Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - GALECTIN THERAPEUTICS INC | d279000d8k.htm |
 Company Overview
January 2012
OTC: GALT
Exhibit 99.1 |
 Forward Looking Statements
This presentation contains, in addition to historical information,
forward-looking statements within the meaning of the Private Securities
Litigation Reform Act of 1995. These statements relate to future events or
future financial performance, and use words such as “may,”
“estimate,”
“could,”
“expect”
and others. They are based on our current expectations
and are subject to factors and uncertainties which could cause actual results to
differ materially from those described in the statements. Factors that could
cause our actual performance to differ materially from those discussed in
the forward-looking statements include, among others: incurrence of
operating losses since our inception, uncertainty as to adequate financing
of our operations, extensive and costly regulatory oversight that could
restrict or prevent product commercialization, inability to achieve commercial
product acceptance, inability to protect our intellectual property,
dependence on strategic partnerships, product competition, and others stated
in risk factors contained in our SEC filings. We cannot assure that we have
identified all risks or that others may emerge which we do not anticipate.
You should not place undue reliance on forward-looking statements.
Although subsequent events may cause our views to change, we disclaim any
obligation to update forward-looking statements.
©
2012 Galectin Therapeutics
OTC:GALT
2 |
 Galectin Therapeutics Highlights
•
Leader in galectin science
•
Pipeline of carbohydrate-based drug compounds that inhibit
galectins
•
Focus on proven galectin activity in fibrosis and cancer
•
Liver Fibrosis
•
Target validated in convincing pre-clinical data
•
Two indications: NASH and Post-transplantation fibrosis
•
Clinical trials expected to begin in end of 2012
•
Cancer Therapy
•
Galectin inhibitor added to chemotherapy
•
Enhancement of immune function activates patient’s own immune
system to kill tumor cells
•
Clinical trial to begin January 2012
3
©
2012 Galectin Therapeutics
OTC:GALT |
 ©
2012 Galectin Therapeutics
OTC:GALT
4
Galectin Proteins Are Normal Cell Proteins
That Promote Interactions Between
Glycoproteins |
 Galectin Proteins Are Important In
Disease Pathogenesis
©
2012 Galectin Therapeutics
OTC:GALT
5
Secreted
Galectin
Proteins
PROMOTE
PATHOLOGY
Markedly Increased in:
1.
Fibrosis
2.
Cancer
3.
Inflammation
1.
Bind to cell
surface and matrix
glycoproteins
2.
Modulate cell
signaling
3.
Promote cell-cell
interactions
4.
Promote cell-
matrix interactions
(galactose residues) |
 Galectin
Proteins
Galectin
Inhibitor
Our Galectin Inhibitors Are Novel
Carbohydrate-Based Drug Compounds
©
2012 Galectin Therapeutics
OTC:GALT
6
•
Target secreted galectins and those
associated with cell membrane
•
Strong binding to multiple galectin proteins
and multiple galectins per drug molecule
•
High molecular weight allows long exposure to
galectin containing compartment
•
Low toxicity potential as a carbohydrate
with no toxic metabolites
•
Low manufacturing costs
•
Strong patent protection with no licensing
encumbrance
•
Two major classes of compounds under
development: GM-CT and GR-MD |
 Galectins Are Involved In The
Pathogenesis Of Many Diseases
•
Fibrosis of organs
•
Nearly all cancers
•
Heart failure
•
Ischemic cardiovascular and cerebrovascular disease
•
Arthritis
•
Allergic disease
•
Eczema and skin inflammation
•
Inflammatory bowel disease
•
Eye inflammation
•
Inflammatory and autoimmune disorders
•
Response to infections
•
Kidney disease
©
2012 Galectin Therapeutics
OTC:GALT
7
Galectins implicated in: |
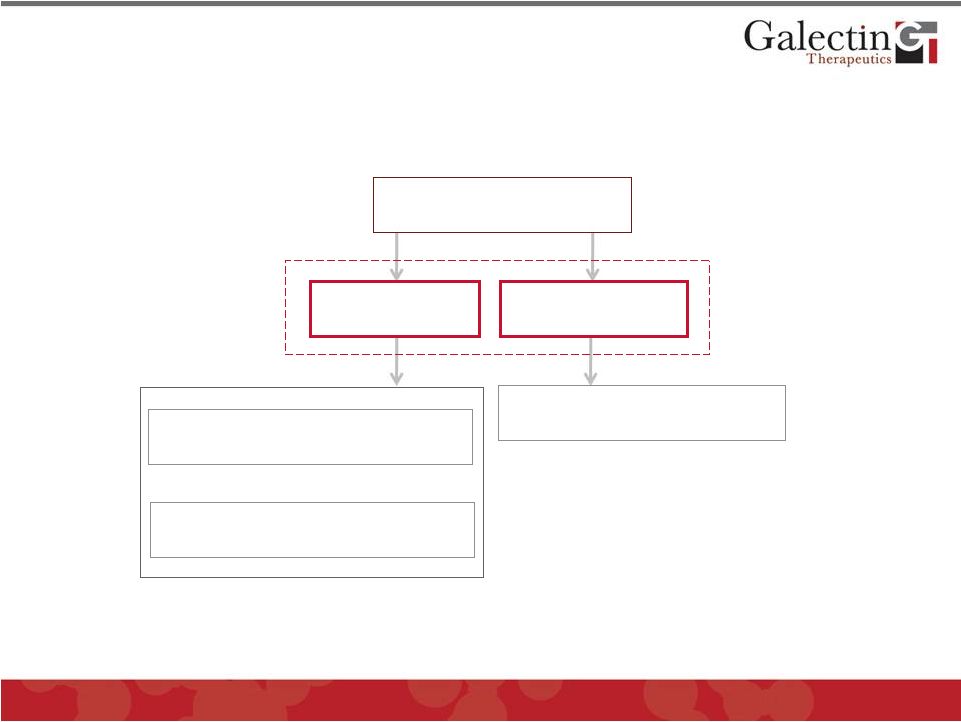 Disease Area Development Programs
Cancer
Fibrosis
Liver Fibrosis
Immunotherapy
Chemotherapy
GALECTINS
©
2012 Galectin Therapeutics
OTC:GALT
8 |
 Disease Area Development Programs
Cancer
Fibrosis
Liver Fibrosis
Immunotherapy
Chemotherapy
GALECTINS
©
2012 Galectin Therapeutics
OTC:GALT
9 |
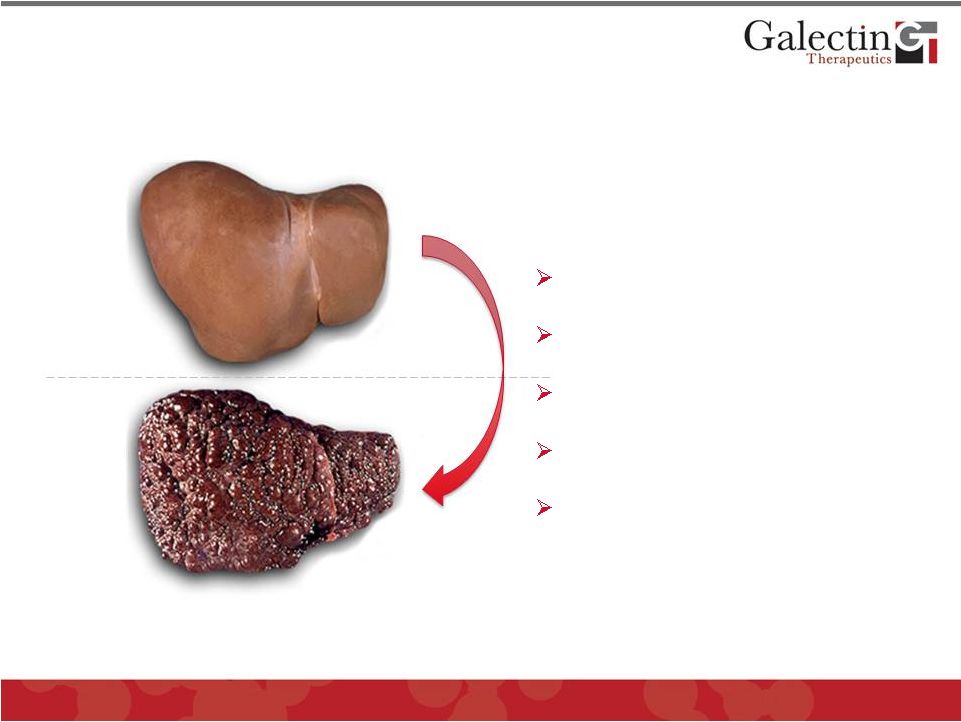 Healthy
Cirrhosis
©
2012 Galectin Therapeutics
OTC:GALT
10
Hepatitis C (57%)
Alcoholic liver disease (24%)
Non-alcoholic fatty liver (9.1%)
Hepatitis B (4.4%)
Miscellaneous (5.5%)
Source: Burden of liver disease in the United States: Summary of a
workshop. American Association for the Study of Liver Disease, May
2001 Multiple Diseases Lead To Liver Fibrosis And
Cirrhosis With Serious Medical Consequences
|
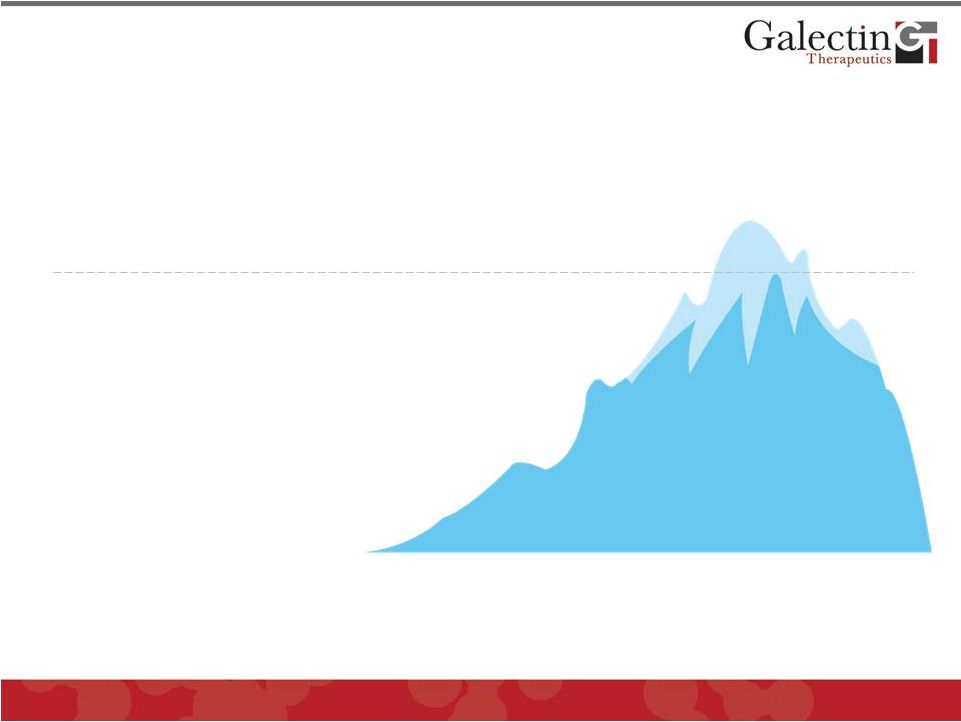 Liver
Cirrhosis Is A Major Problem In The United States
Transplants
(6,291*)
Wait List
(17,000**)
Death
(44,677
#
)
Cirrhosis
(400,000
##
)
Millions of people with liver disease that may progress to cirrhosis
* Performed in US in 2010 (UNOS)
* * Prevalence in US 2010 (UNOS)
The ONLY current therapy is liver transplantation
©
2012 Galectin Therapeutics
OTC:GALT
11
#
Deaths in 1998 (AASLD Workshop, 2001)
##
Prevalence in US 1976-1980 (NIDDK) |
 Galectin-3 Is A Critical Target For
Therapy of Liver Fibrosis
1.
Galectin-3 is produced in large amounts by human fibrotic liver.
2.
Galectin-3 is essential in mice for the development of liver fibrosis.
Fibrosis due to toxin exposure or fatty liver does not occur in mice
that lack the galectin-3 gene.
3.
Galectin inhibitors block production of fibrogenic markers in the key
human cell (stellate cells) responsible for liver fibrosis.
4.
Galectin inhibitors reverse experimental fibrosis in rats induced by
both fibrosis and fatty liver.
©
2012 Galectin Therapeutics
OTC:GALT
12
Key Evidence:
Key Evidence: |
 Galectin Inhibitors Effectively Treat
Toxin-Induced Liver Fibrosis in Rats
Liver Fibrosis, induced by injection
of chemical toxin for 8 weeks
Regression of Fibrosis after 4 weeks
of treatment with GR-MD-02
©
2012 Galectin Therapeutics
OTC:GALT
13
Study performed under contract by Dr. Ji-yao Wang of Fudan University,
Shanghai, China |
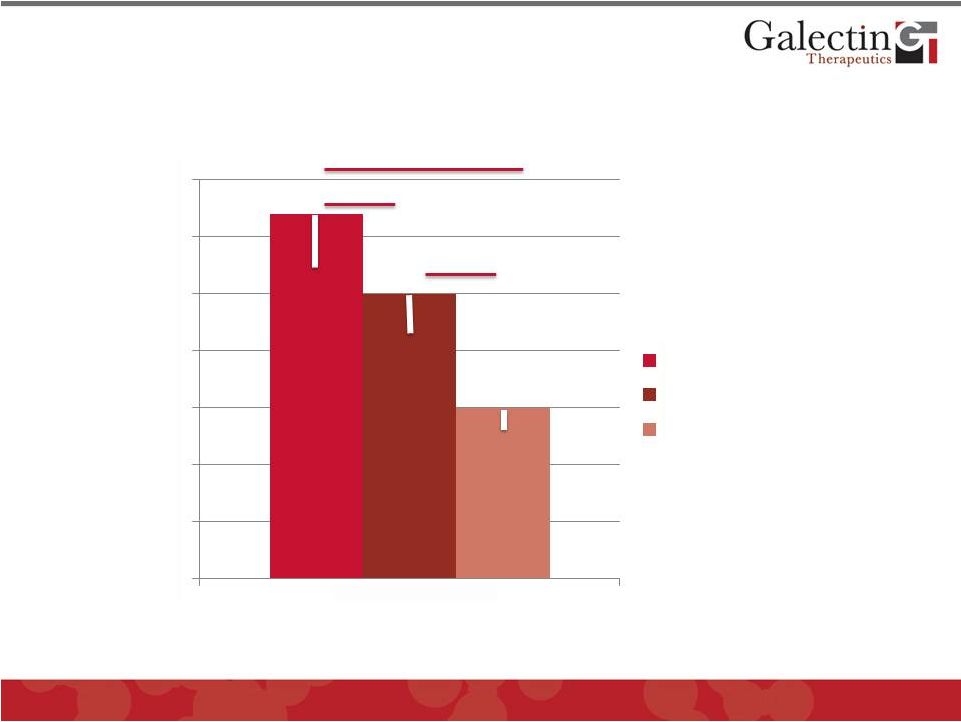 GR-MD-02 is Most Effective in Reducing
Collagen Content in Fibrotic Rats
©
2012 Galectin Therapeutics
OTC:GALT
14
0
1
2
3
4
5
6
7
% Collagen
Vehicle
GR-MD-01
GR-MD-02
N=8
N=8
N=8
One way ANOVA
Bondferroni
P<0.001
P<0.05
P<0.05
P<0.05 |
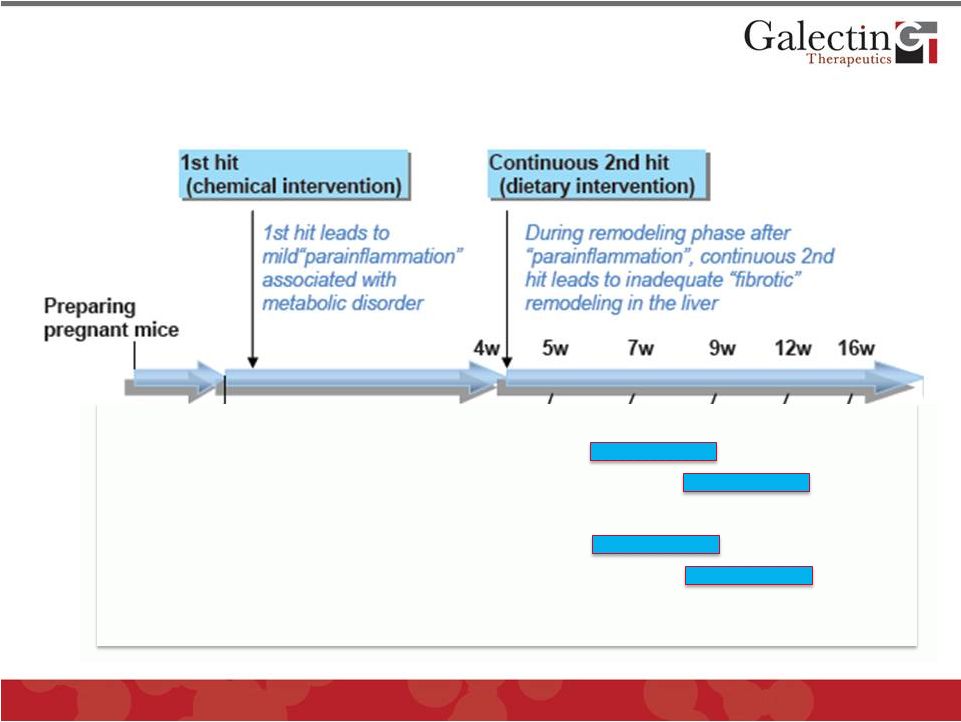 Mouse
NASH Model (Non-Alcoholic Steatohepatitis, “Fatty Liver
Disease”) ©
2012 Galectin Therapeutics
OTC:GALT
15
GM-CT-01
(120 mg/kg 2X/week)
GR-MD-02
(60 mg/kg 2X/week)
Early Rx
Late Rx
Early Rx
Late Rx |
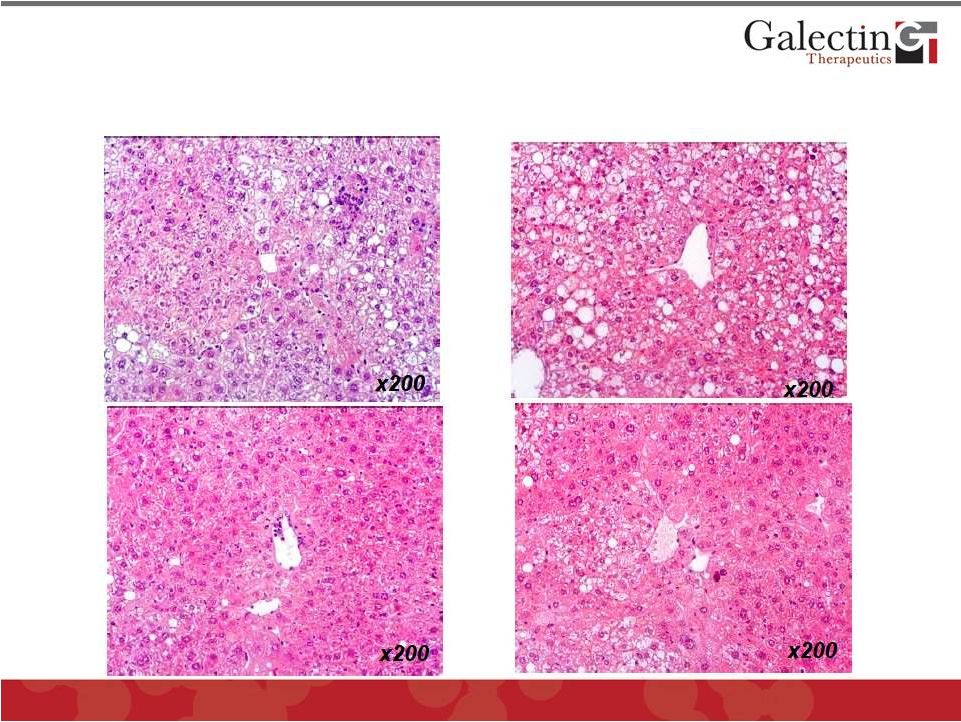 ©
2012 Galectin Therapeutics
OTC:GALT
16
GR-MD-02 Markedly Improves NASH in a Mouse Model When it is
Administered During Development of Disease and After
Establishment of Disease
Early Rx
Vehicle
GR-MD-02
Vehicle
GR-MD-02
Late Rx |
 In a
Mouse NASH Model GR-MD-02 Prevents and Completely Reverses
Fibrosis ©
2012 Galectin Therapeutics
OTC:GALT
17
normal
mouse 0.33
Early Rx
Late Rx |
 Development Program & Markets
•
Pursue two indications for human proof of concept
•
NASH
•
Post-Transplant Hepatitis C Fibrosis
•
NASH
•
NIH estimates 5% of US population has NASH
•
Projected to become the number one reason for liver transplant
•
Multiple candidates in development but none have multiple sites of action,
effect on fibrosis, or safety profile
•
Post-Transplant Hepatitis C Fibrosis
•
Much smaller population but high unmet medical need
•
Orphan disease status possible
•
Expansion to other indications is possible
•
Other liver diseases such as hepatitis and alcoholic disease
•
Pulmonary, renal, and heart fibrosis
©
2012 Galectin Therapeutics
OTC:GALT
18 |
 ©
2012 Galectin Therapeutics
OTC:GALT
19
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
2012
2013
2014
Phase II Post Tx Fibrosis Trial
Phase I DE
NASH
GR-MD-02
Design Phase II Trial
NASH
NASH
Fibrosis Development Program
Preclinical Efficacy Studies
GR-MD-02
First patient
enrolled
Pre-Clinical (Target SQ)
Oral Formulation
Development
Lung fibrosis: Bleomycin
and radiation
Kidney fibrosis: Ureteral
obstruction; diabetic
nephropathy
Heart fibrosis: Heart failure
and arrhythmia
First patient
enrolled
Top line
results
Top line results
Pursue
Partnering
Discussions
GR-MD-02
Post Transplant Fibrosis |
 Summary Of Development Program In
Liver Fibrosis
•
Liver fibrosis represents a very large unmet medical need
•
Galectin-3 protein is proven target
•
Drugs reverse liver fibrosis in animals and show efficacy
in human cell culture models of fibrosis
•
Non toxic drugs with little likelihood of drug interactions
•
Rapid clinical development pathways
•
Evaluation of two clinical indications
©
2012 Galectin Therapeutics
OTC:GALT
20 |
 Disease Area Development Programs
Cancer
Fibrosis
Liver
Fibrosis
Immunotherapy
GALECTINS
Chemotherapy
©
2012 Galectin Therapeutics
OTC:GALT
21 |
 Roles
Of Secreted Galectins In Cancer The vast majority of cancers secrete large
amounts of galectins •
Tumor cell invasion:
extracellular matrix
adhesion & detachment
•
Stromal cell function
•
Metastasis:
cell invasion and
migration
•
Angiogenesis
•
Tumor immunity
©
2012 Galectin Therapeutics
OTC:GALT
22 |
 Clinical Trial Performed In Metastatic
Colorectal Cancer (DAVFU-003)
Cancer Trial
(DAVFU-003)
Day 0
Day 28
GM-CT-01
Chemo (5-FU)
•
Phase 2 trial of 5-FU plus GM-CT-01 in line 3/4 therapy of metastatic
colorectal cancer
showed
6.7
months
median
survival.
In
similar
patients,
Erbitux
®
had
a
6.1 month survival compared to 4.6 months with no therapy
•
Notably, serious adverse events were markedly lower in our studies with
5- FU/GM-CT-01 than in comparison to other studies using
5-FU ©
2012 Galectin Therapeutics
OTC:GALT
23 |
 Development Approach In
Colorectal Cancer
•
Studies demonstrate potential utility of galectin inhibitors in
combination with chemotherapy in cancer
•
FDA has confirmed that preclinical and clinical data are
adequate to proceed with large clinical trials
•
We are deferring new clinical trials pending data from the tumor
immunology clinical trial that may improve the design of future
studies
•
More rapid international registration is an approach that may
provide revenue to support development programs and gain
additional clinical experience with GM-CT-01
©
2012 Galectin Therapeutics
OTC:GALT
24 |
 Tumor-Specific
Cytotoxic T-Cell
Lymphocytes
Blocking Galectins Enhances Tumor
Killing By Immune System
©
2012 Galectin Therapeutics
OTC:GALT
25
Tumor Cells
Cytotoxicity
There are tumor specific cytotoxic T-cells in
patients with cancer that are capable of killing
tumor cells
Experiments performed by Dr. Pierre van der Bruggen of the Ludwig Institute in
Brussels, Belgium as a collaboration with Galectin Therapeutics
|
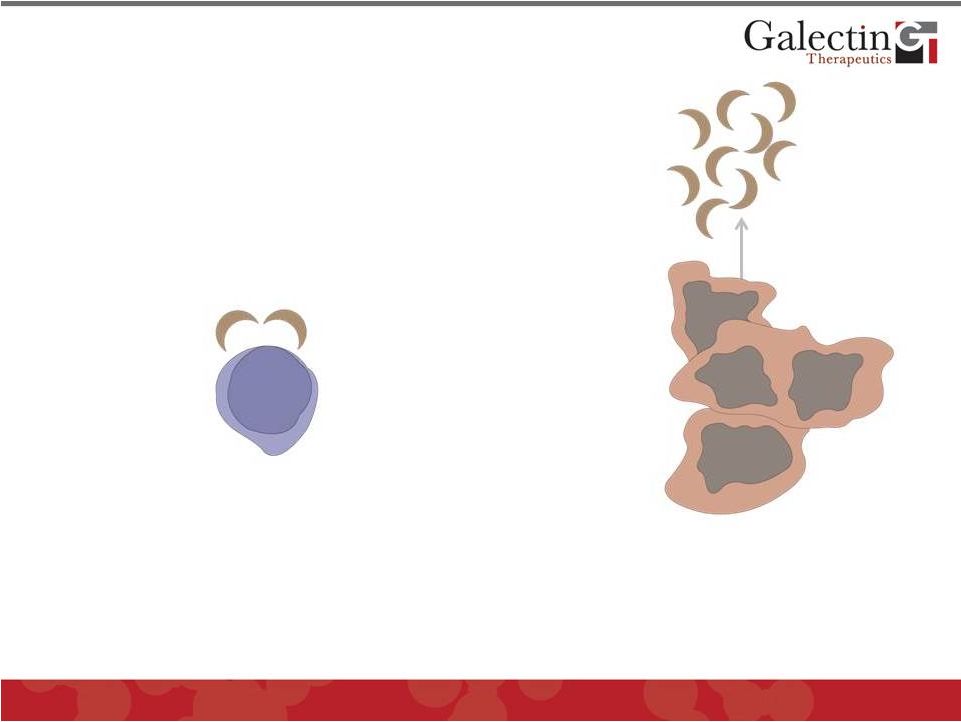 Tumor-Specific
Cytotoxic
T-Cell
Lymphocytes
Blocking Galectins Enhances Tumor
Killing By Immune System
©
2012 Galectin Therapeutics
OTC:GALT
26
Tumor
Cells
However, these T-cells lose the ability to kill
tumor cells. A key reason is that Galectin-3
secreted by tumors bind to T-cells and turn off
their ability to kill tumor cells
Experiments performed by Dr. Pierre van der Bruggen of the Ludwig Institute in
Brussels, Belgium as a collaboration with Galectin Therapeutics
|
 Tumor-Specific
Cytotoxic T-Cell
Lymphocytes
Blocking Galectins Enhances Tumor
Killing By Immune System
©
2012 Galectin Therapeutics
OTC:GALT
27
Tumor Cells
Cytotoxicity
Addition
of
our
drugs,
which
inhibit galectins,
Experiments performed by Dr. Pierre van der Bruggen of the Ludwig Institute in
Brussels, Belgium as a collaboration with Galectin Therapeutics
restore
the
ability
of
T-cells
to
kill tumor
cells |
 GM-CT-01 Restores Ability of Immune
Cells to Kill Tumor Cells
©
2012 Galectin Therapeutics
OTC:GALT
28 |
 Melanoma Clinical Trial Design (I)
©
2012 Galectin Therapeutics
OTC:GALT
29
Phase I/II study of peptide vaccination associated with GM-CT-01,
a galactomannan oligomer that inhibits galectin-3, in patients with
advanced metastatic melanoma
IMPD approved by EMA |
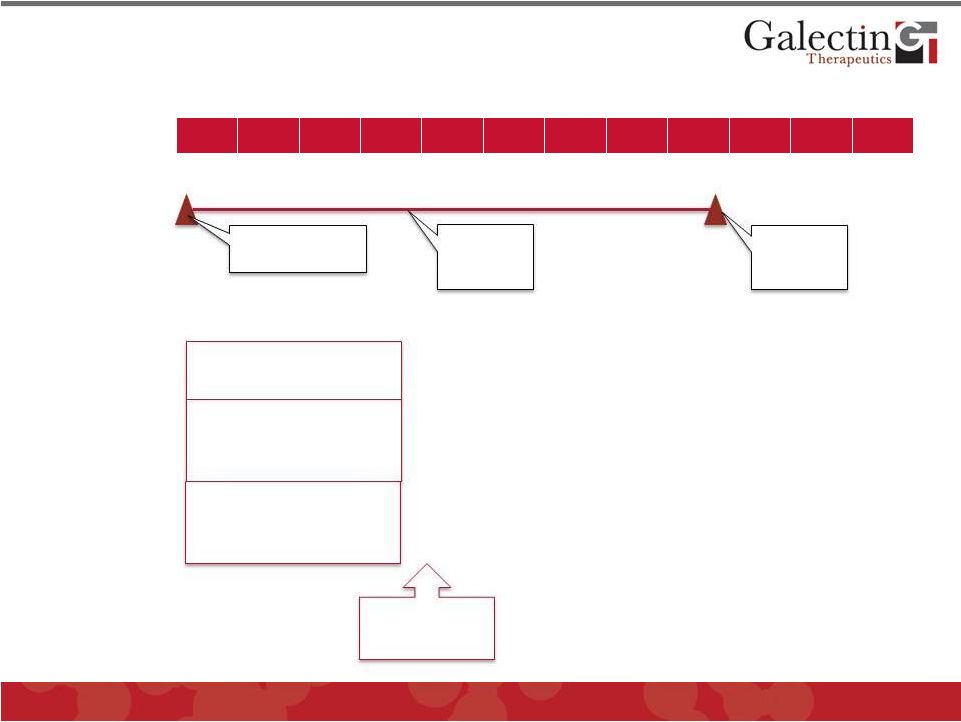 Tumor
Immune Enhancement Development Program ©
2012 Galectin Therapeutics
OTC:GALT
30
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
2012
2013
2014
Preclinical Efficacy Studies
GM-CT-01 & GR-MD-02
GM-CT-01
Phase I/II Melanoma Trial
Top line
results in
Stage 1
First patient
enrolled
Top line
results in
Stage 2
Human CD8 T-Cell/Tumor
assay
Efficacy in immune
competent mice with
syngeneic tumors
Efficacy in combination
with a tumor vaccine
(partnership)
Pursue
Partnering
Discussions |
 Development Program In Cancer
Immunotherapy
•
Galectin proteins secreted by tumor cells are directly
responsible for inhibiting the ability of immune cells to kill
tumors
•
GM-CT-01 restores the ability of immune cells to kill tumor cells
•
Initial clinical trial for treatment of metastatic malignant
melanoma
•
Market for tumor vaccines is expected to grow to $7B by 2015
•
Potential important therapy for many cancers
©
2012 Galectin Therapeutics
OTC:GALT
31 |
 Pipeline
©
2012 Galectin Therapeutics
OTC:GALT
32
Pre-Clinical
Registration
Submitted
Chemotherapy
Colorectal Cancer:
GM-CT-01
•
International (Colombia)
•
United States
Immune Enhancer
Melanoma: GM-CT-01
Liver Fibrosis
Post Tx: GR-MD-02
NASH: GR-MD-02
Phase 1
Phase
3
Phase 2 |
 Galectin Therapeutics Highlights
•
Leader in galectin science
•
Pipeline of carbohydrate-based drug compounds that inhibit
galectins
•
Focus on proven galectin activity in fibrosis and cancer
•
Liver Fibrosis
•
Target validated in convincing pre-clinical data
•
Two indications: NASH and Post-transplantation fibrosis
•
Clinical trials expected to begin in end of 2012
•
Cancer Therapy
•
Galectin inhibitor added to chemotherapy
•
Enhancement of immune function activates patient’s own immune
system to kill tumor cells
•
Clinical trial to begin January 2012
33
©
2012 Galectin Therapeutics
OTC:GALT |
