Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Cardiff Oncology, Inc. | a12-2264_28k.htm |
Exhibit 99.1
|
|
Introduction to TrovaGene, Inc. Antonius Schuh, Ph.D. Chief Executive Officer January 2012 |
|
|
Forward-Looking Statements Statements in this presentation about the Company's expectations, applications of its technology, markets, launch of tests and other statements that are not historical facts are "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on management's current beliefs, assumptions, estimates and projections. Actual results may differ materially from those projected in the forward-looking statements for various reasons, including risks associated with product and test development, test transfer to contracting labs, government regulation, market acceptance, limited commercial experience, dependence on key personnel, obtaining financing and other factors discussed in the Company's periodic reports filed with the Securities and Exchange Commission. DISCLAIMER |
|
|
Contents INTRODUCTION OUR TEAM VALUE PROPOSITION COMPETITIVE ADVANTAGES ADDRESSABLE MARKET TECHNOLOGY & IP APPLICATION AREAS COMMERCIALIZATION STRATEGY FINANCIALS |
|
|
Company Overview Molecular diagnostics company Clinical focus in oncology Collaboration opportunities in prenatal diagnostics, infectious disease and transplant medicine IP protects detection of trans-renal nucleic acids in urine (“DNA in urine”) Founded in 1999 $39M invested over a decade Headquartered in San Diego, CA New CEO, Antonius Schuh, joins Oct 2011 New CFO, Steve Zaniboni, joins Dec 2011 INTRODUCTION COPYRIGHT © 2011, TROVAGENE, Inc. |
|
|
Trovagene: Cell-free Nucleic Acids in Urine Urine-based detection of molecular signal originating from a localized or systemic condition Oncology: Detection of minimal residual disease Earlier approaches include Circulating cells Immunicon, Veridex... Circulating cell-free nucleic acids Sequenom, Verinata, Vogelstein... Trovagene introduces the option to perform diagnostic assays on excreted cell free nucleic acids in urine INTRODUCTION OUR VALUE PROPOSITION COPYRIGHT © 2011, TROVAGENE, Inc. |
|
|
2012 Recent News INTRODUCTION COPYRIGHT © 2011, TROVAGENE, Inc. TrovaGene Acquires CLIA - Certified Laboratory - January 6, 2012 TrovaGene entered into a definitive agreement to acquire MultiGEN Diagnostics, Inc.'s ('MultiGEN') clinical laboratory assets in exchange for the issuance of 750,000 shares of common stock of TrovaGene, with an additional earn-out of up to $3.7 million in cash and common stock, subject to the achievement of specific sales and earnings targets. The acquisition, which is subject to customary closing conditions, is expected to close during the first quarter of 2012. MultiGEN, a privately held molecular diagnostic company located in San Diego, CA, is a subsidiary of Canada-based Bio-ID Diagnostics, Inc. The acquired diagnostic laboratory operations are certified by the State of California in compliance with CLIA (Clinical Laboratory Improvement Amendments), and are accredited by CAP (College of American Pathologists). TrovaGene Obtains Exclusive License to Novel Mutations Associated with Prognosis and Chemotherapy Response in Leukemia - January 3, 2012 TrovaGene announced that it has obtained an exclusive worldwide license to mutations of the SF3B1 splicing factor, which have been shown to be associated with disease progression and chemotherapy response in patients suffering from chronic lymphocytic leukemia (CLL). Research results suggest that SF3B1 mutations represent important incremental diagnostic markers beyond TP53 disruptions and NOTCH1 mutations in CLL patients, and may also provide a therapeutic target for SF3B1 inhibitors, which are currently in pre-clinical development. |
|
|
Experienced Core Management Team OUR TEAM Thomas Adams, PhD Chairman of the Board Corporate VP and CTO, IRIS International, Inc.(2006 to Present) Founder and CEO, Leucadia Technologies (1998-2006) Co-founder and CEO of Genta, Inc. (1989-1997) Co-Founder, Chairman, CEO, Gen-Probe, Inc.(1984-1989) Member of founding team, Sr. VP of R&D, and CTO, Hybritech, Inc. (1980-1984) Antonius Schuh, PhD Chief Executive Officer Co-Founder, CEO, Chairman, Sorrento Therapeutics, Inc. (SRNE.OB) (2008 - 2011) Co-Founder, CEO, Director, AviaraDx, Inc. (acquired by bioMerieux) (2006 - 2008) CEO, Director, Arcturus Biosciences, Inc. (acquired by MDC) (2005 - 2006) CEO, Director, Sequenom, Inc. (NASDAQ: SQNM) (2000 - 2005) Director, Transgenomic, Inc. (TBIO.OB) Director, Diogenix, Inc. (private) Stephen Zaniboni, MBA, CPA Co-Founder, Director, Sorrento Therapeutics, Inc. (SRNE.OB) (2008 – 2011) CFO, Awarepoint, Inc. (2010 – 2011) CFO, Xifin, Inc. (2009 – 2010) CFO, AviaraDx, Inc. (2006 – 2008) CFO, Sequenom Inc. (NADAQ: SQNM) 1997 - 2005 Various financial positions incl, Behring Diagnostics, Aspect Medical, Boston Scientific Practicing CPA with Arthur Andersen |
|
|
Board & Scientific Advisory Board OUR TEAM Dr. Riccardo Dalla-Favera Director, Institute for Cancer Genetics and Herbert Irving Comprehensive Cancer Center, Columbia University, New York Dr. Brunangelo Falini Director – Institute Hematology, University of Perugia, Italy Dr. Sinuhe Hahn Head of Research – Prenatal Medicine, University of Basel, Switzerland Thomas Adams, PhD Chairman of the Board Gary S. Jacob, PhD President & CEO of Synergy Pharmaceuticals, Inc. Gabriele M. Cerrone, MBA Chairman of Callisto Pharmaceuticals, Inc. John P. Brancaccio, CPA CFO of Accelerated Technologies, Inc. Stanley Tennant, MD Cardiologist, Greensboro, NC |
|
|
Clear Advantages Over Competing Technologies Ease of sample collection No physician required Self-sampling possible Virtually no sample volume and sampling frequency limitations Ideal for monitoring applications Enrichment of target for better chance to detect rare events Ideal to detect disease onset early Ideal for monitoring of tumor mutations Sample stability Ideal for central testing VALUE PROPOSITION EASIER...EARLIER...MORE EFFECTIVE |
|
|
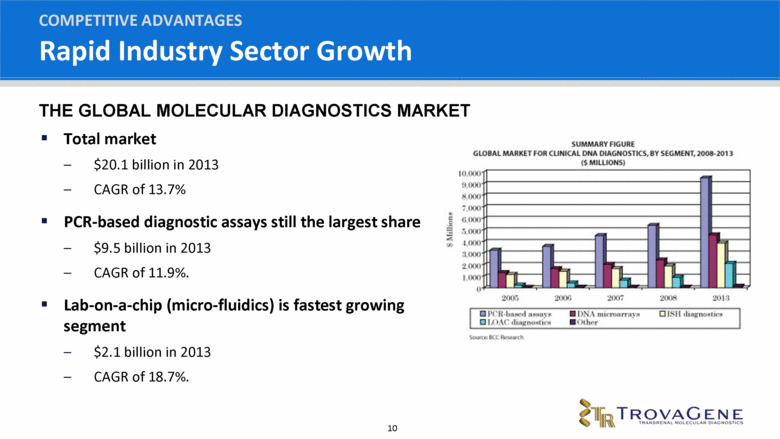
Rapid Industry Sector Growth Total market $20.1 billion in 2013 CAGR of 13.7% PCR-based diagnostic assays still the largest share $9.5 billion in 2013 CAGR of 11.9%. Lab-on-a-chip (micro-fluidics) is fastest growing segment $2.1 billion in 2013 CAGR of 18.7%. COMPETITIVE ADVANTAGES THE GLOBAL MOLECULAR DIAGNOSTICS MARKET |
|
|
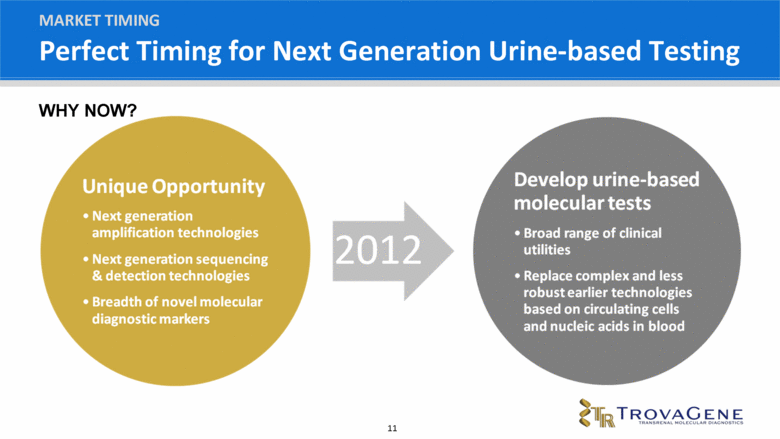
Perfect Timing for Next Generation Urine-based Testing MARKET TIMING WHY NOW? |
|
|
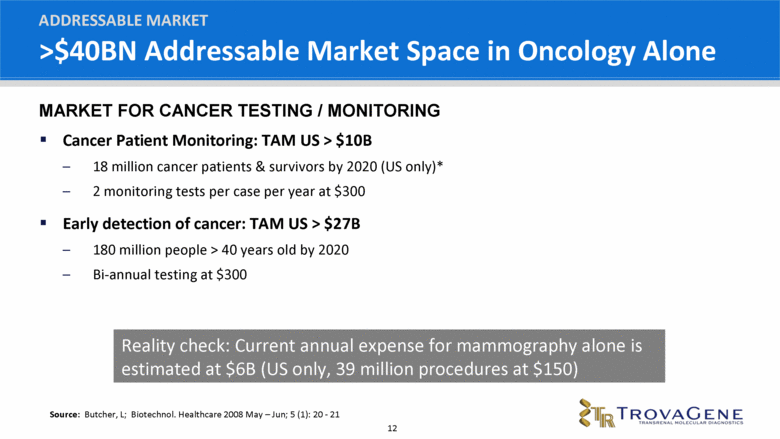
>$40BN Addressable Market Space in Oncology Alone Cancer Patient Monitoring: TAM US > $10B 18 million cancer patients & survivors by 2020 (US only)* 2 monitoring tests per case per year at $300 Early detection of cancer: TAM US > $27B 180 million people > 40 years old by 2020 Bi-annual testing at $300 ADDRESSABLE MARKET Source: Butcher, L; Biotechnol. Healthcare 2008 May – Jun; 5 (1): 20 - 21 Reality check: Current annual expense for mammography alone is estimated at $6B (US only, 39 million procedures at $150) MARKET FOR CANCER TESTING / MONITORING |
|
|
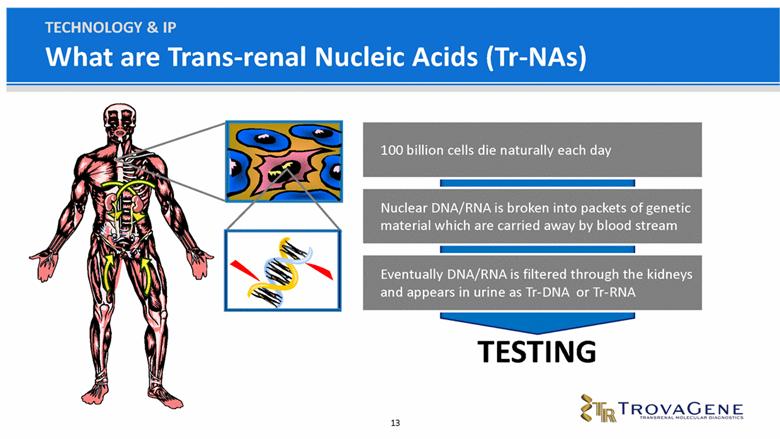
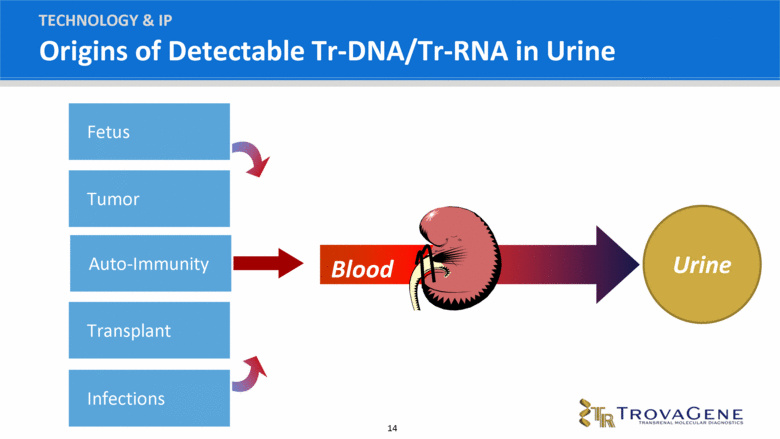
What are Trans-renal Nucleic Acids (Tr-NAs) TECHNOLOGY & IP 100 billion cells die naturally each day Nuclear DNA/RNA is broken into packets of genetic material which are carried away by blood stream Eventually DNA/RNA is filtered through the kidneys and appears in urine as Tr-DNA or Tr-RNA TESTING |
|
|
Origins of Detectable Tr-DNA/Tr-RNA in Urine TECHNOLOGY & IP Urine Blood Fetus Transplant Tumor Infections Auto-Immunity |
|
|
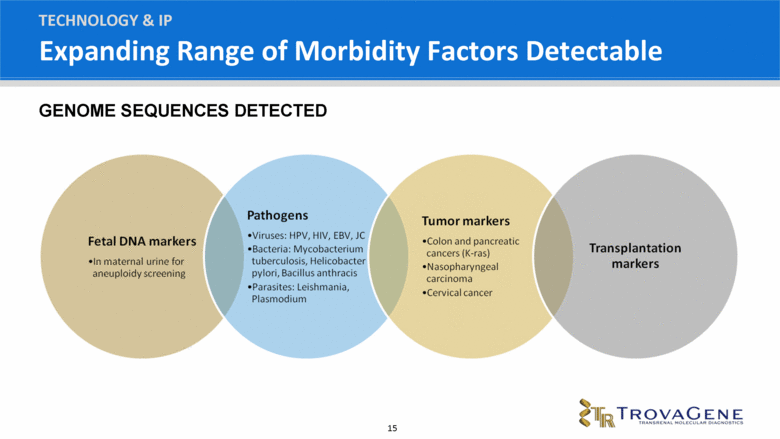
TECHNOLOGY & IP GENOME SEQUENCES DETECTED Expanding Range of Morbidity Factors Detectable |
|
|
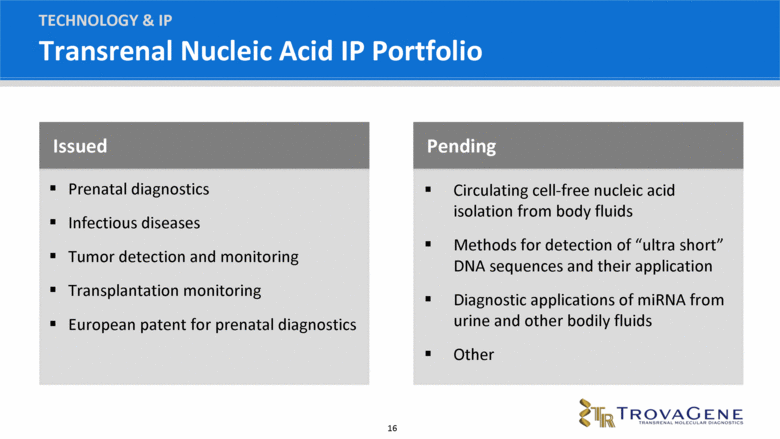
Transrenal Nucleic Acid IP Portfolio Issued Prenatal diagnostics Infectious diseases Tumor detection and monitoring Transplantation monitoring European patent for prenatal diagnostics Pending Circulating cell-free nucleic acid isolation from body fluids Methods for detection of “ultra short” DNA sequences and their application Diagnostic applications of miRNA from urine and other bodily fluids Other TECHNOLOGY & IP |
|
|
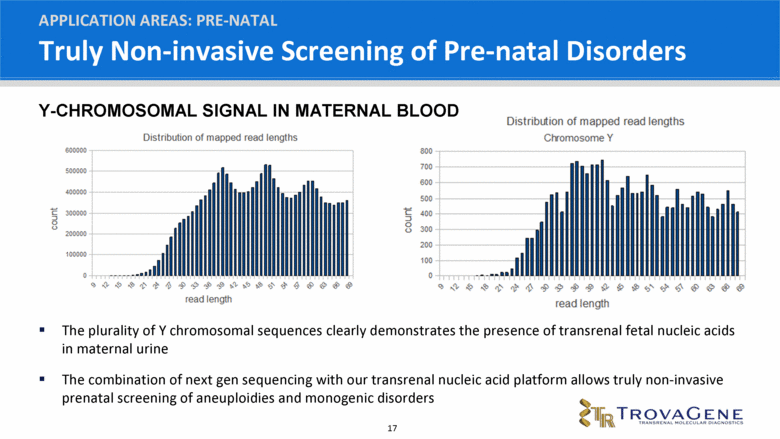
Truly Non-invasive Screening of Pre-natal Disorders APPLICATION AREAS: PRE-NATAL The plurality of Y chromosomal sequences clearly demonstrates the presence of transrenal fetal nucleic acids in maternal urine The combination of next gen sequencing with our transrenal nucleic acid platform allows truly non-invasive prenatal screening of aneuploidies and monogenic disorders Y-CHROMOSOMAL SIGNAL IN MATERNAL BLOOD |
|
|
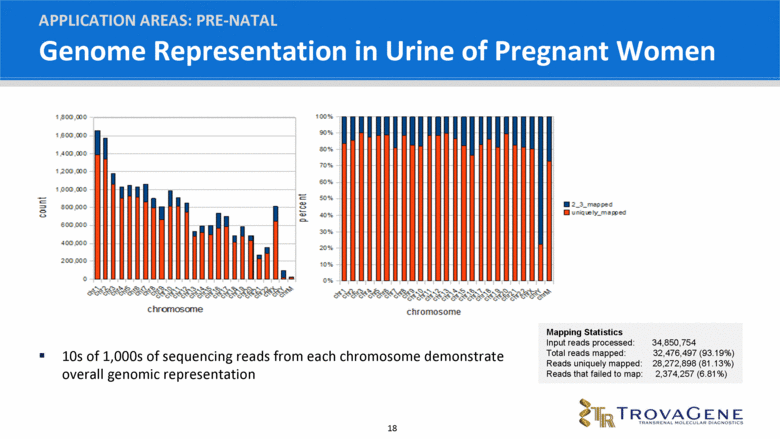
Genome Representation in Urine of Pregnant Women APPLICATION AREAS: PRE-NATAL Mapping Statistics Input reads processed: 34,850,754 Total reads mapped: 32,476,497 (93.19%) Reads uniquely mapped: 28,272,898 (81.13%) Reads that failed to map: 2,374,257 (6.81%) 10s of 1,000s of sequencing reads from each chromosome demonstrate overall genomic representation |
|
|
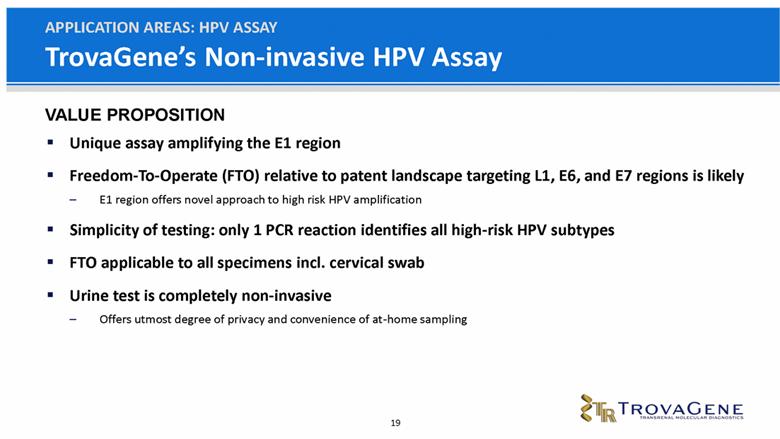
TrovaGene’s Non-invasive HPV Assay Unique assay amplifying the E1 region Freedom-To-Operate (FTO) relative to patent landscape targeting L1, E6, and E7 regions is likely E1 region offers novel approach to high risk HPV amplification Simplicity of testing: only 1 PCR reaction identifies all high-risk HPV subtypes FTO applicable to all specimens incl. cervical swab Urine test is completely non-invasive Offers utmost degree of privacy and convenience of at-home sampling APPLICATION AREAS: HPV ASSAY VALUE PROPOSITION |
|
|
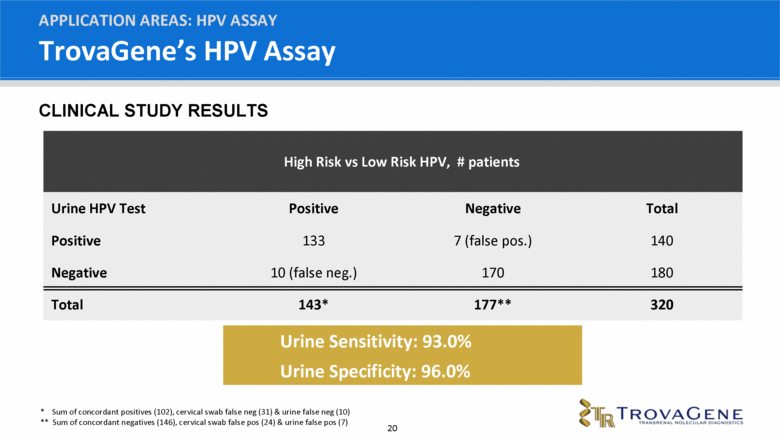
TrovaGene’s HPV Assay APPLICATION AREAS: HPV ASSAY High Risk vs Low Risk HPV, # patients Urine HPV Test Positive Negative Total Positive 133 7 (false pos.) 140 Negative 10 (false neg.) 170 180 Total 143* 177** 320 Urine Sensitivity: 93.0% Urine Specificity: 96.0% * Sum of concordant positives (102), cervical swab false neg (31) & urine false neg (10) ** Sum of concordant negatives (146), cervical swab false pos (24) & urine false pos (7) CLINICAL STUDY RESULTS |
|
|
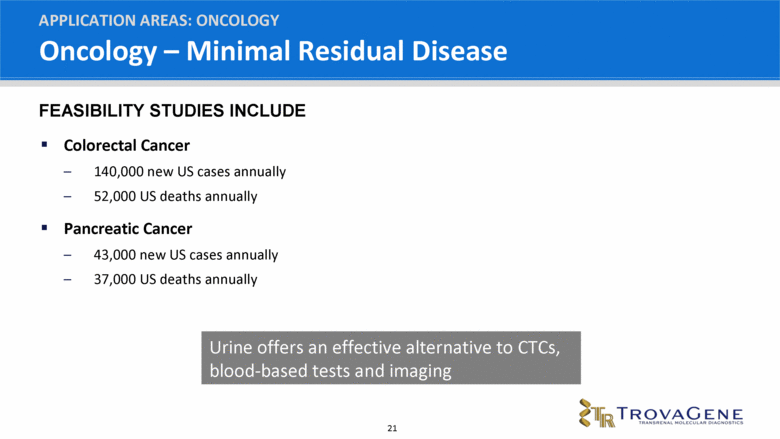
Oncology – Minimal Residual Disease Colorectal Cancer 140,000 new US cases annually 52,000 US deaths annually Pancreatic Cancer 43,000 new US cases annually 37,000 US deaths annually APPLICATION AREAS: ONCOLOGY Urine offers an effective alternative to CTCs, blood-based tests and imaging FEASIBILITY STUDIES INCLUDE |
|
|
Detection of K-ras Mutations in Urine Mutations occur in 35% - 50% of Colorectal Cancers >90% of pancreatic cancers K-ras mutation detection to Determine completeness of surgery Monitor therapy response Detect onset of disease recurrence Feasibility of urine test demonstrated Comparable blood-based test requires min. 50ml sample* * Source: Diehl et al., Analysis of Mutations in DNA Isolated from Plasma and Stool of Colorectal Cancer Patients. Gastroenterology, 2008, 135:489 APPLICATION AREAS: ONCOLOGY |
|
|
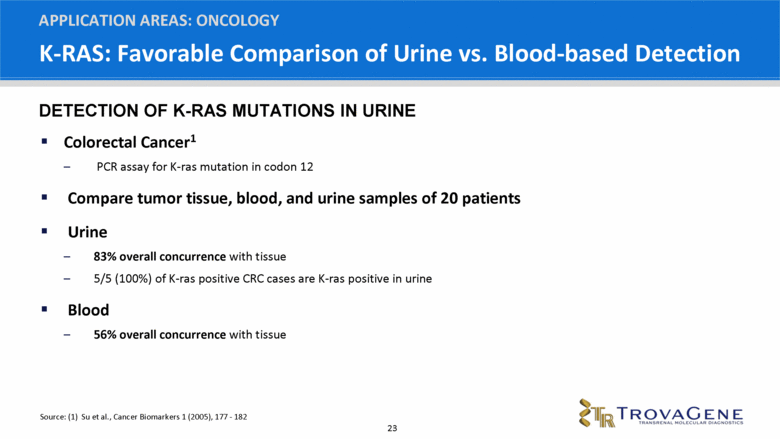
K-RAS: Favorable Comparison of Urine vs. Blood-based Detection Colorectal Cancer1 PCR assay for K-ras mutation in codon 12 Compare tumor tissue, blood, and urine samples of 20 patients Urine 83% overall concurrence with tissue 5/5 (100%) of K-ras positive CRC cases are K-ras positive in urine Blood 56% overall concurrence with tissue APPLICATION AREAS: ONCOLOGY Source: (1) Su et al., Cancer Biomarkers 1 (2005), 177 - 182 DETECTION OF K-RAS MUTATIONS IN URINE |
|
|
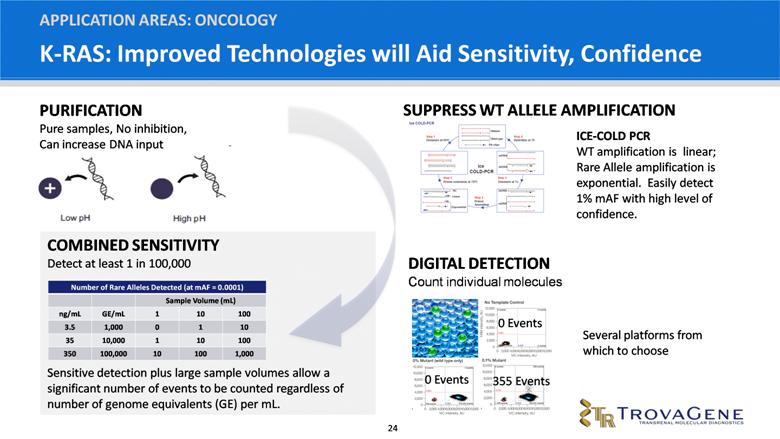
K-RAS: Improved Technologies will Aid Sensitivity, Confidence APPLICATION AREAS: ONCOLOGY COMBINED SENSITIVITY Detect at least 1 in 100,000 Sensitive detection plus large sample volumes allow a significant number of events to be counted regardless of number of genome equivalents (GE) per mL. ICE-COLD PCR WT amplification is linear; Rare Allele amplification is exponential. Easily detect 1% mAF with high level of confidence. 0 Events 0 Events 355 Events PURIFICATION Pure samples, No inhibition, Can increase DNA input DIGITAL DETECTION Count individual molecules Several platforms from which to choose SUPPRESS WT ALLELE AMPLIFICATION Number of Rare Alleles Detected (at mAF = 0.0001) Sample Volume (mL) ng/mL GE/mL 1 10 100 3.5 1,000 0 1 10 35 10,000 1 10 100 350 100,000 10 100 1,000 |
|
|
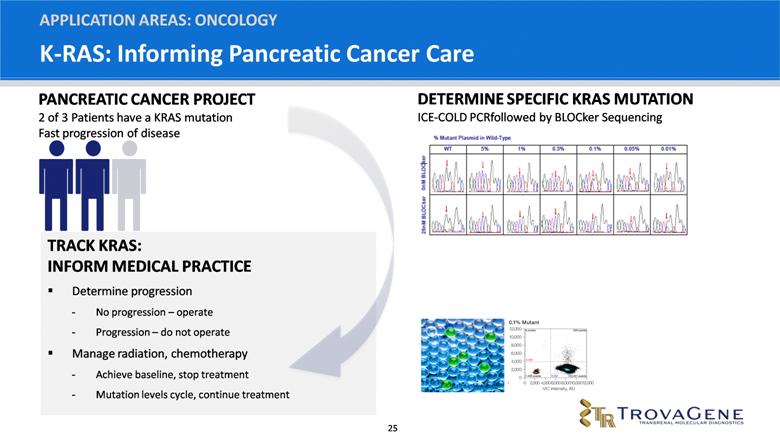
K-RAS: Informing Pancreatic Cancer Care APPLICATION AREAS: ONCOLOGY TRACK KRAS: INFORM MEDICAL PRACTICE Determine progression No progression – operate Progression – do not operate Manage radiation, chemotherapy Achieve baseline, stop treatment Mutation levels cycle, continue treatment PANCREATIC CANCER PROJECT 2 of 3 Patients have a KRAS mutation Fast progression of disease DETERMINE SPECIFIC KRAS MUTATION ICE-COLD PCR followed by BLOCker Sequencing |
|
|
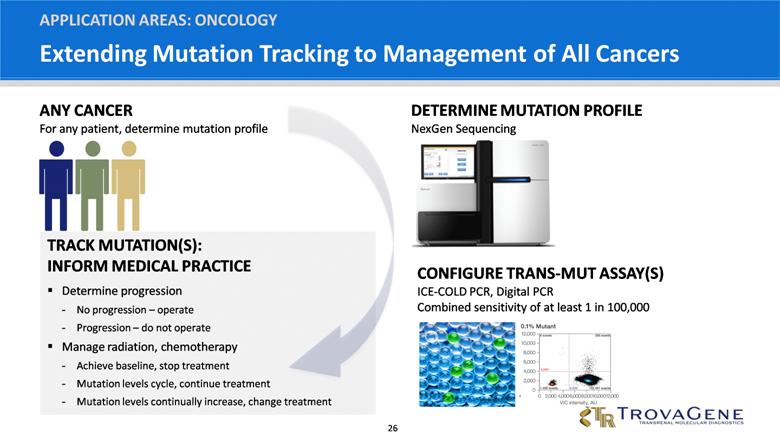
Extending Mutation Tracking to Management of All Cancers APPLICATION AREAS: ONCOLOGY TRACK MUTATION(S): INFORM MEDICAL PRACTICE Determine progression No progression – operate Progression – do not operate Manage radiation, chemotherapy Achieve baseline, stop treatment Mutation levels cycle, continue treatment Mutation levels continually increase, change treatment CONFIGURE TRANS-MUT ASSAY(S) ICE-COLD PCR, Digital PCR Combined sensitivity of at least 1 in 100,000 ANY CANCER For any patient, determine mutation profile DETERMINE MUTATION PROFILE NexGen Sequencing |
|
|
Commercialization Through CLIA lab and Out-licensing COMMERCIALIZATION STRATEGY |
|
|
For More Information Antonius Schuh, PhD Chief Executive Officer Mobile: +1 (858) 205-4193 Office: +1 (858) 496-7462 E-mail: aschuh@trovagene.com TrovaGene Inc. 11055 Flintkote Ave, Suite B San Diego, CA 92121 www.trovagene.com PLEASE CONTACT |
|
|
Thank you We will be pleased to answer your questions CONFIDENTIAL |