreements for its office, manufacturing, warehousing and laboratory space and for office equipment. Rent and operating expenses charged were $774,000, $959,000 and $1,125,000 for the years ended December 31, 2011, 2010, and 2009, respectively. Pursuant to the Company’s lease agreements, a portion of the monthly rental has been deferred. The balance deferred as at December 31, 2011 and 2010 was $212,000 and $134,000, respectively.
Annual future minimum obligations for operating leases as of December 31, 2011 are as follows (in thousands):
| Years Ending December 31, |
Operating leases |
|||
| 2012 |
$ | 546 | ||
| 2013 |
604 | |||
| 2014 |
599 | |||
| 2015 |
618 | |||
| 2016 |
636 | |||
| Thereafter |
710 | |||
|
|
|
|||
| Total minimum lease payments |
$ | 3,713 | ||
|
|
|
|||
Licensing agreement:
On October 20, 2010, we entered into a licensing agreement for intellectual property. The agreement requires minimum payments of €1.0 million in four equal installments over two years and contains provisions for additional licensing fees of €1.25 million and additional royalties based on related product sales. The license terminates upon election by us as defined or termination of every patent and application of patent right included in the agreement or other material breach as defined in the contract. Remaining payments due under the initial agreement are $648,000 as of December 31, 2011. The remaining payments are due in 2012.
95
Table of Contents
7. Inventory-net
Inventory on hand as of December 31, 2011 and December 31, 2010 was comprised of the following (in thousands):
| 2011 | 2010 | |||||||
| Raw materials |
$ | 1,012 | $ | 397 | ||||
| Work-in-process |
706 | 103 | ||||||
| Finished goods |
450 | 397 | ||||||
|
|
|
|
|
|||||
| $ | 2,168 | $ | 897 | |||||
|
|
|
|
|
|||||
8. Property and Equipment, net
Property and equipment was comprised of the following as of December 31, 2011 and 2010 (in thousands):
| 2011 | 2010 | |||||||
| Property and equipment—at cost: |
||||||||
| Plant and machinery |
$ | 2,539 | $ | 2,452 | ||||
| Instruments |
3,918 | 2,822 | ||||||
| Office equipment |
848 | 1,542 | ||||||
| Leasehold improvements |
583 | 596 | ||||||
|
|
|
|
|
|||||
| Total property and equipment—at cost |
7,888 | 7,412 | ||||||
| Less accumulated depreciation |
(5,052 | ) | (4,710 | ) | ||||
|
|
|
|
|
|||||
| Net property and equipment |
$ | 2,836 | $ | 2,702 | ||||
|
|
|
|
|
|||||
The depreciation expense amounted to $1,228,000, $995,000 and $1,404,000 for the years ended December 31, 2011, 2010 and 2009 respectively.
During the year ended December 31, 2010, $289,000 of deposits on systems were transferred from other current assets to property and equipment, net. During the year ended December 31, 2009, $257,000 of systems were transferred out of finished goods inventory into property and equipment, net. These transfers were as a result of a change in the Company’s strategy from outright sales of systems to placing systems with customers for no initial charge and recovering that cost through the sale of test cartridges pursuant to reagent rental agreements.
During the year ended December 31, 2009, due to the anticipated acceleration of the release of future generations of the Company’s products, in particular the NexGen system, the Company assessed all systems for impairment. For systems placed with customers the carrying amount was written down to fair value based on the projected discounted net cash flows to be generated from the sale of test cartridges. Systems that were not expected to generate any future revenues were impaired to $0. The Company recorded an aggregate impairment charge of $865,000 of which $666,000 was charged to cost of sales in respect of systems placed with customers, $70,000 was charged to research and development expenses in respect of systems being used for research purposes, and $130,000 was charged to sales and marketing expenses in respect of systems being
96
Table of Contents
used for demonstration purposes only. Additionally in 2009, the Company revised the estimated useful life of systems from five to three years.
9. Loan Payable and Line of Credit
In March 2010, we entered into a loan and security agreement with Square 1 Bank, pursuant to which we obtained a credit facility consisting of a revolving line of credit in the amount of up to $2 million and an equipment term loan in the amount of up to $2 million. Based upon certain financial covenants, interest on the revolving line of credit will be either (i) the greater of (a) the bank’s prime rate plus 2.75%, or (b) 6%; or (ii) the greater of (a) the bank’s prime rate plus 3.75%, or (b) 7%. In addition, based upon certain financial covenants, interest on the equipment term loan will be either (i) the greater of (a) the bank’s prime rate plus 3.25%, or (b) 6.50%; or (ii) the greater of (a) the bank’s prime rate plus 4.25%, or (b) 7.50%. The revolving line matured in July 2011 and the term loan matures in July 2013. In March 2011, the loan and security agreement was amended, whereby the line of credit availability was increased to $3 million and the maturity was extended to July 2012. The term loan was modified to allow invoices up to 360 days to qualify to be submitted for credit extension. There were no other changes to these two loans.
In March 2011, an additional loan was made available under the amended loan and security agreement for up to $1 million to finance equipment purchases. Based upon certain financial covenants, interest on this equipment term loan will be either (i) the greater of (a) the bank’s prime rate plus 3.25%, or (b) 6.50%; or (ii) the greater of (a) the bank’s prime rate plus 4.25%, or (b) 7.50%. This term loan matures March 2014.
As of December 31, 2011, the Company had no outstanding loans on the line of credit or the 2011 equipment loan and had a balance of $1.6 million used to finance 2010 equipment purchases and tenant improvements to its Carlsbad facility on the original 2010 equipment term loan. The loan bears an interest rate of 6.5%. Interest-only payments at the rate of 6.5% were due monthly from the date of each initial equipment advance until July 12, 2011. Initial equipment advances that were then outstanding are payable in 24 equal monthly installments of principal, plus all accrued and unpaid interest, beginning on August 12, 2011 and continuing on the same day of each month thereafter through July 12, 2013.
Pursuant to the terms of the loan and security agreement, the Company is required to maintain a ratio of liquidity to bank indebtedness equal to at least 1.50 to 1.00. In addition, the loan and security agreement includes several restrictive covenants, including requirements that the Company obtains the consent of Square 1 Bank prior to entering into any change of control event unless all debt is repaid to Square 1 Bank prior to the change of control event, incurring other indebtedness or liens with respect to our property, making distributions to our stockholders, making certain investments or entering into certain transactions with affiliates and other restrictions on storing inventory and equipment with third parties. The agreement also limits the amount the Company can borrow under the term loan to license genetic biomarkers to $500,000. To secure the credit facility, the Company granted Square 1 Bank a first priority security interest in the Company’s assets and intellectual property rights and provided a $500,000 standby letter of credit. The Company is currently in compliance with all ratios and covenants.
97
Table of Contents
10. Employee Benefit Plan
The Company has a 401(k) tax-deferred savings plan, whereby eligible employees may contribute a percentage of their eligible compensation. Company contributions are discretionary. Including administrative fees, the expense was $3,000, $79,000 and $173,000 for the years ended December 31, 2011, 2010 and 2009, respectively. Additionally, the Company made contributions to other defined contribution plans on behalf of its employees amounting to $58,000 for the year ended December 31, 2009. These other defined contribution plans were terminated in 2010.
11. Other current assets and liabilities, and other non-current liabilities consisted of the following as of December 31, 2011 and 2010 (in thousands):
| 2011 | 2010 | |||||||
| Other current assets |
||||||||
| Therapeutic discovery credit receivable |
$ | — | $ | 1,645 | ||||
| Deposits and prepaid expenses |
322 | 291 | ||||||
| Tax receivable |
— | 257 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 322 | $ | 2,193 | ||||
|
|
|
|
|
|||||
| Other non-current assets |
||||||||
| Deposit |
$ | 80 | $ | 55 | ||||
|
|
|
|
|
|||||
| Total |
$ | 80 | $ | 55 | ||||
|
|
|
|
|
|||||
| Other current liabilities |
||||||||
| Accrued professional fees |
$ | 532 | $ | 350 | ||||
| Rental related liabilities |
167 | 330 | ||||||
| Accrued warranties |
92 | 180 | ||||||
| Accrued royalties |
103 | 51 | ||||||
| Note payment received that must be refunded |
345 | — | ||||||
| Accrued intellectual property licenses |
648 | 695 | ||||||
| Other |
772 | 339 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 2,659 | $ | 1,945 | ||||
|
|
|
|
|
|||||
| Other non-current liabilities |
||||||||
| Liability pertaining to uncertain tax position |
$ | 509 | $ | 487 | ||||
| Tax payable |
— | 11 | ||||||
| Accrued liability for intellectual property license |
— | 694 | ||||||
| Rental related liabilities |
79 | 115 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 588 | $ | 1,307 | ||||
|
|
|
|
|
|||||
In July 2010, the Company applied for certification of qualified investments eligible for credits and grants under the qualifying therapeutic discovery project program for the years ending December 31, 2009 and December 31, 2010. The $1.6 million in grant applications were for $561,000 of expenditures for the year ended December 31, 2009 and $1.1 million of expenditures in for the year ended December 31, 2010.
98
Table of Contents
These development projects included the NexGen System, KRAS mutation cancer treatment, Plavix Sensitivity Drug, Warfarin Sensitivity Test, Thrombophilia Risk Test, Respiratory Viral Panel and Cystic Fibrosis Genotyping. In November 2010, the Company was notified that it had been awarded a total of $1.6 million under the program. As of December 31, 2010, the Company recorded the $1.6 million tax credit as an Other Current Assets on the Consolidated Balance Sheet with a corresponding credit to Other Income on the Consolidated Statement of Operations.
In February 2011, the Company requested payment from the U.S. Department of Treasury, and $1.6 million in cash was received.
12. Fair Value of Financial Instruments
The Company’s financial instruments consist of cash equivalents, short-term investments, accounts receivable, and accounts payable. The carrying amounts of accounts receivable and accounts payable are considered reasonable estimates of their fair value, due to the short maturity of these instruments.
Accounting literature provides a fair value hierarchy, which classifies fair value measurements based on the inputs used in measuring fair value. These inputs include: Level 1 defined as observable inputs such as quoted prices for identical instruments in active markets; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable; and Level 3, defined as unobservable inputs for which little or no market data exists, therefore requiring an entity to develop its own assumptions.
Cash and cash equivalents: The carrying amounts reported in the balance sheets for cash and cash equivalents are stated at their fair market value. Cash and cash equivalents are classified as Level 1.
Certificates of deposit: The carrying amounts reported in the balance sheets for certificates of deposit that are reported as short-term investments are stated at their fair market value. Short-term investments are classified as Level 2.
Non-recurring measurements: The Company measures the fair value of its long-lived assets on a periodic basis when it appears that there may be requirement to do so, such as an indication of impairment. During the year ended December 31, 2009, impairment indicators required that an assessment of the fair value of certain intangible assets and systems. These fair value measurements were done on the basis of unobservable Level 3 inputs, for which little or no market data exists. These assumed cash flows were projected based on management’s best estimates for the remaining net cash flow for each item over its the estimated remaining useful life. Due to the relatively short-term period of future cash flows on these items, the use of a discount rate did not have a material impact on the valuation of these items. Impairments recorded during the period as a result of these fair value measurements were $640,253 for intangible assets (note 3), and $865,389 on the laboratory systems (note 8).
99
Table of Contents
The following table presents the Company’s hierarchy for assets measured at fair value on a recurring basis as of December 31, 2011 and 2010, respectively, (in thousands):
| December 31, 2011 | ||||||||||||||||
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Cash equivalents |
$ | 19,225 | $ | — | $ | — | $ | 19,225 | ||||||||
| Certificates of deposit |
— | 5,000 | — | 5,000 | ||||||||||||
| December 31, 2010 | ||||||||||||||||
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Cash equivalents |
$ | 16,707 | $ | — | $ | — | $ | 16,707 | ||||||||
There were no transfers of items between Levels 1, 2 or 3.
13. Selected Quarterly Financial Data (Unaudited)
| 2011 Quarters | ||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| Total revenue |
$ | 758 | $ | 901 | $ | 1,316 | $ | 2,034 | ||||||||
| Gross profit (loss) |
$ | (743 | ) | $ | (393 | ) | $ | (469 | ) | $ | 408 | |||||
| Loss from operations |
$ | (6,649 | ) | $ | (5,715 | ) | $ | (6,105 | ) | $ | (5,394 | ) | ||||
| Net loss |
$ | (6,642 | ) | $ | (5,580 | ) | $ | (6,313 | ) | $ | (5,435 | ) | ||||
| Per share data: |
||||||||||||||||
| Net loss per common share—basic and diluted |
$ | (0.56 | ) | $ | (0.39 | ) | $ | (0.31 | ) | $ | (0.27 | ) | ||||
| 2010 Quarters | ||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| Total revenue |
$ | 410 | $ | 665 | $ | 684 | $ | 804 | ||||||||
| Gross loss |
$ | (89 | ) | $ | (84 | ) | $ | (439 | ) | $ | (802 | ) | ||||
| Loss from operations |
$ | (4,847 | ) | $ | (5,142 | ) | $ | (4,924 | ) | $ | (5,118 | ) | ||||
| Net loss |
$ | (4,849 | ) | $ | (5,137 | ) | $ | (4,917 | ) | $ | (3,500 | ) | ||||
| Per share data: |
||||||||||||||||
| Net loss per common share—basic and diluted |
$ | (0.68 | ) | $ | (0.60 | ) | $ | (0.42 | ) | $ | (0.30 | ) | ||||
14. Subsequent Events
In January 2012, we entered into a lease amendment for adjoining facility space totaling an additional 22,000 square feet. We intend to utilize the additional space for storage initially, and
100
Table of Contents
build out for additional office and warehouse space in 2013. The lease amendment requires an additional security deposit of $22,000 to $77,000, an increase in our standby letter of credit to $858,000, additional rental payments of $16,000 per month until the earlier of July 1, 2013 or we commence operations in the adjoining space, at which time the rent increases approximately $35,000 per month, with annual increases of 3% to 4%. The lease amendment might require Company to pay landlord a liquidated damage of $258,524 in the event a default by Company that results in the early termination of the lease prior to a certain date. The term of the lease is also extended to 91 months after the earlier of July 1, 2013 or we commence operations in the adjoining space and our proportional share of common area maintenance, property management and taxes are increased under the provisions of the amendment to the lease.
101
Table of Contents
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures designed to provide reasonable assurance that information required to be disclosed in reports filed under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) is recorded, processed, summarized and reported within the specified time periods and accumulated and communicated to management, including its Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2011 as required by paragraph (b) of Rule 13a-15 or Rule 15d-15 of the Exchange Act. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of that date, the Company’s disclosure controls and procedures as defined in Rules 13a-15(e) or 15d-15(e) under the Exchange Act were not effective at the reasonable assurance level because of the identification of a material weakness in its internal control over financial reporting, described below, which the Company views as an integral part of its disclosure controls and procedures.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act).
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States. Because of inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions or that the degree of compliance with policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2011 utilizing the criteria described in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this evaluation and the criteria issued by COSO, our management, with the participation of our Chief Executive Officer and Chief Financial Officer, concluded that, as of December 31, 2011, our internal control over financial reporting was not effective because of the material weakness described below.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis by the Company’s internal controls.
102
Table of Contents
During the preparation of our financial statements, there were current period and prior period errors identified by both the external auditors and management, as well as other control deficiencies. These errors and deficiencies resulted in the need to record adjustments that were immaterial individually and in the aggregate; however, due to the quantity of deficiencies, management determined that there was a reasonable possibility that a material misstatement to the annual or interim financial statements might not have been prevented or detected in a timely manner. Specifically, the level of monitoring of our financial closing and reporting process was insufficient to reduce the likelihood of detecting material adjustments to the Company’s books and records. As a result, management identified a material weakness in the Company’s internal control over financial reporting related to the supervision and review of our financial closing and reporting process as of December 31, 2011.
Management’s assessment of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2011 has been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report which is included herein.
Plan for Remediation of Material Weakness
Management is currently addressing the material weaknesses in internal control over financial reporting and is committed to remediating it as expeditiously as possible. Management intends to devote significant time and resources to the remediation effort. Management plans to take the following steps to improve our internal control over financial reporting and to remediate the identified material weakness:
| • | Evaluate the staffing level and qualifications of finance department personnel, and make changes as deemed appropriate; |
| • | Evaluate the utilization of external resources, to provide greater assurance that these resources are effectively managed, and deployed, and make changes as appropriate; |
| • | Evaluate the need to deploy additional software systems to assist in automating and controlling certain processes within the finance function; and |
| • | Enhance our processes and procedures through expanded use of checklists for key tasks to improve effectiveness and efficiency. |
We believe that the actions described above will strengthen our internal control over financial reporting and will, over time, address the material weakness.
We will continue to assess the effectiveness of our remediation efforts in connection with management’s future evaluations of internal control over financial reporting.
Changes in Internal Control over Financial Reporting
An evaluation was also performed under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, of any change in our internal control over financial reporting that occurred during the fourth quarter of 2011 and that has materially affected or is reasonably likely to materially affect, our internal control over
103
Table of Contents
financial reporting. There was no change in internal control over financial reporting that occurred during the fourth quarter of 2011 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting, except as described below:
| • | During the fourth quarter of 2011, we undertook efforts to remediate the previously reported material weaknesses disclosed in our quarterly report on form 10-Q for the quarter ended June 30, 2011. Specifically, we implemented new controls over contract management and provided additional training to our finance personnel. Management believes that this material weakness was fully remediated as of December 31, 2011. |
The material weakness described above was identified after December 31, 2011, and will result in future remediation activities as discussed above.
104
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
GenMark Diagnostics, Inc.
Carlsbad, California
We have audited GenMark Diagnostics, Inc. and subsidiaries’ (the “Company’s”) (formerly Osmetech plc and subsidiaries) internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on that risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
105
Table of Contents
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weakness has been identified and included in management’s assessment: The Company did not maintain effective controls over the preparation of the financial statements. The level of monitoring of the financial closing and reporting process was insufficient to reduce the likelihood of detecting material adjustments to the Company’s books and records. The errors and deficiencies identified resulted in the need to record adjustments that were immaterial individually and in the aggregate; however, due to the quantity of deficiencies, management determined that there was a reasonable possibility that a material misstatement to the annual or interim financial statements might not have been prevented or detected in a timely manner. As a result, management identified a material weakness in the Company’s internal control over financial reporting related to the supervision and review of the financial closing and reporting process as of December 31, 2011. This material weakness was considered in determining the nature, timing, and extent of audit tests applied in our audit of the consolidated financial statements as of and for the year ended December 31, 2011, of the Company and this report does not affect our report on such financial statements.
In our opinion, because of the effect of the material weakness identified above on the achievement of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of December 31, 2011, based on the criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements as of and for the year ended December 31, 2011, of the Company and our report dated March 20, 2012 expressed an unqualified opinion on those consolidated financial statements.
/s/ DELOITTE & TOUCHE LLP
San Diego, California
March 20, 2012
106
Table of Contents
| ITEM 9B. | OTHER INFORMATION |
None.
107
Table of Contents
Certain information required by Part III is omitted from this report because the Company will file a definitive proxy statement within 120 days after the end of its fiscal year pursuant to Regulation 14A (the “Proxy Statement”) for its annual meeting of stockholders to be held on May 25, 2011, and certain information included in the Proxy Statement is incorporated herein by reference.
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
Code of Ethics
We have adopted a code of ethics for our directors, officers and employees, which is available on our website at www.genmarkdx.com in the Investor Information section under “Corporate Governance.” The information on, or that can be accessed from, our website is not incorporated by reference into this report.
| Item 11. | EXECUTIVE COMPENSATION |
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATERS |
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
| Item 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
108
Table of Contents
| Item 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULE |
| 1. | Financial Statements: See “Index to Consolidated Financial Statements” in Part II, Item 8 of this Annual Report on Form 10-K. |
| 2. | Exhibits: The exhibits listed in the accompanying index to exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K. |
109
Table of Contents
SIGNATURES
Pursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized, on March 20, 2012.
| GENMARK DIAGNOSTICS, INC. | ||
| By: |
/s/ HANY MASSARANY | |
| Name: |
Hany Massarany | |
| Title: |
Chief Executive Officer (principal executive officer) | |
March 20, 2012
| By: |
/s/ PAUL ROSS | |
| Name: |
Paul Ross | |
| Title: |
Chief Financial Officer (principal financial officer) | |
March 20, 2012
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Hany Massarany and Paul Ross, jointly and severally, his attorneys-in -fact, each with the power of substitution, for him or her in any and all capacities, to sign any amendments to this Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in -fact, or his substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
110
Table of Contents
| Signature |
Title |
Date | ||
| /s/ HANY MASSARANY Hany Massarany |
President and Chief Executive Officer (principal executive officer) | March 20, 2012 | ||
| /s/ PAUL ROSS Paul Ross |
Chief Financial Officer (principal financial officer and principal accounting officer) | March 20, 2012 | ||
| /s/ DARYL J. FAULKNER Daryl J. Faulkner |
Director | March 20, 2012 | ||
| /s/ JAMES FOX James Fox |
Director | March 20, 2012 | ||
| /s/ CHRISTOPHER GLEESON Christopher Gleeson |
Director | March 20, 2012 | ||
| /s/ KEVIN C O’BOYLE Kevin C O’Boyle |
Director | March 20, 2012 | ||
111
Table of Contents
INDEX TO EXHIBITS
| Exhibit Number |
Description of Exhibits | |||
| 3.1 | Certificate of Incorporation (Incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on March 19, 2010). | |||
| 3.2 | Bylaws (Incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on March 19, 2010). | |||
| 4.1 | Form of Warrant (Incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on March 19, 2010). | |||
| 10.1 | Lease between The Campus Carlsbad, LLC and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, dated February 8, 2010 (Incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on March 19, 2010). | |||
| 10.2 | First Amendment to Lease between The Campus Carlsbad, LLC and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, dated June 29, 2010. | |||
| 10.3 | License Agreement by and between California Institute of Technology and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, dated February 8, 1995 (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on May 21, 2010). | |||
| 10.4 | Amended and Restated License Agreement by and between President and Fellows of Harvard College and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, dated July 14, 1997 (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on May 21, 2010). | |||
| 10.5 | Exclusive License Agreement by and between Marshfield Clinic and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, dated October 15, 2007 (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on May 25, 2010). | |||
| 10.6 | Non-Exclusive Patent License Agreement by and between the University of Washington and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, dated February 28, 2007 (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on May 21, 2010). | |||
| 10.7 | Amended and Restated Chemically Modified Enzymes Kit Patent License Agreement by and between Roche Molecular Systems, Inc., F. Hoffman-La Roche Ltd., and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, dated February 27, 2008 (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on May 21, 2010). | |||
| 10.8 | Non-Exclusive License Agreement by and between The Johns Hopkins University and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, dated December 29, 2006 (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on May 25, 2010). | |||
112
Table of Contents
| Exhibit Number |
Description of Exhibits | |||
| 10.9 | License Agreement by and between the Regents of the University of Michigan, HSC Research and Development Limited Partnership and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, dated March 15, 2006 (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on May 21, 2010). | |||
| 10.10 | License Agreement by and between HSC Research and Development Limited Partnership and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, dated March 15, 2006 (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on May 25, 2010). | |||
| 10.11 | 2010 Equity Incentive Plan (Incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on March 19, 2010).* | |||
| 10.12 | Form of Stock Option Agreement (Incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on March 19, 2010).* | |||
| 10.13 | Form of Director and Officer Indemnification Agreement (Incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on March 19, 2010).* | |||
| 10.14 | Executive Employment Agreement, dated January 1, 2010, by and between Osmetech Technology Inc. and Jon Faiz Kayyem, Ph.D. (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on March 19, 2010).* | |||
| 10.15 | Employment Offer Letter, dated March 11, 2011, by and between Clinical Micro Sensors, Inc. d.b.a. GenMark Diagnostics, Inc. and Paul Ross (Incorporated by reference herein from
Exhibit 10.1 to our Form 8-K as filed with the SEC on March 17, 2011).* | |||
| 10.16 | Separation Agreement and General Release dated March 24, 2011 by and between Pankaj Singhal and Clinical Micro Sensors, Inc. d.b.a. GenMark Diagnostics, Inc. (Incorporated by reference herein from Exhibit 10.1 to our Form 8-K as filed with the SEC on March 28, 2011). | |||
| 10.17 | Executive Employment Agreement, dated as of April 5, 2011, by and between GenMark Diagnostics, Inc. and Hany Massarany (Incorporated by reference herein from Exhibit 10.34 to our Form 10-Q as filed with the SEC on May 13, 2011).* | |||
| 10.18 | Settlement and Release Agreement and Second Amendment to Lease, dated January 19, 2012, by and between the Campus Carlsbad, LLC and Clinical Micro Sensors, Inc. d.b.a. GenMark Diagnostics, Inc. | |||
113
Table of Contents
| Exhibit Number |
Description of Exhibits | |||
| 10.19 | Executive Employment Agreement, dated October 10, 2011, by and between Clinical Micro Sensors, Inc. d.b.a. GenMark Diagnostics, Inc. and Matthew R. Cohen (Incorporated by reference herein from Exhibit 10.31 to our Form 10-Q as filed with the SEC on November 14, 2011).* | |||
| 10.20 | Loan and Security Agreement, dated March 12, 2010, by and among Square 1 Bank and Osmetech Technology Inc., Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, and GenMark Diagnostics, Inc. (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on March 19, 2010). | |||
| 10.21 | First Amendment to Loan and Security Agreement, dated August 17, 2010, by and among Square 1 Bank and Osmetech Technology, Inc., Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, and GenMark Diagnostics, Inc. | |||
| 10.22 | Second Amendment to Loan and Security Agreement, dated September 29, 2010, by and among Square 1 Bank and Osmetech Technology, Inc., Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, and GenMark Diagnostics, Inc. | |||
| 10.23 | Third Amendment to Loan and Security Agreement, dated March 9, 2011, by and among Square 1 Bank and Osmetech Technology, Inc., Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics, and GenMark Diagnostics, Inc. | |||
| 10.24 | Manufacturing Services Agreement, dated February 1, 2007, by and between Aubrey Group, Inc. and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics (incorporated by
reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on April 20, 2010). | |||
| 10.25 | First Amendment to Manufacturing Services Agreement, dated May 7, 2009, by and between Aubrey Group, Inc. and Clinical Micro Sensors, Inc. dba Osmetech Molecular Diagnostics (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on April 20, 2010). | |||
| 10.26 | Executive Employment Agreement, dated February 6, 2012 by and between Clinical Micro Sensors, Inc. d.b.a. GenMark Diagnostics, Inc. and Jorge Garces.* | |||
| 21.1 | List of Subsidiaries (incorporated by reference to our Registration Statement on Form S-1 (File No. 333-165562) filed with the Commission on May 13, 2010). | |||
| 23.1 | Consent of Deloitte & Touche LLP (US).ü | |||
| 23.2 | Consent of Deloitte LLP (UK).ü | |||
| 24.1 | Power of Attorney.ü | |||
| 31.1 | Certification of principal executive officer pursuant to Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended.ü | |||
| 31.2 | Certification of principal financial officer pursuant to Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended.ü | |||
114
Table of Contents
| Exhibit Number |
Description of Exhibits | |||
| 32.1 | Certification of the principal executive officer pursuant to Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. section 1350.ü | |||
| 32.2 | Certification of the principal financial officer pursuant to Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. section 1350.ü | |||
| 101± | XBRL Instance Document | |||
| 101± | XBRL Taxonomy Extension Schema Document | |||
| 101± | XBRL Taxonomy Calculation Document | |||
| 101± | XBRL Taxonomy Definition Linkbase Document | |||
| 101± | XBRL Taxonomy Label Linkbase Document | |||
| 101± | XBRL Taxonomy Presentation Linkbase Document | |||
| * | Indicates a management contract or compensatory plan or arrangement in which any director or named executive officer participates |
| ü | Included in this filing |
| ± | Pursuant to applicable securities laws and regulations, we are deemed to have complied with the reporting obligation relating to the submission of interactive data files in such exhibits and are not subject to liability under any anti-fraud provisions of the federal securities laws as long as we have made a good faith attempt to comply with the submission requirements and promptly amend the interactive data files after becoming aware that the interactive data files fail to comply with the submission requirements. Users of this data are advised that, pursuant to Rule 406T, these interactive data files are deemed not filed and otherwise are not subject to liability. |
115
